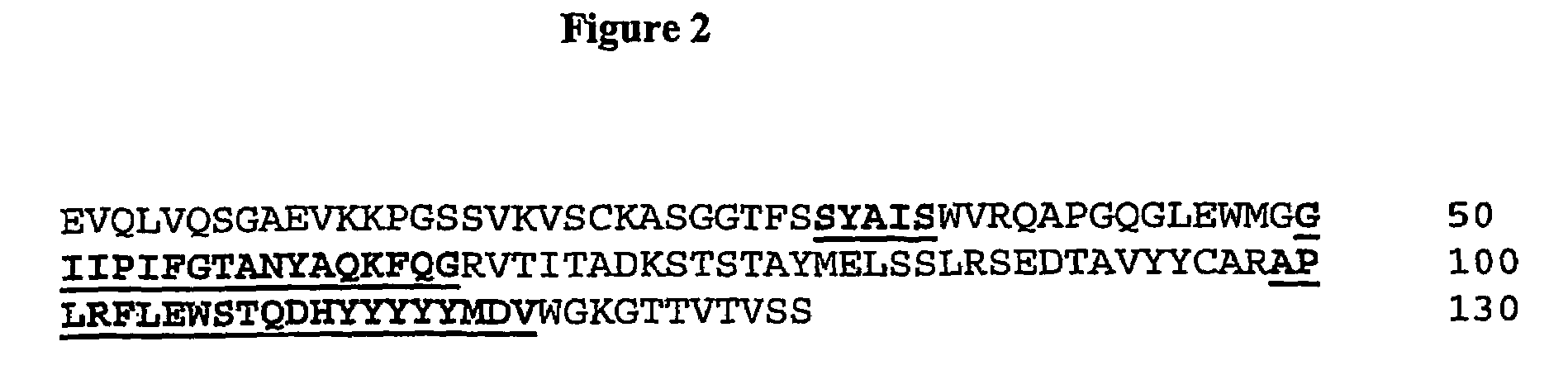Patents
Literature
387 results about "Insulin-like growth factor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
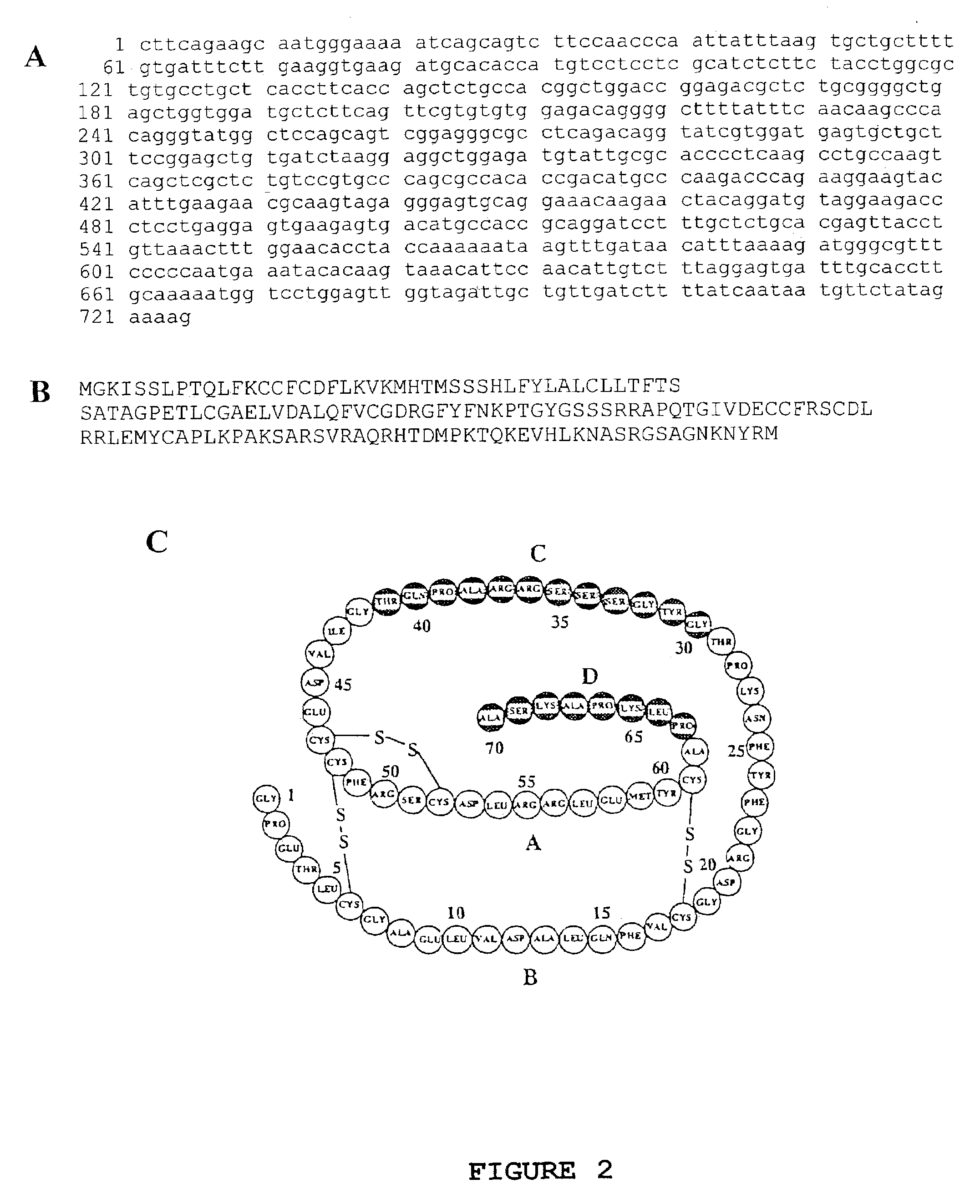
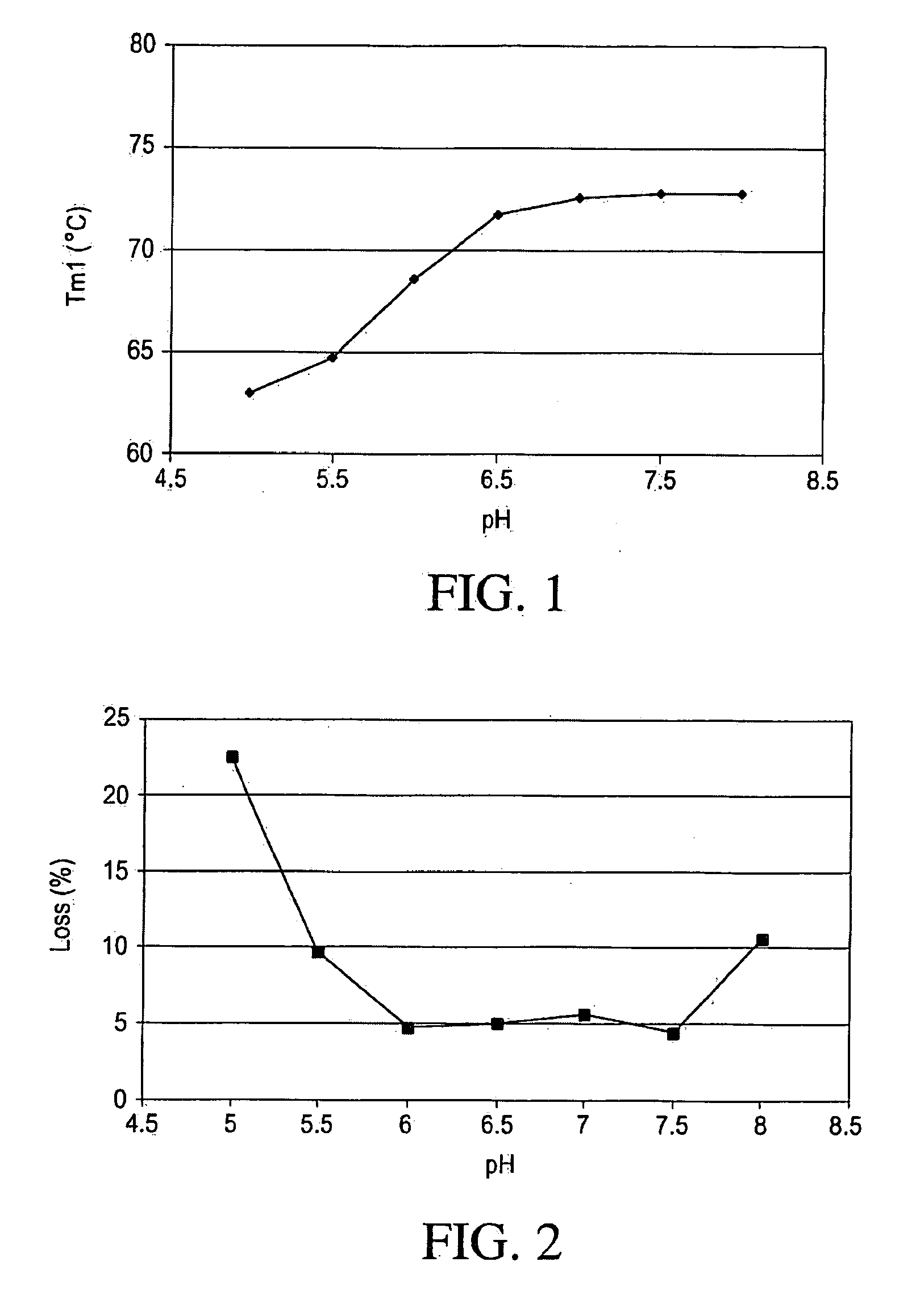
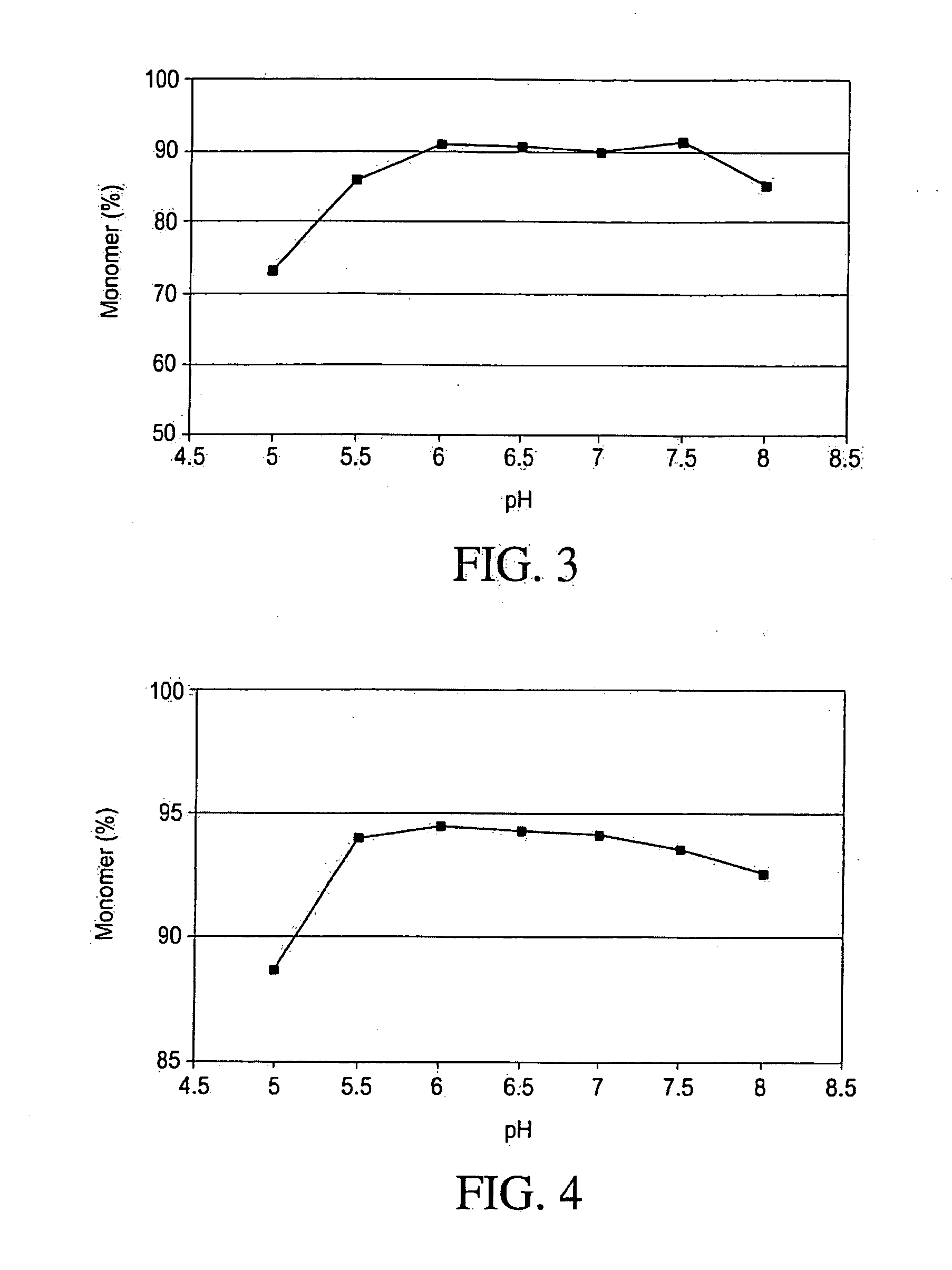
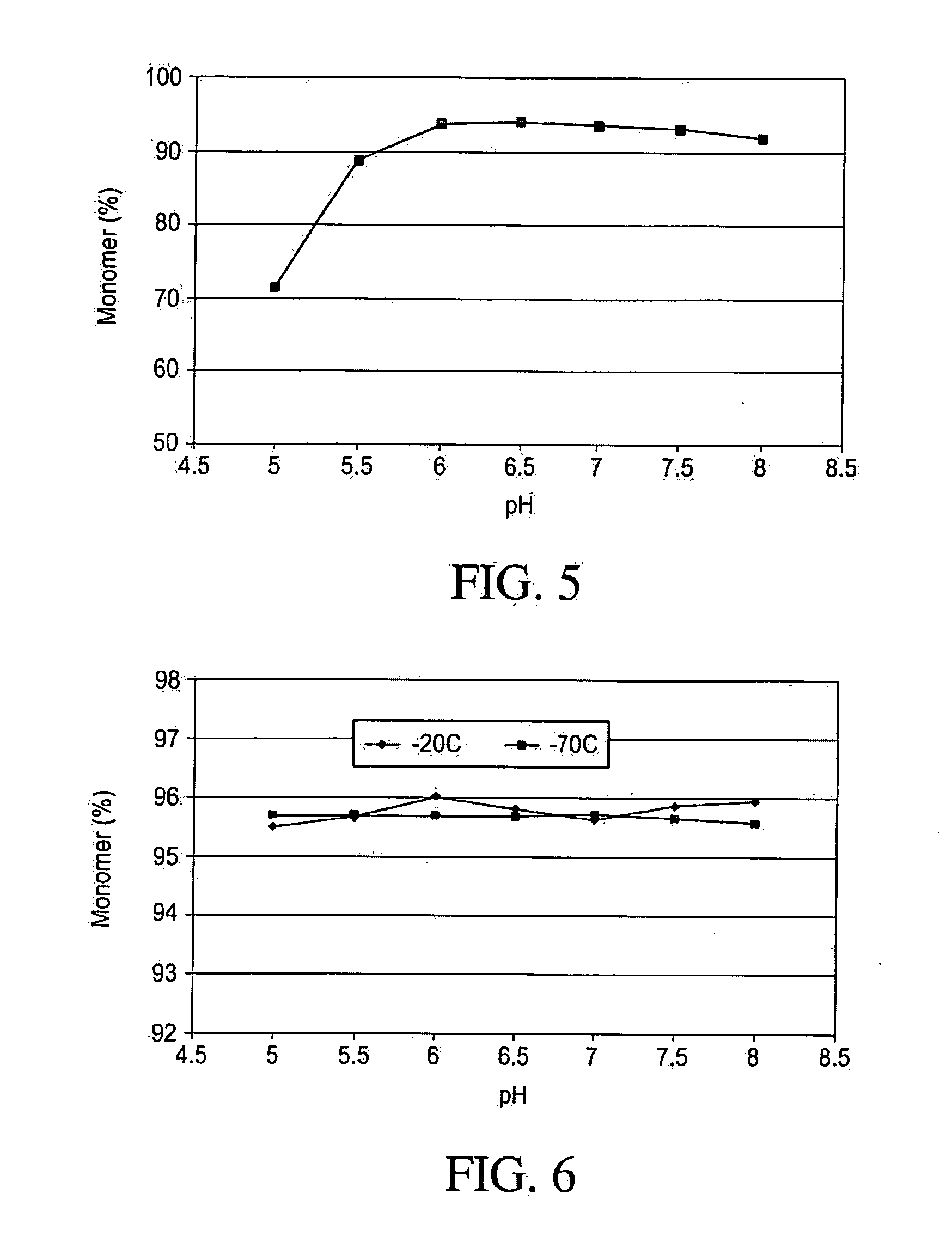
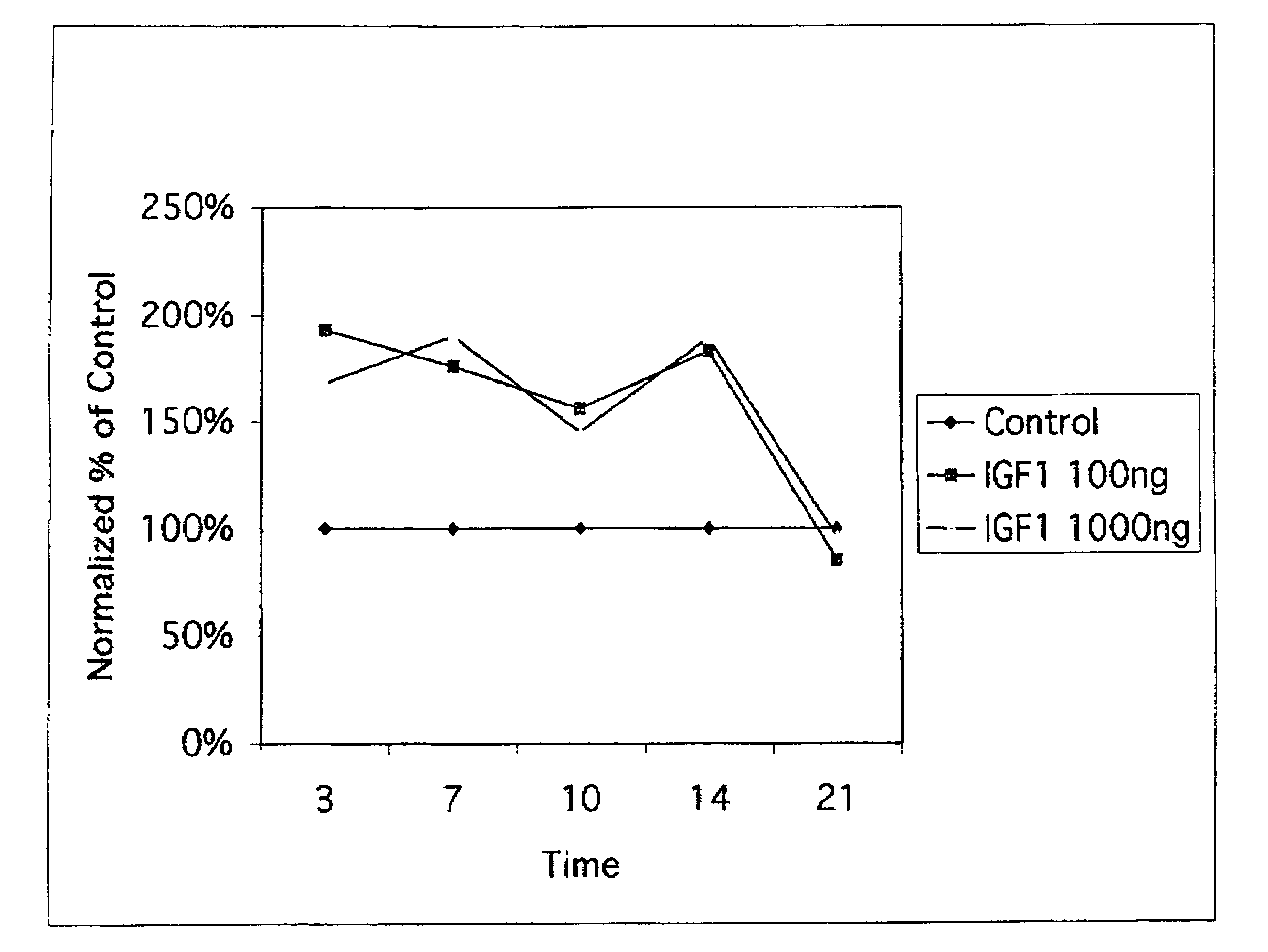
The insulin-like growth factors (IGFs) are proteins with high sequence similarity to insulin. IGFs are part of a complex system that cells use to communicate with their physiologic environment. This complex system (often referred to as the IGF "axis") consists of two cell-surface receptors (IGF1R and IGF2R), two ligands (Insulin-like growth factor 1 (IGF-1) and Insulin-like growth factor 2 (IGF-2)), a family of seven high-affinity IGF-binding proteins (IGFBP1 to IGFBP7), as well as associated IGFBP degrading enzymes, referred to collectively as proteases.
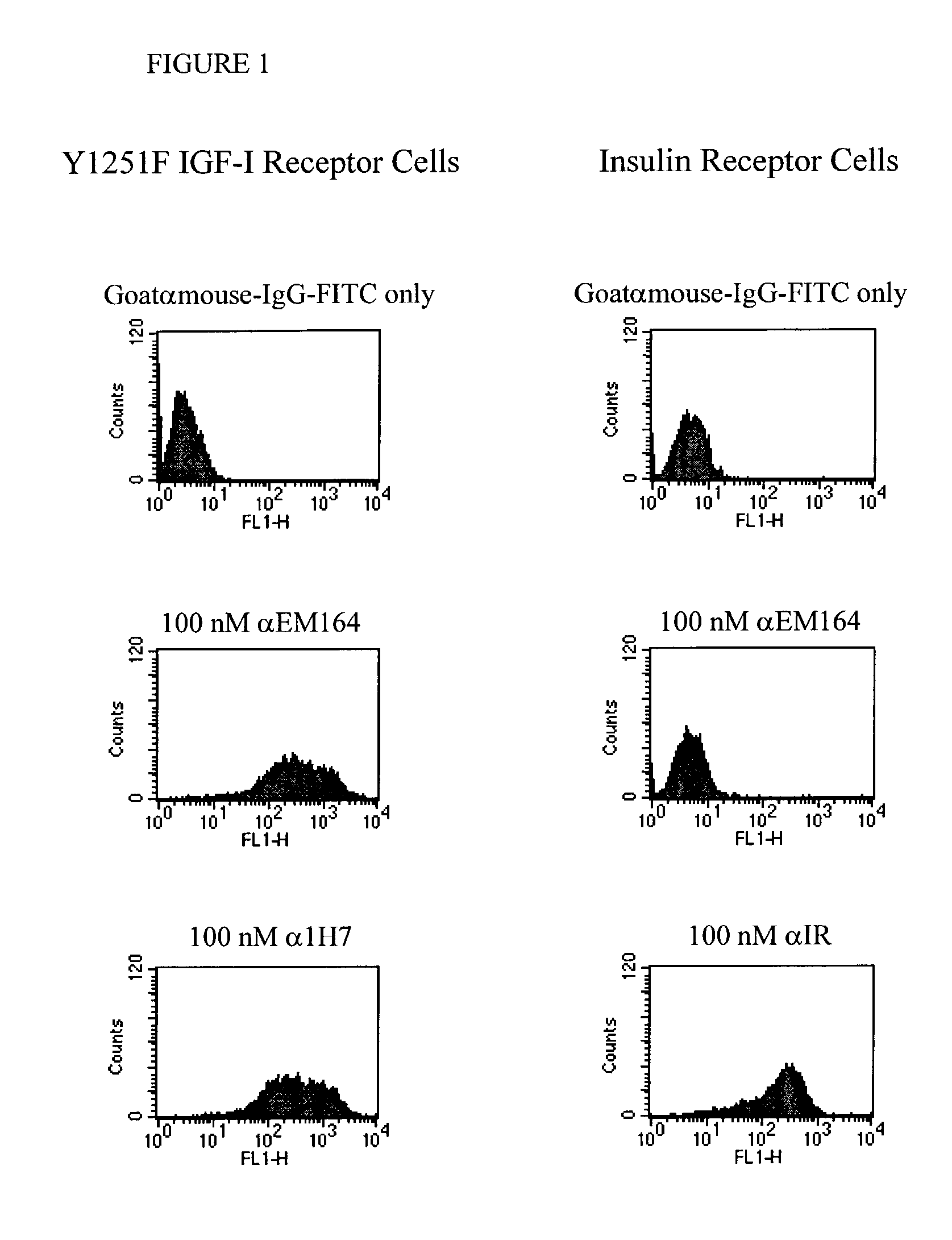
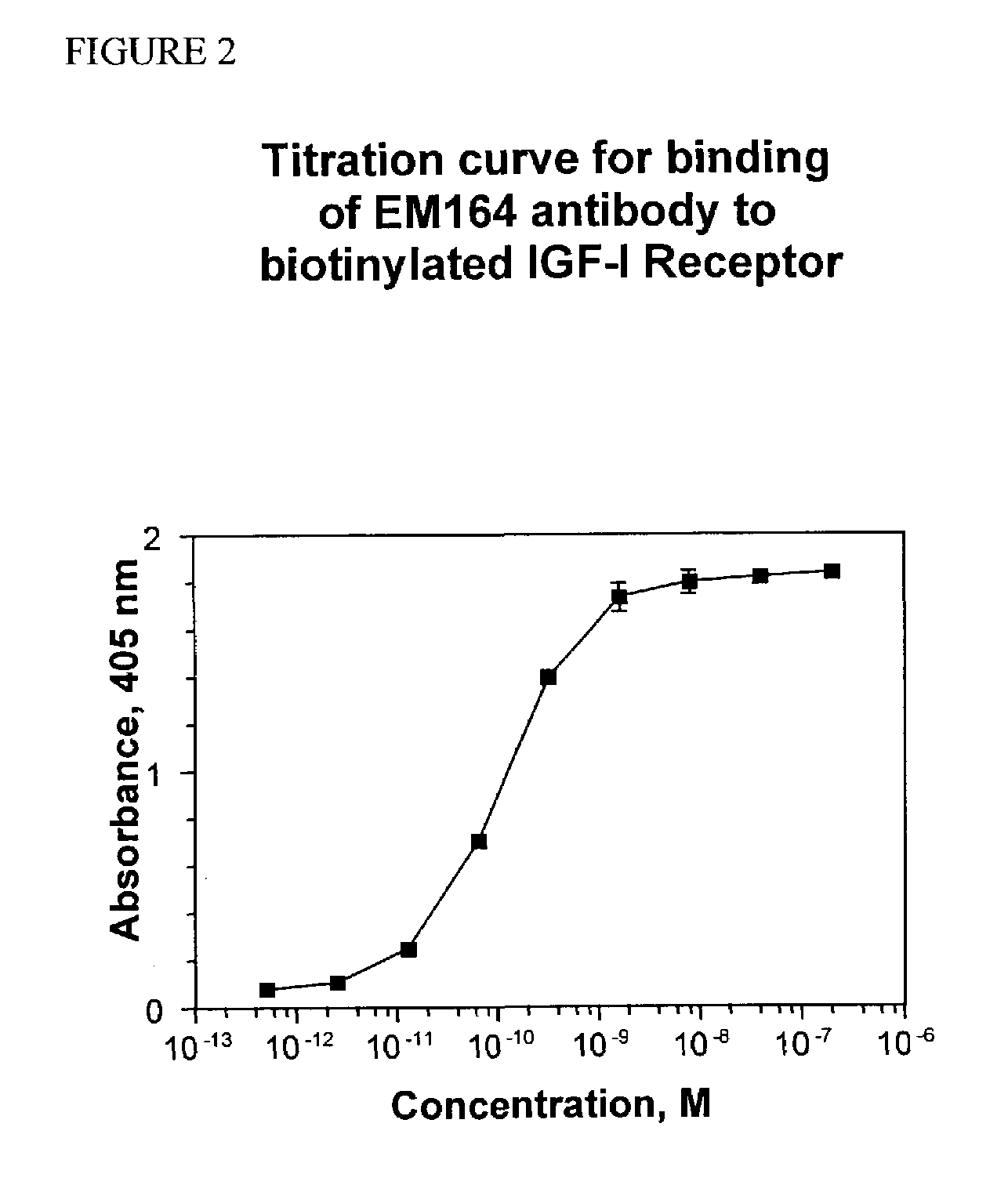
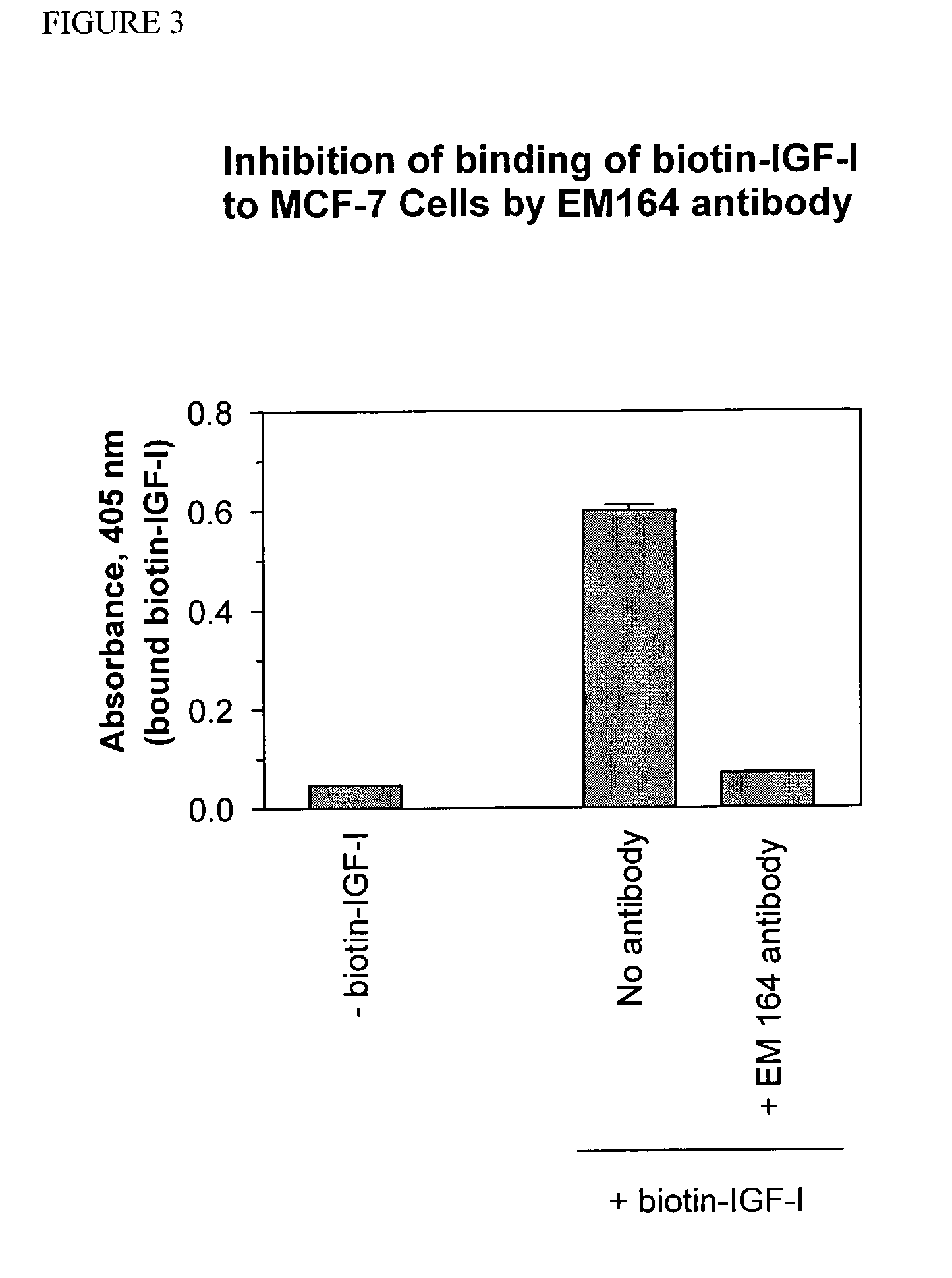
Anti-IGF-I receptor antibody
InactiveUS7538195B2Increase profitOrganic active ingredientsFungiInsulin-like growth factorSynovial sarcoma
Antibodies, humanized antibodies, resurfaced antibodies, antibody fragments, derivatized antibodies, and conjugates of these molecules with cytotoxic agents, which specifically bind to and inhibit insulin-like growth factor-I receptor, antagonize the effects of IGF-I and are substantially devoid of agonist activity toward the insulin-like growth factor-I receptor. These molecules can be conjugated to cytotoxic agents for use in the treatment of tumors that express elevated levels of IGF-I receptor, such as breast cancer, colon cancer, lung cancer, ovarian carcinoma, synovial sarcoma and pancreatic cancer. These molecules can also be labeled for in vitro and in vivo diagnostic uses, such as in the diagnosis and imaging of tumors that express elevated levels of IGF-I receptor.
Owner:IMMUNOGEN INC
Biological markers predictive of anti-cancer response to insulin-like growth factor-1 receptor kinase inhibitors
InactiveUS20070065858A1Restore sensitivityHigh sensitivityCompound screeningApoptosis detectionInsulin-like growth factorPancreatic hormone
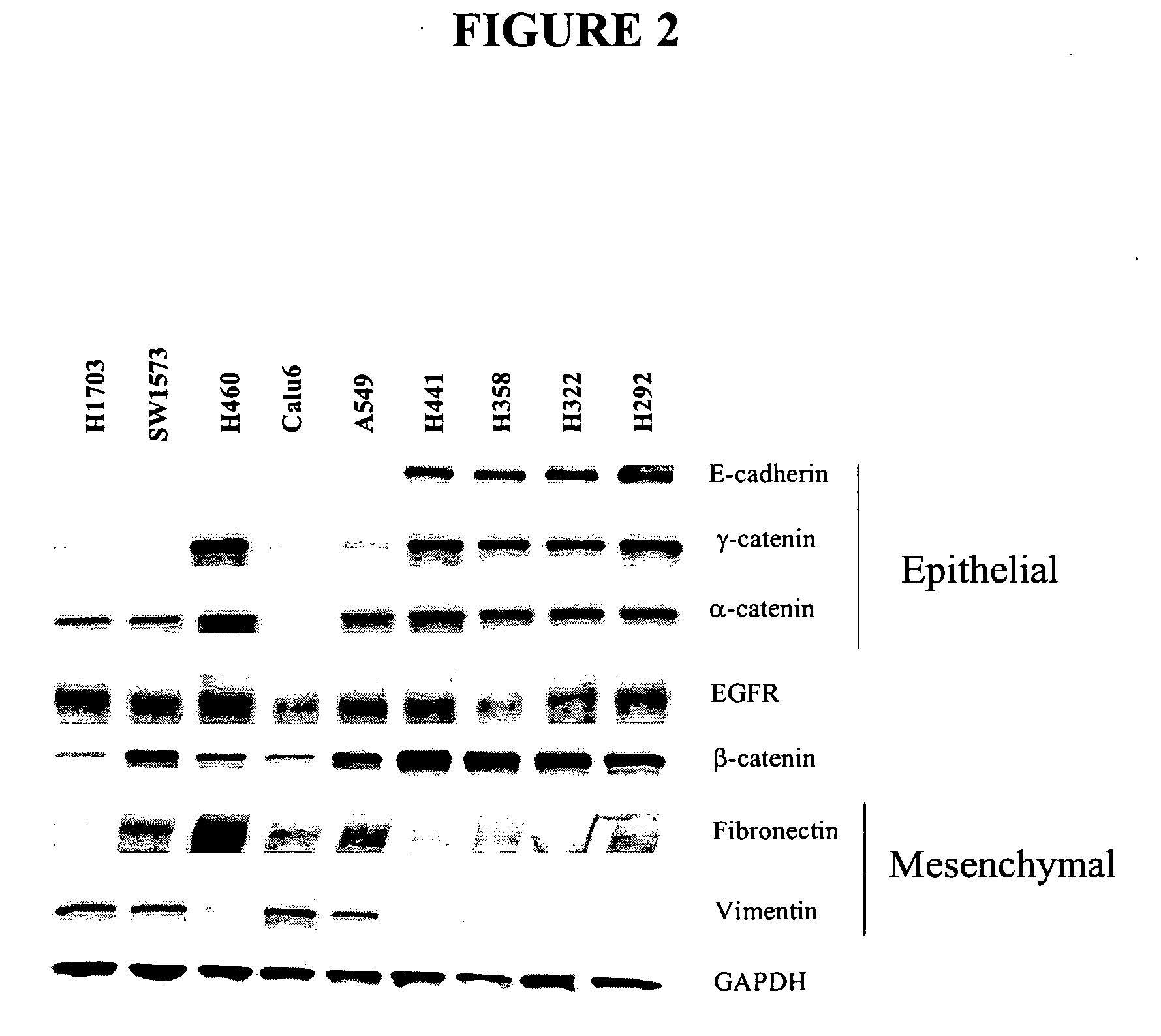
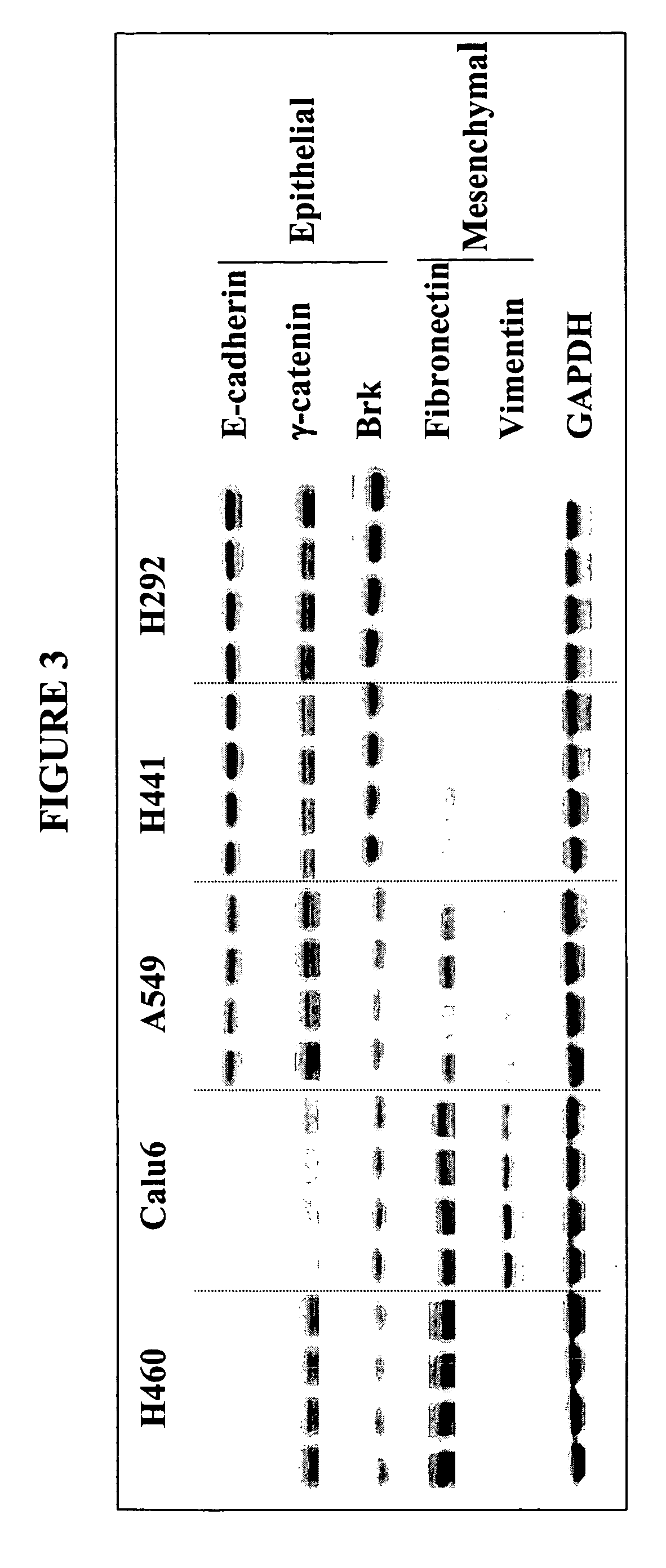
The present invention provides diagnostic and prognostic methods for predicting the effectiveness of treatment of a cancer patient with an IGF-1R kinase inhibitor. Methods are provided for predicting the sensitivity of tumor cell growth to inhibition by an IGF-1R kinase inhibitor, comprising assessing whether the tumor cell has undergone an epithelial to mesenchymal transition (EMT), by determining the expression level of epithelial and / or mesenchymal biomarkers, wherein tumor cells that have undergone an EMT are substantially less sensitive to inhibition by IGF-1R kinase inhibitors. Improved methods for treating cancer patients with IGF-1R kinase inhibitors that incorporate the above methodology are also provided. Additionally, methods are provided for the identification of new biomarkers that are predictive of responsiveness of tumors to IGF-1R kinase inhibitors. Furthermore, methods for the identification of agents that restore the sensitivity of tumor cells that have undergone EMT to inhibition by IGF-1R kinase inhibitors are also provided.
Owner:OSI PHARMA INC
Serum-free culture medium for mesenchymal stem cells
ActiveCN102827807AAvoid instabilityClear chemical compositionSkeletal/connective tissue cellsCell phenotypeSodium bicarbonate
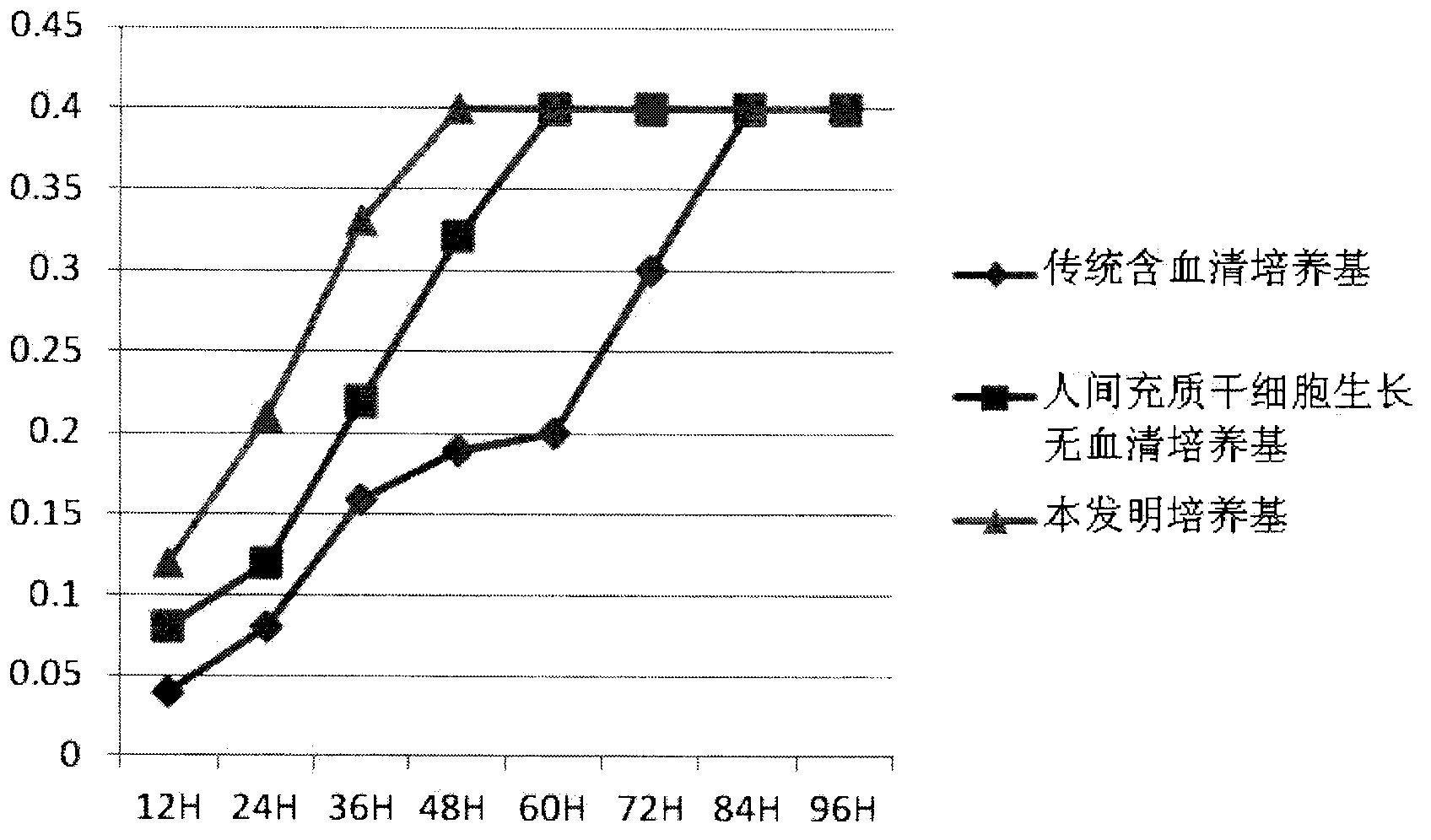
The invention relates to the field of biology, and discloses a serum-free culture medium which essentially comprises an IMDM (Iscove Modified Dulbecco Medium), L-glutamine, sodium bicarbonate, Hepes, recombinant human insulin, recombinant human transferrin, recombinant human albumin, 2-mercaptoethanol, protocatechuic acid, lipid, amino acid, vitamins, trace elements, Pluronic F-68, hydrocortisone, vitamin C, bonding amine or recombinant human fibronectin, progesterone, putrescine, heparin, serotonin, epidermal growth factors (EGFs), b-fibroblast growth factors (FGF), platelet derive growth factor (PDGF)-BB and insulin-like growth factor (IGF)-I. The serum-free culture medium is clear in chemical components, free from animal sources and serum and safe and ideal in cell cultivation and avoids the doped animal components and unstable batches, and the results of the cultured mesenchymal stem cells show that the total cellular score, the cell phenotype and the secretory cell factors are normal, so that the serum-free culture medium has good industrial application prospect.
Owner:内蒙古干细胞医学工程技术研究中心
Compositions comprising reproductive cell media and methods for using such compositions
InactiveUS6849394B2Mammal material medical ingredientsDead animal preservationInsulin-like growth factorCell culture media
Disclosed are compositions for mammalian, avian or piscian reproductive cells and methods for the collection, holding, processing, in vitro fertilization, sexing culturing, or storing (including long-term cryopreservation) of mammalian, avian, or piscian reproductive sperm cells. The compositions comprise a suitable reproductive cell media and a transforming growth factor, an insulin-like growth factor, or zinc, and, optionally, inositol, transferrin, or fructose, or combinations thereof.
Owner:MOFA GRP
Method and composition for restoration of age related tissue loss in the face or selected areas of the body
InactiveUS20060073178A1Restoring age related tissue lossEasy to produceCosmetic preparationsBiocideInsulin-like growth factorThyroid hormones
A treatment method for restoring of age related tissue loss in the face or selected areas of the body is disclosed which includes injecting an injectable composition containing a growth factor and hyaluronic acid as a carrier into the dermis, the hypodermis, or both, in various areas of the face, or selected areas of the body of a person to stimulate collagen, elastin, or fat cell production, thereby restoring age related tissue loss in the face and selected areas of the body. Further disclosed is an injectable composition for restoring of age related tissue loss in the face and selected areas of the body, which contains a growth factor and hyaluronic acid as a carrier for providing time release of the growth factor into tissues. The growth factor can be insulin, insulin-like growth factor, thyroid hormone, fibroblast growth factor, estrogen, retinoic acid, or their combinations.
Owner:CELLHEALTH TECH
Clinical-grade human mesenchymal stem cell serum-free complete medium
ActiveCN103243071APromote growthLow toxicitySkeletal/connective tissue cellsInsulin-like growth factorCuticle
The invention relates to a human mesenchymal stem cell culture medium. According to the culture medium, the basal culture medium comprises the following components based on the final concentration: 1-2g / L of human serum albumin, 5-10mg / L of transferring, 2-8mg / L of fibronectin, 1-4mg / L of laminin, 50g / L of Fe(NO3)3.9H2O, 417g / L of FeSO4.7H2O, 1-3mu g / L of estradiol, 2-5mu g / L of testosterone, 1-3mu g / L of progesterone, 39.25-117.74 mu g / L of dexamethasone, 5-10mg / L of insulin, 376.36mg / L of riboflavin, 80.96-242.87mg / L of coenzyme A, 4.41-6.17mg / L of butanediamine, 1-2mg / L of taurine, 0.61-1.85mg / L of aminoethanol, 8.81-26.42mg / L of pyruvic acid, 3.78-7.56mu g / L of sodium selenate, 292.3-584.6mg / L of L-glutamine, 2-8mu g / L of vascular endothelial growth factor, 4-10mu g / L of epidermal growth factor, 4-10mu g / L of basic fibroblast growth factor, 1-5mu g / L of leukaemia inhibitory factor, 1-5mu g / L of insulin-like growth factor-I and 2-8mu g / L of stem cell factor. The culture medium does not contain the animal serum, the potential animal endogenous endotoxin or virus of the animal serum is eliminated, and the culture medium is conveniently applied to clinics.
Owner:QINGDAO RESTORE BIOTECHNOLOGY CO LTD
Preparation method of allogenic mesenchymal stem cells by CRISPR (clustered regularly interspaced short palindromic repeats) technique editing and IGF (insulin-like growth factor) optimization and application of allogenic mesenchymal stem cells in treating myocardial infarction
ActiveCN105985985AImprove anti-apoptotic abilityPromote homingUnknown materialsFermentationAntigenInflammatory factors
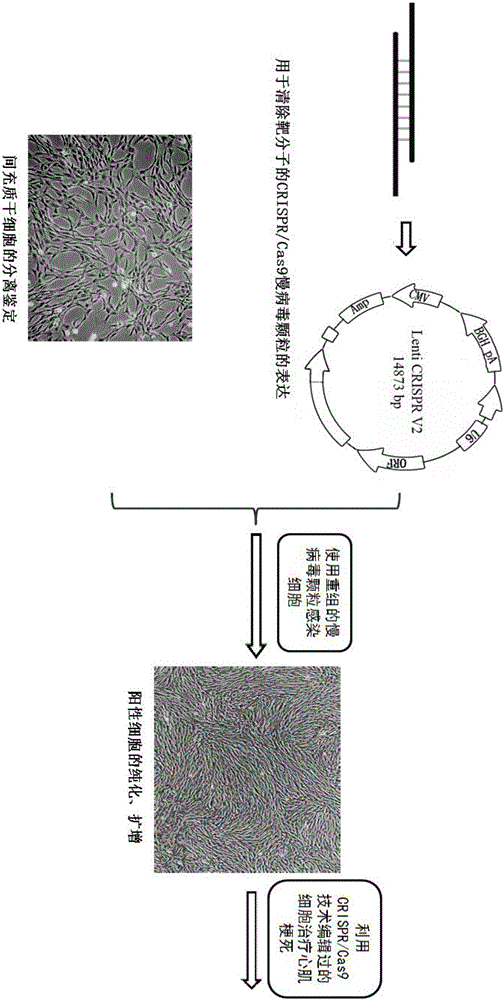
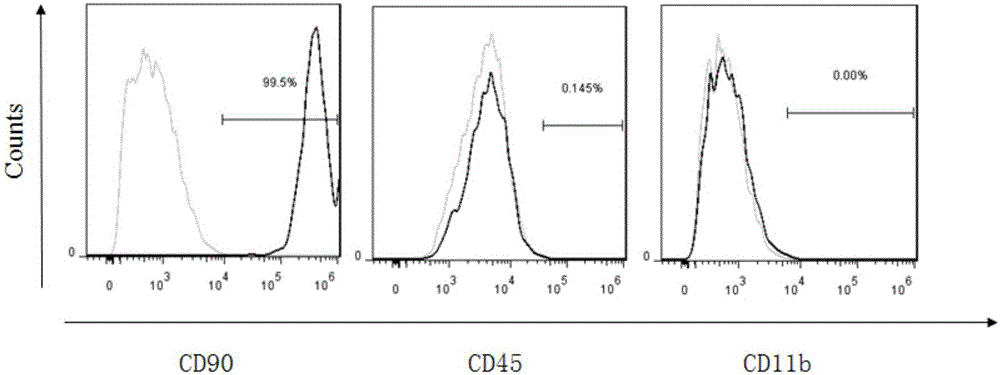
The invention belongs to the field of allogenic mesenchymal stem cells, and particularly relates to a preparation method of allogenic mesenchymal stem cells by CRISPR (clustered regularly interspaced short palindromic repeats) technique editing and IGF (insulin-like growth factor) optimization and application of the allogenic mesenchymal stem cells in treating myocardial infarction. The preparation method comprises the following steps: carrying out separation by density gradient centrifugation to obtain allogenic single karyocytes, and carrying out adherent culture to obtain mesenchymal stem cells; designing a mesenchymal stem cell surface antigen B2M-gRNA and an inflammatory factor TNF-alpha-gRNA; establishing recombinant slow virus particles, and transfecting the mesenchymal stem cells; optimizing the mesenchymal stem cells by using IGF-1; and preparing drugs for treating myocardial infarctions by using the modified and optimized mesenchymal stem cells. The CRISPR / Cas9 technique is utilized to remove the antigens capable of causing immunological rejection and the inflammatory factors capable of causing inflammatory reaction on the mesenchymal stem cell surface, and the IGF-1 is utilized to enhance the apoptosis resistance of the mesenchymal stem cells and promote the homing of the mesenchymal stem cells, thereby providing a new technical scheme for preparing drugs for treating cardiovascular diseases in clinic. The prepared allogenic mesenchymal stem cells can not cause immunological rejection after cell transplantation.
Owner:SUZHOU UNIV
Methods for treatment of insulin-like growth factor-1 (IGF-1) deficiency
ActiveUS7258864B2To promote metabolismIncrease probabilityPeptide/protein ingredientsPeptide preparation methodsInitial treatmentBlood level
Owner:IPSEN BIOPHARMACEUTICALS INC
Pharmaceutical proteins, human therapeutics, human serum albumin, insulin, native cholera toxic b submitted on transgenic plastids
InactiveUS20030204864A1Eliminate needLarge biomassBiocidePeptide/protein ingredientsEscherichia coliInsulin-like growth factor
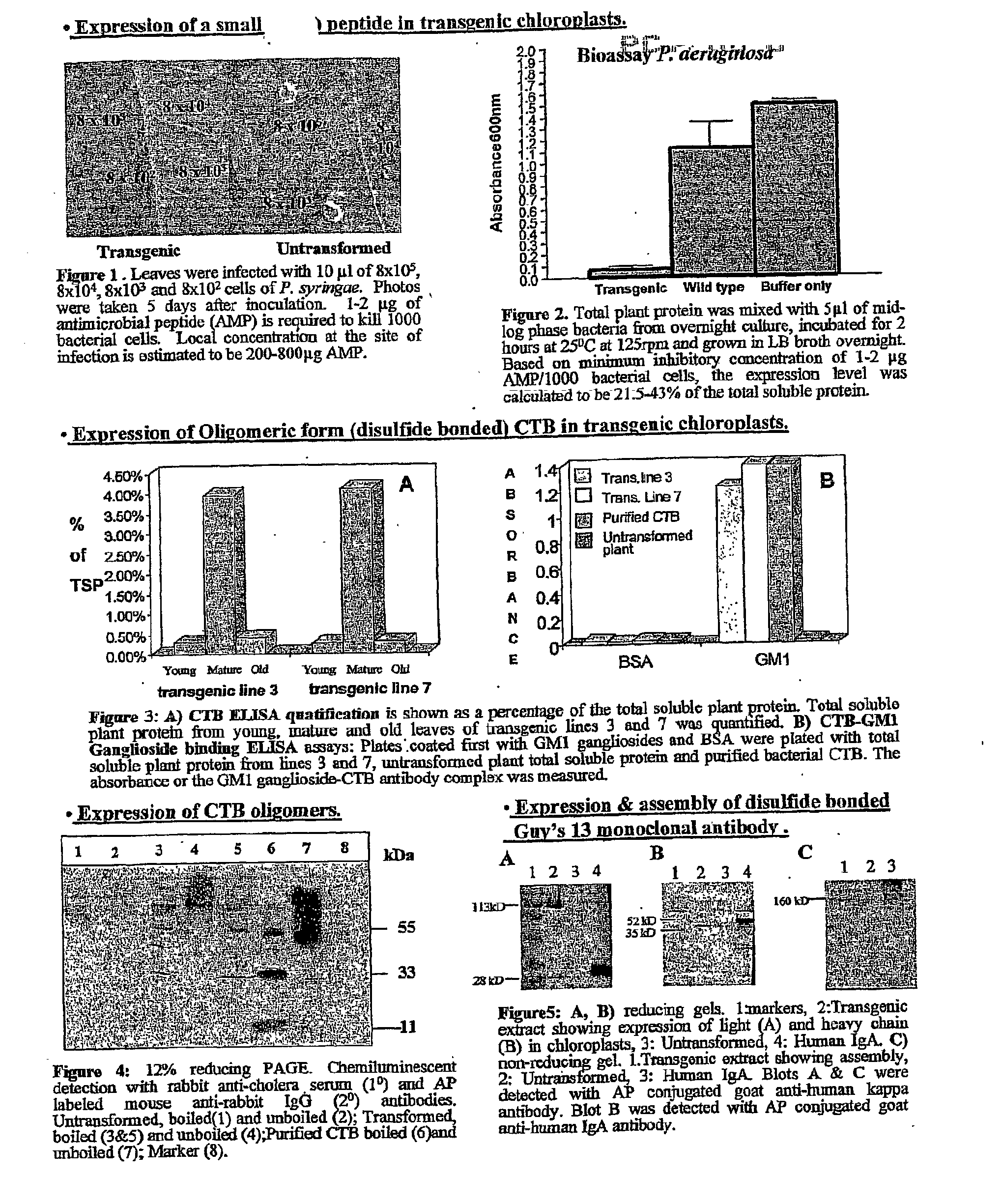
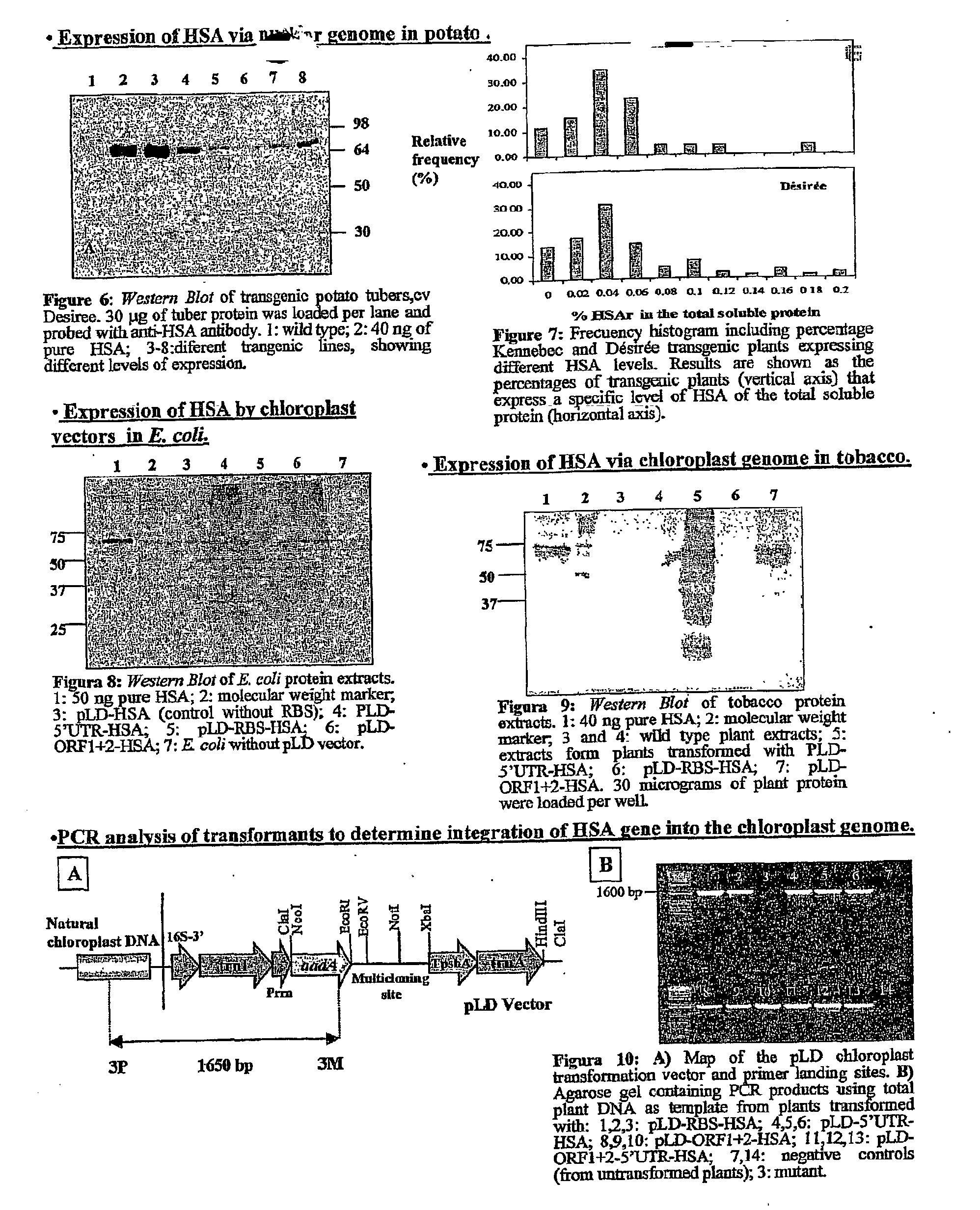
Transgenic chloroplast technology could provide a viable solution to the production of Insulin-like Growth Factor I (IGF-I), Human Serum Albumin (HSA), or interferons (IFN) because of hyper-expression capabilities, ability to fold and process eukaryotic proteins with disulfide bridges (thereby eliminating the need for expensive post-purification processing). Tobacco is an ideal choice because of its large biomass, ease of scale-up (million seeds per plant), genetic manipulation and impending need to explore alternate uses for this hazardous crop. Therefore, all three human proteins will be expressed as follows: a) Develop recombinant DNA vectors for enhanced expression via tobacco chloroplast genomes b) generate transgenic plants c) characterize transgenic expression of proteins or fusion proteins using molecular and biochemical methods d) large scale purification of therapeutic proteins from transgenic tobacco and comparison of current purification / processing methods in E. coli or yeast e) Characterization and comparison of therapeutic proteins (yield, purity, functionality) produced in yeast or E. coli with transgenic tobacco f) animal testing and pre-clinical trials for effectiveness of the therapeutic proteins. Mass production of affordable vaccines can be achieved by genetically engineering plants to produce recombinant proteins that are candidate vaccine antigens. The B subunits of Enteroxigenic E. coli (LTB) and cholera toxin of Vibrio cholerae (CTB) are examples of such antigens. When the native LTB gene was expressed via the tobacco nuclear genome, LTB accumulated at levels less than 0.01% of the total soluble leaf protein. Production of effective levels of LTB in plants, required extensive codon modification. Amplification of an unmodified CTB coding sequence in chloroplasts, up to 10,000 copies per cell, resulted in the accumulation of up to 4.1% of total soluble tobacco leaf protein as oligomers (about 410 fold higher expression levels than that of the unmodified LTB gene). PCR and Southern blot analyses confirmed stable integration of the CTB gene into the chloroplast genome. Western blot analysis showed that chloroplast synthesized CTB assembled into oligomers and was antigenically identical to purified native CTB. Also, GM1,-ganglioside binding assays confirmed that chloroplast synthesized CTB binds to the intestinal membrane receptor of cholera toxin, indicating correct folding and disulfide bond formation within the chloroplast. In contrast to stunted nuclear transgenic plants, chloroplast transgenic plants were morphologically indistinguishable from untransformed plants, when CTB was constitutively expressed. The introduced gene was stably inherited in the subsequent generation as confirmed by PCR and Southern blot analyses. Incrased production of an efficient transmucosal carrier molecule and delivery system, like CTB, in transgenic chloroplasts makes plant based oral vaccines and fusion proteins with CTB needing oral administration a much more practical approach.
Owner:AUBURN UNIV +1
Treating or preventing the early stages of degeneration of articular cartilage or subchondral bone in mammals using carprofen and derivatives
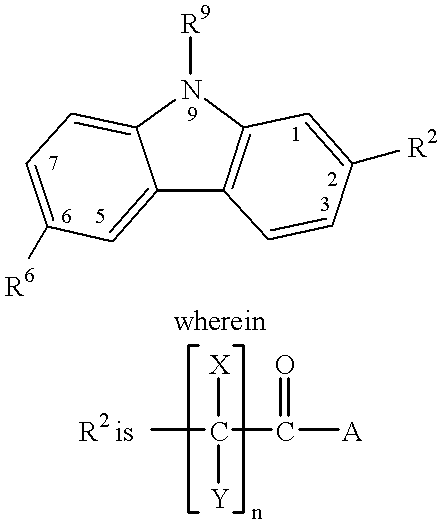
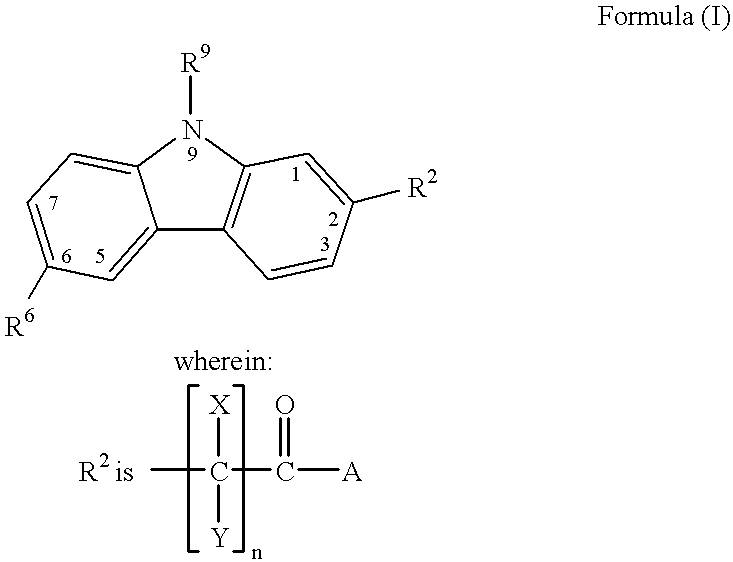
Treating or preventing the early stages of degeneration of articular cartilage or subchondral bone in the affected joint of a mammal is accomplished by administering a chondroprotective compound of Formula (I):where A is hydroxy, (C1-C4)alkoxy, amino, hydroxy-amino, mono-(C1-C2)alkylamino, di-(C1-C2)alkylamino; X and Y are independently H or (C1-C2)alkyl; and n is 1 or 2; R6 is halogen, (C1-C3)alkyl, trifluoromethyl, or nitro; R9 is H; (C1-C2)alkyl; phenyl or phenyl-(C1-C2)alkyl, where phenyl is optionally mono-substituted by fluoro or chloro; -C(=O)-R, where R is (C1-C2)alkyl or phenyl, optionally mono-substituted by fluoro or chloro; or -C(=O)-O-R', where R1 is (C1-C2)alkyl.This treatment ameliorates, diminishes, actively treats, reverses or prevents any injury, damage or loss of articular cartilage or subchondral bone subsequent to said early stage of said degeneration. Whether or not a mammal needs such treatment is determined by whether or not it exhibits a statistically significant deviation from normal standard values in synovial fluid or membrane from the affected joint, with respect to at least five of the following substances: increased interleukin-1 beta (IL-1beta); increased tumor necrosis factor alpha (TNFalpha); increased ratio of IL-1beta to IL-1 receptor antagonist protein (IRAP); increased expression of p55 TNF receptors (p55 TNF-R); increased interleukin-6 (IL-6); increased leukemia inhibitory factor (LIF); decreased insulin-like growth factor-1 (IGF-1); decreased transforming growth factor beta (TGFbeta); decreased platelet-derived growth factor (PDGF); decreased basic fibroblast growth factor (b-FGF); increased keratan sulfate; increased stromelysin; increased ratio of stromelysin to tissue inhibitor of metalloproteases (TIMP); increased osteocalcin; increased alkaline phosphatase; increased cAMP responsive to hormone challenge; increased urokinase plasminogen activator (uPA); increased cartilage oligomeric matrix protein; and increased collagenase.
Owner:PFIZER INC +1
Drugs for treating cancer
InactiveUS20060193772A1Good treatment effectOrganic active ingredientsIn-vivo radioactive preparationsInsulin-like growth factorTreatment effect
The invention aims to provide a medicament for treating cancer in which a cancer therapeutic effect is synergistically increased using a substance inhibiting activities of insulin-like growth factor-I (IGF-I) and insulin-like growth factor-II (IGF-II). According to the invention, there are provided a medicament for treating cancer which comprises a substance inhibiting activities of IGF-I and IGF-II and which is administered in combination with irradiation; and a medicament for treating cancer comprising a combination of a substance inhibiting activities of IGF-I and IGF-II and a substance having an antitumor activity.
Owner:KYOWA HAKKO KOGYO CO LTD +1
Compositions and methods for targeting a polypeptide to the central nervous system
InactiveUS20050100986A1Nervous disorderPeptide/protein ingredientsPseudomonas aeruginosa exotoxin AInsulin-like growth factor
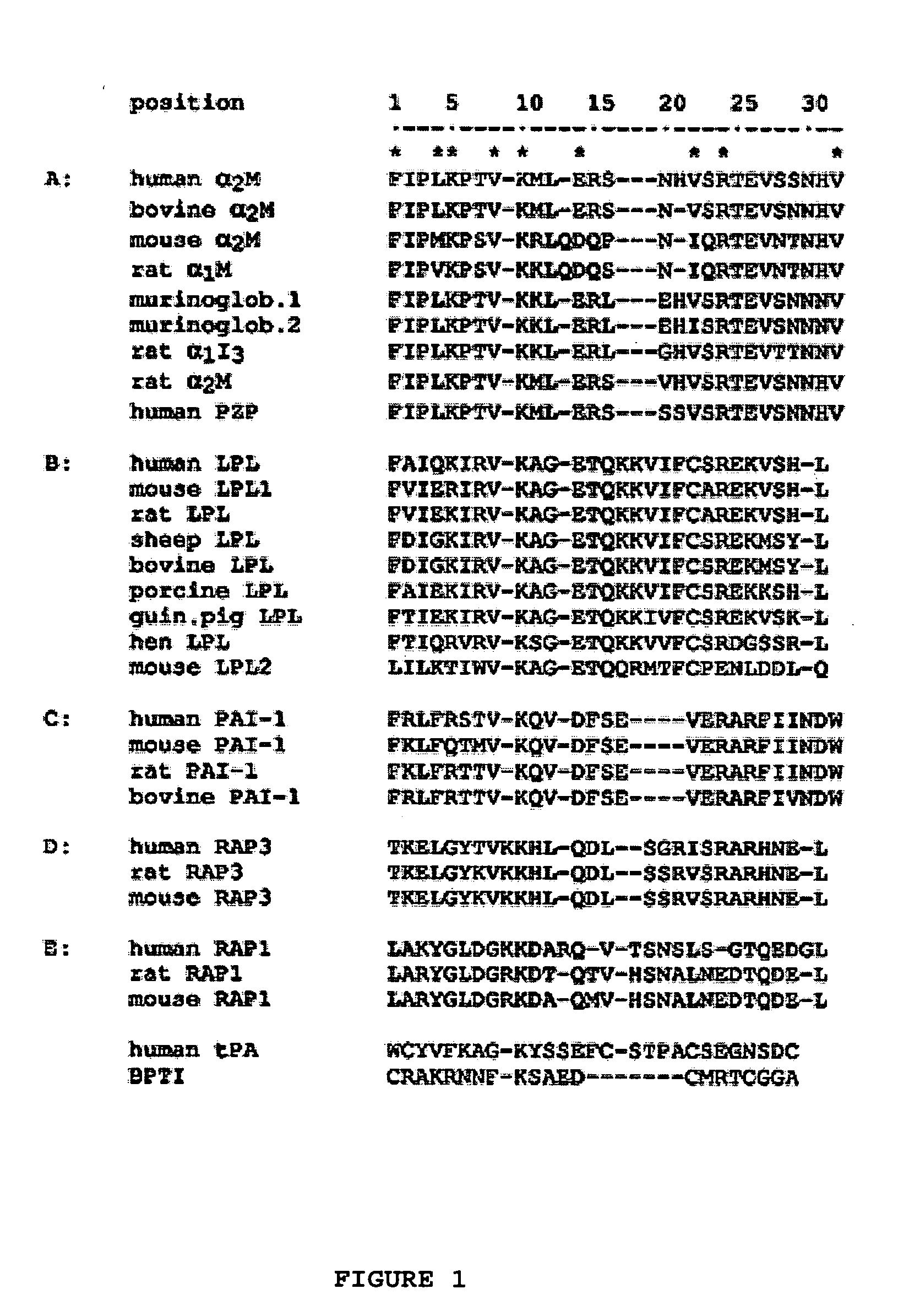
The invention provides a chimeric CNS targeting polypeptide having a BBB-receptor binding domain and a payload polypeptide domain. The chimeric CNS targeting polypeptide can have a BBB-receptor binding domain consisting of a receptor binding domain from ApoB, ApoE, aprotinin, lipoprotein lipase, PAI-1, pseudomonas exotoxin A, transferrin, α2-macroglobulin, insulin-like growth factor, insulin, or a functional fragment thereof. Nucleic acids encoding a chimeric CNS targeting polypeptide are also provided. Further provided is a method of delivering a polypeptide to the CNS of an individual. The method consists of administering to the individual an effective amount of a chimeric CNS targeting polypeptide, said chimeric CNS targeting polypeptide comprising a BBB-receptor binding domain and a payload polypeptide domain. The method also can deliver a polypeptide to the lysosomes of CNS cells.
Owner:SALK INST FOR BIOLOGICAL STUDIES
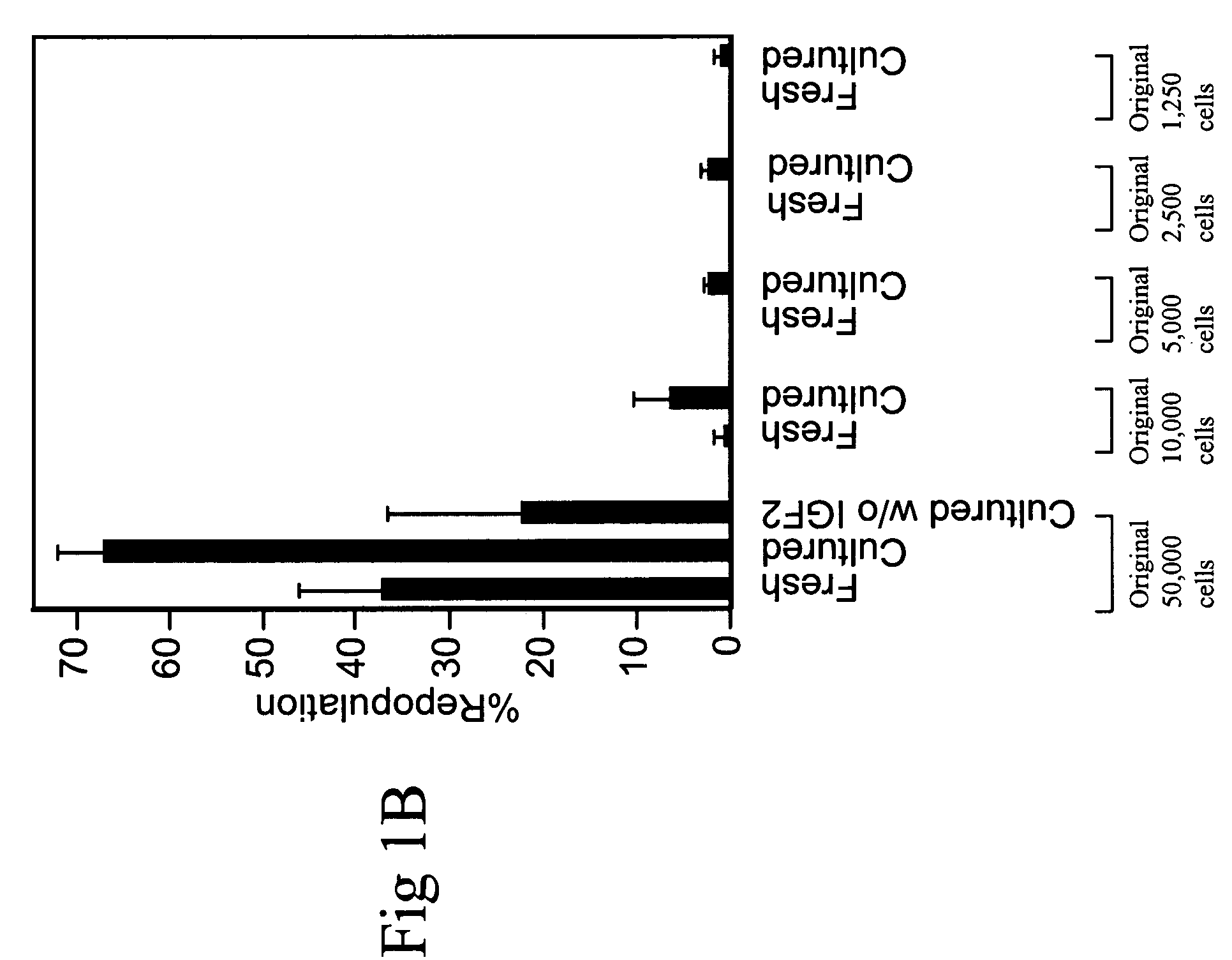
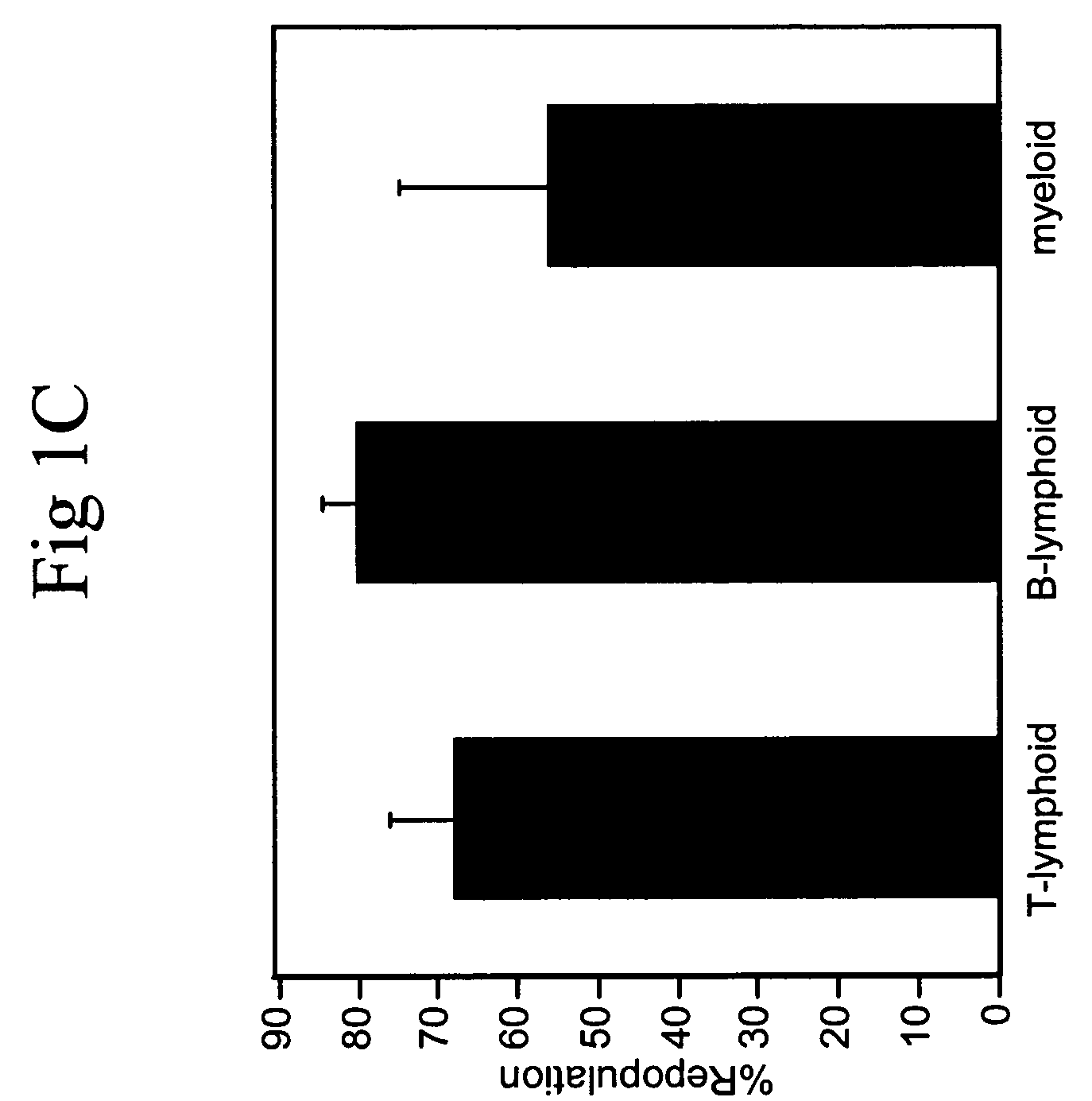
Cultured hematopoietic stem cells and method for expansion and analysis thereof
Hematopoietic stem cells and methods for ex vivo expansion of hematopoietic stem cells are provided. The methods comprise culturing the cells in a media containing an effective amount insulin-like growth factor (IGF), fibroblast growth factor (FGF), thrombopoietin (TPO), and stem cell factor (SCF), under conditions sufficient for expansion of said cells. Methods for identifying expanded hematopoeitc stem cells and kits for ex vivo expansion of hematopoietic stem cells are also provided.
Owner:WHITEHEAD INST FOR BIOMEDICAL RES
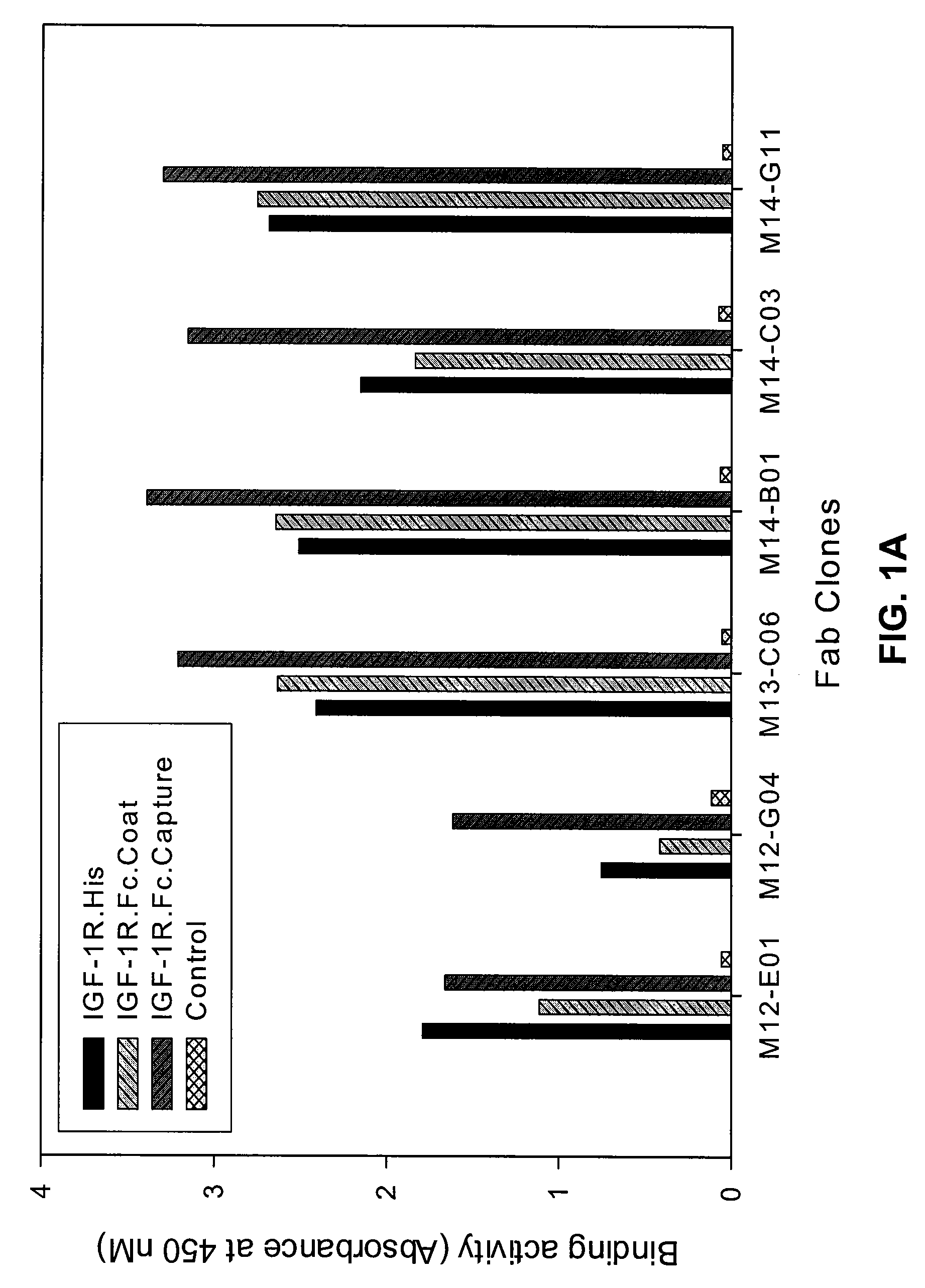
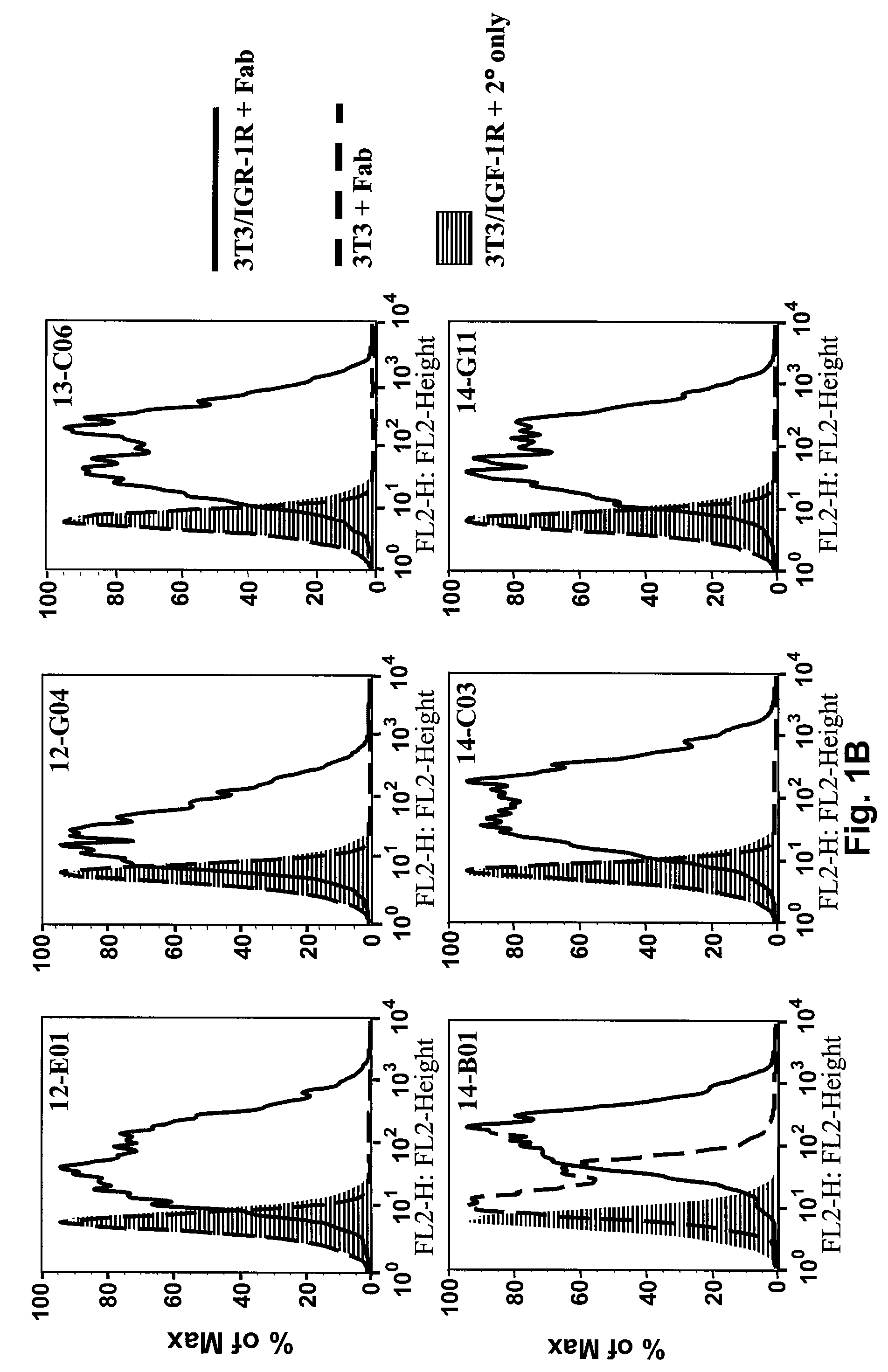
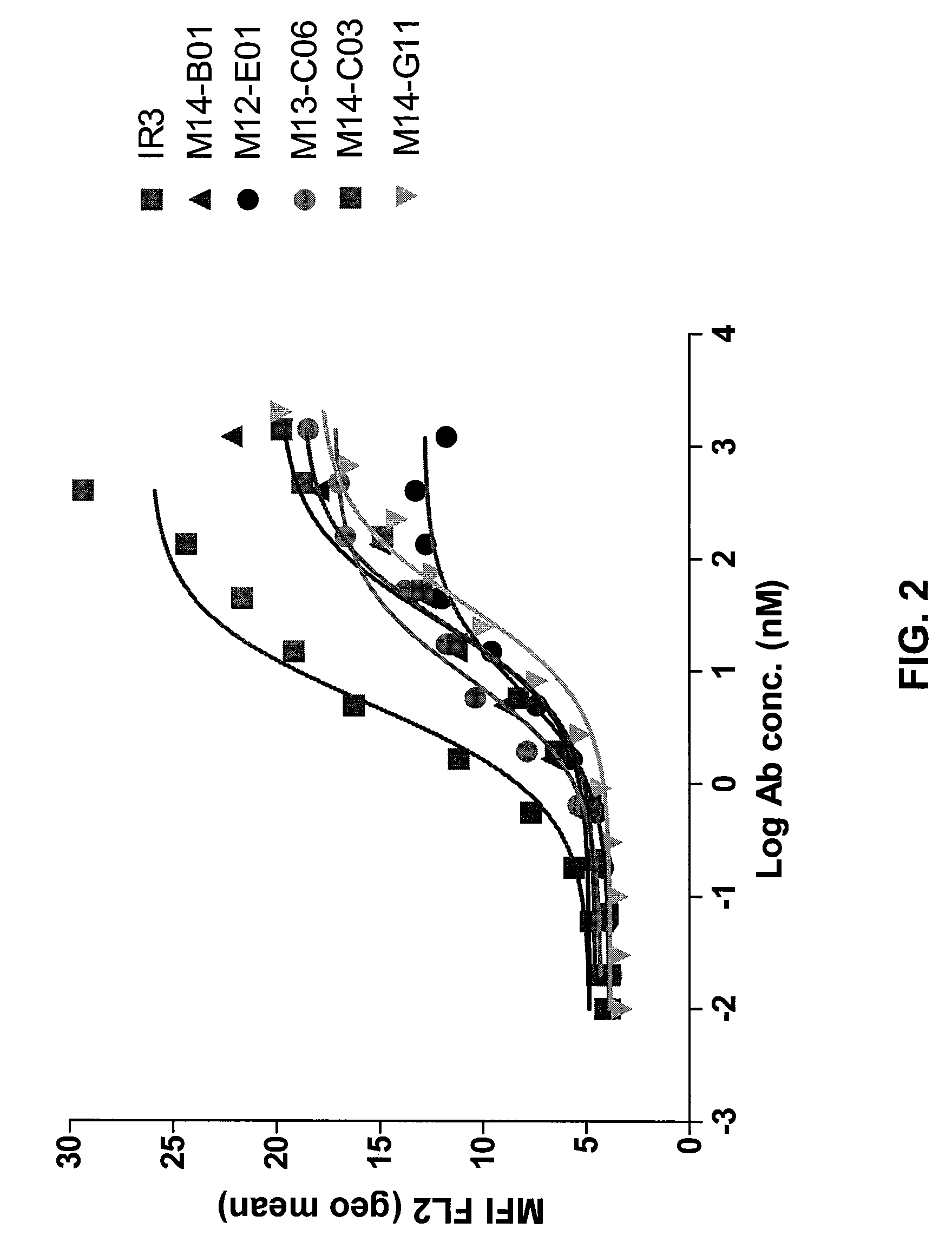
Fully Human Antibodies Directed Against the Human Insulin-Like Growth Factor-1 Receptor
InactiveUS20080025990A1Reduces IGF-IRCut surfaceSenses disorderFungiInsulin-like growth factorSingle-Chain Antibodies
This invention relates to human antibodies that bind to human insulin-like growth factor-1 receptor (IGF-IR), to derivatives of these antibodies (Fabs, single chain antibodies, bi-specific antibodes, or fusion proteins), and to uses of the antibodies and derivatives in therapeutic, and diagnostic methods. The invention relates to nucleic acids encoding the anti-IGF-IR, methods of generating the antibodies and expression. The invention further relates to combination therapies using ant-IGF-IR antibodies with anti-neoplastic drugs.
Owner:IMCLONE SYSTEMS
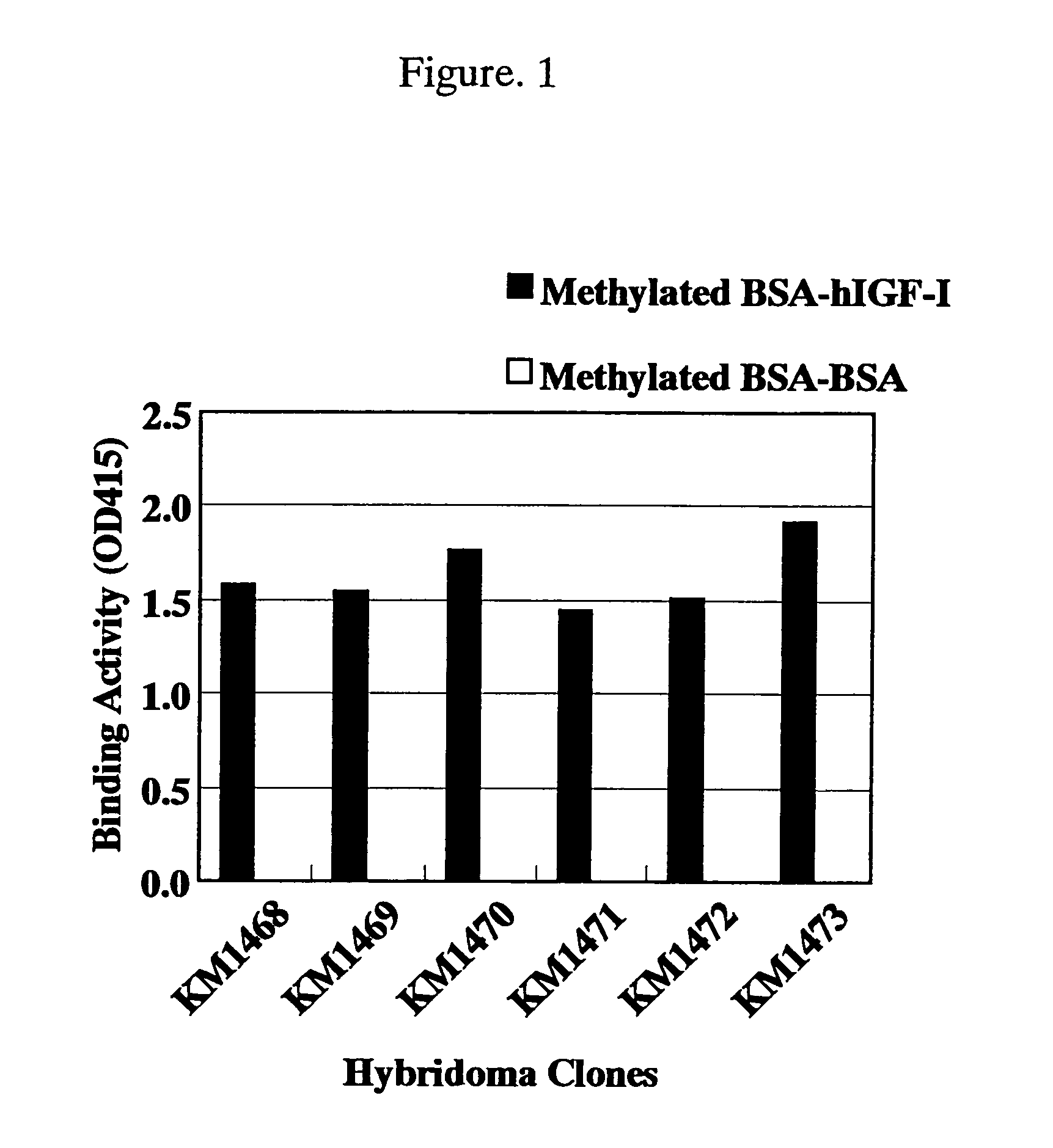
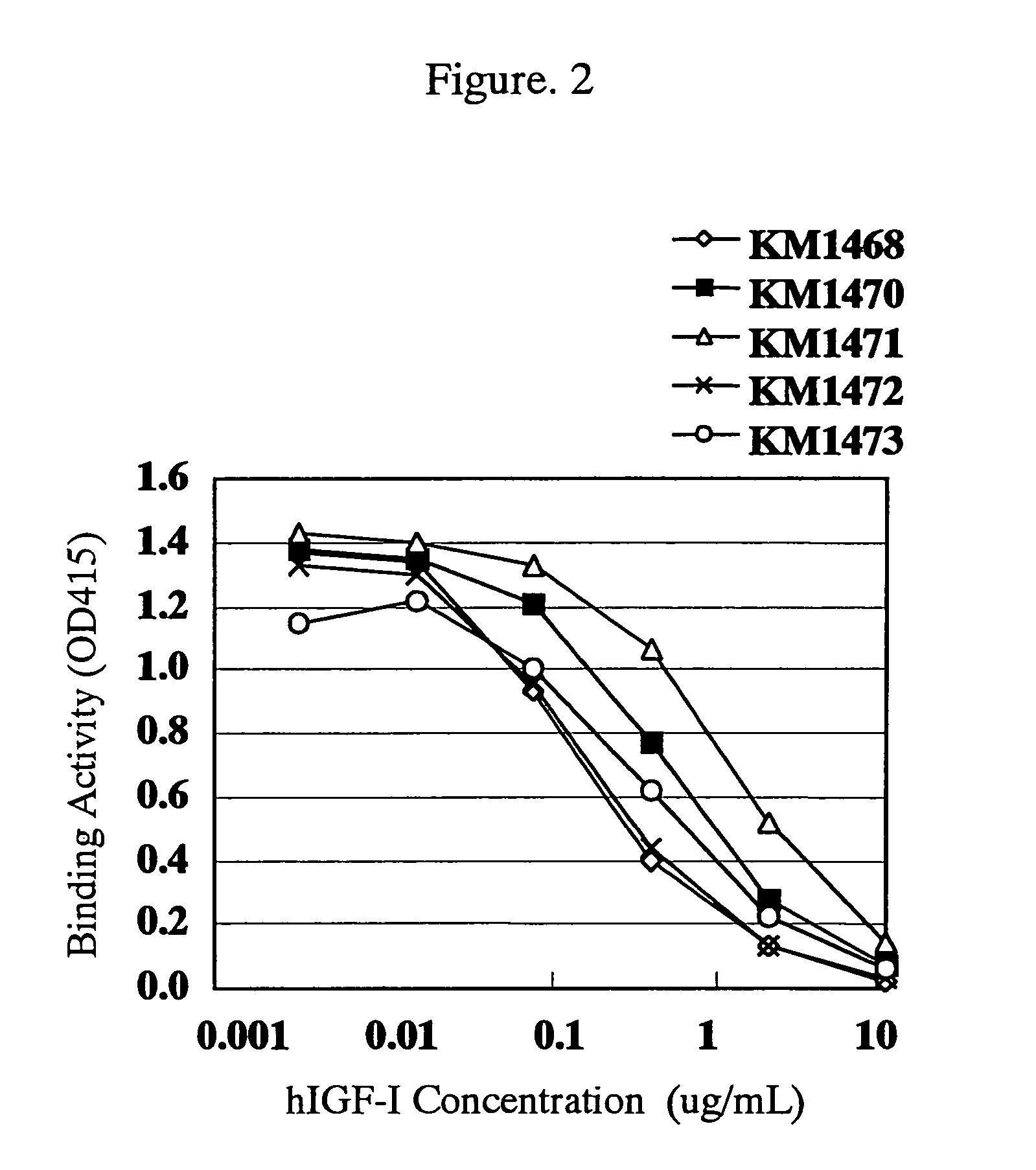
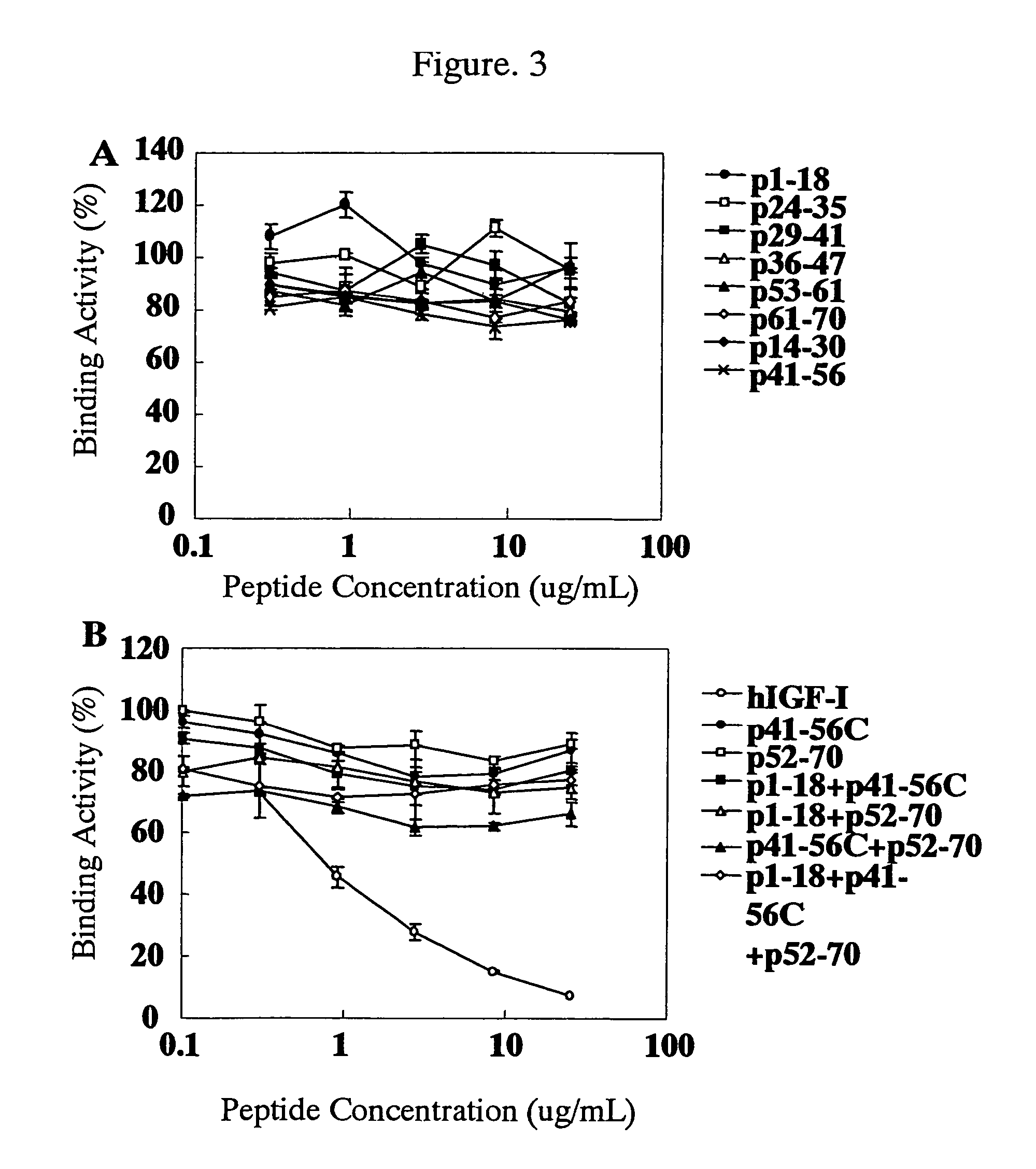
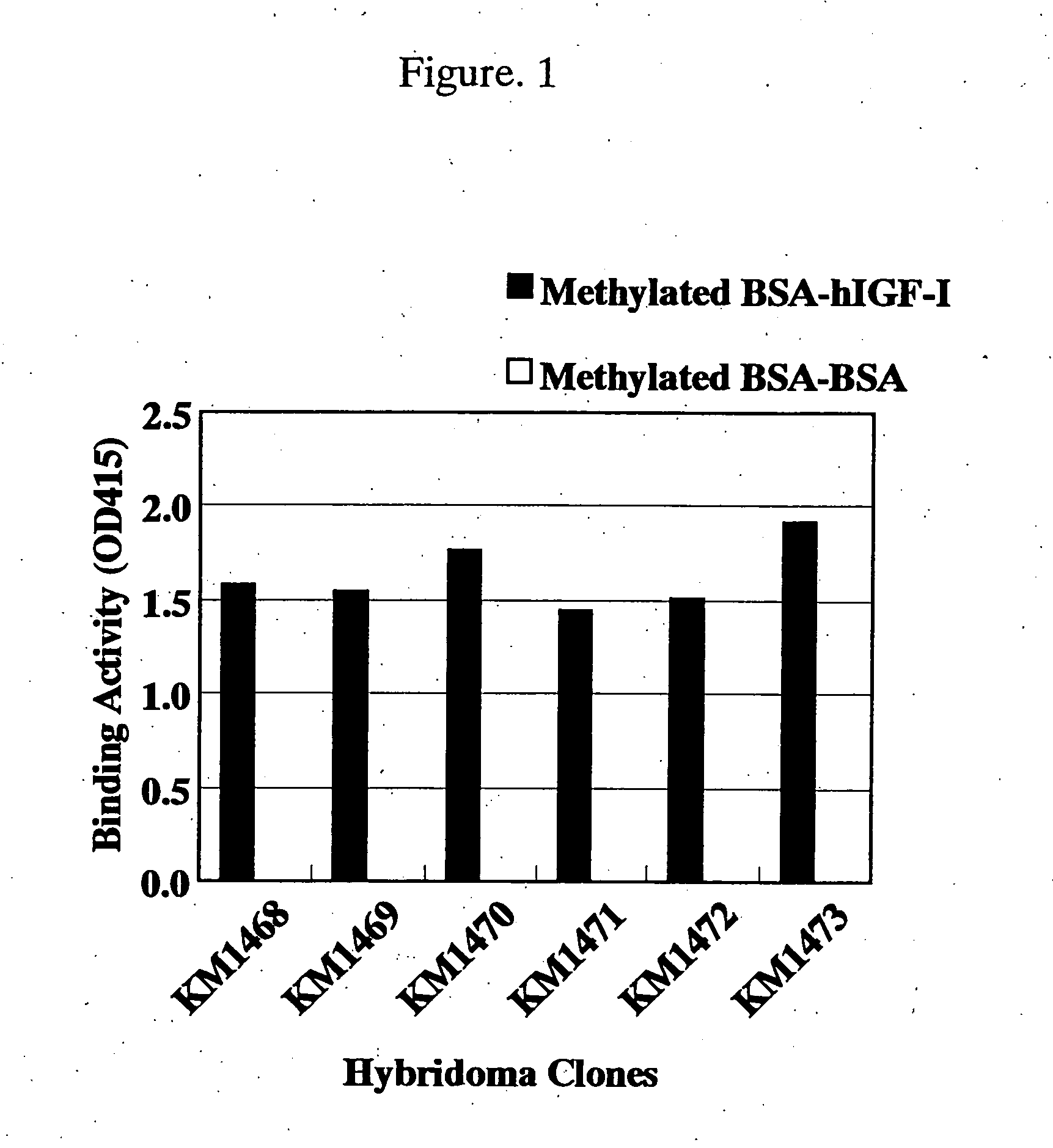
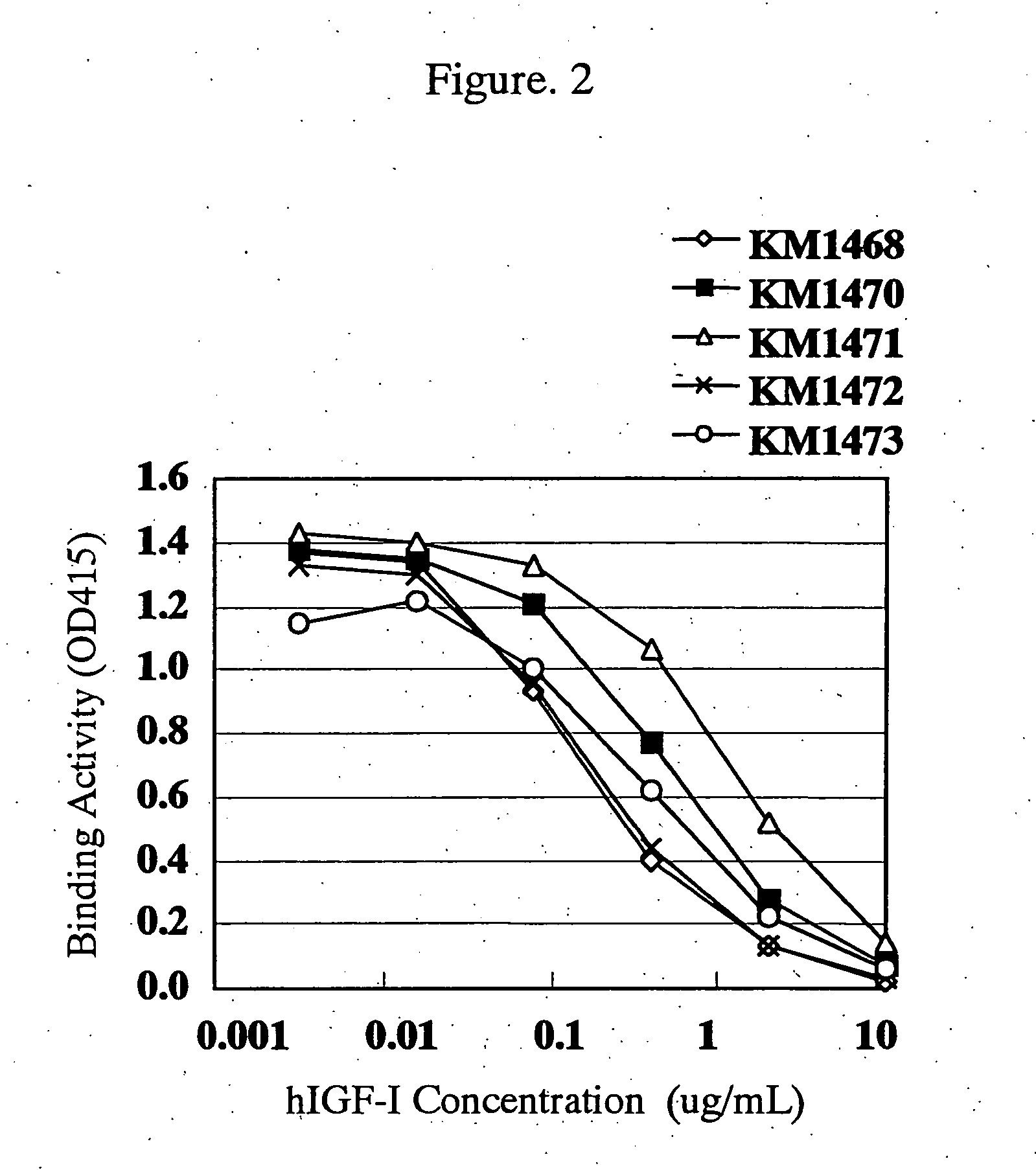
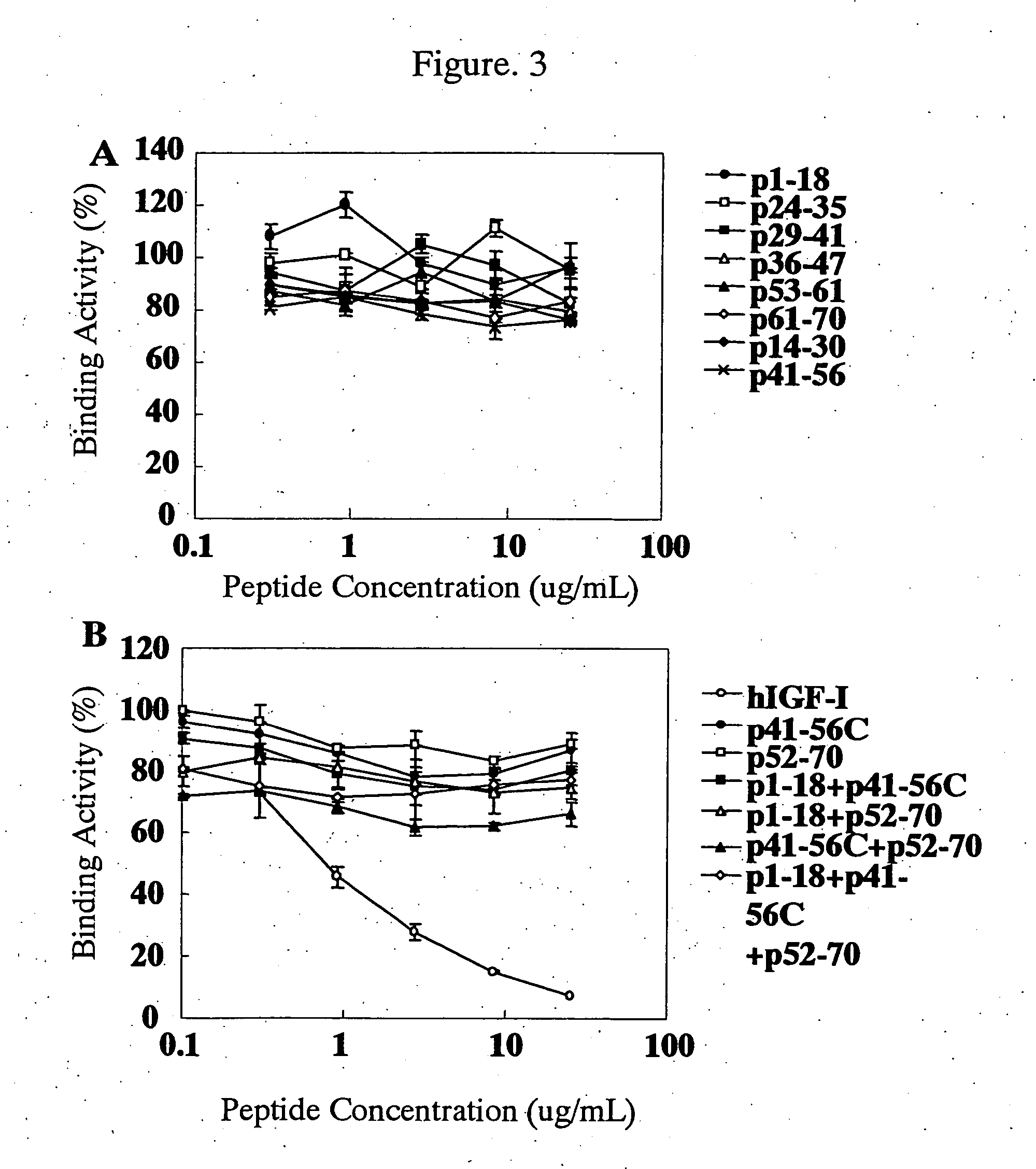
Antibody against human insulin-like growth factor
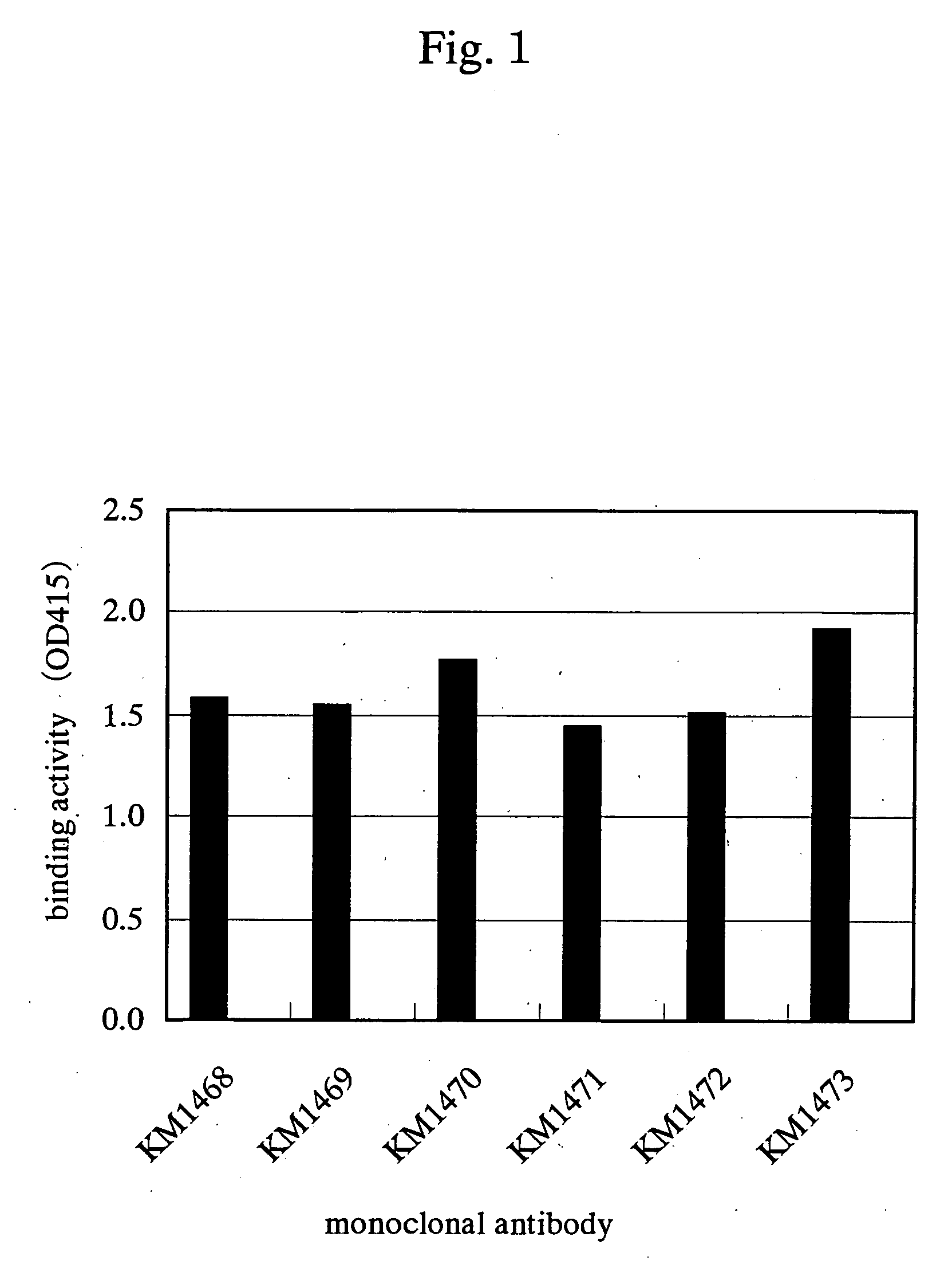
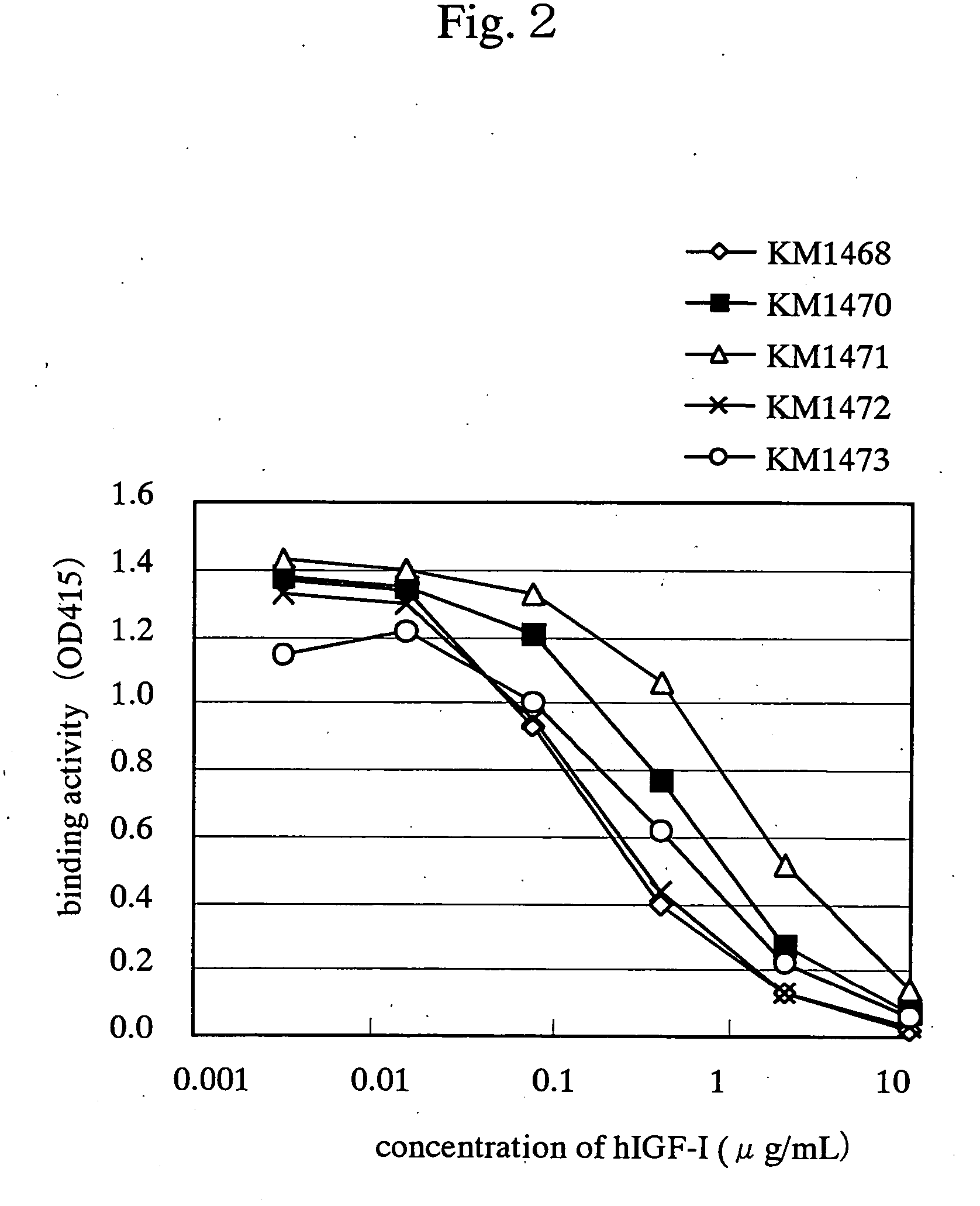
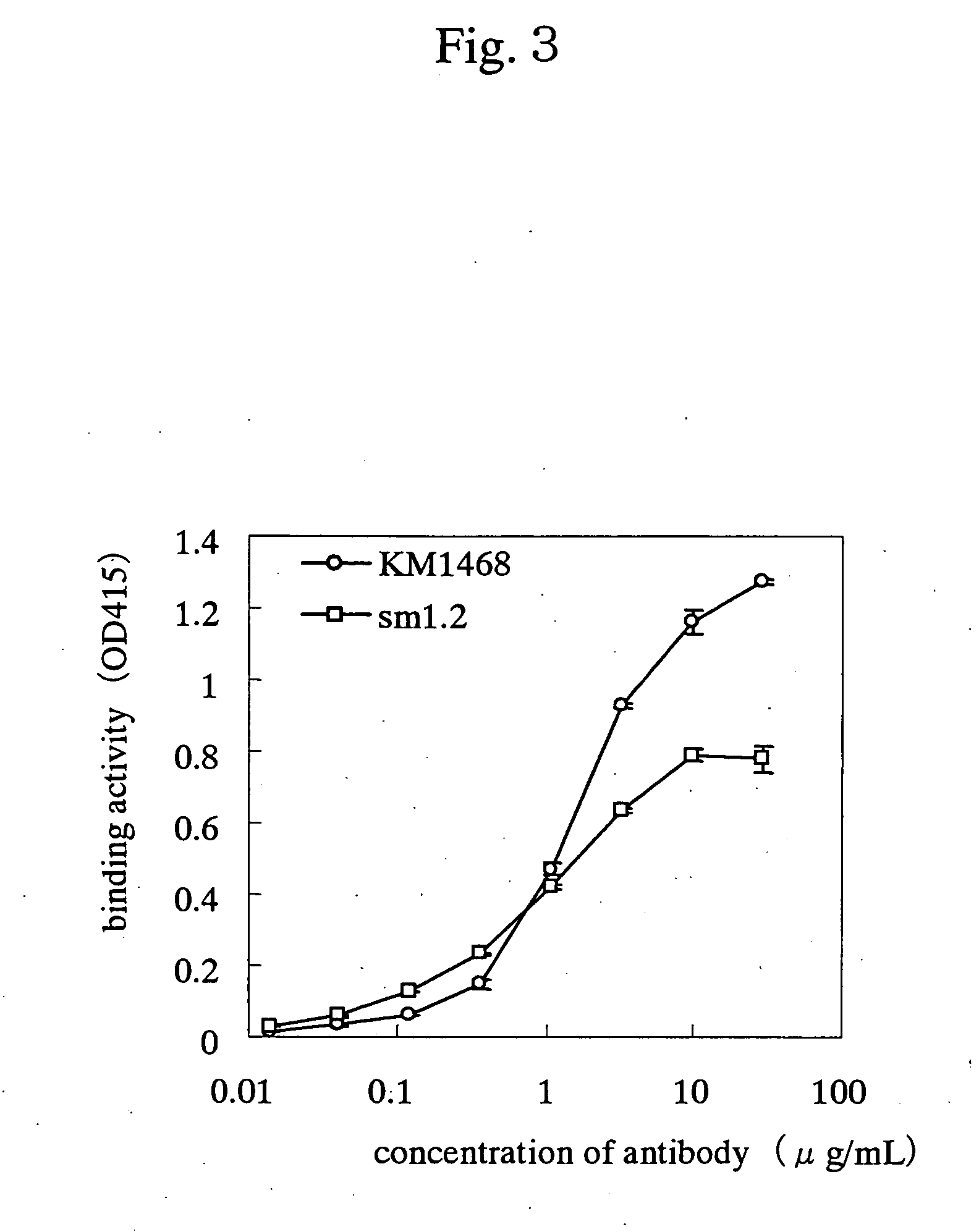
InactiveUS7438911B2Improve stabilitySuppression problemBacteriaAntipyreticInsulin-like growth factorDisease
For the effective treatment of diseases such as cancer in which hIGF participates, there have been desired to be developed antibodies which strongly bind to both factors hIGF-I and hIGF-II and inhibit their functions and fragments of these antibodies. The present invention provides antibodies which have the ability to specifically bind to human IGF-I and IGF-II to thereby inhibit the functions of human IGF-I and IGF-II and have binding activity with a binding constant of 5×109 M−1 or more measured with a biosensor BIACORE. In addition, the present invention provides diagnostics, preventives and remedies for an hIGF-mediated disease and a disease showing pathological progressing due to abnormally promoted hIGF production, which use said antibodies.
Owner:KYOWA HAKKO KIRIN CO LTD
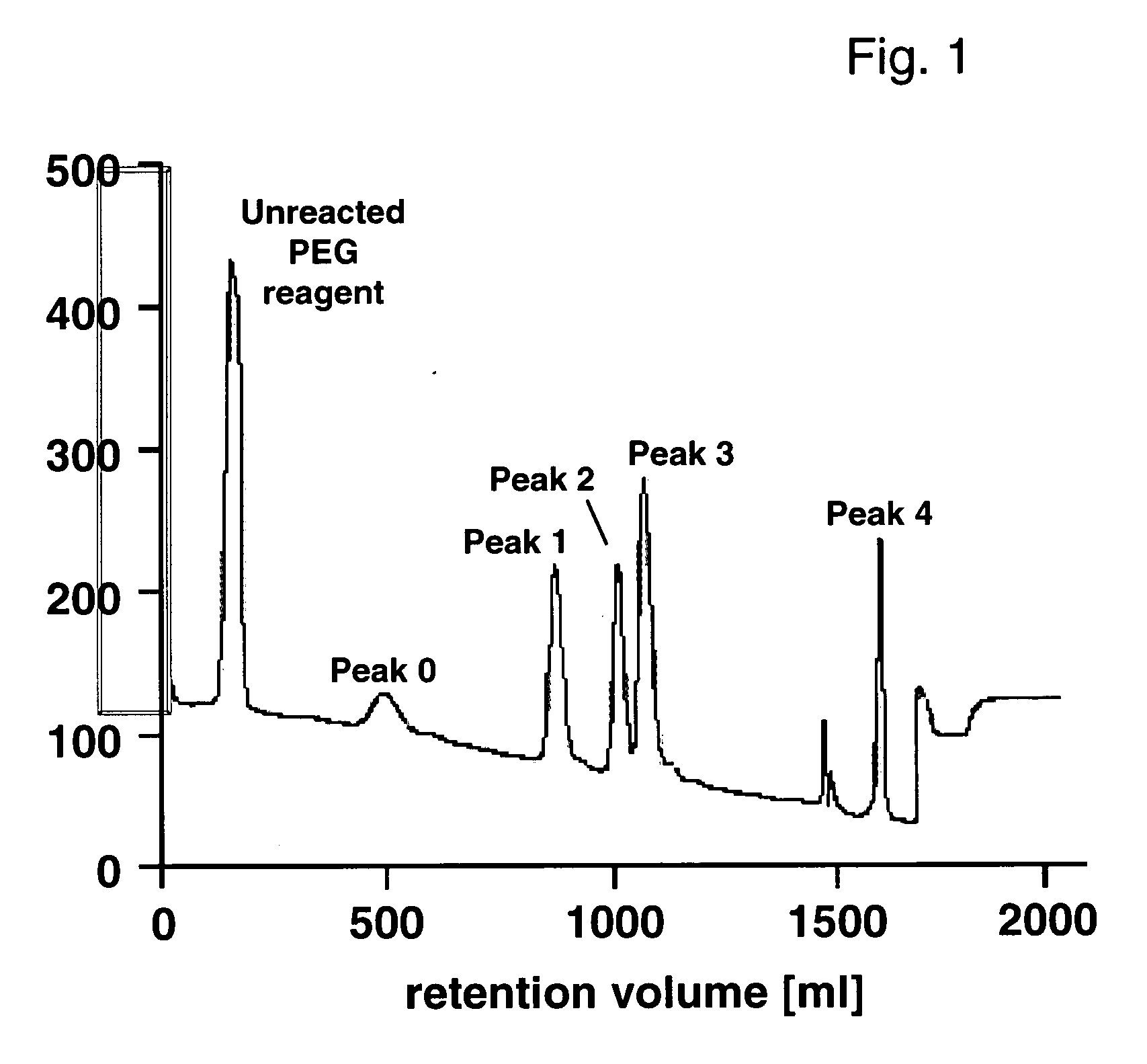
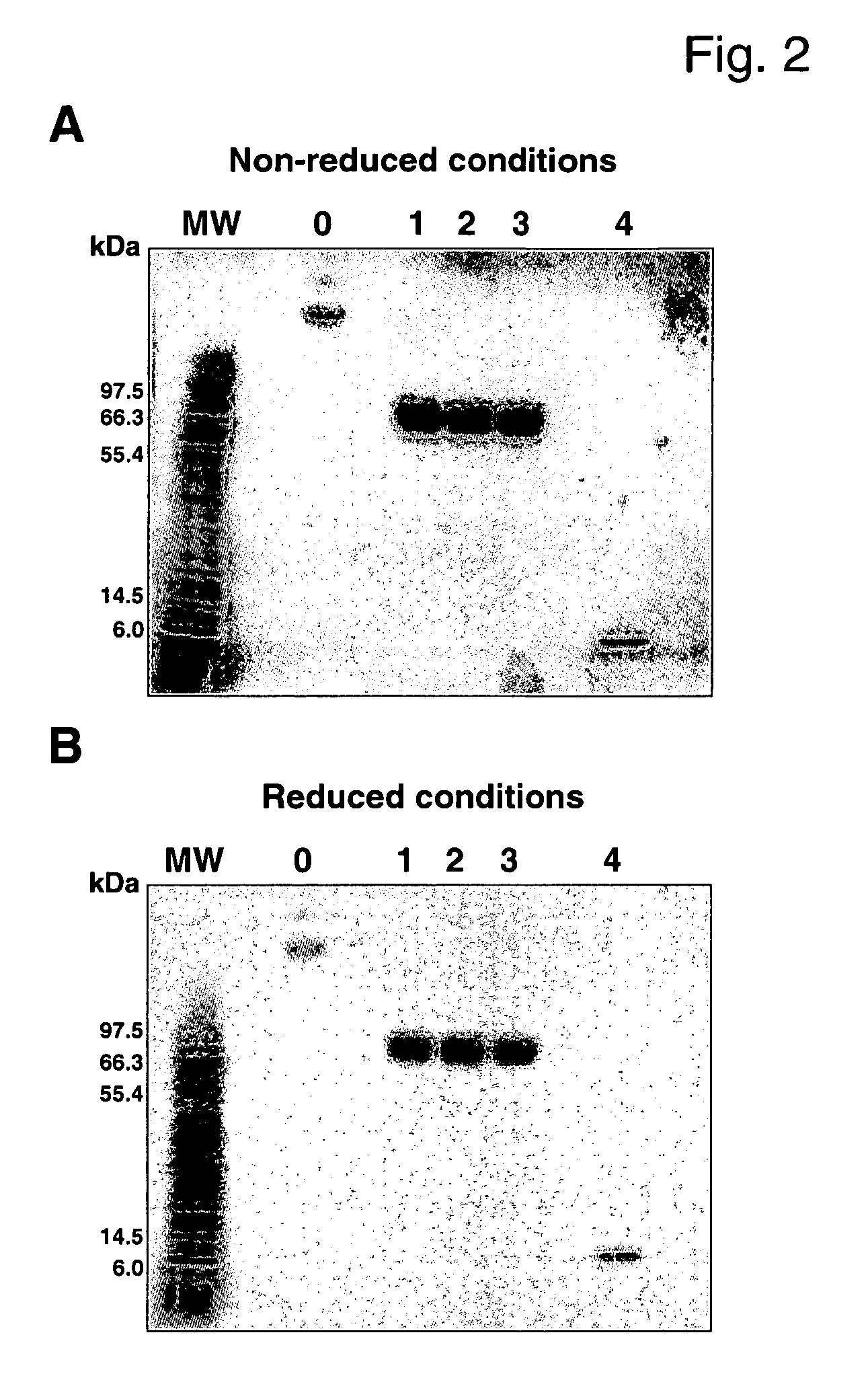
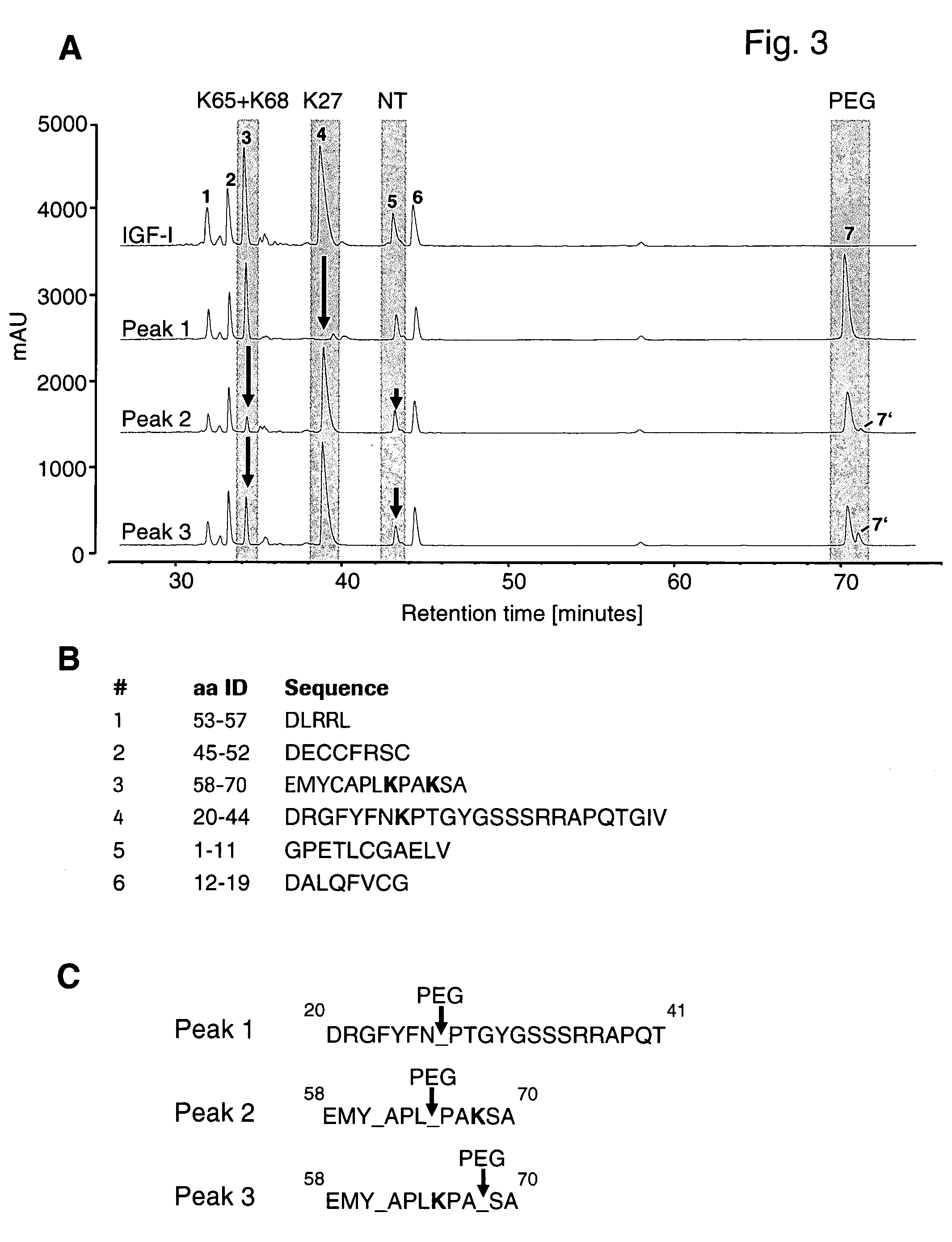
Conjugates of insulin-like growth factor-1 and poly(ethylene glycol)
InactiveUS20060154865A1Maintain good propertiesAntibacterial agentsNervous disorderInsulin-like growth factorPolyethylene glycol
A conjugate consisting of an insulin-like growth factor-1 (IGF-I) variant and one or two poly(ethylene glycol) group(s), characterized in that said IGF-I variant has an amino acid alteration at up to three amino acid positions 27, 37, 65, 68 of the wild-type IGF-I amino acid sequence so that one or two of said amino acids is / are lysine and amino acid 27 is a polar amino acid but not lysine, is conjugated via the primary amino group(s) of said lysine(s) and said poly(ethylene glycol) group(s) have an overall molecular weight of from 20 to 100 kDa is disclosed. This conjugate is useful for the treatment of neurodegenerative disorders like Alzheimer's Disease.
Owner:F HOFFMANN LA ROCHE & CO AG
Antibody against human insulin-like growth factor
InactiveUS20060165695A1Improve stabilitySuppression problemBacteriaAntipyreticInsulin-like growth factorDisease
For the effective treatment of diseases such as cancer in which hIGF participates, there have been desired to be developed antibodies which strongly bind to both factors hIGF-I and hIGF-II and inhibit their functions and fragments of these antibodies. The present invention provides antibodies which have the ability to specifically bind to human IGF-I and IGF-II to thereby inhibit the functions of human IGF-I and IGF-II and have binding activity with a binding constant of 5×109 M−1 or more measured with a biosensor BIACORE. In addition, the present invention provides diagnostics, preventives and remedies for an hIGF-mediated disease and a disease showing pathological progressing due to abnormally promoted hIGF production, which use said antibodies.
Owner:KYOWA HAKKO KIRIN CO LTD
Insulin-like growth factor system and cancer
InactiveUS6448086B1Microbiological testing/measurementDisease diagnosisInsulin-like growth factorAntigen
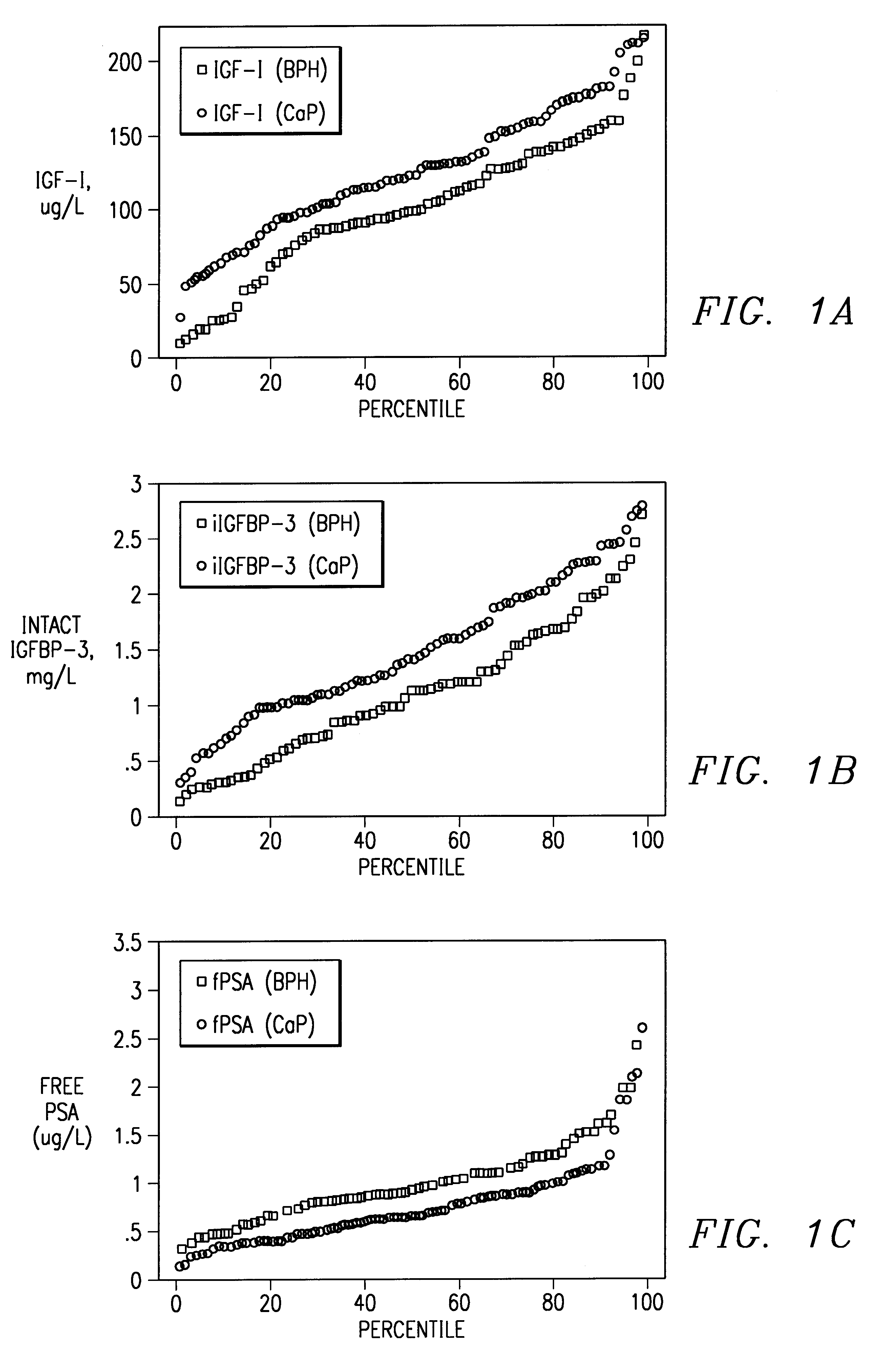
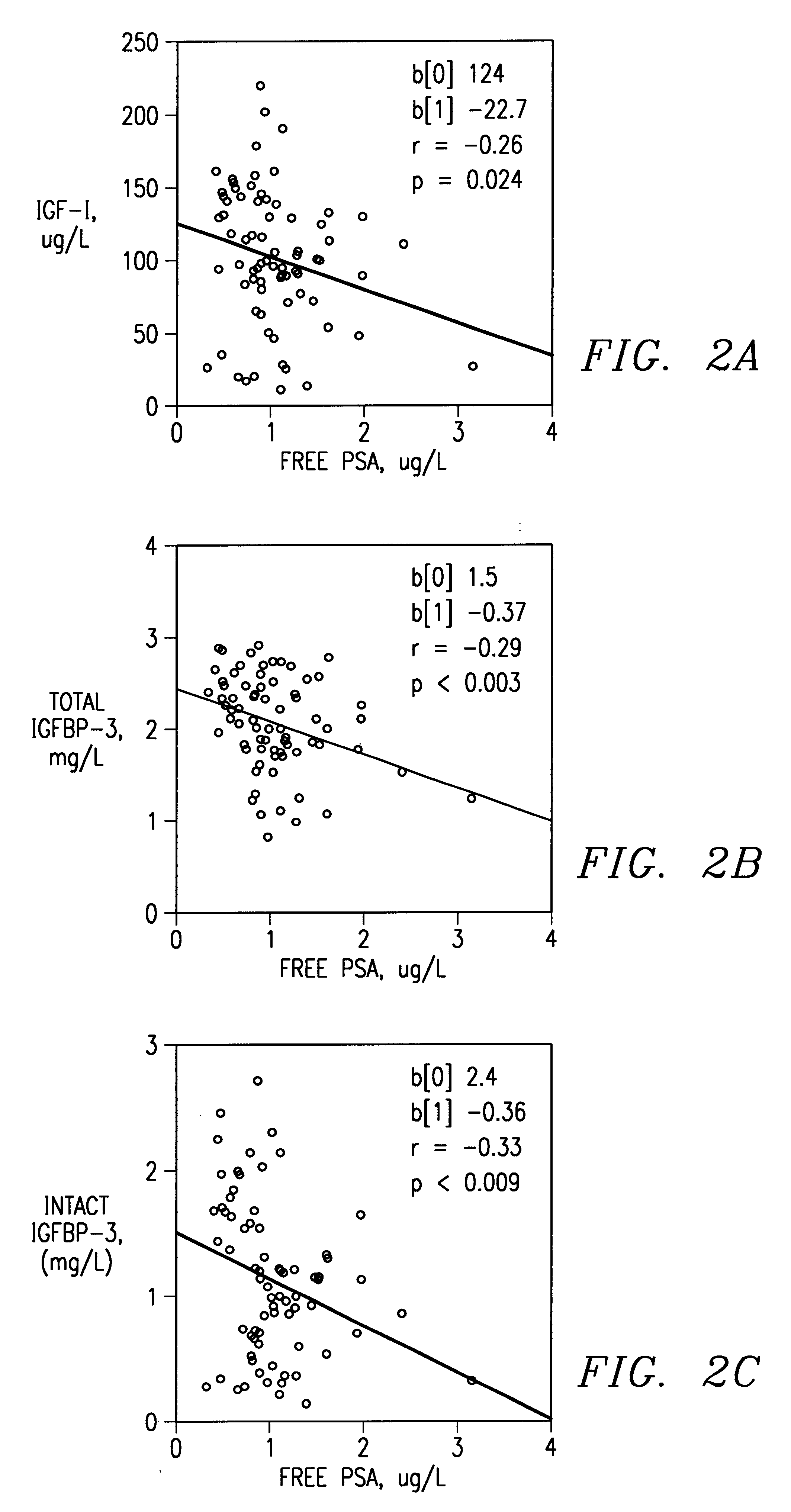
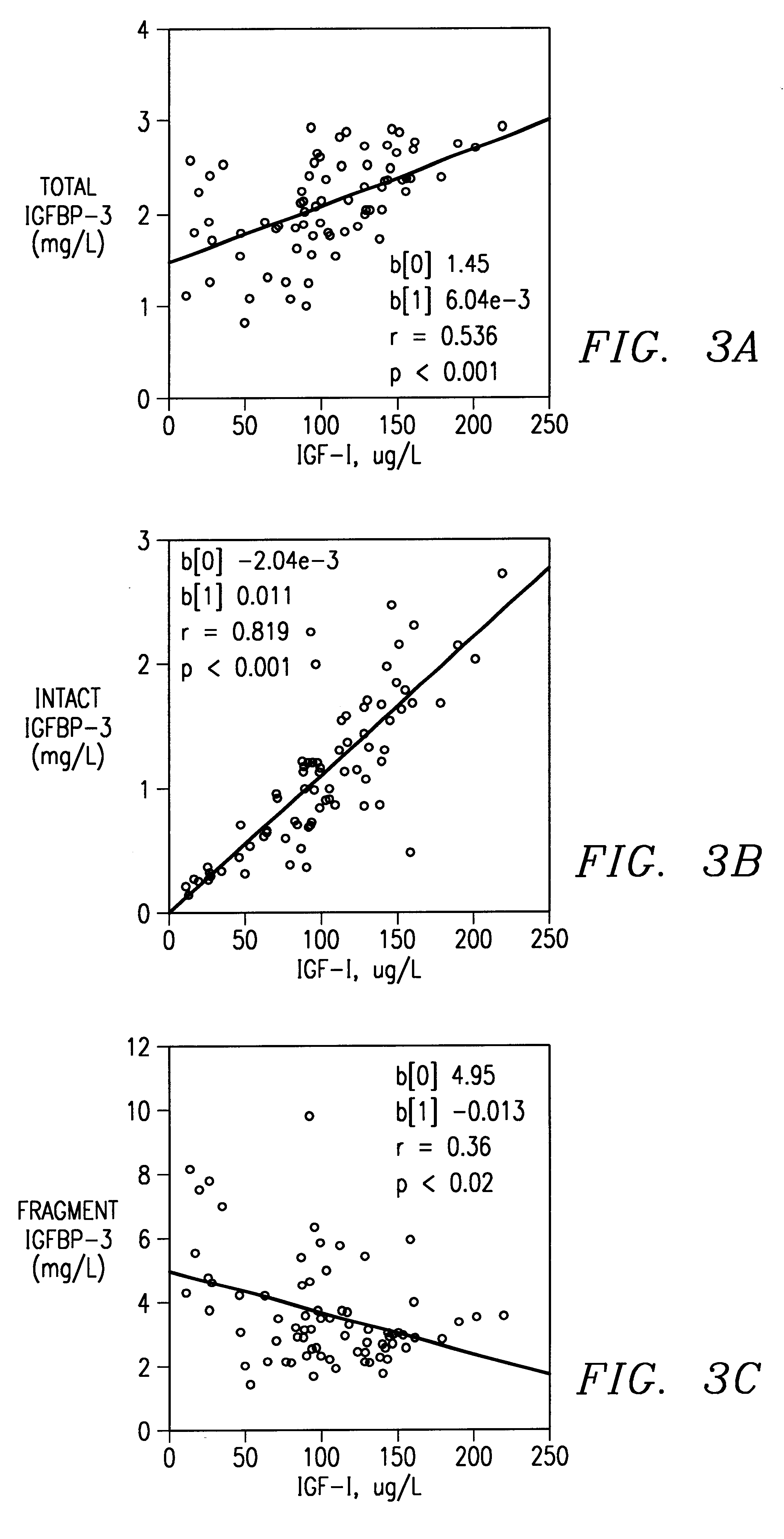
Method of monitoring or diagnosing disease conditions, including disease of the prostate, that involve measuring a combination of tumor markers and at least one component of the IGF axis. The invention is exemplified with prostate cancer and benign prostatic hyperplasia, the tumor marker prostate specific antigen, and the insulin-like growth factors and their binding proteins.
Owner:BECKMAN COULTER INC
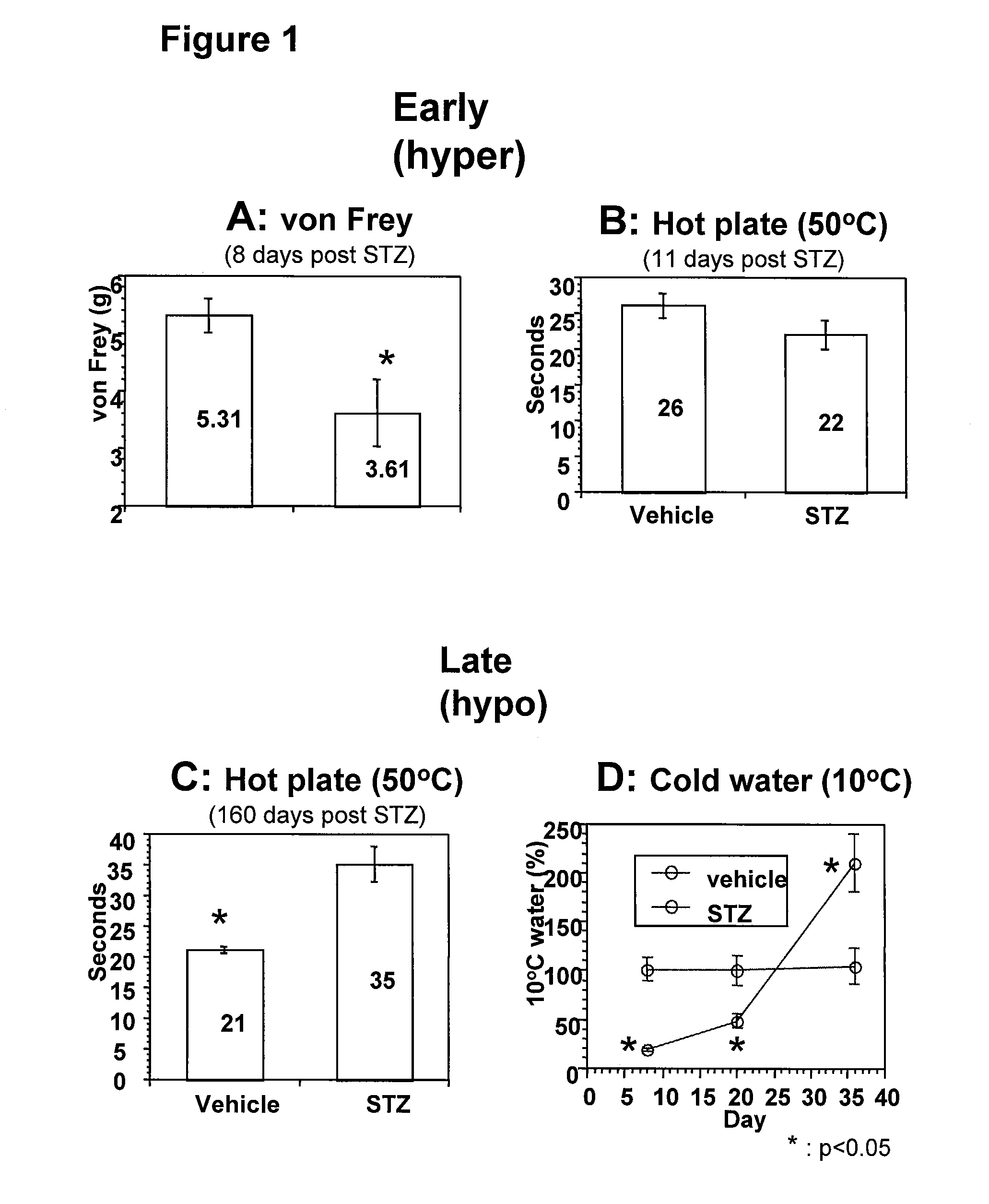
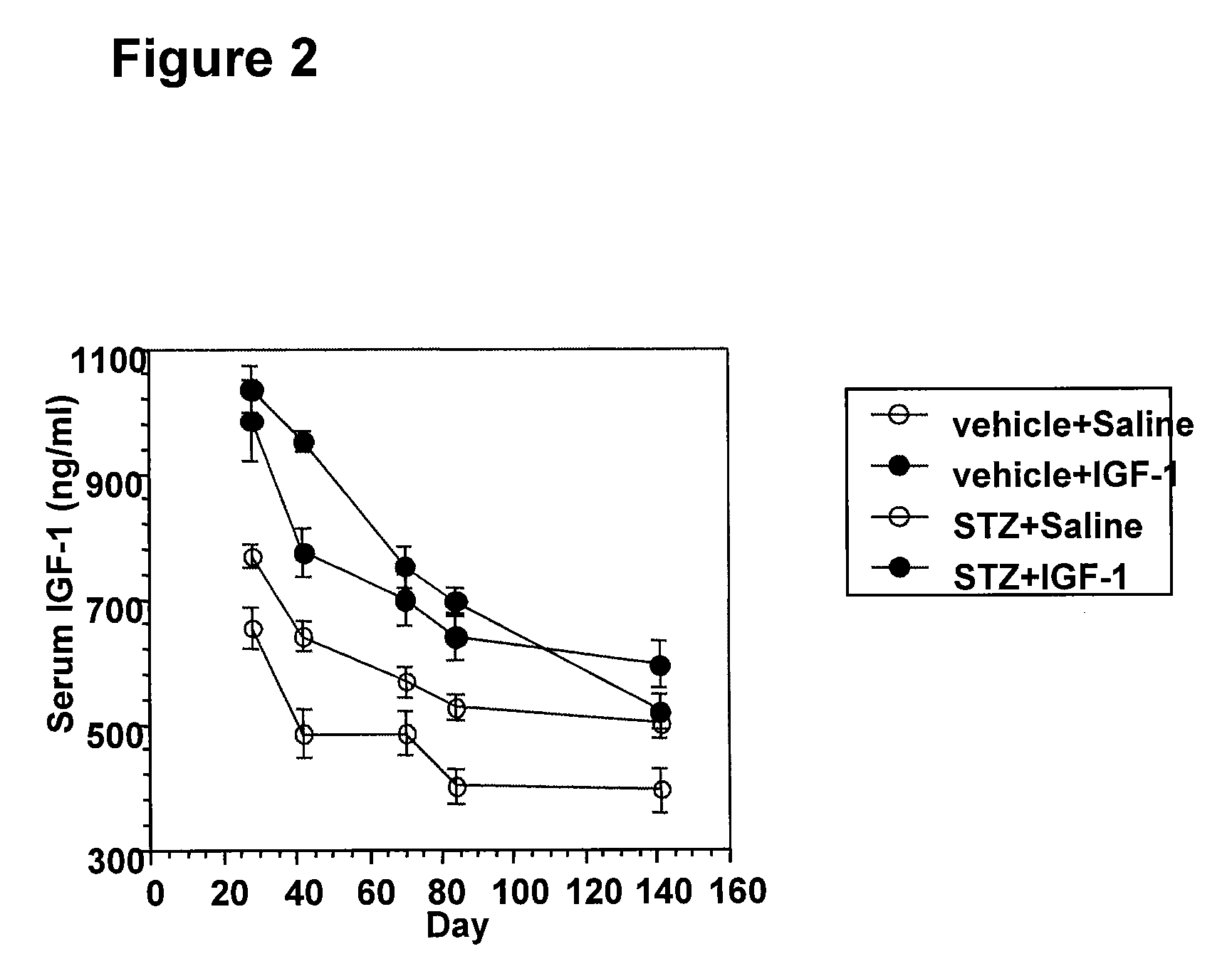
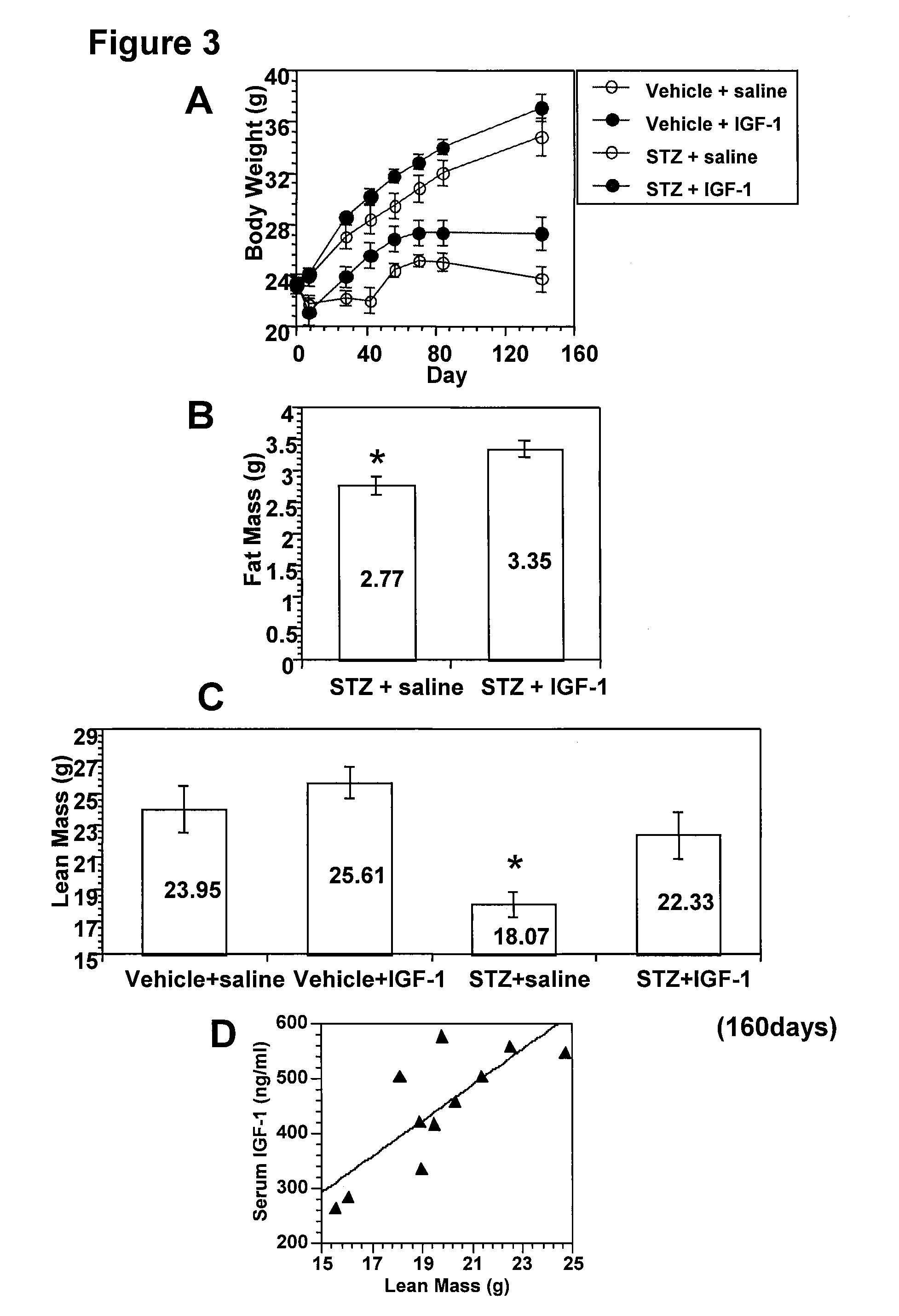
Systemic insulin-like growth factor-1 therapy reduces diabetic peripheral neuropathy and improves renal function in diabetic nephropathy
InactiveUS20100216709A1Prevents subsequent hyposensitivityEasy maintenanceOrganic active ingredientsNervous disorderInsulin-like growth factorHyperglycemic disorder
The present invention provides methods of treatment of patients suffering from the complications of blood sugar disorders: diabetic peripheral neuropathy and diabetic nephropathy by administration of IGF-1 via protein therapy or gene therapy. It relates to methods of treating an individual having a diabetic disorder or a hyperglycemic disorder, comprising administering to the individual an effective amount of a DNA vector expressing IGF-1Eb or IGF-1Ec in vivo or an effective amount of at the IGF-1Eb or IGF-1Ec protein in the early hyperalgesia stage or in patients that have advanced to the hyposensitivity stage. Treatment at the early hyperalgesia stage prevents subsequent hyposensitivity with increases or maintenance of sensory nerve function. IGF-1Eb or IGF-1Ec treatment also increases muscle mass and improves overall mobility, which indicates a treatment-related improvement in motor function. Treatment with IGF-1Eb or IGF-1Ec at the hyposensitivity stage reverses hyposensitivity and improves muscle mass and overall health. Systemic IGF-1 provides a therapeutic modality for treating hyposensitivity associated with DPN. In addition, IGF-1Eb or IGF-1Ec provides a therapeutic modality for treating diabetic nephropathy. IGF-1Eb or IGF-1Ec improves renal function as evidenced by a modulation in serum albumin concentration and a reduction in urine volume and protein levels. IGF-1Eb or IGF-1Ec also reduces diabetic glomerulosclerosis.
Owner:GENZYME CORP
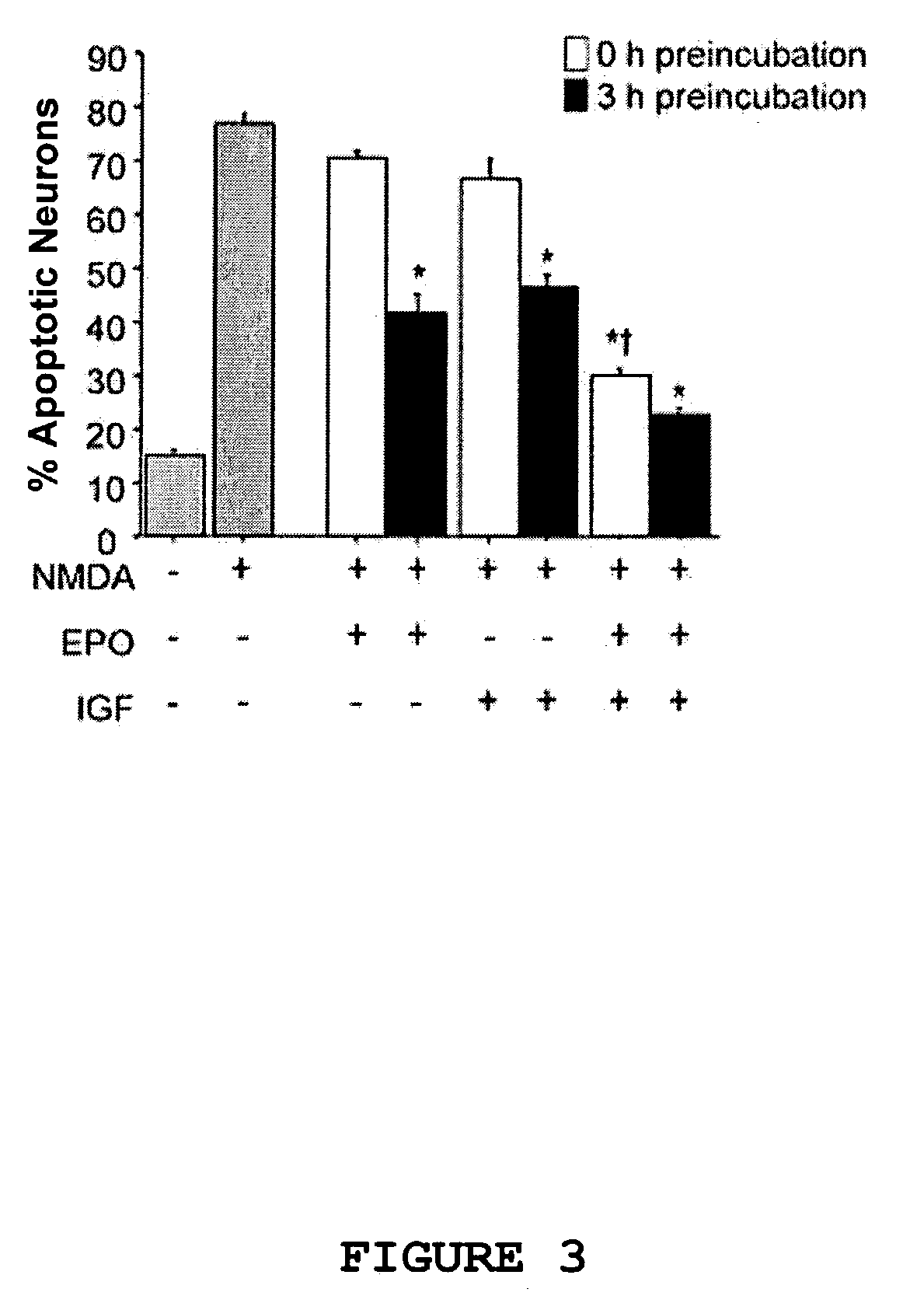
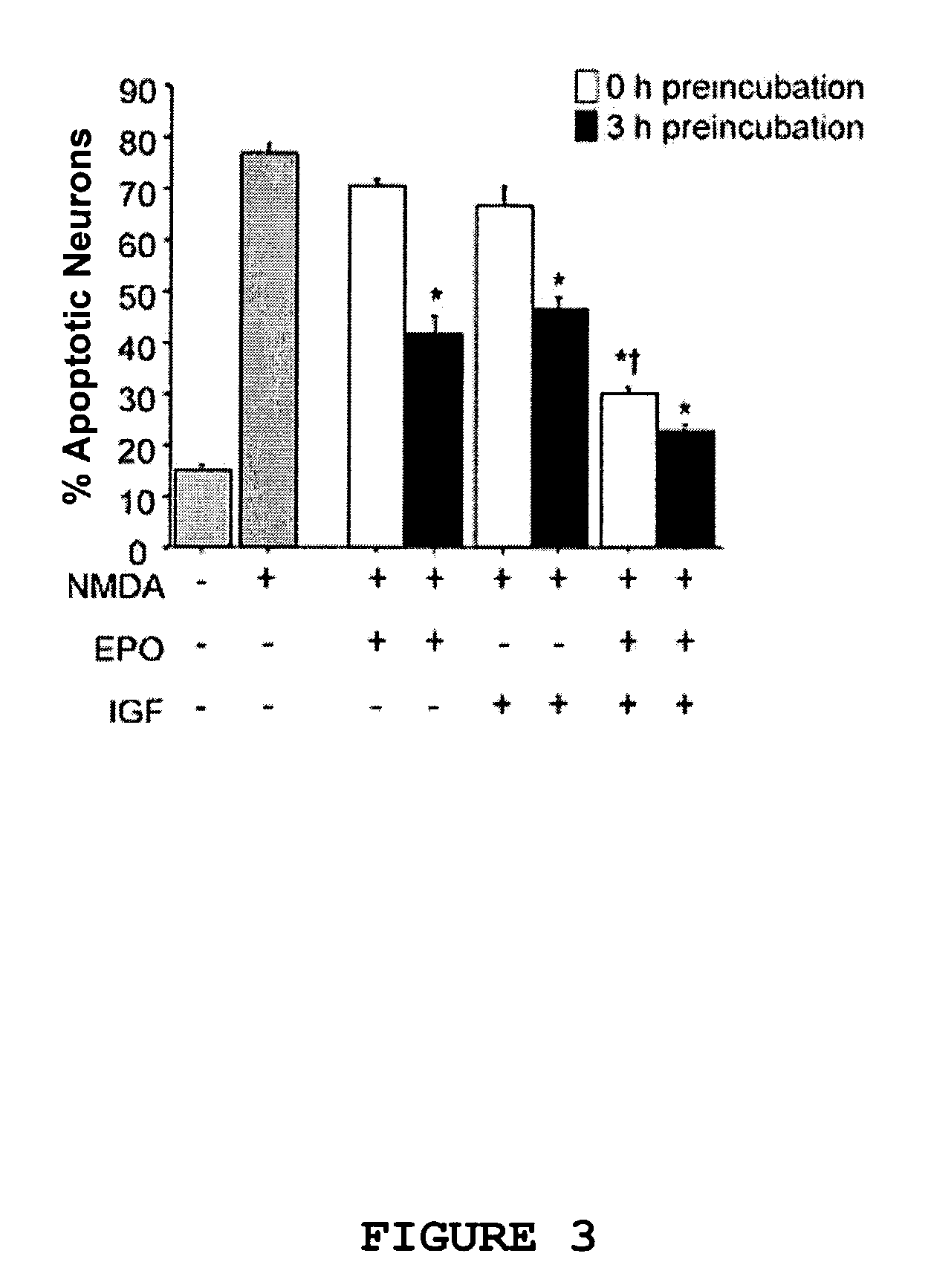
Neuroprotective synergy of erythropoietin and insulin-like growth factor
InactiveUS20040092444A1Prevention and reduction of severityBiocideSenses disorderInsulin-like growth factorHuntingtons chorea
The present invention provides a method of providing acute neuroprotection by inducing the erythropoietin (EPO) signaling pathway in neuronal cells close to or subsequent to the time of excitatory insult; and inducing an insulin-like growth factor (IGF) signaling pathway in the neuronal cells close to or subsequent to the time of excitatory insult, thereby producing a synergistic acute neuroprotective effect in the neuronal cells. The invention also provides a method of preventing or reducing the severity of a neurologic condition in a subject by administering to the subject EPO or an active fragment or analog thereof at a dose of at most 2000 U / kg; and administering to the subject an IGF or an active fragment or analog thereof, thereby providing neuroprotection and preventing or reducing the severity of the neurologic condition. Such a method can be used to prevent or reduce the severity of, for example, Alzheimer's disease, Parkinson's disease, Huntington's disease, epilepsy, amyotrophic lateral sclerosis, multiple sclerosis, a movement disorder, HIV-associated dementia, HIV-associated neuropathy, neuropathic pain, migraine, glaucoma, drug addiction, drug withdrawal, drug dependency, depression or anxiety.
Owner:BURNHAM INST FOR MEDICAL RES +1
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
InactiveUS20110174062A1Eliminate needEasy to adaptDisease diagnosisTesting metalsInsulin-like growth factorRenal Failures
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using assays that detect one or more markers selected from the group consisting of cytochrome c and insulin-like growth factor IA as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
Method of treating glaucoma and intraocular hypertension
InactiveUS20110294730A1Senses disorderPeptide/protein ingredientsInsulin-like growth factorProstaglandin analog
A safe and effective treatment for glaucoma for mammalian species comprises the steps of applying insulin and / or insulin like growth factors (IGF-1) to an eye. In addition to the insulin, another therapeutic agent may be applied to enhance the activity of the insulin. The therapeutic agent may be a pharmaceutical agent or a biochemical pharmaceutical agent. The therapeutic agents include prostaglandin analogs, topical beta-adrenergic receptor antagonists-β blockers, Alpha2-adrenergic agonists hair growth therapeutic agents, beta2-agonist action agents, parasympathomimetic miotic agents, carbonic anhydrase inhibitors, and Physostigmine. In another embodiment, a combination of at least two agents are applied to the eye. To enhance the effect of the insulin, uptake facilitators may be used. Additionally, an antibacterial agent may be applied to control bacterial infection.
Owner:SHANTHA TOTADA R +2
Neuroprotective synergy of erythropoietin and insulin-like growth factors
InactiveUS7439063B2Prevention and reduction of severityBiocideSenses disorderInsulin-like growth factorHuntingtons chorea
The present invention provides a method of providing acute neuroprotection by inducing the erythropoietin (EPO) signaling pathway in neuronal cells close to or subsequent to the time of excitatory insult; and inducing an insulin-like growth factor (IGF) signaling pathway in the neuronal cells close to or subsequent to the time of excitatory insult, thereby producing a synergistic acute neuroprotective effect in the neuronal cells. The invention also provides a method of preventing or reducing the severity of a neurologic condition in a subject by administering to the subject EPO or an active fragment or analog thereof at a dose of at most 2000 U / kg; and administering to the subject an IGF or an active fragment or analog thereof, thereby providing neuroprotection and preventing or reducing the severity of the neurologic condition. Such a method can be used to prevent or reduce the severity of, for example, Alzheimer's disease, Parkinson's disease, Huntington's disease, epilepsy, amyotrophic lateral sclerosis, multiple sclerosis, a movement disorder, HIV-associated dementia, HIV-associated neuropathy, neuropathic pain, migraine, glaucoma, drug addiction, drug withdrawal, drug dependency, depression or anxiety.
Owner:BURNHAM INST FOR MEDICAL RES +1
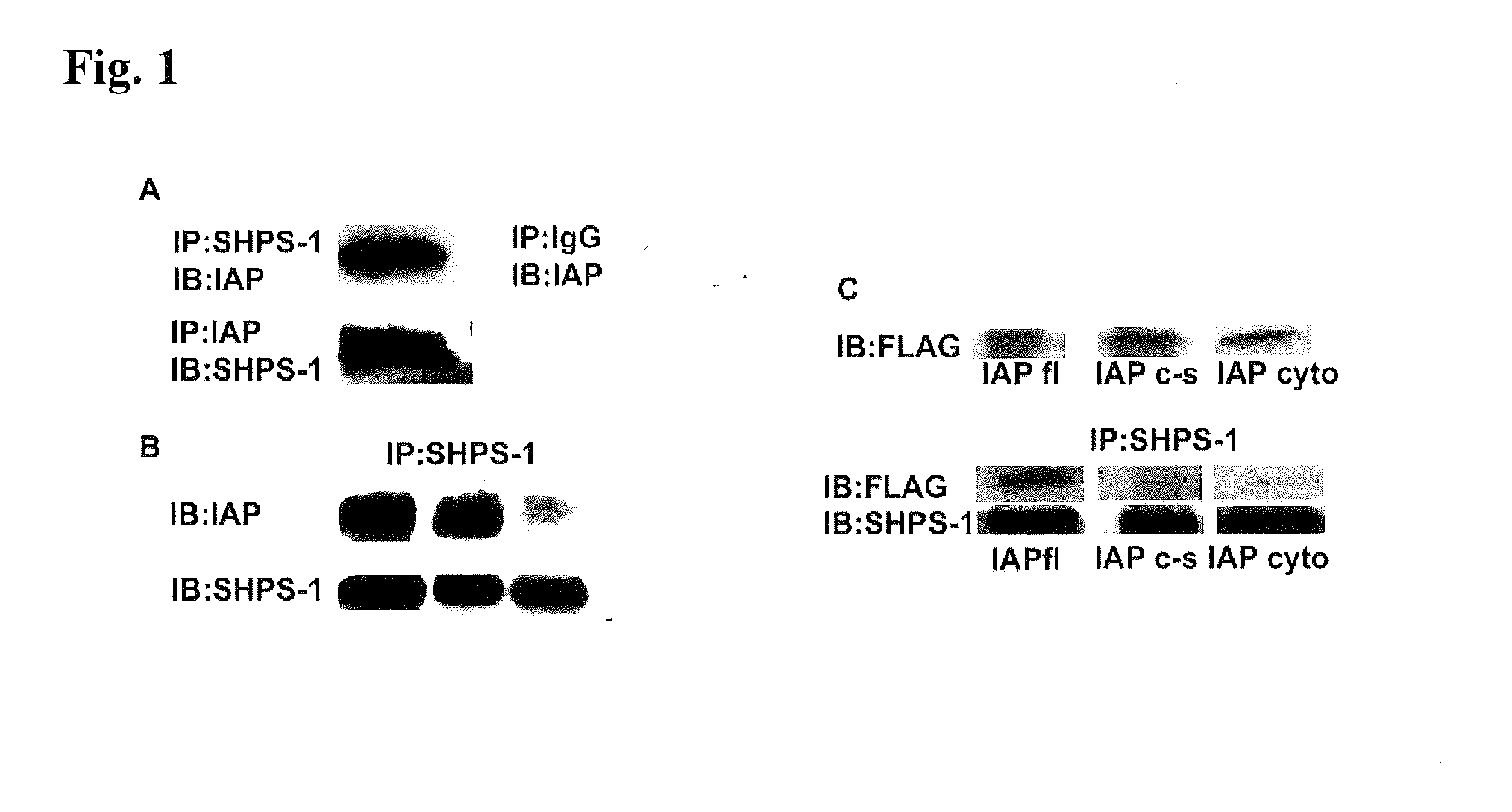
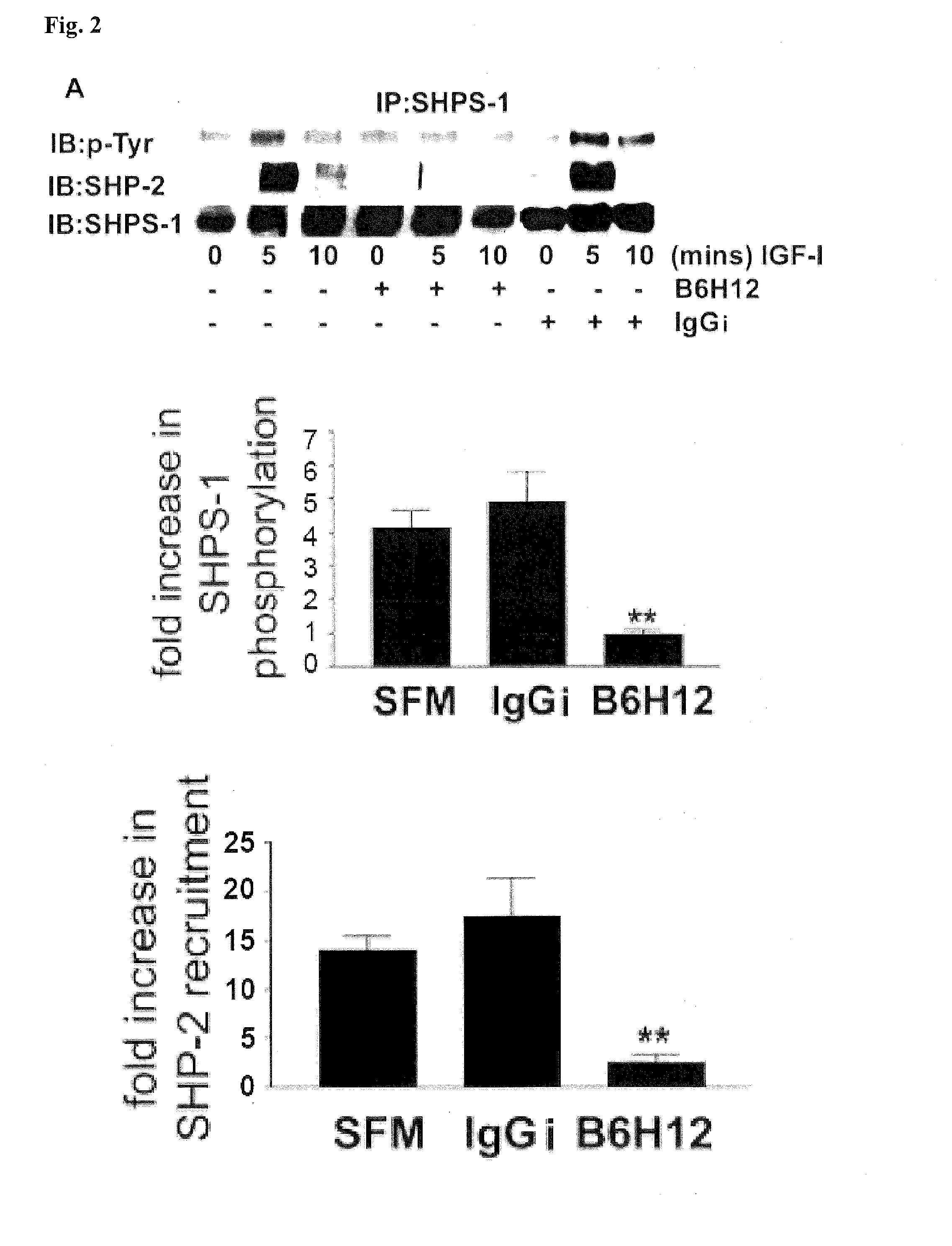
Method for inhibiting cellular activation by insulin-like growth factor-1
InactiveUS20120039896A1Senses disorderAntibody mimetics/scaffoldsInsulin-like growth factorDiabetic retinopathy
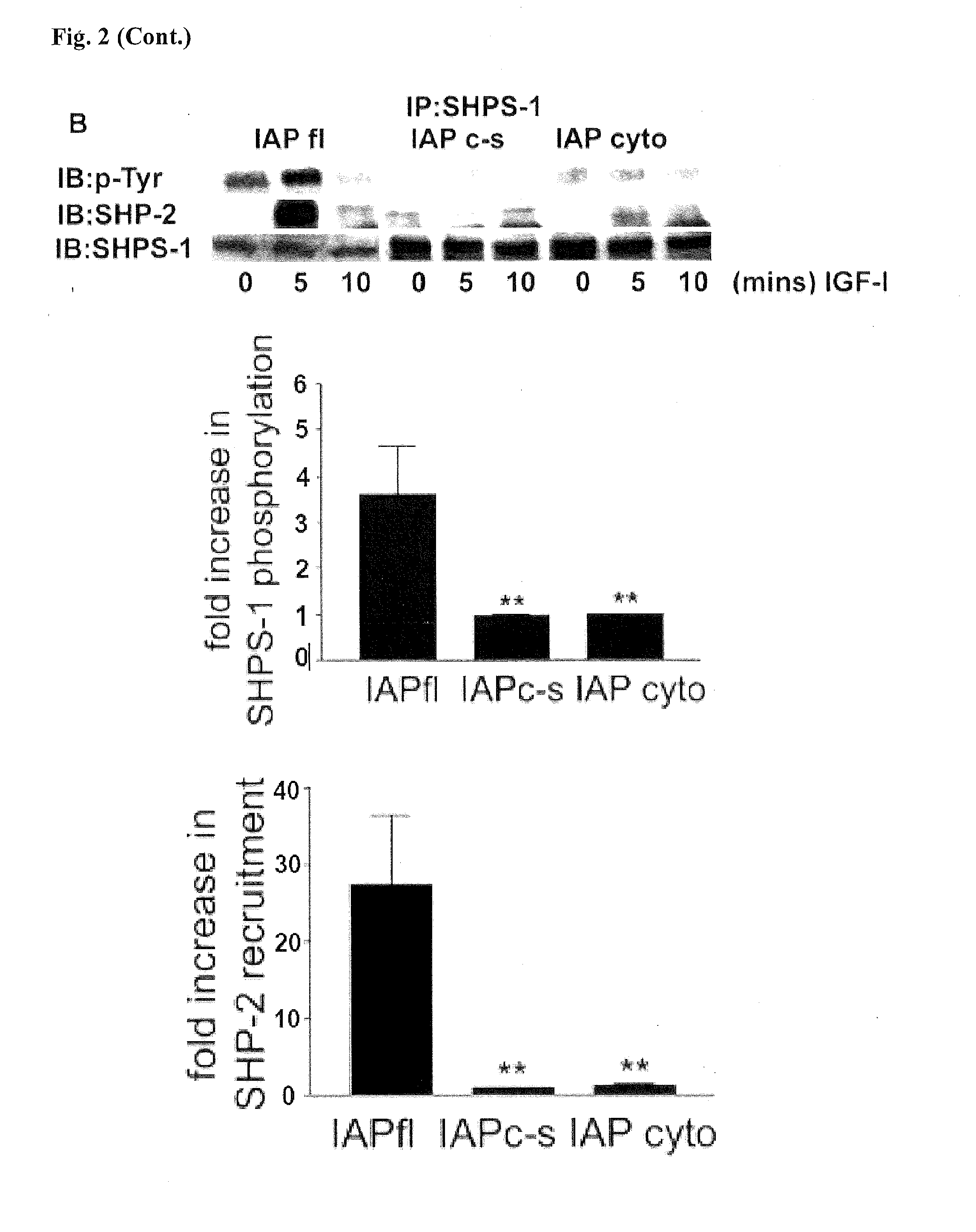
A method of inhibiting cellular activation by Insulin-like Growth Factor-1 (IGF-1) in a subject in need thereof (e.g., a subject afflicted with cancer, atherosclerosis, diabetic retinopathy or other disease) comprises administering an antagonist that inhibits the binding of IAP to SHPS-1 to the subject in an amount effective to inhibit cellular activation by IGF-1. Compounds and compositions for carrying out such methods are also described.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Therapeutic combinations of Anti-igf-1r antibodies and other compounds
InactiveUS20090291088A1Reduce dwell timeHigh binding affinityAntibody mimetics/scaffoldsAntibody ingredientsInsulin-like growth factorAntiendomysial antibodies
The invention relates to methods of treatment using combination therapy wherein a variety of therapeutically useful compounds may be combined with antibodies which bind to insulin-like growth factor receptor-1 (IGF-1R). Specific human and murine monoclonal antibodies which inhibit IGF-1R-mediated pro-survival and tumor proliferation pathways, and variants, fragments, and derivatives thereof are provided. Also provided are specific human and murine monoclonal antibodies which block the ability of the ligands, insulin like growth factor 1 (IGF-1) and insulin like growth factor 2 (IGF-2) to bind to IGF-1R, as well as fragments, variants and derivatives of such antibodies. The invention also includes polynucleotides encoding the above antibodies or fragments, variants or derivatives thereof, as well as vectors and host cells comprising such polynucleotides. The invention particularly includes methods of treating cancer using combination therapies with IGF-1R antibodies.
Owner:BIOGEN MA INC
Stable antibody formulations
InactiveUS20100260766A1Pharmaceutical delivery mechanismAntibody ingredientsInsulin-like growth factorAntibody
The present invention provides formulations and methods for the stabilization of antibodies. In one embodiment, the invention provides the stable solution formulation of an IgG1 antibody that specifically binds to insulin-like growth factor-I receptor. In another embodiment, the invention provides methods of stabilization of IgG1 antibody that specifically binds to insulin-like growth factor-I receptor comprising lyophilizing an aqueous formulation of the antibody. The formulations can be lyophilized to stabilize the antibodies during processing and storage, and then the formulations can be reconstituted for pharmaceutical administration.
Owner:IMCLONE SYSTEMS
Compositions and methods for treating articular cartilage disorders
InactiveUS7141545B2Increase volumeEasy maintenancePeptide/protein ingredientsSkeletal disorderInsulin-like growth factorDisease
A method for treating mammalian articular cartilage disorders, more particularly osteoarthritis, and trauma-related cartilage injuries using insulin-like growth factor I (IGF-I) is provided. The method comprises increasing the amount of IGF-I at the diseased or injured articular site to a therapeutically effective level that is capable of maintenance and / or regeneration of cartilage, which is beneficial to the long-term treatment of osteoarthritis and trauma-related injuries to cartilage tissues. In one embodiment of the invention, single doses of at least 0.01 mg of pharmaceutically effective IGF-I are administered intermittently such that the duration of time off of therapy is greater than the time on therapy, more preferably with a frequency of administration of about once per week or less.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Medium and methods for culturing of avian primordial germ cells
InactiveUS20050282273A1Faster rateKeep for a long timeCulture processArtificial cell constructsInsulin-like growth factorPlant Germ Cells
The invention provides a novel culture medium useful for the proliferation and maintenance of avian primordial germ cells (PGCs) and encompassing a medium base, leukemia inhibitory factor, basic fibroblast growth factor, stem cell factor and insulin-like growth factor, and an avian serum. The invention also provides a method for the proliferation and maintenance of avian primordial germ cells for extended periods encompassing the steps of isolating a population of PGCs from an avian and culturing the PGCs in a culture medium containing the growth factors and avian serum. The invention further provides methods of producing chimeric avians by isolating and culturing PGCs in a culture medium containing avian serum, transferring the PGCs into a recipient avian embryo, and allowing the recipient avian to develop into a chimeric bird. Another aspect of the invention provides a culture of avian PGCs grown and maintained in the culture medium containing avian serum.
Owner:MERIAL LTD
Antibodies against insulin-like growth factor 1 receptor and uses thereof
InactiveUS7378503B2Slow tumor growthExtension of timePeptide/protein ingredientsPeptide sourcesInsulin-like growth factorMedicine
Antibodies which bind to IGF-IR and inhibit the binding of IGF-I and IGF-II to IGF-IR are characterized. These antibodies are implicated in anti-tumor therapy.
Owner:F HOFFMANN LA ROCHE & CO AG
Biological markers for longevity and diseases and uses thereof
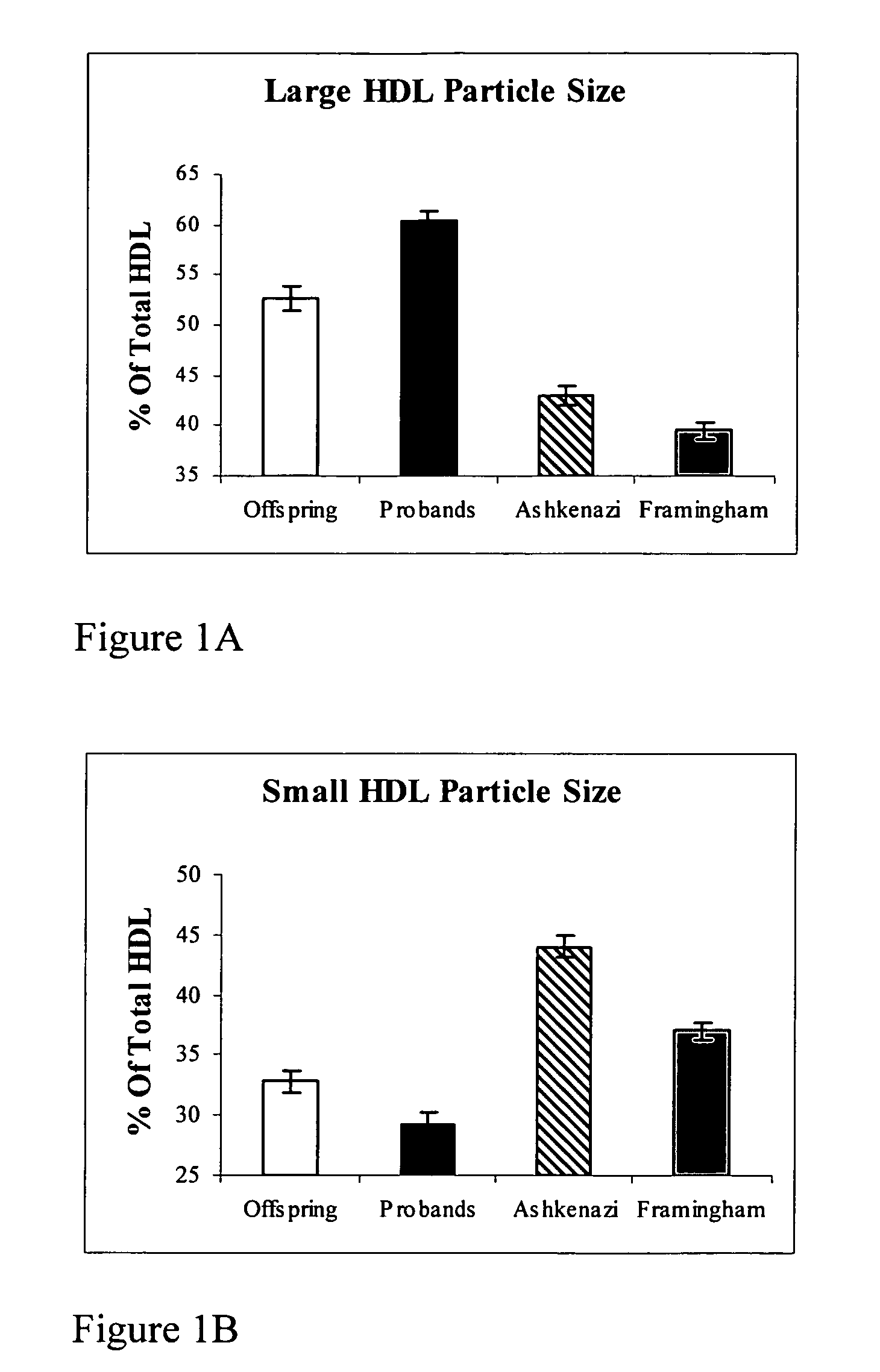
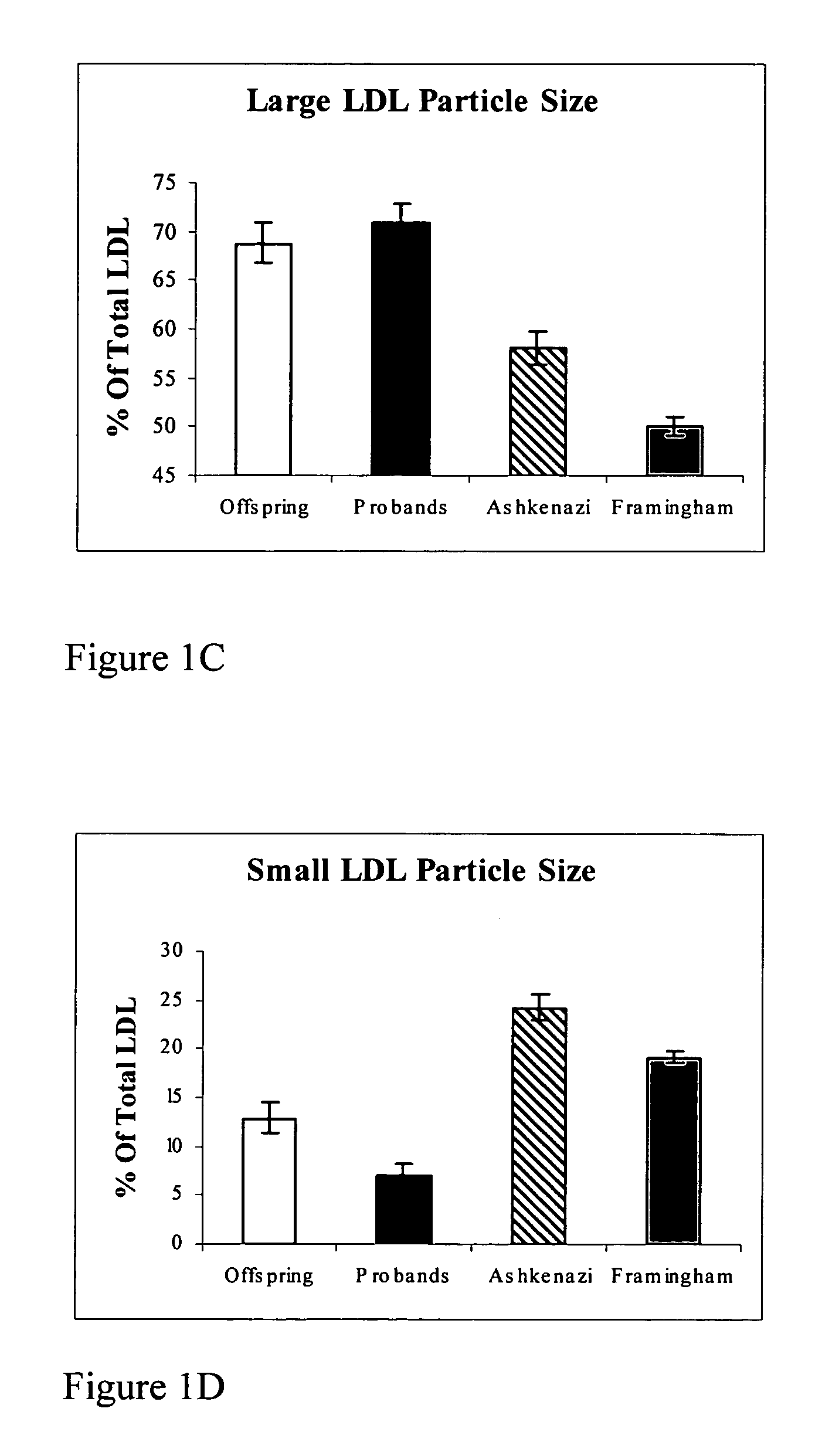
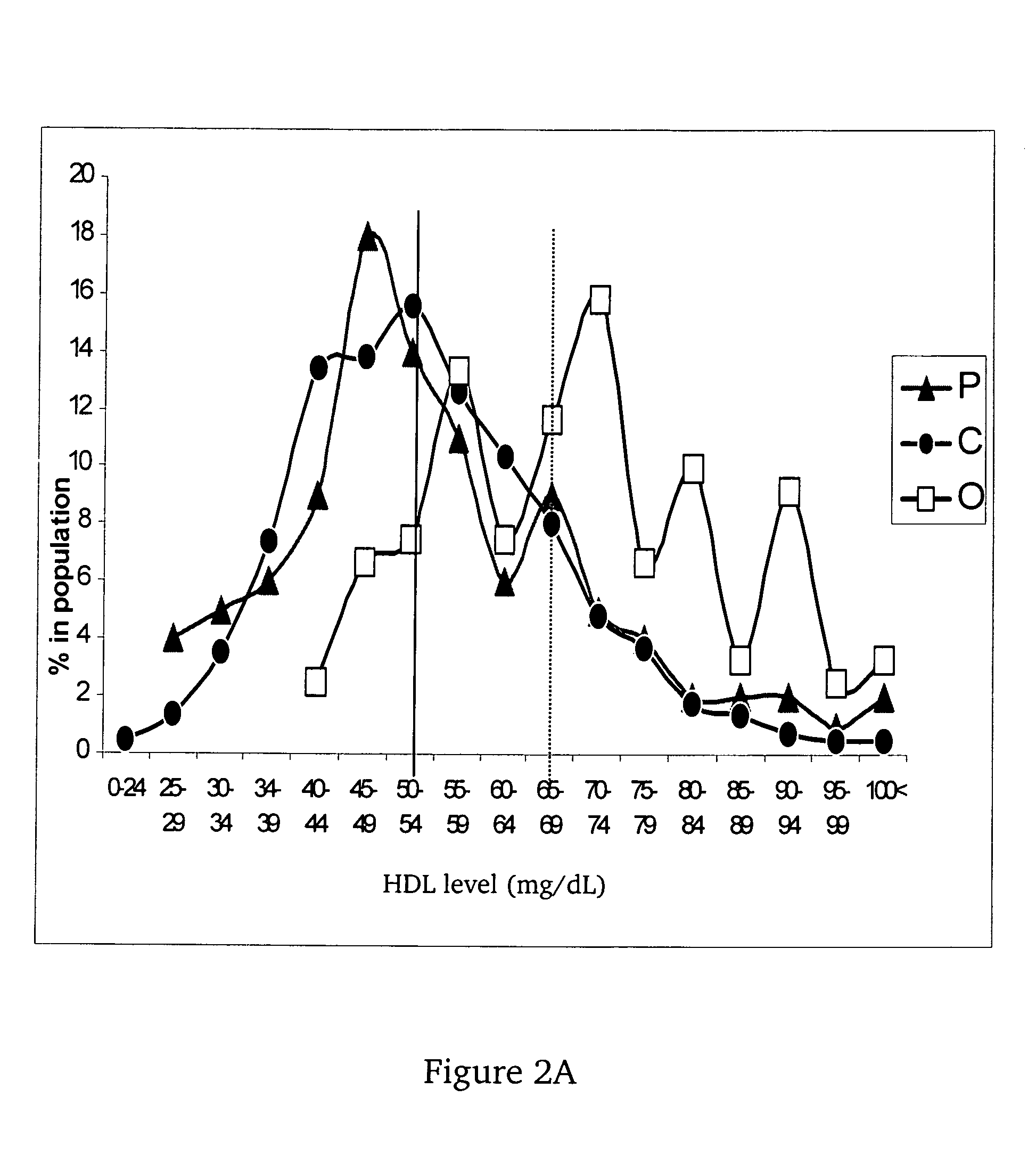
InactiveUS7491543B2Raise the possibilityLikelihood of longevityMicrobiological testing/measurementPeptide preparation methodsInsulin-like growth factorCholesterylester transfer protein
This invention provides methods of using of the sizes and levels of high-density lipoprotein (HDL) and low-density lipoprotein (LDL) particles, the -641 allele of the promoter of the gene encoding apolipoprotein C-3 (APOC-3), the 405 allele of the gene encoding cholesteryl ester transfer protein (CETP), and plasma levels of insulin-like growth factor-1 (IGF-1), adiponectin, CETP and APOC-3, for determining and increasing an individual's likelihood of longevity and of retaining cognitive function during aging, and for determining and decreasing an individual's likelihood of developing a cardiovascular-, metabolic- or age-related disease.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com