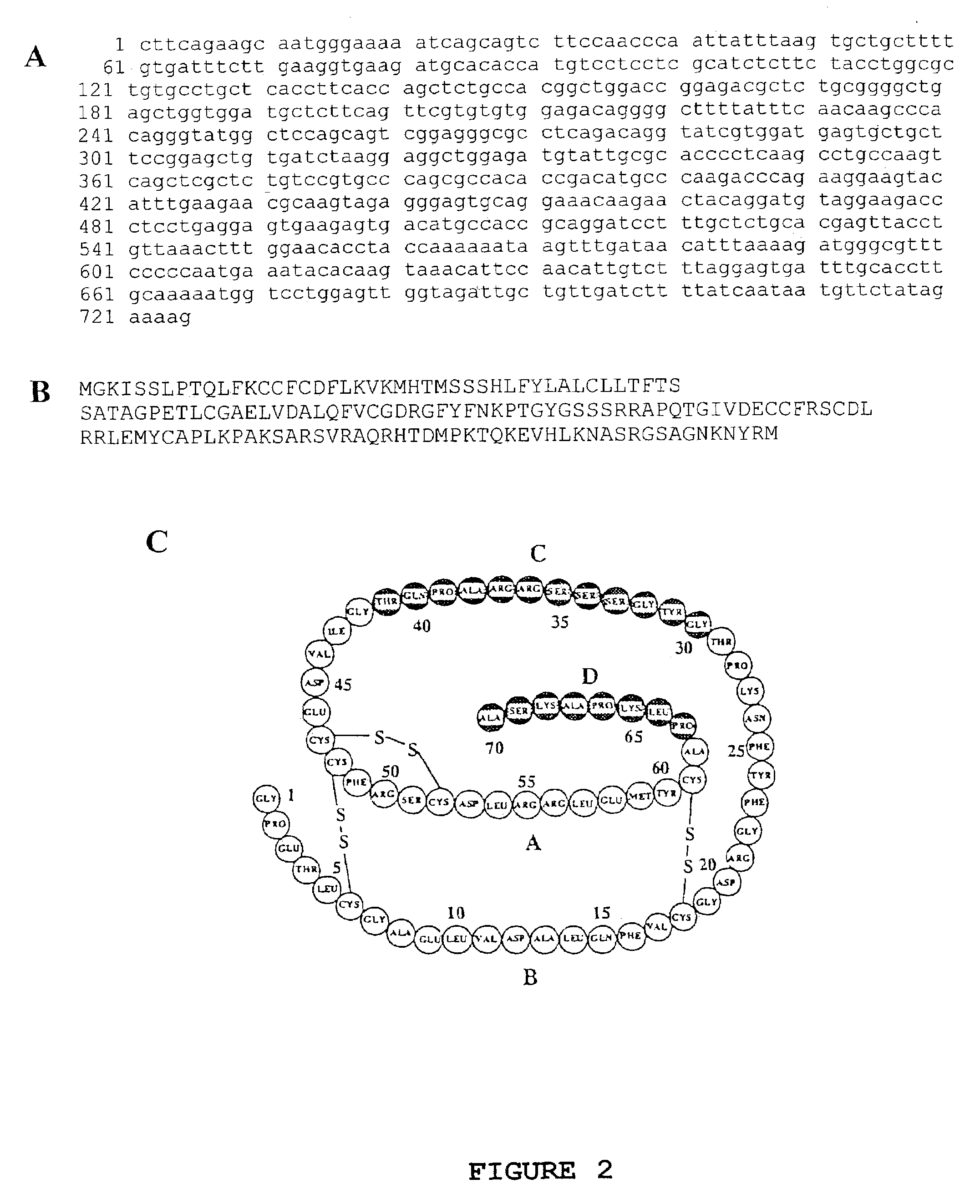
Neuroprotective synergy of erythropoietin and insulin-like growth factors
a technology of erythropoietin and growth factor, which is applied in the field of neuroprotective synergy of erythropoietin and insulinlike growth factor and analogs, can solve the problems of speech impairment and coordination loss, no effective treatment or cure, and reduced the effect of l-dopa treatment typically occurring, so as to prevent or reduce the severity of cerebral neurologic conditions, prevent or reduce the effect of neurologic conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Amelioration Of NMDA-Induced Neurotoxicity by EPO in Combination with IGF-I does not Require Preincubation Before Neurotoxic Insult
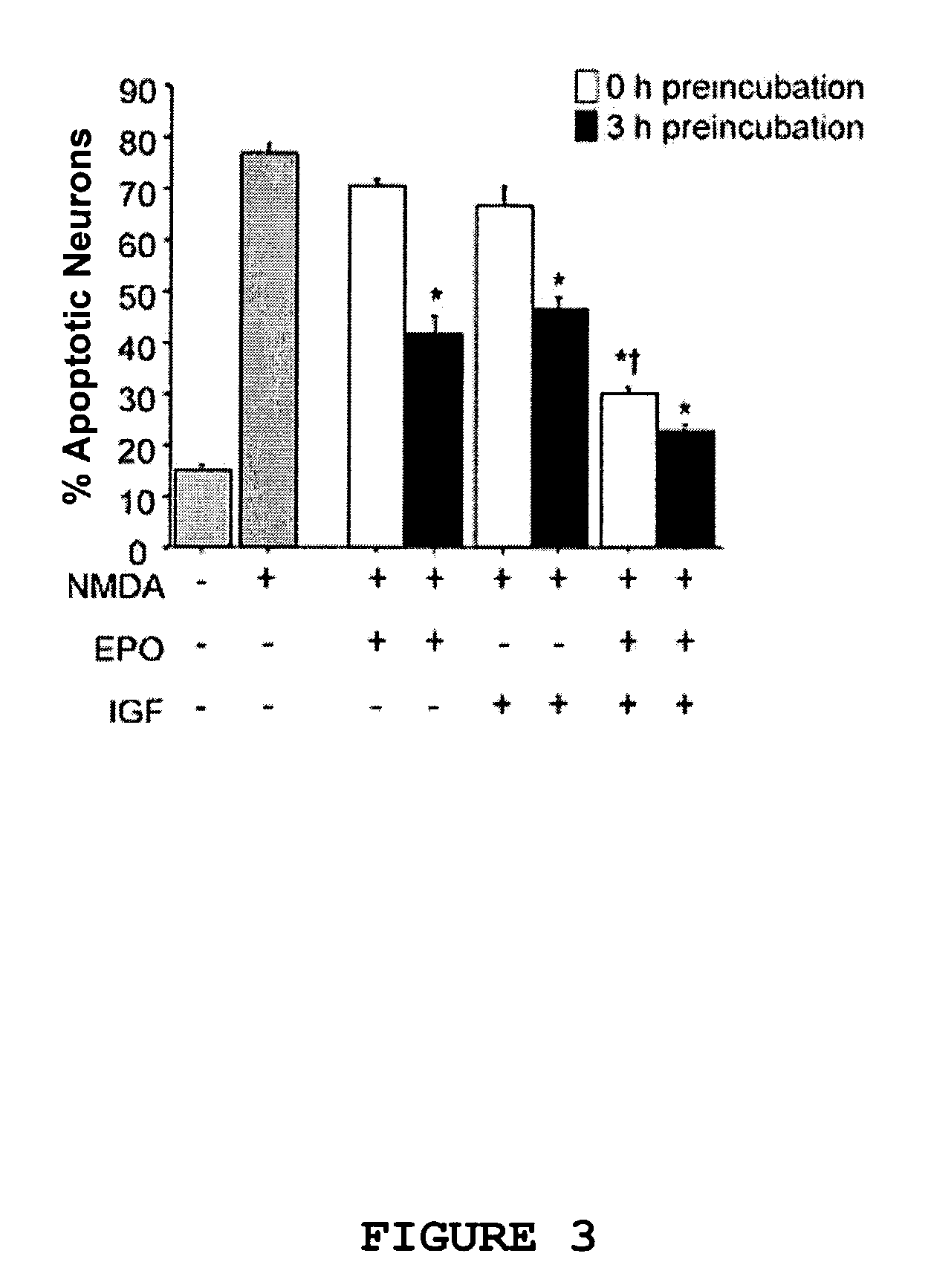
[0162]This example demonstrates that simultaneous application of EPO and IGF-I at the time of neurotoxic insult effectively reduces apoptosis of mature neurons in rat primary cerebrocortical cultures exposed to NMDA.
[0163]The neuroprotective effects of concurrent EPO and IGF-I administration were compared to individual treatment with EPO or IGF-I in rat primary cerebrocortical cultures. Incubation of the cultures with 200 μM NMDA for 20 minutes induced death only in neurons in the mixed neuronal / glial cultures as reported previously (Bonfoco et al., supra, 1995).
[0164]Neuronal apoptosis was quantified by double labeling for TUNEL reactivity, which in conjunction with condensed morphology is indicative of apoptosis, and the neuron-specific protein microtubule associated protein-2 (MAP2) 16 hours after NMDA insult. As shown in FIG. 3, a brief 20 minute exp...
example ii
PI3-Kinase is Required for Neuroprotection by EPO And IGF-I
[0171]This example demonstrates that the PI3 kinase can play a role in mediating the neuroprotective effects of EPO and IGF-I.
[0172]PI3-kinase is involved in IGF-I and EPO signaling (Mayeux et al., supra, 1993; Kermer et al., supra, 2000; and Damen et al., J. Biol. Chem. 270:23402-23408 (1995)). In order to elucidate the role of PI3-kinase in the neuroprotective effects of EPO and IGF-I, rat cerebrocortical neurons were preincubated for three hours with EPO, IGF-I, or EPO in combination with IGF-I (EPO / IGF-I) in the presence or absence of 10 μM LY294002, a specific PI3-kinase inhibitor. As shown in FIG. 4, neuronal apoptosis resulting from NMDA exposure (200 μM NMDA and 5 μM glycine for 20 minutes) decreased in cells preincubated with EPO, IGF-I or EPO / IGF-I. As further shown in FIG. 4, LY294002 abolished the neuroprotective effect of EPO and IGF-I either alone or in combination (p<0.05) but did not itself cause neuronal apo...
example iii
EPO And IGF-I Cooperate in Activating Akt
[0179]This example demonstrates that the Akt kinase can be cooperatively activated by EPO and IGF-I in neuronal cells.
[0180]Akt-kinase is activated downstream of PI3-kinase-mediated production of 3′ phospholipids. In response to production of phosphotidylinositol-3,4,5-trisphosphate, Akt is phosphorylated at two critical sites: serine-473 and threonine-308 (Russell et al., Nuerobiol. 36:455-467 (1998); Scheid and Woodgett, supra, 2001).
[0181]To assess possible activation of the Akt kinase, cerebrocortical-cultures were exposed to EPO or IGF-I for three hours and immunoblotted as described above using anti-phospho Akt (anti-pAkt) and anti-Akt antibodies from Cell Signaling Technologies at 1:2000 dilution. A three hour incubation with EPO or IGF-I resulted in moderate Akt activation, as evidenced by increased phospho-serine-473 Akt detected by western blotting (FIG. 6A). Co-incubation with EPO and IGF-I resulted in a much larger increase in pho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com