Patents
Literature
36 results about "Glomerulosclerosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Glomerulosclerosis is hardening of the glomeruli in the kidney. It is a general term to describe scarring of the kidneys' tiny blood vessels, the glomeruli, the functional units in the kidney that filter urea from the blood.
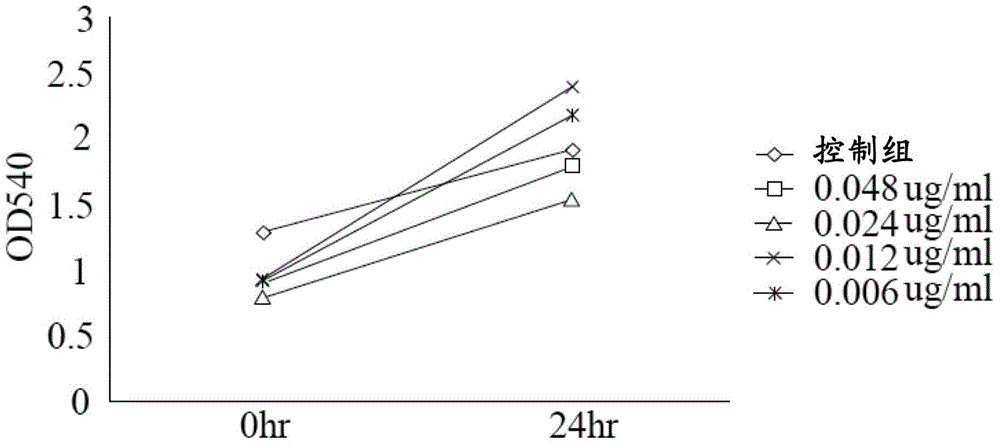
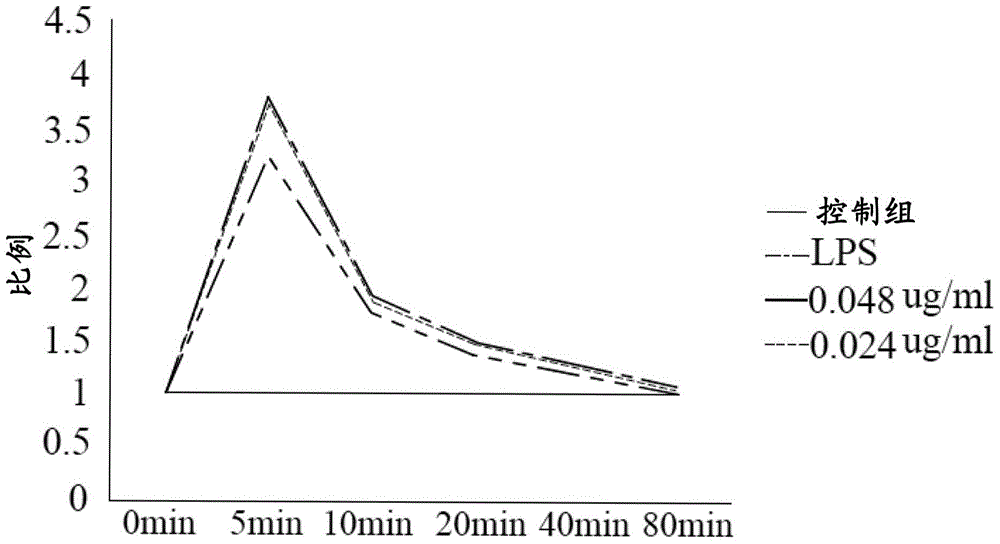
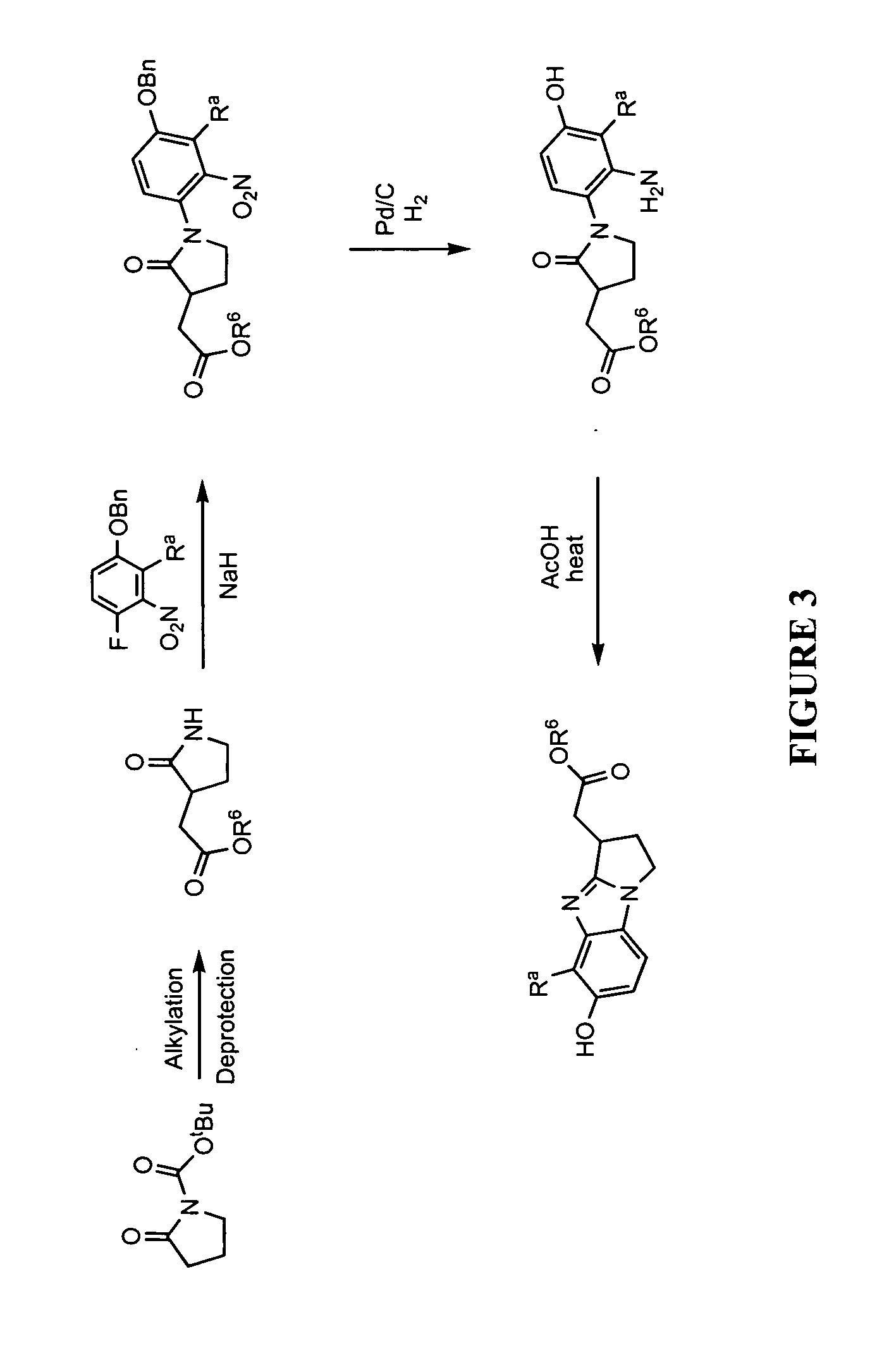
Lactate salt of 4-[6-methoxy-7-(3-piperidin-1-yl-propoxy)quinazolin-4-yl]piperazine-1-carboxylic acid(4-isopropoxyphenyl)-amide and pharmaceutical compositions thereof for the treatment of cancer and other diseases or disorders
InactiveUS20100273808A1High drug loadingOrganic active ingredientsNervous disorderCell survivalCarboxylic acid
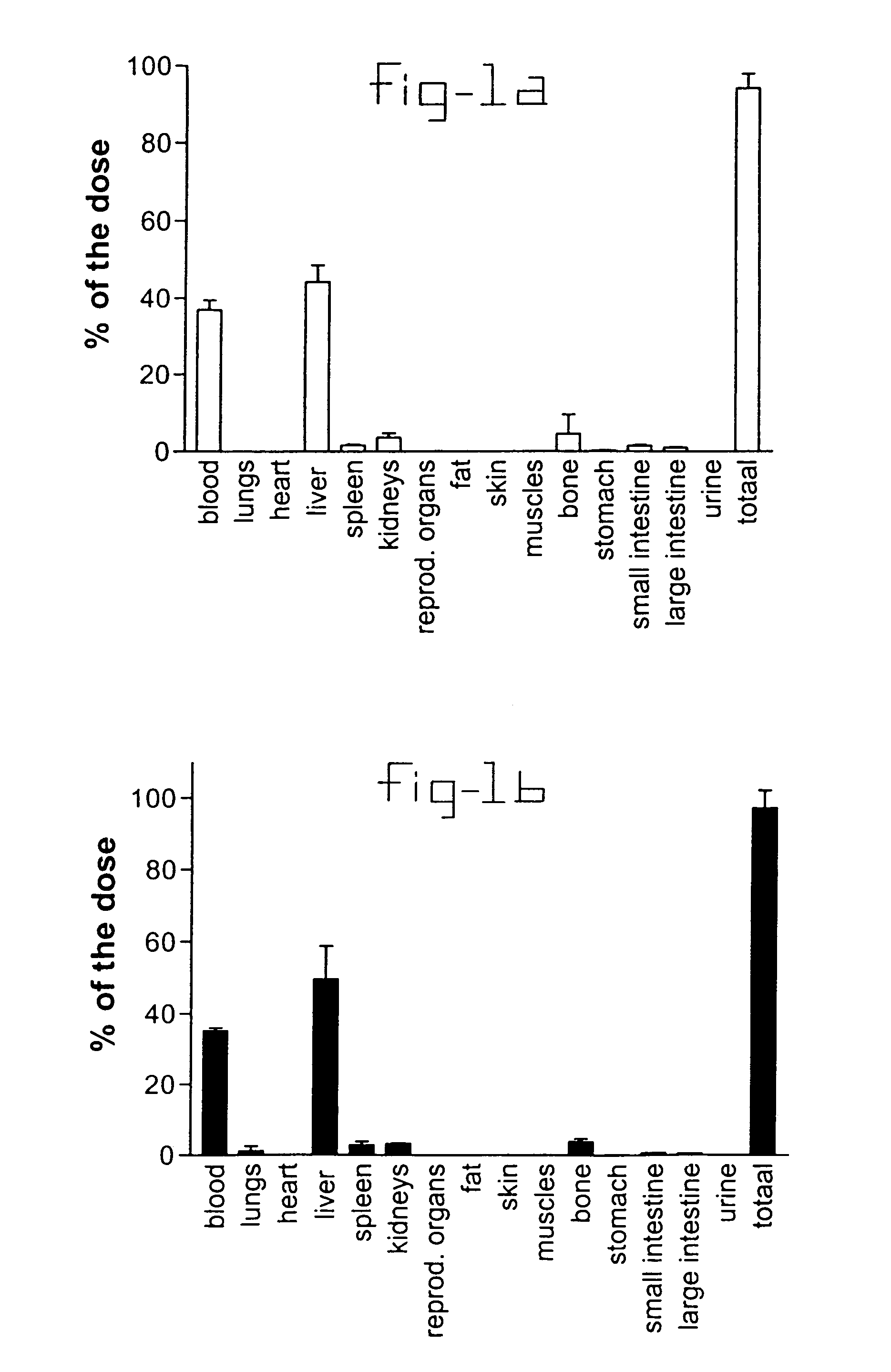
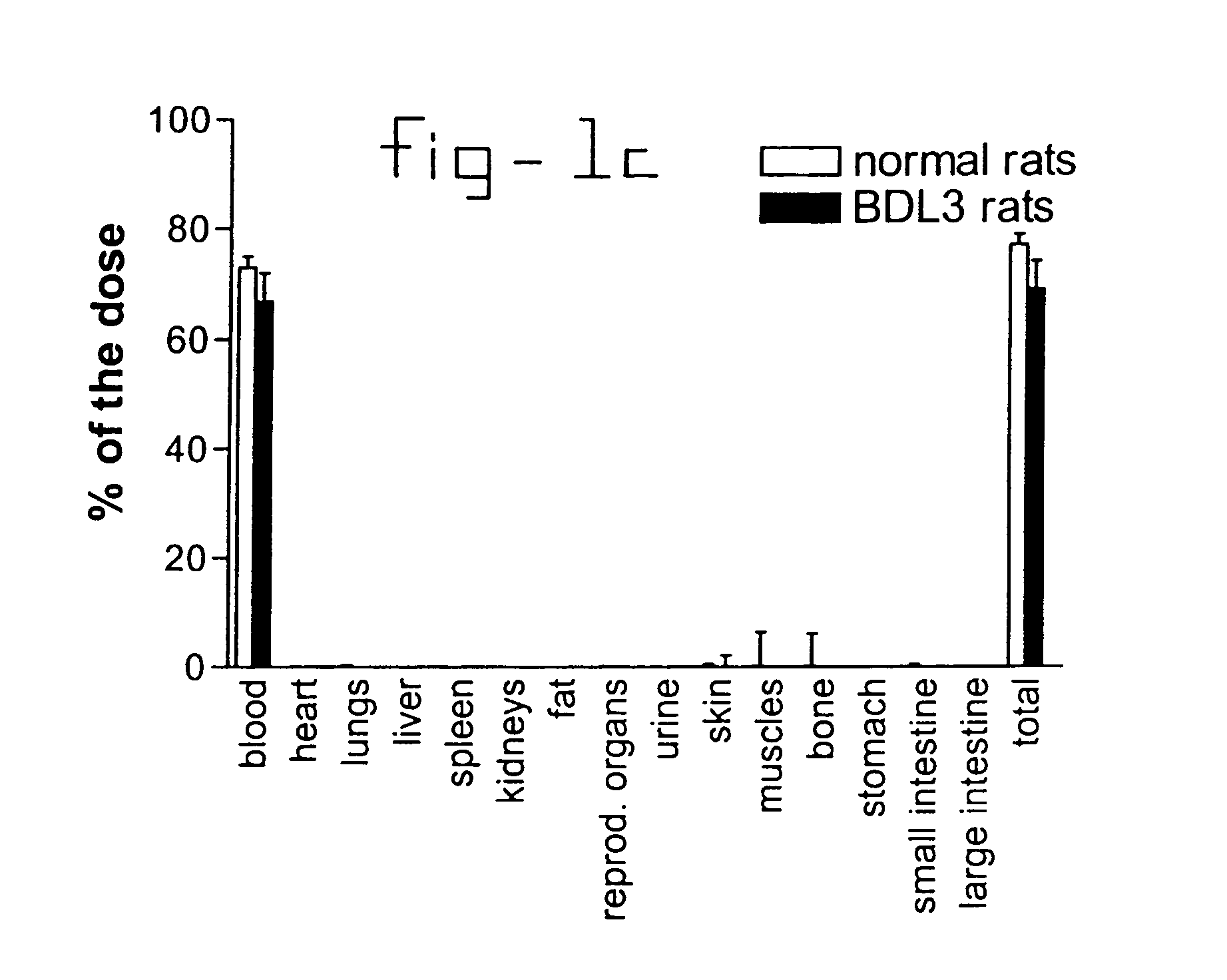
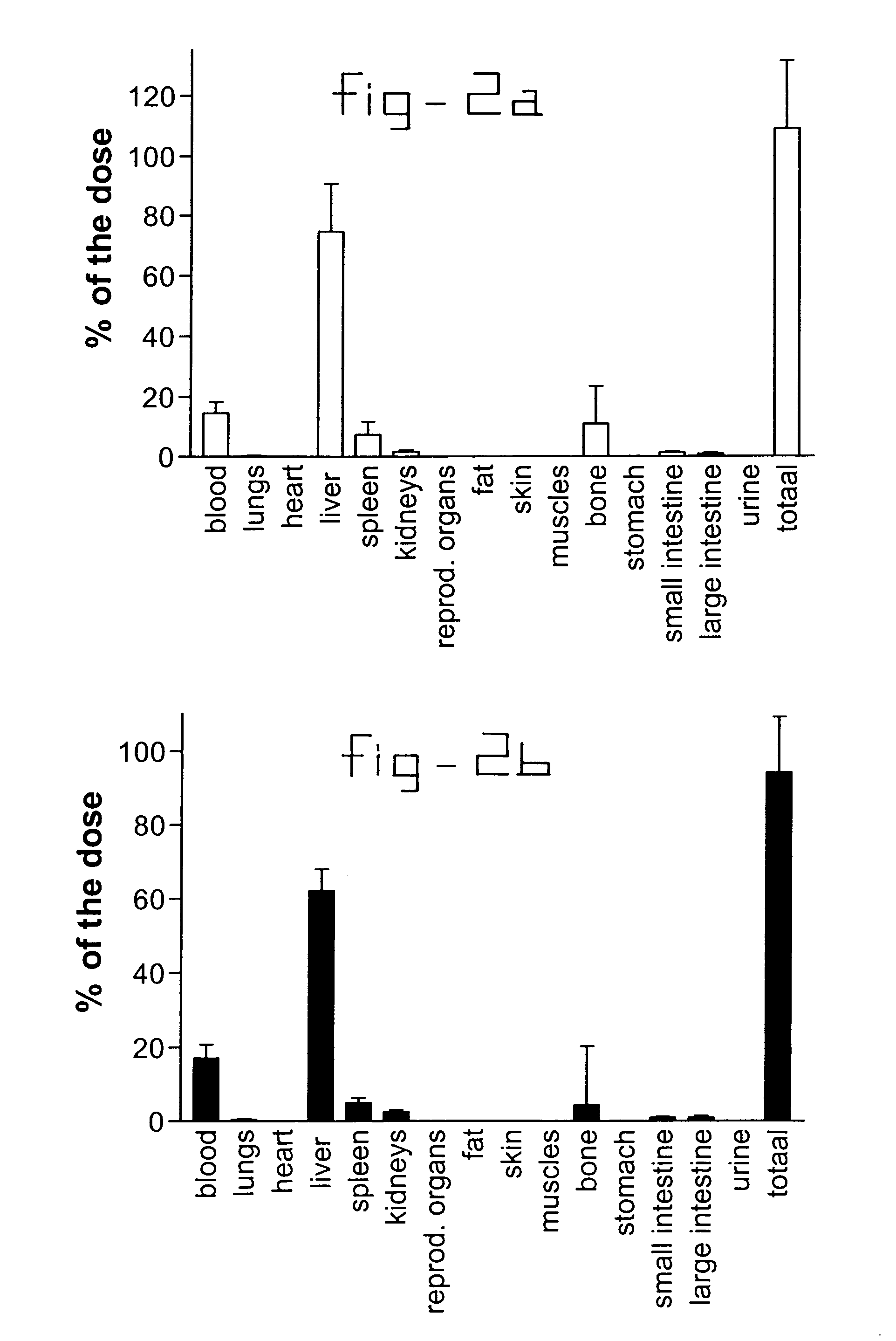
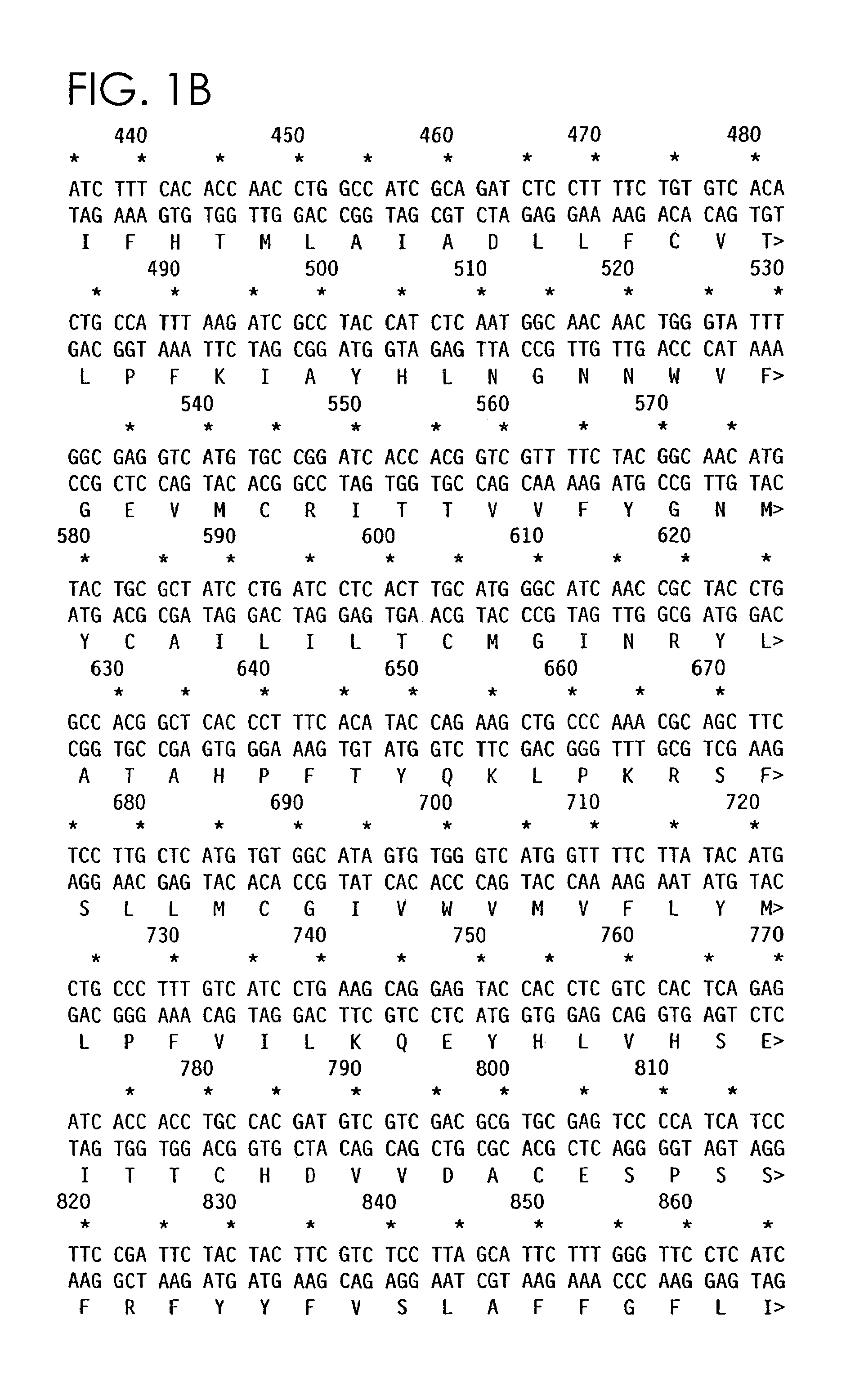
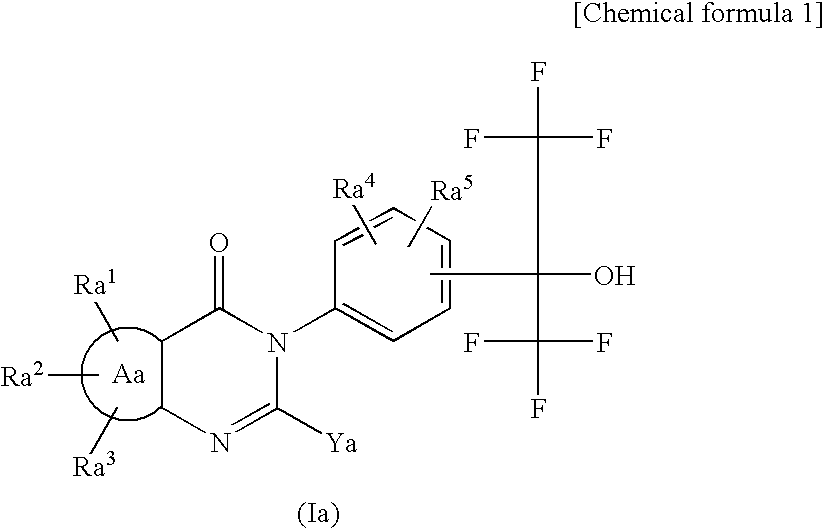
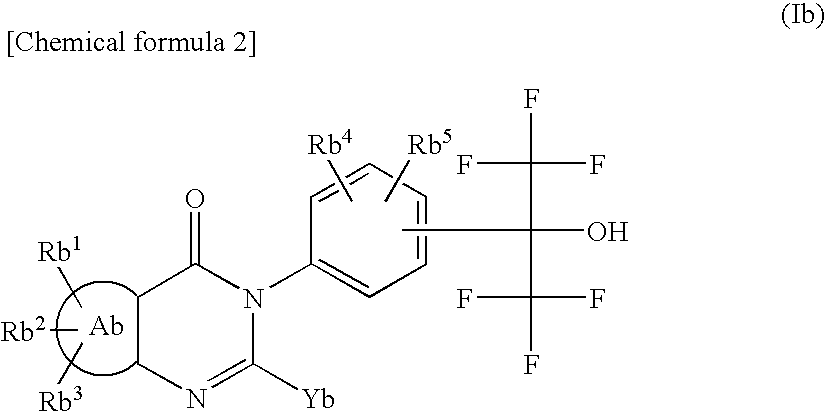
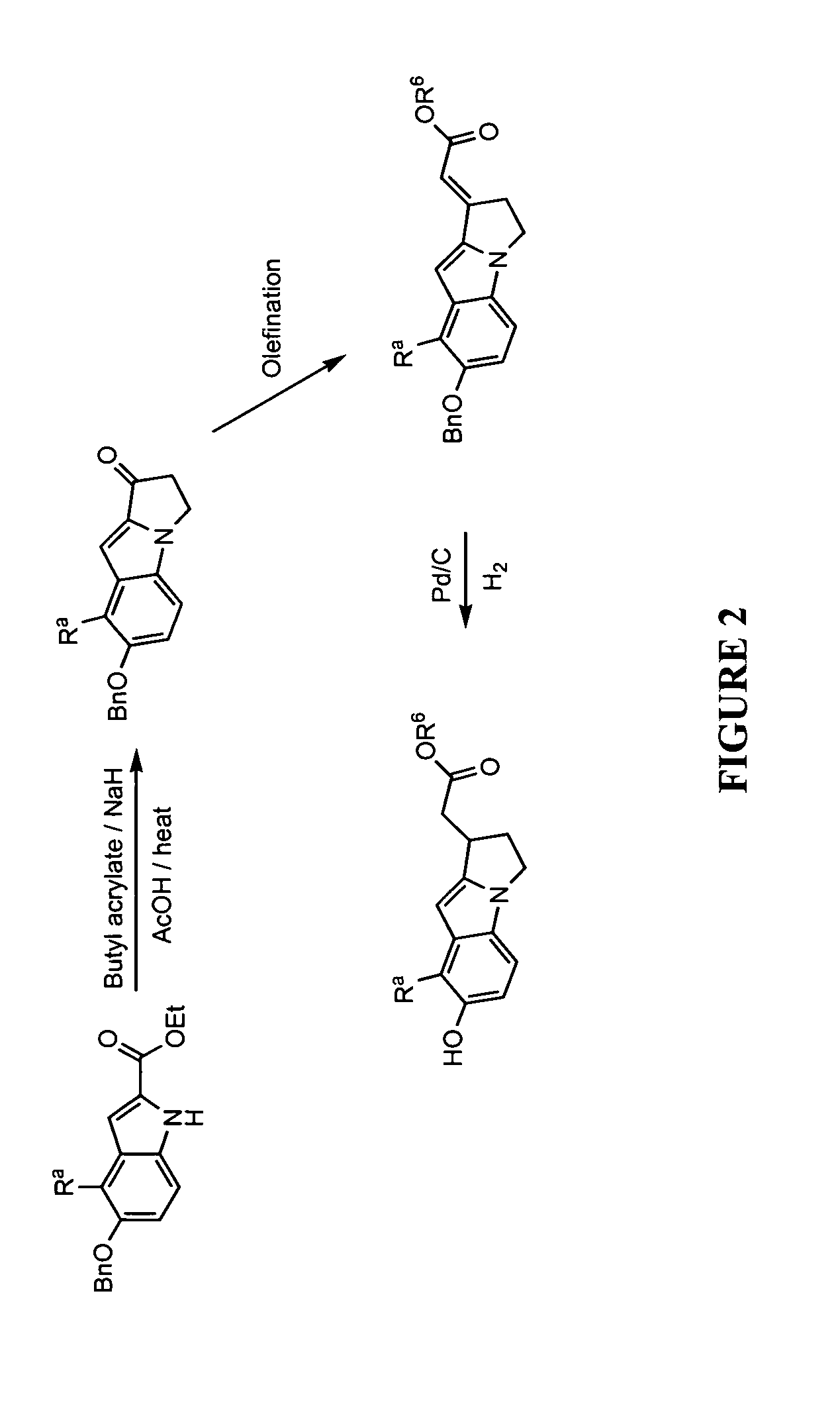
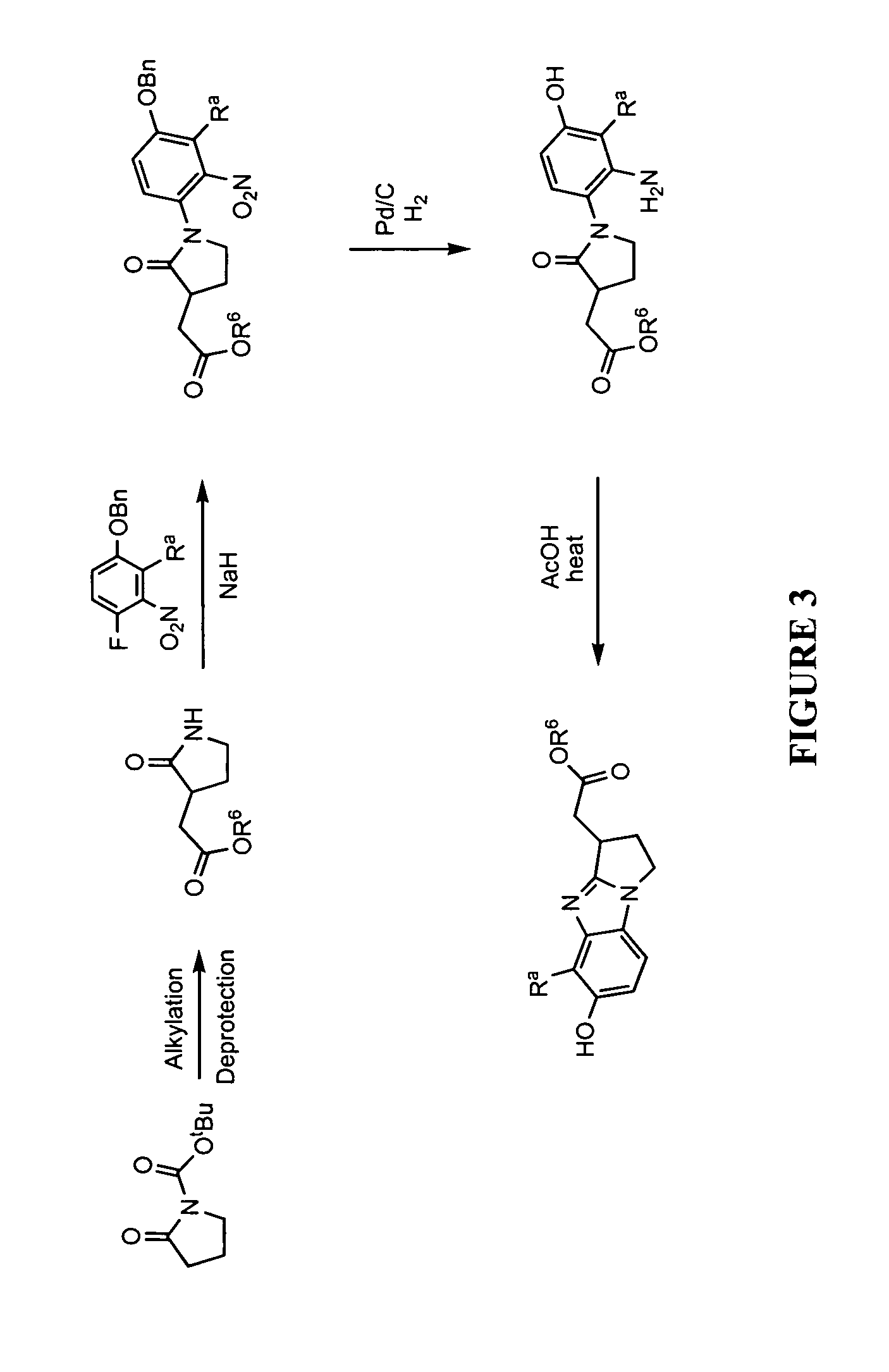
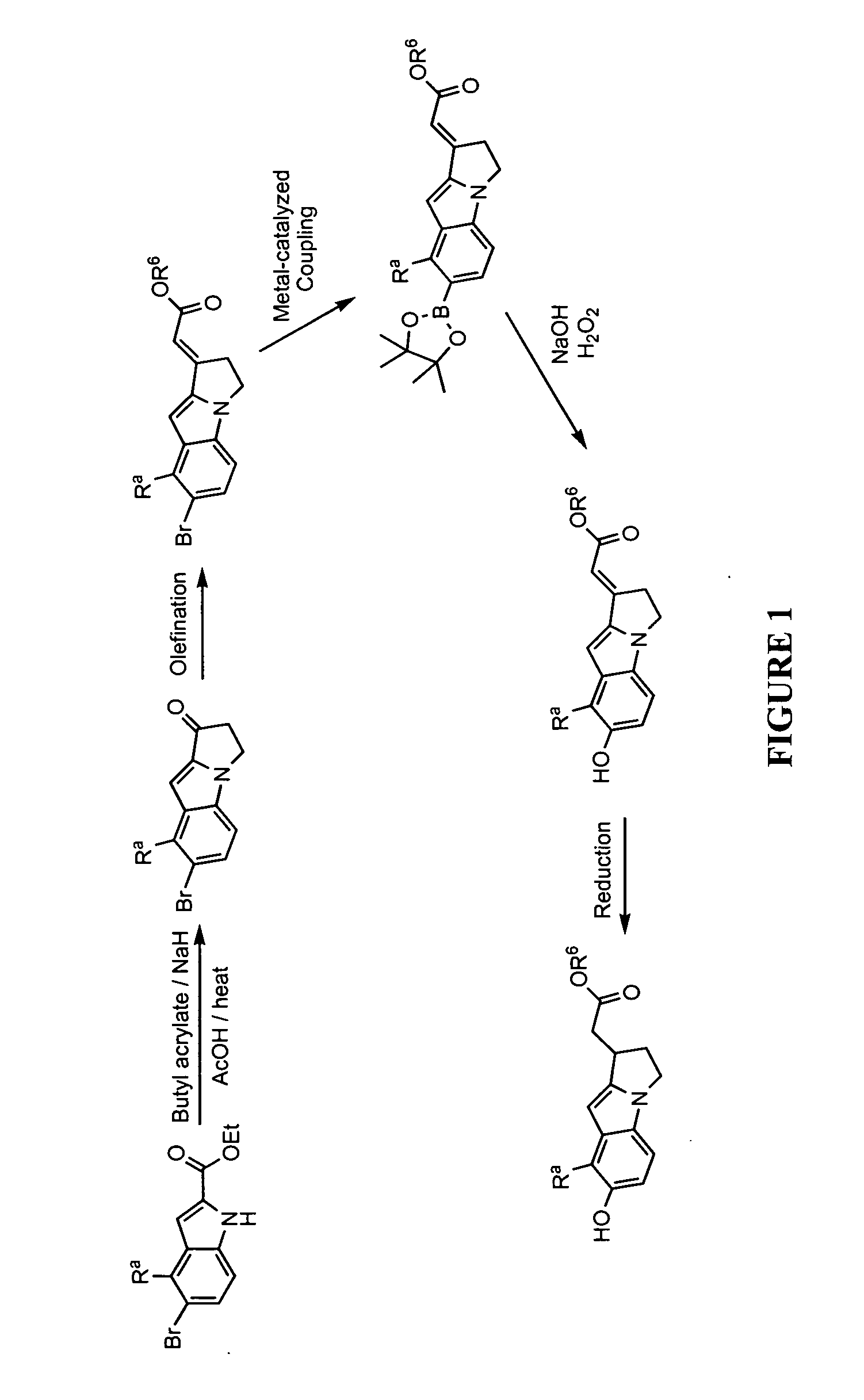
This invention provides a compound of formula (I):or a crystalline form thereof, or a pharmaceutical composition thereof, or an oral pharmaceutical dosage form thereof; processes for the synthesis or manufacture of the compound of formula (I), or a crystalline form thereof, or a pharmaceutical composition thereof, or an oral pharmaceutical dosage form thereof; and the use of said compound, or a crystalline form thereof, or a pharmaceutical composition thereof, or an oral pharmaceutical dosage form thereof, for the treatment of patients suffering from or subject to diseases, disorders or conditions involving cell survival, proliferation, and migration, including cardiovascular disease (e.g., arteriosclerosis and vascular reobstruction), cancer, (e.g., AML and malignant glioma)glomerulosclerosis, fibrotic disease and inflammation.
Owner:MILLENNIUM PHARMA INC
Substituted tricyclic acid derivatives as S1P1 receptor agonists useful in the treatment of autoimmune and inflammatory disorders
Owner:ARENA PHARMA
Substituted tricyclic acid derivatives as s1p1 receptor agonists useful in the treatment of autoimmune and inflammatory disorders
The present invention relates to certain substituted tricyclic acid derivatives of Formula (I) and pharmaceutically acceptable salts thereof, which exhibit useful pharmacological properties, for example, as agonists of the S1P1 receptor. Also provided by the present invention are pharmaceutical compositions containing compounds of the invention, and methods of using the compounds and compositions of the invention in the treatment of S1P1-associated disorders, for example, psoriasis, rheumatoid arthritis, Crohn's disease, transplant rejection, multiple sclerosis, systemic lupus erythematosus, ulcerative colitis, type I diabetes, acne, myocardial ischemia-reperfusion injury, hypertensive nephropathy, glomerulosclerosis, gastritis, polymyositis, thyroiditis, vitiligo, hepatitis, biliary cirrhosis, microbial infections and associated diseases, viral infections and associated diseases, diseases and disorders mediated by lymphocytes, auto immune diseases, inflammatory diseases, and cancer.
Owner:ARENA PHARMA
Pharmaceutical composition containing pirfenidone in sustained-release tablet form
ActiveUS9408836B2Effective in regressionDeleterious effectNervous disorderAntipyreticHepatic fibrosisAnti fibrotic
The instant invention relates to a process for the preparation of a pharmaceutical composition in sustained-release tablet form comprising from 600 milligrams to 2400 milligrams of Pirfenidone (PFD), in such a way that the drug is bioavailable during an extended period of time of 12 hours from its administration. In this way, the anti-fibrotic and anti-inflammatory action of the drug Pirfenidone is optimized. Moreover, the instant invention offers advantages and a higher therapeutic efficacy compared to other pharmaceutical forms of Pirfenidone for oral administration and its therapeutic application in the regression of chronic renal failure secondary to primary glomerulosclerosis; it shows a better activity with regard to the reduction and / or regression of deleterious effects in breast capsular contracture observed after the surgical implantation of breast implants in humans and has an important anti-TNF-α and anti-TGF-β1 action for the treatment of hepatic fibrosis.
Owner:EXCALIBUR PHARM INC
Use of Antagonists of Hepatic Sympathetic Nerve Activity
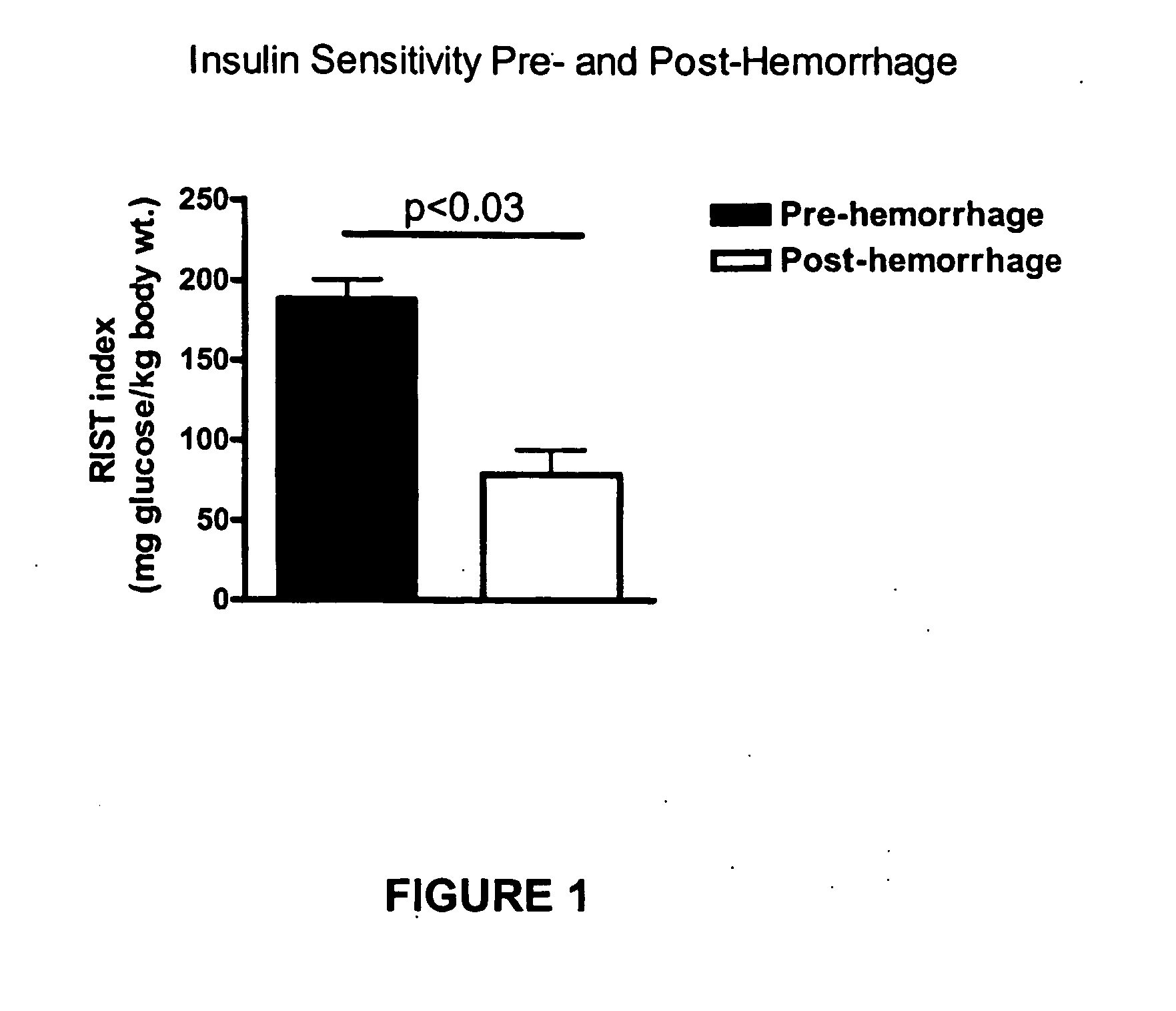
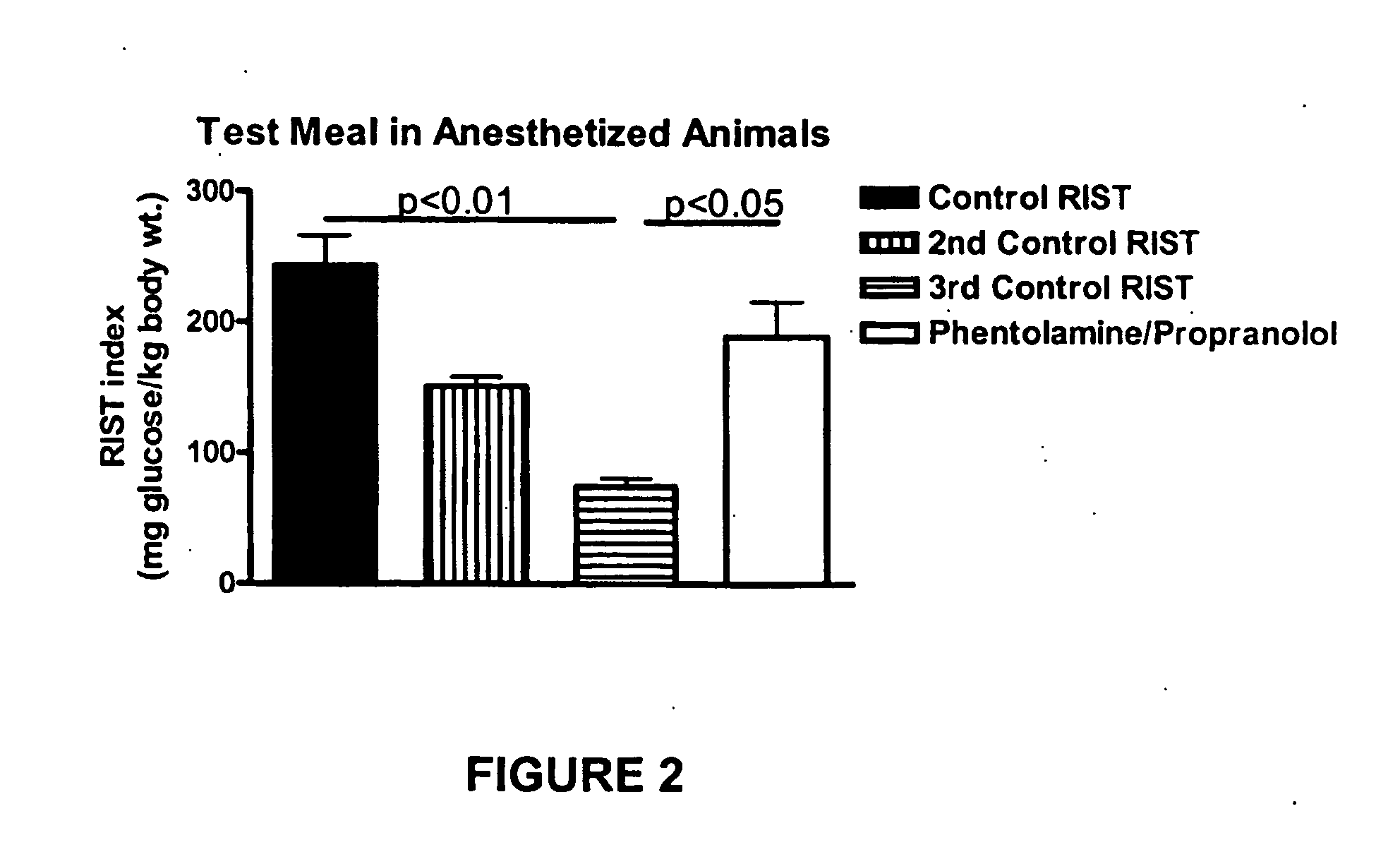
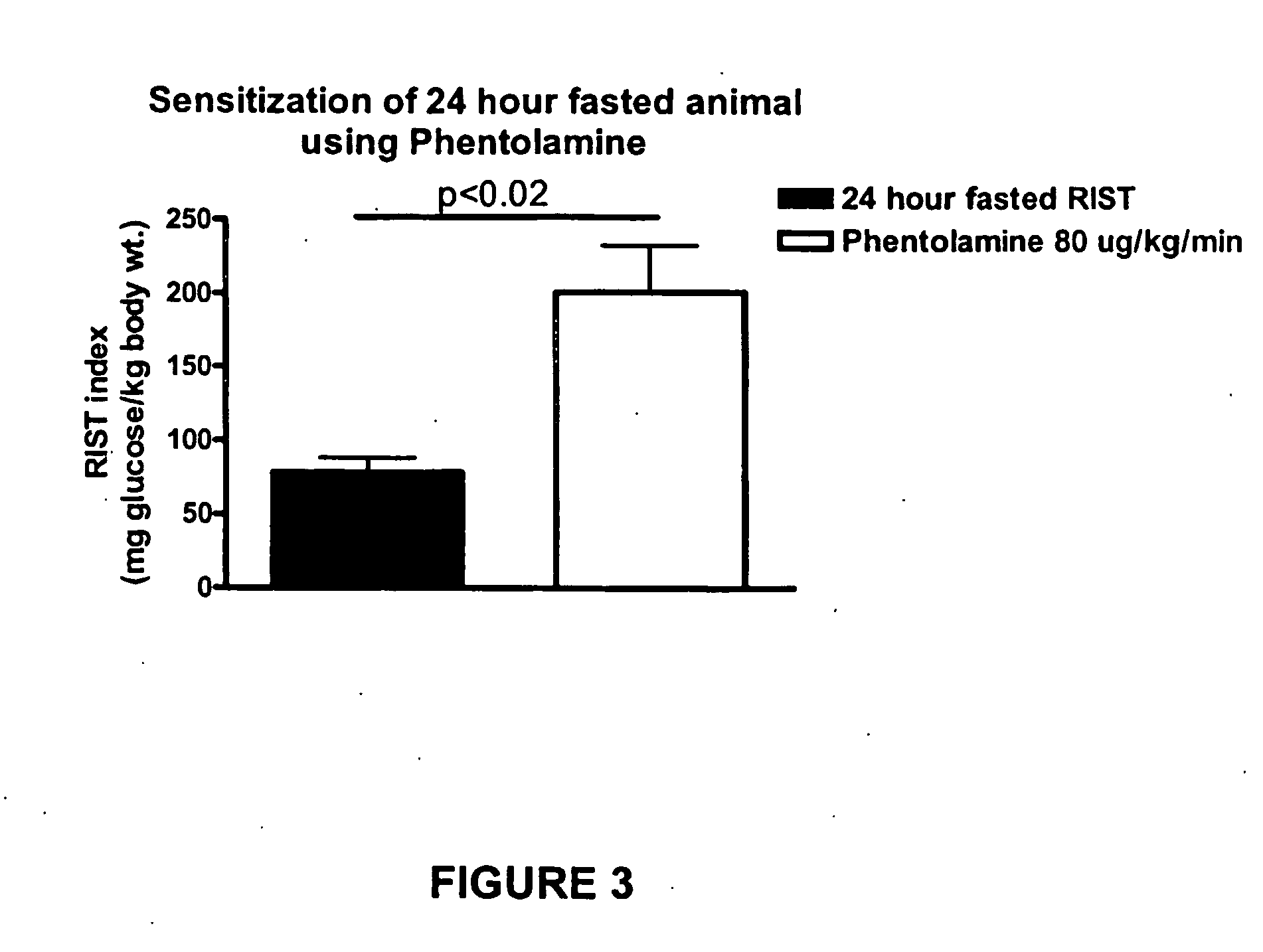
InactiveUS20070238762A1Increasing skeletal muscle glucose uptakeReduce insulin resistanceBiocideMetabolism disorderSympathetic nerve activityDiabetic nephropathy
The present invention provides pharmaceutical compositions comprising antagonists of hepatic sympathetic activity and methods for using said pharmaceutical compositions for treatment of hyperglycemia, hyperinsulinaemia, hyperlipidaemia, hypertriglyceridaemia, diabetes, insulin resistance, impaired glucose metabolism, conditions of impaired glucose tolerance, conditions of impaired fasting plasma glucose, obesity, diabetic retinopathy, diabetic nephropathy, glomerulosclerosis, diabetic neuropathy, syndrome X, renal failure, sexual dysfunction, chronic stress, and anxiety.
Owner:UNIVERSITY OF MANITOBA
Process for the preparation of a pharmaceutical composition containing pirfenidone in sustained-release tablet form and its application in the regression of human chronic renal failure, breast capsular contracture and hepatic fibrosis
ActiveUS20160287567A1Effective in regressionDeleterious effectNervous disorderAntipyreticAnti fibroticLiver fibrosis
The instant invention relates to a process for the preparation of a pharmaceutical composition in sustained-release tablet form comprising from 600 milligrams to 2400 milligrams of Pirfenidone (PFD), in such a way that the drug is bioavailable during an extended period of time of 12 hours from its administration. In this way, the anti-fibrotic and anti-inflammatory action of the drug Pirfenidone is optimized. Moreover, the instant invention offers advantages and a higher therapeutic efficacy compared to other pharmaceutical forms of Pirfenidone for oral administration and its therapeutic application in the regression of chronic renal failure secondary to primary glomerulosclerosis; it shows a better activity with regard to the reduction and / or regression of deleterious effects in breast capsular contracture observed after the surgical implantation of breast implants in humans and has an important anti-TNF-α and anti-TGF-β1 action for the treatment of hepatic fibrosis.
Owner:EXCALIBUR PHARM INC
Chinese medicine composition for treating early and medium-term chronic renal failure
InactiveCN101194984ASignificant effectLower medical costsPowder deliveryUrinary disorderMetaboliteTurbidity
The invention belongs to the traditional medicine technical field, which relates to a traditional Chinese medicine composition for curing early and intermediate chronic renal failure. Under the guidance of a traditional Chinese medicine theory that 'interior accumulation is blood stasis' and 'chronic diseases transforming to collaterals is blood stasis', with the treatment principle of differentiation of symptoms and sighs, differentiation of channels, and based on the principle of strengthening body resistance and reducing turbidity, promoting blood circulation and removing blood stasis, the invention adopts traditional medicine such as lanceolata, epicedium, salvia miltiorrhizae, prepared rhubarb, perilla, peach kernel and the like, which are made into the compositions. Confirmed by clinical and animal experiments, the invention has the renal protecting functions of improving clinical symptoms of patients with early and intermediate chronic renal failure, reducing retention of metabolites of bodies, improving anemia, adjusting lipid and calcium-phosphorus metabolism, reducing the expression of fibrosis factors in nephridial tissues, preventing and treating glomerulosclerosis, lightening renal tubule damage and renal interstitial inflammatory cell infiltration and delaying renal fibrosis. The invention has the advantages of safety, efficiency and low cost, which is applied to the long-term medication for patients with early and intermediate chronic renal failure.
Owner:SHUGUANG HOSPITAL AFFILIATED WITH SHANGHAI UNIV OF T C M
Process for the preparation of a pharmaceutical composition containing pirfenidone in sustained-release tablet form and its application in the regression of human chronic renal failure, breast capsular contracture and hepatic fibrosis
ActiveUS20140296300A1Effective in regressionDeleterious effectBiocideNervous disorderAnti fibroticHepatic fibrosis
The instant invention relates to a process for the preparation of a pharmaceutical composition in sustained-release tablet form comprising from 600 milligrams to 2400 milligrams of Pirfenidone (PFD), in such a way that the drug is bioavailable during an extended period of time of 12 hours from its administration. In this way, the anti-fibrotic and anti-inflammatory action of the drug Pirfenidone is optimized. Moreover, the instant invention offers advantages and a higher therapeutic efficacy compared to other pharmaceutical forms of Pirfenidone for oral administration and its therapeutic application in the regression of chronic renal failure secondary to primary glomerulosclerosis; it shows a better activity with regard to the reduction and / or regression of deleterious effects in breast capsular contracture observed after the surgical implantation of breast implants in humans and has an important anti-TNF-α and anti-TGF-β1 action for the treatment of hepatic fibrosis.
Owner:EXCALIBUR PHARM INC
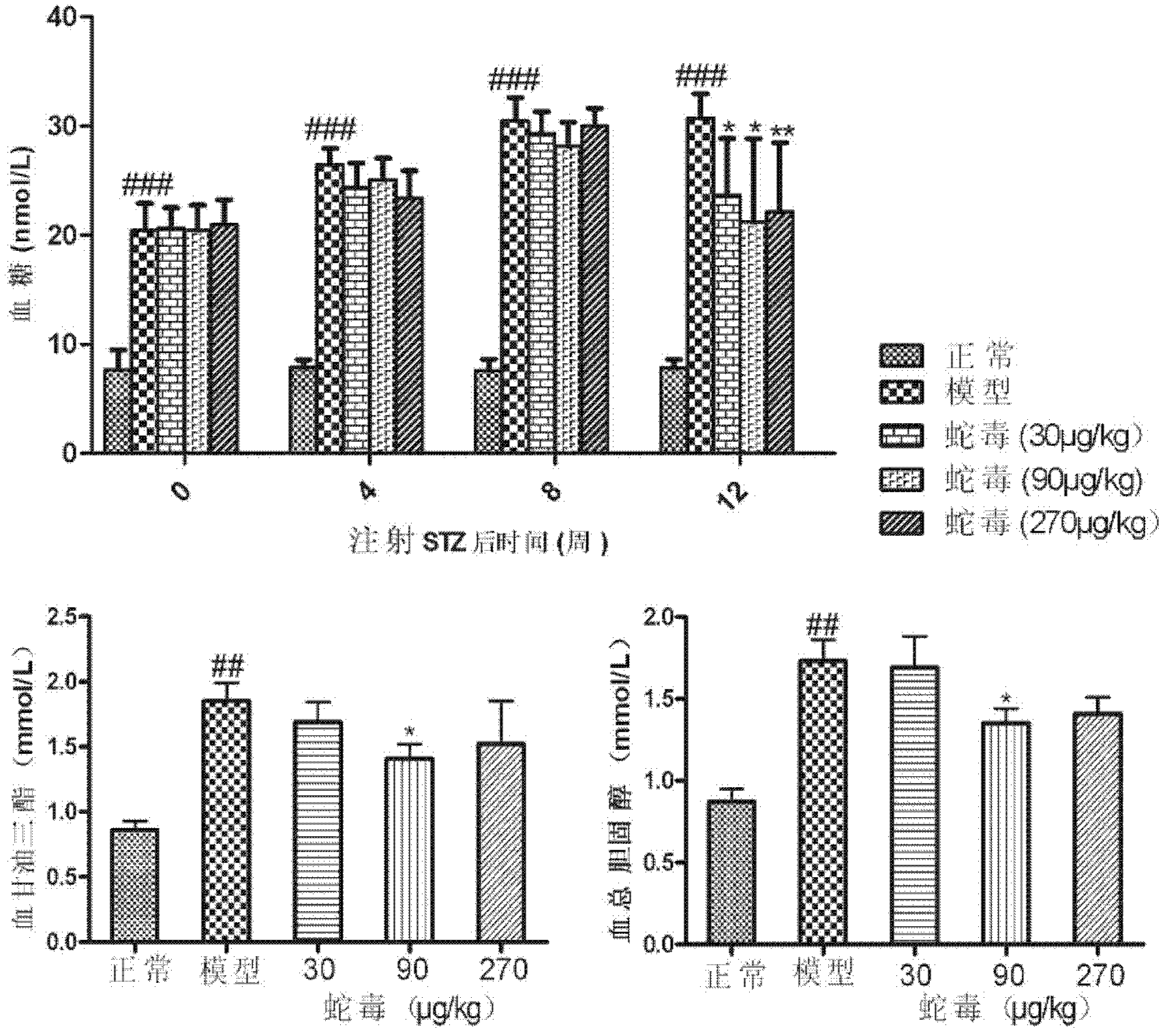
Application of naja atra venin to treatment of diabetes and diabetic nephropathy complicating disease
InactiveCN102526695ALow toxicityImprove efficacyPeptide/protein ingredientsMetabolism disorderDiseaseInflammatory factors
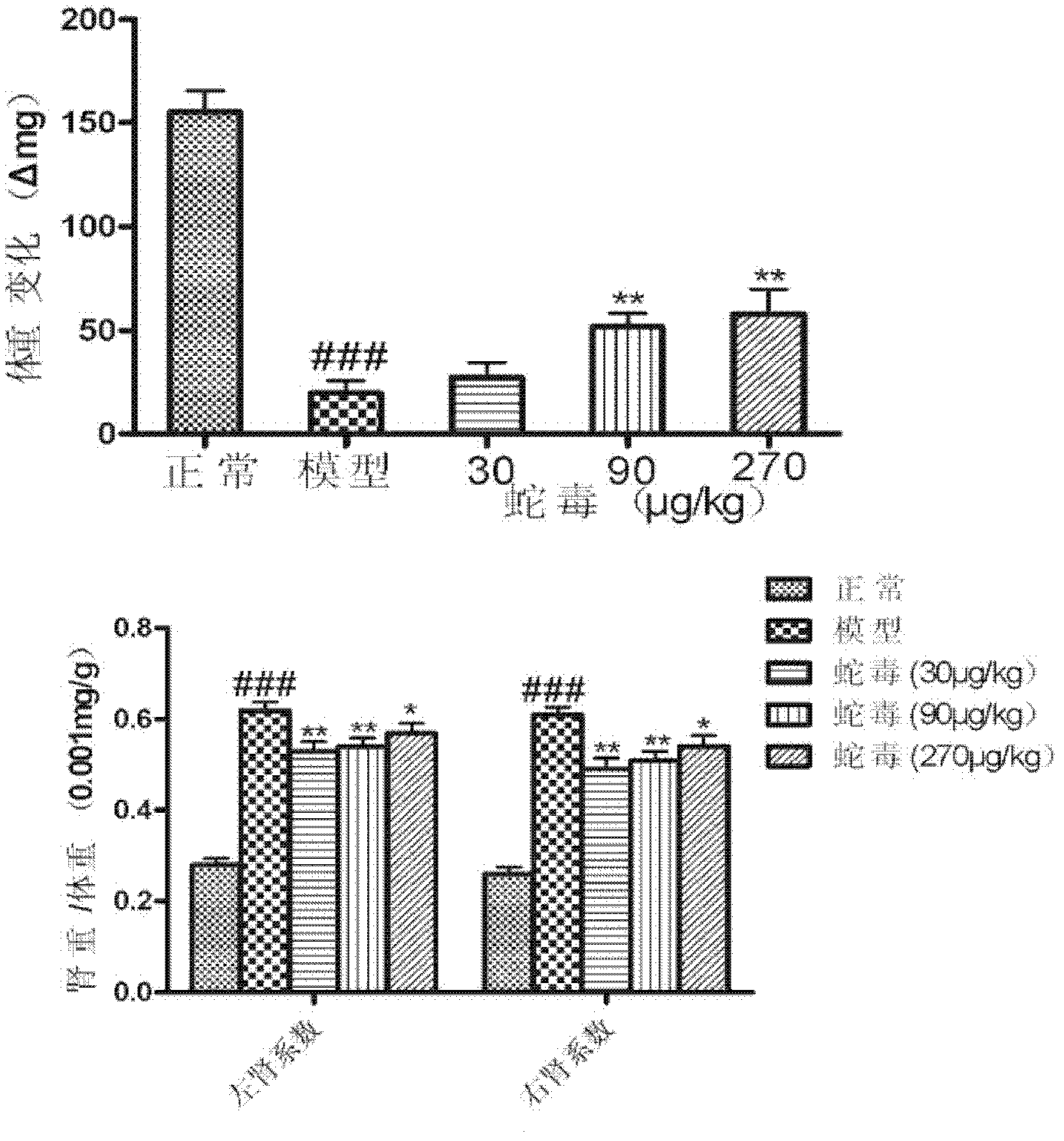
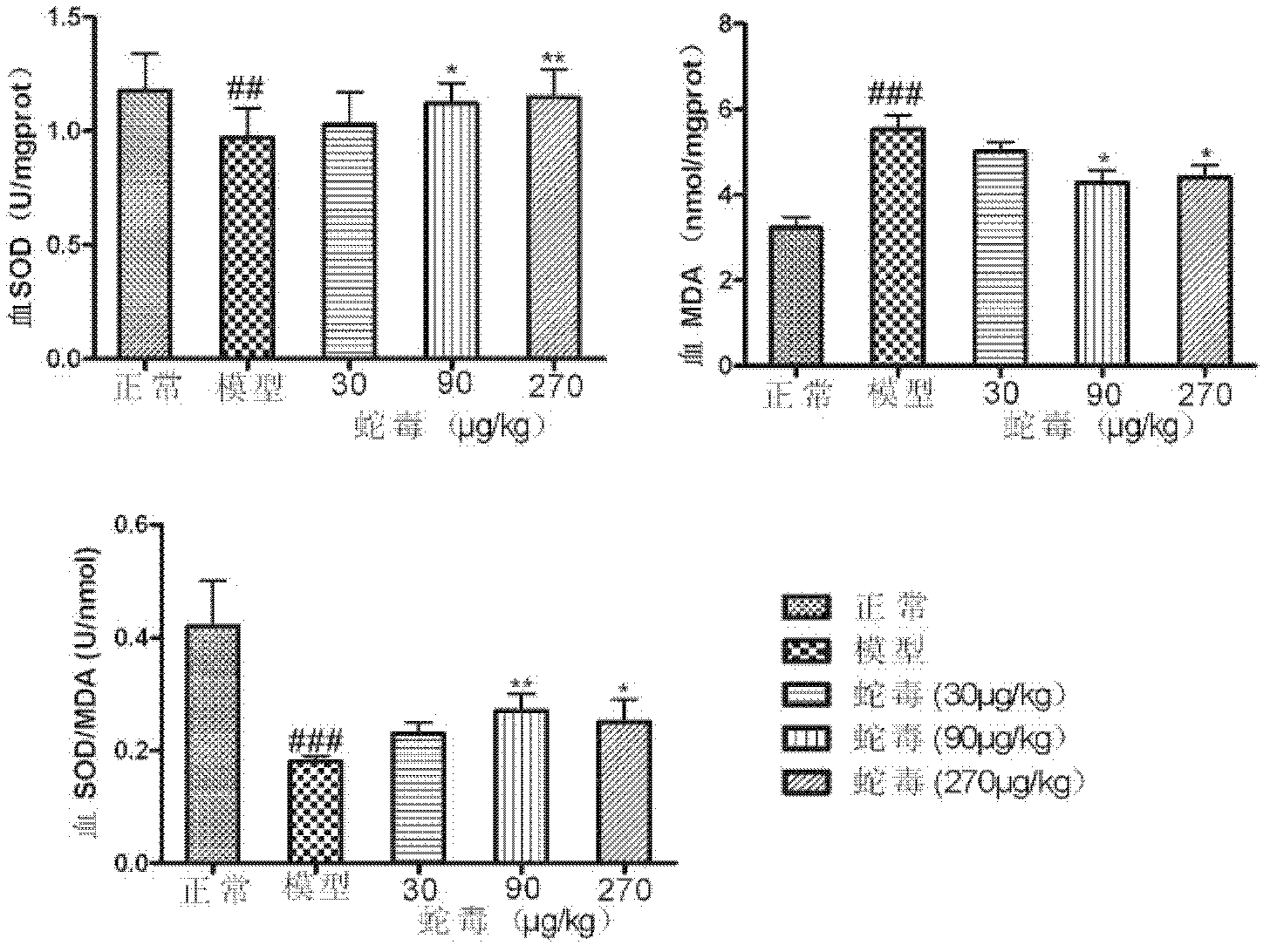
The invention discloses an application of naja atra venin to preparation of a medicament for treating diabetes and a diabetic nephropathy complicating disease. As proved by animal experiments, after the total toxicity of naja atra venin which is modified and renatured physically in a range of 10-3,000 mug / kg is applied, the weight of a mouse suffering from diabetic nephropathy can be reduced, the blood sugar level of a mouse suffering from diabetes can be lowered, urokinase proteins are reduced, plasma albumin is raised, blood plasma triglyeride is reduced, plasma creatinine and urea nitrogen are reduced, the expression of inflammatory factors in kidney is reduced, the oxidative stress level is lowered, glomerulosclerosis is reduced, and nephridial tissue and islet injury are relieved.
Owner:SUZHOU RENBEN PHARMA
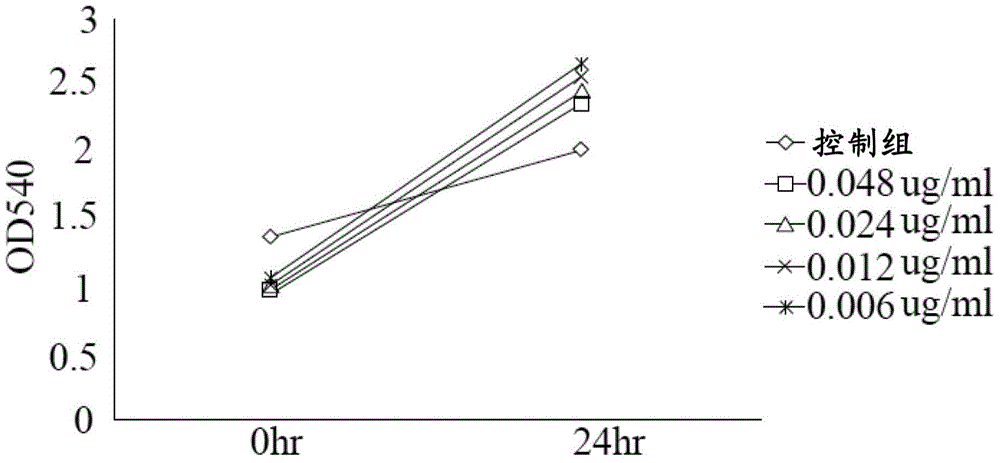
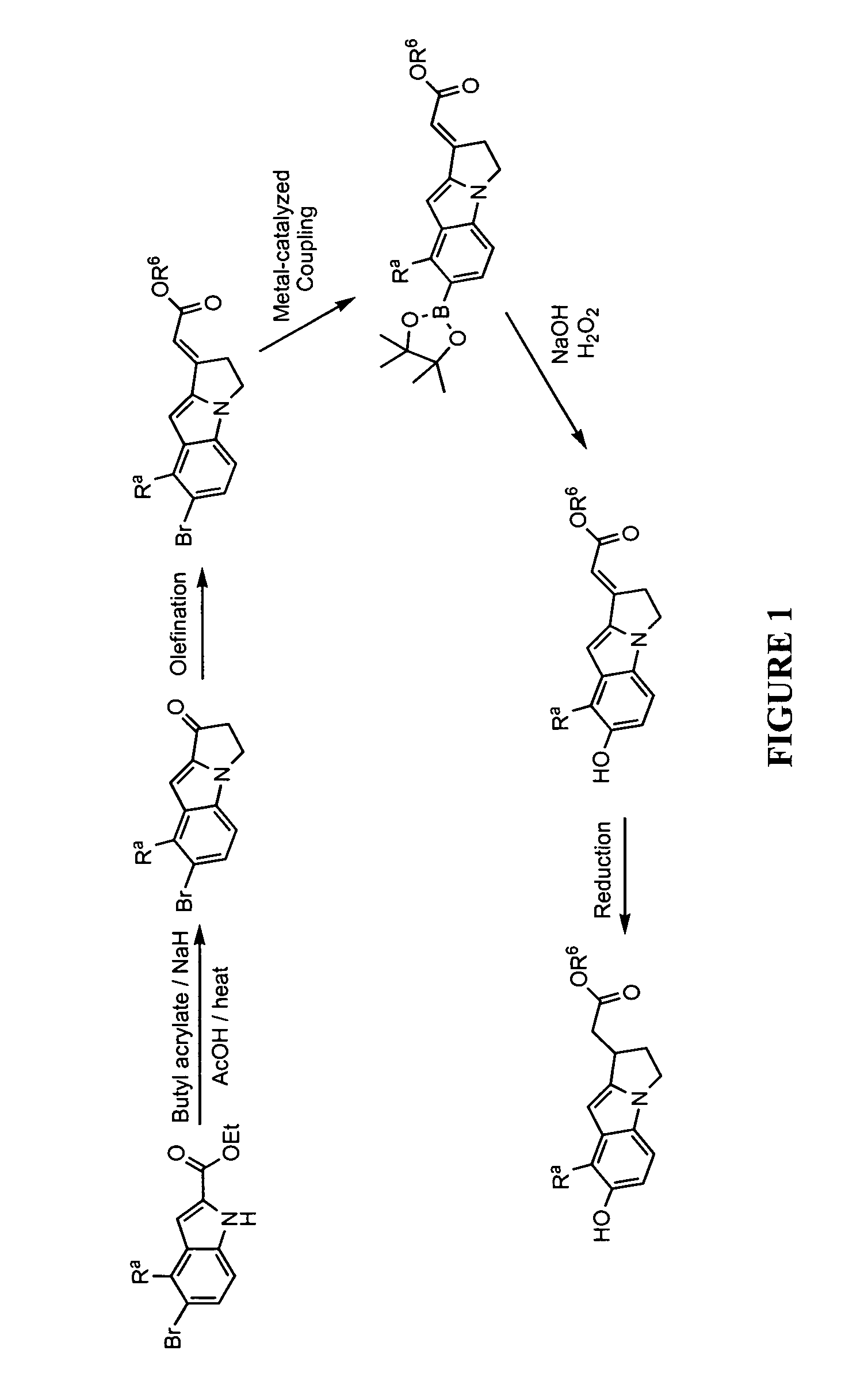
Lactate salt of 4-[6-methoxy-7-(3-piperidin-1-yl-propoxy)quinazolin-4-yl]piperazine-1-carboxylic acid(4-isopropoxyphenyl)-amide and pharmaceutical compositions thereof for the treatment of cancer and other diseases or disorders
InactiveUS20150133460A1High drug loadingOrganic active ingredientsOrganic chemistryCell survivalFibrosis
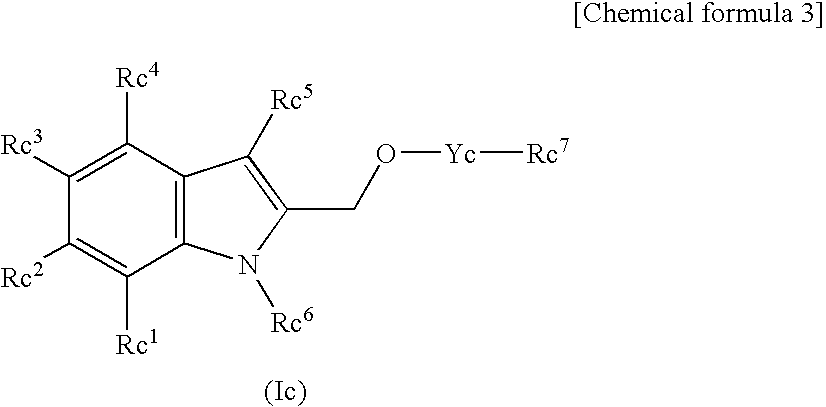
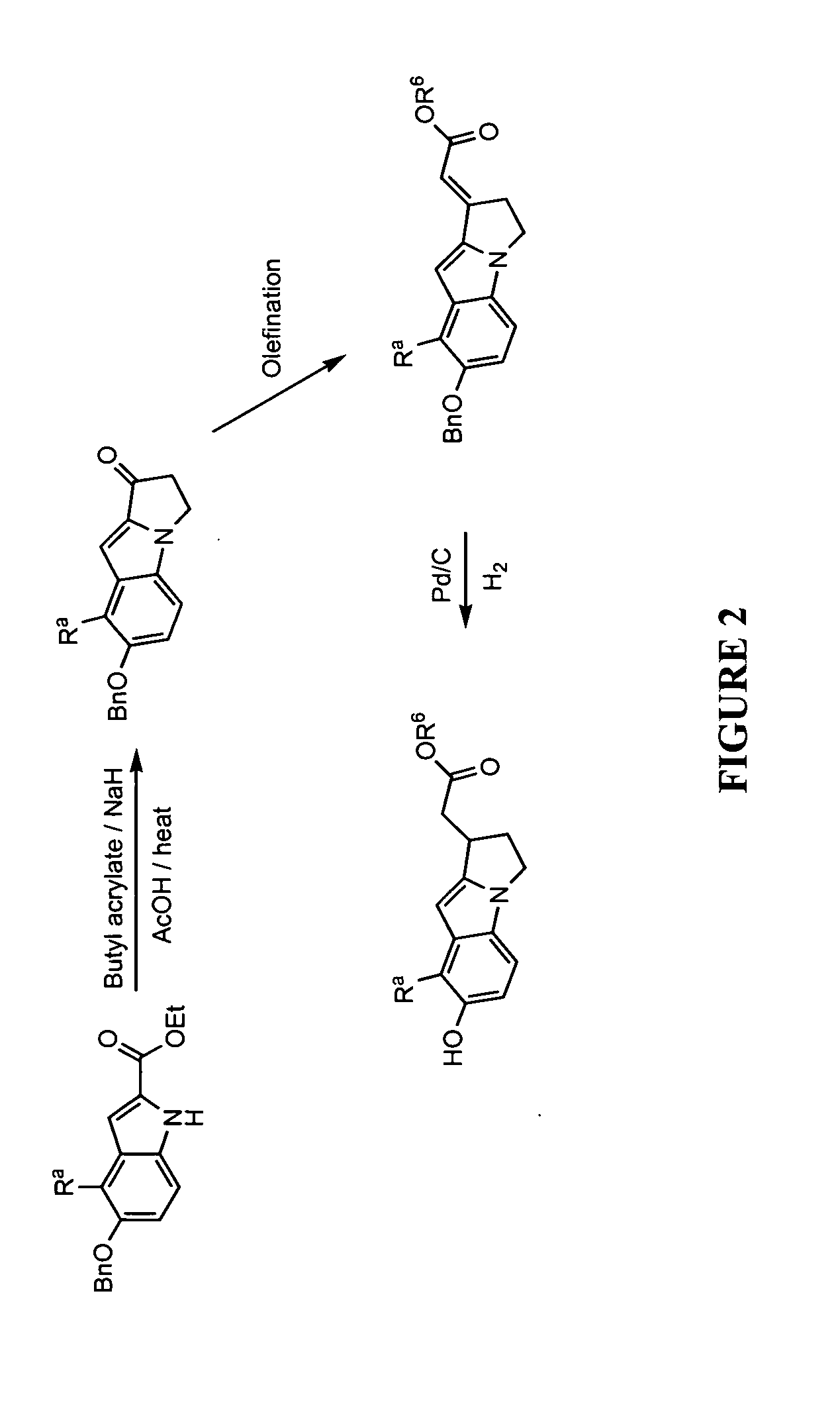
This invention provides a compound of formula (I):or a crystalline form thereof, or a pharmaceutical composition thereof, or an oral pharmaceutical dosage form thereof; processes for the synthesis or manufacture of the compound of formula (I), or a crystalline form thereof, or a pharmaceutical composition thereof, or an oral pharmaceutical dosage form thereof; and the use of said compound, or a crystalline form thereof, or a pharmaceutical composition thereof, or an oral pharmaceutical dosage form thereof, for the treatment of patients suffering from or subject to diseases, disorders or conditions involving cell survival, proliferation, and migration, including cardiovascular disease (e.g., arteriosclerosis and vascular reobstruction), cancer, (e.g., AML and malignant glioma) glomerulosclerosis, fibrotic disease and inflammation.
Owner:MILLENNIUM PHARMA INC
Method for targeting cells involved in sclerotic and/or fibrotic diseases
A compound includes a carrier molecule wherein the carrier molecule is linked to a further molecule, wherein the further molecule is at least one cyclic peptide in which the cyclic peptide portion thereof contains at least one sequence encoding a cell receptor recognizing peptide (RRP) and with the proviso that the compound is not a naturally occurring receptor agonist or antagonist. Preferably, the RRP is a receptor specific for Hepatic Stellate Cells (HSC) or a receptor that is up-regulated on HSC during disease. The RFP may be chosen from among a PDGF receptor, a collagen type VI receptor, cytokine receptor(s) such as TGBβ, INFα and interleukinβ. The cyclic portion of the peptide can contain at least one amino acid sequence RGD or KPT. The compounds can be used as an active targeting ingredient for manufacturing a pharmaceutical composition for therapy, prophylaxis or diagnosis of a disease chosen from fibrotic disease, sclerotic disease, and chronic or acute inflammatory processes including glomeruloscherosis, interstitial fibrosis, lung fibrosis, atherosclerosis, rheumatoid arthritis, Crohns disease, colitis ulcerosa, glomerulonephritis and sepsis, and particularly for targeting HSC. Pharmaceutical compositions contain the above-compound(s).
Owner:BIORION TECH
Protease-activated receptor 3 and uses thereof
Disclosed are cDNAs and genomic DNAs encoding protease-activated receptor 3 (PAR3) from mouse and human, and the recombinant polypeptides expressed from such cDNAs. The recombinant receptor polypeptides, receptor fragments and analogs expressed on the surface of cells are used in methods of screening candidate compounds for their ability to act as agonists or antagonists to the effects of interaction between thrombin and PAR3. Agonists are used as therapeutics to treat wounds, thrombosis, atherosclerosis, restenosis, inflammation, and other thrombin-activated disorders. Antagonists are used as therapeutics to control blood coagulation and thereby treating heart attack and stroke. Antagonists mediate inflammatory and proliferative responses to injury as occur in normal wound healing and variety of diseases including atherosclerosis, restenosis, pulmonary inflammation (ARDS) and glomerulosclerosis. Antibodies specific for a protease-activated receptor 3 (or receptor fragment or analog) and their use as a therapeutic are also disclosed.
Owner:RGT UNIV OF CALIFORNIA
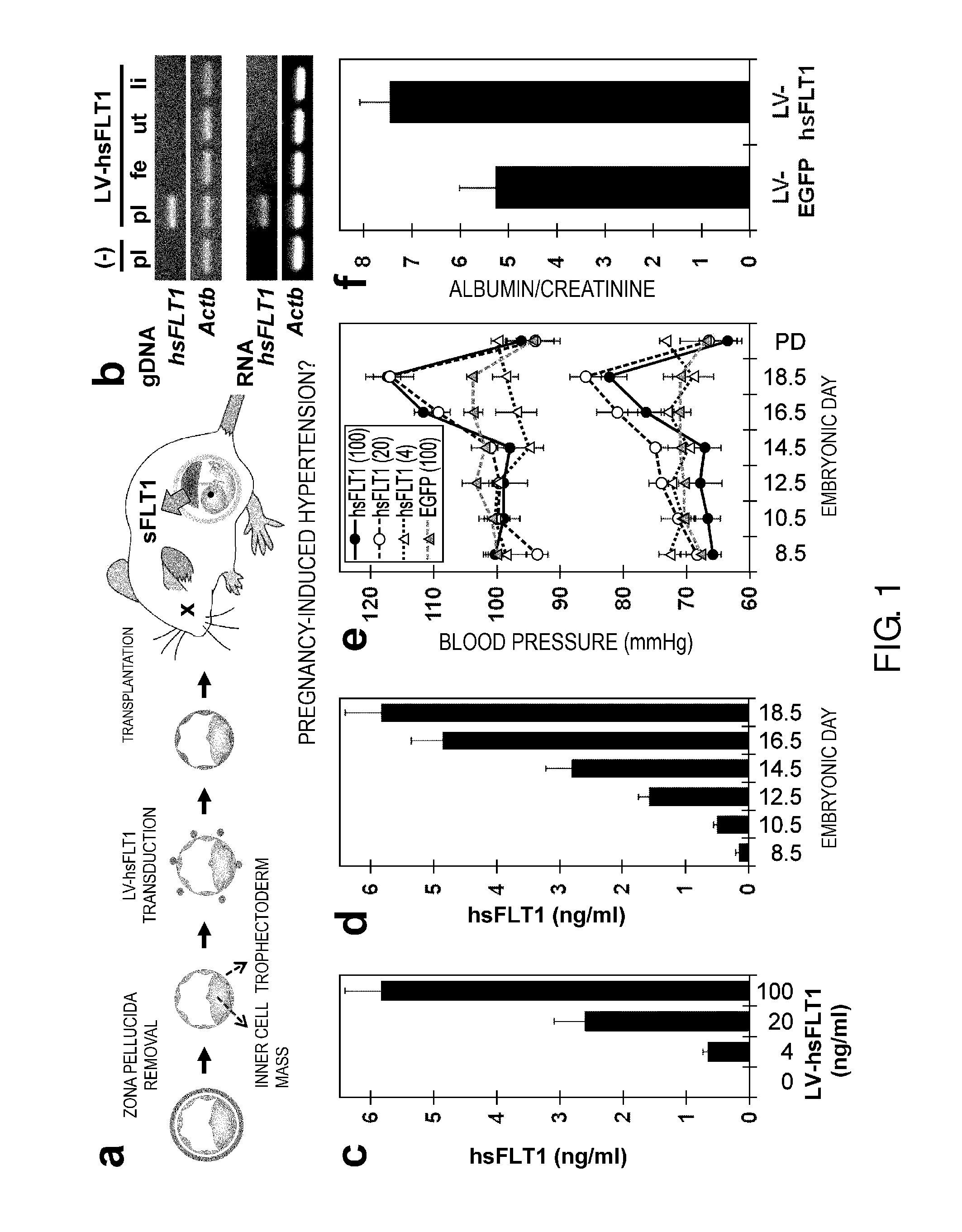
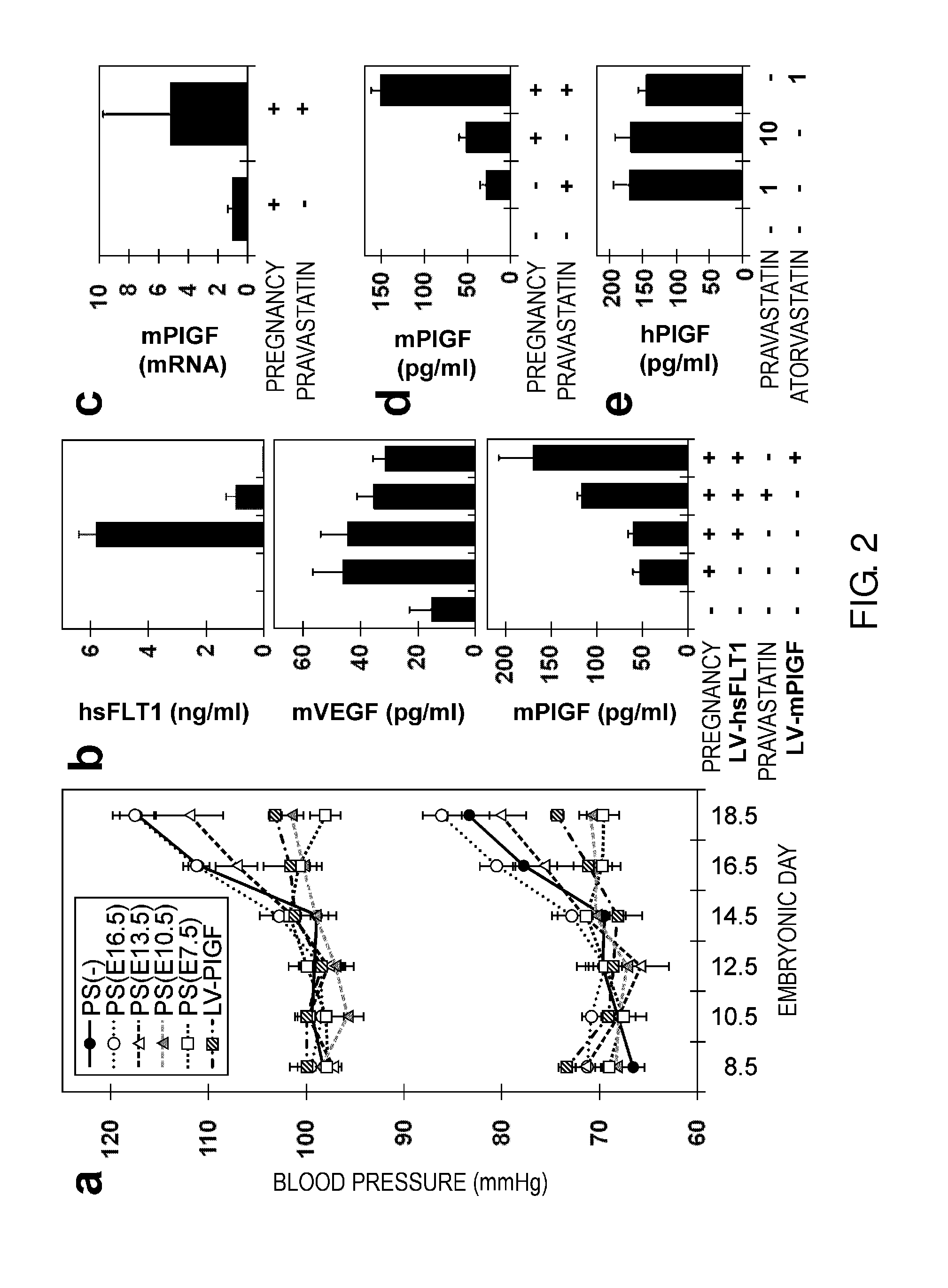
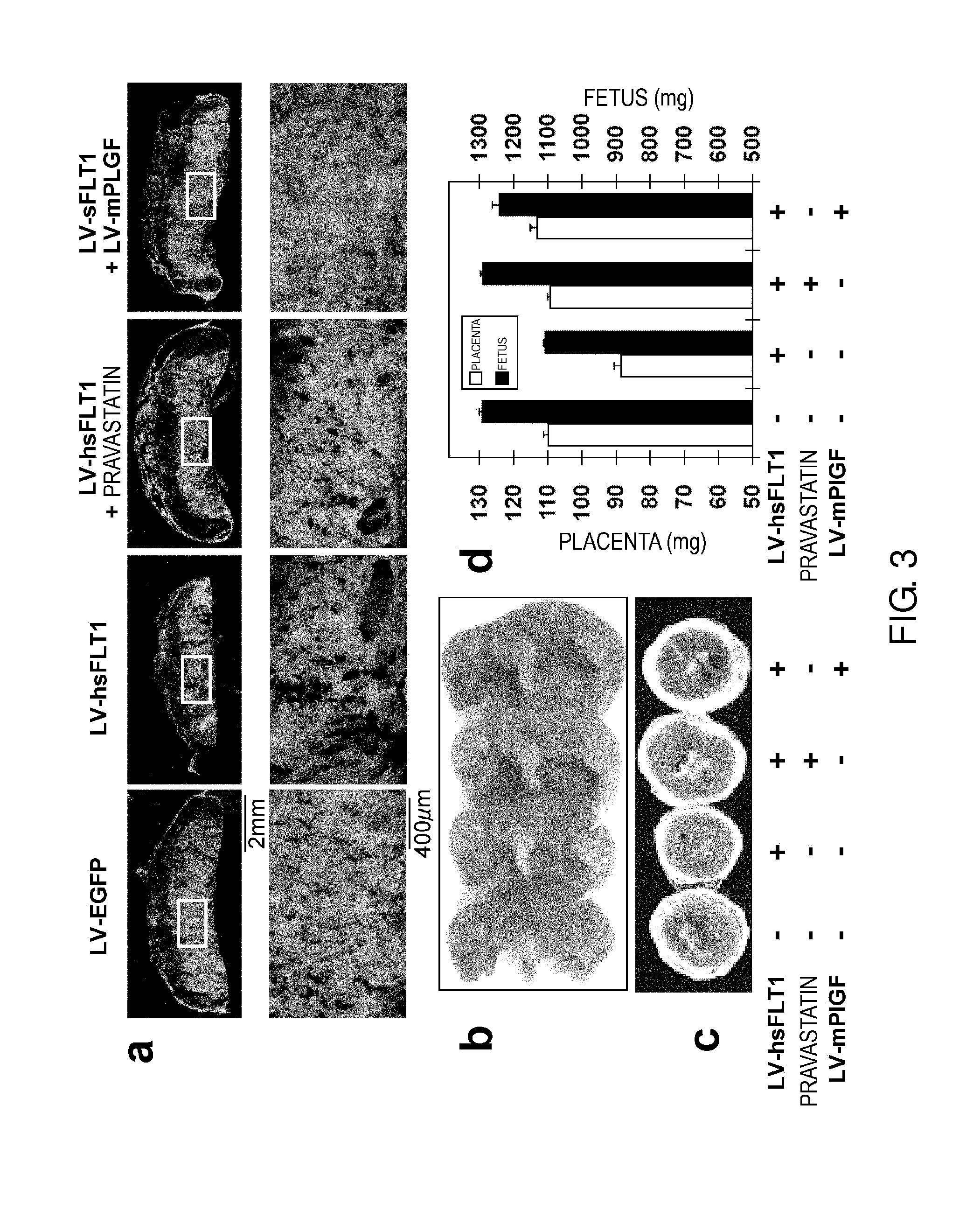
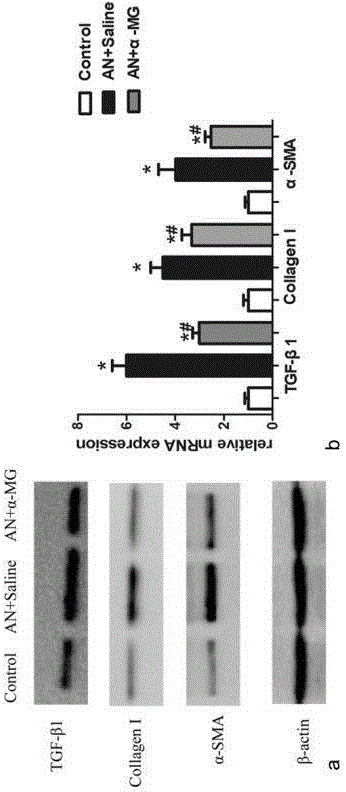
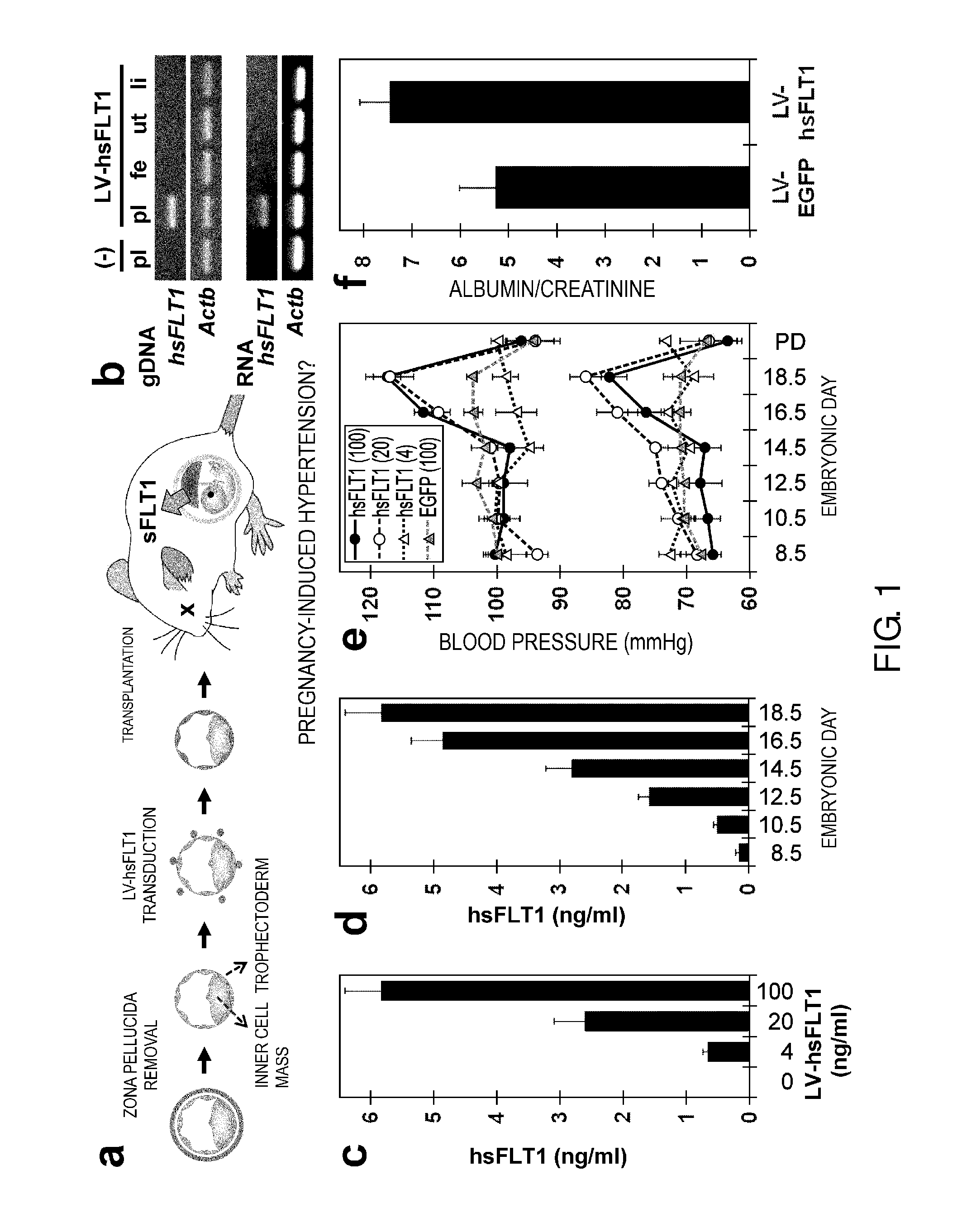
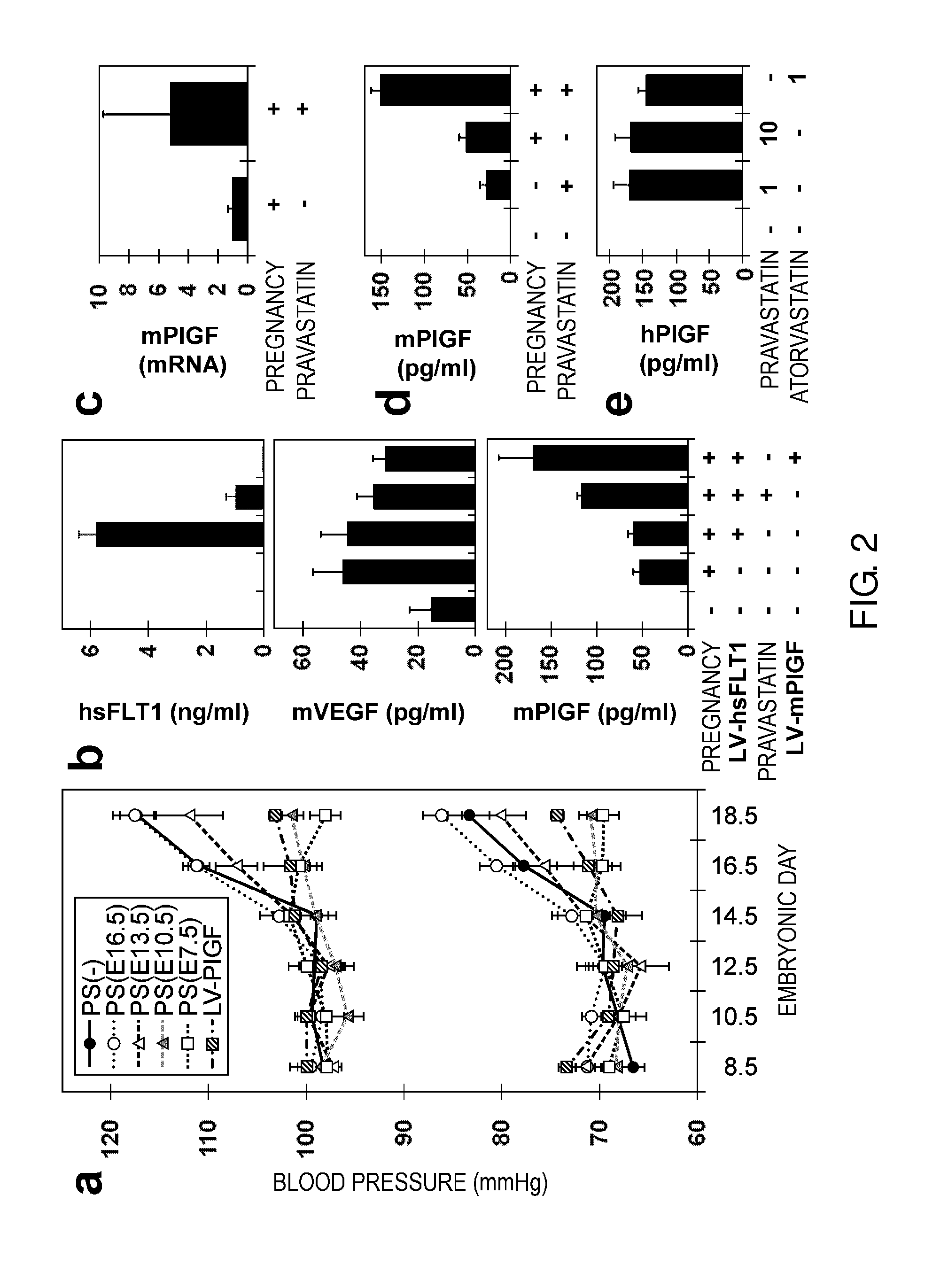
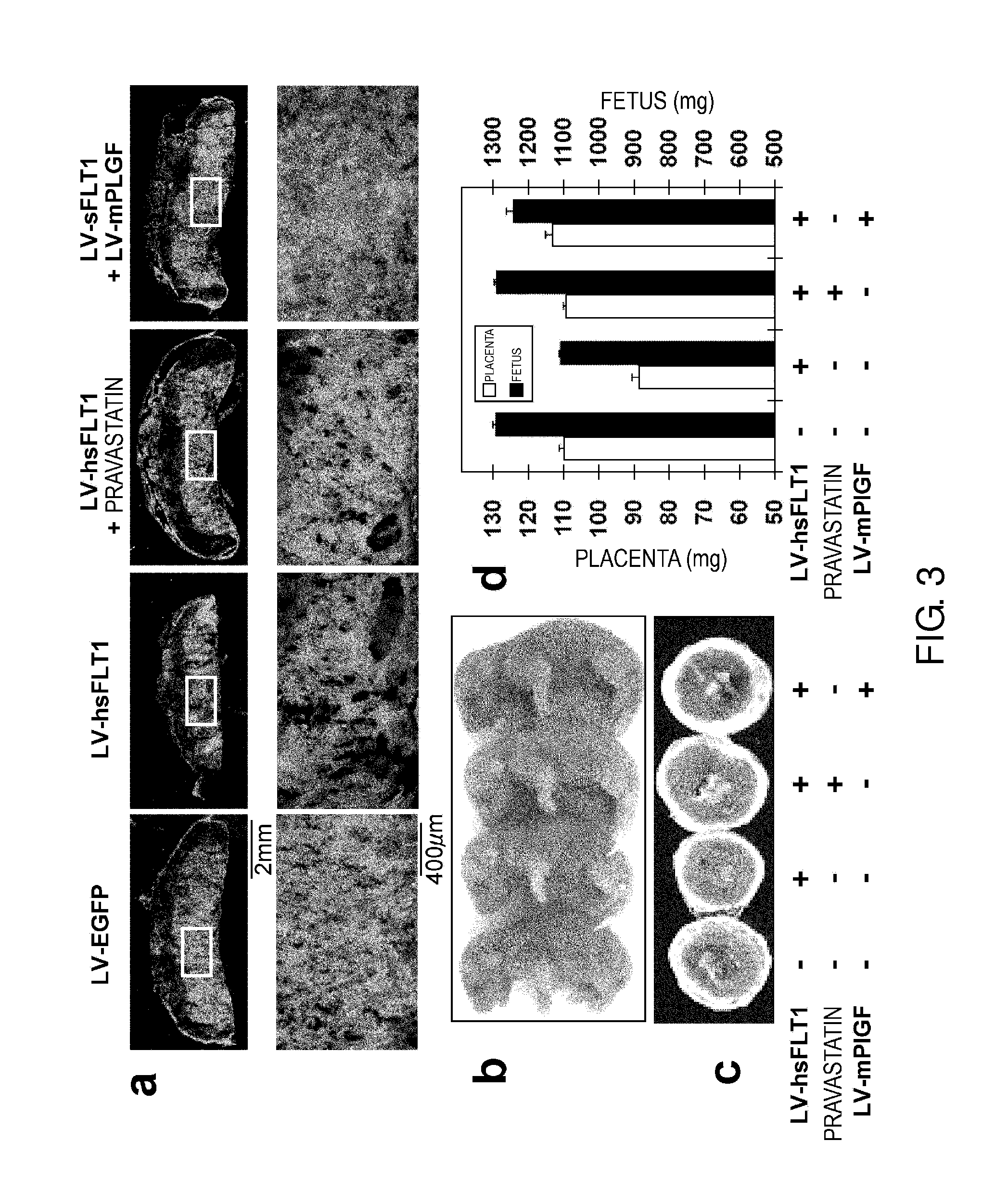
Model Animal for Pregnancy-induced Hypertension Syndrome, and Treatment Method Therefor
ActiveUS20130198874A1Symptoms improvedInduces ectopic expression of PIGFAnimal reproductionPeptide/protein ingredientsBiological activationPregnancy induced
A lentiviral vector was used to produce non-human animals that express human sFLT1 specifically in the murine placenta, to provide model animals of diseases such as pregnancy-induced hypertension syndrome that are close to the clinical conditions, methods for producing the model animals, methods of screening for candidate compounds as therapeutic agents for diseases such as pregnancy-induced hypertension syndrome by using the model animals, and therapeutic agents for diseases such as pregnancy-induced hypertension syndrome. As a result, the model animals were found to exhibit symptoms that are very close to the clinical conditions in human, which are presentation of hypertension as well as placental insufficiency, intrauterine growth retardation, glomerulosclerosis, and proteinuria during pregnancy, and improvement of those symptoms postpartum. Furthermore, when pravastatin was administered to this model animal, it was found that diseases such as pregnancy-induced hypertension syndrome were improved by the activation of placenta-derived growth factor (PIGF) which antagonizes sFLT1.
Owner:FUSO PHARMA INDS
Chinese medicine composition for treating early and medium-term chronic renal failure
InactiveCN101194984BSignificant effectLower medical costsPowder deliveryPill deliveryMetaboliteTurbidity
The invention belongs to the traditional medicine technical field, which relates to a traditional Chinese medicine composition for curing early and intermediate chronic renal failure. Under the guidance of a traditional Chinese medicine theory that 'interior accumulation is blood stasis' and 'chronic diseases transforming to collaterals is blood stasis', with the treatment principle of differentiation of symptoms and sighs, differentiation of channels, and based on the principle of strengthening body resistance and reducing turbidity, promoting blood circulation and removing blood stasis, the invention adopts traditional medicine such as lanceolata, epicedium, salvia miltiorrhizae, prepared rhubarb, perilla, peach kernel and the like, which are made into the compositions. Confirmed by clinical and animal experiments, the invention has the renal protecting functions of improving clinical symptoms of patients with early and intermediate chronic renal failure, reducing retention of metabolites of bodies, improving anemia, adjusting lipid and calcium-phosphorus metabolism, reducing the expression of fibrosis factors in nephridial tissues, preventing and treating glomerulosclerosis, lightening renal tubule damage and renal interstitial inflammatory cell infiltration and delaying renal fibrosis. The invention has the advantages of safety, efficiency and low cost, which is applied tothe long-term medication for patients with early and intermediate chronic renal failure.
Owner:SHUGUANG HOSPITAL AFFILIATED WITH SHANGHAI UNIV OF T C M
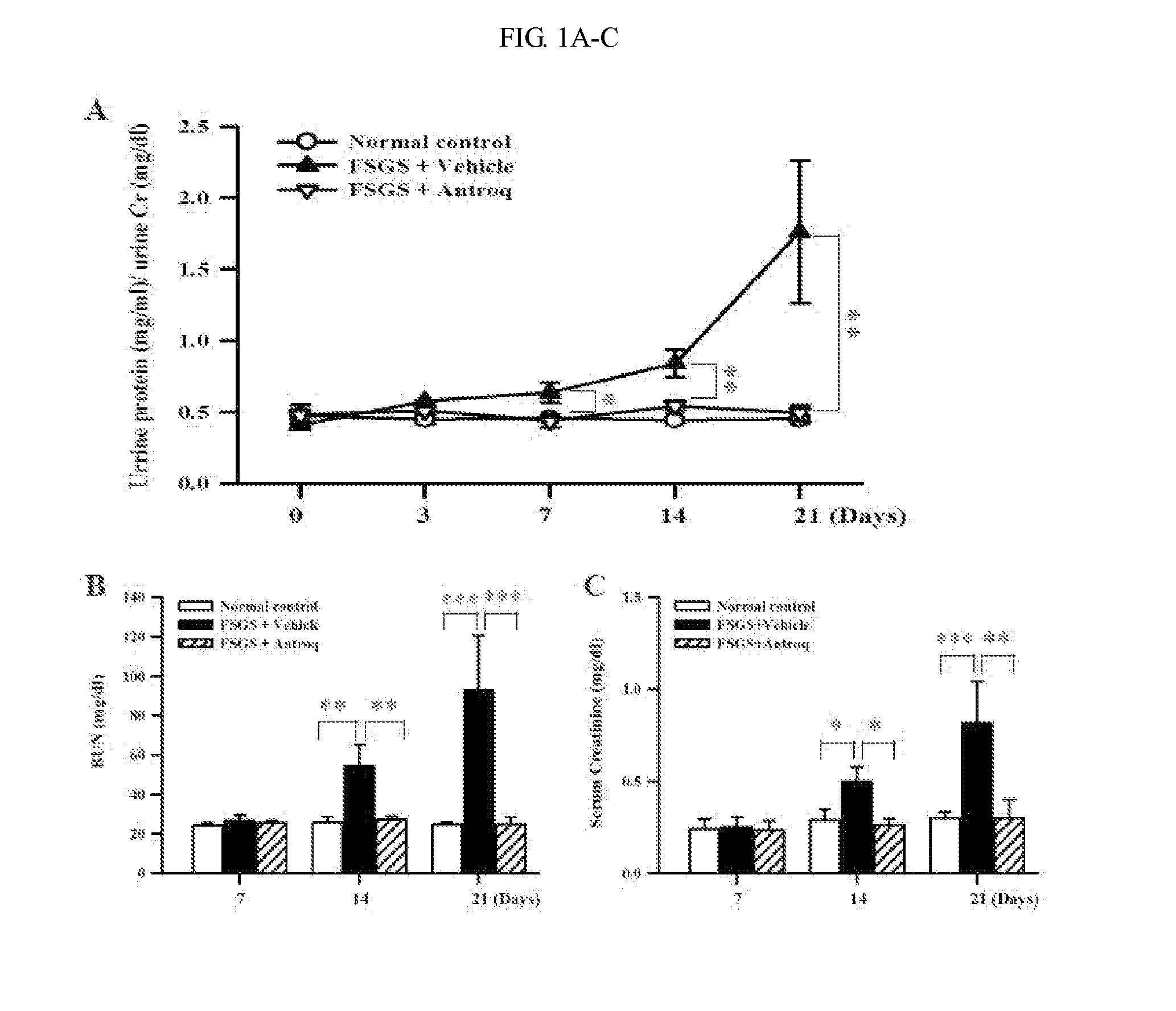
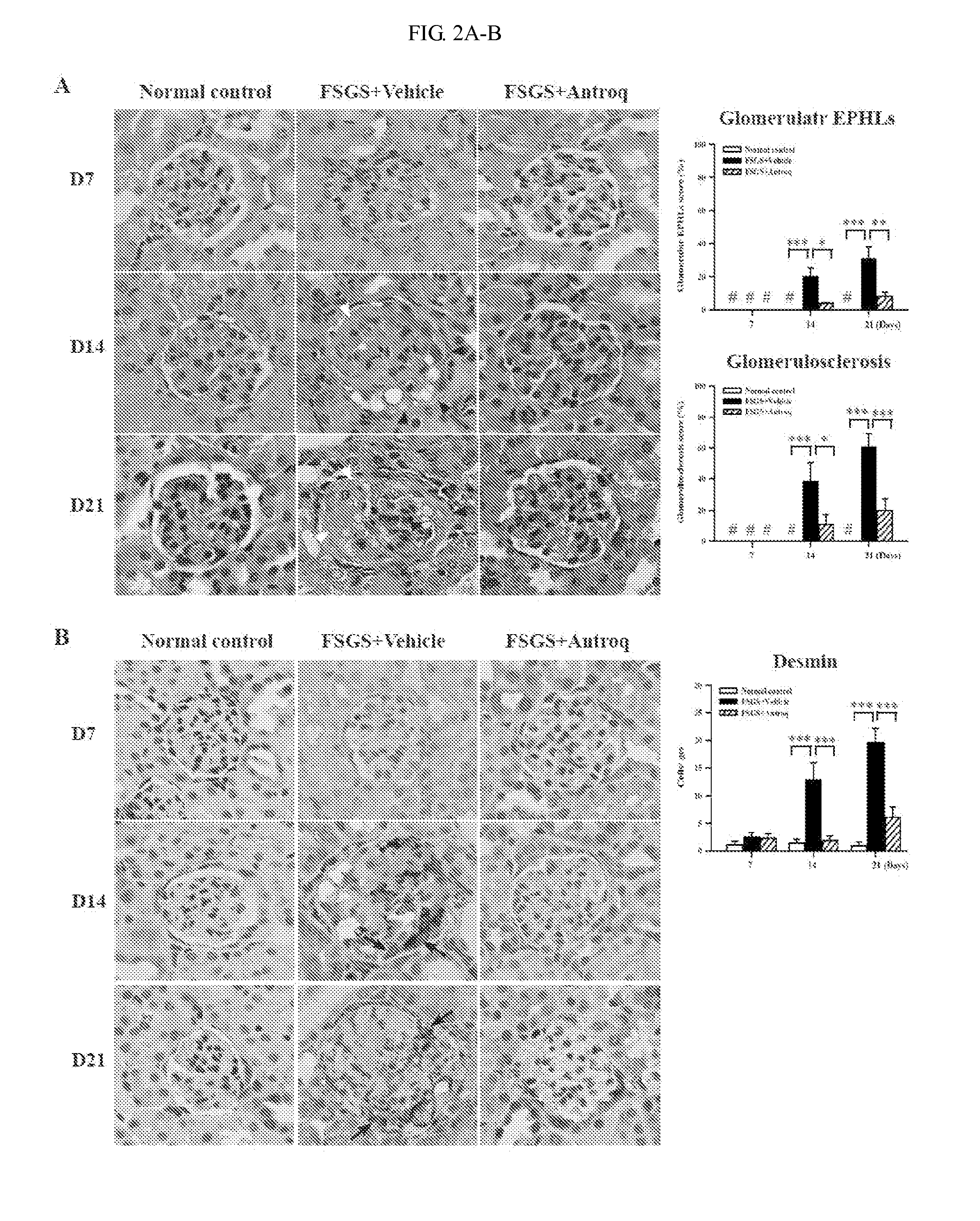
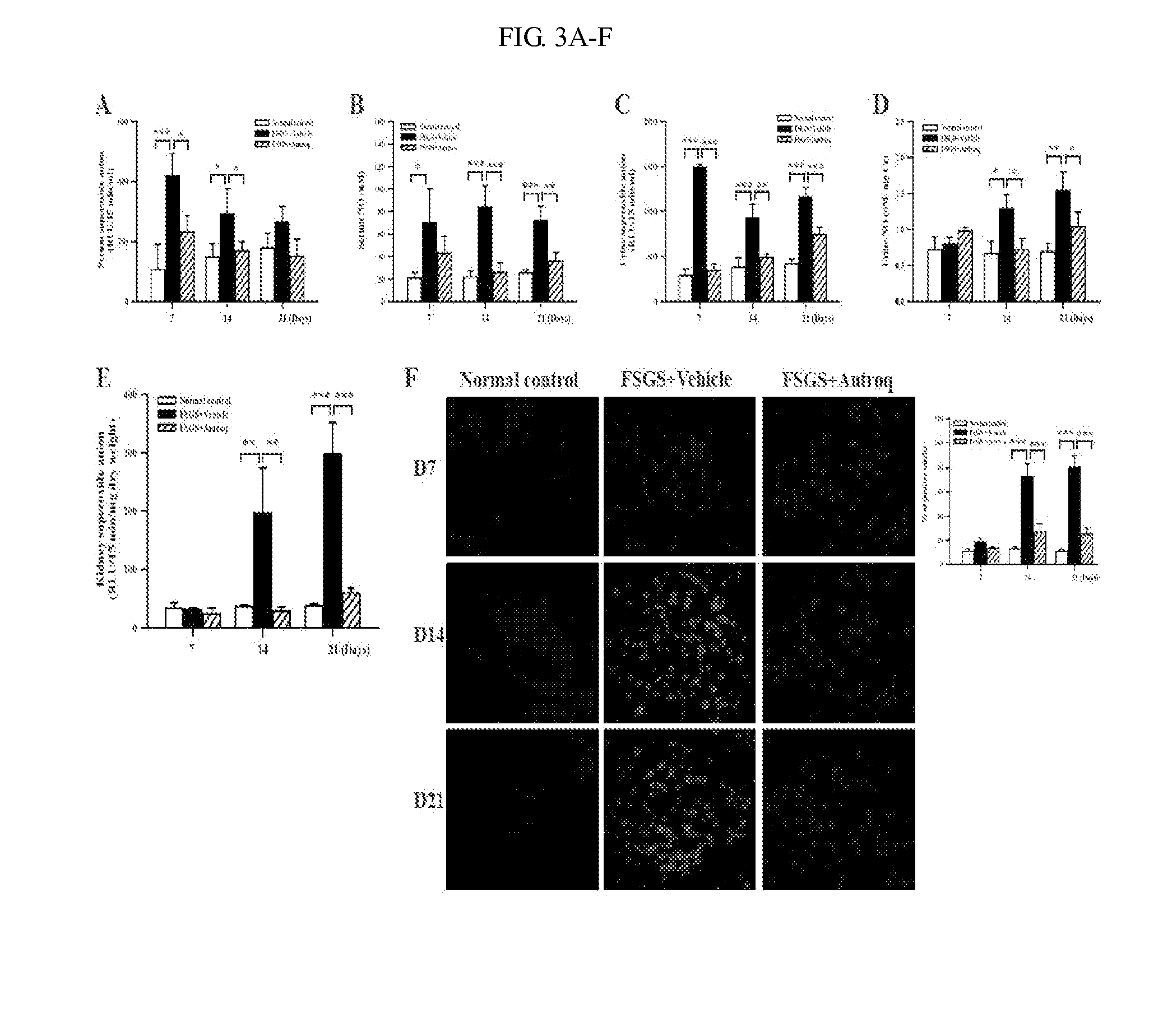
Use of des-Aspartate-angiotensin I as an agent for the treatment and prevention of glomerulosclerosis and renal failure
InactiveUS7553928B2Symptoms improvedPeptide/protein ingredientsVertebrate cellsZona glomerulosaFAILURE KIDNEY
The present invention relates generally to a method for the treatment and / or prophylaxis of renal-related disorders. More particularly, the present invention contemplates a method for the treatment and / or prophylaxis of glomerulosclerosis and / or end stage renal failure and / or related conditions. The method of the present invention is preferably practised by the administration of a derivative of angiotensin I. Generally, but not exclusively, the angiotensin I derivative exhibits anti-angiotensin II properties. In a preferred embodiment, the angiotensin I is des-Aspartate-angiotensin I or a derivative, homologue or analogue thereof. The present invention further contemplates compositions for use in the treatment and / or prophylaxis of renal-related disorders such as but not limited to glomerulosclerosis and / or end stage renal failure.
Owner:NAT UNIV OF SINGAPORE
Methods and compositions for treating kidney disorders
The present invention provides methods for treating glomerulosclerosis such as focal segmental glomerulosclerosis (FSGS) or glomerulonephritis such as immunoglobulin A nephropathy (IgAN) by cyclohexenone compounds.
Owner:GOLDEN BIOTECH
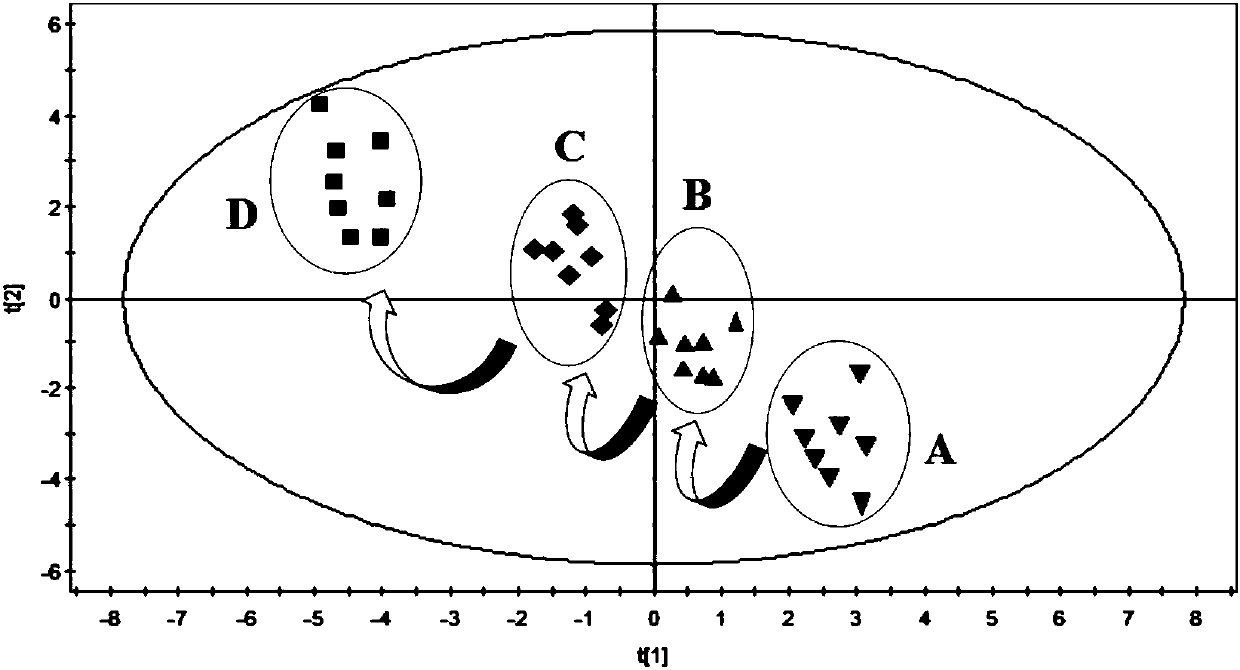
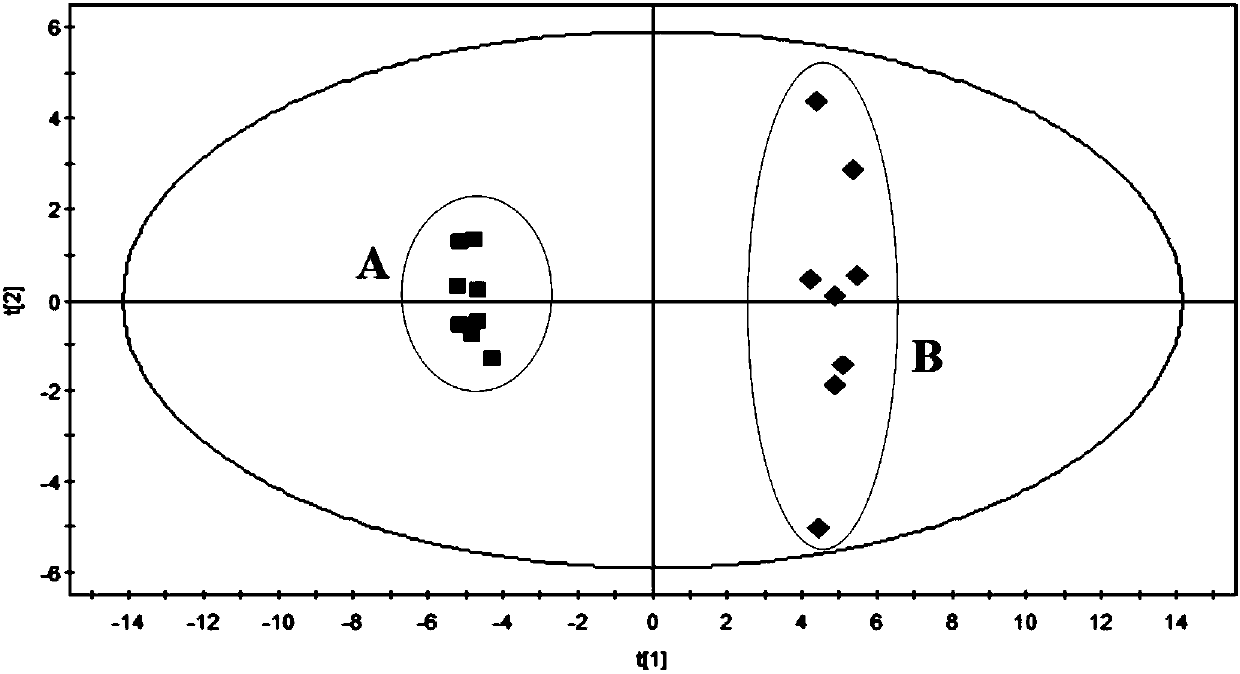
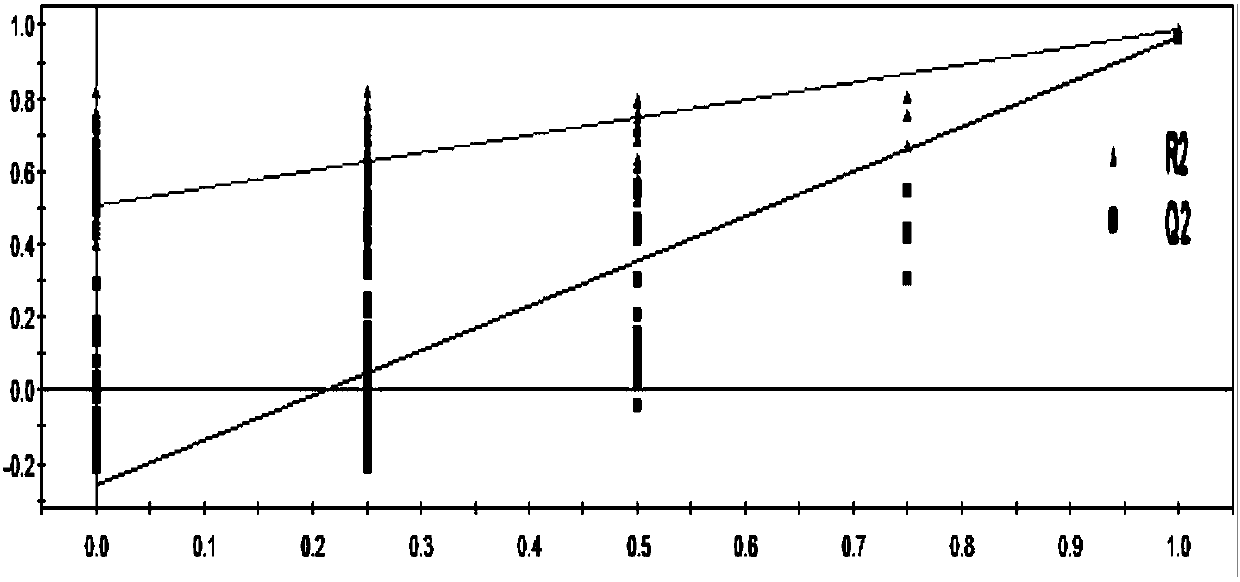
Evaluation method for rat model with glomerulosclerosis
InactiveCN107561175AComponent separationComplex mathematical operationsMultivariate statisticalRat model
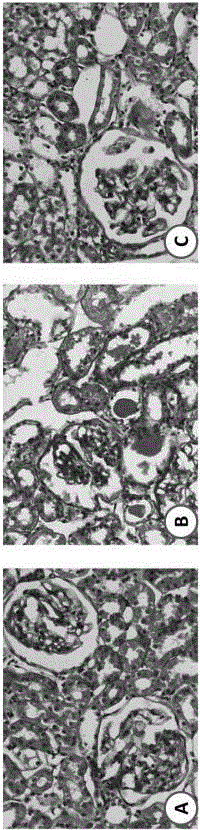
The invention discloses an evaluation method for a rat model with glomerulosclerosis. The evaluation method comprises steps as follows: firstly, urine of the rat model is collected on day 0, on the seventh day, on the tenth day and on the fourteenth day and subjected to LC-MS (liquid chromatography-mass spectrometry) determination and analysis respectively, and an LC-MS spectrogram of the rat model is obtained; an integral data matrix of the LC-MS spectrogram of the rat model is subjected to multivariate statistical analysis, and a profile diagram of the rat model is obtained; the profile diagram of the rat model is subjected to dynamic profile analysis, a dynamic profile change trend diagram of the rat model is obtained, and degree of deviation on the fourteenth day of model constructionis the highest compared with that on day 0; analysis based on Marker-view software indicates that the construction of the rat model with glomerulosclerosis succeeds when content of 15 biomarkers on the fourteenth day of model construction meets the requirement compared with that on day 0; the technical problem that an existing construction and evaluation method for the rat model with glomerulosclerosis is low in accuracy, high in cost, time-consuming and labor-consuming is solved.
Owner:WUHAN UNIV
Rat model of diabetic nephropathy
Owner:THE MEDICAL COLLEGE OF WISCONSIN INC
Tissue Factor Production Inhibitor
InactiveUS20080255111A1Inhibit productionReduce thrombosisBiocideOrganic chemistryAbnormal tissue growthPercent Diameter Stenosis
A medicament which has an activity of inhibiting production of tissue factor and comprises an LXR ligand as an active ingredient; and a medicament for treatment and / or prophylaxis of vascular restenosis following angioplasty, endarterectomy, percutaneous transluminal coronary angioplasty (PTCA) or stent implantation, or treatment and / or prophylaxis of blood coagulation diseases, diseases induced by platelet aggregation including stable or unstable angina pectoris, cardiovascular and cerebrovascular diseases including thromboembolism formation diseases accompanying diabetes, rethrombosis following thrombolysis, cerebral ischemic attack, infarction, stroke, ischemia-derived dementia, peripheral artery disease, thromboembolism formation diseases during use of an aorta-coronary artery bypass, glomerulosclerosis, renal embolism, tumor or cancer metastasis, which comprises an LXR ligand as an active ingredient.
Owner:DAIICHI SANKYO CO LTD
Rat model of diabetic nephropathy
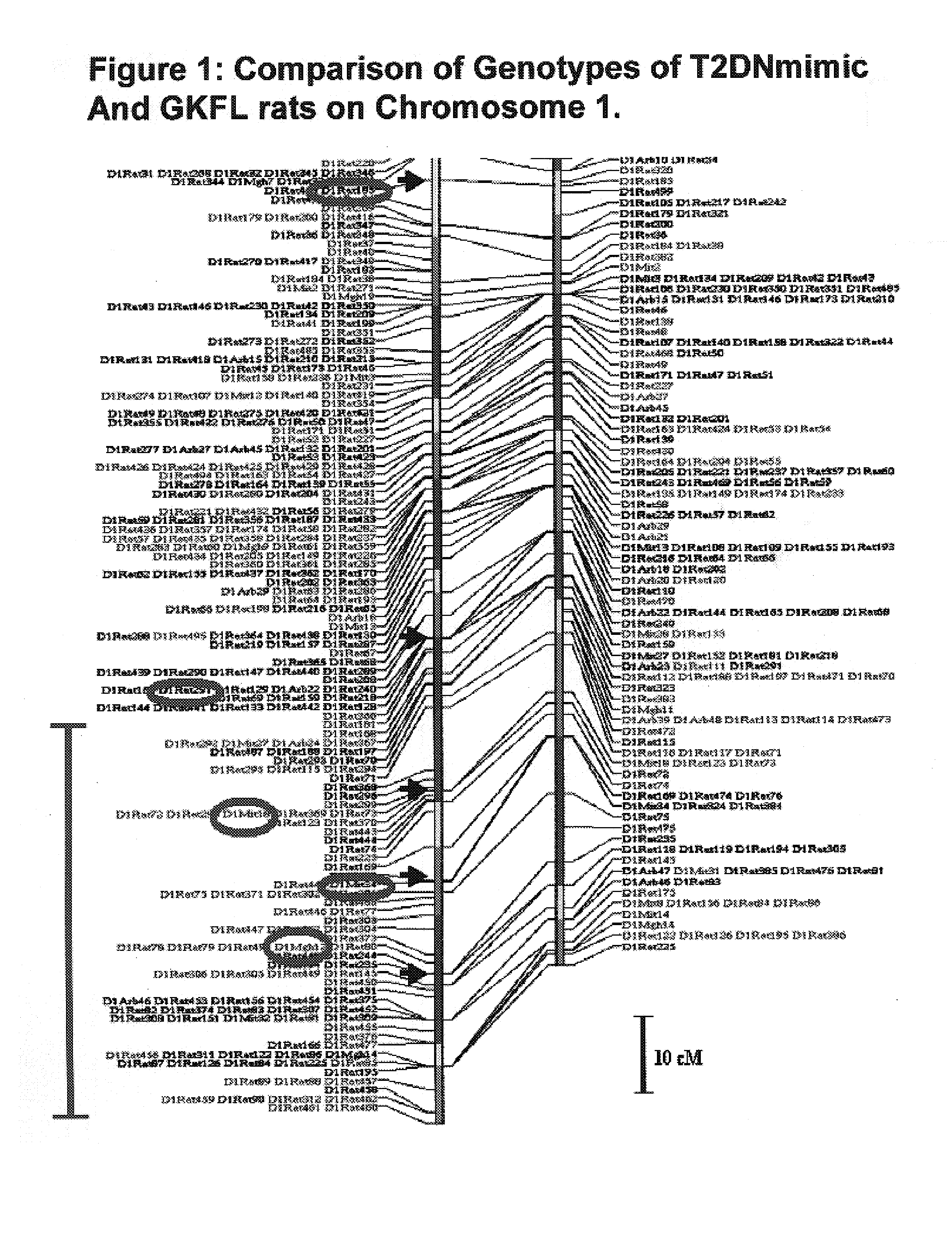
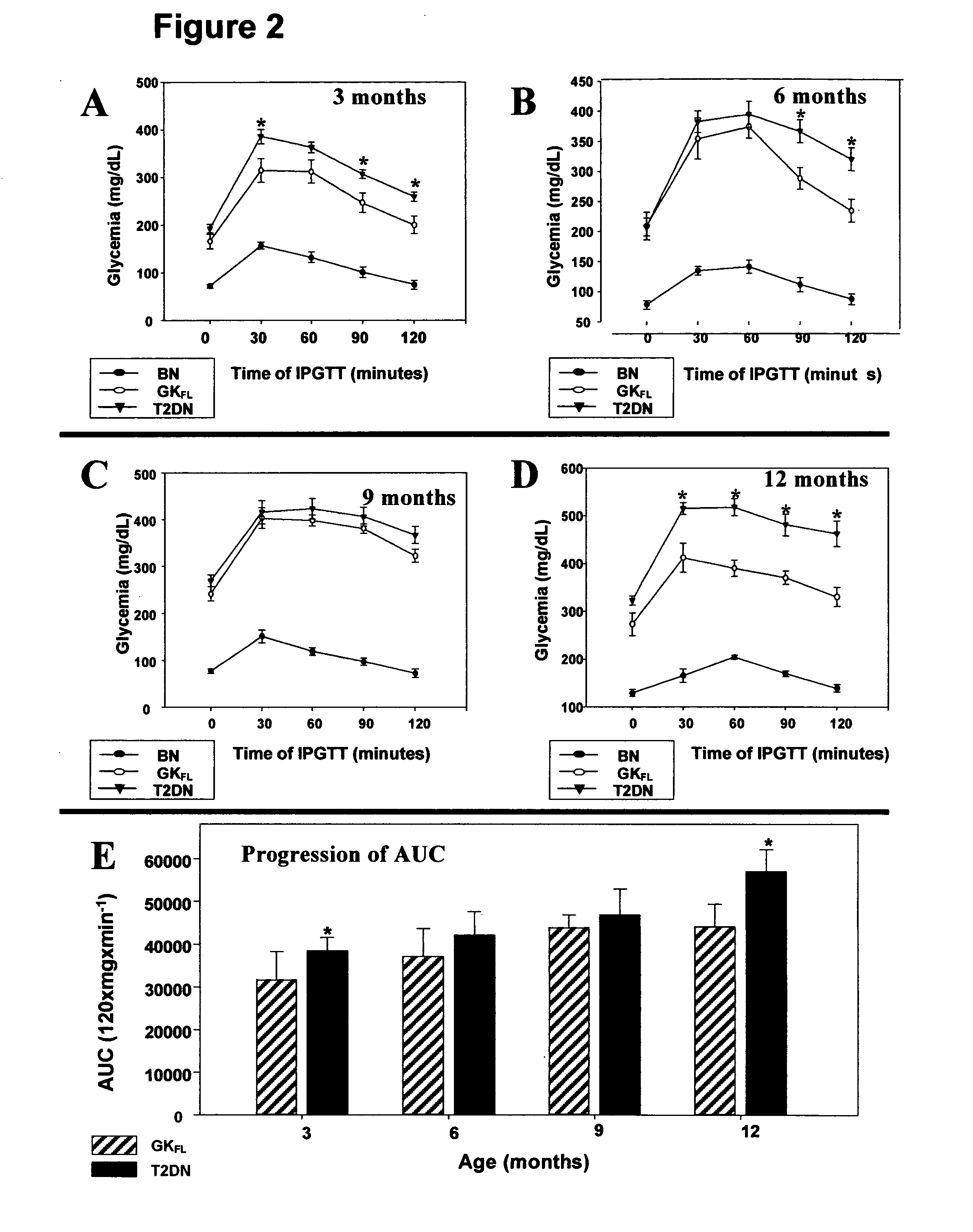
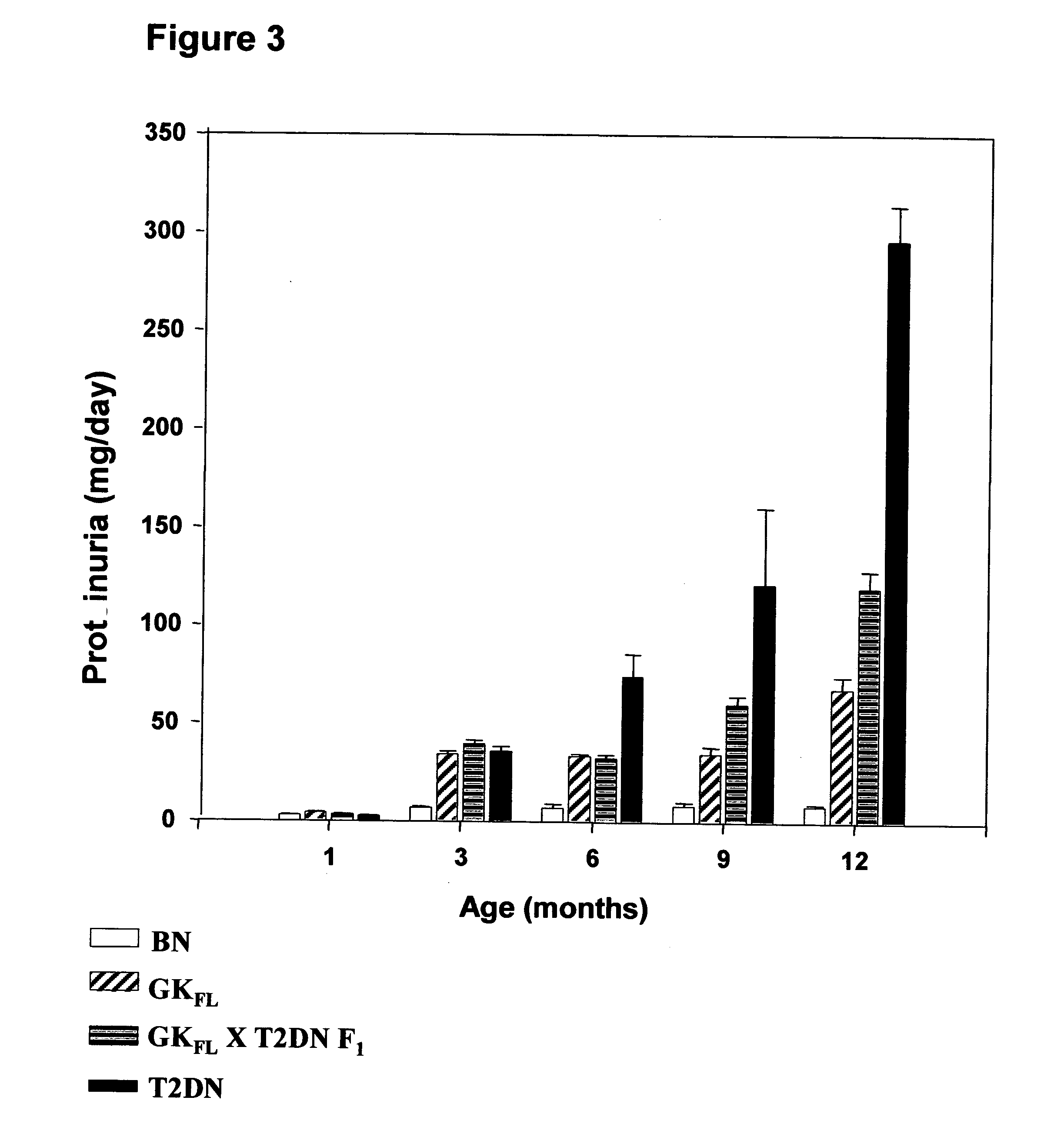
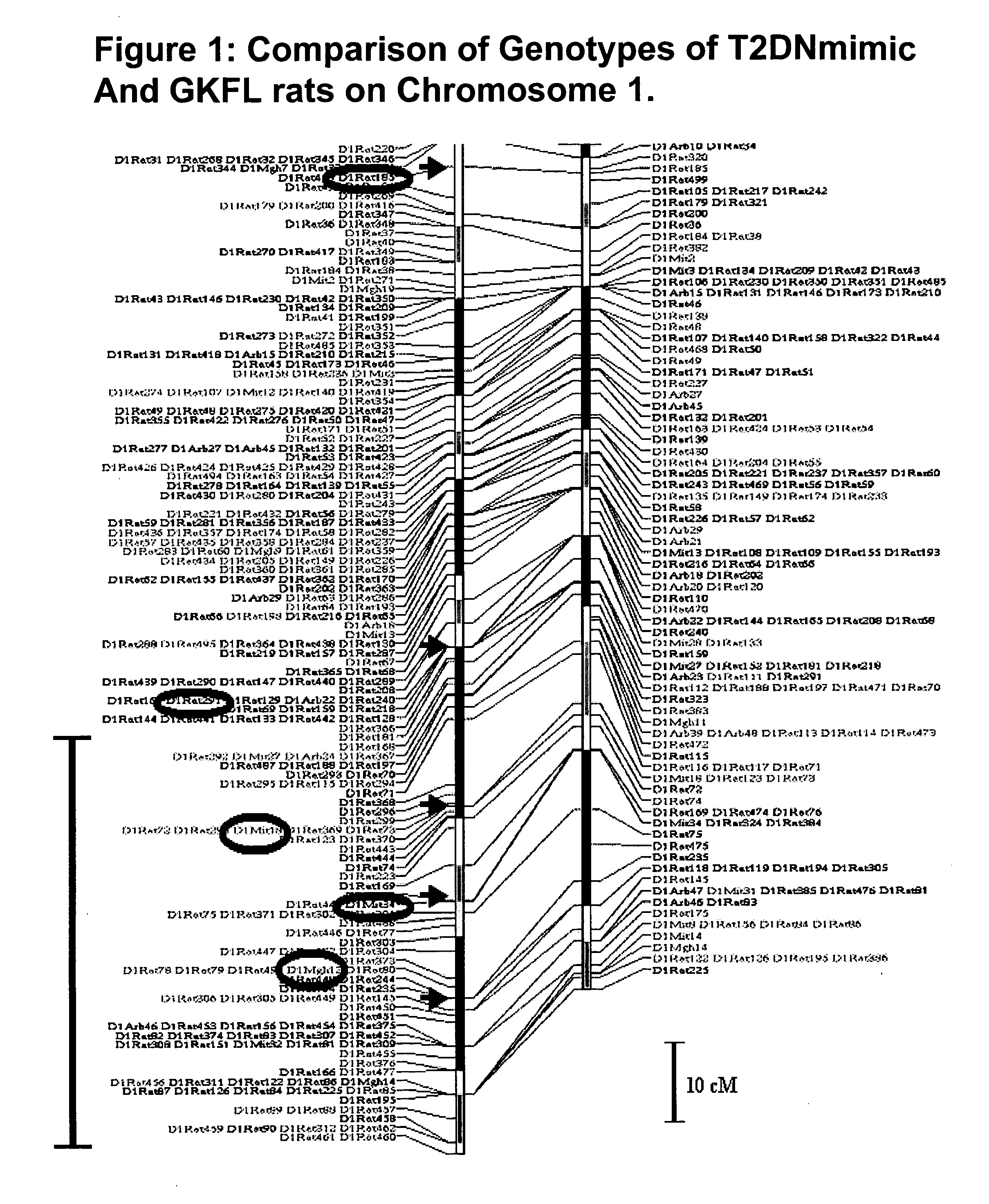
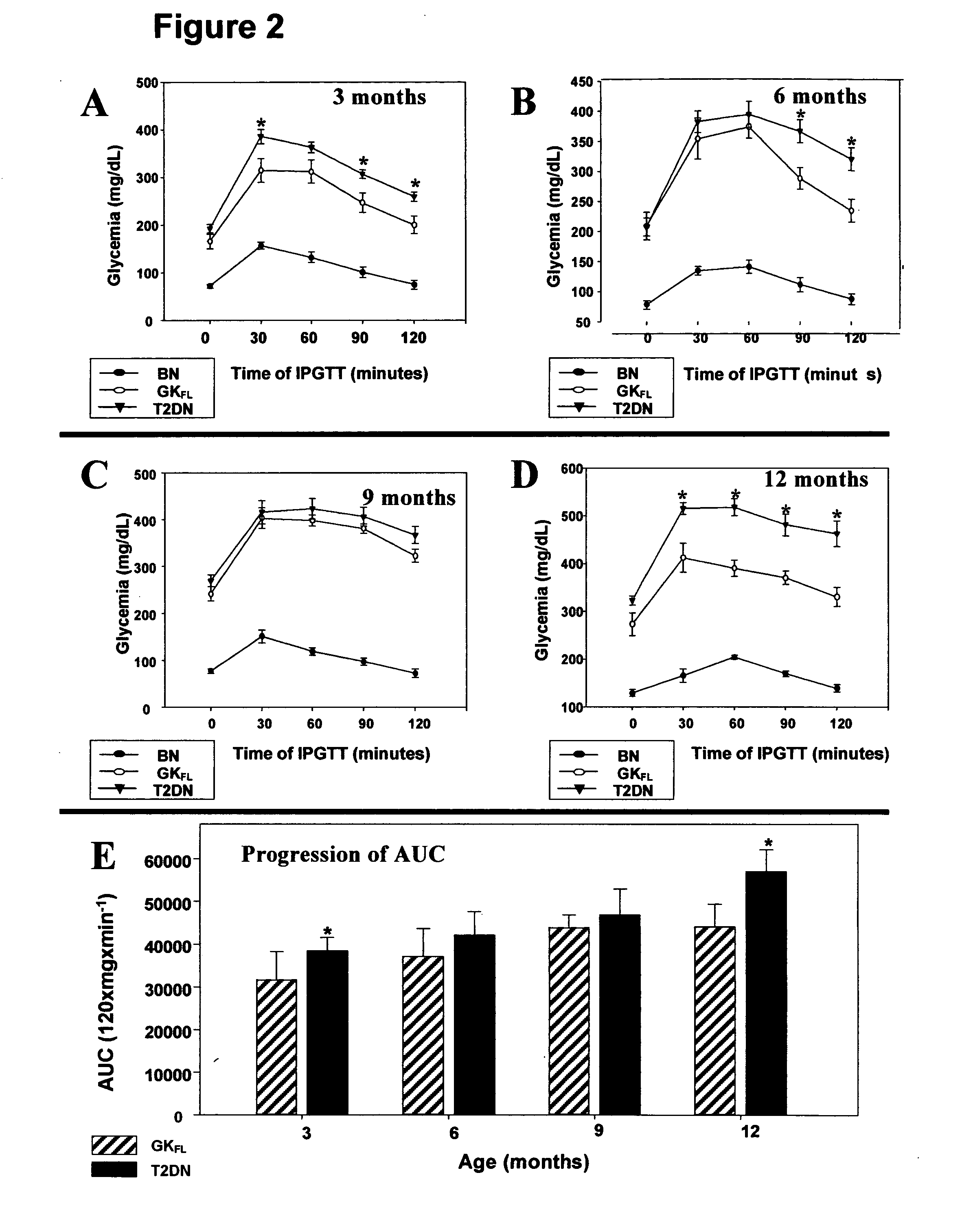
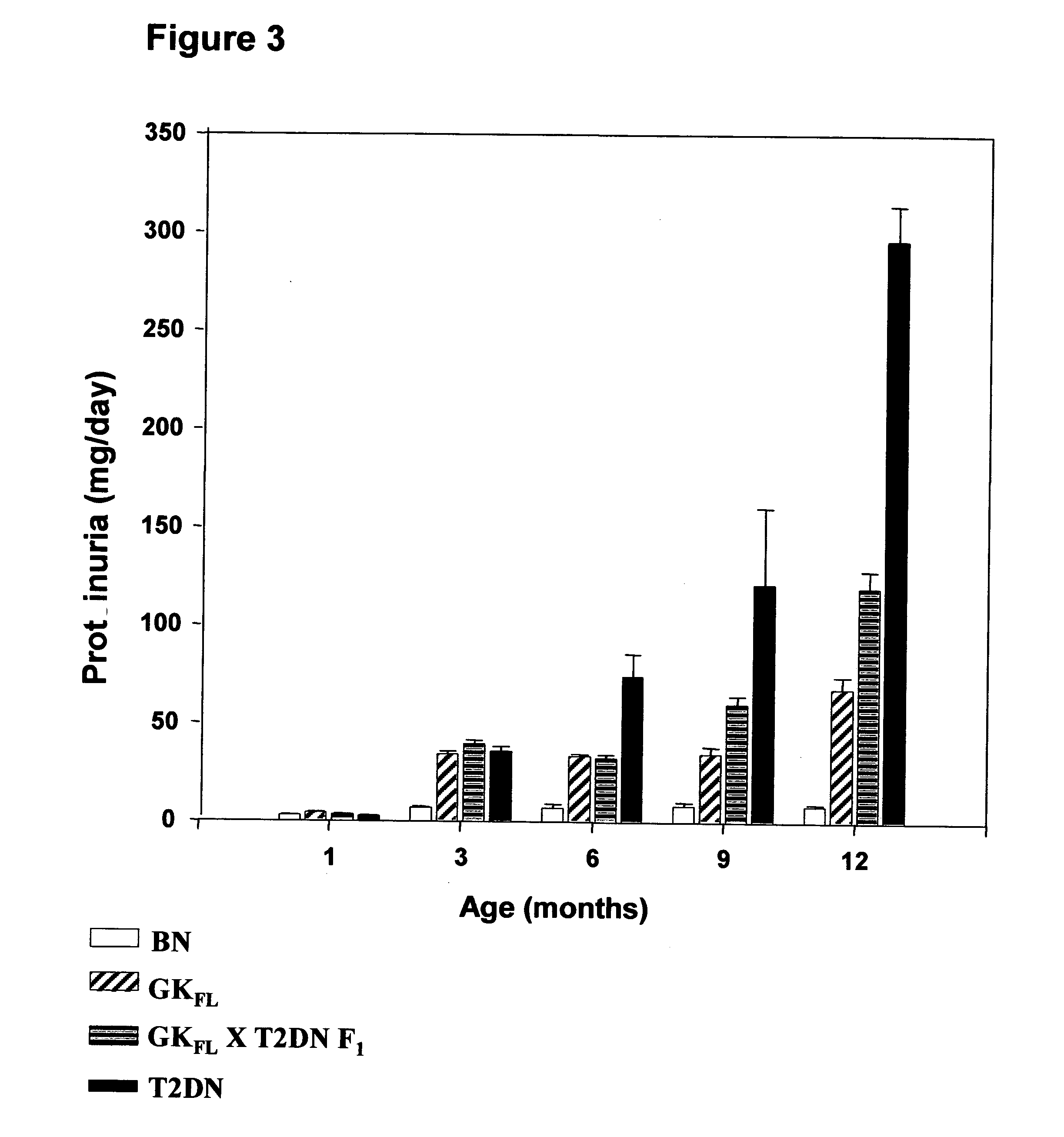
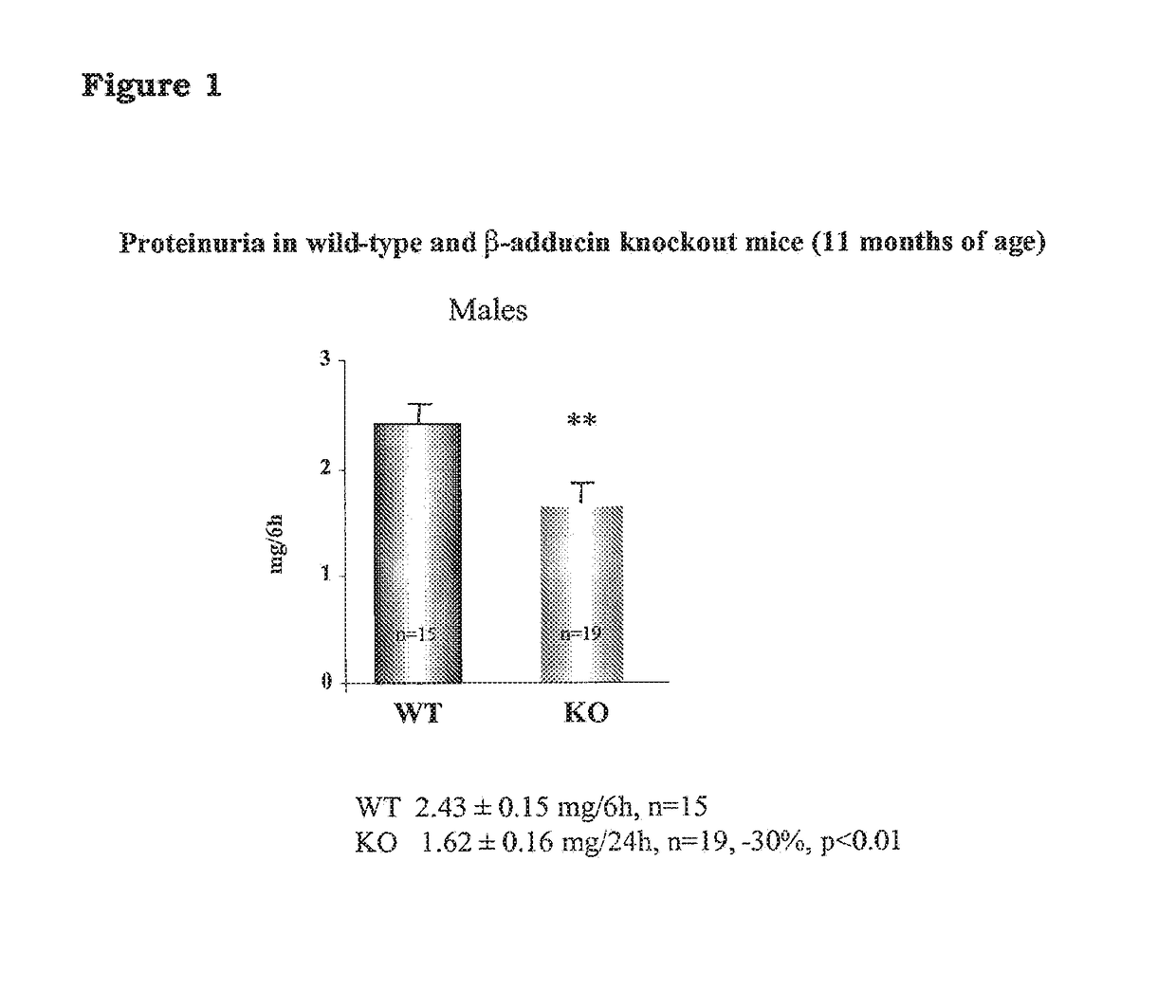
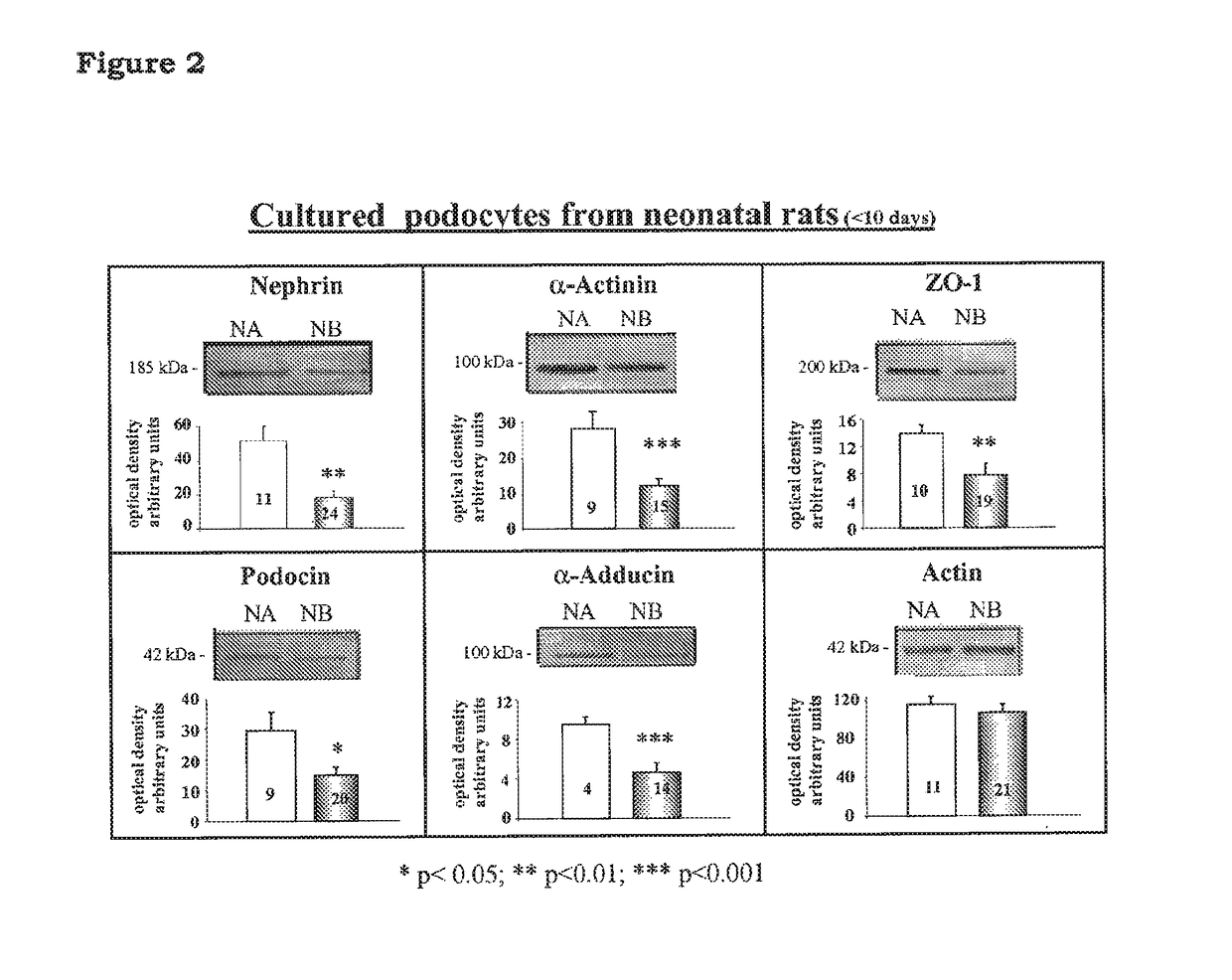
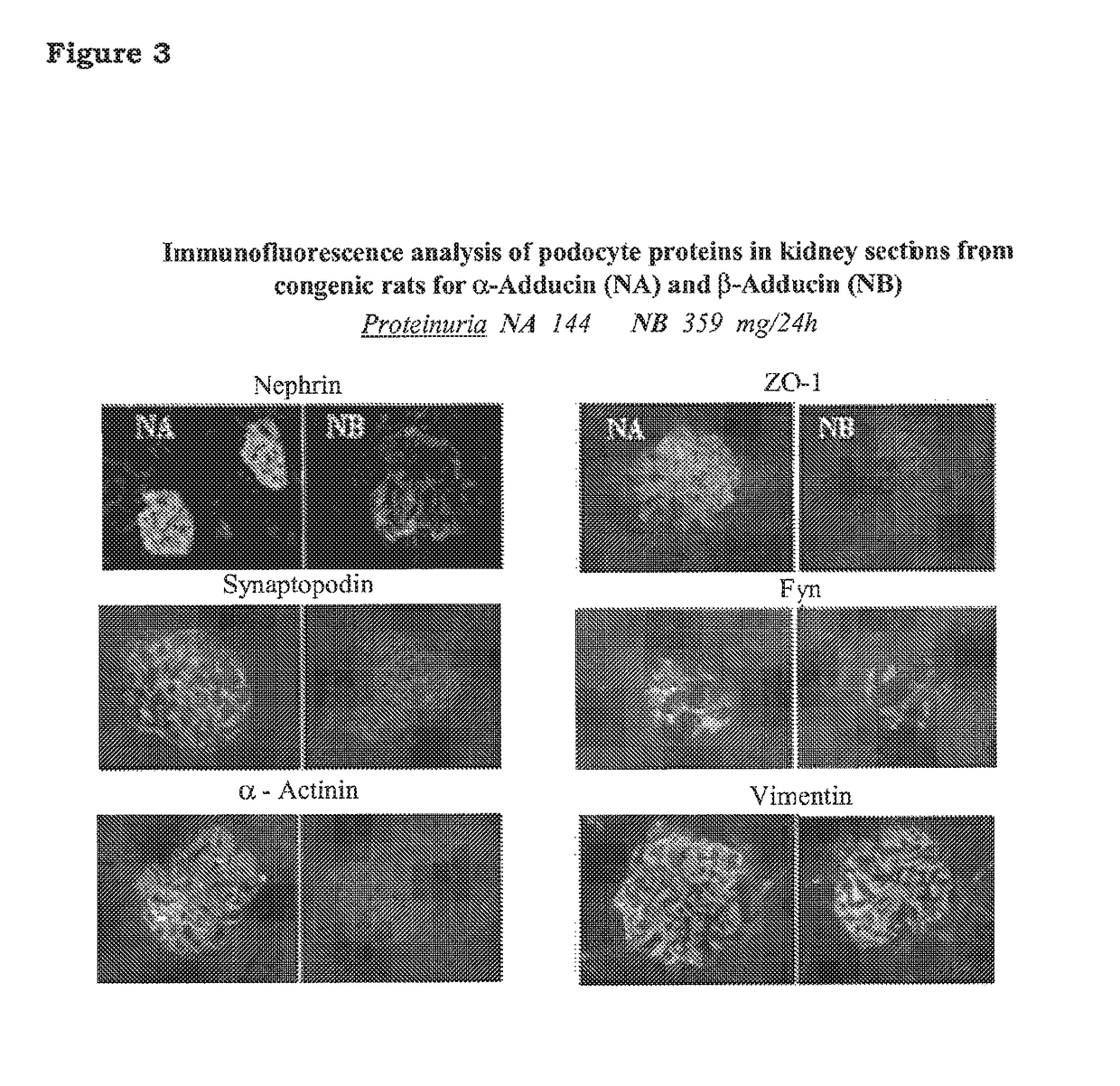
A rat model of diabetic nephropathy is disclosed. In another embodiment of the invention, a method of evaluating a test compound's effect of diabetic nephropathy is disclosed. In one embodiment, this method comprises the steps of (a) exposing the test compound to the rat of claim 1, wherein the rat would develop progressive proteinuria and glomerulosclerosis leading to diabetic nephropathy in the absence of the test compound, and (b) comparing the rat's development of diabetic nephropathy with a control T2DN mimic rat that has not been exposed to the test compound.
Owner:THE MEDICAL COLLEGE OF WISCONSIN INC
5-β, 14-β-androstane derivatives useful for the treatment of proteinuria, glomerulosclerosis and renal failure
Owner:CVIE THERAPEUTICS LTD
New application of mangosteen to aspect of preparing medicines for treating focal segmental glomerulosclerosis
InactiveCN106539843AImprove securityNo side effectsOrganic active ingredientsUrinary disorderAlpha mangostinSide effect
The invention discloses a mangosteen, in particular to new application of alpha-mangostin to an aspect of preparing medicines for treating focal segmental glomerulosclerosis. The alpha-mangostin extracted from mangosteen shells can obviously reduce the glomerulus and renal tubule fibrosis expression level, meanwhile, accelerates SIRT1 expression and inhibits NLRP3 expression, is obvious on effect of treating glomerulosclerosis, is higher in safety of clinical application, has no any side effects, and widens the path of treating the focal segmental glomerulosclerosis.
Owner:刘国勇 +1
Model animal for pregnancy-induced hypertension syndrome, and treatment method therefor
ActiveUS9029627B2Symptoms improvedInduces ectopic expression of PIGFAnimal reproductionPeptide/protein ingredientsBiological activationPregnancy induced
A lentiviral vector was used to produce non-human animals that express human sFLT1 specifically in the murine placenta, to provide model animals of diseases such as pregnancy-induced hypertension syndrome that are close to the clinical conditions, methods for producing the model animals, methods of screening for candidate compounds as therapeutic agents for diseases such as pregnancy-induced hypertension syndrome by using the model animals, and therapeutic agents for diseases such as pregnancy-induced hypertension syndrome. As a result, the model animals were found to exhibit symptoms that are very close to the clinical conditions in human, which are presentation of hypertension as well as placental insufficiency, intrauterine growth retardation, glomerulosclerosis, and proteinuria during pregnancy, and improvement of those symptoms postpartum. Furthermore, when pravastatin was administered to this model animal, it was found that diseases such as pregnancy-induced hypertension syndrome were improved by the activation of placenta-derived growth factor (PIGF) which antagonizes sFLT1.
Owner:FUSO PHARMA INDS
Glomerulosclerosis markers for chronic glomerular diseases and detection kits of glomerulosclerosis markers
PendingCN112048551ADelay progressIncreased interleukinMicrobiological testing/measurementDisease diagnosisChronic glomerular diseaseDisease
The present invention provides glomerulosclerosis markers for chronic glomerular diseases and detection kits of the glomerulosclerosis markers, and relates to the technical field of medicine. The glomerulosclerosis markers for the chronic glomerular diseases are miRNA-196a, miRNA-155, miRNA-490 and interleukin in urine, and the detection kits comprise a kit A and a kit B. An analysis proves that focal segmental glomerulosclerosis (FSGS) is more obvious in renal tubular interstitial injury than other glomerular diseases such as MN and MCD, compared with patients with chronic renal tubular interstitial lesions, interleukin in urine of patients with acute or chronic combined acute renal tubular interstitial lesions is obviously increased. Currently, in many renal tubular functional indexes widely adopted clinically at present, the interleukin in the urine can better reflect the acute renal tubular interstitial lesions, a basis is provided for early and specific clinical discovery of the renal tubular interstitial lesions, and early intervention and treatment of the tubular interstitial injury are one of important links for delaying progression of kidney diseases.
Owner:朱永俊
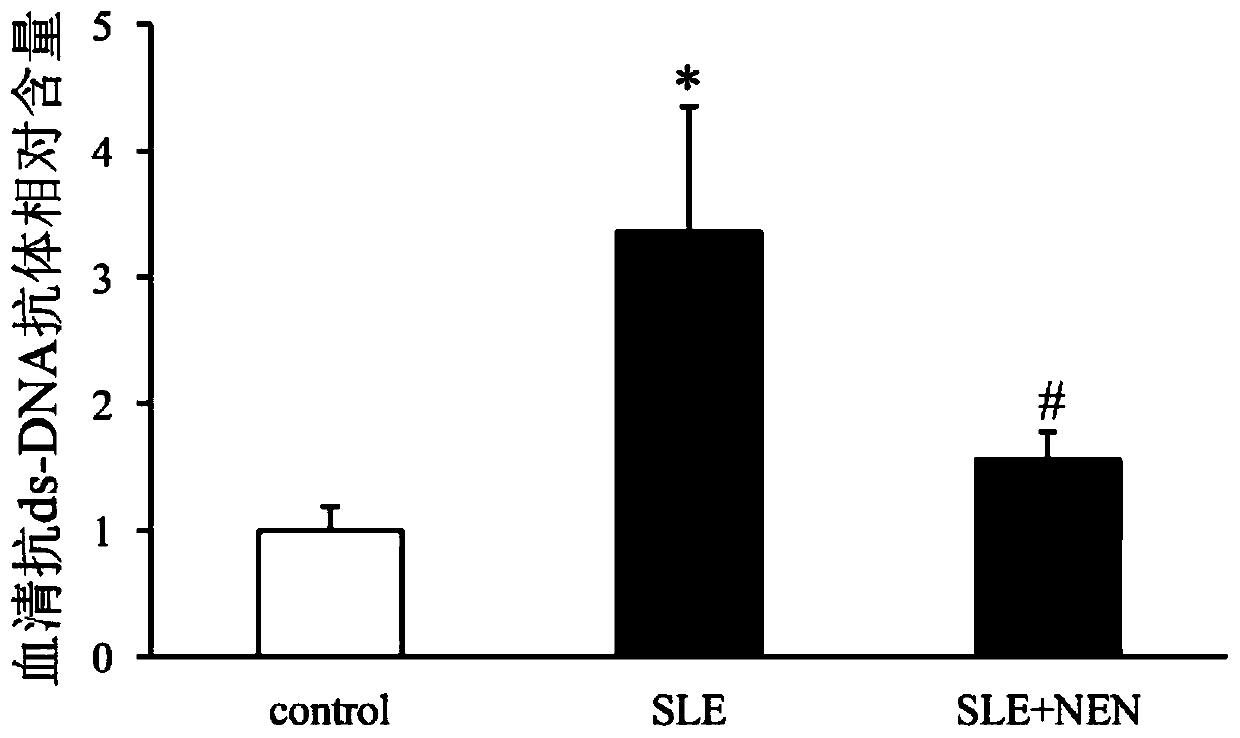
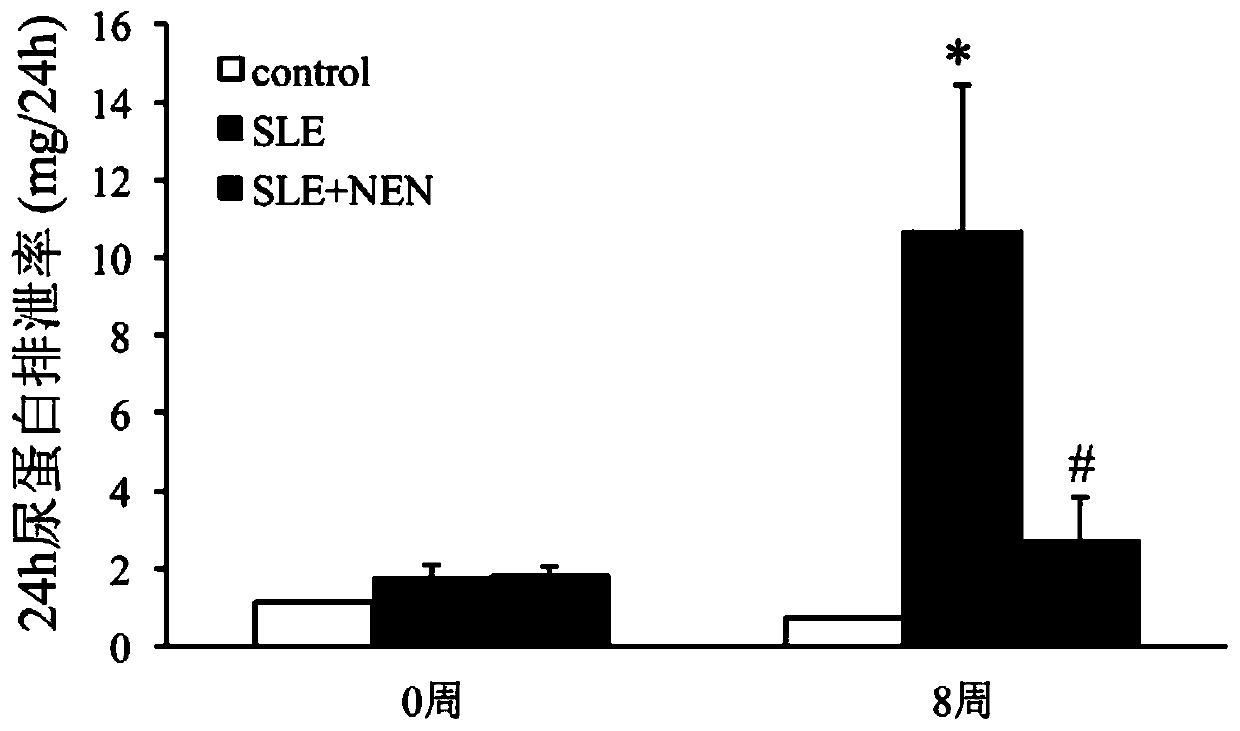
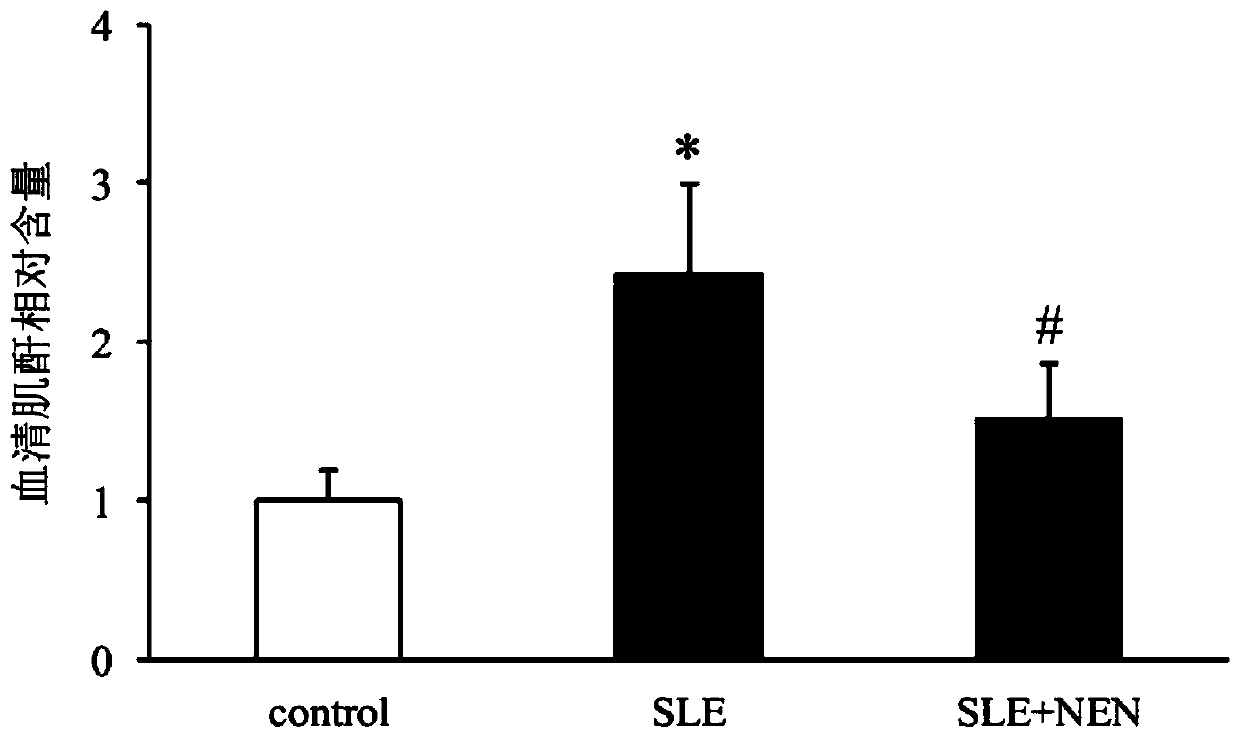
Application of niclosamide ethanolamine salt in preparation of medicine for treating systemic lupus erythematosus and complications thereof
ActiveCN111032030ALower levelReduce excretionOrganic active ingredientsDigestive systemAntiendomysial antibodiesDna antibody
The invention discloses the application of niclosamide ethanolamine salt in preparation of a medicine for treating systemic lupus erythematosus and complications thereof. The niclosamide ethanolaminesalt (NEN) can reduce the serum anti-ds-DNA antibody level, reduce the MRL / lpr mouse proteinuria excretion, reduce the serum creatinine and serum urea nitrogen level, improve glomerulosclerosis and renal tubule interstitial injury, relieve the spleen swelling, relieve lymph node hyperplasia swelling and relieve the skin injury.
Owner:SHENZHEN TRADITIONAL CHINESE MEDICINE HOSPITAL
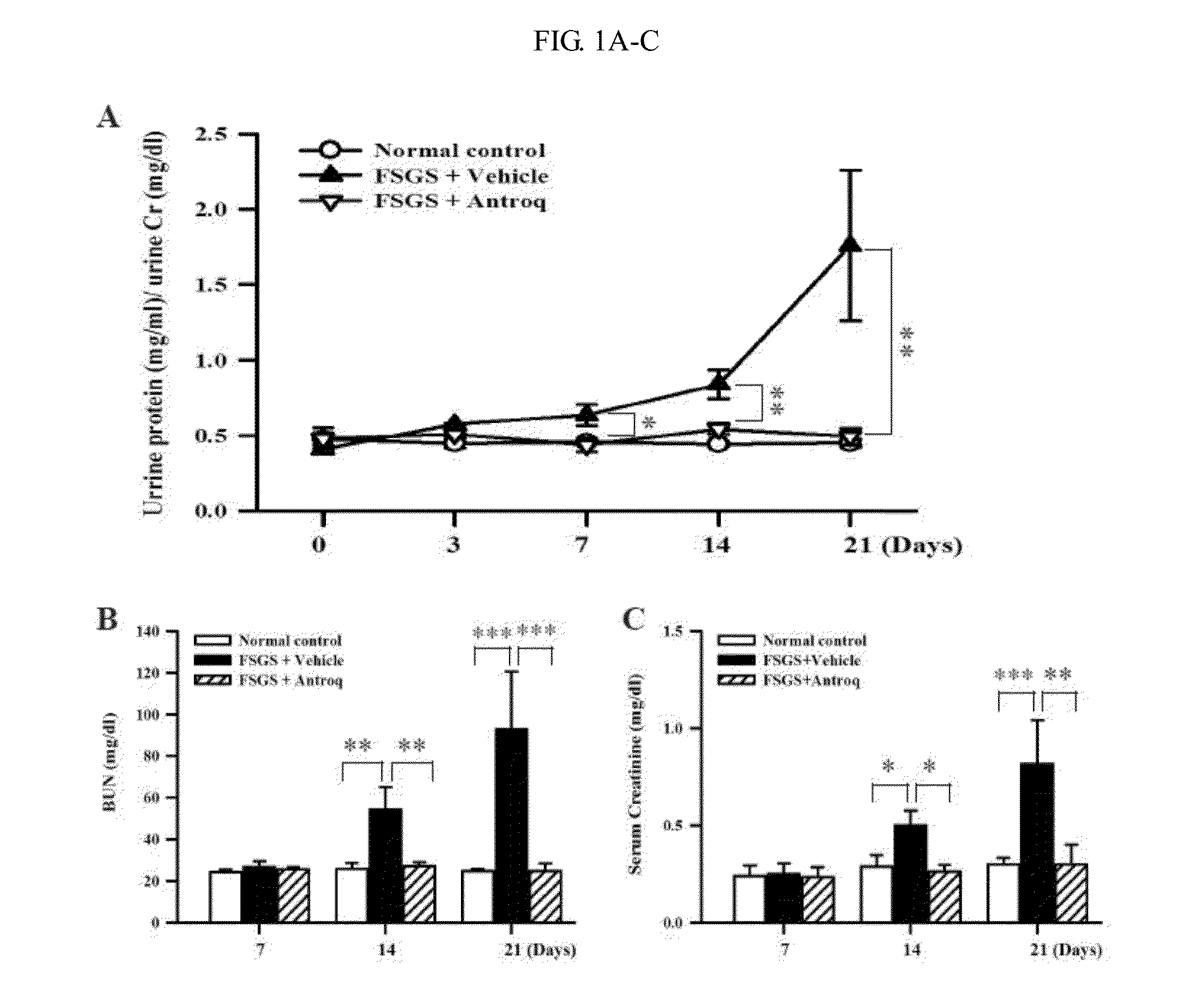
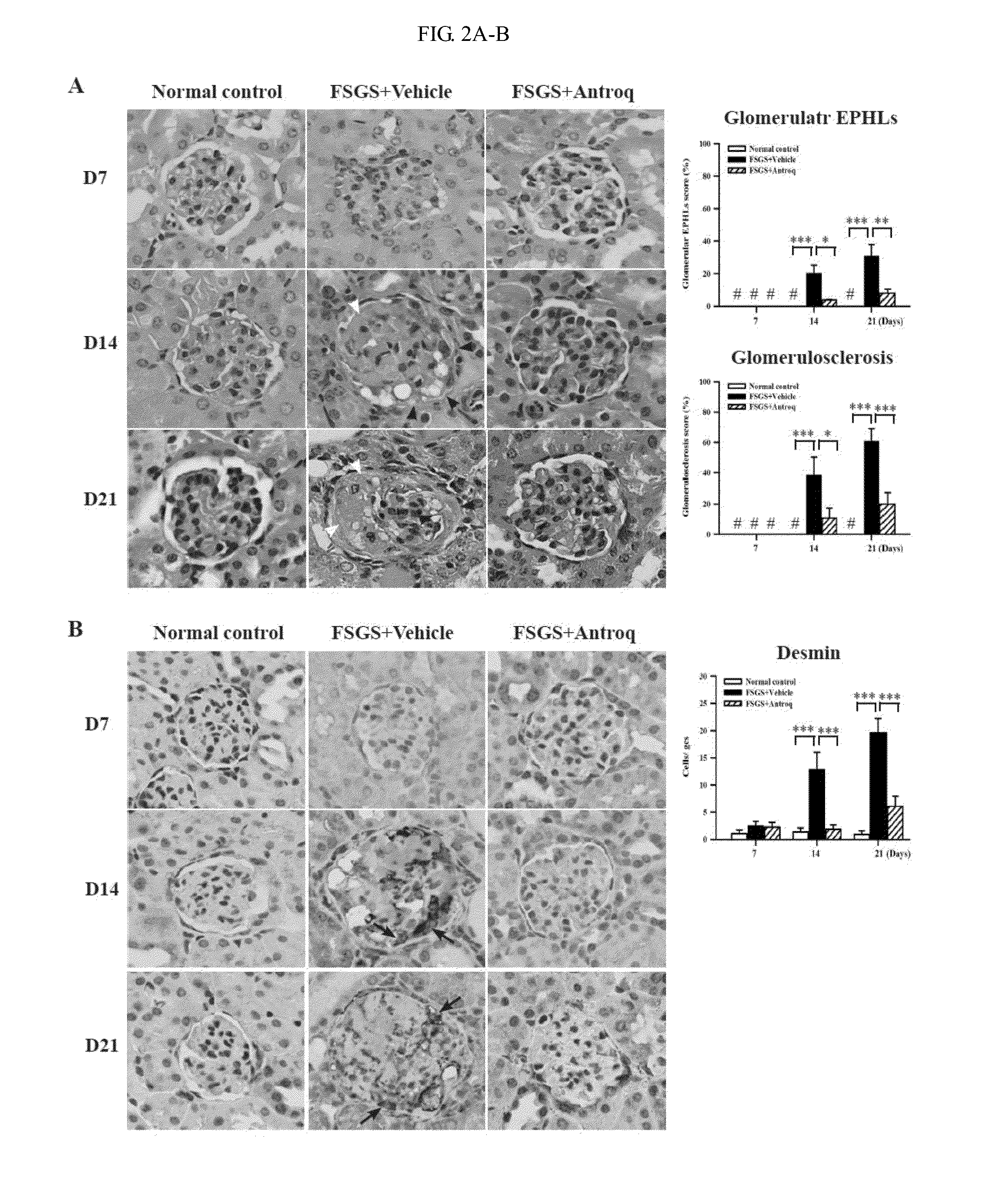
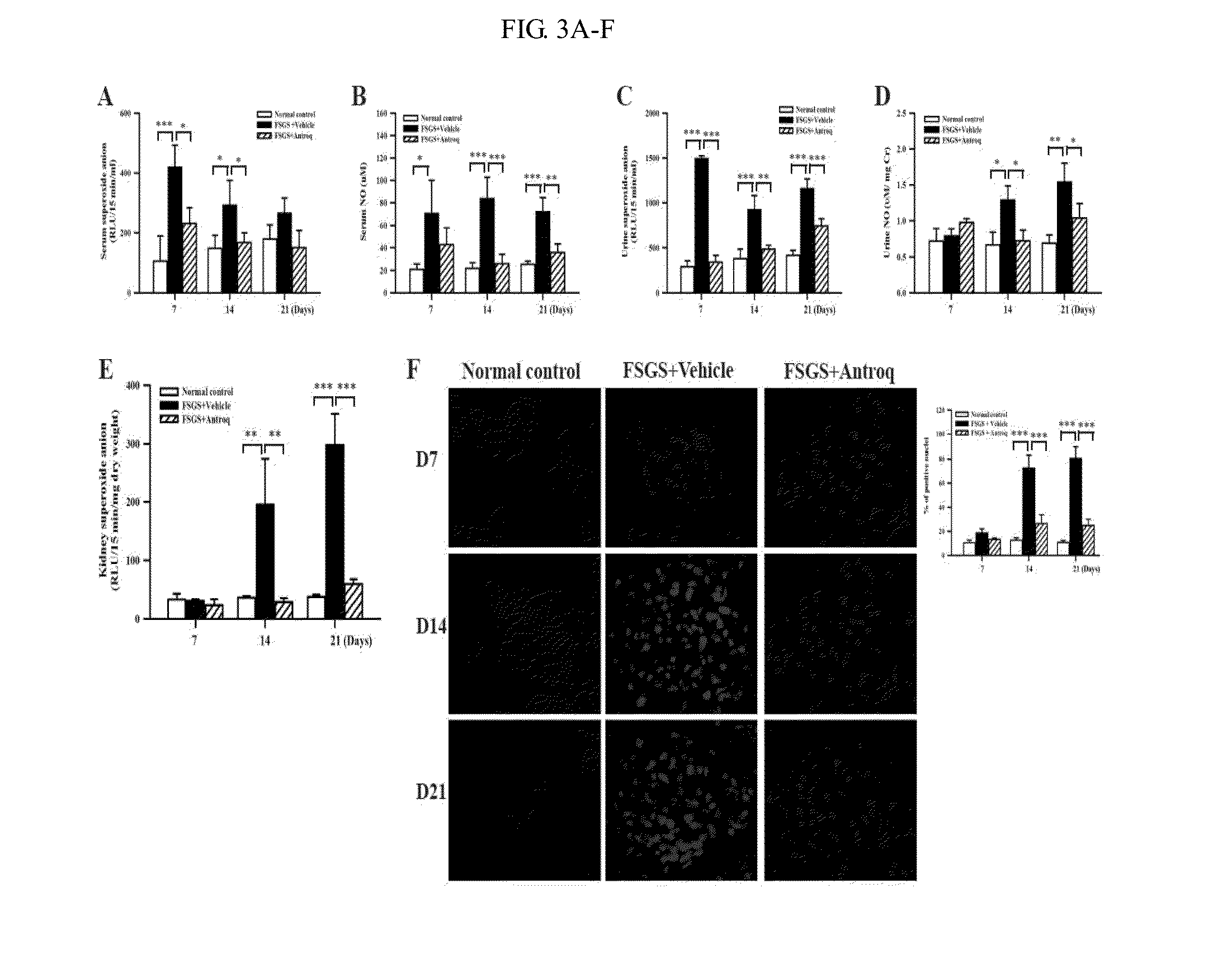
Application of antrodia camphorata compound in preparation of drug for treating kidney diseases
InactiveCN105311005AHas anti-inflammatory propertiesPowerfulOrganic active ingredientsAntipyreticDiseaseNephropathy
The invention discloses an application of an antrodia camphorate compound in preparation of a drug for treating kidney diseases, wherein the compound is represented as the formula (I) and the kidney diseases is glomerulosclerosis or glomerulonephritis.
Owner:曾卉菱
5-Beta, 14-Beta-Androstane Derivatives Useful For The Treatment Of Proteinuria, Glomerulosclerosis And Renal Failure
Compound of formula (I), wherein the symbol have the meaning reported in the text; for preparing a medicament for the prevention and / or treatment of proteinuria, glomerulosclerosis or renal failure.
Owner:CVIE THERAPEUTICS LTD
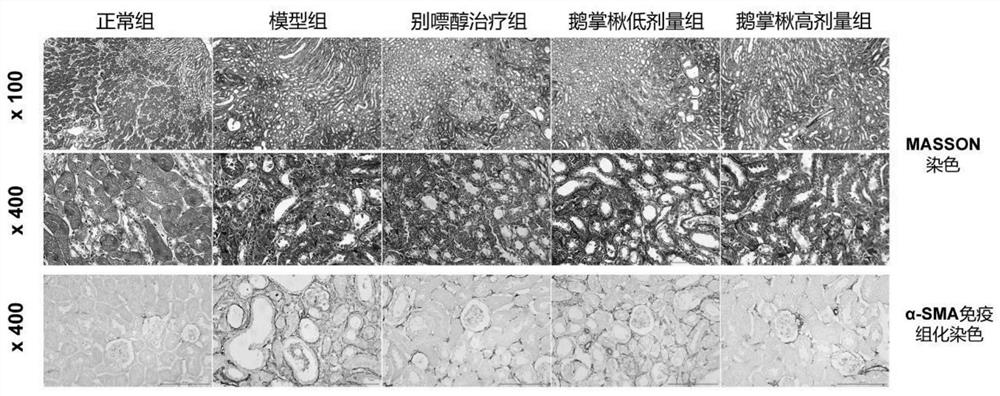
Use of Liriodendron tulipifera or its extract in preparing medicine for reducing serum uric acid level and preventing and treating uric acid nephropathy
ActiveCN112704690BPromote excretionLower serum uric acid levelsSkeletal disorderUrinary disorderRenal glomerulusLiriodendron tulipifera
The invention relates to the application of Liriodendron tulipifera or its extract in the preparation of medicines for reducing serum uric acid level and preventing and treating uric acid nephropathy, which belongs to the field of medicine. One aspect of the present invention provides the application of Liriodendron tulipifera or its extract in the preparation of medicine for lowering blood uric acid level. On the other hand, the present invention also provides the use of Liriodendron tulipifera or its extract in the preparation of medicine for treating and / or preventing uric acid nephropathy. Animal experiments have confirmed that Liriodendron can significantly reduce serum uric acid levels, and at the same time improve the degree of renal tubular dilation, glomerulosclerosis and renal interstitial fibrosis in hyperuricemia mice. Further studies have shown that Liriodendron can reduce serum uric acid levels by promoting renal uric acid excretion. The application of the present invention can provide a new medication option for the clinical treatment of hyperuricemia and the resulting hyperuricemia nephropathy.
Owner:SICHUAN CREATION PHARM TECH CO LTD
Methods and compositions for treating kidney disorders
The present invention provides methods for treating glomerulosclerosis such as focal segmental glomerulosclerosis (FSGS) or glomerulonephritis such as immunoglobulin A nephropathy (IgAN) by cyclohexenone compounds.
Owner:GOLDEN BIOTECH
Application of antrodia camphorata compound in preparation of drugs for treating kidney diseases
InactiveCN105287448AOrganic active ingredientsAntipyreticNephropathyPost-streptococcal glomerulonephritis
The invention discloses application of an antrodia camphorata compound in preparation of drugs for treating kidney diseases. The compound is shown by a formula I as shown in the specification, and the kidney diseases can be glomerulosclerosis or post-streptococcal glomerulonephritis.
Owner:曾卉菱
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Lactate salt of 4-[6-methoxy-7-(3-piperidin-1-yl-propoxy)quinazolin-4-yl]piperazine-1-carboxylic acid(4-isopropoxyphenyl)-amide and pharmaceutical compositions thereof for the treatment of cancer and other diseases or disorders Lactate salt of 4-[6-methoxy-7-(3-piperidin-1-yl-propoxy)quinazolin-4-yl]piperazine-1-carboxylic acid(4-isopropoxyphenyl)-amide and pharmaceutical compositions thereof for the treatment of cancer and other diseases or disorders](https://images-eureka.patsnap.com/patent_img/066cbb96-2b5e-425b-b3cc-1a9460205ec7/US20100273808A1-20101028-D00001.png)
![Lactate salt of 4-[6-methoxy-7-(3-piperidin-1-yl-propoxy)quinazolin-4-yl]piperazine-1-carboxylic acid(4-isopropoxyphenyl)-amide and pharmaceutical compositions thereof for the treatment of cancer and other diseases or disorders Lactate salt of 4-[6-methoxy-7-(3-piperidin-1-yl-propoxy)quinazolin-4-yl]piperazine-1-carboxylic acid(4-isopropoxyphenyl)-amide and pharmaceutical compositions thereof for the treatment of cancer and other diseases or disorders](https://images-eureka.patsnap.com/patent_img/066cbb96-2b5e-425b-b3cc-1a9460205ec7/US20100273808A1-20101028-D00002.png)
![Lactate salt of 4-[6-methoxy-7-(3-piperidin-1-yl-propoxy)quinazolin-4-yl]piperazine-1-carboxylic acid(4-isopropoxyphenyl)-amide and pharmaceutical compositions thereof for the treatment of cancer and other diseases or disorders Lactate salt of 4-[6-methoxy-7-(3-piperidin-1-yl-propoxy)quinazolin-4-yl]piperazine-1-carboxylic acid(4-isopropoxyphenyl)-amide and pharmaceutical compositions thereof for the treatment of cancer and other diseases or disorders](https://images-eureka.patsnap.com/patent_img/066cbb96-2b5e-425b-b3cc-1a9460205ec7/US20100273808A1-20101028-D00003.png)


![Lactate salt of 4-[6-methoxy-7-(3-piperidin-1-yl-propoxy)quinazolin-4-yl]piperazine-1-carboxylic acid(4-isopropoxyphenyl)-amide and pharmaceutical compositions thereof for the treatment of cancer and other diseases or disorders Lactate salt of 4-[6-methoxy-7-(3-piperidin-1-yl-propoxy)quinazolin-4-yl]piperazine-1-carboxylic acid(4-isopropoxyphenyl)-amide and pharmaceutical compositions thereof for the treatment of cancer and other diseases or disorders](https://images-eureka.patsnap.com/patent_img/ecbd2cb0-4888-44ea-8e83-40194d233ef1/US20150133460A1-20150514-D00001.PNG)
![Lactate salt of 4-[6-methoxy-7-(3-piperidin-1-yl-propoxy)quinazolin-4-yl]piperazine-1-carboxylic acid(4-isopropoxyphenyl)-amide and pharmaceutical compositions thereof for the treatment of cancer and other diseases or disorders Lactate salt of 4-[6-methoxy-7-(3-piperidin-1-yl-propoxy)quinazolin-4-yl]piperazine-1-carboxylic acid(4-isopropoxyphenyl)-amide and pharmaceutical compositions thereof for the treatment of cancer and other diseases or disorders](https://images-eureka.patsnap.com/patent_img/ecbd2cb0-4888-44ea-8e83-40194d233ef1/US20150133460A1-20150514-D00002.PNG)
![Lactate salt of 4-[6-methoxy-7-(3-piperidin-1-yl-propoxy)quinazolin-4-yl]piperazine-1-carboxylic acid(4-isopropoxyphenyl)-amide and pharmaceutical compositions thereof for the treatment of cancer and other diseases or disorders Lactate salt of 4-[6-methoxy-7-(3-piperidin-1-yl-propoxy)quinazolin-4-yl]piperazine-1-carboxylic acid(4-isopropoxyphenyl)-amide and pharmaceutical compositions thereof for the treatment of cancer and other diseases or disorders](https://images-eureka.patsnap.com/patent_img/ecbd2cb0-4888-44ea-8e83-40194d233ef1/US20150133460A1-20150514-D00003.PNG)