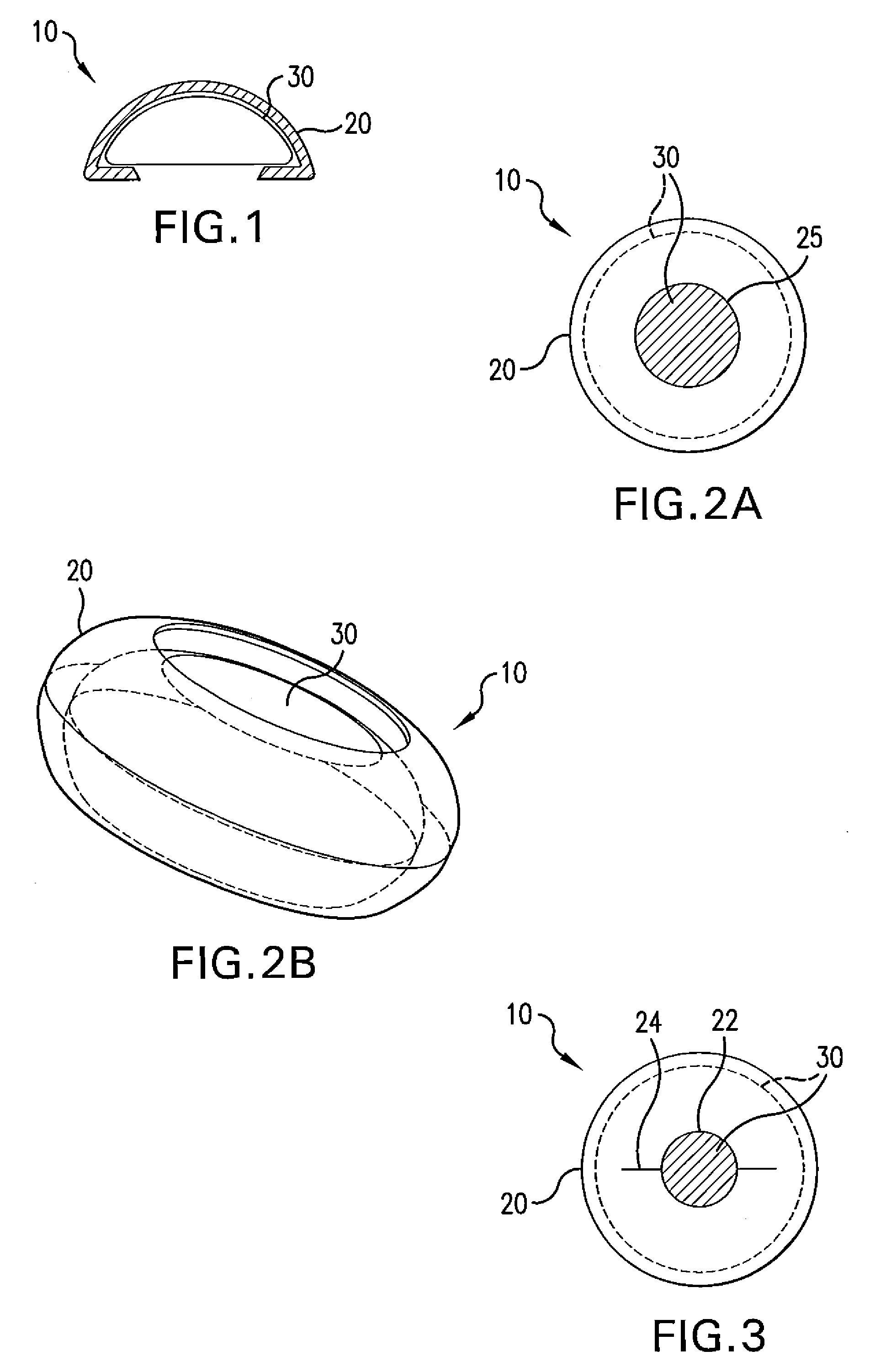
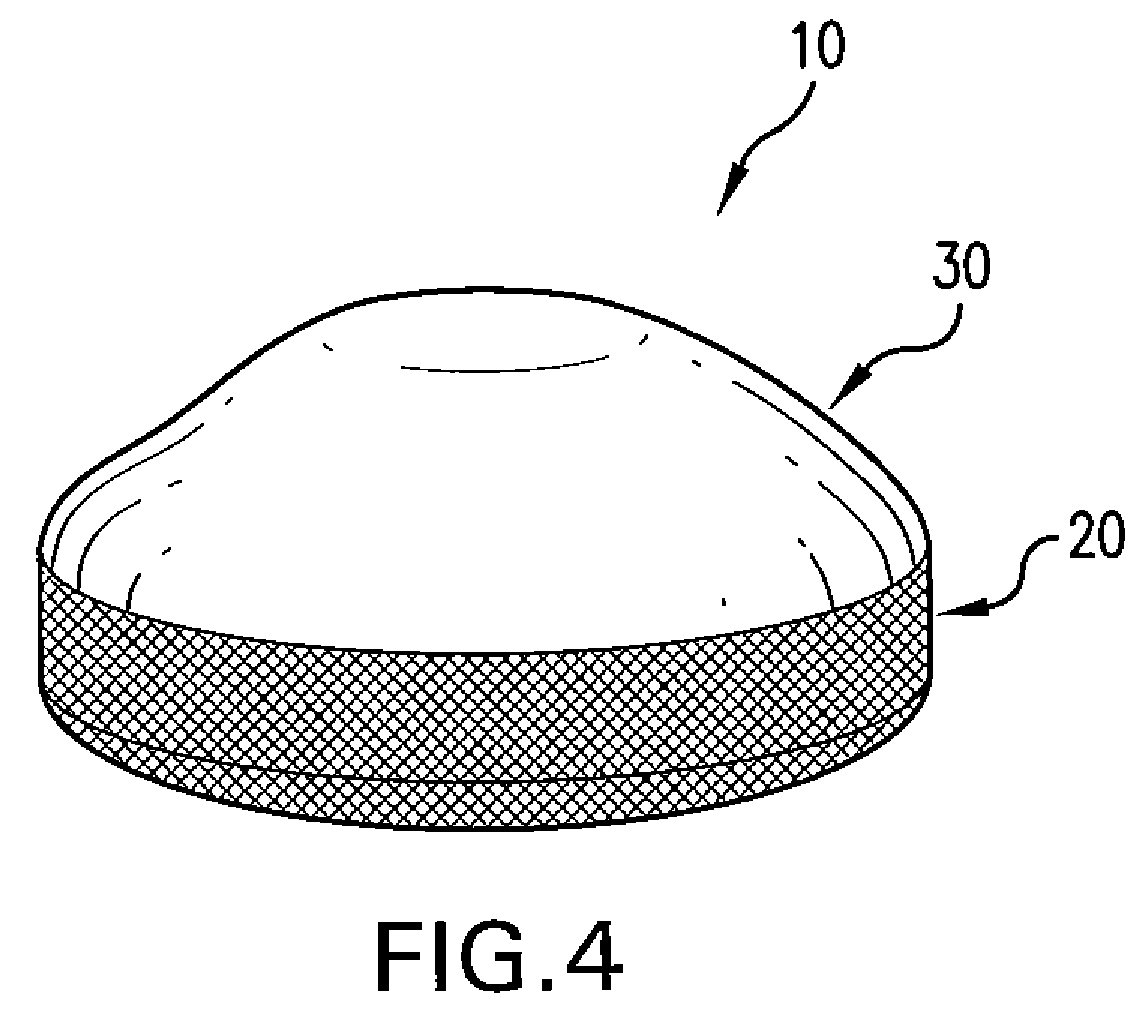
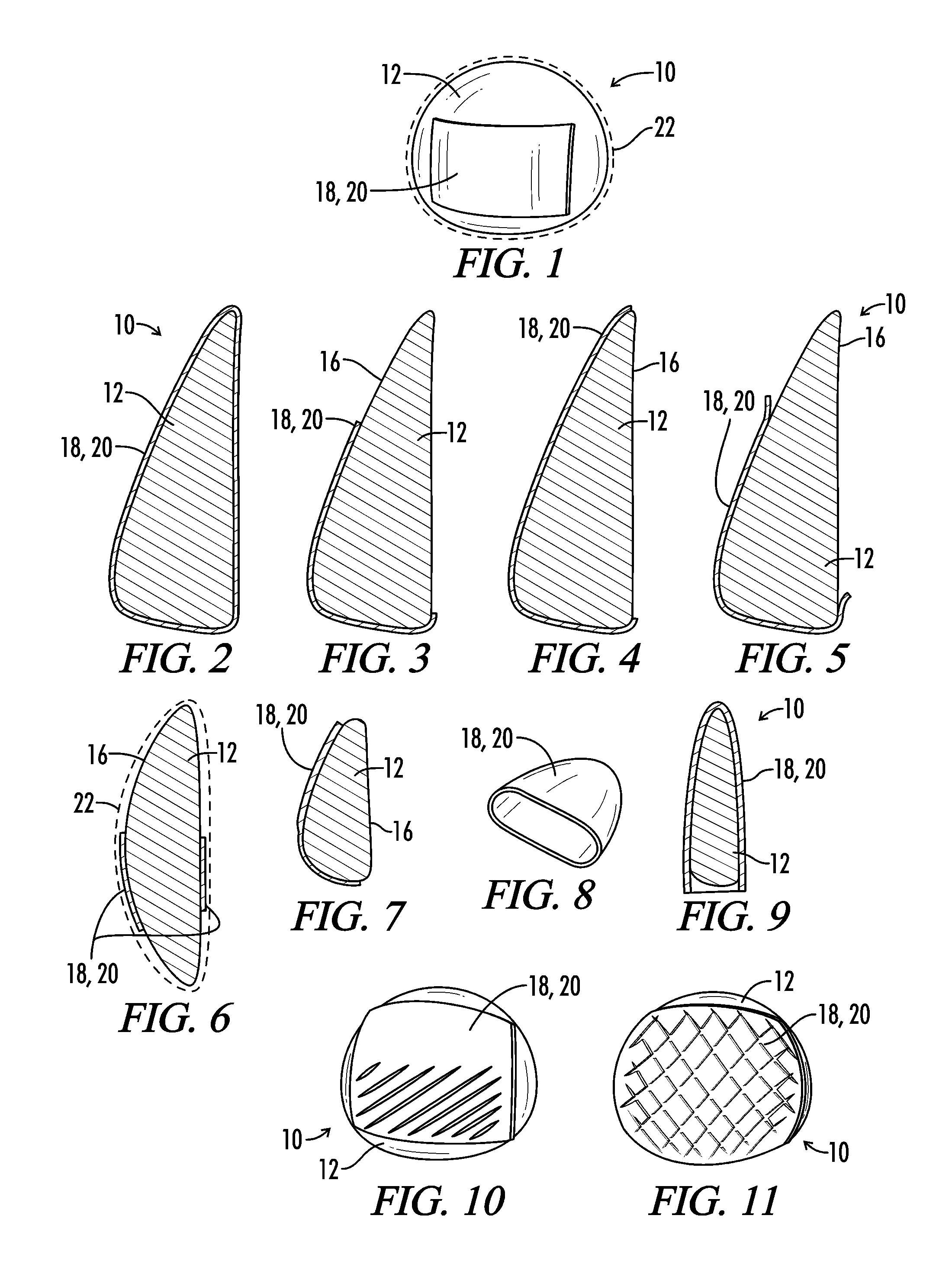
Patents
Literature
52 results about "Capsular contracture" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
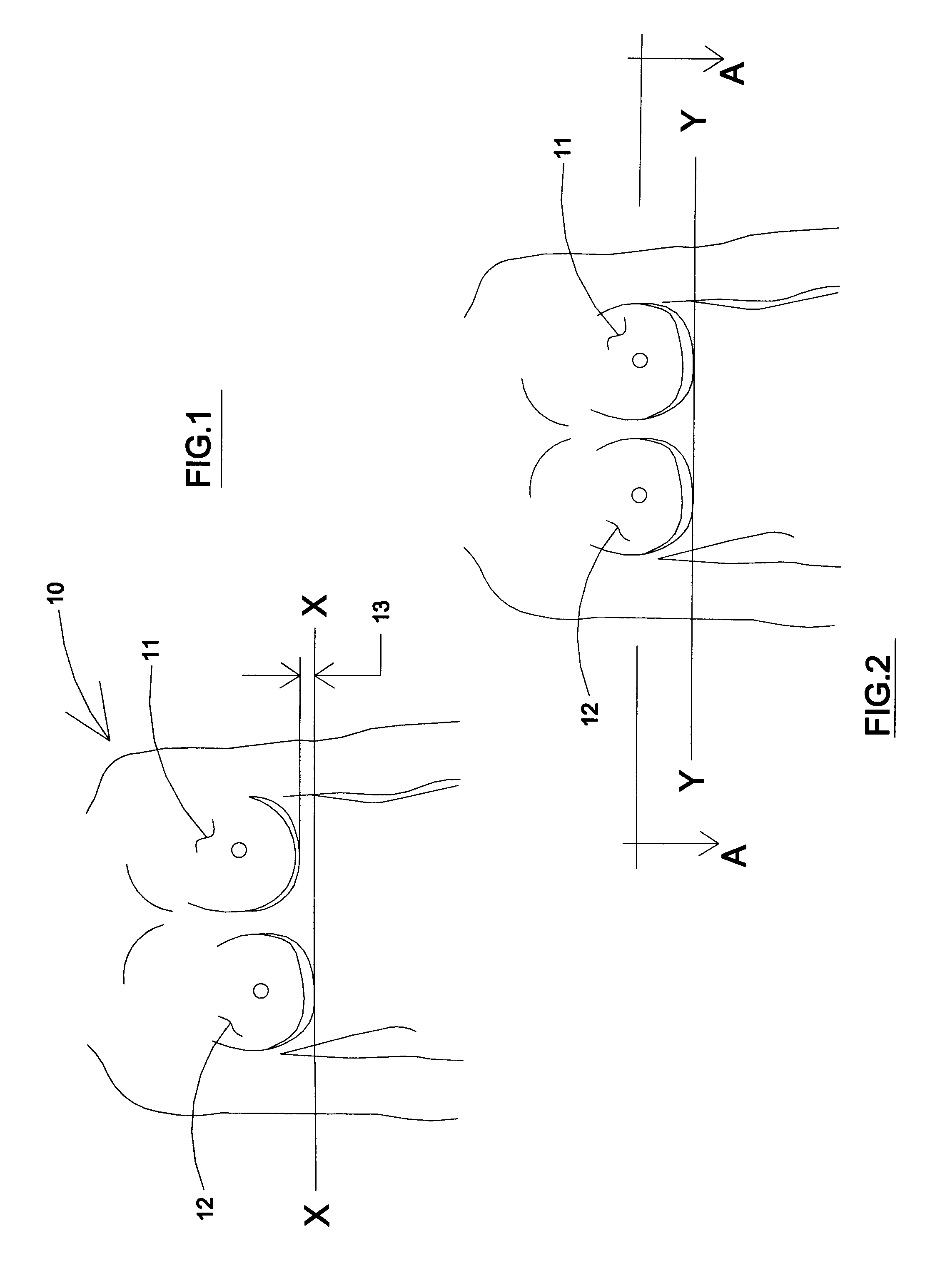
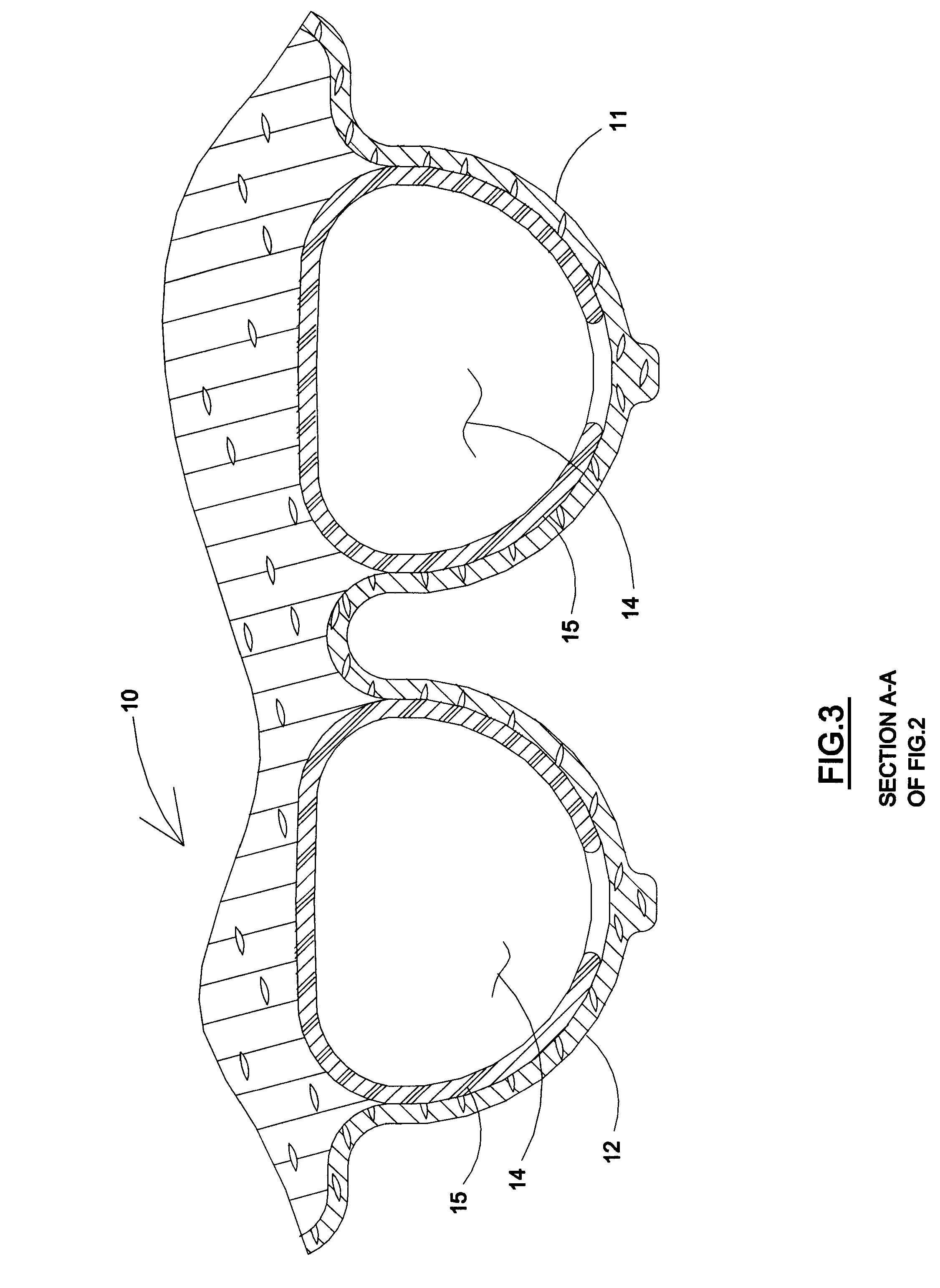
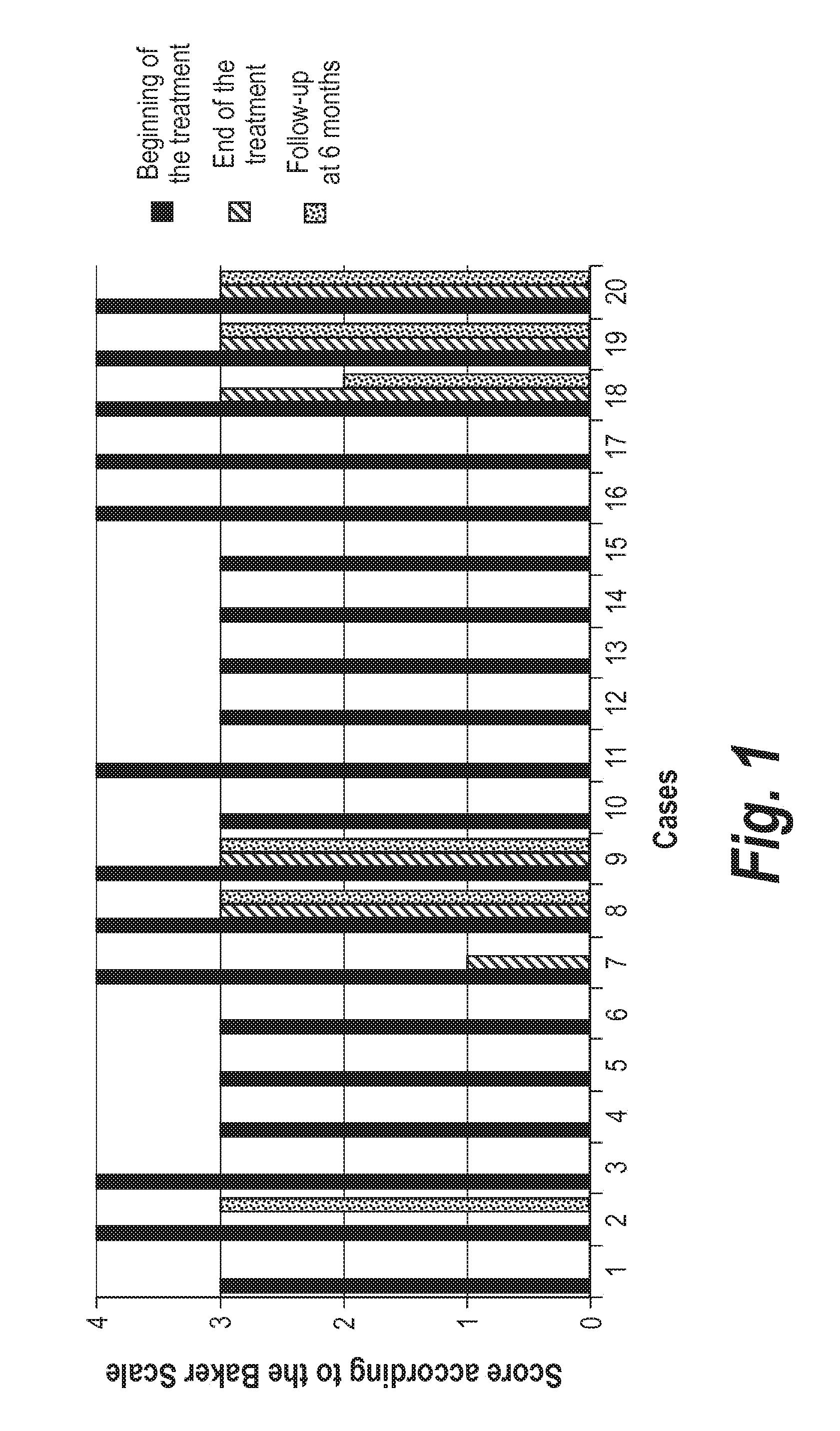
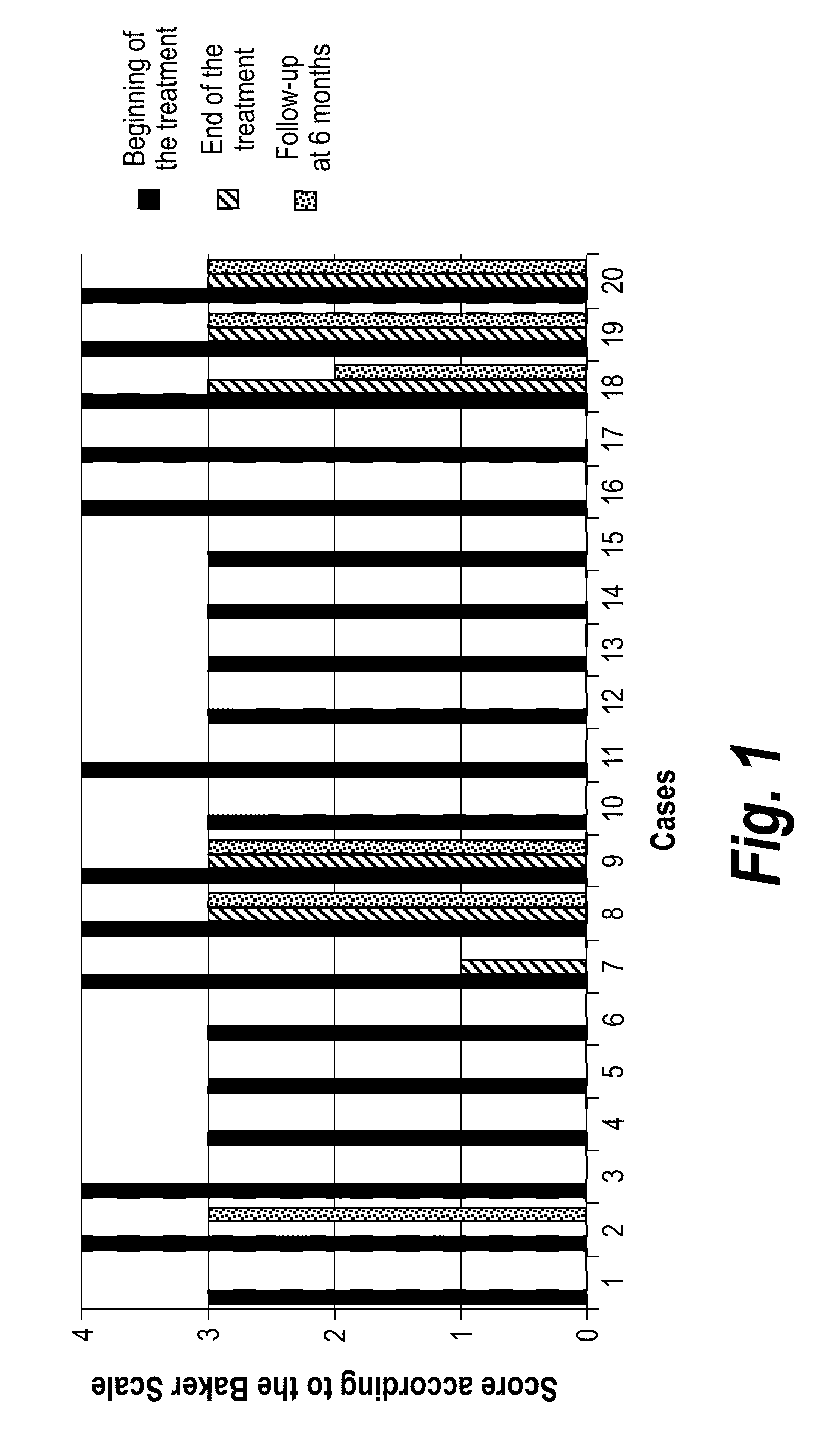
Capsular contracture is a response of the immune system to foreign materials in the human body. Medically, it occurs mostly in context of the complications from breast implants and artificial joint prosthetics.
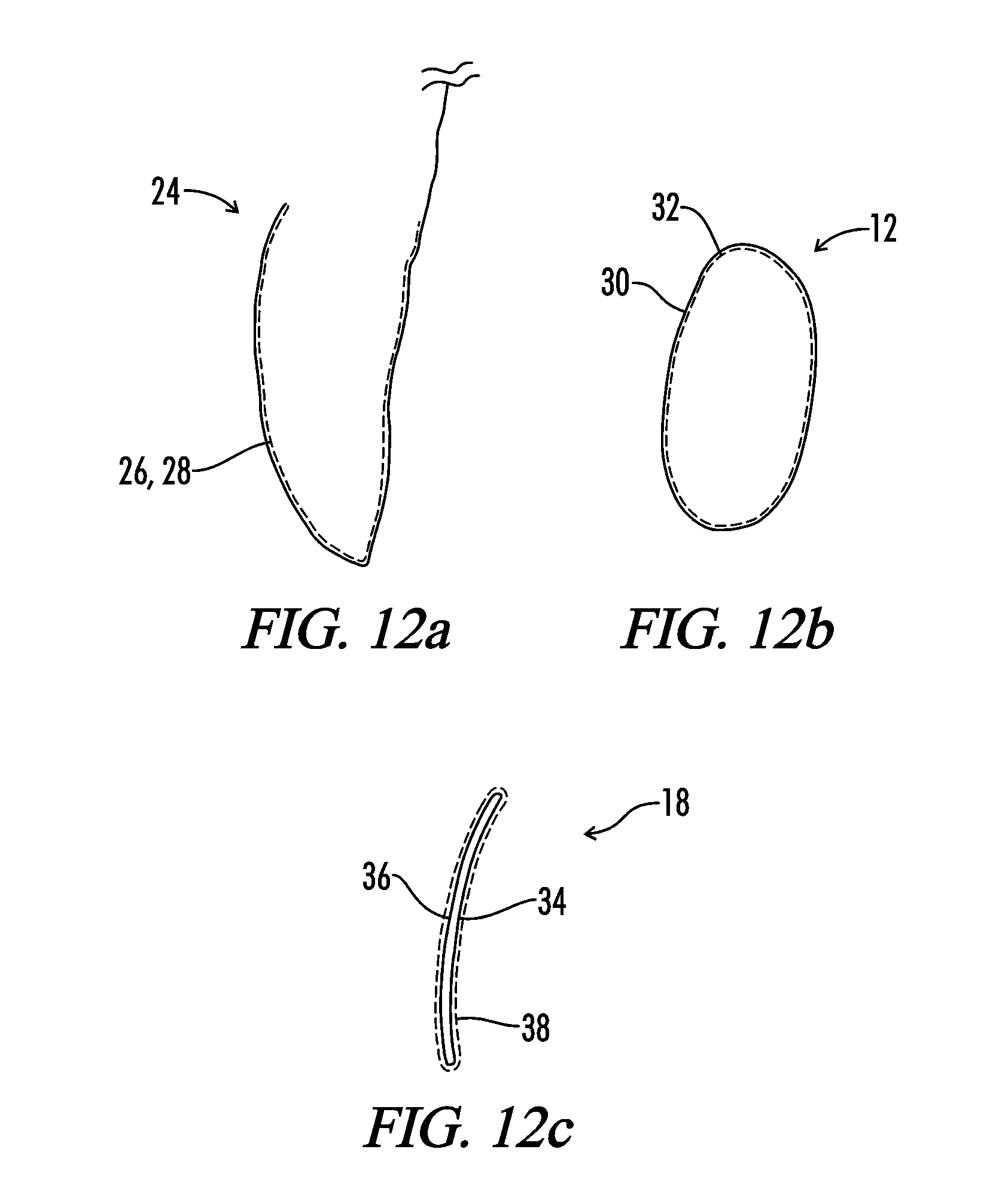
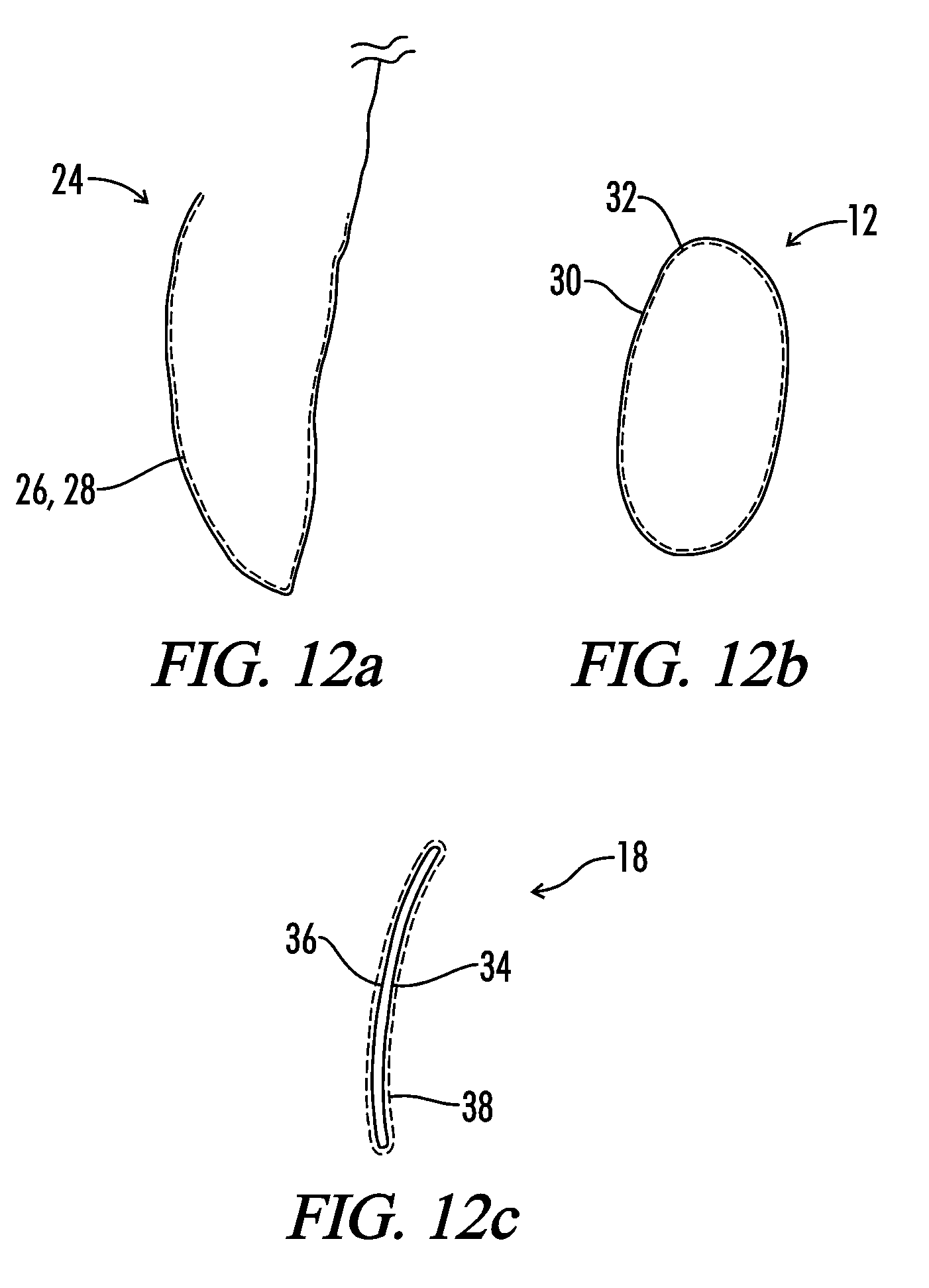
Interfaced medical implant assembly
ActiveUS8425600B2Reduce and eliminate capsular contracturePromote formationMammary implantsTissue regenerationMedicineBreast prostheses
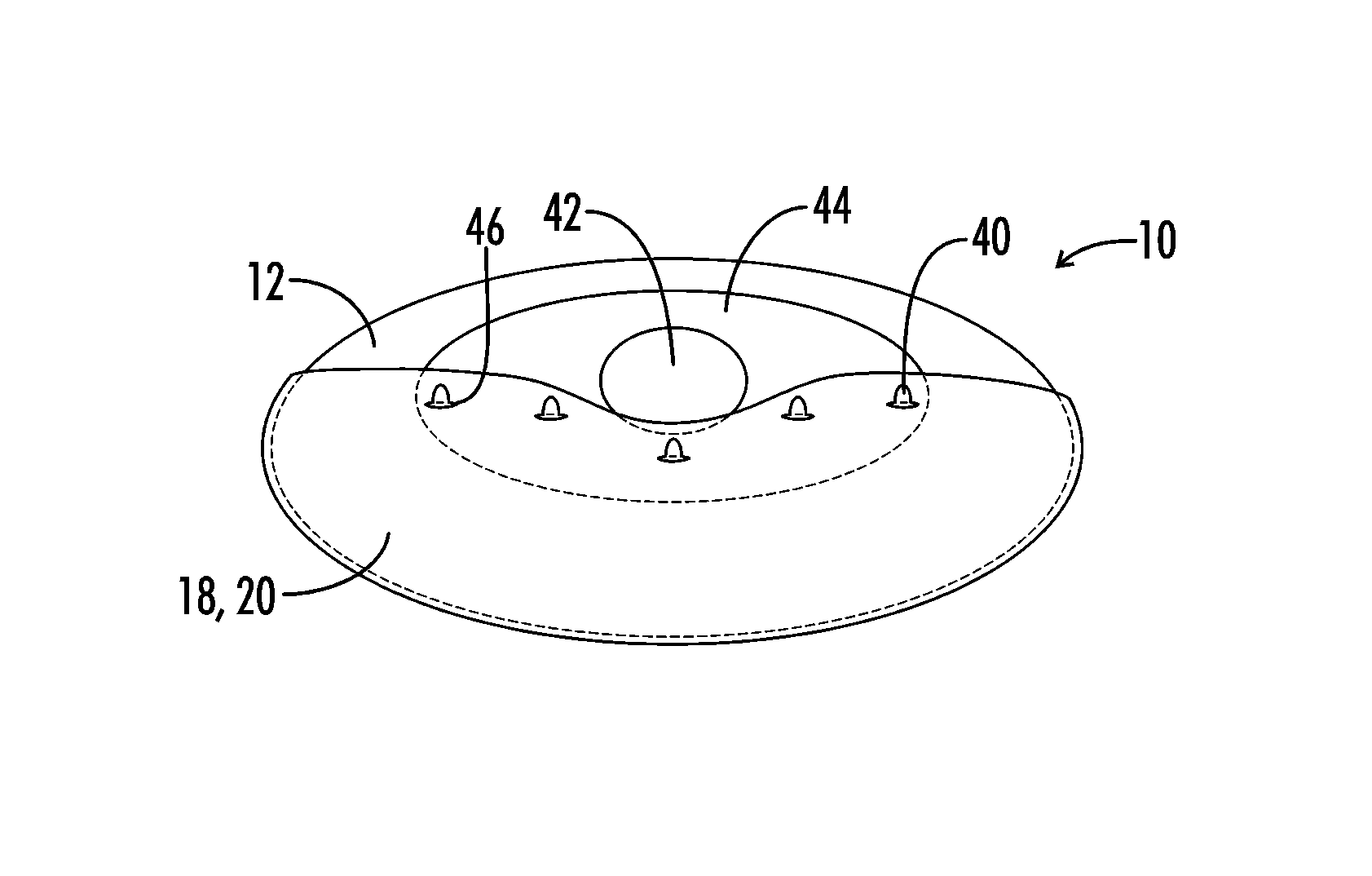
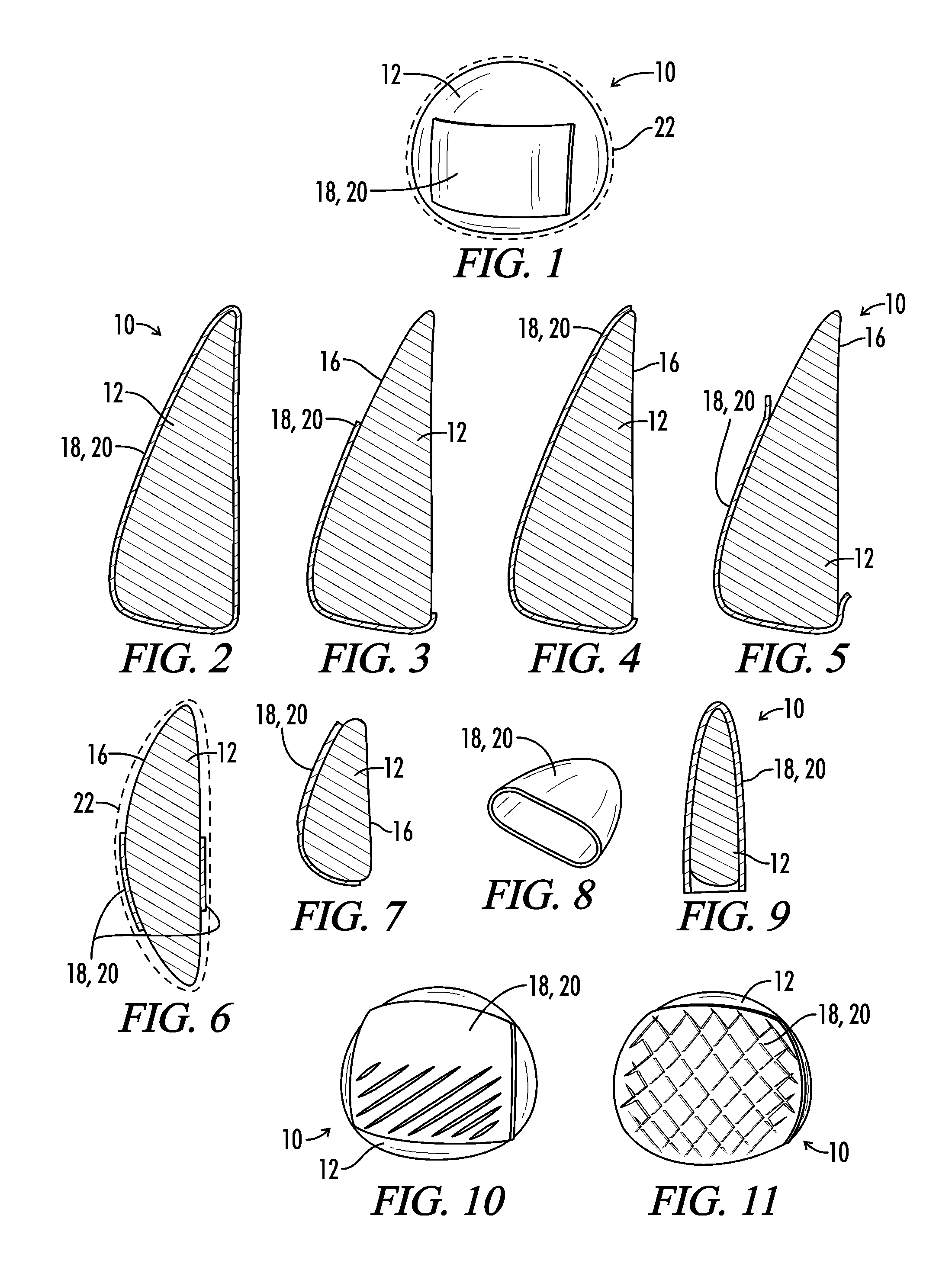
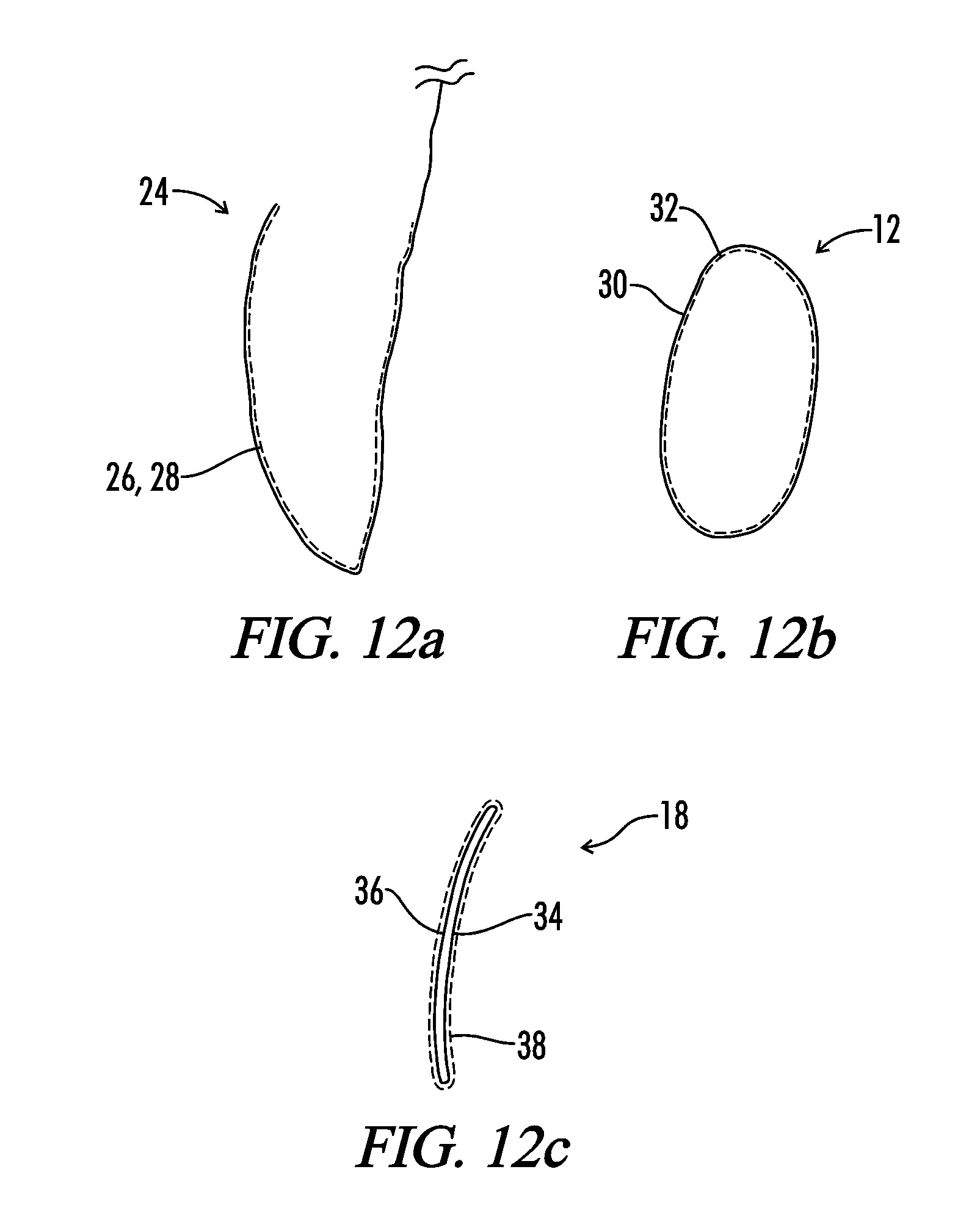
A medical implant assembly and method having a medical implant, e.g. a breast prostheses, attached to a biological interface. The biological interface is comprised of a dermal material with capsular contracture inhibiting properties so that once the medical assembly is inserted into the host, the biological interface, which is intimately coupled to the implant, prevents / reduces capsular contracture formation around the implant. The biological interface comprises a plurality of apertures along its periphery, and attaches to the medical implant by receiving a plurality of attachment flaps or appendages located on the exterior surface of the medical implant within or through the apertures. The attachment of the biological interface is such that the assembly remains intact even where the attachment flaps loosen upon expansion of the implant after insertion into a host, as where the implant is therein injected to a desired dimension.
Owner:MAXWELL G PATRICK
Biodegradable, Polymer Coverings for Breast Implants
ActiveUS20080241212A1Inhibit and reduce formation of scar tissueInhibiting and reducingAntibacterial agentsBiocideBreast implantMedicine
A biodegradable, flexible covering for a breast implant is provided which comprises one or more biodegradable polymer layers dimensioned and shaped to cover at least a portion of the breast implant. The implant can be inserted into an opening of the covering immediately prior to surgery, but alternate configurations and times of insertion are contemplated as well as open or sheet type devices. The coverings can optionally contain one or more drugs for delivery at the surgical site, particularly for treating or preventing infection, pain, inflammation, capsular contracture, scarring or other complications associated with breast augmentation or breast reconstruction.
Owner:MEDTRONIC INC
Interfaced Medical Implant Assembly
ActiveUS20090125107A1Reduce and eliminate capsular contracturePromote formationMammary implantsTissue regenerationBreast prosthesesAppendage
A medical implant assembly and method having a medical implant, e.g. a breast prostheses, attached to a biological interface. The biological interface is comprised of a dermal material with capsular contracture inhibiting properties so that once the medical assembly is inserted into the host, the biological interface, which is intimately coupled to the implant, prevents / reduces capsular contracture formation around the implant. The biological interface comprises a plurality of apertures along its periphery, and attaches to the medical implant by receiving a plurality of attachment flaps or appendages located on the exterior surface of the medical implant within or through the apertures. The attachment of the biological interface is such that the assembly remains intact even where the attachment flaps loosen upon expansion of the implant after insertion into a host, as where the implant is therein injected to a desired dimension.
Owner:MAXWELL G PATRICK
Interfaced medical implant
InactiveUS20110035004A1Promote repairPreventing/reducing capsular contractureMammary implantsBreast prosthesesBiomedical engineering
A medical implant assembly and method having a medical implant, e.g. a breast prostheses, affixed to a biological interface. The biological interface is comprised of a dermal material with capsular contracture inhibiting properties so that once the medical assembly is inserted into the host, the biological interface, which is intimately coupled to the implant, prevents / reduces capsular contracture formation around the implant.
Owner:MAXWELL G
Bioerodible matrix for tissue involvement
InactiveUS20100249924A1Improve efficiencyPromote wound healingBiocideMammary implantsPorosityBreast implant
Disclosed herein are polyurethane polymer matrices with a porosity of from about 20 microns to about 90 microns that are useful in promoting closure and protection of incision sites; supporting the lower pole position of breast implants; and providing a partial or complete covering of breast implants to provide a beneficial interface with host tissue and to reduce the potential for malpositioning or capsular contracture. The disclosed matrices can be seeded with mammalian cells.
Owner:ALLERGAN INC
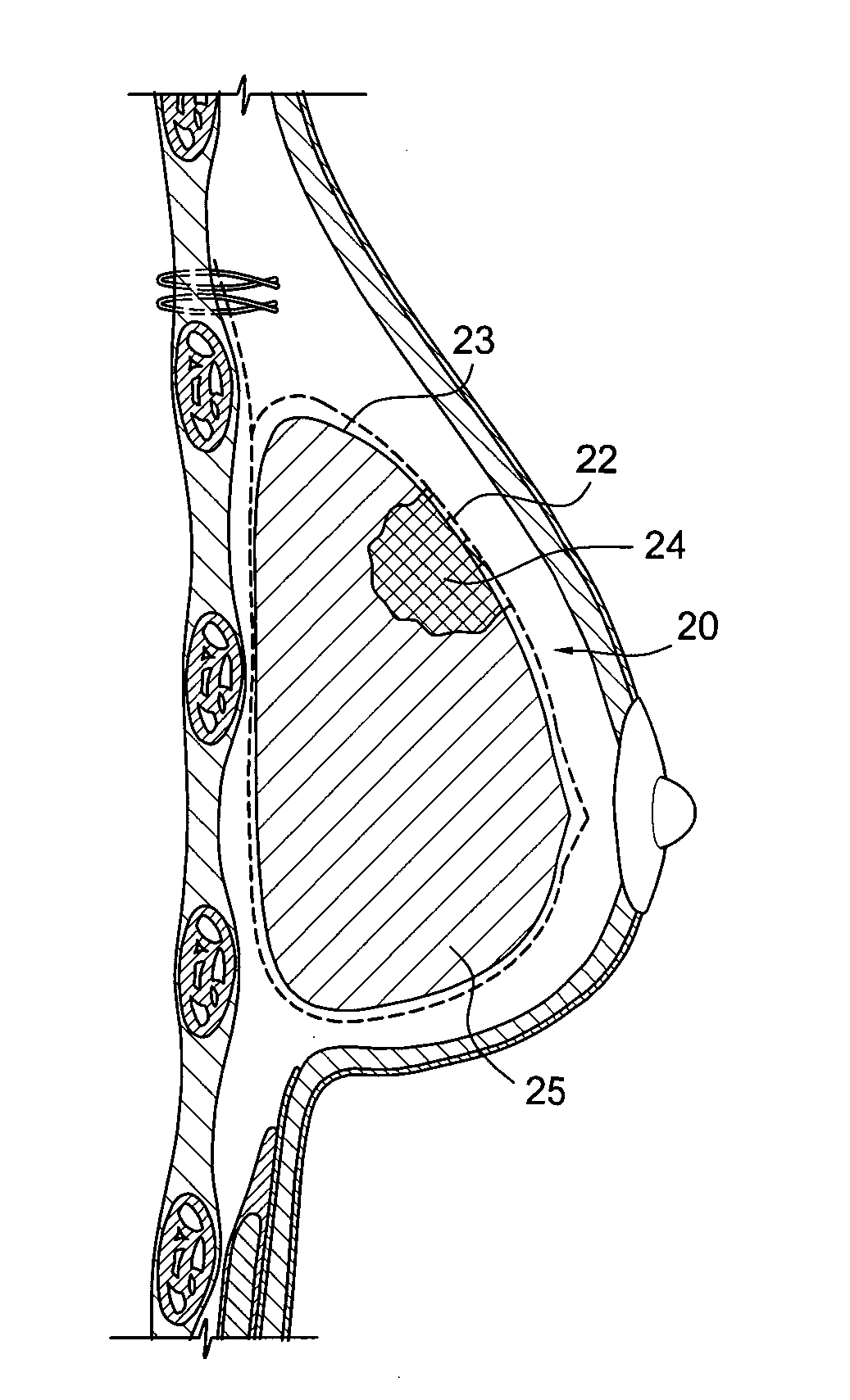
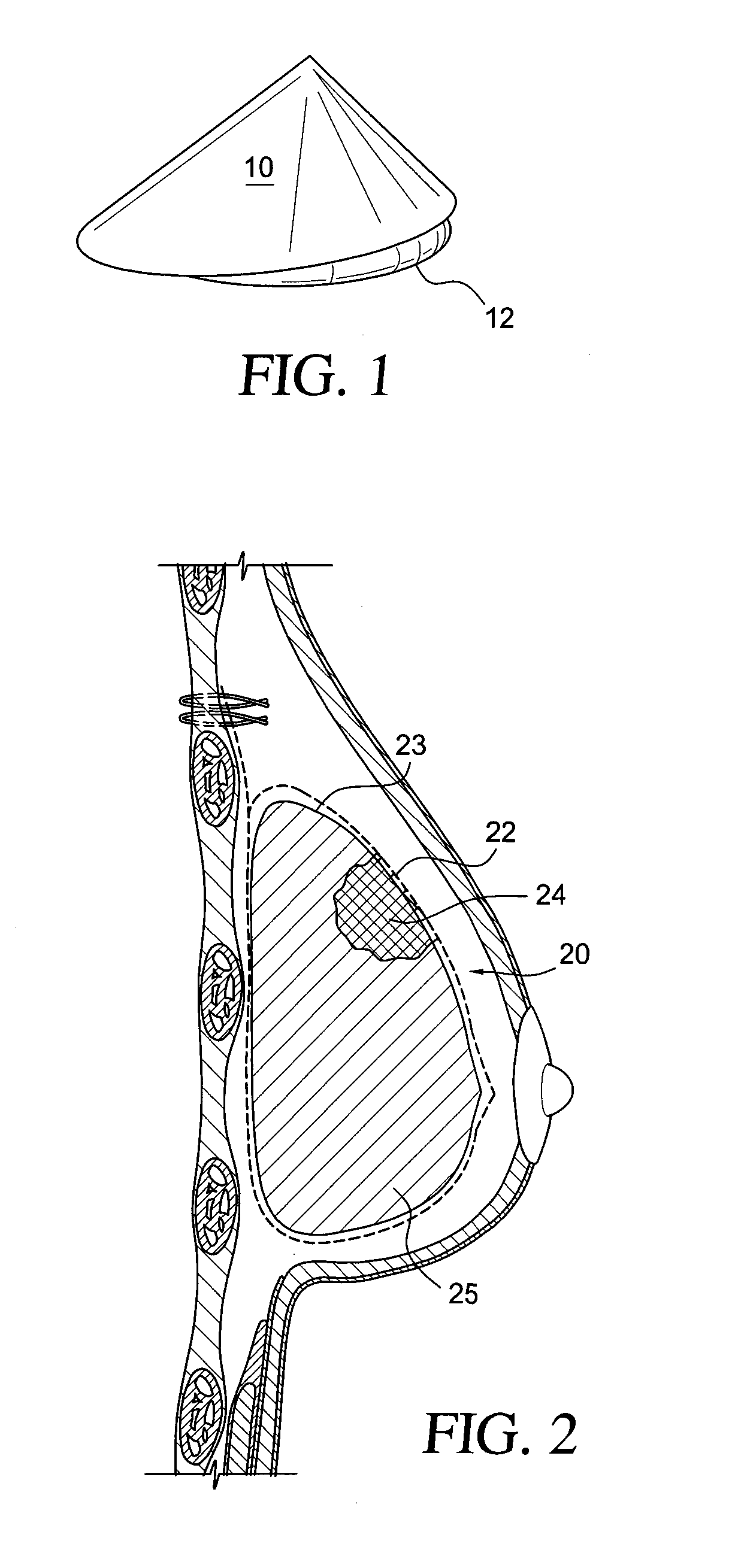
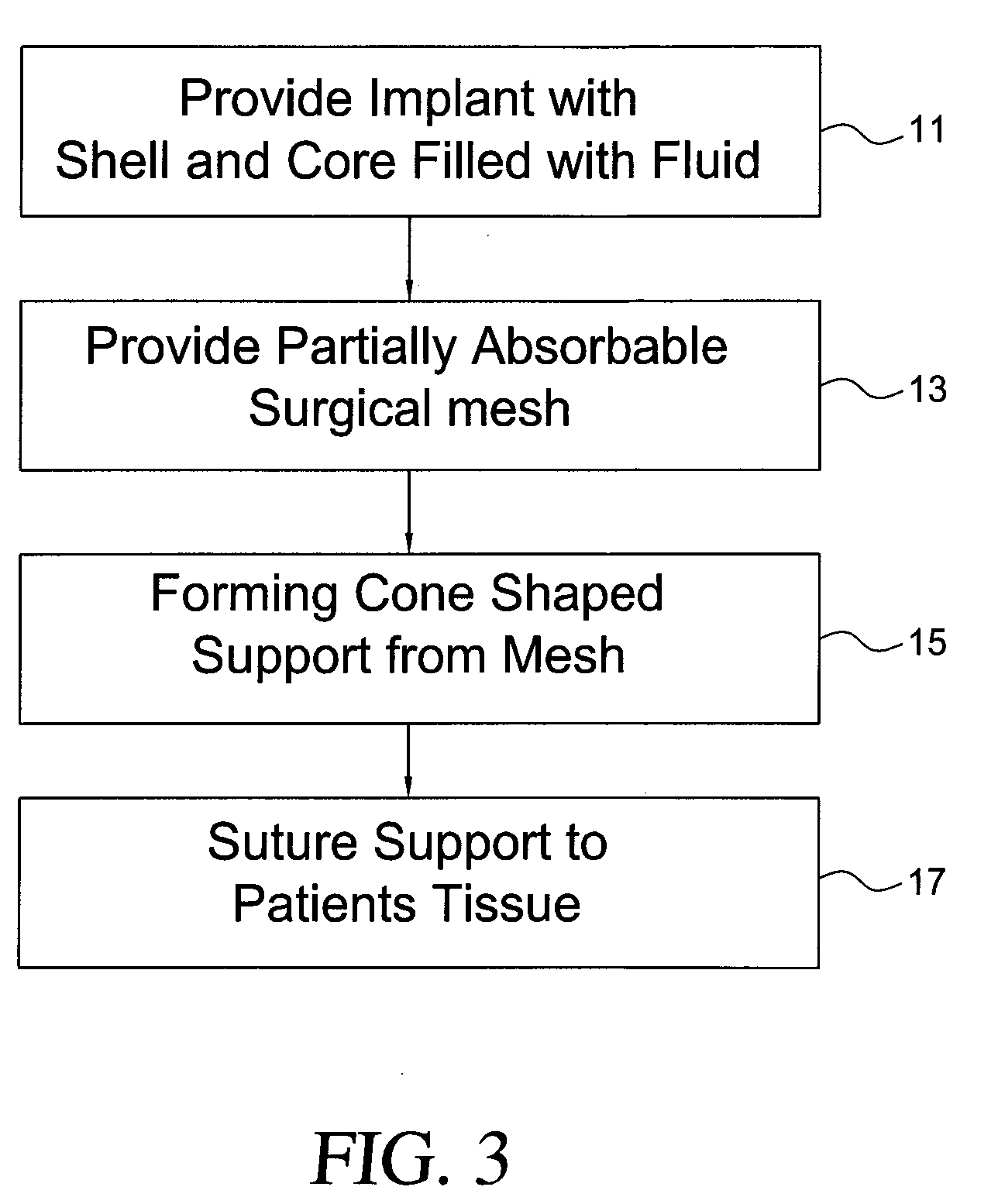
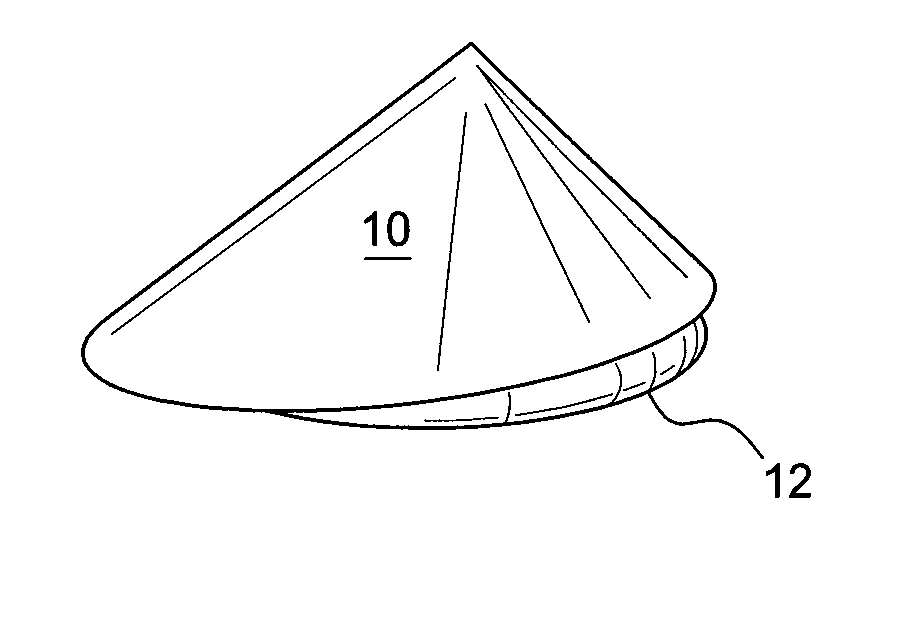
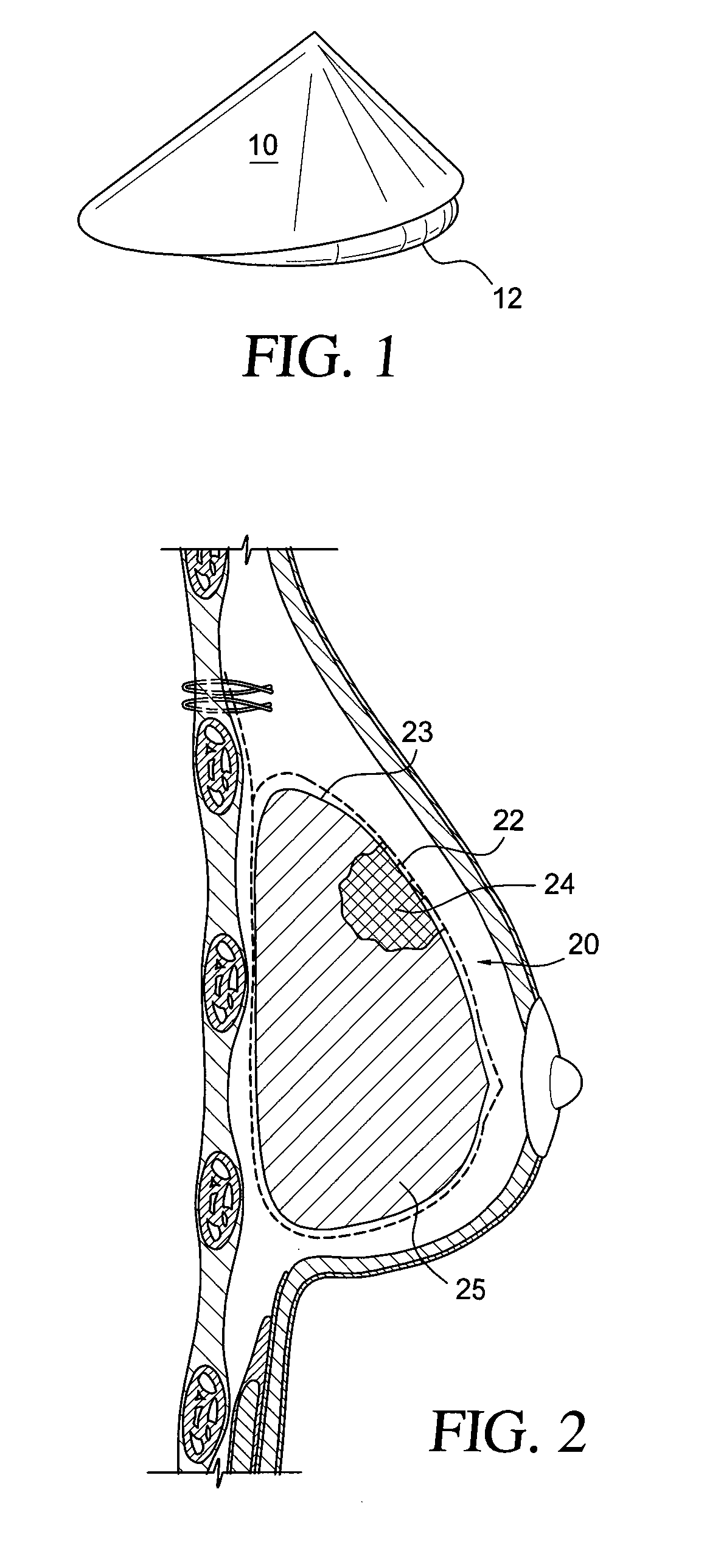
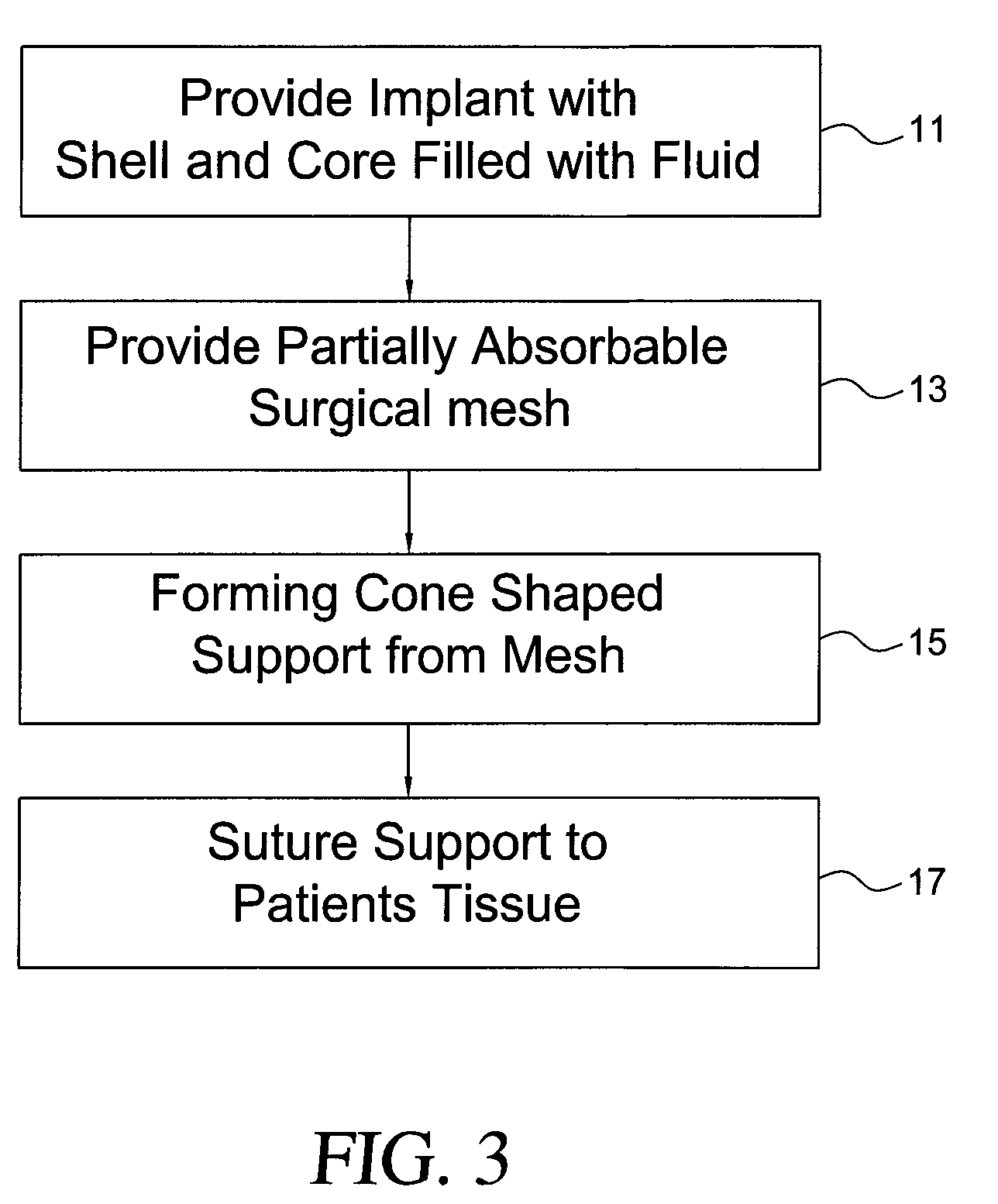
Self supporting implant in a human body and method for making the same without capsular contracture
A self supporting breast implant includes a generally cone shaped partially absorbable medical mesh support member and a silicone shell defining a hollow core that is preferably filled or partially filled with a silicone gel. The support member is made of a polypropolene / poliglecaprone monofilament and may be attached to a patient's tissue by sutures or by absorbable hooks. A textured outer surface or shell is formed around the relatively smooth implant or inner shell and the inner shell is reduced in size to provide a small space between the inner shell and the outer shell to eliminate or at least minimize the adverse effects of capsular contraction.
Owner:TECHNO INVESTMENTS
Method of treating capsular contracture
A method of treating and ameliorating capsular contracture having the steps of providing ultrasound at or near the site of a breast implant, providing specific massage and / or physical manipulation the implant site, and providing a compression bandage at the implant site.
Owner:WEYANT TIM
Drug eluting breast implant cover or coating
InactiveUS20110082545A1Inhibit and reduce formation of scar tissueInhibiting and reducingMammary implantsTissue regenerationPrevention infectionBreast implant
Owner:LIPOSE CORP
Breast Implant Assembly
ActiveUS20140039617A1Promote repairPreventing/reducing capsular contractureMammary implantsBreast implantBreast prostheses
A medical implant assembly and method having a medical implant, e.g. a breast prostheses, attached to a biological interface. The biological interface is comprised of a dermal material with capsular contracture inhibiting properties so that once the medical assembly is inserted into the host, the biological interface, which is intimately coupled to the implant, prevents / reduces capsular contracture formation around the implant. The biological interface comprises a plurality of apertures along its periphery, and attaches to the medical implant by receiving a plurality of attachment flaps or appendages located on the exterior surface of the medical implant within or through the apertures. The attachment of the biological interface is such that the assembly remains intact even where the attachment flaps loosen upon expansion of the implant after insertion into a host, as where the implant is therein injected to a desired dimension.
Owner:MAXWELL G PATRICK
Bioerodible matrix for tissue involvement
InactiveUS20120209381A1Lower potentialImprove efficiencyBiocideMammary implantsPorosityBreast implant
Disclosed herein are polyurethane polymer matrices with a porosity of from about 20 microns to about 90 microns that are useful in promoting closure and protection of incision sites; supporting the lower pole position of breast implants; and providing a partial or complete covering of breast implants to provide a beneficial interface with host tissue and to reduce the potential for malpositioning or capsular contracture. The disclosed matrices can be seeded with mammalian cells.
Owner:ALLERGAN INC
Artificial breast implant provided on the surface threof with silicon open cell foam layer, and method for producing the same
InactiveUS20120245685A1Avoid it happening againIncreased durabilityMammary implantsPharmaceutical containersBreast implantSide effect
Disclosed are an artificial breast implant in which the surface thereof is formed or modified with a silicone open cell (open pore) foam layer, and a method for producing the same. More specifically, disclosed are an artificial breast implant that has a surface including an open cell foam layer made of silicone and thus minimizes side effects such as in vivo rejection, which may occur after implantation of the implant into the body, in particular, the occurrence of capsular contracture to achieve superior biocompatibility and safety, and a method for producing the same.
Owner:YU WON SEOK
Bioerodible matrix for tissue involvement
InactiveUS20120207837A1Lower potentialImprove efficiencyPowder deliveryBiocidePorosityBreast implant
Disclosed herein are polyurethane polymer matrices with a porosity of from about 20 microns to about 90 microns that are useful in promoting closure and protection of incision sites; supporting the lower pole position of breast implants; and providing a partial or complete covering of breast implants to provide a beneficial interface with host tissue and to reduce the potential for malpositioning or capsular contracture. The disclosed matrices can be seeded with mammalian cells.
Owner:ALLERGAN INC
Self supporting implant in a human body and method for making the same without capsular contracture
A self supporting breast implant includes a generally cone shaped partially absorbable medical mesh support member and a silicone shell defining a hollow core that is preferably filled or partially filled with a silicone gel. The support member is made of a polypropolene / poliglecaprone monofilament and may be attached to a patient's tissue by sutures or by absorbable hooks. A textured outer surface or shell is formed around the relatively smooth implant or inner shell and the inner shell is reduced in size to provide a small space between the inner shell and the outer shell to eliminate or at least minimize the adverse effects of capsular contraction.
Owner:TECHNO INVESTMENTS
Biodegradable, polymer coverings for breast implants
ActiveUS8911765B2Inhibit and reduce formation of scar tissueInhibiting and reducingAntibacterial agentsBiocideBreast implantBreast augmentation
Owner:MEDTRONIC INC
Use of leukotriene receptor antagonist for treatment of scarring
InactiveUS20030162828A1Alleviate and eliminate scarAlleviate and eliminate and capsular contractureBiocideAnimal repellantsPsychiatryLeukotriene Receptor Antagonists
A method of preventing or treating either scarring or capsular contractures in subjects in need of such treatment comprising the administration of leukotriene receptor antagonists to said subject in need of treatment.
Owner:SCHLESINGER FAMILY PARTNERS A HAWAII
Breast implant assembly
ActiveUS8876899B2Reduce and eliminate capsular contracturePromote formationMammary implantsBreast implantBreast prostheses
A medical implant assembly and method having a medical implant, e.g. a breast prostheses, attached to a biological interface. The biological interface is comprised of a dermal material with capsular contracture inhibiting properties so that once the medical assembly is inserted into the host, the biological interface, which is intimately coupled to the implant, prevents / reduces capsular contracture formation around the implant. The biological interface comprises a plurality of apertures along its periphery, and attaches to the medical implant by receiving a plurality of attachment flaps or appendages located on the exterior surface of the medical implant within or through the apertures. The attachment of the biological interface is such that the assembly remains intact even where the attachment flaps loosen upon expansion of the implant after insertion into a host, as where the implant is therein injected to a desired dimension.
Owner:MAXWELL G PATRICK
Pharmaceutical composition containing pirfenidone in sustained-release tablet form
ActiveUS9408836B2Effective in regressionDeleterious effectNervous disorderAntipyreticHepatic fibrosisAnti fibrotic
The instant invention relates to a process for the preparation of a pharmaceutical composition in sustained-release tablet form comprising from 600 milligrams to 2400 milligrams of Pirfenidone (PFD), in such a way that the drug is bioavailable during an extended period of time of 12 hours from its administration. In this way, the anti-fibrotic and anti-inflammatory action of the drug Pirfenidone is optimized. Moreover, the instant invention offers advantages and a higher therapeutic efficacy compared to other pharmaceutical forms of Pirfenidone for oral administration and its therapeutic application in the regression of chronic renal failure secondary to primary glomerulosclerosis; it shows a better activity with regard to the reduction and / or regression of deleterious effects in breast capsular contracture observed after the surgical implantation of breast implants in humans and has an important anti-TNF-α and anti-TGF-β1 action for the treatment of hepatic fibrosis.
Owner:EXCALIBUR PHARM INC
Process for the preparation of a pharmaceutical composition containing pirfenidone in sustained-release tablet form and its application in the regression of human chronic renal failure, breast capsular contracture and hepatic fibrosis
ActiveUS20160287567A1Effective in regressionDeleterious effectNervous disorderAntipyreticAnti fibroticLiver fibrosis
The instant invention relates to a process for the preparation of a pharmaceutical composition in sustained-release tablet form comprising from 600 milligrams to 2400 milligrams of Pirfenidone (PFD), in such a way that the drug is bioavailable during an extended period of time of 12 hours from its administration. In this way, the anti-fibrotic and anti-inflammatory action of the drug Pirfenidone is optimized. Moreover, the instant invention offers advantages and a higher therapeutic efficacy compared to other pharmaceutical forms of Pirfenidone for oral administration and its therapeutic application in the regression of chronic renal failure secondary to primary glomerulosclerosis; it shows a better activity with regard to the reduction and / or regression of deleterious effects in breast capsular contracture observed after the surgical implantation of breast implants in humans and has an important anti-TNF-α and anti-TGF-β1 action for the treatment of hepatic fibrosis.
Owner:EXCALIBUR PHARM INC
Process for the preparation of a pharmaceutical composition containing pirfenidone in sustained-release tablet form and its application in the regression of human chronic renal failure, breast capsular contracture and hepatic fibrosis
ActiveUS20140296300A1Effective in regressionDeleterious effectBiocideNervous disorderAnti fibroticHepatic fibrosis
The instant invention relates to a process for the preparation of a pharmaceutical composition in sustained-release tablet form comprising from 600 milligrams to 2400 milligrams of Pirfenidone (PFD), in such a way that the drug is bioavailable during an extended period of time of 12 hours from its administration. In this way, the anti-fibrotic and anti-inflammatory action of the drug Pirfenidone is optimized. Moreover, the instant invention offers advantages and a higher therapeutic efficacy compared to other pharmaceutical forms of Pirfenidone for oral administration and its therapeutic application in the regression of chronic renal failure secondary to primary glomerulosclerosis; it shows a better activity with regard to the reduction and / or regression of deleterious effects in breast capsular contracture observed after the surgical implantation of breast implants in humans and has an important anti-TNF-α and anti-TGF-β1 action for the treatment of hepatic fibrosis.
Owner:EXCALIBUR PHARM INC
Use of leukotriene receptor antagonist for treatment of scarring
A method of preventing or treating either scarring or capsular contractures in subjects in need of such treatment comprising the administration of leukotriene receptor antagonists to said subject in need of treatment.
Owner:SCHLESINGER FAMILY PARTNERS A HAWAII
Application of captopril to inhibition of scar hyperplasia
InactiveCN102335167ALow priceMature technologyOrganic active ingredientsDermatological disorderDiseaseCaptopril
The invention relates to application of captopril to the preparation of medicaments for treating and / or inhibiting scar hyperplastic diseases. Particularly, the hyperplastic diseases comprise hyperplastic scar, cheloid, urethral scar stricture and silicon gel breast augmentation prosthesis postoperative capsular contracture. When the diseases are treated, the captopril is used as an active ingredient, clinically-used emulsifiable paste, gel, injection and the like in different formulations are prepared by the conventional preparation process. The study indicates that the captopril can reduce the capacity of synthesizing pathologic scar fibroblast collagen and secreting the collagen, weaken the multiplication capacity of fibroblasts and reduce the expression of transforming growth factor-beta 1 (TGF-beta 1), and has a good treatment or prevention effect on pathologic scars. Simultaneously, the captopril acts on scar tissue directly, has the advantages of small dose, high curative effect, small side effect, mature raw material process, readily available materials, low cost and the like, and is expected to become a main medicament for treating and preventing the scars clinically.
Owner:PLASTIC SURGERY HOSPITAL CHINESE ACAD OF MEDICAL SCI
Variable double-bag breast implant
InactiveCN101919749ARelieve painReduce exudationMammary implantsBreast implantBiomedical engineering
The invention discloses a variable double-bag breast implant comprising an expansion bag, a breast implant and a self-closing type injection valve. The variable double-bag breast implant is characterized in that the breast implant is arranged in a cavity of the expansion bag to form a double-bag structure, the breast implant and the cavity share the same bottom, and the self-closing type injection valve is arranged on the surface of the expansion bag. The variable double-bag breast implant with the structure can effectively reduce capsular contracture and micromolecule exudation and simultaneously relieve the pains of patients in operation.
Owner:上海威宁整形制品有限公司
Artificial breast implant provided on the surface threof with silicon open cell foam layer and method for producing the same
InactiveUS20130302510A1Minimizes capsular contractureGood biocompatibilityMammary implantsPharmaceutical containersBreast implantSide effect
Disclosed are an artificial breast implant in which the surface thereof is formed or modified with a silicone open cell (open pore) foam layer, and a method for producing the same. More specifically, disclosed are an artificial breast implant that has a surface including an open cell foam layer made of silicone and thus minimizes side effects such as in vivo rejection, which may occur after implantation of the implant into the body, in particular, the occurrence of capsular contracture to achieve superior biocompatibility and safety, and a method for producing the same.
Owner:YU WON SEOK
Methods for Prevention and/or Treatment of Capsular Contracture
InactiveUS20140249490A1Low of affordabilityReduce convenienceCosmetic preparationsBiocideStratum corneumFibrosis
Methods for prevention or treatment of capsular contracture following surgical implants and other fibrosis related conditions comprising applying a topical composition comprising a pharmaceutically acceptable keratolytic, a pharmaceutically acceptable protein denaturant, a hydrating agent, and combinations thereof are disclosed.
Owner:J&E SOLUTIONS
Methods for prevention and/or treatment of capsular contracture
ActiveUS8409600B2Efficient use ofReduce riskBiocideSalicyclic acid active ingredientsFibrosisKeratolytic
Methods for prevention or treatment of capsular contracture following surgical implants and other fibrosis related conditions comprising applying a topical composition comprising a pharmaceutically acceptable keratolytic, a pharmaceutically acceptable protein denaturant, a hydrating agent, and combinations thereof are disclosed.
Owner:FRIEDMAN JEANNE V
Application of microgroove forming mold in preparation of silicone rubber with microgroove pattern on surface
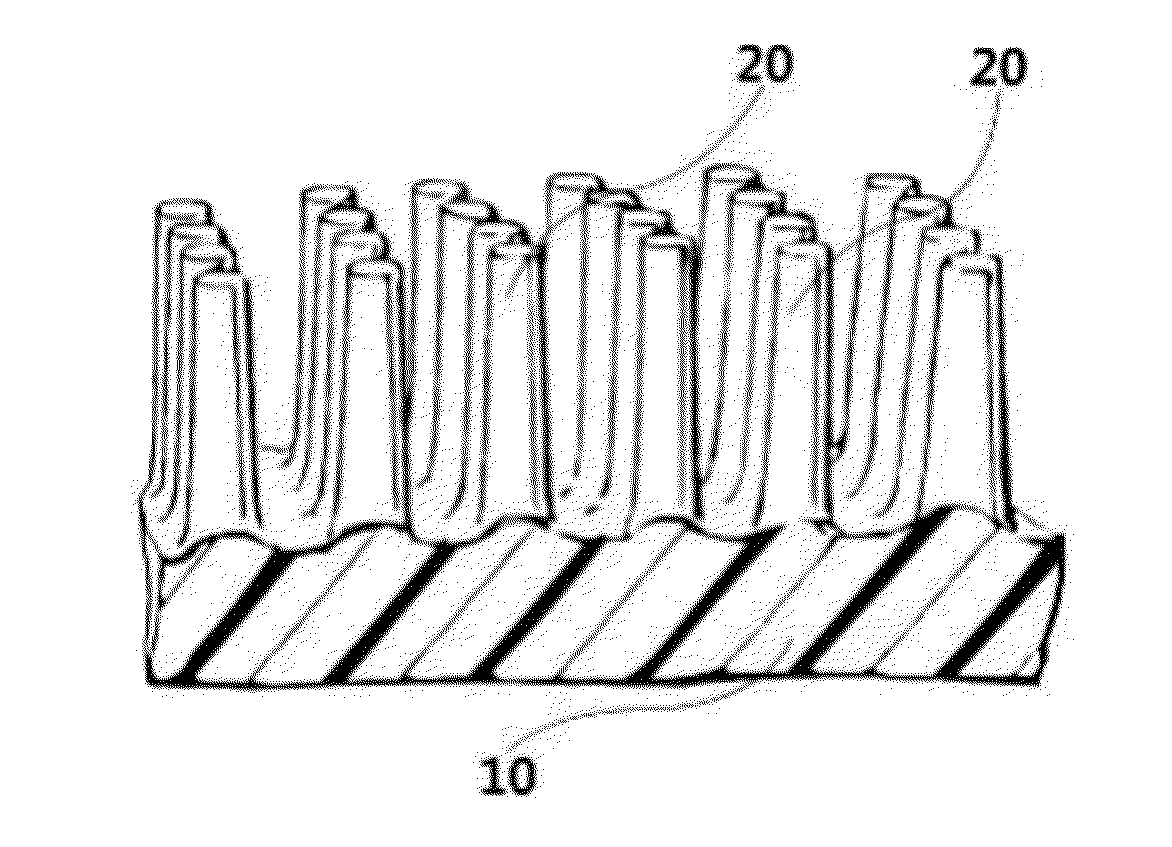
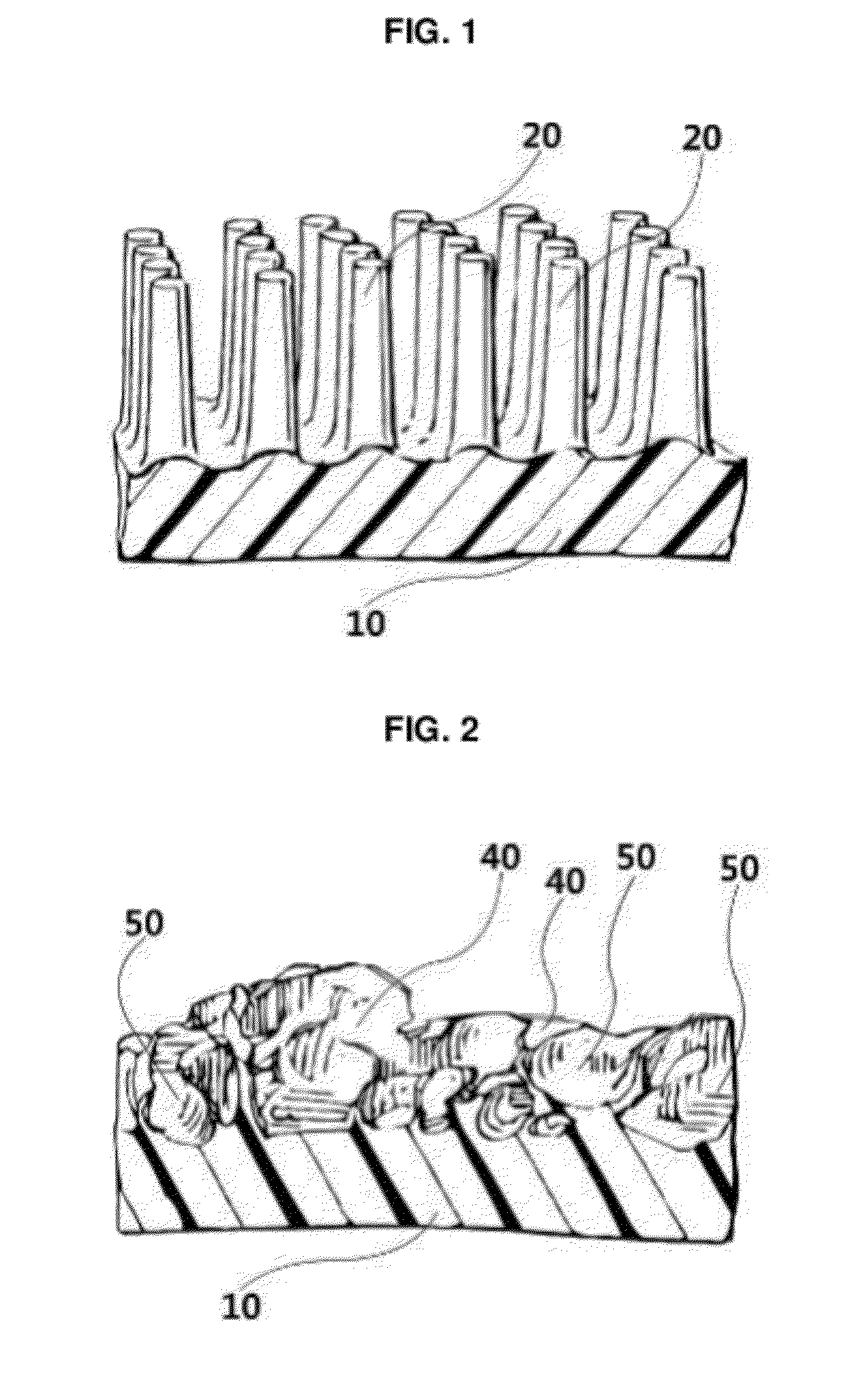
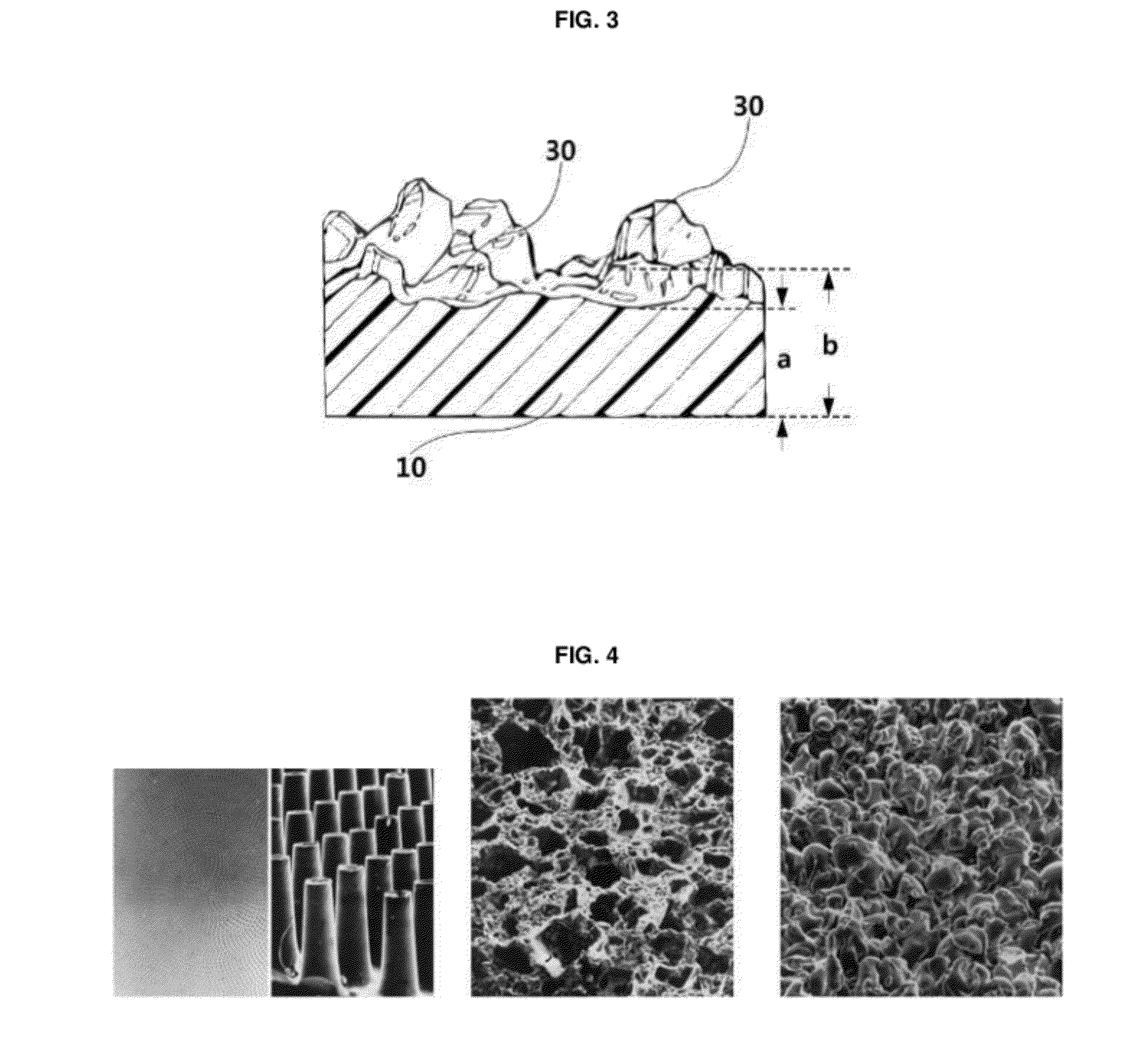
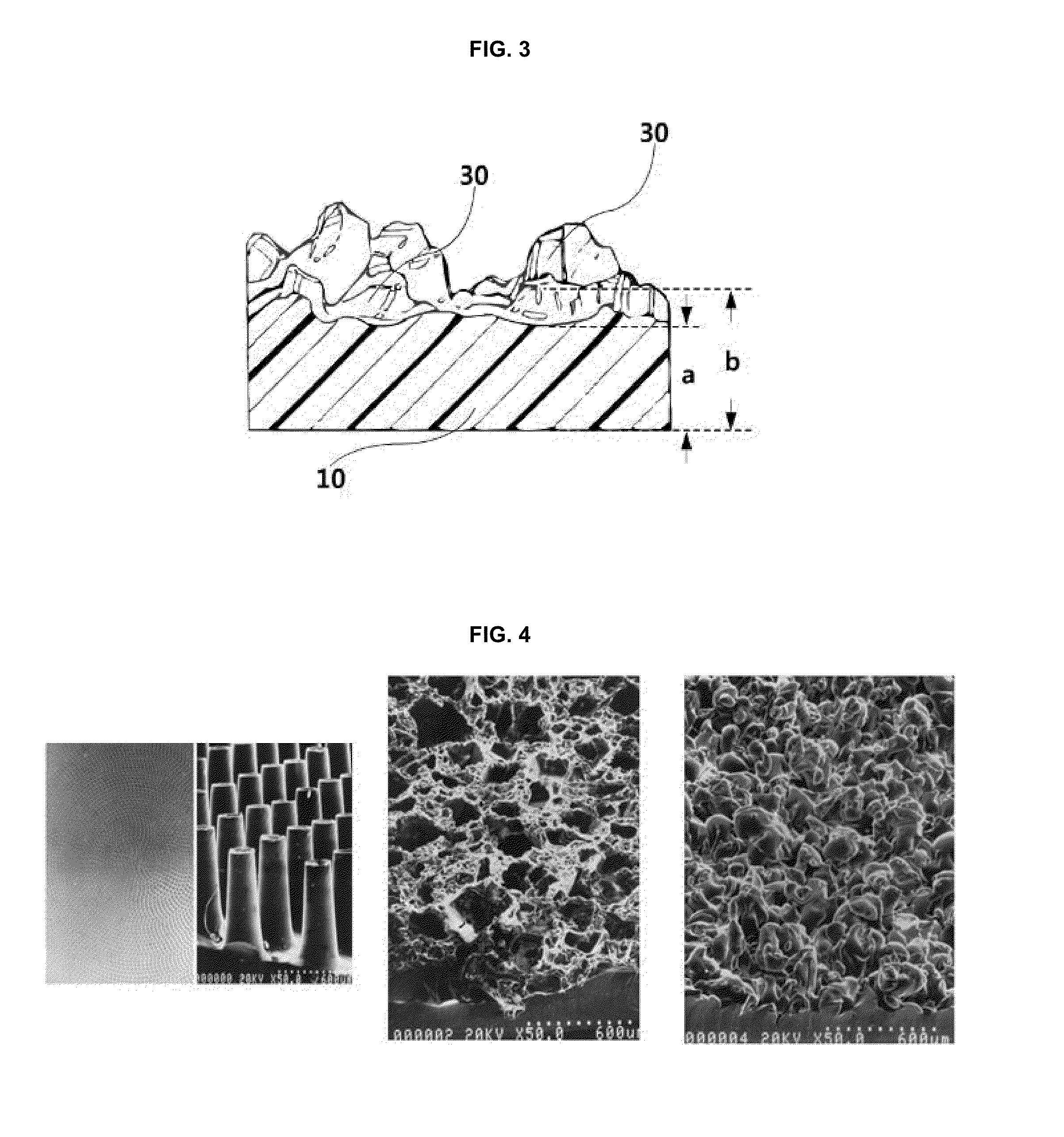
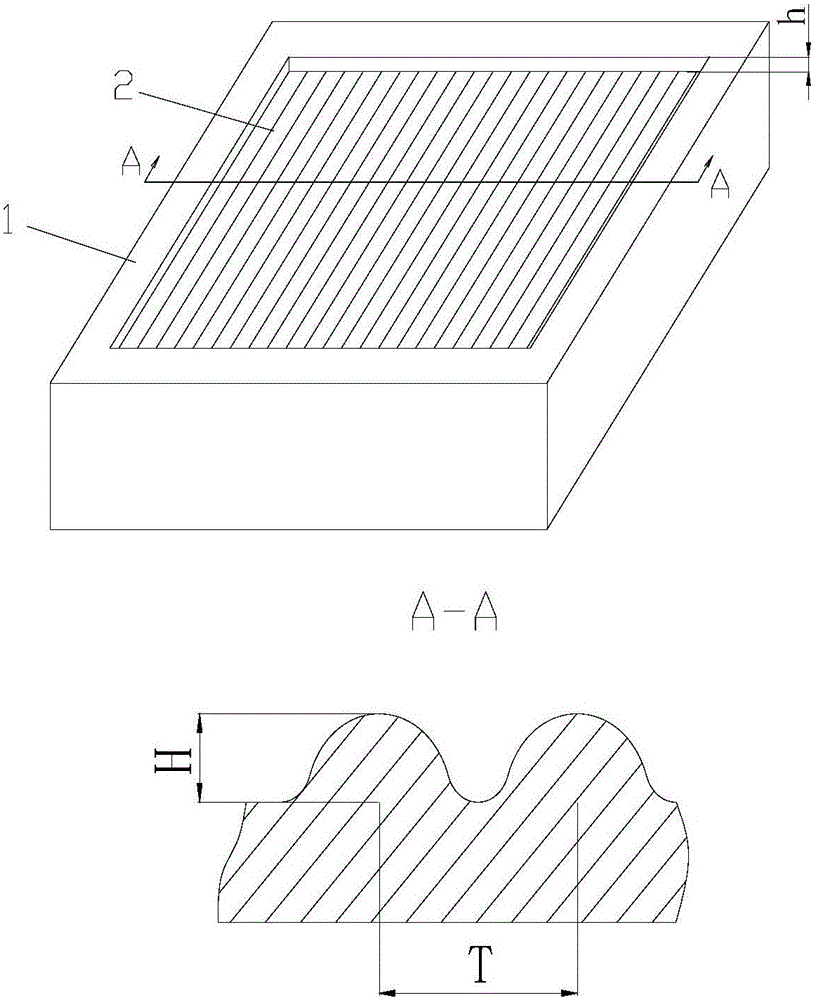
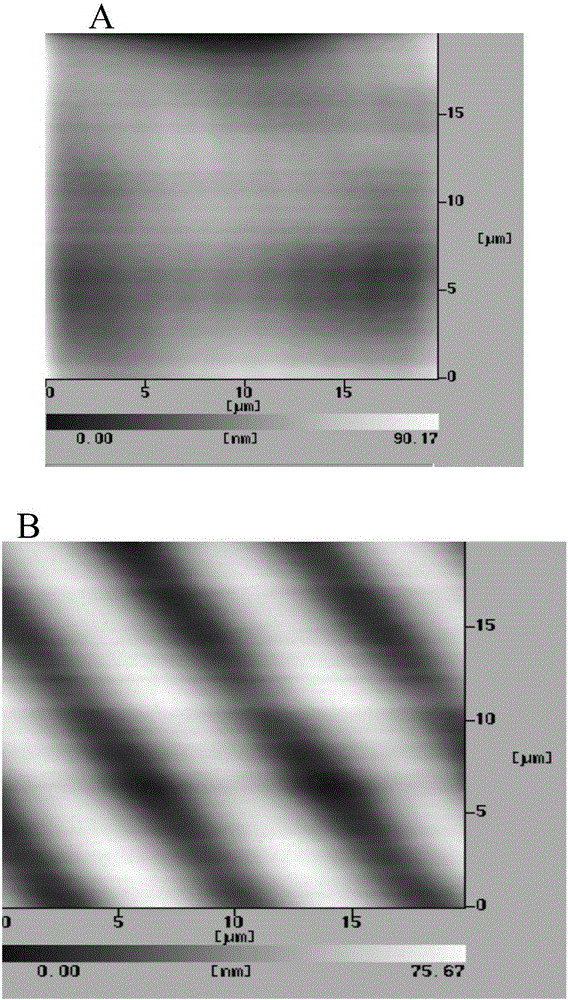
The invention discloses an application of a microgroove forming mold in preparation of silicone rubber with a microgroove pattern on the surface. The microgroove forming mold comprises a female mold, wherein microcosmic bulges used for microgroove forming and arranged in rows or lines are arranged at the bottom of the female mold, the microcosmic bulges are distributed according to the rules that the peak value ranges from 4 mu m to 6 mu m and the period ranges from 5 mu m to 7 mu m, and the depth of the female mold ranges from 0.4 mm to 0.6 mm. The silicone rubber surface is modified by the microgroove forming mold into a structure with the microgroove pattern, the water contact angle of the silicone rubber is increased, the hydrophilicity of the silicone rubber is reduced, growth behaviors of cells on the silicone rubber surface are further affected, the growth direction and space distribution of the cells on the material surface are further affected, finally, the incidence of capsular contracture is reduced, and a novel solution is provided for preparation of implant materials more applicable to clinical application.
Owner:THE SECOND AFFILIATED HOSPITAL ARMY MEDICAL UNIV
Methods for Prevention and/or Treatment of Capsular Contracture
ActiveUS20100189761A1Low of affordabilityReduce convenienceSalicyclic acid active ingredientsBiocideFibrosisKeratolytic
Methods for prevention or treatment of capsular contracture following surgical implants and other fibrosis related conditions comprising applying a topical composition comprising a pharmaceutically acceptable keratolytic, a pharmaceutically acceptable protein denaturant, a hydrating agent, and combinations thereof are disclosed.
Owner:FRIEDMAN JEANNE V
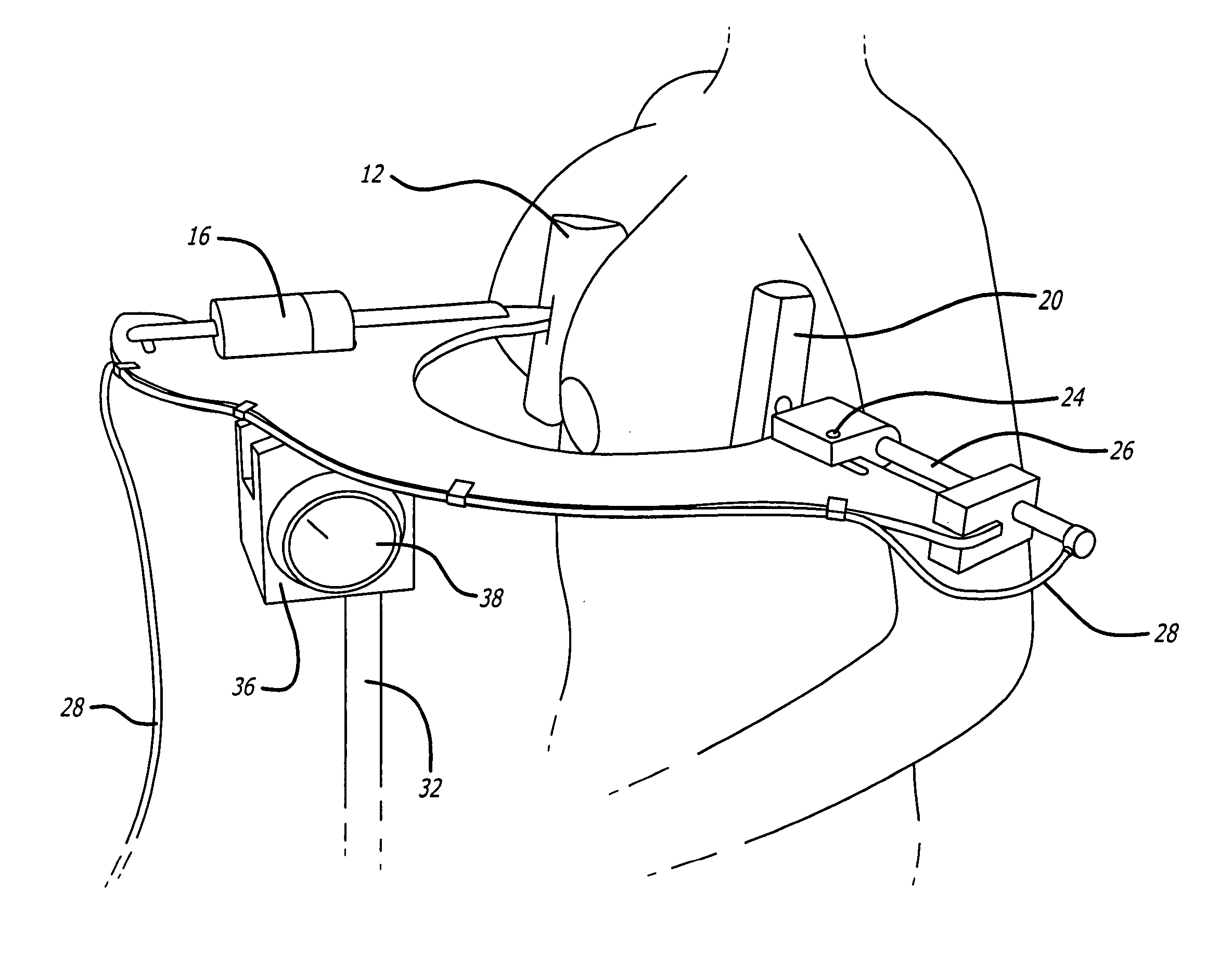
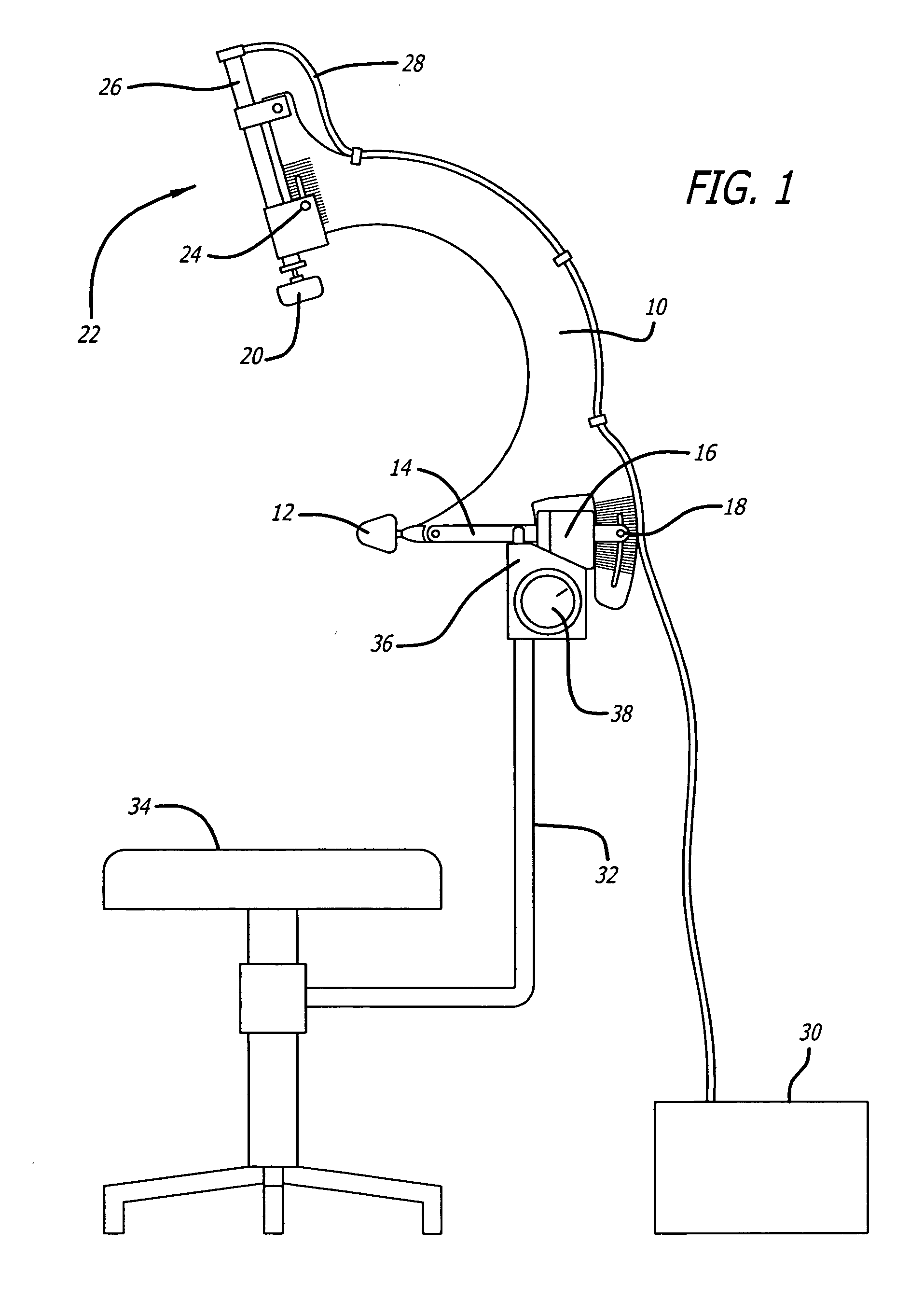
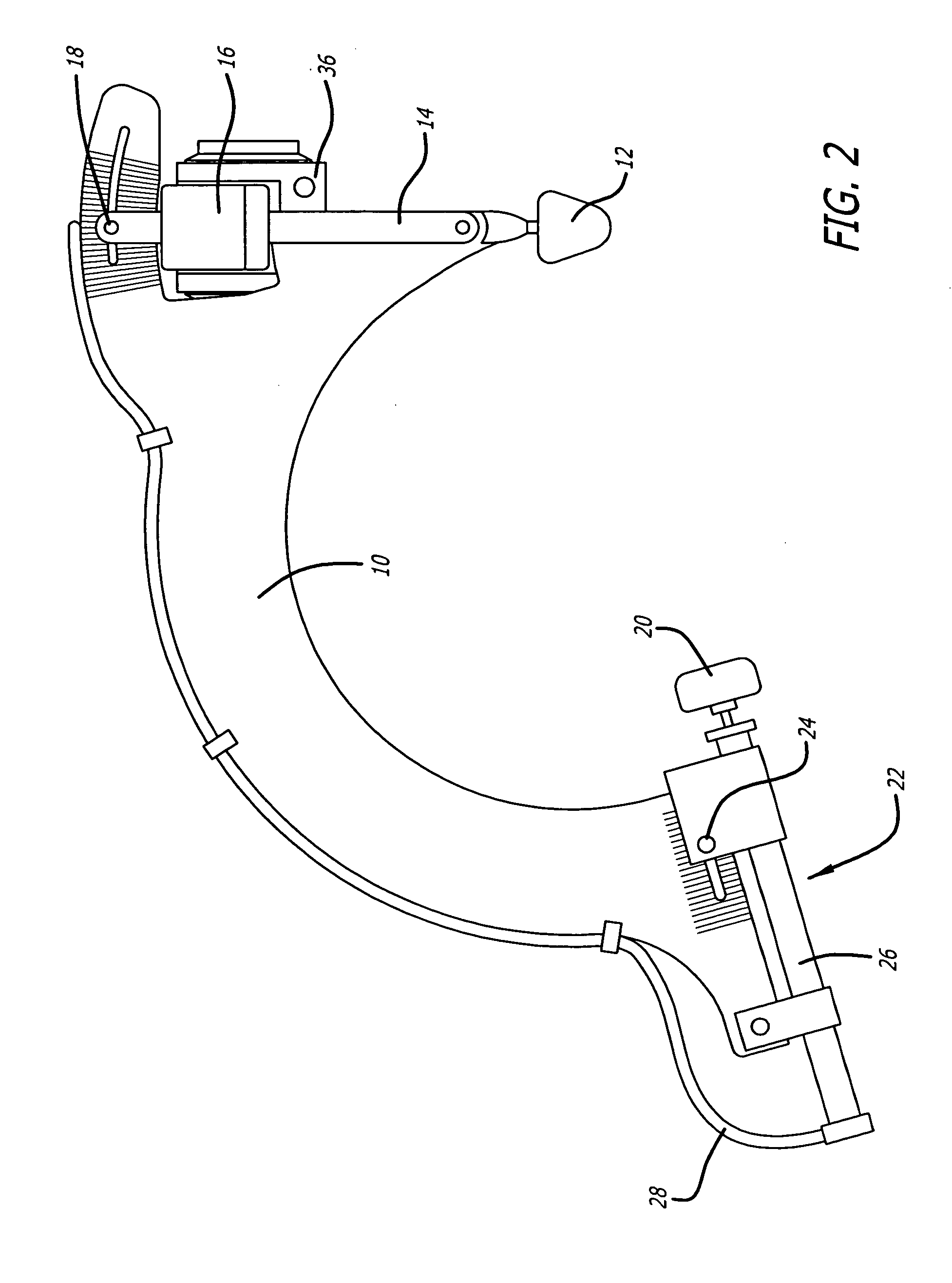
Capsular contracture preventing and relieving device
InactiveUS20050251070A1Prevent and relieve capsular contractureSafe and effective lateral compression forceMammary implantsSurgeryBreast implantBreast Implant Procedure
A device for exerting a compression force on a breast implant located within a user's breast. The device includes a support member adjustably connect to a compression member, the compression member comprising a positioning element on one side of the compression member such that the positioning element is aligned with medial side of the breast and a compression element on the second side of the compression member such that the compression element is positioned on lateral side of the breast, and means extending between the positioning element and the compressing element for retaining the positioning element and the compressing element on opposite sides of the breast, and means for providing pressure to the compressing element causing the compressing element to apply a compression force to the breast implant within the user's breast. Additionally a method for preventing capsular contracture after breast implant surgery is provided.
Owner:VILLANI
Gold particles for use in therapy to prevent or reduce capsular contracture
Gold implant having a cross-section in the range of 20-100, preferably in the range of 20-40 [mu]m for use in therapy to prevent or reduce capsular contracture. Further, the invention relates to a method of producing a gold-coated implant.
Owner:SAFE IMPLANT TECH APS
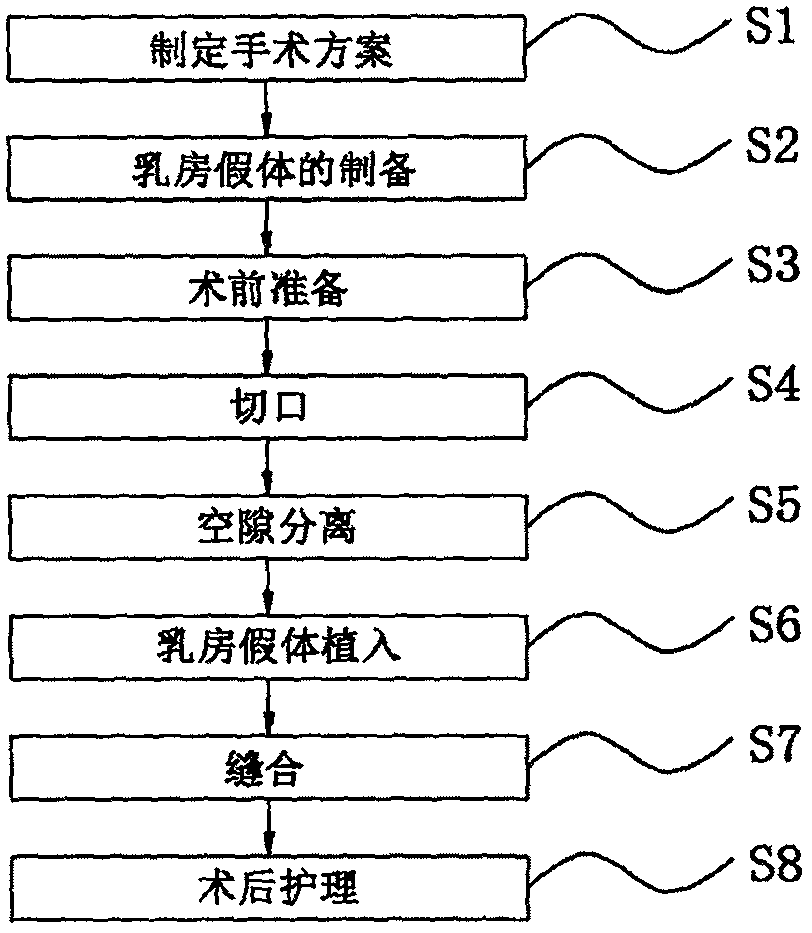
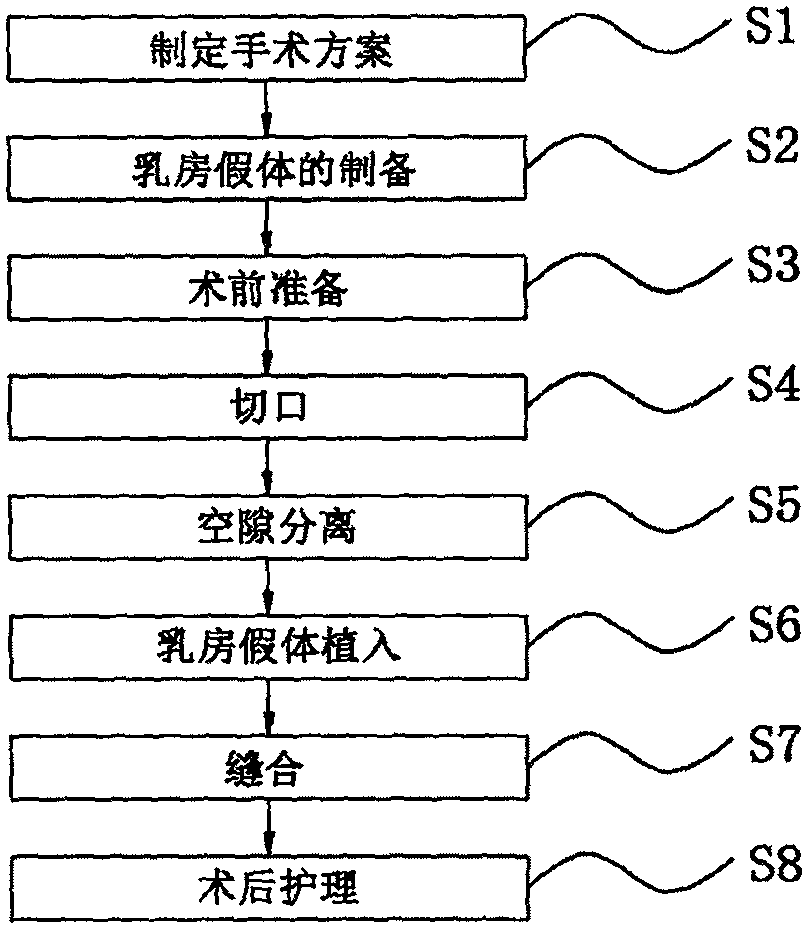
Primary biplanar mammary augumentation method
PendingCN111110285AEasy to integrateRich surgical clinical experienceSurgeryBreast Prosthesis ImplantationEngineering
The invention discloses a primary biplanar mammary augumentation method. The method comprises the following steps: formulation of an operation plane; preparation of breast prosthesis: preparing the breast prosthesis by a 3D printing technology according to various parameters of the breasts of a patient; preoperative preparation; cutting: cutting the breasts according to cuts designed before operation under armpits with a width smaller than 3 cm, perfectly fusing scar with armpit folds to realize traceless conceal; gap separation; breast prosthesis implantation; suturing: suturing the cuts withmedical suture and adopting a pressurized fixed binding manner; and postoperative nursing. According to the biplanar method, after the lower side of pectoralis major is divided, the risk of upward and outward shift of the prosthesis is reduced, the prosthesis deformation caused by contraction of the pectoralis major is also effectively reduced, after part of prosthesis is placed behind the pectoralis major by the biplanar method, the probability of infection and capsular contracture is reduced, the position of lower plica after mammary augumentation can be accurately controlled by the biplanar mammary augumentation operation, the postoperative effect is stable, and shift cannot be caused easily.
Owner:黄惠铭
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com