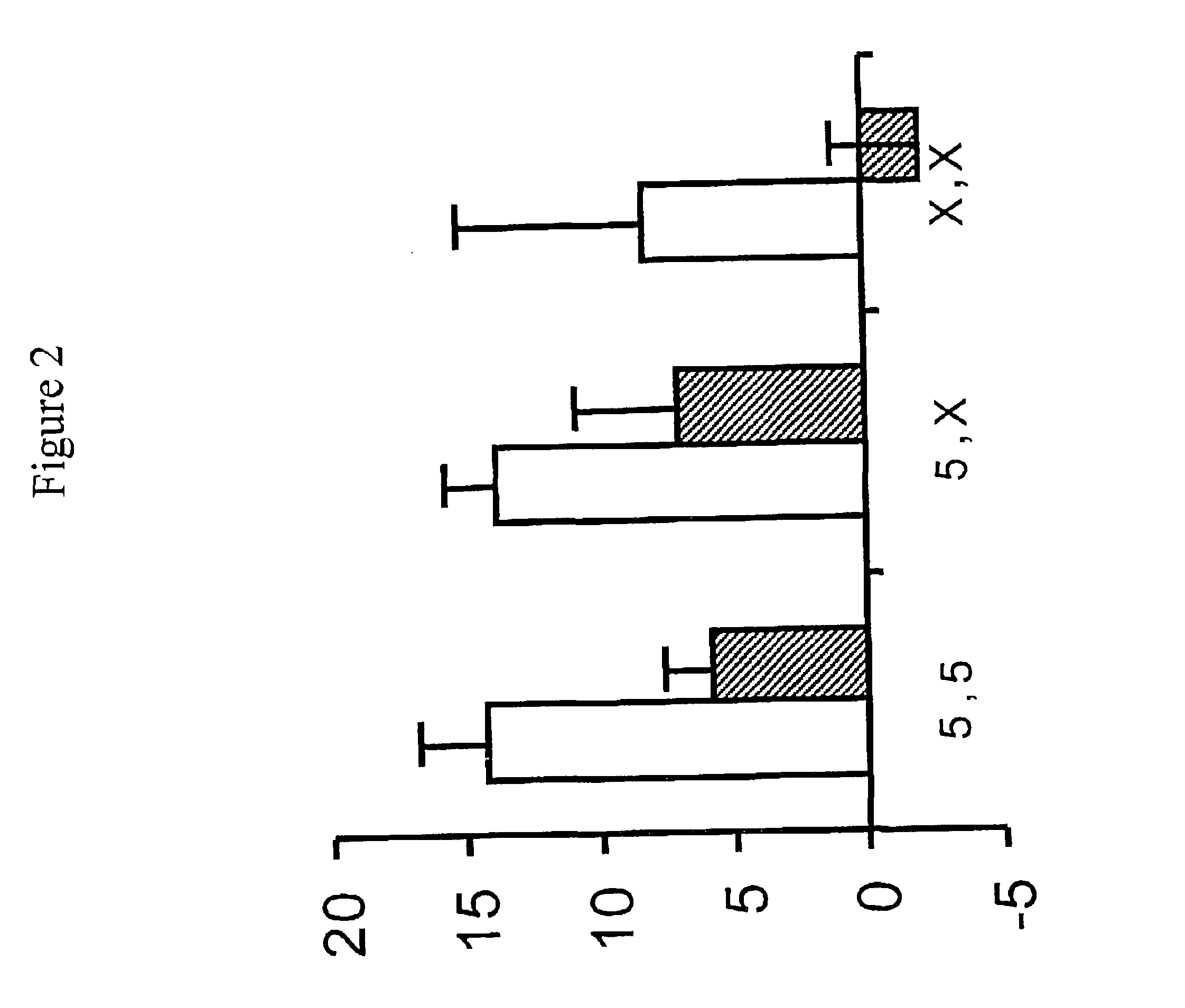
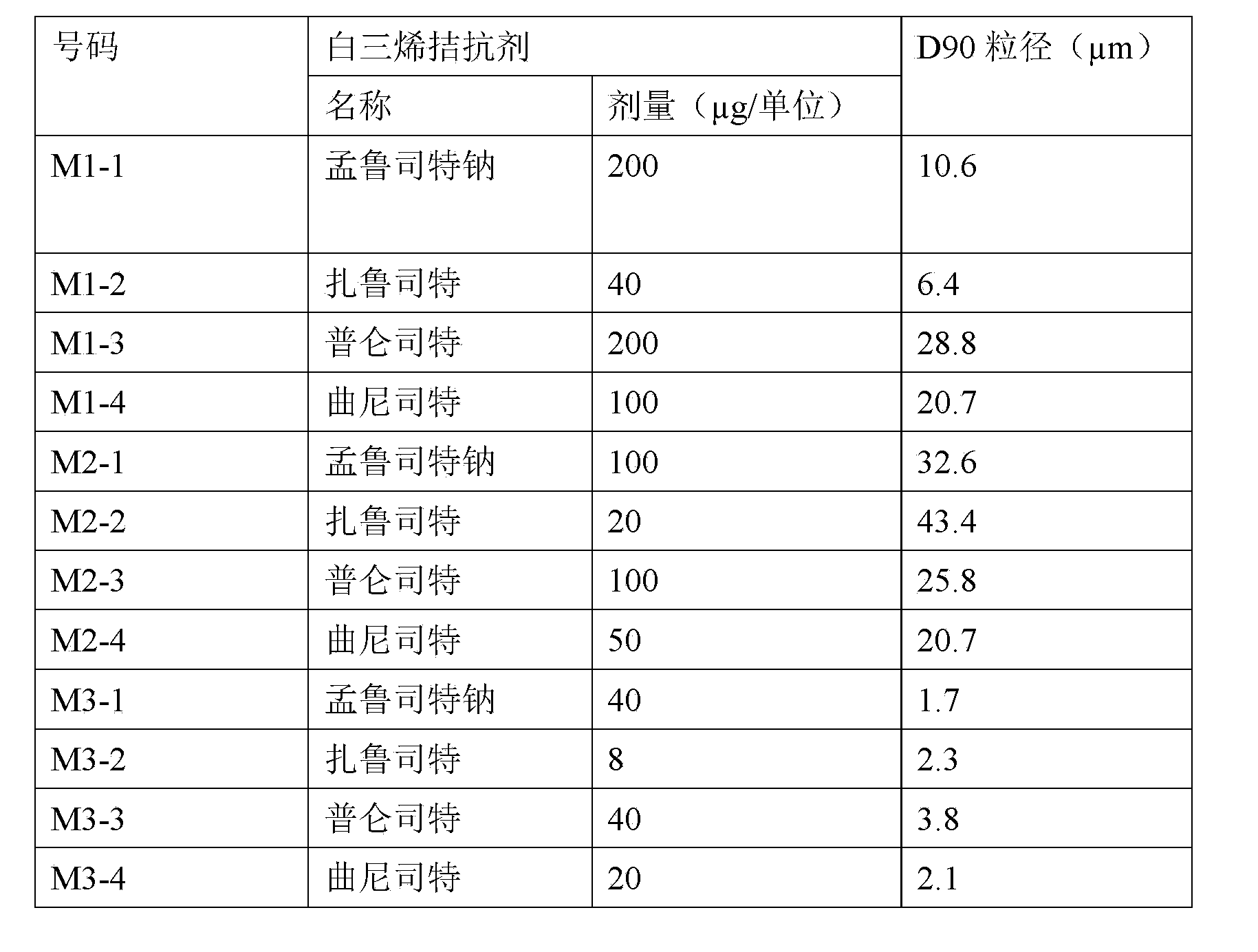
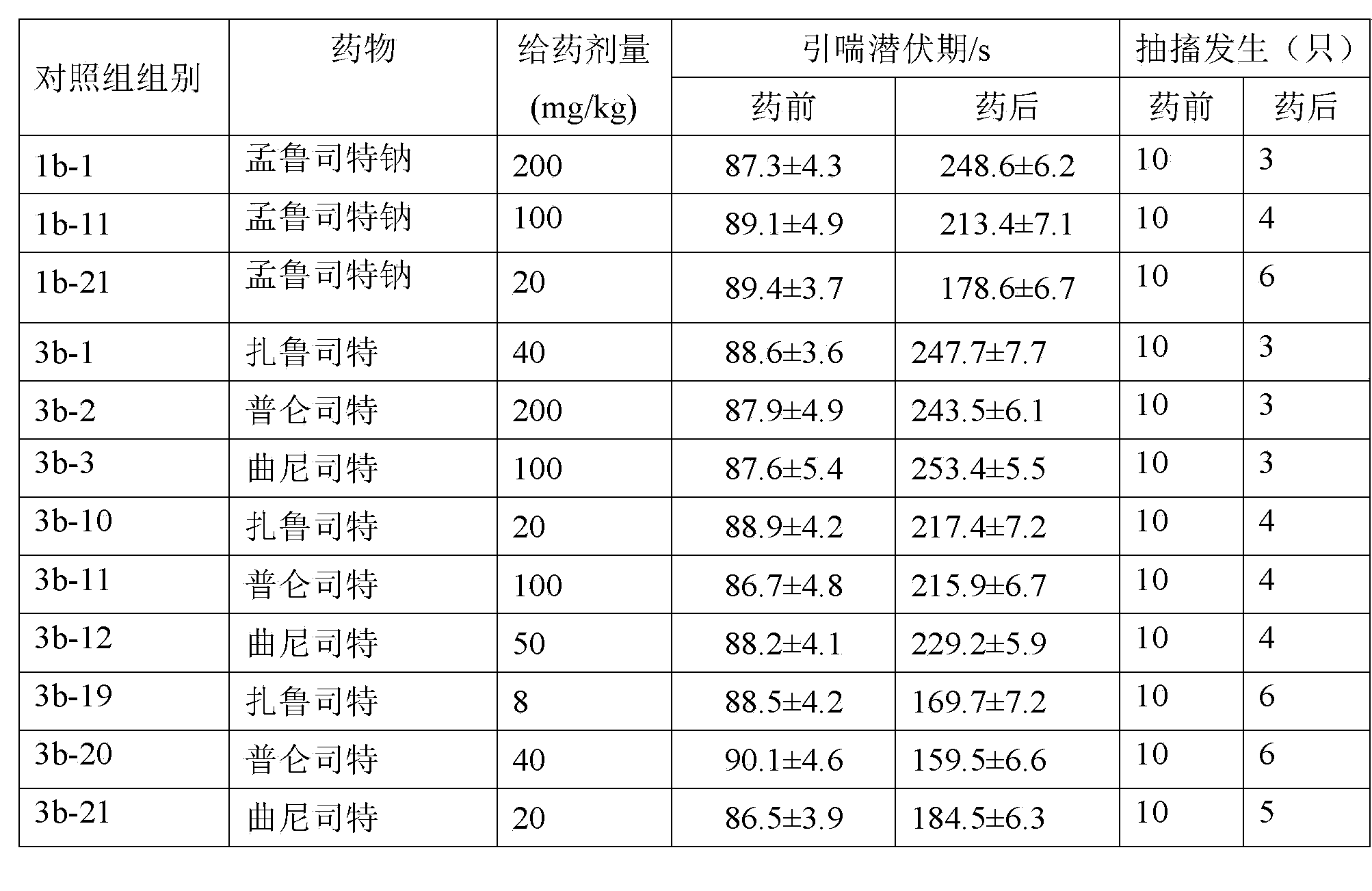
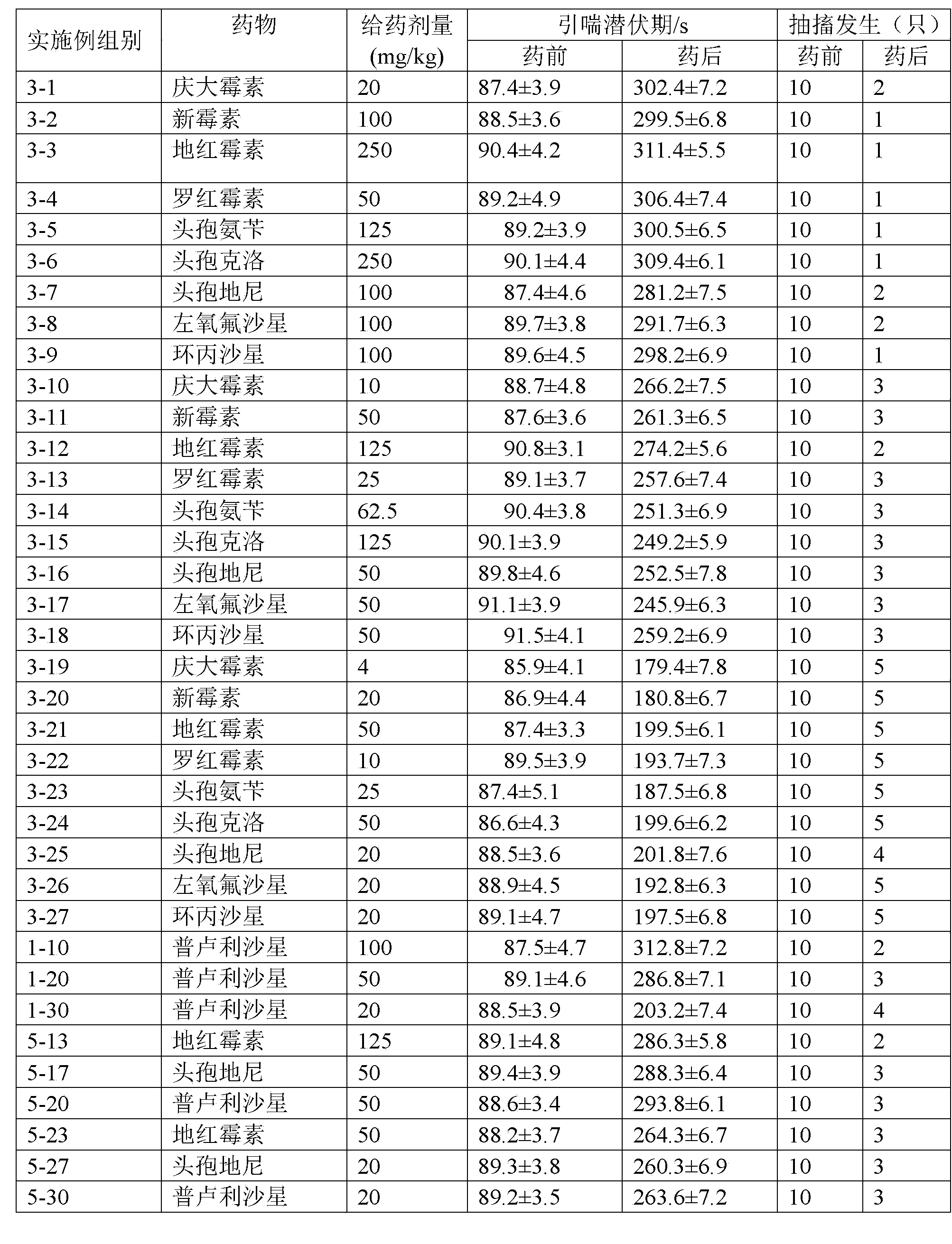
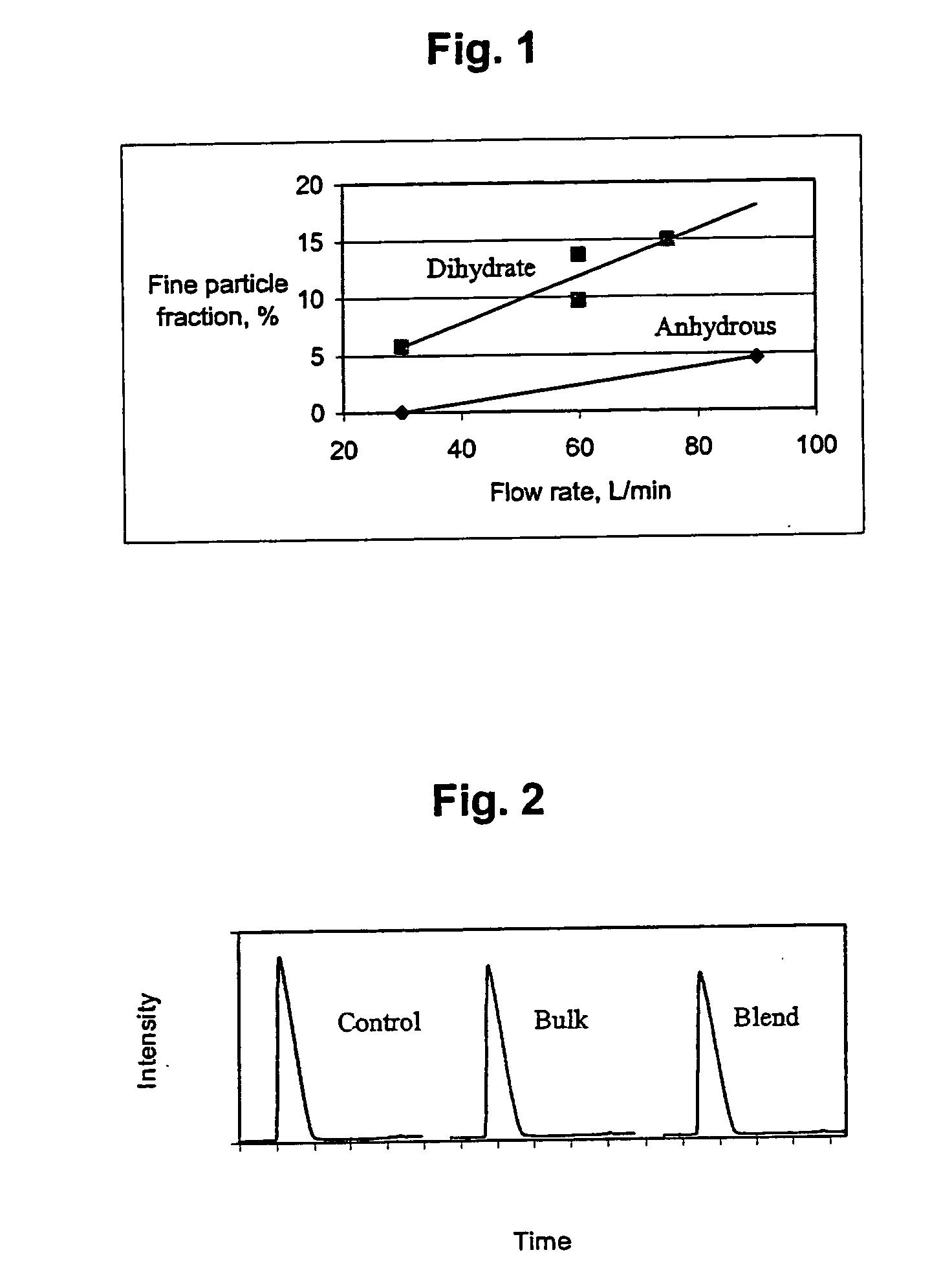
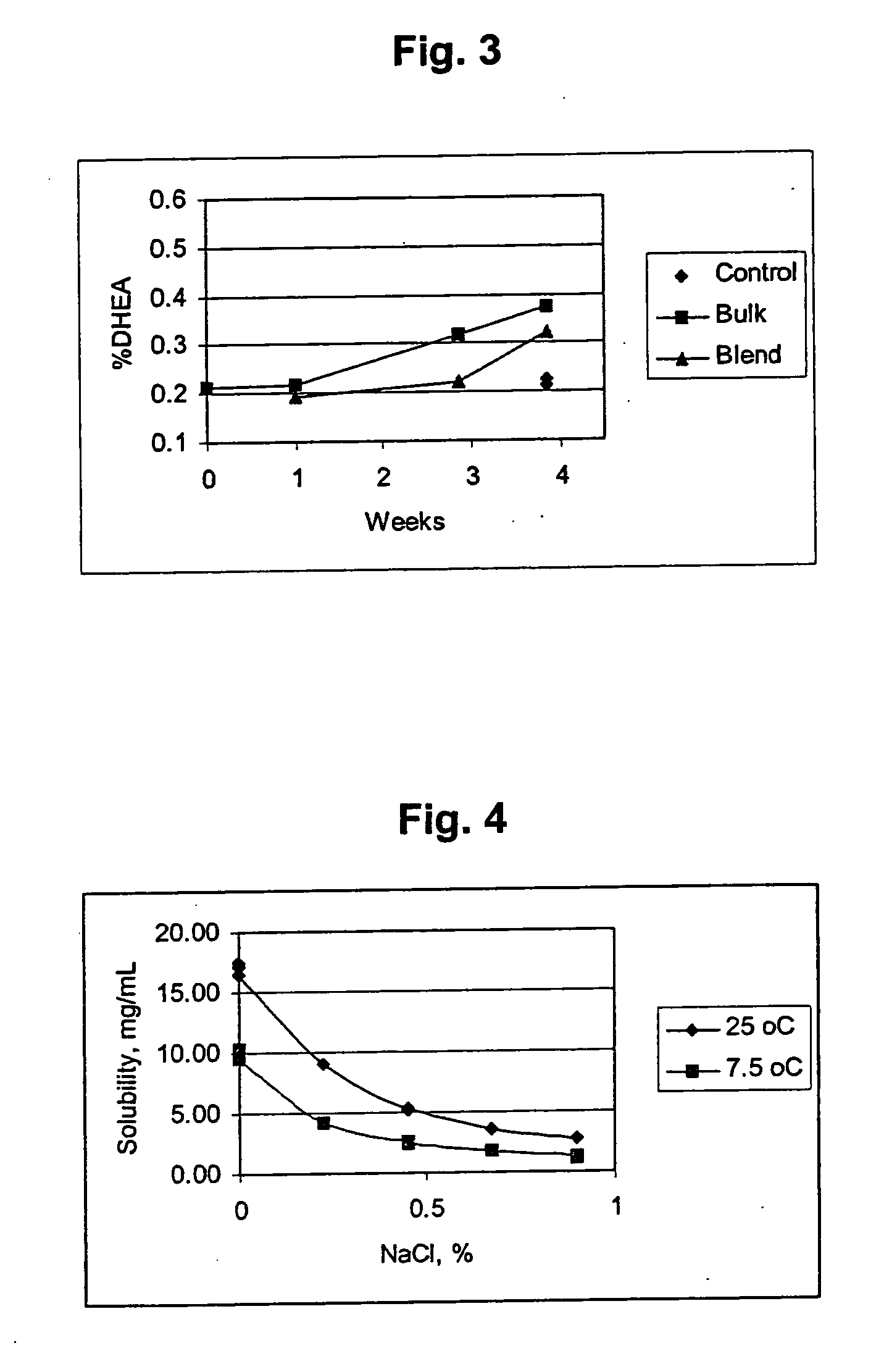
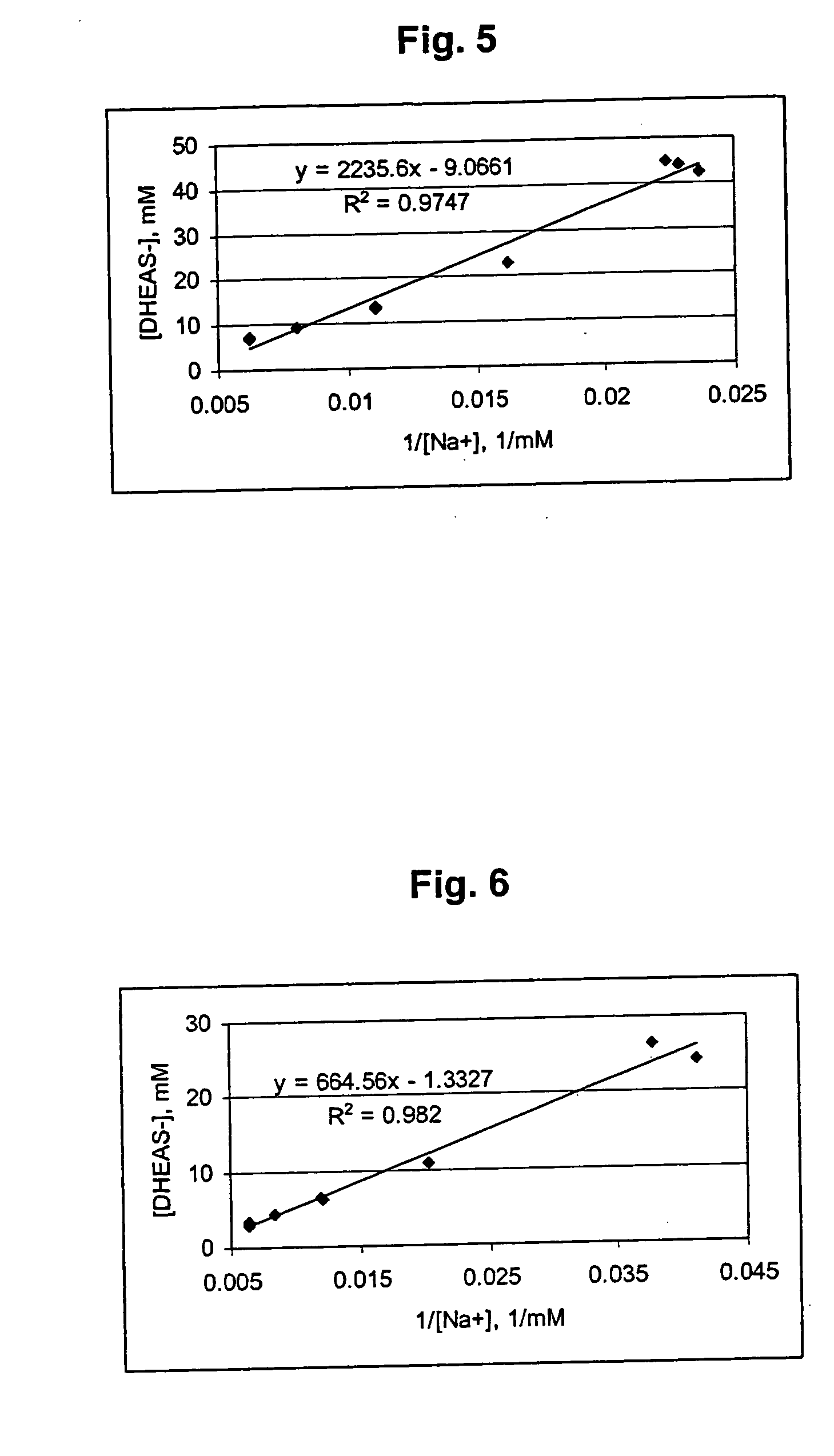
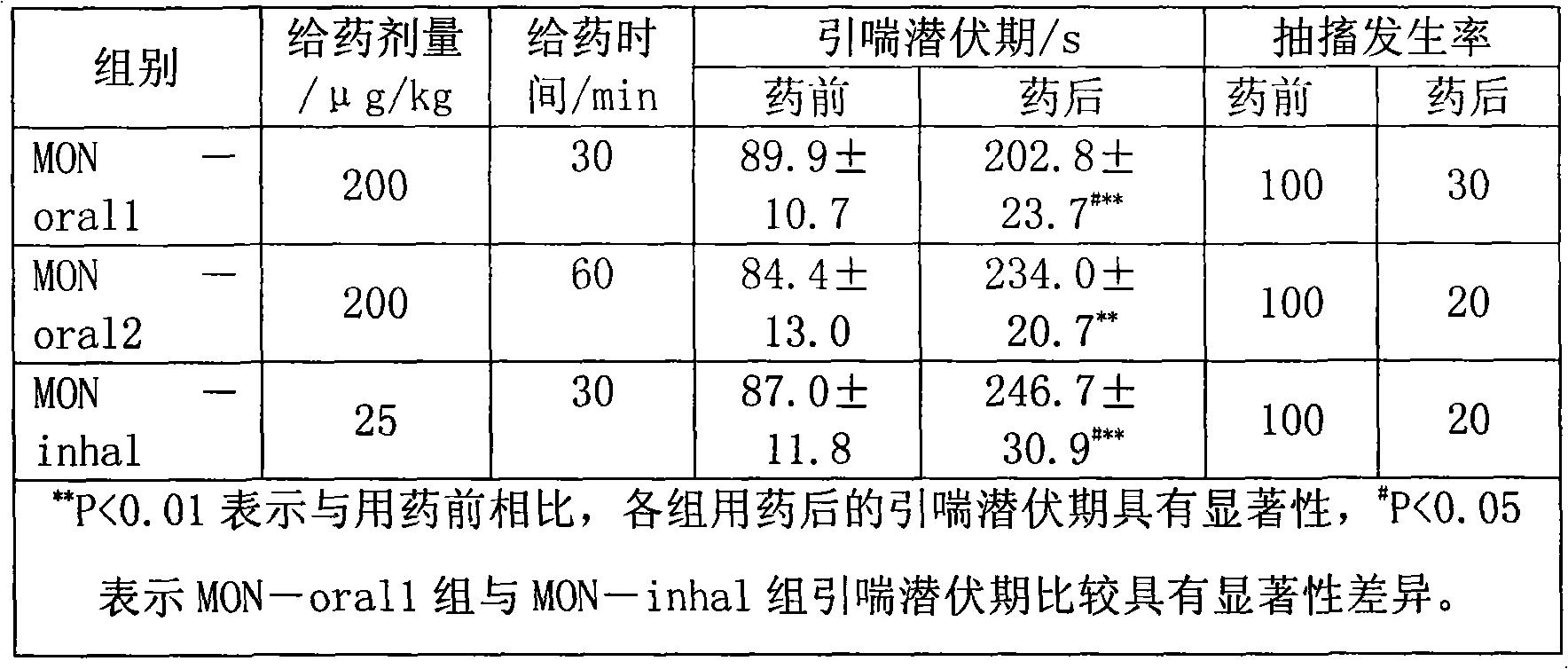
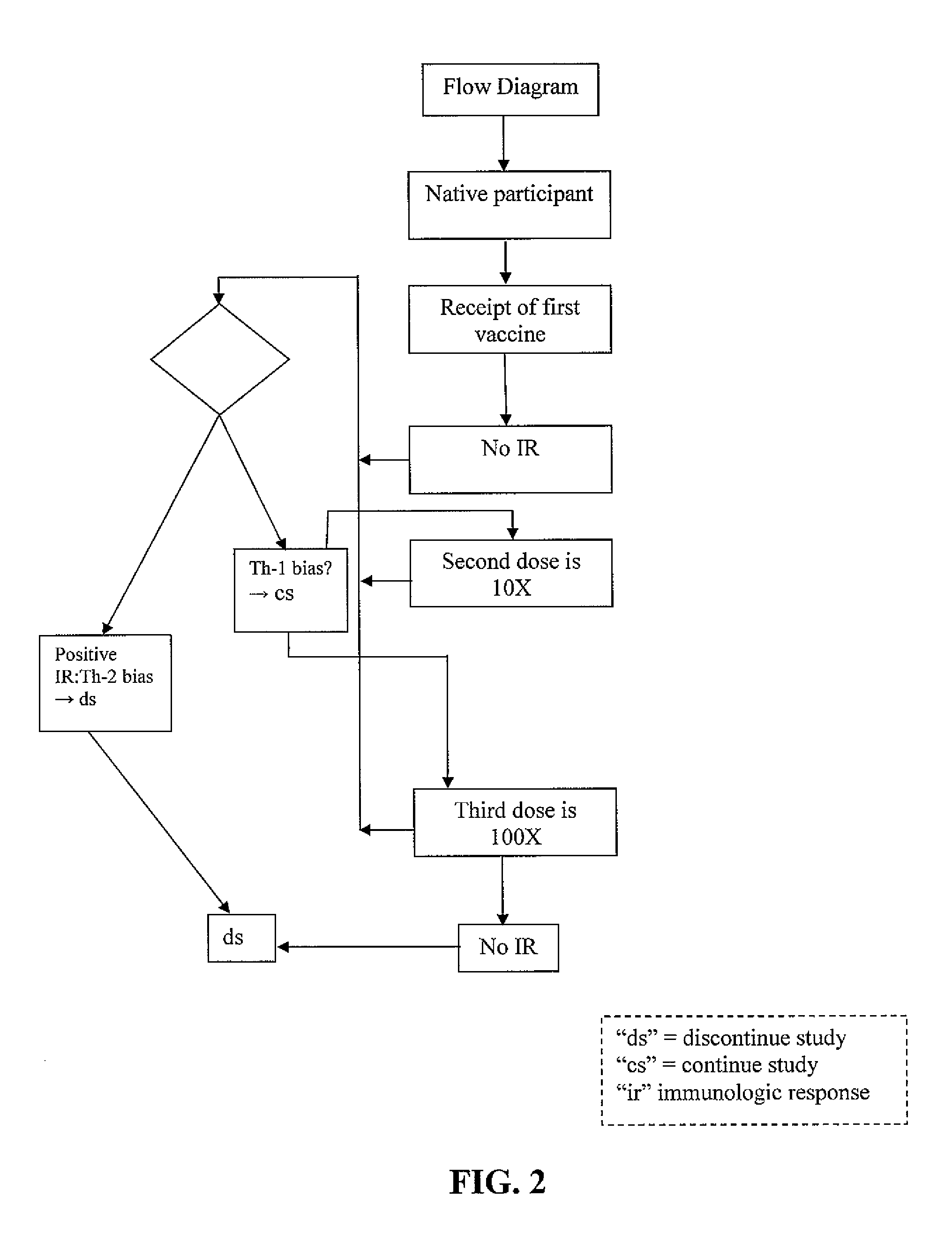
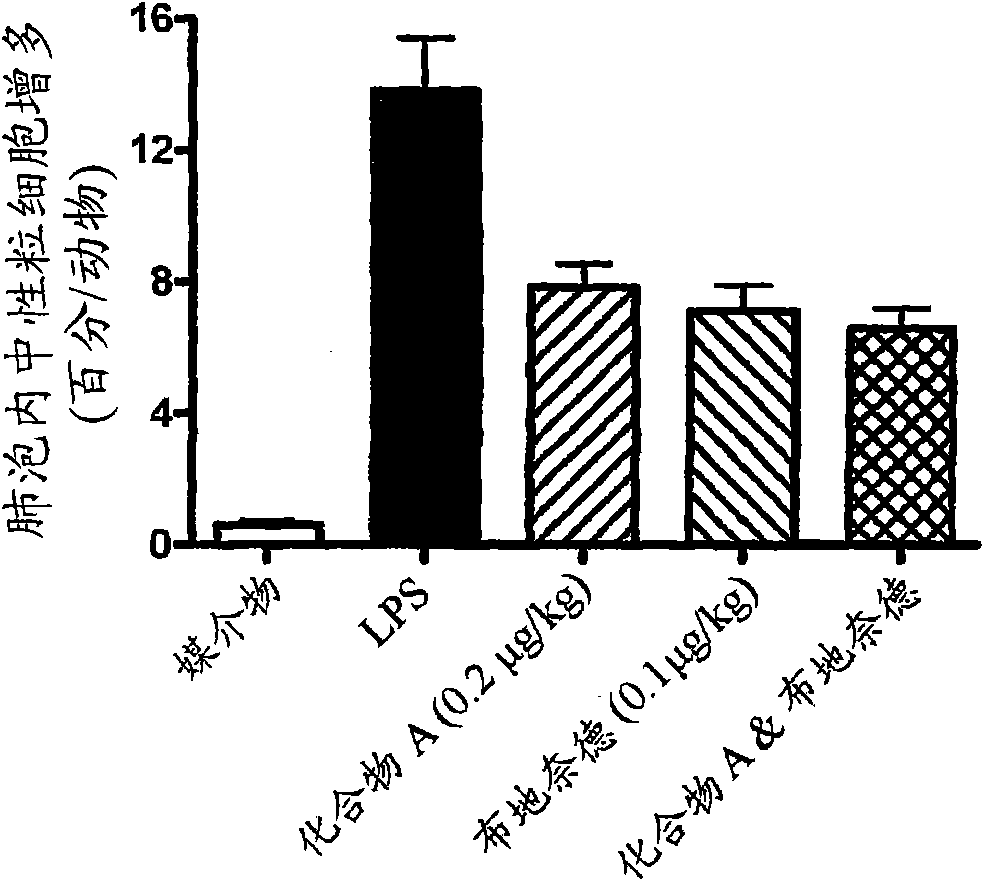
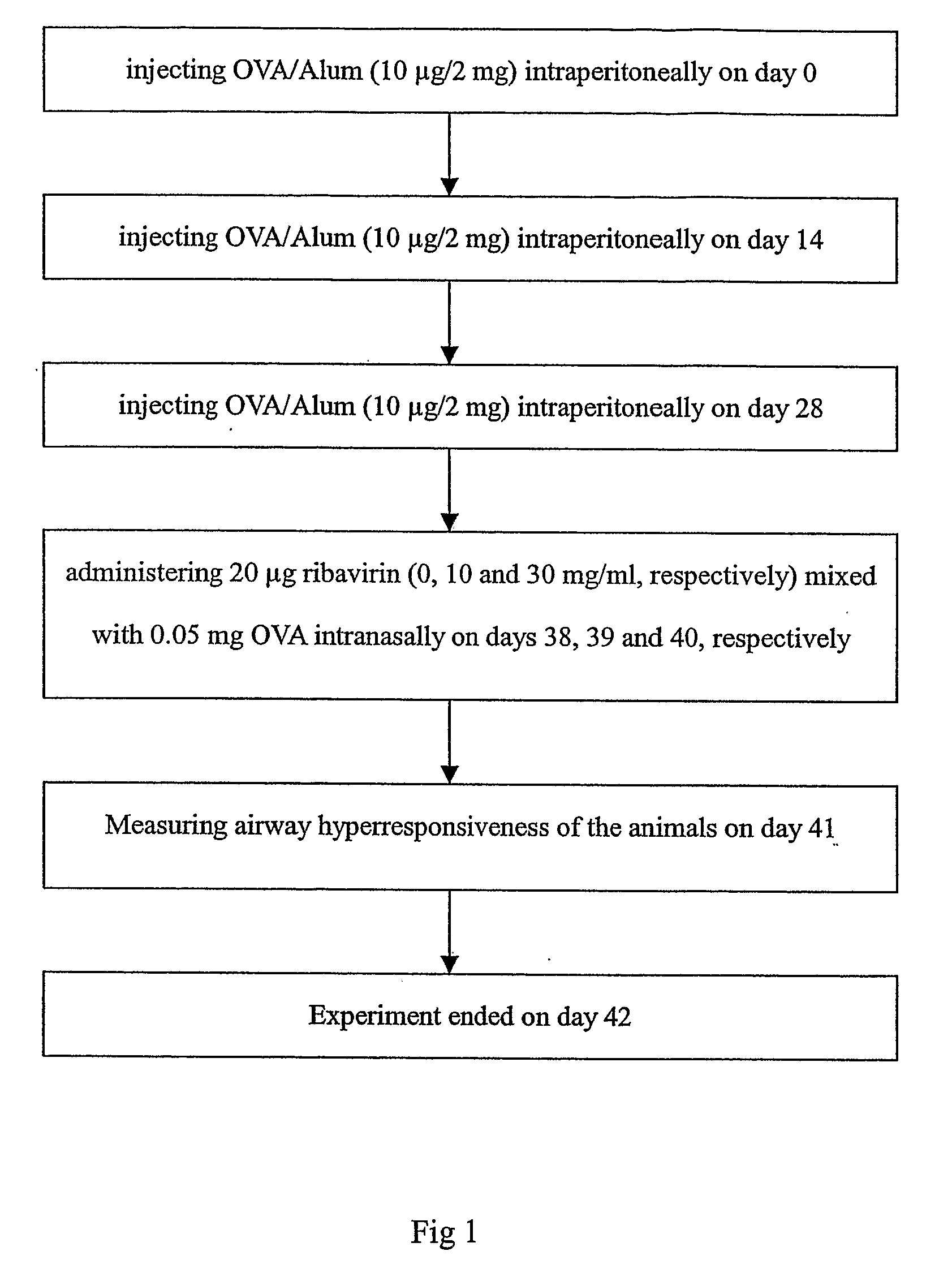
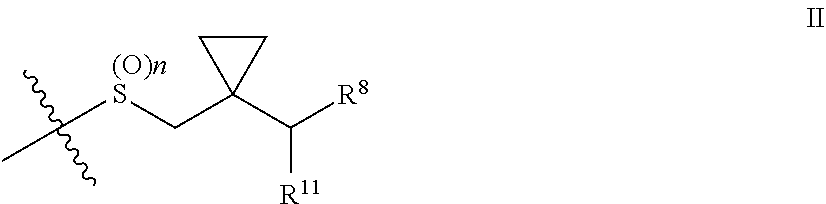
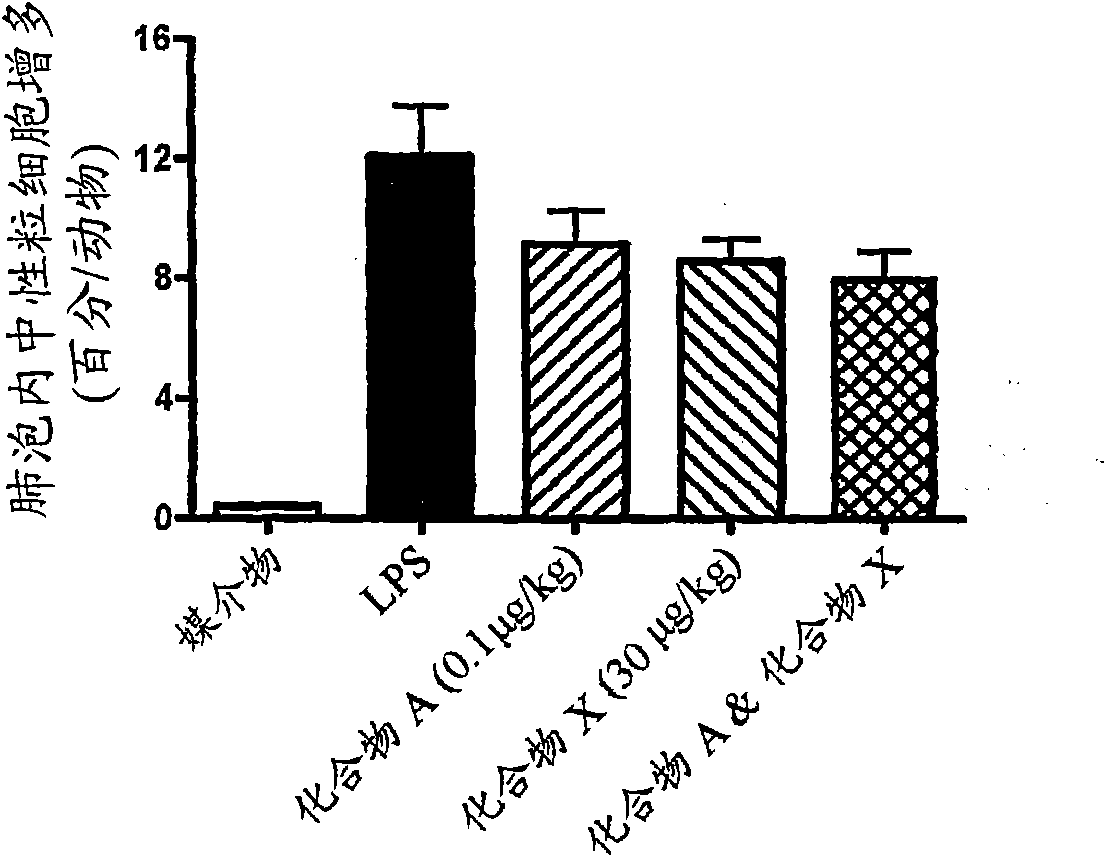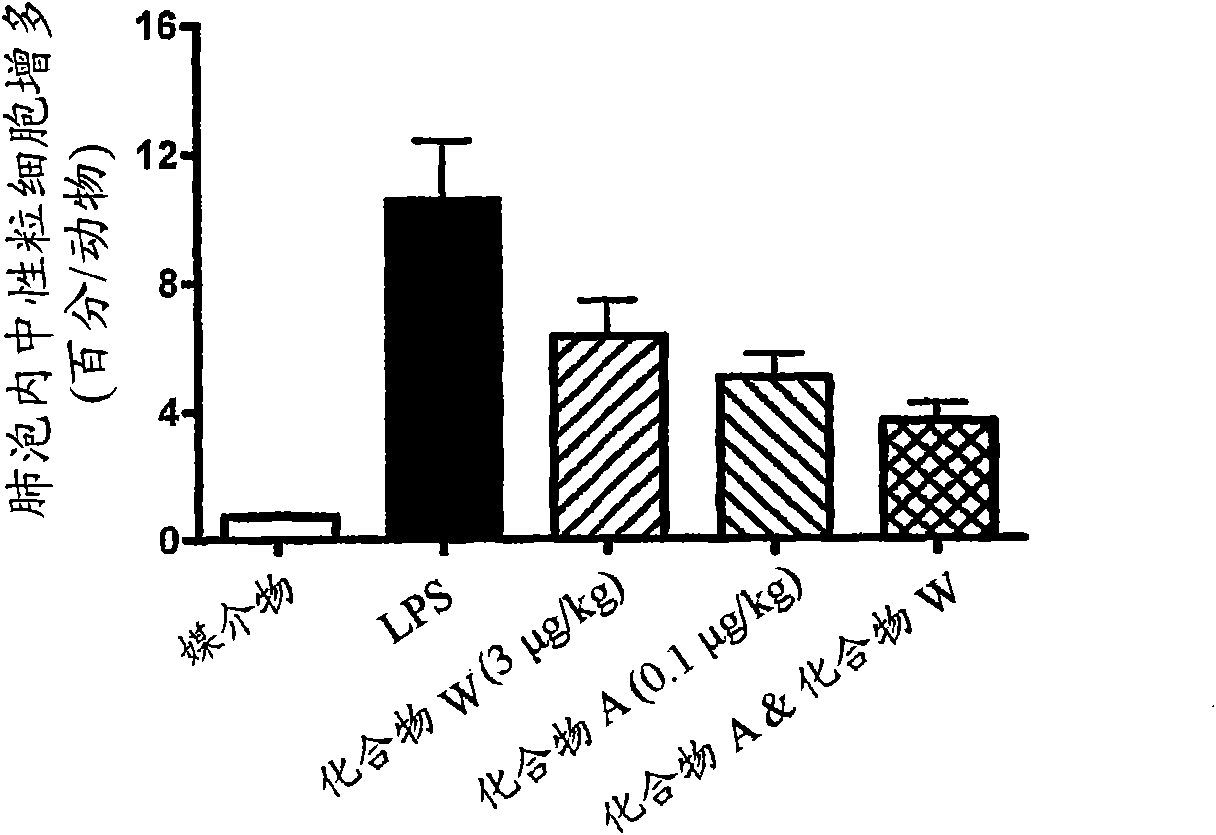Patents
Literature
50 results about "Leukotriene Receptor Antagonists" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Anti-asthma therapy
InactiveUS6224907B1Improve solubilityImprove bioavailabilityPowder deliveryPill deliverySurgeryReceptor antagonist
A dosage form is disclosed for administering a leukatriene-receptor antagonist to a patient over time.
Owner:ENCINAL PHARMA INVESTMENTS
Nanoparticulate leukotriene receptor antagonist/corticosteroid formulations
InactiveUS20070065374A1Useful in prophylaxis and chronic treatment of asthmaGood curative effectPowder deliveryBiocidePediatric patientPatient compliance
Nanoparticulate compositions comprising a corticosteroid and a leukotriene receptor antagonist are described. The compositions are useful in the prophylaxis and chronic treatment of asthma in adults and pediatric patients and for the relief of allergic conjunctivitis, symptoms of seasonal allergic rhinitis in adults and pediatric patients. Combining a leukotriene receptor antagonist with a corticosteroid in a particle size ranges of less than 2000 nm in a single formulation results in improved efficacy. In addition, patient compliance is enhanced since only one dosage form is needed. Furthermore, local administration of the leukotriene receptor antagonist results in less liver toxicity since the liver will be exposed to lower amounts of drug than happens following oral administration. The drug compositions according to the invention can be formulated into inhalation, nasal, or ocular formulations.
Owner:ELAN PHRMA INT LTD
Use of leukotriene receptor antagonist for treatment of scarring
InactiveUS20030162828A1Alleviate and eliminate scarAlleviate and eliminate and capsular contractureBiocideAnimal repellantsPsychiatryLeukotriene Receptor Antagonists
A method of preventing or treating either scarring or capsular contractures in subjects in need of such treatment comprising the administration of leukotriene receptor antagonists to said subject in need of treatment.
Owner:SCHLESINGER FAMILY PARTNERS A HAWAII
Indole compound and use thereof
InactiveUS7728023B2Increased airway hyperreactivityImprove respiratory functionBiocideSenses disorderClinical trialObstructive Pulmonary Diseases
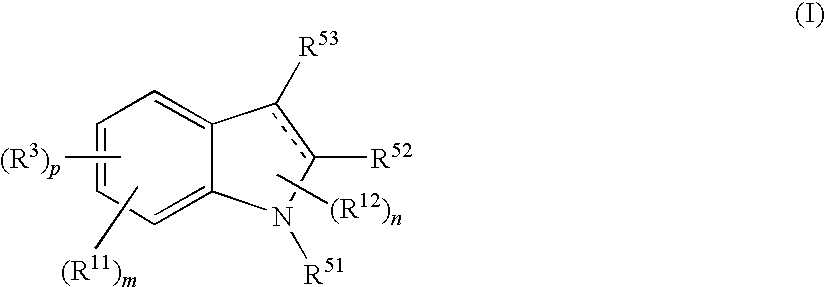
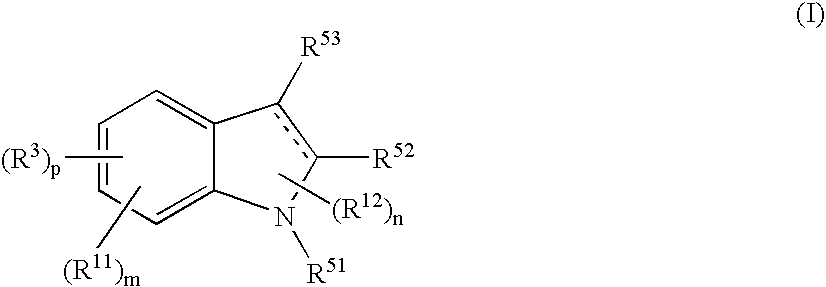
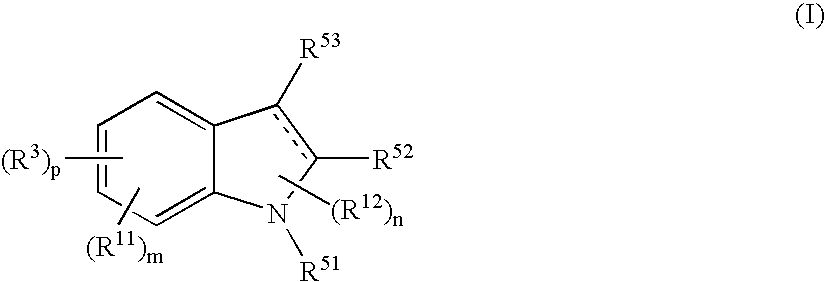
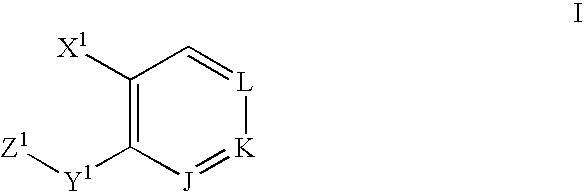
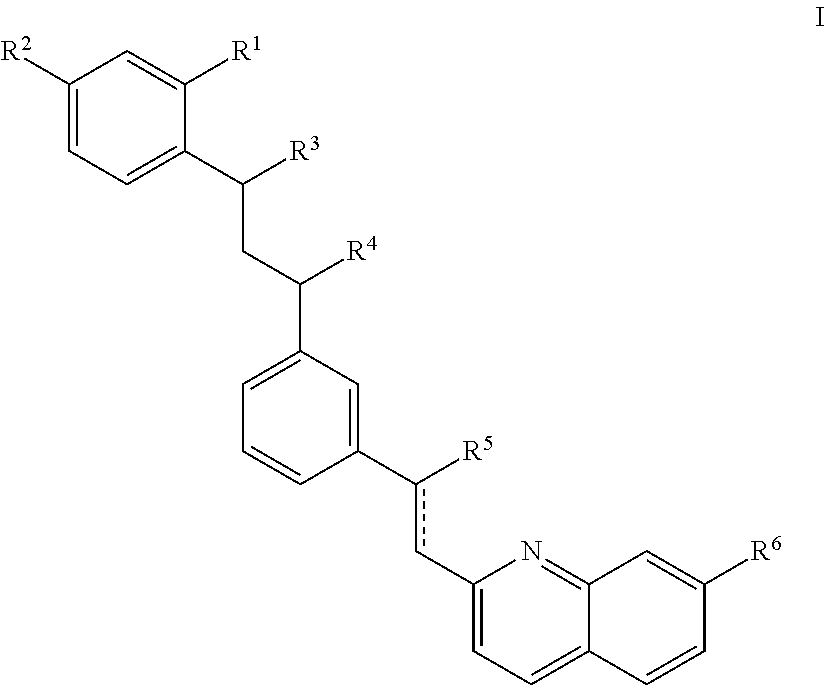
The present invention relates to a compound represented by the formula (I),wherein all symbols are as defined in the description,a salt thereof, a solvate thereof, or a prodrug thereof, which has a leukotriene receptor antagonistic activity which is expected to be more effective than those of the leukotriene receptor antagonists currently used in clinical trials. Therefore, it is useful as an agent for the prevention and / or treatment of a leukotriene-mediated disease such as a respiratory diseases such as bronchial asthma, chronic obstructive pulmonary disease, pulmonary emphysema, chronic bronchitis, pneumonia (e.g. interstitial pneumonia etc.), severe acute respiratory syndrome (SARS), acute respiratory distress syndrome (ARDS), allergic rhinitis, sinusitis (e.g. acute sinusitis, chronic sinusitis, etc.), or the like, or as an expectorant or an antiitussive.
Owner:ONO PHARMA CO LTD
Indole Compound and Use Thereof
InactiveUS20080188532A1Increased airway hyperreactivityImprove respiratory functionBiocideSenses disorderDiseaseBronchial epithelium
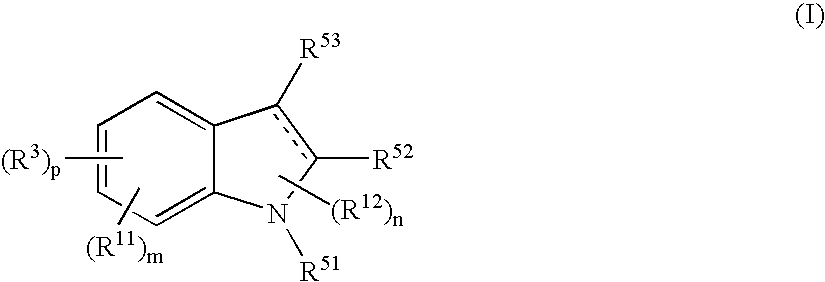
The present invention relates to a compound represented by the formula (I),wherein all symbols are as defined in the description,a salt thereof, a solvate thereof, or a prodrug thereof, which has a leukotriene receptor antagonistic activity which is expected to be more effective than those of the leukotriene receptor antagonists currently used in clinical trials. Therefore, it is useful as an agent for the prevention and / or treatment of a leukotriene-mediated disease such as a respiratory diseases such as bronchial asthma, chronic obstructive pulmonary disease, pulmonary emphysema, chronic bronchitis, pneumonia (e.g. interstitial pneumonia etc.), severe acute respiratory syndrome (SARS), acute respiratory distress syndrome (ARDS), allergic rhinitis, sinusitis (e.g. acute sinusitis, chronic sinusitis, etc.), or the like, or as an expectorant or an antiitussive.
Owner:ONO PHARMA CO LTD
Medicine response assay in respiratory disease
InactiveUS6939674B2Raise the possibilityMinimize the possibilitySugar derivativesMicrobiological testing/measurementLeukotriene synthaseDisease
Correlations between polymorphisms in the 5-lipoxygenase gene, or polymorphisms in the leukotriene C4 synthase gene, and a subject's phenotypic response to treatment with a leukotriene receptor antagonist for respiratory disease are described. Methods of screening subjects to aid in treatment, and methods of screening therapeutic compounds, are presented.
Owner:SMITHKLINE BECKMAN CORP
Compound oral preparation containing antibacterial drugs
InactiveCN104208699ARespiratory disorderPharmaceutical active ingredientsCurative effectTrace Amounts
The invention relates to the components and applications of a compound oral preparation containing antibacterial drugs. When a trace amount of an antibacterial drug is compounded with a drug for treating asthma and chronic obstructive pneumonia, a good effect will be generated. The curative effect is more prominent, when an antibacterial drug is compounded with a leukotriene acceptor antagonist to form an oral compound preparation.
Owner:TIANJIN JINYAO GRP
Method of treating acne
A method of treating inflammatory skin diseases and / or hair loss, comprising administering to a patient in need of such treatment a therapeutically effective amount of a leukotriene receptor antagonist, an antihistamine, or other anti-inflammatory drug, preferably at least twice a day, preferably for at least two months.
Owner:SANDERS JR RICHARD J
Combination of dehydroepiandrosterone or dehydroepiandrosterone-sulfate with a leukotriene receptor antagonist for treatment of asthma or chronic obstructive pulmonary disease
InactiveUS20050026882A1Alleviate different aspectAdvantage of savingBiocidePowder deliveryDiseaseDehydroepiandrosterone sulfate
A pharmaceutical or veterinary composition, comprises a first active agent selected from a dehydroepiandrosterone and / or dehydroepiandrosterone-sulfate, or a salt thereof, and a second active agent comprising a leukotriene receptor antagonist for the treatment of asthma, chronic obstructive pulmonary disease, or any other respiratory disease. The composition is provided in various formulations and in the form of a kit. The products of this patent are applied to the prophylaxis and treatment of asthma, chronic obstructive pulmonary disease, or any other respiratory disease.
Owner:EPIGENESIS PHARMA LLC
Treatment methods of cognitive, emotional and mental ailments and disorders
Methods for the treatment of cognitive, emotional and mental ailments using therapeutically effective amounts of compositions including leukotriene receptor antagonists, leukotriene synthesis inhibitors or leukotriene modifiers, zafirlukasts, montelukasts, other members of the family -lukasts, zileutons.
Owner:SCHULTZ JACK WILLIAM
Use of leukotriene receptor antagonist for treatment of scarring
A method of preventing or treating either scarring or capsular contractures in subjects in need of such treatment comprising the administration of leukotriene receptor antagonists to said subject in need of treatment.
Owner:SCHLESINGER FAMILY PARTNERS A HAWAII
Substituted aryl compounds as novel cyclooxygenase-2 selective inhibitors, compositions and methods of use
InactiveUS20050059665A1Unexpected potential for facilitating wound healingHave antiinflammatory propertiesBiocideSenses disorderHydrolase inhibitorThromboxanes
The invention describes novel substituted aryl compounds that are cyclooxygenase 2 (COX-2) selective inhibitors and novel compositions comprising at least one cyclooxygenase 2 (COX-2) selective inhibitor, and, optionally, at least one compound that donates, transfers or releases nitric oxide, stimulates endogenous synthesis of nitric oxide, elevates endogenous levels of endothelium-derived relaxing factor or is a substrate for nitric oxide synthase, and / or, optionally, at least one therapeutic agent, such as, steroids, nonsterodal anti-inflammatory compounds (NSAID), 5-lipoxygenase (5-LO) inhibitors, leukotriene B4 (LTB4) receptor antagonists, leukotriene A4 (LTA4) hydrolase inhibitors, 5-HT agonists, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) inhibitors, H2 antagonists, antineoplastic agents, antiplatelet agents, thrombin inhibitors, thromboxane inhibitors, decongestants, diuretics, sedating or non-sedating anti-histamines, inducible nitric oxide synthase inhibitors, opioids, analgesics, Helicobacter pylori inhibitors, proton-pump-inhibitors, isoprostane inhibitors, and mixtures thereof. The invention also provides novel kits comprising at least one COX-2 selective inhibitor, and, optionally, at least one nitric oxide donor, and / or, optionally, at least one therapeutic agent. The novel cyclooxygenase 2 selective inhibitors of the invention can be optionally nitrosated and / or nitrosylated. The invention also provides methods for treating inflammation, pain and fever; for treating and / or improving the gastrointestinal properties of COX-2 selective inhibitors; for facilitating wound healing; for treating and / or preventing renal toxicity or other toxicities; for treating and / or preventing other disorders resulting from elevated levels of cyclooxygenase-2; and for improving the cardiovascular profile of COX-2 selective inhibitors.
Owner:NICOX SA
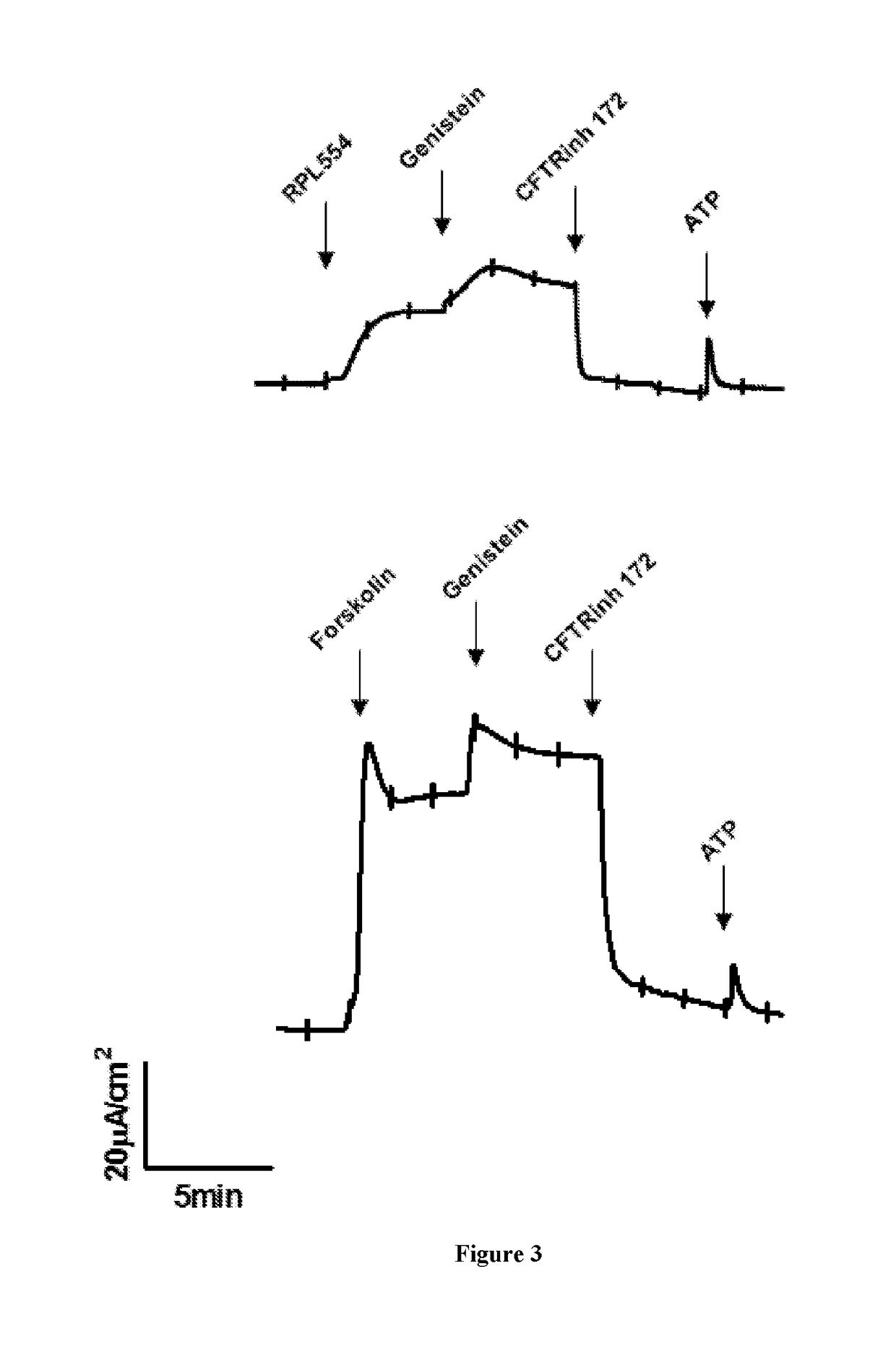
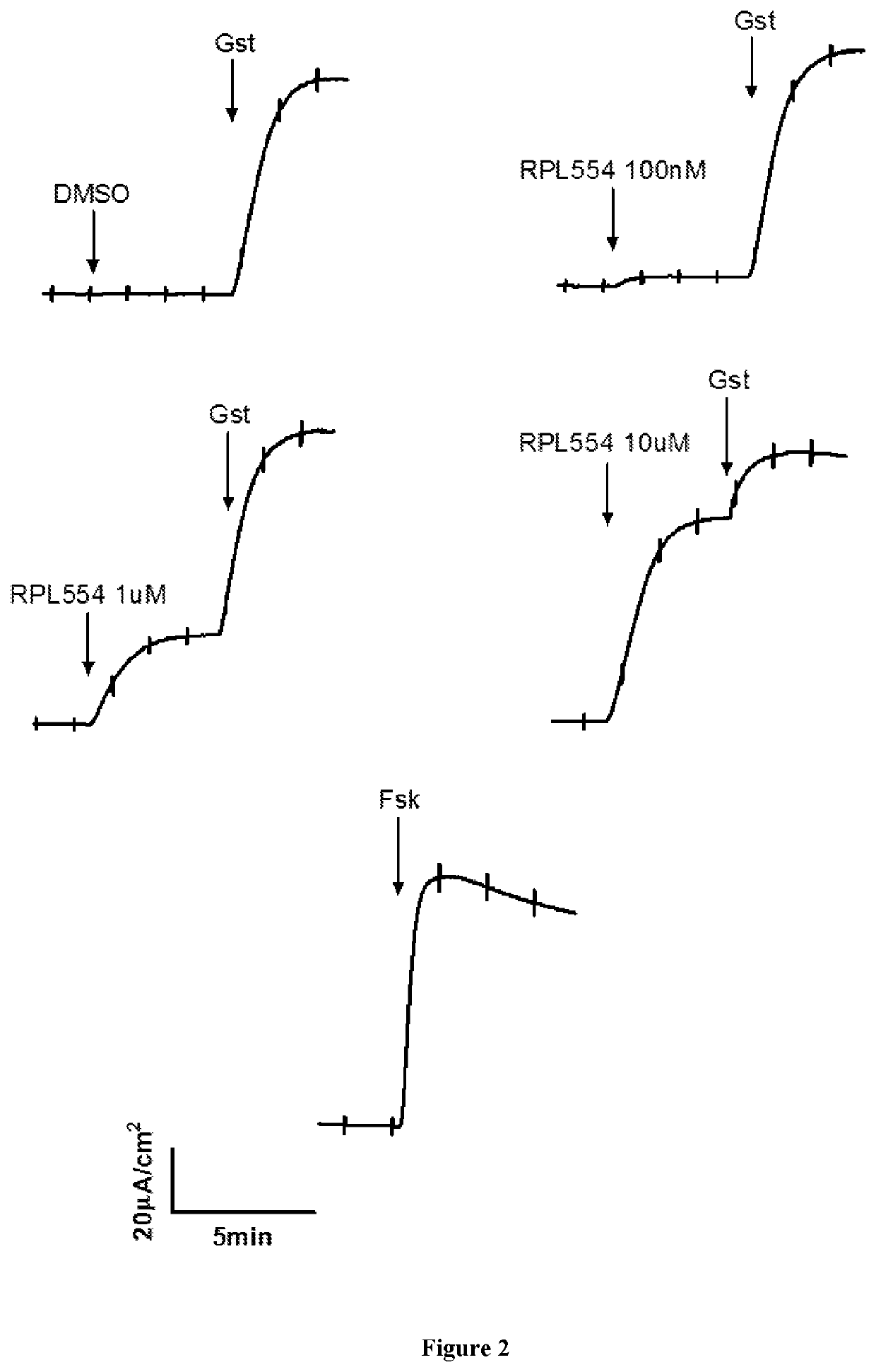
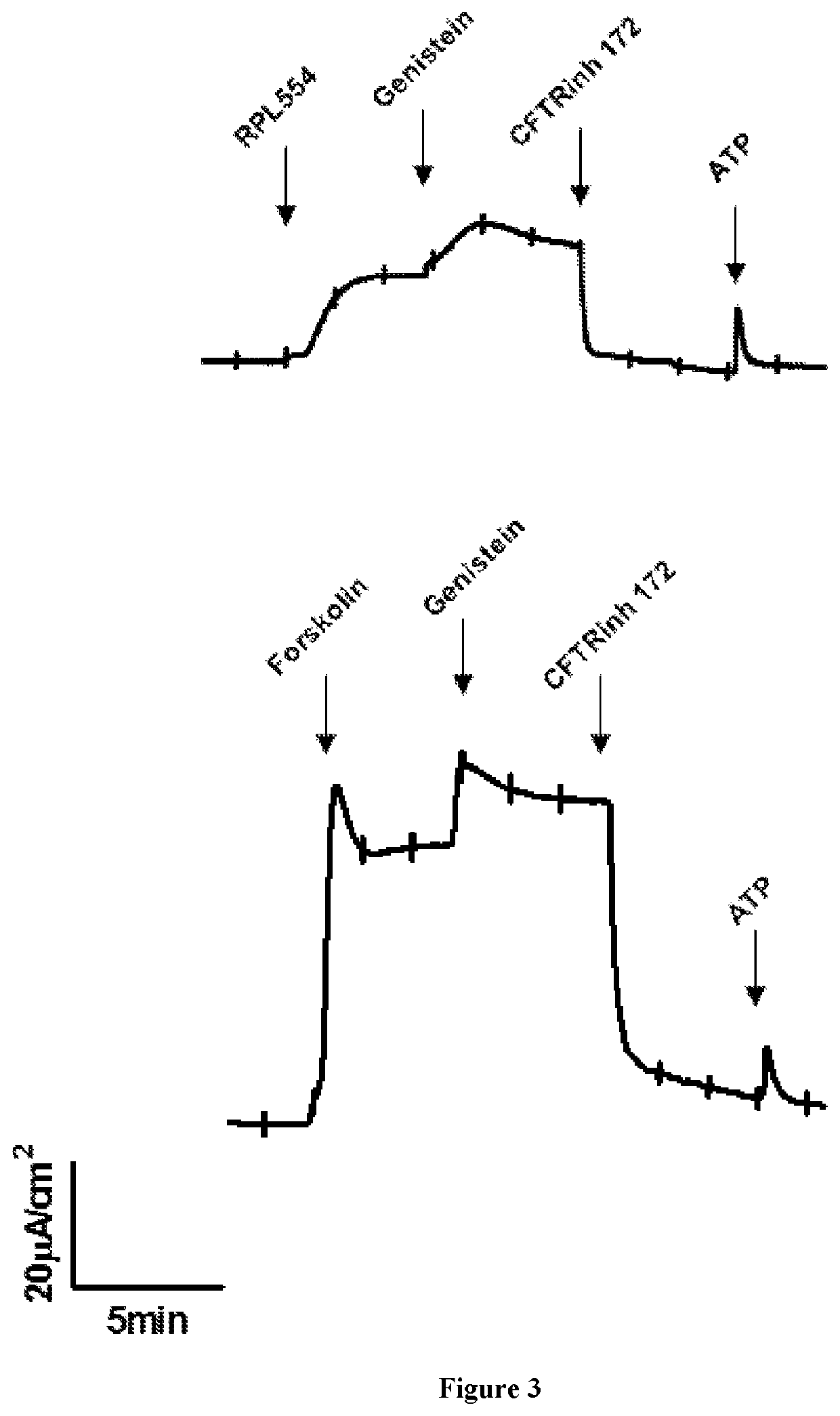
New treatment
The invention provides a compound for use in treating or preventing a disease or condition selected from cystic fibrosis, chronic obstructive pulmonary disease (COPD), asthma, mild pulmonary disease, bronchitis, bronchiectasis, idiopathic bronchiectasis, allergic bronchopulmonary aspergillosis, sinusitis, rhinosinusitis, CFTR-related metabolic syndrome (CRMS), pancreatitis, idiopathic chronic pancreatitis and Sjörgren's syndrome, or for use in preventing male infertility caused by congenital absence of the vas deferens, in a patient by modulating CFTR activity, which compound is 9,10-dimethoxy-2-(2,4,6-trimethylphenylimino)-3-(N-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2H-pyrimido[6,1-a]isoquinolin-4-one or a pharmaceutically acceptable acid addition salt thereof. The invention also provides a composition comprising (i) 9,10-dimethoxy-2-(2,4,6-trimethylphenylimino)-3-(N-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2H-pyrimido[6,1-a]isoquinolin-4-one or a pharmaceutically acceptable acid addition salt thereof and (ii) a leukotriene receptor antagonist. The invention also provides a composition comprising (i) 9,10-dimethoxy-2-(2,4,6-trimethylphenylimino)-3-(N-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2H-pyrimido[6,1-a]isoquinolin-4-one or a pharmaceutically acceptable acid addition salt thereof and (ii) a CFTR potentiator or a CFTR corrector.
Owner:VERONA PHARMA
Novel inhalant containing glucocorticoid and bronchodilator
ActiveCN102247597AImprove stabilityIncreased lung deposition rateGranular deliveryRespiratory disorderStimulantGlucocorticoid
The invention discloses a novel inhalant containing glucocorticoid and bronchodilator, that is, inhalable particles containing glucocorticoid and other medicine, wherein, the other medicine is selected from one of the following medicines: beta 2 receptor stimulant, anticholinergic agent, theophylline agent, leukotriene receptor antagonist, mast cell stabilizer and antihistamine.
Owner:TIANJIN JINYAO GRP
Topical and injectable formulations comprising leukotriene receptor antagonist and uses thereof
Methods of treating, preventing, reducing the occurrence of, or slowing progression of folliculitis, partial or full hair loss, thinning of the hair, changes in the texture of hair, graying or whitening (loss of pigmentation) of the hair, dermatological conditions, and other hair-related conditions, comprising administering topical and injectable formulations containing one or more leukotriene receptor antagonists.
Owner:SCHLESINGER LARRY S
Method of treating hair loss
Owner:SANDERS JR RICHARD J
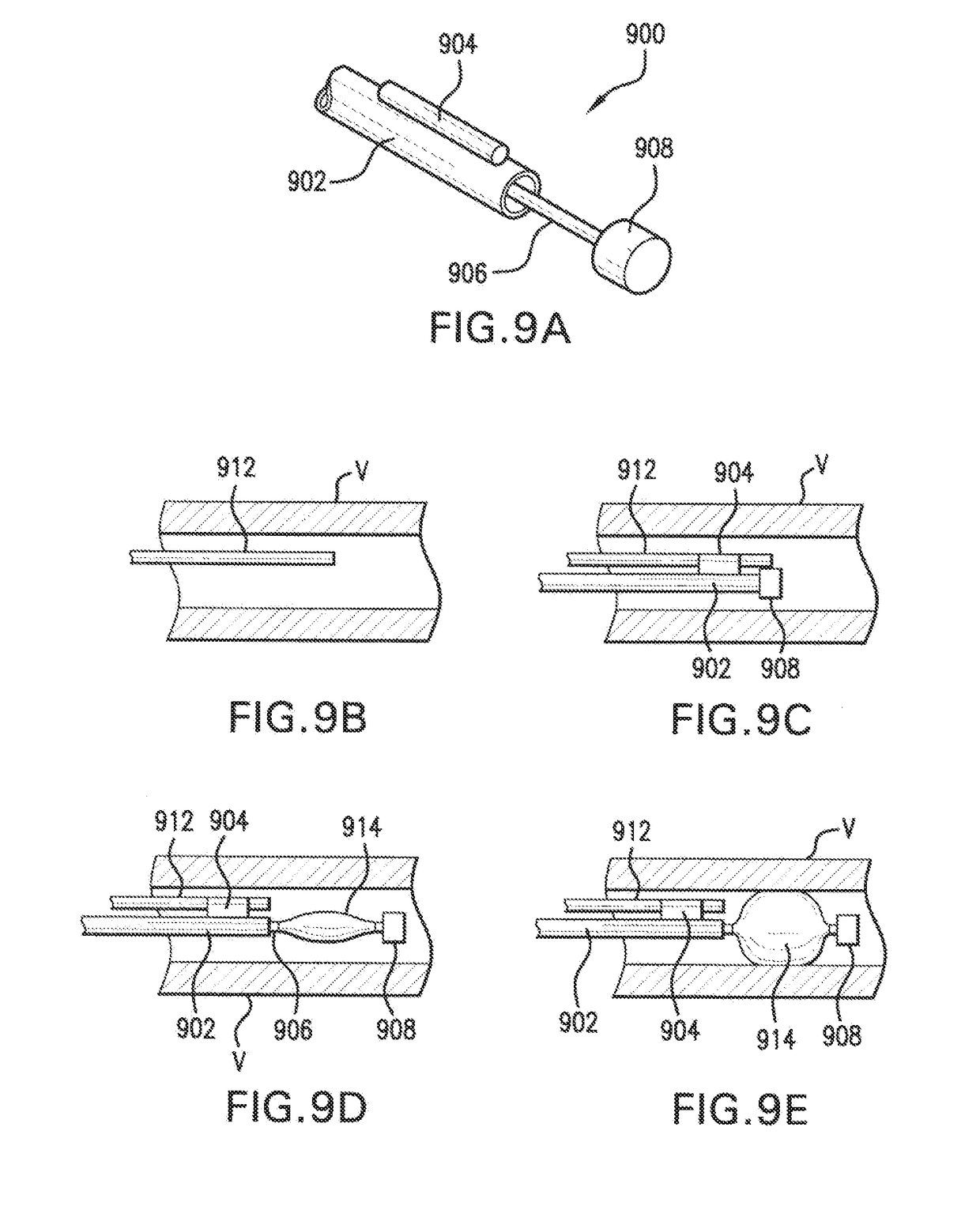
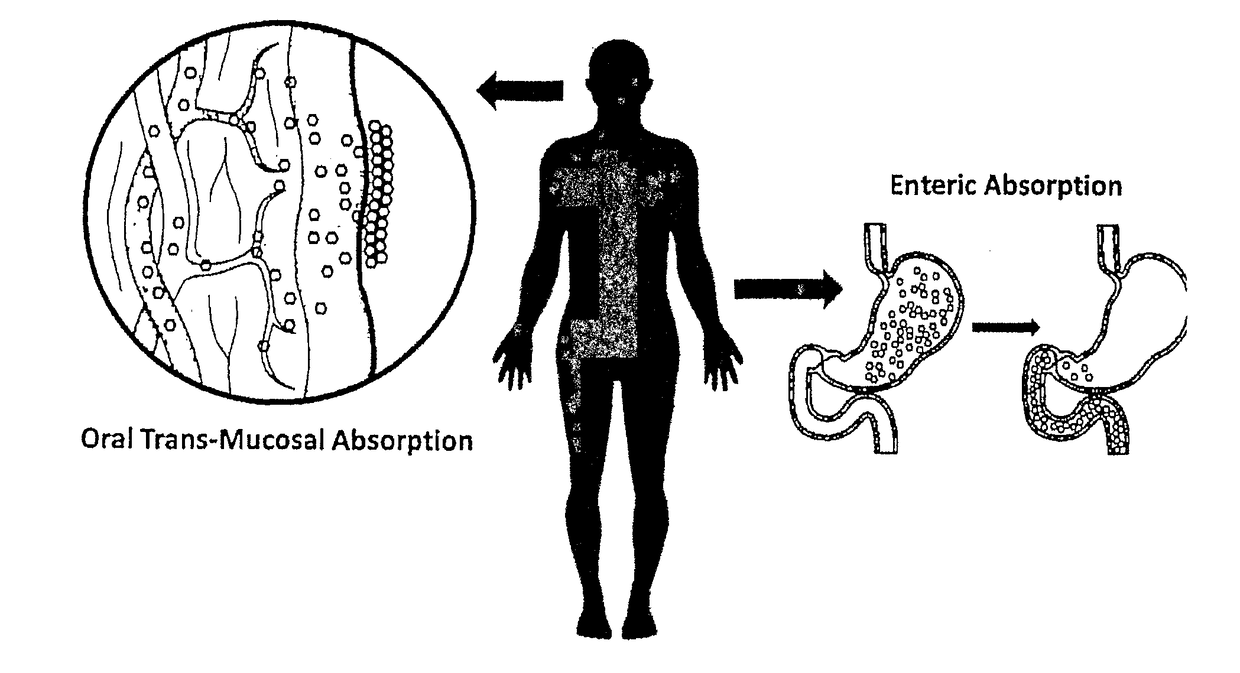
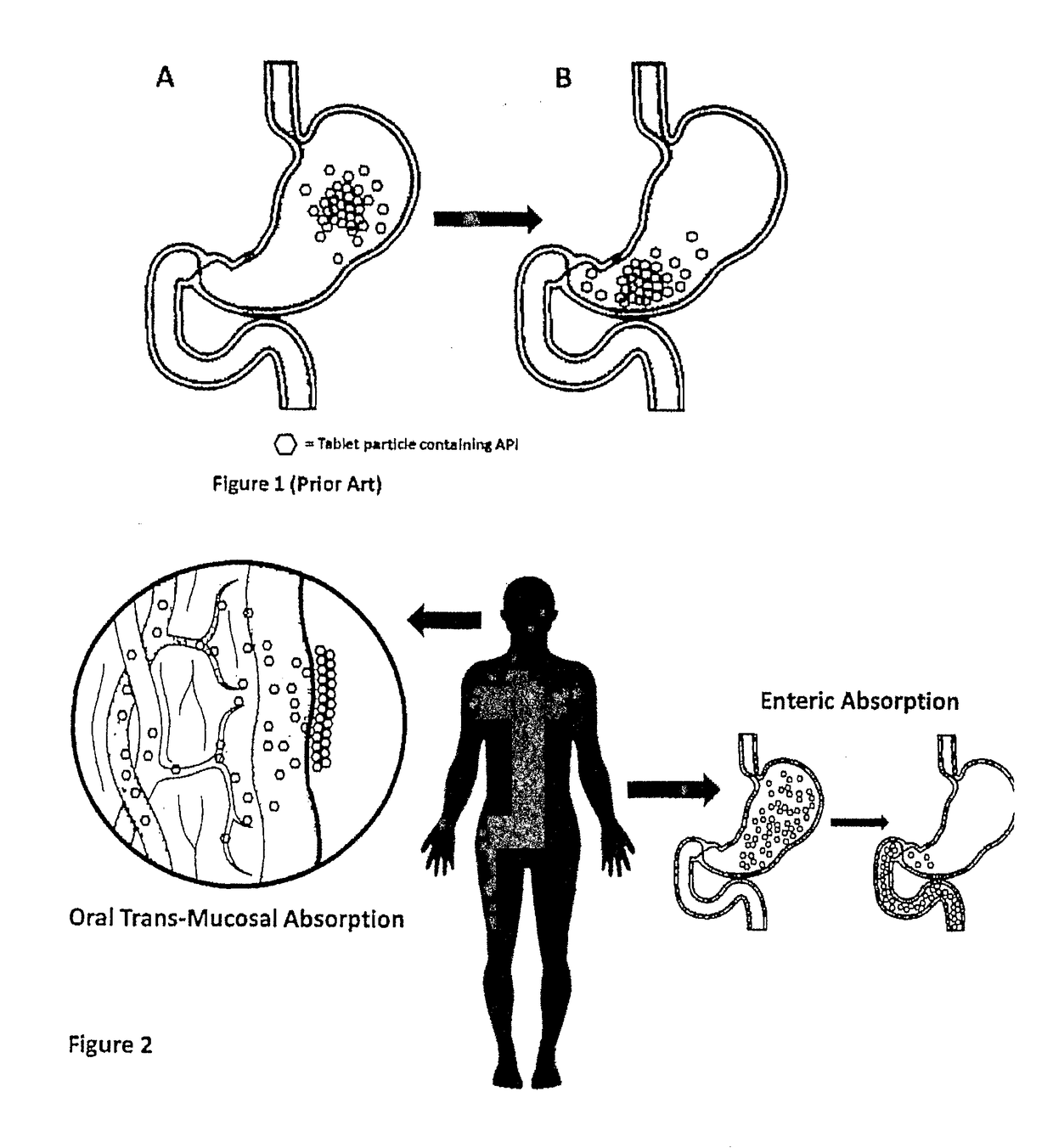
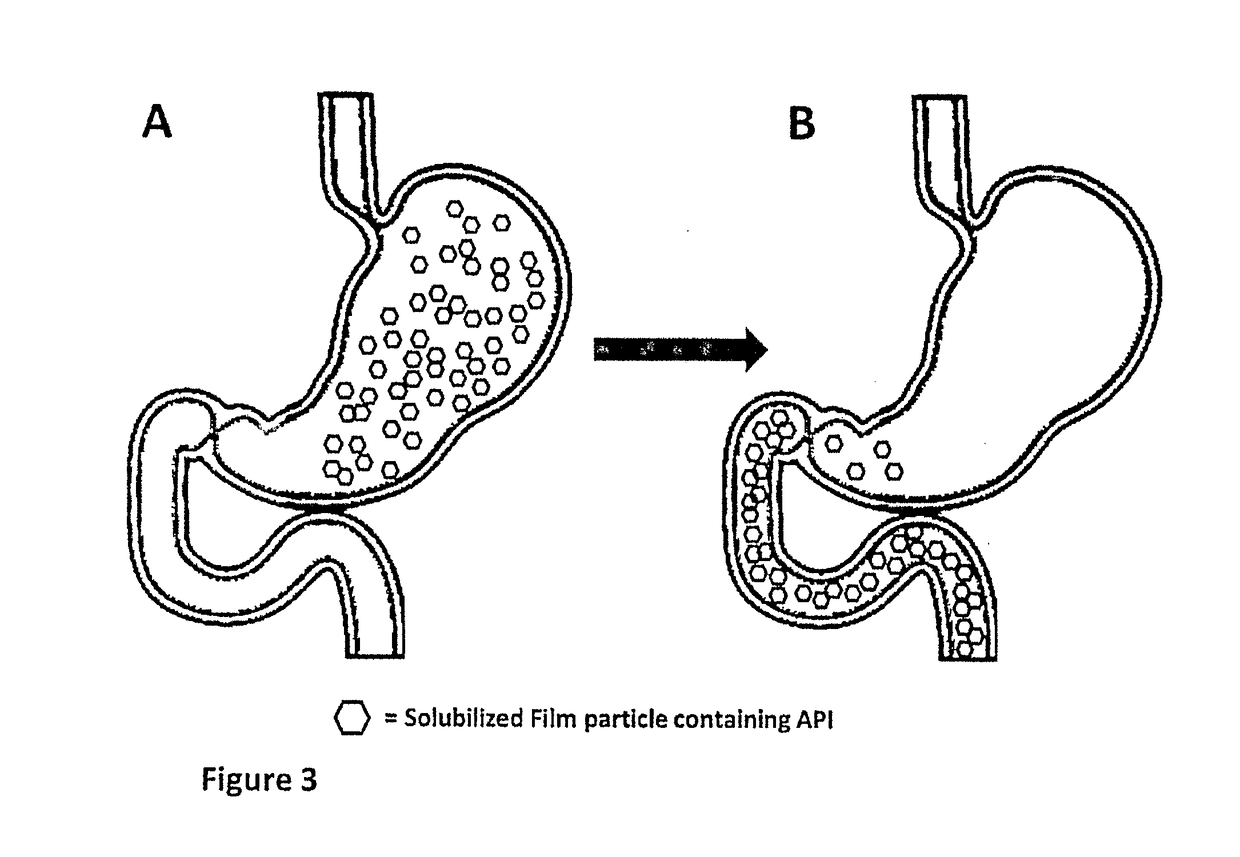
Method of treatment and device for the improved bioavailability of leukotriene receptor antagonists
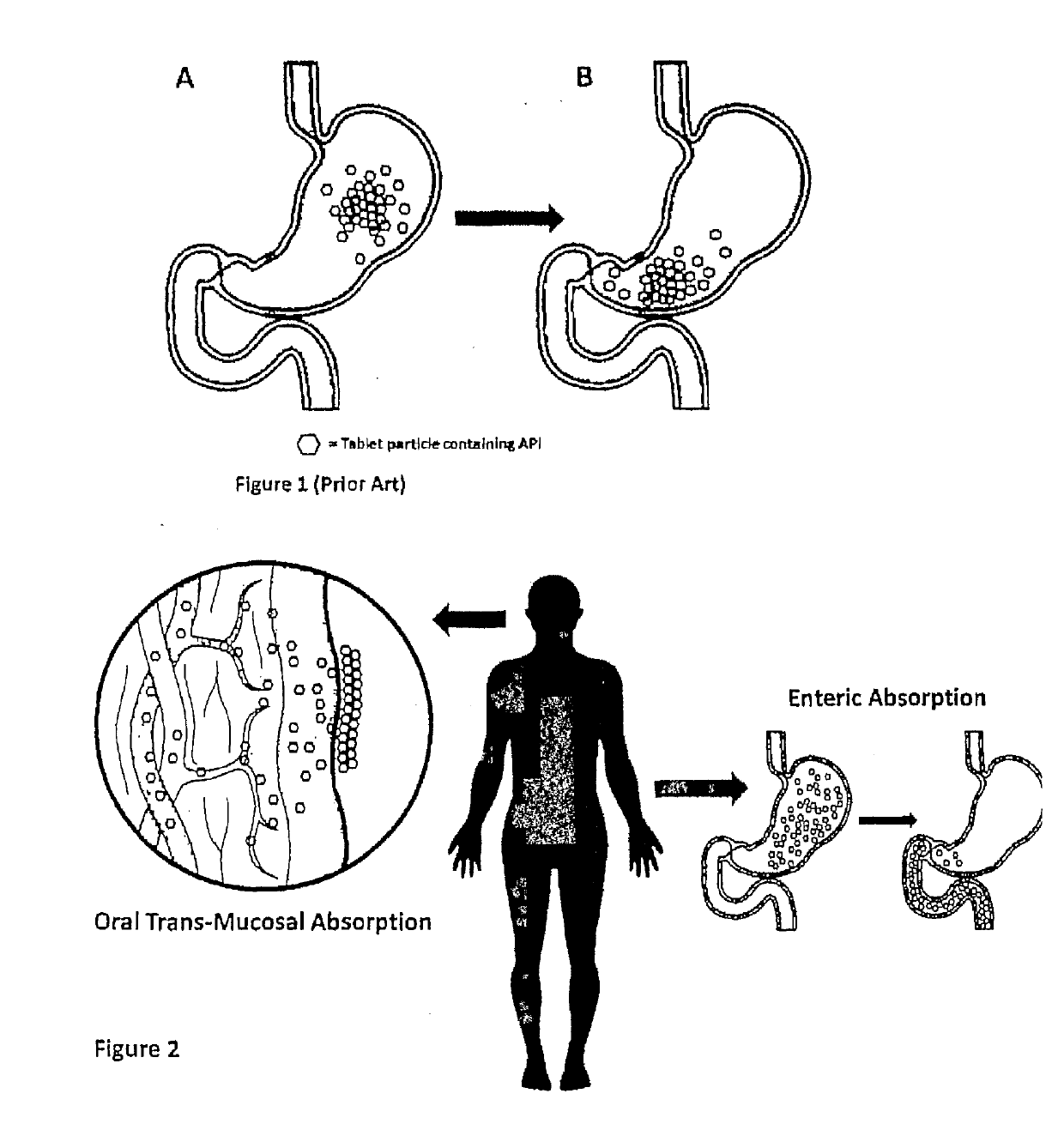
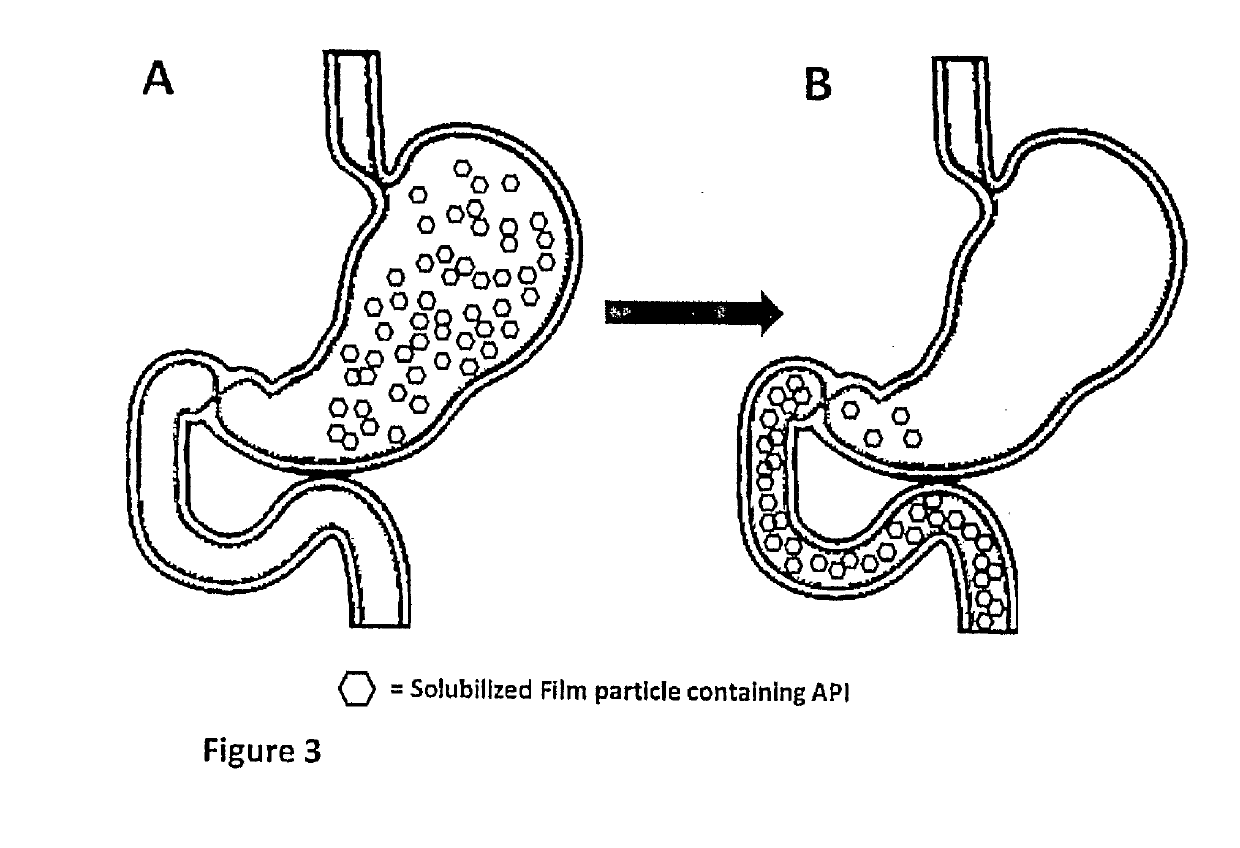
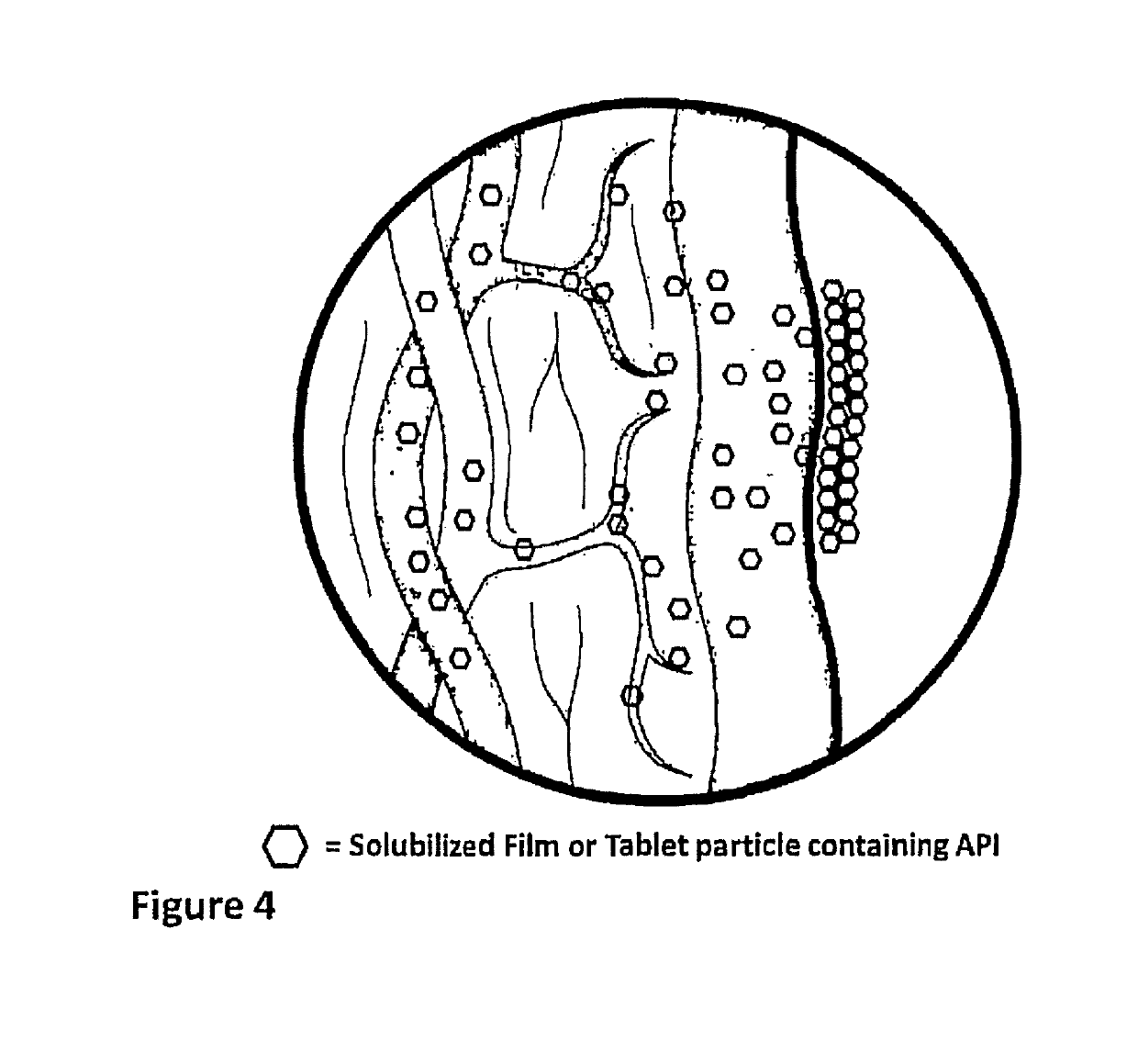
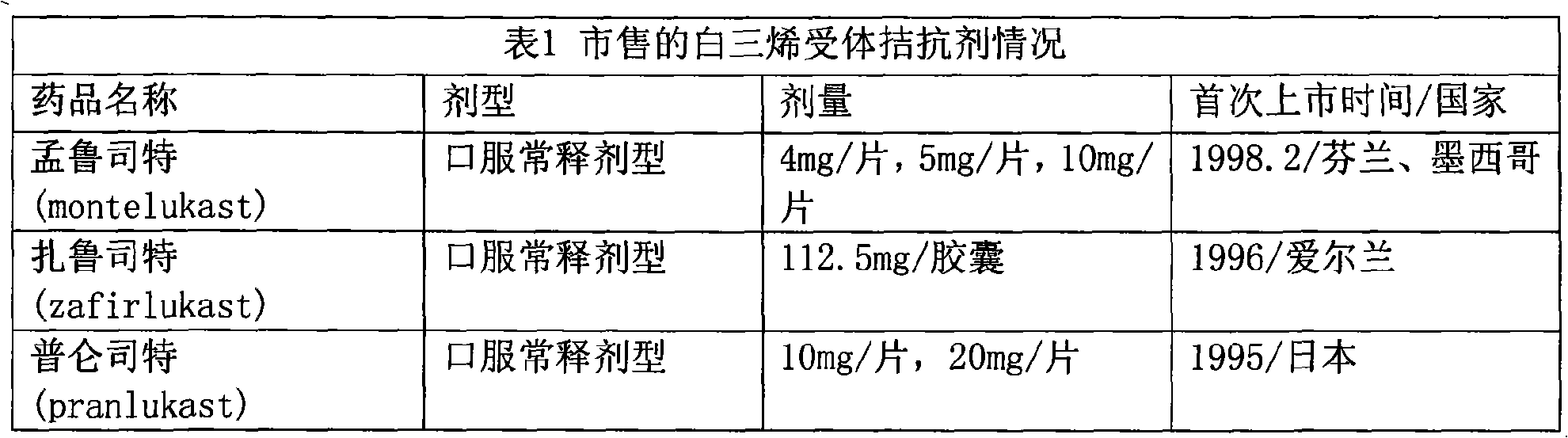
InactiveUS20190133925A1Improve bioavailabilityInorganic non-active ingredientsPharmaceutical delivery mechanismDiseaseFilm Dosage Form
Disclosed is a method of administration and device for the improved bioavailability of leukotriene receptor antagonists. This method and device involve an alkaline surface pH oral film dosage form designed to deliver leukotriene receptor antagonists, such as Montelukast, to the stomach in an amorphous precipitate suspended in aqueous medium. Also disclosed is a device and method for treating a disease, such as a neurodegenerative disease or condition associated with neuroinflammation induced by a leukotriene. The device is a film unit dosage form having an alkaline surface pH film layer and a safe and effective amount of Montelukast. The device is configured and formulated to predominantly achieve enteral delivery of the Montelukast. The method includes enterally delivering to a human or an animal in need of treatment, a safe and effective amount of Montelukast capable of crossing the blood-brain barrier.
Owner:INTELGENX CORP
Medicament composition for treating respiratory disease
InactiveCN101347618AReduce the incidence of side effectsReduce systemic adverse reactionsAerosol deliveryRespiratory disorderDiseaseRespiratory disease
The invention provides a pharmaceutical composition for the treatment of respiratory diseases, in particular asthma, rhinitis or COPD and especially asthma. The composition consists of one or a plurality of LTRAs as active ingredients and one or a plurality of pharmaceutical accessories suitable for respirable aerosol and powder and irrespirable aerosol, pressurized spray and powder.
Owner:天津药业集团有限公司
Nanoparticulate leukotriene receptor antagonist/corticosteroid formulations
InactiveCN101175480AImprove convenienceImprove compliancePowder deliverySenses disorderNasal cavityPediatric patient
Nanoparticulate compositions containing corticosteroids and leukotriene receptor antagonists are described. The composition is used for prevention and long-term treatment of asthma in adults and children, and for relieving symptoms of allergic conjunctivitis and seasonal allergic rhinitis in adults and children. Combining a leukotriene receptor antagonist in the particle size range of less than 2000nm with a corticosteroid in one formulation resulted in increased potency. Additionally, patient compliance is enhanced since only one dosage form is required. Furthermore, topical administration of leukotriene receptor antagonists results in lower hepatotoxicity, since the liver will be exposed to a smaller amount of drug than would be the case after oral administration. The pharmaceutical compositions of the present invention may be formulated for inhalation, nasal or ophthalmic use.
Owner:ELAN PHRMA INT LTD
Methods of inducing TH-1 immune responses to HIV-1 by administering UV/psoralen-treated desialated inactiviated HIV-1 virions deficient in CD55 and CD59
Administration protocols for a fusion protein, matrix protein and psoralen inactivated HIV based immunogenic composition that induces an immune response to HIV. The immunogenic compositions are based on HIV biologically active fusion peptide, matrix peptide, or psoralen inactivated HIV. The number of doses is 3X. The starting dose for an adult is 1x109-1x1010. The starting dose for an adolescent is ½(1x109-1x1010). The starting dose for a pediatric patient is ¼(1x109-1x1010). The second dose will consist of 1 / 10th of starting concentrations. The third dose will consist of 1 / 100th of starting concentrations. This will facilitate a Th-1 response. The days of administration are days 1; 30; and 180. Alternatively the days of administration are days 1; 20-40; and 160-200. The site of administration is one that targets lymphatic tissue. Adjuvant is administered before, simultaneous with or after each dose of the immunogenic compositions. Adjuvants are used to promote a Th-1 immune response and include a leukotriene receptor antagonist such as Montelukast, a mast cell and basophil stabilizer such as Cromolyn, and a prostaglandin synthetase inhibitor such as Indomethacin. Th-1 immune responses to the immunogenic compositions are monitored. The 3X cycle will repeat on until a Th-1 immune response is observed. At that point, the immunogenic composition administered could then decline by a factor of 10 for two more vaccination procedures.
Owner:KARP NELSON M
Treatment
The invention provides a compound for use in treating or preventing a disease or condition selected from cystic fibrosis, chronic obstructive pulmonary disease (COPD), asthma, mild pulmonary disease, bronchitis, bronchiectasis, idiopathic bronchiectasis, allergic bronchopulmonary aspergillosis, sinusitis, rhinosinusitis, CFTR-related metabolic syndrome (CRMS), pancreatitis, idiopathic chronic pancreatitis and Sjörgren's syndrome, or for use in preventing male infertility caused by congenital absence of the vas deferens, in a patient by modulating CFTR activity, which compound is 9,10-dimethoxy-2-(2,4,6-trimethylphenylimino)-3-(N-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2H-pyrimido[6,1-a]isoquinolin-4-one or a pharmaceutically acceptable acid addition salt thereof. The invention also provides a composition comprising (i) 9,10-dimethoxy-2-(2,4,6-trimethylphenylimino)-3-(N-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2H-pyrimido[6,1-a]isoquinolin-4-one or a pharmaceutically acceptable acid addition salt thereof and (ii) a leukotriene receptor antagonist. The invention also provides a composition comprising (i) 9,10-dimethoxy-2-(2,4,6-trimethylphenylimino)-3-(N-carbamoyl-2-aminoethyl)-3,4,6,7-tetrahydro-2H-pyrimido[6,1-a]isoquinolin-4-one or a pharmaceutically acceptable acid addition salt thereof and (ii) a CFTR potentiator or a CFTR corrector.
Owner:VERONA PHARMA
Free-standing biodegradable patch
InactiveUS20190070342A1Increase flexibilityImprove mechanical propertiesPowder deliveryBalloon catheterMonoclonal antibodyTherapeutic effect
Methods and apparatus for a free-standing biodegradable patch suitable for medical applications, especially nasal applications are provided, wherein the patch comprises a free-standing film containing at least one therapeutic agent selected from corticosteroids, antihistamines, monoclonal antibodies, leukotriene receptor antagonists, anti-inflammatory cytokines, vasoconstrictive agents, and any combination thereof. The patch may take a non-adherent form during delivery to a target location within a vessel or tissue, and thereafter may be activated to adhere to vessel wall or tissue, and release the therapeutic content in a sustained fashion. The patch may be micronized and administered in a powdered form.
Owner:BIOINSPIRE TECH
Method of treatment and device for the improved bioavailability of leukotriene receptor antagonists
InactiveUS20180250240A1Improve bioavailabilityPharmaceutical non-active ingredientsSheet deliveryDiseaseBioavailability
Disclosed is a method of administration and device for the improved bioavailability of leukotriene receptor antagonists. This method and device involve an alkaline surface pH oral film dosage form designed to deliver leukotriene receptor antagonists, such as Montelukast, to the stomach in an amorphous precipitate suspended in aqueous medium. Also disclosed is a device and method for treating a disease, such as a neurodegenerative disease or condition associated with neuroinflammation induced by a leukotriene. The device is a film unit dosage form having an alkaline surface pH film layer and a safe and effective amount of Montelukast. The device is configured and formulated to predominantly achieve enteral delivery of the Montelukast. The method includes enterally delivering to a human or an animal in need of treatment, a safe and effective amount of Montelukast capable of crossing the blood-brain barrier.
Owner:INTELGENX CORP
Combinations of beta- 2 -adrenoceptor agonistic benzothiazolone
The invention provides a pharmaceutical product comprising a first active ingredient which is N-[2-(Diethylamino)ethyl]-N-(2-{[2-(4-hydroxy-2-oxo-2,3-dihydro-1,3-benzothiazol-7-yl)ethyl]amino}ethyl)-3-[2-(1-naphthyl)ethoxy]propan amide or a salt thereof, and a second active ingredient selected from: a non-steroidal Glucocorticoid Receptor (GR Receptor) Agonist; an antioxidant; a CCR1 antagonist; achemokine antagonist (not CCR1); a corticosteroid; a CRTh2 antagonist; a DP1 antagonist; an Histone Deacetylase Inducer; an IKK2 inhibitor; a COX inhibitor; a lipoxygenase inhibitor; a leukotriene receptor antagonist; an MPO inhibitor; a muscarinic antagonist which is Aclidinium bromide, Glycopyrrolate, Oxitropium bromide, Pirenzepine, telenzepine, Tiotropium bromide,3(R)-(2-hydroxy-2,2-dithien-2-ylacetoxy)-1-(3-phenoxypropyl)-1-azoniabicyclo[2.2.2]octane bromide,3(R)-1-phenethyl-3-(9H-xanthene-9-carbonyloxy)-1-azoniabicyclo[2.2.2]octane bromide or (3R)-3-[(2S)-2-cyclopentyl-2-hydroxy-2-thien-2-ylacetoxy]-1-(2-phenoxyethyl)-1-azoniabicyclo[2.2.2]actane bromide; a p38 inhibitor; a PDE inhibitor; a PPARy agonist; a protease inhibitor; a Statin; a thromboxane antagonist; a vasodilator; or, an ENAC blocker (Epithelial Sodium-channel blocker); and its use in the treatment of respiratory disease.
Owner:ASTRAZENECA AB
Treatment methods of cognitive, emotional and mental ailments and disorders
Methods for the treatment of cognitive, emotional and mental ailments using therapeutically effective amounts of compositions including leukotriene receptor antagonists, leukotriene synthesis inhibitors or leukotriene modifiers, zafirlukasts, montelukasts, other members of the family—lukasts, zileutons.
Owner:SCHULTZ JACK WILLIAM
Method and Composition for Treating Allergic Diseases
A method and composition for treating allergic diseases and / or airway inflammation including pollinosis, bronchial asthma, allergic rhinitis, atopic dermatitis and anaphylactic shock with the administration to a subject an amount of an anti-infectious agent such as ribavirin and optionally combined with an anti-inflammatory agent selected from inhaled steroids, leukotriene receptor antagonists and beta-2 receptor agonist.
Owner:WHOLESOME BIOPHARM
New formulations containing leukotriene receptor antagonists
PendingUS20220105082A1Wide range of activitiesLess side effectsPowder deliveryDispersion deliveryAtopic dermatitisPharmaceutical formulation
There is provided pharmaceutical formulations that may be used topically comprising a leukotriene receptor antagonist, a salt or a solvate thereof. Particular leukotriene receptor antagonists that may be mentioned include montelukast styrene. The formulations find particular utility in direct topical administration for the treatment of inflammation, of inflammatory disorders and / or of condition characterized by inflammation, including wounds, burns, psoriasis, haemorrhoids, acne and atopic dermatitis.
Owner:ENLITISA (SHANGHAI) PHARMACEUTICAL CO LTD
Combination of dehydroepiandrosterone or dehydroepiandrosterone-sulfate with a leukotriene receptor antagonist for treatment of asthma or chronic obstructive pulmonary disease
InactiveUS20090317476A1Effectively prophylacticallyEffectively therapeutically treatPowder deliveryBiocideDiseaseDehydroepiandrosterone sulfate
A pharmaceutical or veterinary composition, comprises a first active agent selected from a dehydroepiandrosterone and / or dehydroepiandrosterone-sulfate, or a salt thereof, and a second active agent comprising a leukotriene receptor antagonist for the treatment of asthma, chronic obstructive pulmonary disease, or other respiratory diseases. The composition is provided in various formulations and in the form of a kit. The products of this patent are applied to the prophylaxis and treatment of asthma, chronic obstructive pulmonary disease, or other respiratory diseases.
Owner:EPIGENESIS PHARMA LLC
Compositions and methods for treating proliferative diseases
InactiveUS20120178782A1Treating and preventing psoriasisBiocidePill deliveryAcyl Coenzyme A SynthetasesAcyl-CoA synthetase
Synergistic pharmaceutical compositions and the methods for preventing and treating proliferative diseases such as cancer and psoriasis. The compositions comprise synergistic combinations of: (i) an acyl-CoA-synthetase enzyme inhibitor (AcsI4), (ii) a compound having an inhibiting effect on enzymes with cyclooxygenase activity (COX); and (iii) a compound selected from a 5-lypoxygenase enzyme (LOX-5) inhibitor and a leukotriene receptor antagonist.
Owner:PODESTA ERNESTO JORGE
Antitumor agent
Owner:TOCHIGI INST OF CLINICAL PATHOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com