Patents
Literature
475 results about "Bronchial epithelium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bronchial epithelium is a key element of the respiratory airways. It constitutes the interface between the environment and the host. It is a physical barrier with many chemical and immunological properties. The bronchial epithelium is abnormal in asthma, even in children.
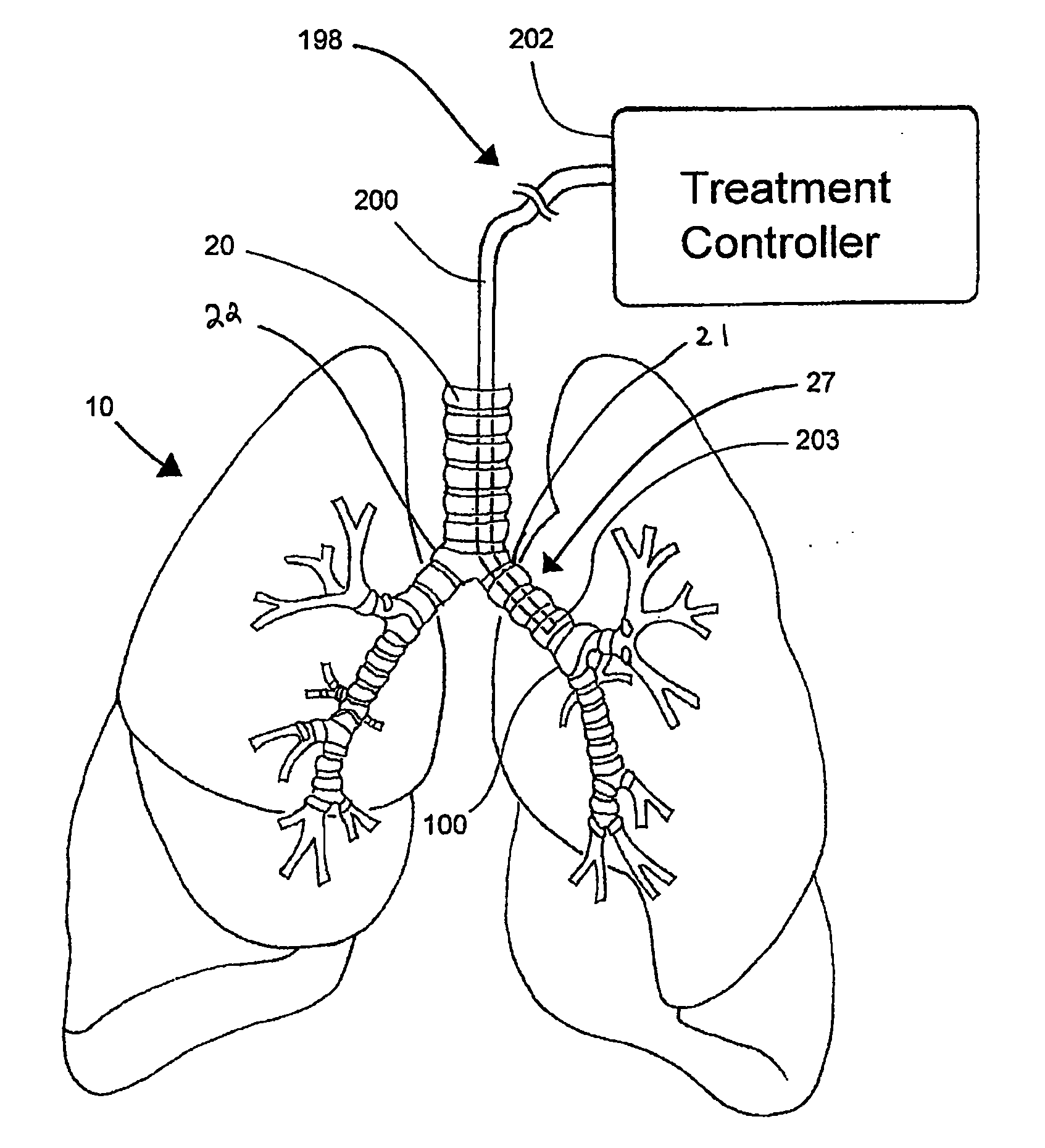
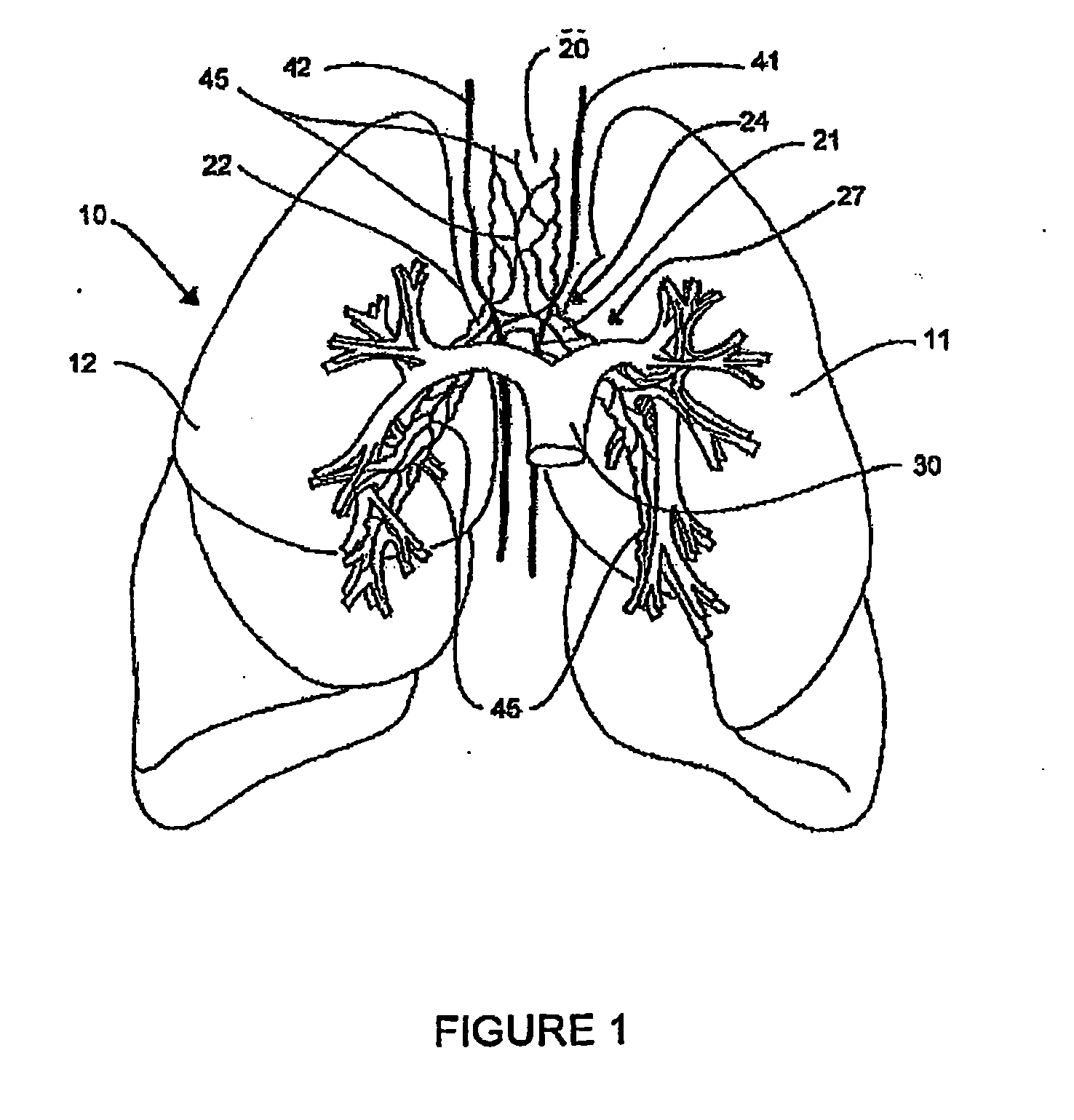
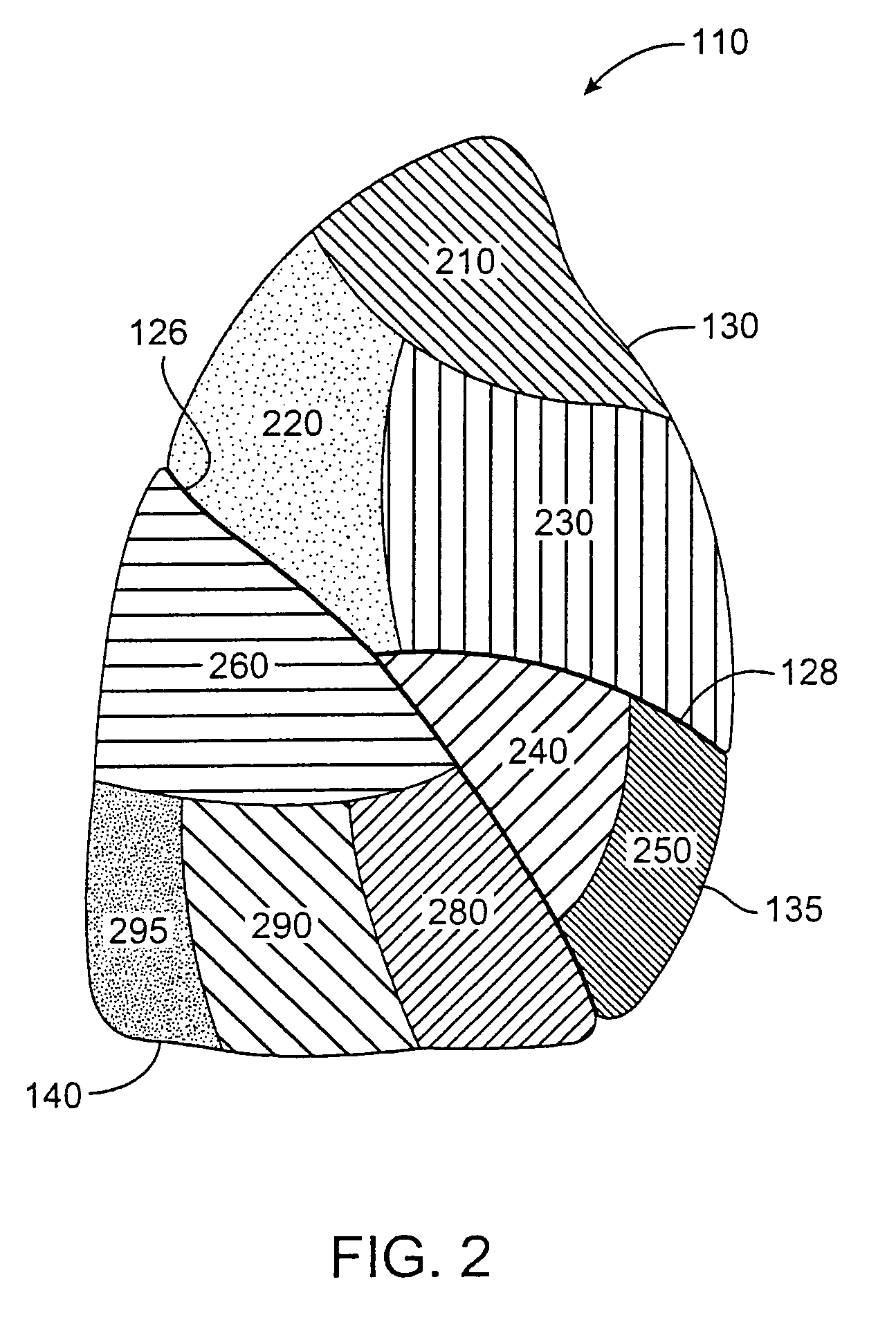
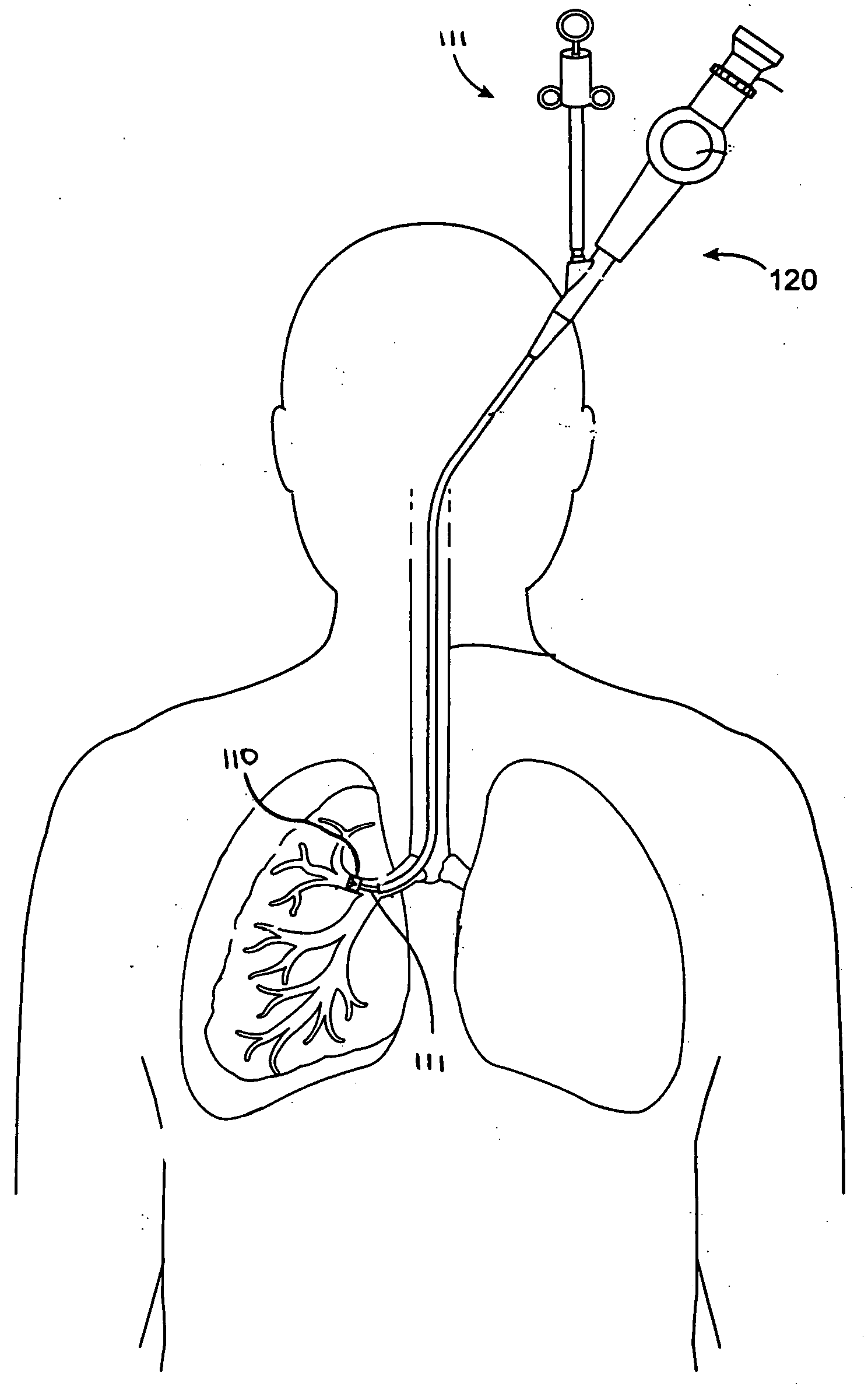
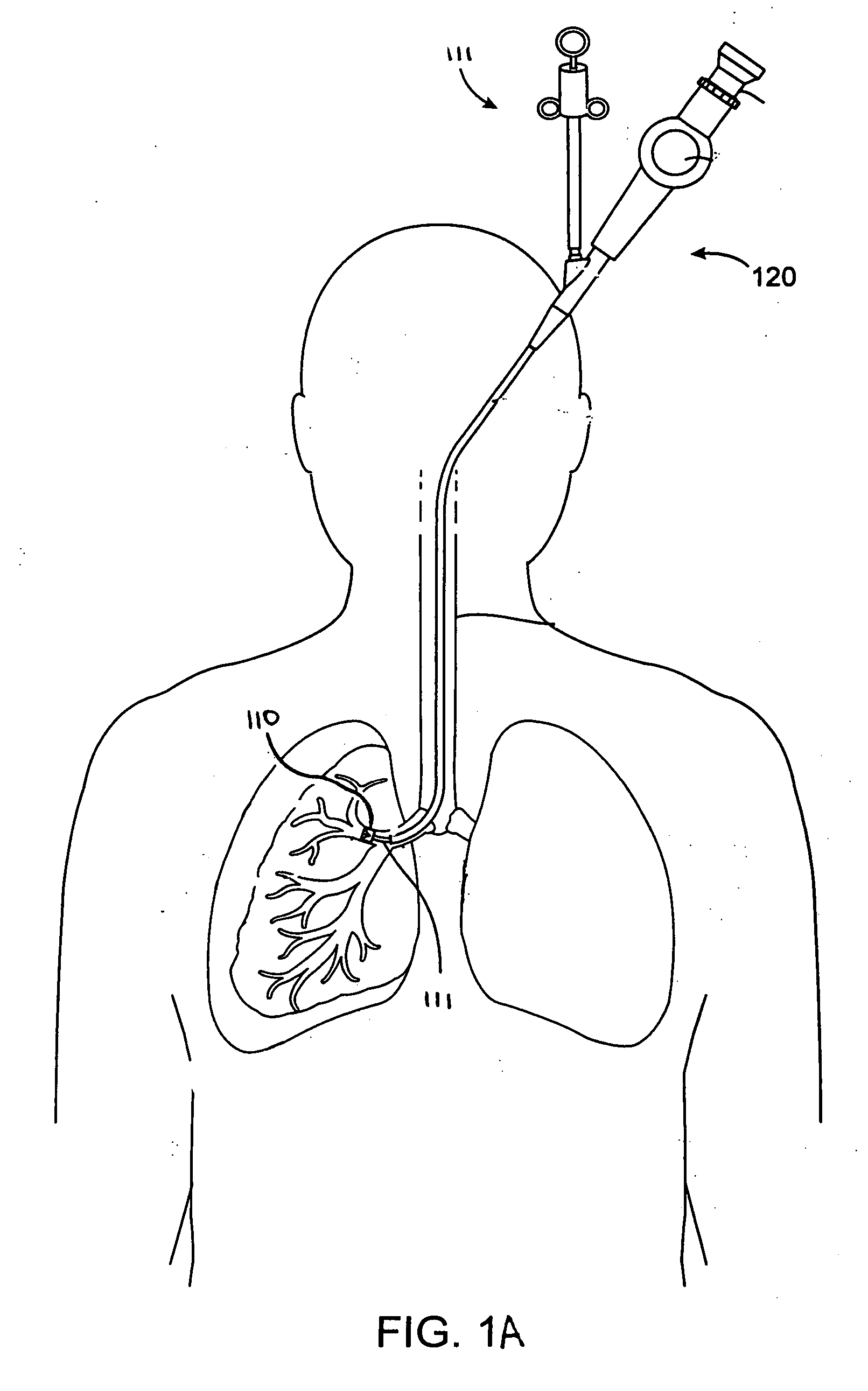
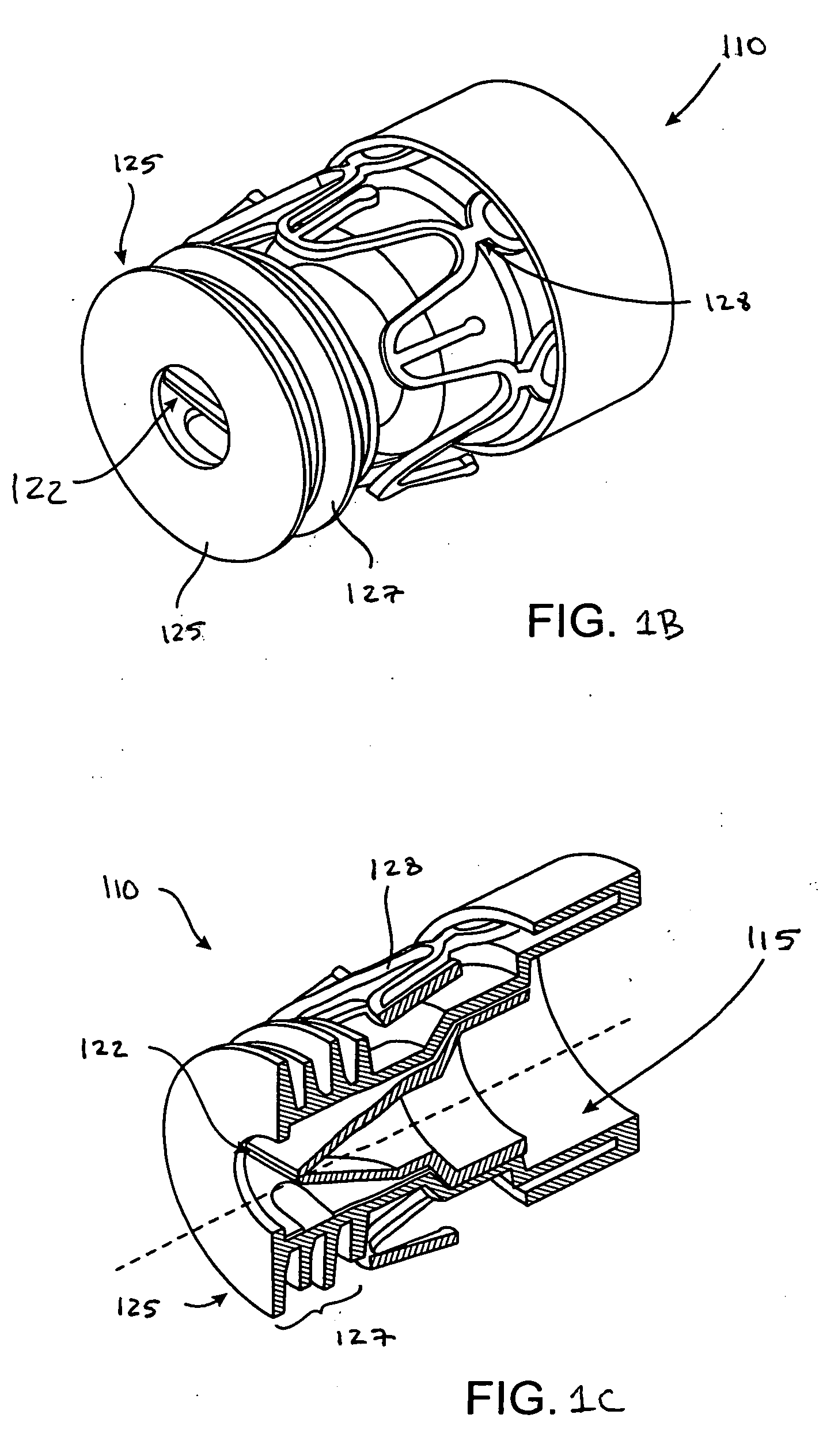
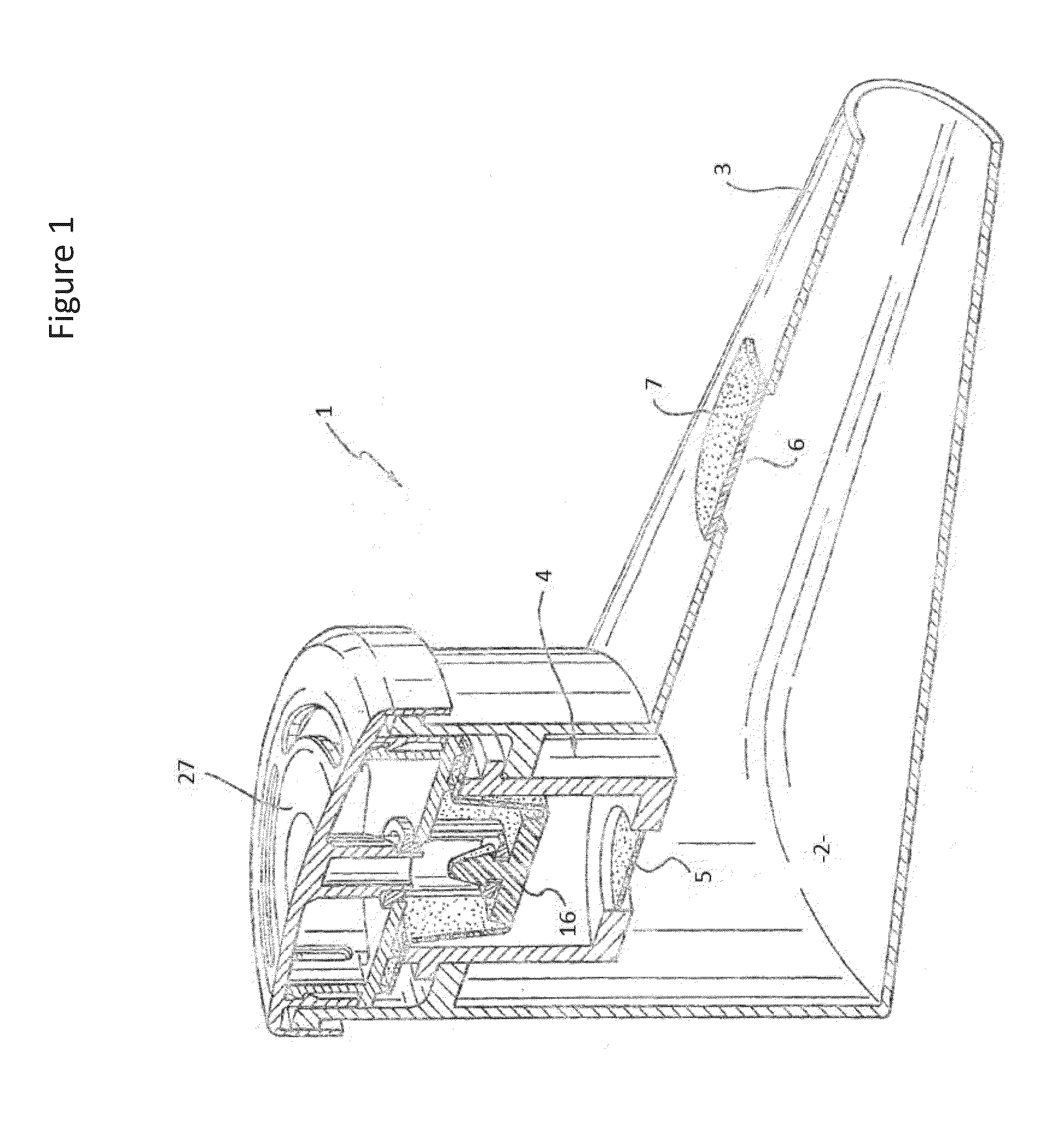
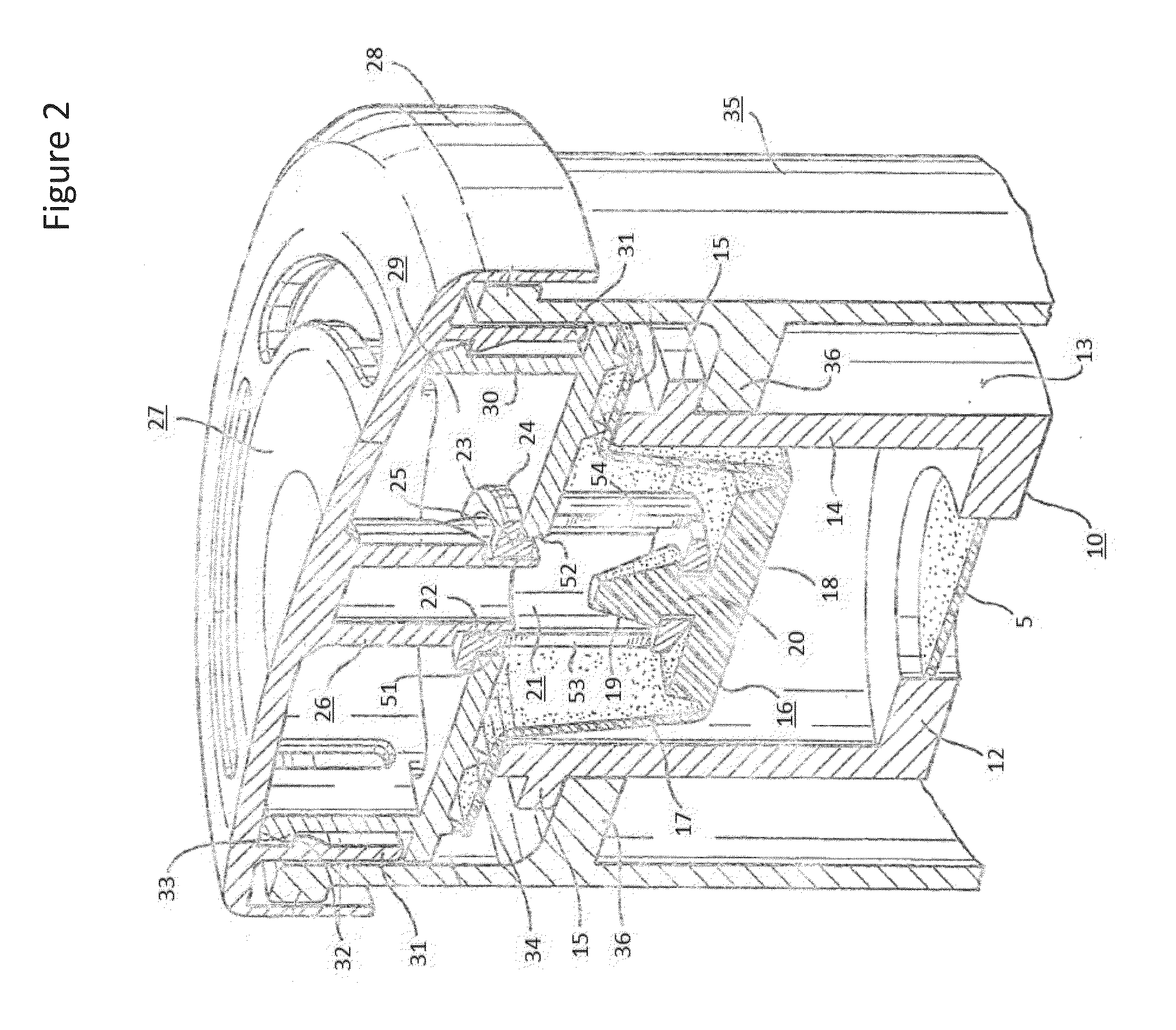
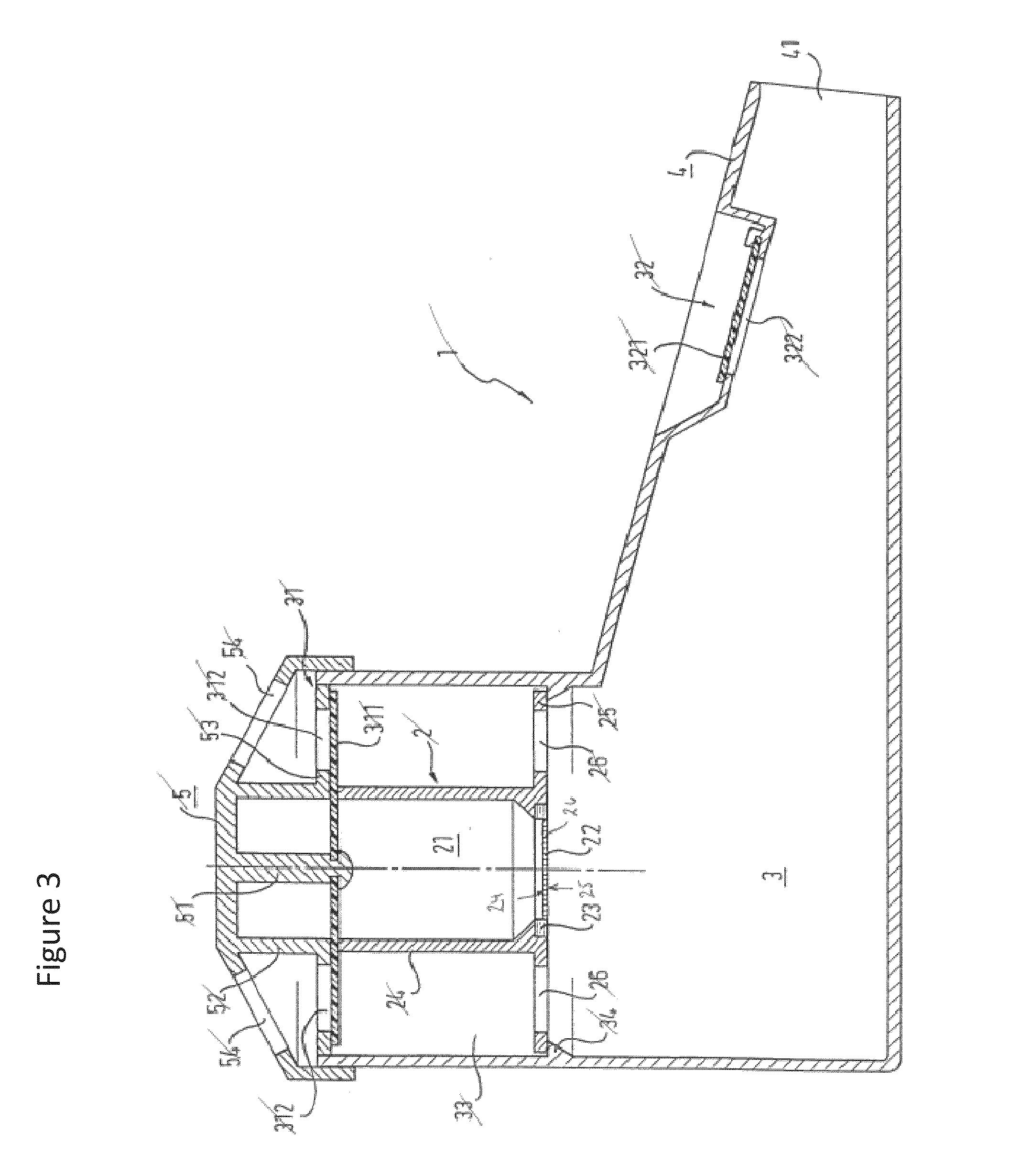
Systems, assemblies, and methods for treating a bronchial tree
ActiveUS20090306644A1Improve the immunityWithout eliminating smooth muscle toneUltrasound therapyDiagnosticsNervous systemCell membrane
Systems, assemblies, and methods to treat pulmonary diseases are used to decrease nervous system input to distal regions of the bronchial tree within the lungs. Treatment systems damage nerve tissue to temporarily or permanently decrease nervous system input. The treatment systems are capable of heating nerve tissue, cooling the nerve tissue, delivering a flowable substance that cause trauma to the nerve tissue, puncturing the nerve tissue, tearing the nerve tissue, cutting the nerve tissue, applying pressure to the nerve tissue, applying ultrasound to the nerve tissue, applying ionizing radiation to the nerve tissue, disrupting cell membranes of nerve tissue with electrical energy, or delivering long acting nerve blocking chemicals to the nerve tissue.
Owner:NUVAIRA INC
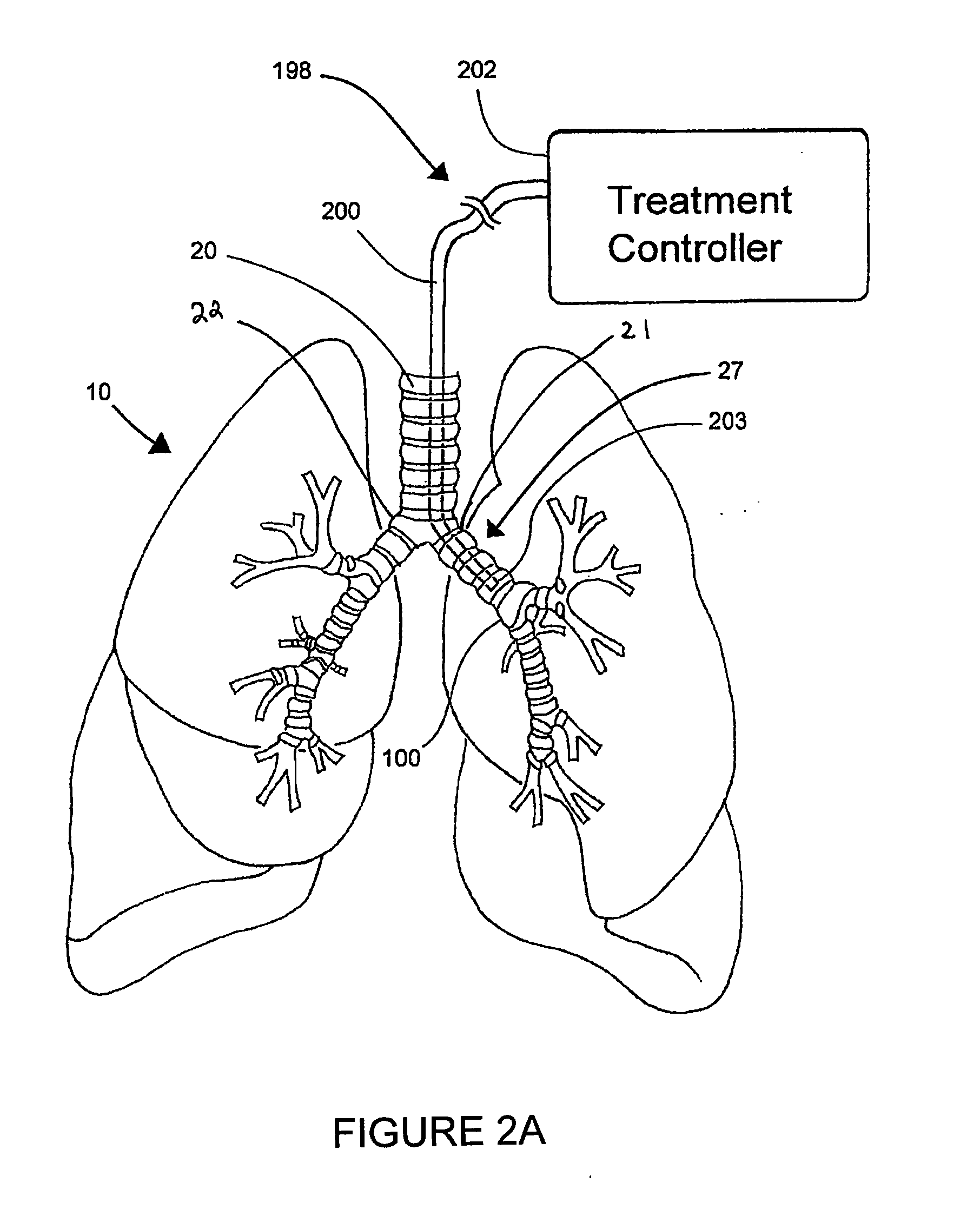
Methods and devices for inducing collapse in lung regions fed by collateral pathways
Disclosed are methods and devices for regulating fluid flow in one or more lung regions that are supplied fluid through one or more collateral pathways. An identified region of the lung is targeted for volume reduction or collapse. The targeted lung region is then bronchially isolated to inhibit air from flowing into the targeted lung region through bronchial pathways that directly feed air to the targeted lung region. If the targeted lung region does not collapse after bronchially isolating the targeted lung region, then it is possible that a collateral pathway is feeding air to the targeted lung region, thereby preventing the targeted lung region from collapsing. In such a case, the collateral pathway is identified and air flow into the targeted lung region via the collateral pathway is reduced or eliminated.
Owner:PULMONX
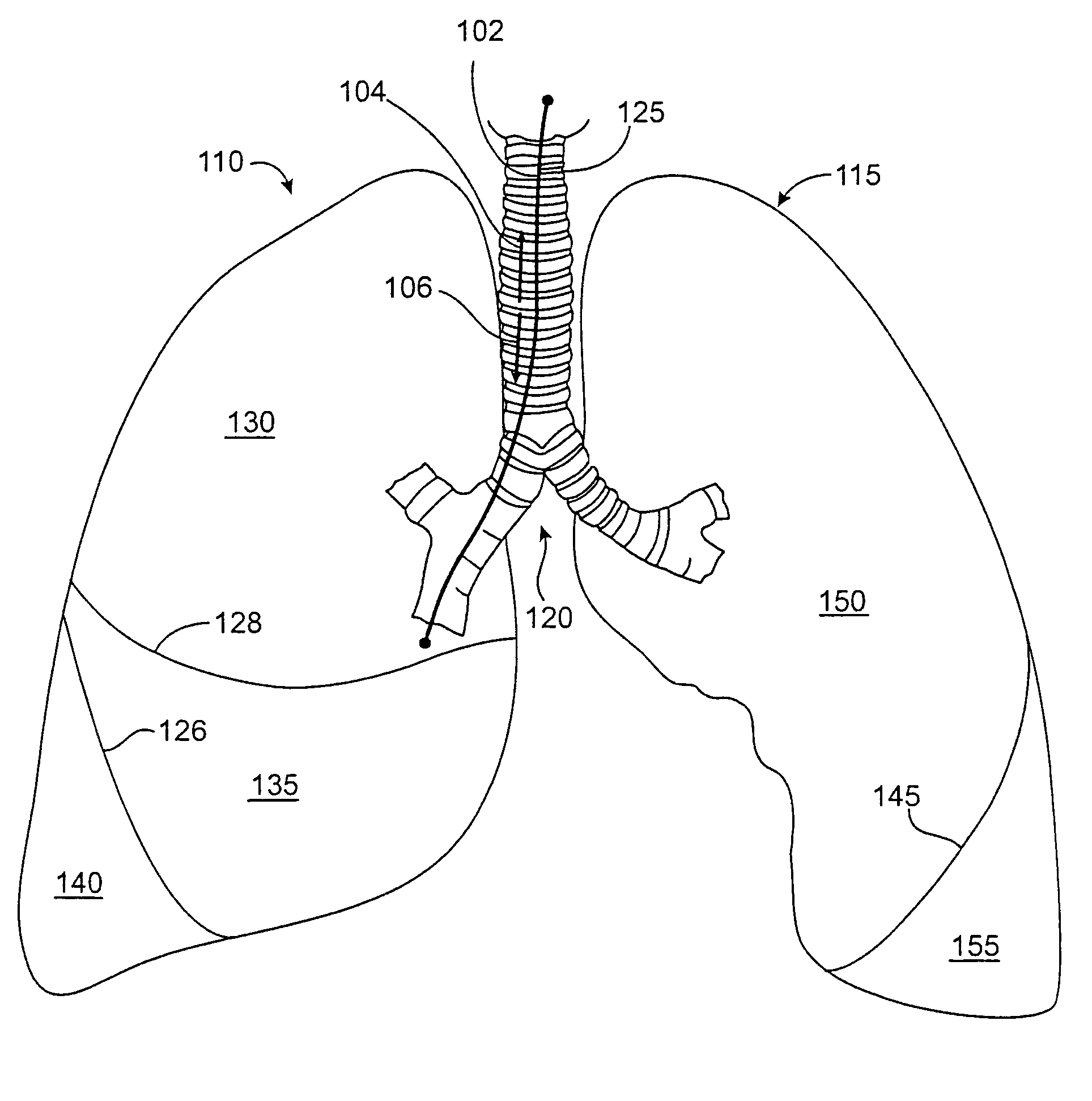
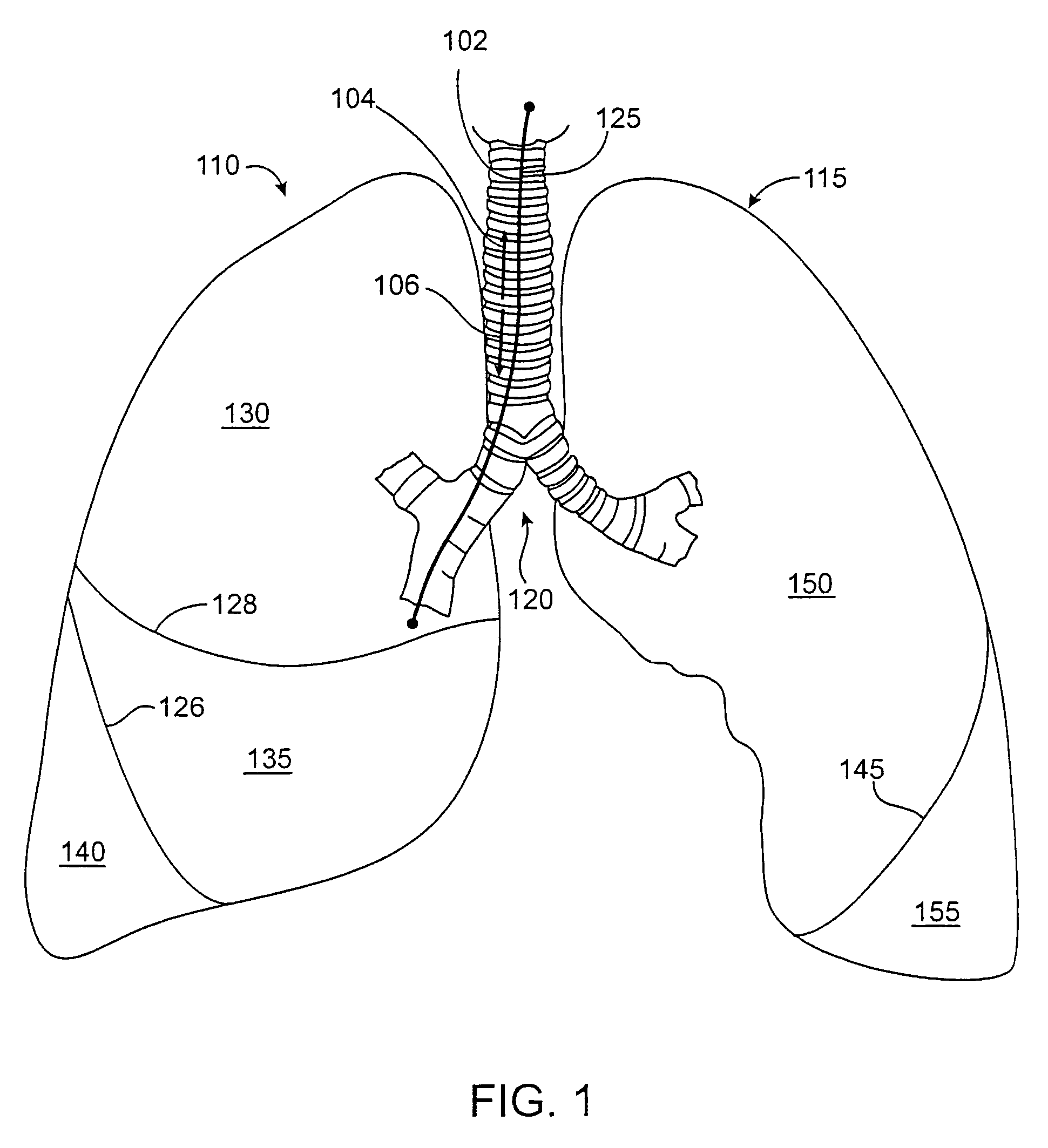
Treatment planning with implantable bronchial isolation devices
Disclosed is a treatment planning method that can be used to maximize the effectiveness of minimally invasive treatment on a patient. Pursuant to the treatment planning method, the presence of lung disease, such as emphysema, is first identified, followed by a determination of the distribution and extent of damage of the disease, followed by a determination of whether the patient is suitable for treatment, and a determination of the appropriate strategy for treatment for a suitable patient.
Owner:PULMONX
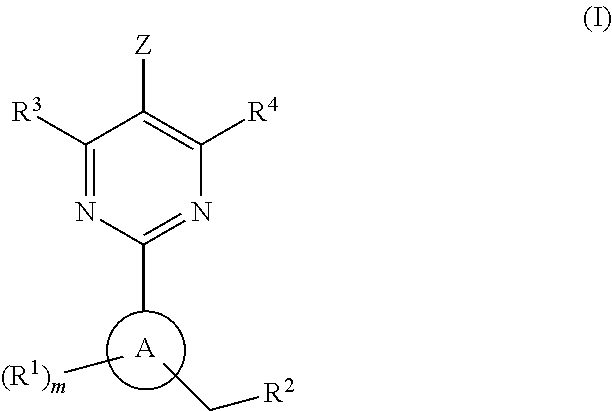
Soluble Guanylate Cyclase Activators
This inventions relates to compounds having the structure Formula Iand pharmaceutically acceptable salts thereof which are soluble guanylate cyclase activators. The compounds are useful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, pulmonary hypertension, angina pectoris, thromboses, restenosis, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver.
Owner:MERCK SHARP & DOHME LLC
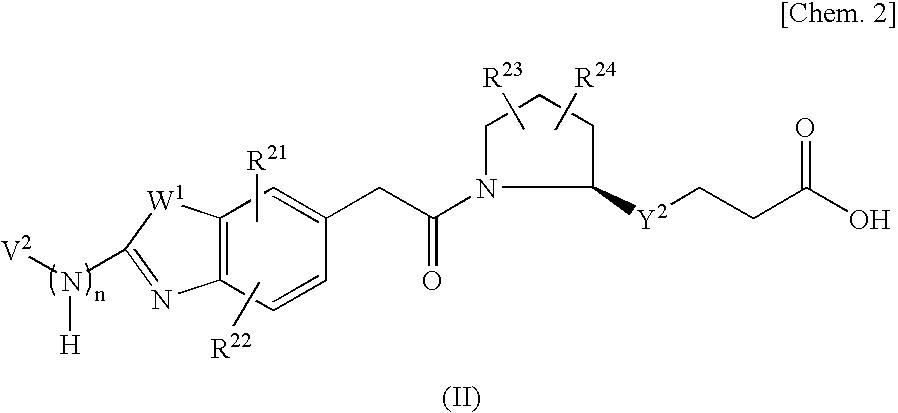
Nitrogenated heterocyclic derivative , and pharmaceutical agent comprising the derivative as active ingredient
InactiveUS20090131403A1Prevention and/or treatmentEasy to useBiocideSenses disorderAcquired immunodeficiencyAutoimmune condition
The compound represented by formula (I), a salt thereof, an N-oxide thereof, a solvate thereof, or a prodrug thereof specifically binds CCR5, so it is useful for preventing and / or treating CCR5-related diseases, for example, various inflammatory diseases (asthma, nephritis, nephropathy, hepatitis, arthritis, rheumatoid arthritis, rhinitis, conjunctivitis, ulcerative colitis, etc.), immunological diseases (autoimmune diseases, rejection in organ transplantation, immunosuppression, psoriasis, multiple sclerosis, etc.), infectious diseases (infection with human immunodeficiency virus, acquired immunodeficiency syndrome, etc.), allergic diseases (atopic dermatitis, urticaria, allergic bronchopulmonary aspergillosis, allergic eosinophilic gastroenteritis, etc.), ischemic reperfusion injury, acute respiratory distress syndrome, shock accompanying bacterial infection diabetes cancer metastasis and so on.Wherein all symbols in formula are as defined in the specification
Owner:ONO PHARMA CO LTD
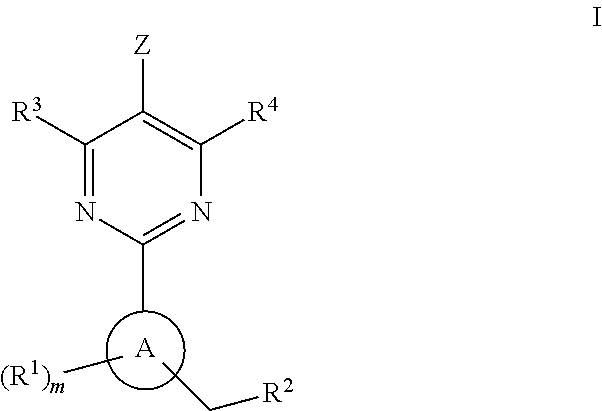
Soluble guanylate cyclase activators
A compound having the structureuseful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, angina pectoris, thromboses, restenoses, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver in a human or animal patient.
Owner:MERCK SHARP & DOHME CORP
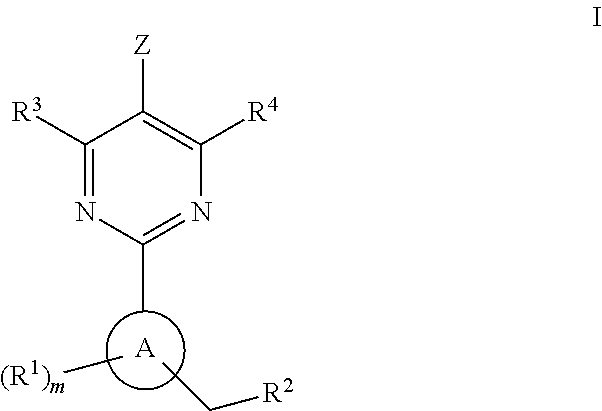
Soluble guanylate cyclase activators
A compound having the structureuseful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, angina pectoris, thromboses, restenoses, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver in a human or animal patient.
Owner:MERCK SHARP & DOHME LLC
Soluble guanylate cyclase activators
The invention relates to compounds having the structure of Formula (I) and pharmaceutically acceptable salts thereof, which are soluble guanylate cyclase activators. The compounds are capable of modulating the body's production of cyclic guanosine monophosphate (“cGMP”) and are generally suitable for the therapy and prophylaxis of diseases which are associated with a disturbed cGMP balance. The compounds are useful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, pulmonary hypertension, angina pectoris, thromboses, restenosis, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver.
Owner:MERCK SHARP & DOHME LLC
Soluble guanylate cyclase activators
This inventions relates to compounds having the structure Formula Iand pharmaceutically acceptable salts thereof which are soluble guanylate cyclase activators. The compounds are useful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, pulmonary hypertension, angina pectoris, thromboses, restenosis, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver.
Owner:MERCK SHARP & DOHME LLC
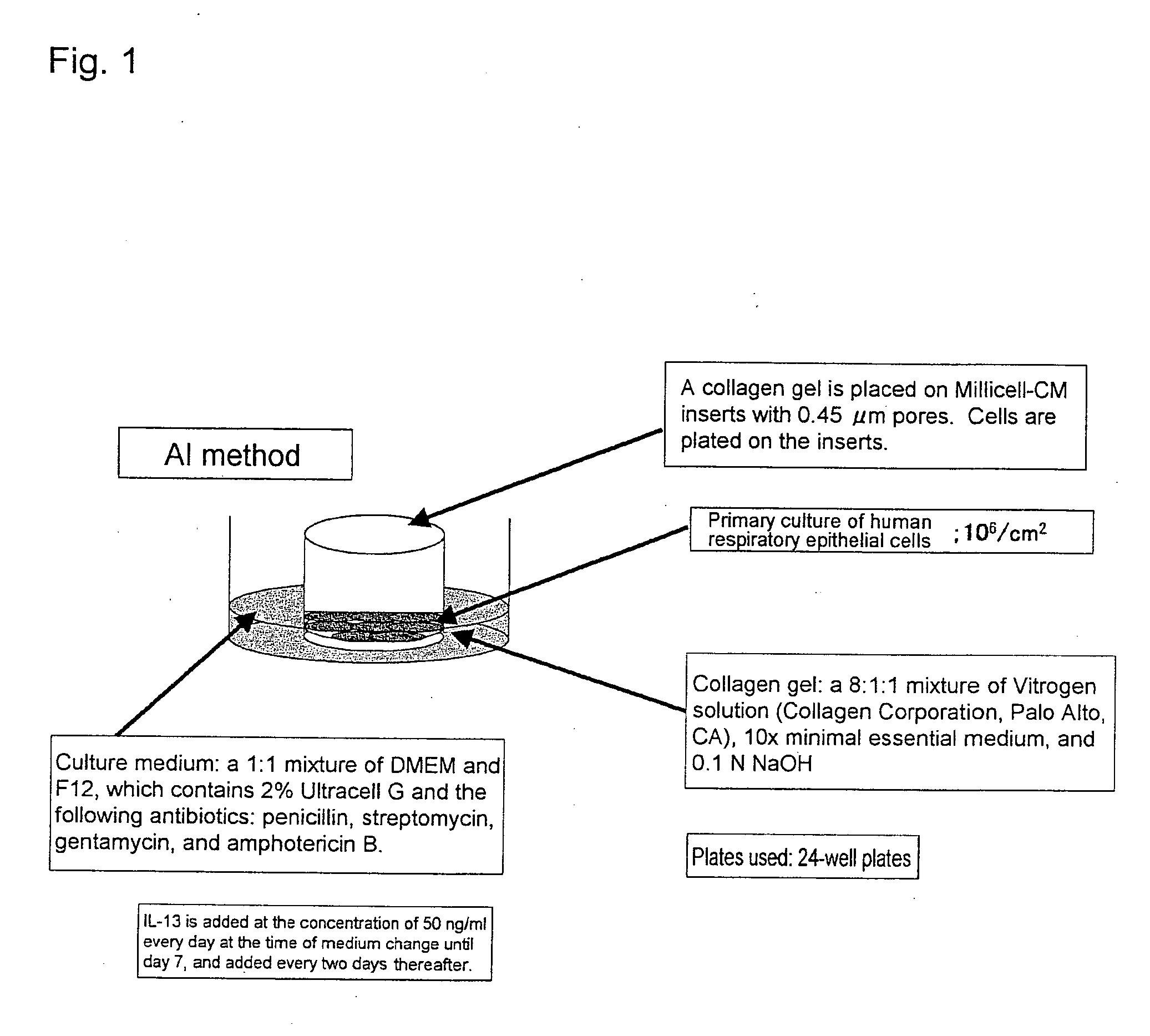
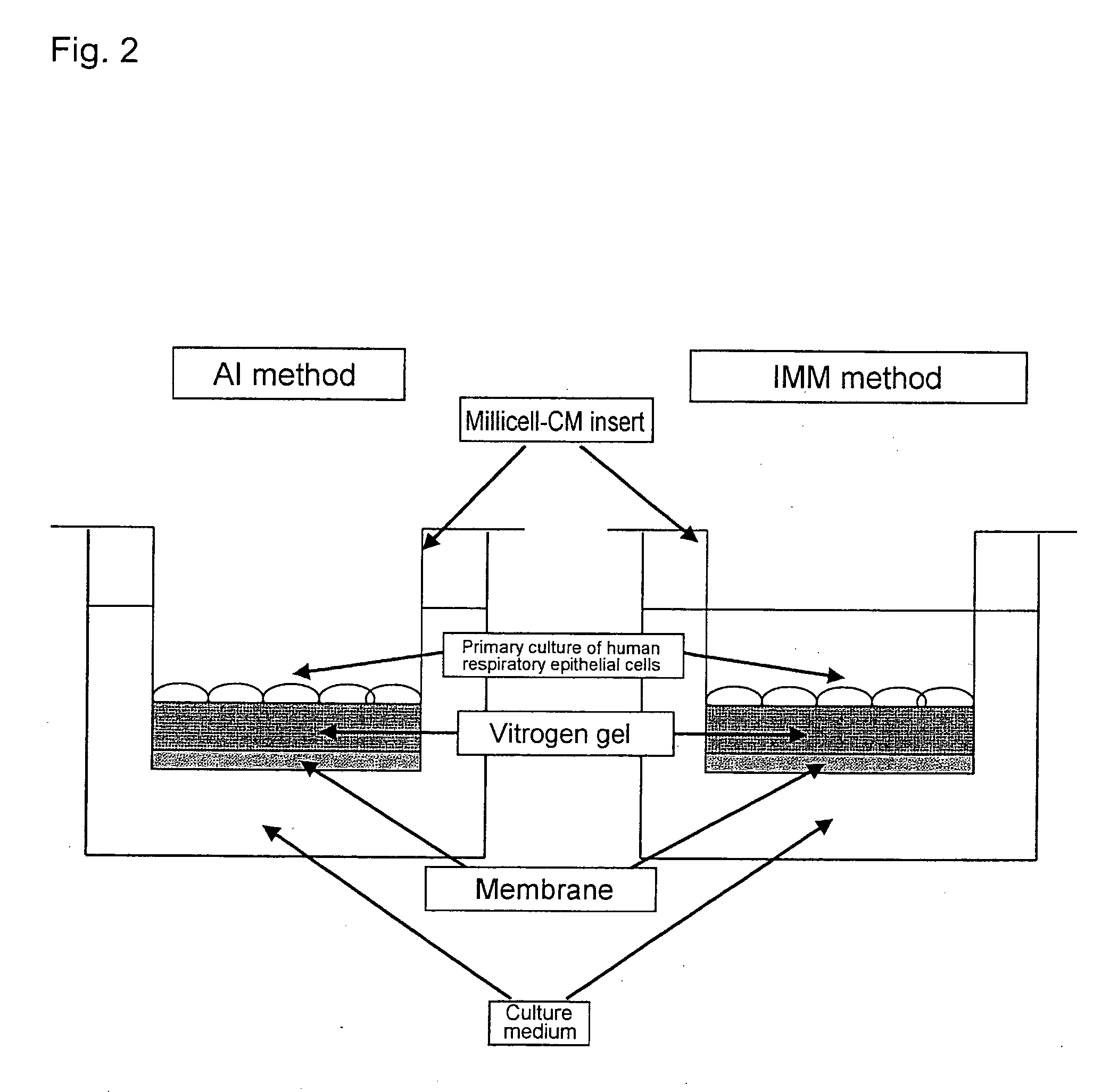
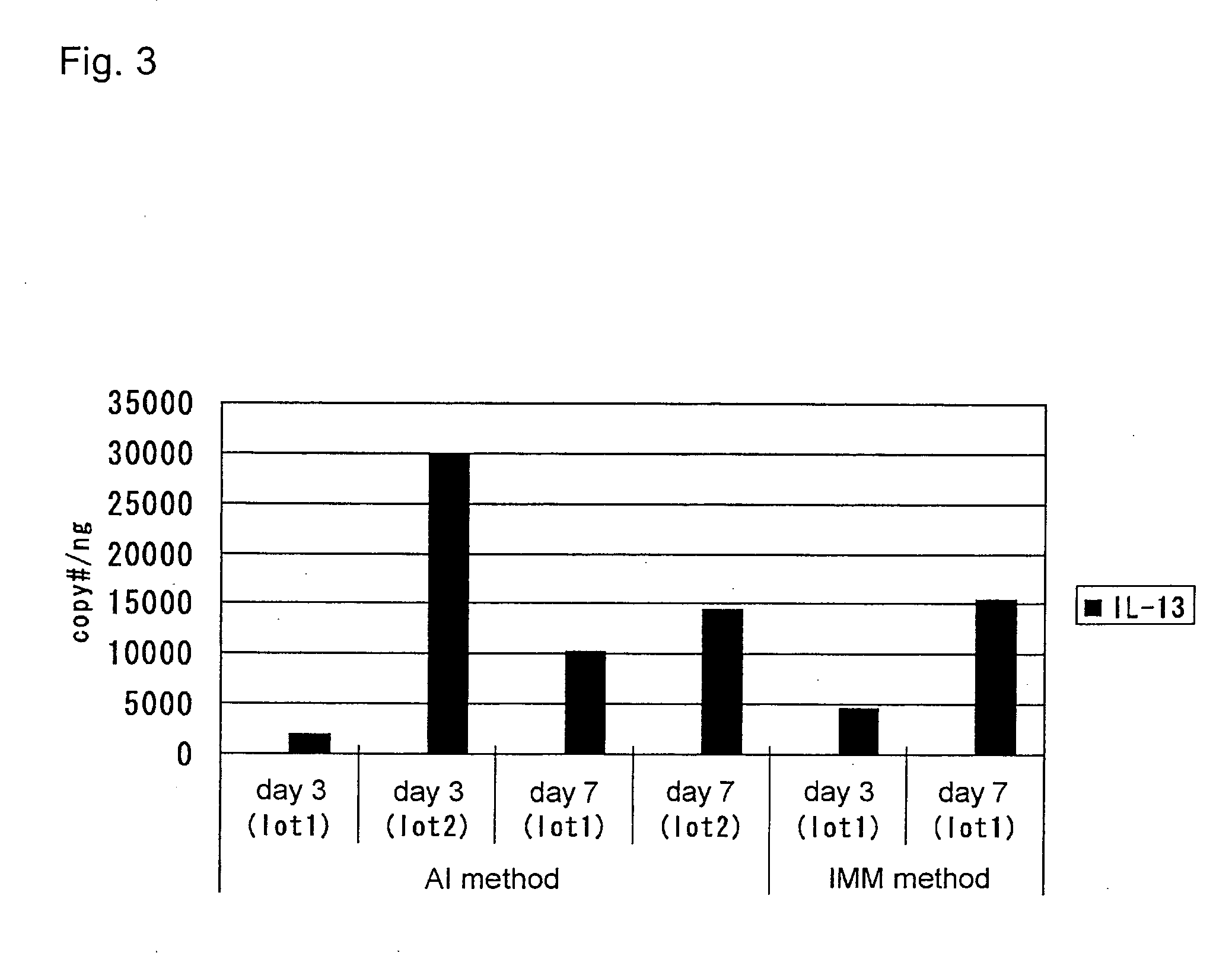
Methods of testing for bronchial asthma or chronic obstructive pulmonary disease
An objective of the present invention is to provide a method of testing for bronchial asthma or chronic obstructive pulmonary disease, a method of screening for candidate compounds for treating bronchial asthma or chronic obstructive pulmonary disease, and a pharmaceutical agent for treating bronchial asthma or chronic obstructive pulmonary disease. The present invention identified genes whose expression levels varied between respiratory epithelial cells that had been stimulated by IL-13 to induce the goblet cell differentiation, and unstimulated respiratory epithelial cells. The respiratory epithelial cells were cultured according to the air interface method. The genes were revealed to be useful as markers for testing for bronchial asthma or chronic obstructive pulmonary disease and screening for therapeutic agents for such diseases. Specifically, the present invention provides methods of testing for bronchial asthma or chronic obstructive pulmonary disease and methods of screening for compounds to treat the diseases based on the comparison of the expression levels of marker genes identified as described above.
Owner:GENOX RES
Soluble guanylate cyclase activators
This inventions relates to compounds having the structure Formula I and pharmaceutically acceptable salts thereof which are soluble guanylate cyclase activators. The compounds are useful for treatment or prevention of cardiovascular diseases, endothelial dysfunction, diastolic dysfunction, atherosclerosis, hypertension, pulmonary hypertension, angina pectoris, thromboses, restenosis, myocardial infarction, strokes, cardiac insufficiency, pulmonary hypertonia, erectile dysfunction, asthma bronchiale, chronic kidney insufficiency, diabetes, or cirrhosis of the liver.
Owner:MERCK SHARP & DOHME LLC
Systems for treating pulmonary infections
Provided herein are systems for treating a subject with a pulmonary infection, for example, a nontuberculous mycobacterial pulmonary infection, a Burkholderia pulmonary infection, a pulmonary infection associated with bronchiectasis, or a Pseudomonas pulmonary infection. The system includes a pharmaceutical formulation comprising a liposomal aminoglycoside dispersion, and the lipid component of the liposomes consist essentially of electrically neutral lipids. The system also includes a nebulizer which generates an aerosol of the pharmaceutical formulation at a rate greater than about 0.53 gram per minute. The aerosol is delivered to the subject via inhalation for the treatment of the pulmonary infection.
Owner:INSMED INC
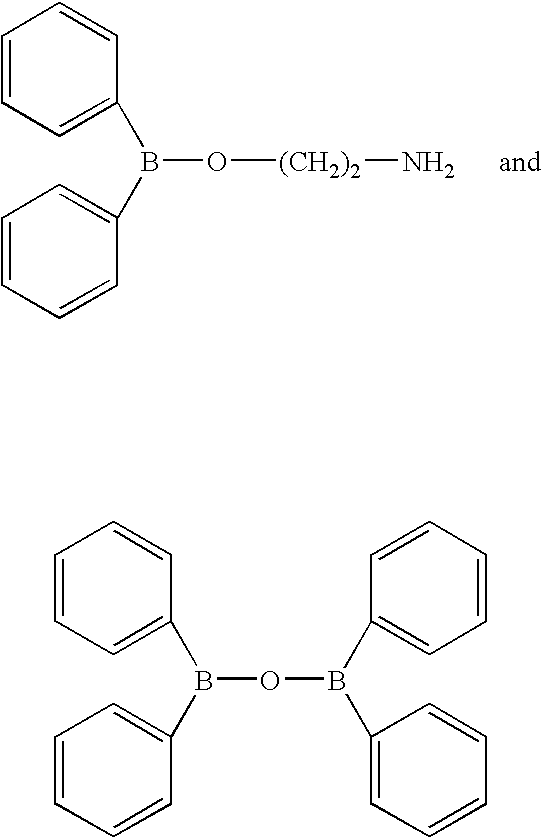
Intracellular calcium concentration increase inhibitors
InactiveUS7217701B2Effective controlImprove effective controlBiocideSenses disorderBronchial epitheliumBULK ACTIVE INGREDIENT
An intracellular calcium concentration increase inhibitor containing as the active ingredient (1) a boron compound represented by the formula (I).The compound represented by the formula (I) inhibits the increase of the intracellular calcium concentration, and therefore it is deemed to be useful as an agent for the prophylaxis and / or treatment of platelet aggregation, ischemic diseases in hearts and brains, immune deficiency diseases, allorgosis, bronchial asthma, hypertension, cerebrovascular spasm, various renal diseases, pancreatitis, Alzheimer's disease, etc.
Owner:MIKOSHIBA KATSUHIKO
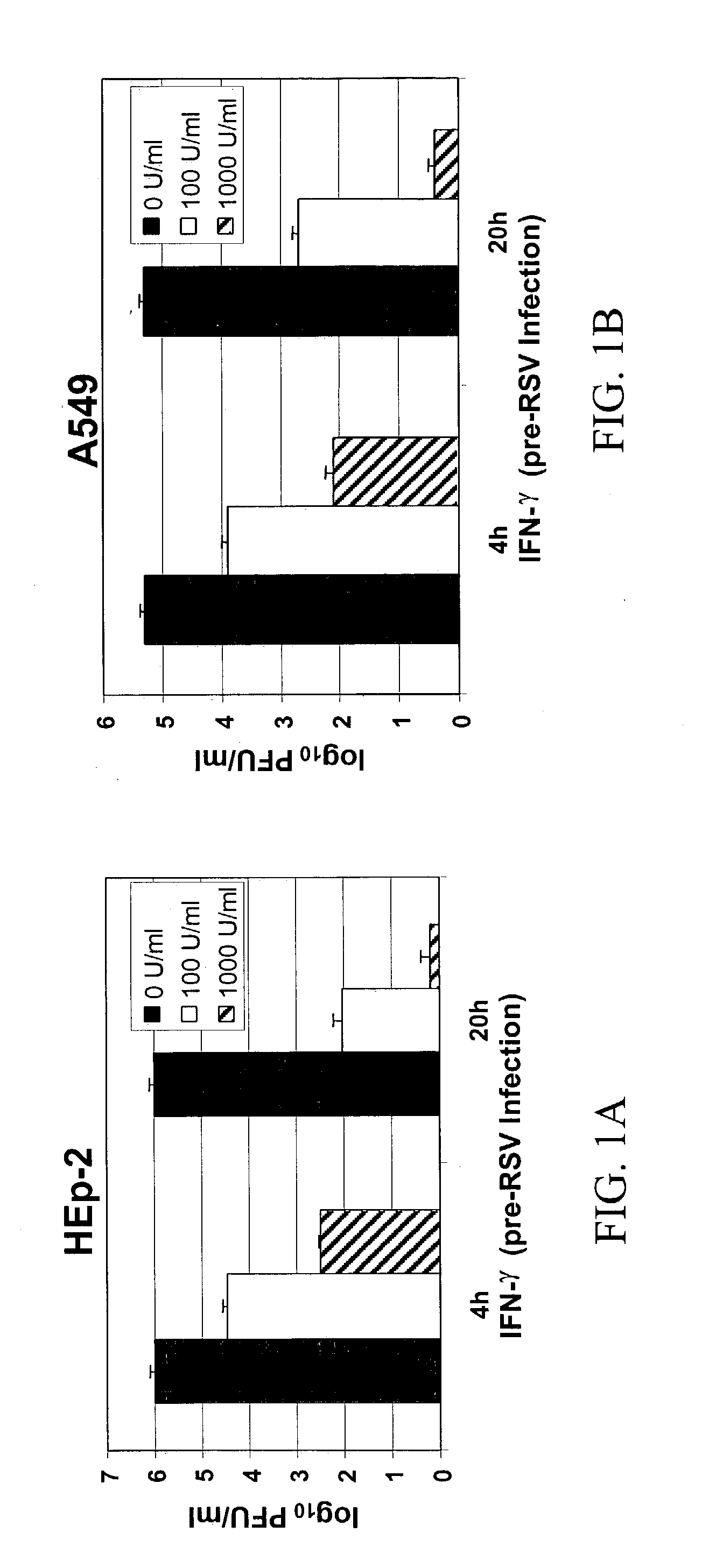
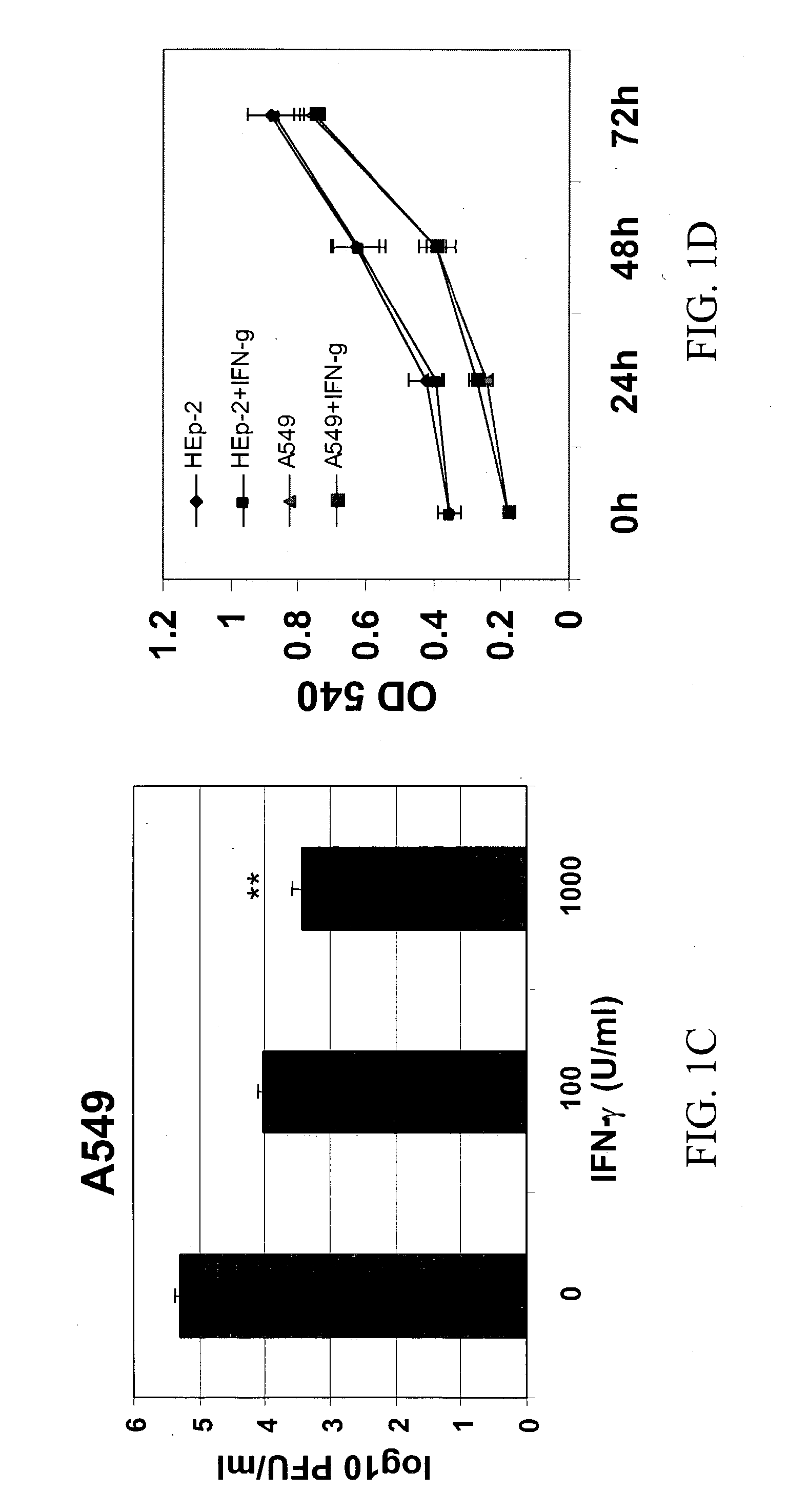
Materials and methods for prevention and treatment of RNA viral diseases
InactiveUS20040009152A1Preventing and decreasing severity of symptomBiocidePeptide/protein ingredientsDiseaseMononuclear cell infiltration
The subject invention concerns a method of inhibiting an RNA virus infection within a patient by increasing the amount of 2-5 oligoadenylate synthetase (2-5 AS) activity within the patient. Preferably, the preventative and therapeutic methods of the present invention involve administering a nucleotide encoding 2-5 AS, or at least one catalytically active fragment thereof, such as the p40, p69, p100 subunits, to a patient in need thereof. The present inventors have determined that overexpression of 2-5AS causes a reduction in epithelial cell damage, reduction in infiltration of mononuclear cells in the peribronchiolar and perivascular regions, and reduction in thickening of the septa in the lungs. Levels of chemokines, such as MIP1-alpha, are also reduced upon overexpression of 2-5AS. The subject invention also pertains to pharmaceutical compositions containing a nucleotide sequence encoding 2-5 AS and a pharmaceutically acceptable carrier, as well as vectors for delivery of the 2-5 AS nucleotide sequence.
Owner:IB SECURITYHOLDERS +1
Stable amorphous ambroxol hydrochloride compound
The invention belongs to the technical field of medicine, and particularly relates to an amorphous ambroxol hydrochloride compound and a preparation method thereof. The amorphous ambroxol hydrochloride compound has high purity and stability, does not have obvious moisture absorption and weight increase even under the condition of high humidity, ensures that relative substances do not grow, and has higher dissolution rate than crystalline ambroxol hydrochloride. The invention also relates to an application of the amorphous ambroxol hydrochloride compound in preparation of medicine for the treatment of acute and chronic pulmonary diseases with the symptom of ropy sputum and difficult expectoration, phlegm eliminating treatment of acute exacerbation of chronic bronchitis, asthmatic bronchitis and bronchial asthma, prophylactic treatment of lung complication after an operation, and treatment of infant respiratory distress syndrome (IRDS) of premature infants and neonates.
Owner:天津梅花生物医药科技有限公司
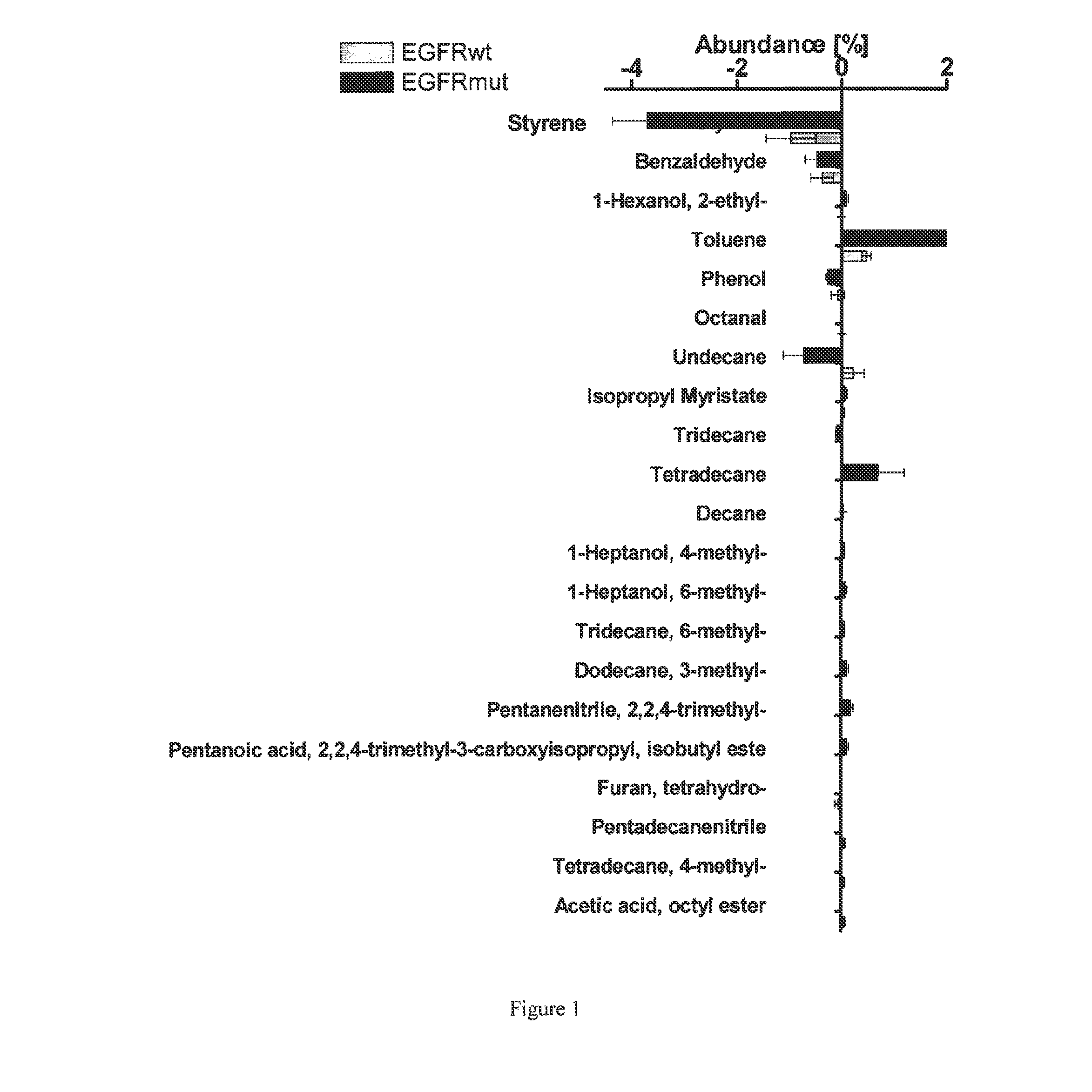
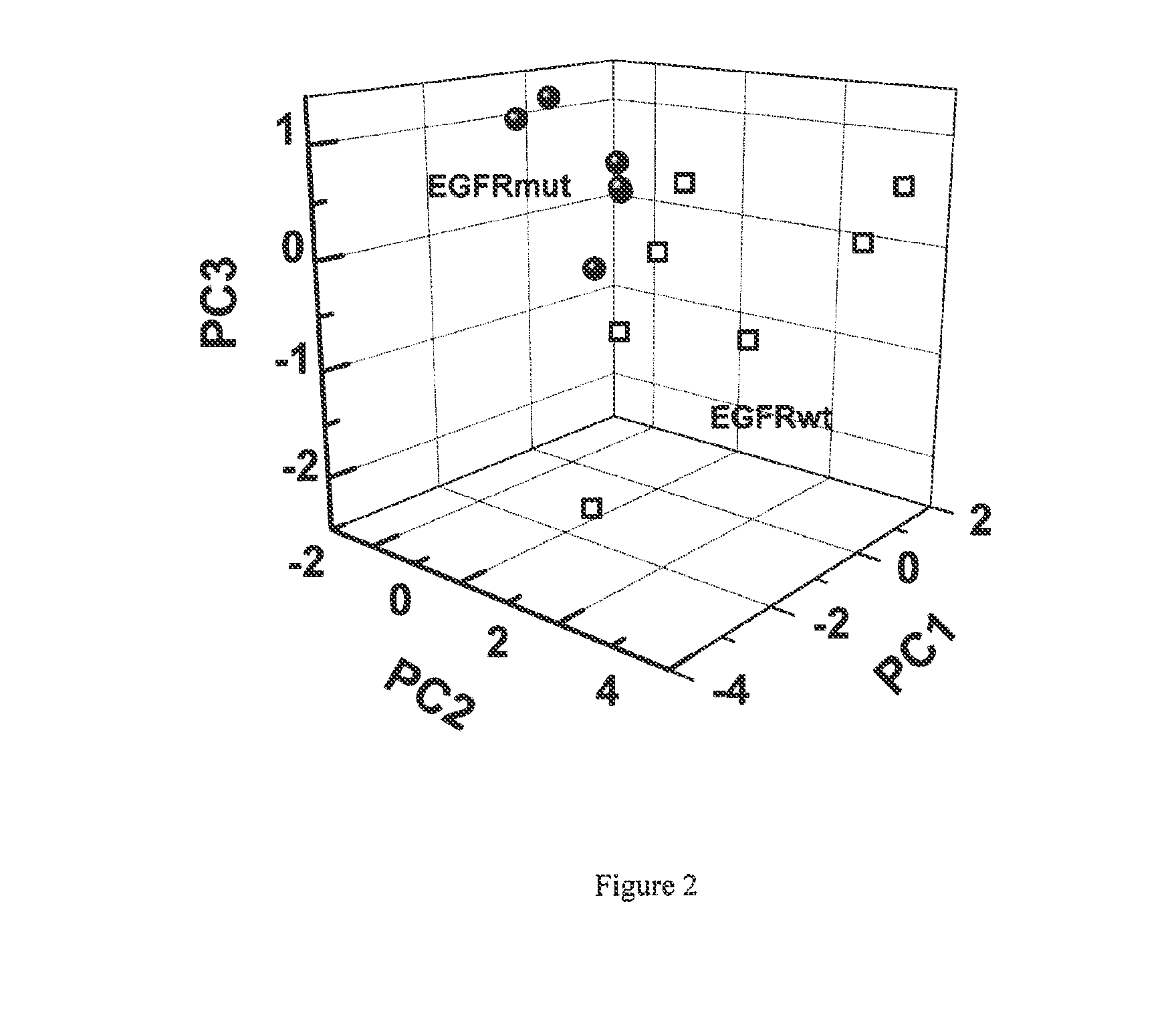
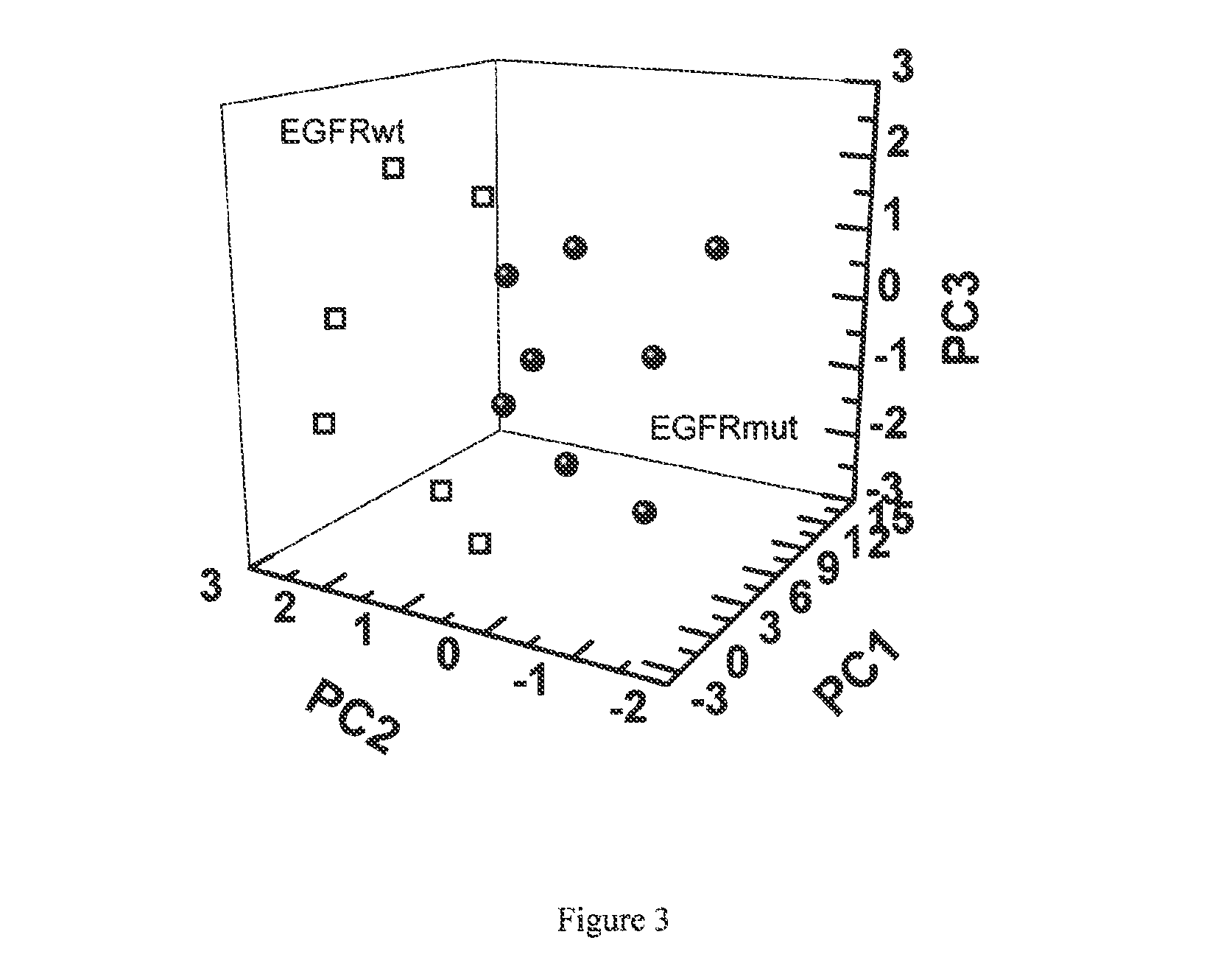
Volatile organic compounds for detecting cell dysplasia and genetic alterations associated with lung cancer
ActiveUS20130143247A1Increased riskComponent separationMicrobiological testing/measurementBronchial epitheliumOncology
The present invention provides methods of identifying a genetic abnormality such as mutation in EGFR or KRAS or ALK which is associated with the management of lung cancer or diagnosing, prognosing or monitoring the treatment of pre-cancerous conditions of the lung, such as bronchial dysplasia or atypical alveolar hyperplasia (AAH), through the detection of at least one volatile organic compound indicative of these states.
Owner:UNIV OF COLORADO THE REGENTS OF +1
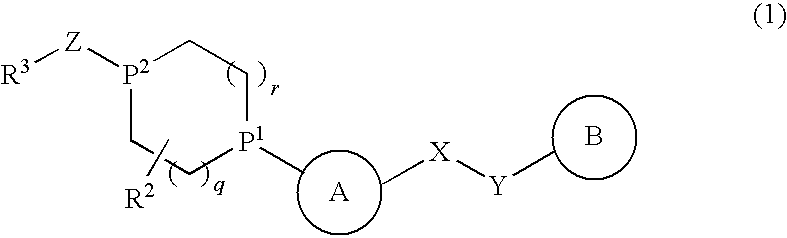
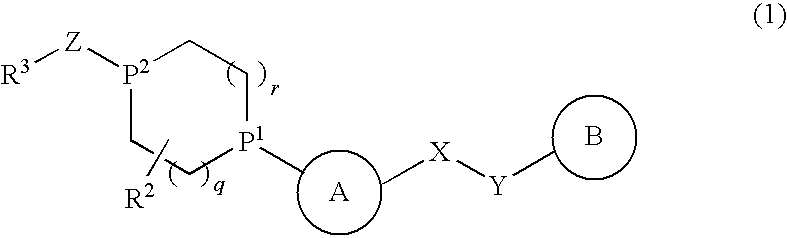
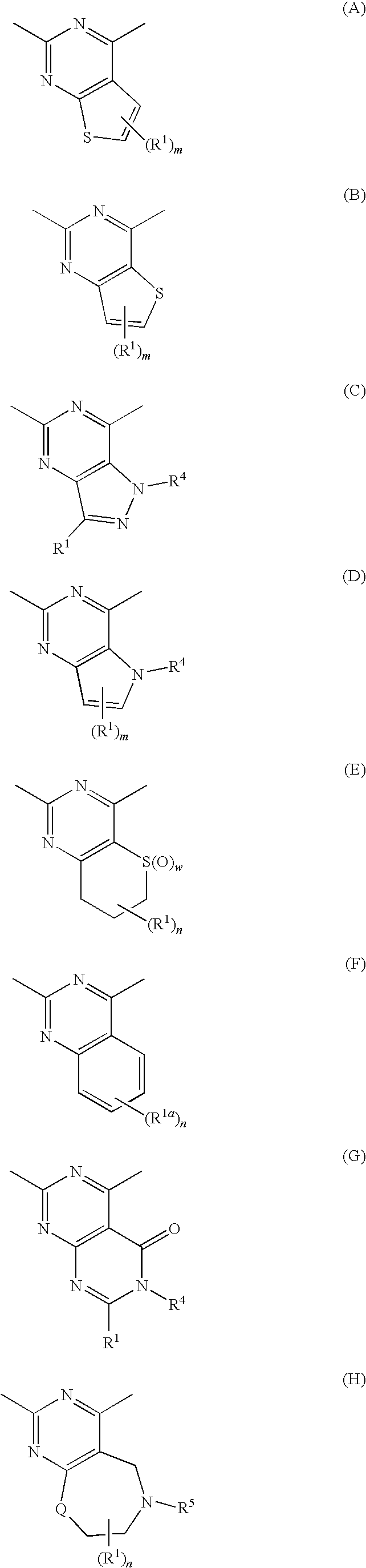
Alicyclic Heterocyclic Compound
InactiveUS20090182140A1Useful in treatmentOrganic chemistryAntipyreticAtopic dermatitisBronchial epithelium
An alicyclic heterocyclic compound represented by the following formula or a pharmaceutically acceptable salt thereof:wherein ring A is a heterocyclic ring, ring B is a carbocyclic ring, a heterocyclic ring etc., P1 and P2 are CH or N, q and r are 0 to 2, X is —NH—, —O—, —CH2—, etc., Y is —CH2—, —CO—, —SO2—, etc., Z is —CO—, —SO2—, etc., and R3 is carbocyclic group, heterocyclic group, hydroxyl, alkoxy or amino,is useful as a controlling agent of the function of CCR4 useful for the prevention or treatment for bronchial asthma, atopic dermatitis, etc.
Owner:MITSUBISHI TANABE PHARMA CORP
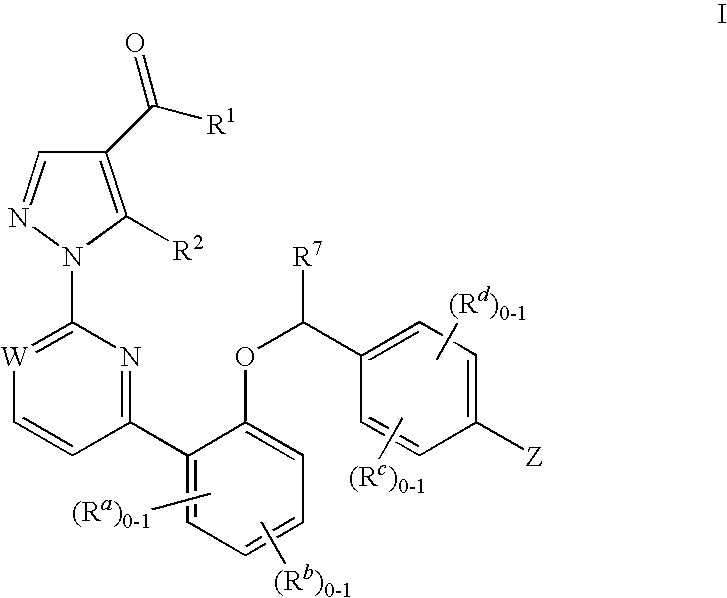
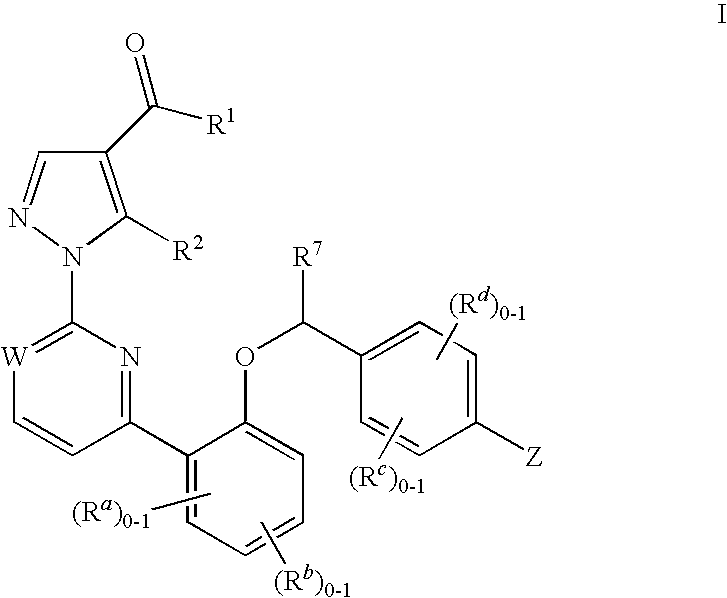
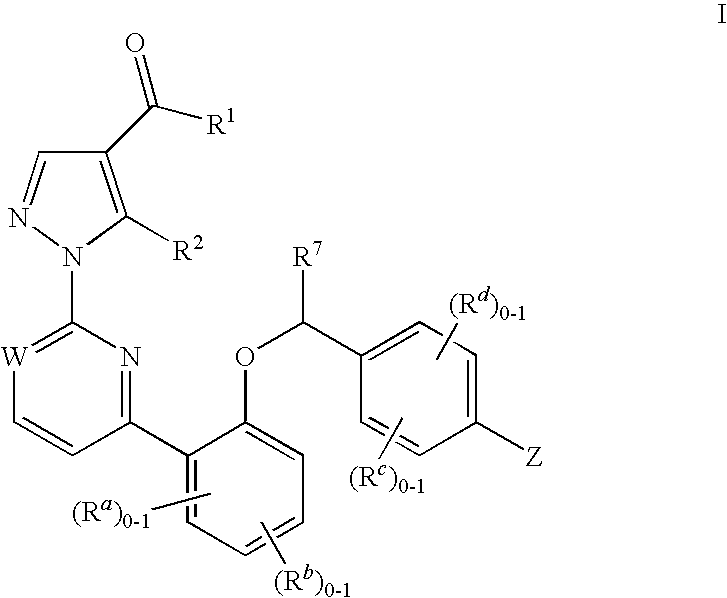
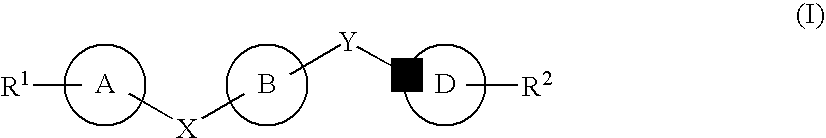
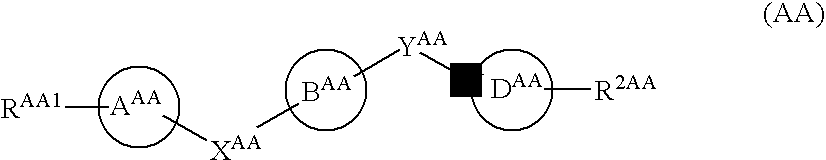
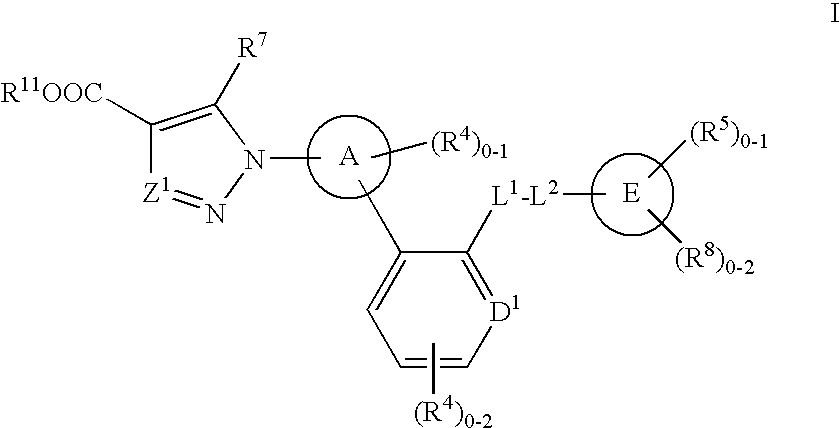
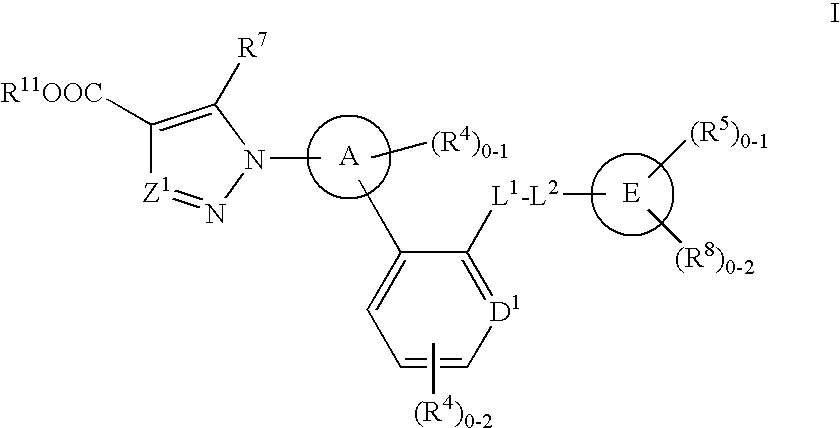
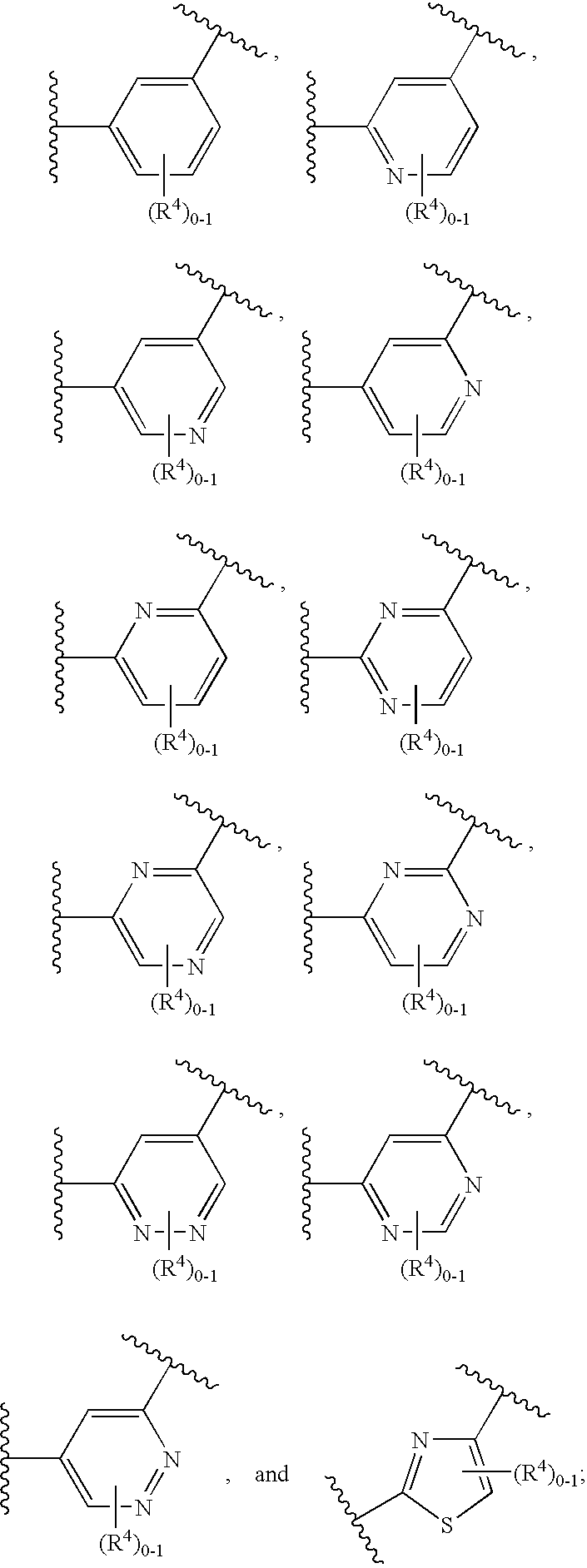
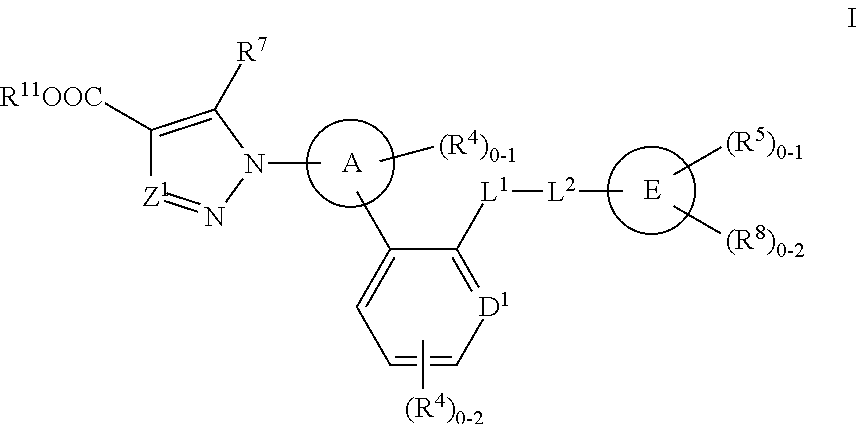
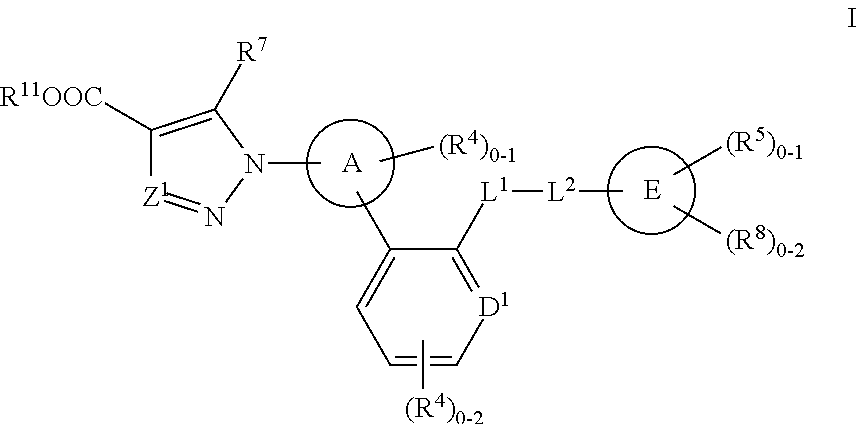
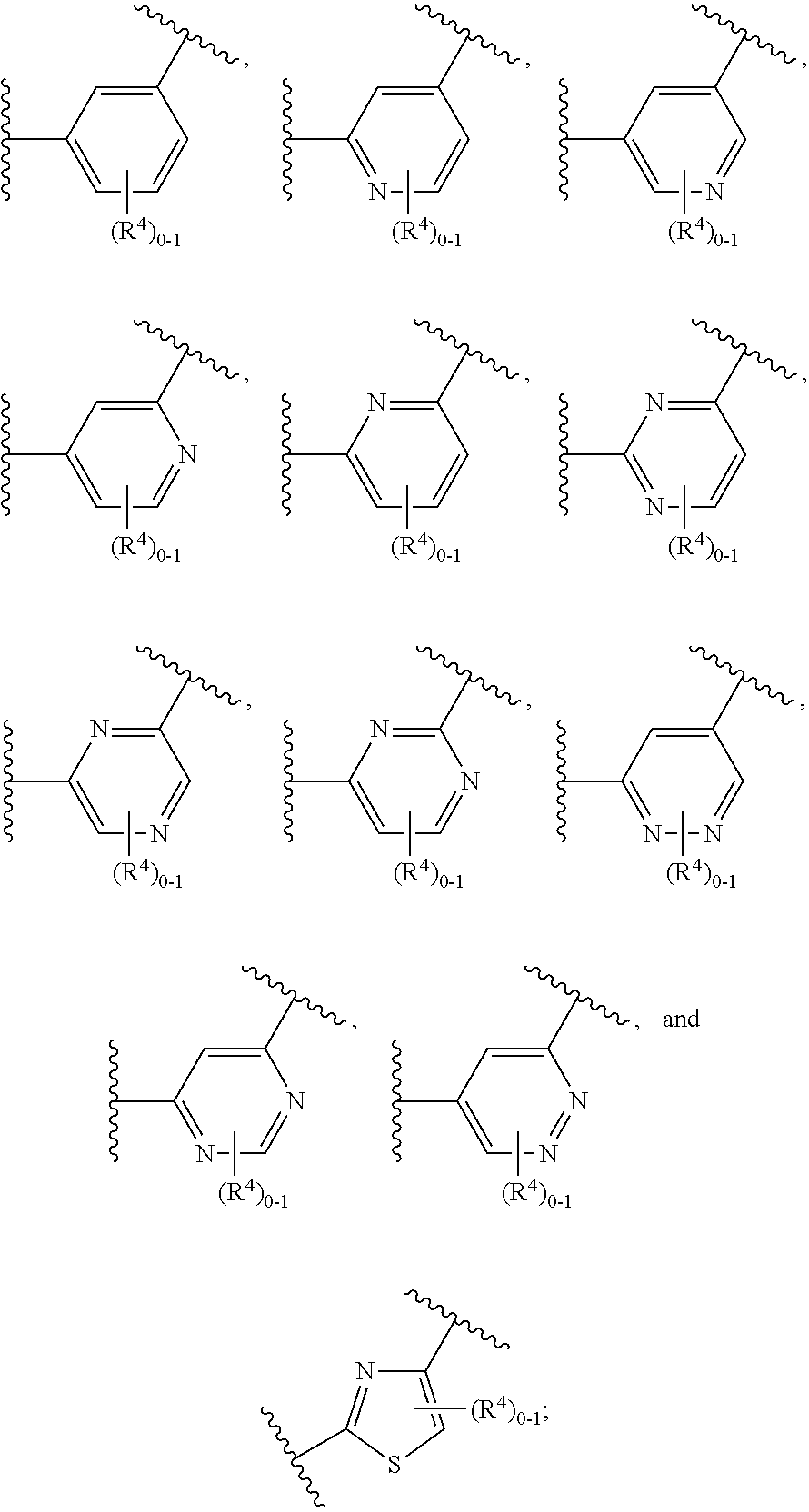
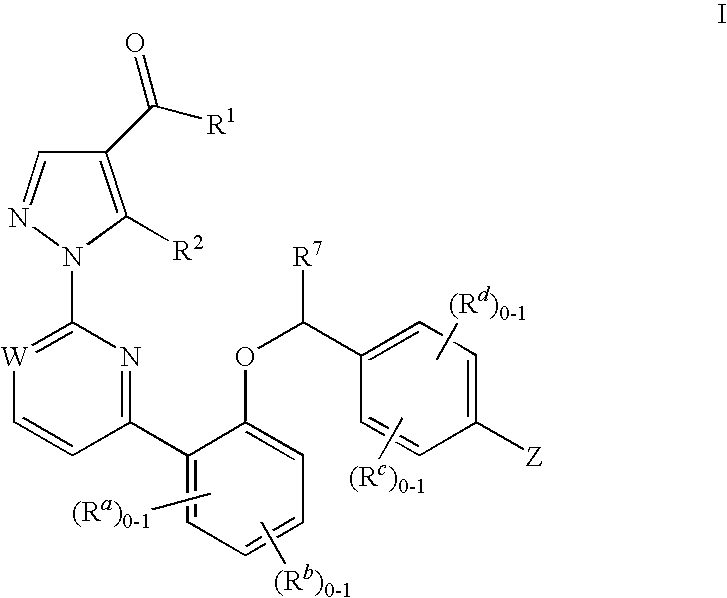
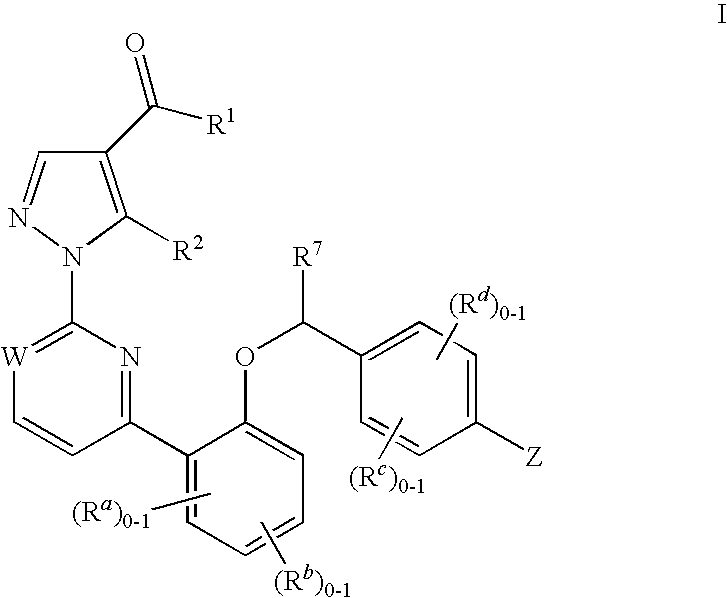
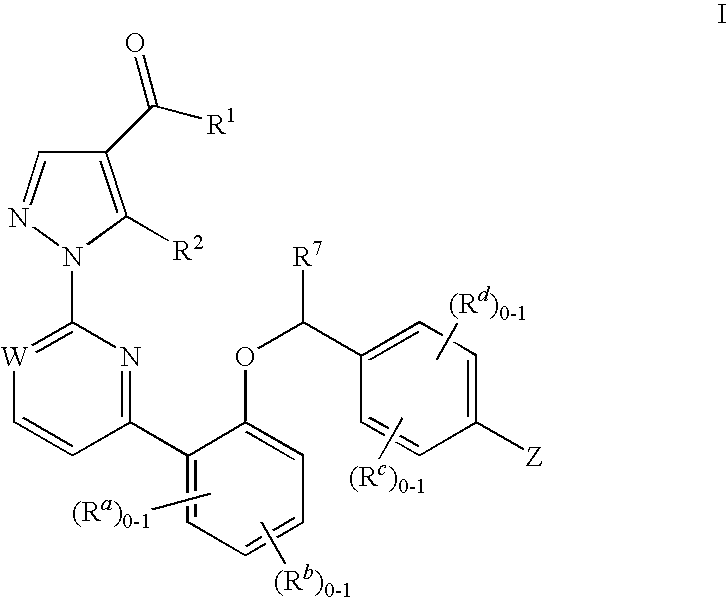
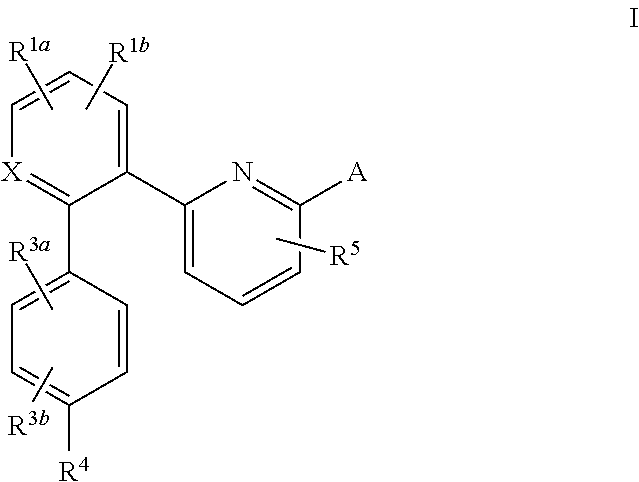
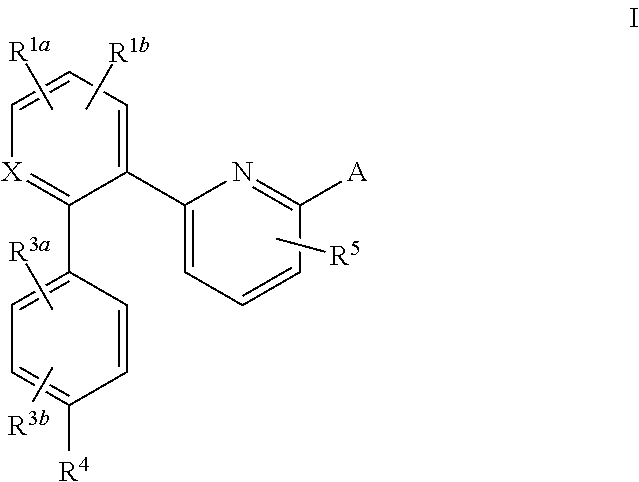
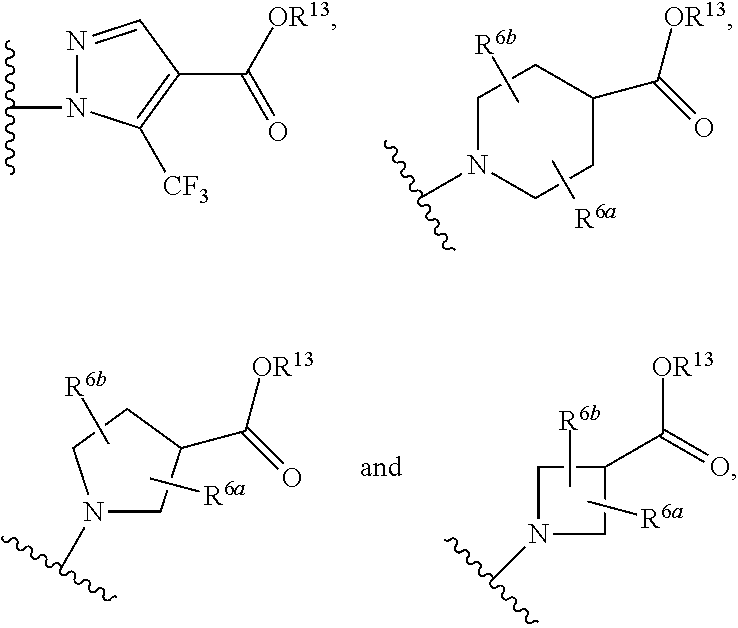
Certain (2S)-n-[(1S)-1-cyano-2-phenylethyl]-1,4-oxazepane-2-carboxamides as dipeptidyl peptidase 1 inhibitors for treating bronchiectasis
InactiveUS20180028541A1Increase the length of timeReduce probabilityAntibacterial agentsOrganic active ingredientsBenzoxazoleDipeptidyl peptidase
The present disclosure relates to methods for treating bronchiectasis, for example, non-cystic fibrosis bronchiectasis with compositions comprising an effective amount of certain (2S)—N-[(1S)-1-cyano-2-phenylethyl]-1,4-oxazepane-2-carboxamide compounds of Formula (I), including pharmaceutically acceptable salts thereof,that inhibit dipeptidyl peptidase 1 (DPP1) activity. Methods provided herein are useful for prophylaxis, increasing the lung function in a patient, and / or and / or decreasing the rate of pulmonary exacerbation in a patient. In one embodiment, the compound of Formula (I) is (2S)—N-{(1S)-1-cyano-2-[4-(3-methyl-2-oxo-2,3-dihydro-1,3-benzoxazol-5-yl)phenyl]ethyl}-1,4-oxazepane-2-carboxamide.
Owner:ASTRAZENECA AB +1
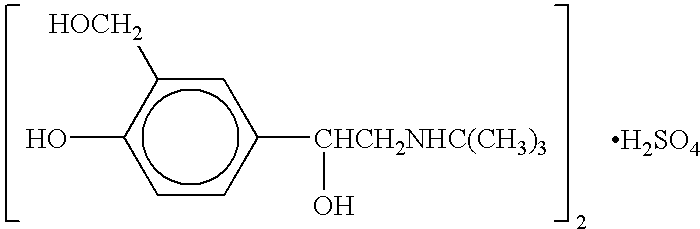
Albuterol inhalation solution, system, kit and method for relieving symptoms of pediatric asthma
The present invention relates to an albuterol inhalation solution, system, kit and method for relieving bronchospasm in children suffering from asthma. In one alternative embodiment, the solution of the present invention is a sterile, premixed, premeasured single unit dose of albuterol for asthmatic patients 2 to 12 years of age. The present solution may be free of anti-microbial preservatives, such as benzalkonium chloride. In another alternative embodiment, the solution of the present invention comprises about 0.63 mg or about 1.25 mg albuterol.
Owner:MYLAN SPECIALTY
Traditional Chinese medicinal composition for treating bronchitis and bronchial asthma
InactiveCN103920106AImprove antibacterial propertiesImprove immunityRespiratory disorderAluminium/calcium/magnesium active ingredientsFritillaria cirrhosaLicorice roots
The invention discloses a traditional Chinese medicinal composition for treating bronchitis and bronchial asthma. The composition is characterized in that the composition comprises, by weight, 12 parts of Cordyceps sinensis, 15 parts of Radix Codonopsis, 15 parts of Rhizoma Atractylodis Macrocephalae (stir-fried with bran), 15 parts of Poria cocos, 10 parts of Radix Ophiopogonis, 12 parts of Malaytea Scurfpea Fruit (stir-fried with salt), 10 parts of Chinese magnoliavine (processed with vinegar), 12 parts of Chinese angelica, 9 parts of Rhizoma Pinelliae Preparatum, 12 parts of snakegourd peel, 9 parts of bitter apricot kernel, 9 parts of Rhizoma Coptidis, 6 parts of jujube, 10 parts of perilla fruit, 10 parts of Aster tataricus, 10 parts of lily, 6 parts (calcined) gypsum, 9 parts of Cortex Magnoliae Officinalis, 10 parts of Radix Peucedani, 6 parts of dried orange peel, 9 parts of Chinese Eaglewood, 6 parts of ginger, 9 parts of loquat leaf, 10 parts of Fritillaria cirrhosa, 12 parts of prepared rehmannia root, 10 parts of Radix Scrophulariae, 6 parts of Arisaema Cum Bile, 10 parts of Radix Platycodonis, 10 parts of White Mulberry Root-bark (processed with honey), 9 parts of Rhizoma Anemarrhenae, 12 parts of Common Coltsfoot Flower, 15 parts of honeysuckle flower, 12 parts of Herba Schizonepetae, 12 parts of Weeping Forsythia and 6 parts of honey-fried licorice root.
Owner:赵庆军
Ambroxol hydrochloride oral solution and preparation method thereof
InactiveCN101352417AWilling to takeImprove complianceOrganic active ingredientsPharmaceutical delivery mechanismSucroseAcute bronchitis
The invention discloses an ambroxol hydrochloride oral solution and a preparation method thereof, and relates to a preparation method of western medicine, in particular to a medical production formula used for curing bronchitis and a preparation method thereof; the preparation method comprises the following steps: sucrose, preservatives and purified water are dissolved in a mixing mode so as to prepare simple syrup; the ambroxol hydrochloride, corrigents and preservatives are dissolved by the purified water so as to prepare ambroxol hydrochloride solution; the ambroxol hydrochloride solution and the simple syrup are stirred and mixed evenly, the corrigents and the preservatives are added successively and discontinuously and then the purified water is added to adjust the concentration. The invention has simple and reasonable process, and is easy to operate practically and the produced products have good stability; the products can used as common phlegm-eliminating drugs, has functions of increasing the liquid level of the respiratory tract, reducing mucus secretion, enhancing the secretion of pulmonary surfactant and the movement of cilium and preventing cough to a certain extent, and is applicable to acute respiratory disease and chronic respiratory disease, such as ropy sputum and difficult cough caused by acute bronchitis and chronic bronchitis, bronchial asthma and pulmonary tuberculosis, etc.
Owner:扬州市三药制药有限公司
Aromatic Compound
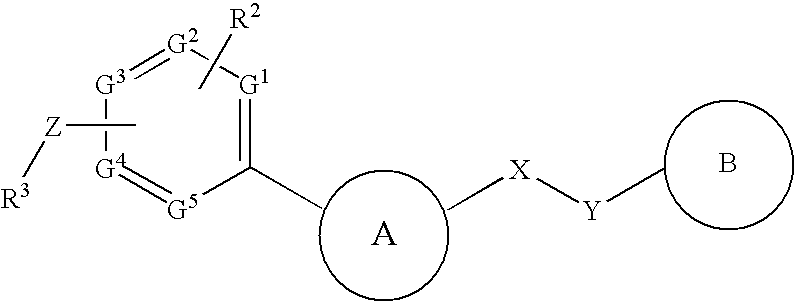
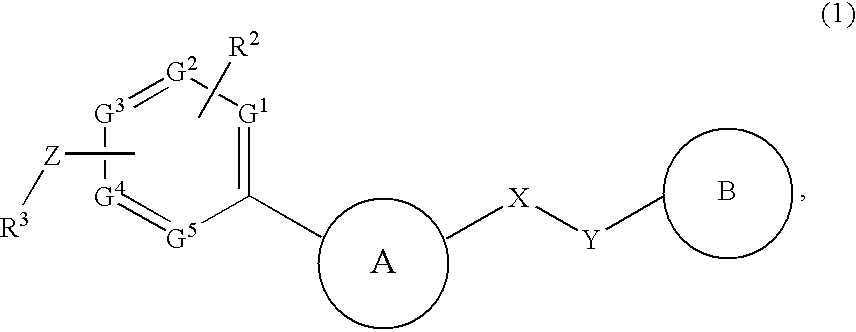
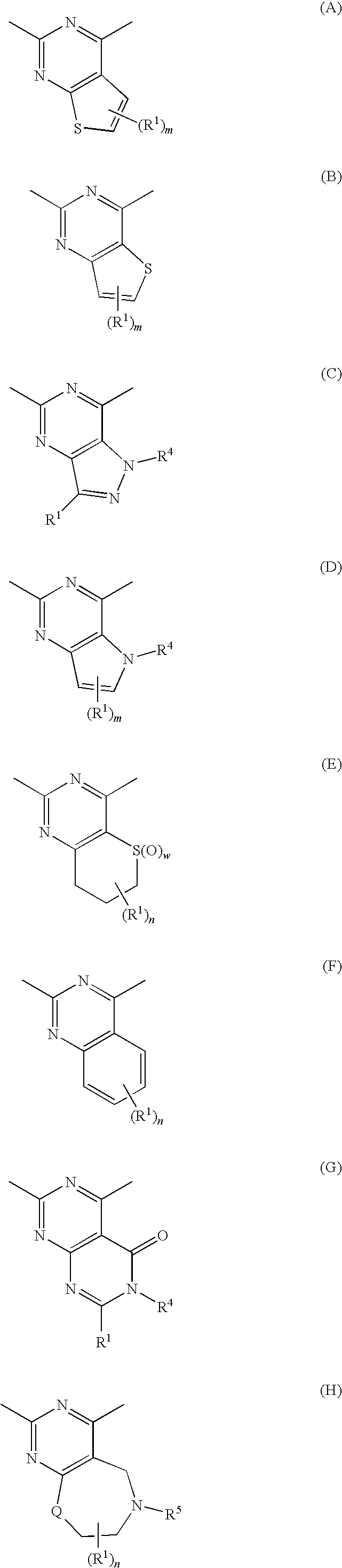
InactiveUS20090182142A1Useful in treatmentOrganic chemistryAntipyreticChemical compoundBronchial epithelium
An aromatic compound represented by the following formula or a pharmaceutically acceptable salt thereof:, wherein ring A is a heterocyclic ring, ring B is a carbocyclic ring, a heterocyclic ring etc., G1, G2, G3, G4 and G5 are CH or N, X is —NH—, —O—, —CH2—, etc., Y is —CH2—, —CO—, —SO2—, etc., Z is a single bond, —CO—, —SO2—, —NH—, —O—, —S—, —CONH—, —SO2NH—, etc., R2 is hydrogen, alkyl, alkoxy, halogen, etc., and R3 is carbocyclic group, heterocyclic group, alkyl, etc.,is useful as a controlling agent of the function of CCR4 useful for the treatment or therapy for bronchial asthma, atopic dermatitis, etc.
Owner:FURUKUBO SHIGERU +1
Recombinant anti-interleukin-9 antibodies
The application describes neutralizing chimeric and humanized anti-human IL-9 antibodies, and the use thereof to identify neutralizing epitopes on human IL-9 and as medicaments to prevent and treat asthma, bronchial hyperresponsiveness, atopic allergy, and other related disorders. Particularly disclosed are recombinant antibodies derived from three murine anti-human IL-9 antibodies identified infra as MH9A3, MH9D1, and MH9L1.
Owner:LUDWIG INST FOR CANCER RES +1
Nanoparticles for targeted delivery of active agents to the lung
InactiveUS20110064652A1Fast and safe and easy deliveryEnhanced local lung deliveryOrganic active ingredientsBiocideActive agentDelivery system
The present invention concerns a delivery system administered to the lung preferably by inhalation comprising a polymer-based nanoparticle; and a linker comprising a first portion non-covalently anchored to said nanoparticle, wherein at least part of said first portion comprises a hydrophobic / lipophilic segment embedded in said nanoparticle; and a second portion comprising a coupling group, preferably a maleimide compound, exposed at the outer surface of said nanoparticle. In accordance with one embodiment, the delivery system comprises one or more targeting agents, each covalently bound to said coupling group, preferably maleimide compound, and is administered as an aerosol in the therapy or diagnosis of lung cancer or bronchial dysplasia. In accordance with yet another embodiment, the delivery system comprises a drug and / or a radiopharmaceutical and / or a contrasting agent. A specific example for a linker in accordance with the invention is octadecyl-4-(maleimideomethyl)cyclohexane-carboxylic amide (OMCCA).
Owner:BORLAK JURGEN +3
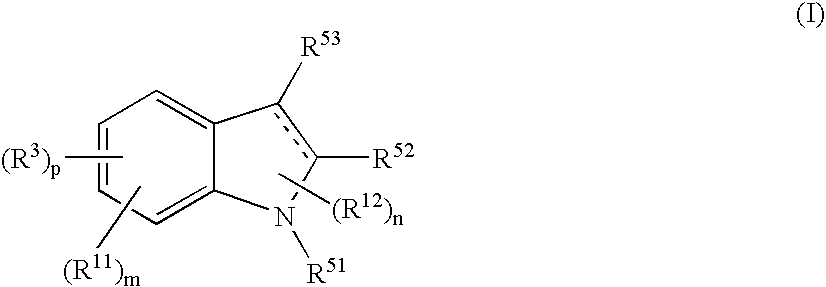
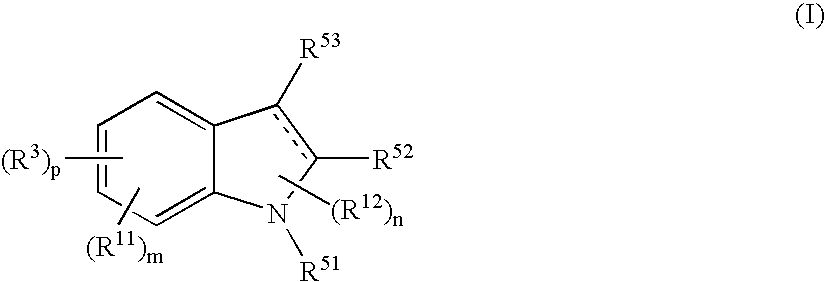
Indole Compound and Use Thereof
InactiveUS20080188532A1Increased airway hyperreactivityImprove respiratory functionBiocideSenses disorderDiseaseBronchial epithelium
The present invention relates to a compound represented by the formula (I),wherein all symbols are as defined in the description,a salt thereof, a solvate thereof, or a prodrug thereof, which has a leukotriene receptor antagonistic activity which is expected to be more effective than those of the leukotriene receptor antagonists currently used in clinical trials. Therefore, it is useful as an agent for the prevention and / or treatment of a leukotriene-mediated disease such as a respiratory diseases such as bronchial asthma, chronic obstructive pulmonary disease, pulmonary emphysema, chronic bronchitis, pneumonia (e.g. interstitial pneumonia etc.), severe acute respiratory syndrome (SARS), acute respiratory distress syndrome (ARDS), allergic rhinitis, sinusitis (e.g. acute sinusitis, chronic sinusitis, etc.), or the like, or as an expectorant or an antiitussive.
Owner:ONO PHARMA CO LTD
Diagnosis, prognosis and treatment of pulmonary diseases
InactiveUS20060078558A1Reducing airway resistance responseImprove responsivenessOrganic active ingredientsPeptide/protein ingredientsDiseaseObstructive Pulmonary Diseases
The present invention provides methods to protect a subject from a respiratory disorder involving an airway obstructive disease such as asthma or chronic obstructive pulmonary disease. Provided are methods to protect a subject from an airway obstructive disease using gene therapy. Methods are provided for supplying FoxA2 function to cells of the lung and airway, such as smooth muscle and epithelial cells, by FoxA2 gene therapy. The FoxA2 gene, a modified FoxA2 gene, or a part of the gene may be introduced into the cell in a vector such that the gene remains extrachromosomal or may be integrated into the subjects chromosomal DNA for expression. These methods provide for administering to a subject in need of such treatment a therapeutically effective amount of a FoxA2 gene, or pharmaceutically acceptable composition thereof, for overexpressing the FoxA2 gene. Such methods of expressing the administered FoxA2 gene in the lungs and airway provide for: (1) preventing or alleving bronchial hyperresponsiveness; (2) preventing or alleving of an airway obstructive disease, e.g., bronchial hyperreactivity, airway hyperresponsiveness, asthma or chronic obstructive pulmonary disorder (“COPD”); (3) reducing the airway resistance response to inhaled natural or synthetic bronchoconstrictors or allergens or to exercise; and (4) enhancing responsiveness (relaxation) of airway tissues to β-agonists.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
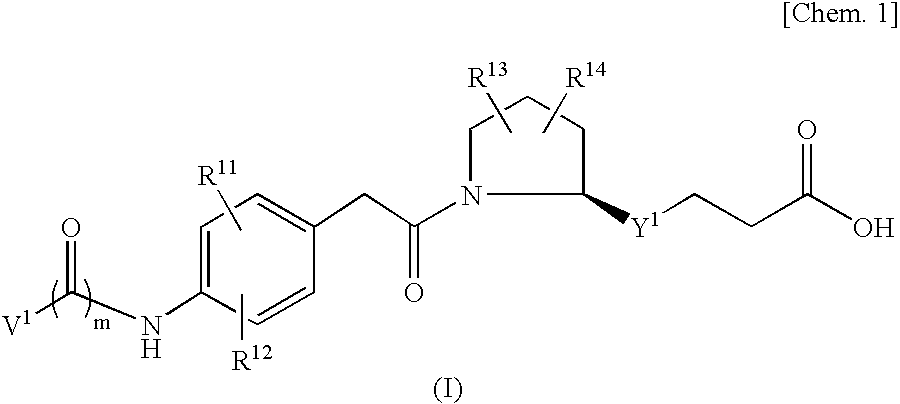
Vla-4 Inhibitor
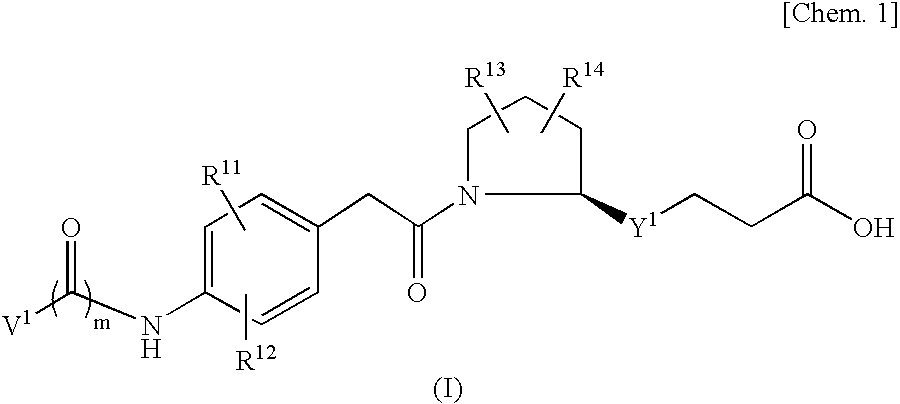
An object of the present invention is to provide a compound which selectively inhibits binding of a ligand and α4β1 integrin (VLA-4), a process for producing the compound, and a medicament containing the compound. A compound represented by the formula (I) etc. or a salt thereof, a process for producing the compound or a salt thereof, a medicament containing the compound or a salt thereof, as well as a preventive and / or a therapeutic agent for a disease caused by cell adhesion, for example, inflammatory reaction, autoimmune disease, cancer metastasis, bronchial asthma, nasal obstruction, diabetes, arthritis, psoriasis, multiple sclerosis, inflammatory bowel disease and rejection reaction at transplantation, containing the compound or a salt thereof as a primary component. [wherein Y1 represents a divalent aryl group etc., V1 represents an aryl group etc., and R11 to R14 represent H, OH or a halogen atom etc.]
Owner:DAIICHI PHARMA CO LTD
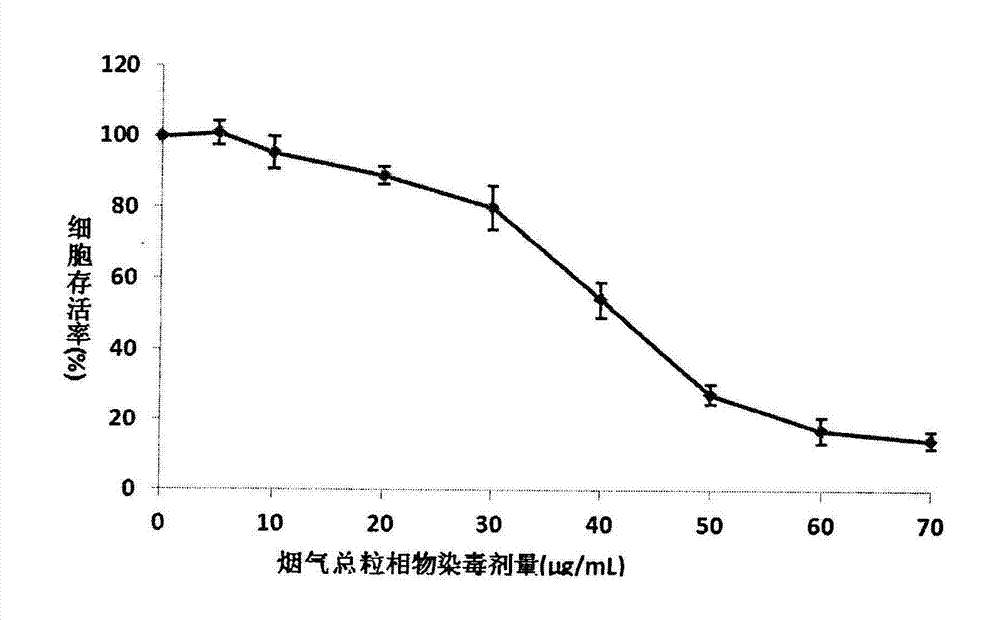
MTT (thiazolyl blue) cell toxicity test method of biological assessment of total particle matter in cigarette smoke
InactiveCN103088105AReduce the number of steps to wash cellsStrong targetingMicrobiological testing/measurementColor/spectral properties measurementsBronchial epitheliumCell system
The invention relates to an MTT (thiazolyl blue) cell toxicity test method of biological assessment of a total particle matter in cigarette smoke and belongs to the technical field of safety assessment of tobacco and cigarette smoke. The MTT cell toxicity test method is characterized by comprising the following steps of: inoculating and culturing immortalized human bronchial epithelial cells (BEAS-2B cells) and contaminating the total particle matter in the cigarette smoke; detecting the cell survival rate by adopting an MTT method; and analyzing and evaluating the cell toxicity of the total particle matter in the cigarette smoke according to a test result. Compared with the prior art, the MTT cell toxicity test method has the following characteristics that BEAS-2B cells are human cells and are target organ source cells acting on a human body by the smoke; a BEAS-2B cell system is used for carrying out cell toxicity assessment on the cigarette smoke and has the strong pertinence; in a testing process, steps of washing the cells for a plurality of times are reduced; and a formaldehyde fixing step does not need to be carried out so that a testing period is shortened, the operation is rapid and convenient, the sensitivity is high and the result is stable and reliable. Moreover, the MTT test method disclosed by the invention is further applicable to a smoke cell toxicity test of various cell systems and the commonality is strong.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
Lung bronchial segmentation and calibration method
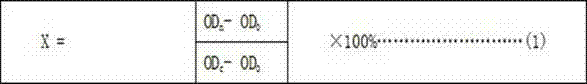
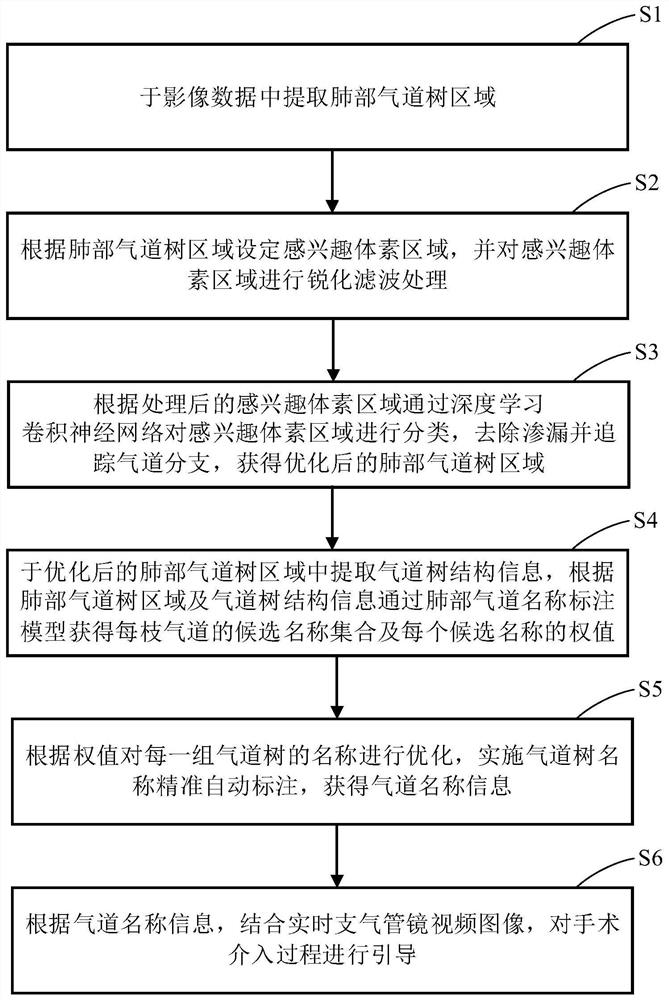
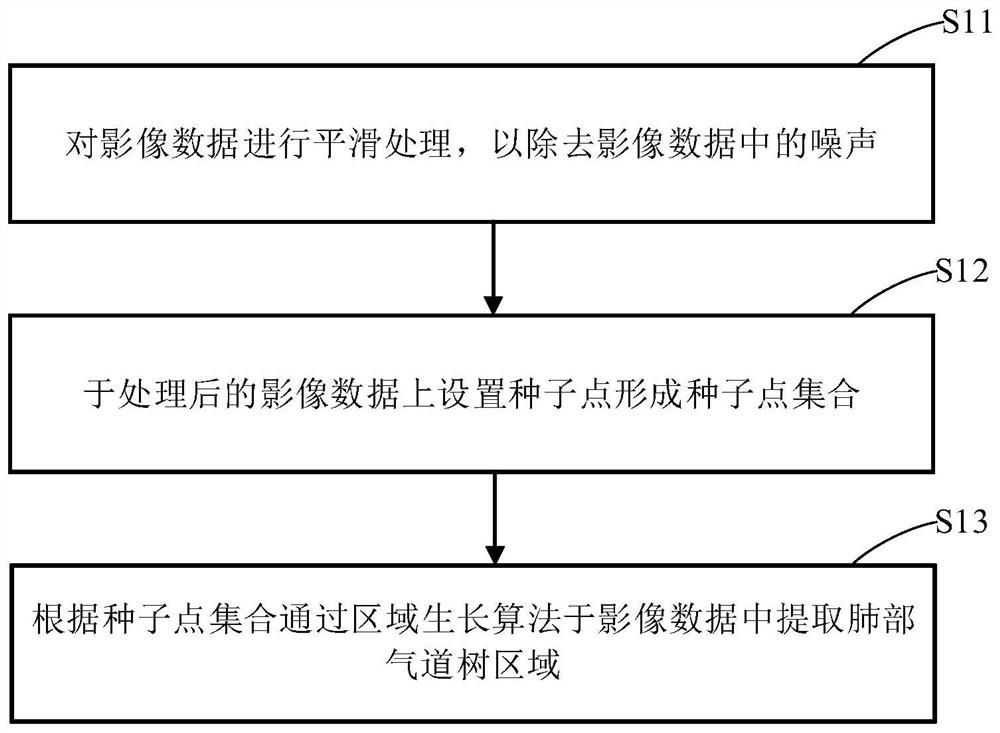
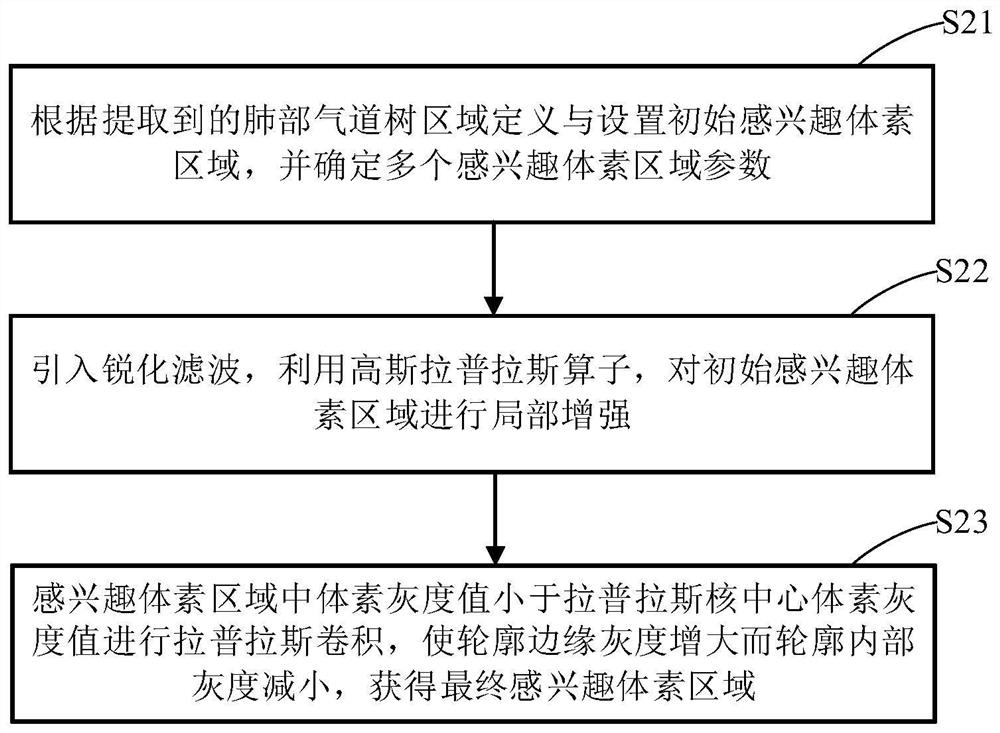
PendingCN112330686AReduce volume effectReduce noiseImage enhancementImage analysisVoxelBronchial epithelium
The invention discloses a lung bronchial segmentation and calibration method. The method comprises the following steps of S1, extracting a lung airway tree region from image data; S2, setting an interested voxel region according to the lung airway tree region, and performing sharpening filtering processing on the interested voxel region; S3, classifying the voxel regions of interest through a deeplearning convolutional neural network according to the processed voxel regions of interest, removing leakage and tracking airway branches to obtain an optimized lung airway tree region; S4, extracting airway tree structure information from the optimized lung airway tree region, and obtaining a candidate name set of each airway and a weight of each candidate name through a lung airway name labeling model according to the lung airway tree region and the airway tree structure information; and S5, optimizing the names of each group of airway trees according to the weights, and implementing accurate and automatic labeling of the names of the airway trees to obtain airway name information.
Owner:罗雄彪 +3
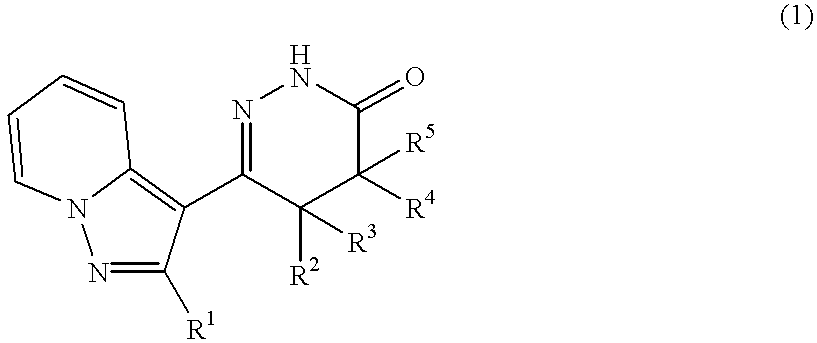
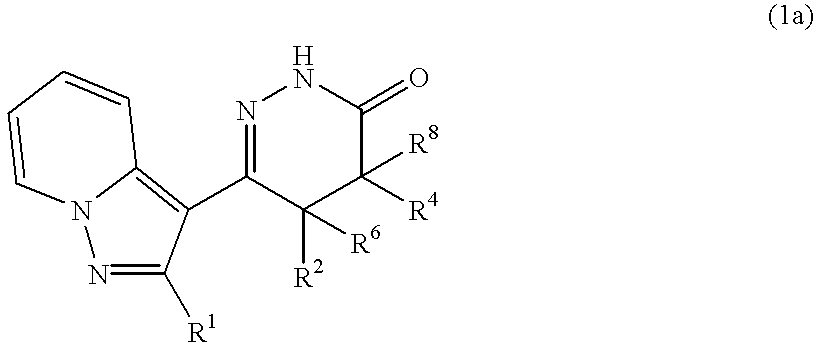
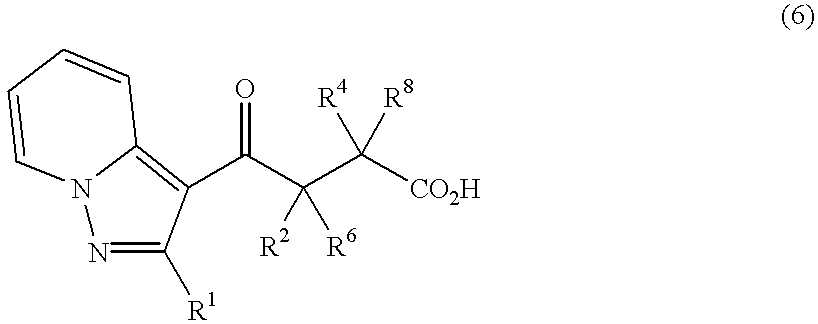
Pyrazolopyridylpyridazinone derivatives and process for the preparation thereof
Novel pyrazolopyridylpyridazinone derivatives characterized by being represented by general formula (1) and pharnacologically acceptable salts thereof, which exhibit a phosphodiesterase inhibiting activity and have a selective potent bronchodilating effect on the respiratory tract; a process for the preparation of them; and bronchodilators containing the same as the active ingredient; wherein R1 is C1-C4 lower alkyl or C3-C6 cycloalkyl; and R2, R3, R4 and R5 are each independently hydrogen, C1-C4 lower alkyl or phenyl, or alternatively R3 and R5 may be united to form a double bond.
Owner:KYORIN PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
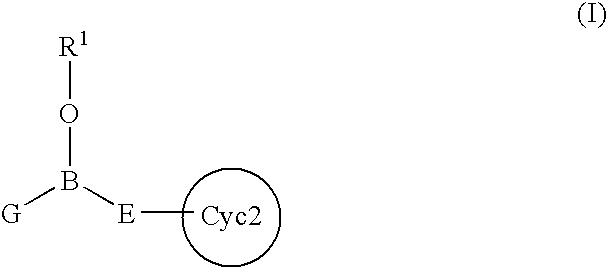
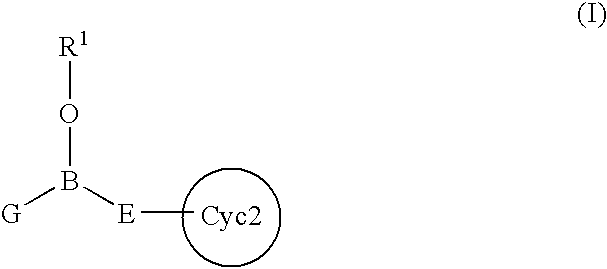
![Certain (2S)-n-[(1S)-1-cyano-2-phenylethyl]-1,4-oxazepane-2-carboxamides as dipeptidyl peptidase 1 inhibitors for treating bronchiectasis Certain (2S)-n-[(1S)-1-cyano-2-phenylethyl]-1,4-oxazepane-2-carboxamides as dipeptidyl peptidase 1 inhibitors for treating bronchiectasis](https://images-eureka.patsnap.com/patent_img/4b914f3c-69a9-4bcc-8c7e-af7950ba3cba/US20180028541A1-20180201-C00001.png)
![Certain (2S)-n-[(1S)-1-cyano-2-phenylethyl]-1,4-oxazepane-2-carboxamides as dipeptidyl peptidase 1 inhibitors for treating bronchiectasis Certain (2S)-n-[(1S)-1-cyano-2-phenylethyl]-1,4-oxazepane-2-carboxamides as dipeptidyl peptidase 1 inhibitors for treating bronchiectasis](https://images-eureka.patsnap.com/patent_img/4b914f3c-69a9-4bcc-8c7e-af7950ba3cba/US20180028541A1-20180201-C00002.png)
![Certain (2S)-n-[(1S)-1-cyano-2-phenylethyl]-1,4-oxazepane-2-carboxamides as dipeptidyl peptidase 1 inhibitors for treating bronchiectasis Certain (2S)-n-[(1S)-1-cyano-2-phenylethyl]-1,4-oxazepane-2-carboxamides as dipeptidyl peptidase 1 inhibitors for treating bronchiectasis](https://images-eureka.patsnap.com/patent_img/4b914f3c-69a9-4bcc-8c7e-af7950ba3cba/US20180028541A1-20180201-C00003.png)