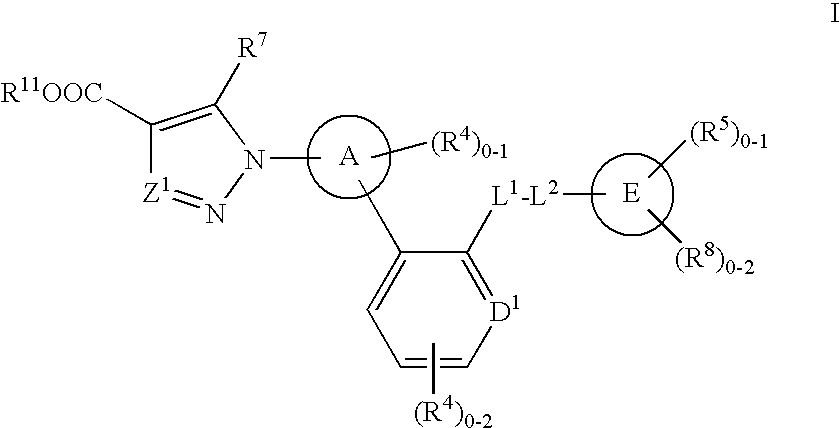
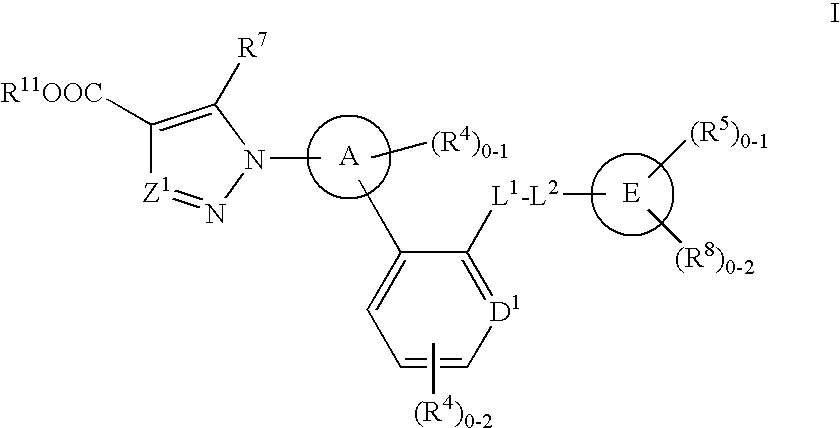
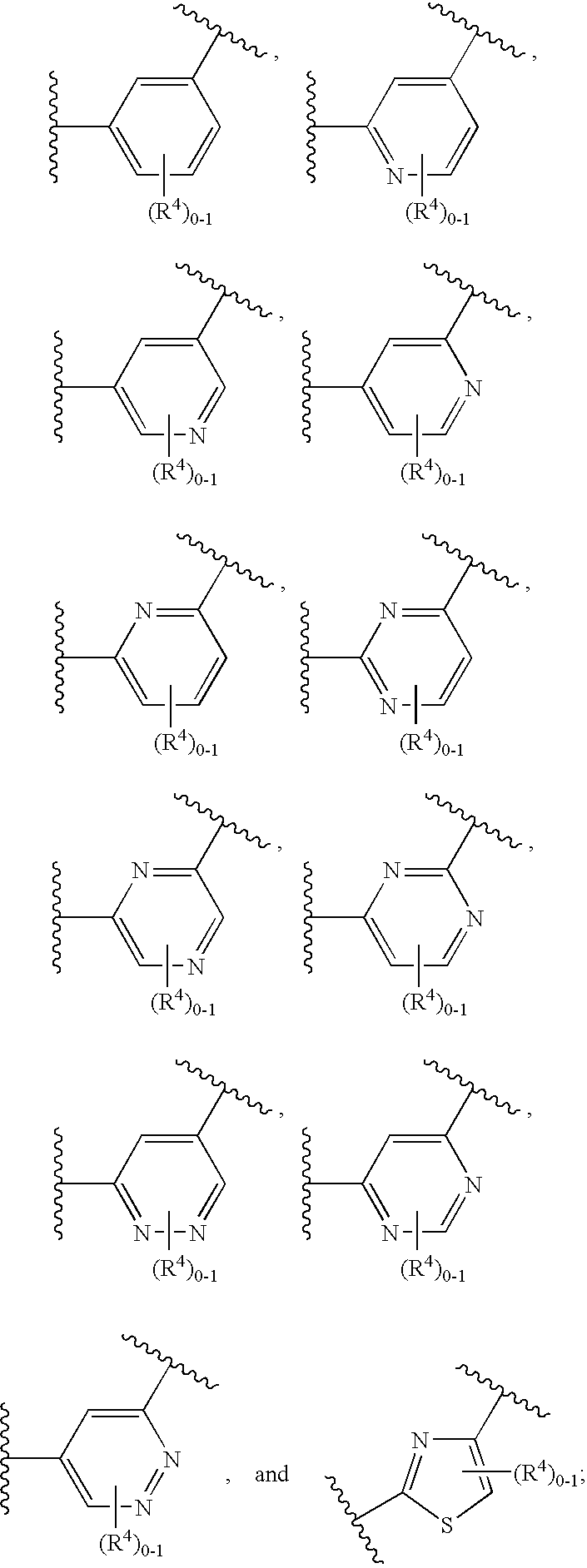
Soluble guanylate cyclase activators
a technology of guanylate cyclase and activator, which is applied in the field of soluble guanylate cyclase activator, can solve the problems of increased dosage, increased tolerance and activity, and poisons that have a predominantly weak effect on cgmp formation, and achieve the effect of suitable for disease therapy and prophylaxis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0184]
Step A. Ethyl-1-(6-chloropyridin-2-yl)-5-trifluoromethyl-1H-pyrazole-4-carboxylate
[0185]To a solution 2-chloro-6-hydrazinopyridine (1.00 g, 6.97 mmol) and triethylamine (0.971 mL, 6.97 mmol) in acetonitrile (35 mL) was added ethyl 2-(ethoxymethylene)-4,4,4-trifluoro-3-oxobutyrate (1.36 mL, 6.97 mmol). After 20 min, the reaction mixture was placed in a 60° C. oil bath. After 30 min, the reaction mixture was allowed to cool to ambient temperature, then was concentrated in vacuo. Purification by flash chromatography on silica gel (0 to 30% EtOAc in hexanes, then 30 to 100% EtOAc in hexanes) gave the title compound: LCMS m / z 319.9 [M+H]+; 1H NMR (500 MHz, CDCl3) δ 8.10 (s, 1H), 7.88 (t, J=7.5 Hz, 1H), 7.58 (d, J=8.0 Hz, 1H), 7.47 (d, J=8.0 Hz, 1H), 4.38 (q, J=7.0 Hz, 2H), 1.38 (t, J=7.0 Hz, 3H).
Step B. Ethyl 1-[6-(2-hydroxylphenyl)pyridine-2-yl]-5-trifluoromethyl-1H-pyrazole-4-carboxylate
[0186]To a flask containing the title compound from the Example 1 Step A (500 mg, 1.56 mmol) w...
example 2
[0189]
Step A. Ethyl 1-(6-{2-[(4-bromobenzyl)oxy]phenyl}pyridin-2-yl)-5-(trifluoromethyl)-1H-pyrazole-4-carboxylate
[0190]To a solution of the title compound from Example 1 Step B (682 mg, 1.81 mmol) in DMF (10 mL) were added 4-bromobenzyl bromide (678 mg, 2.71 mmol) and cesium carbonate (1.77 g, 5.42 mmol). After 1.5 h, the reaction mixture was poured into sat. aq. NH4Cl and extracted with EtOAc. The organic phase was separated, dried over sodium sulfate, filtered, and concentrated in vacuo. Purification by flash chromatography on silica gel (0 to 25% EtOAc in hexanes, then 25 to 100% EtOAc in hexanes) provided the title compound: LCMS m / z 548.0 [M+H]+; 1H NMR (500 MHz, CDCl3) δ 8.13 (s, 1H), 8.08 (d, J=8.0 Hz, 1H), 7.95 (dd, J=7.5, 1.5 Hz, 1H), 7.88 (t, J=8.0 Hz, 1H), 7.54 (d, J=8.0 Hz, 1H), 7.48 (d, J=8.0 Hz, 2H), 7.39-7.36 (m, 1H), 7.23 (d, J=8.0 Hz, 2H), 7.12 (t, J=7.5 Hz, 1H), 7.03 (d, J=7.5 Hz, 1H), 5.10 (s, 2H), 4.39 (q, J=7.0 Hz, 2H), 1.39 (t, J=7.0 Hz, 3H).
Step B. Ethyl 5-(t...
example 3
[0193]
Step A. Ethyl 5-(trifluoromethyl)-1-(6-{2-[(4-{(E)-2-[4-(trifluoromethyl)phenyl]vinyl}benzyl)oxy]phenyl}pyridin-2-yl)-1H-pyrazole-4-carboxylate
[0194]To a vial containing the title compound from Example 2 Step A (50.0 mg, 0.092 mmol) were added 2-(4-trifluoromethylphenyl)vinyl boronic acid (29.6 mg, 0.137 mmol) and trans-dichlorobis(triphenylphosphine) palladium (II) (6.4 mg, 0.009 mmol). Acetonitrile (0.400 mL) and sodium carbonate (0.229 mL, 1.0 M aqueous, 0.229 mmol) were added, and the resulting mixture was degassed via nitrogen sparge. The reaction vial was capped and placed in a pre-heated oil bath (70° C.). After 18 h, the reaction mixture was allowed to cool to ambient temperature and was poured into water. The mixture was extracted with DCM, and the organic phase was concentrated in vacuo. Purification by chromatography on silica gel (0 to 30% EtOAc in hexanes, then 30 to 100% EtOAc in hexanes) provided the title compound: LCMS m / z 638.4 [M+H]+; 1H NMR (500 MHz, CDCl3)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
| Electrical conductance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com