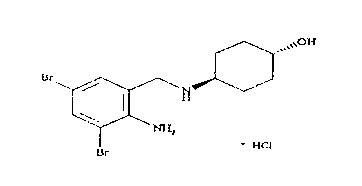
Stable amorphous ambroxol hydrochloride compound
A ambroxol hydrochloride, amorphous technology, applied in the field of medicine, can solve problems affecting the quality and safety of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
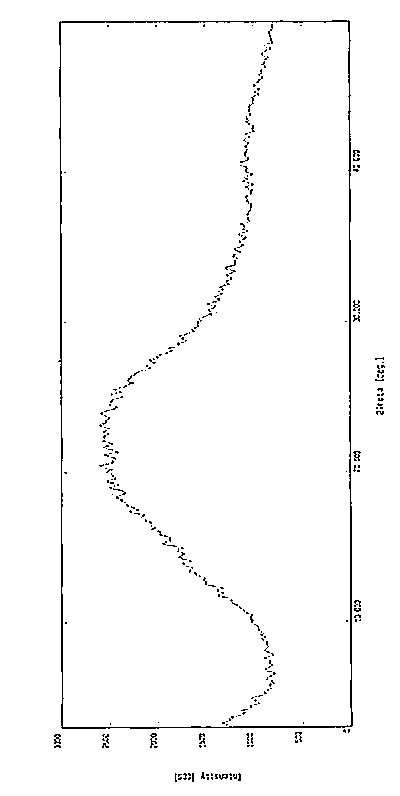
[0058] The X-ray diffraction pattern of the powder is shown in figure 1 . Instrument model and measurement conditions: Rigaku D / max 2500 diffractometer; CuKa 40Kv 100mA; 2θ scanning range: 0-50 ° .
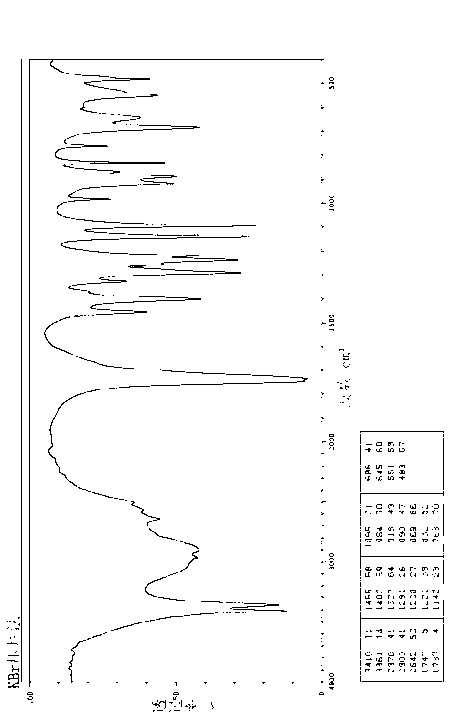
[0059] The infrared spectrum of the crystal is shown in figure 2 , Determination with KBr tablet.
[0060] Example 2
Embodiment 2
[0062] prescription:
[0063] Ambroxol Hydrochloride Amorphous 15g
[0064] Lactose 500g
[0065] Microcrystalline Cellulose 240g
[0066] Aspartame 15g
[0067] Croscarmellose Sodium 80g
[0068] Hydroxypropyl Methyl Cellulose 135g
[0070] Appropriate amount of water, made into 1000 bags.
[0071] Process:
[0072] 1) Preparation: dry lactose, microcrystalline cellulose, aspartame, croscarmellose sodium, hydroxypropyl methylcellulose, and magnesium stearate at 80°C for 4 hours, and set aside;
[0073] 2) Weighing: Weigh the prescription amount of amorphous ambroxol hydrochloride, lactose, microcrystalline cellulose, aspartame, croscarmellose sodium, hydroxypropyl methylcellulose, magnesium stearate ;
[0074] 3) Mixing: Put the prescribed amount of amorphous ambroxol hydrochloride, lactose, microcrystalline cellulose, aspartame, croscarmellose sodium, and hydroxypropyl methylcellulose into a high-efficiency mixing granulator in, mix...
Embodiment 3
[0082] prescription:
[0083] Ambroxol Hydrochloride Amorphous 15g
[0084] Lactose 60g
[0085] Microcrystalline Cellulose 50g
[0086] Croscarmellose Sodium 30g
[0087] Hydroxypropyl Methyl Cellulose 15g
[0089] Appropriate amount of water, made into 1000 capsules.
[0090] Process:
[0091] 1) Preparation: dry lactose, microcrystalline cellulose, croscarmellose sodium, hydroxypropyl methylcellulose, and magnesium stearate at 80°C for 4 hours, and set aside;
[0092] 2) Weighing: Take amorphous ambroxol hydrochloride, lactose, microcrystalline cellulose, croscarmellose sodium, hydroxypropyl methylcellulose, magnesium stearate of prescription quantity;
[0093] 3) Mixing: put the prescription amount of amorphous ambroxol hydrochloride, lactose, microcrystalline cellulose, croscarmellose sodium, and hydroxypropyl methylcellulose into a high-efficiency mixing granulator, and mix evenly;
[0094] 4) Granulation: Add an appropriate amoun...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com