Patents
Literature
402 results about "Rhinitis allergic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Allergic rhinitis is a diagnosis associated with a group of symptoms affecting the nose. These symptoms occur when you breathe in something you are allergic to, such as dust, animal dander, or pollen.
Semi-soft C-class immunostimulatory oligonucleotides
The invention relates to specific C-Class semi-soft CpG immunostimulatory oligonucleotides that are useful for stimulating an immune response. In particular the oligonucleotides are useful for treating allergy, such as allergic rhinitis and asthma, cancer and infectious disease, such as hepatitis B and hepatitis C.
Owner:COLEY PHARMA GRP INC +1
Compositions and methods for treating asthma and other lung disorders
InactiveUS20100008997A1Powder deliveryOrganic active ingredientsObstructive Pulmonary DiseasesOxygen
Provided are compositions and methods for treating lung or respiratory disorders or conditions characterized by airflow obstruction or limitation, or a symptom thereof (e.g., asthma, rhinitis, allergic rhinitis, and chronic obstructive pulmonary disease (COPD) and COPD-associated conditions (e.g., bronchitis, emphysema, asthma), emphysema, pneumonia, bronchitis, influenza, SARS, tuberculosis, and whooping cough (pertussis), and the like) in a subject in need thereof by administering a therapeutic composition comprising at least one electrokinetically altered fluid (gas-enriched (e.g., oxygen-enriched) electrokinetic fluids) comprising an ionic aqueous solution of charge-stabilized oxygen-containing nanostructures as disclosed herein. In certain aspects, the methods comprise regulating intracellular signal transduction by modulation of at least one of cellular membranes, membrane potential, membrane proteins (e.g., membrane receptors, (e.g., to G protein coupled receptors, and intercellular junctions)). Additional aspects include therapeutic compositions, and combination treatment methods comprising administration of electrokinetically generated fluid in combination with at least one additional therapeutic agent.
Owner:REVALESIO CORP
Compounds
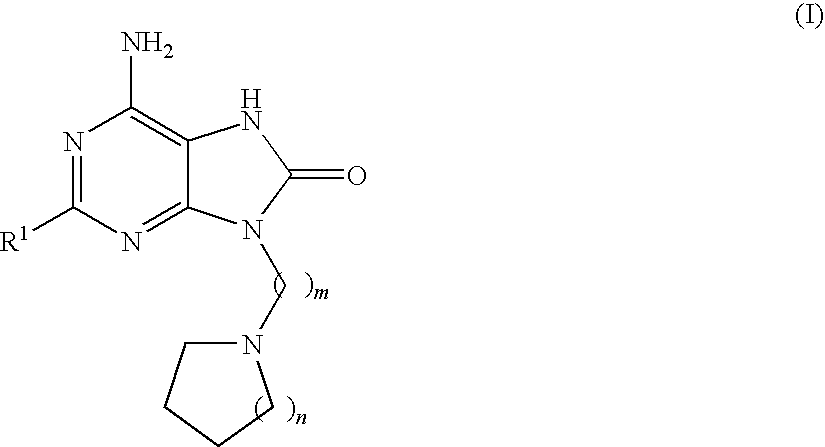
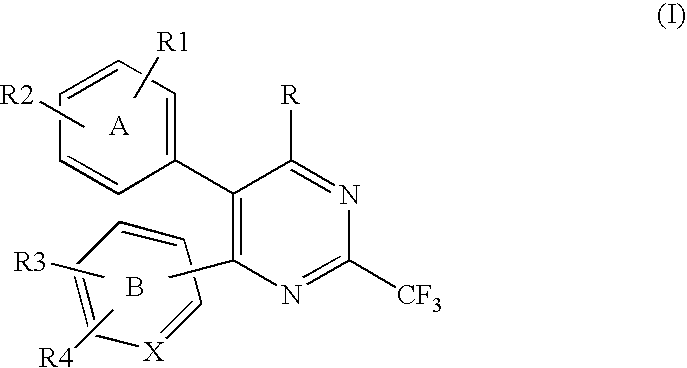
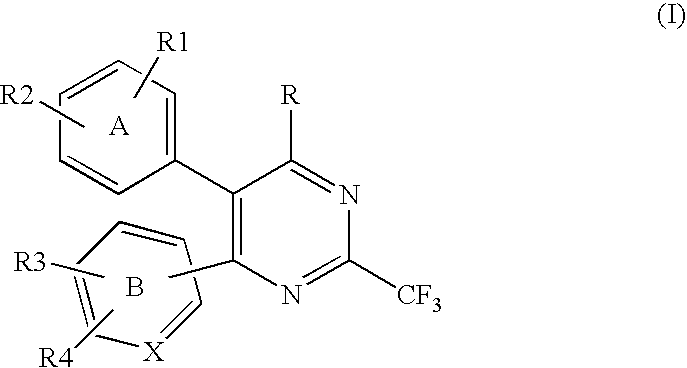
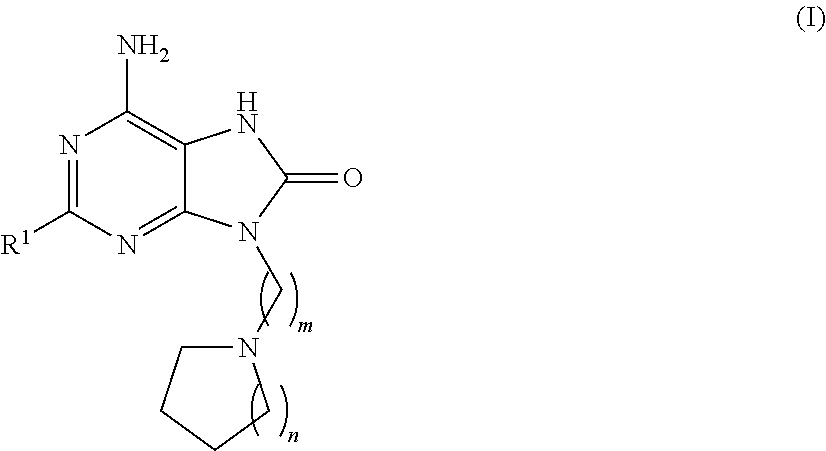
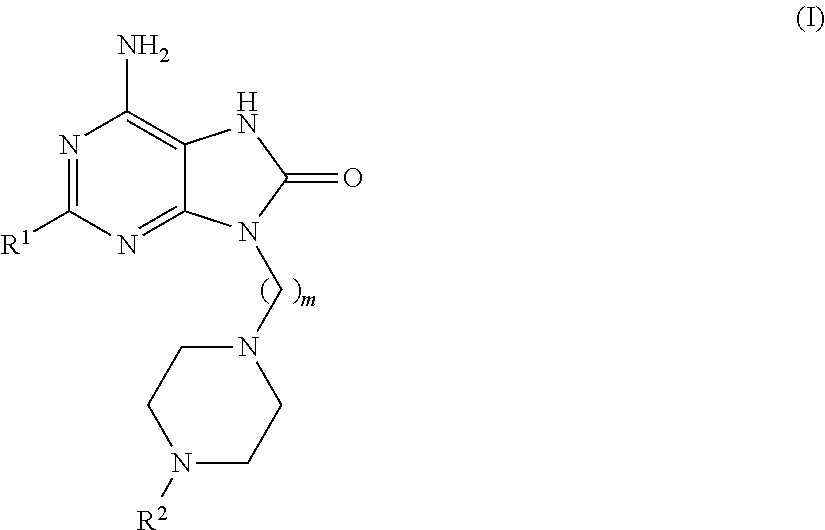
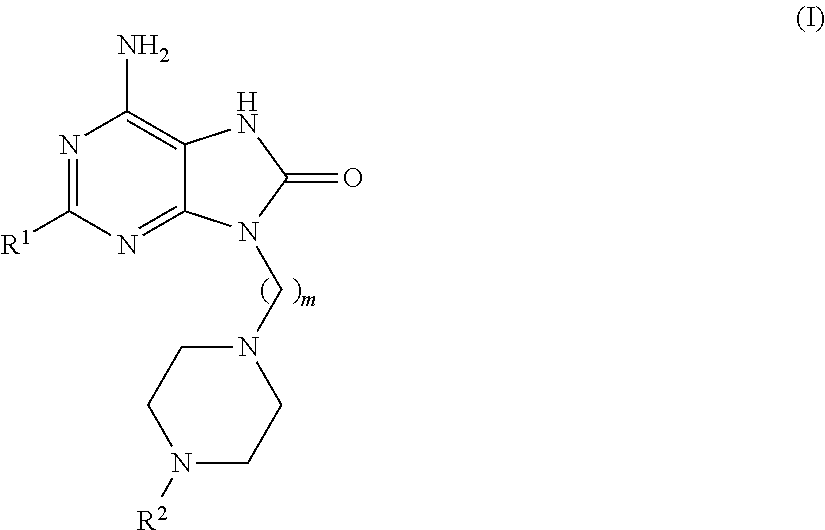
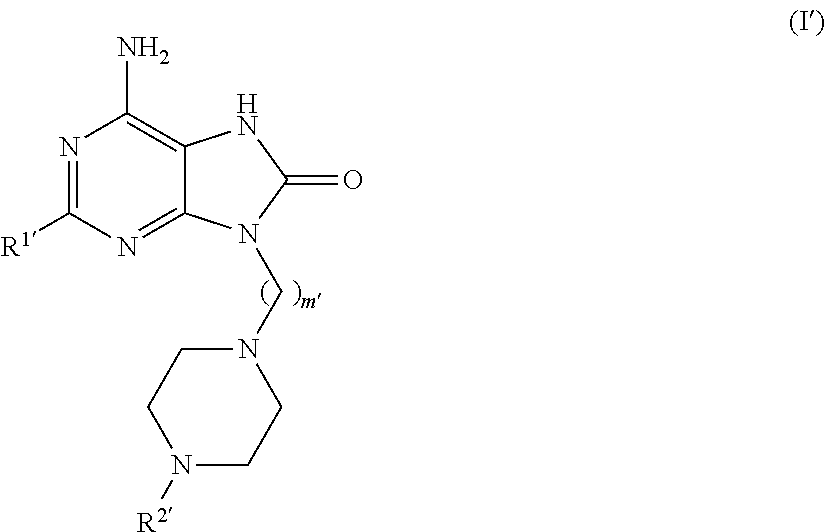
Compounds of formula (I):wherein R1 is C1-6alkylamino, C1-6alkoxy, or C3-7cycloalkyloxy; m is an integer having a value of 3 to 6; n is an integer having a value of 0 to 4; and salts thereof are inducers of human interferon. Compounds which induce human interferon may be useful in the treatment of various disorders, for example the treatment of allergic diseases and other inflammatory conditions for example allergic rhinitis and asthma, the treatment of infectious diseases and cancer, and may also be useful as vaccine adjuvants.
Owner:GLAXO SMITHKLINE LLC
Compositions and methods for treating asthma and other lung disorders
Provided are compositions and methods for treating or preventing lung or respiratory disorders or conditions characterized by airflow obstruction or limitation, or a symptom thereof (e.g., asthma, rhinitis, allergic rhinitis (e.g. nose respiratory tract), and chronic obstructive pulmonary disease (COPD) and COPD-associated conditions (e.g., bronchitis, emphysema, asthma), emphysema, pneumonia, bronchitis, influenza, SARS, tuberculosis, and whooping cough (pertussis), and the like) in a subject in need thereof by administering a therapeutic composition comprising at least one electrokinetically generated fluid (including gas-enriched electrokinetically generated fluids) as disclosed herein, the electrokinetically altered aqueous fluid suitable to alter cellular membrane structure or function sufficient to provide for modulation of intracellular signal transduction, wherein treating a lung disorder or a symptom thereof is thereby afforded. Additional aspects relate to therapeutic compositions, and combination treatment methods comprising administration of at least one electrokinetically generated fluid in combination with at least one additional therapeutic agent.
Owner:REVALESIO CORP
Compositions and methods for treating asthma and other lung disorders
Provided are compositions and methods for treating lung or respiratory disorders or conditions characterized by airflow obstruction or limitation, or a symptom thereof (e.g., asthma, rhinitis, allergic rhinitis, and chronic obstructive pulmonary disease (COPD) and COPD-associated conditions (e.g., bronchitis, emphysema, asthma), emphysema, pneumonia, bronchitis, influenza, SARS, tuberculosis, and whooping cough (pertussis), and the like) in a subject in need thereof by administering a therapeutic composition comprising at least one electrokinetically altered fluid (gas-enriched (e.g., oxygen-enriched) electrokinetic fluids) comprising an ionic aqueous solution of charge-stabilized oxygen-containing nanostructures as disclosed herein. In certain aspects, the methods comprise regulating intracellular signal transduction by modulation of at least one of cellular membranes, membrane potential and / or conductance, membrane proteins (e.g., membrane receptors, (e.g., to G protein coupled receptors, and intercellular junctions)). Additional aspects include therapeutic compositions, and combination therapies comprising administration of the electrokinetically generated fluids with at least one additional therapeutic agent.
Owner:REVALESIO CORP
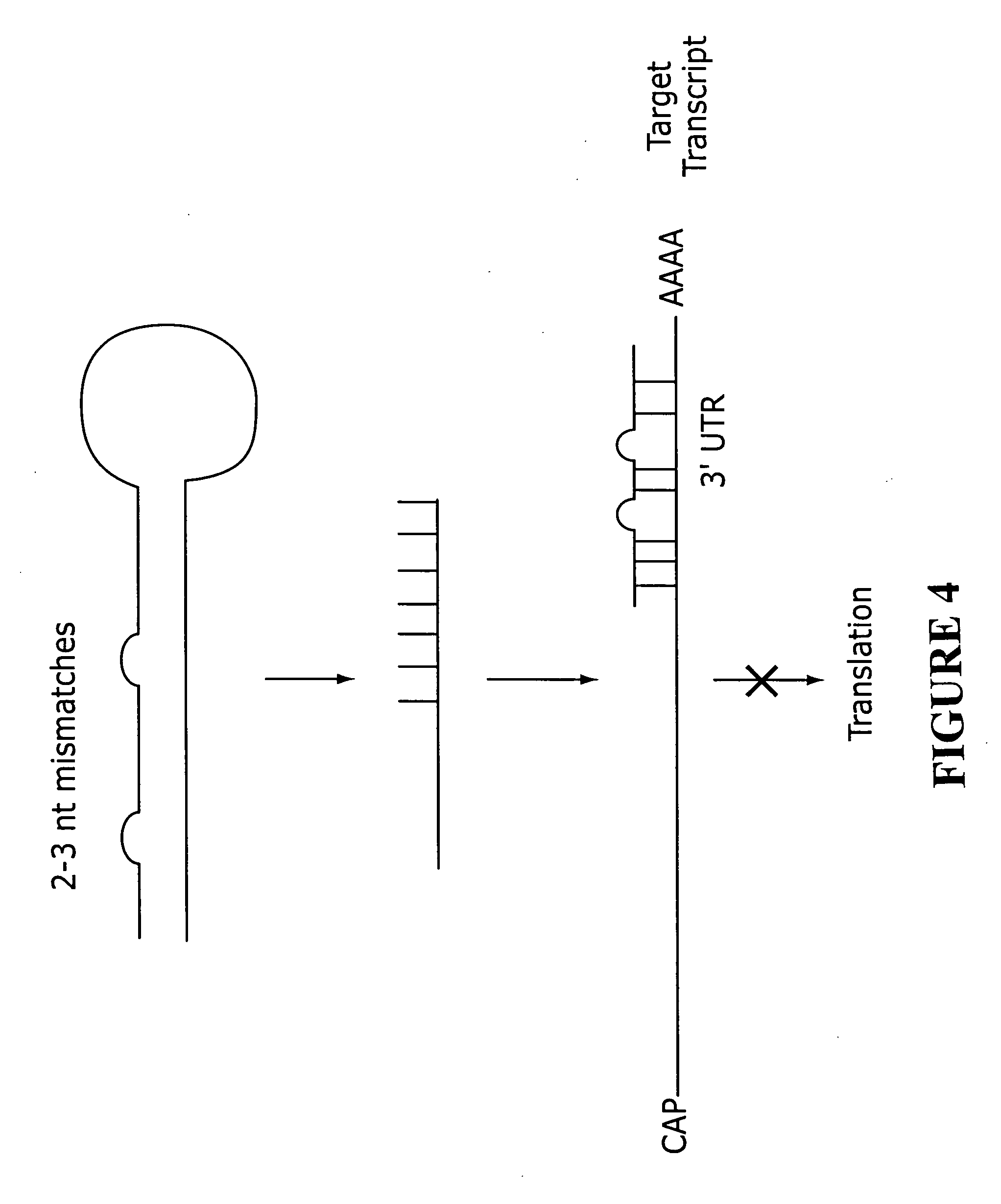
RNAi-based therapeutics for allergic rhinitis and asthma
InactiveUS20060058255A1Suppress gene expressionGood curative effectAntibacterial agentsSpecial deliveryTLR8Disease
The present invention provides compositions comprising one or more RNAi agents (e.g., siRNAs, shRNAs, or RNAi vectors) for the treatment of conditions and diseases mediated by (e.g., featuring IgE-mediated hypersensitivity), as well as systems for identifying RNAi agents effective for this purpose. The compositions are suitable for the treatment of allergic rhinitis and / or asthma. In certain embodiments of the invention the RNAi agent is targeted to a transcript that encodes a protein selected from the group consisisting of the FCεRIα chain, the FCεRIβ chain, c-Kit, Lyn, Syk, ICOS, OX40L, CD40, CD80, CD86, Re1A, Re1B, 4-1BB ligand, TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, TLR8, TLR9, CD83, SLAM, common γ chain, and COX-2. In addition, the invention provides RNAi agent / delivery agent compositions and methods of use. In certain embodiments of the invention compositions comprising an RNAi agent are delivered by the respiratory route.
Owner:MASSACHUSETTS INST OF TECH
Method For Treating Nasal Irritation
A method for treating nasal cavity irritation, such as symptoms of allergic rhinitis. The method includes inserting a plug of a frozen liquid into a nasal cavity of a patient experiencing nasal irritation. The method also includes holding the plug against a lining of the nasal cavity to reduce the irritation.
Owner:JACOBSON ABBY NOMA +1
Nanoparticulate leukotriene receptor antagonist/corticosteroid formulations
InactiveUS20070065374A1Useful in prophylaxis and chronic treatment of asthmaGood curative effectPowder deliveryBiocidePediatric patientPatient compliance
Nanoparticulate compositions comprising a corticosteroid and a leukotriene receptor antagonist are described. The compositions are useful in the prophylaxis and chronic treatment of asthma in adults and pediatric patients and for the relief of allergic conjunctivitis, symptoms of seasonal allergic rhinitis in adults and pediatric patients. Combining a leukotriene receptor antagonist with a corticosteroid in a particle size ranges of less than 2000 nm in a single formulation results in improved efficacy. In addition, patient compliance is enhanced since only one dosage form is needed. Furthermore, local administration of the leukotriene receptor antagonist results in less liver toxicity since the liver will be exposed to lower amounts of drug than happens following oral administration. The drug compositions according to the invention can be formulated into inhalation, nasal, or ocular formulations.
Owner:ELAN PHRMA INT LTD
Semi-soft c-class immunostimulatory oligonucleotides
The invention relates to specific C-Class semi-soft CpG immunostimulatory oligonucleotides that are useful for stimulating an immune response. In particular the oligonucleotides are useful for treating allergy, such as allergic rhinitis and asthma, cancer and infectious disease, such as hepatitis B and hepatitis C.
Owner:COLEY PHARMA GMBH +1
Herbal cough formulations and process for the preparation thereof
The present invention relates to herbal composition for the treatment of chronic respiratory disorders such as cold, cough, allergic asthma, seasonal allergic rhinitis, pharyngitis, laryngitis and the like and a process for preparing the same. The composition comprises extracts derived from Ayurvedic plants selected from the following group: The process for preparing the herbal composition of the present invention comprises procuring, cleaning, grading of specified herbal plants as per the standard specification, disintegrating and pulverizing separately to form the coarse powder, then macerating individual powders separately with aqueous solution of preservatives and then extracting, filtering, concentrating and spray drying to make dry extract powder or semisolid soft extract. Further dissolving each of the extracts separately in aqueous solution containing preservatives and then allowing to maturate after mixing. Then adding filtered extracts to the sugar syrup containing preservatives, glycerin and appropriate excipients to obtain a homogenous cough syrup. The herbal composition of the present invention is non alcoholic, non sedating and non-freezing in nature and the process of preparation as described above does not employ pharmaceutically unacceptable organic solvents.
Owner:J B CHEM & PHARMA
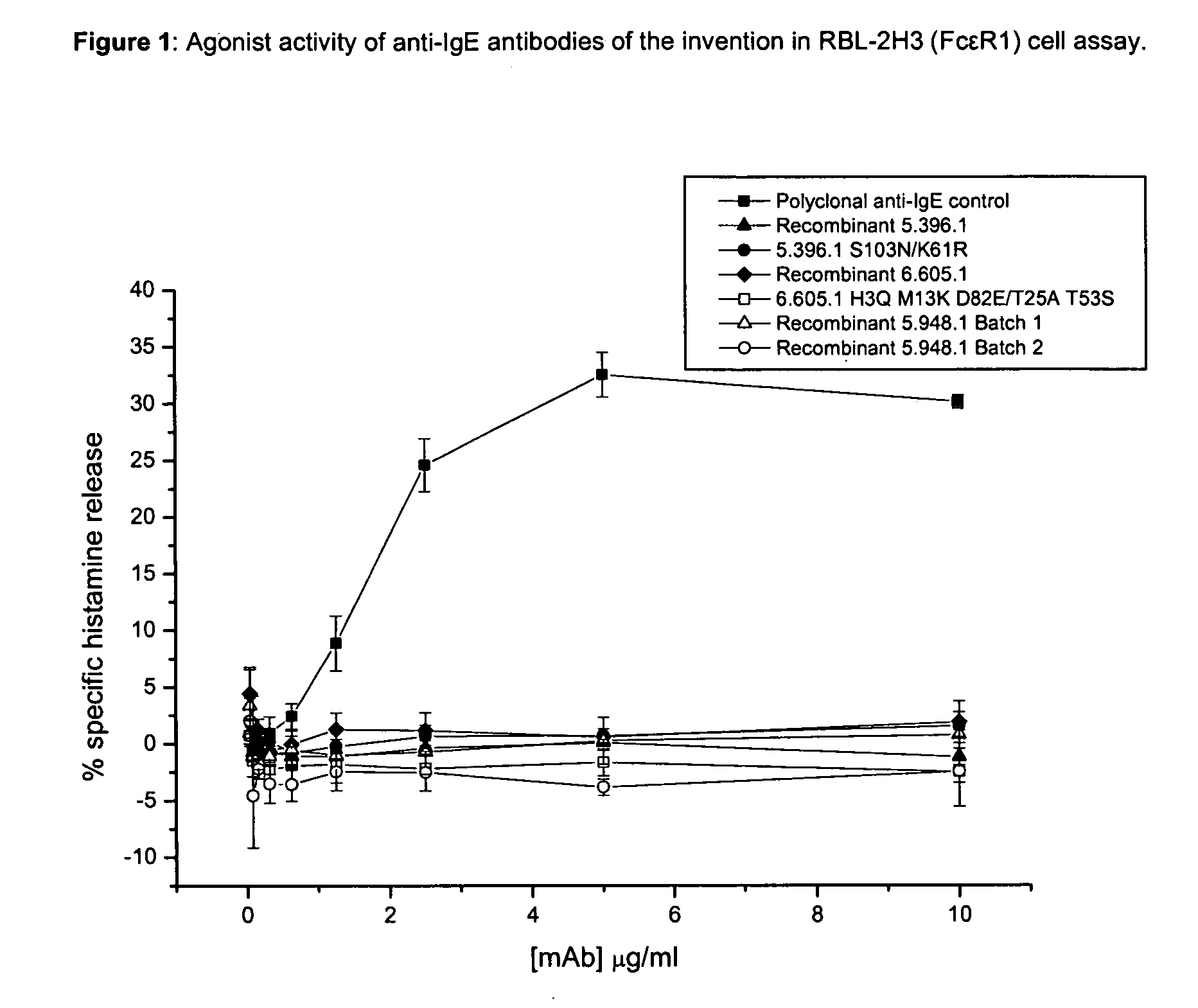
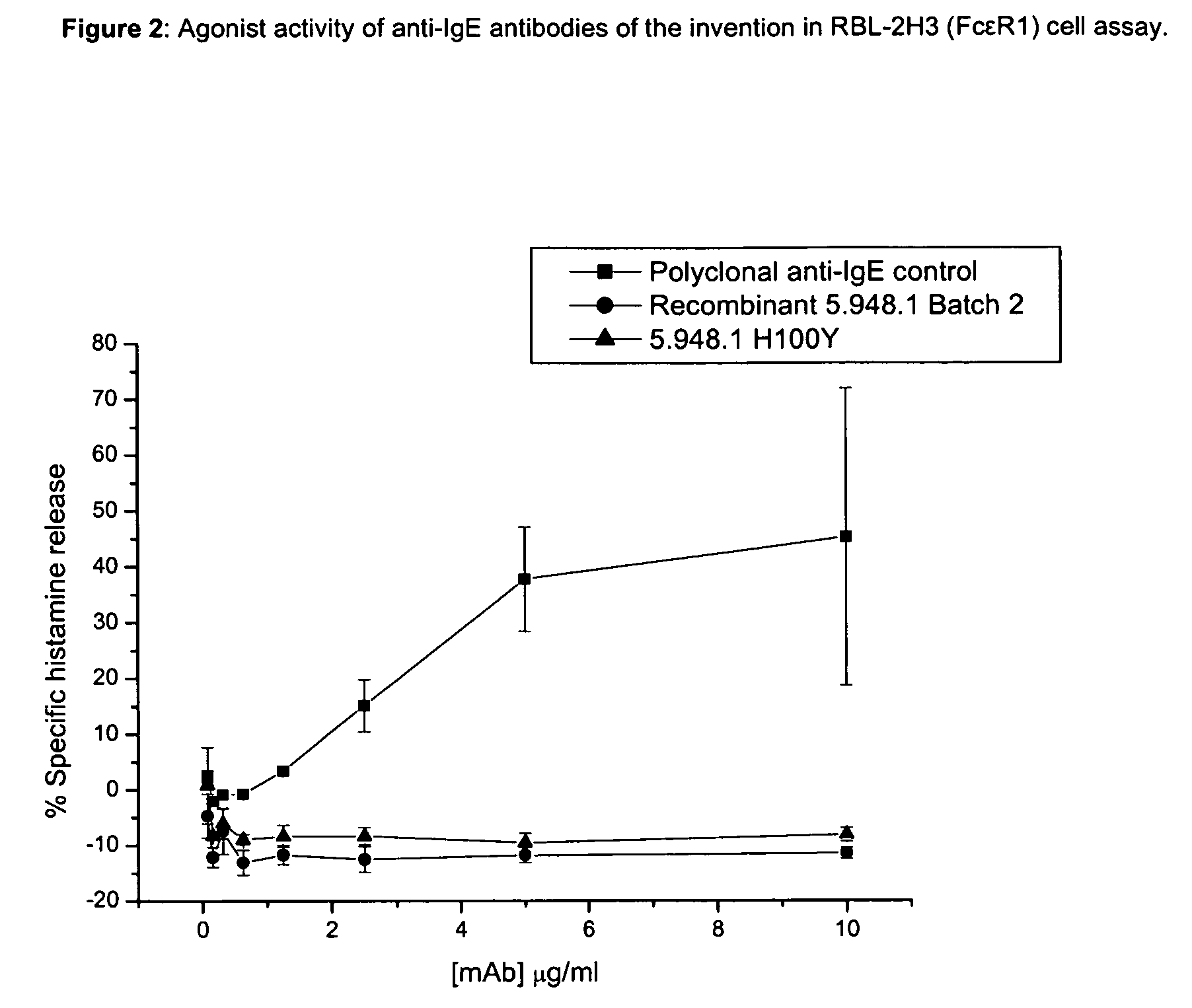
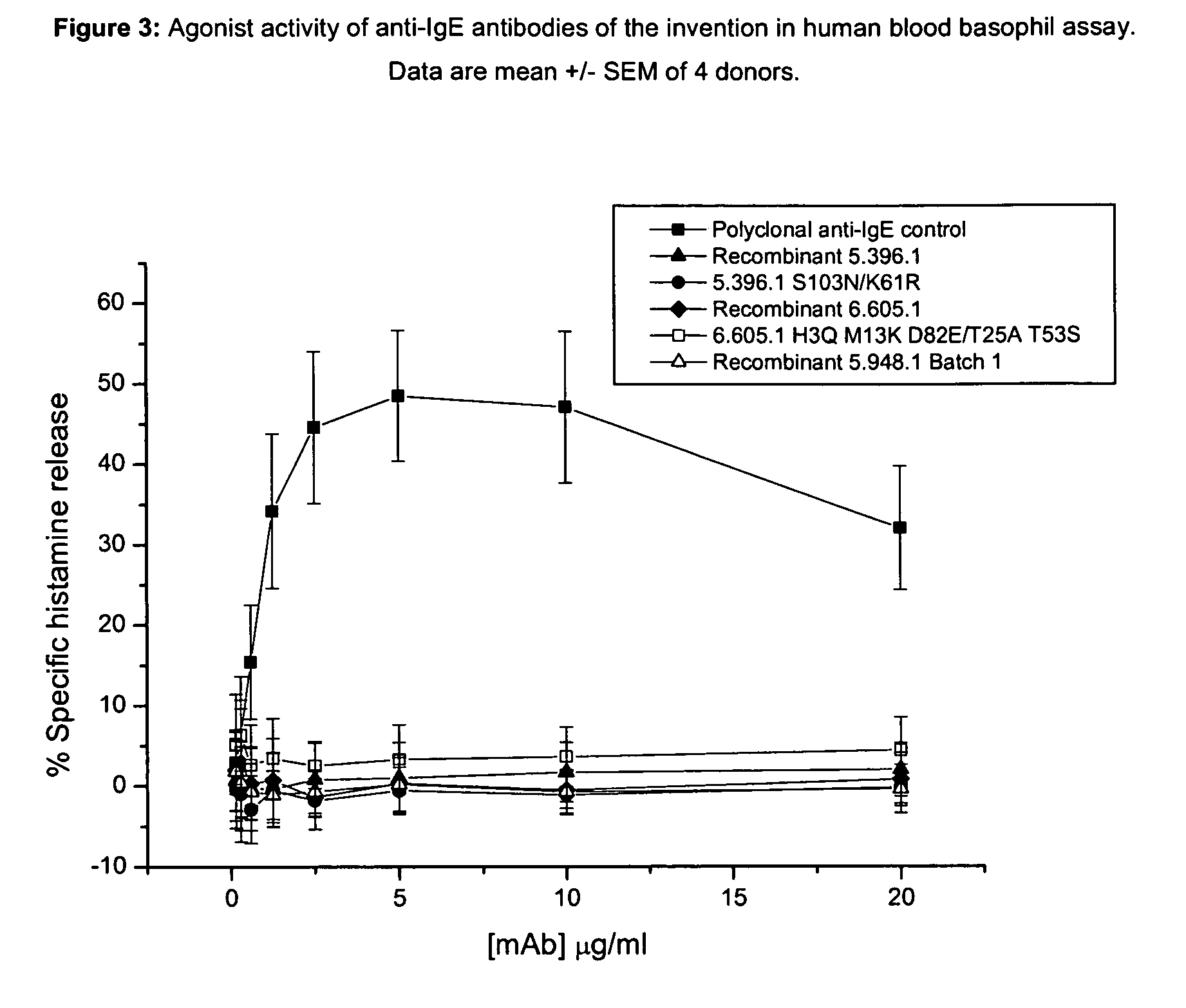
Anti-IgE antibodies
The present invention relates to novel human antibodies specifically directed against human immunoglobulin E (anti-IgE). The present invention also relates to pharmaceutical compositions and methods for treating asthma, in particular allergic asthma, as well as other IgE-mediated disorders including allergic rhinitis and food allergies.
Owner:PFIZER INC +1
Aqueous liquid preparation containing 2-amino-3-(4-bromobenzoyl)phenylacetic acid
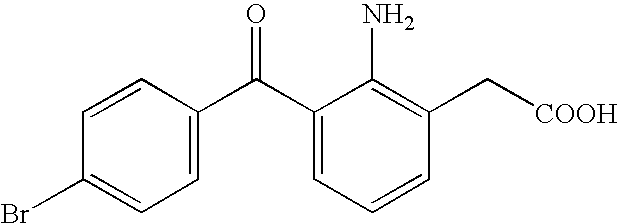
ActiveUS20050239895A1No irritationInhibit deteriorationBiocideOrganic active ingredientsScleritisPhenylacetic acid
An aqueous liquid preparation of the present invention containing 2-amino-3-(4-bromobenzoyl)phenylacetic acid or its pharmacologically acceptable salt or a hydrate thereof, an alkyl aryl polyether alcohol type polymer such as tyloxapol, or a polyethylene glycol fatty acid ester such as polyethylene glycol monostearate is stable. Since even in the case where a preservative is incorporated into said aqueous liquid preparation, the preservative exhibits a sufficient preservative effect for a long time, said aqueous liquid preparation in the form of an eye drop is useful for the treatment of blepharitis, conjunctivitis, scleritis, and postoperative inflammation. Also, the aqueous liquid preparation of the present invention in the form of a nasal drop is useful for the treatment of allergic rhinitis and inflammatory rhinitis (e.g. chronic rhinitis, hypertrophic rhinitis, nasal polyp, etc.).
Owner:SENJU PHARMA CO LTD
Nanoparticulate ebastine formulations
The invention is directed to compositions comprising at least one nanoparticulate H1-histamine receptor antagonist, such as ebastine or a salt or derivative thereof, having improved dissolution rate providing a faster onset of drug availability. The nanoparticulate H1-histamine receptor antagonist particles, such as ebastine, have an effective average particle size of less than about 2000 nm and are useful in the treatment of seasonal and perennial allergic rhinitis and related diseases.
Owner:ELAN PHRMA INT LTD
Combination of loteprednol and antihistamines
The present invention relates to a novel combination of a soft steroid, in particular loteprednol, and at least one antihistamine, such as, for example, azelastine and / or levocabastine, for simultaneous, sequential or separate administration in the local treatment of allergies and airway disorders, for example of allergic rhinitis (rhinoconjunctivitis).
Owner:VIATRIS GMBH & CO KG
Orally disintegrating tablets comprising diphenhydramine
The compositions of the present invention comprise a therapeutically effective amount of particles consisting of diphenhydramine or pharmaceutically acceptable salts thereof, optionally in combination with another drug such as pseudoephedrine, or phenylephrine and hydrocodone, in combination with rapidly-dispersing microgranules comprising a disintegrant and a sugar alcohol and / or a saccharide. These compositions are useful in treating the symptoms of one or more diseases or conditions in which diphenhydramine (alone or in combination with one or two other drugs) is a therapeutically effective, e.g. allergic rhinitis, sinusitis, upper respiratory tract infections, motion sickness, Parkinson's disease, insomnia, the common cold, and nighttime pain management, particularly for subjects or patients with dysphagia, and people ‘on the move’.
Owner:ADARE PHARM INC
Compositions for use in treating ige-associated disorders
InactiveUS20050031609A1Inhibitory activityReduced activityAllergen ingredientsUnknown materialsAntigenDisease
The present invention provides methods of treating IgE-associated disorders and products for use therein. The methods comprise administering to a subject an amount of a first composition comprising an immunogenie antigen and an amount of a second composition that inhibits the activity of IgE. The methods are particularly useful in treatment of allergies such as allergic rhinitis. These combination methods offer significant advantages, such as improving the efficacy of therapy while showing a good safety profile.
Owner:HULTSCH THOMAS +1
Novel heterocycles
InactiveUS20070167413A1Useful in treatmentOrganic active ingredientsBiocideRESPIRATORY DISTRESS SYNDROME ADULTContact dermatitis
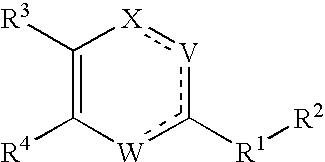
The present invention relates to novel heterocyclic compounds of the general formula (I), their derivatives, analogs, tautomeric forms, stereoisomers, polymorphs, hydrates, solvates, pharmaceutically acceptable salts and compositions, metabolites and prodrugs thereof. The present invention more particularly provides novel hetereocycles of the general formula (I). Also included is a method of treatment of immunological diseases, inflammation, pain disorder, rheumatoid arthritis; osteoporosis; multiple myeloma; uveititis; acute and chronic myelogenous leukemia; ischemic heart disease; atherosclerosis; cancer; ischemic-induced cell damage; pancreatic beta cell destruction; osteoarthritis; rheumatoid spondylitis; gouty arthritis; inflammatory bowel disease; adult respiratory distress syndrome (ARDS); psoriasis; Crohn's disease; allergic rhinitis; ulcerative colitis; anaphylaxis; contact dermatitis; muscle degeneration; cachexia; asthma; bone resorption diseases; ischemia reperfusion injury; brain trauma; multiple sclerosis; sepsis; septic shock; toxic shock syndrome; fever, and myalgias due to infection in a mammal comprising administering an effective amount of a compound of formula (I) as described above.
Owner:ORCHID RES LAB +1
Purine derivatives for use in the treatment of allergic, inflammatory and infectious diseases
The present invention relates to compounds of formula (I):wherein R1 is C1-6alkylamino, C1-6alkoxy, or C3-7cycloalkyloxy; m is an integer having a value of 3 to 6; n is an integer having a value of 0 to 4; and salts thereof are inducers of human interferon. Compounds which induce human interferon may be useful in the treatment of various disorders, for example the treatment of allergic diseases and other inflammatory conditions for example allergic rhinitis and asthma, the treatment of infectious diseases and cancer, and may also be useful as vaccine adjuvants.
Owner:GLAXO SMITHKLINE LLC
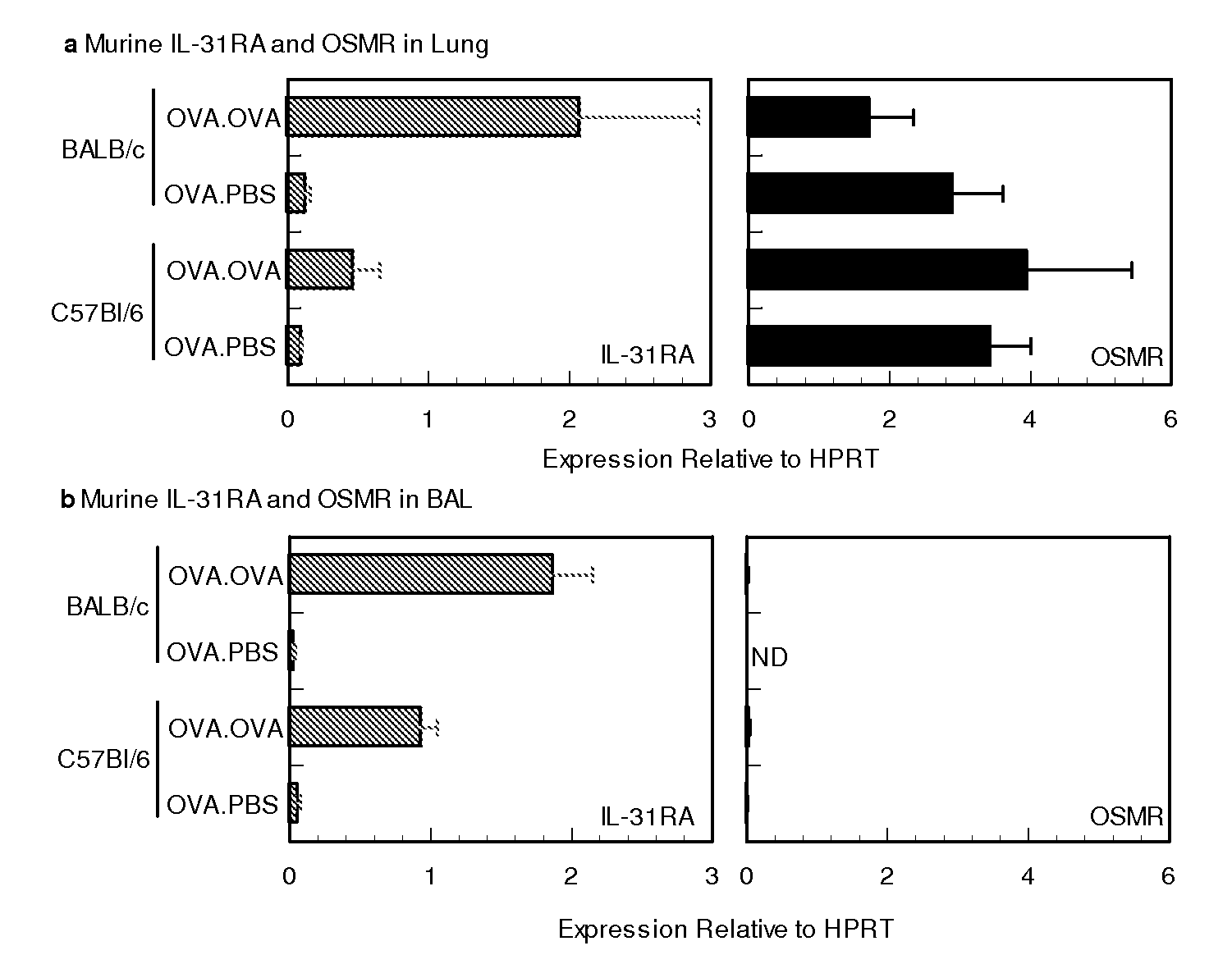
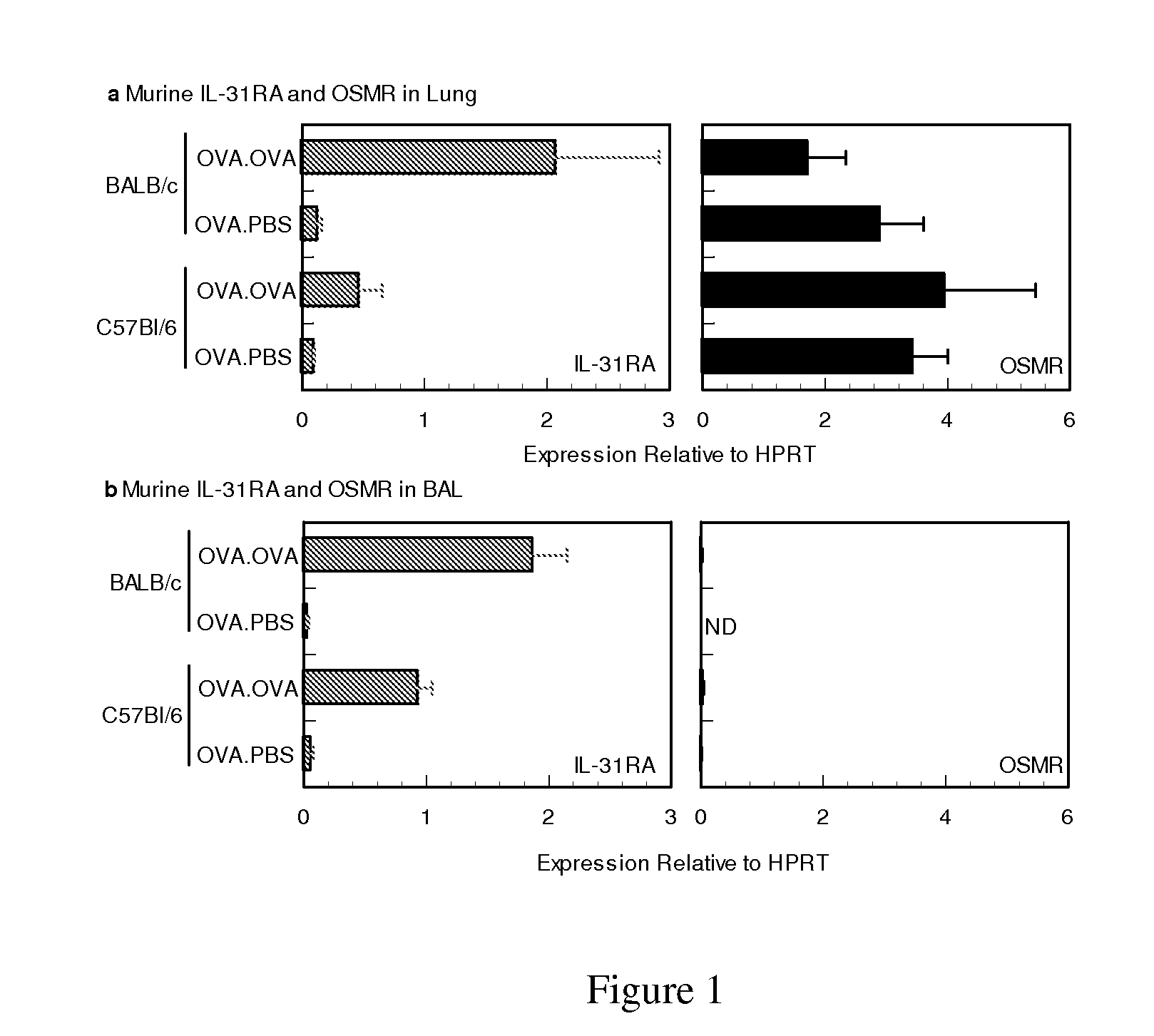
Methods of using il-31 to treat airway hyper-responsiveness and asthma
Use of IL-31 agonists, including IL-31, are used to treat agonists are used to treat asthma, airway hyper-responsiveness or allergic rhinitis. The method comprise inhibiting, reducing, limiting or minimizing production of proimflammatory cytokines and include administration of the IL-31 agonsit during sensitization or challenge resulting in the asthma, airway hyper-responsiveness or allergic rhinitis state.
Owner:ZYMOGENETICS INC
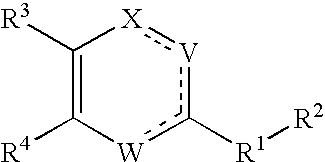
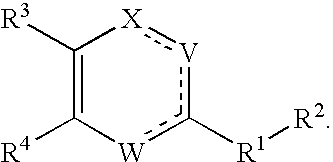
Substituted heterocyclic compounds and methods of use
The present invention relates to compounds having the general formula or a pharmaceutically acceptable salt thereof, wherein R1 is a saturated or unsaturated 5-, 6- or 7-membered, ring containing 0, 1, 2 or 3 atoms selected from N, O and S, wherein the ring may be fused with a benzo group, and is substituted by 0, 1 or 2 oxo groups, and wherein R1 is additionally substituted; and R2 is a substituted C1-6alkyl. Also included is a method of prophylaxis or treatment of inflammation, rheumatoid arthritis, Pagets disease, osteoporosis, multiple myeloma, uveititis, acute or chronic myelogenous leukemia, pancreatic β cell destruction, osteoarthritis, rheumatoid spondylitis, gouty arthritis, inflammatory bowel disease, adult respiratory distress syndrome (ARDS), psoriasis, Crohn's disease, allergic rhinitis, ulcerative colitis, anaphylaxis, contact dermatitis, asthma, muscle degeneration, cachexia, Reiter's syndrome, type I diabetes, type II diabetes, bone resorption diseases, graft vs. host reaction, Alzheimer's disease, stroke, myocardial infarction, ischemia reperfusion injury, atherosclerosis, brain trauma, multiple sclerosis, cerebral malaria, sepsis, septic shock, toxic shock syndrome, fever, myalgias due to HIV-1, HIV-2, HIV-3, cytomegalovirus (CMV), influenza, adenovirus, the herpes viruses or herpes zoster infection in a mammal comprising administering an effective amount a compound as described above.
Owner:AMGEN INC
Arylsulfonamide derivatives for use as ccr3 antagonists in the treatment of inflammatory and immunological disorders
InactiveUS20050070582A1Effective treatmentBiocideSenses disorderAtopic dermatitisBULK ACTIVE INGREDIENT
The present invention relates to a sulfonamide derivative which is useful as an active ingredient of pharmaceutical preparations. The sulfonamide derivatives of the present invention have CCR3 (CC type chemokine receptor) antagonistic activity, and can be used for the prophylaxis and treatment of diseases associated with CCR3 activity, in particular for the treatment of asthma, atopic dermatitis, allergic rhinitis and other inflammatory / immunological disorders.
Owner:AXIKIN PHARMA +1
Herbal cough formulations and process for the preparation thereof
InactiveUS20060257507A1Safely and significantly treatPromote balance between supply and demandBiocideAnimal repellantsDiseaseGlycerol
The present invention relates to an herbal composition which is non-alcoholic, non-sedating and non-freezing in nature for the treatment of chronic respiratory disorders such as cold, cough, allergic asthma, seasonal allergic rhinitis, pharyngitis, laryngitis and the like and a process for preparing the same. The process comprises procuring, cleaning, grading of specified herbal plants per the standard specification, disintegrating and pulverizing separately to form coarse powder, then macerating individual powders separately with aqueous solution of preservatives, then extracting, filtering, concentrating and spray drying to make dry extract powder or semisolid soft extract. Further dissolving each of the extracts separately in aqueous solution containing preservatives and then allowing to maturate after mixing. Then adding filtered extracts to the sugar syrup containing preservatives, glycerin and appropriate excipients to obtain a homogenous cough syrup.
Owner:J B CHEM & PHARMA
Immune fusion protein and gene encoding same and application thereof
ActiveCN101633698AImprove bindingHigh affinityPeptide/protein ingredientsMicroorganismsHalf-lifeAsthma
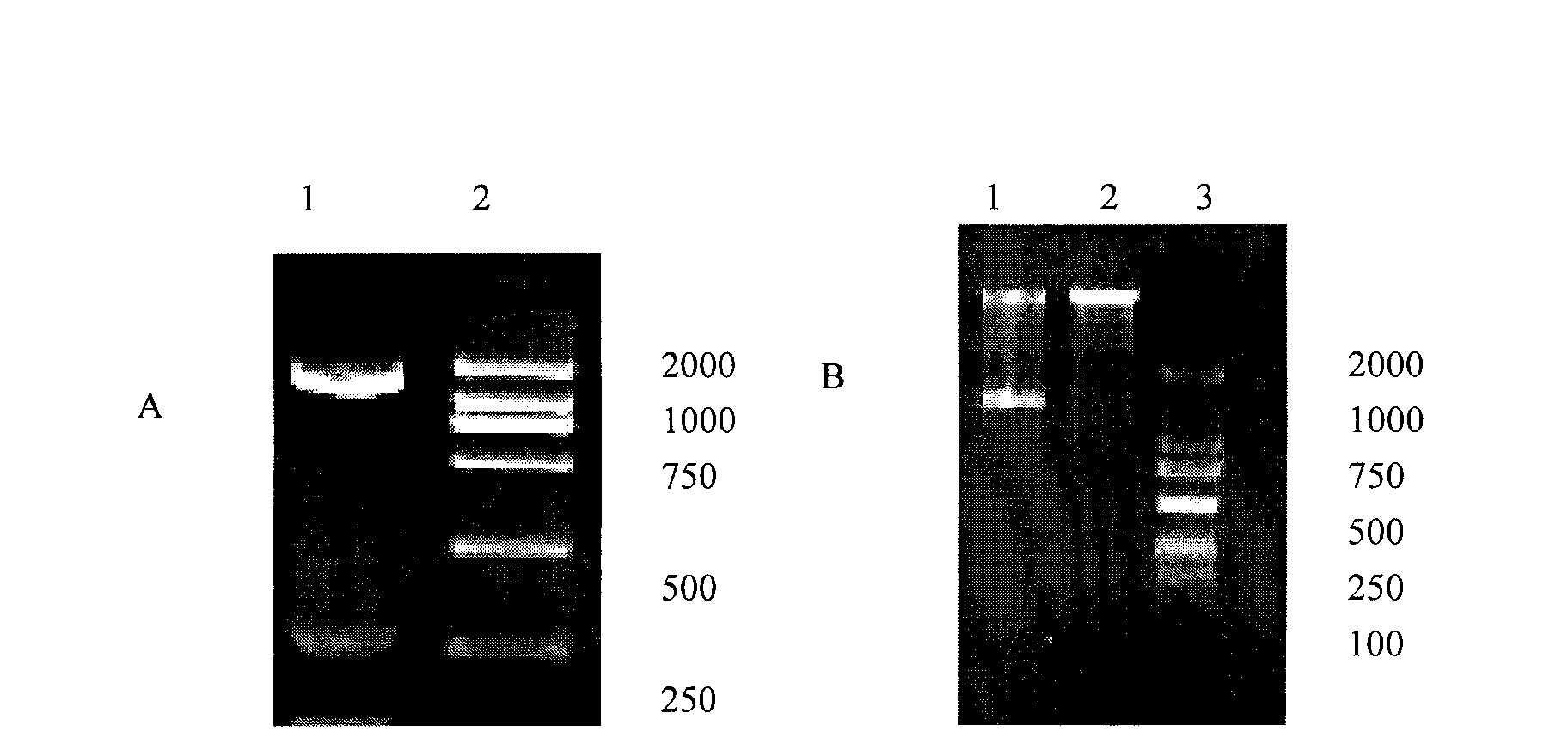
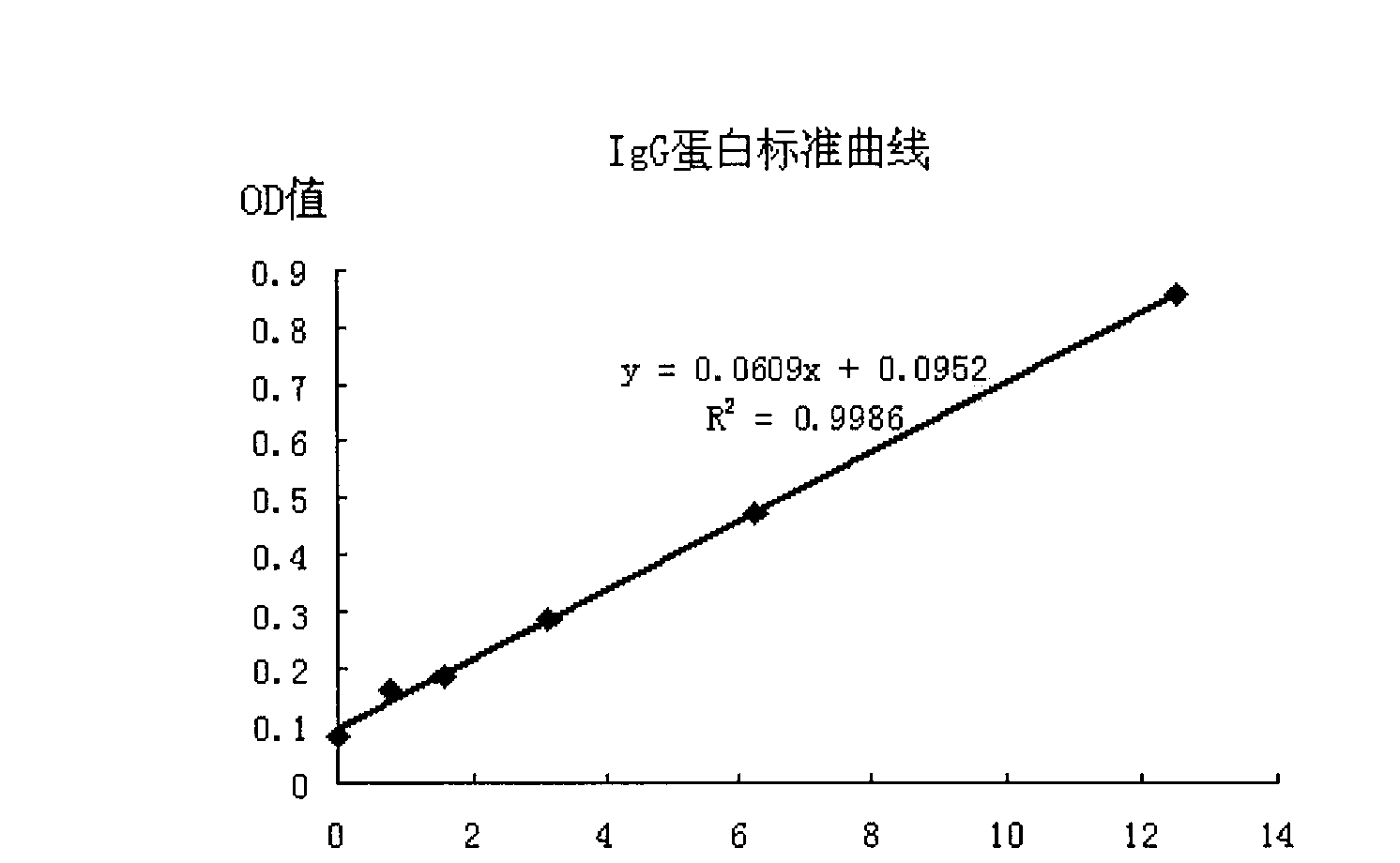
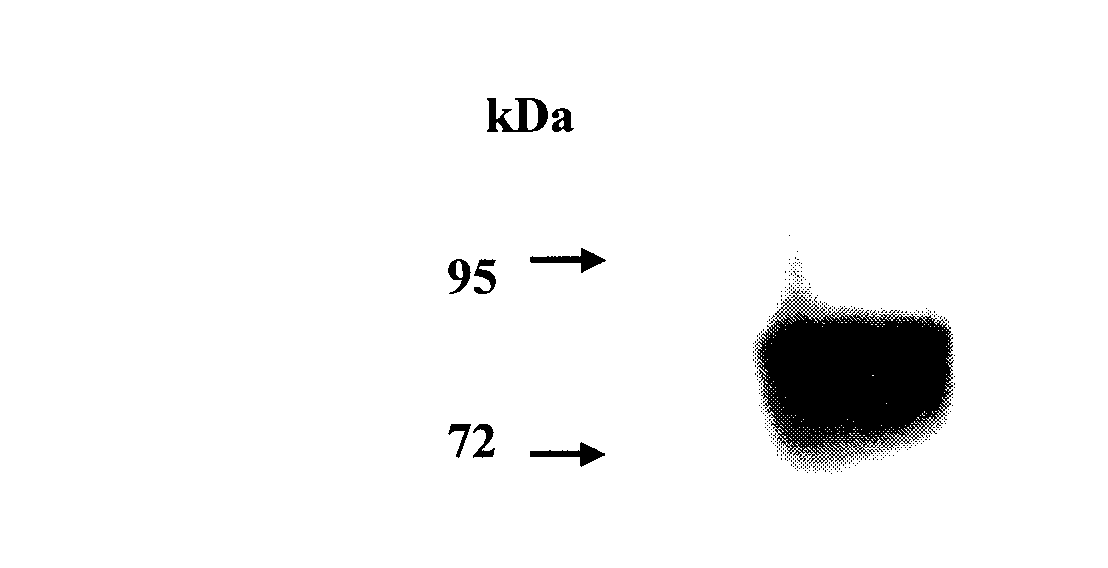
The invention discloses a protein. The provided protein is the following protein (1) or (2): the protein (1) is formed by an amino acid sequence shown by a sequence 2 in a sequence table; and the protein (2) is formed by the amino acid sequence of the sequence 2 in the sequence table through the substitution and / or deletion and / or addition of one or several amino acid residues, can be combined with IgE molecules and is derived from the protein (1). The protein has higher affinity with the IgE; Fc epsilonR I alpha and IgG2 are both derived from humanized protein, and the protein has no antigenicity, does not need humanized reconstruction, has a longer half life, and has the molecular weight of 170kDa which is larger than the molecular weight of sFcepsilonR I alpha. The protein has potential value on treating anaphylactic diseases including asthma, allergic rhinitis, atopic dermatitis, food allergy and the like.
Owner:BEIJING JINGYI TAIXIANG TECH DEV +1
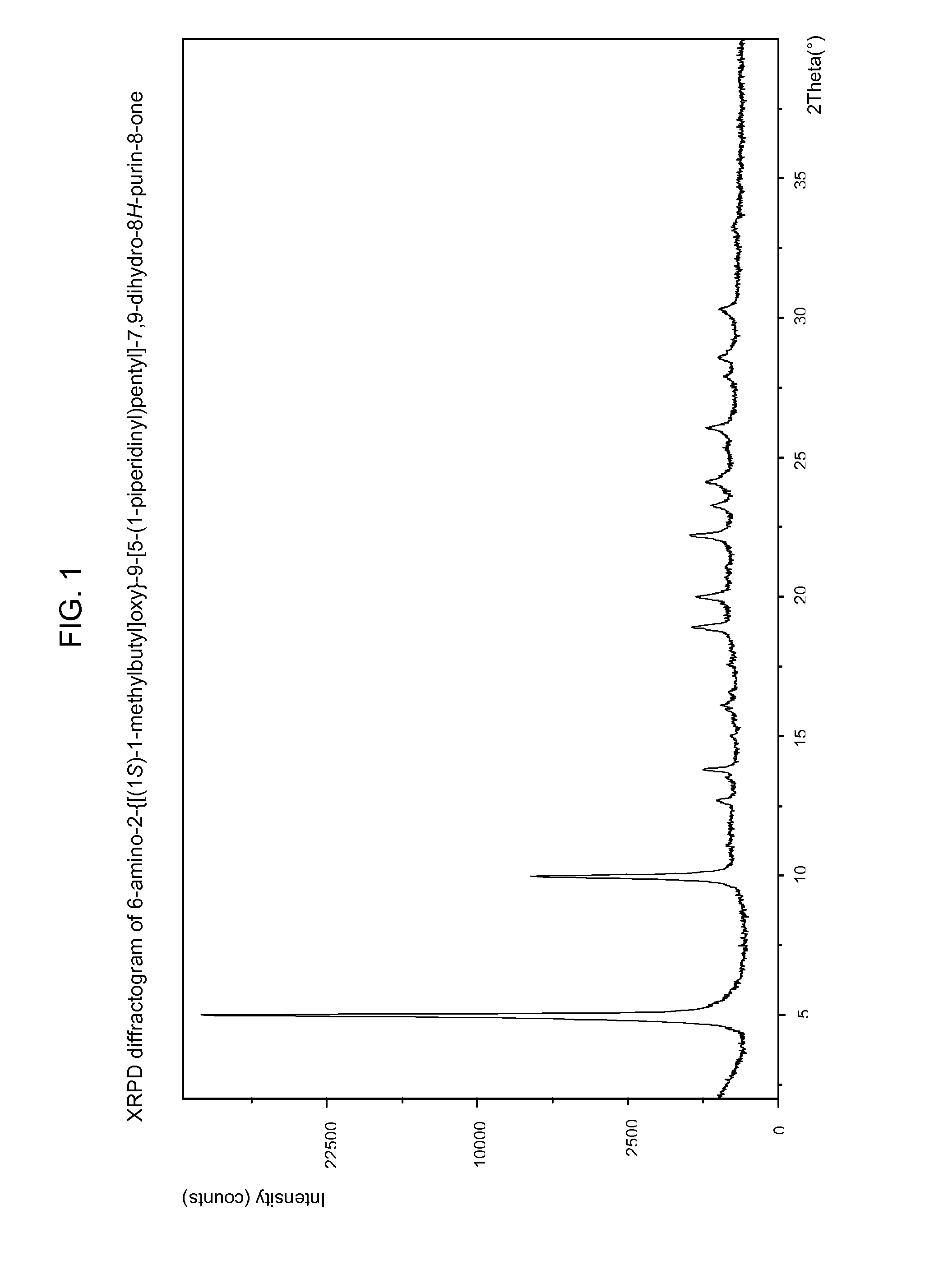
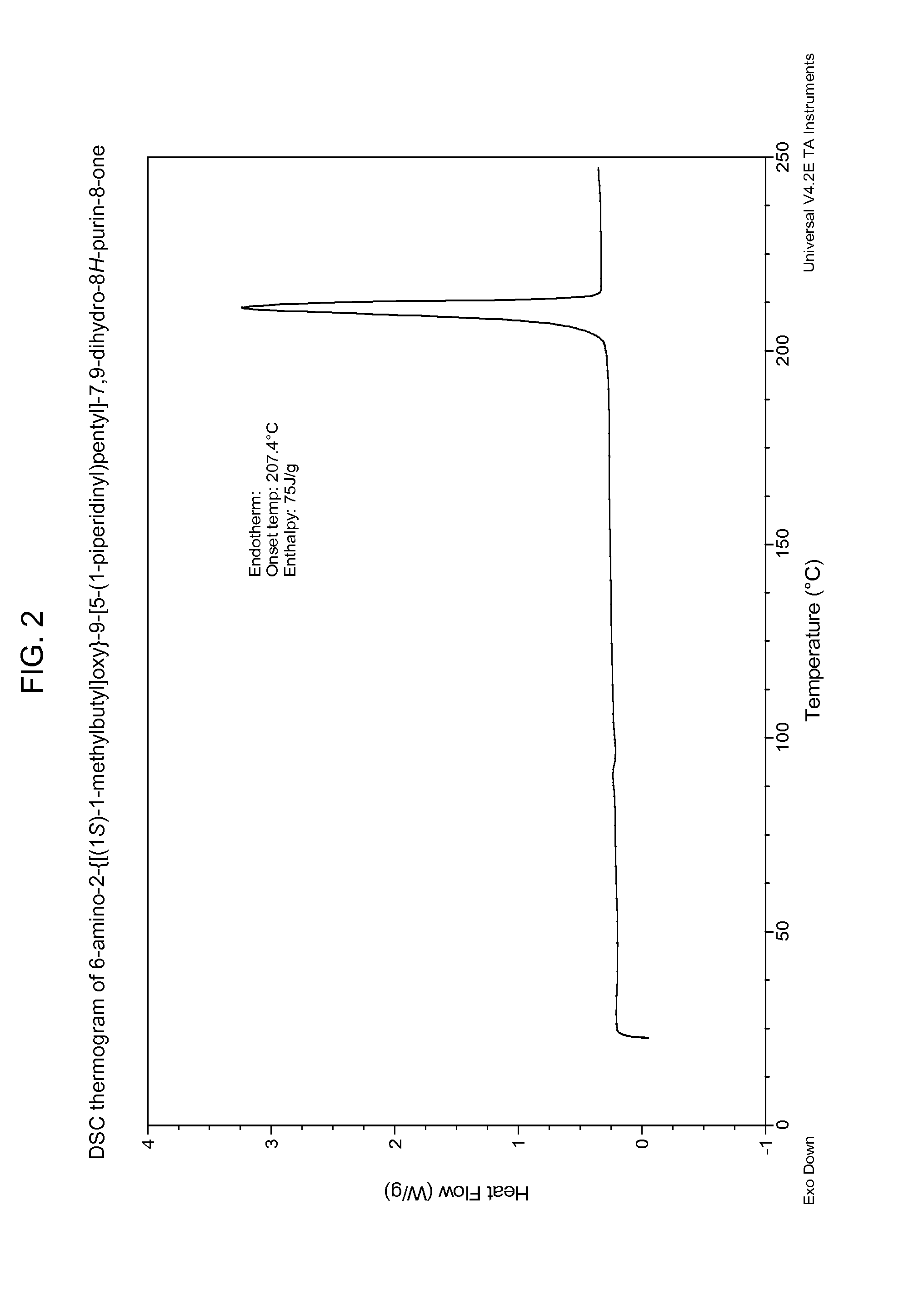
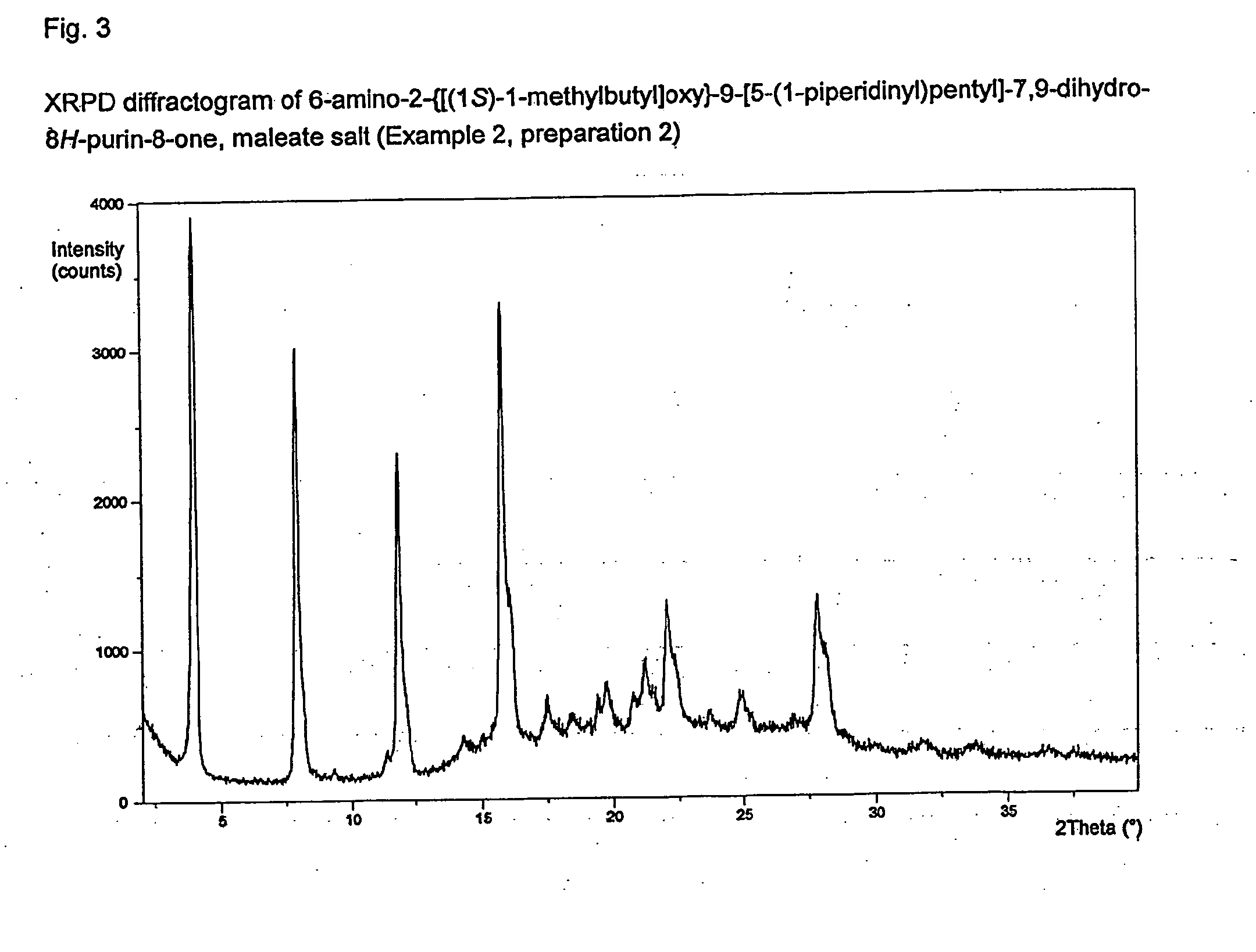
6-amino-2--9-[5-(1-piperidinyl)-7,9-dihydro-8h-purin-8-one maleate
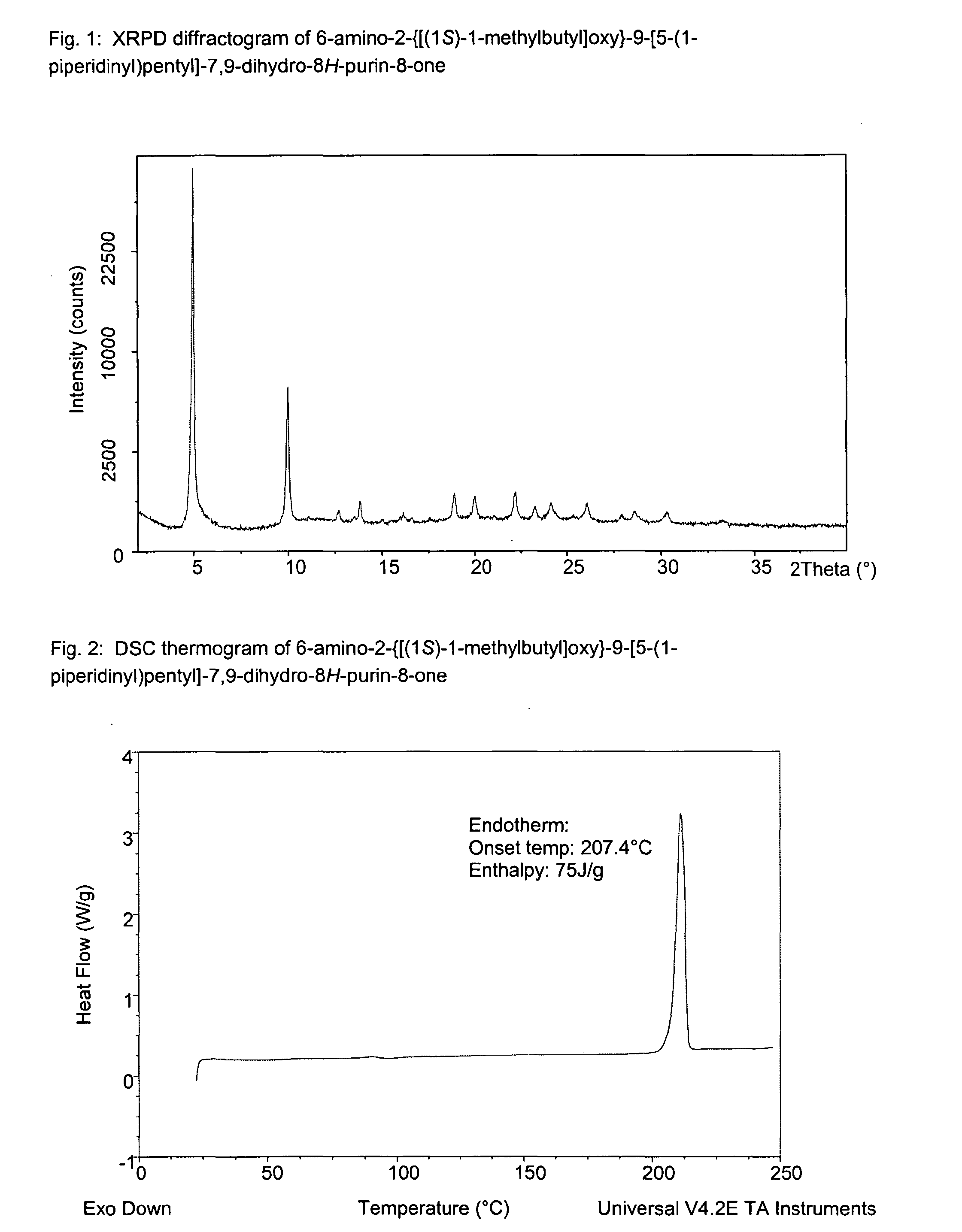
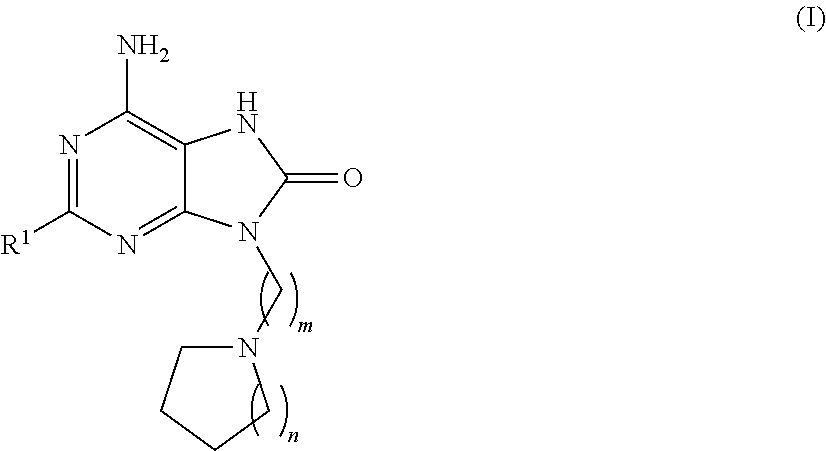
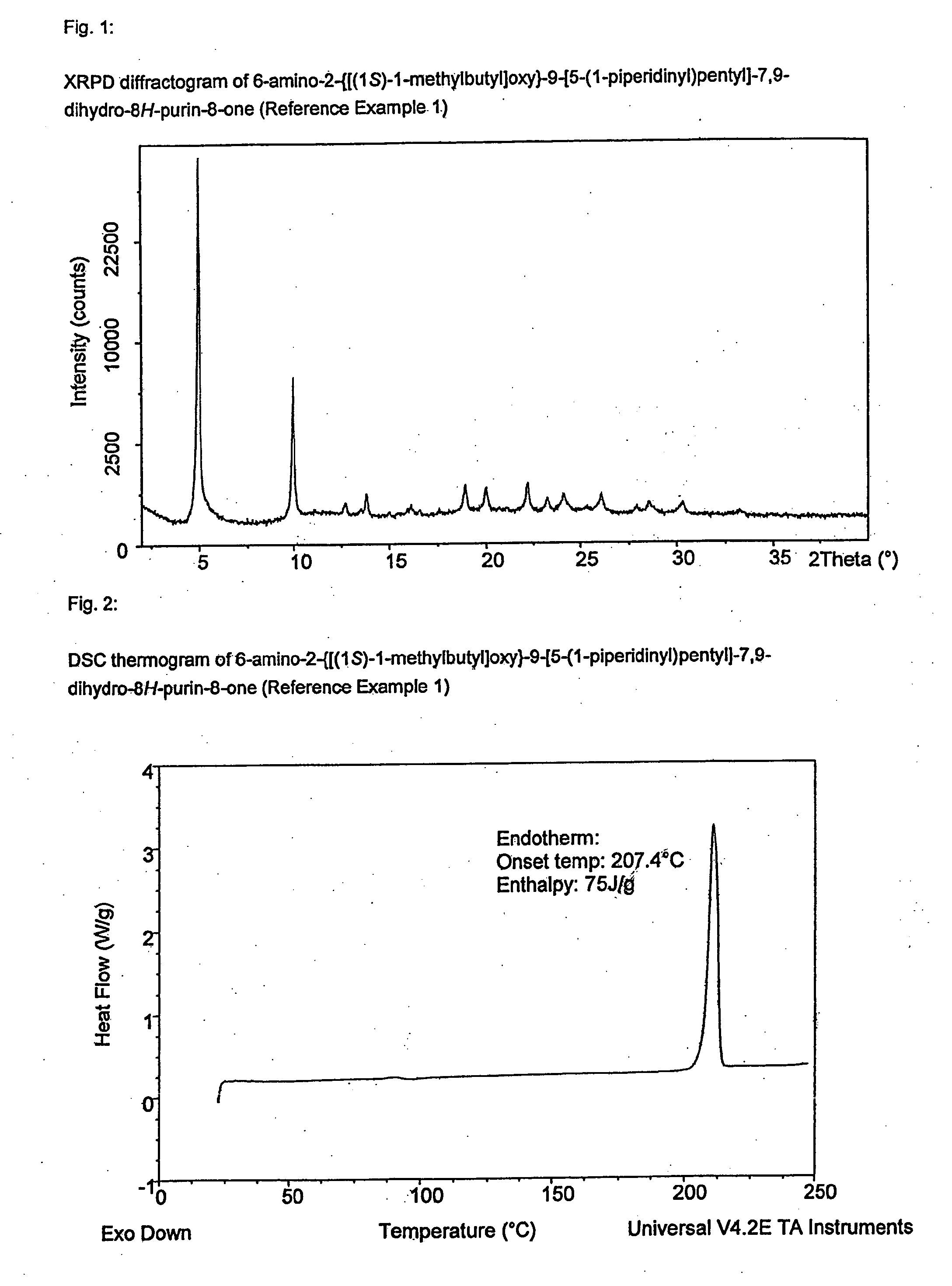
InactiveUS20120308609A1Sufficient solubilityImprove stabilityBiocideAntipyreticDisease causeInflammation
The present invention relates to a compound which is 6-amino-2-{[(1S)-1-methylbutyl]oxy}-9-[5-(1-piperidinyl)pentyl]-7,9-dihydro-8H-purin-8-one:in the form of a maleate salt, may be useful in the treatment of various disorders, for example the treatment of allergic diseases and other inflammatory conditions for example allergic rhinitis and asthma, the treatment of infectious diseases and cancer, and may also be useful as vaccine adjuvants.
Owner:GLAXO SMITHKLINE LLC
Methods for Treating Diseases and Conditions with Inverse Agonists and for Screening for Agents Acting as Inverse Agonists
InactiveUS20070276024A1Reduce the populationPrevents decrease in populationBiocideCompound screeningScreening methodTherapeutic effect
The present invention describes a method for treating a disease or condition associated with the activity of a G protein coupled receptor (GPCR) comprising administering an inverse agonist for the GPCR, alone or in combination with an agonist for the GPCR, to an organism with a disease or condition associated with the activity of the GPCR in a quantity and for a period that causes an increase in the population of spontaneously active GPCRs associated with that physiological function, thereby producing a therapeutic effect to ameliorate the disease or condition. This provides a basis for so-called “paradoxical pharmacology.” These methods can be used to treat pulmonary airway diseases, including asthma and chronic allergic rhinitis, among other diseases and conditions, including obesity. The present invention further describes a screening method for screening a compound for inverse agonist activity to a GPCR.
Owner:INVION LTD
Liquid medicine for preventing and treating allergic rhinitis and wet tissue or tissue prepared by applying liquid medicine
ActiveCN104013677AImprove anti-allergic effectGood anti-inflammatory effectPharmaceutical delivery mechanismRespiratory disorderNosePharmaceutical drug
The invention relates to the technical field of medicine application, and particularly relates to a liquid medicine for preventing and treating allergic rhinitis and wet tissue or tissue prepared by applying the liquid medicine. The liquid medicine is prepared from the following raw materials in parts by weight: 10-90 parts of magnolia flower, 5-40 parts of centipeda minima and 5-40 parts of cocklebur fruit. The wet tissue and tissue for preventing and treating allergic rhinitis by applying the liquid medicine are prepared from natural skin-friendly non-irritative materials, comprise a plurality of traditional Chinese medicinal effective components with the effects of resisting rhinitis and restoring nasal cavity, and are used for preventing and relieving discomfort of the nose of a patient with allergic rhinitis in daily life and non-invasively cleaning snot and other excretions when rhinitis attacks. Traditional Chinese medicine is developed to the tissue mode which is concise and convenient, the tissue has good application value and market prospect, is advantageous to popularize modern application of traditional Chinese medicines, and is beneficial to pain relieving of patients with allergic rhinitis.
Owner:张大威 +1
Purine derivatives for use in the treatment of allergic, inflammatory and infectious diseases
The present invention relates to compounds of formula (I):wherein R1 is C1-6alkylamino, C1-6alkoxy, or C3-7cycloalkyloxy; m is an integer having a value of 2 to 6; R2 is hydrogen, C1-6alkyl, or C3-7cycloalkylC0-6alkyl; and salts thereof are inducers of human interferon. Compounds which induce human interferon may be useful in the treatment of various disorders, for example the treatment of allergic diseases and other inflammatory conditions for example allergic rhinitis and asthma, the treatment of infectious diseases and cancer, and may also be useful as vaccine adjuvants.
Owner:GLAXO SMITHKLINE LLC
Treatment of behavioral disorders
InactiveUS20050192290A1Ameliorate behavioral disorderSufficient amountBiocideNervous disorderTherapeutic ACTHFexofenadine
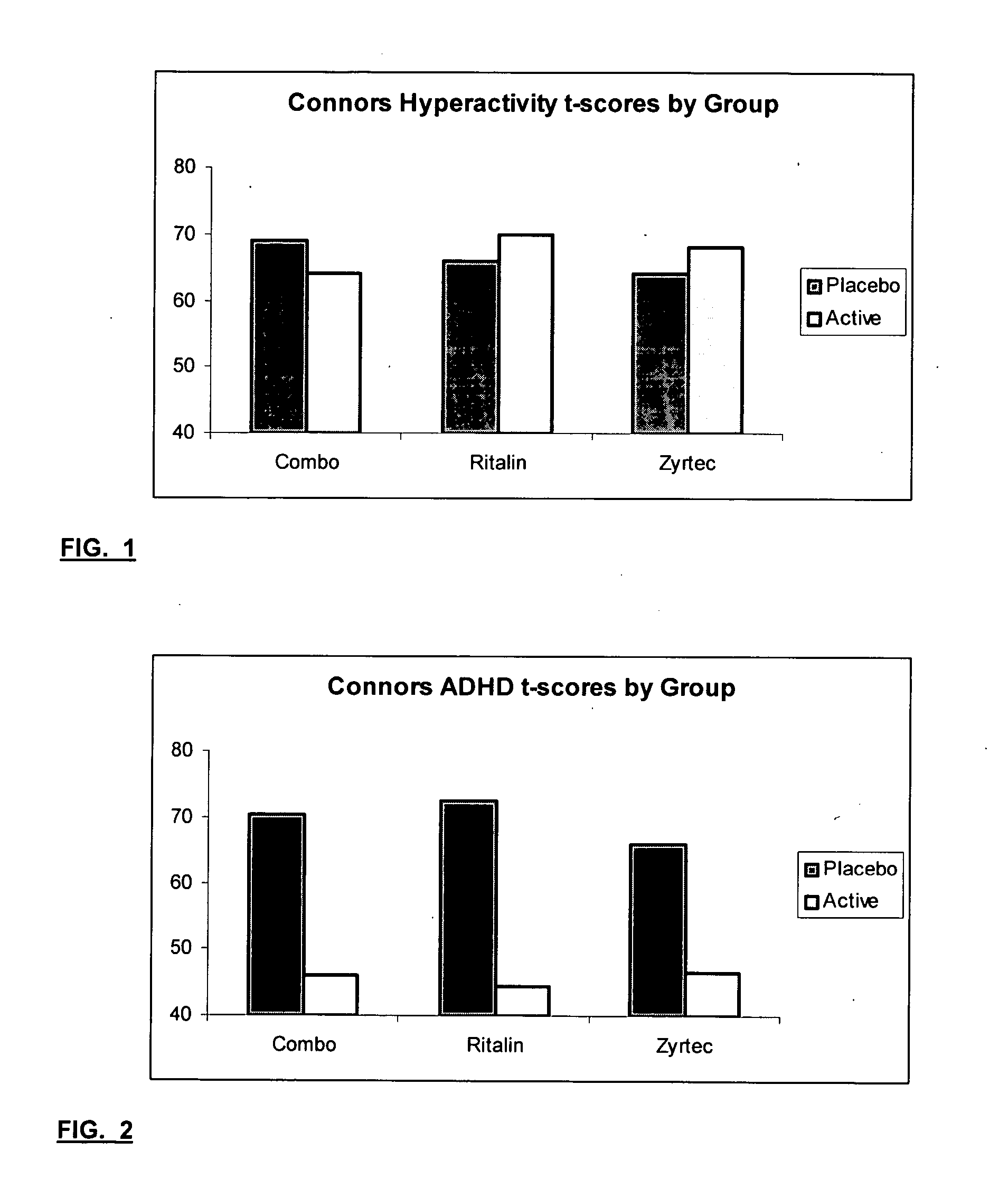
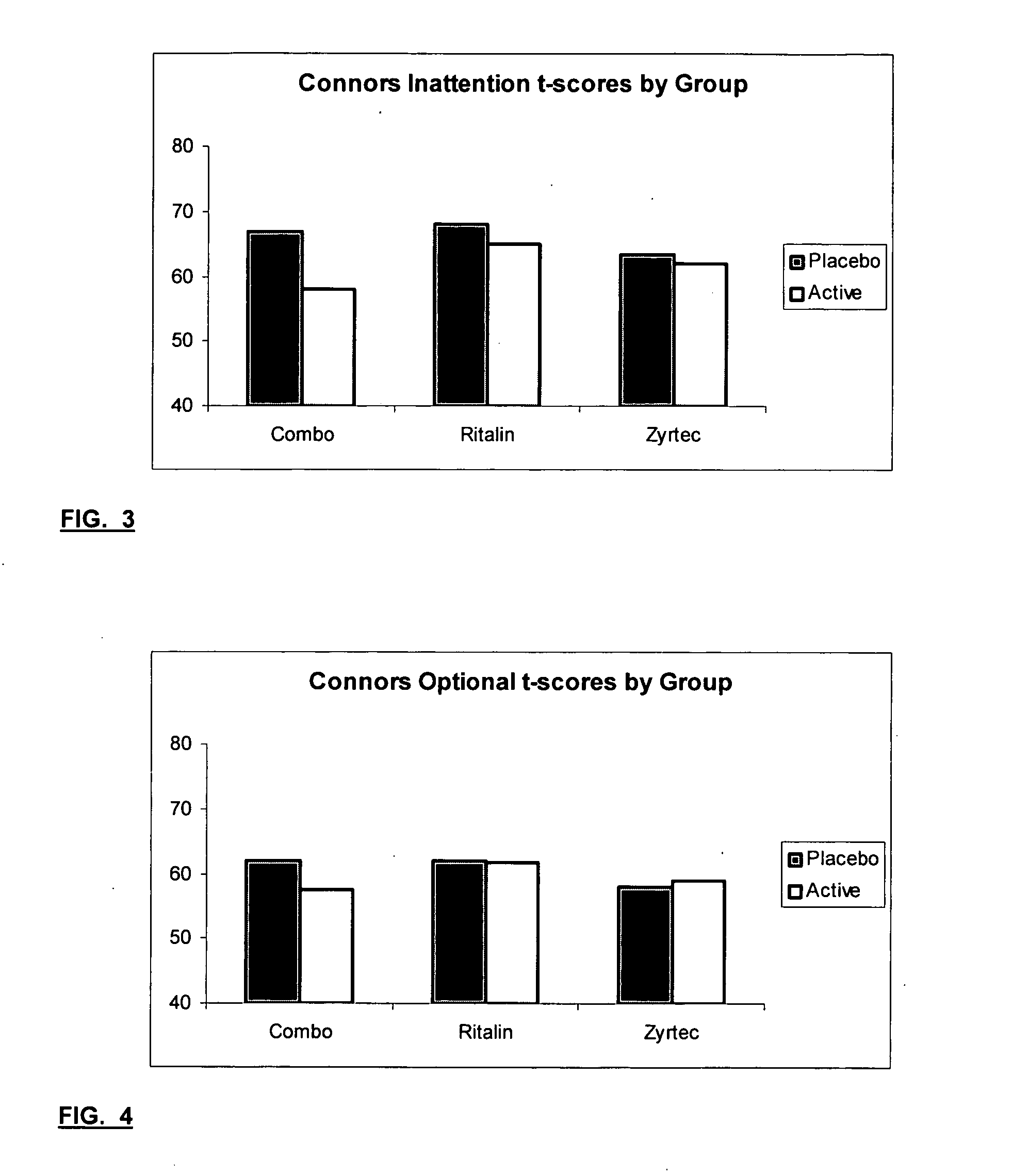
The present invention relates to a method for treating a behavior disorder comprising the administration of a therapeutically effective amount of antihistamine, such as ceterizine, fexofenadine; loratadine, and desloratadine. The behavioral disorders may include ADHD, anxieity, depression, and autism. The method may include the administration of the antihistamine in combination with a stimulant medication, such as methylphenidate, thereby to achieve a synergistic effect. In any event, the amount of antihistamine and / or stimulant is effective to downregulate neurotrophic factors such as nerve growth factor or CD40. The invention is also directed to a method of preventing the onset of behavior disorders in patients presenting with symptoms of allergic rhinitis.
Owner:MELAMED ISAAC
6-amino-2-{[(1S)-1-methylbutyl]oxy}-9-[5-(1-piperidinyl)-7,9-dihydro-8H-purin-8-one maleate
The present invention relates to a compound which is 6-amino-2-{[(1S)-1-methylbutyl]oxy}-9-[5-(1-piperidinyl)pentyl]-7,9-dihydro-8H-purin-8-one:in the form of a maleate salt, may be useful in the treatment of various disorders, for example the treatment of allergic diseases and other inflammatory conditions for example allergic rhinitis and asthma, the treatment of infectious diseases and cancer, and may also be useful as vaccine adjuvants.
Owner:GLAXO SMITHKLINE LLC
Polymer-modified bioactive synthetic chemokines, and methods for their manufacture and use
The invention relates to polymer-modified bioactive synthetic chemokines and to methods for their production and use. The bioactive synthetic chemokines of the invention comprises a polymer modified polypeptide chemokine backbone. The compounds and methods or the invention are useful for the treatment of disorders involving naturally occurring chemokines, such as for the treatment of HIV and AIDS related disorders and for the treatment of asthma, allergic rhinitis, atopic dermatitis, atheroma / atherosclerosis, organ transplant rejection, and rheumatoid arthritis.
Owner:AMYLIN PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
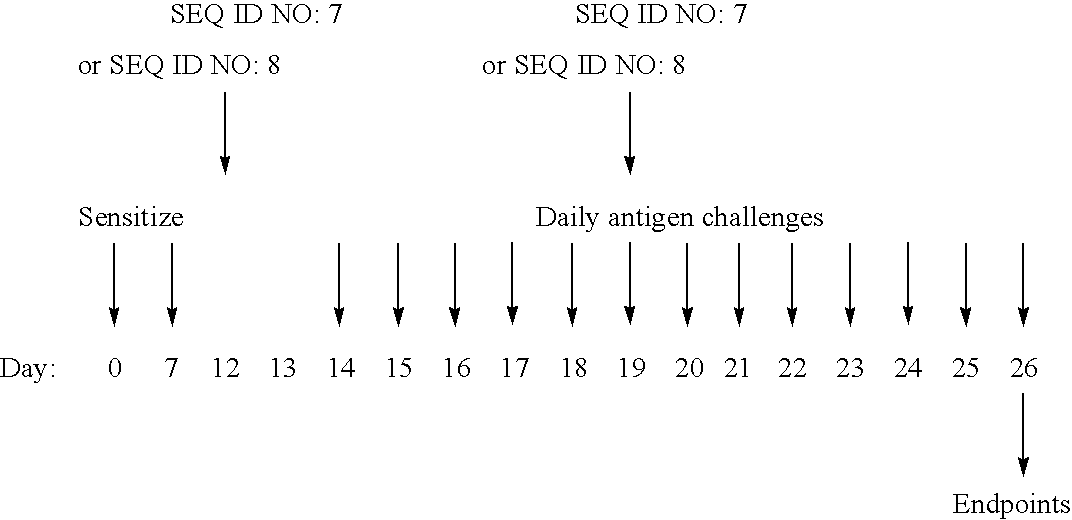
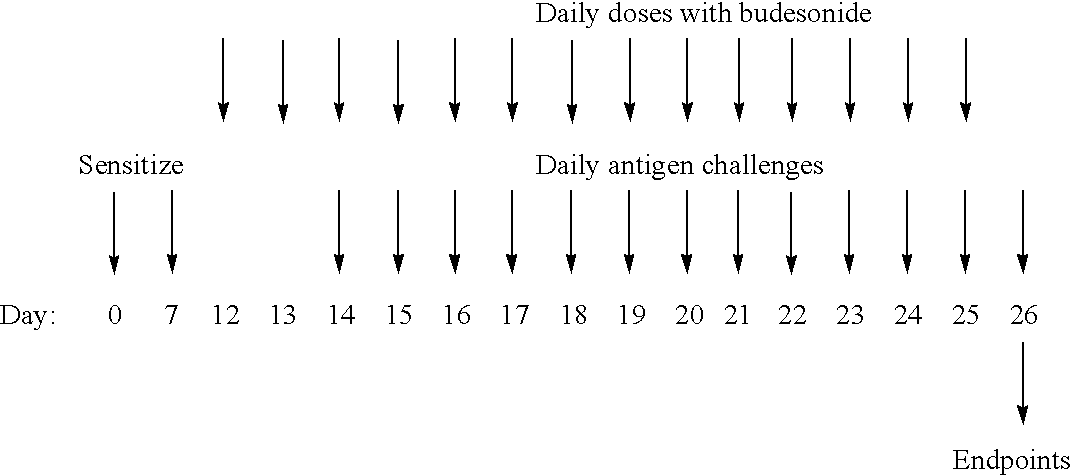
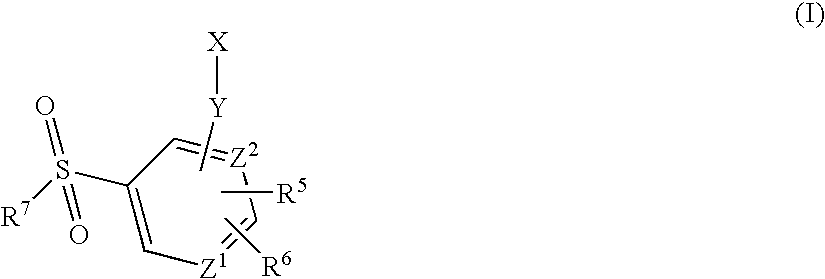
![6-amino-2-{[(1<i>S</i>)-1-methylbutyl]oxy}-9-[5-(1-piperidinyl)-7,9-dihydro-8<i>H</i>-purin-8-one maleate 6-amino-2-{[(1<i>S</i>)-1-methylbutyl]oxy}-9-[5-(1-piperidinyl)-7,9-dihydro-8<i>H</i>-purin-8-one maleate](https://images-eureka.patsnap.com/patent_img/d5e289da-081c-43b1-b690-39fbdd04bca2/US08703754-20140422-D00001.png)
![6-amino-2-{[(1<i>S</i>)-1-methylbutyl]oxy}-9-[5-(1-piperidinyl)-7,9-dihydro-8<i>H</i>-purin-8-one maleate 6-amino-2-{[(1<i>S</i>)-1-methylbutyl]oxy}-9-[5-(1-piperidinyl)-7,9-dihydro-8<i>H</i>-purin-8-one maleate](https://images-eureka.patsnap.com/patent_img/d5e289da-081c-43b1-b690-39fbdd04bca2/US08703754-20140422-D00002.png)
![6-amino-2-{[(1<i>S</i>)-1-methylbutyl]oxy}-9-[5-(1-piperidinyl)-7,9-dihydro-8<i>H</i>-purin-8-one maleate 6-amino-2-{[(1<i>S</i>)-1-methylbutyl]oxy}-9-[5-(1-piperidinyl)-7,9-dihydro-8<i>H</i>-purin-8-one maleate](https://images-eureka.patsnap.com/patent_img/d5e289da-081c-43b1-b690-39fbdd04bca2/US08703754-20140422-C00001.png)