Immune fusion protein and gene encoding same and application thereof
A gene and protein technology, applied in the field of immune fusion protein and its coding gene and application, can solve the problem of weak complement fixation ability, achieve high affinity, huge economic and social benefits, and broad clinical application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
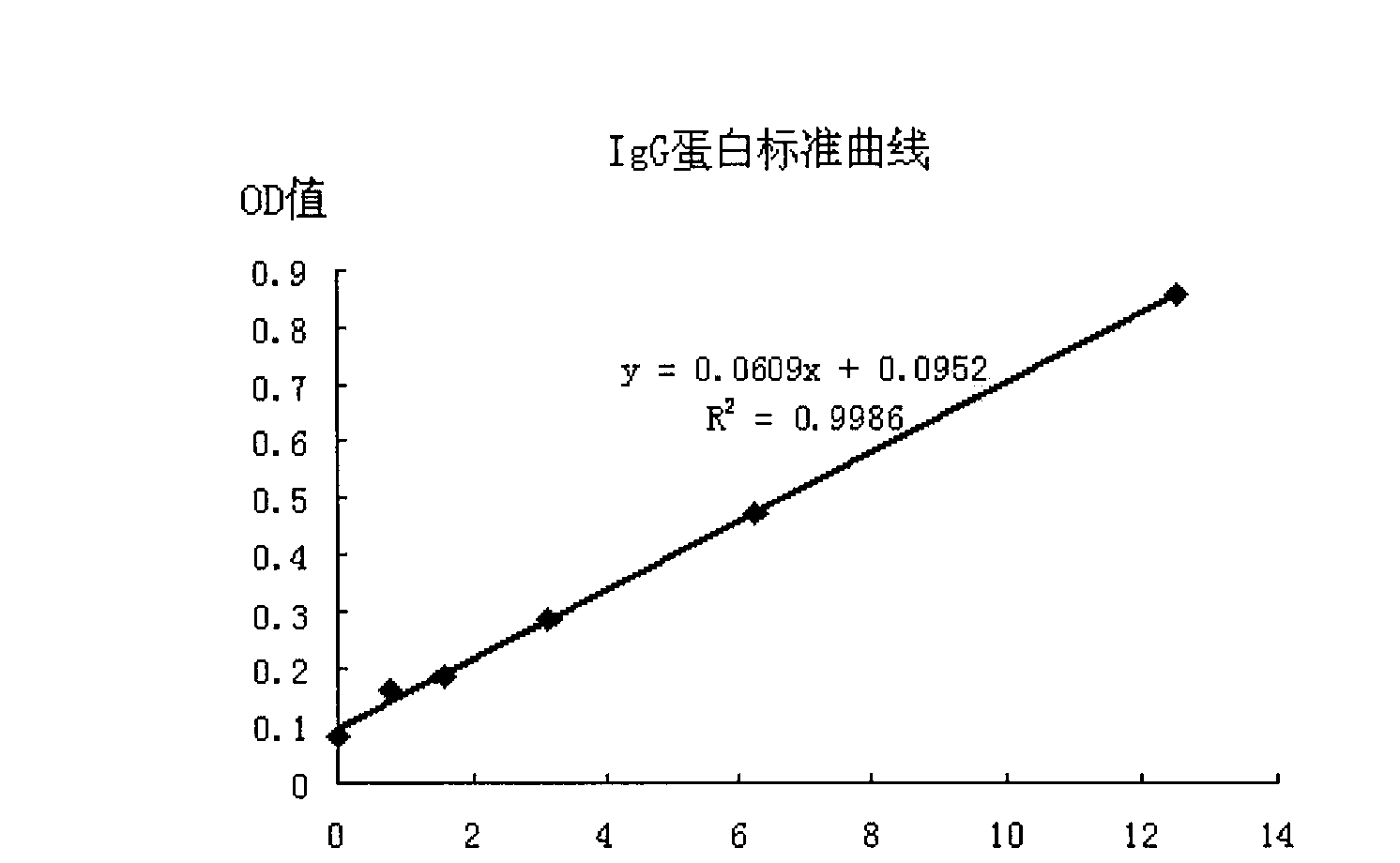
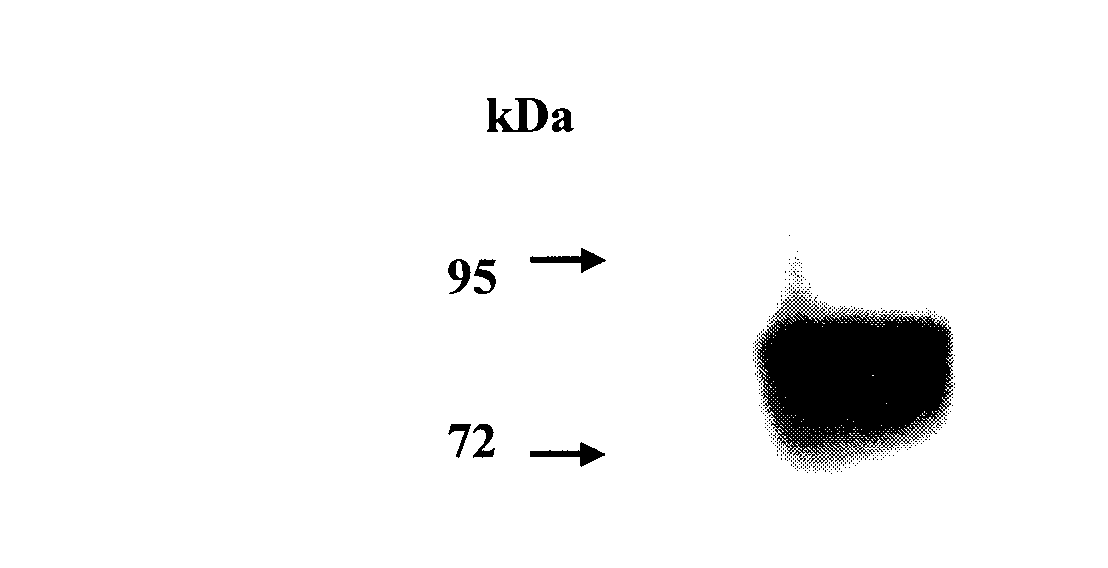
[0042] Example 1. Acquisition of the immune fusion protein FcεRIα / IgG2 and its functional analysis
[0043] 1. Acquisition of the immune fusion protein FcεRIα / IgG2
[0044] 1. Optimization and synthesis of expression gene of immune fusion protein FcεRIα / IgG2
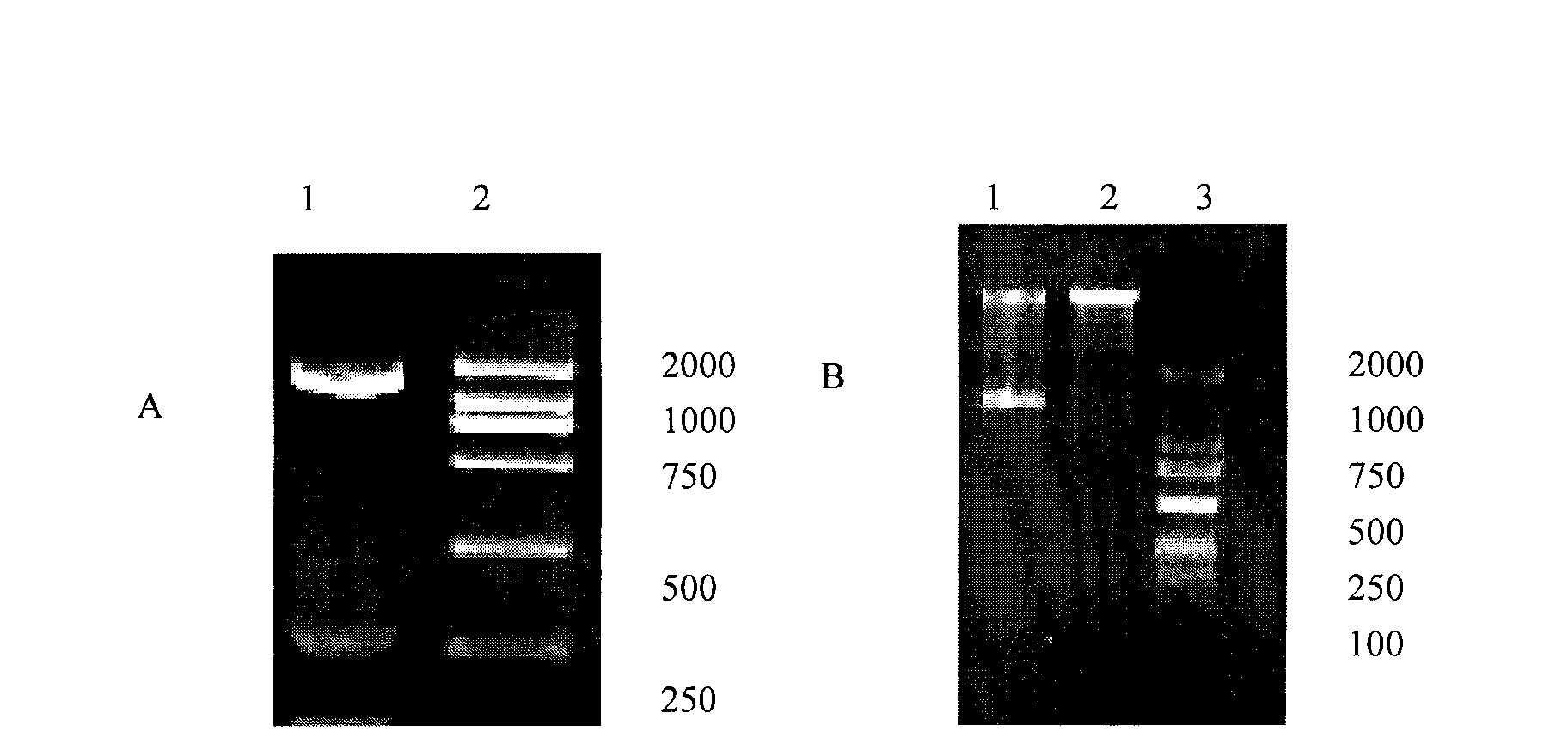
[0045] Artificially synthesize the DNA fragment shown in Sequence 1, use the DNA fragment as a template, and perform PCR amplification with the following primers:
[0046] Upstream primer: 5-CCG CTCGAG CCGCCACCATGGAGAC-3;
[0047] Downstream primer 5-CCG GAATTC TTACTTTTCCAGGAGACAGGGACAGG-3,
[0048] The underlined parts are the restriction sites of XhoI and EcoRI respectively, which were synthesized by Beijing Aoke Biotechnology Co., Ltd.
[0049] The reaction conditions of PCR amplification were: pre-denaturation at 95°C for 5 minutes; denaturation at 94°C for 45 s, annealing at 56°C for 45 s, extension at 72°C for 2 min, and 30 cycles of amplification; and extension at 72°C for 10 minutes. PCR amplified product...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com