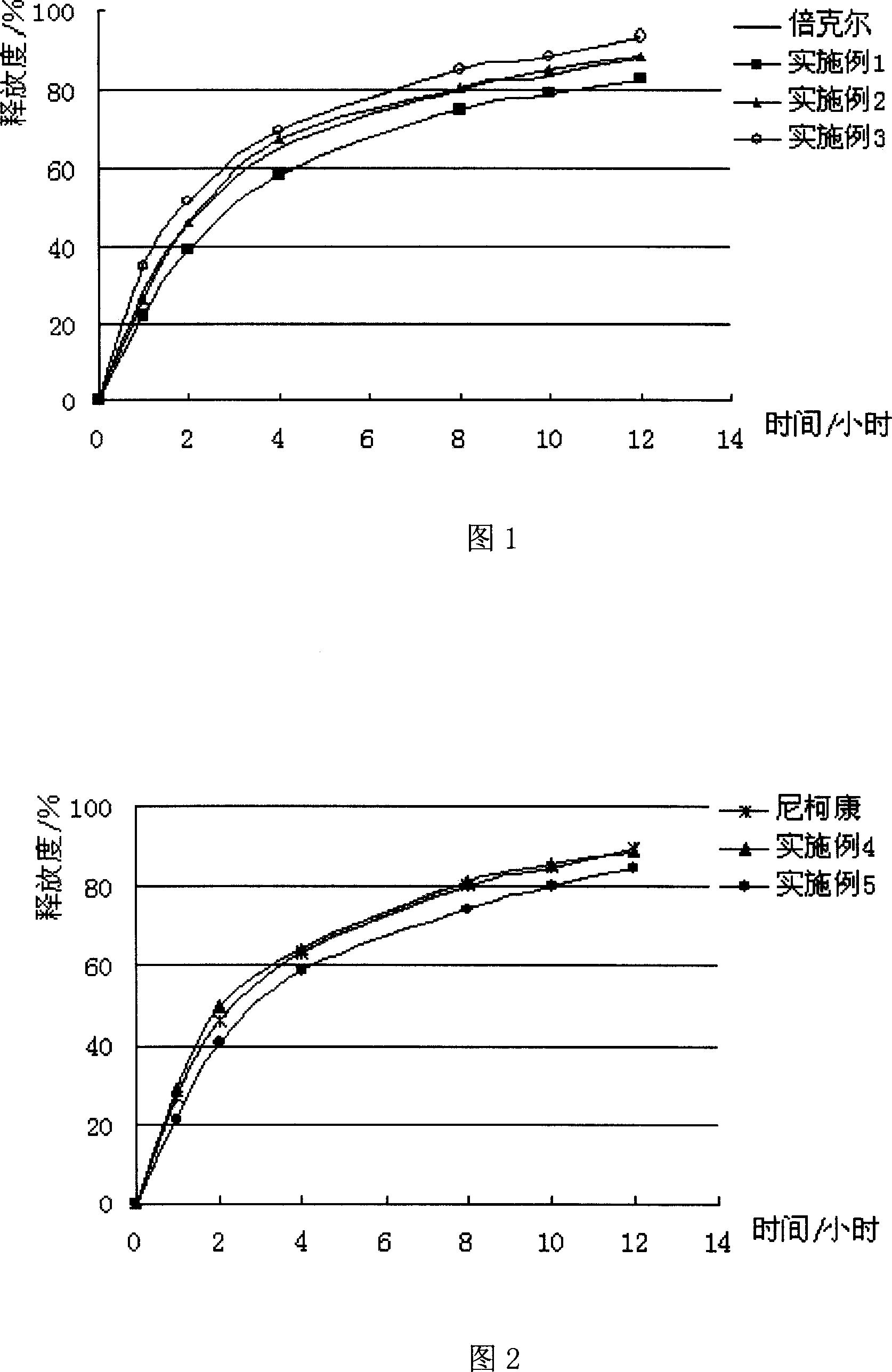
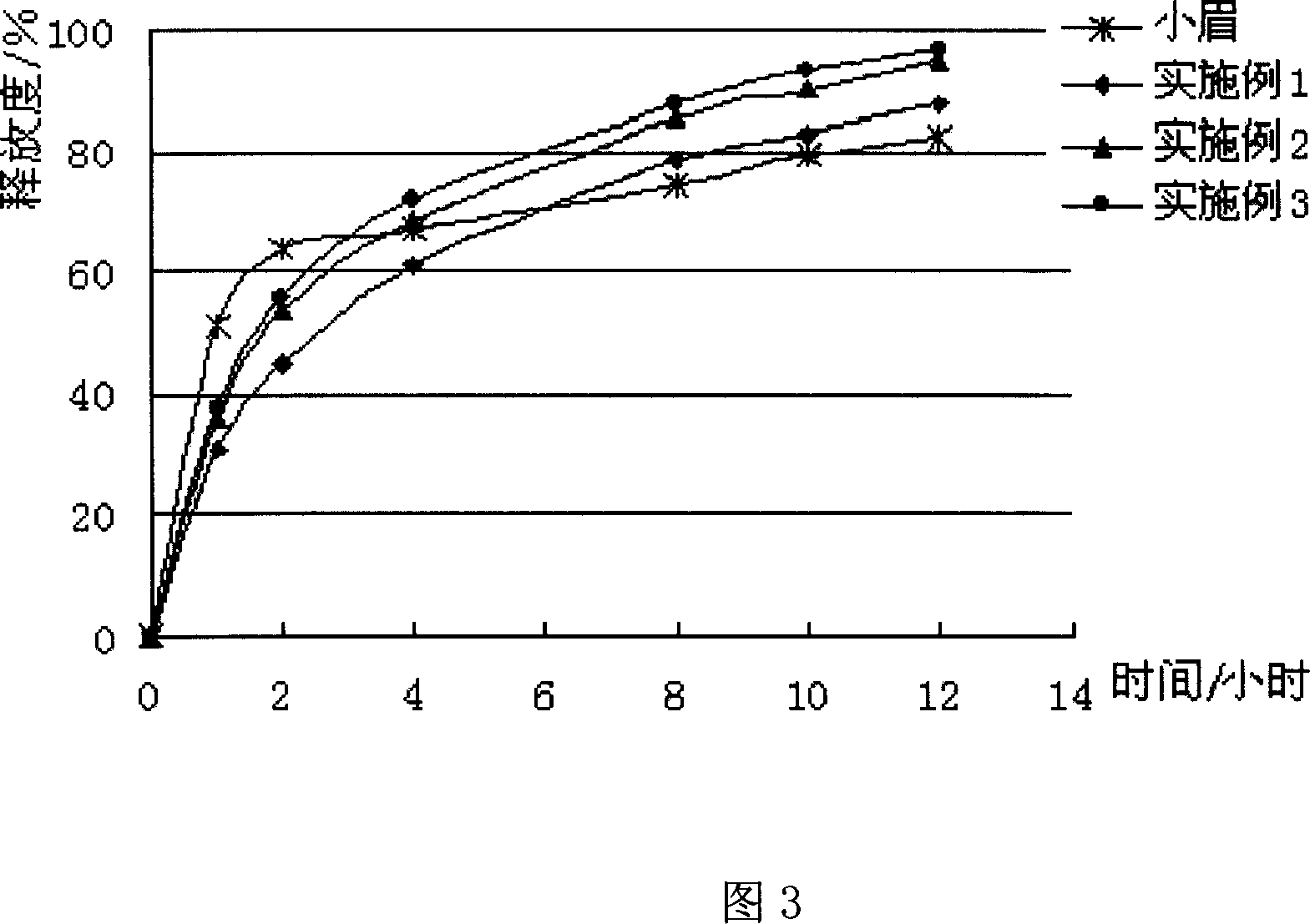
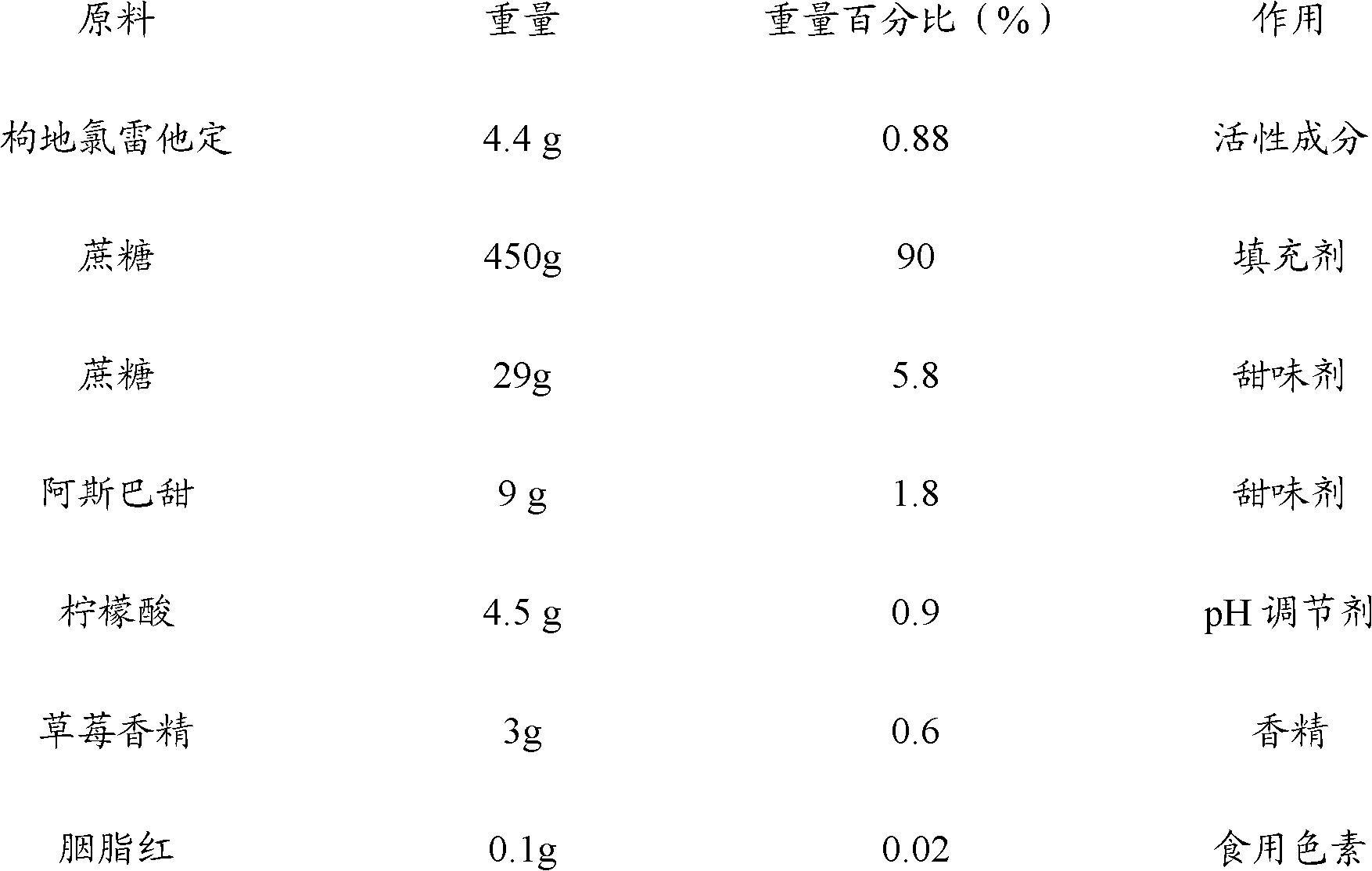
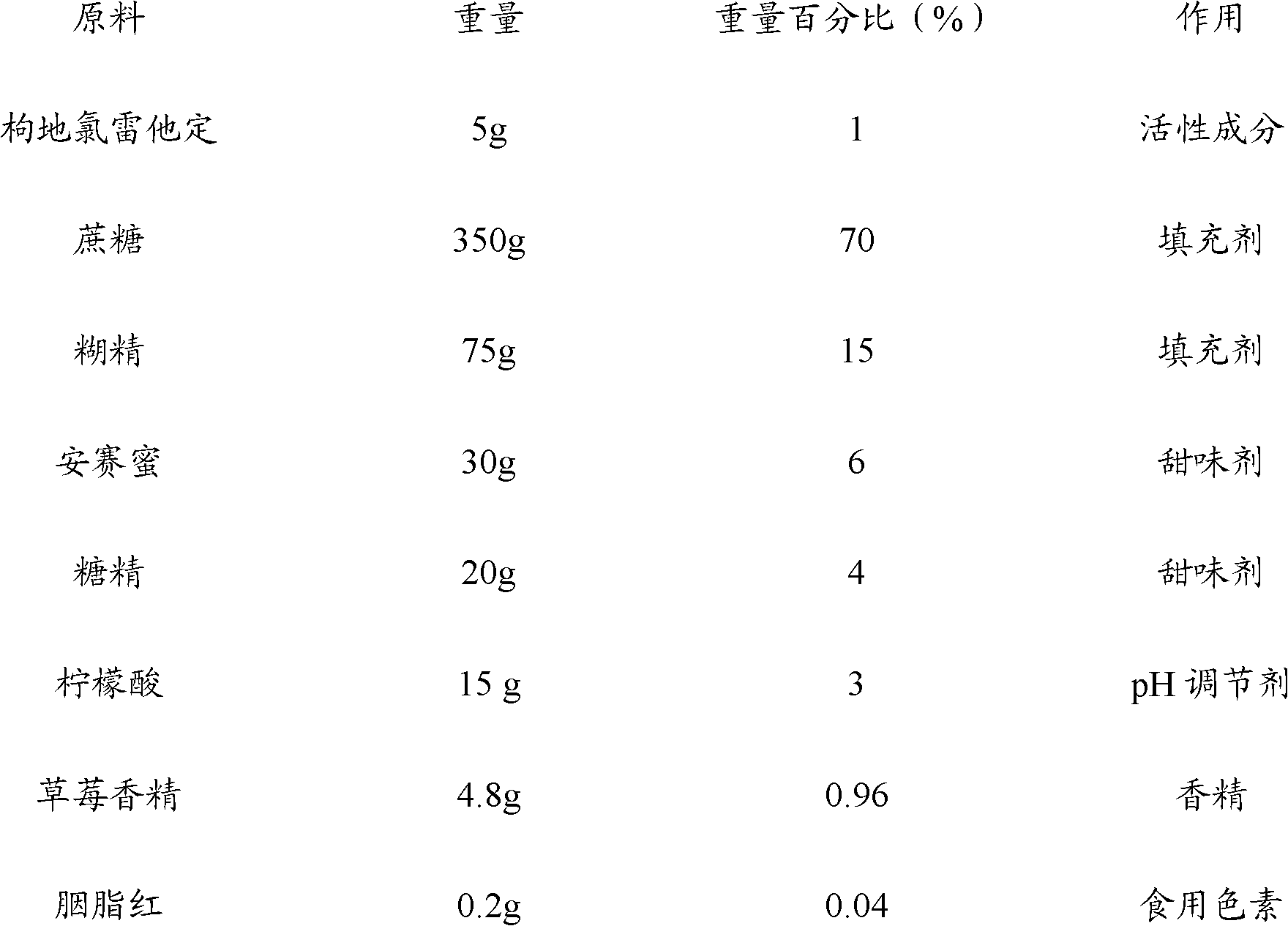
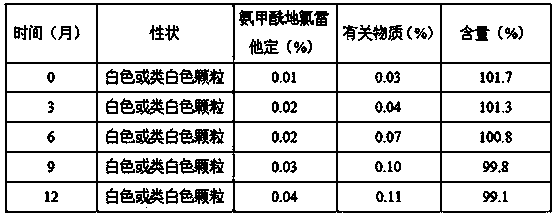
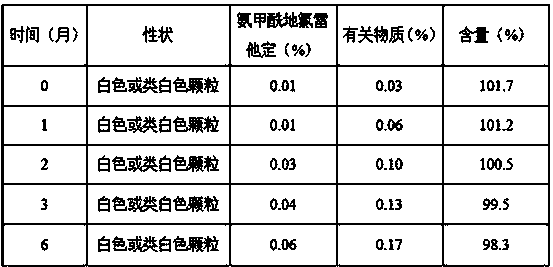
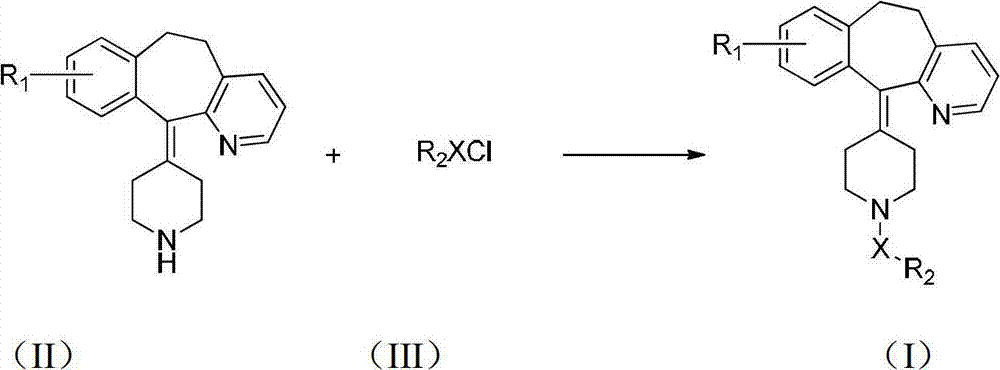Patents
Literature
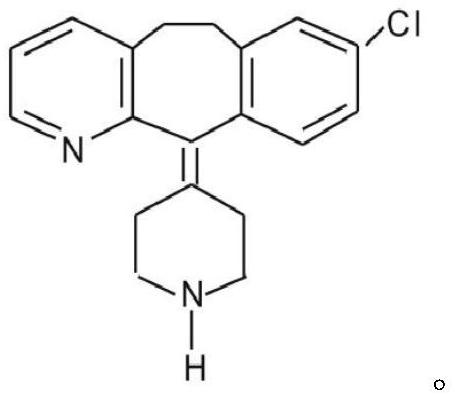
160 results about "Desloratadine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
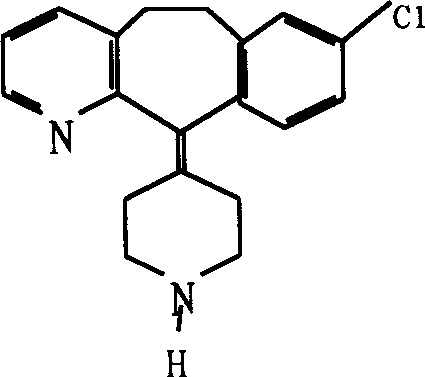
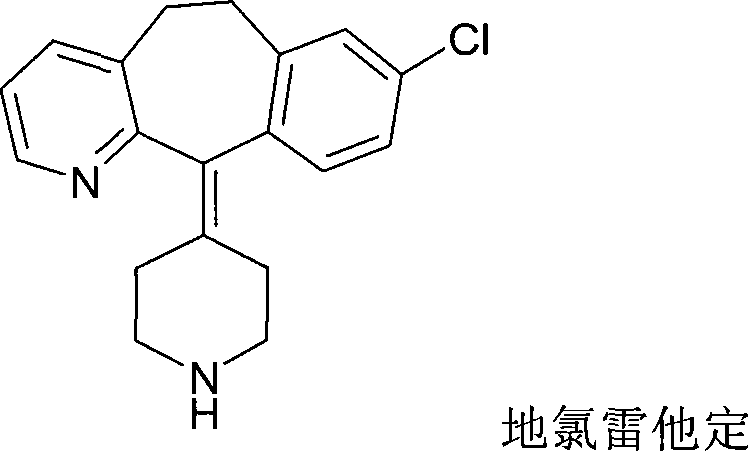
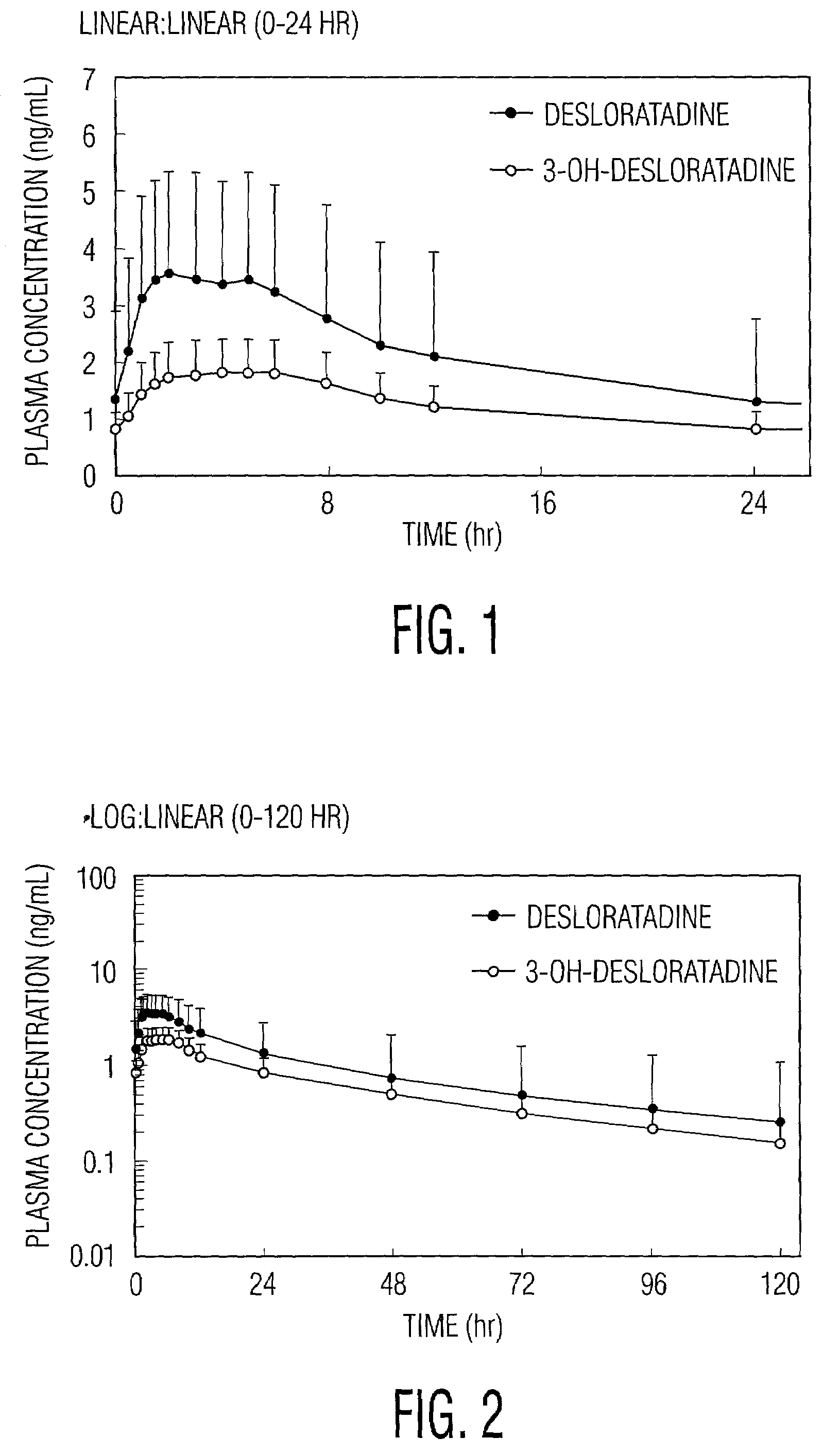
Desloratadine is an antihistamine used to relieve allergy symptoms such as watery eyes, runny nose, itching eyes/nose, sneezing, hives, and itching.
[Instant dissolving tablet composition for loratidine and desloratidine]
Disclosed here is a tablet formulation of loratidine and desloratidine, non-sedating antihistaminic agents, that allows fast dissolution of tablets in the mouth allowing administration of these drugs without the aid of water. The formulation has pleasing taste and texture.
Owner:GULF PHARMA INDS
Desloratadine grain and preparation method thereof
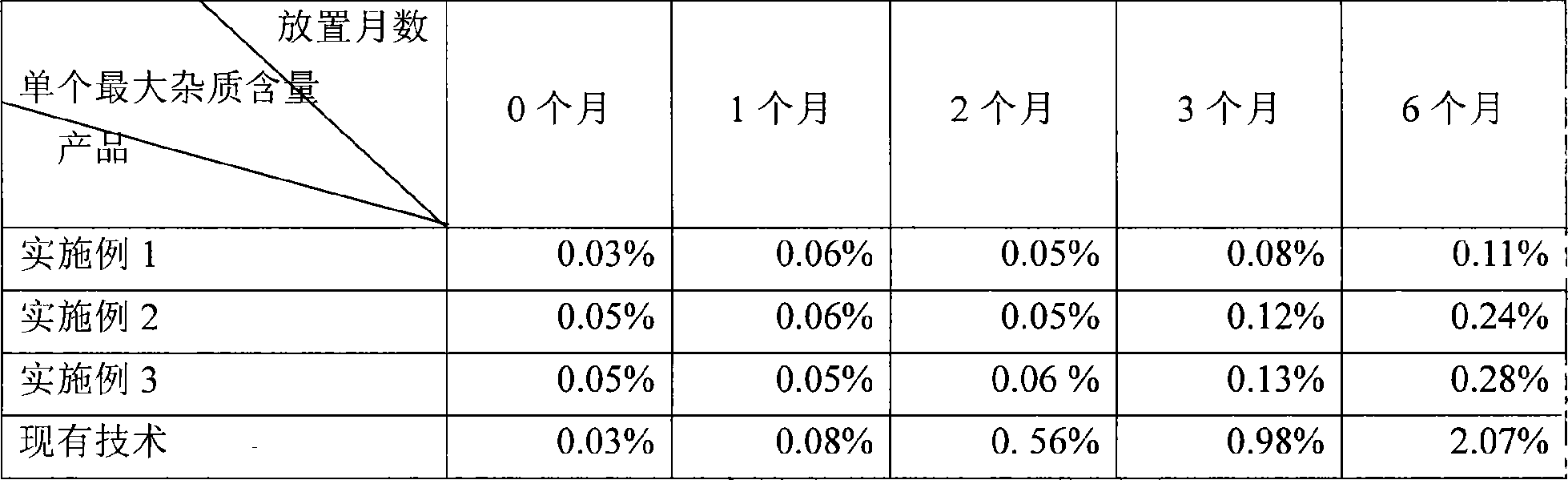
ActiveCN102038645ADoes not affect releaseEasy to takeOrganic active ingredientsPharmaceutical non-active ingredientsMedicineRoom temperature
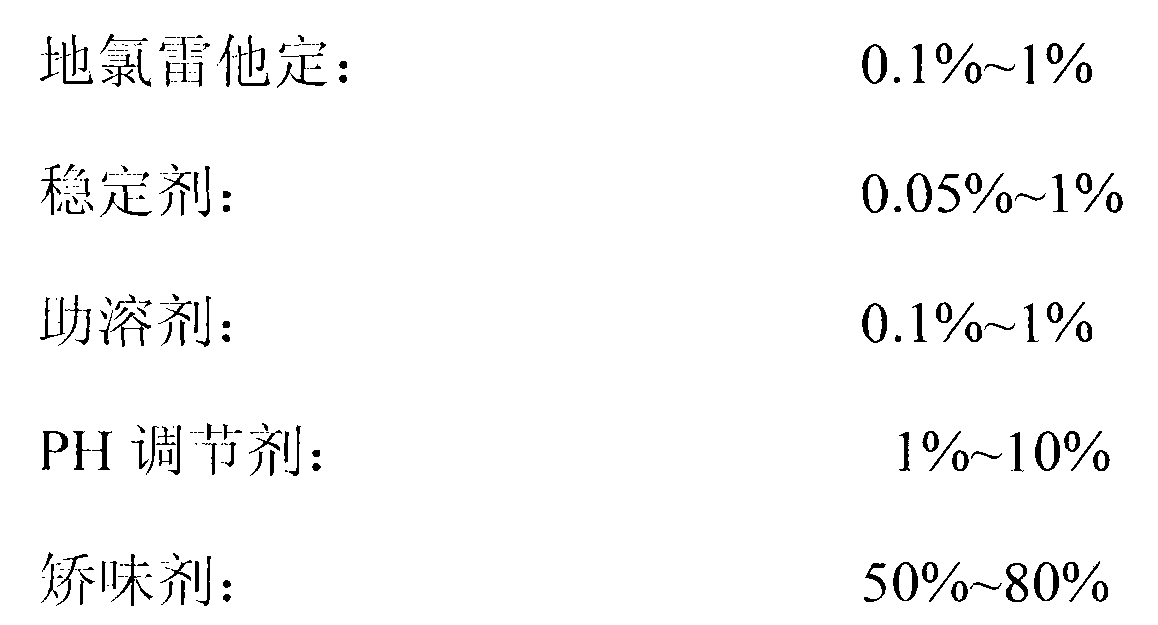
The invention relates to a desloratadine grain and a preparation method thereof. The preparation method comprises the steps: fully coating a medicine and proper auxiliary materials (such as a stabilizing agent, a cosolvent, a PH adjusting agent, a filling agent and the like) into a water-soluble high molecular material; and adding a proper opacifier into a coating material, so that the medicine can be isolated from the light and the external moist air when the medicine is stored, and the stability of the medicine can be adequately guaranteed when the medicine is stored under the room temperature; mixing the coated grain with a proper flavoring agent and soluble auxiliary materials to obtain the desloratadine granular formulation; and properly adding a small quantity of edible coloring agent and fruity essence to increase the novelty to the children, and improve the taking compliance.
Owner:HAINAN PULIN PHARMA +1
Medicinal composition for stabilizing delotadine in preparation
InactiveCN1552324ASolve the defect of brown-red substanceGuaranteed stabilityOrganic active ingredientsImmunological disordersLactoseActive ingredient
A composite medicine in which desloratadine is stabilized features that after desloratadine is mixed with medicinal auxiliaries, an antioxidizing agent is added to the mixture.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
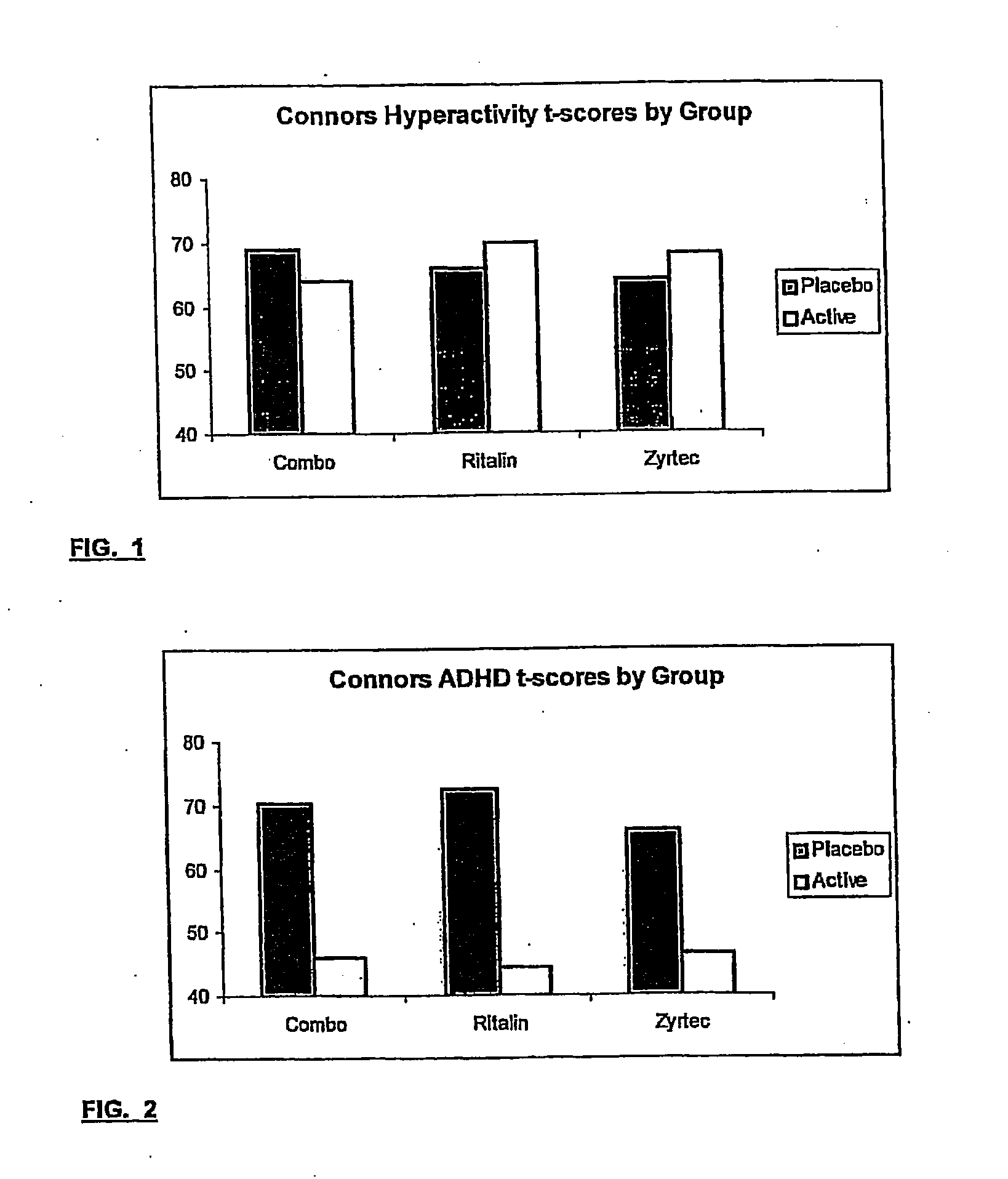
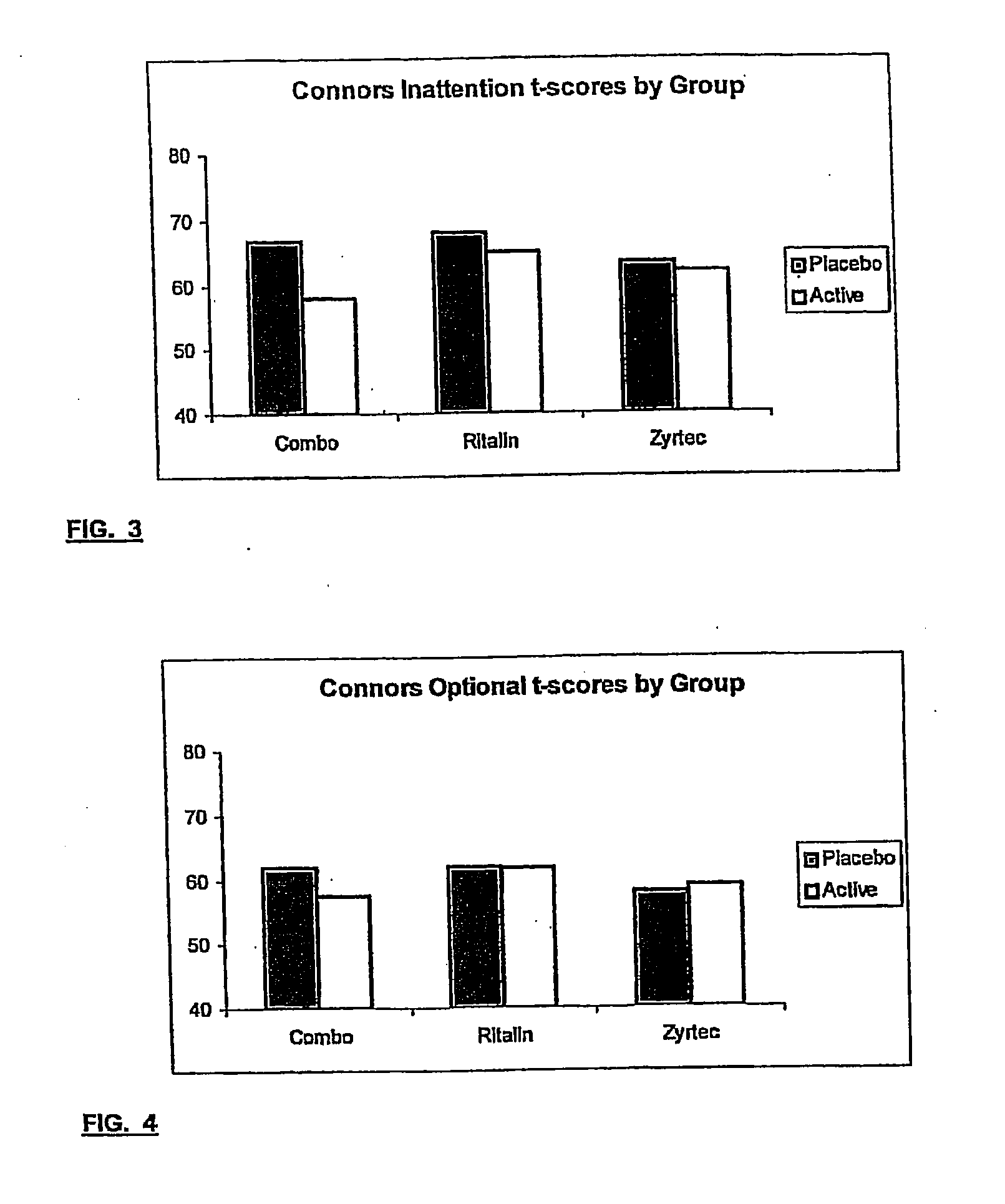
Treatment of behavioral disorders
InactiveUS20050192290A1Ameliorate behavioral disorderSufficient amountBiocideNervous disorderTherapeutic ACTHFexofenadine
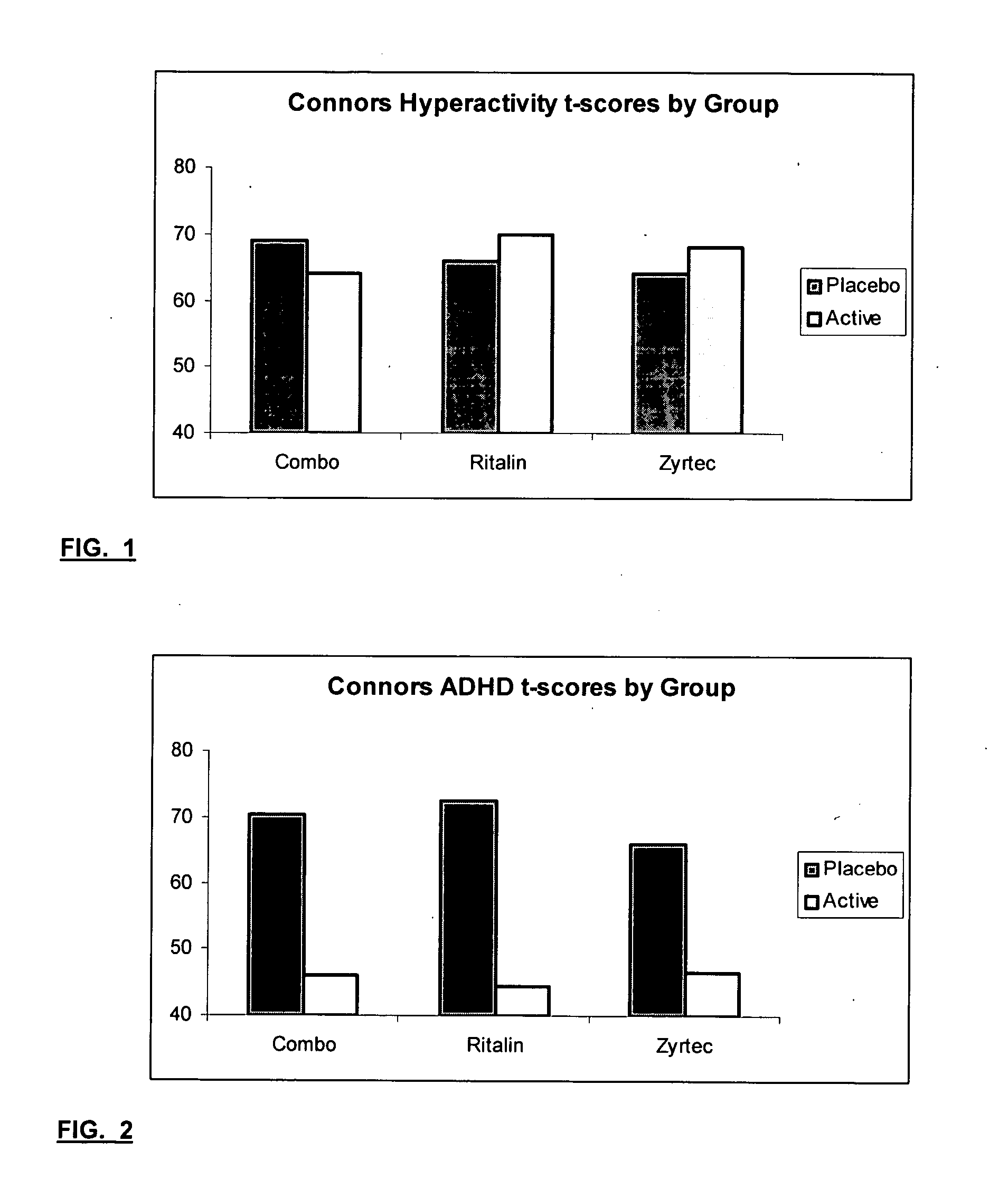
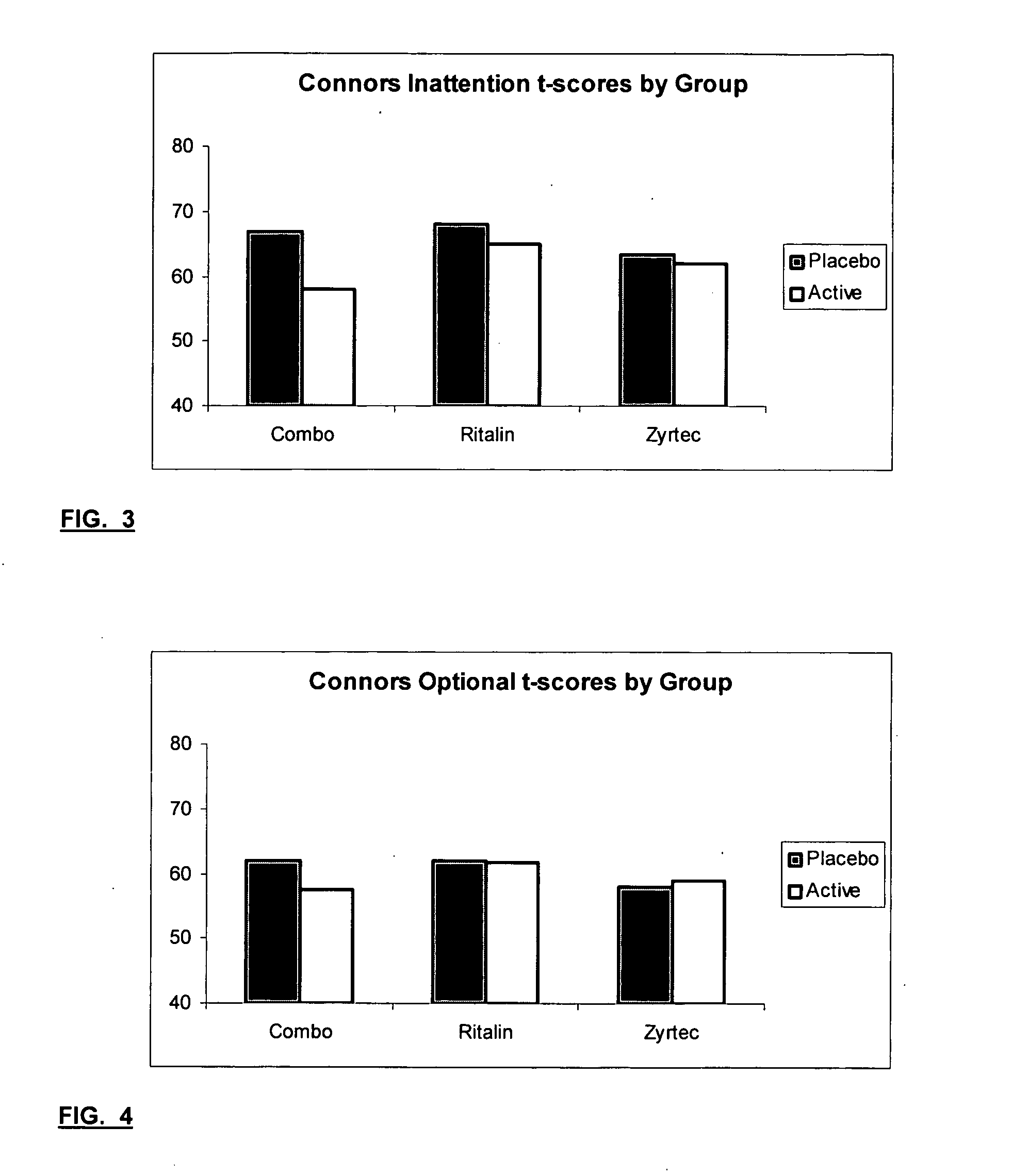
The present invention relates to a method for treating a behavior disorder comprising the administration of a therapeutically effective amount of antihistamine, such as ceterizine, fexofenadine; loratadine, and desloratadine. The behavioral disorders may include ADHD, anxieity, depression, and autism. The method may include the administration of the antihistamine in combination with a stimulant medication, such as methylphenidate, thereby to achieve a synergistic effect. In any event, the amount of antihistamine and / or stimulant is effective to downregulate neurotrophic factors such as nerve growth factor or CD40. The invention is also directed to a method of preventing the onset of behavior disorders in patients presenting with symptoms of allergic rhinitis.
Owner:MELAMED ISAAC
Desloratadine dry-mixed suspensoid and preparing method thereof
The present invention discloses a new dosage form of dichloroleitadine. It includes coated dichloroleitadine, and its content is 0.01-1%, and its coating material is methyl aminoalkyl acrylate copolymer type E and II acrylic resin. The dichloroleitadine raw material is sensitive to light heat and humidity, in order to ensure its stability, said raw material is coated so as to improve its non-stability, at the same time can hide its taste.
Owner:HAINAN PULIN PHARMA +1
Desloratadine syrup and preparation method thereof
InactiveCN101390860ASolve solubilityFix stability issuesOrganic active ingredientsPharmaceutical delivery mechanismChemistryAntihistamine
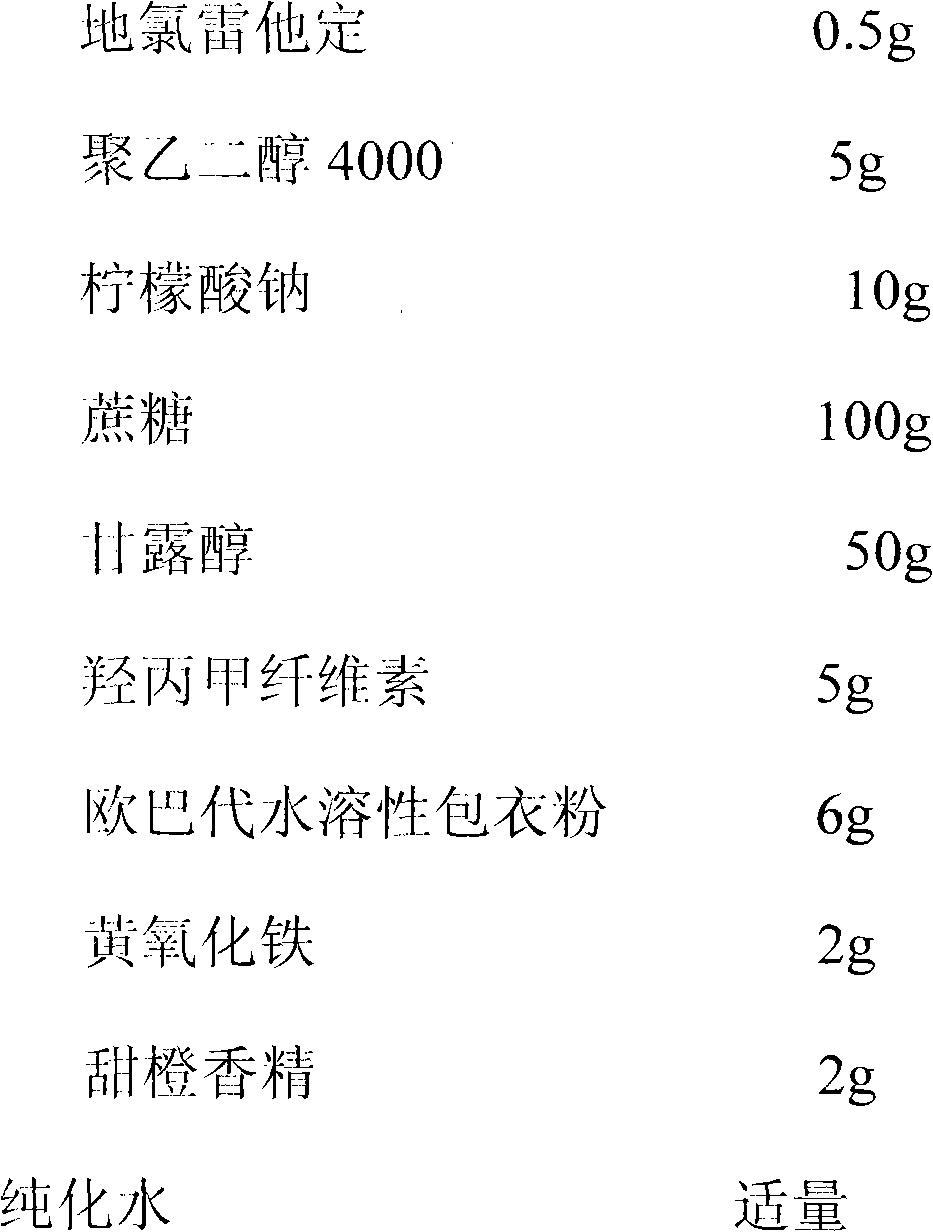
The invention discloses a syrup of and the preparation method thereof. The insolubleness and instability problems of desloratadine are successfully solved through addition of cosolvent and stabilizer, and the control of pH value. The syrup of antihistamines desloratadine can facilitate the oral-taking of patients with weak swallowing capability, has good taste and good stability.
Owner:AVENTIS PHARMA HAINAN
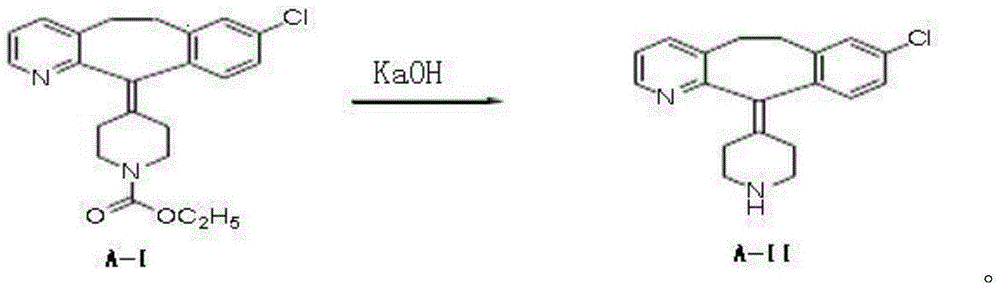
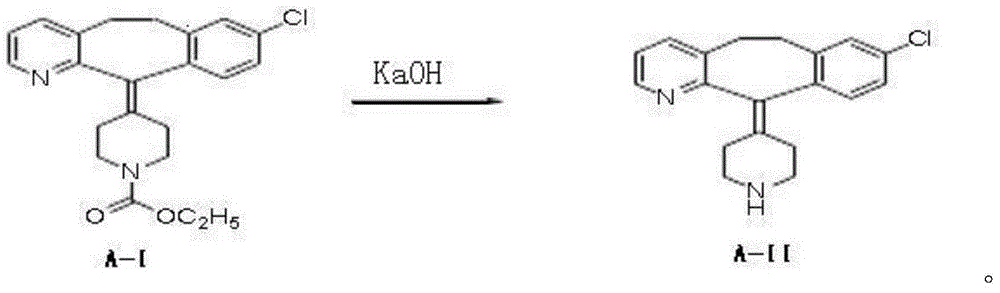
Process for the preparation of desloratadine
The present invention provides a process for the preparation of desloratadine comprising contacting loratadine with a mixture of a weak inorganic base and sodium or potassium hydroxide in a ratio, ranging from 0.01 to 0.15 equivalents of sodium or potassium hydroxide per equivalent of weak inorganic base, in one or more suitable solvents) followed by isolation.
Owner:RANBAXY LAB LTD
Desloratadine nitric oxide donor, and preparation method and application thereof
InactiveCN104292211AGood inhibitory effectEnhanced inhibitory effectOrganic active ingredientsOrganic chemistryHydrogenNitric oxide
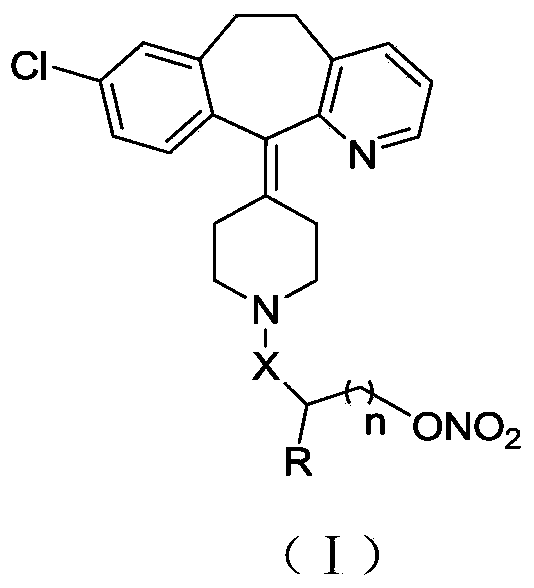
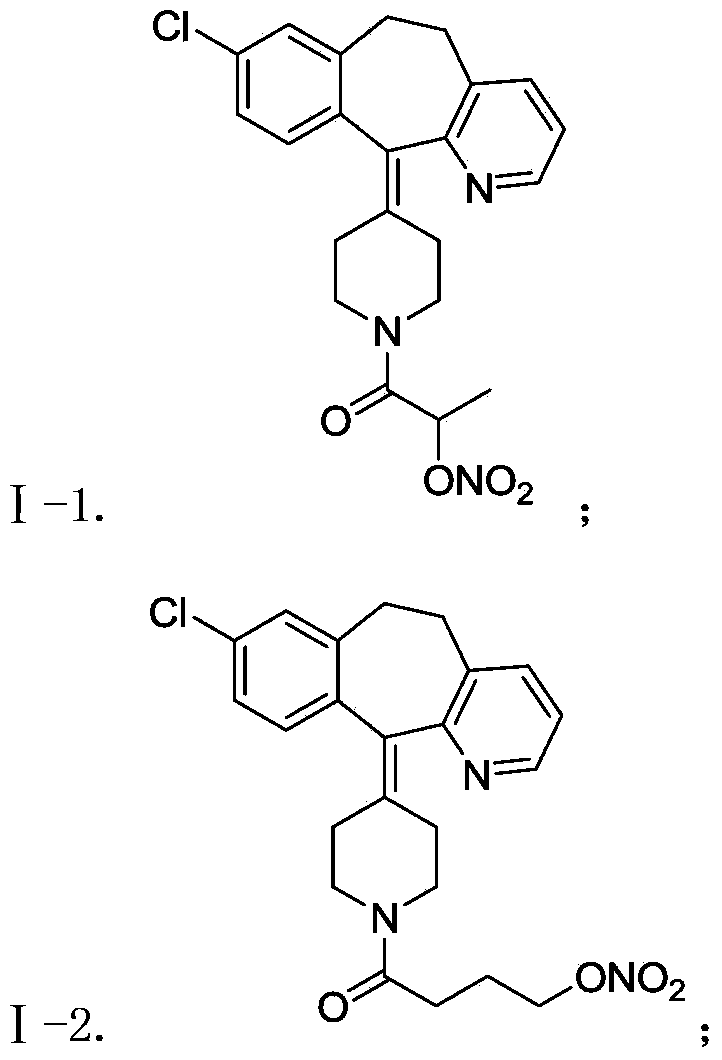
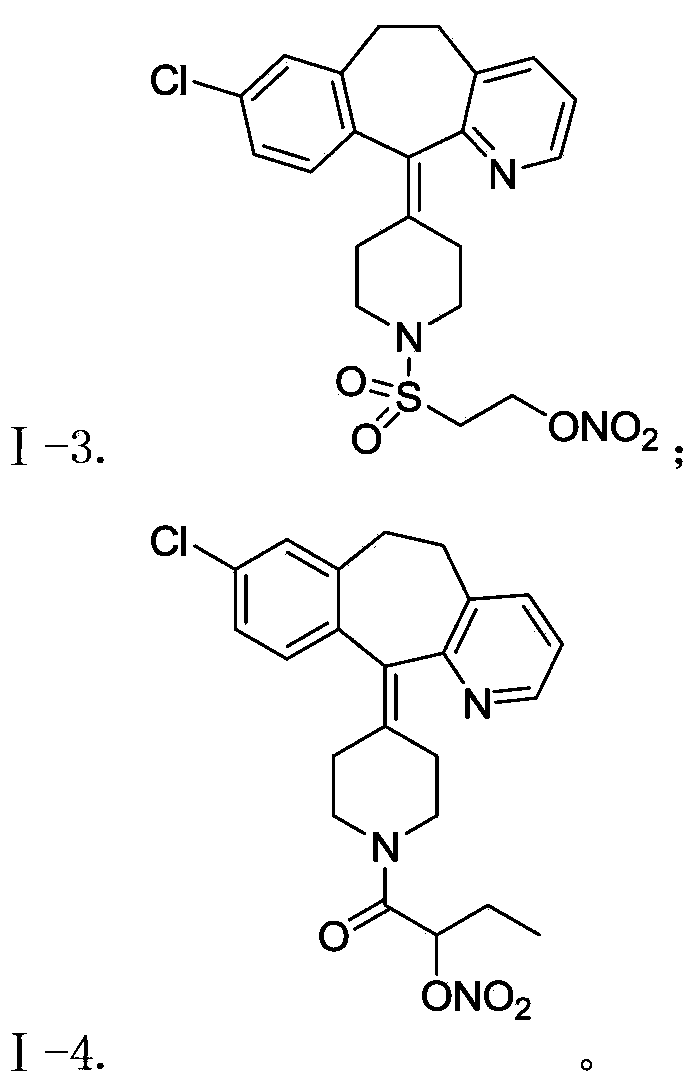
The invention belongs to the technical field of medicine, and discloses a desloratadine nitric oxide donor with novel structure shown as a formula I and pharmaceutically acceptable salts thereof, wherein X is carbonyl or sulfonyl, n equals to 0, 1 or 2, and R is hydrogen and C1-C4 alkyl. The invention also relates to a preparation of the compound, and also discloses a pharmaceutical composition using the compound or the pharmaceutically acceptable salt thereof as an active ingredient, and application thereof as antitumor drug, especially application thereof to preparation of drugs for the treatment of breast cancer, lung cancer and stomach cancer.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
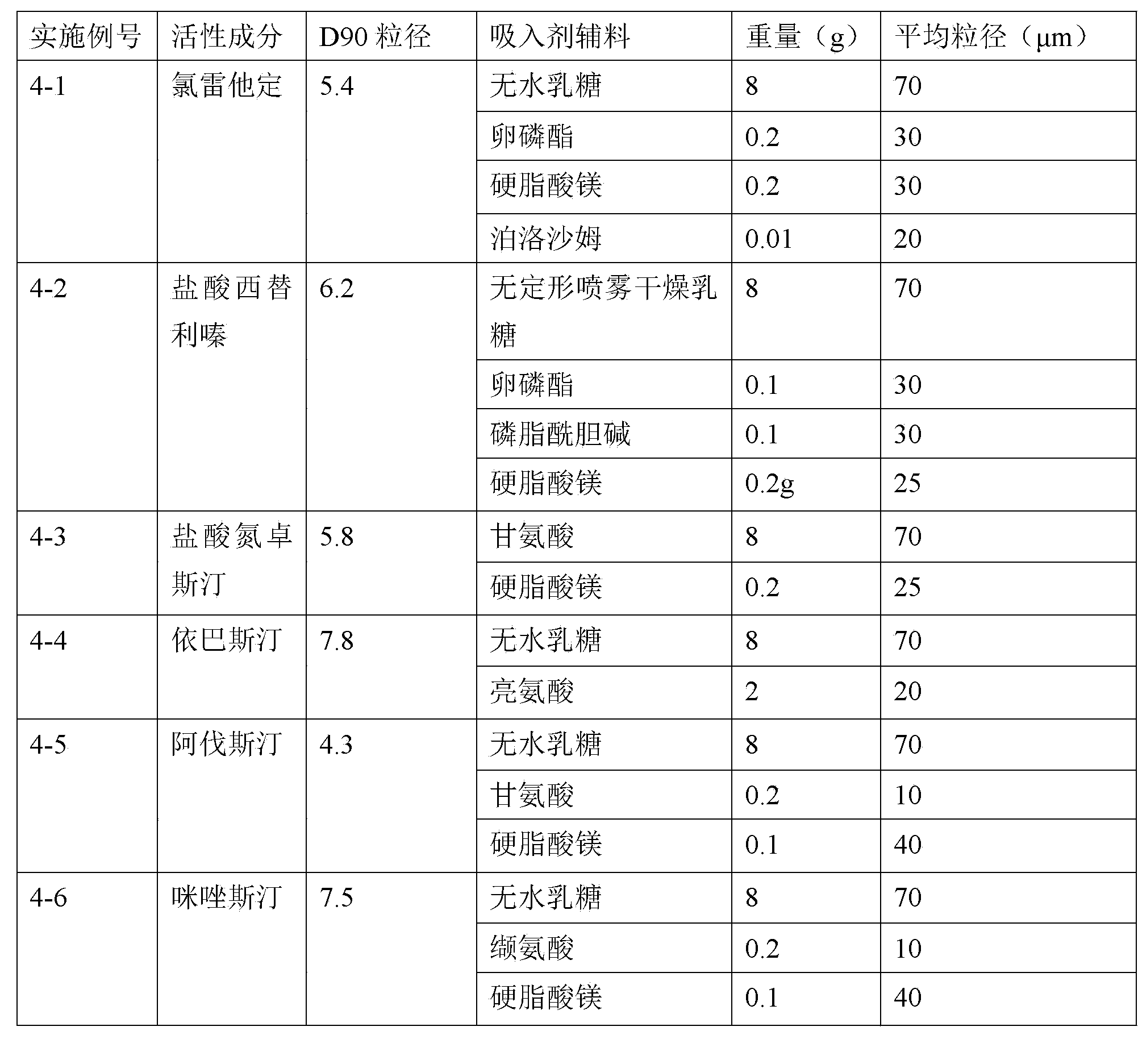
Coated tablet containing desloratadine and preparation method thereof
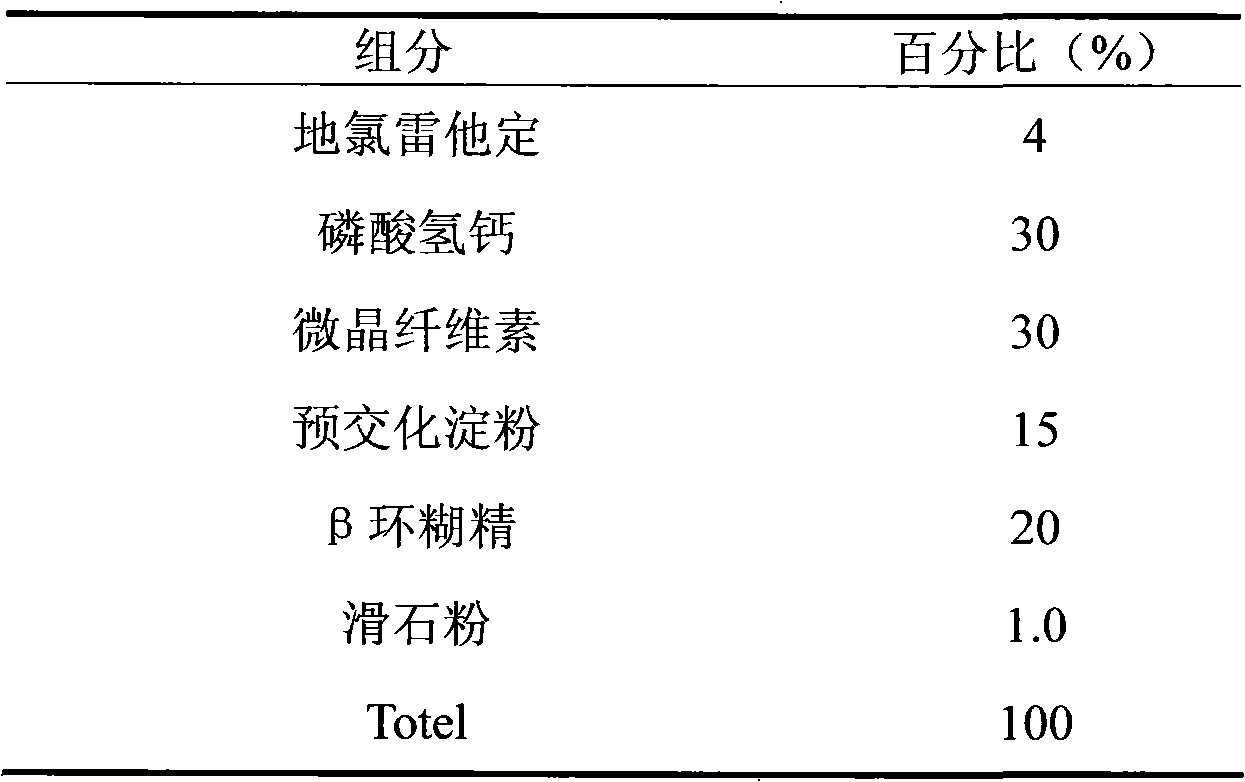
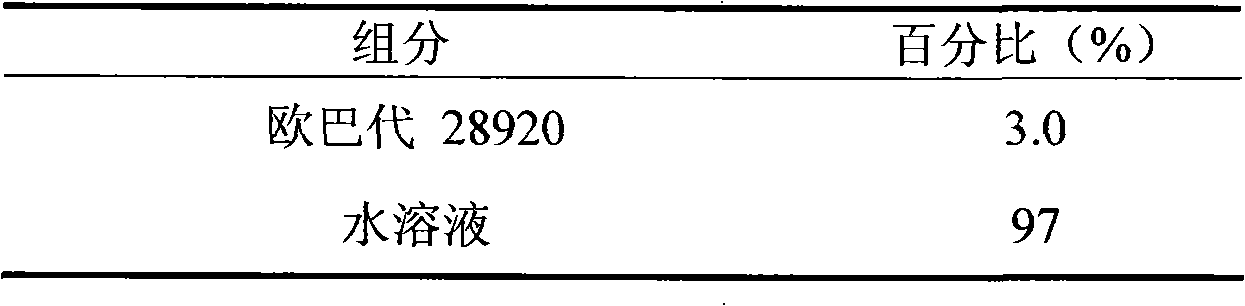
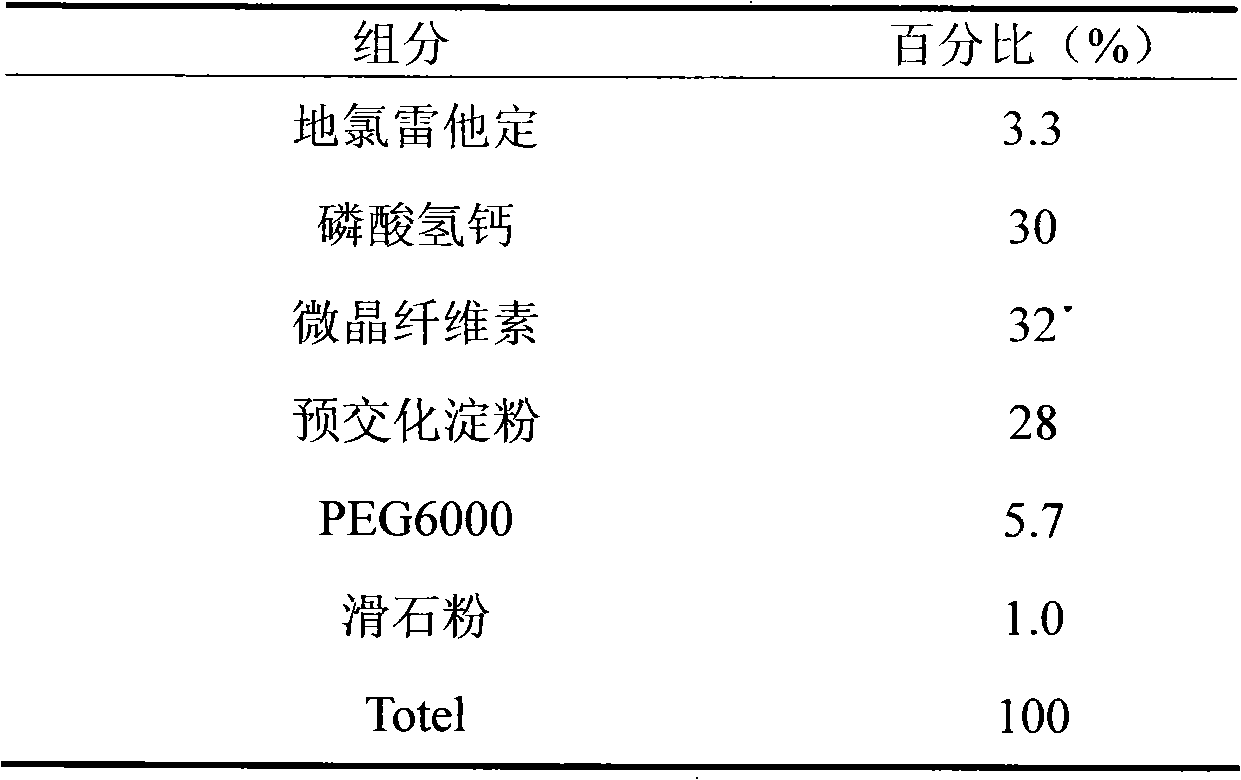
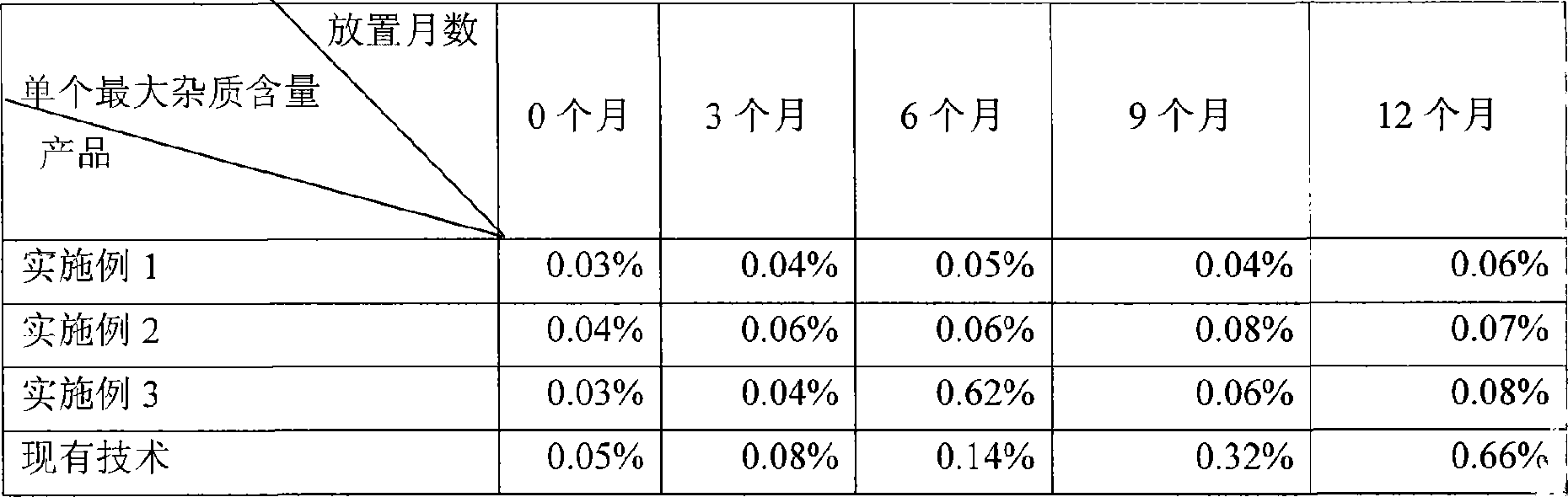
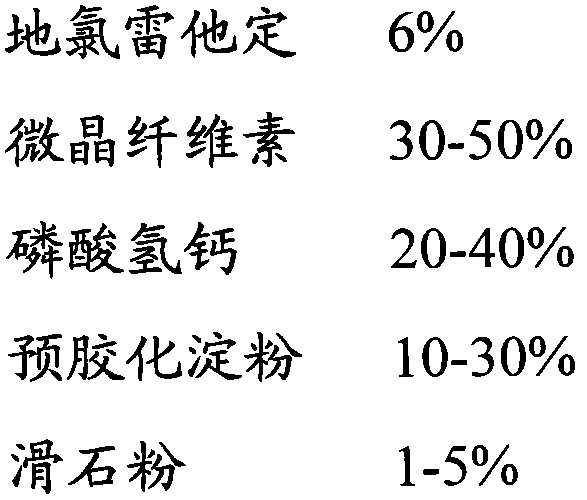
ActiveCN101548959AFix stability issuesDissolution qualifiedOrganic active ingredientsRespiratory disorderCoated tabletsHydrogen phosphate
The invention discloses a coated tablet containing desloratadine and a preparation method thereof. The coated tablet applies a combination of calcium hydrogen phosphate, microcrystalline cellulose and pregelatinized starch of specific proportion, performs inclusion to the desloratadine by applying the solid dispersion technology, ensures the crushability of the particles so as to be improved in the dissolution rate, reduces the degradation of the desloratadine in the oxygen and the moisture and effectively increases the stability.
Owner:AVENTIS PHARMA HAINAN
Wrapped tablets dichlororeytadin and its preparing method
InactiveCN101045040ASolve the problem of rapid material riseReduce the impactOrganic active ingredientsRespiratory disorderOxygenMoisture
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
Desloratadine oral liquid preparation and preparation method thereof
ActiveCN102697711AImprove stabilityNo degradationOrganic active ingredientsAntipyreticBeta-CyclodextrinsImpurity
The invention discloses a desloratadine oral liquid preparation which comprises desloratadine, cyclodextrin, EDTA and water, wherein the mole ratio of desloratadine to cyclodextrin is 1:1-1:10, the concentration of desloratadine in the oral liquid preparation is 0.1mg / ml-1mg / ml, and the cyclodextrin is hydroxypropyl-beta-cyclodextrin or sulfobutyl-beta-cyclodextrin. According to the invention, cyclodextrin is added to the desloratadine oral liquid preparation prescription, the stability of the oral liquid preparation is greatly increased, and no degradation impurity is produced.
Owner:GUANGZHOU NEWORLD PHARMA CO LTD
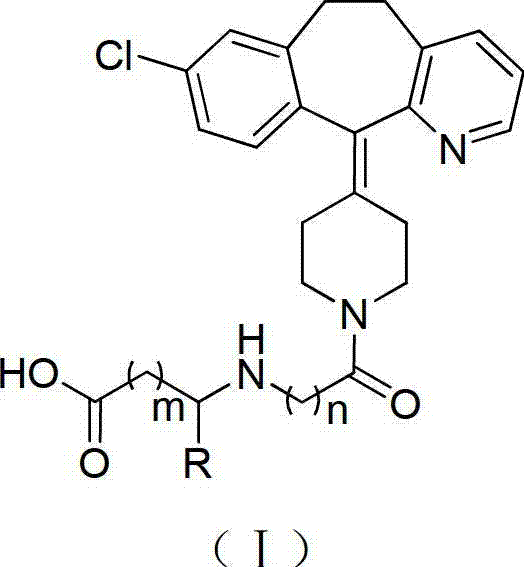
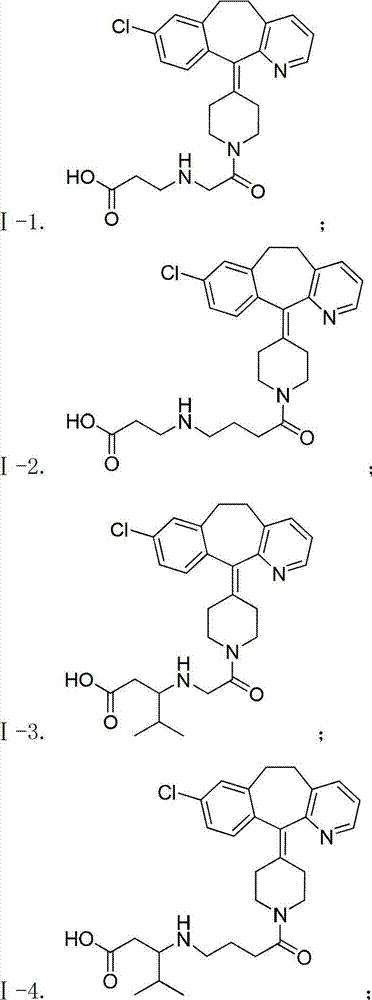
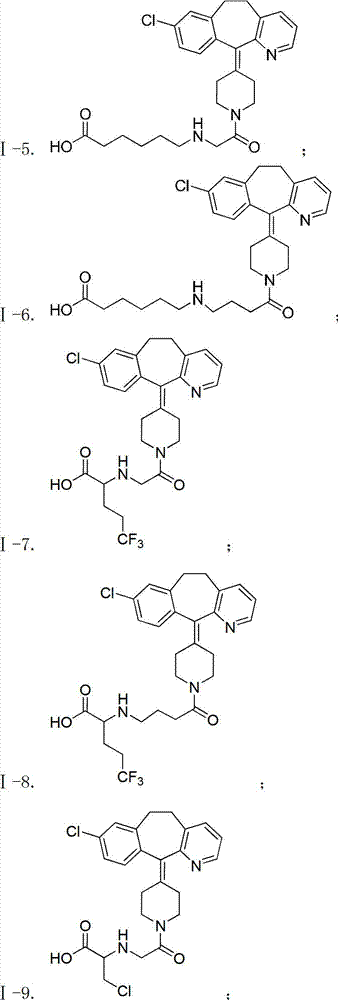
Desloratadine-containing amino acid derivative as well as preparation method and application thereof
ActiveCN103044395AEnhanced inhibitory effectOrganic active ingredientsOrganic chemistryHydrogenChemical compound
The invention discloses a desloratadine-containing amino acid compound with a structure of formula I and a pharmaceutically acceptable salt thereof. In the formula, n is 1, 2 or 3, m is 0, 1, 2, 3, 4, 5 or 6, and R is hydrogen, methyl, ethyl, isopropyl, fluorine-substituted C1-C4 alkyl or chloromethyl. The invention further discloses a preparation method for the compound and a pharmaceutical composition taking the compound or the pharmaceutically acceptable salt of the compound as an active ingredient at the same time, as well as the application of the compound, the pharmaceutically acceptable salt of the compound and the pharmaceutical composition serving as anti-tumor drugs, especially the application in preparation of drugs for treating breast cancer, lung cancer and gastric cancer.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Preparation method of desloratadine
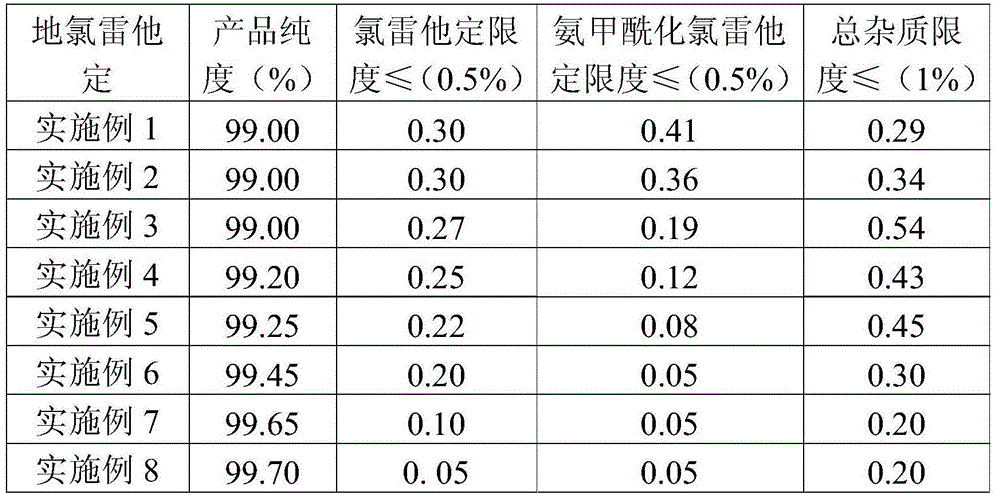
The invention relates to a preparation method of desloratadine. The preparation method comprises the following steps: (1) dissolving loratadine into an alcohol solvent with the volume concentration of 55-75% under the protection of nitrogen, adding potassium hydroxide, heating to 70-75 DEG C, beginning reflux reaction, controlling the temperature of the reflux reaction to keep a gradient change within 70-100 DEG C, and refluxing till complete reaction of the loratadine; (2) adding an ethyl acetate solvent into a completely reacted reaction solution for extracting, draining out a water layer, repeating the extracting step for 2-3 times, washing by using water, and crystallizing, decolorizing and recrystallizing to obtain desloratadine. By the preparation method, the technical problems that in the prior art, the product desloratadine cannot meet a requirement for purity, contains many impurities, and cannot meet requirements on clinical application are solved.
Owner:GUANGDONG JIUMING PHARMA
Desloratadine patch and preparation method thereof
InactiveCN101933914AImprove solubilityGood curative effectOrganic active ingredientsImmunological disordersCross-linkSolubility
The invention relates to a desloratadine patch and a preparation method thereof. The desloratadine patch comprises a patch film and medicament components on the patch film, wherein the medicament components comprise main component desloratadine and auxiliary materials; and the auxiliary materials comprise a solubilizing agent, a substrate, a cross-linking agent, an excipient and an osmosis reinforcing agent. The invention overcomes the bias that in the prior art, the desloratadine is considered to be difficult to externally penetrate through the skin cuticle and not to reach effective tissue concentration in the skin tissue so as not to exert the curative effects of allergic resistance and inflammation resistance, solves the problem of poor solubility of the desloratadine, improves the permeability of the desloratadine on the skin cuticle, ensures that the desloratadine rapidly arrives at the affected part and takes the effect rapidly and solves the problems of inconspicuous effect-taking and obvious first pass effect on gastrointestinal tract of the traditional patch. The desloratadine patch has the advantages of good curative effect, rapid action, less toxic or side effect and convenient use.
Owner:HANGZHOU SHARPLY PHARM R&D INSTIT +1
Treatment of Behavioral Disorders
The present invention relates to a method for treating a behavior disorder comprising the administration of a therapeutically effective amount of antihistamine, such as cetirizine, fexofenadine; loratadine, and desloratadine. The behavioral disorders may include ADHD, anxiety, depression, and autism. The method may include the administration of the antihistamine in combination with a stimulant medication, such as methylphenidate, thereby to achieve a synergistic effect. In any event, the amount of antihistamine and / or stimulant is effective to downregulate neurotrophic factors such as nerve growth factor or CD40. The invention is also directed to a method of preventing the onset of behavior disorders in patients presenting with symptoms of allergic rhinitis.
Owner:MELAMED ISAAC
Loratadine oral compound medication composition
InactiveCN101015519AOrganic active ingredientsPharmaceutical delivery mechanismAntitussive drugsDrug effect
This invention relates to an orally adminstered compound Chinese medicinal composition of loratadine for treating cough. The composition comprises quick-release part containing loratadine and slow-release part containing centrum antitussive drug, wherein the centrum antitussive drug is preferably dextromethorphan. The invention maintains the quick and long lasting action of loratadine or desloratadine, overcomes the quick releasing and short action on relieving cough of dextromethorphan, slow releases dextromethorphan, and maintains the drug effect to arrive the purpose of relieving cough for a long time.
Owner:CHONGQING PHARMA RES INST
Desloratadine critrate disodium particles and preparation method for same
InactiveCN102525944AEasy to takeFast absorptionOrganic active ingredientsGranular deliveryBioavailabilityBULK ACTIVE INGREDIENT
The invention discloses desloratadine critrate disodium particles. Based on 100 percent, the desloratadine critrate disodium particles comprise the following raw materials in percentage by weight: 0.1 to 2 percent of desloratadine critrate disodium, 20 to 90 percent of filler, 1 to 10 percent of sweetener, 0.1 to 5 percent of PH conditioner, 0.1 to 2 percent of essence and 0 to 0.05 percent of food coloring, wherein the desloratadine critrate disodium serves as an active ingredient; and the filler, the sweetener, the PH conditioner, the essence and the food coloring serve as minor ingredients. The prepared desloratadine critrate disodium particles have good mouth-feel, and are fresh red, attractive, convenient to take, fast in absorption and high in bioavailability; and a child can compliantly take the particles.
Owner:YANGTZE RIVER PHARM GRP GUANGZHOU HAIRUI PHARM CO LTD
Oral desloratadine drops and preparation method thereof
InactiveCN106619504AEasy to acceptClear pharmacological propertiesOrganic active ingredientsSenses disorderNoseDisease patient
The invention belongs to the technical field of medicines and relates to a preparation method of oral desloratadine drops. The oral desloratadine drops are prepared from a loratadine raw material medicine, a preservative, a stabilizer, a coloring agent, a corrigent, a thickening agent, a cosolvent and the like. 10 mg of drops is orally taken every time for adults and adolescents of 12 years old or above, and infants are administrated according to kilogram and body weight. The oral desloratadine drops are syrup or a solution and has the following advantages in overall effect that the drug exists in a molecular state in liquid, can be rapidly absorbed after administration, plays a drug action and is high in initial effect time; the process is simple in operation, the cost is low, and the drops are convenient to take, stable in quality, reliable in treating effect and easy to absorb; the drops are good in taste, are conveniently accepted by patients, are suitable for patients difficult to take orally and infant and old diseased patients and has strong anti-inflammatory and antiallergic effects and rapid oral taking and absorbing effects, and the nose and eye symptoms and signs are relieved after the drug is orally taken.
Owner:BEIJING VENTUREPHARM BIOTECH
Compound composition of intal and Statins
InactiveCN101766617ASolve the irritatingOrganic active ingredientsSenses disorderDiseaseAdditive ingredient
The invention relates to a compound composition containing intal and statins claritin. The compound composition consists of the following components: a) a certain amount of intal; b) a certain amount of one of olopatadine hydrochloride, ioratadine, desloratadine, degreasing ioratadine, desloratadine, rupatadine and betahistine; c) other medicinal excipients. The compound composition can be made into external preparations such as eye drops, nose drops, aerosol, spray, inhalant, gelata, eye ointments, ointments or patch and the like. The compound composition can be used for curing the diseases such as anaphylactic eye diseases, anaphylactic rhinitis, skin urtication, urticaria, allergic asthma and the like, can significantly improve allergic symptoms, and has the characteristics of high efficiency, stability, safety, low adverse reaction rate, convenient use and the like.
Owner:北京华禧联合科技发展有限公司
Composition containing desloratadine citrate disodium
InactiveCN103721267AGood water solubilityOvercome bitternessOrganic active ingredientsAntipyreticCyclodextrinPharmaceutical drug
The invention relates to a composition containing desloratadine citrate disodium. The composition adopts the dosage form of granules; the combination of cyclodextrin and desloratadine citrate disodium in a specific proportion is adopted; the cyclodextrin is used for wrapping the desloratadine citrate disodium, so as to decrease the degradation of the desloratadine citrate disodium in active auxiliary materials such as monose, and biose, and the stability of the desloratadine citrate disodium is effectively enhanced. Accelerated tests and long term tests prove that the stability of the medicine during the storage process can be soundly ensured, in addition, due to the addition of a proper corrigent, the taste of the medicine is improved. Therefore, the composition is particularly suitable for children or patients suffering from dysphagia.
Owner:YANGTZE RIVER PHARM GRP GUANGZHOU HAIRUI PHARM CO LTD
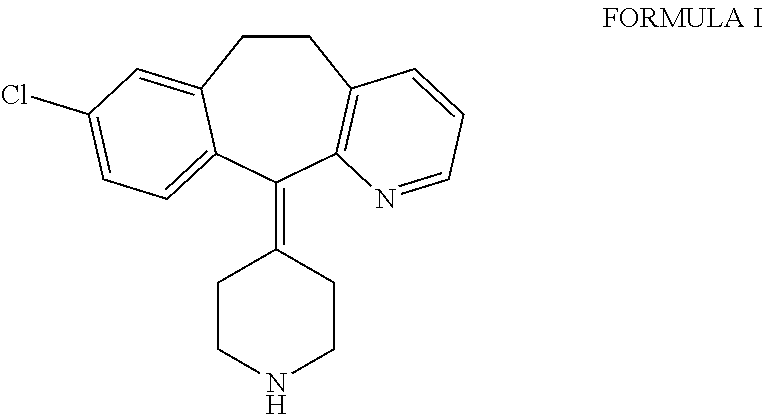
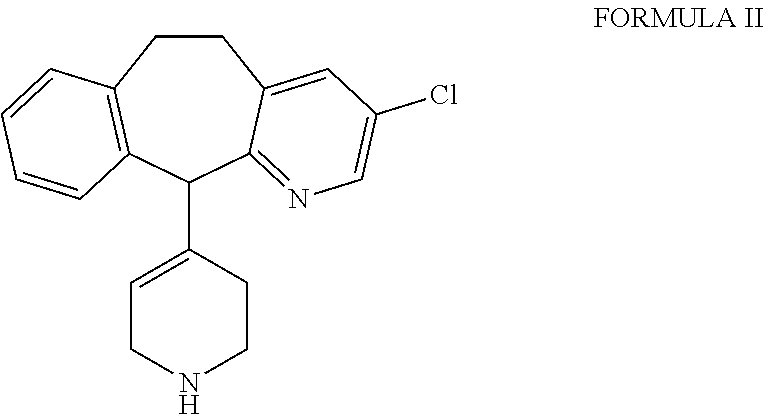
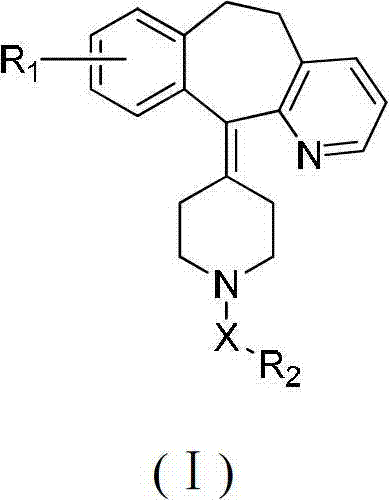
Desloratadine derivatives, and preparation method and application thereof
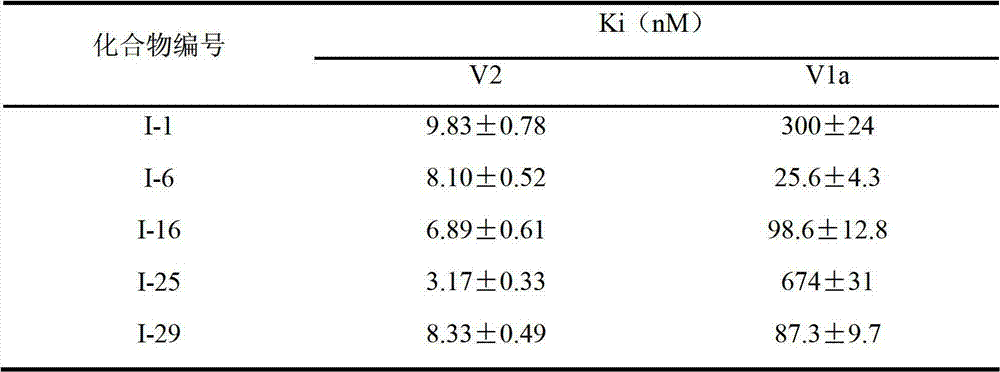
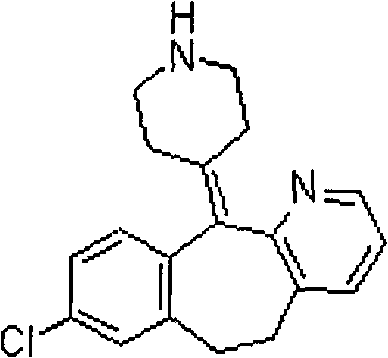
The invention relates to desloratadine derivatives, and a preparation method and application thereof, particularly desloratadine derivatives disclosed as Formula I and pharmaceutically acceptable salts thereof and a preparation method thereof, and application of the desloratadine derivatives disclosed as Formula I and pharmaceutically acceptable salts thereof as active effective ingredients of active pharmaceutical compositions in preventing and treating diseases related to arginine vasopressin V1a receptor, arginine vasopressin V1b receptor, arginine vasopressin V2 receptor, sympathetic nervous system or renin-angiotensin-aldosterone system.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Soft capsule containing desloratadine and preparation method thereof
ActiveCN102813640AEffectiveGood effectOrganic active ingredientsCapsule deliveryMedicineSuspending Agents
The invention belongs to the technical field of medicines and particularly relates to a soft capsule containing desloratadine and a preparation method thereof. Content of the soft capsule is prepared by esloratadine, antioxygen, dispersion media and suspending agents according to certain proportion, safety and stability of the soft capsule are improved by means of the soft capsule mode, so that the soft capsule is fast in dissolving, high in bioavailability, accurate in dose and fast in effect, the preparation method is simple, and the soft capsule is easy to produce and wide in application prospect.
Owner:AVENTIS PHARMA HAINAN
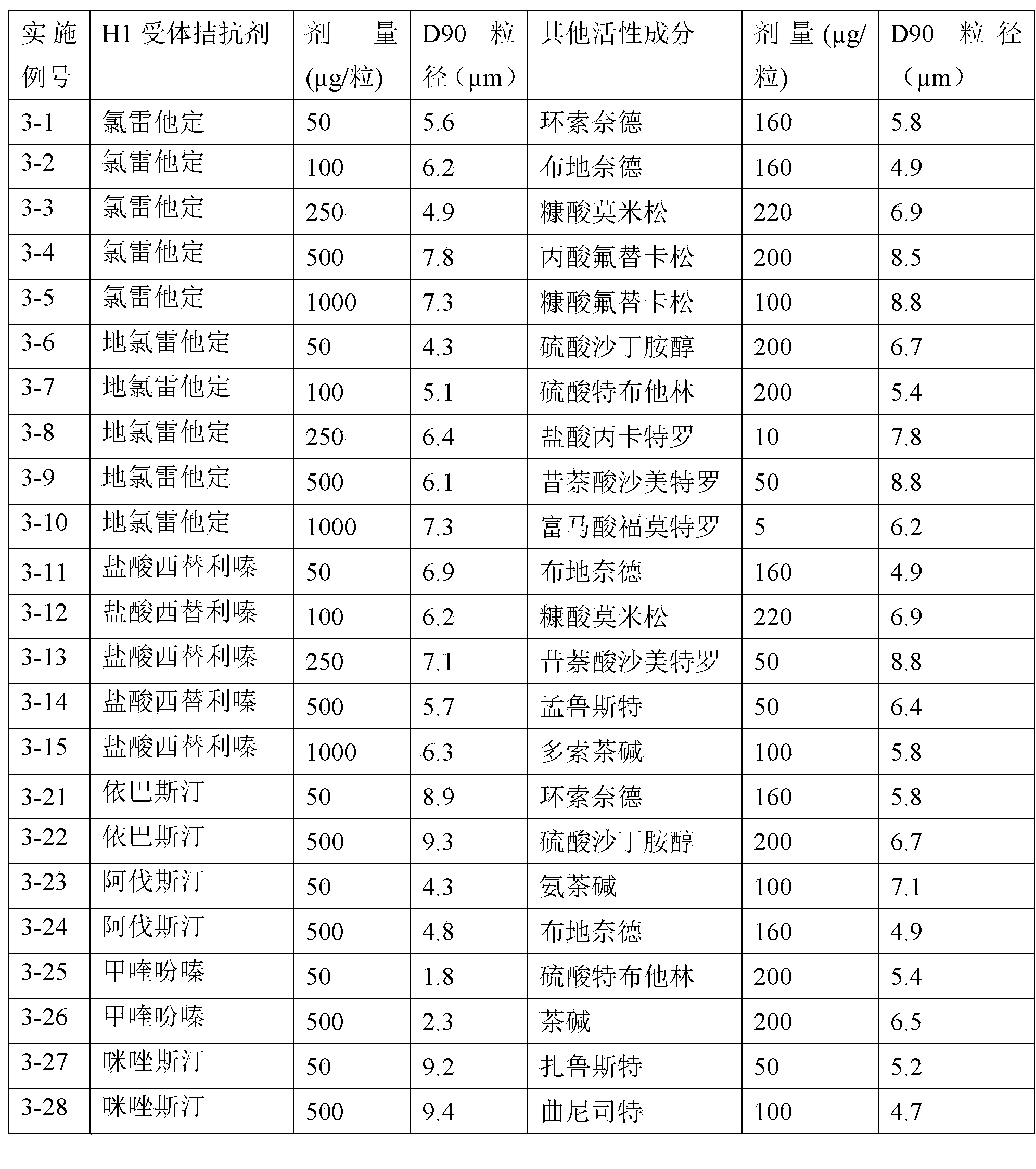
H1-receptor-antagonist-containing inhalation preparation
The invention relates to an H1-receptor-antagonist-containing inhalation preparation which contains an H1 receptor antagonist and one or more pharmaceutical auxiliary materials suitable for inhalation administration. The H1 receptor antagonist is one or more of loratadine, desloratadine, cetirizine, levocetirizine, astemizole, ketotifen, ebastine, fexofenadine, avastin, mequitazine, mizolastine and salts thereof, and preferably one or more of loratadine, desloratadine, cetirizine, levocetirizine, ebastine, mizolastine, avastin, mequitazine, ketotifen and hydrochlorides or fumarates thereof.
Owner:TIANJIN JINYAO GRP
Desloratadine citrate disodium freeze-dried oral instant tablets and preparing method thereof
ActiveCN105769795AEasy to carryQuality improvementOrganic active ingredientsPill deliveryCITRATE ESTERMedicine
The invention discloses desloratadine citrate disodium freeze-dried oral instant tablets and a preparing method thereof, and belongs to the field of preparation of desloratadine citrate disodium freeze-dried oral instant tablets. The desloratadine citrate disodium freeze-dried oral instant tablets are prepared from, by weight, 5-20 parts of desloratadine citrate disodium, 30-80 parts of framework materials, 10-30 parts of cyclodextrin, 1-10 parts of a suspending agent, 0.1-5 parts of a flavoring agent, 0.1-2 parts of a pH regulating agent and 0.001-0.01 part of a coloring agent. The desloratadine citrate disodium freeze-dried oral instant tablets are good in appearance, small in friability, good in taste, short in melting time, capable of taking effect fast, convenient to carry and good in taking compliance.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
Desloratadine oral liquid preparation and preparation method thereof
ActiveCN112220748AImprove bioavailabilityImprove stabilityOrganic active ingredientsDispersion deliveryPatient compliancePharmaceutical medicine
The invention belongs to the field of pharmacy, particularly relates to the field of oral liquid preparations, and more particularly relates to a desloratadine oral liquid preparation and a preparation method thereof. The desloratadine oral liquid preparation is an aqueous solution and is composed of a desloratadine active component, a stabilizer, pharmaceutically acceptable auxiliary materials and solvent water. According to the desloratadine oral solution and the preparation method thereof disclosed by the invention, the preparation process is simple, and the patient compliance is good. It can be known through influence factor tests and acceleration tests that products with stable quality can be prepared through the formula technology.
Owner:JIANGSU ALPHA PHARM CO LTD
Desloratadine citrate disodium oral rapidly disintegrating film and preparation method thereof
InactiveCN105616389AHigh mechanical strengthImprove wear resistanceOrganic active ingredientsPharmaceutical non-active ingredientsMedicineFiller Excipient
The invention discloses a desloratadine citrate disodium oral rapidly disintegrating film and a preparation method thereof, belonging to the preparation field of desloratadine citrate disodium oral rapidly disintegrating films. The invention firstly discloses a desloratadine citrate disodium oral rapidly disintegrating film which comprises the following components in parts by weight: 2-10 parts of desloratadine citrate disodium, 30-60 parts of film forming material, 10-30 parts of cyclodextrin, 2-8 parts of a plasticizer, 0.2-1.0 part of a flavoring agent, 0.5-1.0 part of a pH regulator and 0.001-0.01 part of a coloring agent. Preferably, the desloratadine citrate disodium oral rapidly disintegrating film disclosed by the technical scheme of the invention further comprises a filling agent. The desloratadine citrate disodium oral rapidly disintegrating film is good in appearance, moderate in flexibility, good in taste, short in disintegrating time, high in effect taking speed, convenient to carry, good in taking compliance, simple in preparation technology and low in cost.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
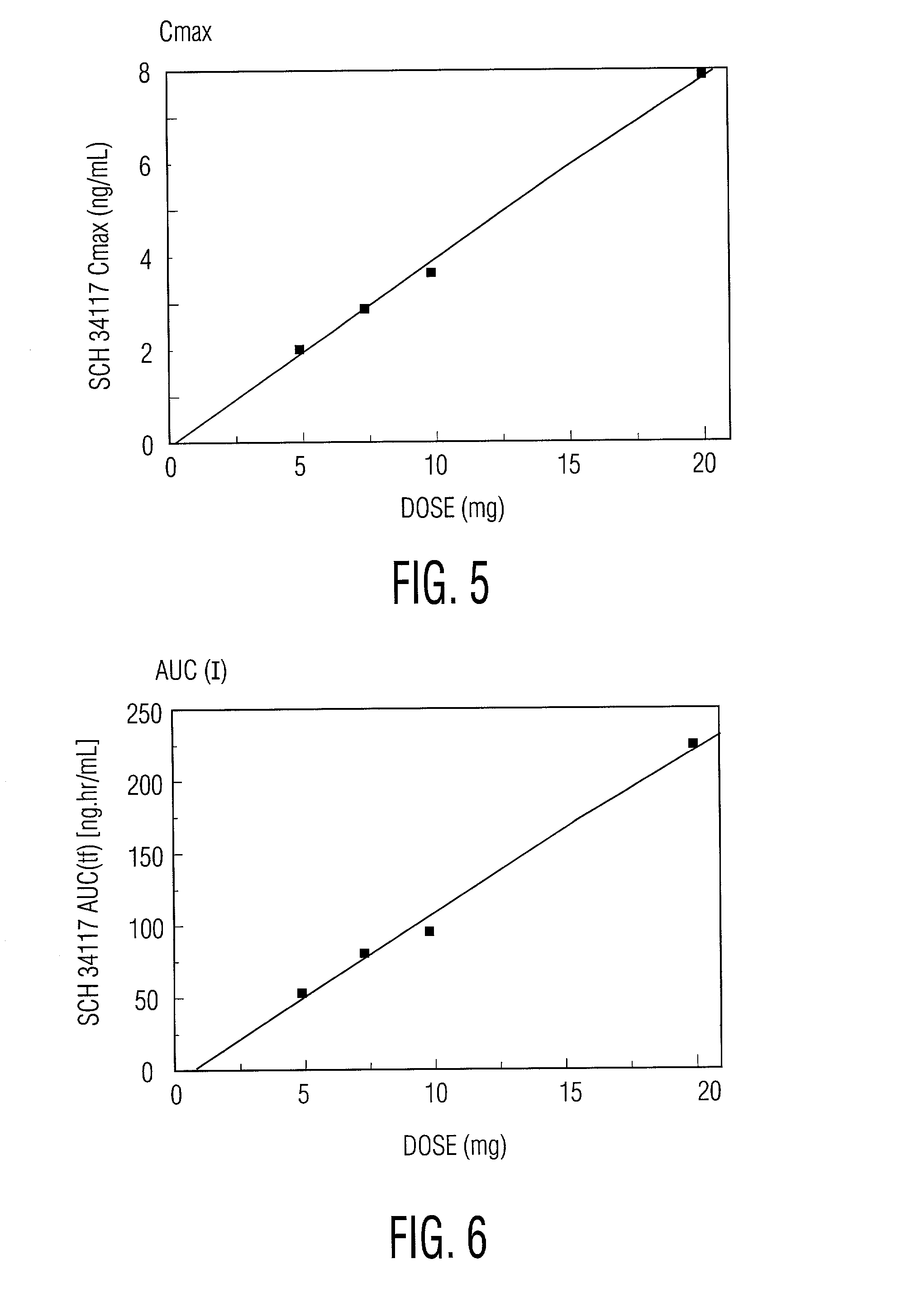
Treating allergic and inflammatory conditions
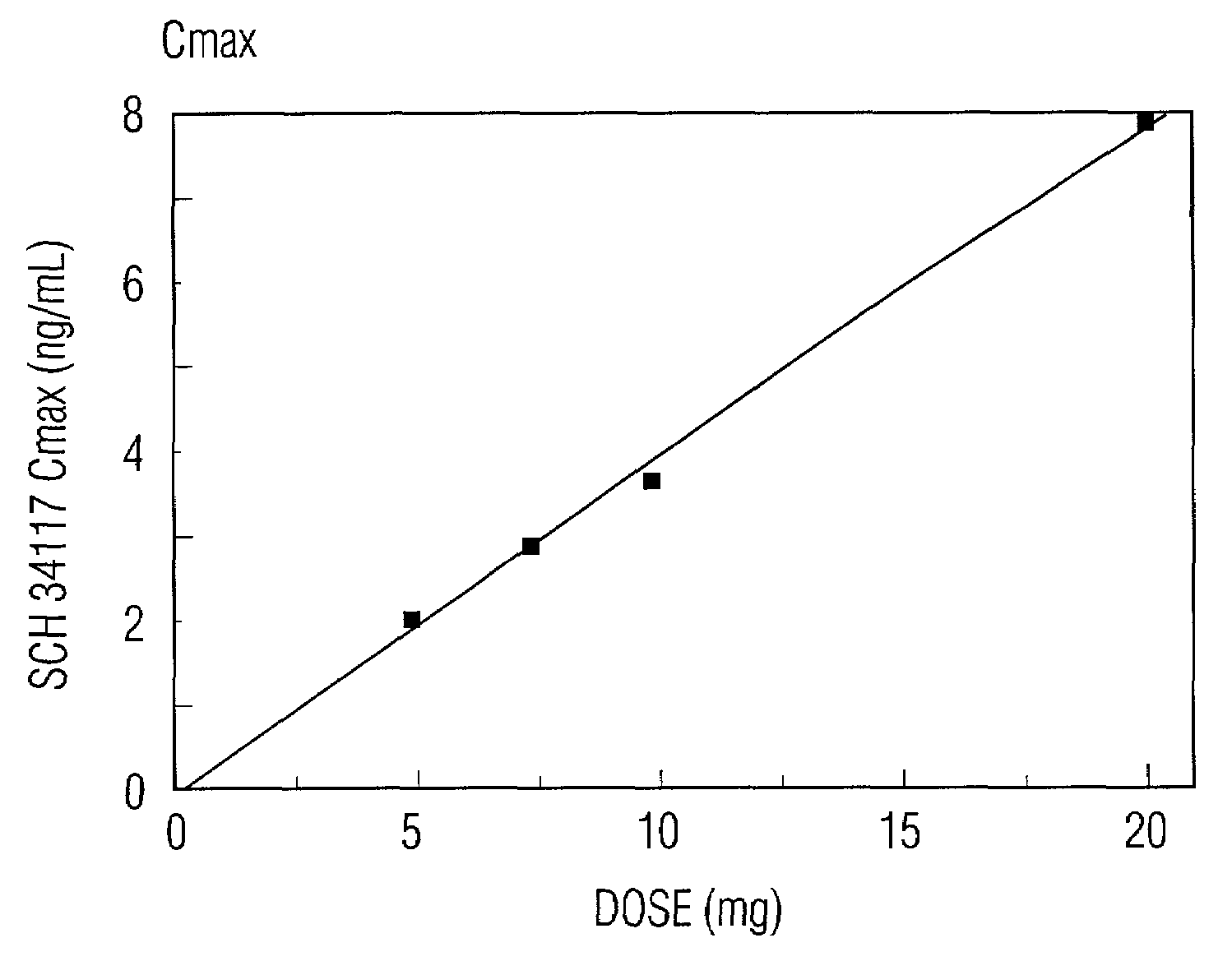
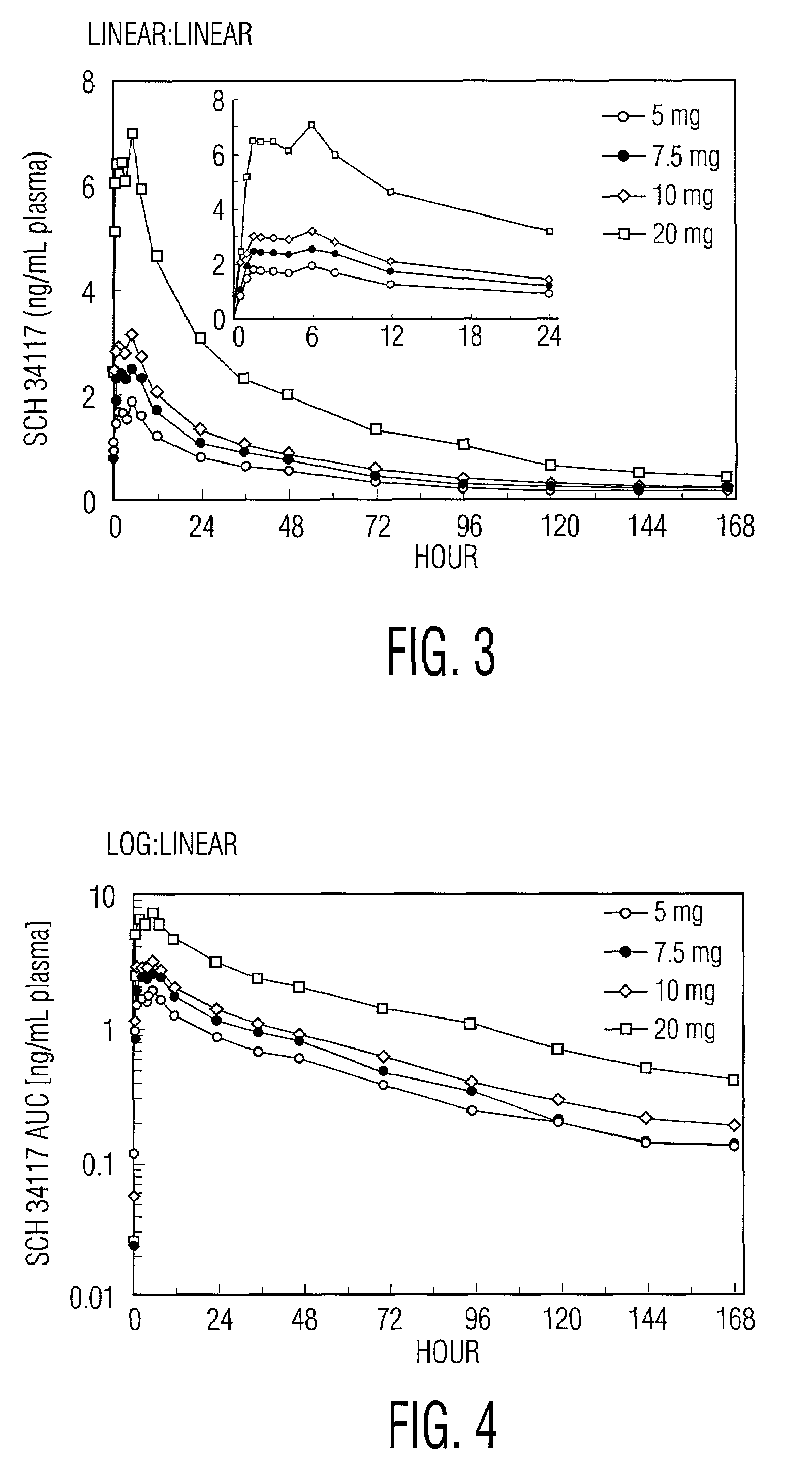
A method of treating and / or preventing allergic and inflammatory conditions of the skin or upper and lower airway passages, e.g. seasonal allergic rhinitis, pernninal allergic rhinitis, or chronic idopathic urticaria, in a human more 12 years old, by administering an amount of desloratadine, e.g. 2x2.5 mg or 5 mg / day for a time sufficient to produce a geometric mean steady state maximum plasma concentration of desloratadine in the range of about 2.90 ng / mL to about 4.54 ng / mL, or a arithmetic mean steady state maximum plasma concentration of desloratadine in the range of about 3.2 ng / mL to about 5.0 ng / mL is disclosed.
Owner:MERCK SHARP & DOHME CORP
Desloratadine capsule and preparation method thereof
ActiveCN103948562AImprove instabilityAvoid contactOrganic active ingredientsSenses disorderHydrogen phosphateMoisture absorption
The invention discloses a desloratadine capsule and a preparation method thereof. The desloratadine capsule is prepared from desloratadine as an active ingredient and auxiliary materials. The desloratadine capsule comprises 6% of desloratadine, 30-50% of microcrystalline cellulose, 20-40% of calcium hydrogen phosphate, 10-30% of pregelatinized starch and 1-5% of talcum powder. According to the preparation method, a pregelatinized starch aqueous solution having alkalescence coats desloratadine by a spray drying method and the pregelatinized starch effectively wrap desloratadine so that moisture absorption and contact with oxygen in air are effectively avoided, instability of desloratadine in an acid environment is effectively overcome and good stability of a desloratadine preparation is realized. The preparation method has simple and effective processes and is suitable for industrial production.
Owner:GUANGDONG JIUMING PHARMA +1
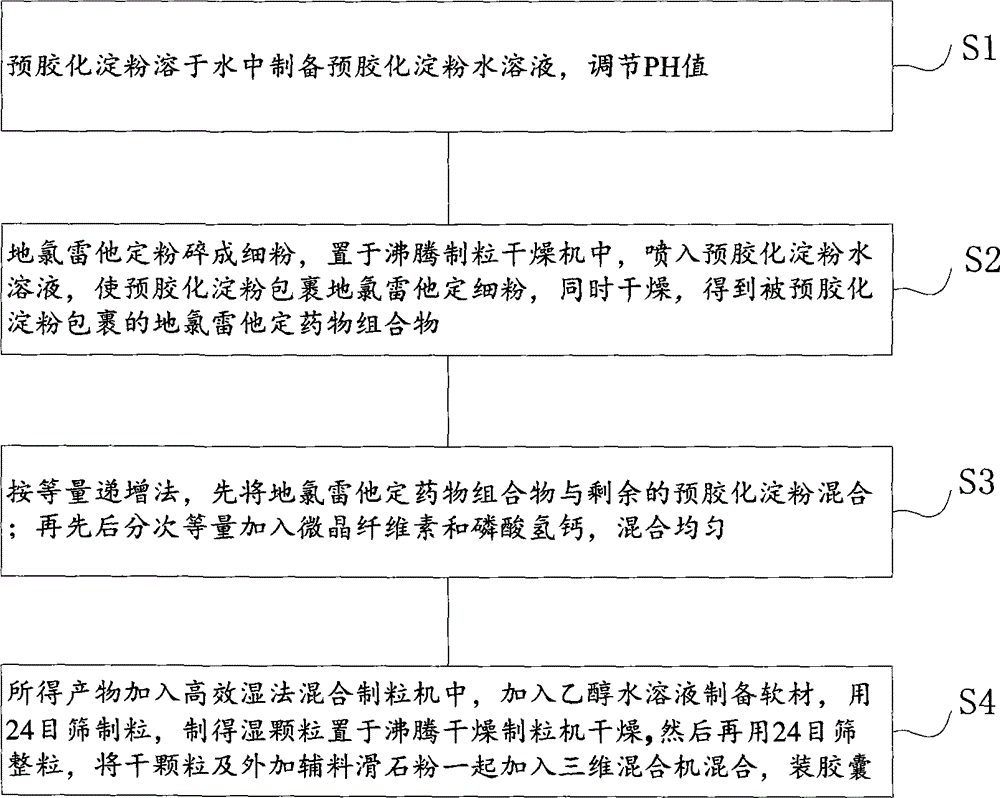
Treating allergic and inflammatory conditions
A method of treating and / or preventing allergic and inflammatory conditions of the skin or upper and lower airway passages, e.g. seasonal allergic rhinitis, perennial allergic rhinitis, or chronic idopathic urticaria, in a human more 12 years old, by administering an amount of desloratadine, e.g. 2×2.5 mg or 5 mg / day for a time sufficient to produce a geometric mean steady state maximum plasma concentration of desloratadine in the range of about 2.90 ng / mL to about 4.54 ng / mL, or a arithmetic mean steady state maximum plasma concentration of desloratadine in the range of about 3.2 ng / mL to about 5.0 ng / mL is disclosed.
Owner:MERCK SHARP & DOHME CORP
Desloratadine oral dispersible film
InactiveCN102940617ADisintegrates quicklyQuick effectOrganic active ingredientsImmunological disordersAllergic dermatitisIrritation
The invention relates to a desloratadine oral dispersible film which comprises desloratadine, biologically-acceptable water-soluble polymers, plasticizers, purified water and the like, can be dispersed or dissolved quickly in the oral cavity, and is used for treating allergic rhinitis, allergic rhinitis and asthma syndrome, allergic nasal conjunctivitis, allergic dermatitis, allergic asthma and the like. The desloratadine oral dispersible film is free of irritation, convenient to take and carry, fast in effect, good in taste and particularly suitable for being used by the olds and children.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com