Preparation method of desloratadine
A technology of desloratadine and loratadine, which is applied in the field of drug synthesis, can solve the problems of low product purity and unsuitability for clinical application, etc., and achieves the effects of improving the purity, reducing the occurrence of side reactions, and increasing the content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
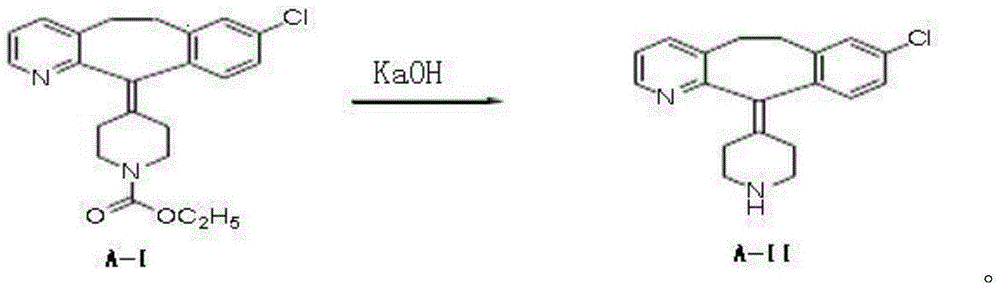
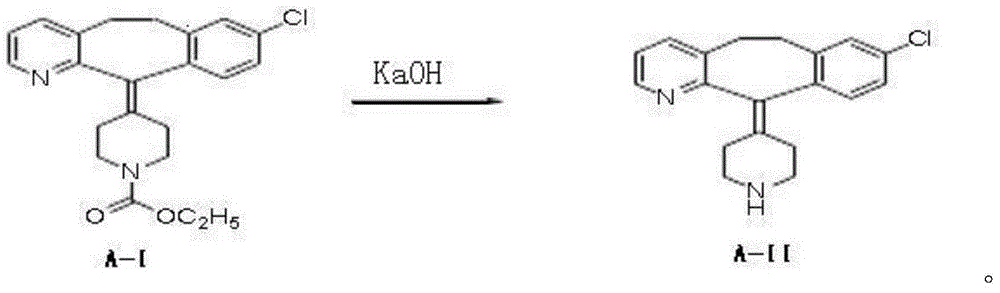
[0028] The preparation routes of the following examples of the present invention are as follows:
[0029]
Embodiment 1
[0031] The preparation method of desloratadine described in the present embodiment comprises the following steps:
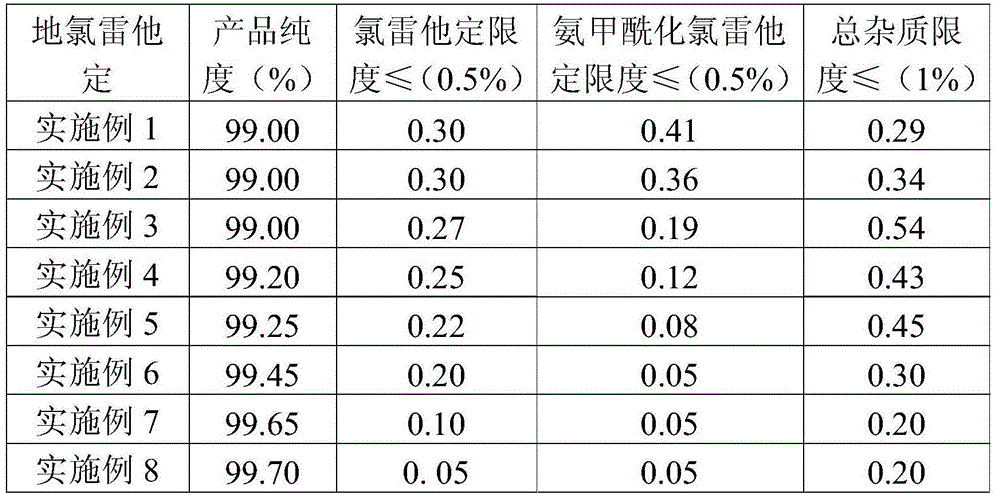
[0032](1) get loratadine 15kg (39.17mol) shown in formula A-I and be dissolved in the ethanol solvent that volume concentration is 75% in the ethanol solvent of 138L under nitrogen protection, then add potassium hydroxide 30kg (535.7mol) and stir evenly, and Rapidly raise the temperature to 70-75°C to start the reflux reaction, and then control the reflux reaction to show a gradient change within 70-100°C until the loratadine reaction is complete. The gradient temperature change program of the reflux reaction is specifically: Rapidly raise the reaction temperature from 75°C to 80°C, and keep the reaction for 3h; then quickly raise the temperature to 100°C, and keep the reaction for 2h; then quickly cool down to 85°C, and keep the reaction for 3h; then quickly cool down to 70°C, until the reaction completely;
[0033] Detect whether the loratadine reaction is com...
Embodiment 2
[0039] The preparation method of desloratadine described in the present embodiment comprises the following steps:
[0040] (1) 15kg (39.17mol) of loratadine shown in formula A-I is dissolved in the methanol solvent of 55% volume concentration in 163L of methanol solvent under the protection of nitrogen, then add potassium hydroxide 60kg (1071.4mol) and stir evenly, and Rapidly raise the temperature to 70-75°C to start the reflux reaction, and then control the reflux reaction to show a gradient change within 70-100°C until the loratadine reaction is complete. The gradient temperature change program of the reflux reaction is specifically: Rapidly raise the reaction temperature from 75°C to 100°C, and keep the reaction for 2h; then quickly cool down to 85°C, and keep the reaction for 2h; then quickly raise the temperature to 100°C, and keep the reaction for 3h; Complete response;
[0041] Detect whether the loratadine reaction is complete with thin-layer chromatography, specific...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com