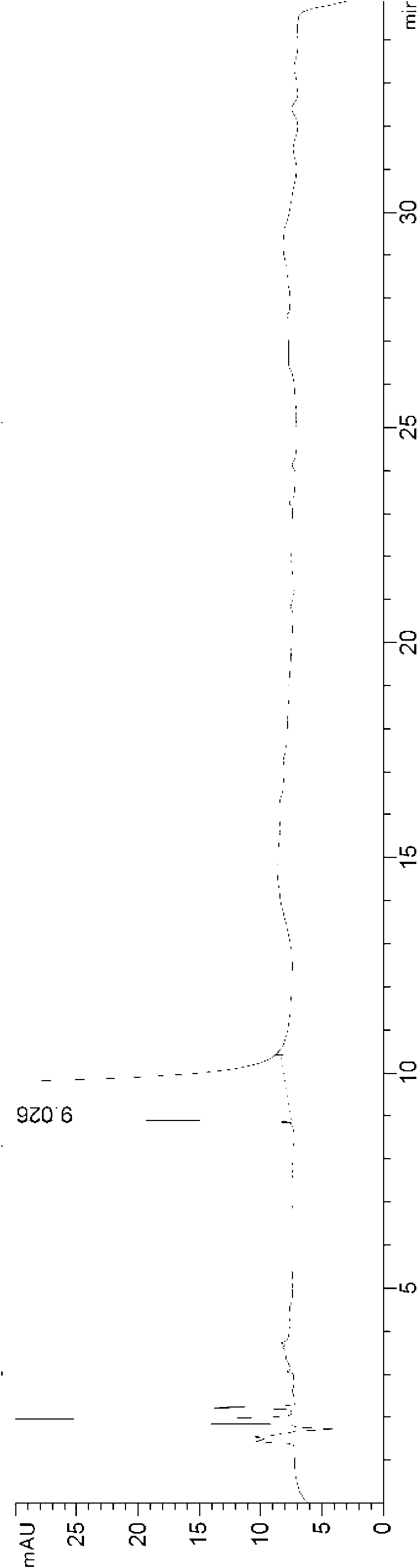Desloratadine oral liquid preparation and preparation method thereof
A technology for desloratadine and liquid preparations, which is applied in the field of pharmacy, and can solve the problems of degraded impurities, poor stability of desloratadine, and unsatisfactory effects, and achieve the effect of improving stability and not producing degraded impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation method of the oral liquid preparation of desloratadine in this example is as follows (wherein the molar ratio of desloratadine to cyclodextrin is 1:4):
[0048] (1) Dissolve 960mg of HP-β-CD (hydroxypropyl-β-cyclodextrin), 25mg of EDTA-2NaCa, 100mg of sodium benzoate (preservative), and 150mg of citric acid (organic acid) in 60ml of water , To obtain a cyclodextrin solution;
[0049] (2) Dissolve 50 mg of desloratadine in 10 ml of propylene glycol to obtain a desloratadine solution;
[0050] (3) Dissolve the desloratadine solution obtained in step (2) in the cyclodextrin solution obtained in step (1), adjust the pH to 5.5, add water to the volume to 100ml, mix well, and sterilize with moist heat at 115°C for 30 minutes The oral liquid preparation of desloratadine described in this example was obtained.
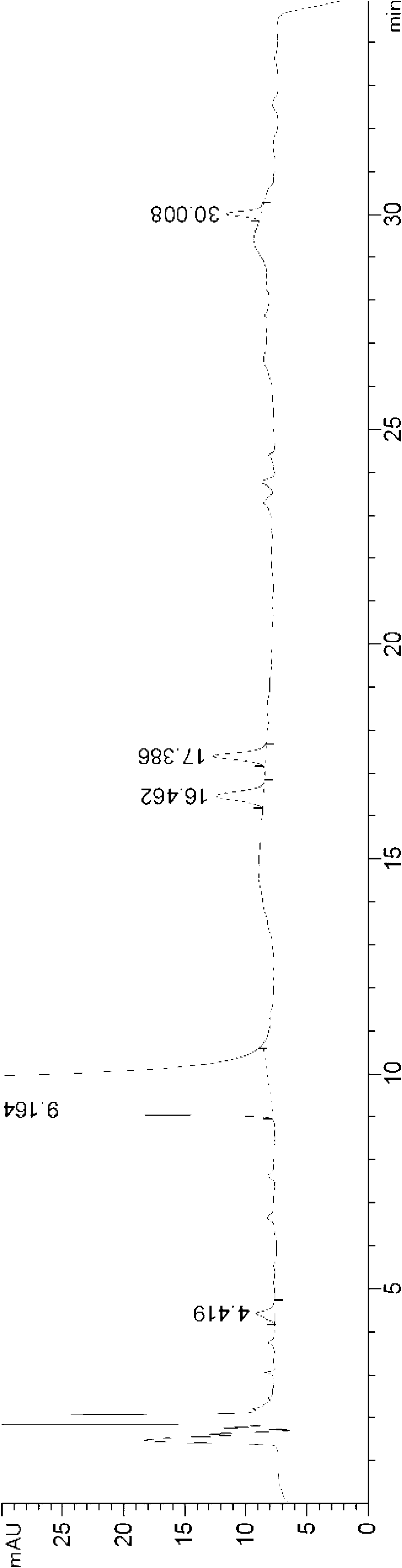
[0051] The HPLC chart of desloratadine oral liquid preparation obtained in this example is as follows image 3 As shown, it can be seen from the figure that th...
Embodiment 2
[0053] The preparation method of the oral liquid preparation of desloratadine in this example is as follows (wherein the molar ratio of desloratadine to cyclodextrin is 1:2):
[0054] (1) Dissolve 680 mg of SBE-β-CD (sulfobutyl-β-cyclodextrin) in 60 ml of water to obtain a cyclodextrin solution;
[0055] (2) Dissolve 50 mg of desloratadine in 10 ml of propylene glycol to obtain a desloratadine solution;
[0056] (3) Dissolve the desloratadine solution obtained in step (2) in the cyclodextrin solution obtained in step (1), add water to make the volume to 100ml, mix well, and sterilize with moist heat at 115°C for 30 minutes to obtain this example The oral liquid preparation of desloratadine.
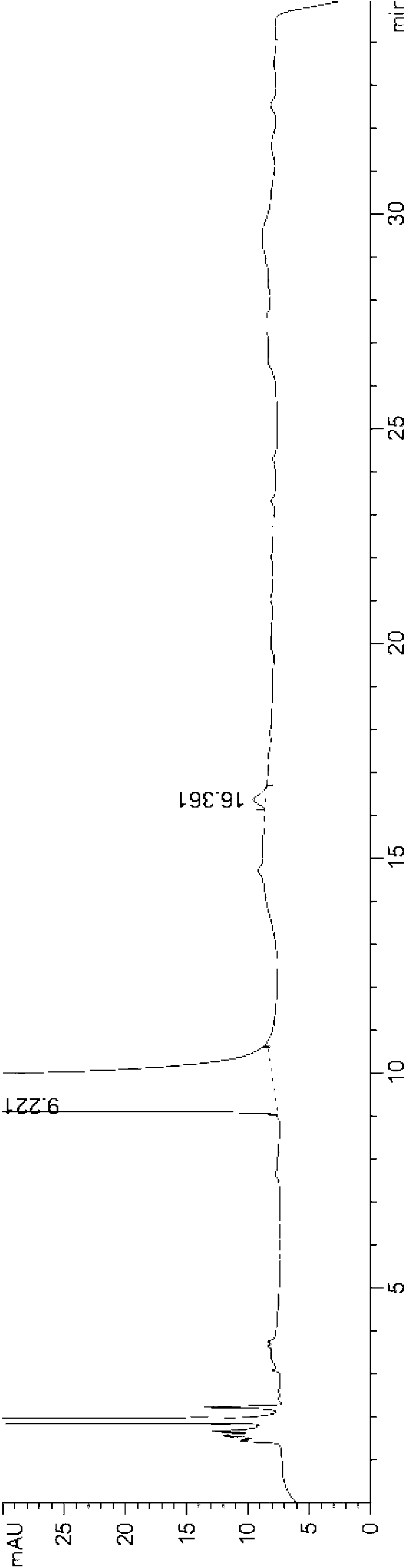
[0057] The HPLC chart of desloratadine oral liquid preparation obtained in this example is as follows Figure 4 As shown, it can be seen from the figure that there is only a desloratadine peak at 8.740 minutes, no impurity peaks, and no degraded impurities exist.
Embodiment 3
[0059] The preparation method of the oral liquid preparation of desloratadine in this example is as follows (the molar ratio of desloratadine to cyclodextrin is 1:1):
[0060] (1) Dissolve 240mg of HP-β-CD (hydroxypropyl-β-cyclodextrin), 100mg of sodium benzoate (preservative), and 150mg of citric acid (organic acid) in 60ml of water to obtain a cyclodextrin solution ;
[0061] (2) Add 50 mg of desloratadine to the cyclodextrin solution obtained in step (1), dissolve ultrasonically for 15 minutes to obtain a transparent solution, adjust the pH to 6.0, add water to the volume to 100ml, mix and filter to obtain The oral liquid formulation of desloratadine described in this embodiment.
[0062] The HPLC chart of desloratadine oral liquid preparation obtained in this example is as follows Figure 5 As shown, it can be seen from the figure that there is only a desloratadine peak at 8.707 minutes, no impurity peaks, and no degraded impurities exist.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com