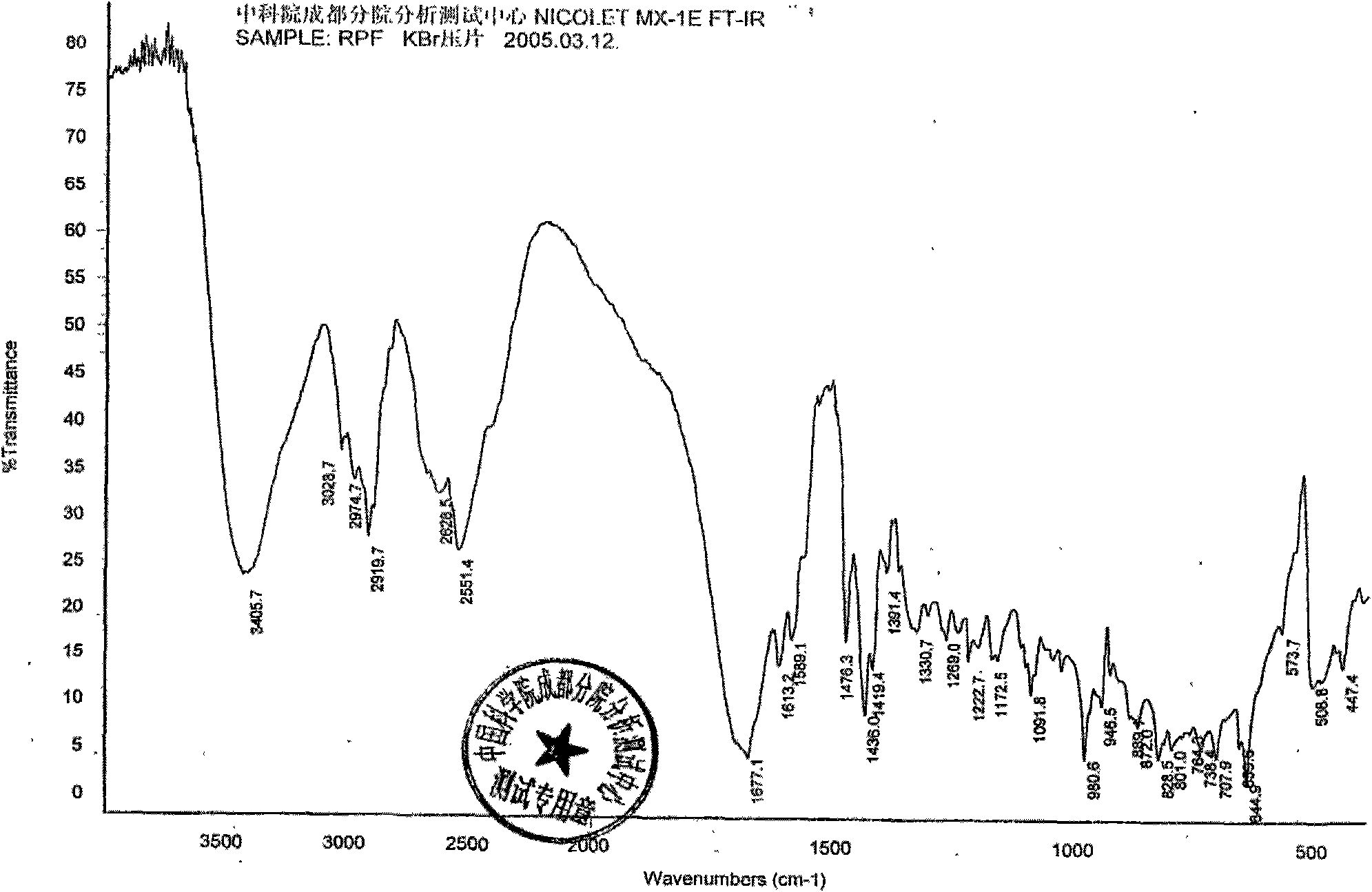
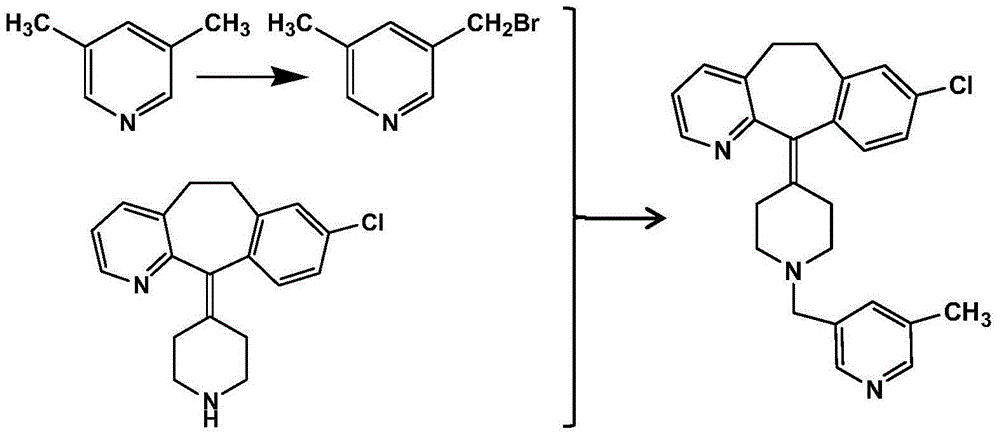
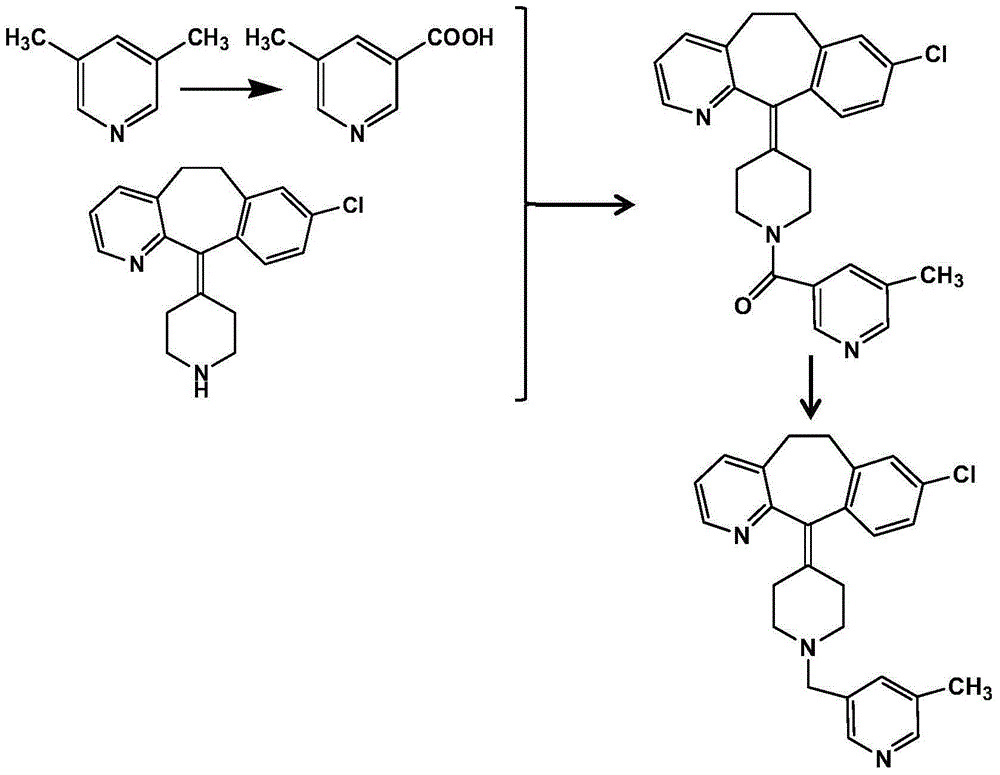
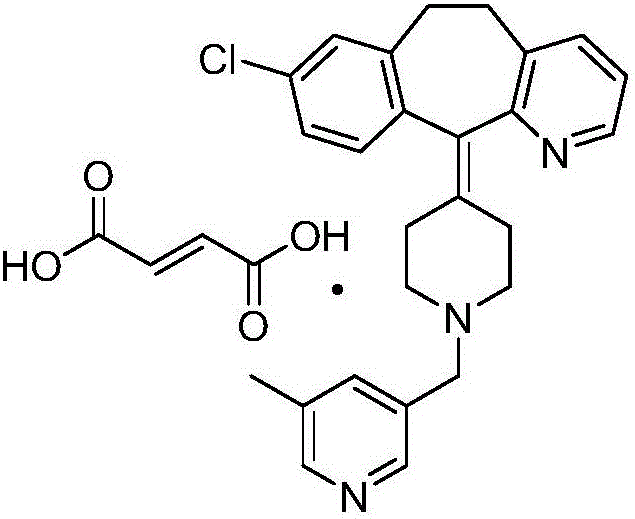
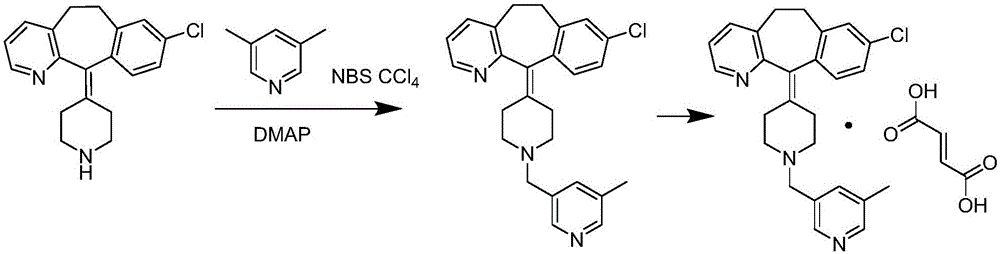
Patents
Literature
72 results about "Rupatadine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
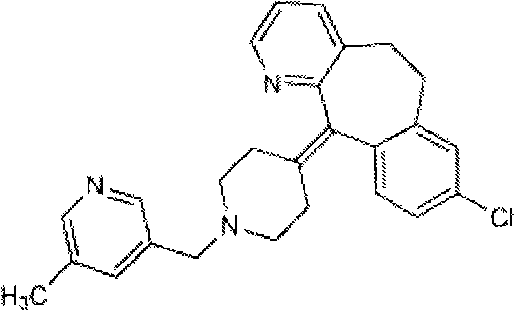
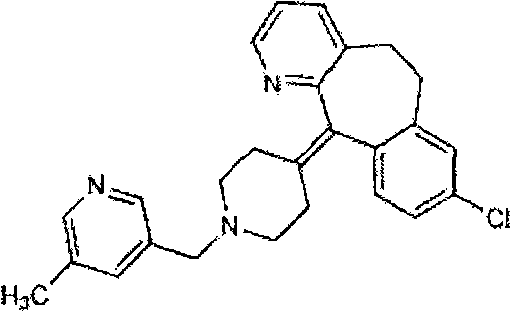
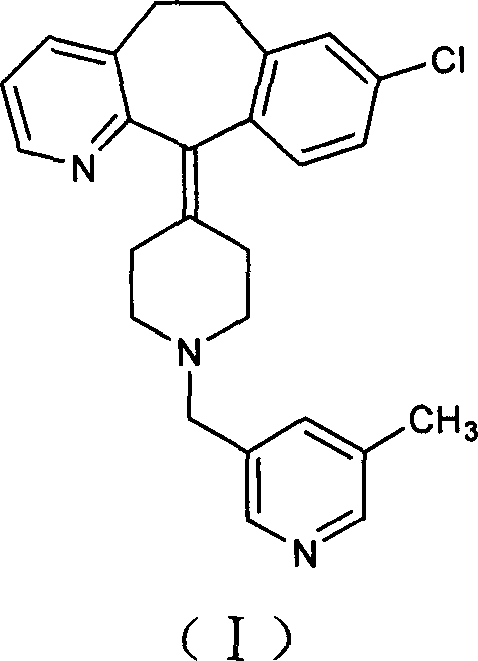
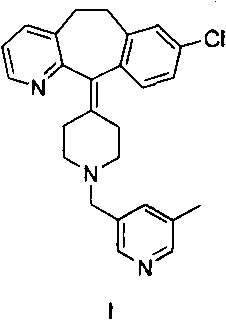
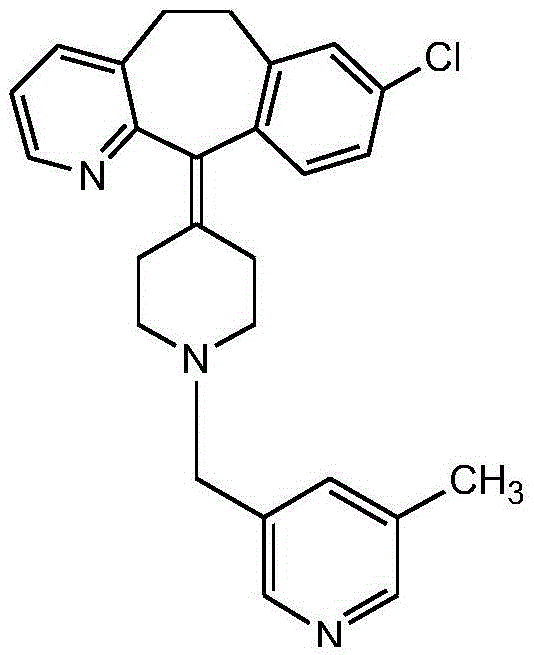
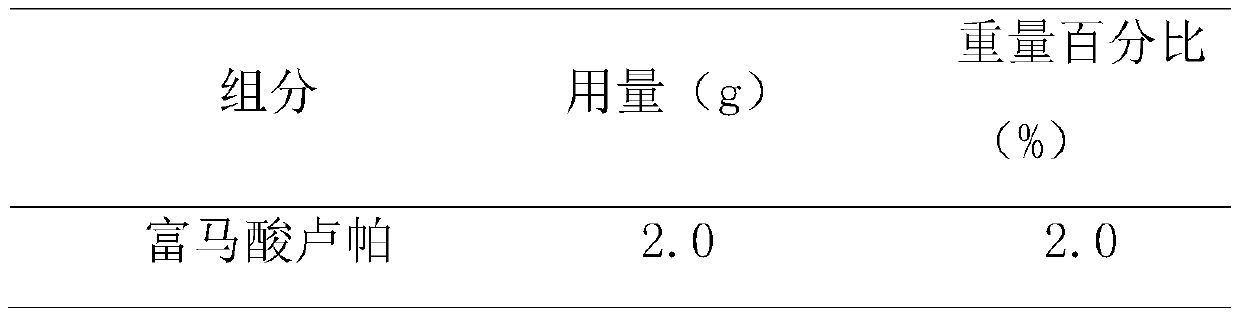
Rupatadine is a second generation antihistamine and PAF antagonist used to treat allergies. It was discovered and developed by J. Uriach y Cia and is marketed as Rupafin and under several other trade names.
Liquid preparation for dosing nasal cavities and method for making the same
InactiveCN101669926AEasy to useFast drug absorptionOrganic active ingredientsPharmaceutical delivery mechanismNasal cavityPatient compliance
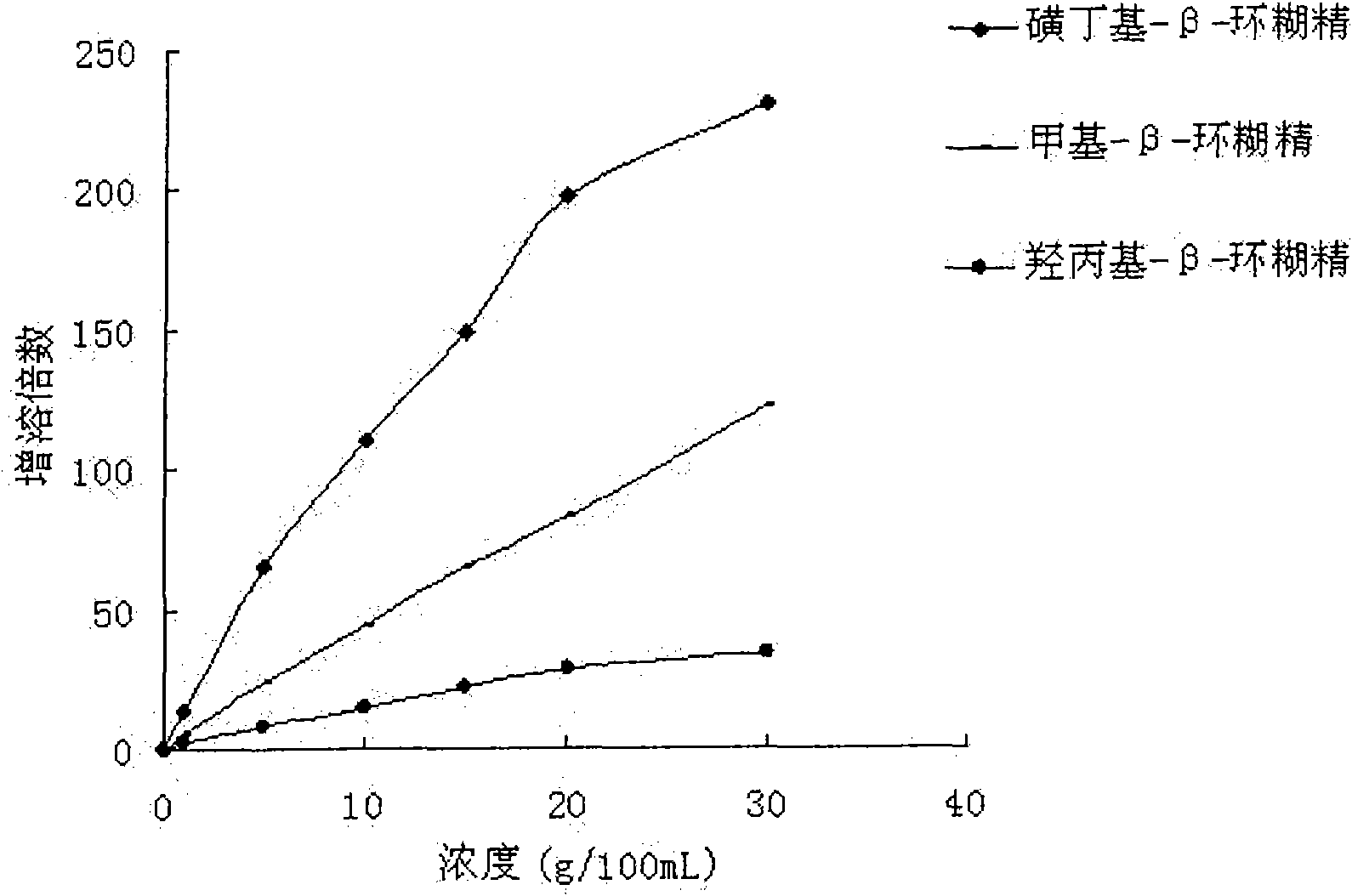
The invention relates to a liquid preparation for dosing nasal cavities, which comprises 0.1 to 25 g / 100 mL of rupatadine and 0.5 to 30 g / 100 mL of cyclodextrin compound. The invention also relates toa method for making the liquid preparation. The liquid preparation of the invention has the characteristics of convenient use, high patient compliance and rapid absorption speed after dosing.
Owner:广州达信生物技术有限公司
Liquid preparation for dosing eyes and method for making the same
The invention relates to a liquid preparation for dosing eyes, which comprises 0.1 to 25 g / 100 mL of rupatadine and 0.5 to 30 g / 100 mL of cyclodextrin compound. The invention also relates to a methodfor making the liquid preparation. The liquid preparation of the invention has the characteristics of rapid absorption, convenient use, obvious curative effect and stability, and has excellent curative effect for treating conjunctivitis, in particular for treating allergic conjunctivitis.
Owner:广州达信生物技术有限公司
Process for preparing piperidine derivative
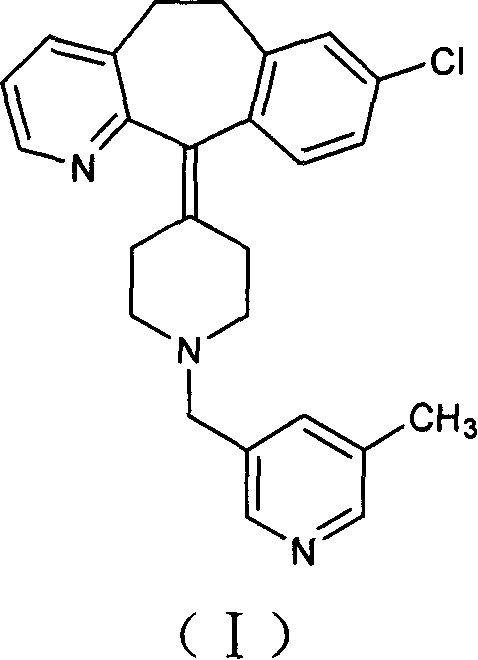
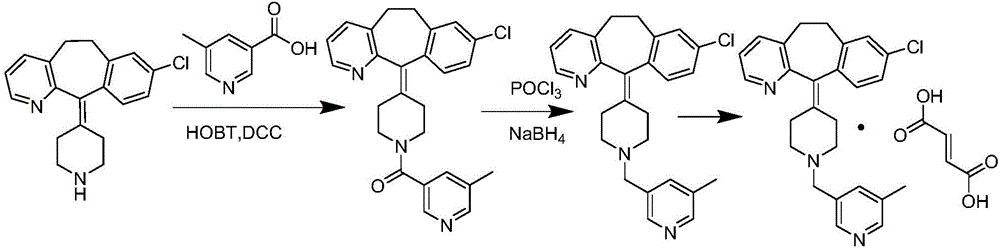
The invention provides a process for preparing Rupatadine or its salts, wherein the Rupatadine has a chemical formula (I).
Owner:BEIJING SIHUAN KEBAO PHARM CO LTD
Liquid formulations of rupatadine fumarate
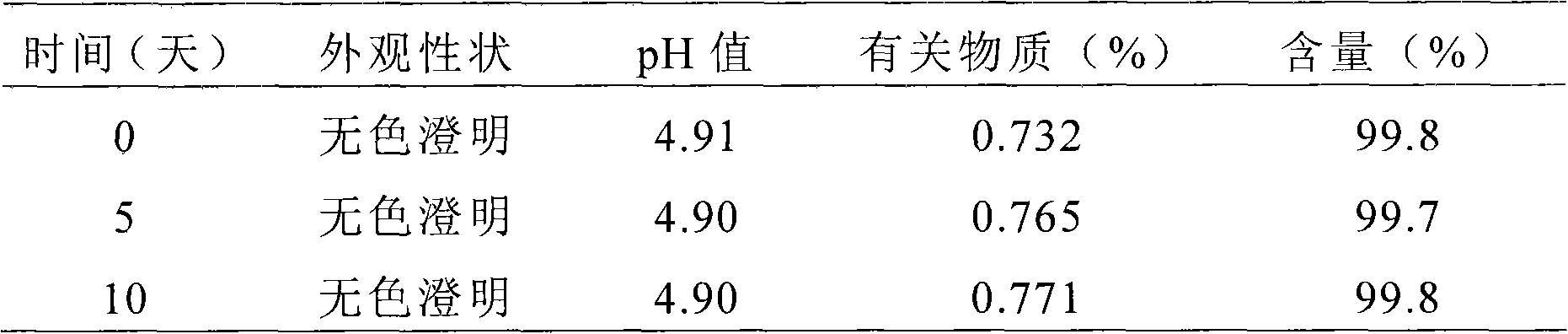
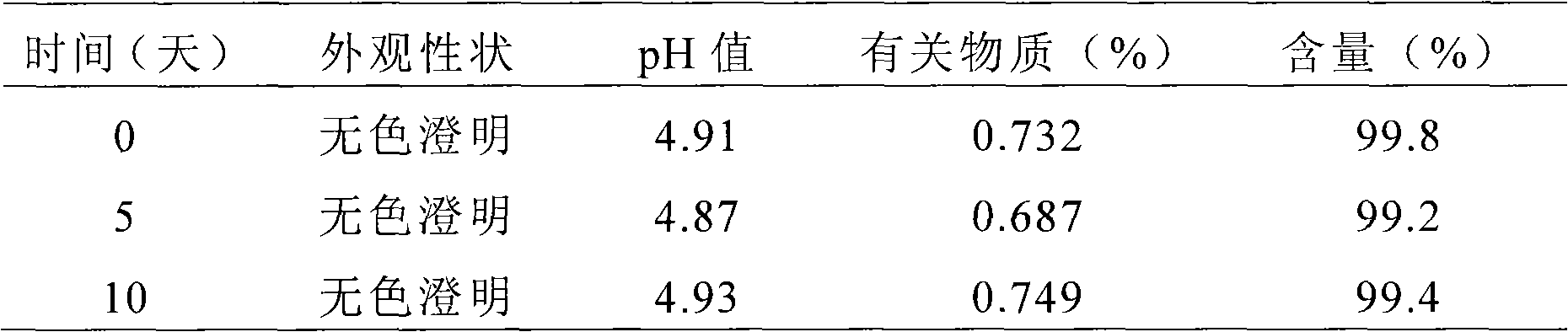
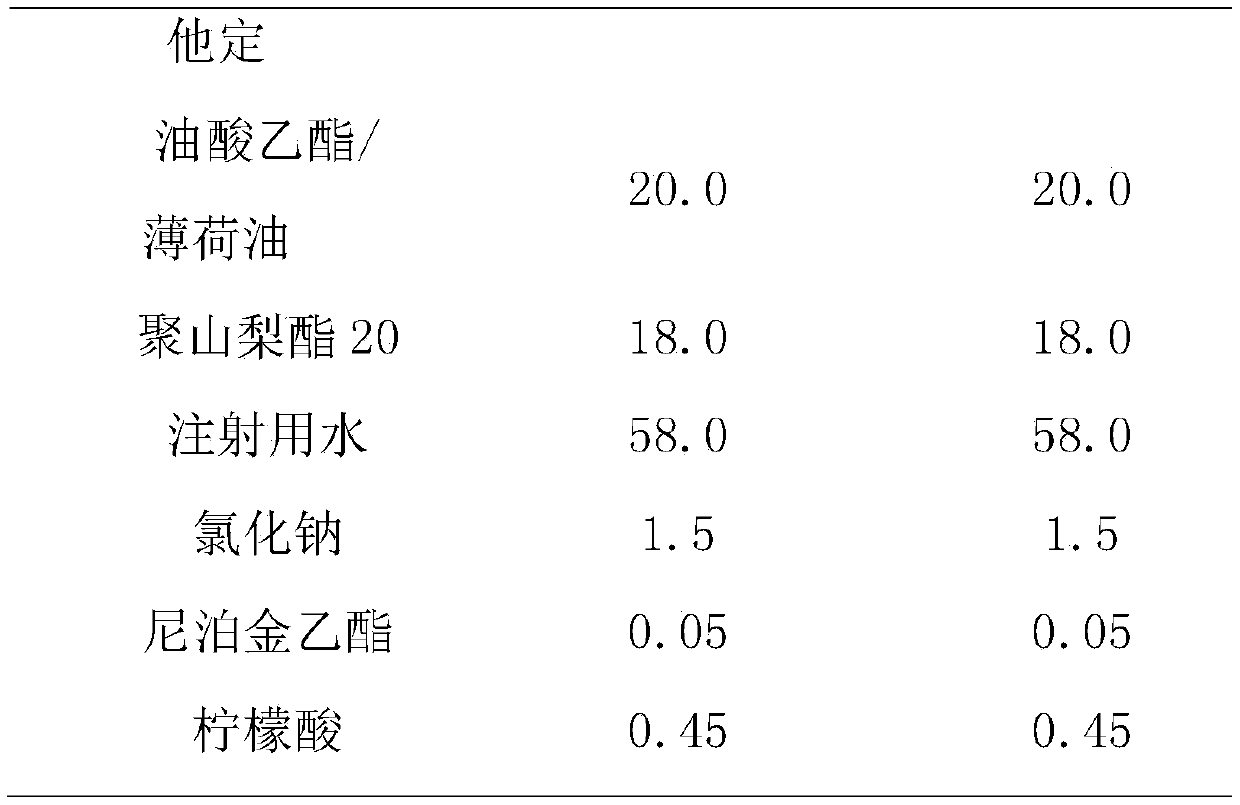
The present invention provides cyclodextrin-free aqueous liquid formulations of rupatadine fumarate, useful for the treatment of allergic rhinitis and urticaria. Said formulations comprise rupatadine fumarate, one or more cosolvents and one or more pH regulating agents wherein the composition has a pH between 4 and 6.5.
Owner:J·乌里亚奇·Y股份有限公司
Preparation method of rupatadine fumarate
The invention relates to the technical field of pharmaceutical synthesis, and in particular relates to a preparation method of rupatadine fumarate. The preparation method comprises the following steps of: adding 235-237g of 3-chloromethyl-5-pyridine hydrochloride and 465-475ml of tertiary butanol in a reaction container; stirring and heating to 55-65 DEG C; dropping 235-245ml of concentration sulfuric acid, controlling the temperature of the reaction liquid to be 55-65 DEG C and reacting for 8-10 hours; cooling to room temperature, diluting with 234-245ml of water, then adding 345-355ml of methylbenzene, and adjusting pH value of the liquor by stronger ammonia water to 7.8-8.2 to separate out an organic phase; and after water layer extraction, combining the organic phases, washing the organic phase, drying and evaporating to remove the solvent to obtain an oily liquid product. The preparation method of rupatadine fumarate is simple and quick in preparation process, so that the preparation method is suitable for industrialized production. The yield is higher, and the reaction time is effectively shortened. Meanwhile, the refining process is simpler and the product purity is higher.
Owner:ANHUI YOUCARE KAIYUE PHARMA
Rupatadine fumarate eye drops and preparation method thereof
ActiveCN101926762AEasy to prepareSignificant effectOrganic active ingredientsSenses disorderDiseaseSide effect
The invention relates to rupatadine fumarate eye drops. The eye drops are prepared from rupatadine fumarate serving as a primary medicament, and a thickening agent, buffer salt, an isotonic agent, a bacteriostatic agent and a solubilizer serving as auxiliary materials. A preparation method A comprises the following steps of: dissolving the thickening agent by using a certain amount of water, and dissolving the primary medicament, the buffer salt, the isotonic agent, the bacteriostatic agent and the solubilizer by using a certain amount of water for injection; and mixing the two kinds of solution, and adding a proper amount of water for injection to reach the needed concentration. A preparation method B comprises the following steps of: stirring the thickening agent, the rupatadine fumarate, the buffer salt, the bacteriostatic agent, the isotonic agent, the solubilizer and the water for injection for a long time until the solution is clarified; and supplying the water for injection to fix the volume. The rupatadine fumarate eye drops have the advantages of simple preparation method and use for treating ocular surface allergic diseases, and have the characteristics of obvious curative effect, no irritation, no toxic or side effect, high stability and the like.
Owner:HAICHANG CONTACT LENSES +1
Oral disintegrated rupatadine tablet and its preparing method
InactiveCN1985816AMask bad tasteDisintegrates quicklyOrganic active ingredientsPill deliveryDiseaseOlder people
The present invention provides a kind of oral disintegrated rupatadine tablet and its preparation process. The oral disintegrated rupatadine tablet has recipe comprising rupatadine in 5-40 mg each as main medicine component as well as filler, disintegrant, corrective, antioxidant, lubricant, etc. The present invention is used in treating allergic rhinitis, hay fever and other diseases. The medicine of the present invention has simple preparation process, convenient taking, fast acting and obvious curative effect, and is especially suitable for old people, children and patient with dysphagia.
Owner:SHANTOU UNIV MEDICAL COLLEGE
Compound composition of intal and Statins
InactiveCN101766617ASolve the irritatingOrganic active ingredientsSenses disorderDiseaseAdditive ingredient
The invention relates to a compound composition containing intal and statins claritin. The compound composition consists of the following components: a) a certain amount of intal; b) a certain amount of one of olopatadine hydrochloride, ioratadine, desloratadine, degreasing ioratadine, desloratadine, rupatadine and betahistine; c) other medicinal excipients. The compound composition can be made into external preparations such as eye drops, nose drops, aerosol, spray, inhalant, gelata, eye ointments, ointments or patch and the like. The compound composition can be used for curing the diseases such as anaphylactic eye diseases, anaphylactic rhinitis, skin urtication, urticaria, allergic asthma and the like, can significantly improve allergic symptoms, and has the characteristics of high efficiency, stability, safety, low adverse reaction rate, convenient use and the like.
Owner:北京华禧联合科技发展有限公司
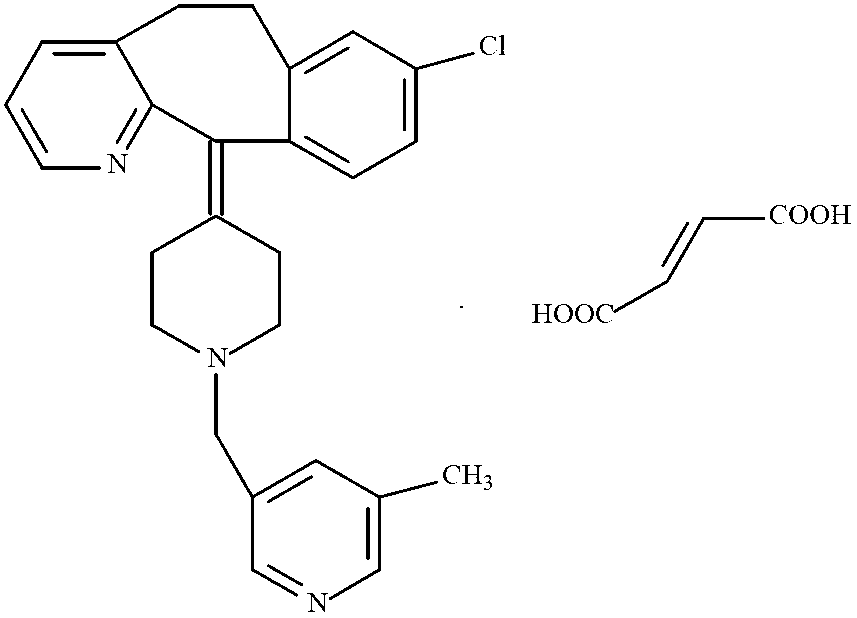
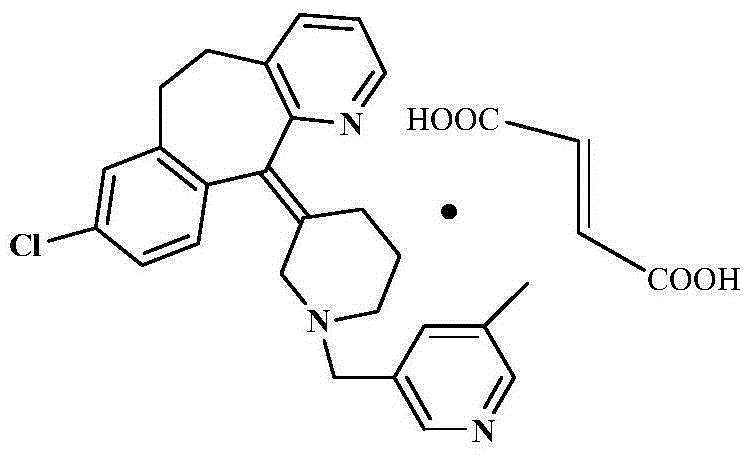
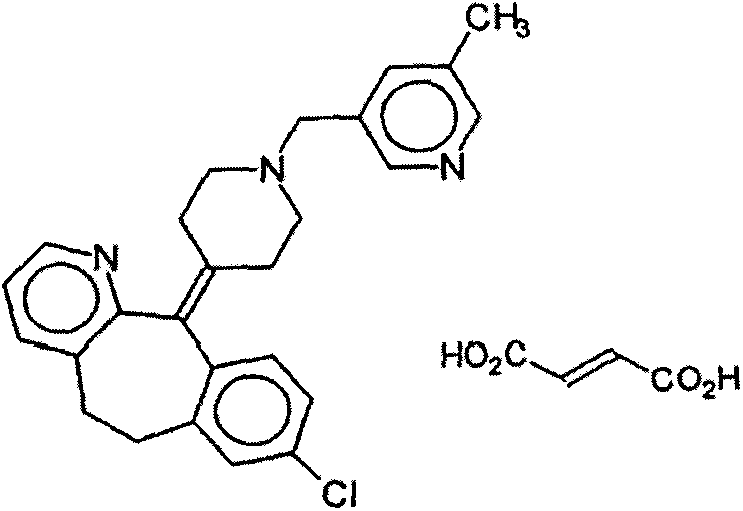
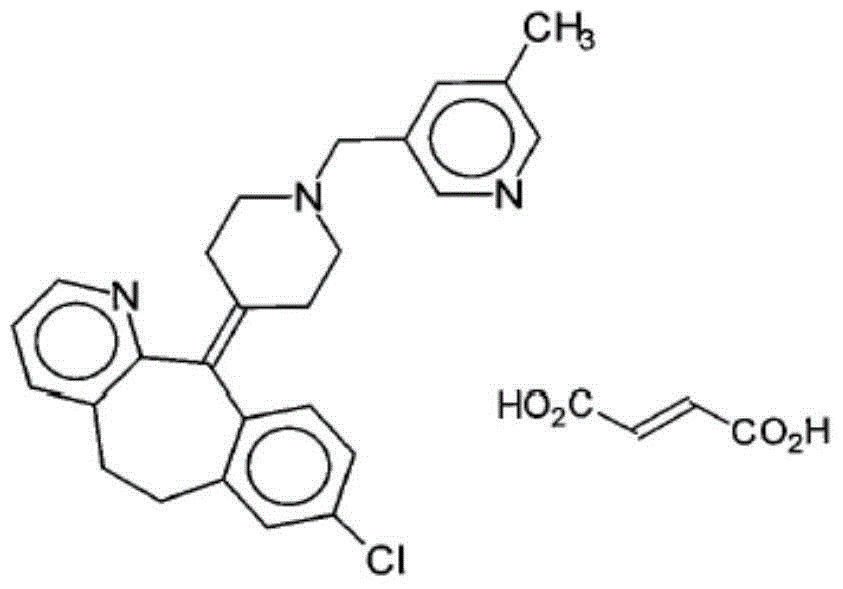
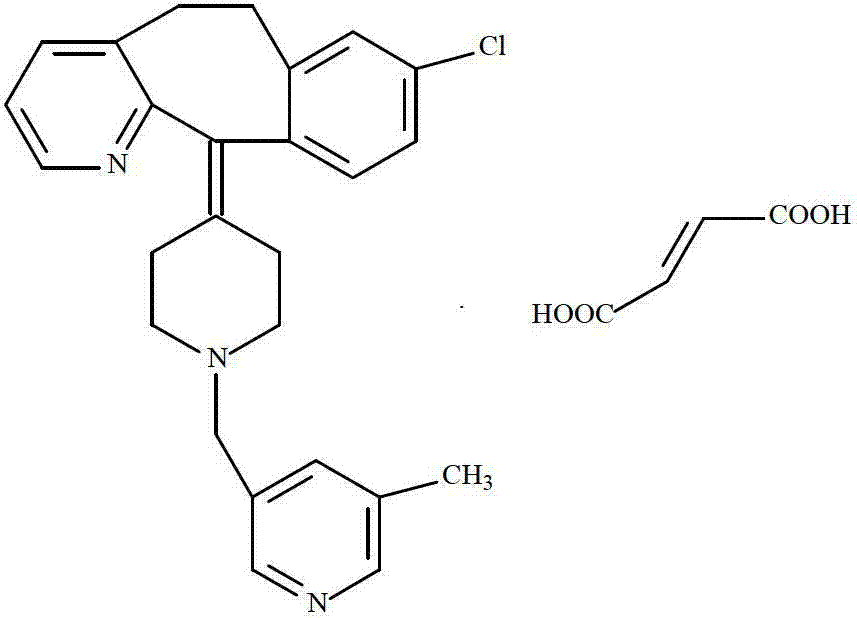
Rupatadine fumarate compound
The invention provides a rupatadine fumarate compound which has dual effects of antihistamine and antagonistic platelet activating factor (PAF). According to the research, allergy and inflammatory diseases are multi-factor complex processes caused by generation and release of various different media; histamine is the most inflammatory medium appearing in allergy early symptom, and the disease symptoms including sneezing, rhinocnesmus, tearing, running nose, skin itch, wheal and the like are mostly caused by histamine H1 receptor. And PAF also can cause bronchial constriction and increased permeability of the vessel, so that running nose, nasal congestion, wheal and itch are caused; meanwhile, the PAF is also a main cause of asthma. The antiallergic drug which is clinically used only has an antihistamine activity effect, but does not have a PAF antagonistic effect.
Owner:海思科制药(眉山)有限公司
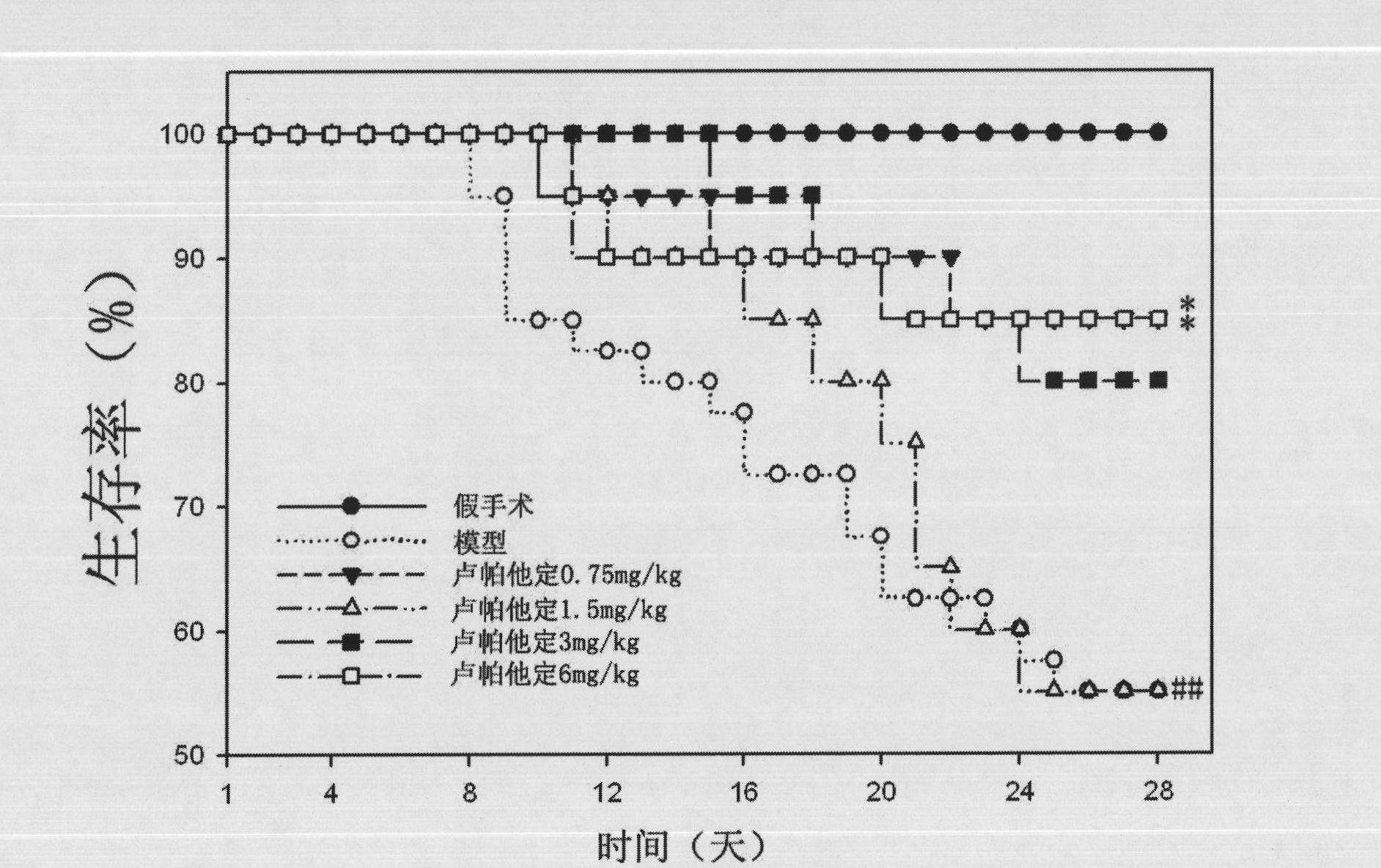
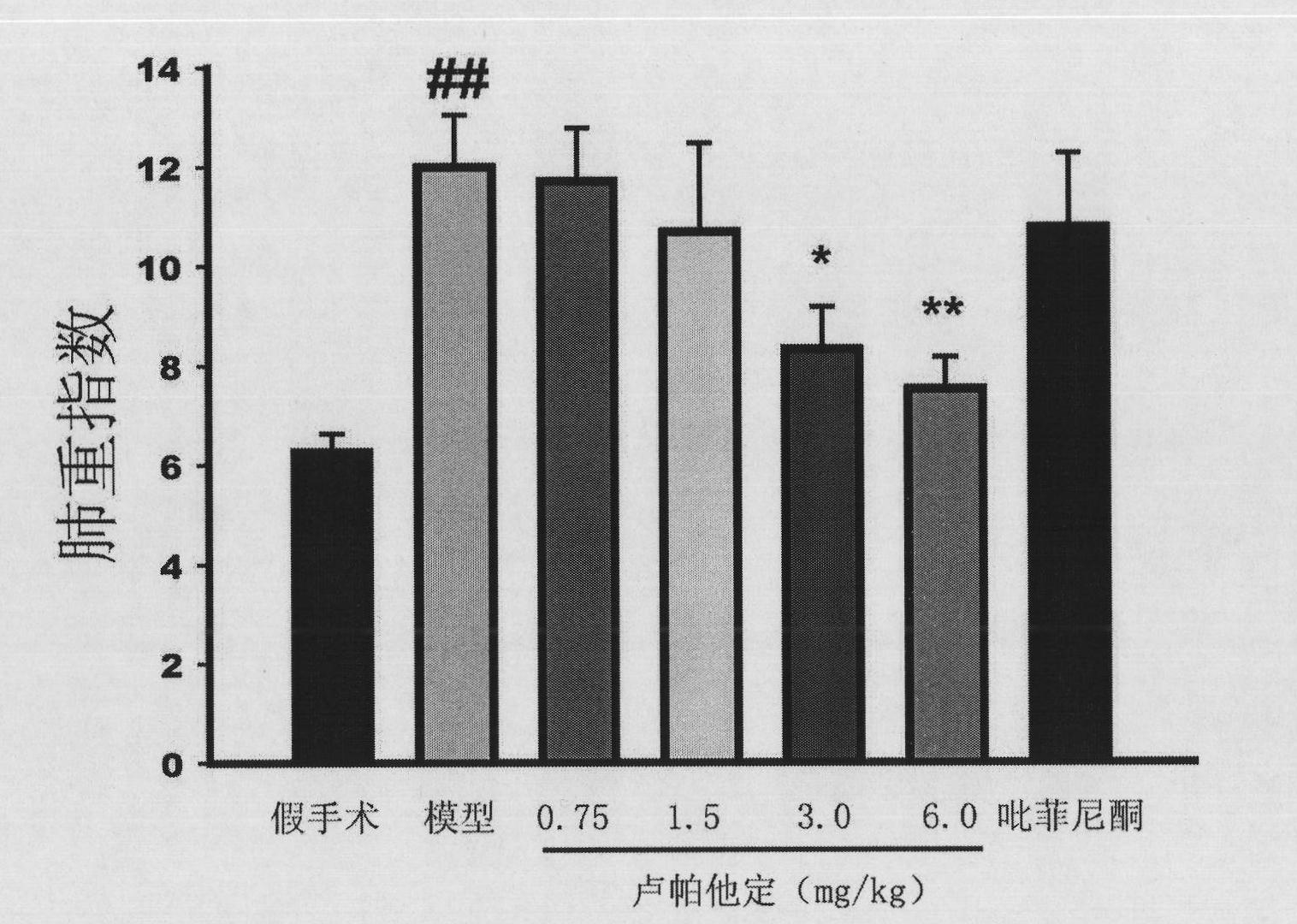
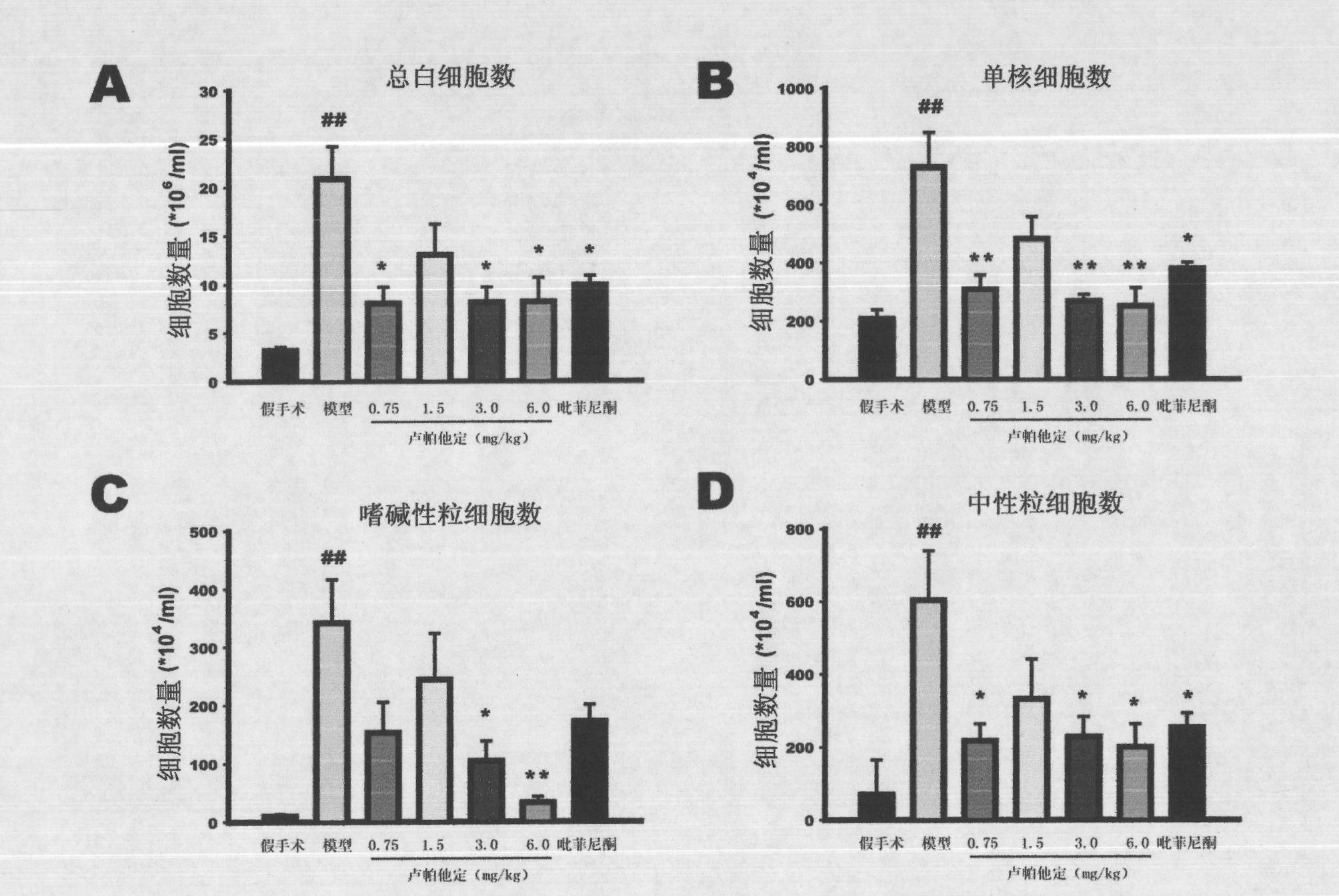
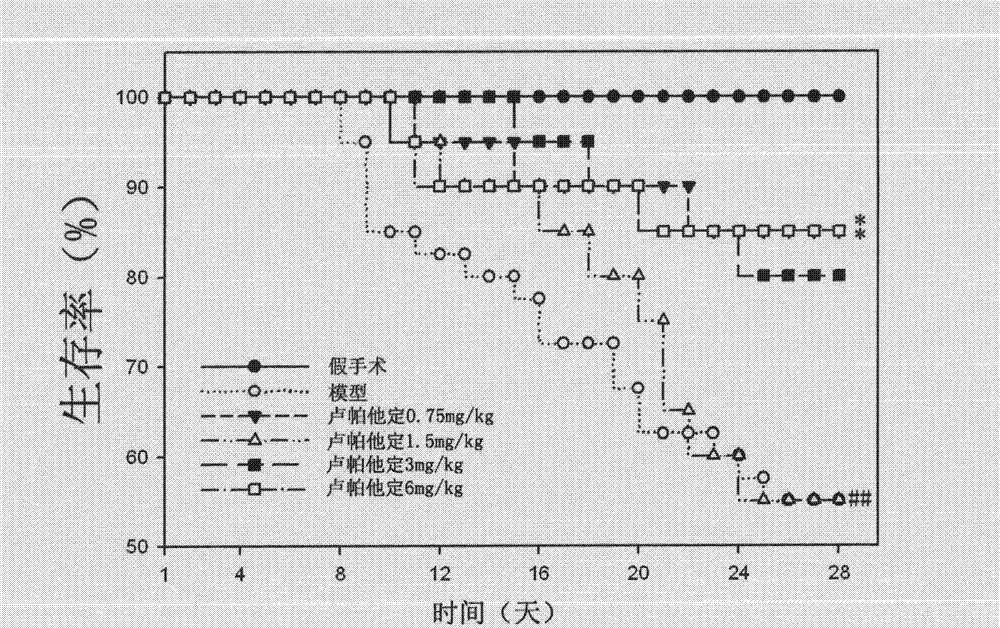
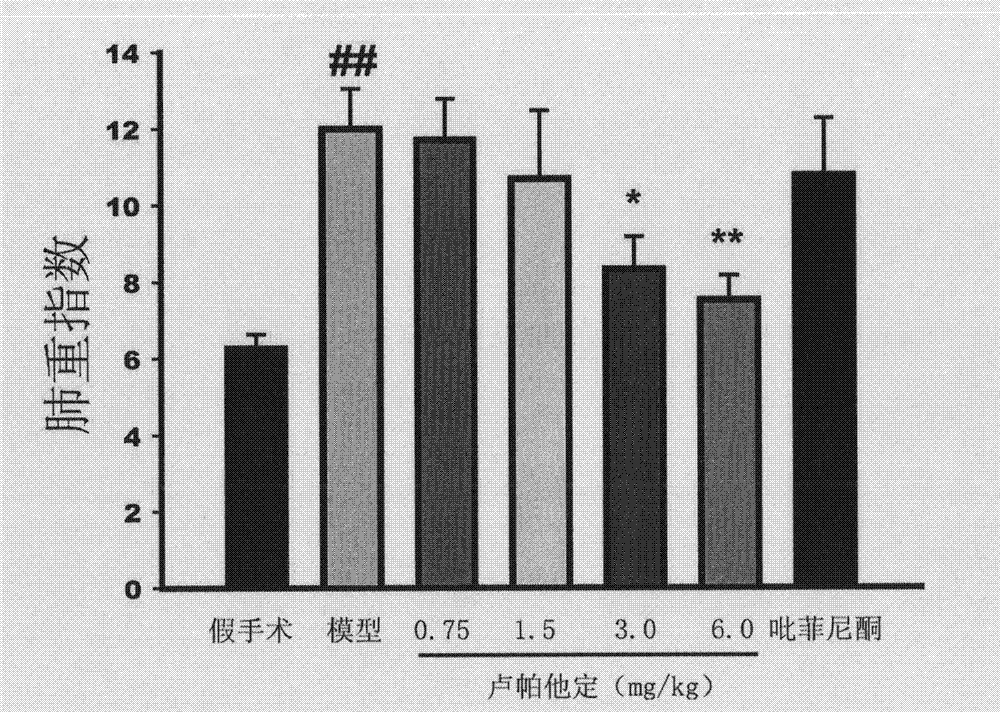
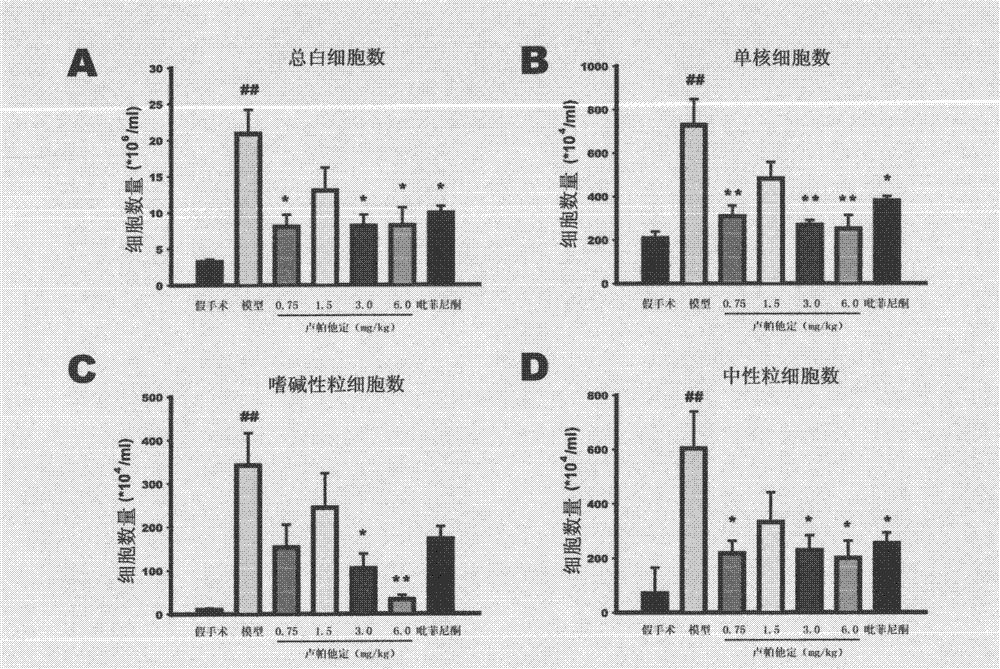
Use of rupatadine for preventing or treating pulmonary fibrosis
ActiveCN102145002ASignificant effectSmall toxicityOrganic active ingredientsRespiratory disorderSide effectCurative effect
The invention discloses novel use of rupatadine for preventing or treating pulmonary fibrosis. The rupatadine is remarkable in curative effect for treating the pulmonary fibrosis, little toxic and side effect and safe in use.
Owner:BEIJING WEIFENG YIMIN TECH +2
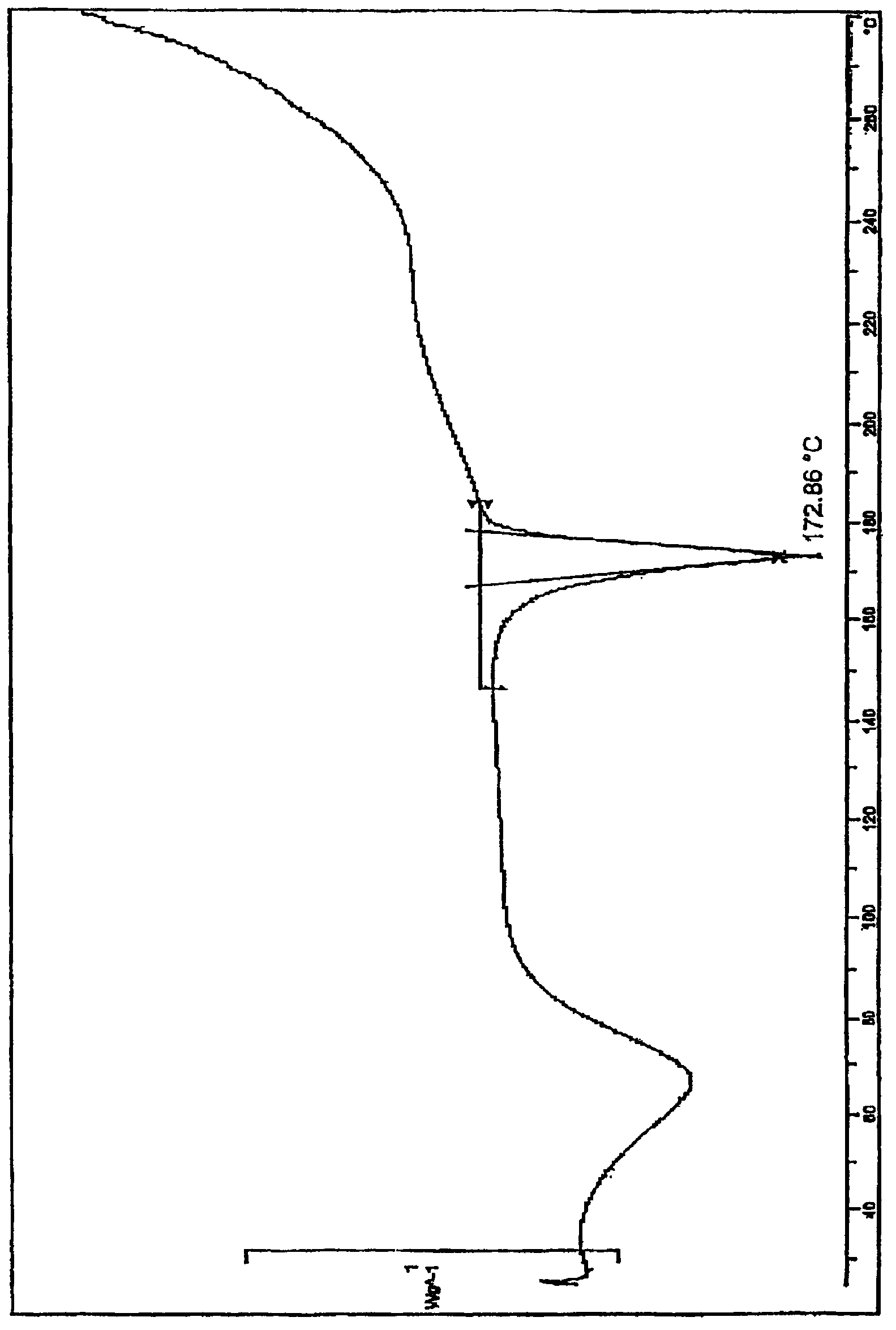
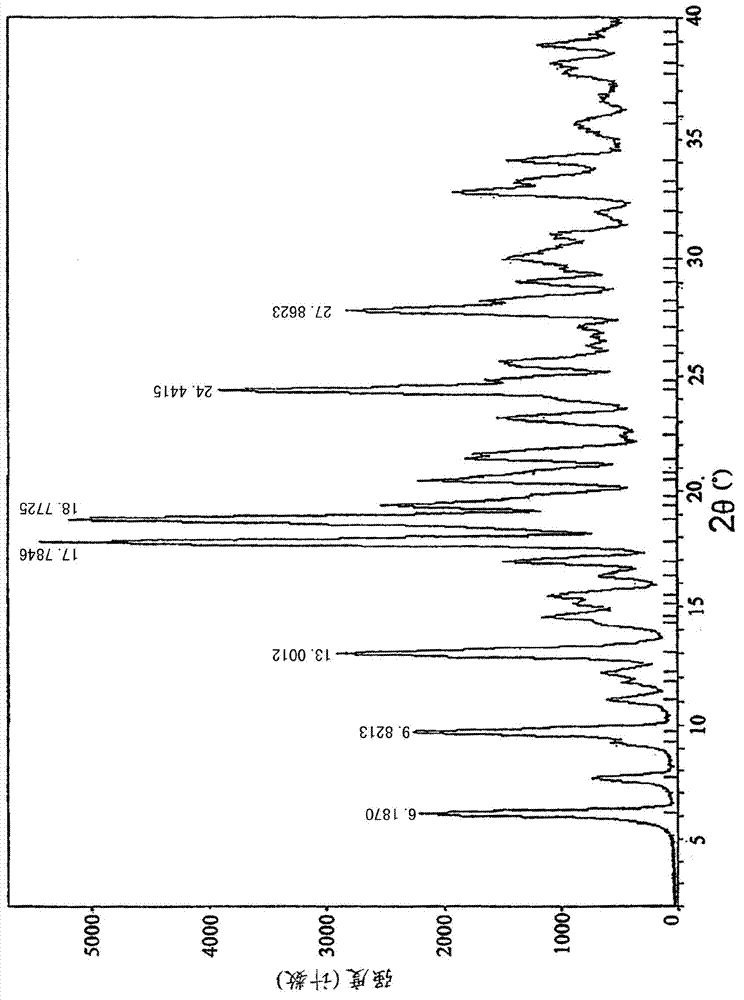
Method for salifying or recrystallization of rupatadine fumarate
The invention provides a method for preparing rupatadine fumarate, which is suitable for actual large-scale industrial production and has high yield and quality and excellent stability. A preparation method for the salifying of the rupatadine fumarate comprises the following step of performing a salifying reaction on rupatadine and fumaric acid in a mixed solvent, wherein the mixed solvent is a mixed solvent of acetone and water in a volume ratio of 5-20:1. In addition, the rupatadine fumarate also can be subjected to recrystallization in the mixed solvent.
Owner:NANJING VARSAL MEDICINE TECH DEV +1
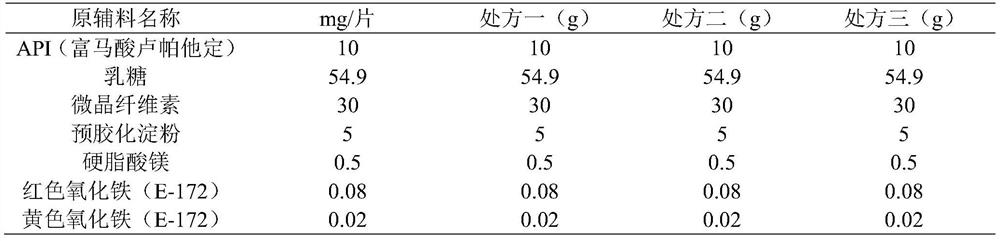
Rupatadine fumarate tablets and preparation method thereof
InactiveCN103751141AImprove discoloration, etc.Promote dissolutionOrganic active ingredientsPharmaceutical product form changePharmaceutical drugCurative effect
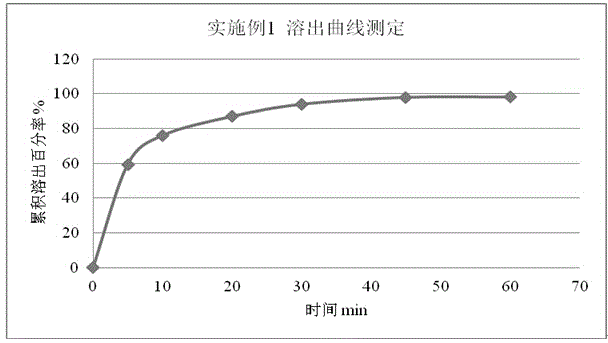
The invention discloses rupatadine fumarate tablets and a preparation method thereof, belonging to the technical field of medicine. The tablets are prepared by making granules from rupatadine fumarate as active ingredient, and pharmaceutical adjuvants including a filler, a binder and a disintegrant, adding a lubricant, and making into tablets. Through the screening of the type and use amount of the filler, disintegrant, binder and lubricant and the use of specific sieving mesh to sieve raw materials during a preparation process, the prepared tablets have stable properties, good content uniformity, good dissolution, small tablet weight difference and high stability, so as to ensure in vivo bioavailability and clinical curative effect.
Owner:YANGTZE RIVER PHARM GRP NANJING HAILING PHARM CO LTD +1
Preparation of rupatadine or salt thereof
InactiveCN101497606ASimple and fast operationShort reaction timeOrganic chemistryRespiratory disorderReagentAlkali metal
The invention relates to a method for preparing rupatadine and salt thereof, which comprises the following steps: directly adding acyloxy alkali metal borohydride or generating the acyloxy alkali metal borohydride through an in-situ reaction; and performing a reduction reaction of the acyloxy alkali metal borohydride and a compound shown in a formula (I) to prepare the rupatadine. The method has the advantages of simple and convenient operation, short reaction time, cheap adopted reagents, and lower production cost.
Owner:FOSHAN DAYI TECH LTD
Preparation method of rupatadine fumarate
The invention provides a preparation method of rupatadine fumarate, which includes the steps of (1) reducing an amide compound 1 with a borane reagent to obtain rupatadine 2, represented as the following formula; and (2) performing a salt-forming reaction to the rupatadine with fumaric acid to prepare the rupatadine fumarate. The method is simple in operations, is reduced in use amount of reagents and is increased in production yield when compared with a method in the prior art.
Owner:SHANGHAI NEW ASIA PHARMA
Preparation method of rupatadine fumarate
The invention provides a preparation method of rupatadine fumarate shown as a formula (VII). The preparation method comprises the following steps: (1) 5-methylnicotinic acid is taken as a raw material and subjected to a reaction in presence of methanol and a catalyst, and a corresponding compound shown as a formula (II) is obtained; (2) the compound shown as the formula (II) is subjected to a reaction in presence of a reducing agent and a solvent, and a corresponding compound shown as a formula (III) is obtained; (3) the compound shown as the formula (III) is subjected to a reaction in presence of a brominating agent and a solvent, and a corresponding compound shown as a formula (IV) is obtained; (4) the compound shown as the formula (IV) and desloratadine (V) are subjected to condensation under the actions of an acid binding agent and a solvent, and rupatadine (VI) is obtained; (5) rupatadine (VI) is subjected to a reaction in presence of fumaric acid and a solvent, and corresponding rupatadine fumarate (VII) is obtained.
Owner:杭州仟源保灵药业有限公司
Oral liquid preparation of rupatadine fumarate and preparation method of oral liquid preparation
InactiveCN111110625AOvercome the defect of poor stabilityOrganic active ingredientsPharmaceutical delivery mechanismPreservativePharmaceutical Substances
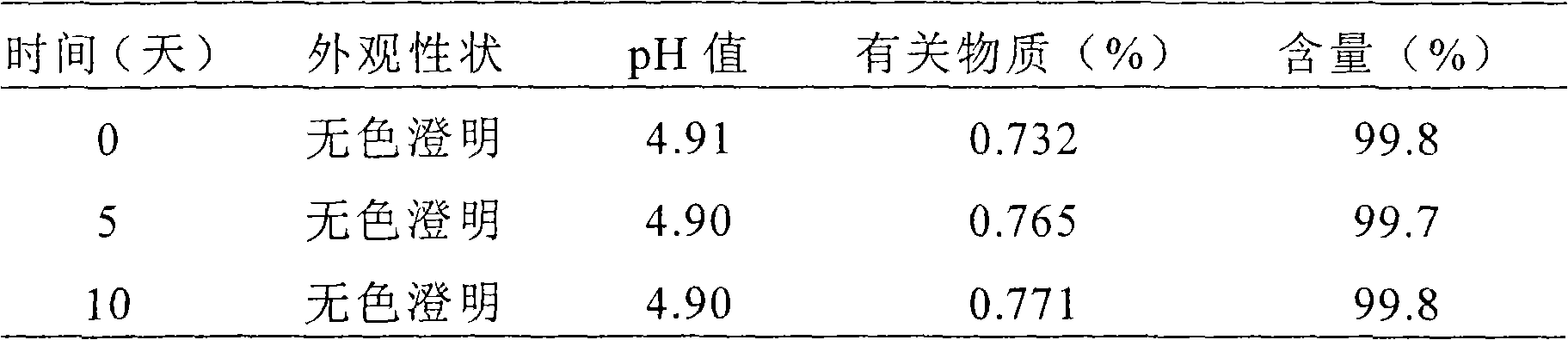
The invention relates to the fields of medicine and dosage forms of medicine, in particular to an oral liquid preparation of rupatadine fumarate and a preparation method of the oral liquid preparation, and discloses an oral liquid preparation of rupatadine fumarate. The oral liquid preparation per 100 mL contains 0.128 g of rupatadine fumarate, 1-20 g of a solubilizer, 1-30 g of a sweetening agent, 0-0.2 g of a flavoring agent, 0-0.01 g of a coloring agent, 0-0.20 g of a preservative and a pH regulator for adjusting the pH value to 4.6-5.6. The oral liquid preparation of rupatadine fumarate has good stability in the study of accelerated stability.
Owner:扬子江药业集团江苏紫龙药业有限公司
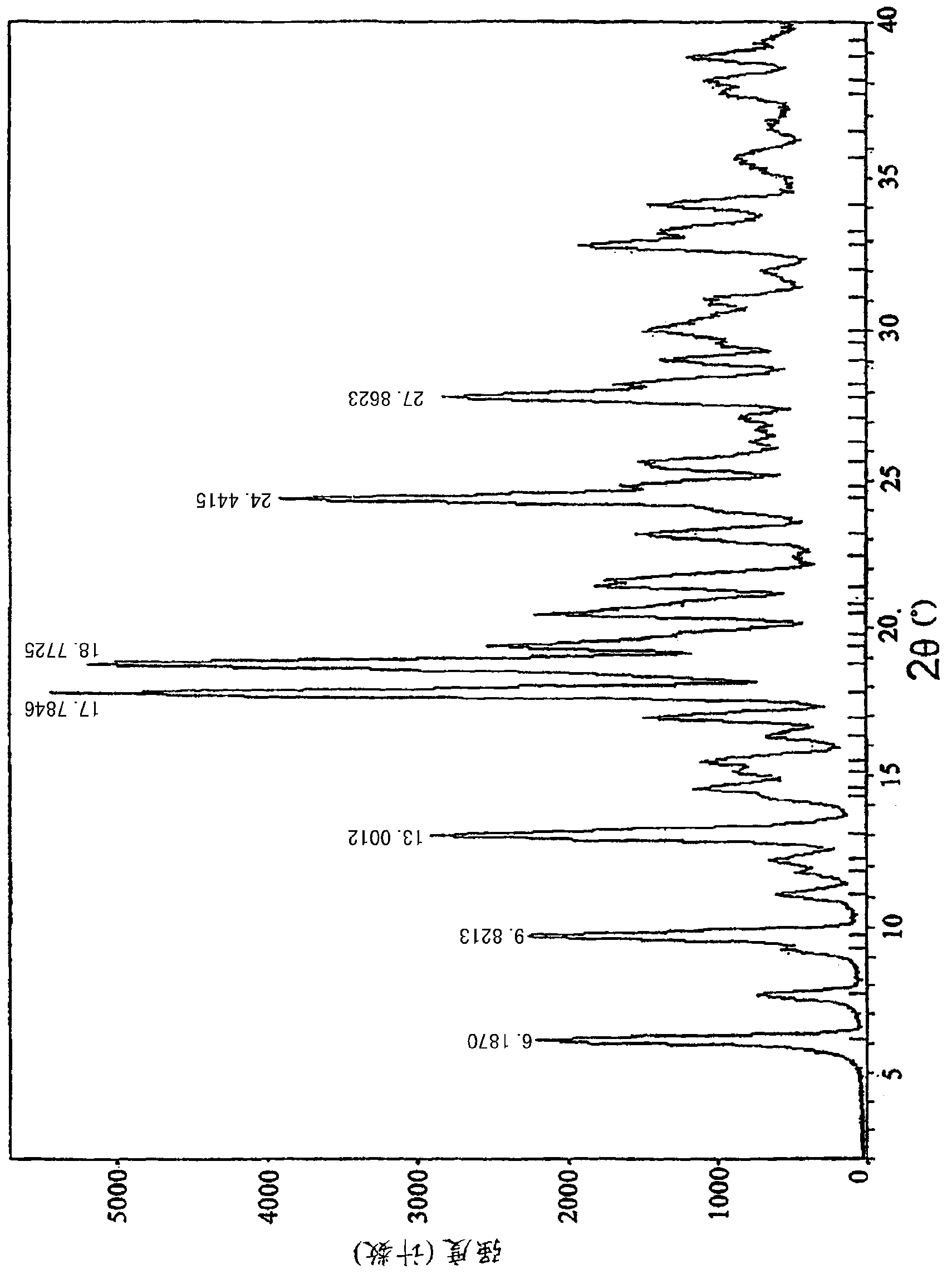
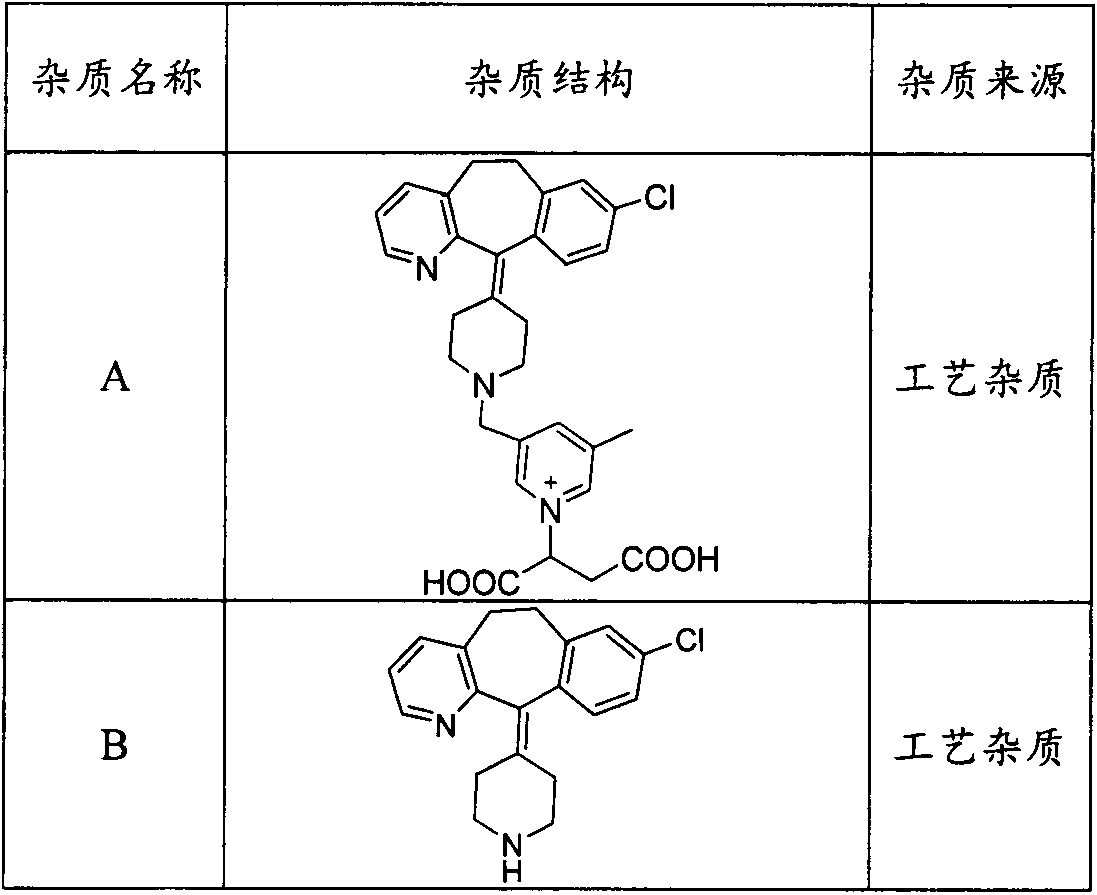
Preparation method of rupatadine fumarate impurity S
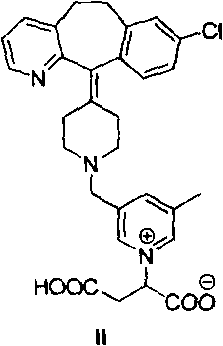
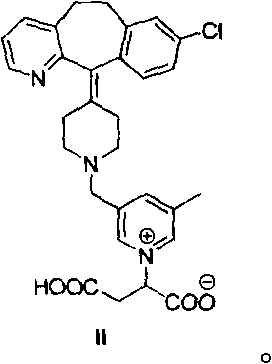
The invention provides a preparation method of a rupatadine fumarate impurity S. The preparation method comprises the following steps: performing a substitution reaction on a raw material A and a rawmaterial B to obtain a substitution product; halogenating the substitution product by a halogenating reagent to obtain a halogenation product; and performing a condensation reaction on the halogenation product and a raw material C to obtain the rupatadine fumarate impurity S. The preparation method has the advantages of wide sources and low prices in the raw materials, increase in the yield of therapamycin fumarate impurity S through a short synthesis route, few side reactions and good reproducibility. A reference substance with excellent quality and high purity can be provided for the qualitative and quantitative analysis of rupatadine fumarate through the preparation and structural identification of the rupabadine fumarate impurity S in order to guide the preparation and the applicationof the rupatadine fumarate. The structure of the rupatadine fumarate impurity S is shown in the description, and X in the structure is a halogen atom.
Owner:杭州仟源保灵药业有限公司
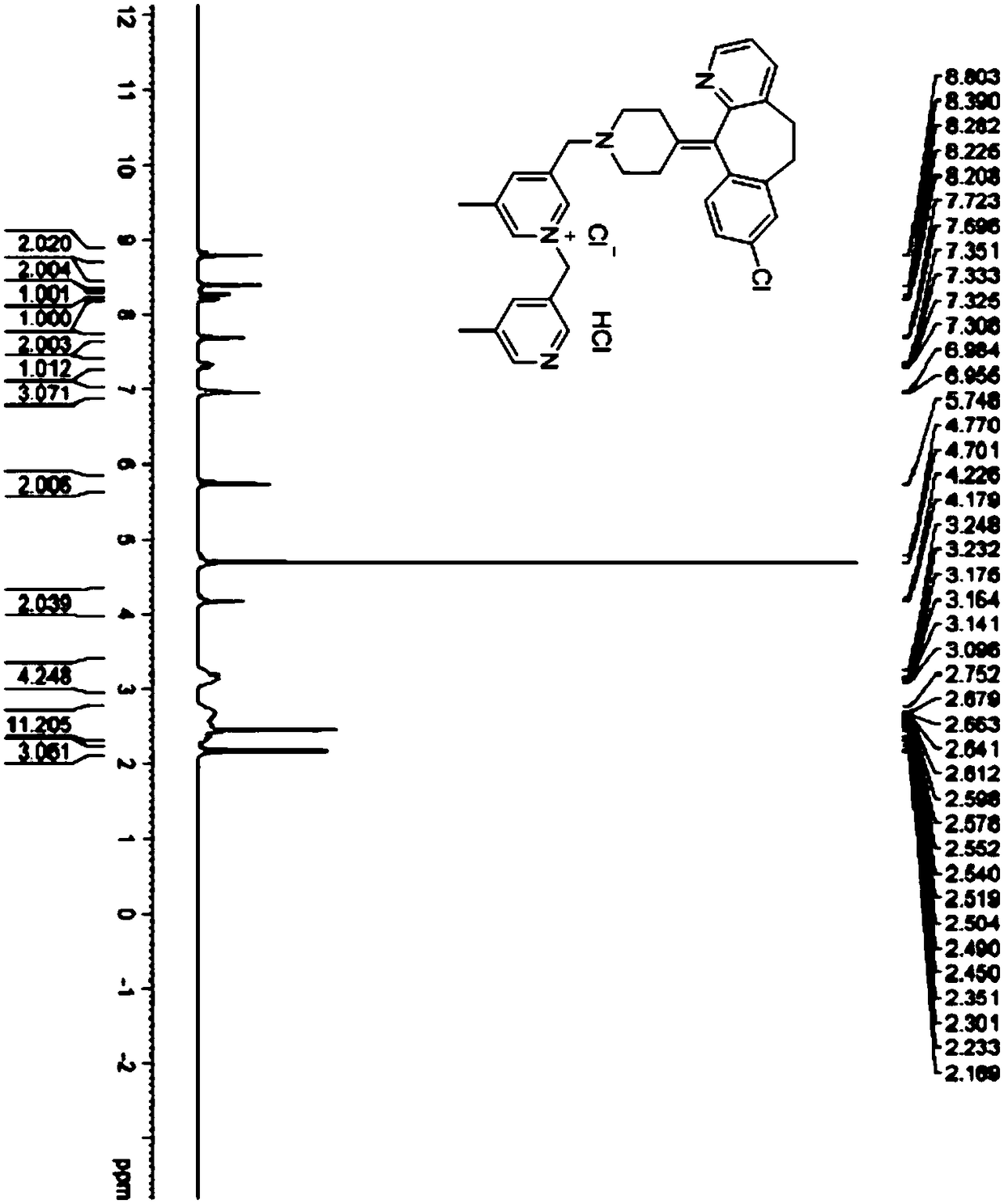
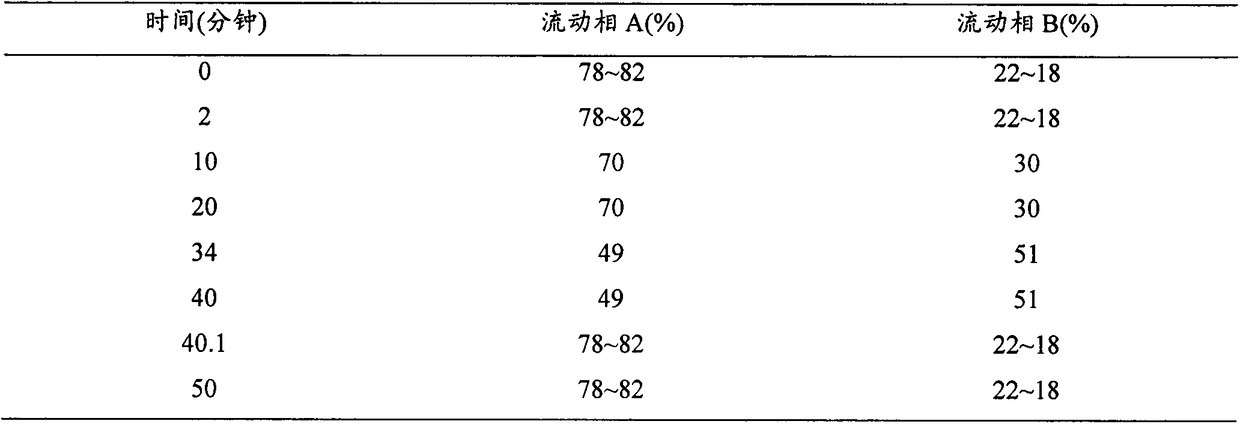
Method for detecting content of rupatadine fumarate by liquid chromatography
InactiveCN102680622AQuality is easy to controlThe method is simple and fastComponent separationPhosphatePhosphoric acid
The invention relates to the technical field of content detection of biomedical preparations, in particular to a method for detecting the content of rupatadine fumarate by liquid chromatography. According to the method, a chromatographic column is used, gradient elution is adopted, a flowing phase consists of a flowing phase A and a flowing phase B, the flowing phase A is a buffer solution which consists of 0.05 to 0.30 volume percent triethylamine and 0.01 to 0.05mol / L phosphate and phosphoric acid and has the pH of 3.0 to 5.0, and the flowing phase B is an organic modifier. By the method, related substances such as the rupatadine fumarate are controlled, and the content of the rupatadine fumarate can be accurately measured, the quality of the rupatadine fumarate is controllable, the method is simple and quick, can be used for the quality control of the rupatadine fumarate and has great practical significance for quality control in the synthesis and preparation processes, analysis sensitivity is high, a result is accurate and reliable, the quality of Chinese herbal medicines and a preparation is controllable, and the requirement of quick analysis during production is met.
Owner:FOSHAN DAYI TECH LTD
Medicine for treating allergic rhinitis and preparation method thereof
InactiveCN101590050ARound shapeImprove liquidityOrganic active ingredientsPharmaceutical non-active ingredientsCarboxymethyl starchTreatment effect
The invention relates to a medicine for treating allergic rhinitis. The medicine comprises the following raw materials according to parts by weight: 90-110 parts of fumarate rupatadine, 100-130 parts of pregelatinized starch, 30-50 parts of microcrystalline cellulose, 20-30 parts of hydroxypropyl methylcellulose solution of 3 percent, 3-6 parts of sodium carboxymethyl starch and 0.2-2 parts of magnesium stearate. The medicine has simple recipe, reasonable composition of the raw materials and proper proportion, and the dissolution degree is enhanced to more than 90 percent from 70 percent; and the effective components can be farthest absorbed by a human body, and a favorable therapeutic effect is achieved.
Owner:JIAOZUO XIANDA TRADE
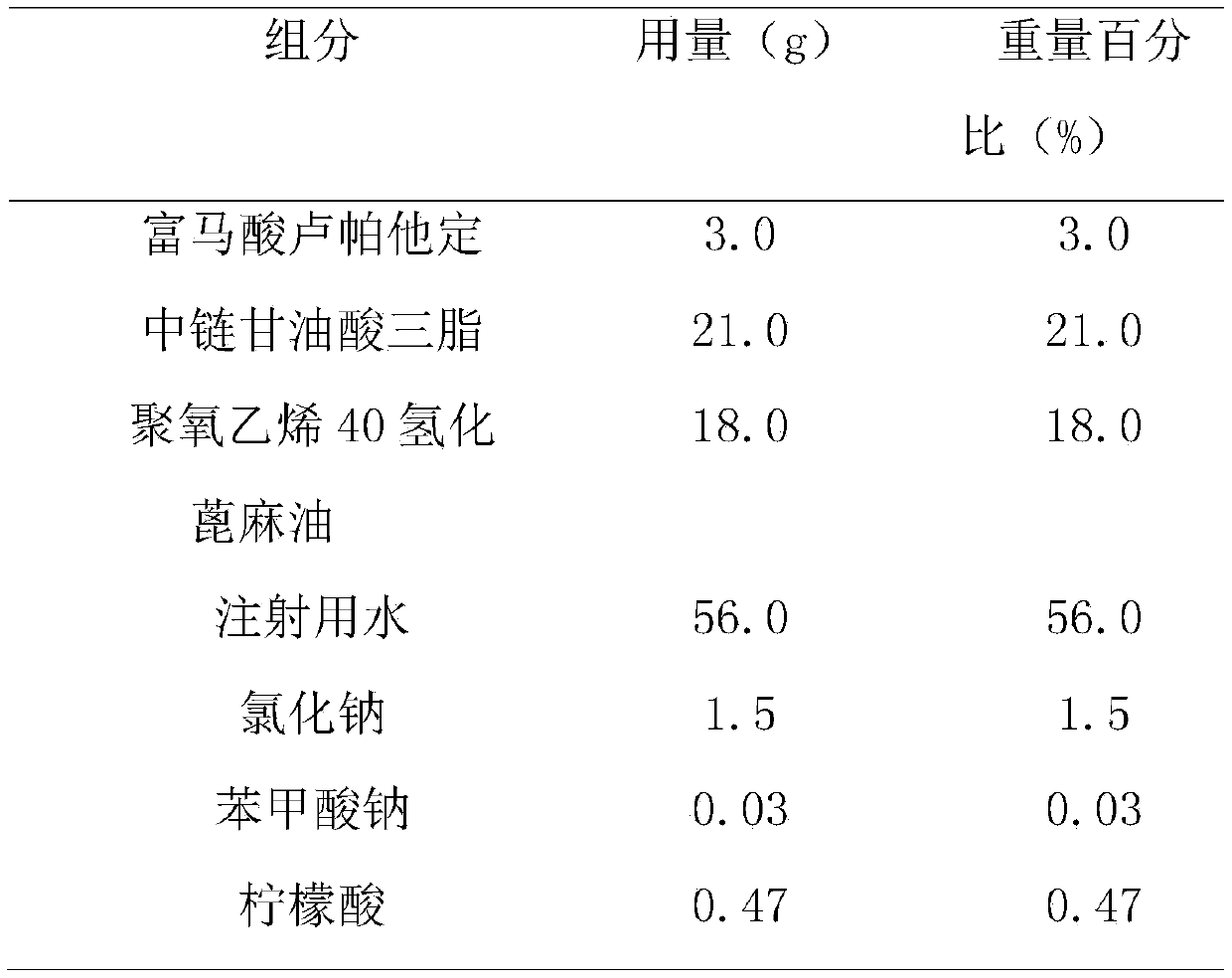
Rupatadine fumarate granule and preparation method thereof
InactiveCN104997734ASolve hygroscopicityFix stability issuesPowder deliveryOrganic active ingredientsOrganic acidPreservative free
The invention relates to a rupatadine fumarate granule and a preparation method thereof. The rupatadine fumarate granule can be used for treating allergic rhinitis and urticaria. The granule comprises rupatadine fumarate, organic acid, a water-soluble polymer material, a flavoring agent, an artificial sweetening agent and solid fruity essence and does not contain antiseptic. The rupatadine fumarate granule is prepared by using the proper preparation method. The preparation method effectively overcomes the problems of dissolving-out speed and hygroscopicity, improves stability of the granule and enables the granule to have good taste and to be easily taken by patients.
Owner:广州艾格生物科技有限公司
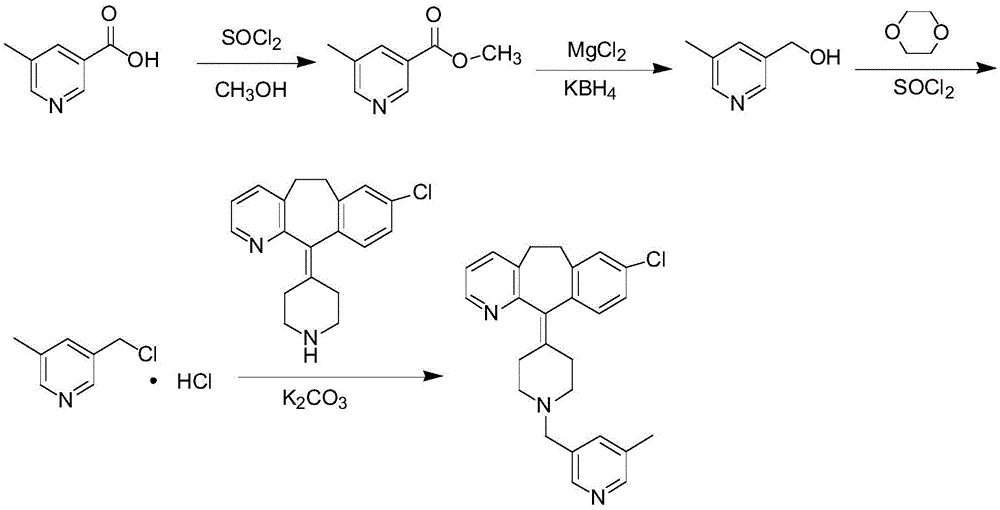
Preparation process of Rupatadine
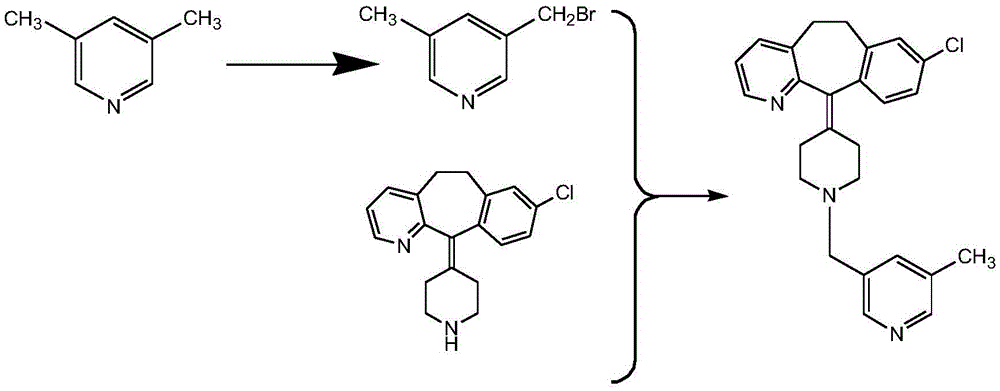
The invention discloses a rupatadine preparation process, which comprises: S1, preparing methyl 5-methylnicotinate; S2, preparing 5-methyl-3-pyridinemethanol; S3, preparing 3-methyl-5-chloromethylpyridine hydrochloride; and S4, preparing rupatadine. According to the present invention, the preparation process has advantages of low cost, mild reaction condition, simple operation, low requirement onequipment, good product purity and high yield, and is suitable for large-scale industrial production.
Owner:HEFEI PINGGUANG PHARMA
Rupatadine-containing patch
ActiveUS20200121612A1Improve skinPromote absorptionOrganic active ingredientsAntipyreticPolymer scienceActive agent
An external patch that contains rupatadine as a second-generation antihistamine, has excellent plaster physical properties, good adhesion to applied skin, and good transdermal absorption of rupatadine as an active ingredient is provided. The external patch containing rupatadine uses an acrylic adhesive as an adhesive base. Specifically, the external patch containing rupatadine uses an acrylic adhesive as an adhesive base and further contains an organic acid having 2 to 7 carbon atoms as a solubilizer, a fatty acid ester as a softener, and / or a surfactant.
Owner:NOUCOR HEALTH SA +1
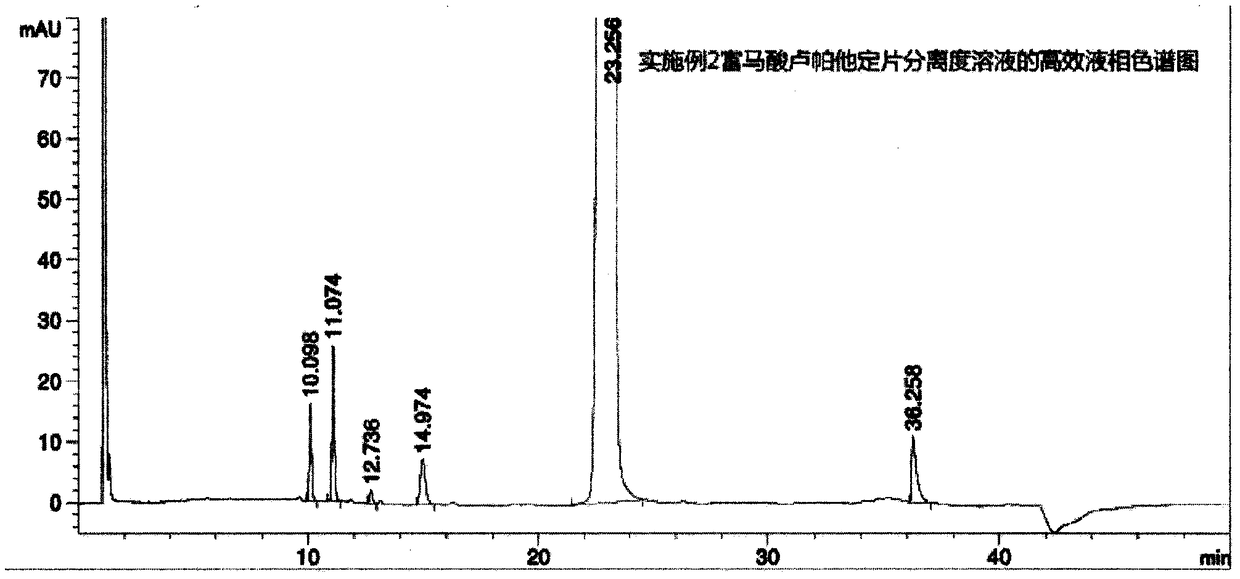
Rupatadine fumarate tablet related substance control method
The invention discloses a high performance liquid chromatography determination method for rupatadine fumarate tablet related substances, wherein impurities are quantified by using a calibration factorcontained main component self-contrasted method. According to the present invention, the operation of the method is simple and easy to perform; and the test results prove that the selected chromatographic conditions can well separate the main component and the related substances so as to well control the related substances of the rupatadine fumarate tablet.
Owner:扬子江药业集团江苏紫龙药业有限公司
A bioadhesive nasal gel product of compound rupatadine and its preparation method
ActiveCN106265664BRapid relief of various symptomsRelieve various symptomsOrganic active ingredientsAntipyreticMetizolineXylometazoline hydrochloride
The invention discloses a compound rupatadine bioadhesion nasal gel product and a preparation method. The compound rupatadine bioadhesion nasal gel product is characterized by being prepared from, 10-100 parts of rupatadine and salts thereof, 5-20 parts of xylometazoline hydrochloride / naphazoline / pseudoephedrine hydrochloride, 1-4 parts of furacilin, 300-5000 parts of polymer gel materials, 2-200 parts of a biological adhesive, 180-200 parts of a buffer salt, 800-1600 parts of a wetting agent and 20000 parts of sterile water for injection. The wetting agent is dissolved into 2 / 3 of the sterile water for injection to obtain a solution A; the polymer gel material is stirred and added into the solution A to obtain a gel system B; the biological adhesive is dispersed into the gel system B to obtain a gel system C; the rupatadine and salts thereof, the xylometazoline hydrochloride / naphazoline / pseudoephedrine hydrochloride, the furacilin and the buffer salt are successively added into 1 / 3 of the sterile water for injection, and sonication is performed to obtain a solution D; the solution D is dispersed into the gel system C, and stirring is performed to obtain the final product.
Owner:重庆市人民医院
Rupatadine fumarate emulsion type nasal spray agent and preparation method thereof
PendingCN110200915APlay an anti-allergic effectImprove bioavailabilityOrganic active ingredientsAerosol deliveryMedicineNasal spray
The invention relates to the biomedical technical field, and in particular, relates to a rupatadine fumarate emulsion type nasal spray agent and a preparation method thereof. A prescription of nasal drops contains the following medicinal-grade components by mass: 2-5% of rupatadine fumarate, 5-25% of an oil phase, 10-20% of an emulsifier, 40-65% of an aqueous phase, 0.5-5% of an osmotic pressure regulation agent, 0.05-0.1% of a bacteriostatic agent, and 0.5-5% of a pH regulator. A nose drop liquid is prepared by emulsification with a high-speed shearing homogenizer, aseptic operation is carried out, and the nose drop liquid are sub-packed in a special spray device.
Owner:扬子江药业集团江苏紫龙药业有限公司
Rupatadine fumarate compound
The invention provides a rupatadine fumarate compound which has dual effects of antihistamine and antagonistic platelet activating factor (PAF). According to the research, allergy and inflammatory diseases are multi-factor complex processes caused by generation and release of various different media; histamine is the most inflammatory medium appearing in allergy early symptom, and the disease symptoms including sneezing, rhinocnesmus, tearing, running nose, skin itch, wheal and the like are mostly caused by histamine H1 receptor. And PAF also can cause bronchial constriction and increased permeability of the vessel, so that running nose, nasal congestion, wheal and itch are caused; meanwhile, the PAF is also a main cause of asthma. The antiallergic drug which is clinically used only has an antihistamine activity effect, but does not have a PAF antagonistic effect.
Owner:海思科制药(眉山)有限公司
Methods and compositions for prevention of feedlot bovine respiratory disease
ActiveUS20180185344A1Organic active ingredientsMicrobiological testing/measurementBovine respiratory diseaseIntensive care medicine
Methods for preventing feedlot bovine respiratory diseases employing an anti-inflammatory drug rupatadine are disclosed. Compositions are further disclosed. Beneficially, the methods and compositions provide safe and cost-effective management of a costly disease.
Owner:IOWA STATE UNIV RES FOUND
Rupatadine fumarate intermediate and preparation method of rupatadine fumarate
PendingCN114133353AHigh purityHigh yieldOrganic compound preparationCarboxylic acid salt preparationChemical synthesisCombinatorial chemistry
The invention belongs to the technical field of chemical synthesis, and particularly relates to a rupatadine fumarate intermediate and a preparation method of rupatadine fumarate. The method comprises the following steps: (1) reacting a compound shown in a formula VI with a halogenating reagent to obtain a rupatadine fumarate intermediate shown in a formula V; (2) adding the intermediate of formula V and a compound of formula IV into an acid-binding agent for reaction to obtain a compound of formula III; (3) reacting the compound in the formula III with a reducing agent to obtain a compound in a formula II; and (4) reacting the compound shown in the formula II with fumaric acid to obtain rupatadine fumarate. According to the preparation method, 3, 5-dimethylpyridine-N-oxide is used for carrying out bromination or chlorination, and compared with bromination or chlorination carried out by using 3, 5-dimethylpyridine, the purity and the yield of a halogenated product are higher. Moreover, after the compound shown in the formula V reacts with the compound shown in the formula IV, column chromatography is not needed, the compound shown in the formula III is obtained through solvent refining, post-treatment operation is simple, and industrial production is facilitated.
Owner:CHONGQING HUAPONT PHARMA
Use of rupatadine for preventing or treating pulmonary fibrosis
ActiveCN102145002BSignificant effectSmall toxicityOrganic active ingredientsRespiratory disorderSide effectCurative effect
The invention discloses novel use of rupatadine for preventing or treating pulmonary fibrosis. The rupatadine is remarkable in curative effect for treating the pulmonary fibrosis, little toxic and side effect and safe in use.
Owner:BEIJING WEIFENG YIMIN TECH +2
Preparation method of rupatadine fumarate tablet
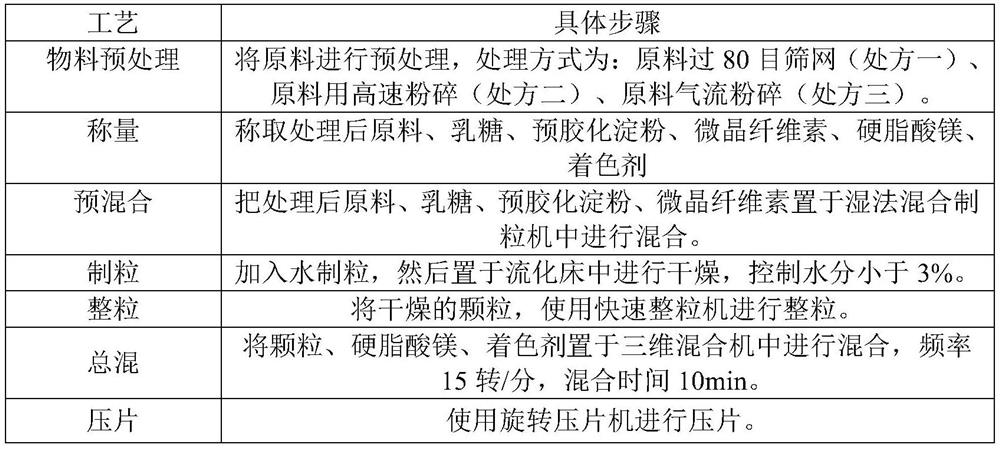
InactiveCN113081980AImprove solubilityImprove bioavailabilityOrganic active ingredientsPharmaceutical non-active ingredientsFluidized bedMagnesium stearate
The invention discloses a preparation method of a rupatadine fumarate tablet. The preparation method comprises the following steps of: S1, mixing rupatadine fumarate with a part of microcrystalline cellulose, and then performing jet milling to obtain mixed powder; S2, mixing the mixed powder, pregelatinized starch, lactose and the remaining microcrystalline cellulose, performing wet granulation, and drying the mixture in a fluidized bed, adopting water as a wetting agent; and S3, carrying out size stabilization on particles obtained in the step S2, mixing the particles with magnesium stearate and a coloring agent, and tabletting to obtain the rupatadine fumarate tablet. According to the preparation method of the rupatadine fumarate tablet of the invention, the rupatadine fumarate and a part of microcrystalline cellulose are mixed and then are subjected to airflow pulverization, so that the operability in production is improved, the solubility of the raw materials can also be improved, and the absorption speed of the product in vivo is greatly increased; the wet granulation process is adopted, the process is stable and controllable; and the stability of the prepared sample is relatively good, and large-scale production and quality control during large-scale production are facilitated.
Owner:北京阳光诺和药物研究股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com