Liquid formulations of rupatadine fumarate
A liquid technology of rupatadine fumarate, which is applied in the direction of medical preparations containing non-active ingredients, medical preparations containing active ingredients, dispersion liquid delivery, etc. It can solve stability problems and is not suitable for use as drugs, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 2
[0168] Reference Example 2: Formation Rate of Compound II in Rupatadine Fumarate Aqueous Solution
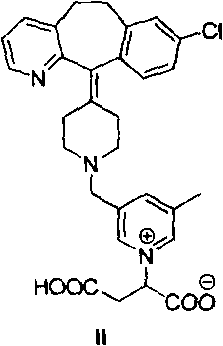
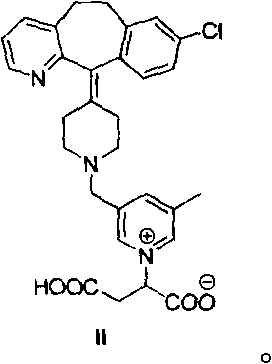
[0169] It has been found that rupatadine reacts with fumaric acid under acidic conditions to produce an adduct (compound II):
[0170]
[0171] Different aqueous solutions of different pH (3.41, 4.38 and 5.00) containing rupatadine fumarate at a concentration of 1 g (rupatadine fumarate) / L (the required amount of HCl (适量) or NaOH (适量) The solution was added to a solution containing only water and rupatadine fumarate) stored under different temperature conditions (room temperature and 40°C / 75%HR) for two months and at the beginning, one month and the end of the period , use method 2 analysis, as shown in the table below.
[0172]
[0173] These results show that the rate of formation of compound II is pH dependent. The lower the pH, the higher the rate of compound II formation.
example 3
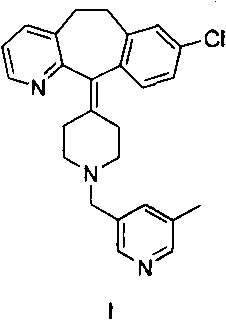
[0174] Reference Example 3: Preparation of Compound II
[0175]
[0176] In a 1000 mL flask, 35 g (0.084 mol) of rupatadine (I) was dissolved in 260 mL of ethanol. To this solution was added 16.1 g (0.087 mol) of bromosuccinic acid, and the mixture was reacted at room temperature overnight. The mixture was concentrated to half volume and allowed to react at room temperature for 5 days.
[0177] The solvent was removed in vacuo and the solid was washed with ethanol and with a mixture of ethanol:water:ammonia. It was then purified by flash chromatography using a 9:1:1 ethanol:ammonia:water mixture.
[0178] Finally, 4.51 g (0.0085 mol, 9.7% yield) of the desired product (compound II) were obtained.
[0179] 1 H-NMR (300MHz, CD 3 OD): 8.71(broad peak signal, 2H), 8.31(m, 2H), 7.63(d, J=7.31Hz, 1H), 7.18(m, 4H), 5.48(dd, J=3.29Hz, J=11.70 Hz,1H),3.69(s,2H),3.41(m,3H),3.11(dd,J=11.7Hz,J=17.2Hz,1H),2.8(m,4H),2.54(s,3H), 2.45(m,2H),2.26(m,4H).
[0180] 13 C-NMR (75.43MH...
Embodiment 1
[0186] Embodiment 1: Preparation of nasal liquid formulation of rupatadine fumarate (1.00g (rupatadine) / L)
[0187] The quantitative composition of this formulation is disclosed in the table below.
[0188] Rupatadine fumarate was dissolved in polyethylene glycol 400. This is the "active solution".
[0189] Dissolve anhydrous citric acid and anhydrous disodium phosphate in pure water and stir until completely dissolved. This is the "carrier solution".
[0190] Mix and homogenize the "active solution" with the "carrier solution". Add preservatives to the final solution. Finally, pure water was added to the resulting mixture to the desired volume.
[0191]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com