Rupatadine fumarate tablet related substance control method
A technology for rupatadine fumarate and related substances, which is applied in the field of drug analysis and can solve problems such as shortening the service life of a chromatographic column and damage to the chromatographic column.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Instrument and Chromatographic Conditions
[0025] Agilent 1260 high-performance liquid chromatography; the chromatographic column is a C18 chromatographic column, 150mm×4.6mm×5μm, and the column temperature is 30°C;
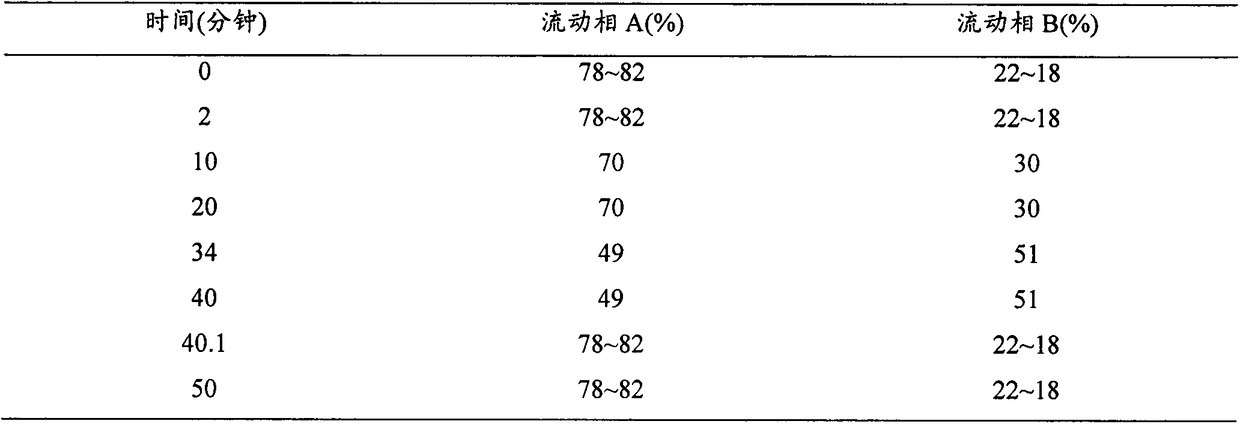
[0026] Using 20 mmol phosphate aqueous solution [adding 0.1% (v / v) triethylamine, adjusting the pH to 5.0 with dilute phosphoric acid] as mobile phase A, using acetonitrile as mobile phase B, the gradient elution procedure is as follows:
[0027]
[0028] The mobile phase flow rate is 1.2ml / min;
[0029] Using a UV detector, the detection wavelength is set to 210nm;
[0030] Experimental procedure
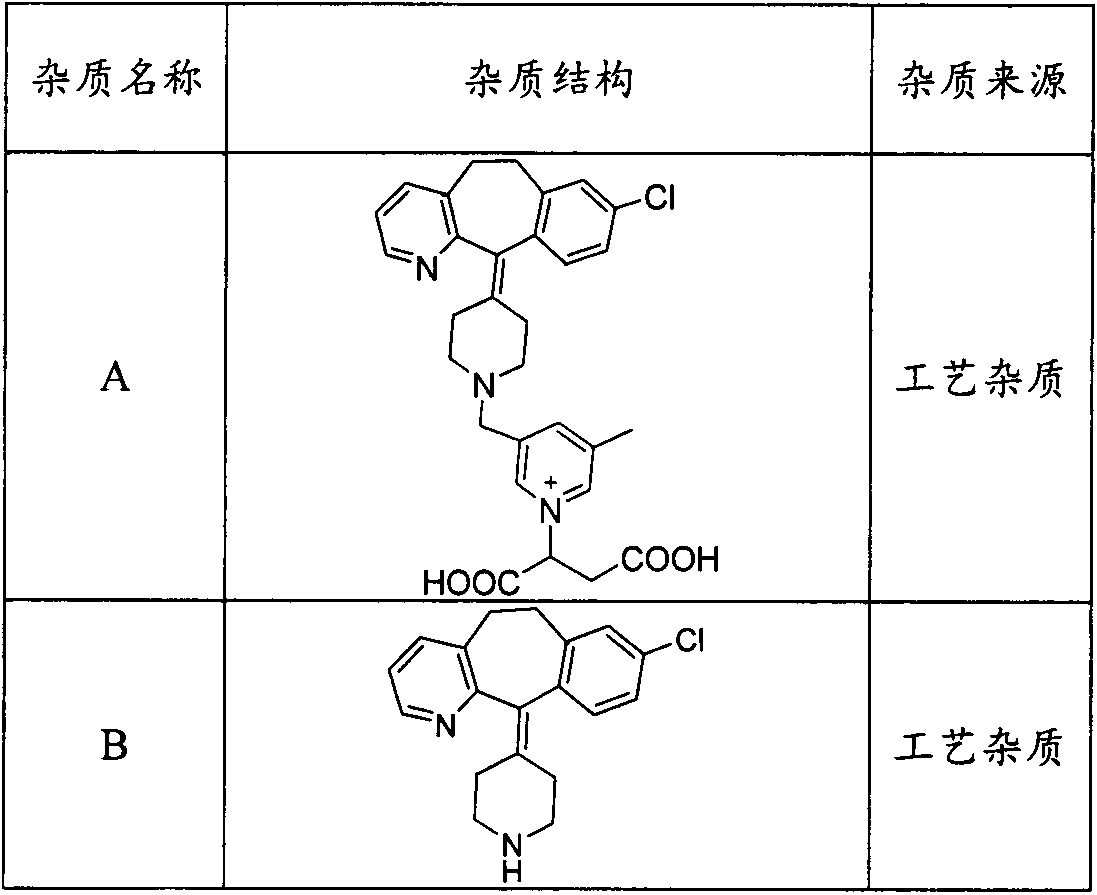
[0031] Take an appropriate amount of rupatadine fumarate reference substance, impurities A, B, C, and F, dissolve and dilute with a diluent to make a mixed solution containing 200 μg of each impurity and rupatadine per 1 ml, as a linear test stock solution. Precisely measure 1.0ml, 1.0ml, 1.0ml, 3.0ml, 2ml of linear test stock solution, put them in 50ml...
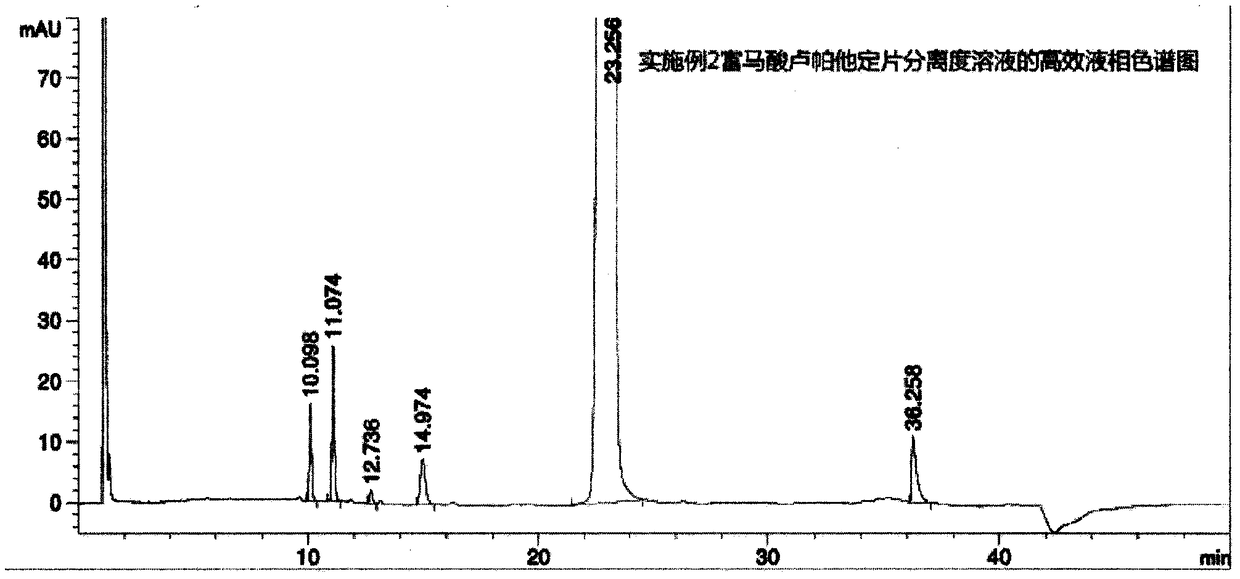
Embodiment 2
[0035] Instrument and Chromatographic Conditions
[0036] Agilent 1260 high-performance liquid chromatography; the chromatographic column is a C18 chromatographic column, 150mm×4.6mm×5μm, and the column temperature is 30°C;
[0037] Using 20 mmol phosphate aqueous solution [adding 0.1% (v / v) triethylamine, adjusting the pH to 5.0 with dilute phosphoric acid] as mobile phase A, using acetonitrile as mobile phase B, the gradient elution procedure is as follows:
[0038]
[0039] The mobile phase flow rate is 1.2ml / min;
[0040] Using a UV detector, the detection wavelength is set to 210nm;
[0041] Experimental procedure
[0042] Take an appropriate amount of fine powder of this product (approximately equivalent to rupatadine 10mg), put it in a 20ml measuring bottle, add an appropriate amount of diluent (mobile phase A-acetonitrile=80:20, V:V), dissolve it by ultrasonic, and dilute with diluent to the mark, shake well, filter, and take the filtrate as the test solution; ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com