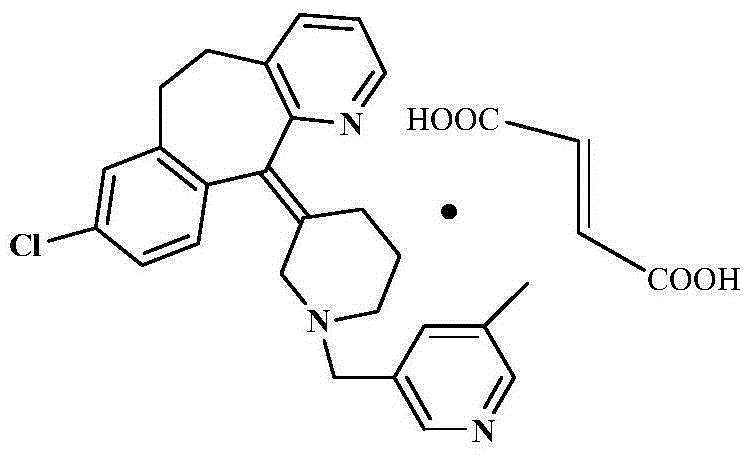
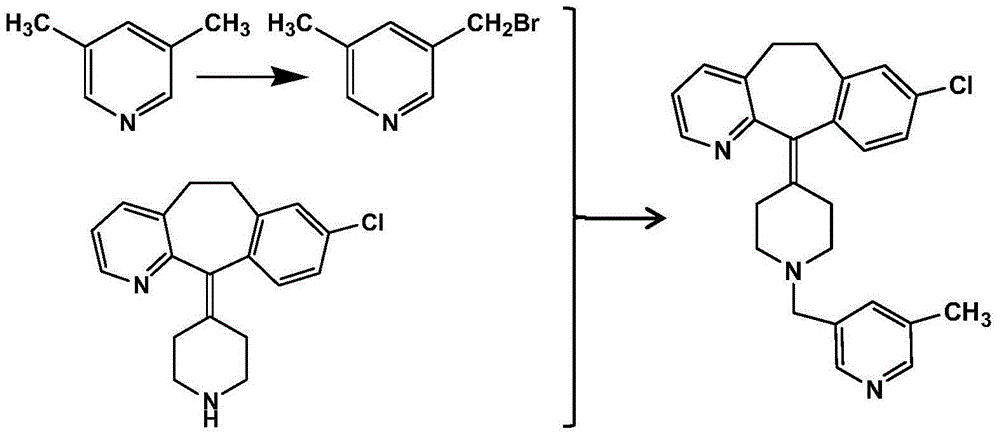
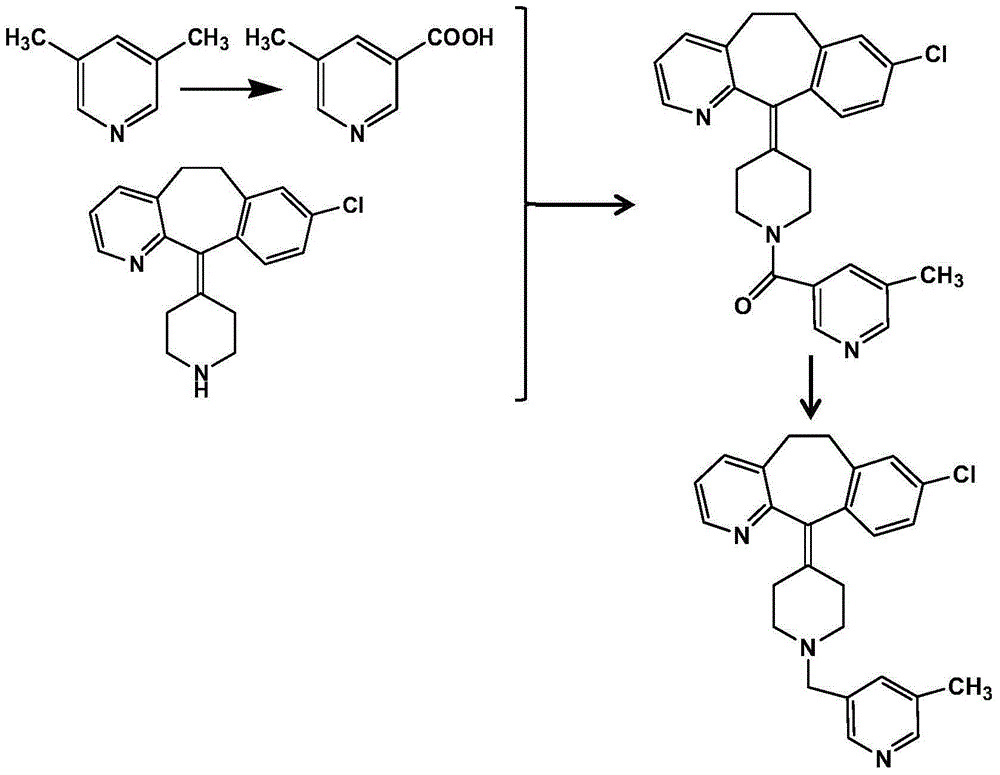
Preparation method of rupatadine fumarate
A kind of rupatadine fumarate and preparation technology, applied in the field of medicine and chemical industry, can solve the problems of long reaction time, complicated side reactions, large consumption of acid water, etc., and achieve the reduction of reagent consumption, shortening of reaction time, and the production of Effect of Yield Improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0037] Taking rupatadine amide (i.e. the amide compound 1) and borane tetrahydrofuran complex as an example: add 43g (0.1mol) rupatadine amide in a 1000ml three-necked bottle, and 400ml borane tetrahydrofuran complex ( 1mol / L), add 300ml of tetrahydrofuran, cool and stir under nitrogen protection. Slowly raise the temperature and stir overnight. After overnight, cool the solution to below zero. Start to add 9% HCl dropwise. After the drop is completed, concentrate until the tetrahydrofuran is evaporated to dryness. Add an equal volume of water, raise the temperature to 100°C and stir for 2 hours, turn off the heating, and stir overnight. The next day, add 800ml of ethyl acetate to the solution, stir to lower the temperature, and adjust the pH of the solution to 8-9 with 20% NaOH. Wash with water, combine the organic layers, add appropriate amount of activated carbon, silica gel and anhydrous magnesium sulfate to the organic layer, stir the reaction, filter to obtain the filtra...
example 2
[0039] Take rupatadine amide and borane tetrahydrofuran complex as an example: add 4.3g (0.01mol) rupatadine amide and 40ml borane tetrahydrofuran complex (1mol / L) into a 250ml three-necked bottle, and add 30ml of tetrahydrofuran, cooled and stirred under nitrogen protection. Slowly raise the temperature and stir overnight. After overnight, cool the solution to below zero. Start to add 9% HCl dropwise. After the drop is completed, concentrate until the tetrahydrofuran is evaporated to dryness. Add an equal volume of water, raise the temperature to 100°C and stir for 2 hours, turn off the heating, and stir overnight. The next day, add 80ml of ethyl acetate to the solution, stir to lower the temperature, and adjust the pH of the solution to 8-9 with 20% NaOH. Wash with water, combine the organic layers, add appropriate amount of activated carbon, silica gel and anhydrous magnesium sulfate to the organic layer, stir the reaction, filter to obtain the filtrate, concentrate to obta...
example 3
[0041]Take rupatadine amide, sodium borohydride and boron trifluoride as an example: add 43g (0.1mol) rupatadine amide and 18.9g (0.5mol) sodium borohydride to a 2000ml three-necked bottle, and then add 560ml tetrahydrofuran, cooled and stirred under nitrogen protection. Another 98ml of boron trifluoride ether solution (46.5% BF3) was added dropwise to the above-mentioned three-necked bottle within 10 minutes. 9% HCl, dropwise, concentrated until the tetrahydrofuran was evaporated to dryness, added an equal volume of water, heated to 100°C and stirred for 2 hours, then turned off the heating, stirred overnight, the next day, added 800ml ethyl acetate to the solution, stirred to cool down, and used 20% NaOH is used to adjust the pH of the solution to 8-9. After adjustment, let stand to separate layers, wash the water layer with ethyl acetate, wash the ethyl acetate layer with water, combine the organic layers, and add an appropriate amount of activated carbon, Silica gel and a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com