Liquid preparation for dosing nasal cavities and method for making the same
A liquid preparation and nasal administration technology, applied in the field of medicine, can solve the problems of poor patient compliance and slow onset of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
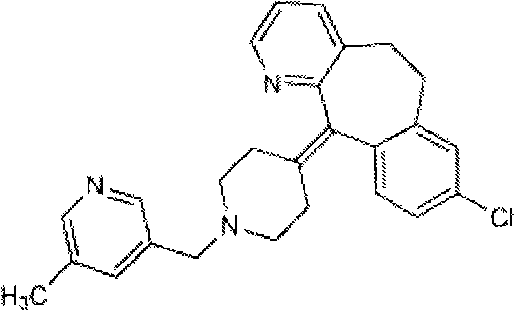
[0055] Example 1 Preparation of Rupatadine Nasal Spray
[0056] Main drug and excipient name dosage
[0057] Rupatadine 0.625g
[0058] Sulfobutyl ether-β-cyclodextrin 1.50g
[0059] 5% Benzalkonium Bromide 0.20mL
[0060] Appropriate amount of distilled water, and add to 100mL
[0061] Preparation method: Dissolve sulfobutyl ether-β-cyclodextrin and 5% benzalkonium bromide in about 90mL of water, then add rupatadine, dissolve it by ultrasonication, add distilled water to 100mL, micropores of 0.45μm Filtrate through a filter membrane and sterilize by circulating steam to obtain the medicinal liquid of rupatadine nasal spray, with a pH of 4.5-5.0. It is obtained by distributing the above-mentioned medicinal liquid into a multi-dose delivery device.
Embodiment 2
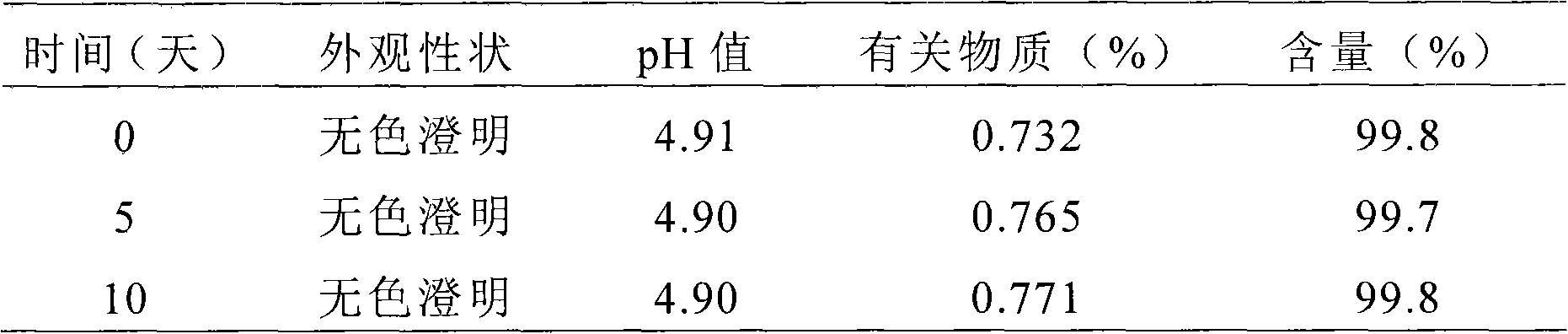
[0062] The preparation of embodiment 2 rupatadine nasal drops
[0063] Main drug and excipient name dosage
[0064] Rupatadine 25.0g
[0065] Chlorobutanol 0.50g
[0066] Sodium Glycocholate 0.05g
[0067] Potassium dihydrogen phosphate 0.56g
[0068] Sulfobutyl ether-β-cyclodextrin 30.0g
[0069] Potassium hydroxide appropriate amount, adjust pH to 4.5
[0070] Appropriate amount of distilled water, and add to 100mL
[0071] Preparation method: Put potassium dihydrogen phosphate, sodium glycocholate, and chlorobutanol in a beaker, add 90 mL of distilled water to dissolve, and adjust the pH to 4.5-5.0 with potassium hydroxide solution. Add sulfobutyl ether-β-cyclodextrin to the above solution and mix evenly, slowly add rupatadine and ultrasonically dissolve it, and add distilled water to 100 mL. Filter through a 0.45 μm microporous membrane and sterilize by circulating steam to obtain the medicinal solution of rupatadine nasal drops. It is obtained by distributing the ab...
Embodiment 3
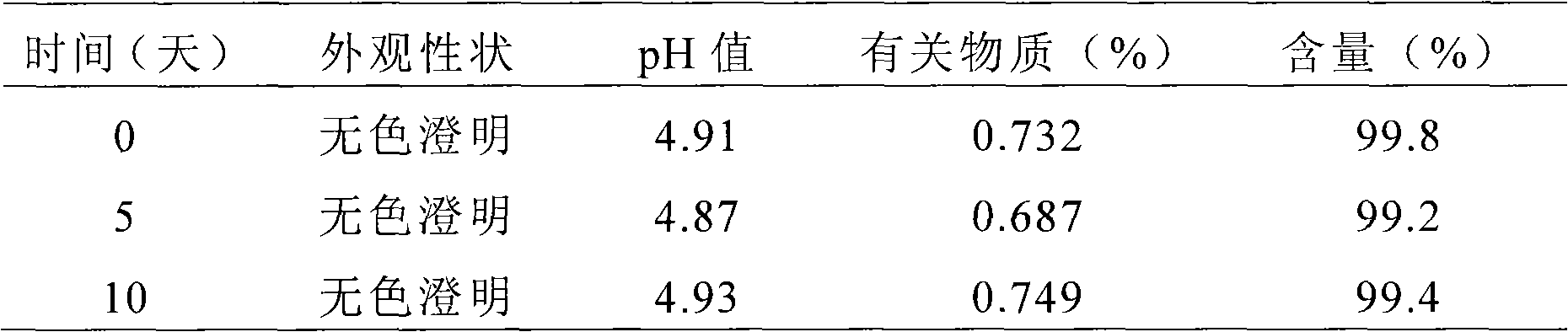
[0072] Example 3. Preparation of Rupatadine Nasal Spray
[0073] Main drug and excipient name dosage
[0074] Rupatadine 0.38g
[0075] Dimethyl-β-cyclodextrin 2.50g
[0076] Ethylparaben 0.50g
[0077] Distilled water to 100mL
[0078] Preparation method: Dissolve ethylparaben and dimethyl-β-cyclodextrin in 90mL water, then add rupatadine, dissolve it by ultrasonication, add distilled water to 100mL, filter through a 0.45μm microporous membrane, Sterilization by circulating steam to obtain the medicinal solution of rupatadine nasal spray, the measured pH is 4.5-5.0. It is obtained by distributing the above-mentioned medicinal liquid into a multi-dose delivery device.
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com