Preparation process of Rupatadine
A preparation process and solution technology, which is applied in the field of rupatadine preparation process, can solve the problems of low solubility, high cost, and low product yield, and achieve the effects of mild reaction conditions, low equipment requirements, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
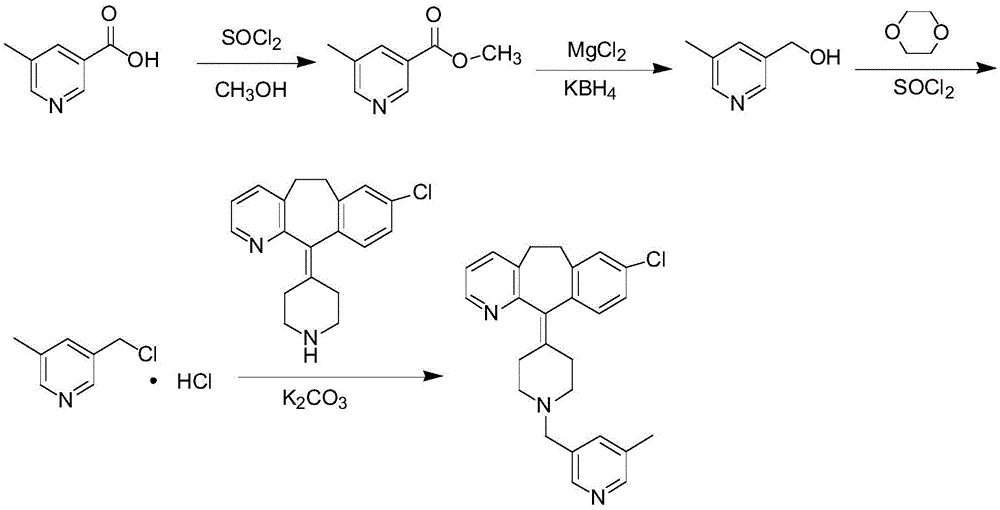
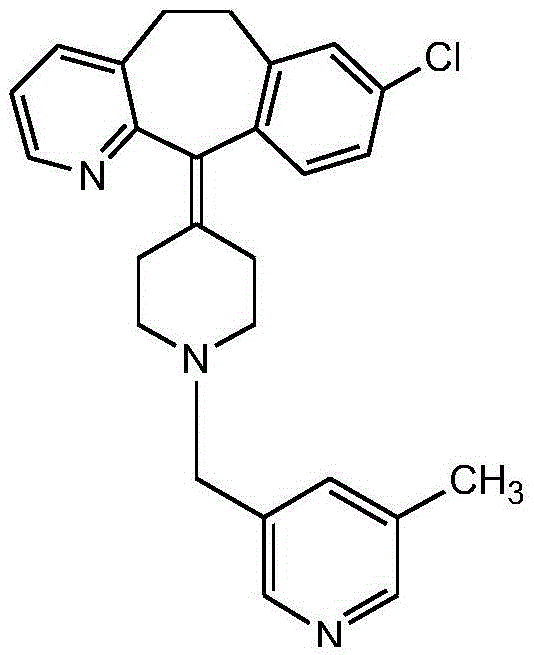
[0053] A preparation process for rupatadine, comprising the steps of:
[0054] S1. Preparation of methyl 5-methylnicotinate: mix 5-methylnicotinic acid and methanol evenly, add thionyl chloride dropwise, raise the temperature to 40°C, keep warm for 7.5h, keep stirring during the dropping and keeping warm, Adjust the temperature to 50°C, remove methanol and thionyl chloride by evaporation under reduced pressure, cool to room temperature, add ice water to obtain solution A; in an ice bath, adjust the pH of solution A to 9, add ethyl acetate for extraction, and obtain ethyl acetate Ester phase rotary evaporation obtains methyl 5-methylnicotinate;
[0055] S2. Preparation of 5-methyl-3-pyridinemethanol: mix magnesium chloride, potassium borohydride, and tetrahydrofuran, heat up to 65°C, reflux for 4.5 hours, and cool to room temperature to obtain solution B; Dissolve the methyl ester in tetrahydrofuran, adjust the temperature to 0°C, add solution B dropwise, keep warm for 1.5h, s...
Embodiment 2
[0059] A preparation process for rupatadine, comprising the steps of:
[0060] S1. Preparation of methyl 5-methylnicotinate: mix 5-methylnicotinic acid and methanol evenly, add thionyl chloride dropwise, heat up to 100°C, keep warm for 2.5h, and keep stirring during the process of dropping and keeping warm. Adjust the temperature to 60°C, remove methanol and thionyl chloride by evaporation under reduced pressure, cool to room temperature, add ice water to obtain solution A; in an ice bath, adjust the pH of solution A to 7, add ethyl acetate for extraction, and obtain ethyl acetate Ester phase rotary evaporation obtains methyl 5-methylnicotinate;
[0061] S2. Preparation of 5-methyl-3-pyridinemethanol: mix magnesium chloride, potassium borohydride, and tetrahydrofuran, heat up to 70°C, reflux for 0.5h, and cool to room temperature to obtain solution B; Dissolve the methyl ester in tetrahydrofuran, adjust the temperature to 80°C, add solution B dropwise, keep warm for 0.5h, sti...
Embodiment 3
[0065] A preparation process for rupatadine, comprising the steps of:
[0066] S1. Preparation of methyl 5-methylnicotinate: Mix 1 part of 5-methylnicotinic acid and 10 parts of methanol in molar parts, add 5 parts of thionyl chloride dropwise, and the addition of thionyl chloride is completed within 10 minutes , heat up to 90°C, keep warm for 3h, keep stirring during the dropwise addition and heat preservation, adjust the temperature to 58°C, remove methanol and thionyl chloride by evaporation under reduced pressure, cool to room temperature, add ice water to obtain solution A; , use ammonia water to adjust the pH of solution A to 7.5, add ethyl acetate to extract, add anhydrous sodium sulfate to the ethyl acetate phase to dry to a moisture content of 0.5wt%, filter, take the ethyl acetate phase and rotary evaporate to obtain 5-formaldehyde methyl nicotinate;
[0067] S2. Preparation of 5-methyl-3-pyridinemethanol: Mix 1 part of magnesium chloride, 1 part of potassium borohy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com