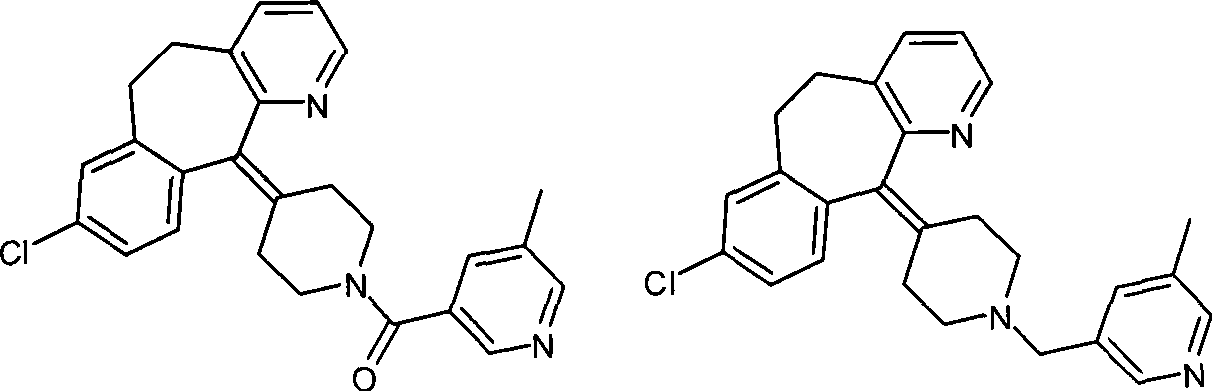
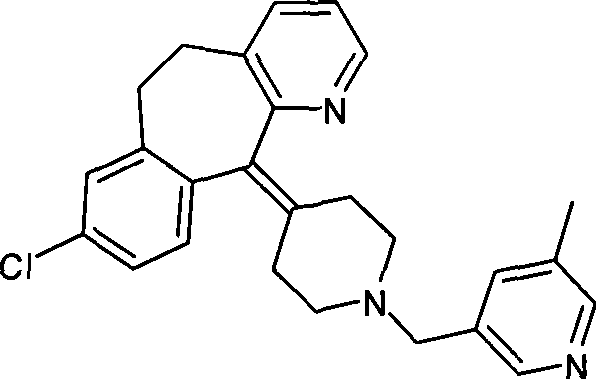
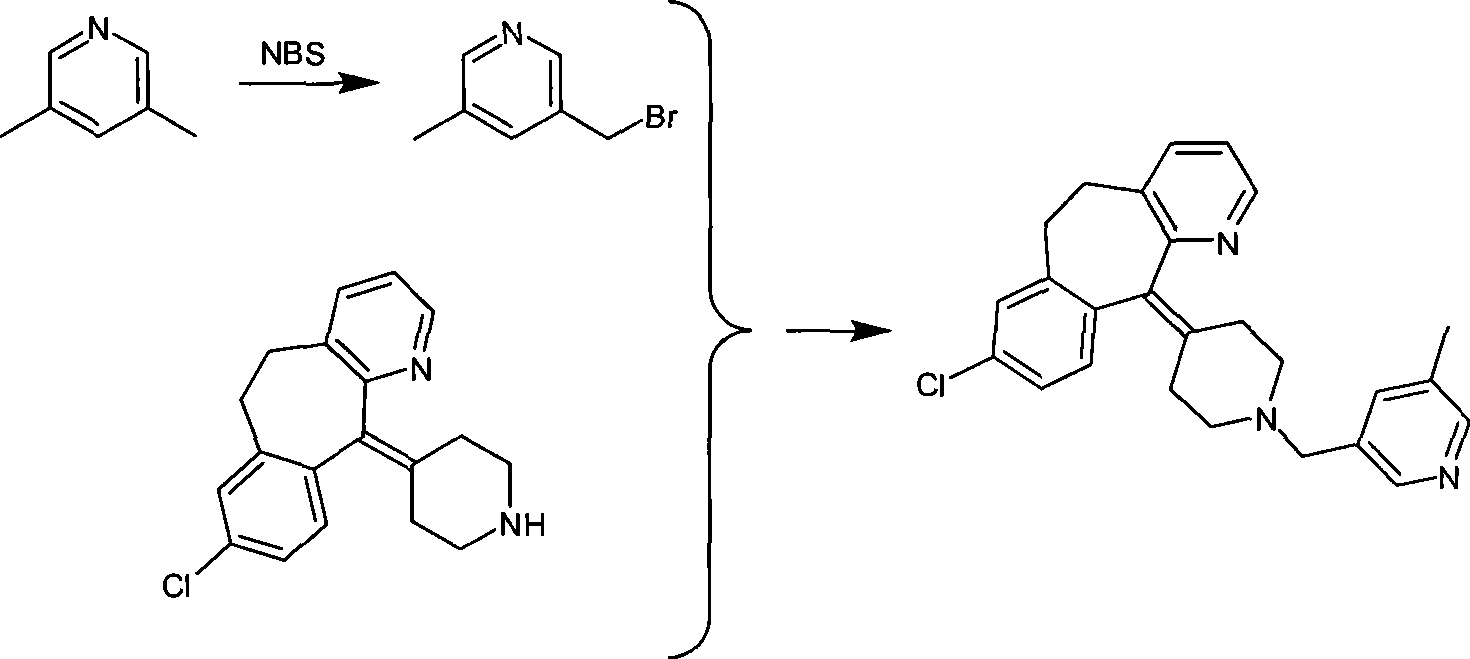
Preparation of rupatadine or salt thereof
The technology of a compound and acyloxy base is applied in the field of preparation of rupatadine or its salt, which can solve the problems of harsh operating conditions, long reaction time, expensive reagent sodium dihydroaluminate, etc., and achieve low production cost and high reaction rate. The effect of short time and cheap reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 3.78g (0.1 mole) of sodium borohydride to a 100ml single-necked round bottom flask, then add 50ml of tetrahydrofuran, place it on an ice bath, and stir it electromagnetically. Another 11.40 g of trifluoroacetic acid was dissolved in 10 ml of tetrahydrofuran to form a solution, and the solution was dropped into the above-mentioned flask within 30 minutes to prepare sodium trifluoroacetoxyborohydride. Get another 250ml single-necked round bottom flask, add 4.30g (0.01 moles) of the compound of formula I, add 10ml tetrahydrofuran again, electromagnetic stirring, the sodium trifluoroacetoxyborohydride that above-mentioned generation is added dropwise, then material temperature is raised to 30°C, keep for 2 hours. Cool the material to 0°C with an ice bath, slowly drop into 50ml of 10% hydrochloric acid solution, measure the pH to 1, then evaporate most of the tetrahydrofuran on a rotary evaporator, and precipitate a solid, then add 100ml of water, heat to reflux, and kee...
Embodiment 2
[0035]Add 4.30 g (0.01 mol) of the compound of formula I, 3.78 g (0.1 mol) of sodium borohydride, and 50 ml of tetrahydrofuran into a 250 ml single-necked round bottom flask, place it on an ice bath, and stir it electromagnetically. Another 11.40 g of trifluoroacetic acid was dissolved in 10 ml of tetrahydrofuran to form a solution. The solution was dropped into the above-mentioned flask within 30 minutes, and then the temperature of the material was raised to 30° C. and kept for 2 hours. Cool the material to 0°C with an ice bath, slowly drop into 50ml of 10% hydrochloric acid solution, measure the pH to 1, then evaporate most of the tetrahydrofuran on a rotary evaporator, and precipitate a solid, then add 100ml of water, heat to reflux, and keep For 10 minutes, dissolve the precipitated solid, cool to 0°C, add 6 mol / L sodium hydroxide solution until the pH value reaches about 8, extract with ethyl acetate, then dry with a desiccant, evaporate the solvent to obtain Lu Patadine...
Embodiment 3
[0037] Carry out in the same manner as in Example 2, the difference is that the acyloxy alkali metal borohydride is potassium trifluoroacetoxy borohydride, the aprotic solvent is diethyl ether, and the reaction temperature is the heating reflux temperature of diethyl ether, heating The reflux time was 1.5 hours, and 3.01 g of rupatadine product was obtained with a yield of 72.36%. HPLC purity 97.9%. The melting point is 58-60 degrees.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com