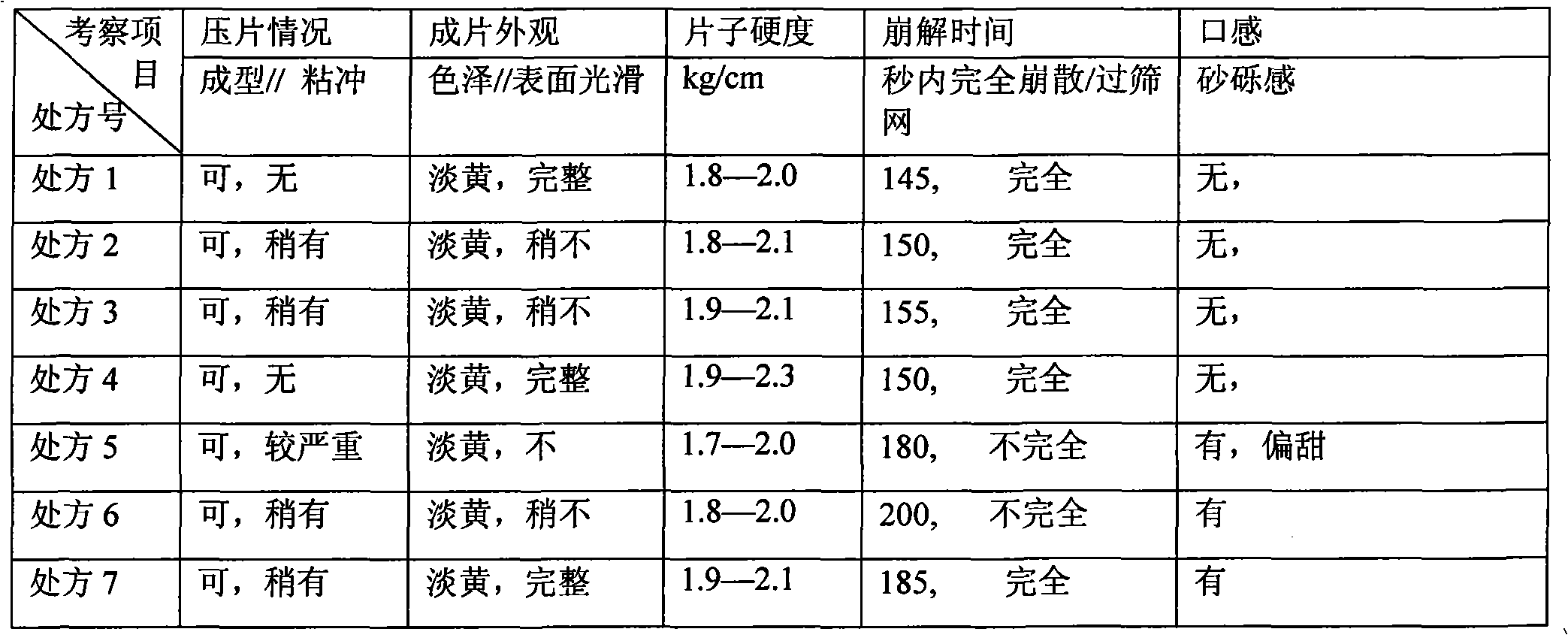
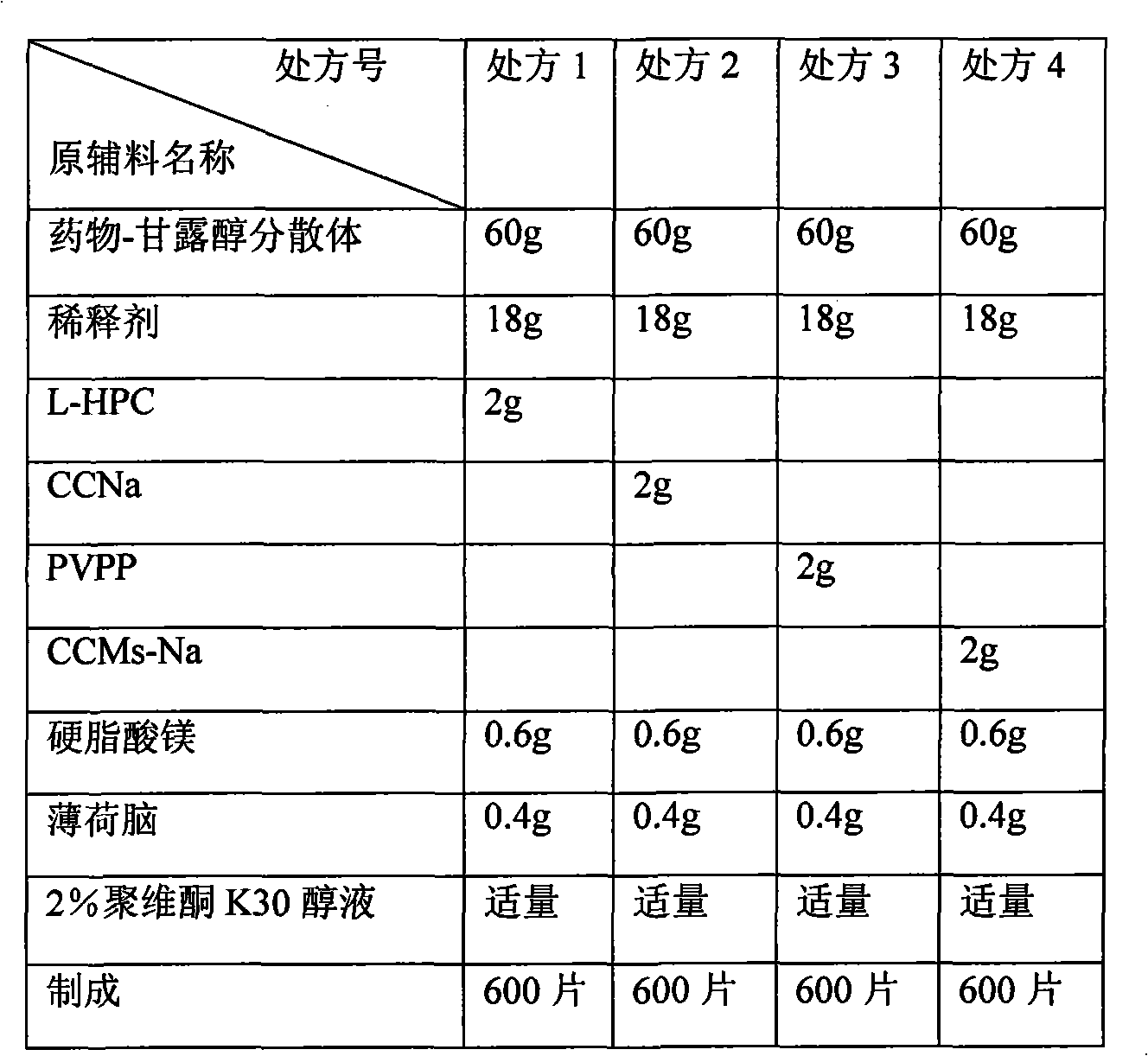
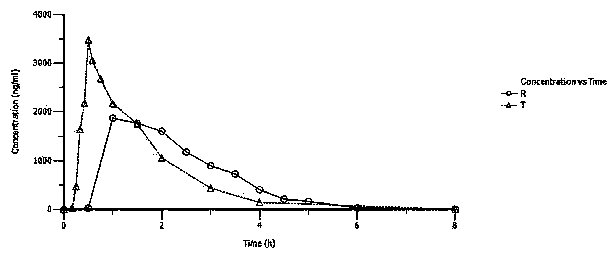
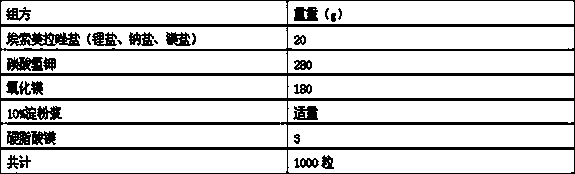
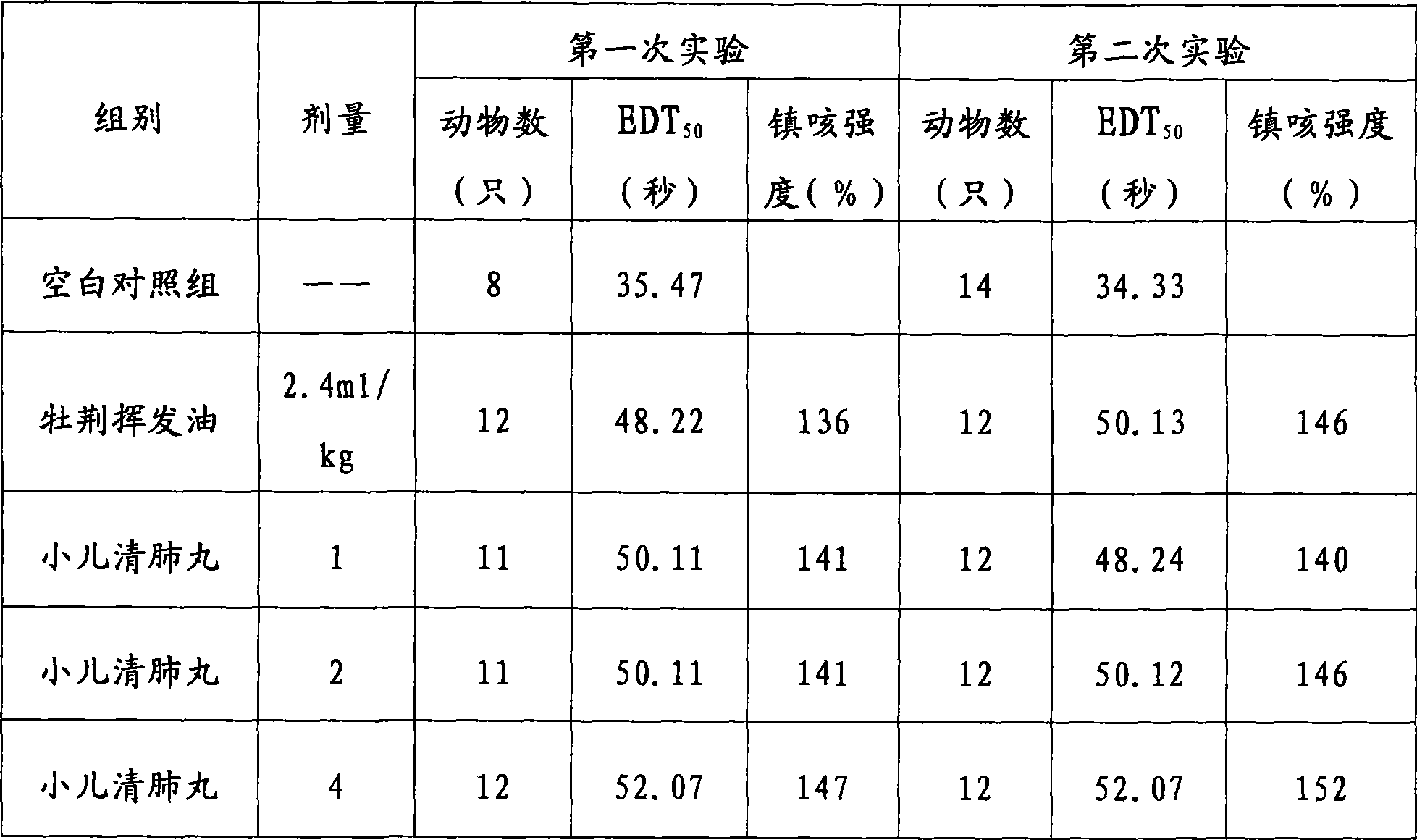
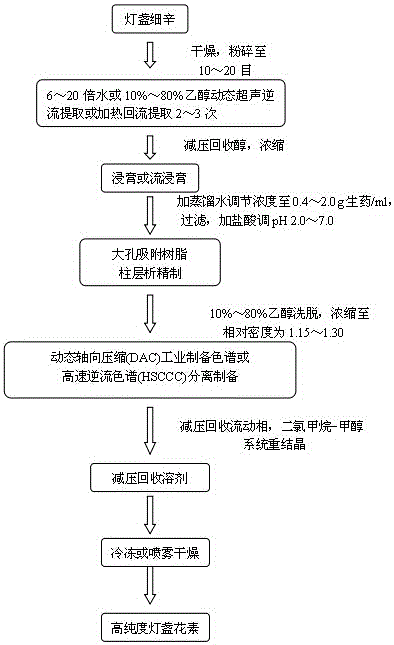
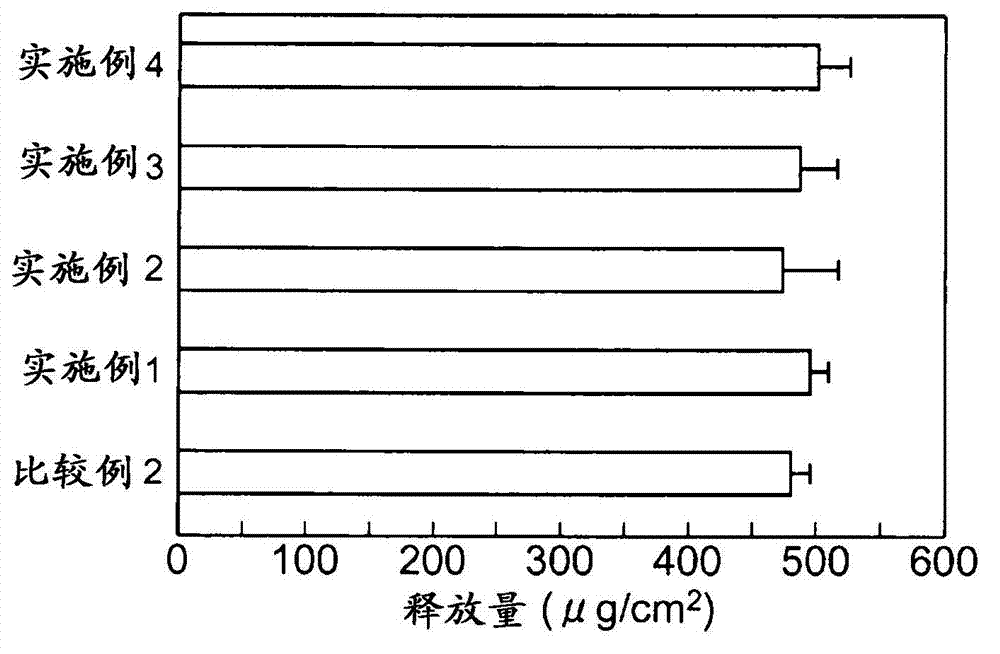
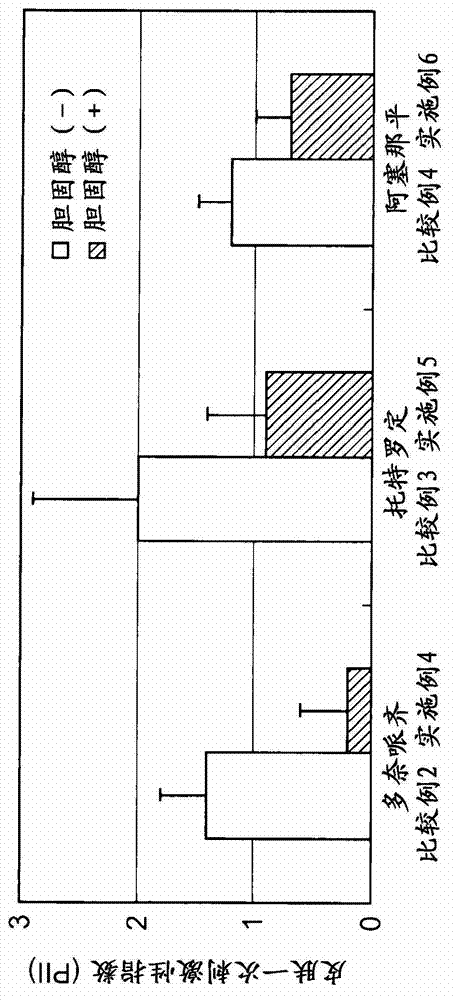
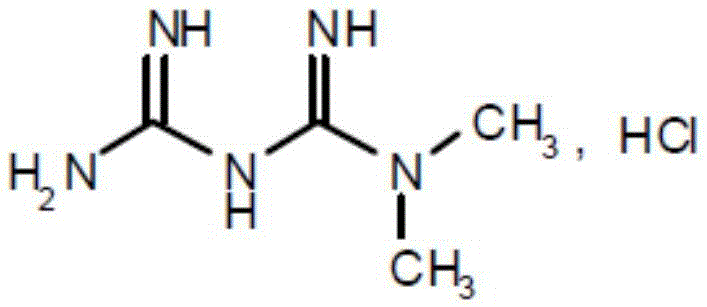
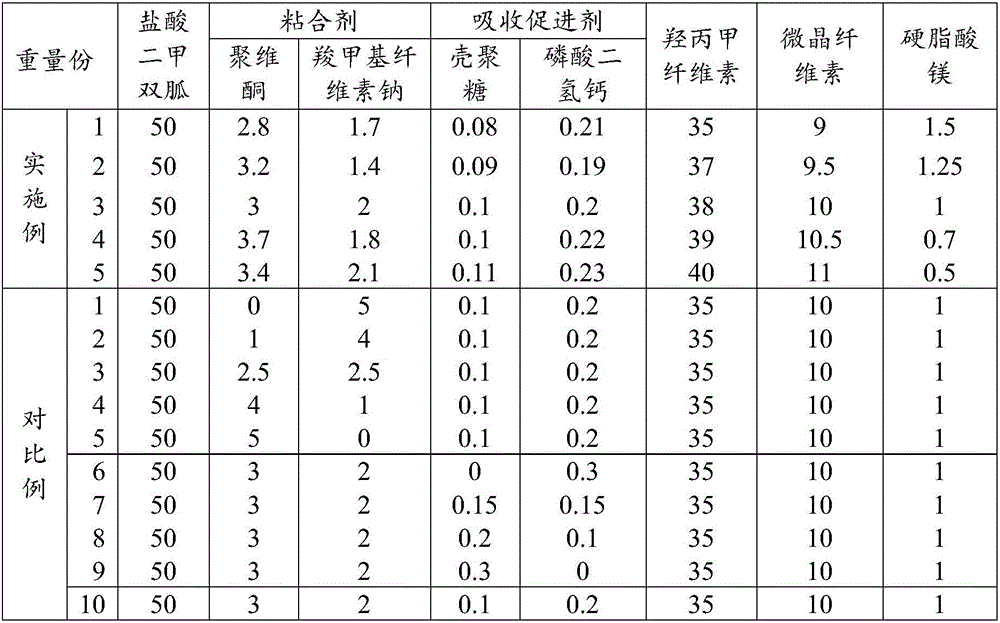
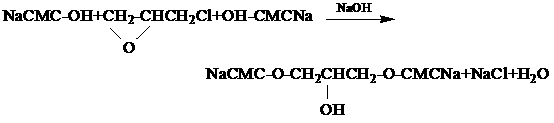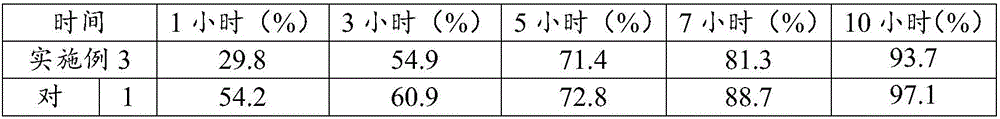Patents
Literature
407 results about "Pharmaceutical Adjuvants" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hyperin extraction method and preparation and use thereof
InactiveCN101386634AMeet the requirements of different dosage formsGuarantee product qualityOrganic active ingredientsSugar derivativesFreeze-dryingSolvent
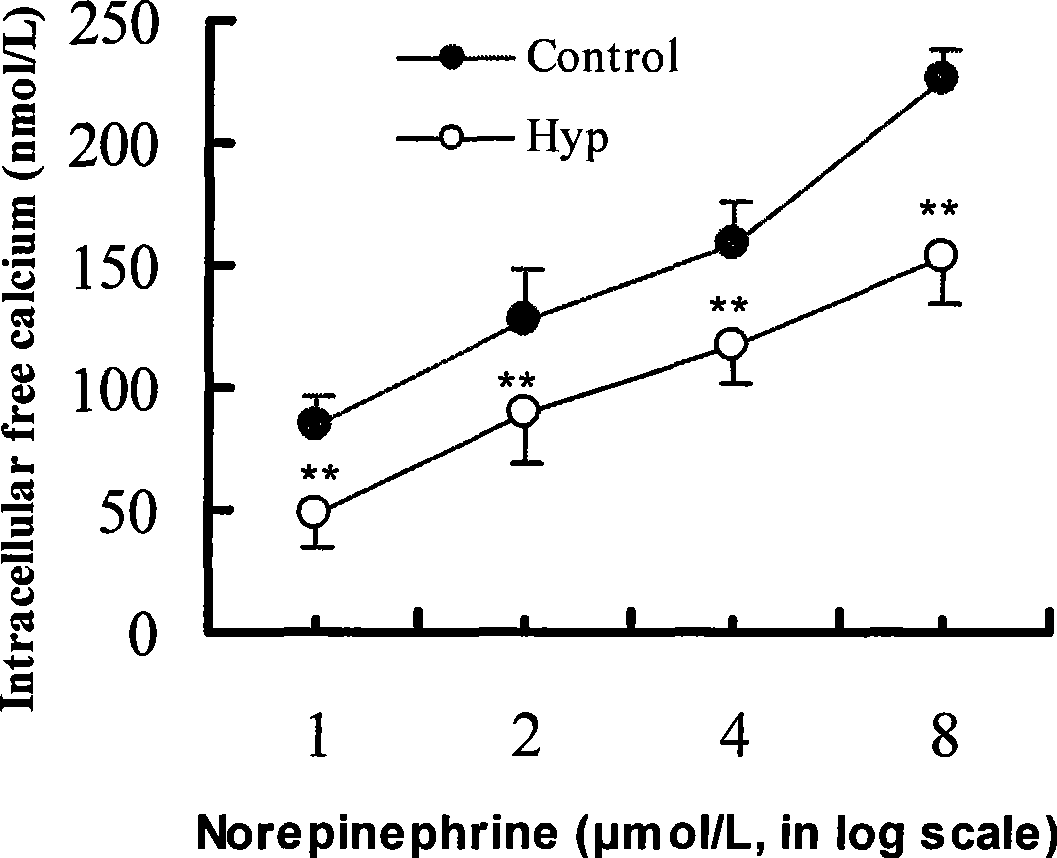
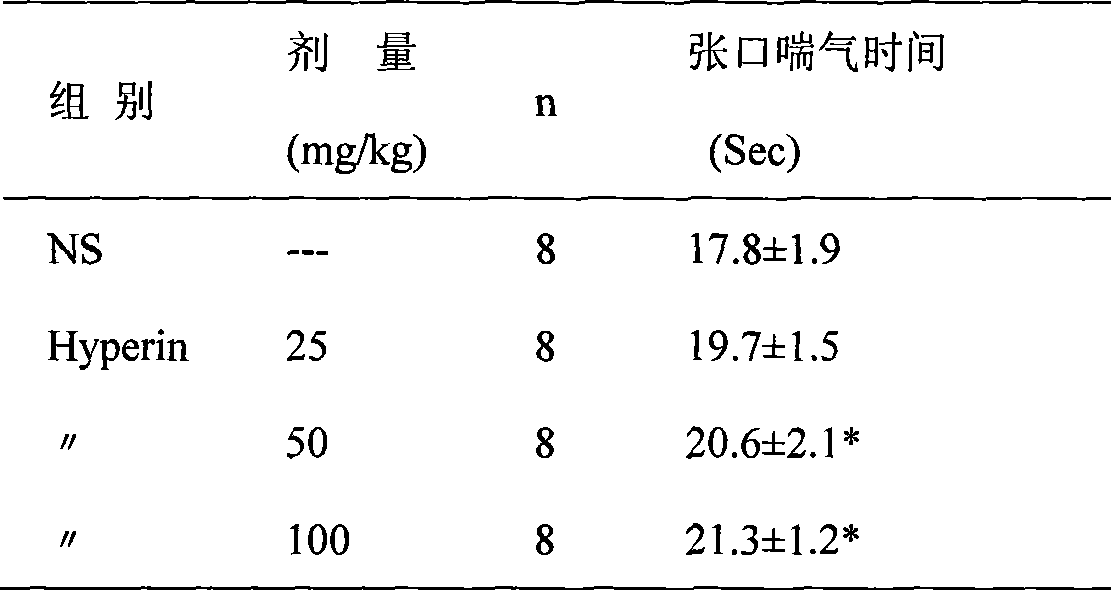
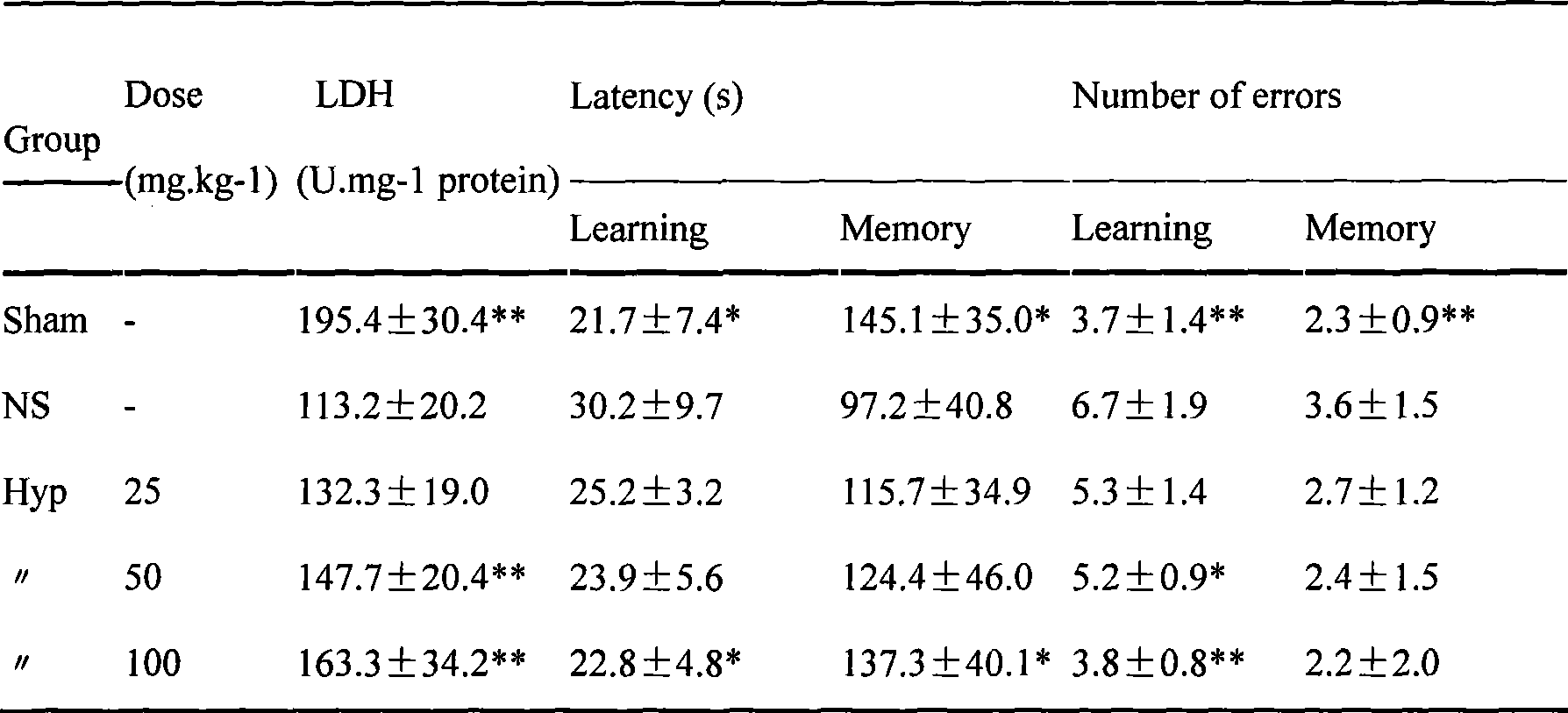
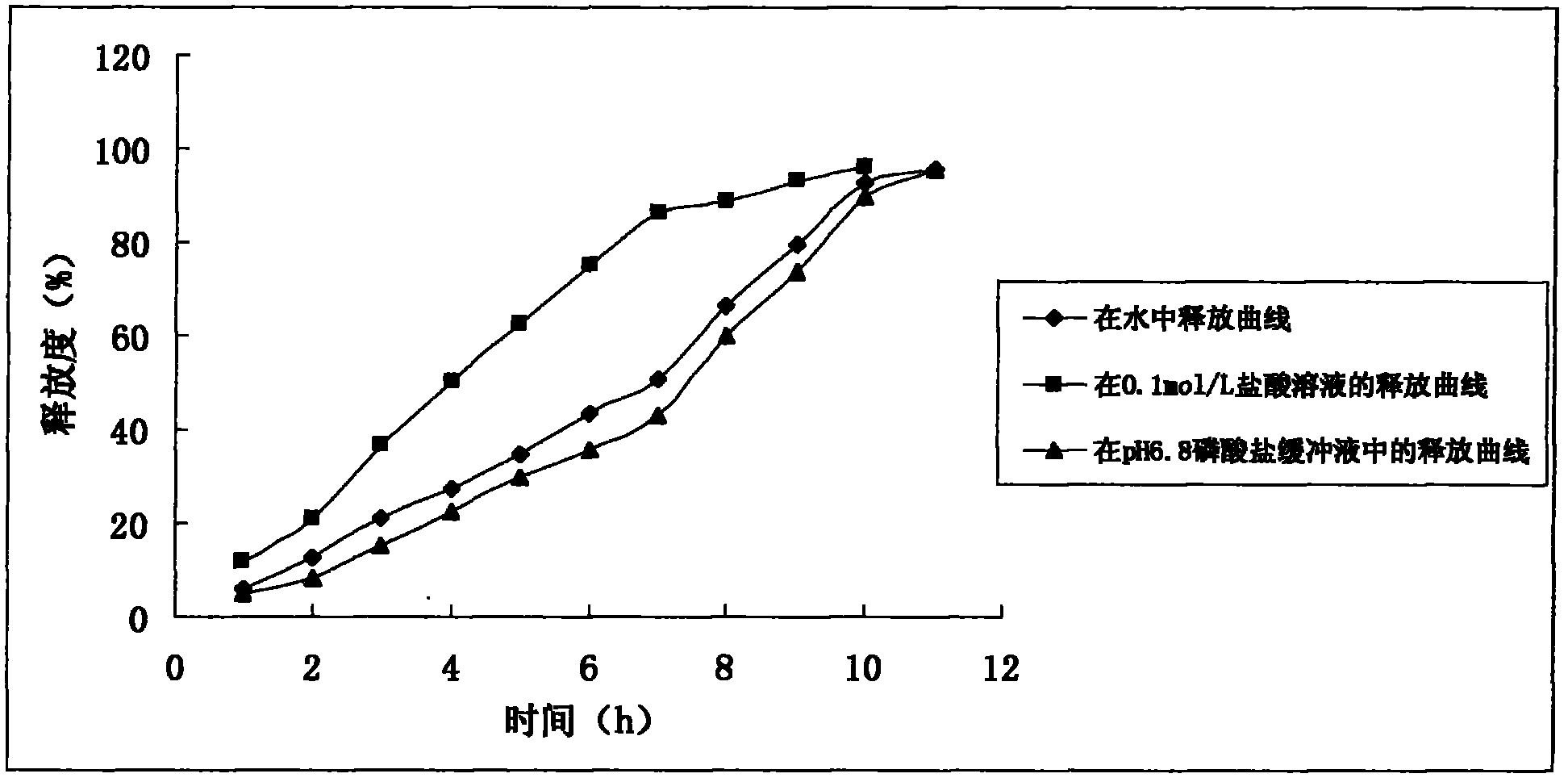
A method for extracting hyperin comprises the following steps: a crude product of an extracting solution of a maniod eibish flower is obtained through extracting the concentrated solution of a maniod eibish flow ethanol extracting solution by normal butanol or ethyl acetate or through the column chromatography of an HPD100 or HPD600 macroscopic absorbent resin; and then hyperin with the content of between 90 and 98 percent in weight percentage is obtained through purification and recrystallization by using a solvent. Hyperin preparations comprise freeze-dried powder injection, liquid drugs injection, transfusions, dropped pills, tablets, capsules, or soft capsules prepared by using hyperin as an active component and pharmaceutical adjuvant. The hyperin is used for preparing the medicines for treating cerebral ischemia.
Owner:周春晓 +1
Plant cellulose hard empty capsule preparation method
ActiveCN103301086AStable molecular structureGood film formingPharmaceutical non-active ingredientsCapsule deliveryCelluloseFiber
The invention provides a plant cellulose hard empty capsule preparation method, and belongs to the technical field of pharmaceutical adjuvants. The plant cellulose is obtained from plant derivatives such as pine trees, purified cotton and the like. Prepared plant cellulose hard empty capsules comprise colorless and transparent hard empty capsules, colored hard empty capsules and light-shielding hard empty capsules. The preparation method comprises steps of: mixing the plant cellulose, a surfactant, a coagulant aid, a coloring agent and water, forming and drying. The plant cellulose hard empty capsule has performance stability, low water content and no interference with human body metabolism, and is green, environment-friendly, safe and suitable for being filled with various contents.
Owner:SHAOXING KANGKE CAPSULE
Cinobufotalin lyophilized powder for injection and its preparation method
A cinobufotalin freeze dried injection and preparation process thereof are disclosed. It is prepared by cinobufotalin effective part and pharmaceutical adjuvant extracted from dry toad skin. The effective part of cinobufotalin is obtained by alcohol extraction, centrifugation and macropore polymeric adsorbent separation and purification. Wherein, the main effective component indole total alkaloid content is over than 30%. The content assay result and pharmaceutical examination result indicates that the cinobufotalin freeze dried injection has a higher content of effective part and stronger pharmaceutical function.
Owner:张平
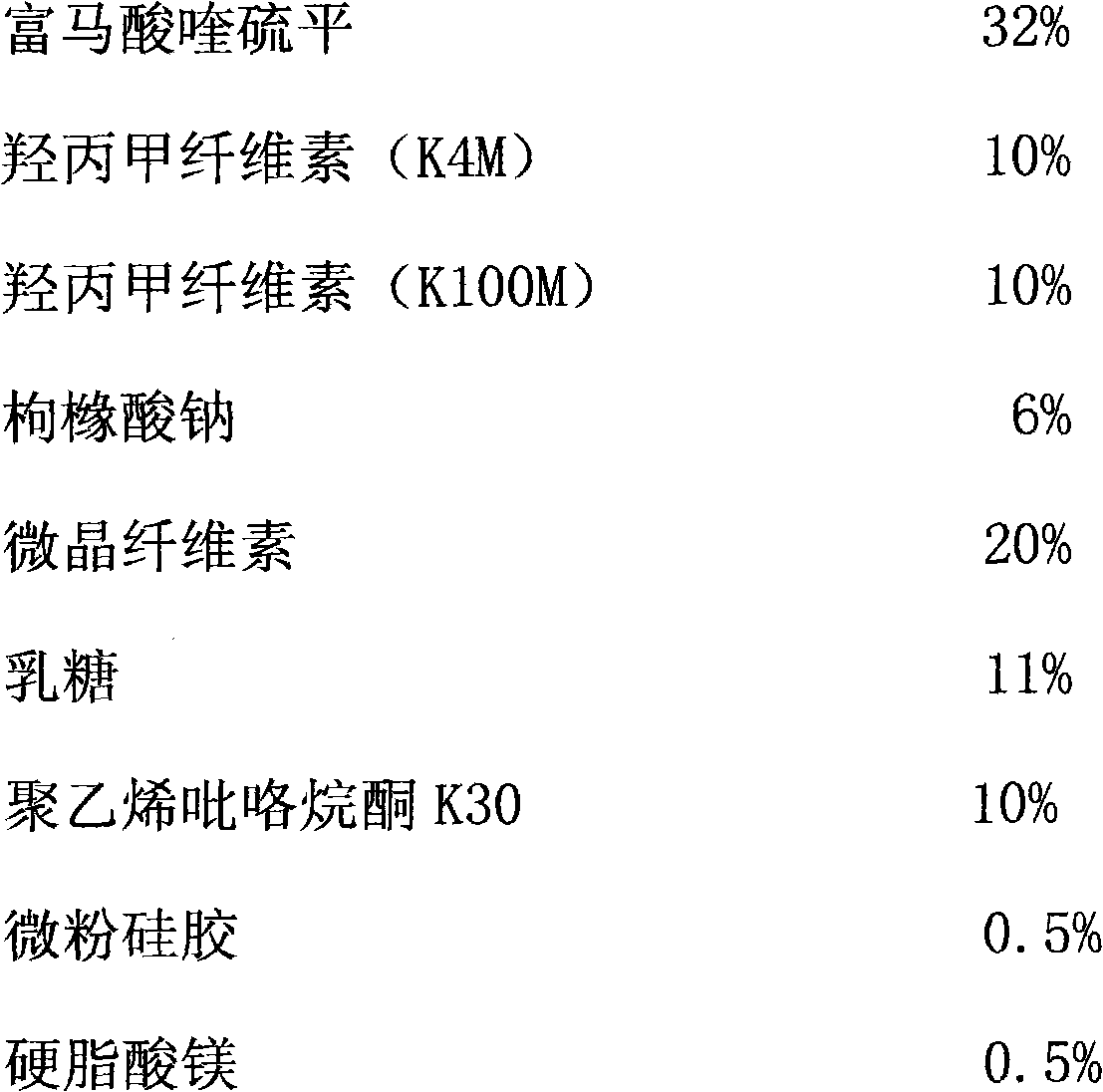
Sustained release tablet of quetiapine fumarate composition and preparation method of sustained release tablet
InactiveCN102218042AReduce weightIncrease toleranceOrganic active ingredientsNervous disorderSustained Release TabletOrganic acid
The invention discloses a sustained release tablet of a quetiapine fumarate composition, comprising the following components in percentage by weight: 25 to 40% of quetiapine fumarate, 2 to 8% of organic acid salt, 5 to 30% of a sustained release material and the balance of other pharmaceutical adjuvants, wherein the sustained release material is K type hydroxypropyl methylcellulose. The sustained release material is the K type hydroxypropyl methylcellulose and the organic acid salt is added, therefore, a stable sustained release skeleton can be formed by few sustained release materials, the raw material can be saved, the weight of unit preparation can be reduced, and the problem of difficulty in swallowing caused by large size of the preparation can be released and / or solved; the hydroxypropyl methylcellulose with different viscosities are used as the material of the skeleton so that the preparation can reach formulated sustained release effects and has the characteristics of strong controllability and stability in storage. By use of the preparation method capable of granulating in one step, the operation is simplified and the production efficiency can be improved.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Chinese medicinal composition for treating digestive system diseases and preparation method thereof
The invention provides a Chinese medicinal composition for treating digestive system diseases, which is mainly prepared from Chinese medicinal materials such as phellodendron, spreading hedyotis herb, dried ginger, seabuckthorn and the like according to a certain weight ratio. The Chinese medicinal composition can be prepared into any common oral preparation with appropriate pharmaceutical adjuvants according to the conventional preparation process. The Chinese medicinal composition has the effects of clearing heat and detoxicating and subdhing swelling and stopping ulcer. Clinical observation indicates that the Chinese medicinal composition can be widely used for treating the digestive system diseases, and particularly has a good treatment effect on peptic ulcer.
Owner:HUNAN JIUDIAN PHARMA
Enzalutamide soft capsule and preparation method thereof
ActiveCN104857517AHigh dissolution rateImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsPolyethylene glycolBHA - Butylated hydroxyanisole
The invention discloses an enzalutamide soft capsule and a preparation method thereof. The enzalutamide soft capsule comprises capsule content and a capsule shell, wherein the capsule content comprises enzalutamide and pharmaceutical adjuvants; the capsule shell is formed by gelatin, glycerin, sorbitol, titanium dioxide, purified water and the like. The soft capsule can be prepared with the method comprising following steps: Labrasol, butylated hydroxyanisole, butylated hydroxytoluene and enzalutamide are mixed to obtain the capsule content material of the soft capsule; the gelatin, the glycerin, the sorbitol, titanium dioxide and the purified water are mixed to obtain the capsule shell material of the soft capsule; the capsule content material and the capsule shell material of the soft capsule are pelleted on a soft capsule making machine to obtain the enzalutamide soft capsule. The enzalutamide soft capsule and the preparation method have the following advantages: the soft capsule is convenient to administrate and carry, the drug stability is good, effective components are dissolved quickly, and the bioavailability is high.
Owner:NANJING HEALTHNICE MEDICAL TECH +1
Preparation of walnut shell extract and applications thereof in preparations of cardiotonic medicine and cardio-cerebrovascular medicine
The invention discloses a preparation method for extracting ethanol from an extract of a natural walnut shell, a pharmaceutical composition using the extract as the active component and research results of relevant pharmacology. A walnut shell is ground and boiled for extracting the ethanol and then is concentrated and dried for obtaining the brown red extract. The extracting method is characterized by short process, relatively high extracting rate and is easy for industrialized production. The discovery of the medical value of the walnut shell and the development of the product can prevent great waste of natural pharmaceutical resources. The research of the pharmacological activity shows that the walnut shell extract has a certain activity for strengthening heart and improving myocardial blood supply and is possible to be developed into a new pharmaceutical of natural traditional Chinese medicine for curing cardio-cerebrovascular disease, having a plurality of pharmacological activities, low toxicity and high efficiency. Various oral preparations of different pharmaceutical compositions can be prepared by mixing the extract of effective curing amount with pharmaceutical adjuvant.
Owner:INST OF RADIATION MEDICINE CHINESE ACADEMY OF MEDICAL SCI +1
Vildagliptin composition
ActiveCN104161752AImprove stabilityExcellent Tablet PropertiesMetabolism disorderPharmaceutical non-active ingredientsMagnesium saltDiluent
The invention relates to a vildagliptin composition. Specifically the composition comprises vildagliptin, (a) at least one medicinal diluent, (b) at least one disintegrating agent, and (c) lubricant, optionally comprises other pharmaceutical adjuvant, and does not use fatty acid magnesium salts as the lubricant. The composition has a better stability and drug dissolution.
Owner:JIANGSU HANSOH PHARMA CO LTD
Pain relieving bilayer controlled-release tablet and preparation method thereof
ActiveCN103655505AImprove physical stabilityTightly boundAntipyreticAnalgesicsSide effectBlood concentration
The invention provides a pain relieving bilayer controlled-release tablet which comprises a quick release layer and a slow release layer, wherein holes are formed in the slow release layer; the holes are filled with quick release particles; the quick release layer and the quick release particles consist of pain relieving drugs and pharmaceutical adjuvant; the slow release layer consists of pain relieving drugs, slow release materials and pharmaceutical adjuvant. The pain relieving bilayer controlled-release tablet has the following technical effects: 1) the bilayer tablet has better physical stability than a common bilayer tablet, so that the storage and the transportation are convenient; 2) the disintegration time limit of the quick release layer of the bilayer controlled-release tablet is 10-30 seconds through the detection of the dissolving experiment; the slow release layer presents the zero-level release mode; the medicine taking effectiveness and safety of patients are largely improved. In the preparation process of the bilayer tablet, the quick release particles are filled in the holes. The quick disintegration of the quick release layer after the medicine taking is guaranteed through the drug release mode of combining the quick release with the slow release, so that the blood concentration can quickly achieve the range of a therapeutic window; the slow release layer is slowly released in a longer time period to continuously maintain the treatment effect; the toxic or side effects are effectively controlled.
Owner:越洋医药(广州)开发有限公司
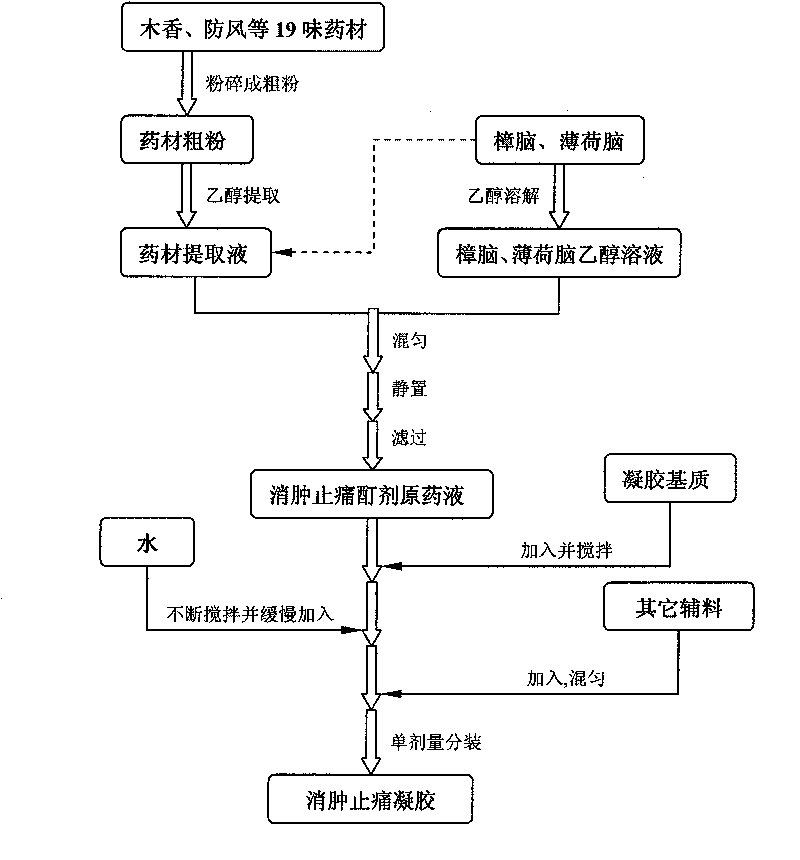
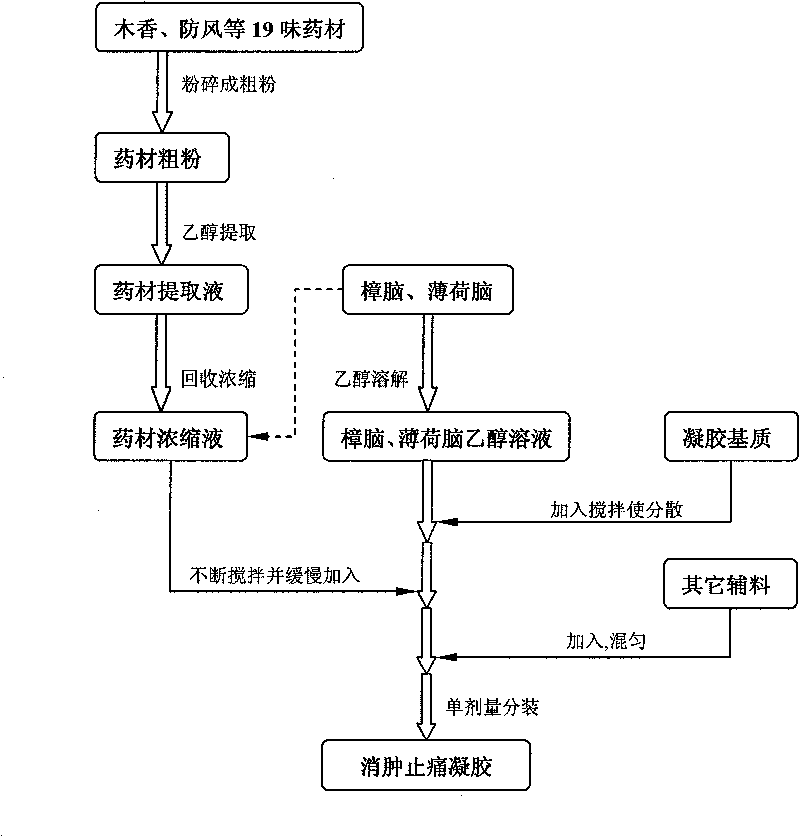
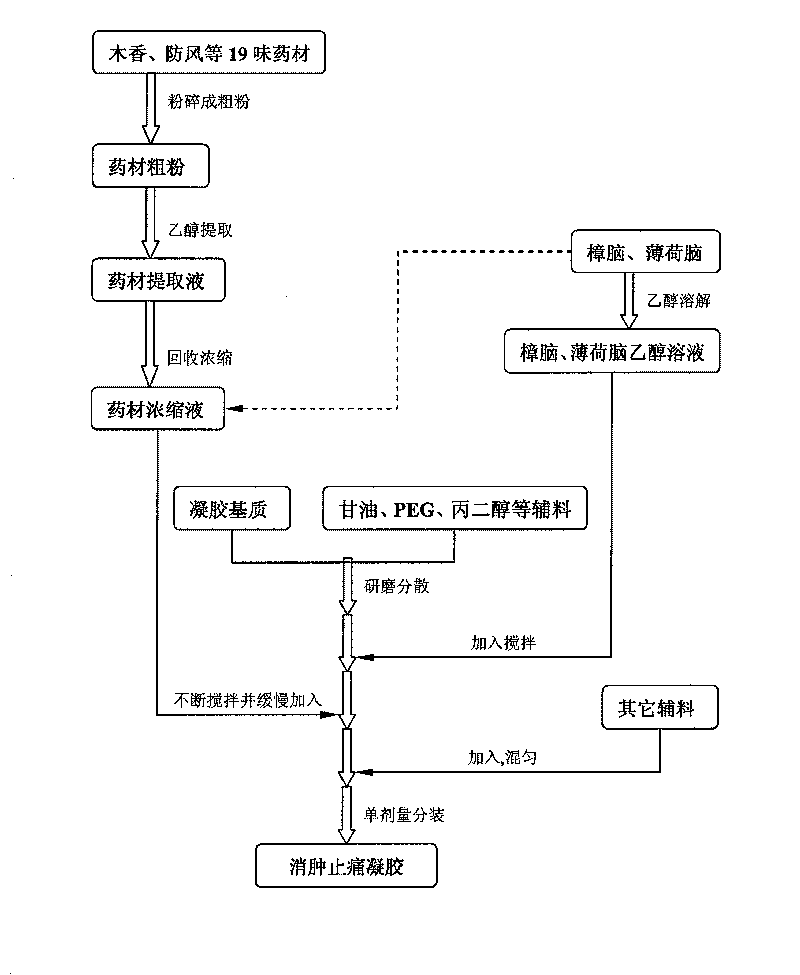
Swelling reducing and pain easing gel and preparation method thereof
InactiveCN101757522AReduce manufacturing costReduce dosageOrganic active ingredientsAntipyreticRheumatismPharmaceutical Adjuvants
The invention relates to swelling reducing and pain easing gel and preparation method thereof. The gel mainly contains swelling reducing and pain easing tincture liquid medicine, gel substrate and other pharmaceutical adjuvant and has the characteristics of small dosage of the adjuvant and good stability. The gel acts locally, has the functions of stimulating blood circulation and causing the muscles and joints to relax, reducing swelling and easing pain, and is used for curing injuries from falls, rheumatism, unknown swelling and toxin, and parotitis swelling. The gel has the advantages of long dwell time, durable effect, simple formulation process, low production cost and strong operability in industrial production.
Owner:CHONGQING PHARMA RES INST
Sildenafil citrate sublingual tablet and preparation method thereof
The invention discloses a sildenafil citrate sublingual tablet and a preparation method thereof. The sildenafil citrate sublingual tablet is prepared from 1 part by weight of sildenafil citrate and 4 to 8 parts by weight of pharmaceutical adjuvant , wherein the pharmaceutical adjuvant comprises 2 to 4 percent of disintegrant, 94 to 96 percent of diluter, 0.5 to 1.2 percent of lubricator, 0.3 to 1 percent of corrective and 0.1 to 0.2 percent of binder, which are based on the total weight of the pharmaceutical adjuvant. Test results show that the prepared sublingual tablet has shorter disintegration time, higher dissolving speed in vitro and higher body bioavailability than the conventional tablets.
Owner:张睿
Cefixime compound and pharmaceutical composition thereof
InactiveCN103193798AUniform particle size distributionWell mixedAntibacterial agentsOrganic active ingredientsDrug compoundPharmaceutical Adjuvants
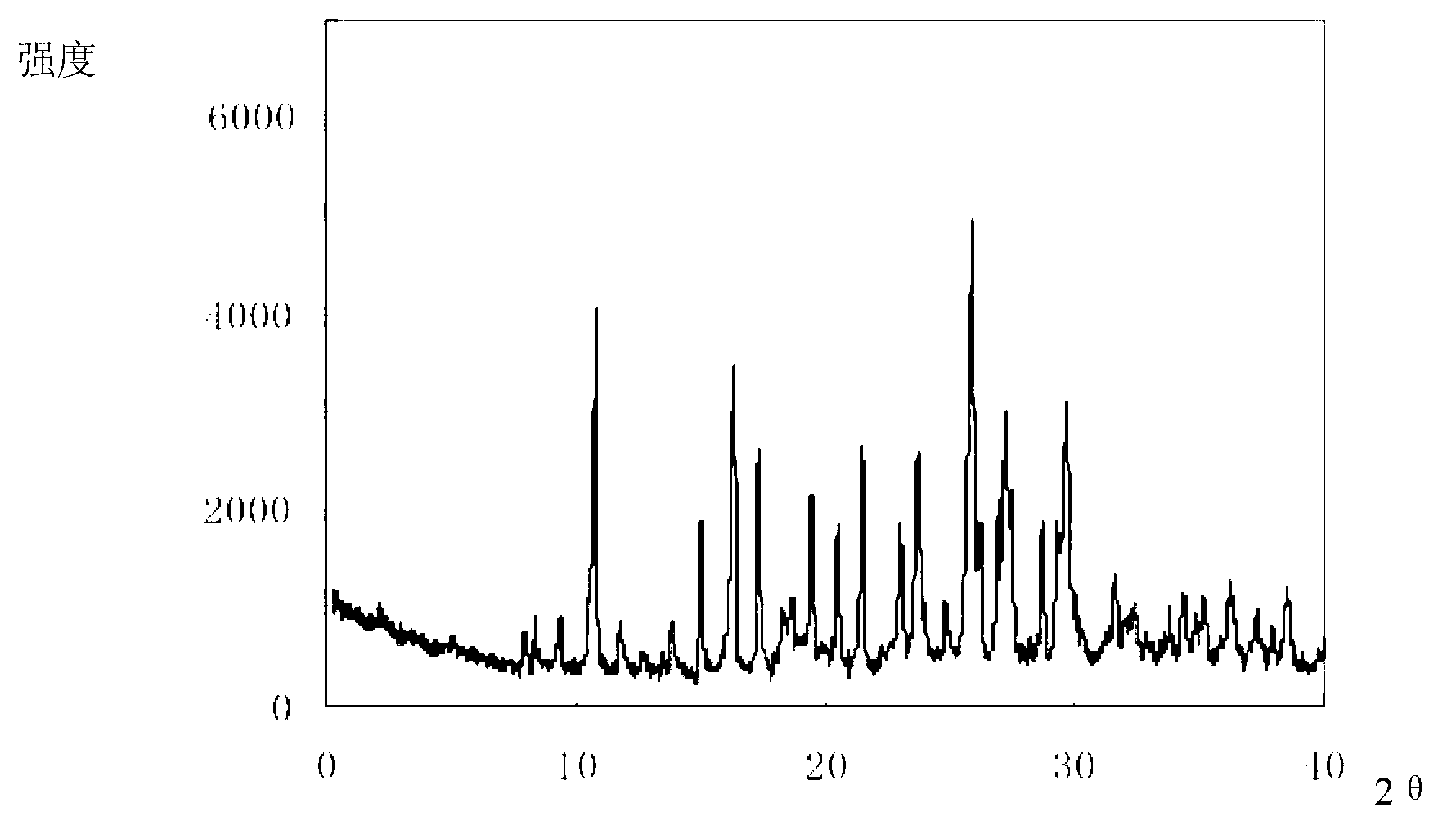
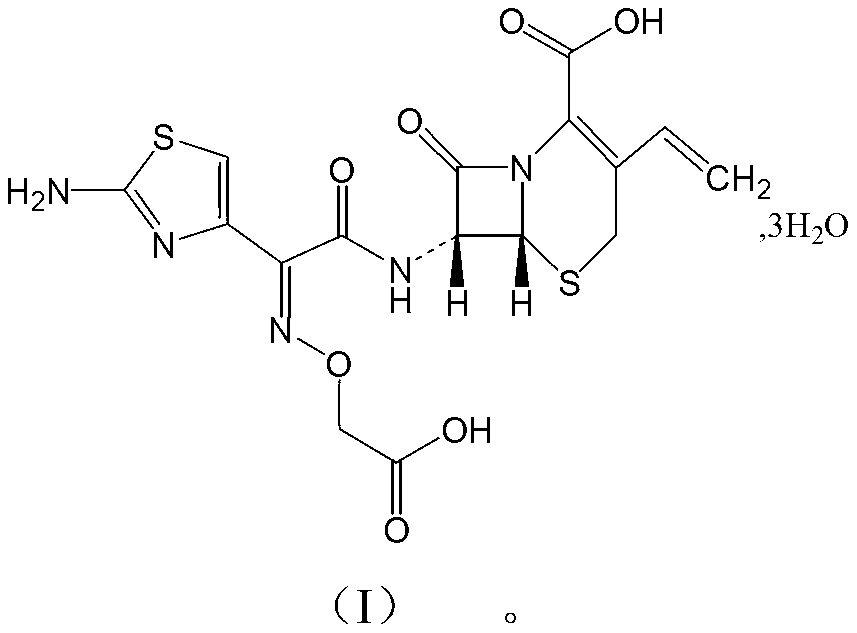
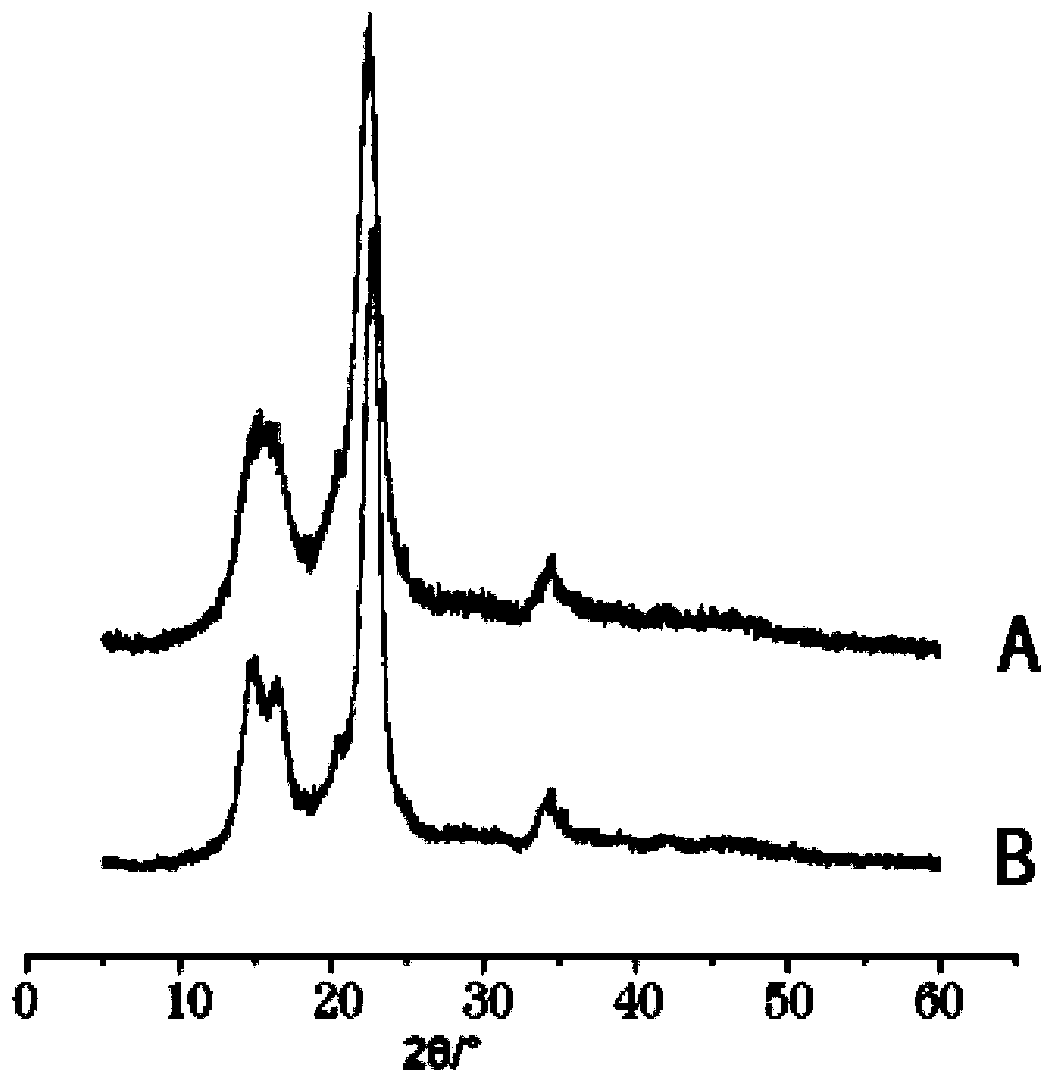
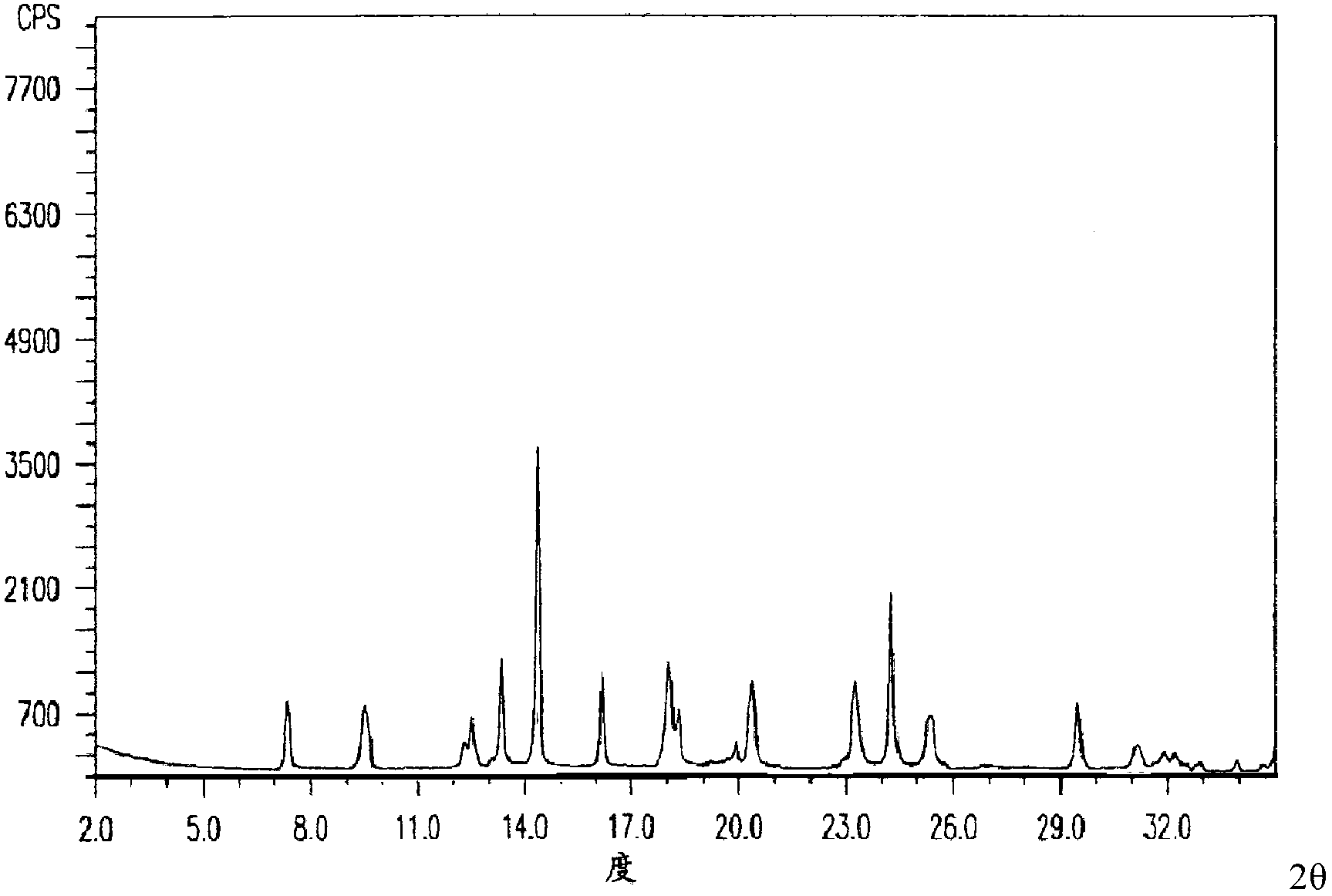
The invention relates to a drug compound, and particularly relates to a cefixime compound. An X-ray powder diffraction pattern of the cefixime compound measured through a Cu-K alpha ray is shown in a figure I. The invention also relates to a pharmaceutical composition containing the cefixime compound. The pharmaceutical composition comprises the cefixime compound and a pharmaceutical adjuvant; the pharmaceutical composition is an oral preparation including an oral normal release preparation and a controlled release preparation; and the oral normal release preparation is selected from a tablet, an enteric-coated tablet, a capsule, a dispersible tablet, dry suspension, a chewable tablet or granules. The cefixime compound disclosed by the invention is high in purity, high in bioavailability and suitable for clinical application.
Owner:四川省惠达药业有限公司
Method for processing traditional Chinese medicinal ultramicro wall-broken oral tablet slices
InactiveCN102772444AReduce sulfur dioxideReduce pesticide residuesInorganic active ingredientsUnknown materialsMeal powderMedicinal herbs
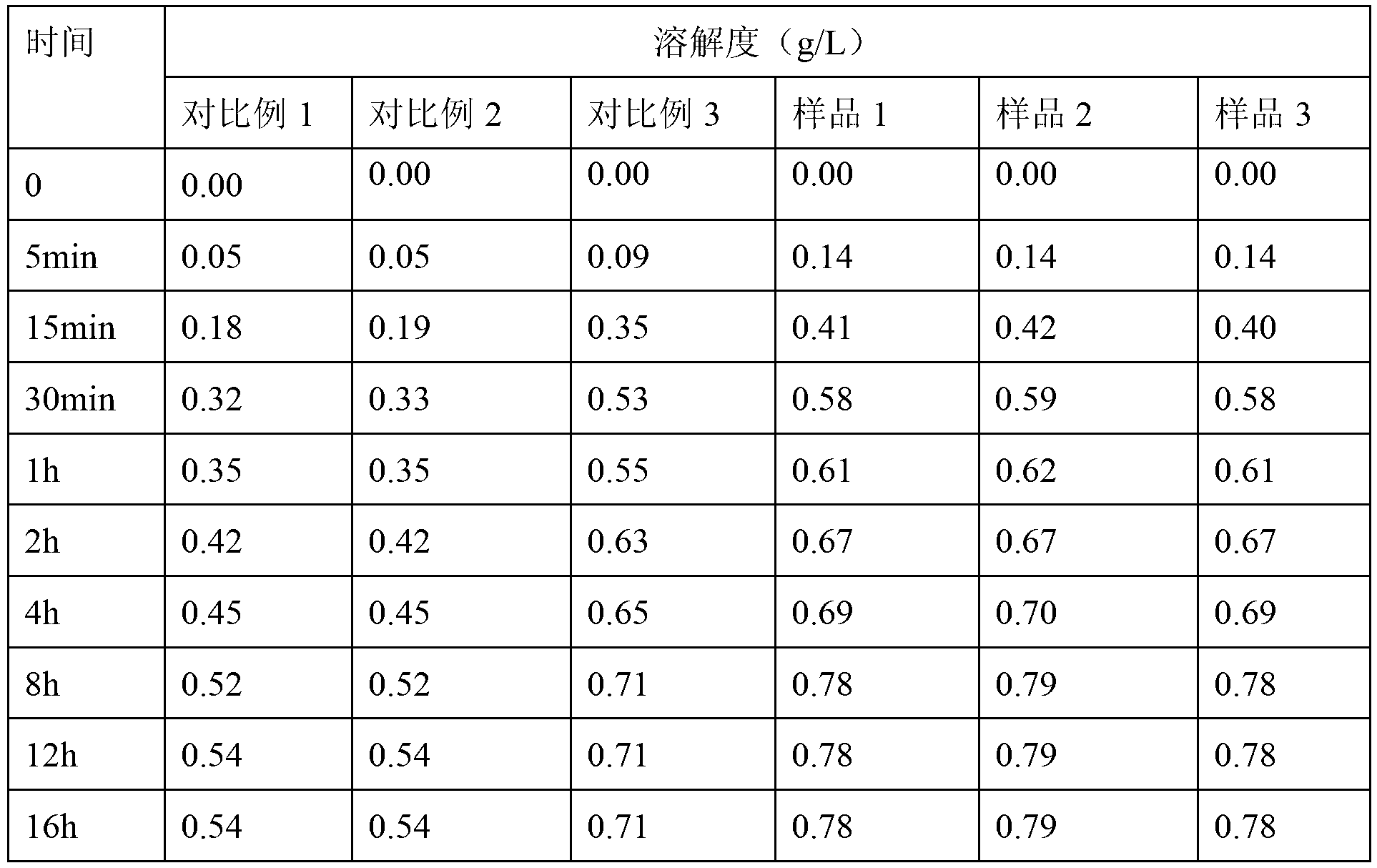
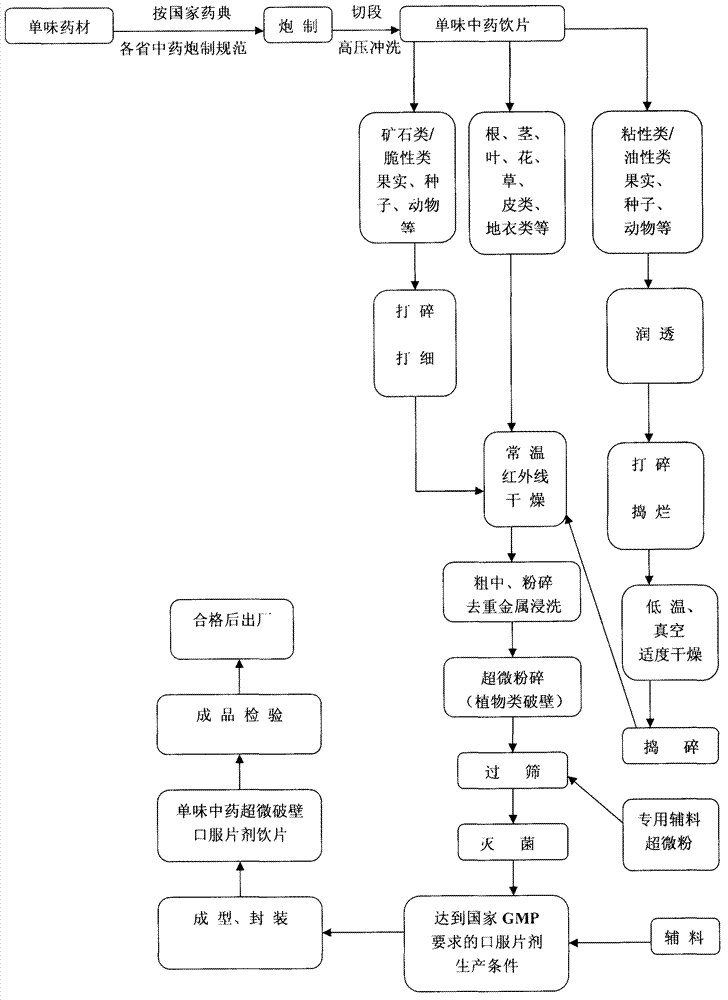
The invention discloses a method for processing traditional Chinese medicinal ultramicro wall-broken oral tablet slices. The method mainly comprises the steps of standardizing, processing and cutting single traditional Chinese medicinal material, then removing pesticide residues through high-pressure cleaning, drying, processing the cut traditional Chinese medicinal materials into coarse powder, dipping to remove heavy metals, processing the coarse powder into ultramicro wall-broken powder through ultramicro grinding, mixing the ultramicro wall-broken powder with pharmaceutical adjuvant powder according to a certain ratio and sterilizing after reaching the standard of quality control to finally obtain the tablet slices. The new tablet slices are prepared from single traditional Chinese medicinal slice, can be directly swallowed without brewing, have various flavors and integrate the advantages of ultramicro power and oral tablets, so that the effective time can be controlled, the usage amount of the pharmaceutical adjuvants is less, the drug-loading rate is high, and a new method is innovated for the modernization of traditional Chinese medicines.
Owner:周明千
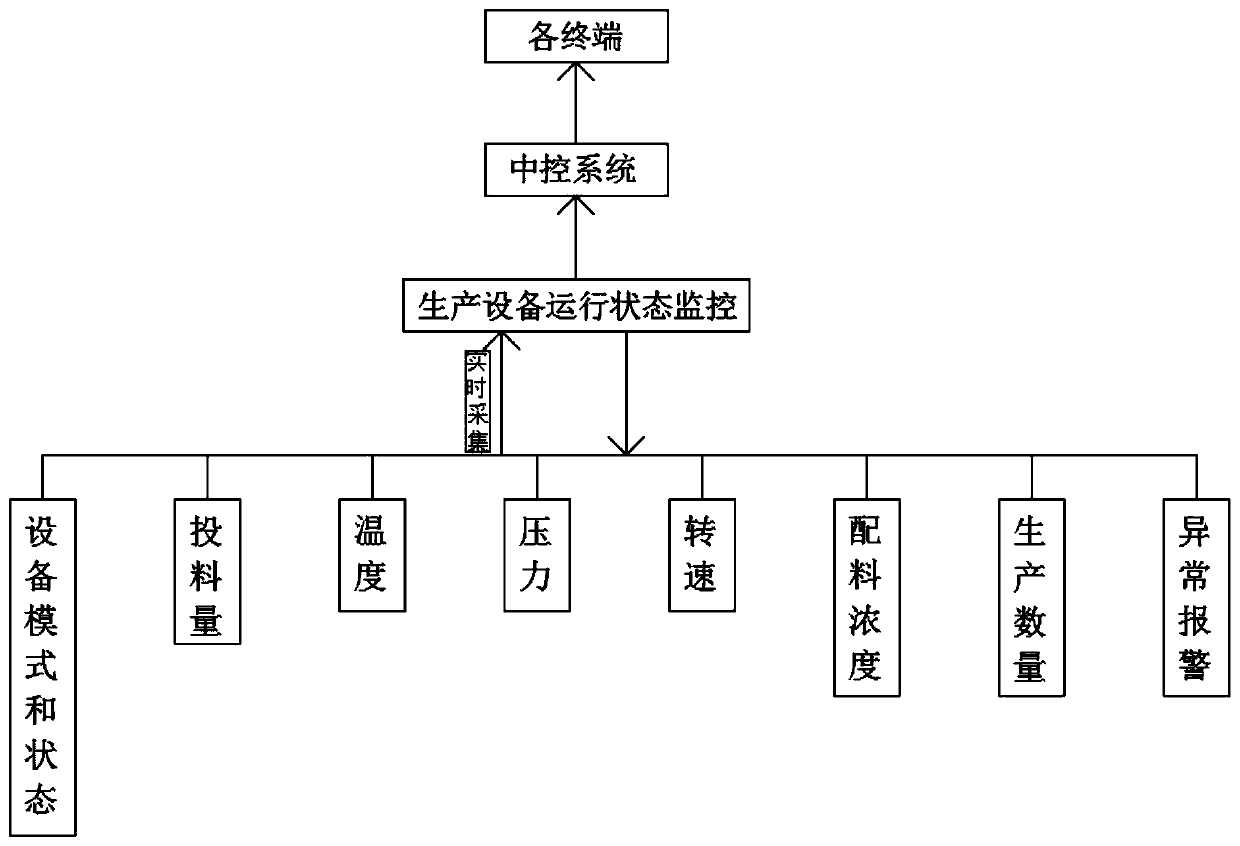
Pharmaceutical adjuvant automatic production line control system
PendingCN111459105AEasy to controlEasy to storeTotal factory controlProgramme total factory controlData acquisitionPharmaceutical Aids
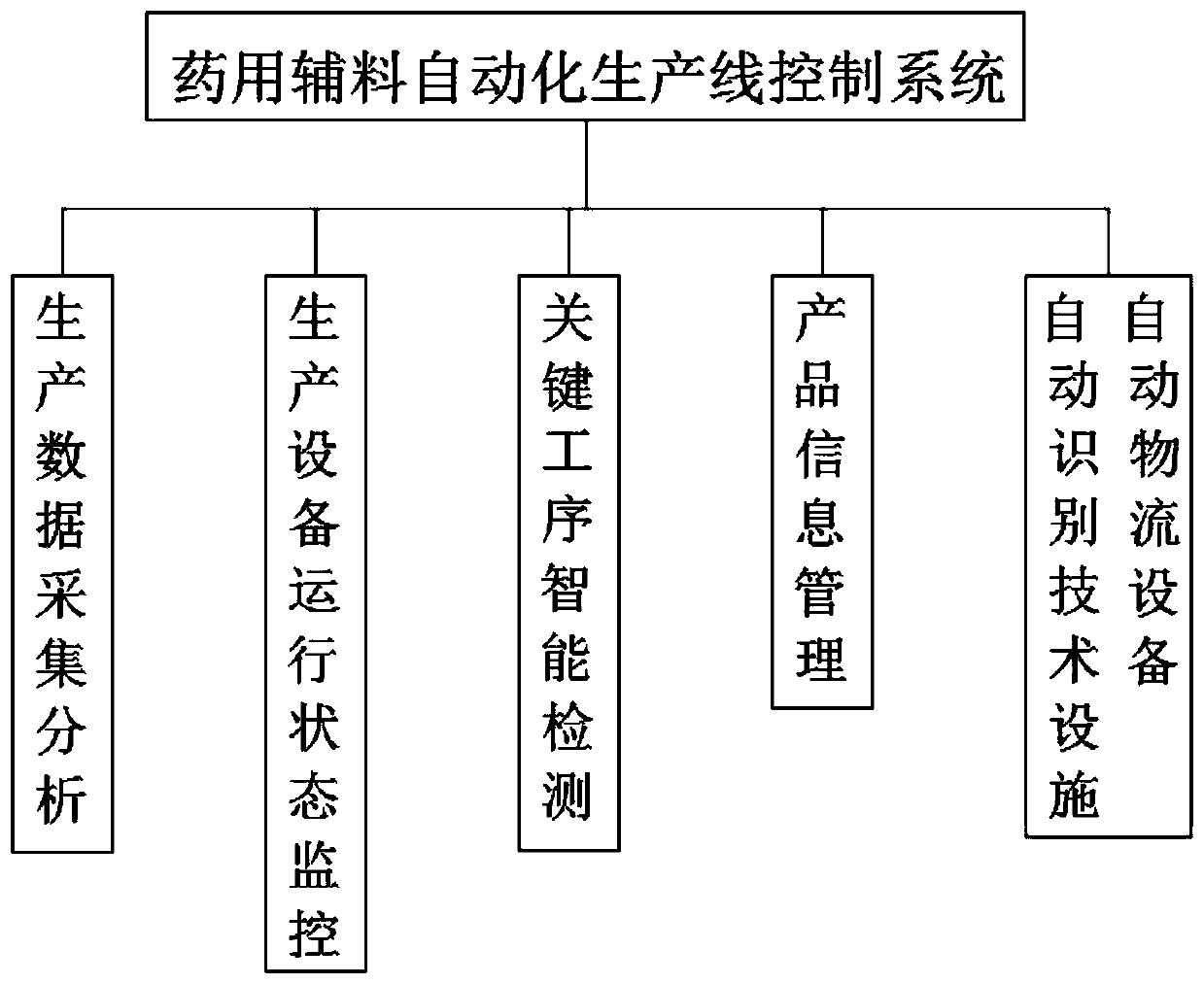
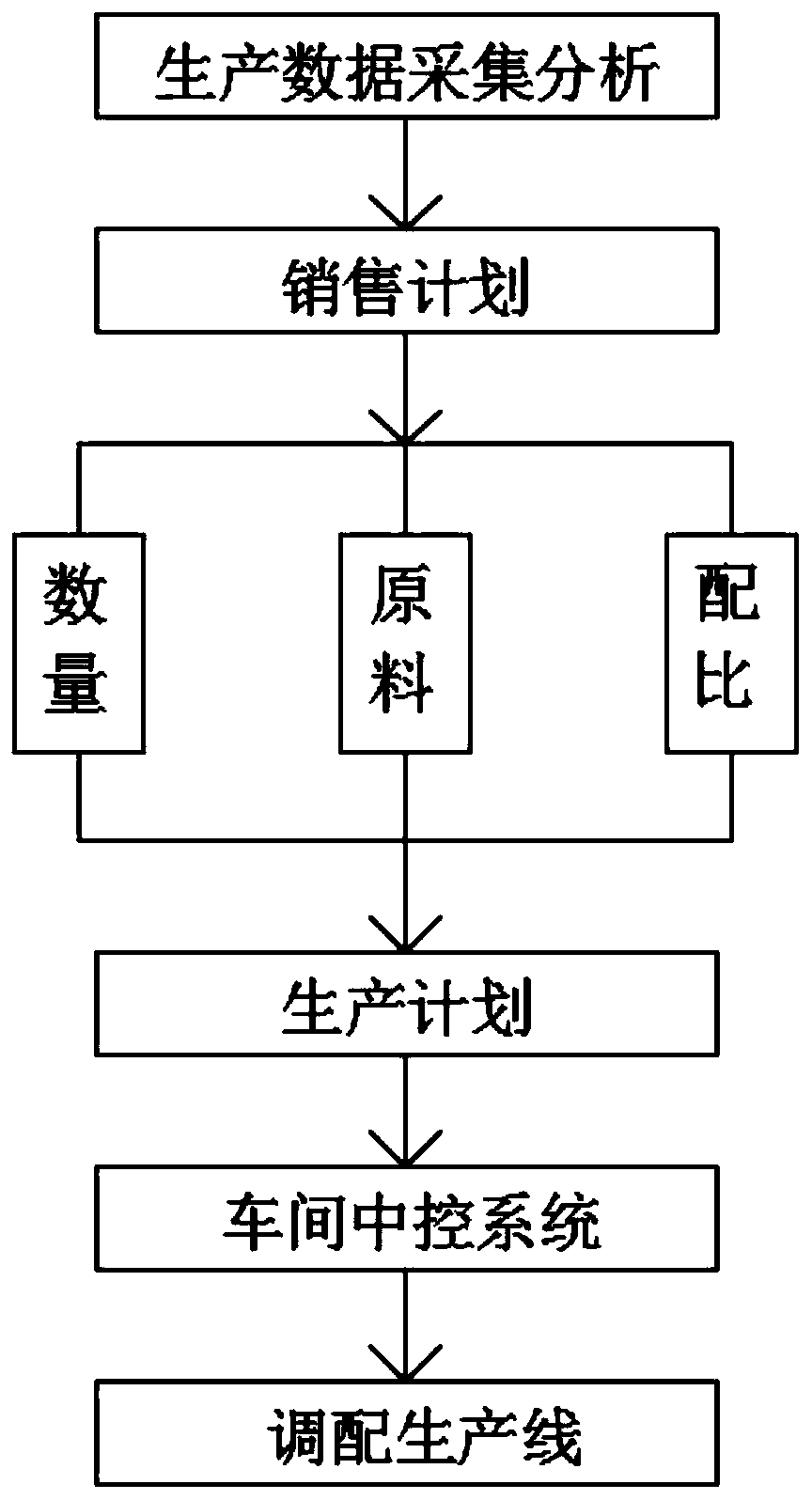
The invention discloses a pharmaceutical adjuvant automatic production line control system and relates to the field of pharmaceutical adjuvant production. The system comprises production data acquisition and analysis, production equipment operation state monitoring, key process intelligent quality detection, product information management, and automatic identification technical facility and automatic logistics equipment use; according to the production data acquisition and analysis, a production plan is automatically generated according to the content of a sales plan. According to the system of the invention, overall control is carried out based on the automatic production line control system; a production plan is automatically generated according to the sales plan and issued to a workshopcentral control system; the central control system plans a and deploys a production line; equipment operation states are collected in real time and fed back to the central control system; the centralcontrol system performs analysis and then processes the information and sends a result to each terminal; production conditions are collected and transmitted to the central control system in real timein a production process; an alarm is automatically given when reaction parameters deviate from a set standard; automatic or manual adjustment is performed in a PLC system to perform exception removal; and therefore, the real-time feedback rate can be increased, production quality can be controlled more easily, and production efficiency can be improved.
Owner:ANHUI SUNHERE PHARMA EXCIPIENTS
Method for preparing total ginkgo flavone glycosides slow-release capsules by applying attapulgite
InactiveCN101716198AShorten the production cycleReduced elutionInorganic non-active ingredientsPharmaceutical delivery mechanismDesorptionGinkgo biloba
The invention discloses a method for preparing total ginkgo flavone glycosides slow-release capsules by applying attapulgite, which comprises the following steps of: washing, drying and pulverizing gingko leaves into coarse powder; adding the gingko leaf coarse powder and petroleum ether with the weight ten times of the gingko leaf coarse powder into a container, refluxing and extracting for 1 hour, degreasing, filtering and drying; adding water with the weight ten times of the degreased gingko leaf coarse powder into the degreased gingko leaf coarse powder, boiling, decocting for 3 times, combining decocting liquids, filtering and concentrating to the relative density of 1.10; adding ethanol with the mass concentration of 95 percent into a concentrated liquid until the ethanol concentration is 70 percent, depositing, settling and filtering; adsorbing a filtrate through modified attapulgite to obtain the attapulgite loading total ginkgo flavone glycosides; and adding a pharmaceutical adjuvant material into the attapulgite loading total ginkgo flavone glycosides, and encapsulating into empty capsules to obtain the total ginkgo flavone glycosides slow-release capsules. The invention adopts the principle of adsorption separation and selects the modified attapulgite with strong adsorbability for the total ginkgo flavone glycosides to directly prepare a preparation without elution, the total ginkgo flavone glycosides slow-release capsules are slowly desorbed under the desorption action of a body fluid, steps are simplified, the production period is shortened, and the yield of products is improved.
Owner:HUAIYIN INSTITUTE OF TECHNOLOGY
Kelp microcrystalline cellulose and preparation method thereof as well as application of prepared kelp microcrystalline cellulose
Owner:QINGDAO HENGSHENG BIOLOGICAL PHARMA TECH DEV
Preparation method of pharmaceutical adjuvant sodium carboxymethyl starch
The invention provides a preparation method of pharmaceutical adjuvant sodium carboxymethyl starch. The preparation method comprises the following chemical reactions: carrying out etherification reaction on starch and sodium hydroxide solution, after the reaction, washing and centrifugally separating so as to obtain the qualified sodium carboxymethyl starch, wherein the washing process is used for desalting. The preparation method provided by the invention has the advantages that the step is simple and reasonable, and the cost of the obtained pharmaceutical adjuvant sodium carboxymethyl starch is lower. The obtained sodium carboxymethyl starch is dried under 75 DEG C-95 DEG C so as to obtain the product with the qualified water content, and then the product is pulverized and sieved, and is introduced into a weighing and packaging process. The main component of the supernatant liquid, washing liquid and filtered solution generated in the processing step is ethanol and ethanol can be recycled by a fractionating tower.
Owner:BENGBU COLLEGE
New furan derivative and application thereof
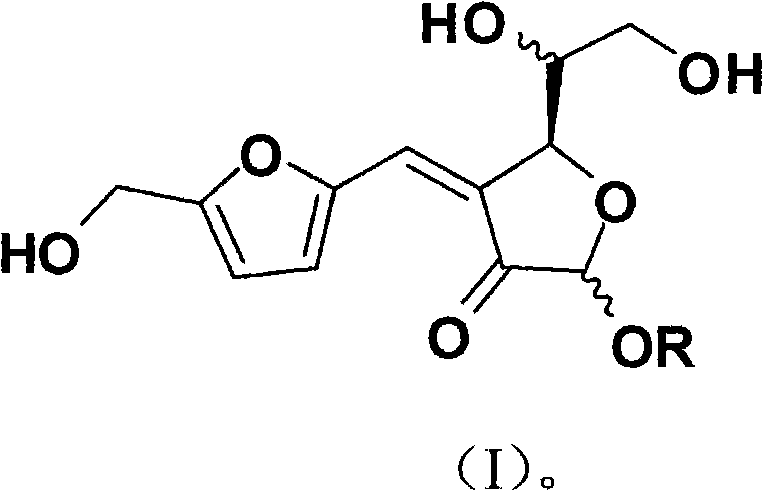
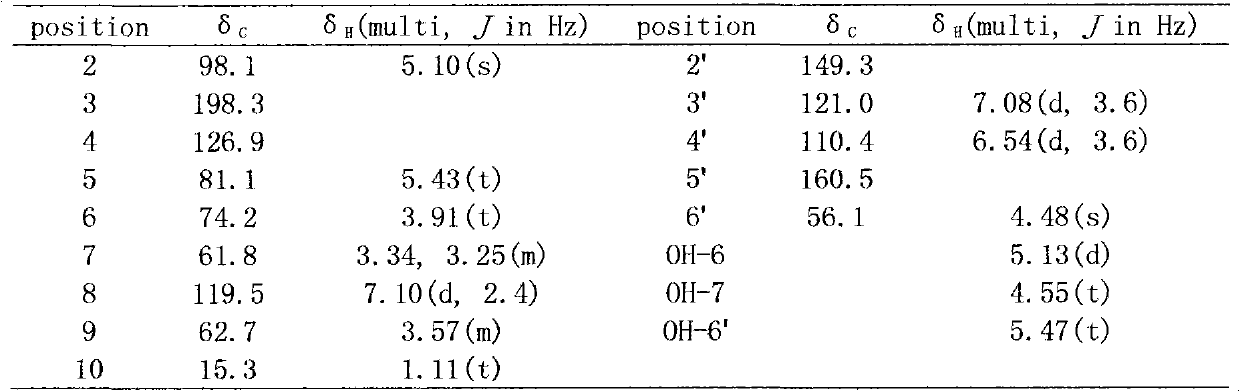
InactiveCN101830871AStable structureStrong anti-complement activityOrganic active ingredientsOrganic chemistryFuranDisease
The invention belongs to the technical field of medicaments and relates to a furan derivative and application thereof, in particular to a new furan derivative for treating immunologic diseases. The structural formula of the furan derivative is shown in the specifications, wherein an R group can be linear chain alkyl and branched chain alkyl having 2 to 11 carbon atoms. A compound has a stable structure and various clinically acceptable formulations can be prepared by matching the compound with an appropriate amount of pharmaceutical adjuvant. An immunology experimental result indicates that the compound has relatively high anti-complement activity and no activity difference between two formulations, so the compound has the advantages of relatively high activity, little medicament dosage and the like. The furan derivative and a preparation which is possibly developed by the furan derivative are mainly used for treating various diseases caused by over activation of a complement system. The R group is linear chain alkyl and branched chain alkyl having 2 to 11 carbon atoms.
Owner:SHENYANG PHARMA UNIVERSITY
Preparation method of cross-linking sodium carboxymethylcellulose pharmaceutical adjuvant
ActiveCN103059321AAvoid hydrolysisNo residuePharmaceutical non-active ingredientsCross-linkOrganic solvent
The invention relates to a preparation method of a cross-linking sodium carboxymethylcellulose pharmaceutical adjuvant. The method comprises the following steps: firstly, adjusting the pH value of a dispersion liquid of sodium carboxymethylcellulose with degree of substitution of 0.65-0.80 by a degree of substitution adjusting system to 10-14; continuously stirring till the system is uniformly dispersed; then, stirring and adding an ethanol liquid of epoxy chloropropane as a cross-linking agent, wherein epoxy chloropropane in the ethanol liquid of epoxy chloropropane accounts for 6-40wt% of ethanol liquid of epoxy chloropropane; stirring at 10-60 DEG C and reacting for 2-12 hours; after reaction, adding acid to neutralize; filtering, washing and drying; and smashing and sieving to obtain the cross-linking sodium carboxymethylcellulose, wherein the physicochemical properties of the cross-linking sodium carboxymethylcellulose meet the Chinese pharmacopoeia (2) (2010 Edition). The reaction condition is mild, the degree of crosslinking is controllable, the reaction solvent is mild, and no residual organic solvents are left after reaction. In cross-linking reaction under a cross-linking reaction, sodium carboxymethylcellulose is prevented from being hydrolyzed.
Owner:HUBEI GEDIAN HUMANWELL PHARMA EXCIPENTS
Nutrition preparation for kidney cell repair and preparation method of nutrition preparation
InactiveCN106110291AReasonable collocationSimple processing technologyPeptide/protein ingredientsMetabolism disorderBiotechnologyPlant sterol
The invention belongs to a nutrition preparation and a preparation method thereof, in particular to a nutrition preparation for kidney cell repair and a preparation method of the nutrition preparation. The nutrition preparation is characterized by consisting of the following natural functional ingredients in parts by weight: 1-20 parts of a rhizoma polygonati extract, 1-10 parts of a ginseng extract, 1-20 parts of a raspberry extract, 1-30 parts of an oyster extract, 1-10 parts of a maca extract, 1-30 parts of refined peptide powder, 1-30 parts of soybean peptide, 1-10 parts of phytosterol and the like; and one or more pharmaceutical adjuvant material is added on the basis of the composition. According to the nutrition preparation for kidney cell repair disclosed by the invention, such functional ingredients as the rhizoma polygonati extract, the ginseng extract, the raspberry extract, the oyster extract, the maca extract, the refined peptide powder, the soybean peptide, the phytosterol and the like are scientifically combined and reasonably matched for the first time.
Owner:CASHESU TIANJIN PHARM TECH CO LTD
Esomeprazole pharmaceutical composition and preparation thereof
InactiveCN103845734AAvoid degradation damageRaise the pHOrganic active ingredientsDigestive systemAluminium hydroxideStrong acids
The invention provides an esomeprazole pharmaceutical composition with stronger acid resistance and a preparation thereof. The esomeprazole pharmaceutical composition comprises esomeprazole, one or more antacids and common pharmaceutical adjuvants, wherein the esomeprazole comprises esomeprazole salt, particularly esomeprazole magnesium salt and esomeprazole lithium salt; and the antacids comprise sodium bicarbonate, sodium carbonate, potassium carbonate, potassium bicarbonate, calcium carbonate, aluminum carbonate, magnesium carbonate, magnesium hydroxide, magnesium oxide, aluminium hydroxide, magnesium aluminum carbonate and the like. The preparation of the esomeprazole pharmaceutical composition is prepared by adding the antacids via interior addition and exterior addition; and in an optimized preparation method, the weight ratio of the antacids in the interior addition and the exterior addition is 2: 3, so that the antacids play double efficacies, the dosage of acid neutralization agent is reduced and the acid resisting effect is better.
Owner:LIAONING YILING KECHUANG BIOLOGICAL MEDICAL TECH +1
Chinese medicinal composition for diffusing lung, relieving exterior syndrome, suppressing cough and reducing phlegm and preparation method thereof
The invention relates to a traditional Chinese medicine combination capable of ventilating the lung, relieving exterior syndrome, relieving cough and reducing sputum, composed of hogfennel root, radix trichosanthis, peppermint, mulberry bark, bitter almond (fried), perilla seed, balloonflower, purple common perilla, glycyrrhiza, inula flower, citrus aurtantium, pummelo pee, Chinese radish seed, scullcap, pumice (calcinied) and an amount of pharmaceutical adjuvant, wherein the hogfennel root is capable of relieving cough and eliminating sputum, the mulberry bark is capable of clearing away the lung-heat and sputum, the Chinese radish seed, purple common perilla, citrus aurtantium, perilla seed, pumice, bitter almond, pummelo pee are capable of eliminating sputum and clearing away the heat-evil, the balloonflower, glycyrrhiza and inula flower are capable of regulating vital energy and eliminating sputum. The scullcap is capable of clearing away lung-heat. The peppermint is capable of dispelling wind and heat from the body, relieving the sore-throat, clearing away the head and eyes. The traditional Chinese medicine combination(Xiao'er qingfeiwan) is capable of ventilating the lung, relieving exterior syndrome, relieving cough and reducing sputum and mainly treats acute tracheitis, wind-heat type common cold, cough, white sticky sputum or yellow thick sputum. The traditional Chinese medicine combination has features of convenient administration, small dose, no side-effect and is much friendly for children.
Owner:津药达仁堂集团股份有限公司达仁堂制药厂
Preparation method for high-purity breviscapine extract as well as preparations and application thereof
ActiveCN106432385AHigh purityNo pollution in the processOrganic active ingredientsNervous disorderChromatographic separationDisease
The invention provides a preparation method for high-purity breviscapine extract as well as preparations and application thereof. The method disclosed by the invention comprises the following steps: taking erigeron breviscapus as a raw material, performing dynamic ultrasonic countercurrent extraction or heating reflux extraction, filtering, centrifuging, adjusting the pH value to 2.0-7.0, performing macroporous resin column chromatography, preparing by using dynamic axial compression industrial chromatographic separation, or preparing by adopting high-speed counter-current chromatography, freezing or performing spray drying, thereby obtaining the high-purity breviscapine extract. According to the method disclosed by the invention, extraction can be completed in a short time at a low temperature, the separation cycle is short, the efficiency is high, the occupied volume for extraction is reduced, the production cost is lowered, the energy consumption and environmental pollution can be reduced, and the purity of the obtained breviscapine can be 95% or higher. Added with various added pharmaceutical adjuvants, the high-purity breviscapine extract can be prepared into ordinary tablets, thin membrane coated tablets, capsules, dispersible tablets, dropping pills, granules, sustained-release preparations and other solid oral preparations, as well as spray, aerosol, lyophilized powder for injection and injection, and the preparations can be used for treating cardiovascular and cerebrovascular diseases and are obvious in curative effects, safe and reliable.
Owner:黑龙江华弘成生物科技有限公司
Etoposide cubic liquid crystal as well as preparation method and application thereof
InactiveCN107233312AImprove bioavailabilityImprove stabilityOrganic active ingredientsSuppositories deliveryCrystallographySide effect
The invention belongs to the technical field of medicines and in particular relates to an etoposide cubic liquid crystal as well as a preparation method and application thereof. The preparation method comprises the following steps: firstly selecting a lipid material / water system and a stabilizer for preparing the etoposide cubic liquid crystal, wherein the average particle size of the prepared etoposide cubic liquid crystal is 208nm, and the encapsulation efficiency is 60-65%; and secondly preparing the etoposide cubic liquid crystal into a hollow suppository by adopting a special pharmaceutical adjuvant combination, so that drug bioavailability and drug stability are improved, toxic and side effects are reduced, and targeted drug delivery is realized; and a preparation process is simple, instruments are available, and the preparation time is short.
Owner:GUANGDONG PHARMA UNIV
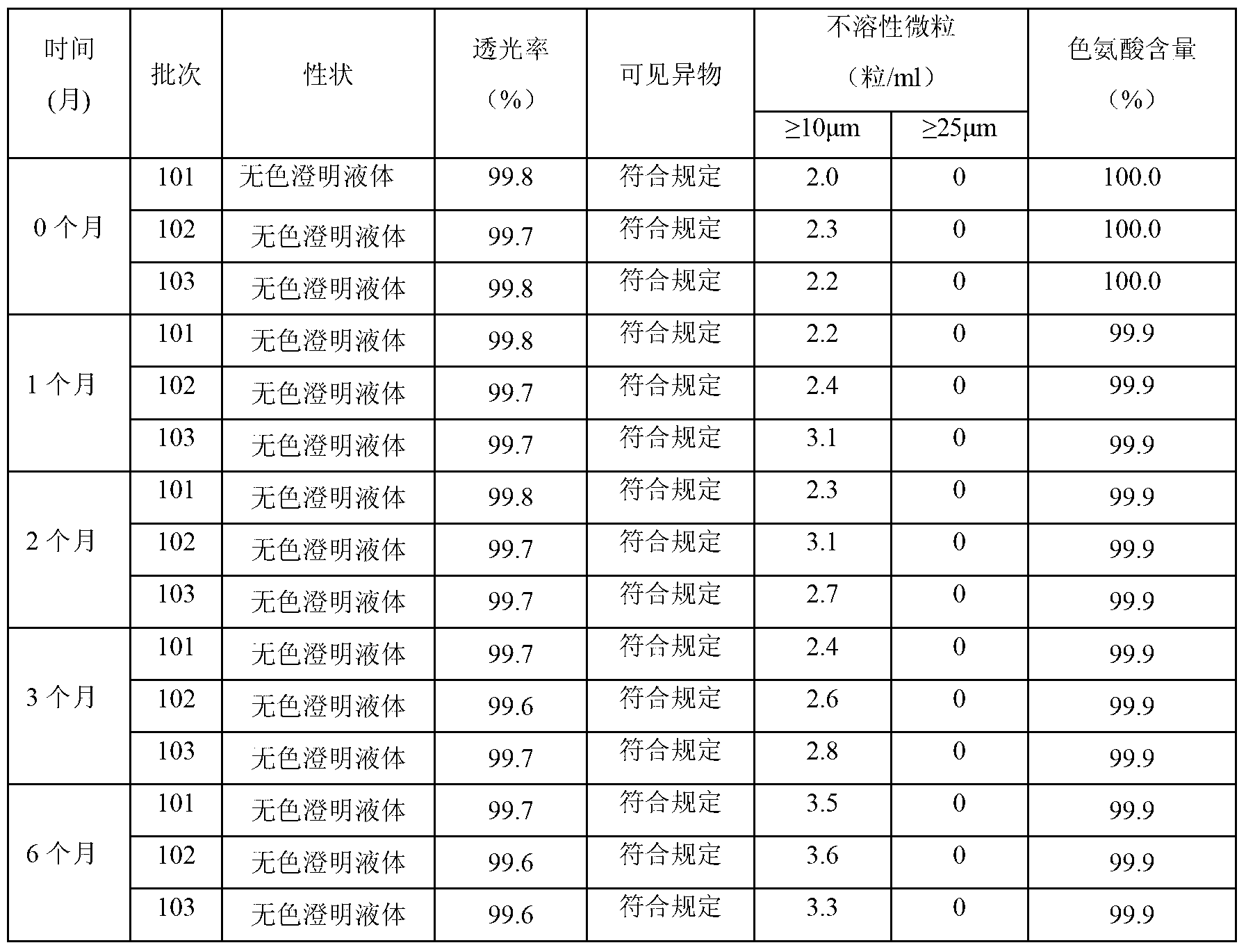
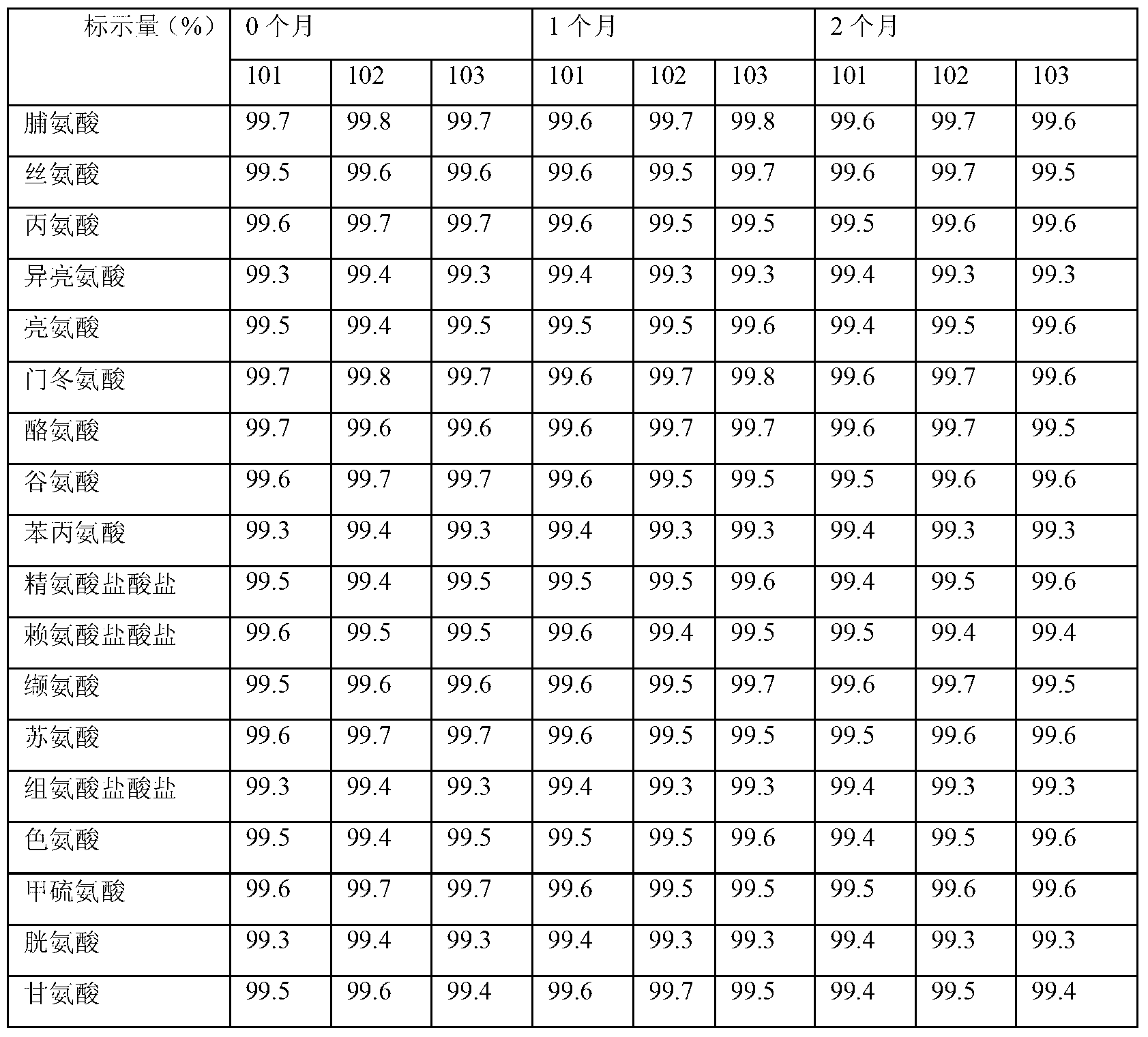
Compound amino acid composition and preparation method thereof
InactiveCN103315999AEasy to manufactureThe prepared L-tryptophan is convenient to prepareOrganic active ingredientsOrganic chemistryTryptophanCombinatorial chemistry
The invention relates to a pharmaceutical composition, and particularly relates to a compound amino acid composition and a preparation method thereof. The compound amino acid composition contains 18 amino acids and pharmaceutical adjuvants; tryptophan is a compound as shown in an X-ray powder diffraction figure I, which is obtained by measurement with a Cu-Kalpha ray. The preparation of the compound amino acid composition, which is prepared from the tryptophan, has good stability and is very suitable for clinical application. The compound amino acid composition has very good mobility and is convenient for preparing the preparation in the medicine preparation process.
Owner:四川省惠达药业有限公司
Compound preparation for treating II type diabetes mellitus and preparation method of compound preparation
InactiveCN104473920AThere are few varieties to chooseHigh dissolution rateOrganic active ingredientsMetabolism disorderIpragliflozinMetformin Hydrochloride
The invention provides a compound preparation for treating II type diabetes mellitus and a preparation method of the compound preparation. The pharmaceutical composition for treating II type diabetes mellitus is prepared from active components ipragliflozin and metformin hydrochloride as well as pharmaceutical adjuvants including a filler, an adhesive, a disintegrant and a lubricant. The compound preparation for treating II type diabetes mellitus prepared by the method has no special requirements on particle sizes of the active components, is free of superfine grinding, low in energy consumption, high in dissolution rate and high in bioavailability, and has a market development prospect; and the defects of poor dissolution rate and low bioavailability of the active components are solved.
Owner:CHANGSHA BAISHUN BIOTECH
Skin irritation suppressant and transdermal preparation
InactiveCN102858372ALess irritatingOrganic active ingredientsNervous disorderCholesterol derivativePharmaceutical Adjuvants
Owner:HISAMITSU PHARM CO INC
Tannic acid preparation for treating burn, bedsore and diaper dermatitis and preparation method thereof
InactiveCN103040849AReduce exudationInhibit growthOrganic active ingredientsDermatological disorderMonoglycerideGlycerol
The invention relates to a tannic acid preparation and a preparation method, and the tannic acid preparation is characterized by comprising, by weight, 2-30% of tannic acid, 40-75% of pharmaceutical adjuvants, and 20-50% of water. The pharmaceutical adjuvants used in the preparation comprise glycerin, monoglyceride stearate, beeswax, Vaseline, paraffin, tween, span, sodium bisulfate, ethylparaben, etc. The preparation method comprises the following steps: dissolving tannic acid by a little water or ethanol, heating to 55-85 DEG C to prepare an oil phase and a water phase, finally adding the water phase into the oil phase under uniform stirring, and performing condensation and split charging to obtain the tannic acid preparation. The preparation comprises paste agents (ointment, plaster), cream, emulsion, oil agents, water aqua, and the like, and the tannic acid content can be determined by a spectrophotometric method. The prepared tannic acid preparation conforms with medicine standards, and is suitable for the treatment of burn, bedsore and diaper dermatitis (neonatal red buttock) and the like.
Owner:XINXIANG MEDICAL UNIV
Metformin hydrochloride sustained-release tablet and preparation method thereof
ActiveCN106176652ABroaden the absorption windowImprove bioavailabilityOrganic active ingredientsMetabolism disorderDiseaseSustained Release Tablet
The invention provides a metformin hydrochloride sustained-release tablet and a preparation method thereof, and belongs to the field of pharmacy. The metformin hydrochloride sustained-release tablet comprises metformin hydrochloride, a binding agent, an absorption accelerant, hydroxypropyl methylcellulose and a pharmaceutical adjuvant. The weight ratio of the metformin hydrochloride, the binding agent and the absorption accelerant is 500:(45-55):(2.5-3.5). The preparation method of the sustained-release tablet includes the steps: mixing and palletizing the absorption accelerant, the metformin hydrochloride and the binding agent; mixing palletized materials, the hydroxypropyl methylcellulose and the pharmaceutical adjuvant; pressing the mixture to obtain the metformin hydrochloride sustained-release tablet. The sustained-release tablet can widen absorbing windows of the metformin hydrochloride is high in bioavailability, blood concentration of the metformin hydrochloride can be maintained at treatment level in long time, and diseases are effectively treated. According to the method, the hydroxypropyl methylcellulose is added after palletizing, the sustained-release performance of the sustained-release tablet is greatly improved, and better treatment effects are achieved.
Owner:NANHAI PHARMA CHONGQING
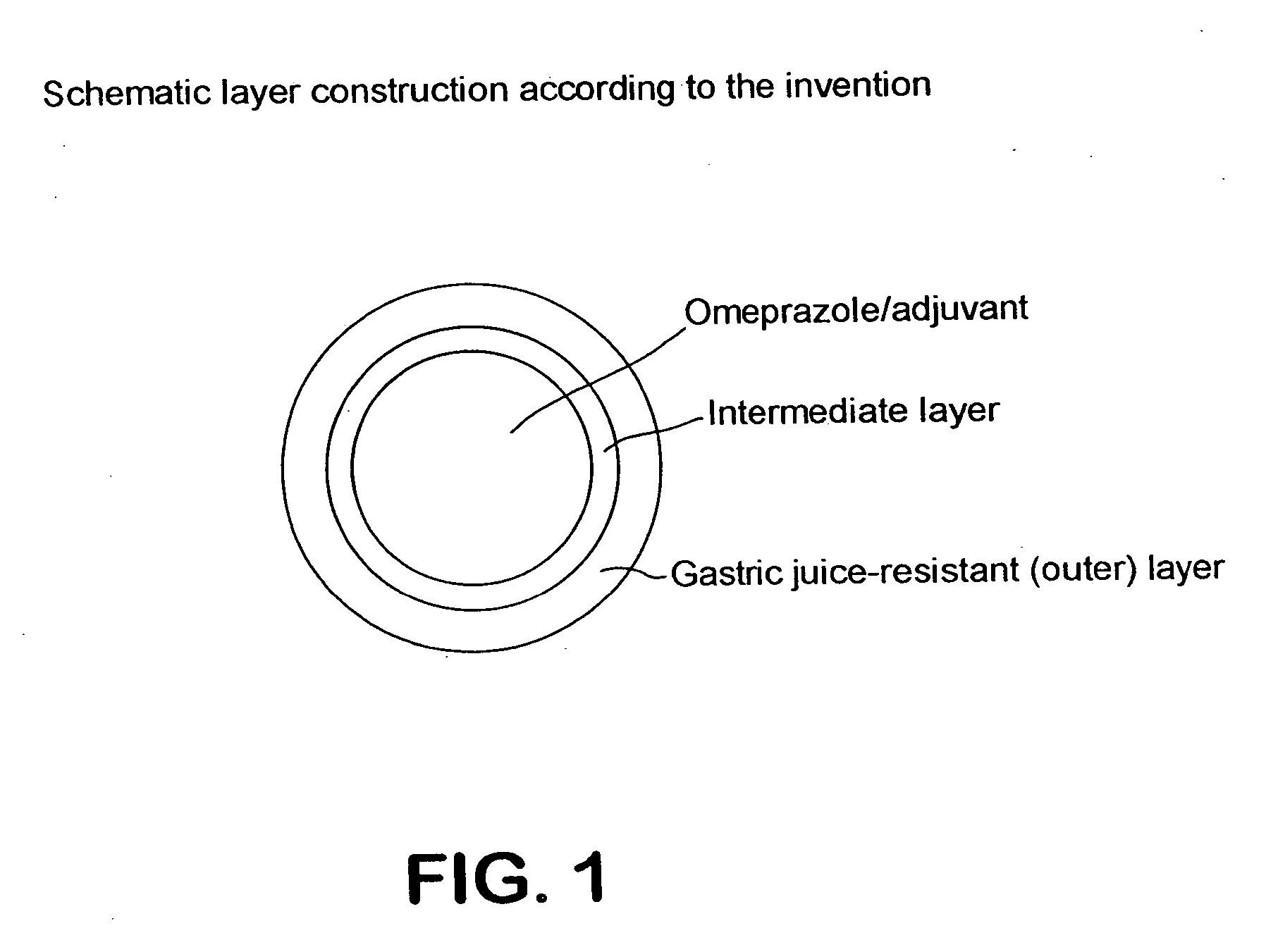
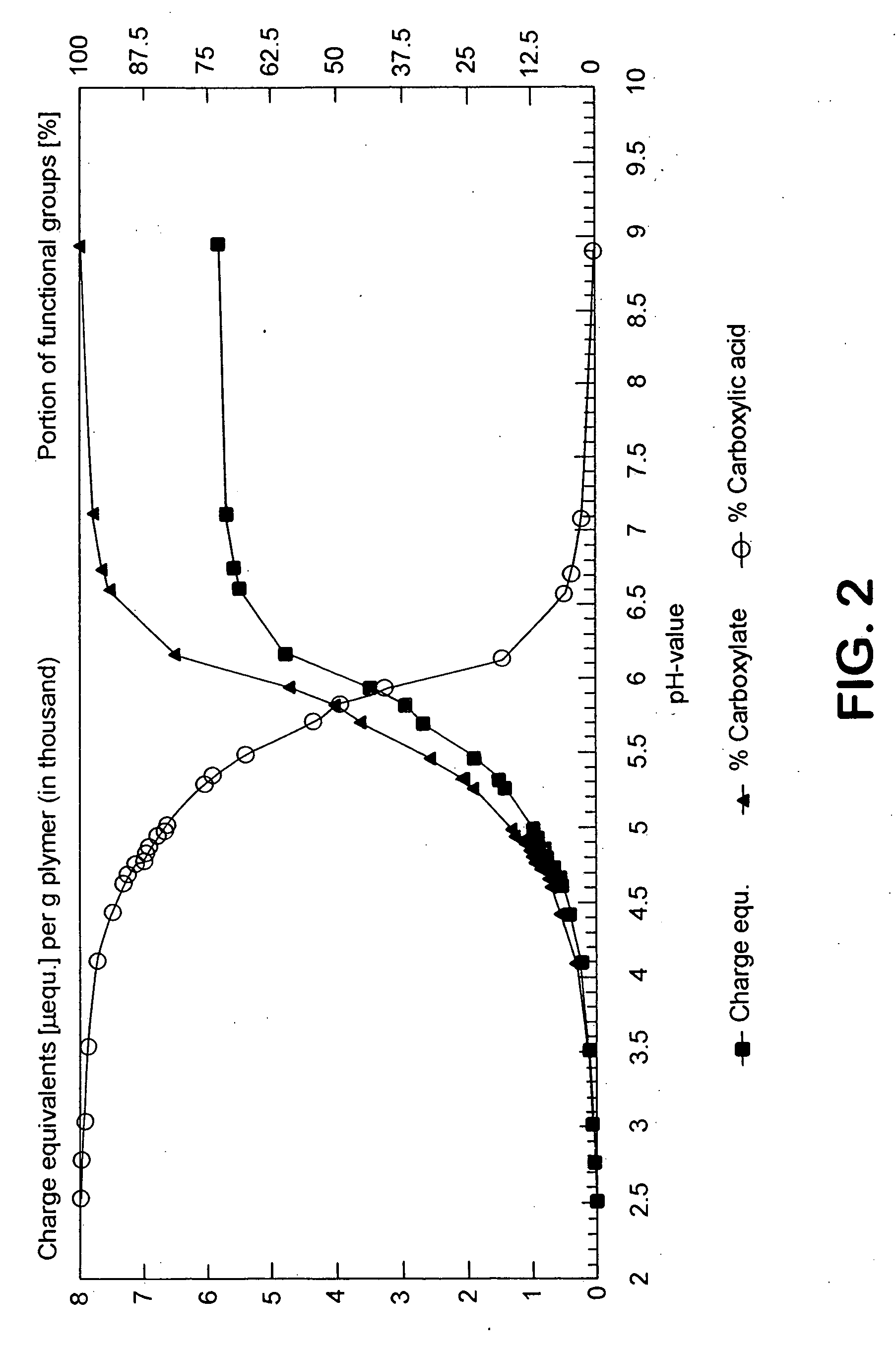
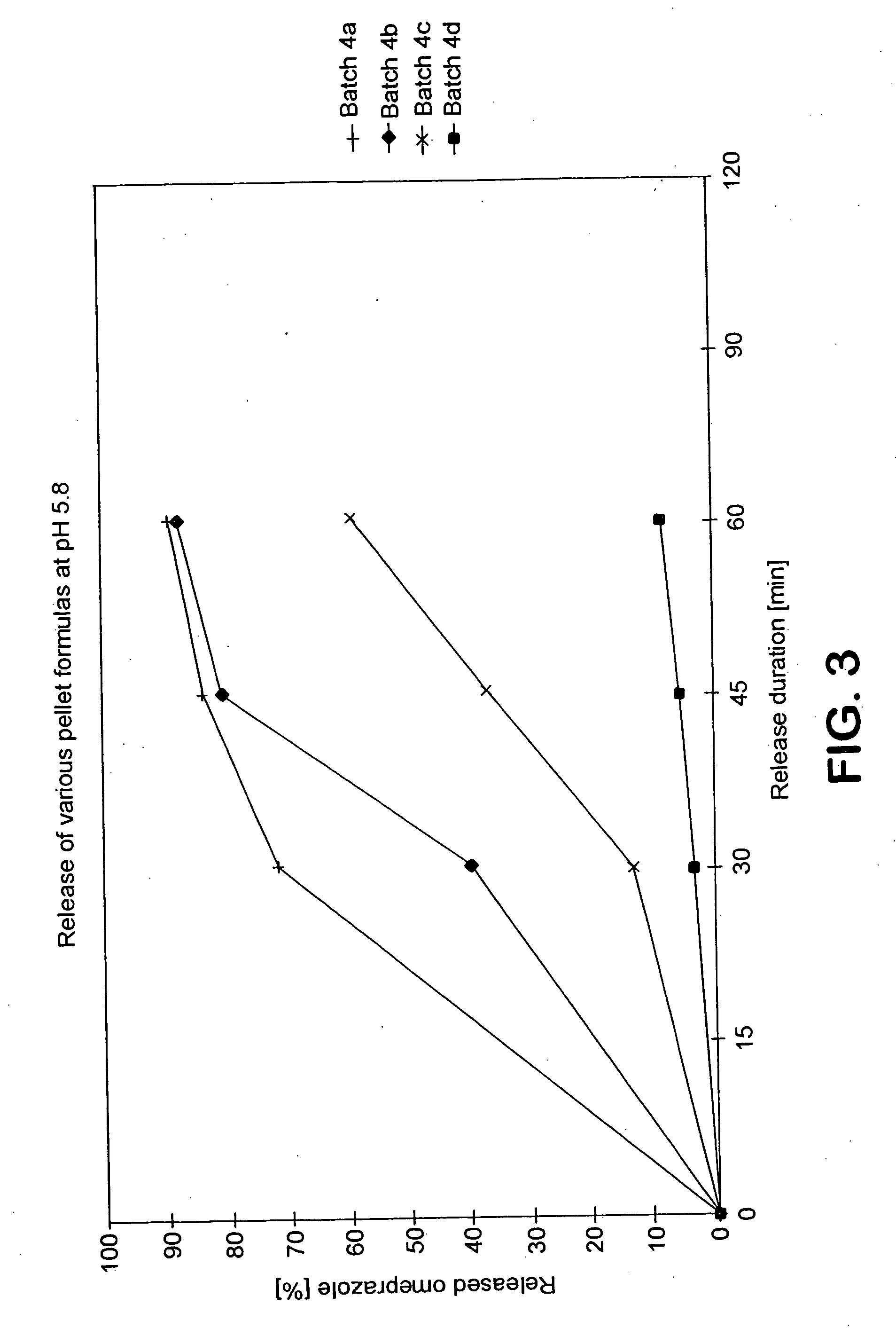
Stable drug form for oral administration with benzimidazole derivatives as active ingredient and process for the preparation thereof
The invention relates to a stable medicament for oral administration which comprises (a) a core which contains an active ingredient selected from Omeprazole, Lansoprazole and Pantoprazole, together with customary pharmaceutical adjuvants, (b) an intermediate layer applied onto the core, and (c) a gastric juice-resistant outer layer. The intermediate layer in (b) is formed as a reactive layer in which a gastric juice-resistant polymer layer material partially neutralized with alkali with cation exchange capacity is present. Further, a method for the production of the stable medicament is disclosed.
Owner:HEESE GERD ULFERT +5
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com