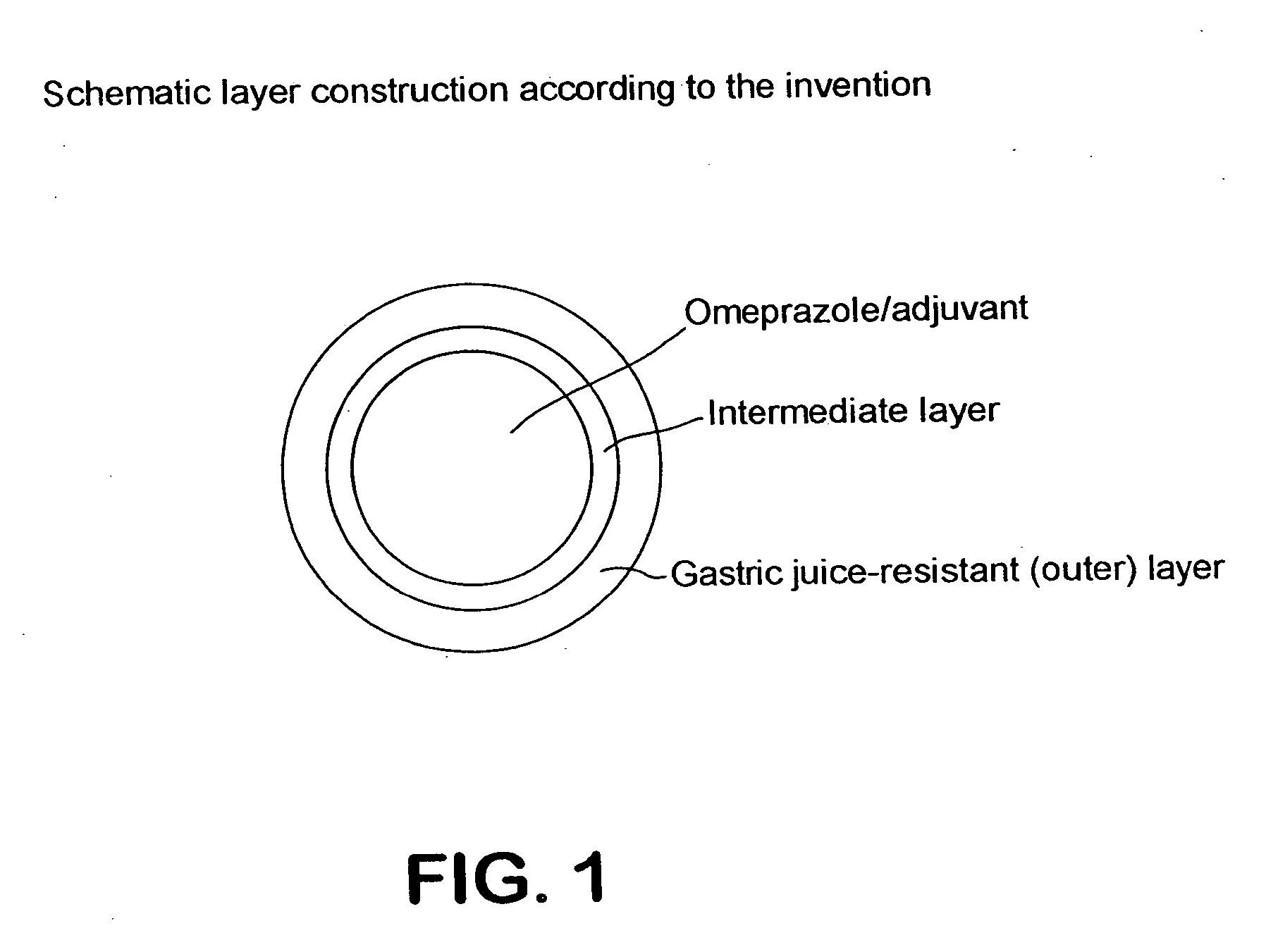
Stable drug form for oral administration with benzimidazole derivatives as active ingredient and process for the preparation thereof
a technology of benzimidazole and active ingredient, which is applied in the field of stable drug form for oral administration with benzimidazole derivatives as active ingredient and the preparation thereof, can solve the problems of destroying omeprazole, deteriorating storage stability, and not being suitable for omeprazol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 2
Stability of Pellet Formulations:
In a further series of experiments, the medicament according to the invention was compared with the state of the art (EP 0 247 983). For this, various colored batches were produced which have a three-layer construction: core, with the active ingredient Omeprazole in the presence of an alkaline buffering substance (Na2HPO4, according to the state of the art) and without alkaline buffering substance (according to the invention). intermediate layer either consisting of a enteric layer material partially neutralized with alkali to a pH 6.0 and / or 7.0 according to the invention or inert layer material which contains sodium acetate as a buffering substance according to the state of the art. The reference example contains non-neutralized enteric layer material and sodium acetate as a buffering substance. Outer layer of Eudragit L 100-55.
Additionally, a medicament was tested in the series of experiments in which the intermediate layer was omitted.
T...
example 3
“Self-Repair-Mechanism” of the Reactive Intermediate Layer:
Pellets with the following construction were compared: without intermediate layer (so-called pellet core) with the reactive intermediate layer according to the invention with an inert intermediate layer of HPMC (reference example)
For better judgement of the “self-repair-mechanism”, the pellets were not provided with the outer enteric coat. All pellet types were tested in artificial gastric juice (pH 1.2) in a release model of the European Pharmacopoeia (basket). The intermediate layer was partially nuetralized to pH 7.0, the upper limit of the preferred range.
The results (pellet cores without intermediate layer: not shown) are summarized in the following Table 3:
TABLE 3Intermediate layer according to the inventionReference exampleEudragit ® L100-55, pH 7.0, partially neutralized(HPMC)5% Intermediate10% Intermediate15% Intermediate20% Intermediate20% Intermediatelayer 3 alayer 3 blayer 3 clayer 3 dlayer 3 eTimePell...
example 4
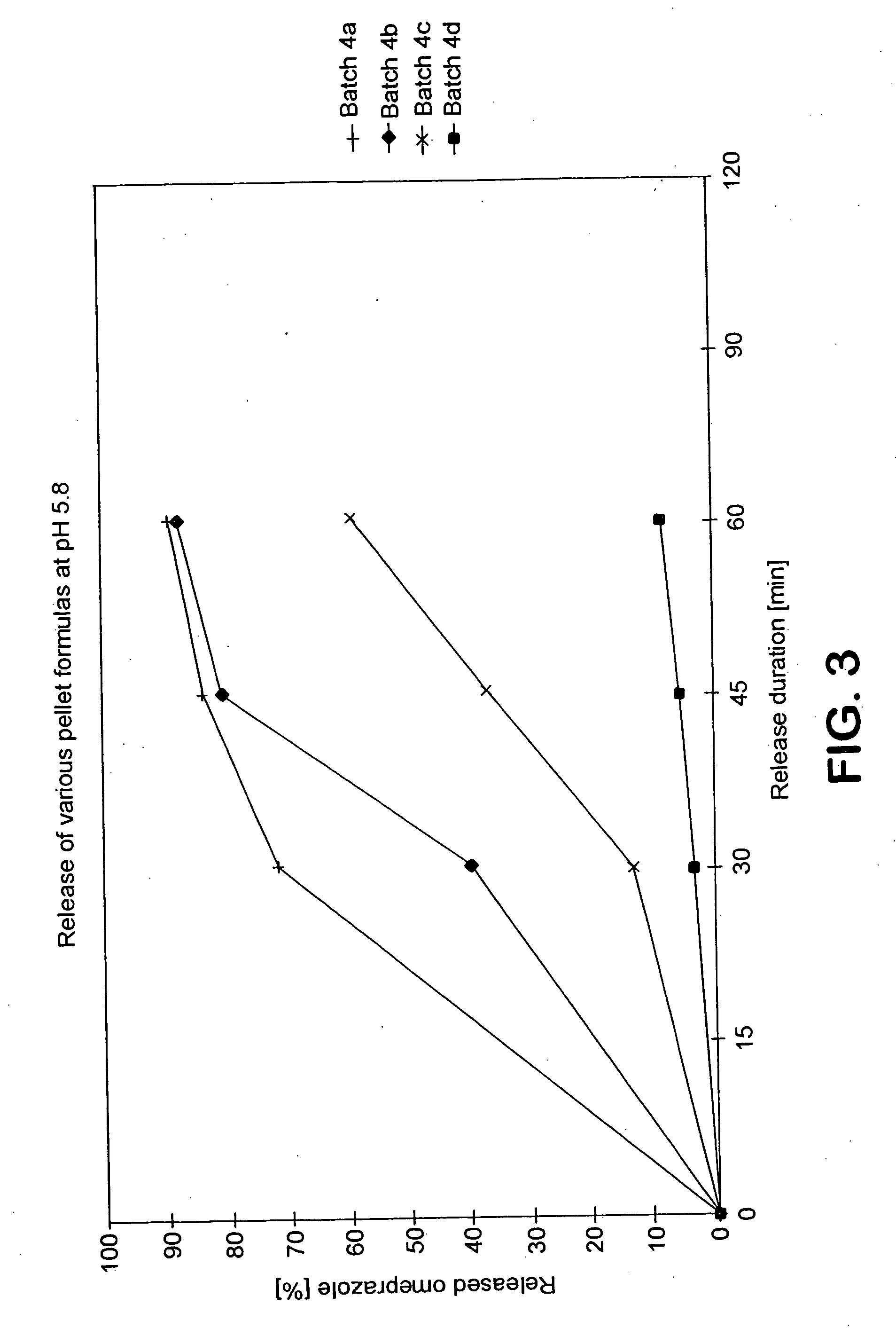
Release Behaviour of Various Pellet Formulas:
Essential for the good bio-availability of the active ingredient is its release as quickly as possible in the upper small intestine region, i.e. in a weakly acid / neutral environment. To investigate the release behaviour pellets with various formulas were introduced into an aqueous medium with a pH value of 5.8 as an in vitro model for the upper small intestine (artificial intestinal fluid) and the Omeprazole released under stirring into the surroundings was determined as a function of time with HPLC (analogously to the Pharmacopia).
The examined pellet formulas and the release results are reproduced in Table 4:
TABLE 4Pellet formulaReleased Omeprazole [%]Batchrelease period30 min45 min60 minaccording to the invention4 aOmeprazole-Core + Adjuvant*,728489IL.: 3% E. L 100-55 pH 7.0gjr: 30% E. L 100-554 bOmeprazole-Core + Adjuvant*,408188IL.: 3% E. L 100-55 pH 6.0gjr: 30% E. L 100-55comparative examples4 cOmeprazole-Core + Adjuvant*, 3 5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com