Etoposide cubic liquid crystal as well as preparation method and application thereof
A technology of etoposide and cube, which is applied in the field of etoposide cubic liquid crystal and its preparation, can solve the problem of low incidence of vomiting and achieve the effects of short preparation time, short preparation process time and encapsulation efficiency measurement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
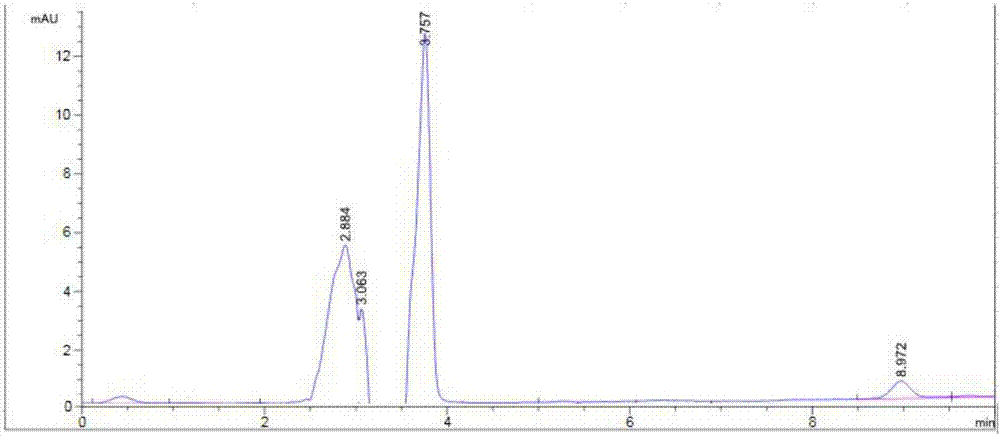
[0057] An etoposide cubic liquid crystal, the average particle size of the etoposide cubic liquid crystal is 208nm, and the encapsulation rate of the etoposide cubic liquid crystal is 65%.
[0058] Preparation method: Weigh 0.1g of GMO, add 5mg of etoposide and 1ml of acetone into a 50ml Erlenmeyer flask to dissolve, and use an ultrasonic cleaner to sonicate for 15min, as phase A; accurately weigh 0.02g of F1270.02g, add 20ml of water, Heat in a water bath to fully dissolve, stir, and prepare a solution with a concentration of 0.1% F127 to form phase B. Phase A was slowly added dropwise to phase B, and stirred at room temperature for 1 h. Then ultrasonic treatment was performed at 300w for 5 minutes in a cell disruptor, once every 5 seconds (with an interval of 5 seconds), to obtain a cubic liquid crystal solution of etoposide.
[0059] The application of etoposide cubic liquid crystal is made into a hollow suppository; its preparation is specifically:
[0060] (1) Take an a...
Embodiment 2
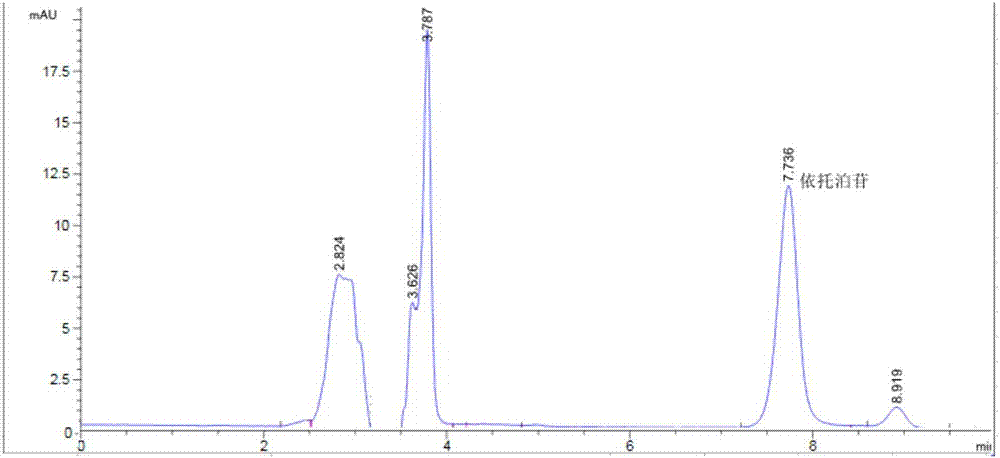
[0064] An etoposide cubic liquid crystal, the average particle size of the etoposide cubic liquid crystal is 180nm, and the encapsulation rate of the etoposide cubic liquid crystal is 75%.
[0065] Preparation method: Weigh 0.08g of phytantriol, add 6mg of etoposide and 0.8ml of acetone into a 50ml Erlenmeyer flask to dissolve, and use an ultrasonic cleaner to sonicate for 13min, as phase A; accurately weigh 0.018g of lecithin , add 22ml of water, heat in a water bath to fully dissolve, stir, and prepare a solution with a concentration of 0.08% lecithin to form phase B. Phase A was slowly added dropwise to phase B, and stirred at room temperature for 1.2h. Then ultrasonic treatment was performed at 280w for 4 minutes in a cell disintegrator, once every 5 seconds (with an interval of 5 seconds), to obtain a cubic liquid crystal solution of etoposide.
[0066] The application of etoposide cubic liquid crystal is made into hollow suppository; its preparation is specifically the ...
Embodiment 3
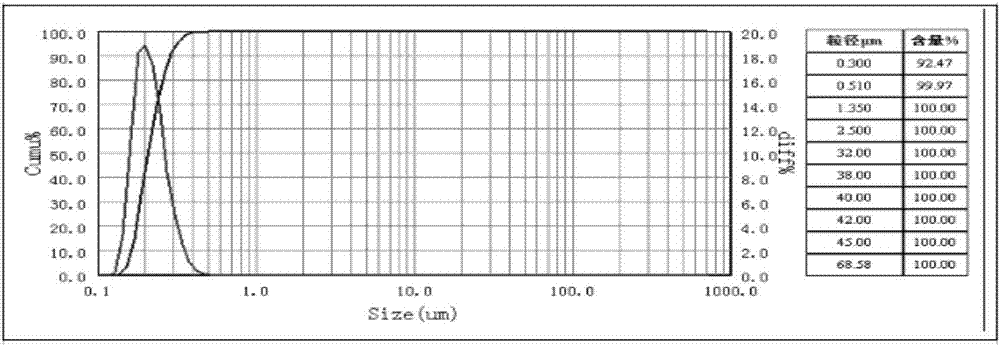
[0068] An etoposide cubic liquid crystal, the average particle size of the etoposide cubic liquid crystal is 100nm, and the encapsulation rate of the etoposide cubic liquid crystal is 90%.
[0069] Preparation method: Weigh 0.12g of glycerol monolauryl ester, add 4mg of etoposide and 1.2ml of acetone into a 50ml Erlenmeyer flask to dissolve, and use an ultrasonic cleaner to sonicate for 17min, as phase A; accurately weigh Cremophor RH400. 022g, add 22ml of water, heat in a water bath to fully dissolve, stir, and prepare a solution with a concentration of 0.1% Cremophor RH40 to form a B phase. Phase A was slowly added dropwise to phase B, and stirred at room temperature for 0.8h. Then ultrasonic treatment was performed at 320w for 5 minutes in a cell disruptor, once every 5 seconds (with an interval of 5 seconds), to obtain a cubic liquid crystal solution of etoposide.
[0070] The application of etoposide cubic liquid crystal is made into hollow suppository; its preparation i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com