Sustained release tablet of quetiapine fumarate composition and preparation method of sustained release tablet
A technology for quetiapine fumarate and sustained-release tablets, which is applied in the direction of drug combinations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve the problem of tablet weight, controllability and storage Problems such as stability defects, gel skeleton damage, and slow-release effect effects, etc., to achieve stable storage, simplified operation, and good tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
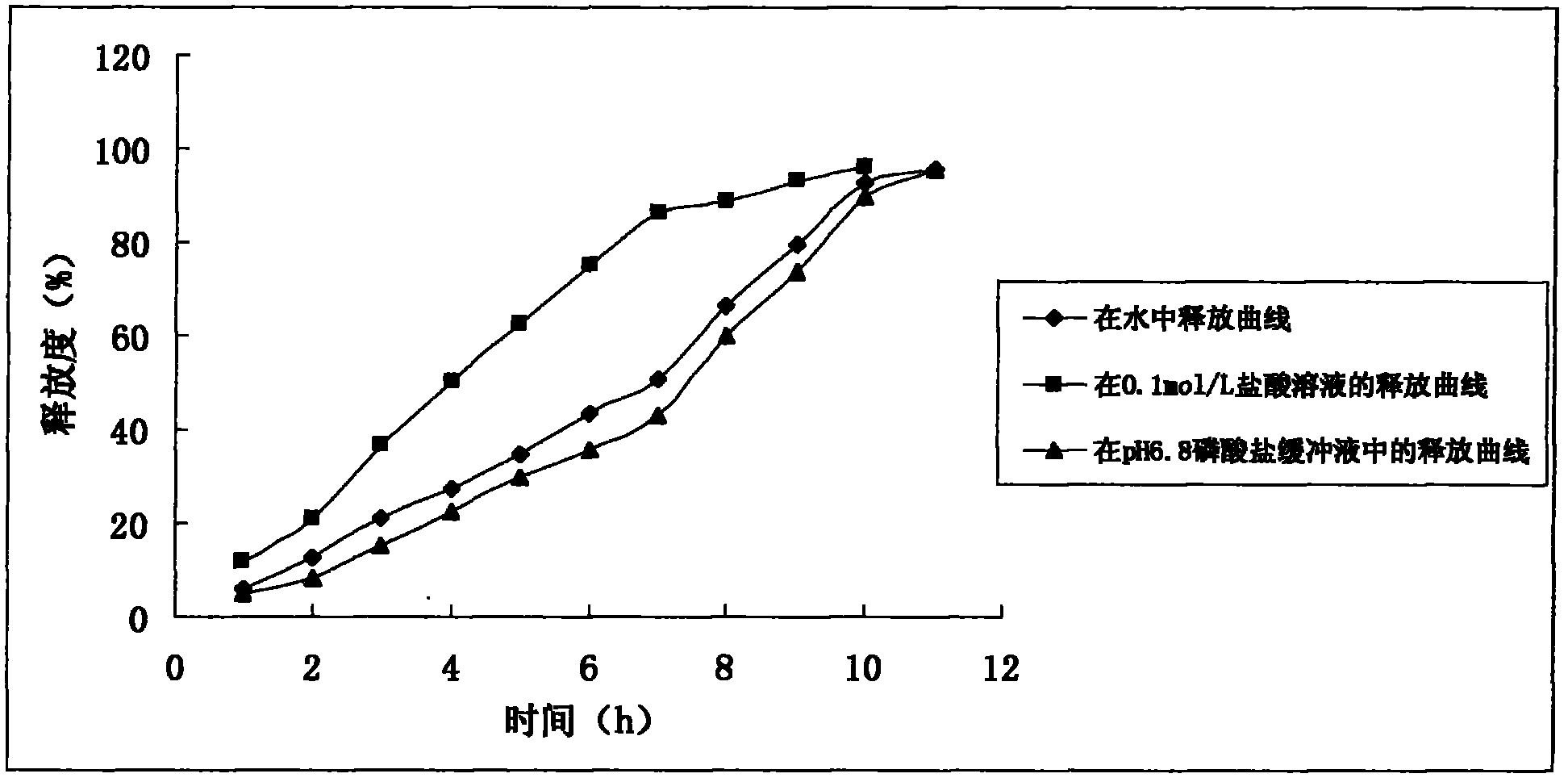
Image
Examples
Embodiment 1
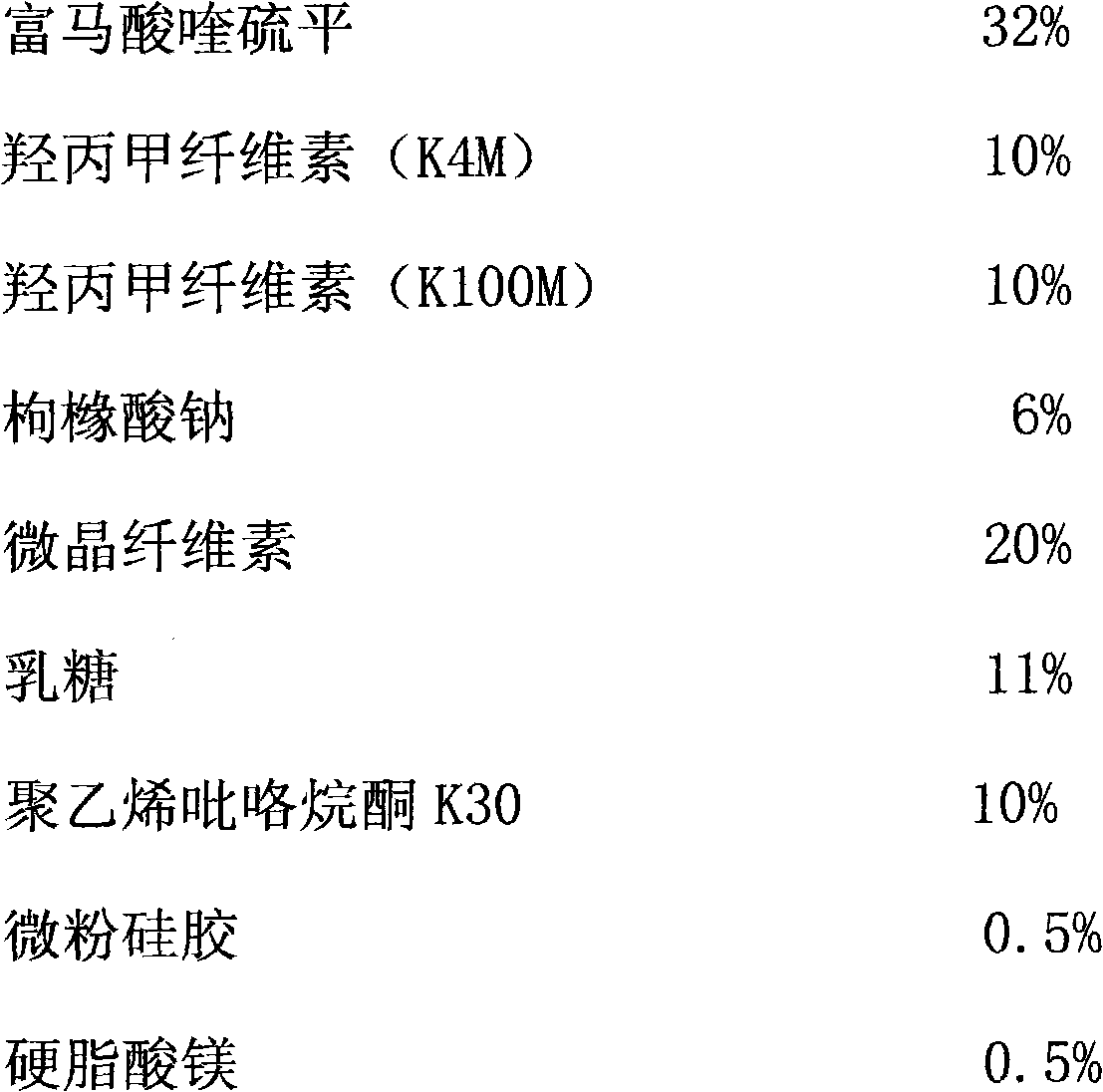
[0018] Sustained-release tablet of the present invention is formulated by the raw and auxiliary materials of following weight ratio:
[0019]
[0020] Among them, hypromellose (K4M) and hypromellose (K100M) are sustained-release materials, sodium citrate is an organic acid salt, lactose, and microcrystalline cellulose are fillers, and polyvinylpyrrolidone K30 is a binder. agent, micronized silica gel and magnesium stearate are lubricants.
[0021] The raw materials are crushed according to the pharmaceutical grade and passed through a 80-mesh sieve; quetiapine fumarate, organic acid salts, slow-release materials and other pharmaceutical excipients except binders and lubricants are mixed according to the formula amount and placed in one step In the granulator, evenly spray the formulated amount of binder to carry out one-step granulation. After the granulation is completed, the granules are sieved with a 22-mesh sieve, and the prepared granules and the formulated amount of m...
Embodiment 2
[0023] Sustained-release tablet of the present invention is formulated by the raw and auxiliary materials of following weight ratio:
[0024]
[0025] Among them, hypromellose (K15M) and hypromellose (K100M) are slow-release materials, sodium tartrate is an organic acid salt, lactose and microcrystalline cellulose are fillers, polyvinylpyrrolidone K30 is a binder, Magnesium stearate is a lubricant.
[0026] The raw materials are crushed according to the pharmaceutical grade and passed through a 80-mesh sieve; quetiapine fumarate, organic acid salts, slow-release materials and other pharmaceutical excipients except binders and lubricants are mixed according to the formula amount and placed in one step In the granulator, evenly spray the formulated amount of binder to carry out one-step granulation. After the granulation is completed, the granules are sieved with a 22-mesh sieve, and the prepared granules and the formulated amount of micropowder silica gel and magnesium stear...
Embodiment 3
[0028] Sustained-release tablet of the present invention is formulated by the raw and auxiliary materials of following weight ratio:
[0029]
[0030] Among them, hypromellose (K4M) and hypromellose (K100M) are slow-release materials, sodium citrate is organic acid salt, microcrystalline cellulose, lactose and powdered sugar are fillers, polyvinylpyrrolidone K90 As a binder, magnesium stearate is a lubricant.
[0031] The raw materials are crushed according to the pharmaceutical grade and passed through a 100-mesh sieve; quetiapine fumarate, organic acid salts, slow-release materials and other pharmaceutical excipients except binders and lubricants are mixed according to the formula amount and placed in one step In the granulator, evenly spray the formulated amount of adhesive to carry out one-step granulation. After the granulation is completed, 22 mesh sieves are used to sieve the granules, and the prepared granules and the formulated amount of micropowder silica gel and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com