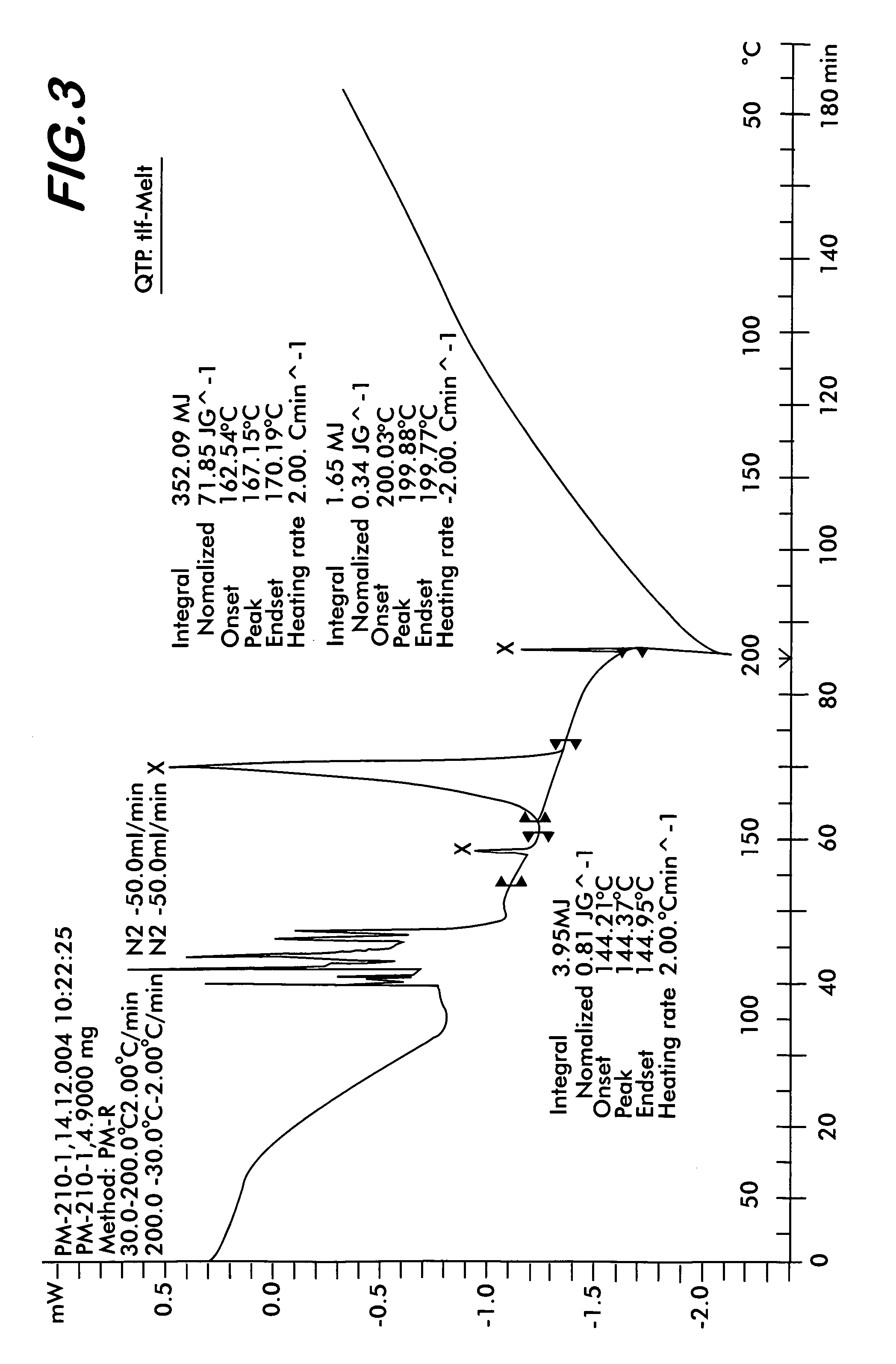
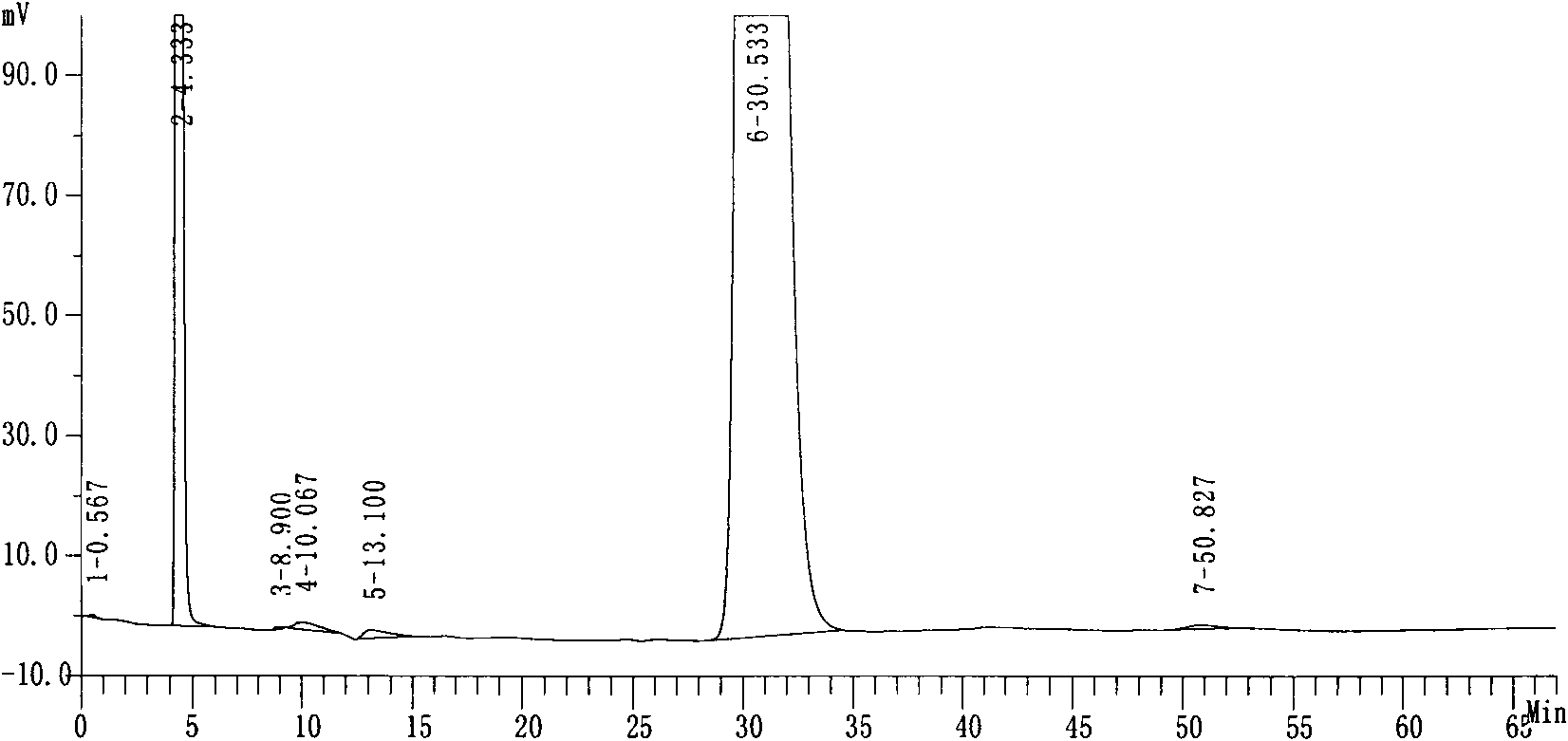
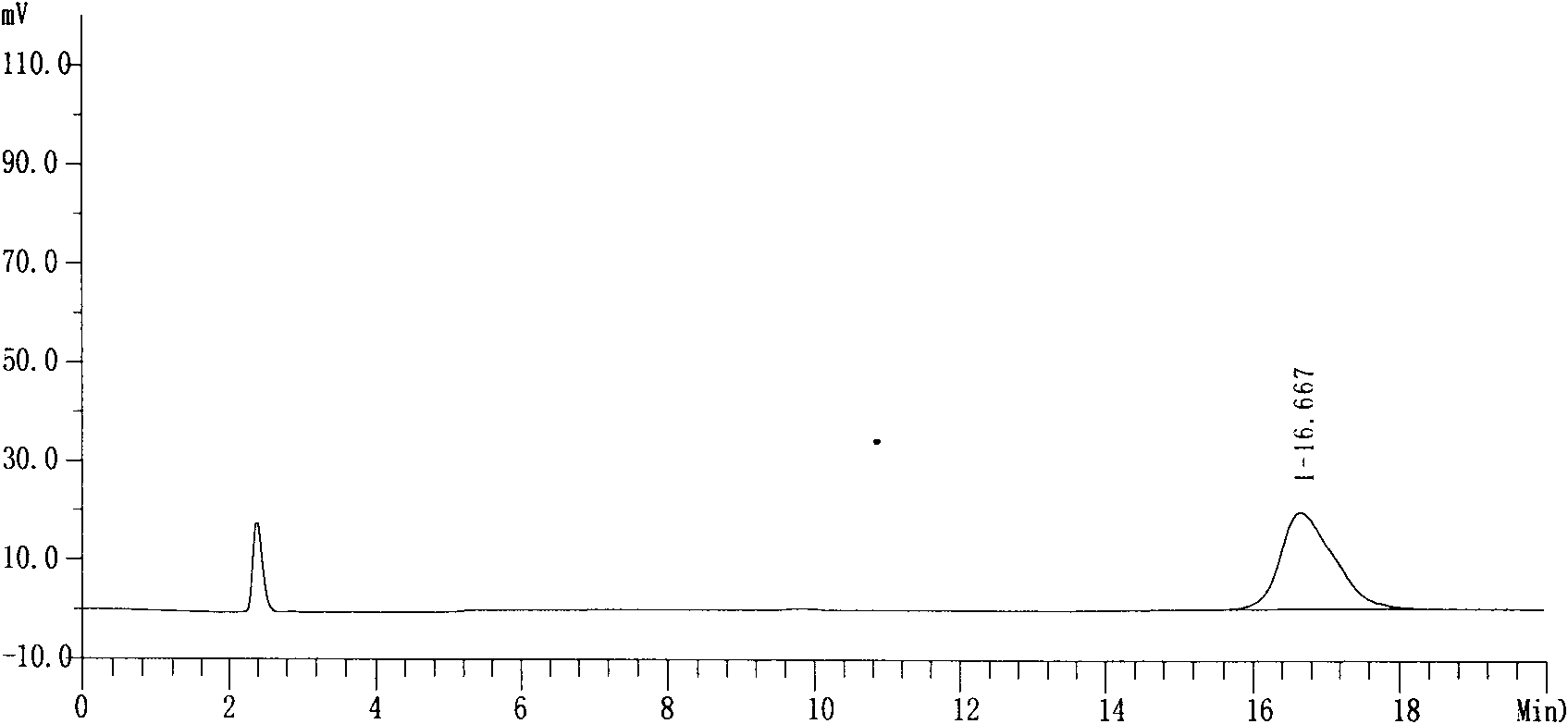
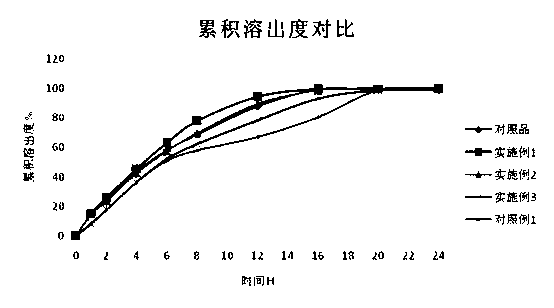
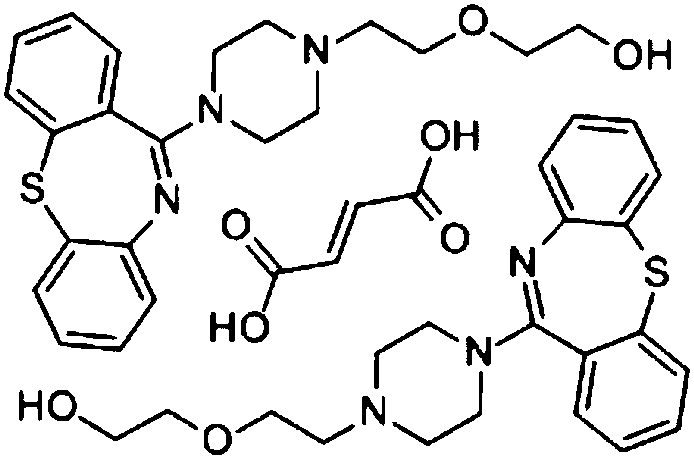
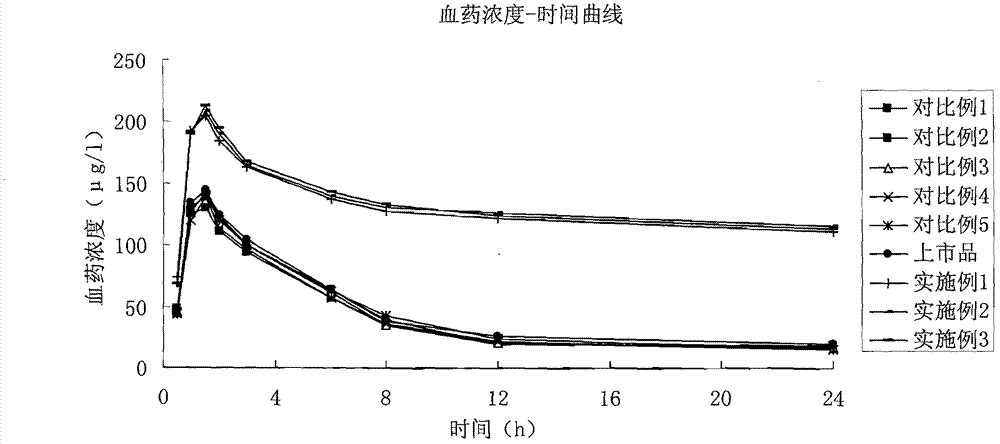
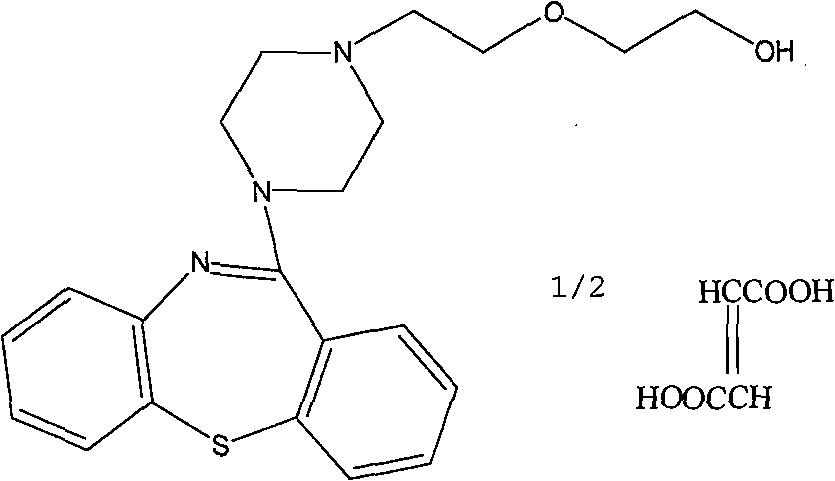
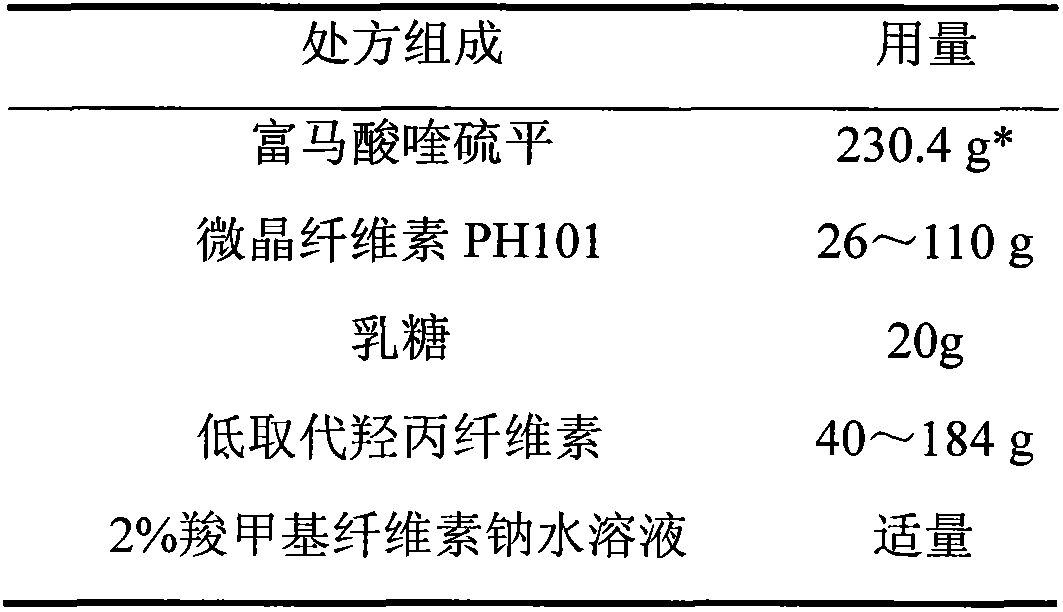
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
72 results about "Quetiapine Fumarate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
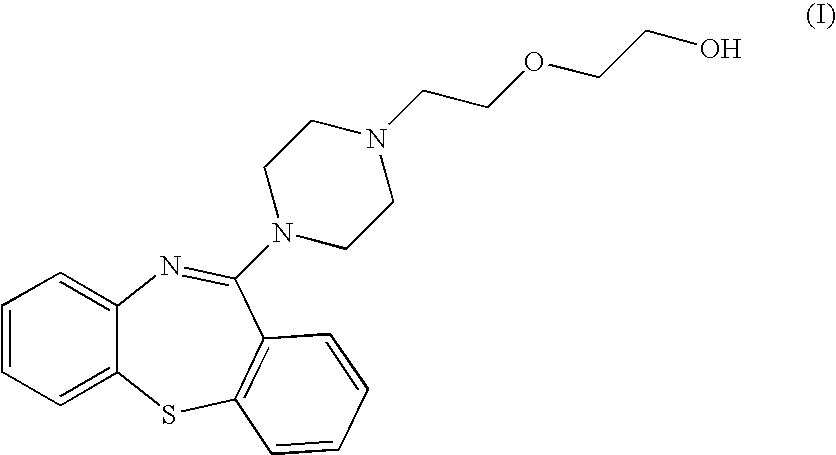
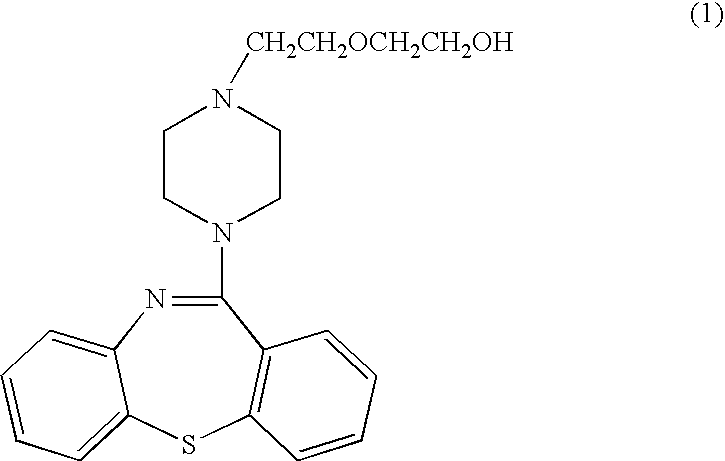
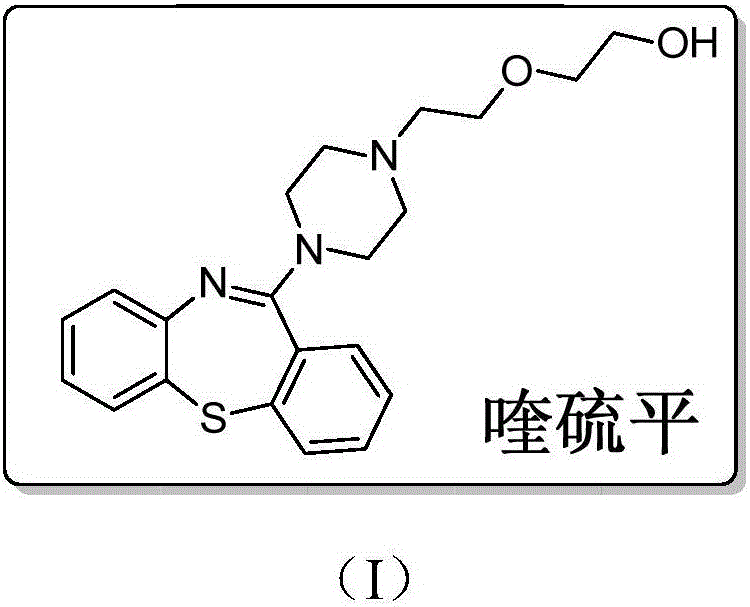
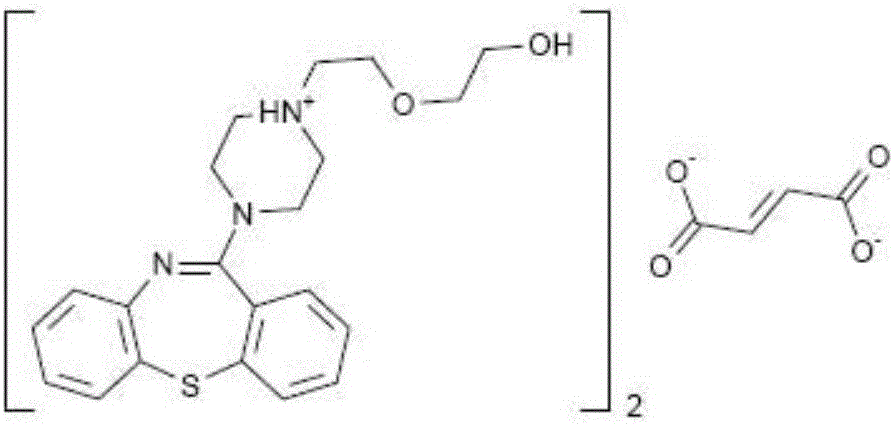
A dibenzothiazepine and ANTIPSYCHOTIC AGENT that targets the SEROTONIN 5-HT2 RECEPTOR; HISTAMINE H1 RECEPTOR, adrenergic alpha1 and alpha2 receptors, as well as the DOPAMINE D1 RECEPTOR and DOPAMINE D2 RECEPTOR. It is used in the treatment of SCHIZOPHRENIA; BIPOLAR DISORDER and DEPRESSIVE DISORDER.
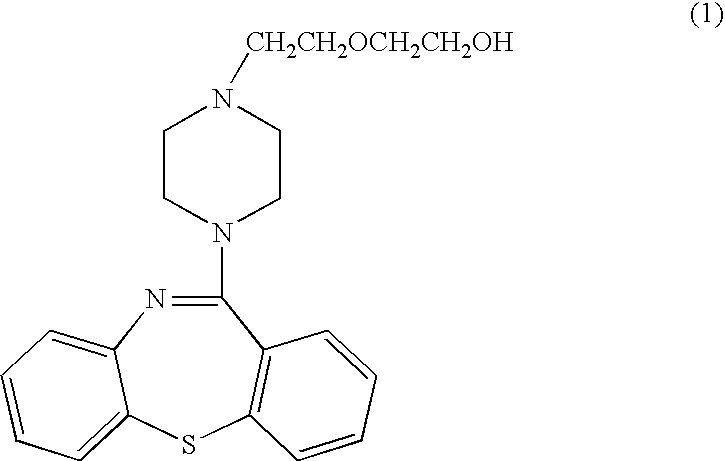
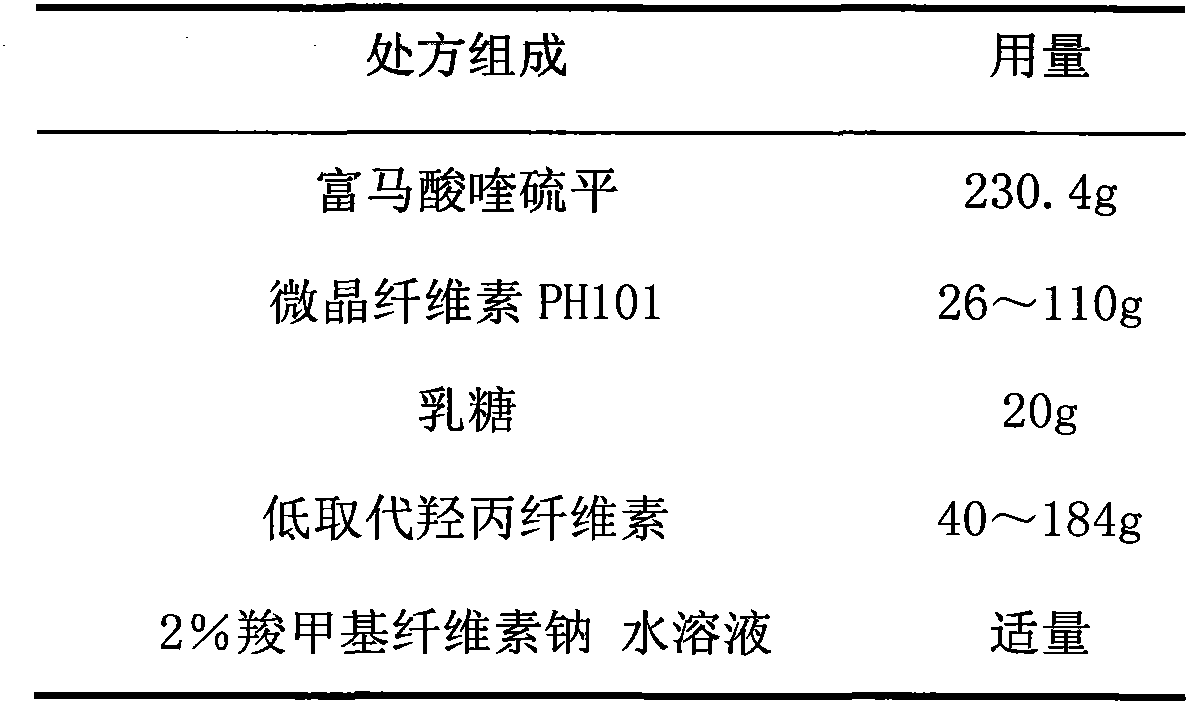
Composition of slow (controled) releasing preparation of Quetiadine Hemifumarate, and application
InactiveCN101091700ATo achieve the purpose of sustained (controlled) releaseOrganic active ingredientsPowder deliveryOrganic acidWax
The present invention discloses a quithiopine fumarate slow (controlled) release preparation and its application. Its preparation composition includes (by wt%) 27-50% of quithiopine fumarate, 2-7% of organic acid, 40-50% of water-soluble macromolecule, 2-7% of enteric solubility material, 0.1-2% of wax and 2-7% of water-insoluble macromolecule. Said preparation can be effectively used for curing schizophrenia.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT +1
Sustained release tablet of quetiapine fumarate composition and preparation method of sustained release tablet
InactiveCN102218042AReduce weightIncrease toleranceOrganic active ingredientsNervous disorderSustained Release TabletOrganic acid
The invention discloses a sustained release tablet of a quetiapine fumarate composition, comprising the following components in percentage by weight: 25 to 40% of quetiapine fumarate, 2 to 8% of organic acid salt, 5 to 30% of a sustained release material and the balance of other pharmaceutical adjuvants, wherein the sustained release material is K type hydroxypropyl methylcellulose. The sustained release material is the K type hydroxypropyl methylcellulose and the organic acid salt is added, therefore, a stable sustained release skeleton can be formed by few sustained release materials, the raw material can be saved, the weight of unit preparation can be reduced, and the problem of difficulty in swallowing caused by large size of the preparation can be released and / or solved; the hydroxypropyl methylcellulose with different viscosities are used as the material of the skeleton so that the preparation can reach formulated sustained release effects and has the characteristics of strong controllability and stability in storage. By use of the preparation method capable of granulating in one step, the operation is simplified and the production efficiency can be improved.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Method for simultaneously detecting seven sleep chemical medicines
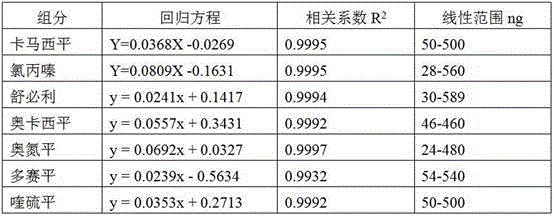
The invention discloses a supplemental detection method of seven chemical medicines including carbamazepine, chlorpromazine hydrochloride, olanzapine, doxepin hydrochloride, quetiapine fumarate, oxcarbazepine and sulpiride which are illegally added into health-care food or Chinese patent medicine for improving sleep. After a sample is subjected to ultrasonic extraction with methyl alcohol, chromatogram column separation is conducted, a mobile phase is eluted, and DAN detector is used for detection. By means of a built detection method, methodological verification is conducted, and parameters of results are shown in the description. It is verified that the method is quick, high in specificity and suitable for detection of chemical medicine added to the health-care food or Chinese patent medicine for improving sleep.
Owner:SHANXI PROVINCE FOOD & DRUG INSPECTION INST
Quetiapine fumarate tablet and preparation method thereof
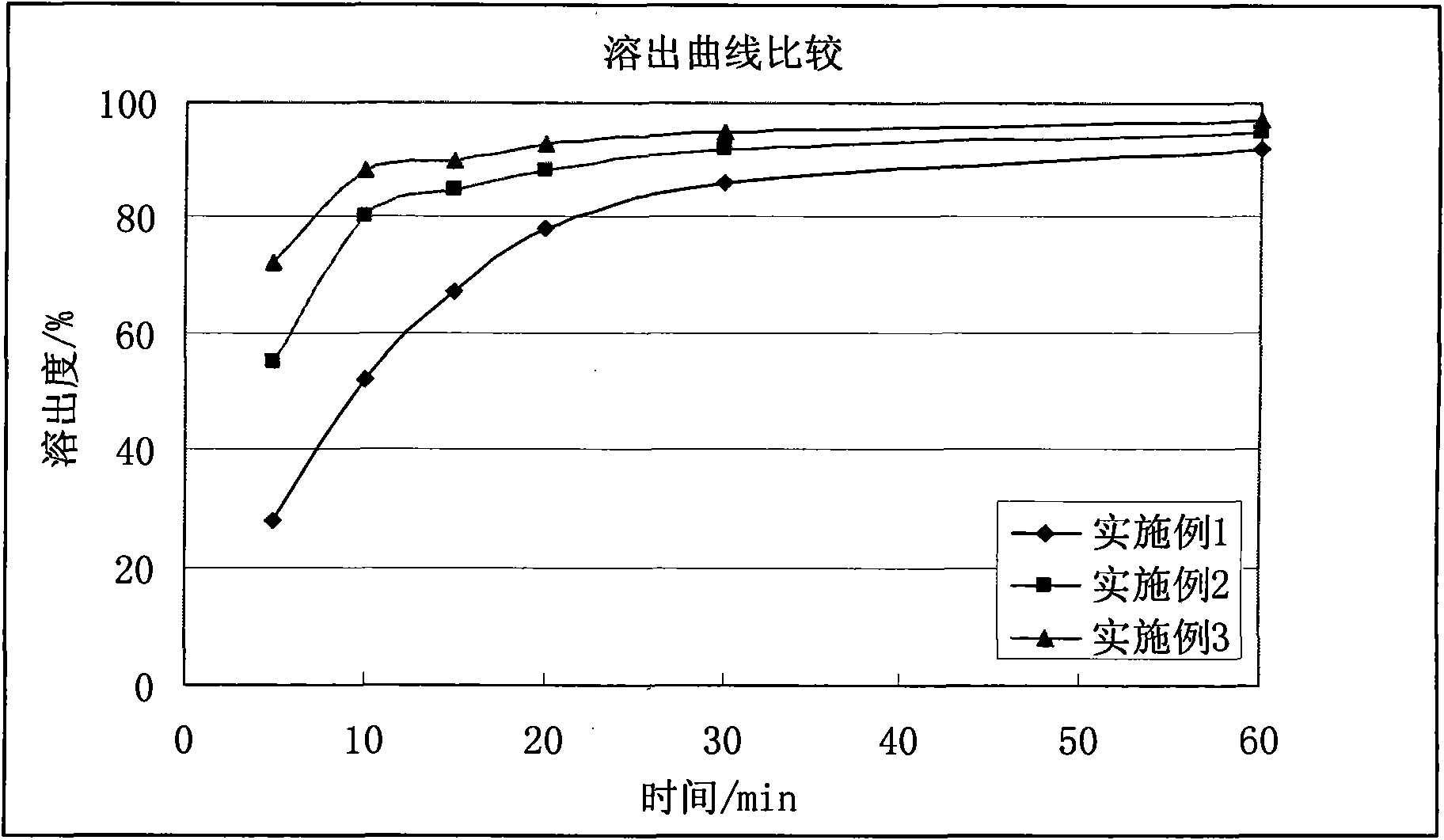
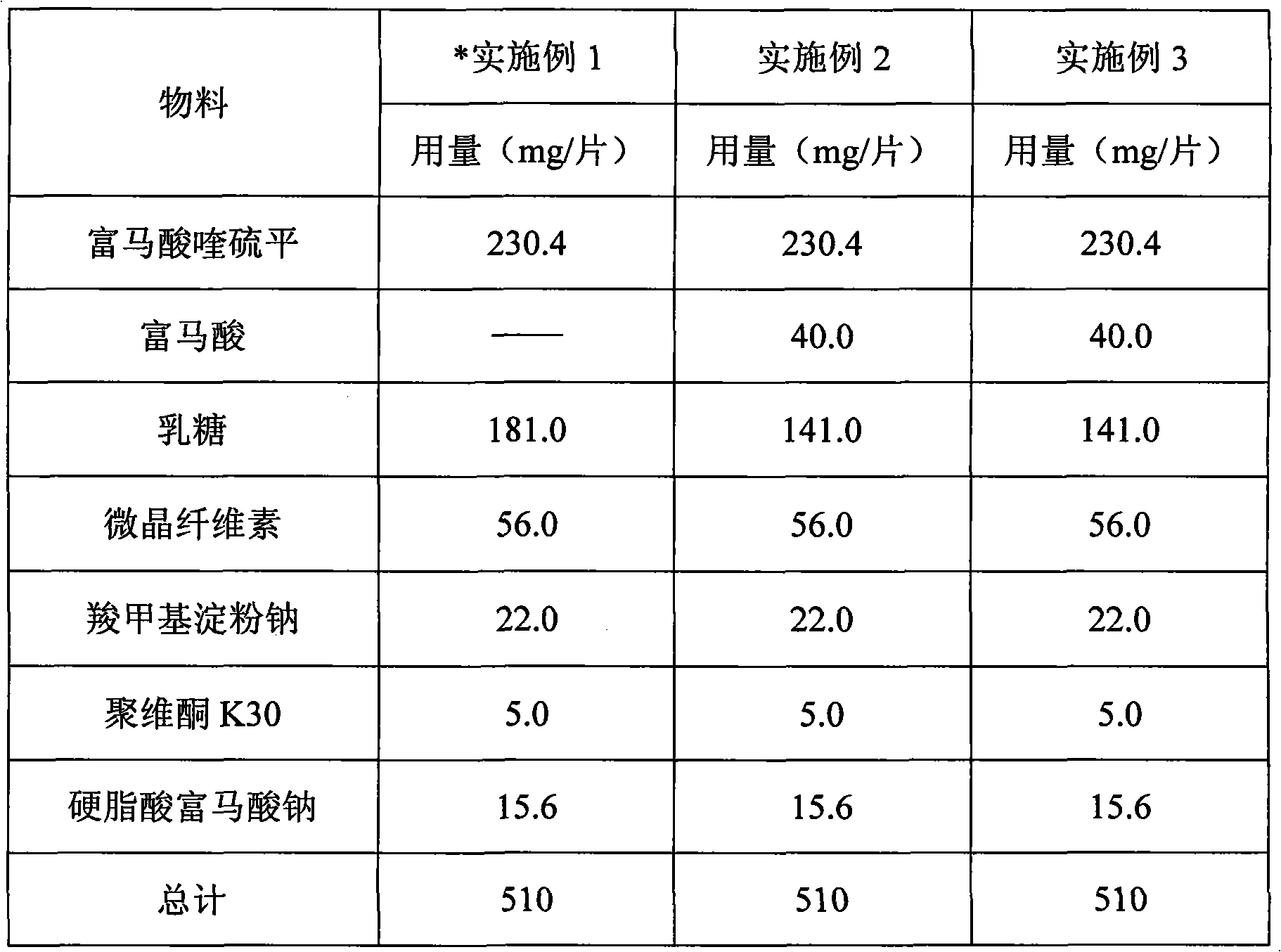
InactiveCN101940561APromote dissolutionSolve the problem of poor dissolutionOrganic active ingredientsNervous disorderQuetiapineBULK ACTIVE INGREDIENT
The invention provides a quetiapine fumarate tablet which comprises active ingredient quetiapine fumarate or pharmaceutically acceptable salt, and at least one pharmaceutic adjuvant; the quetiapine fumarate tablet also comprises one or more specific acids which can promote a medicine to be rapidly dissolved out; and fumaric acid is preferentially selected. The invention also relates to a preparation method of the quetiapine fumarate tablets. By the invention, the product which can be rapidly dissolved out and has good stability can be acquired, and the quality of the product can be improved.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Pharmaceutical compositions containing quetiapine fumarate
InactiveUS20080193527A1Improved dissolution profileReduce energy costsOrganic active ingredientsBiocideQuetiapineBULK ACTIVE INGREDIENT
A granule formulation useful for preparation of pharmaceutical compositions. The granule formulation includes a core containing quetiapine or a pharmaceutically acceptable salt thereof as an active ingredient, and a binder agent. The core is coated with a coating layer including a lubricant agent. Solid pharmaceutical compositions containing quetiapine, and their preparation, are described.
Owner:LAB LESVI SL
Quetiapine fumarate sustained-release tablets and preparation method thereof
InactiveCN102335155ASimple processGood slow releaseOrganic active ingredientsNervous disorderSustained Release TabletQuetiapine
The invention belongs to the field of medicinal preparations, and discloses quetiapine fumarate sustained-release tablets and a preparation method thereof. The sustained-release tablets are mainly prepared from the following raw materials in part by mass: 1 part of quetiapine fumarate (based on quetiapine), 0.1 to 1 part of acrylic resin framework material, 0 to 0.5 part of filling agent, 0.04 to0.05 part of adhesive and 0.01 to 0.02 part of lubricating agent. The sustained-release tablets can comprise hydrophilic gel framework material for adjusting the release rate of the main medicament, and the mass ratio of the quetiapine to the hydrophilic gel framework material is 1: (0.1-0.5). The prepared quetiapine fumarate sustained-release tablets can be used for treating schizophrenia. The sustained-release tablets have the advantages of reducing the administration frequency, improving the administration compliance of a patient, improving the medicinal effect, ensuring application safetyand the like, and are more suitable for patients. Meanwhile, the preparation method of the sustained-release tablets is simple and favorable for industrialized large-scale production.
Owner:SUZHOU UNIV
Method for preparing pharmaceutical pure quetiapine fumarate
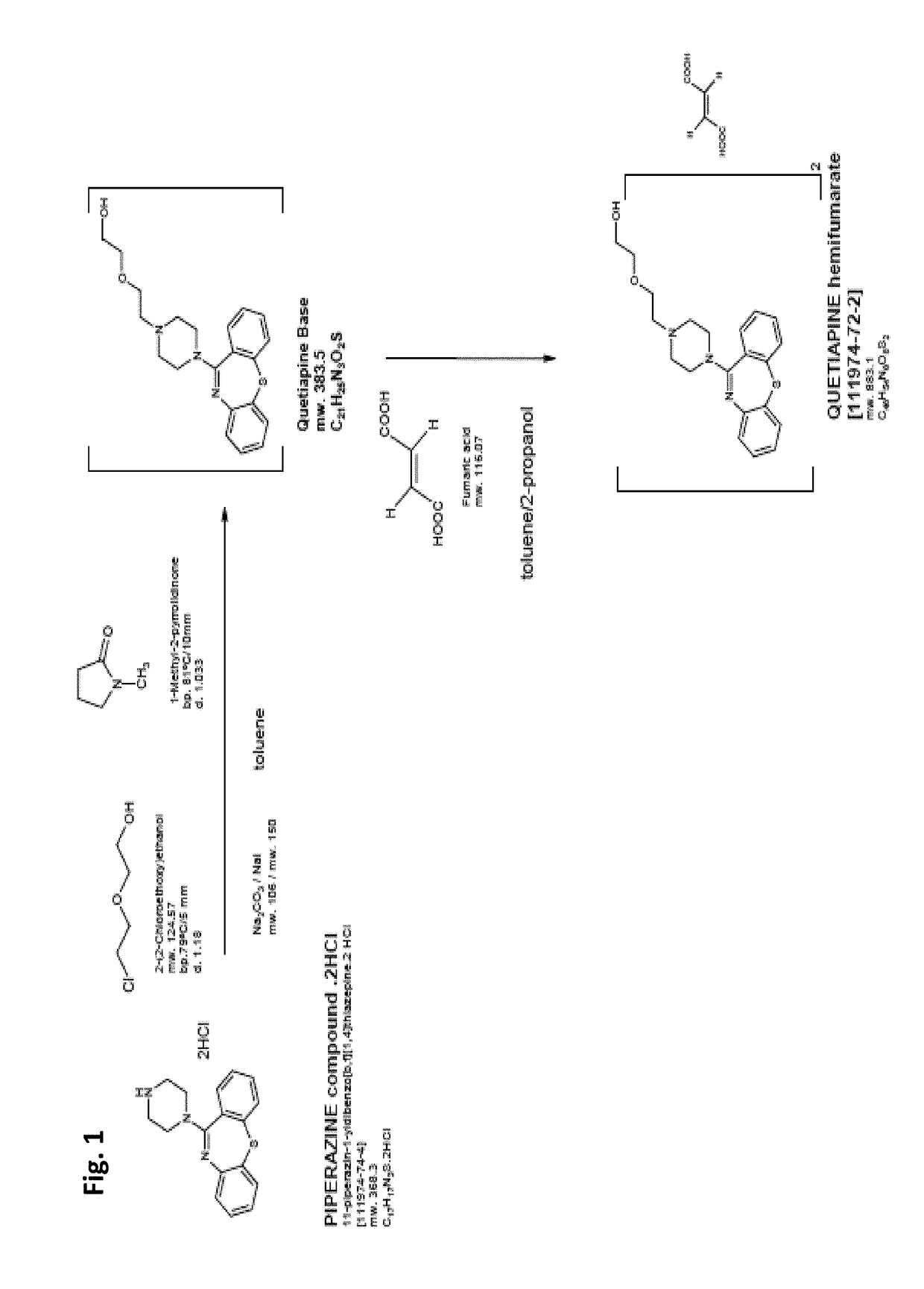
ActiveCN101190902ASolve technical problems with strong side reactionsSolve technical problems of side reactionsOrganic chemistryDimethylaniline N-oxideThiazepine
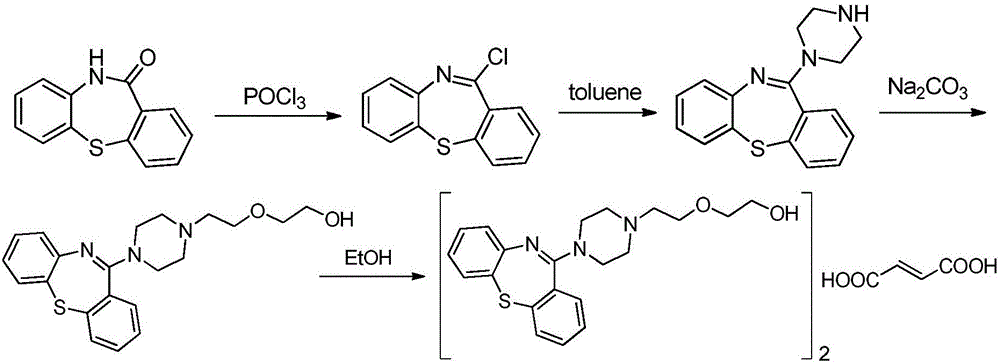
The invention relates to a preparation method of pure quetiapine fumarate and the steps of the process thereof are that: (1)chlorination: four materials of dibenzo(b,f)(1,4)thiazepine-11-(10H)-ketone, chlorinated oxidant, N,N-Dimethylaniline and toluol react to obtain toluol solution of chloride; (2) addition and salification: the toluol solution of chloride reacts to obtain quetiapine according to the ratio of 1:0.5-1:0.5-0.8 of cyclic product (kg) to N-(2-(2-Hydroxyethoxy)ethyl)piperazine (kg) to anhydrous sodium carbonate; the mixture of the quetiapine (kg) and fumaric acid (kg) and ethanol (kg) is then salified to obtain the quetiapine fumarate according to the respective ratio 1:0.1-0.3:2-4. The invention effectively controls the impurity content of the quetiapine fumarate below 0.1 percent, improves the quality of products and achieves the standard of pharmaceutical purity and causes little side effects to patients. In addition, the invention also solves the problems of recovery of phosphorus oxychloride and environment pollution, shortens the cycle of reaction, improves the yield and reduces the cost.
Owner:HUNAN DONGTING PHARMA
Quetiapine fumarate tablet and preparation method thereof
The invention relates to a quetiapine fumarate tablet and a preparation method thereof. The tablet of the invention comprises quetiapine fumarate, a filler, a disintegrant, povidone and magnesium stearate in certain proportions. The preparation method comprises the following steps: granulating the quetiapine fumarate, the filler and the disintegrant by a wet method; then adding the povidone and granulating into a soft material; screening, drying and finishing the soft material; adding the magnesium stearate and mixing; measuring the content and calculating the tablet weight; and respectively tabletting and coating to obtain the quetiapine fumarate tablet. The tablet can be used for treating schizophrenia. By using the formula provided by the invention, the defect of poor particle mobility during granulating in the prior art is overcome.
Owner:SHANGHAI CHENPON PHARM TECH CO LTD
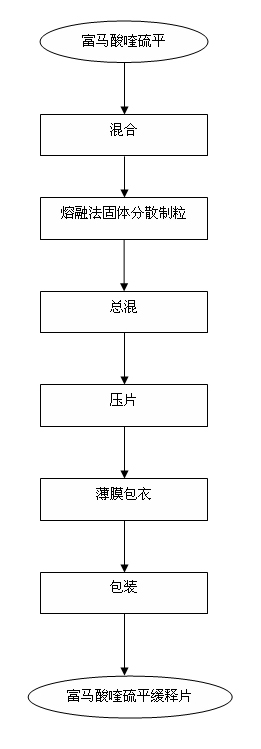
Preparation process of quetiapine fumarate slow release tablet
InactiveCN102198113AReduce the number of dosesImprove complianceOrganic active ingredientsNervous disorderMedicineFilm-coated tablet
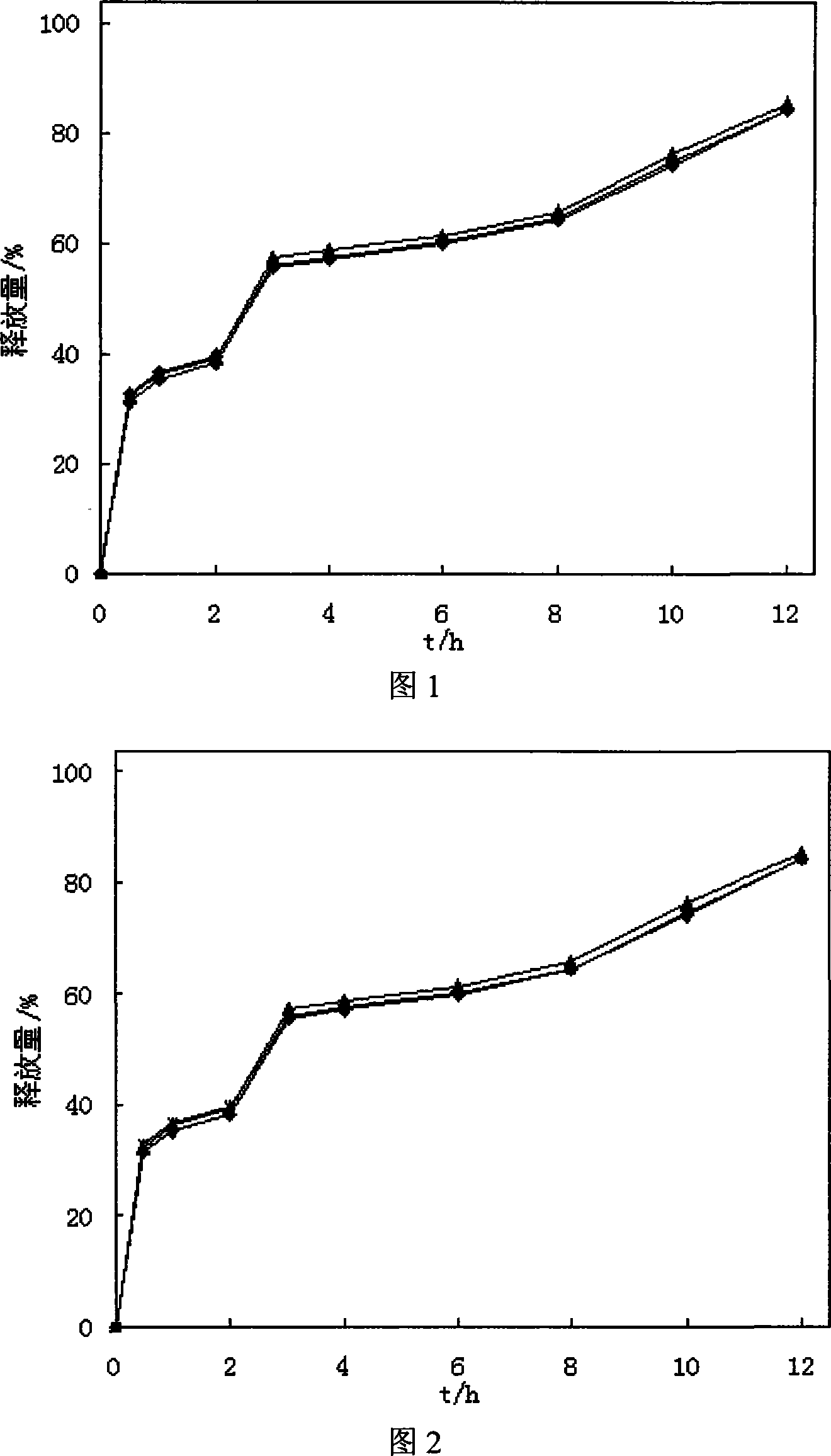
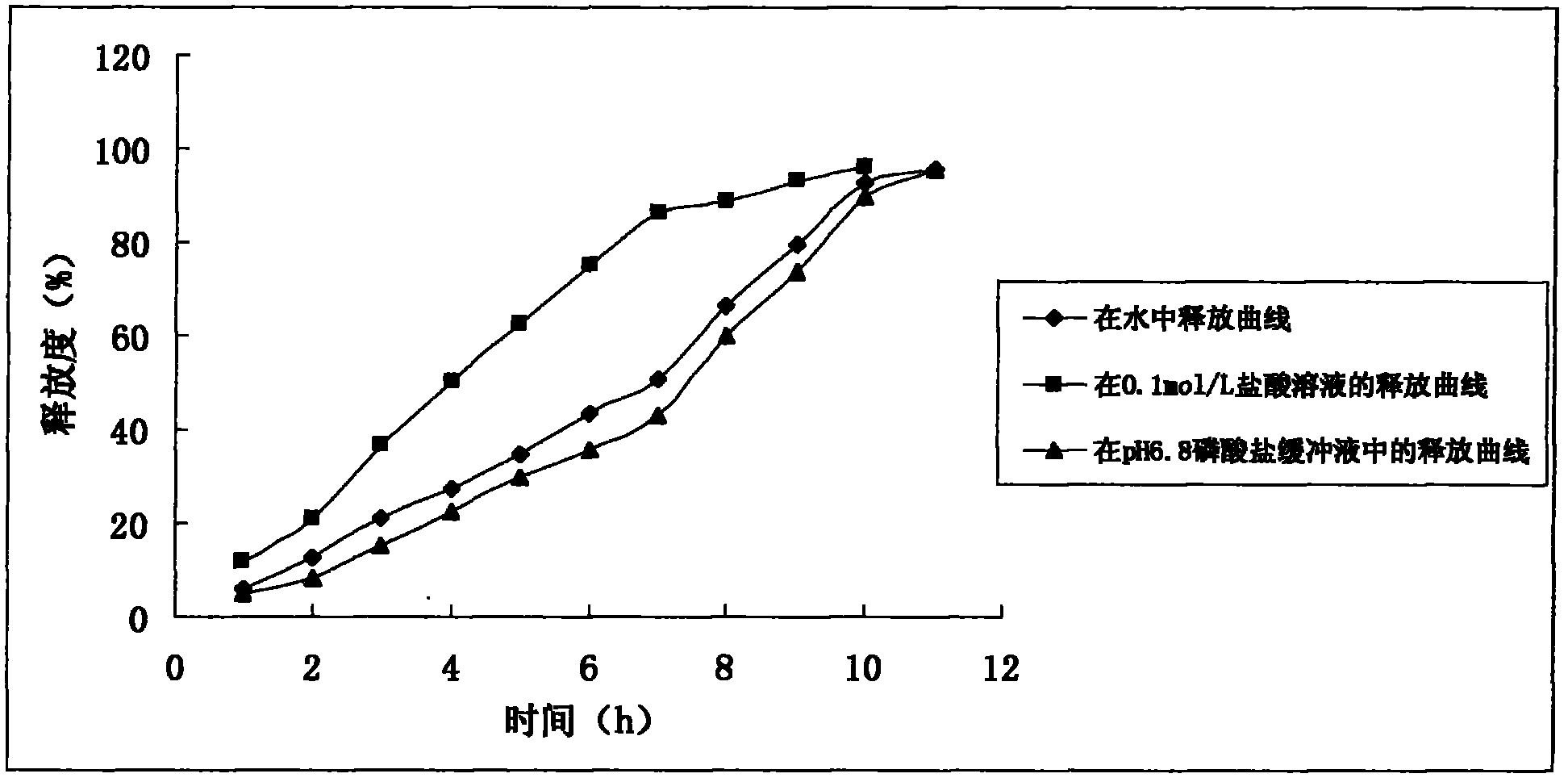
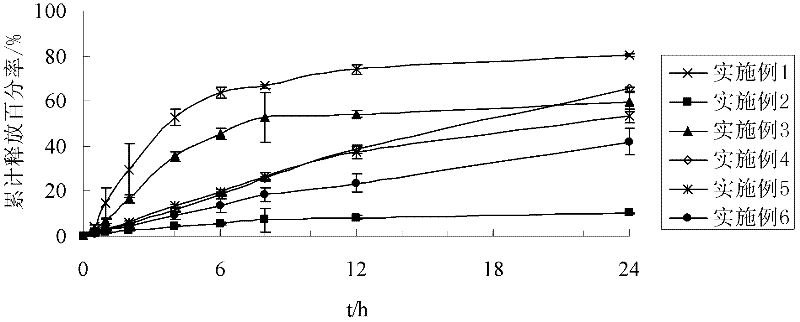
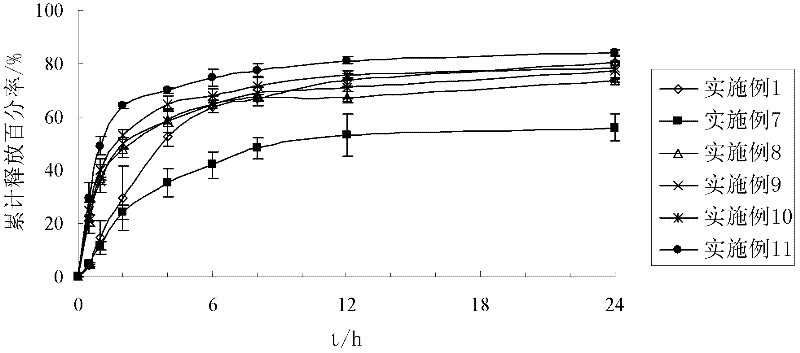
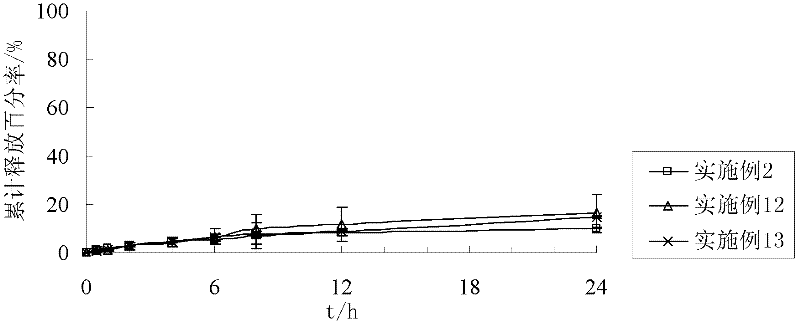
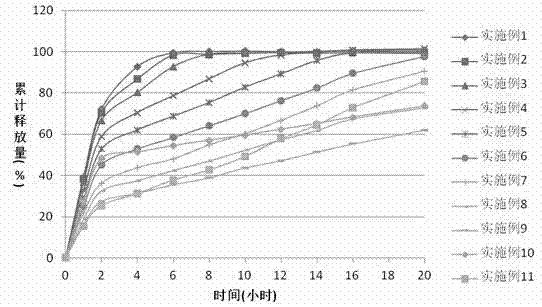
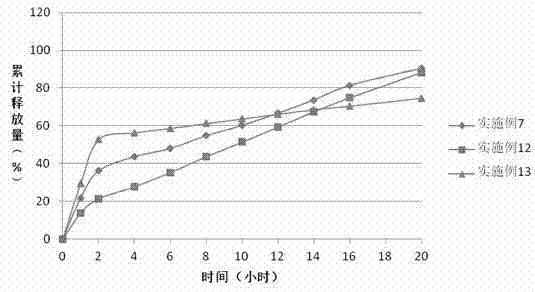
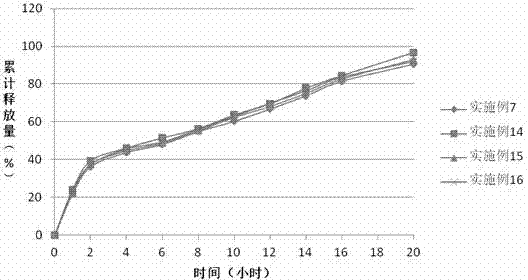
The invention discloses a preparation process of a quetiapine fumarate slow release tablet, belonging to the technical field of preparing medicinal slow / controlled-release preparations. In the process, a soluble slow-release accessory is used as a retarding agent, and slow-release particles are prepared by using a fusion-method solid dispersion technology and are tabletted. The quetiapine fumarate slow release tablet is the slow-release matrix tablet prepared by using a process of externally covering the tablet with a quick-release thin-film coating. The quetiapine fumarate in the invention has the in-vitro release characteristics as follows: after 750ml of 0.1NHCl is added for 2 hours, the release amount of quetiapine fumarate within 0-2 hours is 0-35%, and after 250ml of K3PO4 is added, the release amounts of the quetiapine fumarate within 2-8 hours, 8-12 hours and 12-20 hours are respectively 35-60%, 60-80% and 80-100%. The process has the advantages of simple and safe flow, controllable quality, low cost, short production period and great suitability for large-scale industrial production.
Owner:SICHUAN UNIV
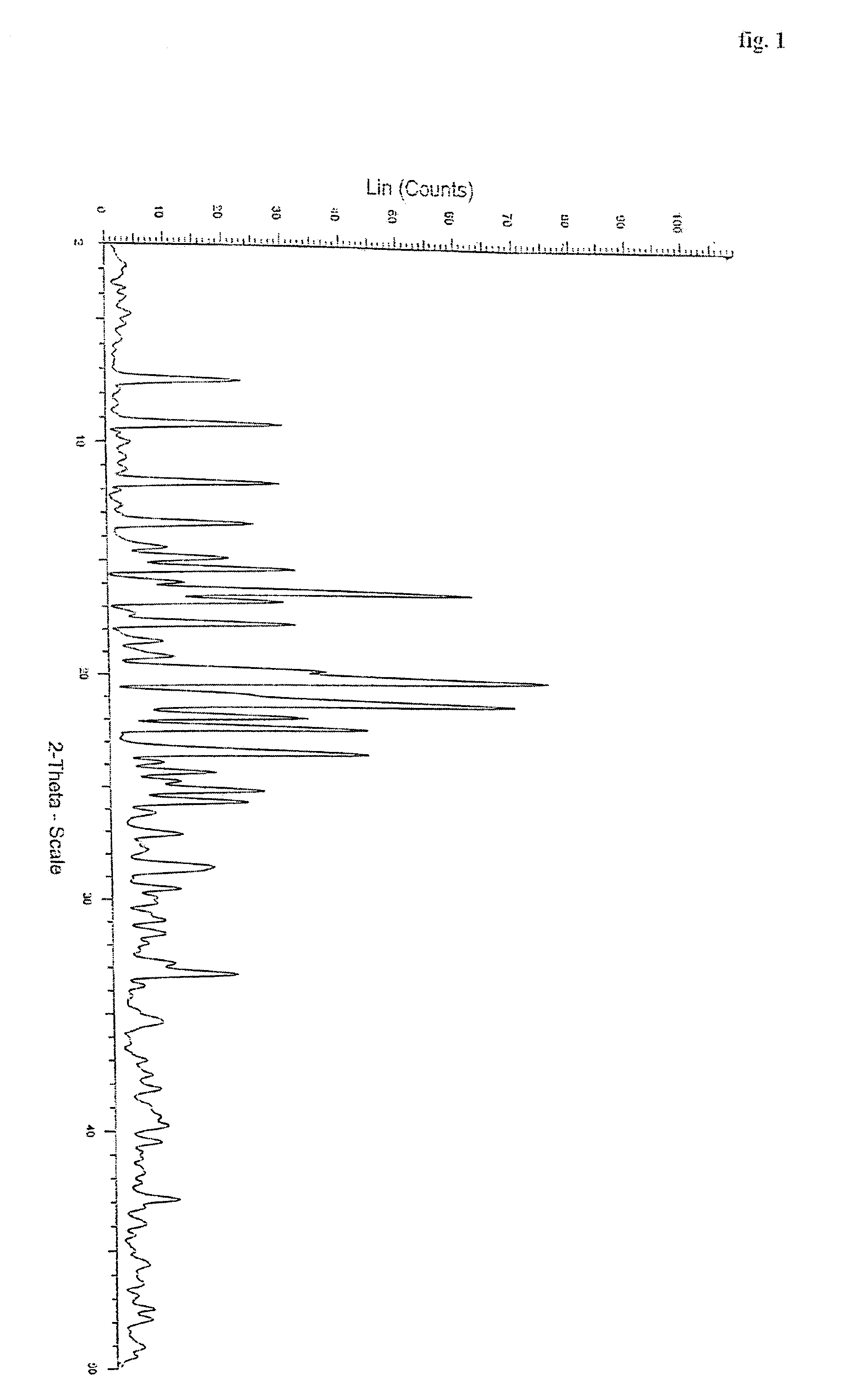
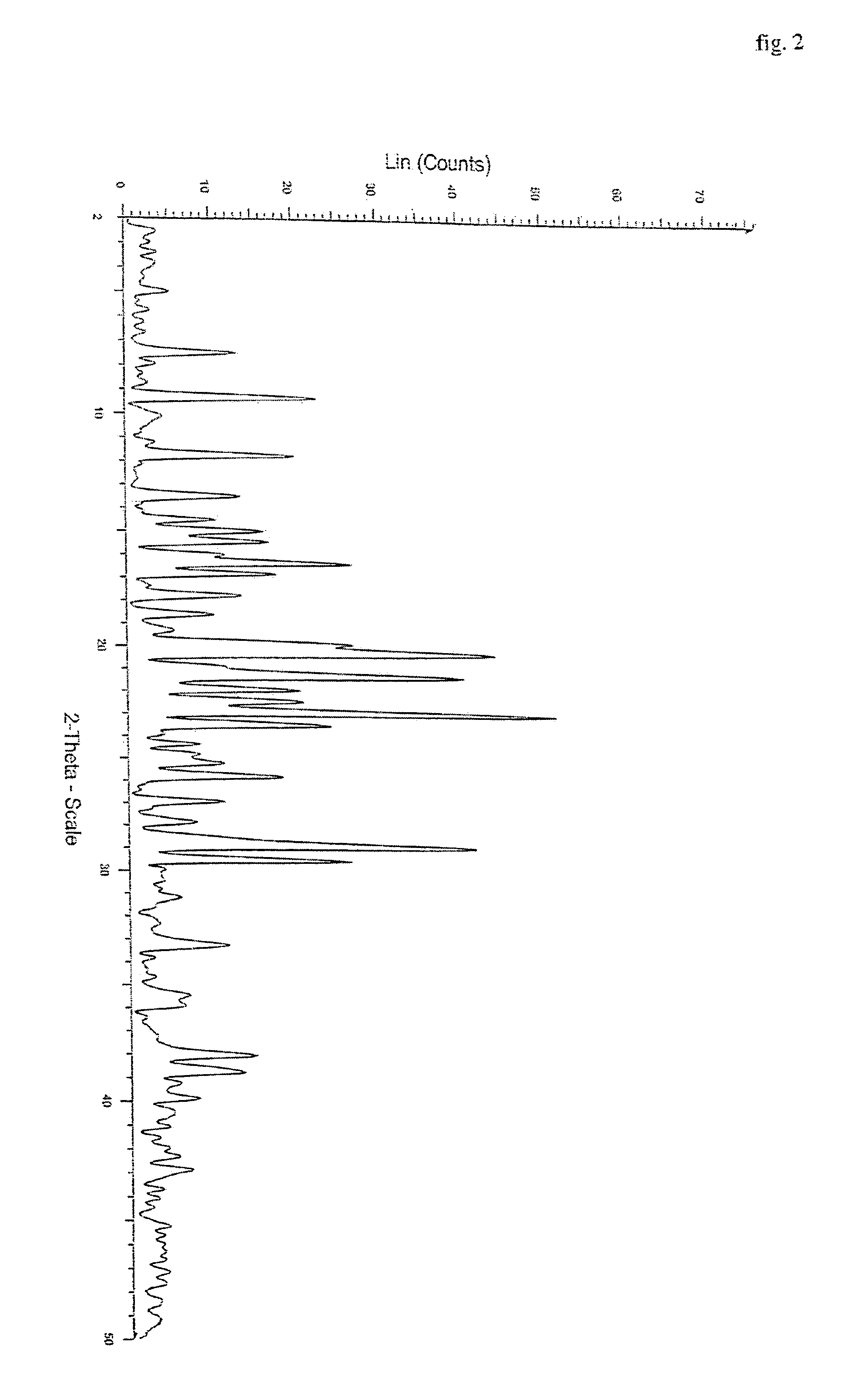
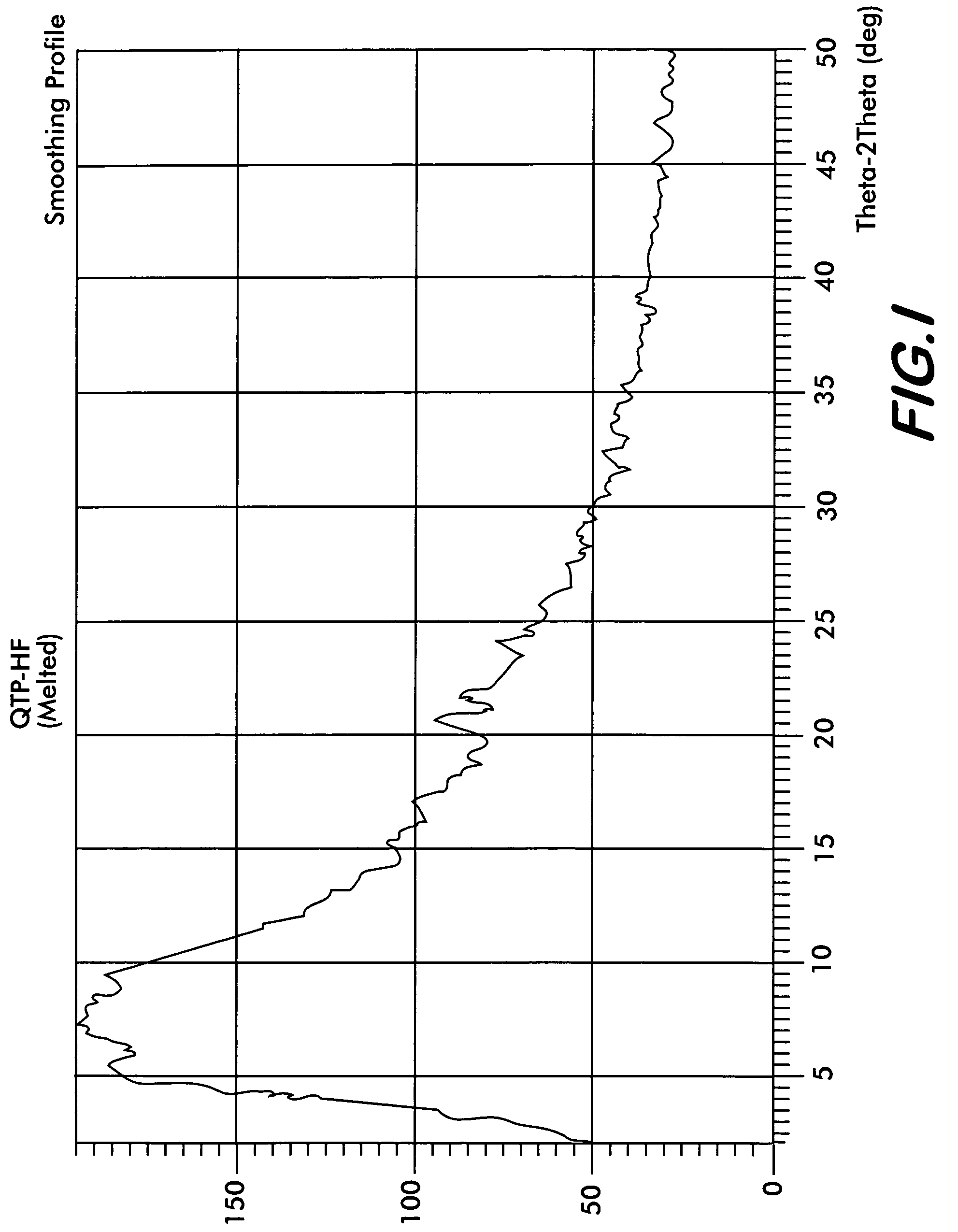
Polymorphs of quetiapine fumarate
InactiveUS7238686B2Improve stabilityImprove solubilityBiocideOrganic chemistryMedicineQuetiapine Fumarate
The present invention relates to novel polymorphic forms of quetiapine fumarate, processes for their preparation and pharmaceutical compositions containing them.
Owner:HETERO DRUGS LTD
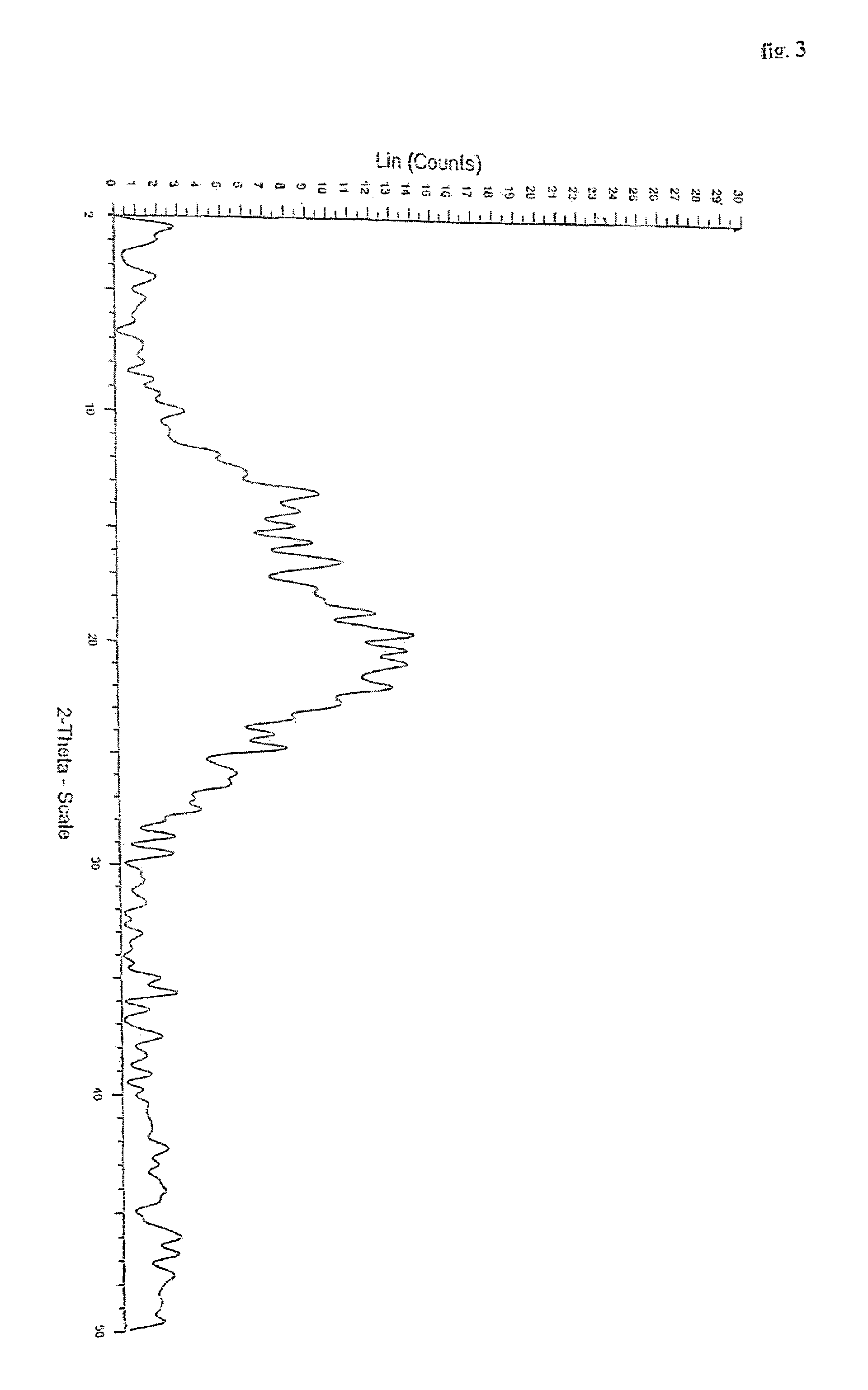
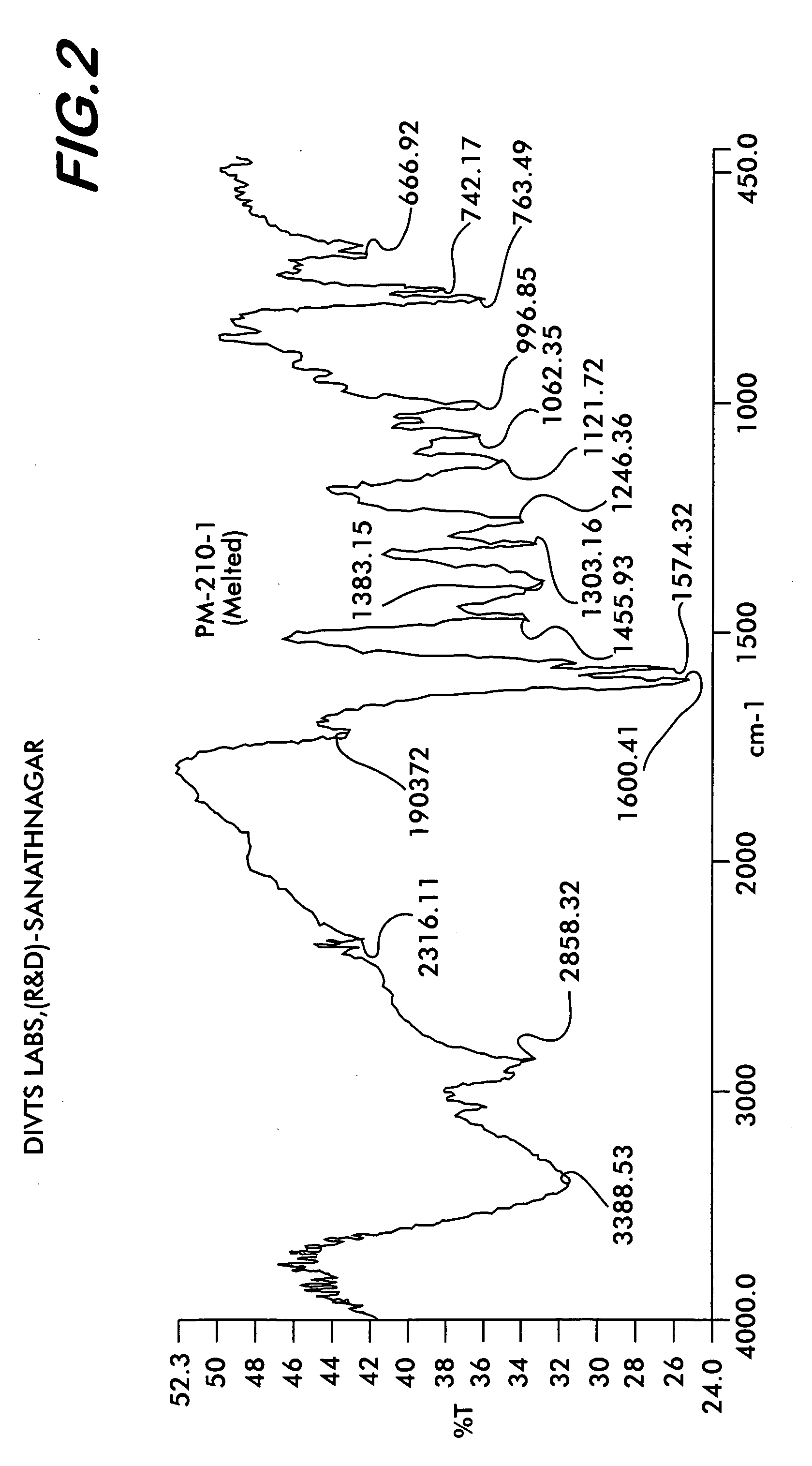
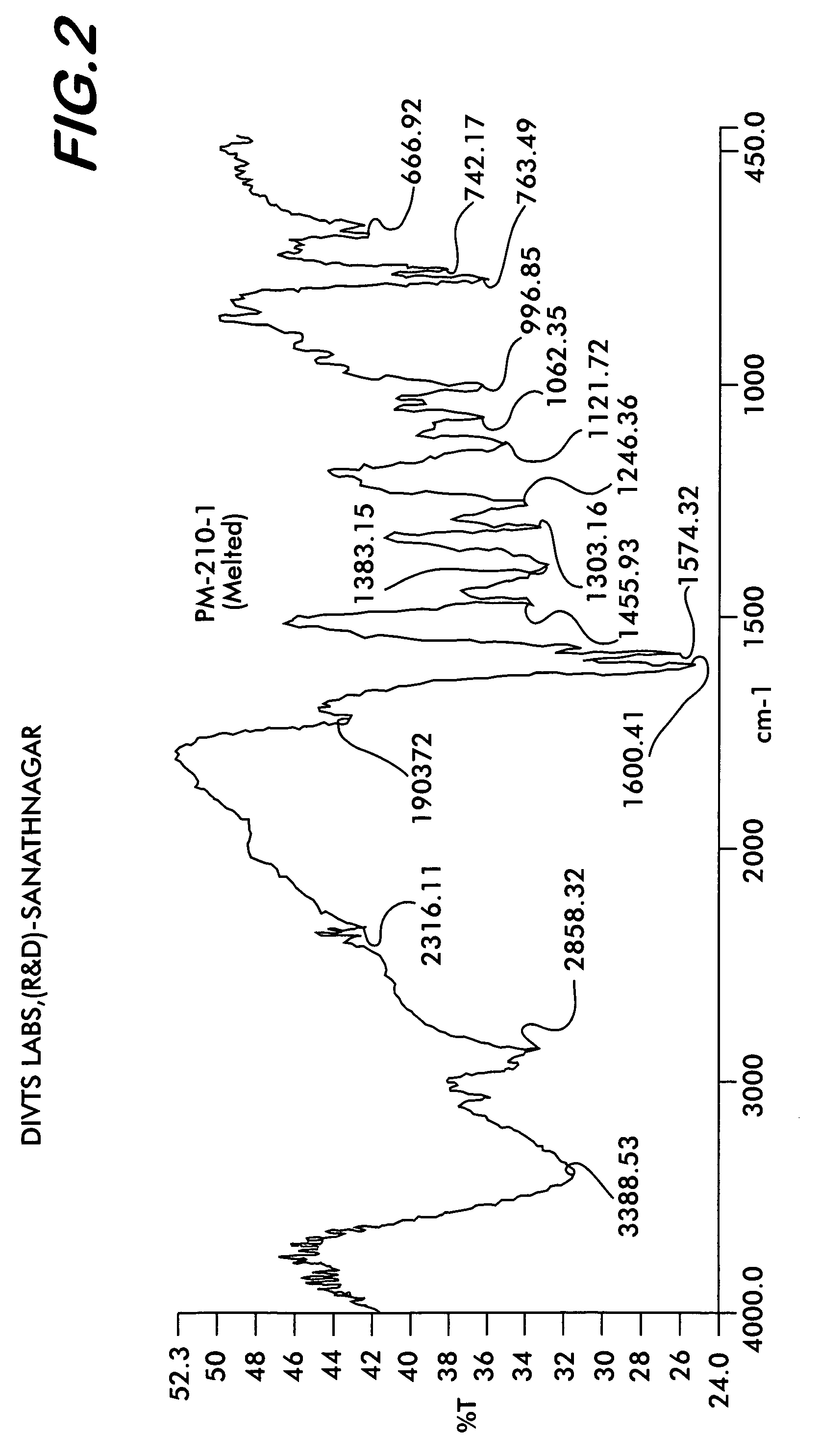
Novel polymorph of Quetiapine fumarate and a process for its preparation
ActiveUS20060223994A1Economical and simpleAcceptable stabilityOrganic chemistryChemistryQuetiapine Fumarate
The present invention relates to a novel polymorph of Quetiapine fumarate and a simple method for its preparation.
Owner:DIVI S LAB LTD
Pharmaceutical composition of quetiapine fumarate
A pharmaceutical composition comprising (i) quetiapine or a pharmaceutically acceptable salt thereof, especially quetiapine fumarate; (ii) a mixed excipient comprising an intimate admixture of polyvinylacetate and polyvinylpyrrolidone in a weight ratio from 5:2 to 10:2; and, optionally (iii) an acid especially fumaric acid.
Owner:SYNTHON BV
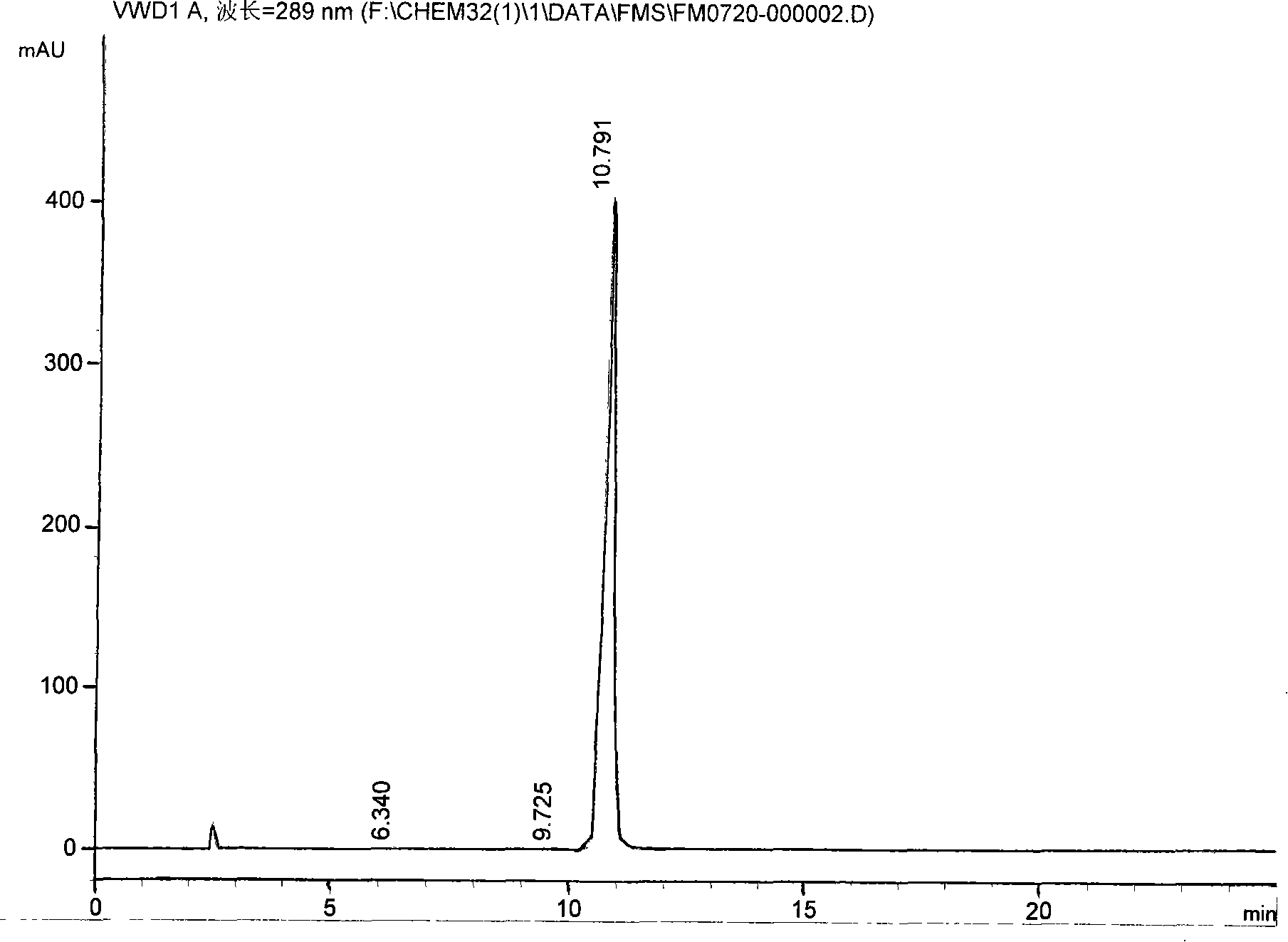
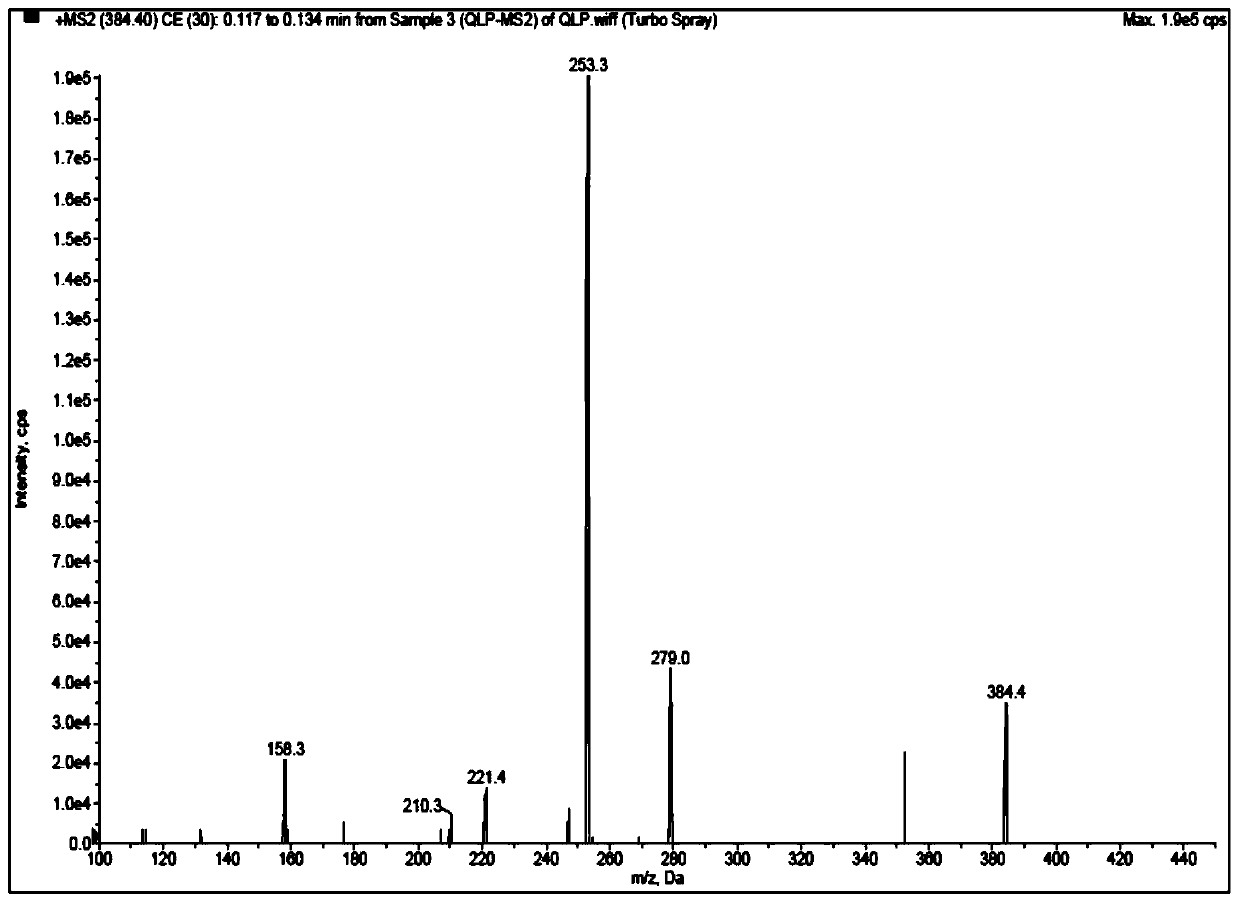
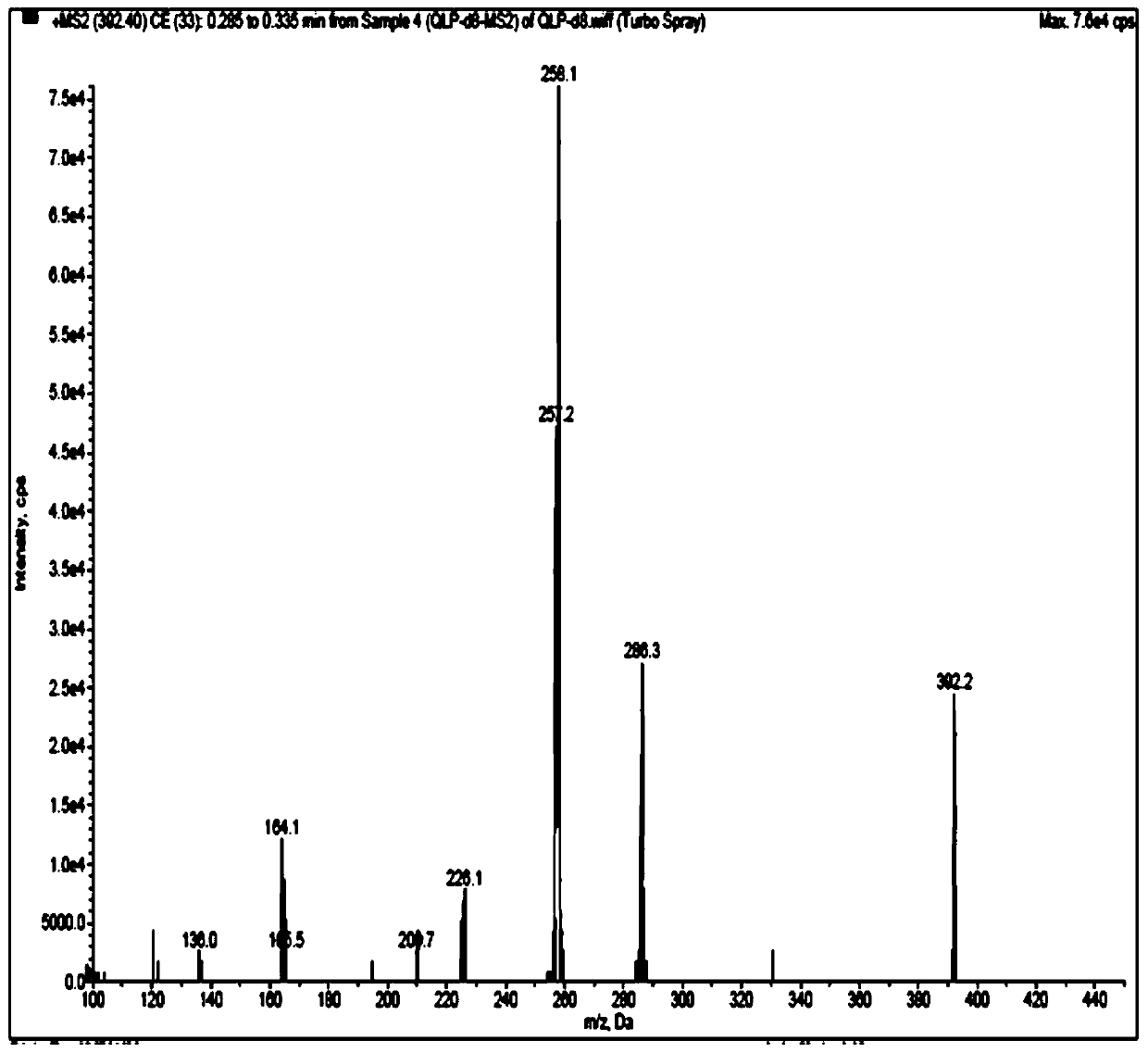
Method for detecting quetiapine in human plasma by HPLCMS-MS combination
InactiveCN109900820ASame retention timeGood reproducibilityComponent separationQuetiapineRetention time
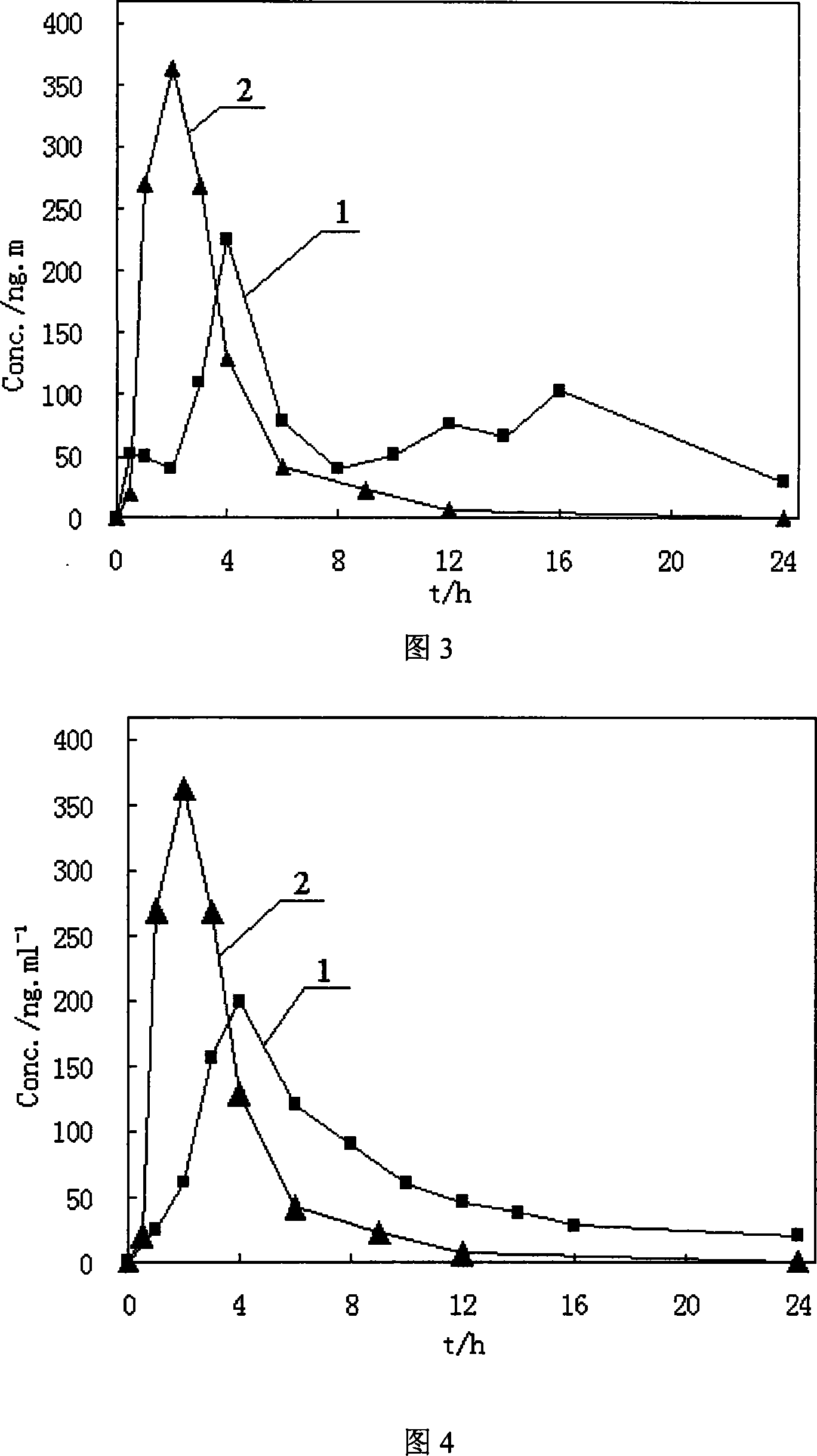
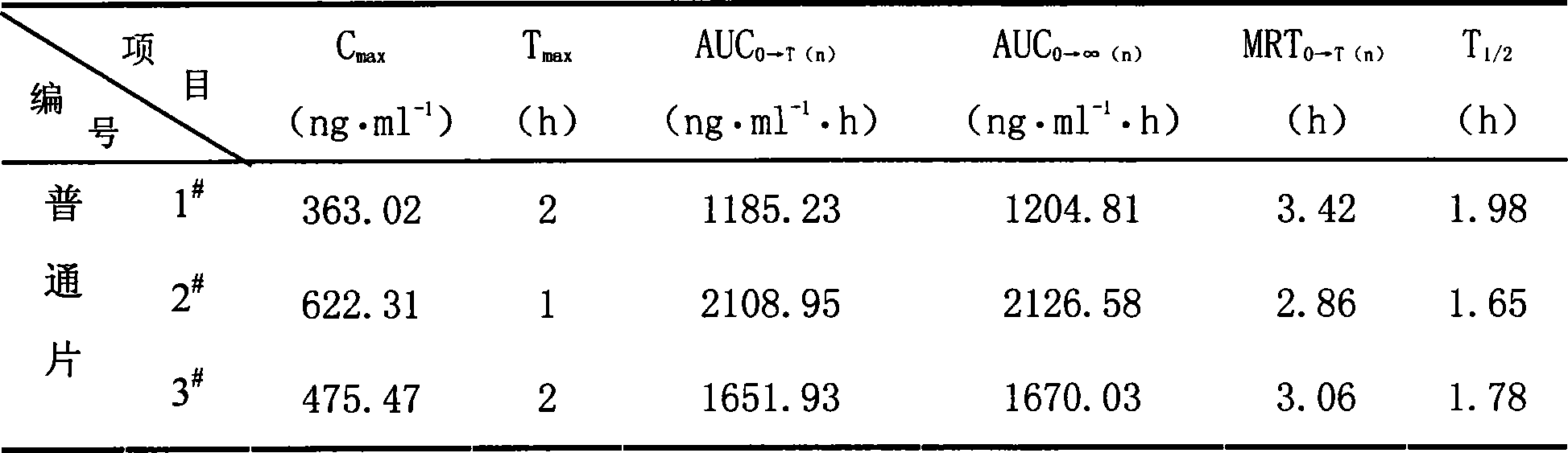
The invention belongs to the field of biological analysis and particularly relates to a method for detecting quetiapine in human plasma by HPLCMS-MS combination. The method comprises steps of (1), human plasma sample pretreatment; (2), liquid chromatography-mass spectrum combined detection, mobile phase A and mobile phase B are utilized as a mixed mobile phase for gradient elution, the mobile phase A is acetonitrile-water, the mobile phase B is ammonium acetate-water; and (3), determination of the concentration of the quetiapine in the human plasma. The method is advantaged in that deuteratedfumaric acid quetiapine is used as an internal marker, gradient elution is performed through utilizing an InertSustain C18 column, the deuterated internal marker and a determinand have the same retention time, chemical properties and matrix effects, and reproducibility and accuracy of concentration detection of the deuterated fumaric acid quetiapine in plasma are good. The method is used for evaluating bioequivalence of each quetiapine dosage form.
Owner:NANJING HEALTHNICE MEDICAL TECH +2
Pharmaceutical composition of quetiapine fumarate
Owner:SYNTHON BV
Solid quetiapine fumarate liposome preparation
InactiveCN102406606AGood curative effectLong retention timeOrganic active ingredientsNervous disorderSide effectCholesterol
The invention provides a solid quetiapine fumarate liposome preparation and a preparation method thereof. According to the preparation method, quetiapine fumarate, yolk lecithin, cholesterol, soyasterol and sorbitam stearate are selected according to a specific weight ratio and prepared into high-quality quetiapine fumarate liposome, and the quetiapine fumarate liposome is prepared into a solid preparation according to a common preparation method. Compared with the traditional preparation, the preparation provided by the invention has the advantages of greatly improved stability, bioavailability and quality and reduced toxic and side effects.
Owner:HAINAN MEIDA PHARMA
Quetiapine fmarate dispersible tablets and preparation method thereof
InactiveCN101416948AFast absorptionEasy to takeOrganic active ingredientsNervous disorderMedicineQuetiapine
The invention discloses a quetiapine fumarate dispersible tablet preparation and a preparation method thereof. The quetiapine fumarate dispersible tablet preparation is prepared by the following materials according to weight part: 1 part to 300 parts of quetiapine fumarate (calculated by quetiapine), 2 parts to 400 parts of disintegrating agent, 5 parts to 900 parts of filling agent, 0 part to 100 parts of flow agent and 0.2 part to 50 parts of lubricant. The quetiapine fumarate dispersible tablet preparation has the following advantages: 1. the preparation can rapidly disintegrate and conceal the disagreeable taste of drugs; 2. the preparation can be taken by being dispersed in water, also can be swallowed, chewed and sucked, and is convenient to be taken; and 3. the preparation has fast absorption and high biological availability.
Owner:张宏宇
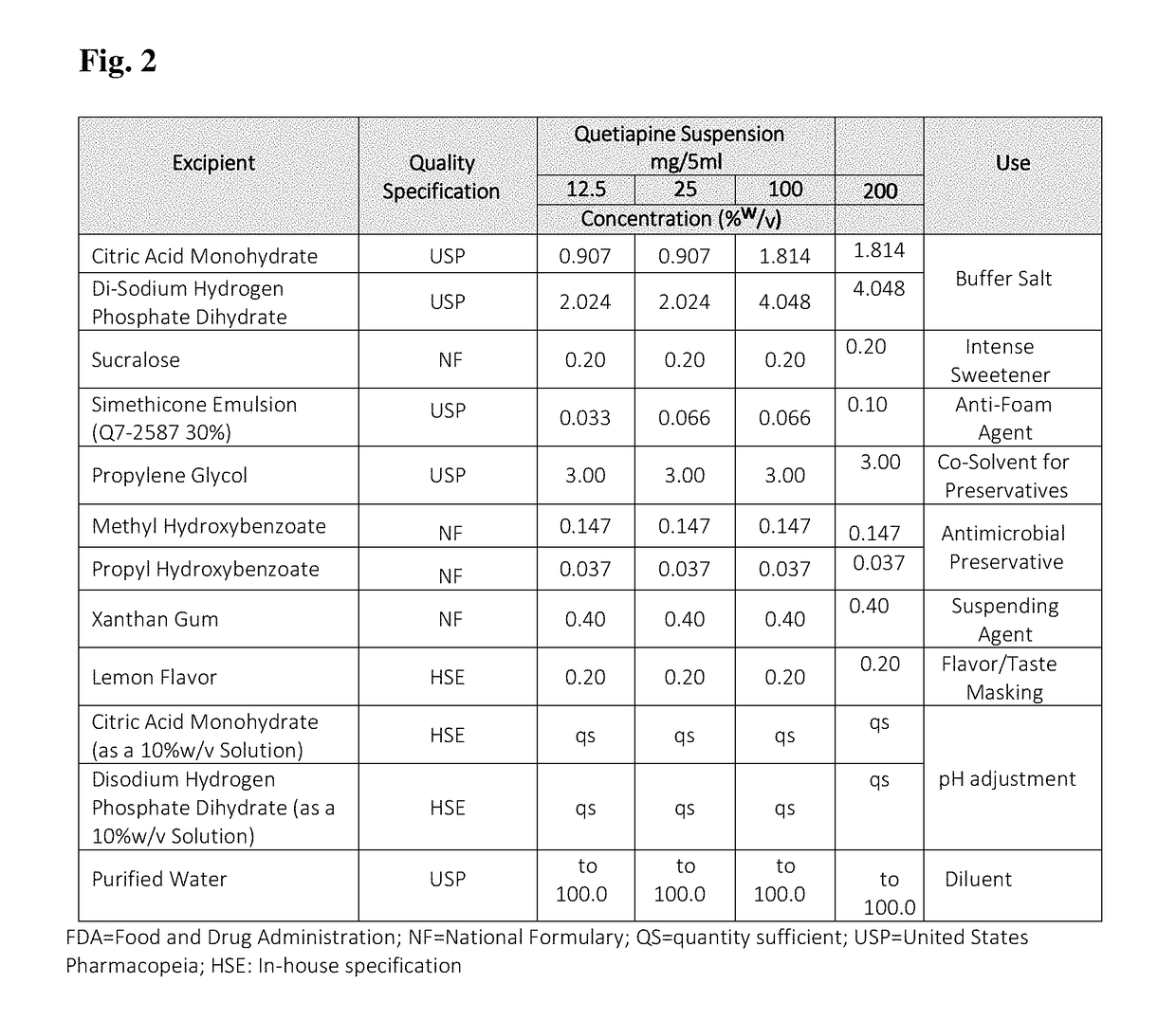
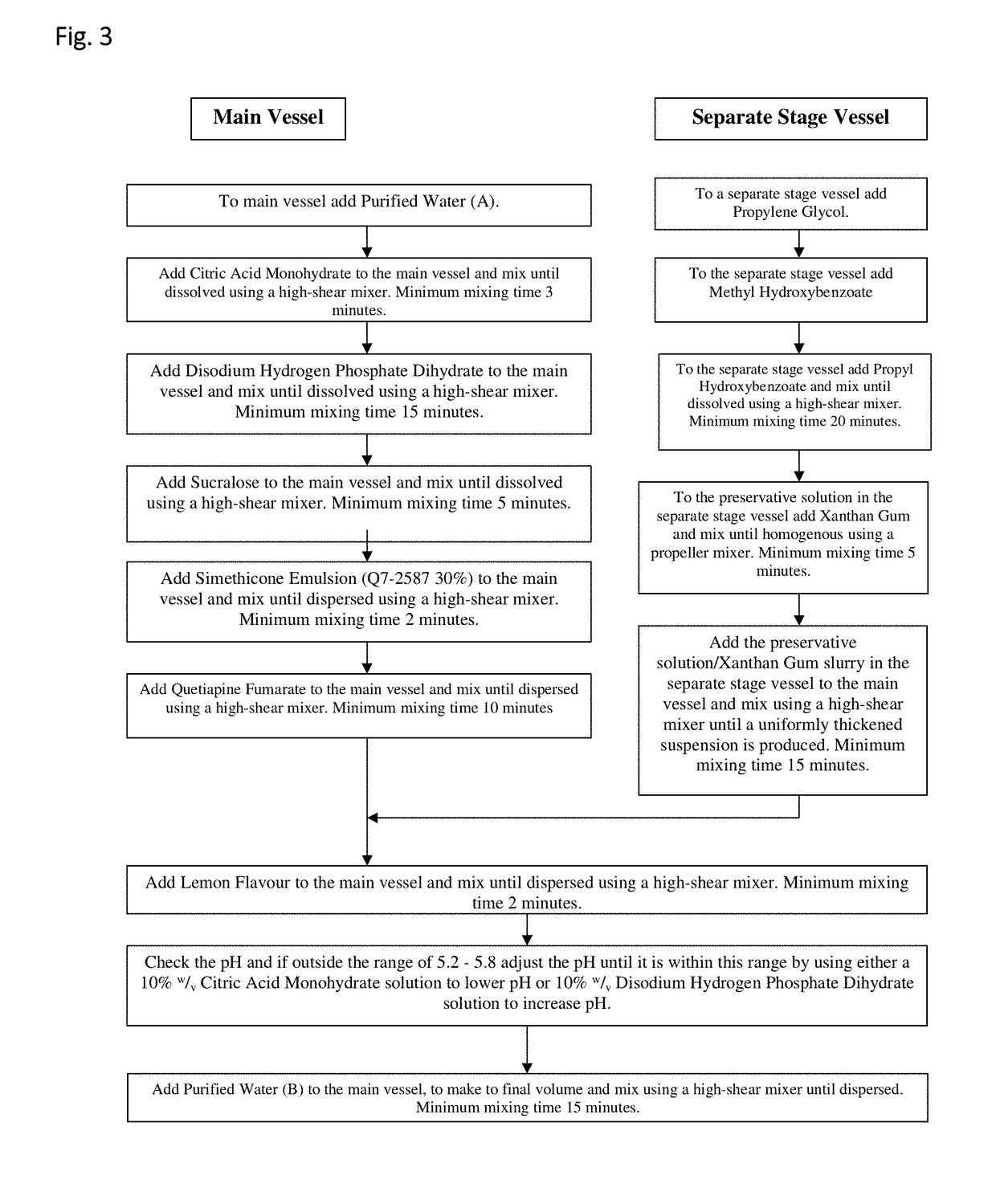
Oral quetiapine suspension formulations with extended shelf life and enhanced bioavailability
A quetiapine fumarate composition for oral administration is provided comprising a pharmaceutically acceptable salt or solvate of quetiapine existing as a suspension in an aqueous carrier agent. The inventive liquid formulation demonstrates high bioavailability consistent with approved dosage forms, low agglomeration, reduced content of excipients commonly used in solid oral dosage forms and extended shelf life stability. Also provided is a method of manufacturing a liquid quetiapine suspension composition for oral administration and methods of administering therapeutically effective dosages of an oral liquid quetiapine suspension composition to patients in need thereof.
Owner:TLC THERAPEUTICS LLC
Quetiapine fumarate sustained-release tablet and preparation method thereof
InactiveCN104586805AGood slow releaseRaw materials are easy to getOrganic active ingredientsNervous disorderOrganic acidSustained Release Tablet
The invention provides a quetiapine fumarate sustained-release tablet. The sustained-release tablet comprises the following components in percentage by weight: 5%-50% of quetiapine fumarate, 2%-12% of hydroxypropyl methylcellulose K4M, 0.4%-5% of hydroxypropyl methylcellulose K100M, 6%-35% of hydroxypropyl methylcellulose K100LV, 5%-20% of organic acid salt, 10%-60% of a filling agent and 0.2-2% of a lubricant. According to the sustained-release tablet, by adopting hydroxypropyl methylcellulose compositions with different viscosities as a sustained-release framework material, the preparation can reach the expected sustained-release effect. By measuring in-vitro release, results show that the preparation is smoothly released at 6 hours, 12 hours and 20 hours and illustrates that the product has better sustained-release property. The raw materials are easily available, the preparation method is simple and the sustained-release tablet is conductive to scale operation, is suitable for industrial production and has better application value.
Owner:SHANGHAI ZHONGXI SUNVE PHARMA +1
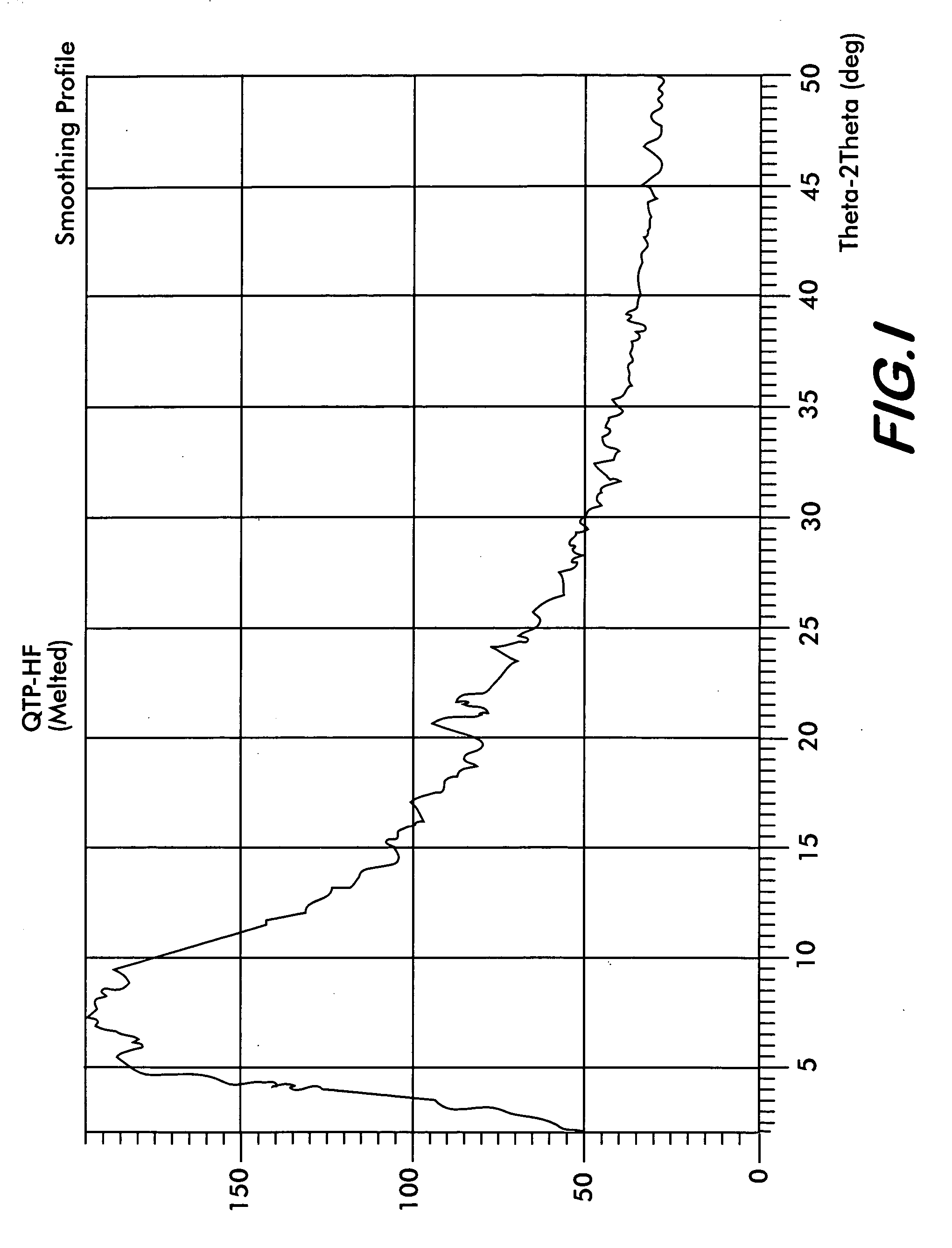
Crystalline quetiapine fumarate and pharmaceutical compositions thereof
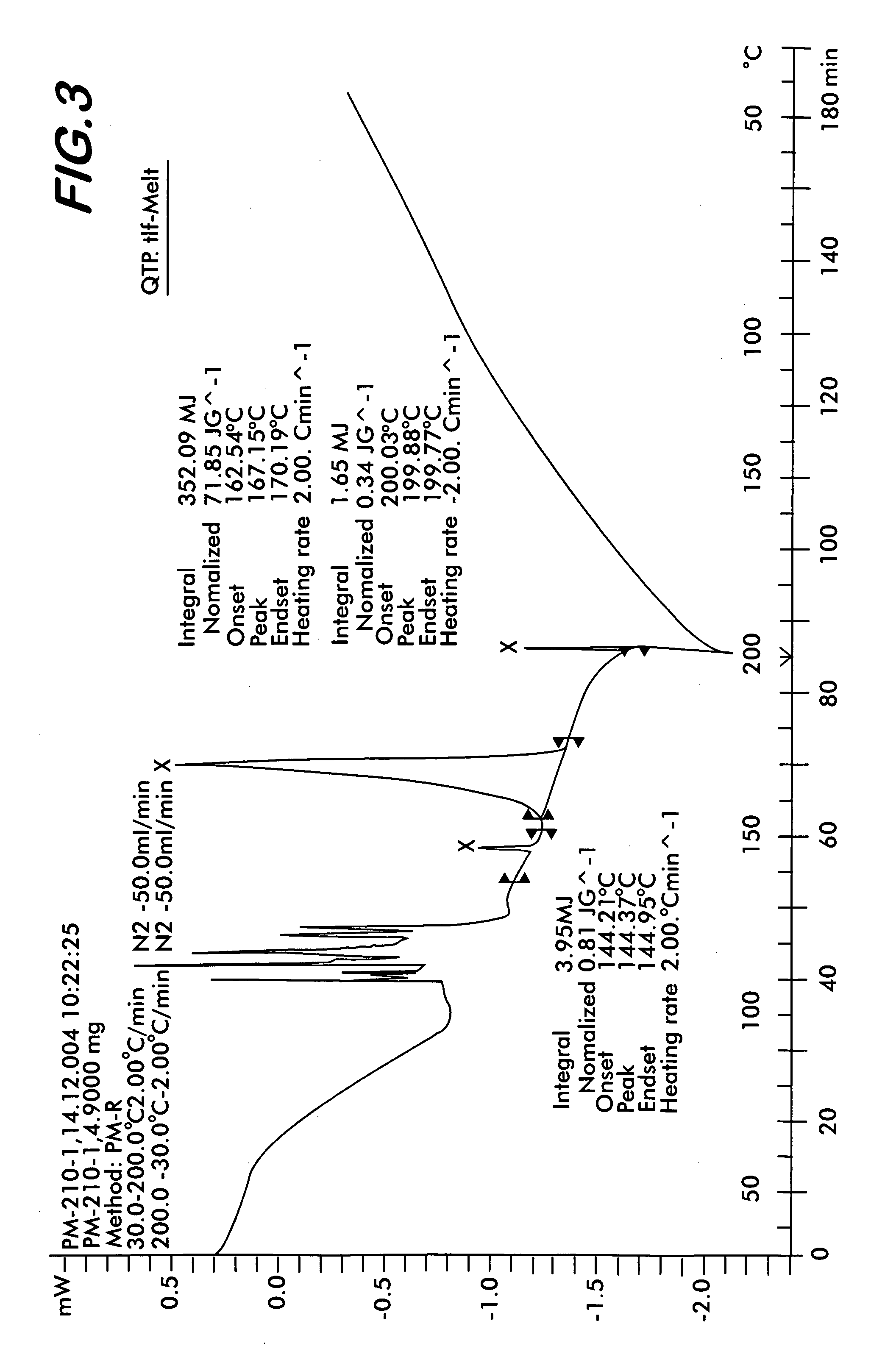
ActiveCN102206195AReduce the temperatureAvoid pollutionOrganic active ingredientsNervous disorderSolventBioavailability
The invention relates to a method for producing crystalline quetiapine fumarate which is produced by using water as a solvent, heating, dissolving and cooling crystallization. The invention also discloses a preparation using crystalline quetiapine fumarate prepared by the method. A quetiapine fumarate tablet which is prepared by mixing the crystalline quetiapine fumarate with auxiliary materials, granulating the mixture, adding a lubricant into the granulated mixture and tabletting, has fast soluble speed, and is capable of effectively improving product bioavailability.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Oral quetiapine suspension formulations with extended shelf life and enhanced bioavailability
A quetiapine fumarate composition for oral administration is provided comprising a pharmaceutically acceptable salt or solvate of quetiapine existing as a suspension in an aqueous carrier agent. The inventive liquid formulation demonstrates high bioavailability consistent with approved dosage forms, low agglomeration, reduced content of excipients commonly used in solid oral dosage forms and extended shelf life stability. Also provided is a method of manufacturing a liquid quetiapine suspension composition for oral administration and methods of administering therapeutically effective dosages of an oral liquid quetiapine suspension composition to patients in need thereof.
Owner:TLC THERAPEUTICS LLC
Boletic acid quetiapine oral preparation and preparation method thereof
InactiveCN101375852ADisintegrates quicklyFast absorptionOrganic active ingredientsNervous disorderQuetiapineDrug administration
The invention discloses a quetiapine fumarate oral preparation and a preparation method thereof. The quetiapine fumarate oral preparation is mainly prepared by raw materials with the following proportion by weight: 25-300 parts of quetiapine fumarate counted by quetiapine, 2-100 parts of disintegrating agent and 5-400 parts of filling agent. The preparation method of the oral preparation comprises the following process steps: (1) the quetiapine fumarate is taken, smashed, screened and evenly mixed with flavoring agent and the disintegrating agent, and wetting agent is added for preparing soft materials; (2) the soft materials are screen through a 20-mesh sieve for carrying out the granulation and the drying, the 20-mesh sieve is used for carrying out the size stabilization, the filling agent, an odor correcting agent, glidant and lubricant are added for even mixing; (3) the content of the drug which is evenly mixed is measured, the tablet weight is calculated, and the tablet pressing is carried out for the preparation. The oral preparation can be rapidly disintegrated in oral cavity without the use of water for the drug administration; the absorption is rapid and the bioavailability is high; and the taste is good.
Owner:张宏宇
Polymorph of Quetiapine fumarate and a process for its preparation
ActiveUS7488821B2Acceptable stabilitySafe, environmentally friendly and commercially viableOrganic chemistryPharmacologyQuetiapine Fumarate
The present invention relates to a novel polymorph of Quetiapine fumarate and a simple method for its preparation.
Owner:DIVI S LAB LTD
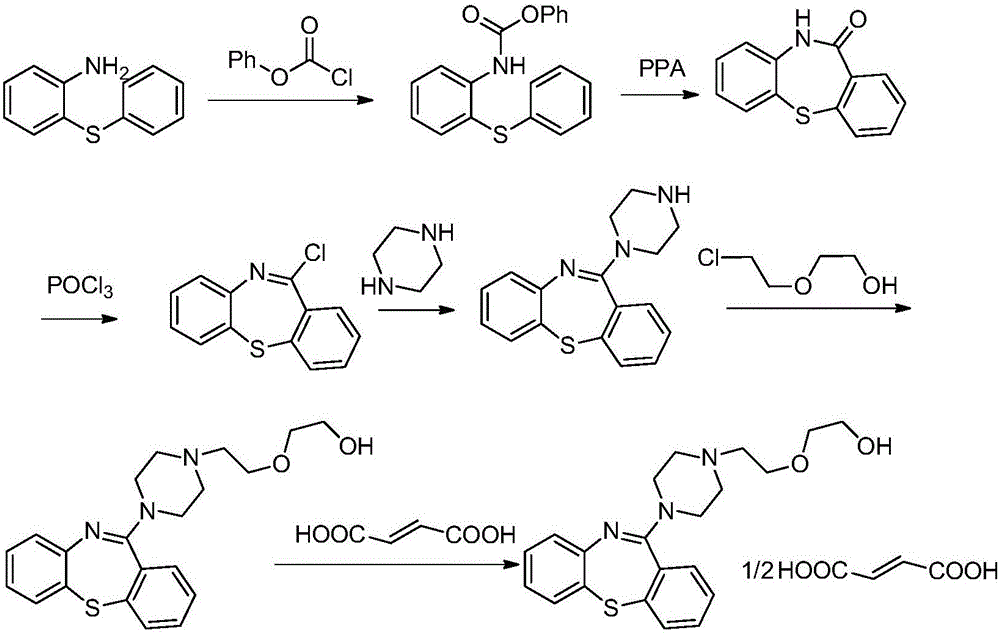
Quetiapine synthesizing method
ActiveCN105859653ASuitable for mass productionPreparation cost Raw materials are cheap and easy to getOrganic chemistryBeckmann rearrangementThiazepine
The invention discloses a quetiapine synthesizing method. O-chlorobenzoic acid with the low price is adopted as a starting material to react with thiophenol, and then ring closure is performed to obtain thioxanthone. Hydroxyl amination and Beckmann rearrangement are performed to obtain a key intermediate dibenzo[b,f][1,4]thiazepines-11-(10H)one, chlorination is performed, then, a reaction is performed on 1-(2-hydroxyethoxy)ethylpiperazine with the existence of acid-binding agent to obtain quetiapine, and the quetiapine and fumaric acid form a salt in an absolute ethyl alcohol system to obtain a product. According to the quetiapine synthesizing method, raw materials are low in price and easy to obtain, the steps are simple, operation is easy, and the cost can be effectively lowered. According to the method, the high-purity quetiapine can be obtained, the liquid phase purity of the obtained semi-fumaric acid quetiapine obtained through salt forming is 99% or above, and the quetiapine synthesizing method can be applied to the field of medicine.
Owner:JIAXING UNIV
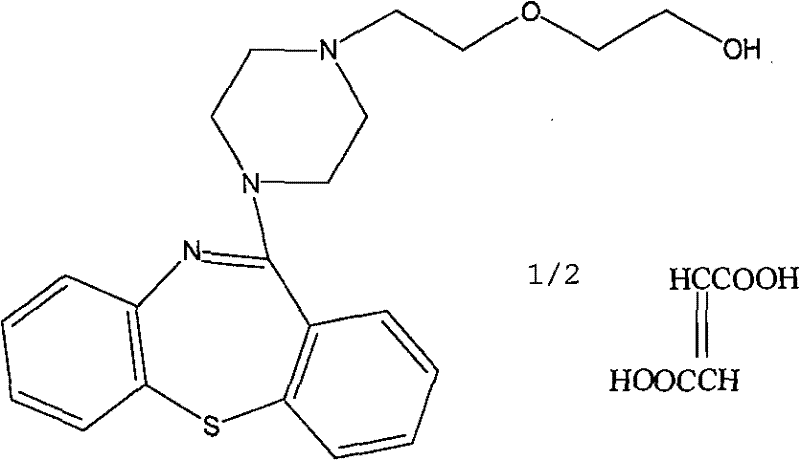
Quetiapine hemifumarate synthesis technology
The invention discloses a quetiapine hemifumarate synthesis technology. The technology comprises the following steps: mixing a compound of formula (II) and C1-C4 alkyl acid, heating the compound of formula (II) and the C1-C4 alkyl acid to 80-100 DEG C, adding zinc powder or iron powder, and carrying out a heat insulation reaction for 4-6 h to obtain quetiapine; and carrying out salt formation on the obtained quetiapine and fumaric acid to obtain quetiapine hemifumarate, wherein a feeding molar ratio of quetiapine to fumaric acid is 1:0.5. The technology has the advantages of simplicity in operation, high yield, high purity of the above obtained product, and easy realization of industrialization.
Owner:广安凯特制药有限公司
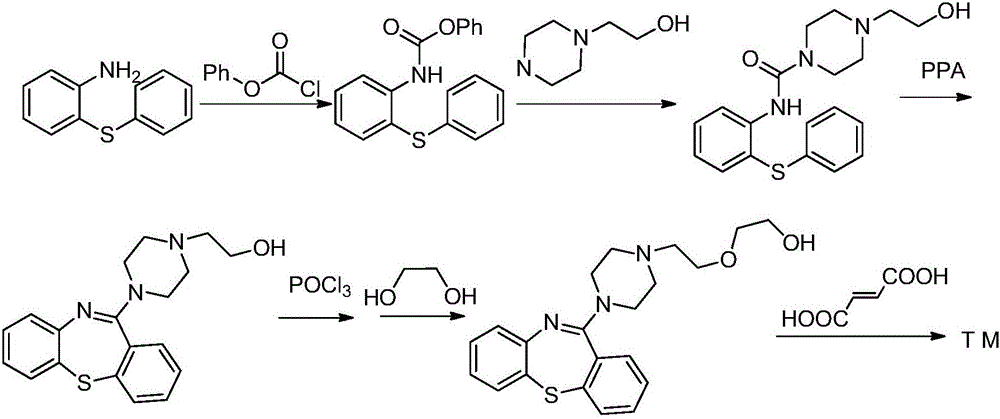
Preparation method of quetiapine fumarate
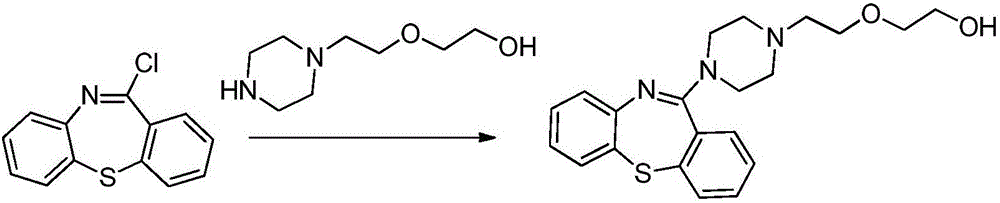
The invention discloses a preparation method of quetiapine fumarate with high purity, which is suitable for industrialization and includes: taking 11-piperazine-dibenzo[B, F][1, 4]thiazepine dihydrochloride as the initial raw material, N-substituting and salt-forming to generate quetiapine fumarate. The highly finished product obtained by the method has high purity, the operating method is simple, the production cost is low, the yield is high, the preparation method is more suitable for industrialization, and the reaction time is shortened by adding a phase-transfer catalyst.
Owner:HAINAN SHENGKE LIFE SCI RES INST
Quetiapine fumarate sustained-release tablet and preparation method thereof
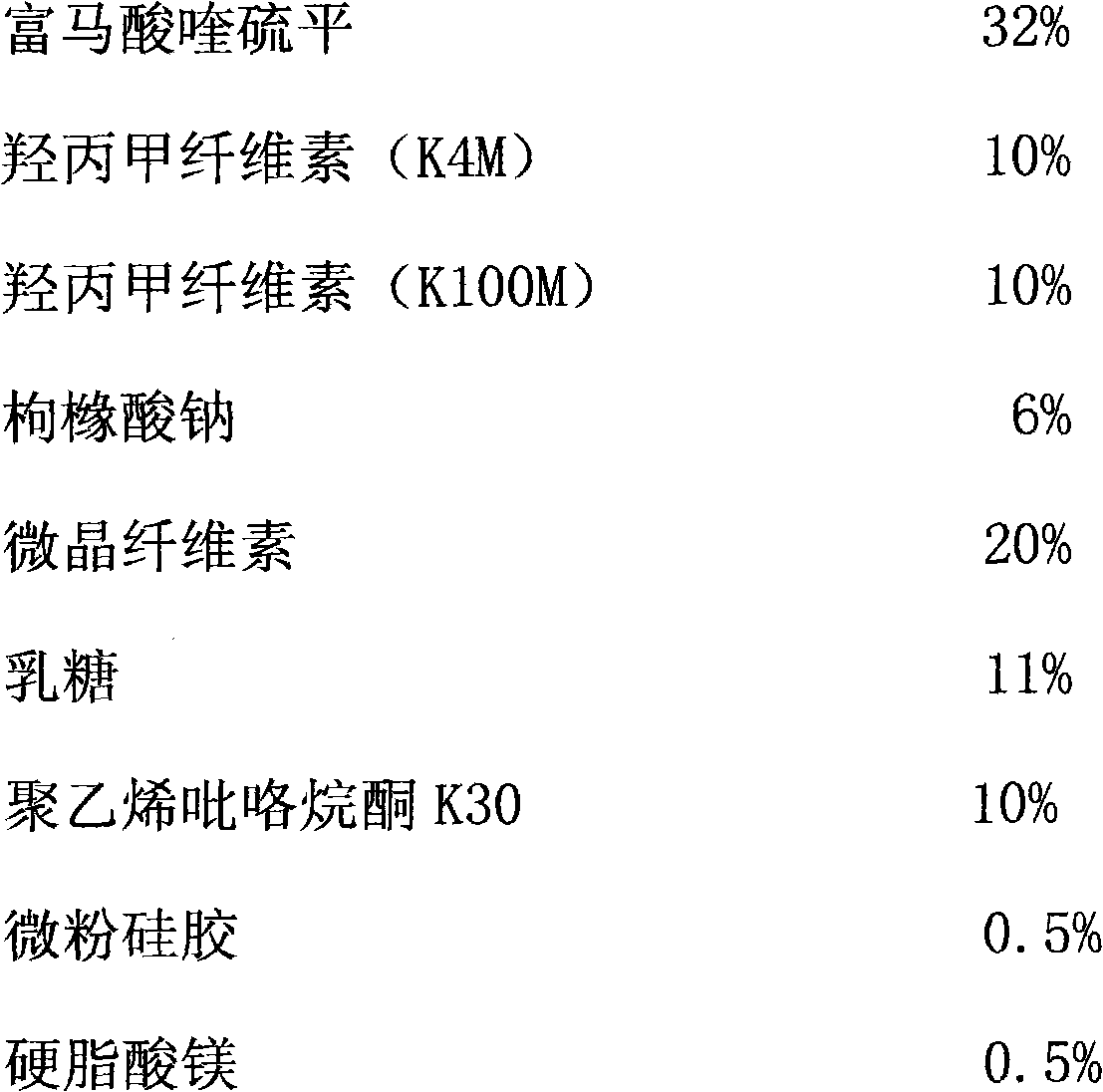
InactiveCN107854447AReduce contact timeImprove liquidityOrganic active ingredientsNervous disorderSustained Release TabletContact time
The invention relates to a quetiapine fumarate sustained-release tablet and a preparation method thereof, and belongs to the technical field of pharmaceutical preparations. The technical scheme of theinvention is that the quetiapine fumarate sustained-release tablet comprises the following components in percentage by weight: 40.15% of quetiapine fumarate, 25.00% of hydroxypropyl methylcellulose,10.50% of lactose, 17.00% of microcrystalline cellulose, 6.35% of sodium citrate, and 1.00% of magnesium stearate, and the preparation method comprises the steps of sieving, granulating, straighteningand the like, wherein the granulating step uses 30-50% ethanol aqueous solution as a wetting agent for granulation. The preparation process of the quetiapine fumarate sustained-release tablet provided by the invention reduces the contact time between water and raw materials and ensures the dissolution quality of the product to the greatest extent.
Owner:北京满格医药科技有限公司 +1
Solid quetiapine fumarate liposome preparation
InactiveCN102406606BGood curative effectLong retention timeOrganic active ingredientsNervous disorderYolkSide effect
The invention provides a solid quetiapine fumarate liposome preparation and a preparation method thereof. According to the preparation method, quetiapine fumarate, yolk lecithin, cholesterol, soyasterol and sorbitam stearate are selected according to a specific weight ratio and prepared into high-quality quetiapine fumarate liposome, and the quetiapine fumarate liposome is prepared into a solid preparation according to a common preparation method. Compared with the traditional preparation, the preparation provided by the invention has the advantages of greatly improved stability, bioavailability and quality and reduced toxic and side effects.
Owner:HAINAN MEIDA PHARMA
Quetiapine fumarate film-controlled slow-release pellet capsule
InactiveCN103211794AAccelerated agingReduce permeabilityOrganic active ingredientsNervous disorderSustained release pelletsMedicine
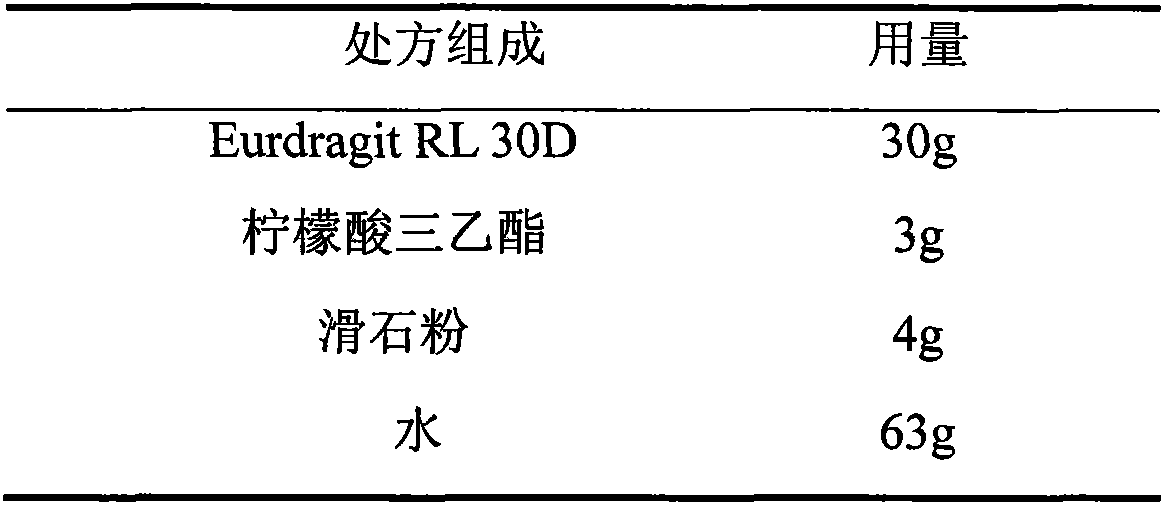
The invention relates to a quetiapine fumarate film-controlled slow-release pellet capsule. A slow-release film of the quetiapine fumarate film-controlled slow-release pellet utilizes Eurdragit RL 30D as a film-formation material. A pellet core of the quetiapine fumarate film-controlled slow-release pellet contains low-substituted hydroxypropyl cellulose having high expansibility, and also contains a pharmaceutically-acceptable common excipient for the slow-release pellet, wherein preferably, the excipient comprises microcrystalline cellulose, and the pellet core comprises 10 to 40wt% of the low-substituted hydroxypropyl cellulose. The slow-release film comprises Eurdragit RL 30D, triethyl citrate as a plasticizer and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit RS 30D to triethyl citrate to talcum powder is 30: 3: 4 and a film weight increasing ratio is in a range of 19 to 35%. The quetiapine fumarate film-controlled slow-release pellet comprises the pellet core containing low-substituted hydroxypropyl cellulose having high water expansibility and thus after absorbing water, the quetiapine fumarate film-controlled slow-release pellet expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the quetiapine fumarate film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:内蒙古天衡医院管理有限公司
Sustained-release tablet containing quetiapine fumarate and preparation method of sustained-release tablet
ActiveCN104840442AGood slow releaseSimple preparation processOrganic active ingredientsNervous disorderSustained Release TabletQuetiapine
The invention provides a sustained-release tablet containing quetiapine fumarate. The sustained-release tablet comprises the following components according to weight percent: 12.5% to 60% of the quetiapine fumarate according to quetiapine, 10% to 40% of sustained-release material and other pharmaceutic adjuvants as the rest, wherein the sustained-release material is a composition of carbomer and hydroxyethyl cellulose. After being dosed, the sustained-release tablet can slowly and continuously release as required, so as to maintain the effective treatment concentration, thereby achieving a long-acting effect by dosing once a day. Additionally, according to the sustained-release tablet, a dry granulation tableting process is adopted, the preparation process is simple, large-scale industrial production is facilitated, and the production cost can be effectively reduced.
Owner:ZHEJIANG YONGNING PHARMA +1
Synthetic method of thiazepine compound
InactiveCN103772319AAvoid it happening againAdvanced process routeOrganic chemistryThiazepineThiosalicylic acid
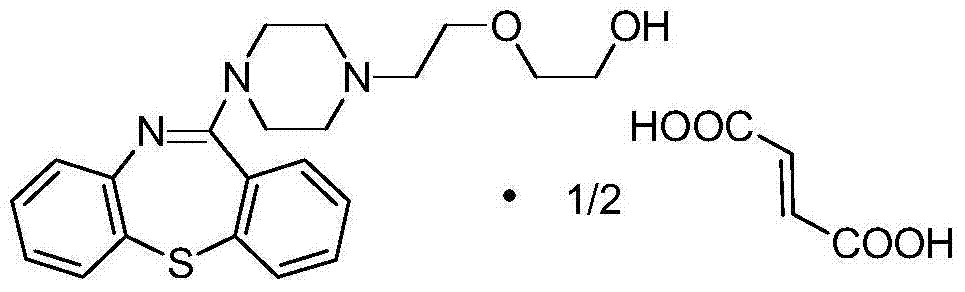
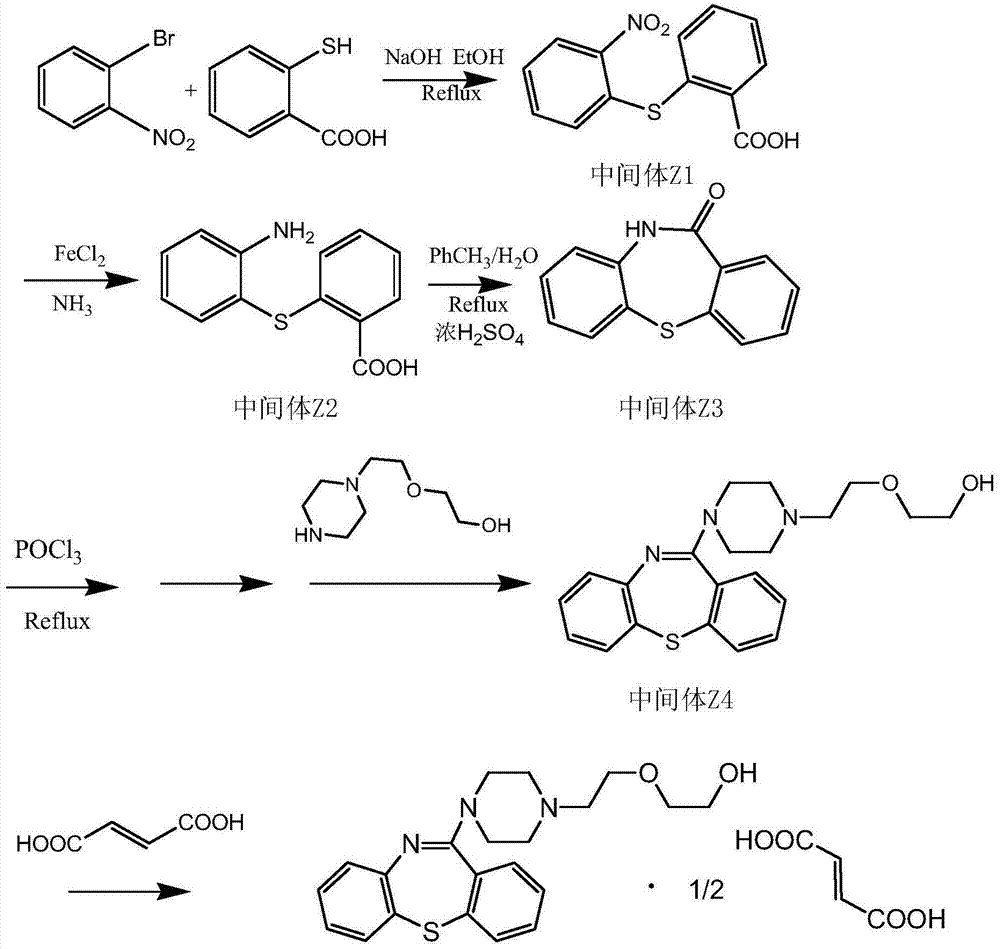
The invention discloses a synthetic method of a thiazepine compound, in particular to a production process of quetiapine hemifumarate. O-bromonitrobenzene and thiosalicylic acid are used as starting materials and are subjected to displacement, reduction, condensation, halogenation and re-condensation, and then salt formation is performed with fumaric acid to obtain quetiapine hemifumarate. The production process has simple reaction operation and mild conditions and is easy for industrialized production.
Owner:CHENGDU YILUKANG MEDICAL TECH & SERVICE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com