Composition of slow (controled) releasing preparation of Quetiadine Hemifumarate, and application
A technology of quetiapine fumarate and quinolone fumarate, which is applied in the field of quetiapine fumarate oral sustained-release preparations, which can solve the problems of inconvenience to patients and their families, and short action time of preparations.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
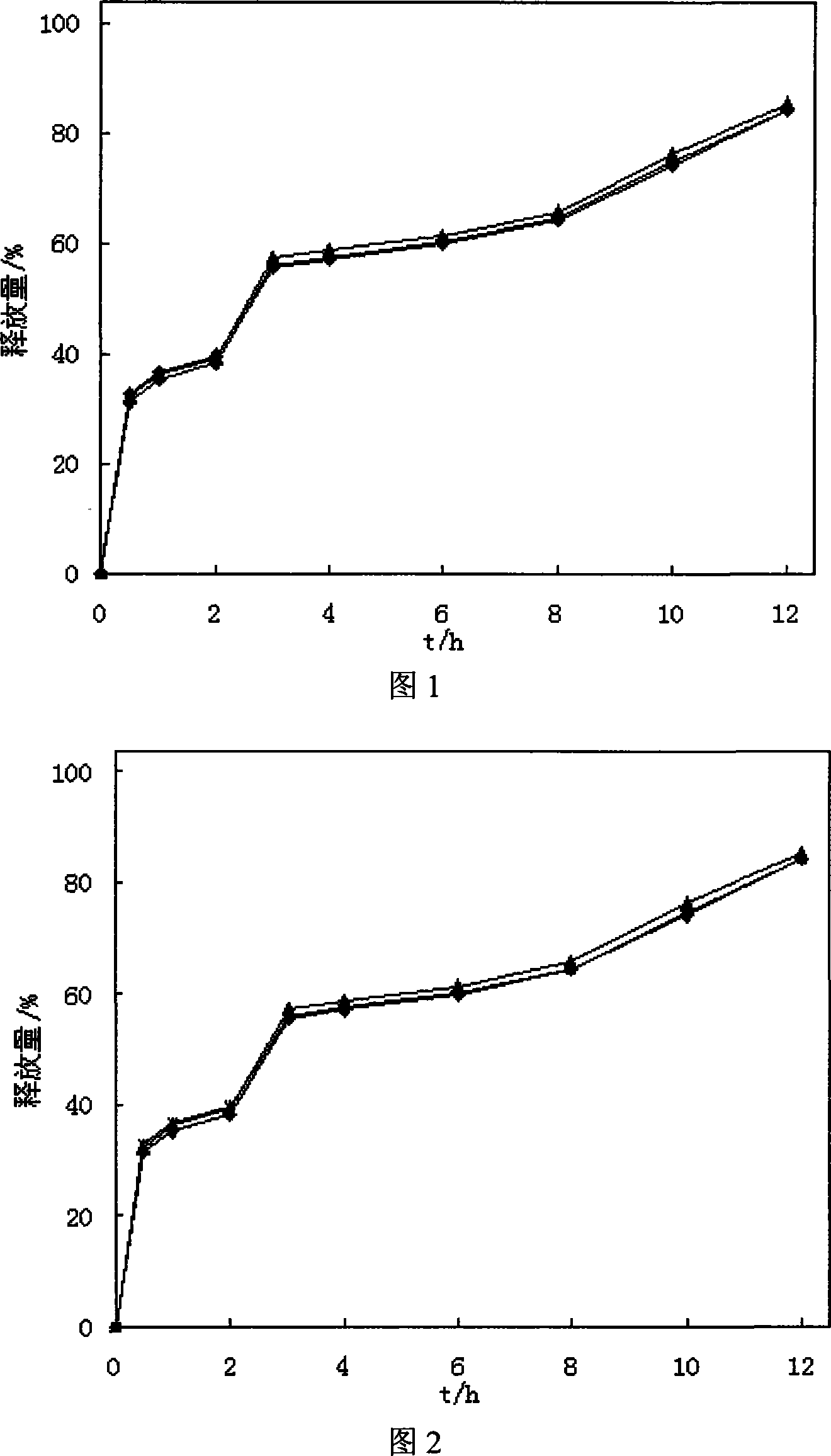
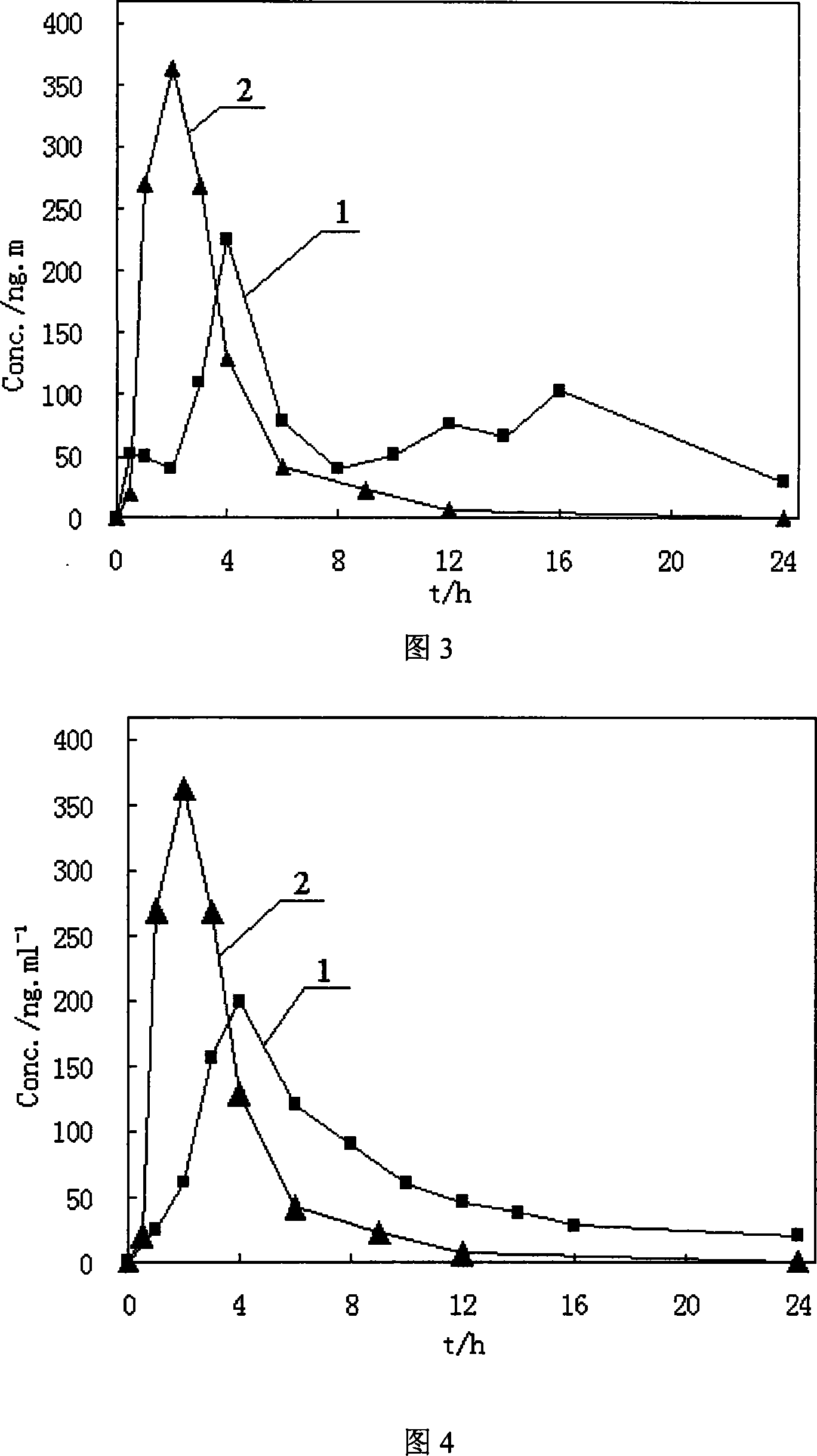
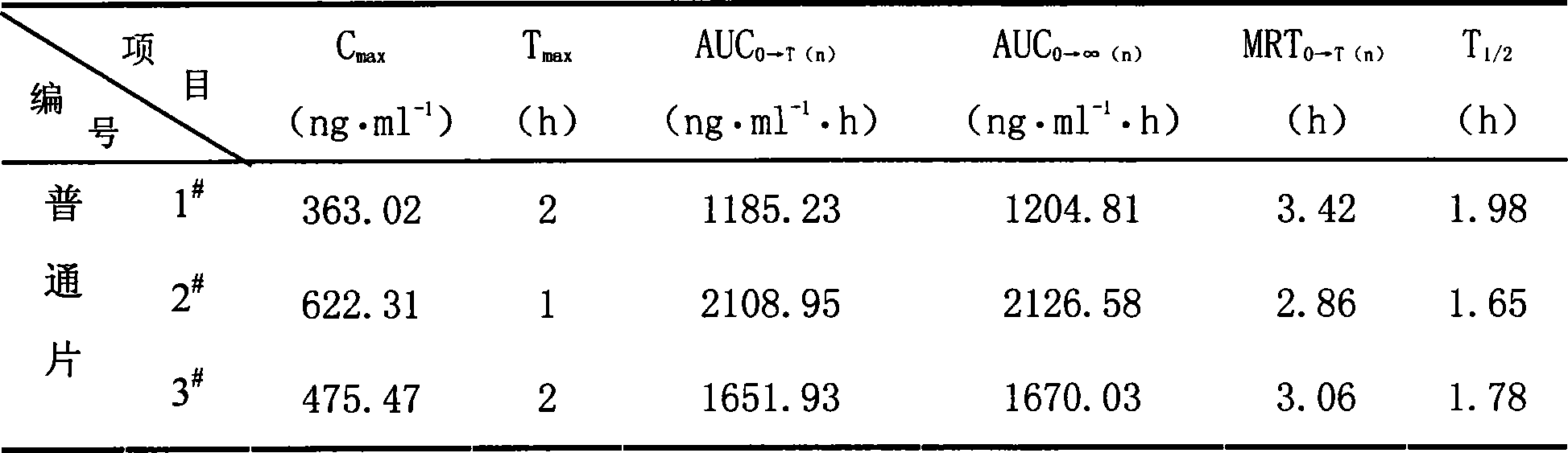
[0068] Mix 100 grams of quetiapine fumarate, 10 grams of citric acid, 90 grams of water-soluble macromolecular material polyvinylpyrrolidone and 2 grams of waxy stearyl alcohol, and add hydroxypropyl methylcellulose with a weight concentration of 3%. The slurry is made into a soft material, then granulated to obtain the granules, put in a coating machine, use 400ml of enteric-coated material and water-insoluble polymer material for ethanol mixture slurry coating, and dry at 60°C to obtain quetiapine fumarate ( Controlled) release granules.
[0069]The enteric-coated material is hydroxypropylmethylcellulose phthalate (HPMCP-55), the water-insoluble polymer material is ethyl cellulose, the enteric-coated material and the water-insoluble polymer material are in the ethanol mixture slurry, and the enteric-coated material is The content of the water-insoluble polymer material is 0.035g / ml, the content of the water-insoluble polymer material is 0.015g / ml, and the volume concentratio...
Embodiment 2
[0071] Mix 100 grams of quetiapine fumarate, 5 grams of tartaric acid, 50 grams of water-soluble macromolecular material polyvinyl alcohol, and 1 gram of cetyl alcohol, and add 3% hydroxypropyl methylcellulose slurry to make a soft Material, then granulated, to obtain granules, add 1.5 grams of talcum powder and mix evenly, punch a tablet with a shallow concave of 9mm. Take the tablet and place it in a coating pan, coat it with 100ml of ethanol mixture slurry of enteric material and water-insoluble polymer material, and dry at 60°C to obtain a tablet.
[0072] The enteric material is methacrylic acid-methacrylic acid copolymer (II), and the water-insoluble polymer material is trimethylammonium ethyl methacrylate-methacrylate copolymer (RS-100). In the ethanol mixture slurry of the molecular material, the content of the enteric material is 0.075g / ml, and the content of the water-insoluble polymer material is 0.025g / ml. The volume concentration of ethanol used was 85%.
[007...
Embodiment 3
[0075] The granules of Example 1 are packed into capsules to obtain capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com