Patents
Literature
1013results about How to "Obstruct passage" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
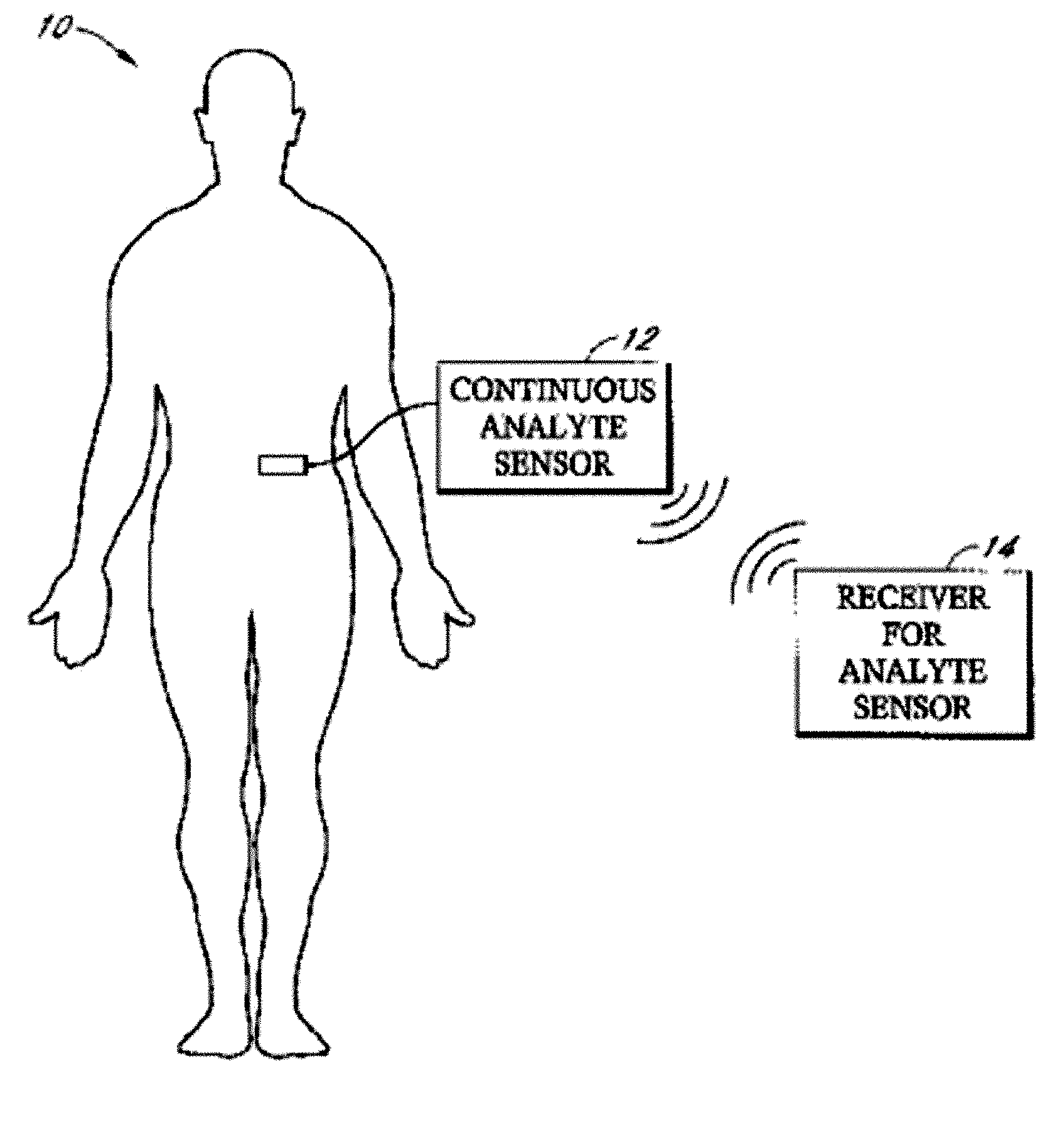
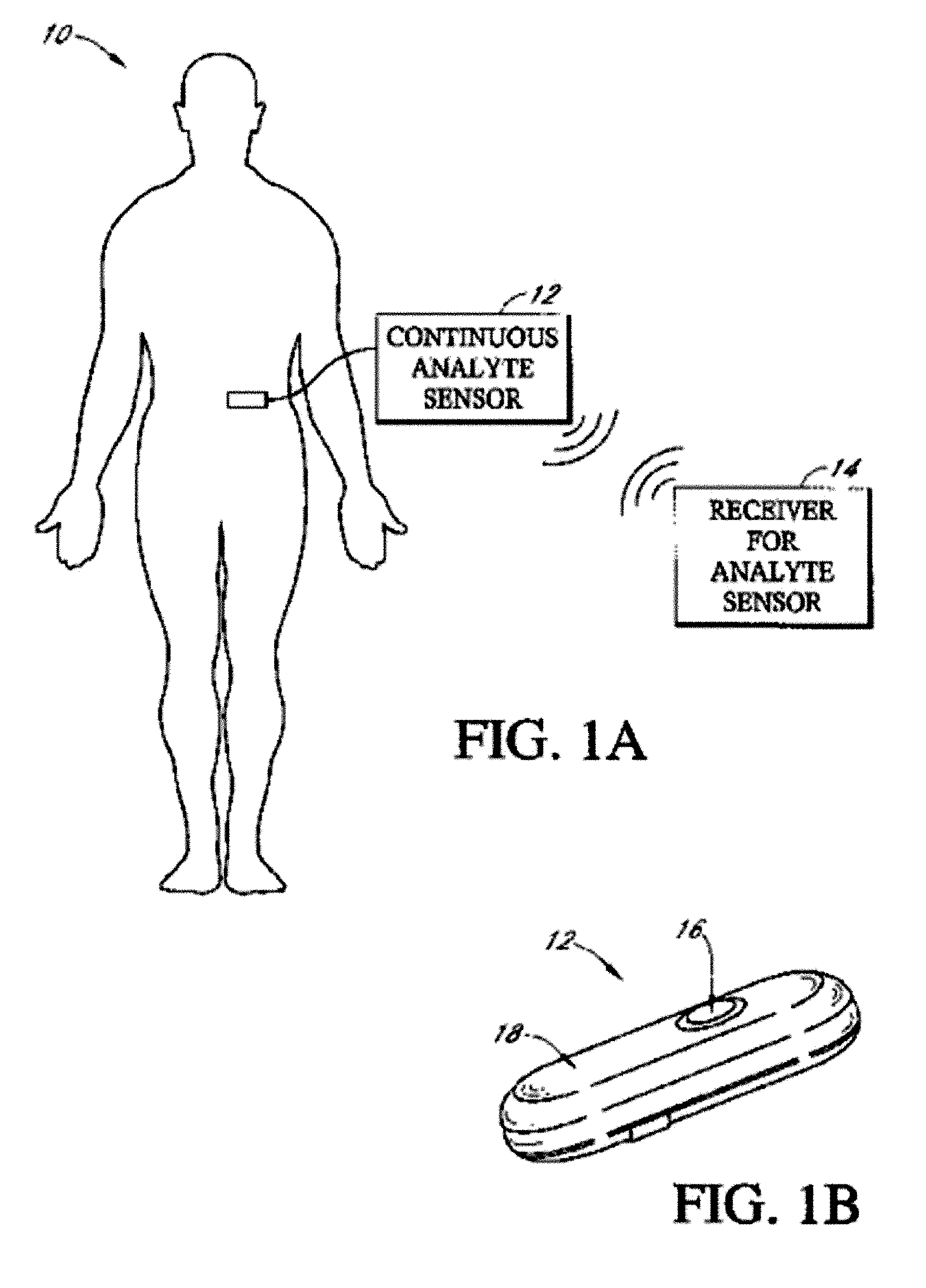
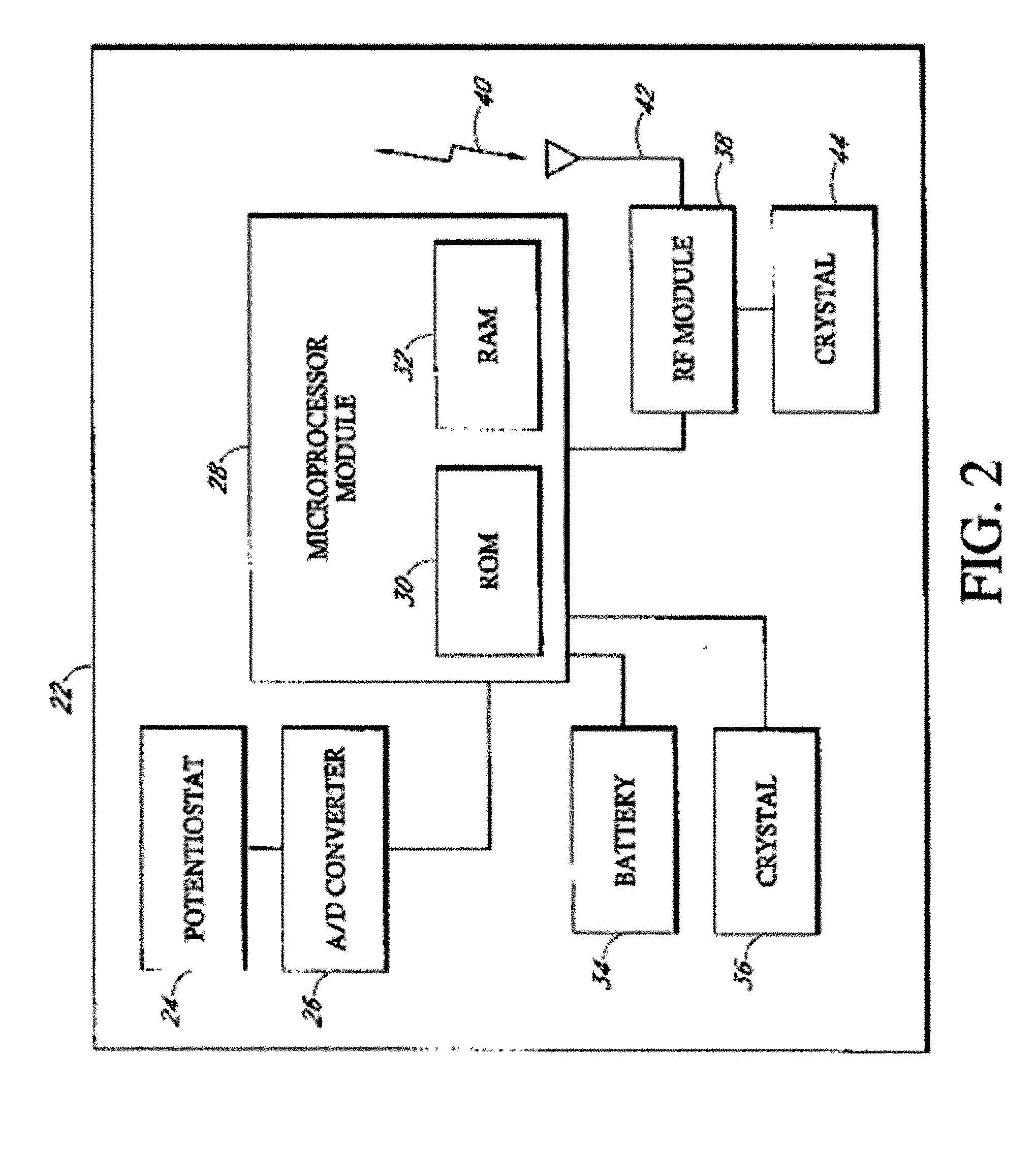
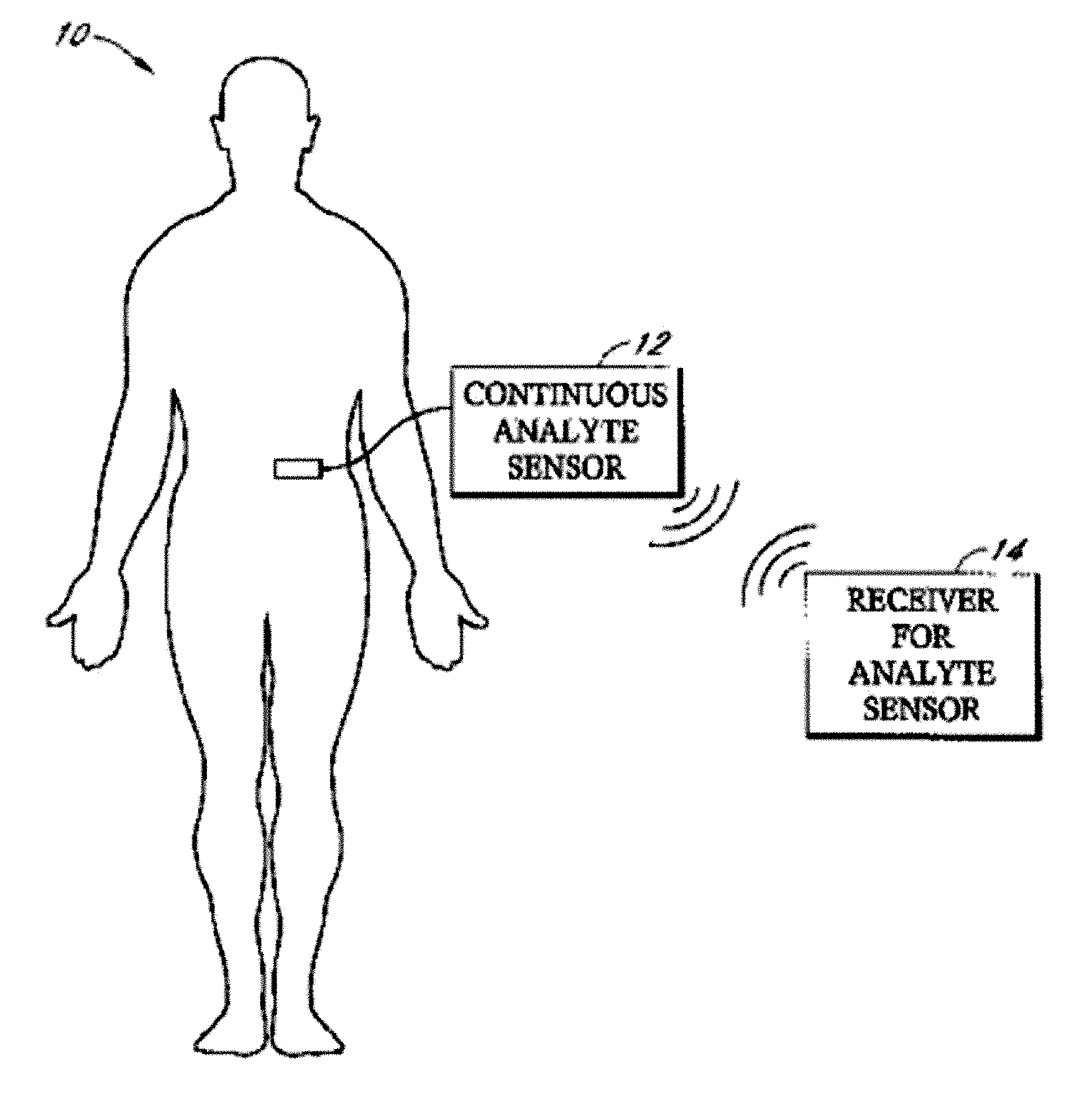
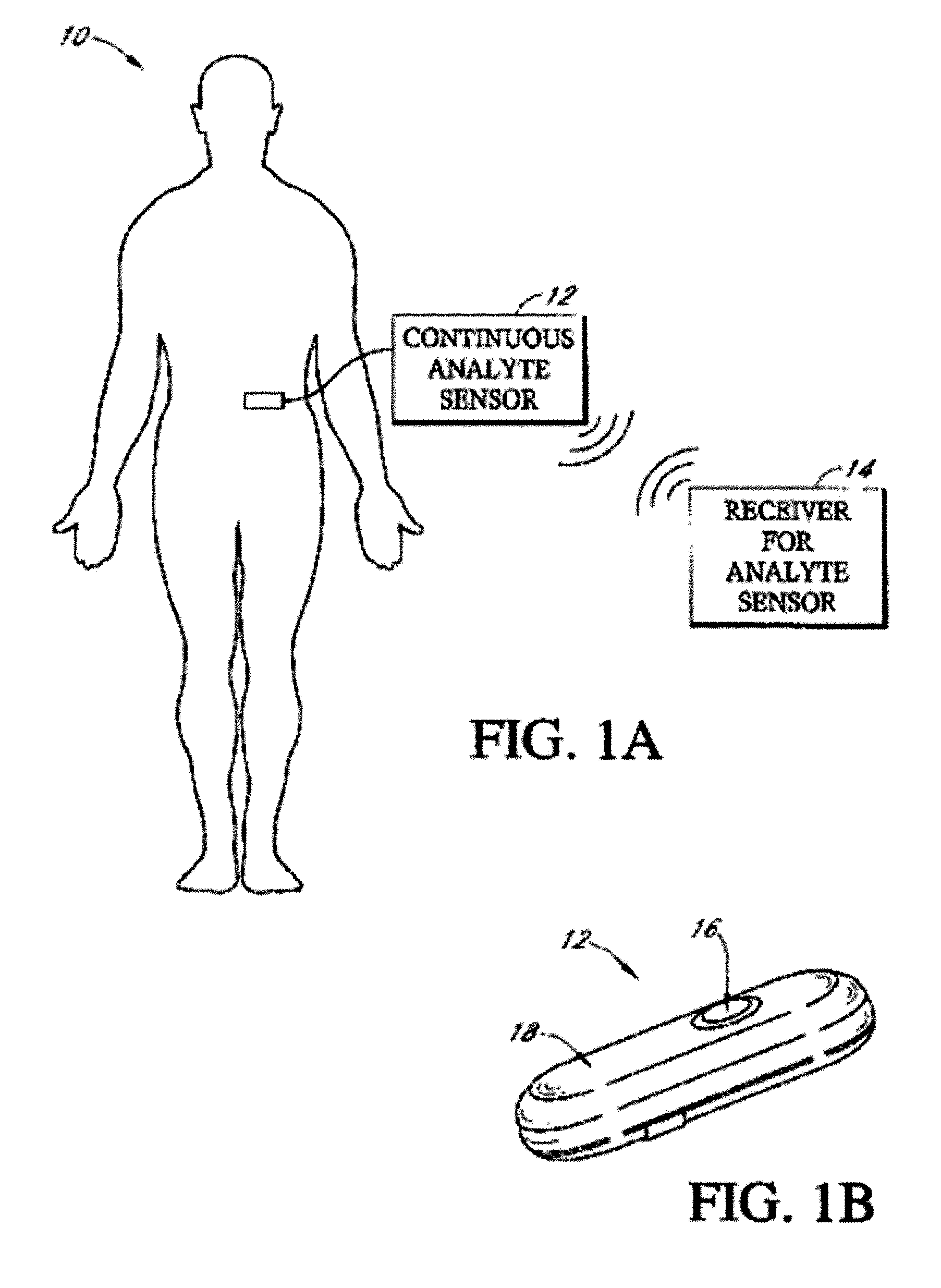
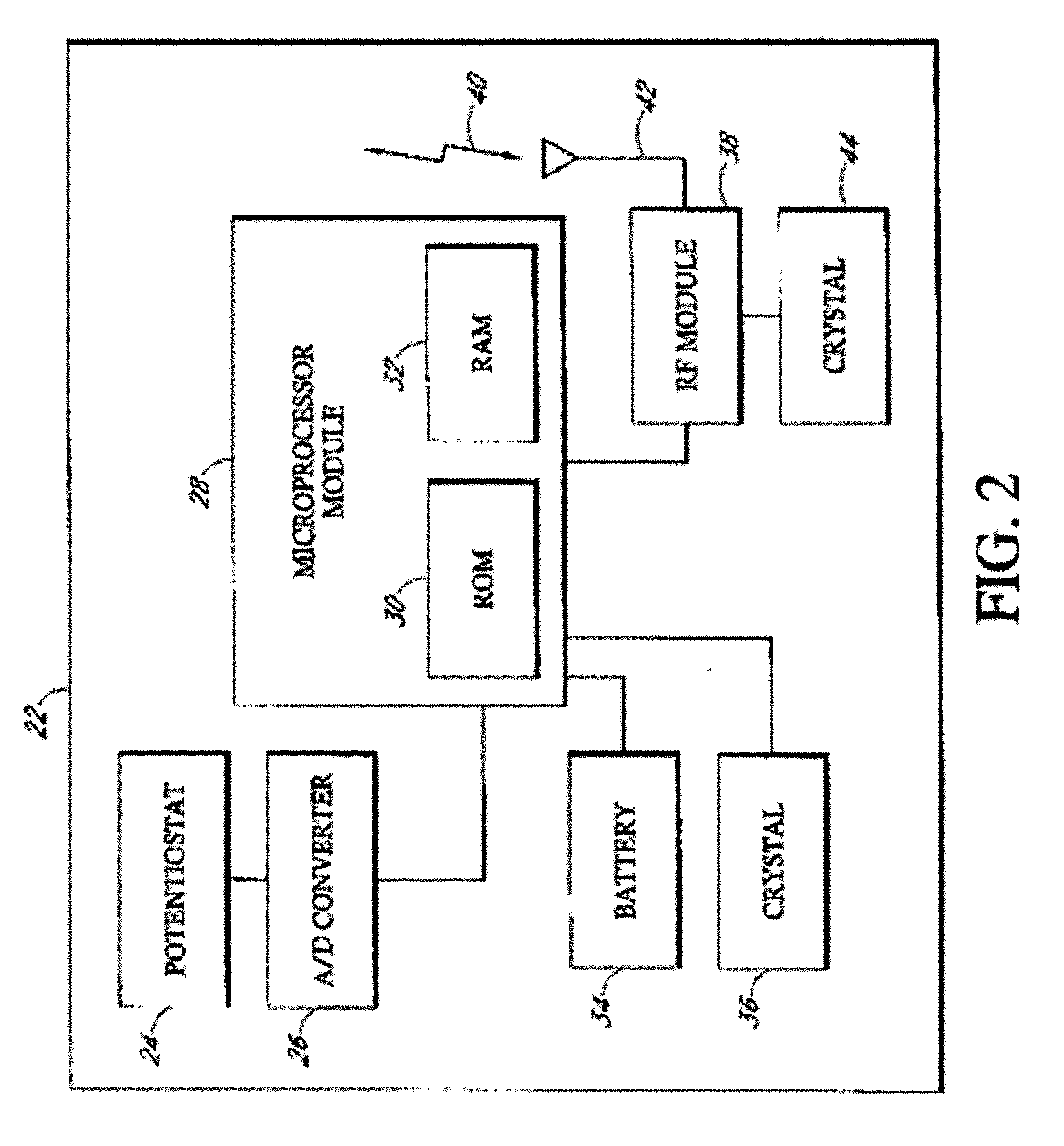
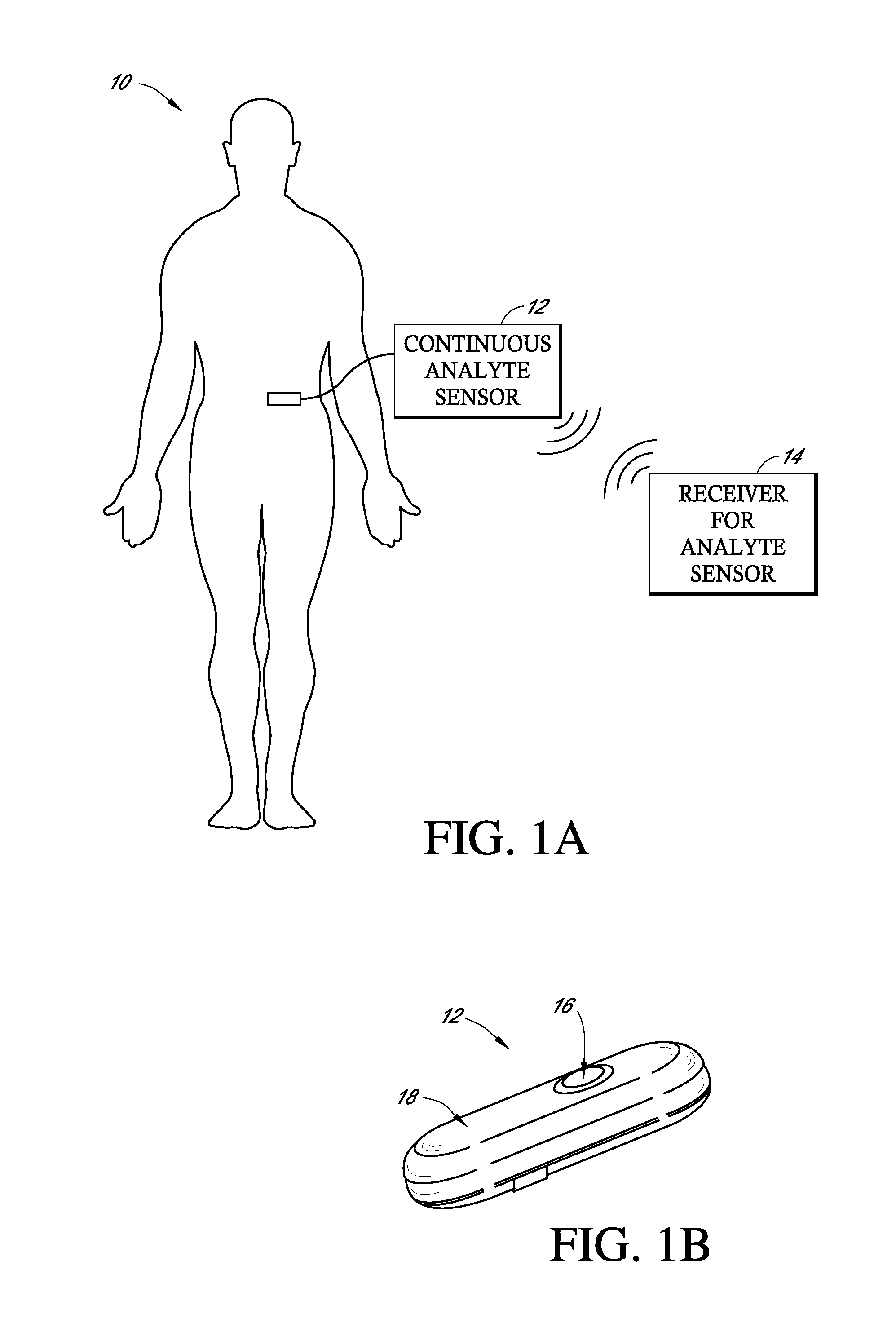
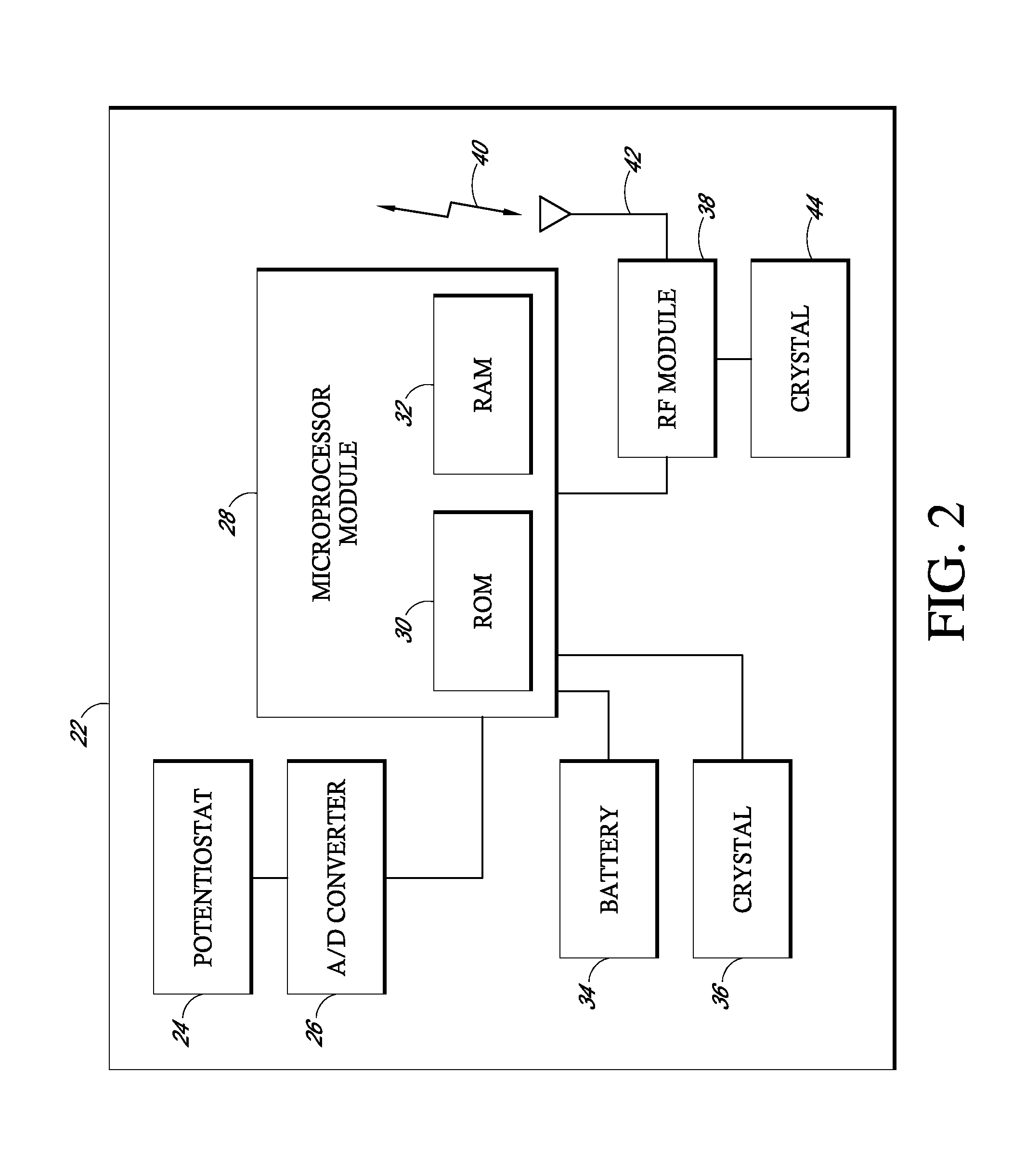
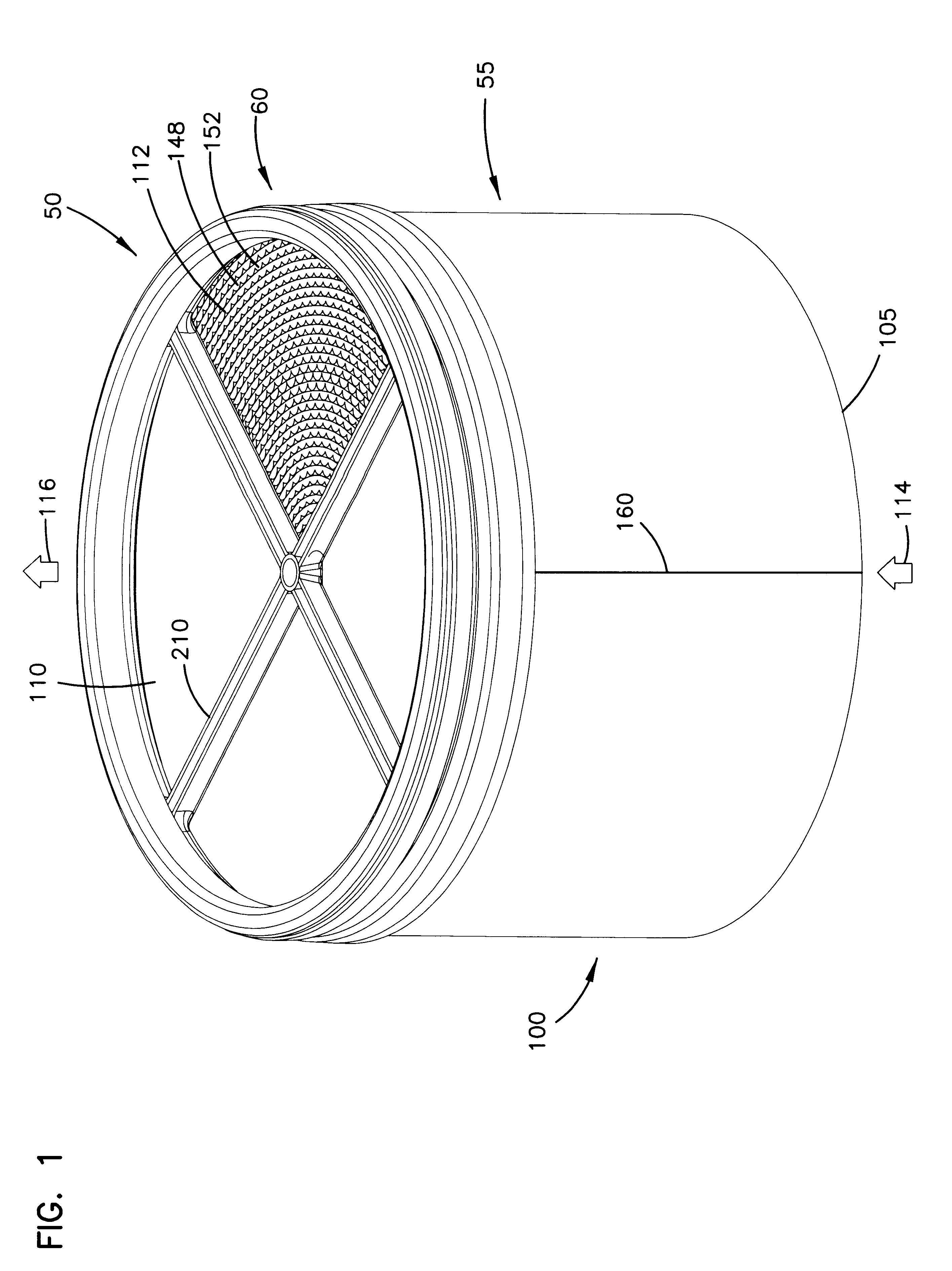
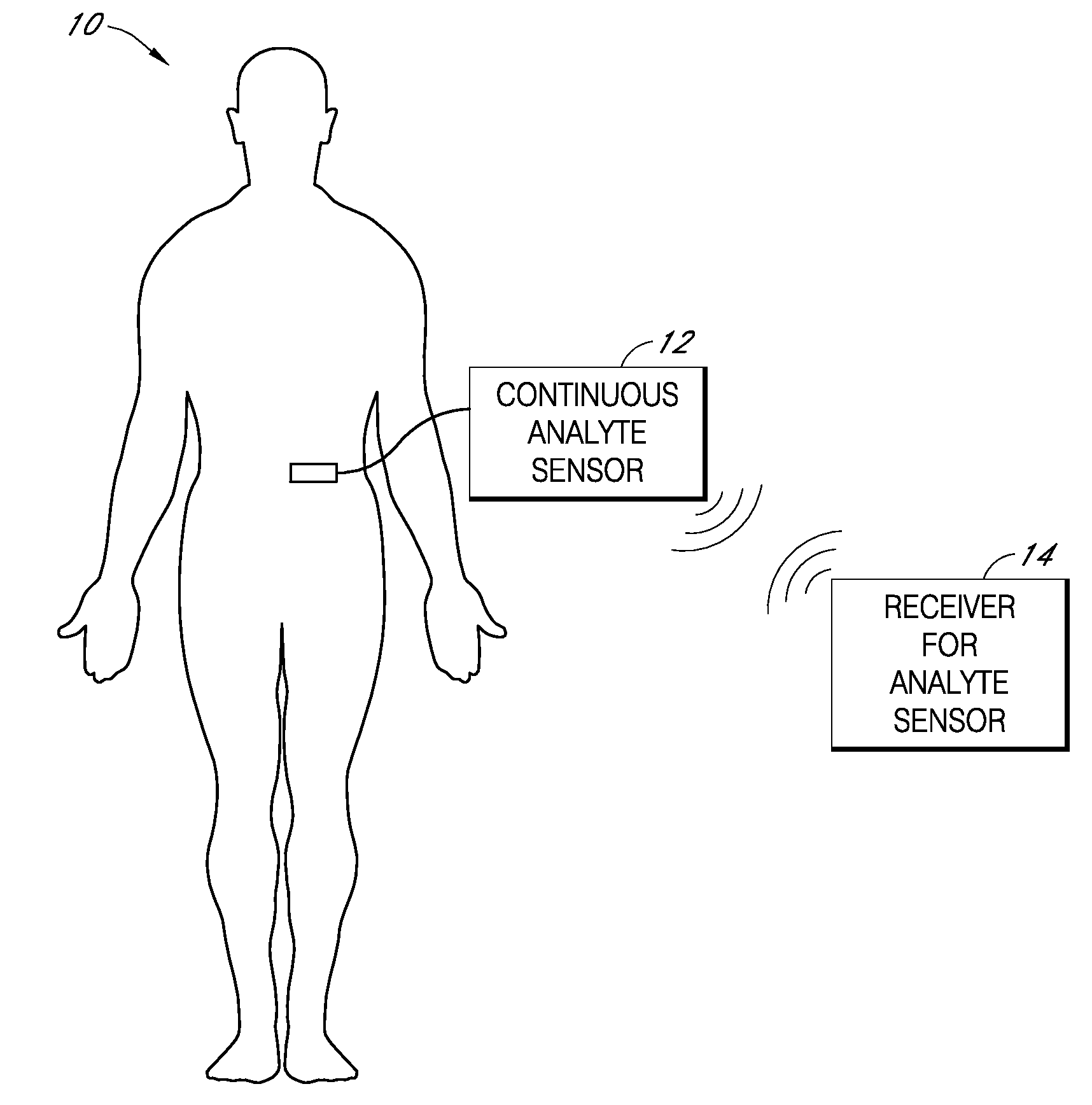
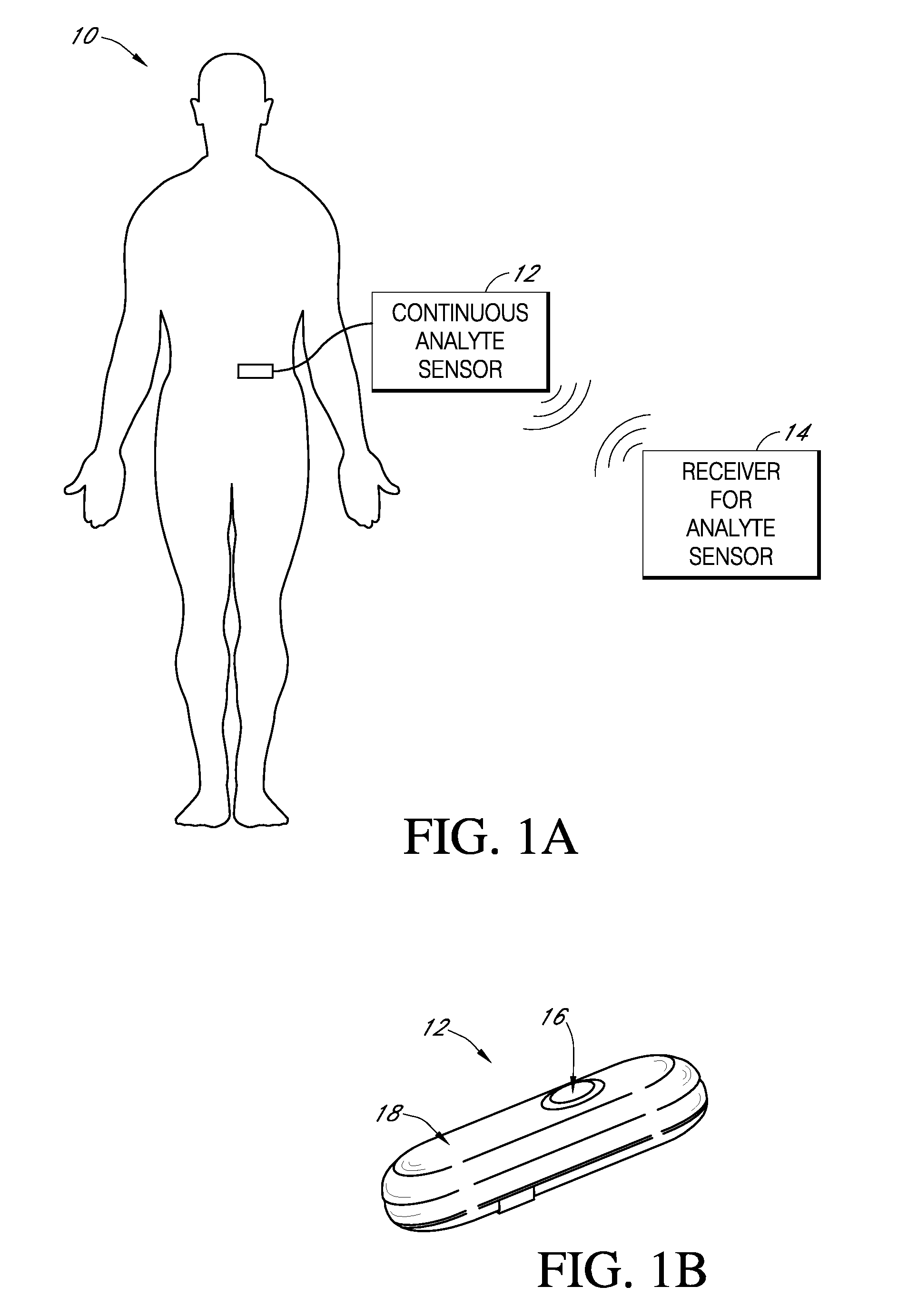
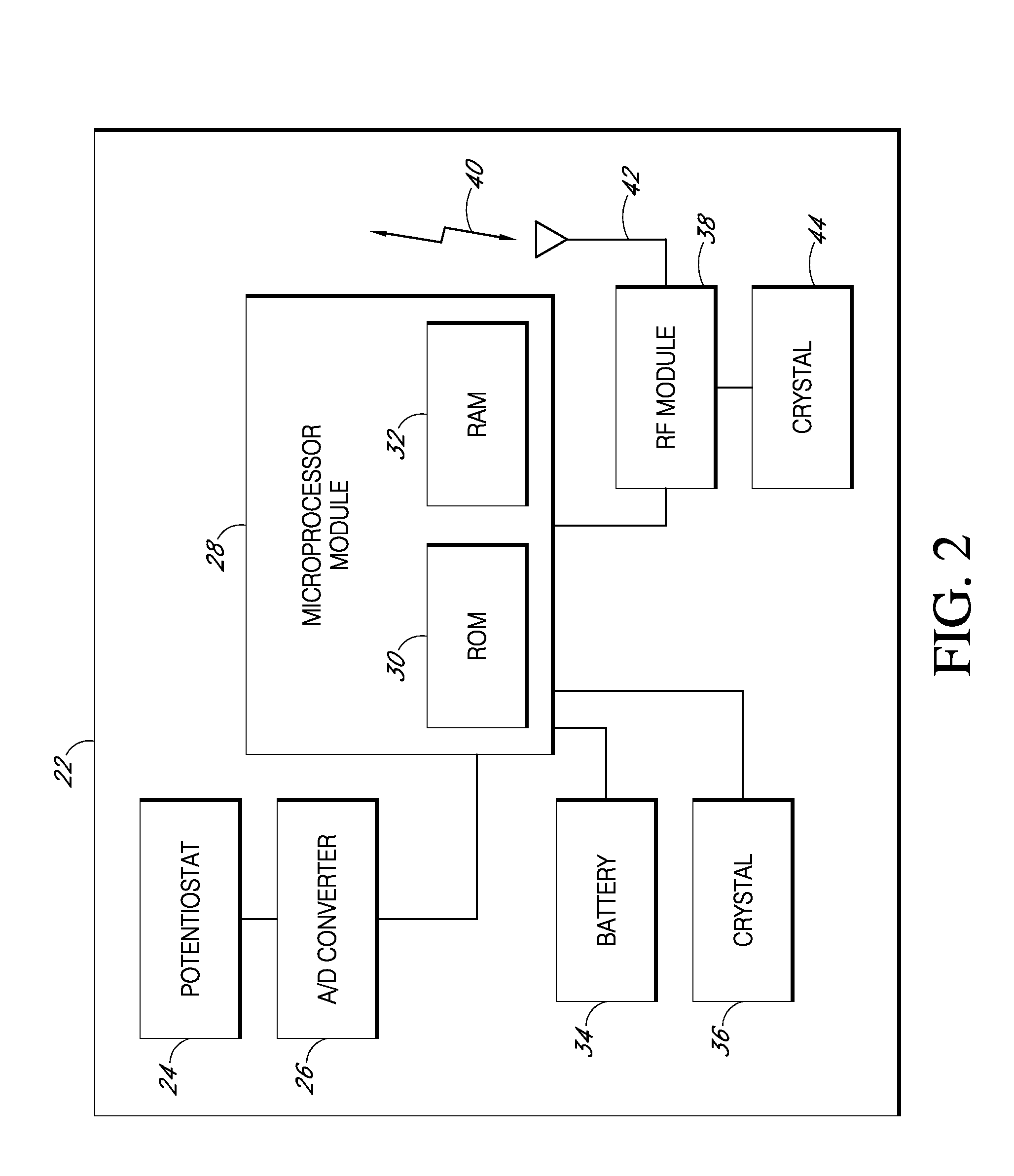
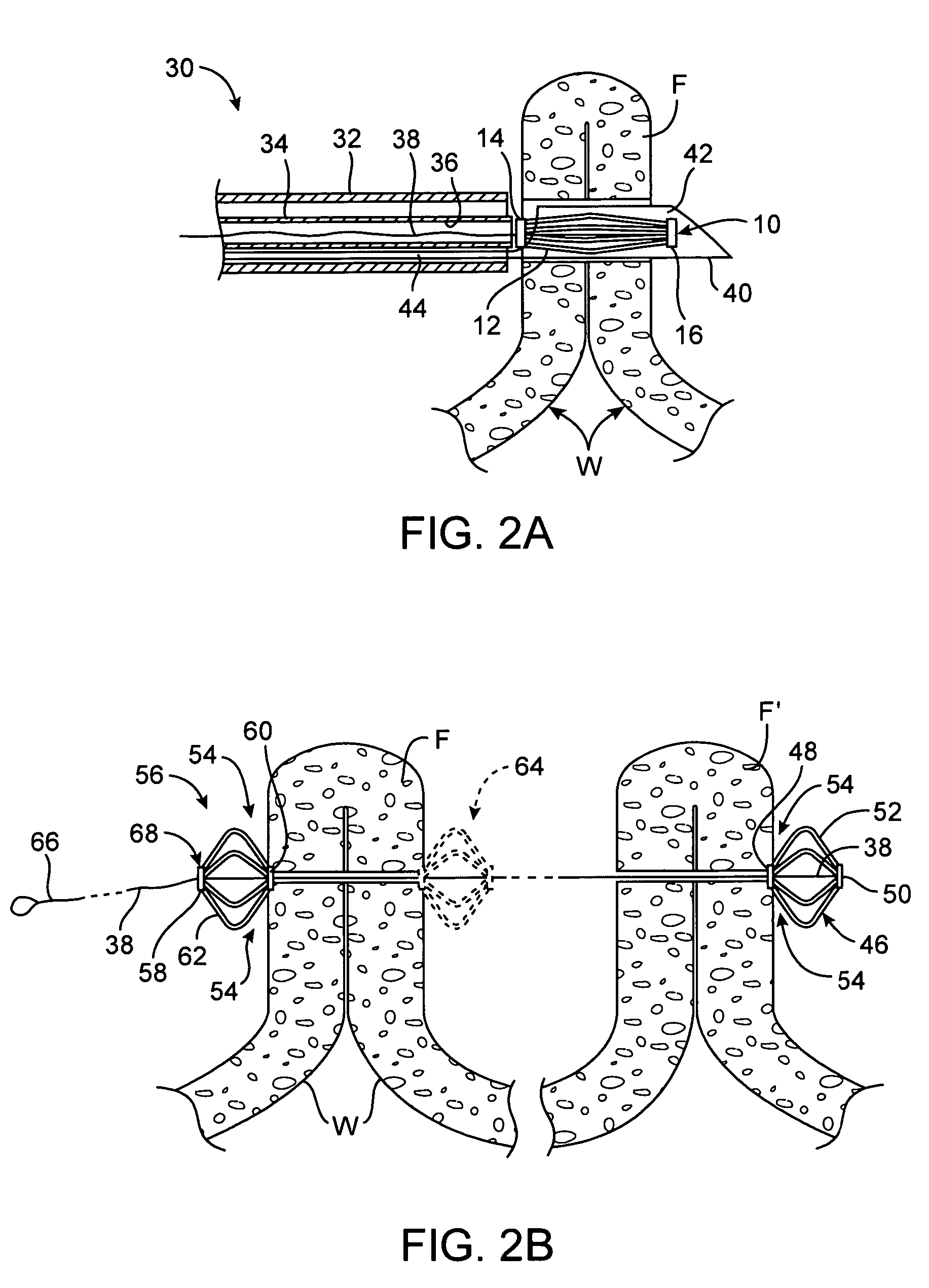
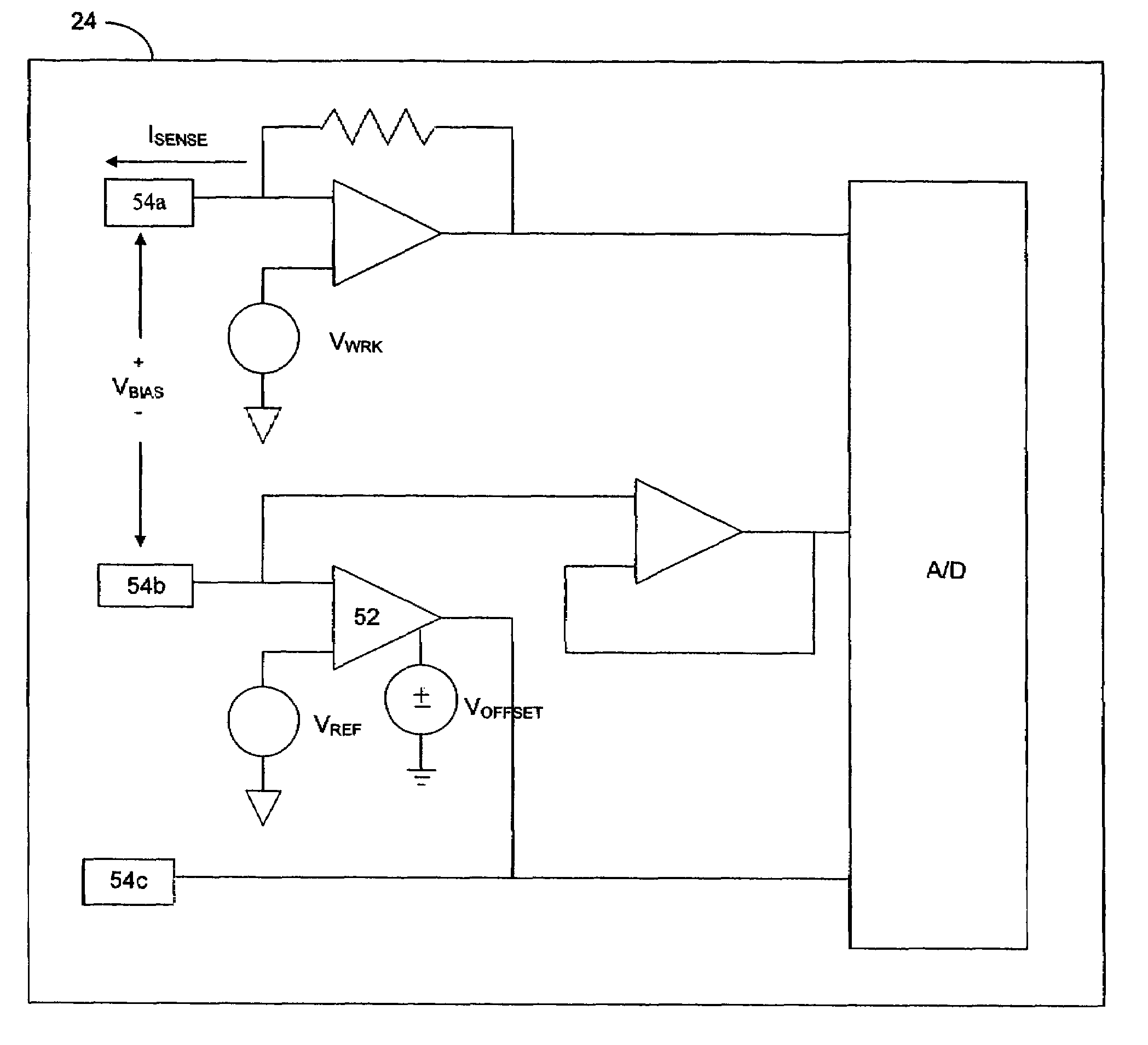
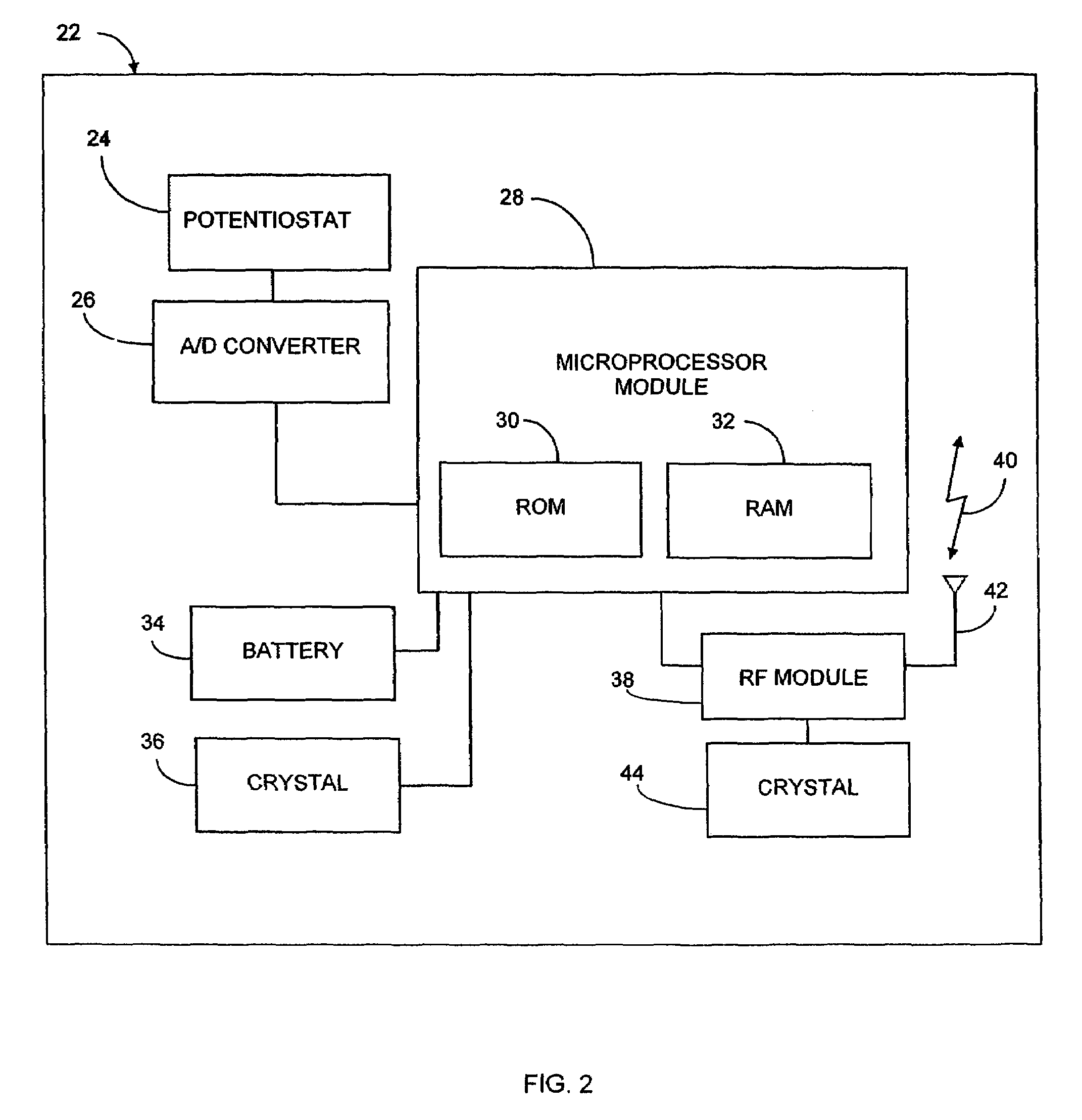
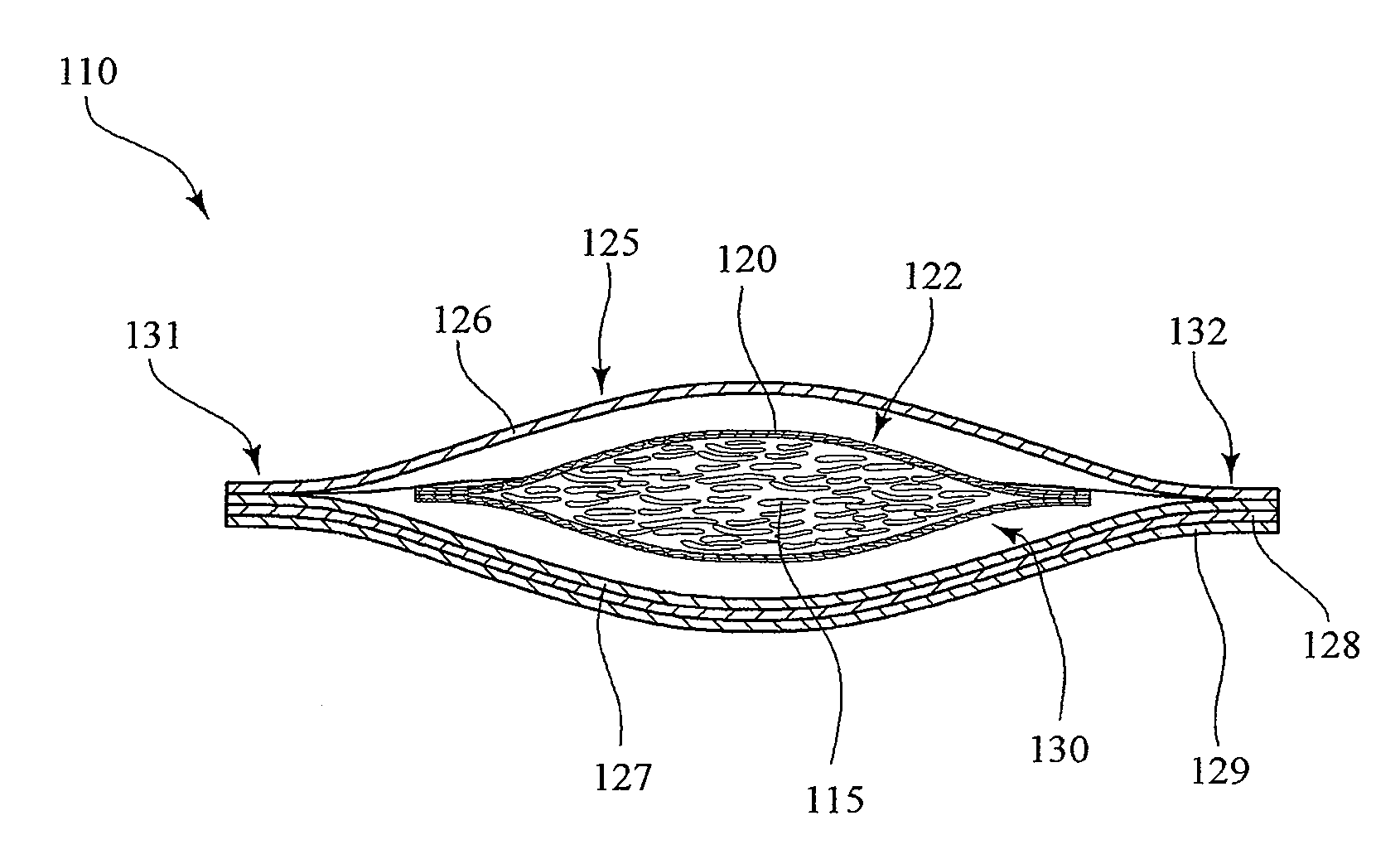
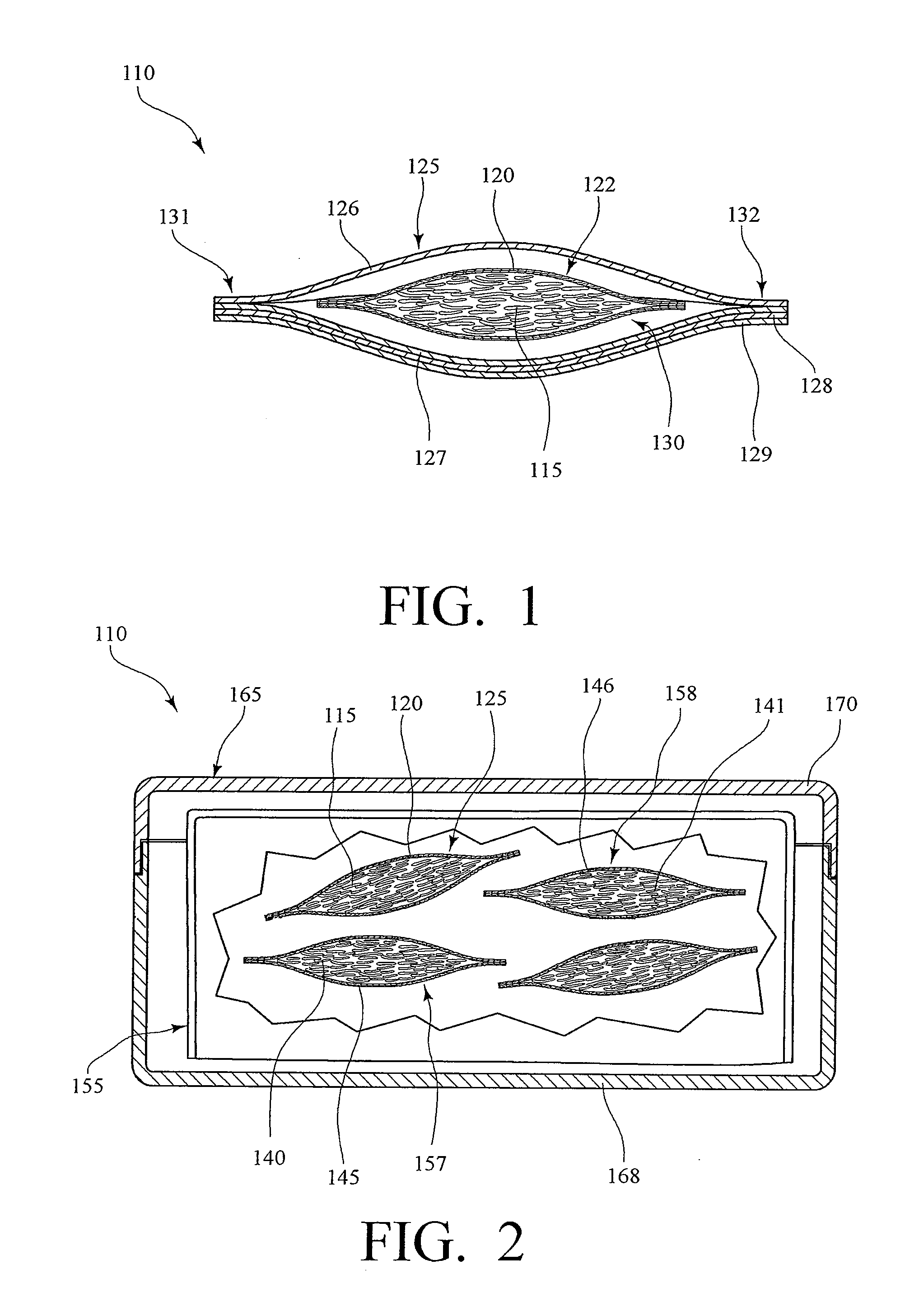
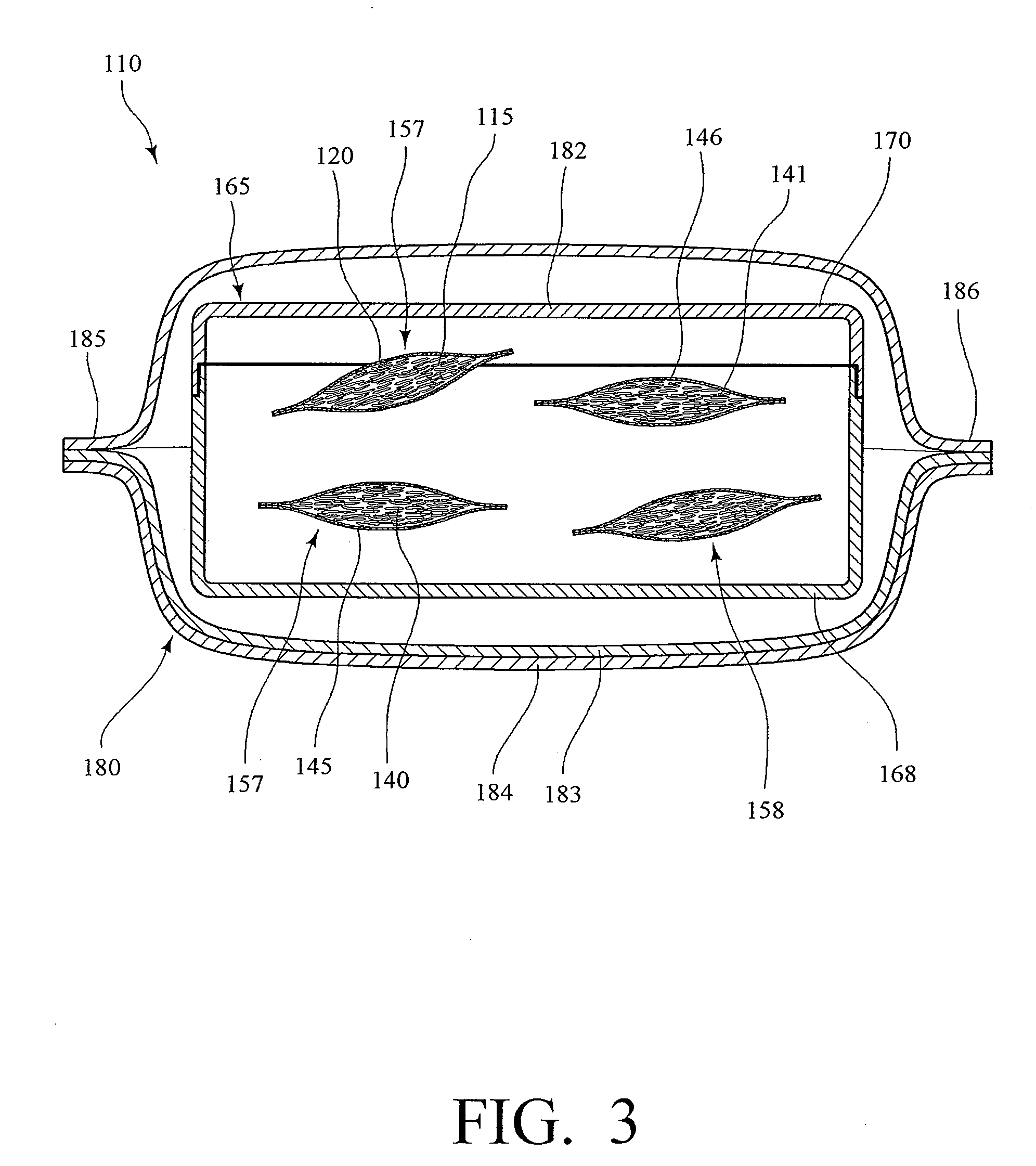
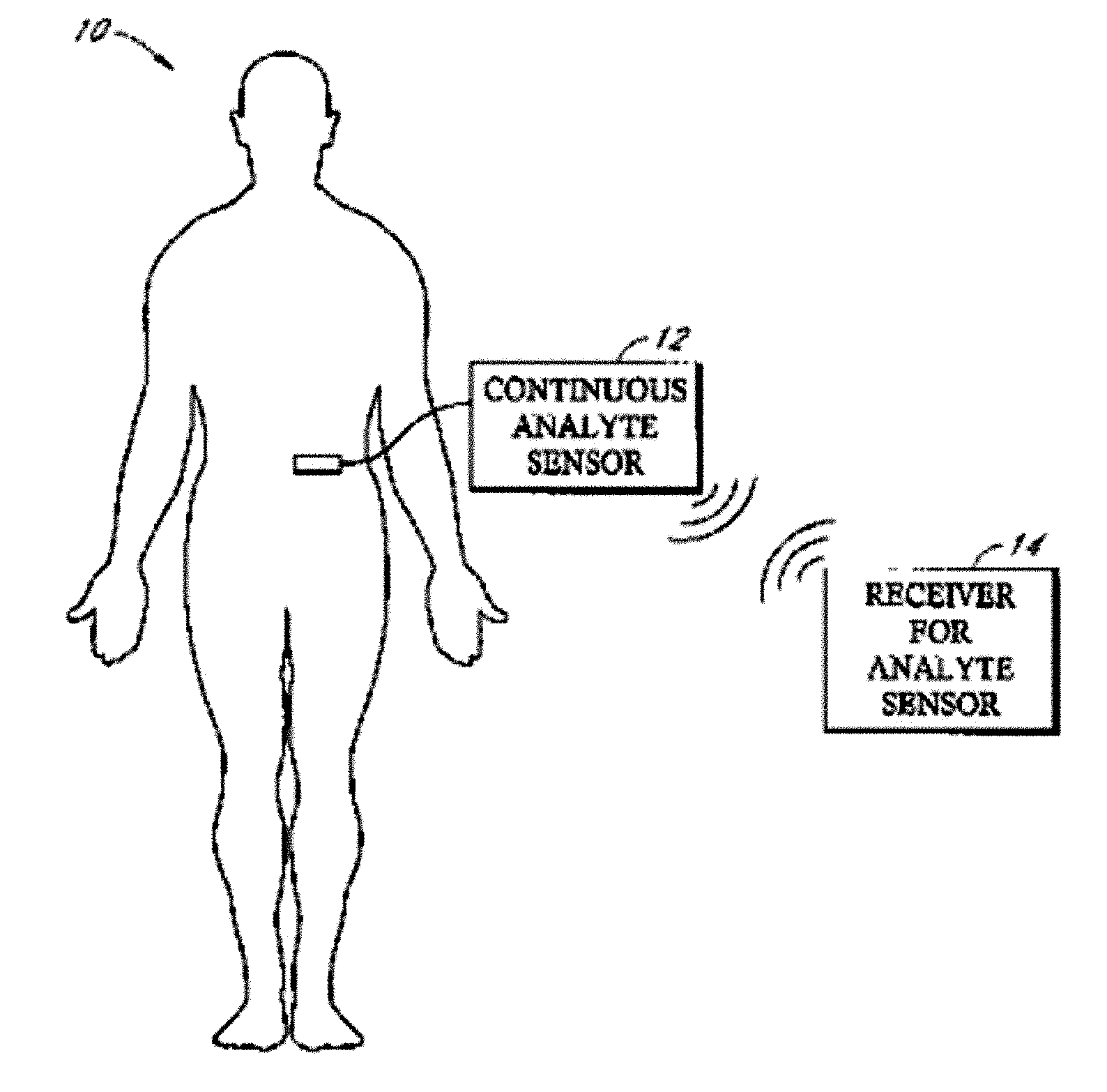
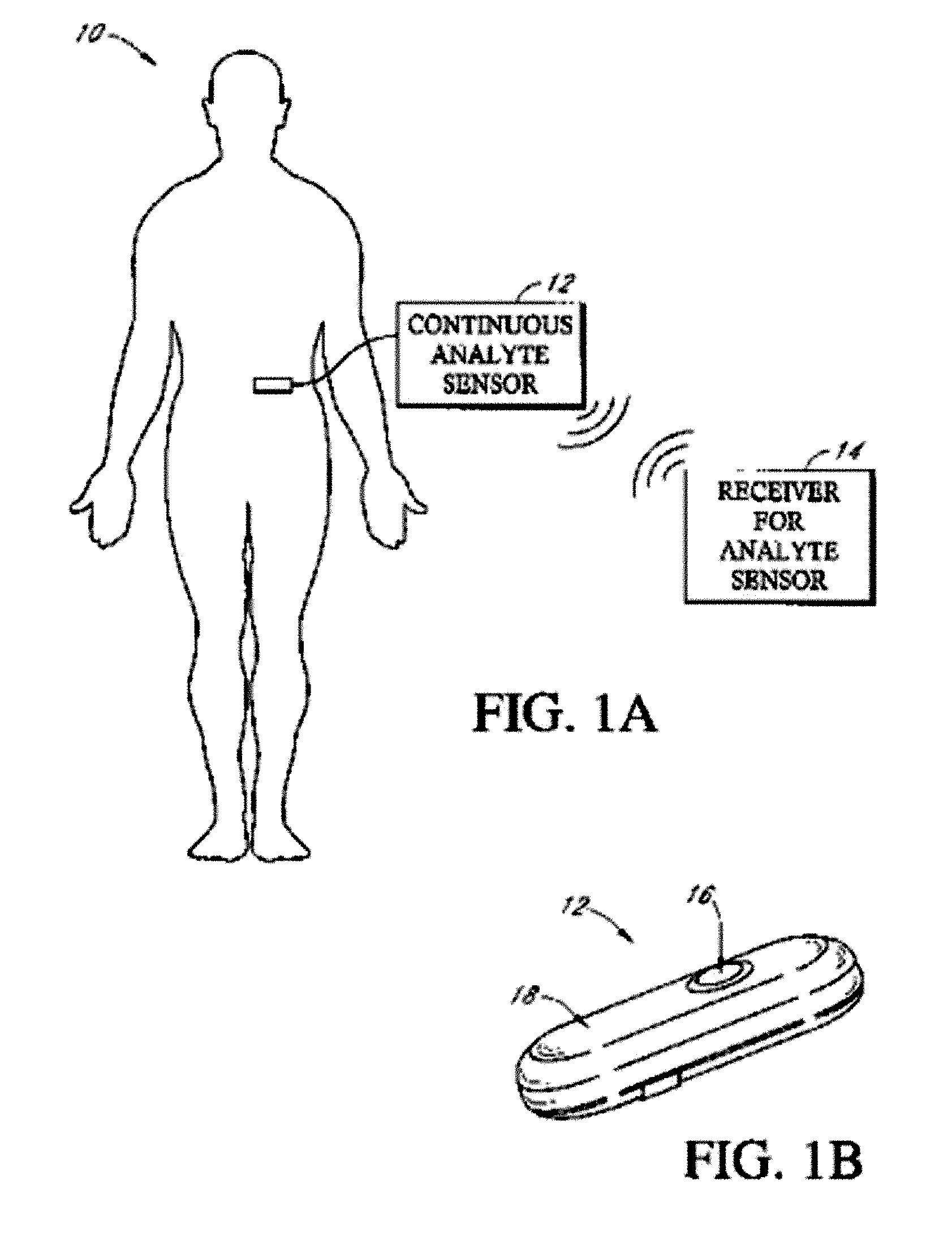
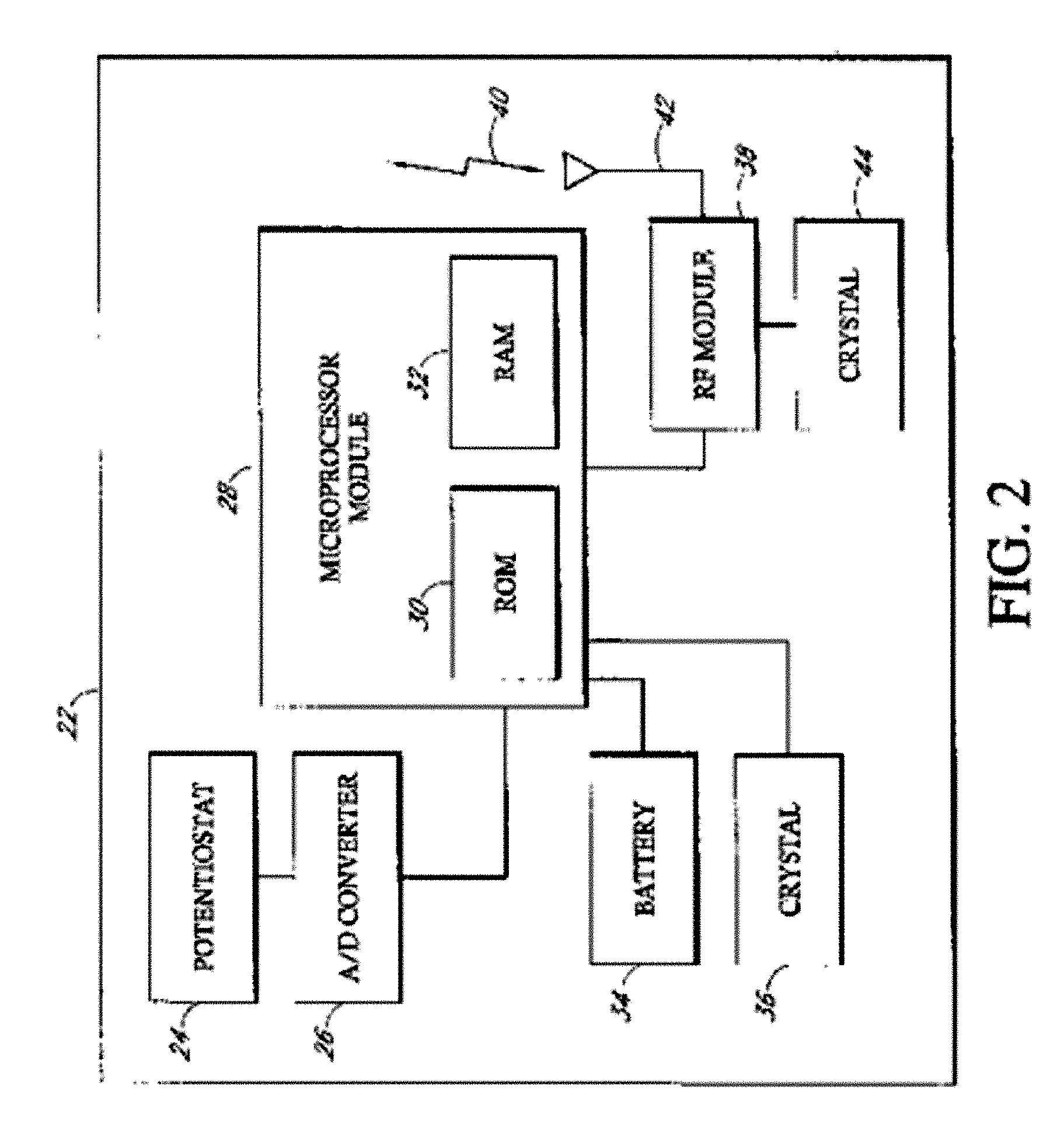
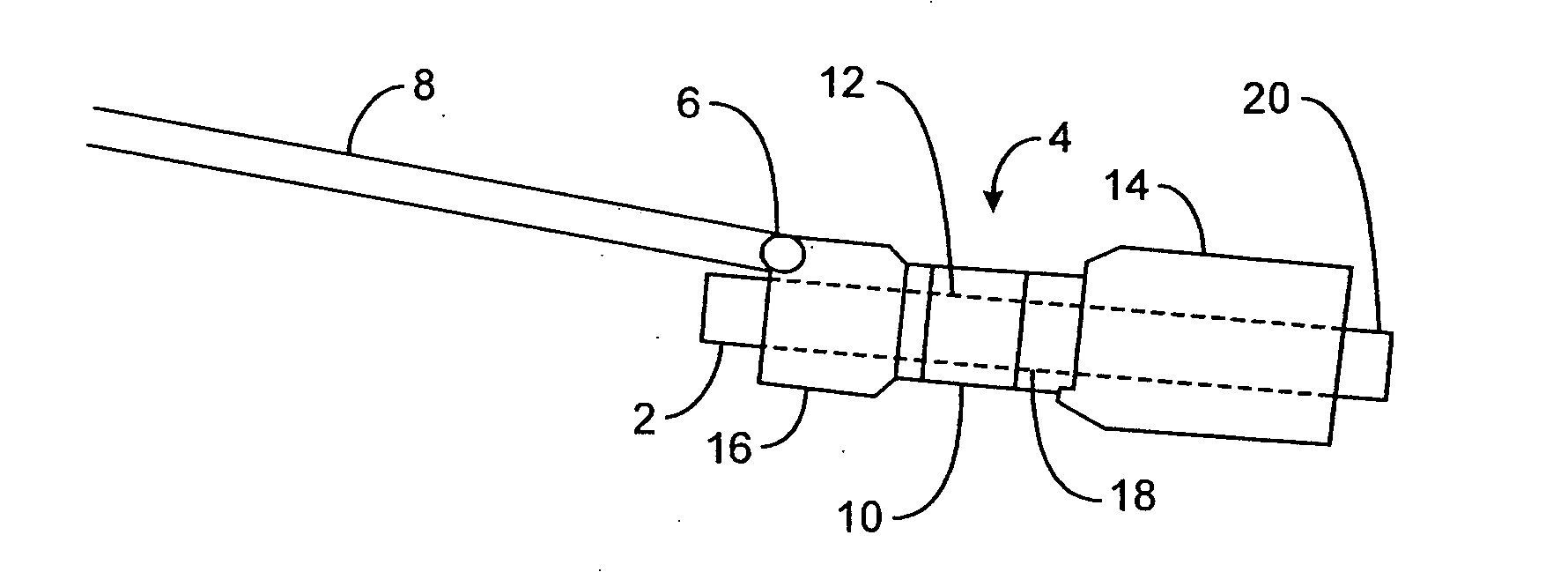
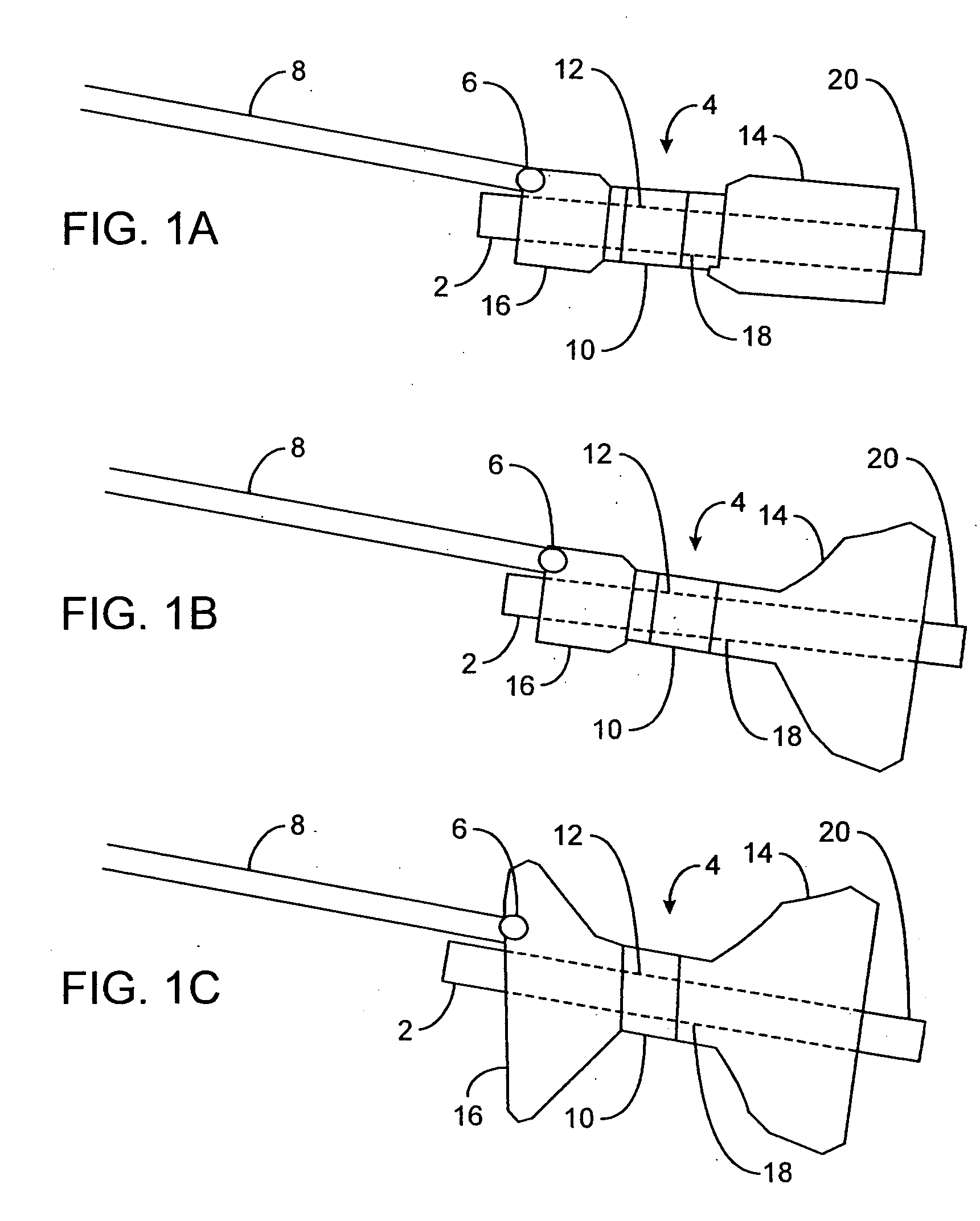
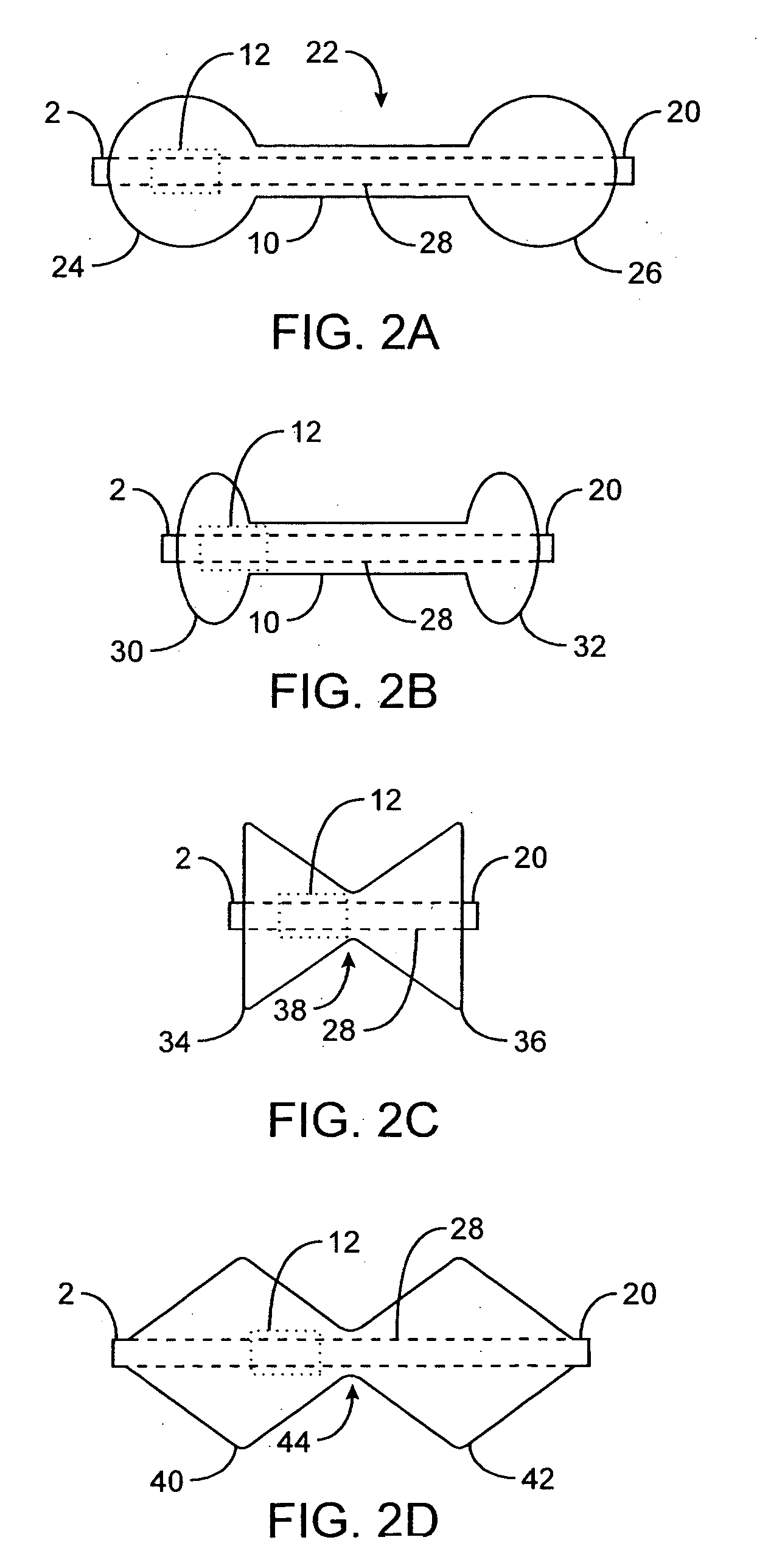
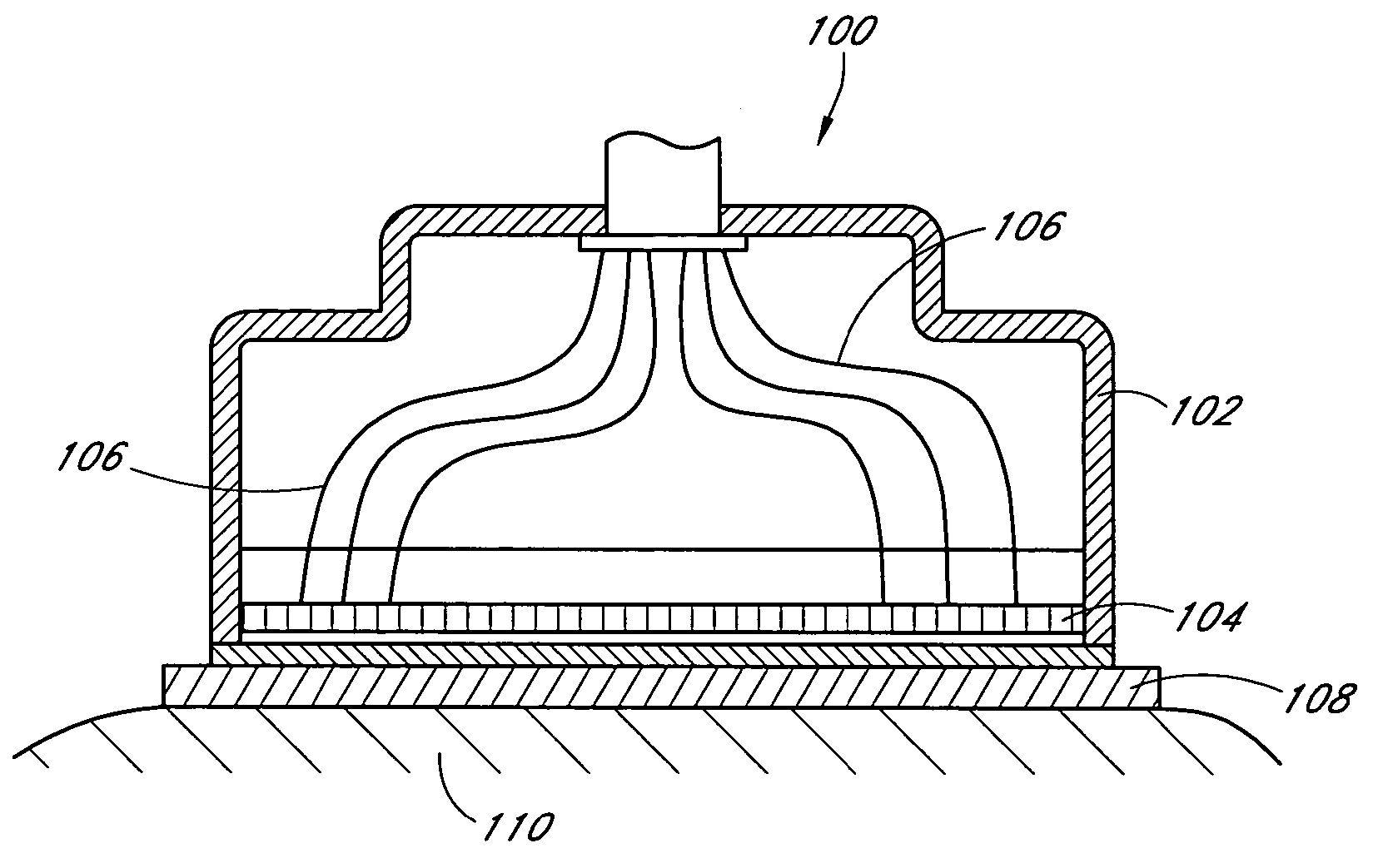
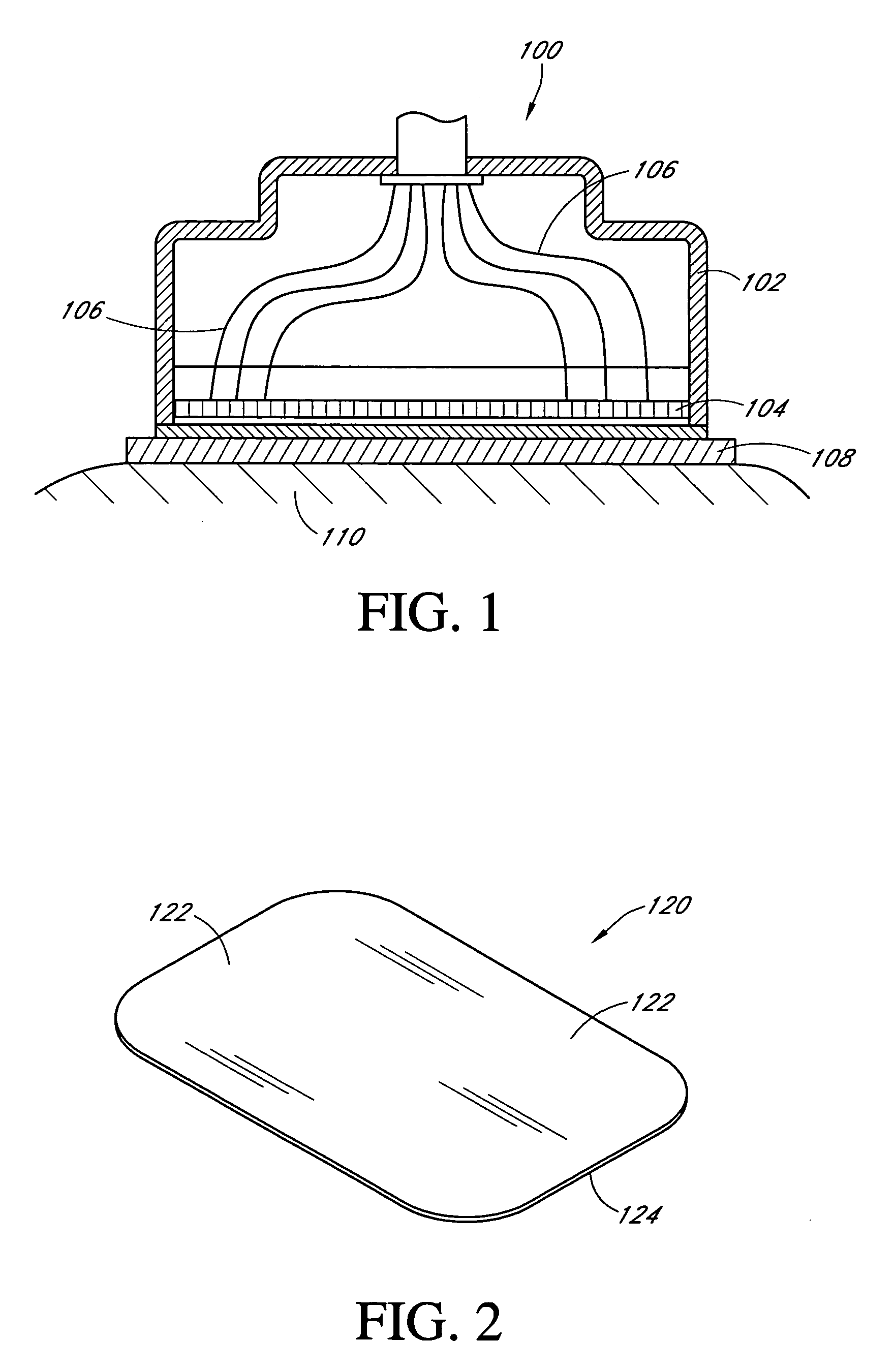
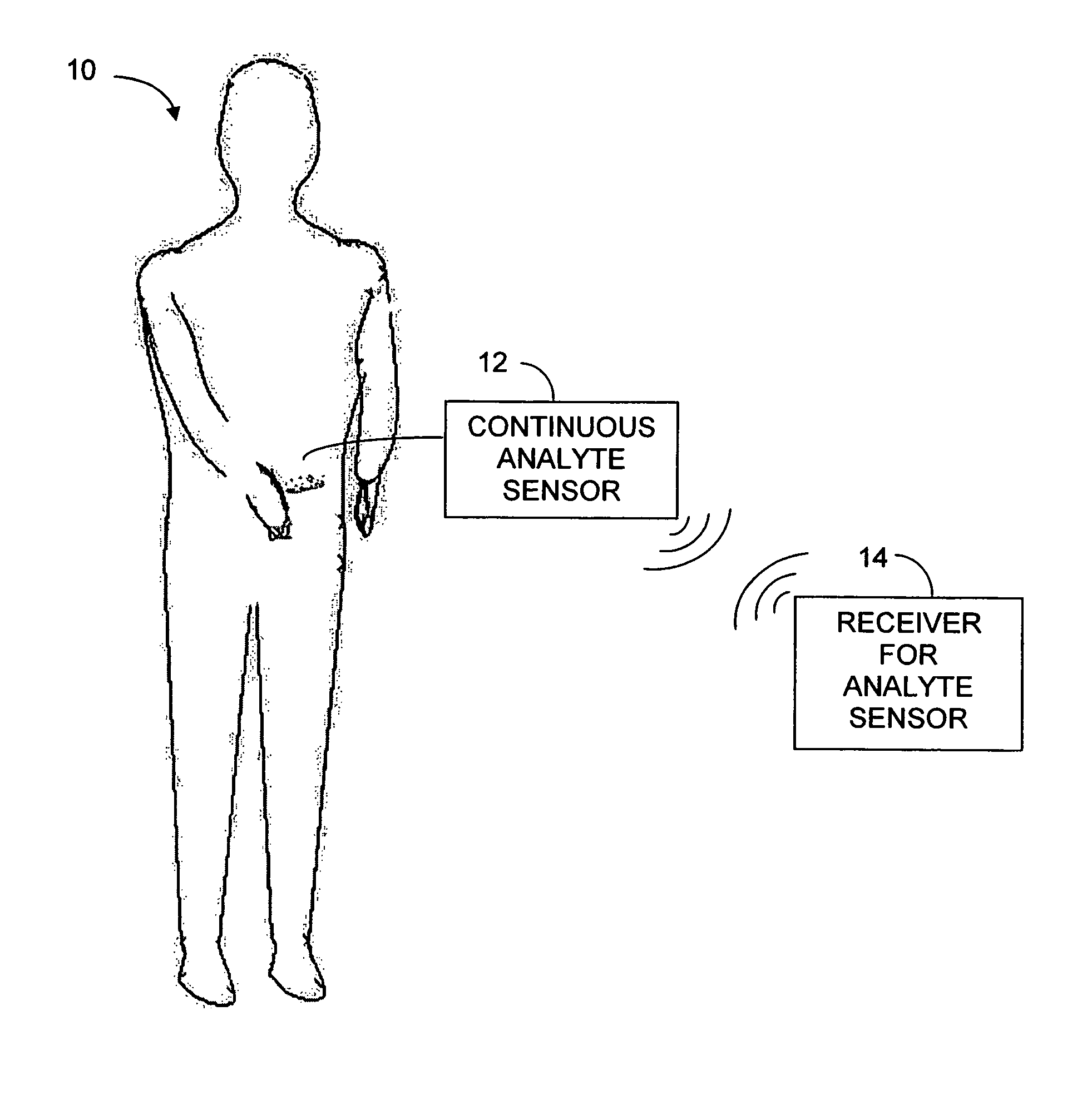
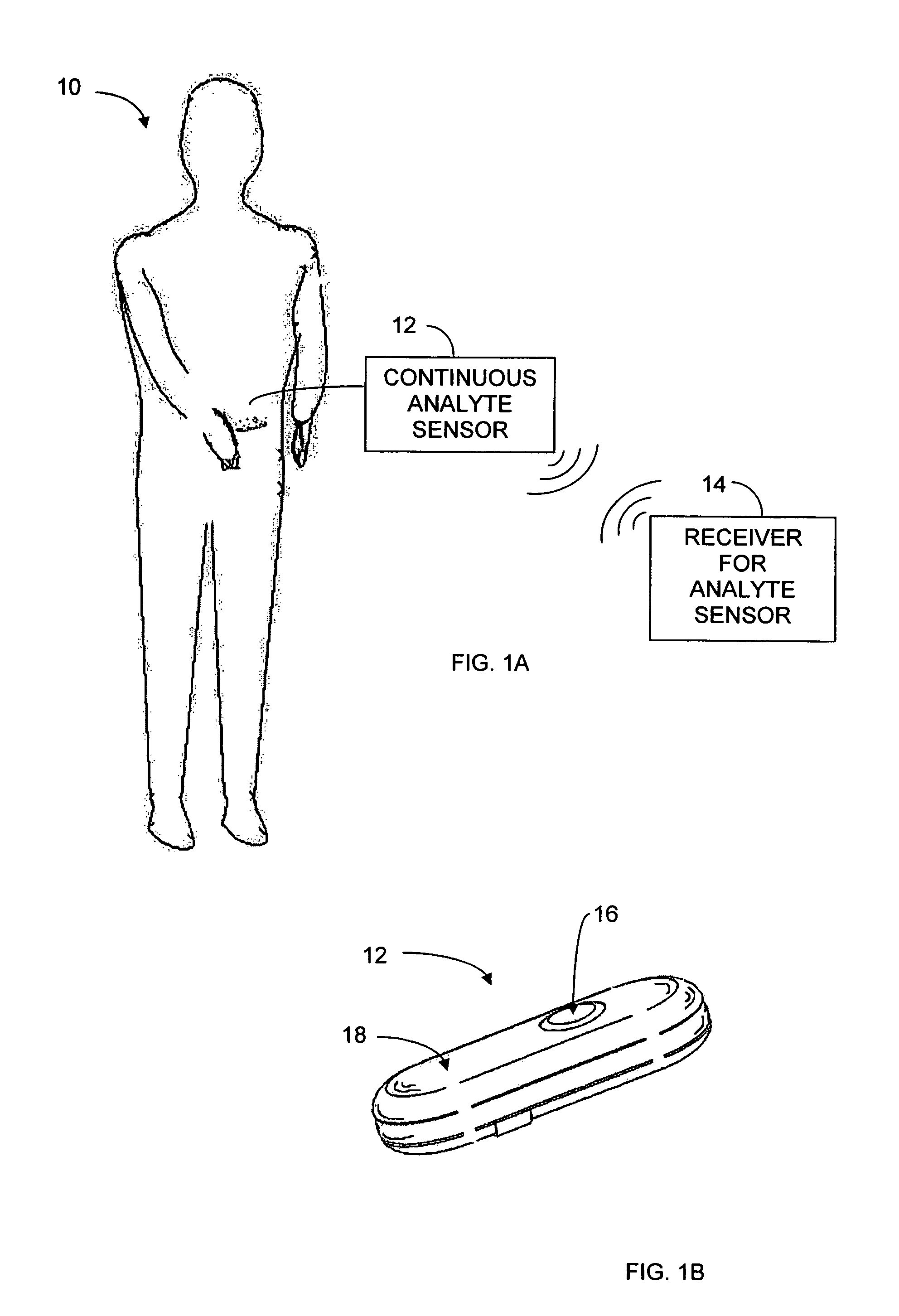
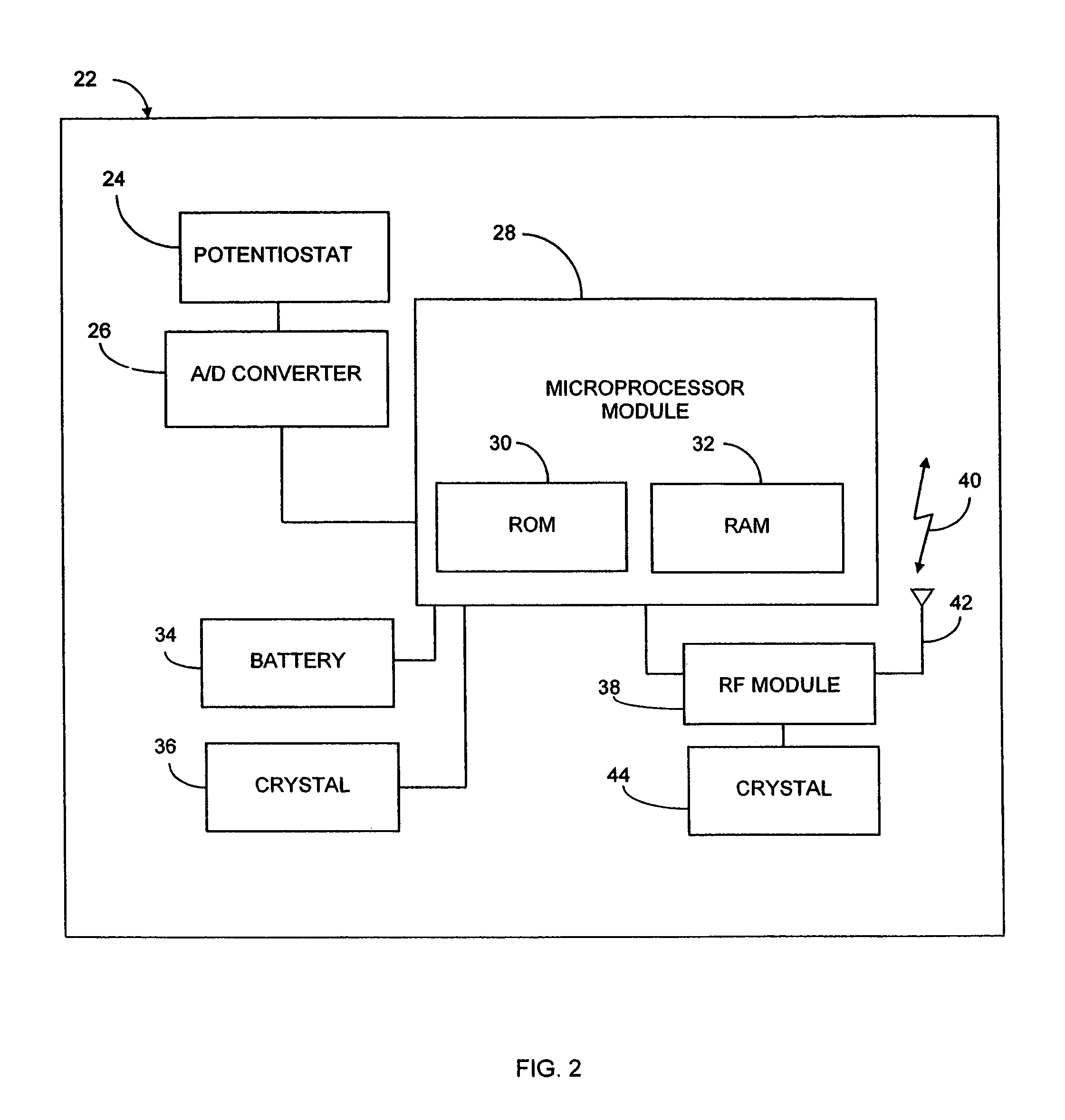
Implantable analyte sensor
InactiveUS20050245799A1Improved patient convenienceConvenient careCatheterDiagnostic recording/measuringAnalyteMiniaturization
Abstract of the DisclosureAn implantable analyte sensor including a sensing region for measuring the analyte and a non-sensing region for immobilizing the sensor body in the host. The sensor is implanted in a precisely dimensioned pocket to stabilize the analyte sensor in vivo and enable measurement of the concentration of the analyte in the host before and after formation of a foreign body capsule around the sensor. The sensor further provides a transmitter for RF transmission through the sensor body, electronic circuitry, and a power source optimized for long-term use in the miniaturized sensor body.
Owner:DEXCOM
Implantable analyte sensor
ActiveUS20050245795A1Improved patient convenienceConvenient careImmobilised enzymesBioreactor/fermenter combinationsAnalyteEngineering
Abstract of the DisclosureAn implantable analyte sensor including a sensing region for measuring the analyte and a non-sensing region for immobilizing the sensor body in the host. The sensor is implanted in a precisely dimensioned pocket to stabilize the analyte sensor in vivo and enable measurement of the concentration of the analyte in the host before and after formation of a foreign body capsule around the sensor. The sensor further provides a transmitter for RF transmission through the sensor body, electronic circuitry, and a power source optimized for long-term use in the miniaturized sensor body.
Owner:DEXCOM
Implantable analyte sensor
InactiveUS20090030294A1Improve convenienceMinimize movementBioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteMiniaturization
An implantable analyte sensor including a sensing region for measuring the analyte and a non-sensing region for immobilizing the sensor body in the host. The sensor is implanted in a precisely dimensioned pocket to stabilize the analyte sensor in vivo and enable measurement of the concentration of the analyte in the host before and after formation of a foreign body capsule around the sensor. The sensor further provides a transmitter for RF transmission through the sensor body, electronic circuitry, and a power source optimized for long-term use in the miniaturized sensor body.
Owner:DEXCOM INC
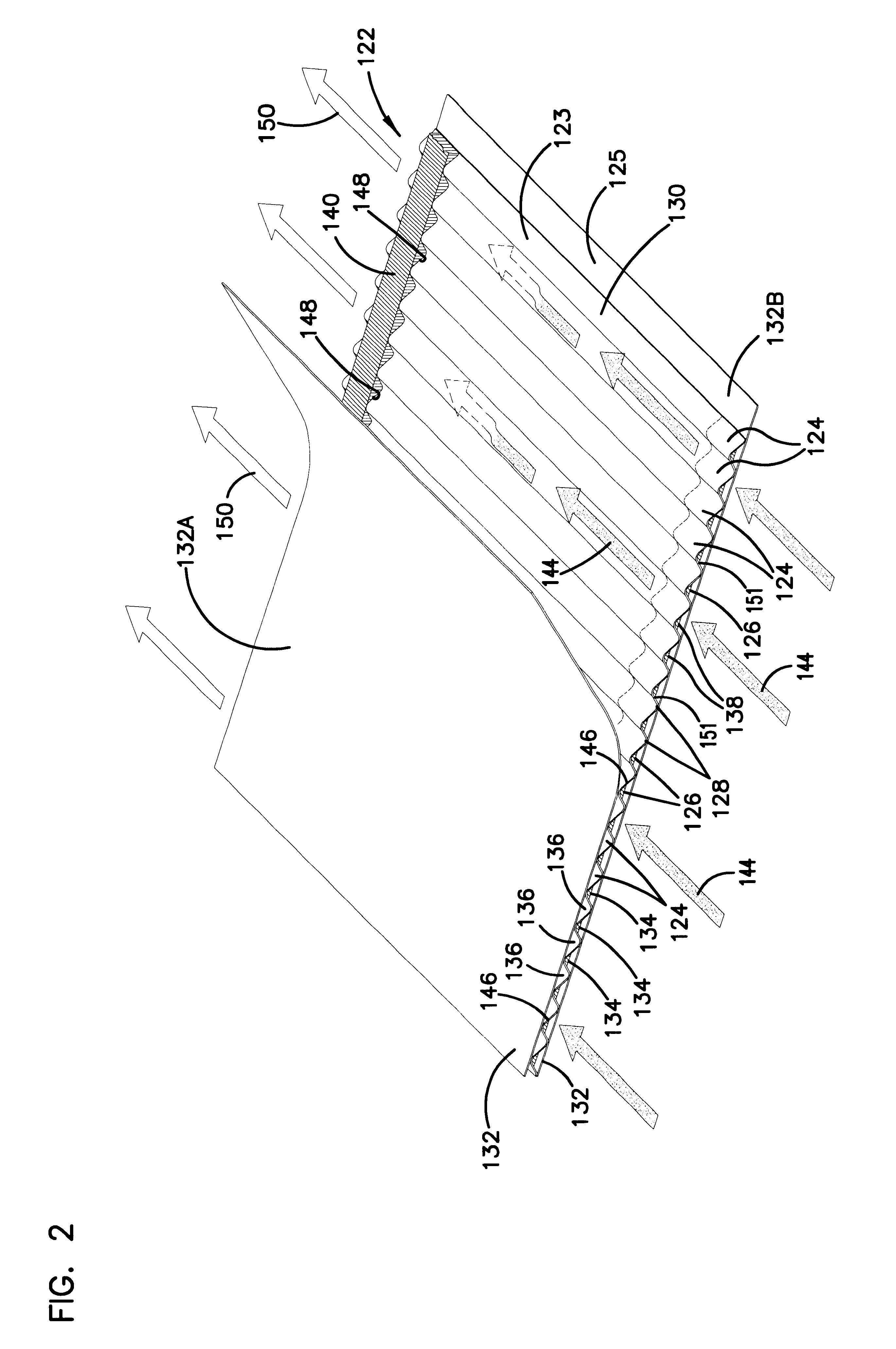
Filter arrangement; sealing system; and methods
InactiveUS6190432B1Prevent crashAvoid passingHuman health protectionCombination devicesEngineeringMechanical engineering
A filter pack includes a filter construction and a sealing system for sealing the construction within a duct or housing. The filter construction has first and second opposite flow faces and is configured for a straight-through flow. The sealing system includes a frame construction and a compressible seal member. The compressible seal member is molded around a portion of the frame construction. The compressible seal member is sufficiently compressible to form a radial seal between and against the frame construction and a surface of a housing when the filter pack is inserted within the housing.
Owner:DONALDSON CO INC
Implantable analyte sensor
An implantable analyte sensor including a sensing region for measuring the analyte and a non-sensing region for immobilizing the sensor body in the host. The sensor is implanted in a precisely dimensioned pocket to stabilize the analyte sensor in vivo and enable measurement of the concentration of the analyte in the host before and after formation of a foreign body capsule around the sensor. The sensor further provides a transmitter for RF transmission through the sensor body, electronic circuitry, and a power source optimized for long-term use in the miniaturized sensor body.
Owner:DEXCOM INC
System for optimizing anchoring force
ActiveUS7695493B2Constant force against the tissuePrevent overcompressionSuture equipmentsDiagnosticsConstant forceStrain gauge
Systems for optimizing anchoring force are described herein. In securing tissue folds, over-compression of the tissue directly underlying the anchors is avoided by utilizing tissue anchors having expandable arms configured to minimize contact area between the anchor and tissue. When the anchor is in its expanded configuration, a load is applied to the anchor until it is optimally configured to accommodate a range of deflections while the anchor itself exerts a substantially constant force against the tissue. Various devices, e.g., stops, spring members, fuses, strain gauges, etc., can be used to indicate when the anchor has been deflected to a predetermined level within the optimal range. Moreover, other factors to affect the anchor characteristics include, e.g., varying the number of arms or struts of the anchor, positioning of the arms, configuration of the arms, the length of the collars, etc.
Owner:USGI MEDICAL
Implantable analyte sensor
ActiveUS7657297B2Improve convenienceMinimize movementImmobilised enzymesBioreactor/fermenter combinationsAnalyteMiniaturization
An implantable analyte sensor including a sensing region for measuring the analyte and a non-sensing region for immobilizing the sensor body in the host. The sensor is implanted in a precisely dimensioned pocket to stabilize the analyte sensor in vivo and enable measurement of the concentration of the analyte in the host before and after formation of a foreign body capsule around the sensor. The sensor further provides a transmitter for RF transmission through the sensor body, electronic circuitry, and a power source optimized for long-term use in the miniaturized sensor body.
Owner:DEXCOM INC
Smokeless tobacco
InactiveUS20080173317A1Improve barrier propertiesObstruct passageTobacco preparationTobacco treatmentSodium bicarbonateEngineering
A smokeless tobacco product is provided. A water-permeable pouch containing a tobacco formulation and configured for insertion into the mouth of a user of that product is provided. The tobacco formulation includes granular tobacco and a buffer comprised of sodium carbonate and sodium bicarbonate. An outer packaging material enveloping the pouch is provided and is sealed so as to allow a controlled environment to be maintained within.
Owner:R J REYNOLDS TOBACCO COMPANY
Implantable analyte sensor
ActiveUS20050242479A1Improved patient convenienceConvenient careImmobilised enzymesBioreactor/fermenter combinationsAnalyteMiniaturization
Abstract of the DisclosureAn implantable analyte sensor including a sensing region for measuring the analyte and a non-sensing region for immobilizing the sensor body in the host. The sensor is implanted in a precisely dimensioned pocket to stabilize the analyte sensor in vivo and enable measurement of the concentration of the analyte in the host before and after formation of a foreign body capsule around the sensor. The sensor further provides a transmitter for RF transmission through the sensor body, electronic circuitry, and a power source optimized for long-term use in the miniaturized sensor body.
Owner:DEXCOM
Filter arrangement; sealing system; and methods
InactiveUS6350291B1Avoid passingObstruct passageHuman health protectionCombination devicesEngineeringMechanical engineering
A filter pack includes a filter construction and a sealing system for sealing the construction within a duct or housing. The filter construction has first and second opposite flow faces and is configured for a straight-through flow. The sealing system includes a frame construction and a compressible seal member. The compressible seal member is molded around a portion of the frame construction. The compressible seal member is sufficiently compressible to form a radial seal between and against the frame construction and a surface of a housing when the filter pack is inserted within the housing.
Owner:DONALDSON CO INC
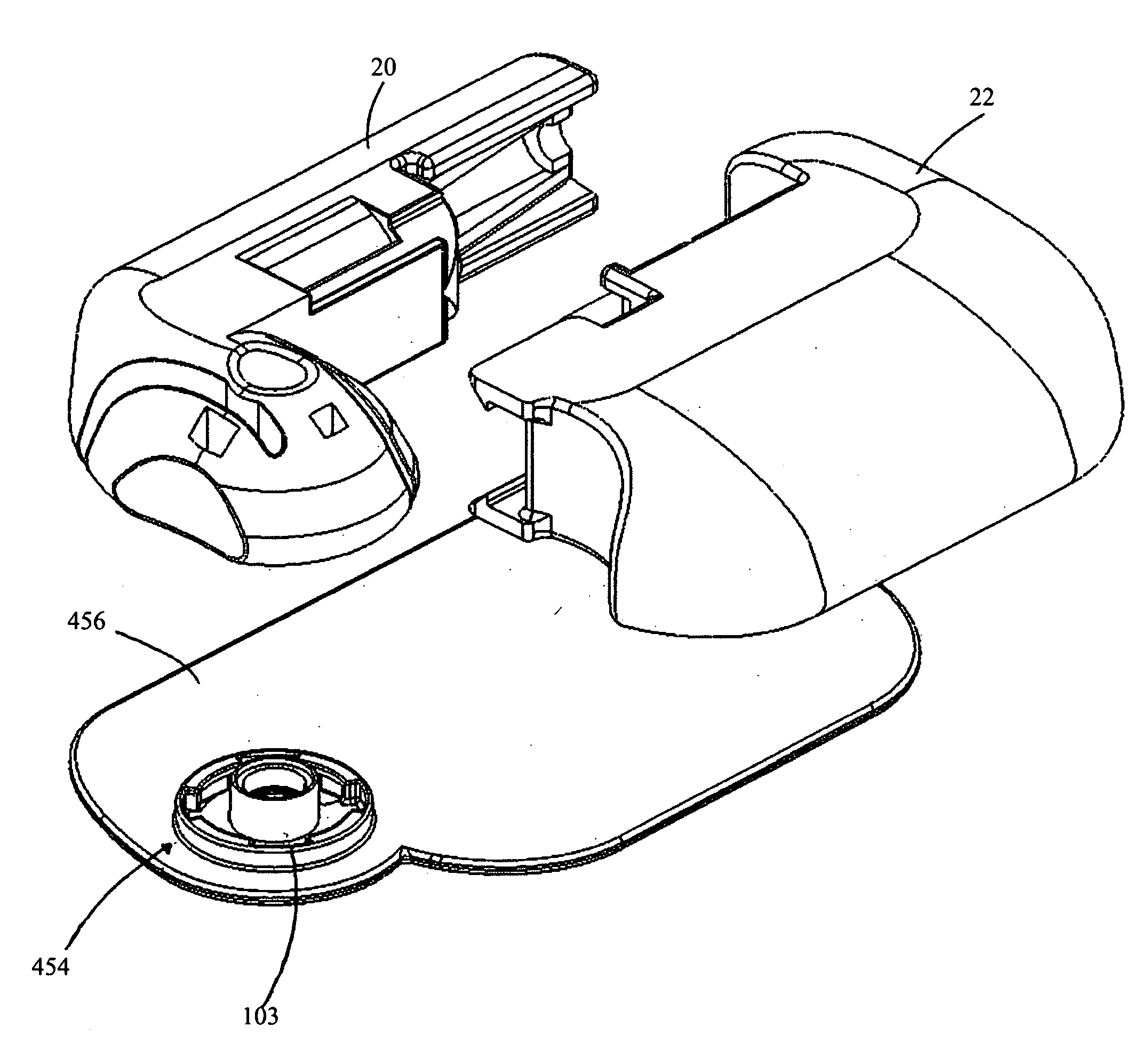
Infusion medium delivery device and method with drive device for driving plunger in reservoir
ActiveUS20080097381A1Prevent rotationObstruct passageMedical devicesPressure infusionSingle useElectronics
A delivery device includes a durable housing portion and a separable disposable portion that selectively engage and disengage from each other. The disposable housing portion secures to the patient-user and may be disposed of after it has been in use for a prescribed period. Components that normally come into contact with a patient-user or with infusion medium are supported by the disposable housing portion, while the durable housing portion supports other components such as electronics and a drive device. A reservoir is supported by the disposable housing portion and has a moveable plunger that operatively couples to the drive device, when the disposable and durable housing portions are engaged.
Owner:MEDTRONIC MIMIMED INC
Methods and products for producing lattices of EMR-treated islets in tissues, and uses therefor
InactiveUS20060004306A1Big advantageSufficient amountElectrotherapyChiropractic devicesElectromagnetic radiationBiomedical engineering
Methods of treatment of tissue with electromagnetic radiation (EMR) to produce lattices of EMR-treated islets in the tissue are disclosed. Also disclosed are devices and systems for producing lattices of EMR-treated islets in tissue, and cosmetic and medical applications of such devices and systems.
Owner:PALOMAR MEDICAL TECH
Smokeless tobacco products and processes
InactiveUS20100018539A1Improve barrier propertiesObstruct passageTobacco preparationTobacco treatmentSmokeless tobaccoEngineering
An improved pouching machine is provided. The improved pouching machine comprises a feed hopper with a first and a second end and a feed screw with a plurality of pins extending from the circumference. The feed screw is connected to a first shaft and the first shaft is connected to a motor to rotate the first shaft. An agitator screw is positioned adjacent to the feed screw and has a plurality of pins extending from the circumference. The agitator screw is connected to a second shaft.
Owner:R J REYNOLDS TOBACCO COMPANY
Rechargeable lithium/water, lithium/air batteries
InactiveUS20070221265A1Improve protectionEasy to controlFinal product manufacturePV power plantsHigh energyOptoelectronics
Electrochemical cells, and more specifically, rechargeable batteries comprising lithium anodes for use in water and / or air environments, as well as non-aqueous and non-air environments, are presented. In one embodiment, an electrochemical cell includes an anode comprising lithium and a multi-layered structure positioned between the anode and an electrolyte of the cell. A multi-layered structure can include at least a first single-ion conductive material layer (e.g., a lithiated metal layer), and at least a first polymeric layer positioned between the anode and the single-ion conductive material. The invention also can provide an electrode stabilization layer positioned within the electrode, i.e., between one portion and another portion of an electrode, to control depletion and re-plating of electrode material upon charge and discharge of a battery. Advantageously, electrochemical cells comprising combinations of structures described herein are not only compatible with environments that are typically unsuitable for lithium, but the cells may be also capable of displaying long cycle life, high lithium cycling efficiency, and high energy density.
Owner:SION POWER CORP
Mechanical tissue device and method
InactiveUS20080119886A1Rigid enoughLength of device being increasedDilatorsOcculdersVeinVenous blood
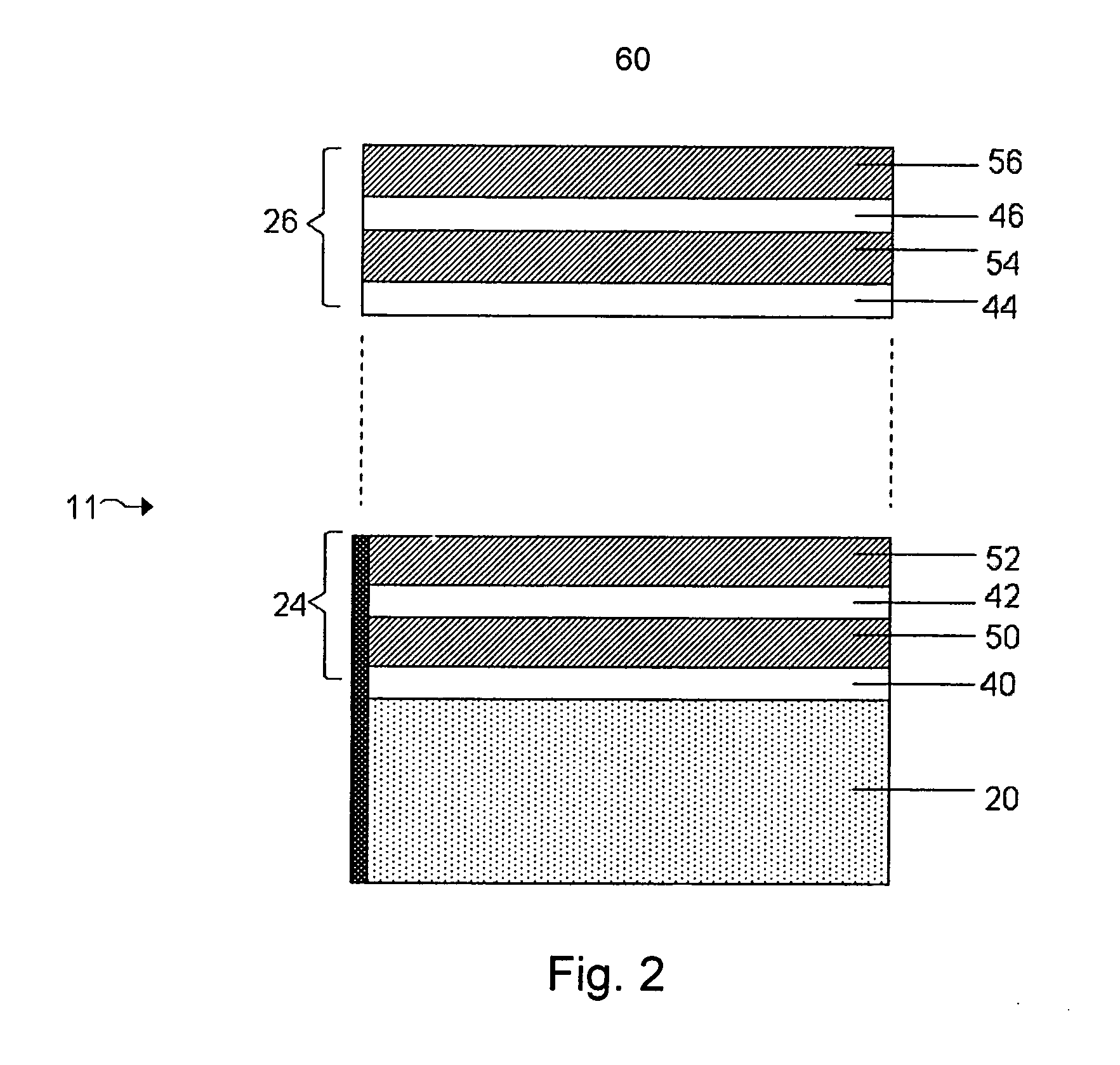
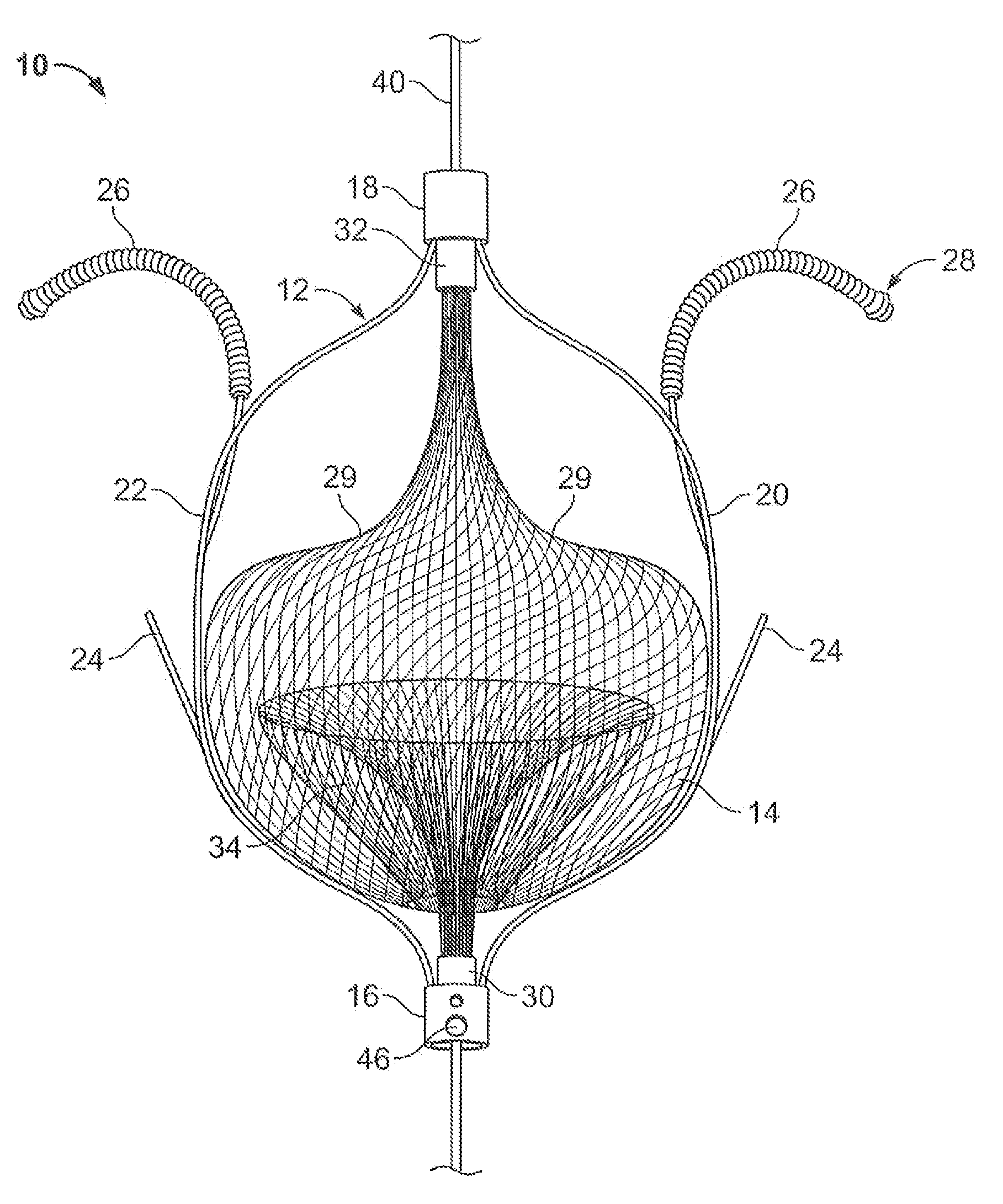
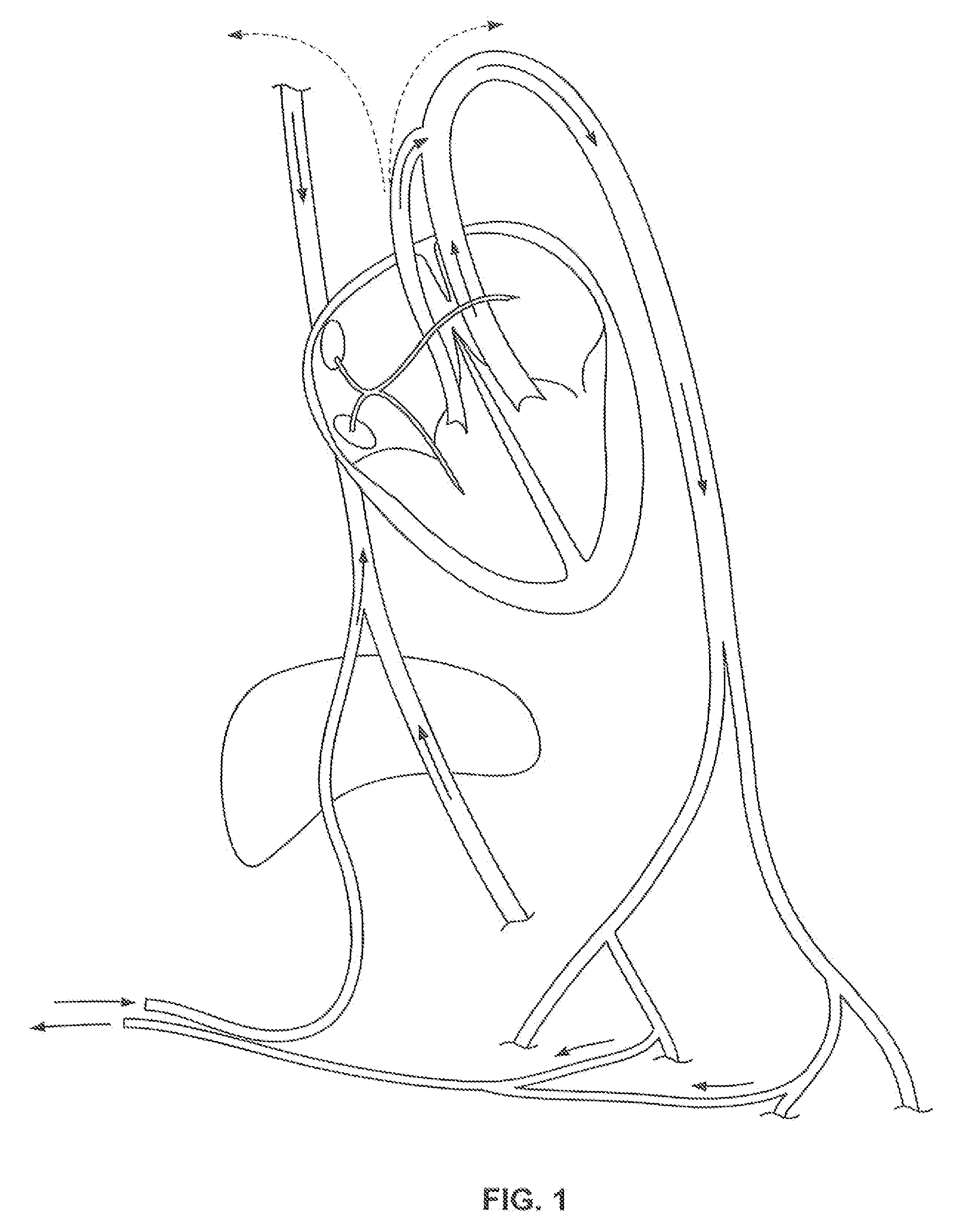
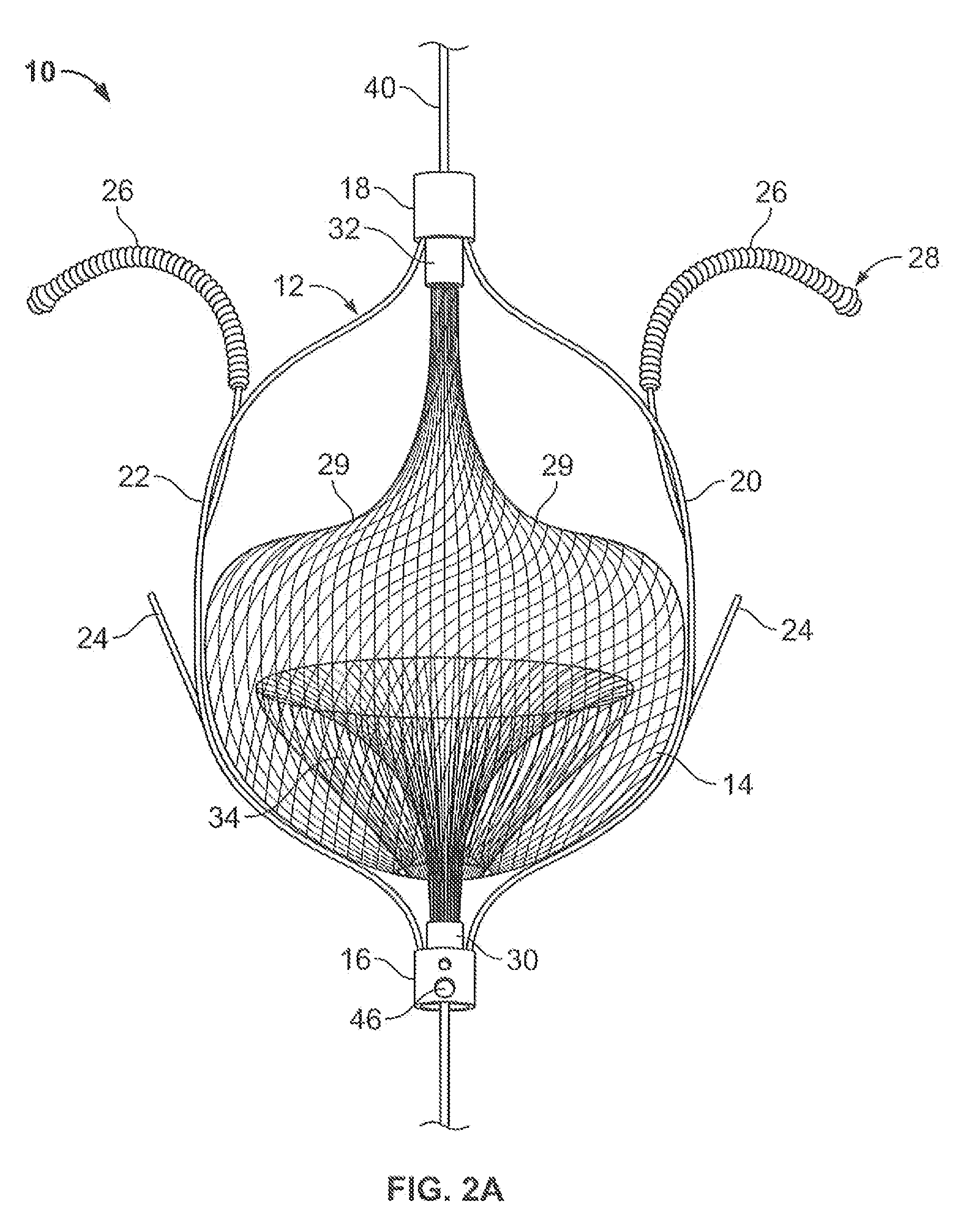
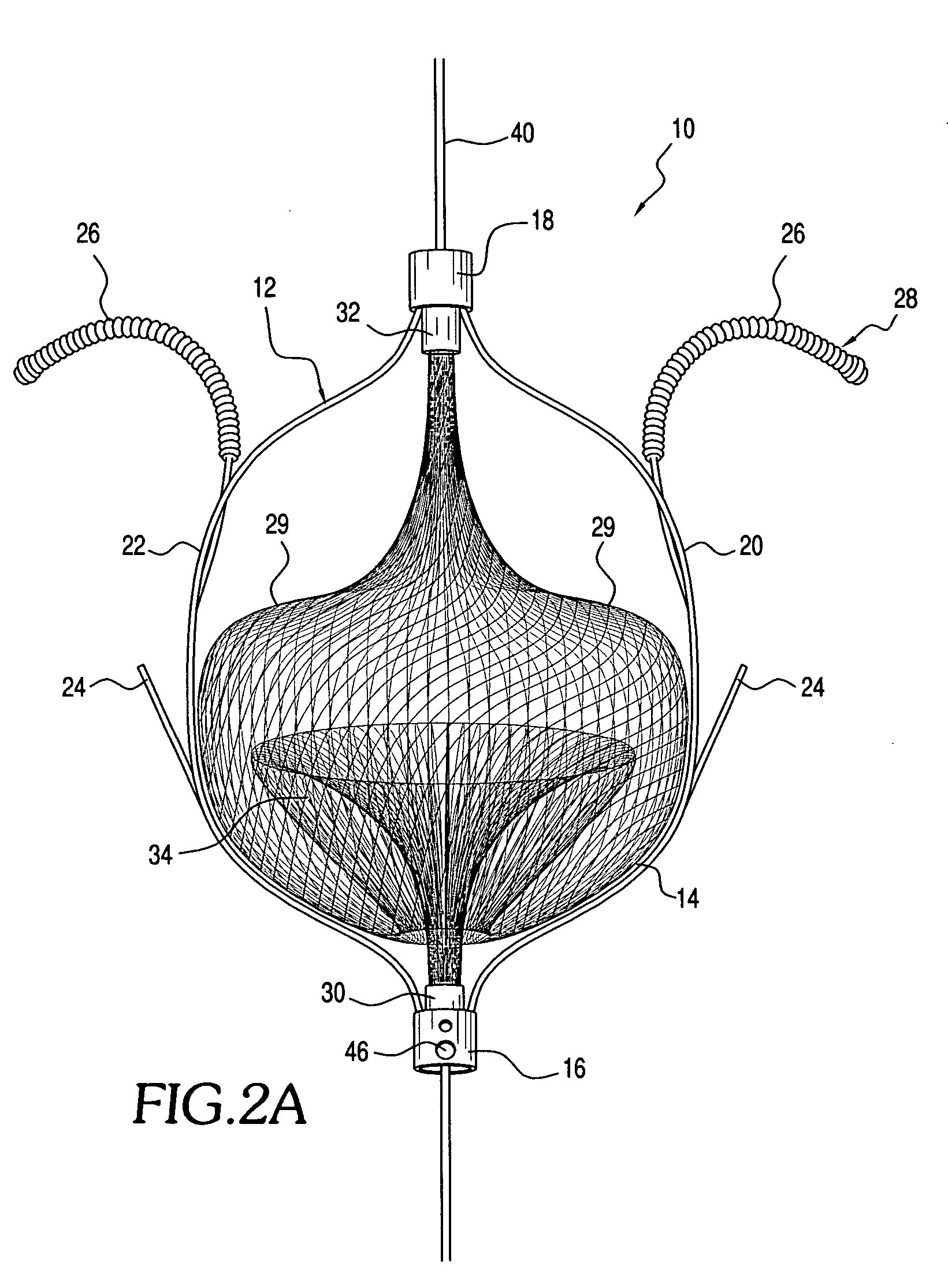
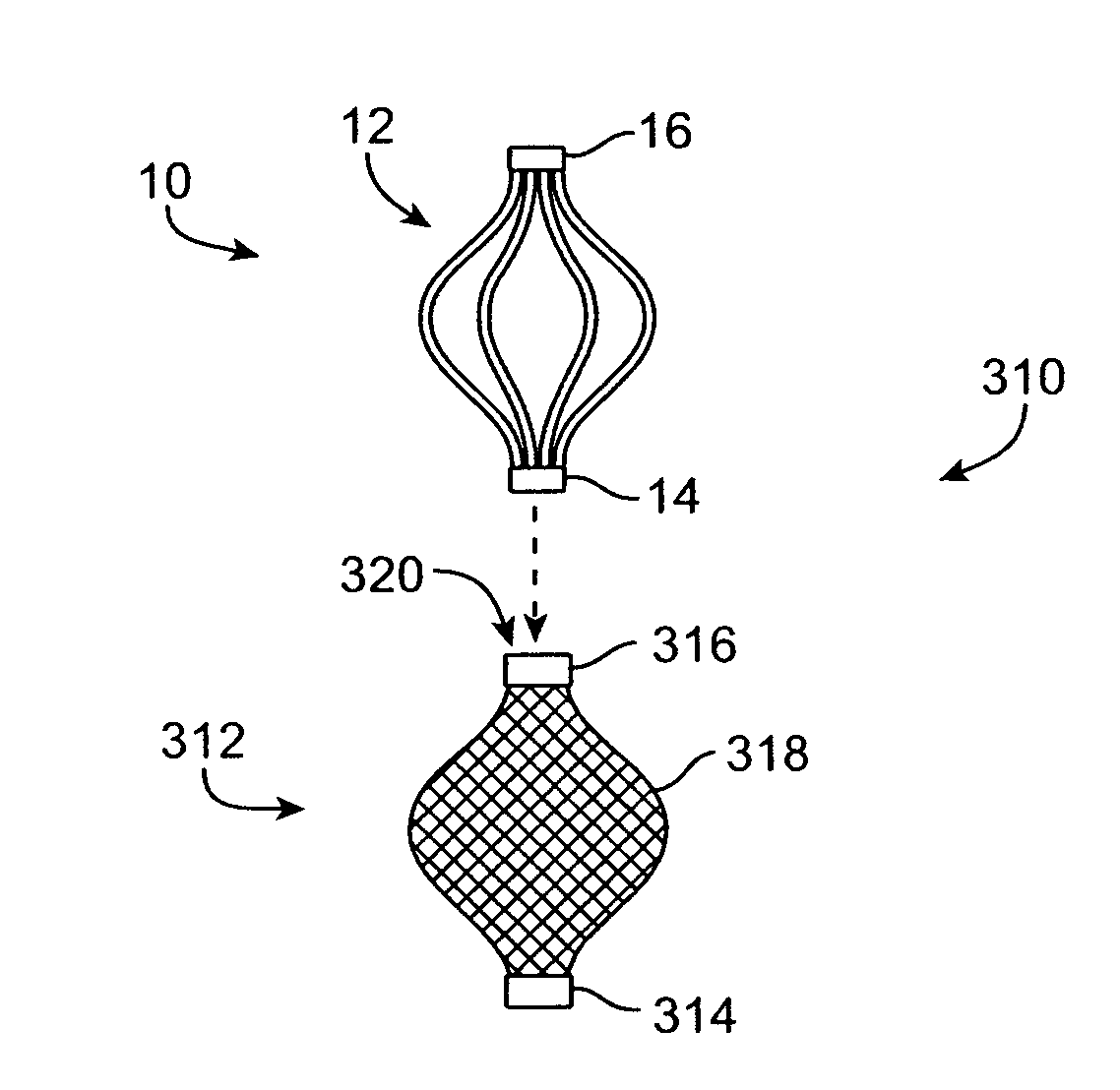
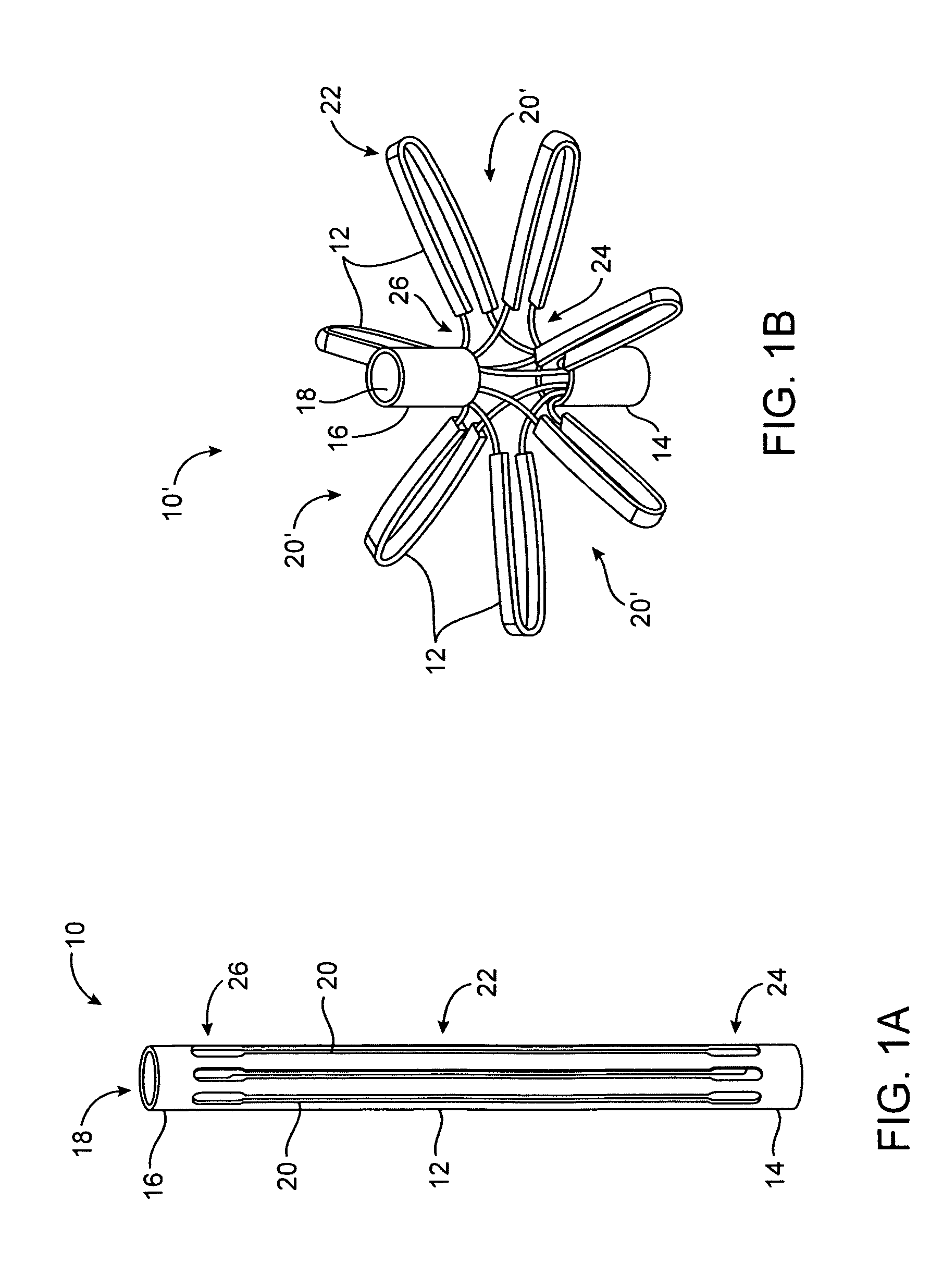
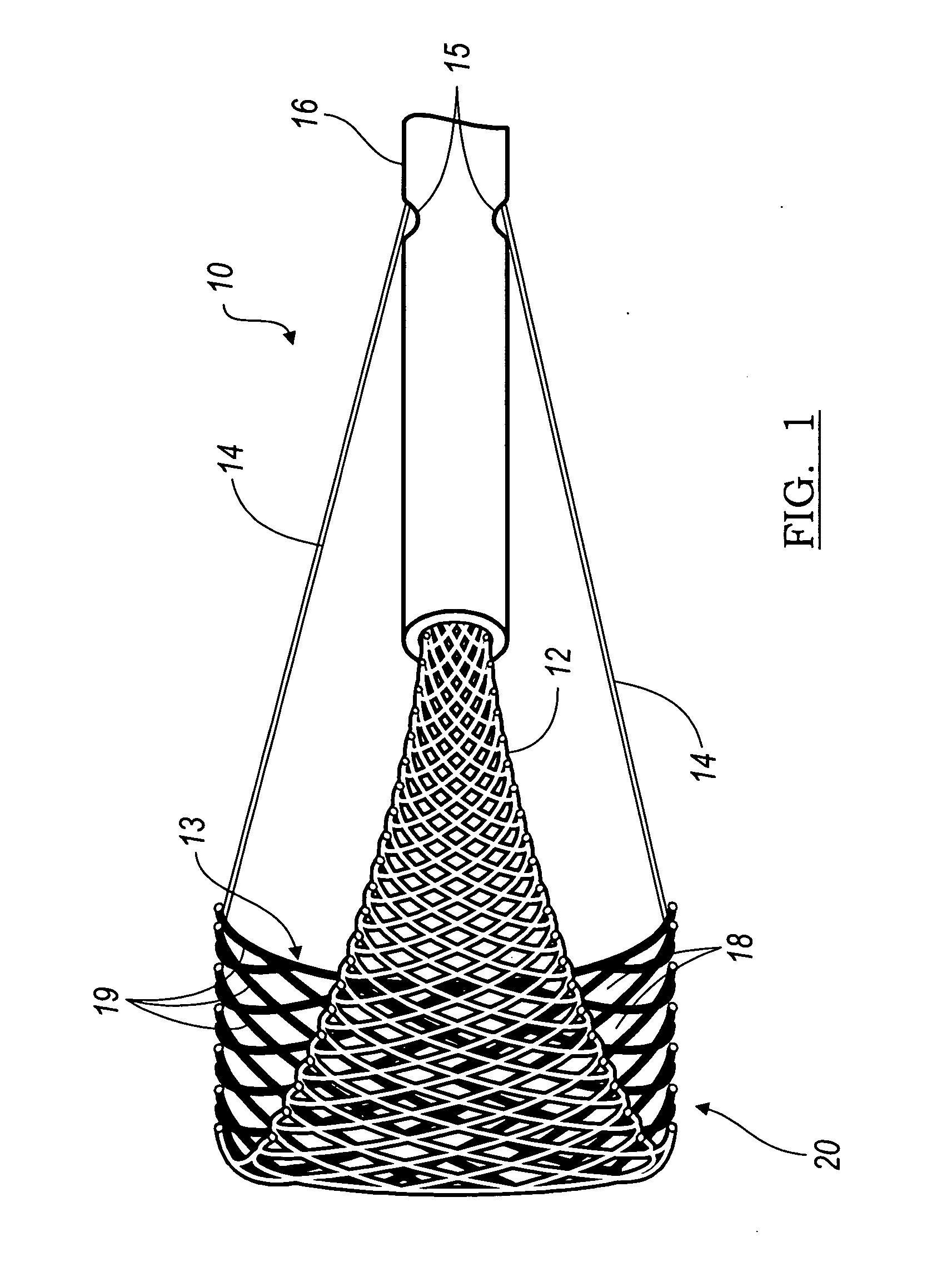
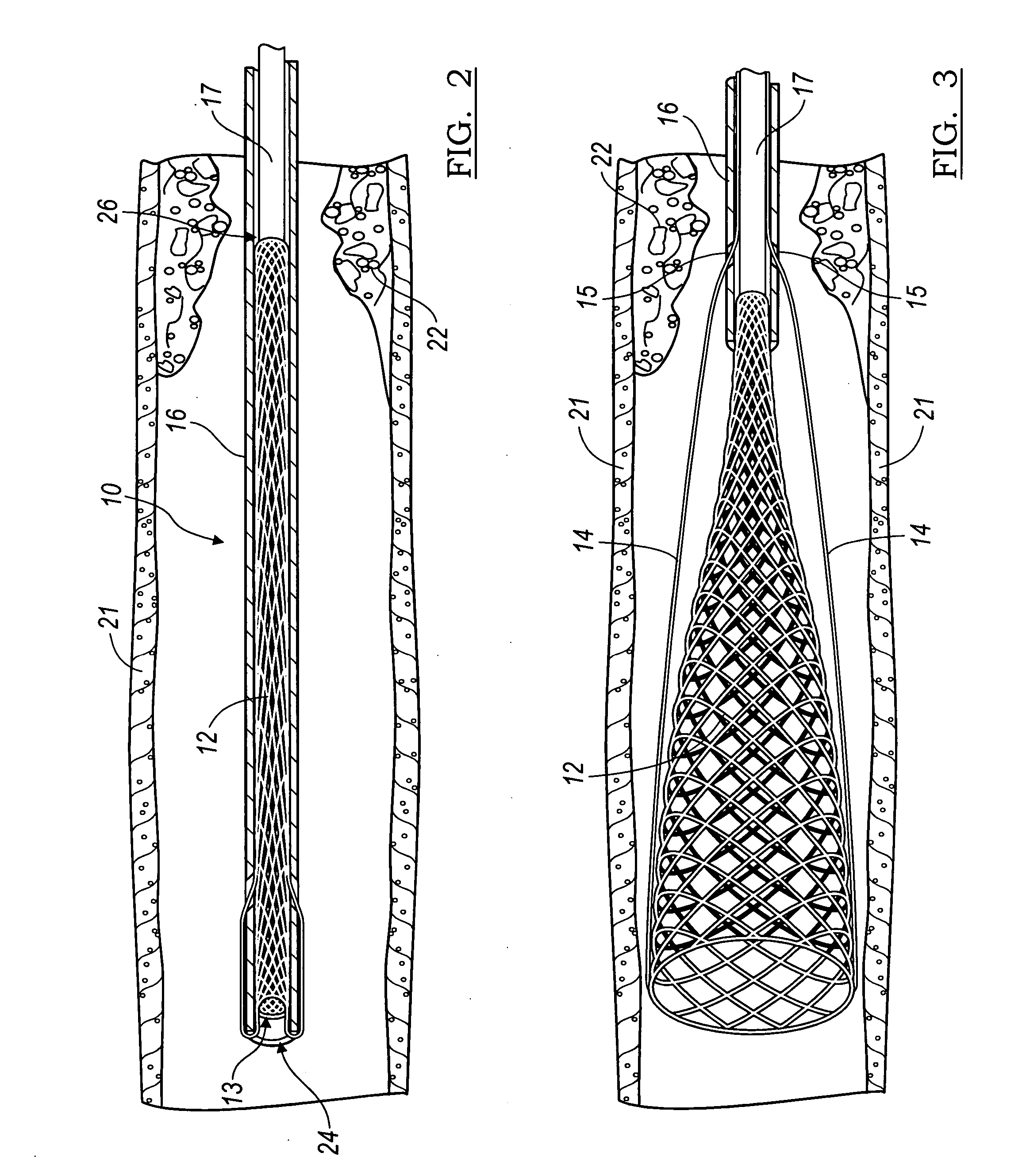
The present invention relates generally to a device and method for preventing the undesired passage of emboli from a venous blood pool to an arterial blood pool. The invention relates especially to a device and method for treating certain cardiac defects, especially patent foramen ovales and other septal defects, through the use of an embolic filtering device capable of instantaneously deterring the passage of emboli from the moment of implantation. The device consists of a frame, and a braided mesh of sufficient dimensions to prevent passage of emboli through the mesh. The device is preferably composed of shape memory allow, such as Nitinol, which conforms to the shape and dimension of the defect to be treated.
Owner:SEPTRX
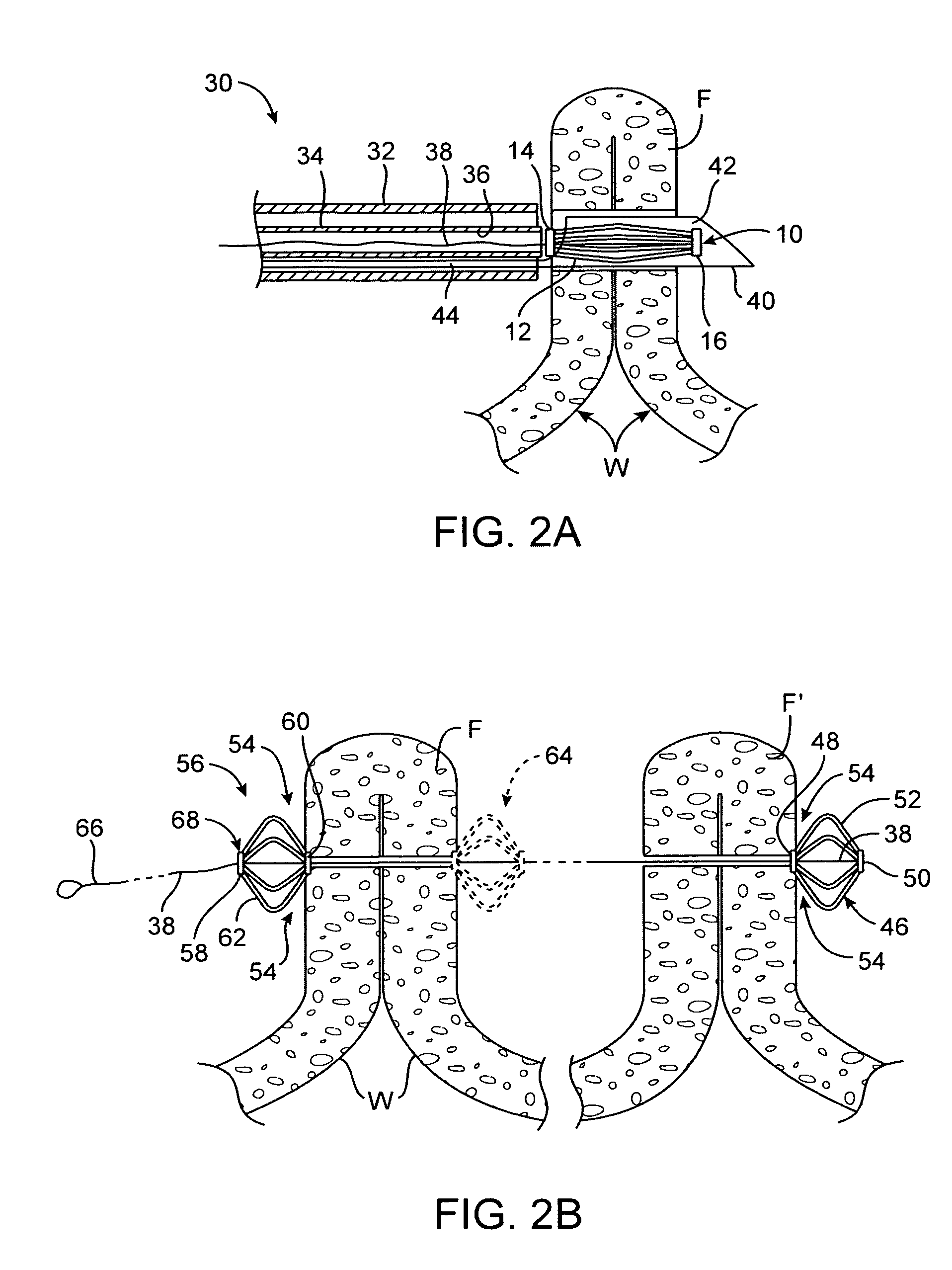
Pyloric valve obstructing devices and methods
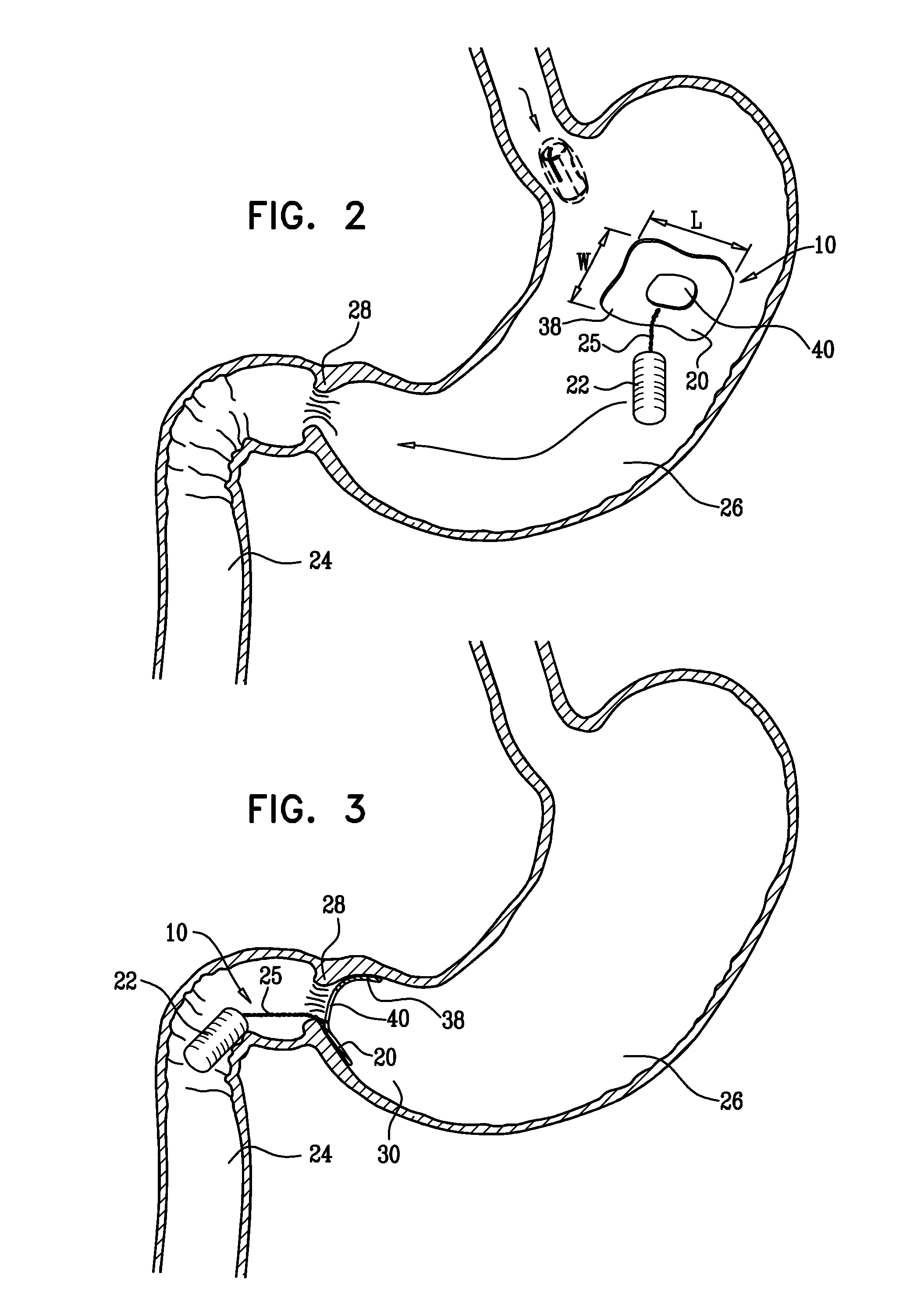
ActiveUS20050033331A1Treat and ameliorate obesityReduced food intakeSuture equipmentsEndoradiosondesPylorusPartial obstruction
Methods, devices and systems facilitate intermittent and / or partial obstruction of a pyloric valve. Devices generally include a support portion for preventing the device from passing through the pyloric valve and a tissue engagement portion for contacting tissue adjacent the pyloric valve to obstruct the valve. Some embodiments also include a positioning member extending from the tissue engagement portion for helping position the device for obstructing the valve. A retaining member may optionally be included on the distal end of the positioning member for further maintaining a position of the device in the stomach. Some embodiments are deliverable into the stomach through the esophagus, either by swallowing or through a delivery tube or catheter. Some embodiments are fully reversible. Some embodiments self-expand within the stomach, while others are inflated or otherwise expanded.
Owner:BARONOVA
Electron beam layer manufacturing
ActiveUS20110061591A1Obstruct passageProgramme controlAdditive manufacturing apparatusFree formClosed loop
A process and apparatus for free form fabrication of a three-dimensional work piece comprising (a) feeding raw material in a solid state to a first predetermined location; (b) depositing the raw material onto a substrate as a molten pool deposit under a first processing condition; (c) monitoring the molten pool deposit for a preselected condition; (d) comparing information about the preselected condition of the monitored molten pool deposit with a predetermined desired value for the preselected condition of the monitored molten pool deposit; (e) solidifying the molten pool deposit; (f) automatically altering the first processing condition to a different processing condition based upon information obtained from the comparing step (d); and repeating steps (a) through (f) at one or more second locations for building up layer by layer a three-dimensional work piece. The apparatus is characterized by a detector that monitors a preselected condition of the deposited material and a closed loop electronic control device for controlling operation of one or more components of the apparatus in response to a detected condition by the detector.
Owner:SCIAKY SA
Embolic filtering method and apparatus
InactiveUS20060009799A1Obstruct passageEncourage and facilitate growth of tissueAnnuloplasty ringsDilatorsVenous bloodCardiac defects
The present invention relates generally to a device and method for preventing the undesired passage of emboli from a venous blood pool to an arterial blood pool. The invention relates especially to a device and method for treating certain cardiac defects, especially patent foramen ovales and other septal defects, through the use of an embolic filtering device capable of instantaneously deterring the passage of emboli from the moment of implantation. The device consists of a frame, and a braided mesh of sufficient dimensions to prevent passage of emboli through the mesh. The device is preferably composed of shape memory allow, such as nitinol, which conforms to the shape and dimension of the defect to be treated.
Owner:SEPTRX
Methods and products for producing lattices of EMR-treated islets in tissues, and uses therefor
InactiveUS20060020309A1Sufficient amountReduce riskElectrotherapySurgical instrument detailsElectromagnetic radiationBiomedical engineering
Methods of treatment of tissue with electromagnetic radiation (EMR) to produce lattices of EMR-treated islets in the tissue are disclosed. Also disclosed are devices and systems for producing lattices of EMR-treated islets in tissue, and cosmetic and medical applications of such devices and systems.
Owner:PALOMAR MEDICAL TECH
Compressible tissue anchor assemblies
ActiveUS7736379B2Constant force against the tissuePrevent overcompressionSuture equipmentsDiagnosticsConstant forceStrain gauge
Apparatus & methods for optimizing anchoring force are described herein. In securing tissue folds, over-compression of the tissue directly underlying the anchors is avoided by utilizing tissue anchors having expandable arms configured to minimize contact area between the anchor and tissue. When the anchor is in its expanded configuration, a load is applied to the anchor until it is optimally configured to accommodate a range of deflections while the anchor itself exerts a substantially constant force against the tissue. Various devices, e.g., stops, spring members, fuses, strain gauges, etc., can be used to indicate when the anchor has been deflected to a predetermined level within the optimal range. Moreover, other factors to affect the anchor characteristics include, e.g., varying the number of arms or struts of the anchor, positioning of the arms, configuration of the arms, the length of the collars, etc.
Owner:USGI MEDICAL
Filter arrangement; sealing system; and methods
InactiveUS6610117B2Avoid passingObstruct passageCombination devicesGas treatmentMechanical engineeringStructural engineering
A filter pack includes a filter construction and a sealing system for sealing the construction within a duct or housing. The filter construction has first and second opposite flow faces and is configured for a straight-through flow. The sealing system includes a frame construction and a compressible seal member. The compressible seal member is molded around a portion of the frame construction. The compressible seal member is sufficiently compressible to form a radial seal between and against the frame construction and a surface of a housing when the filter pack is inserted within the housing.
Owner:DONALDSON CO INC
Controlled electroporation and mass transfer across cell membranes
InactiveUS20060121610A1High levelImprove efficiencyBioreactor/fermenter combinationsBiological substance pretreatmentsControl mannerCell membrane
Electroporation is performed in a controlled manner in either individual or multiple biological cells or biological tissue by monitoring the electrical impedance, defined herein as the ratio of current to voltage in the electroporation cell. The impedance detects the onset of electroporation in the biological cell(s), and this information is used to control the intensity and duration of the voltage to assure that electroporation has occurred without destroying the cell(s). This is applicable to electroporation in general. In addition, a particular method and apparatus are disclosed in which electroporation and / or mass transfer across a cell membrane are accomplished by securing a cell across an opening in a barrier between two chambers such that the cell closes the opening. The barrier is either electrically insulating, impermeable to the solute, or both, depending on whether pore formation, diffusive transport of the solute across the membrane, or both are sought. Electroporation is achieved by applying a voltage between the two chambers, and diffusive transport is achieved either by a difference in solute concentration between the liquids surrounding the cell and the cell interior or by a differential in concentration between the two chambers themselves. Electric current and diffusive transport are restricted to a flow path that passes through the opening.
Owner:RGT UNIV OF CALIFORNIA
Shaving apparatus with pivot-actuated valve for delivery of shaving aid material
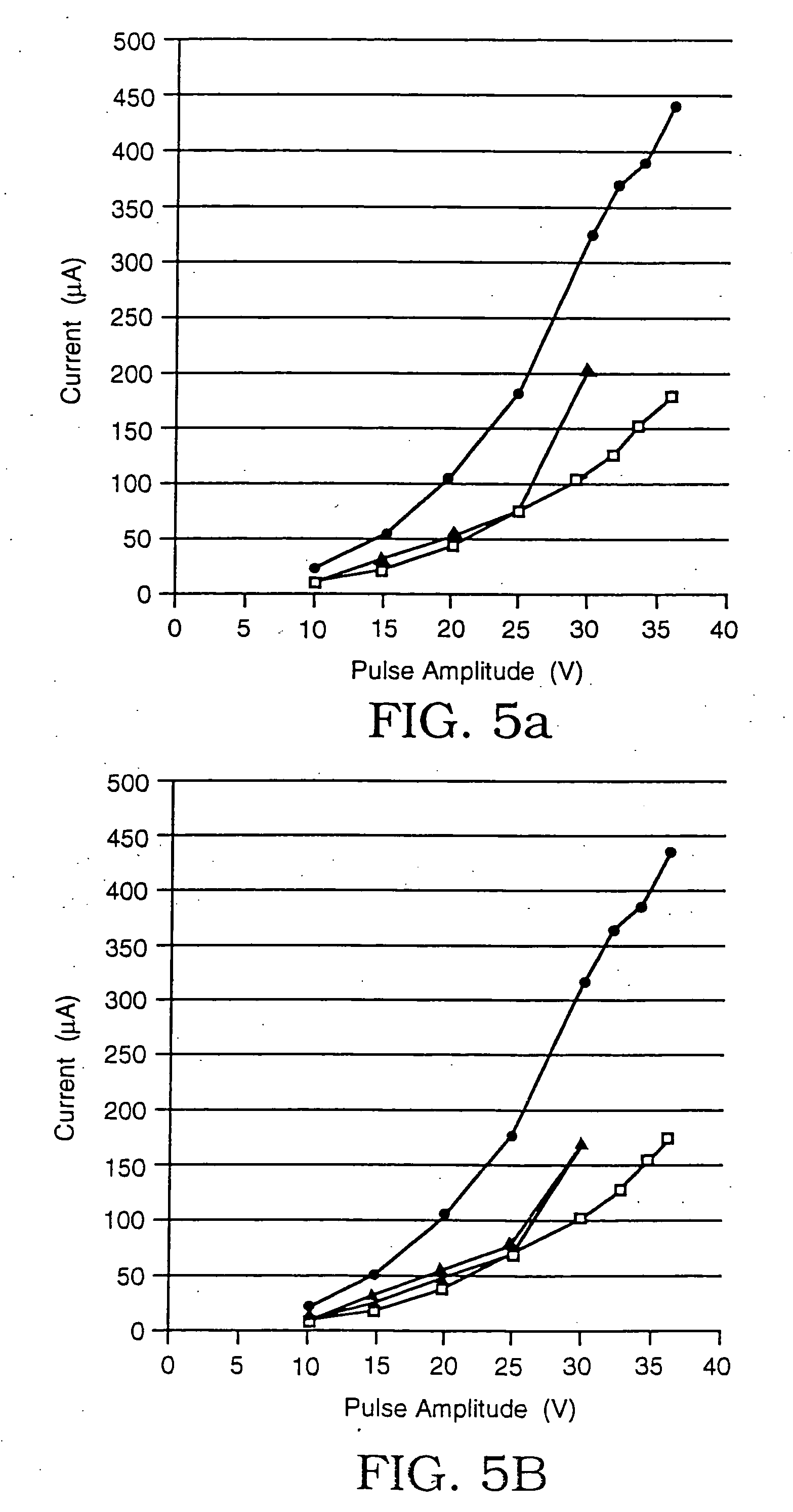
InactiveUS7121754B2Prevent materialObstruct passageCarpet cleanersFloor cleanersEngineeringRazor Blade
A shaving apparatus includes a reservoir for storing a shaving aid material, a razor cartridge having at least one razor blade, and a valve selectively actuatable by movement of the razor cartridge between a first position and a second position. In the first position the valve permits passage of a shaving aid material from the reservoir, and in the second position the valve substantially prevents the passage of the shaving aid material from the reservoir.
Owner:EDGEWELL PERSONAL CARE BRANDS LLC
Compressible tissue anchor assemblies
ActiveUS20060217762A1Constant force against the tissuePrevent overcompressionSuture equipmentsDiagnosticsStrain gaugeBiomedical engineering
Apparatus & methods for optimizing anchoring force are described herein. In securing tissue folds, over-compression of the tissue directly underlying the anchors is avoided by utilizing tissue anchors having expandable arms configured to minimize contact area between the anchor and tissue. When the anchor is in its expanded configuration, a load is applied to the anchor until it is optimally configured to accommodate a range of deflections while the anchor itself exerts a substantially constant force against the tissue. Various devices, e.g., stops, spring members, fuses, strain gauges, etc., can be used to indicate when the anchor has been deflected to a predetermined level within the optimal range. Moreover, other factors to affect the anchor characteristics include, e.g., varying the number of arms or struts of the anchor, positioning of the arms, configuration of the arms, the length of the collars, etc.
Owner:USGI MEDICAL
Invertible filter for embolic protection
A medical device including a guiding member and a filter portion is disclosed. The guiding member includes a lumen configured to slidably engage the filter portion. The filter portion forms a tubular geometry that extends distally from the guiding member. The filter portion is configured to evert to form a proximally facing concave geometry for capturing emboli. Further, the filter portion includes filter openings that are sized to allow blood cells to pass therethrough while preventing the passage of emboli.
Owner:COOK MEDICAL TECH LLC
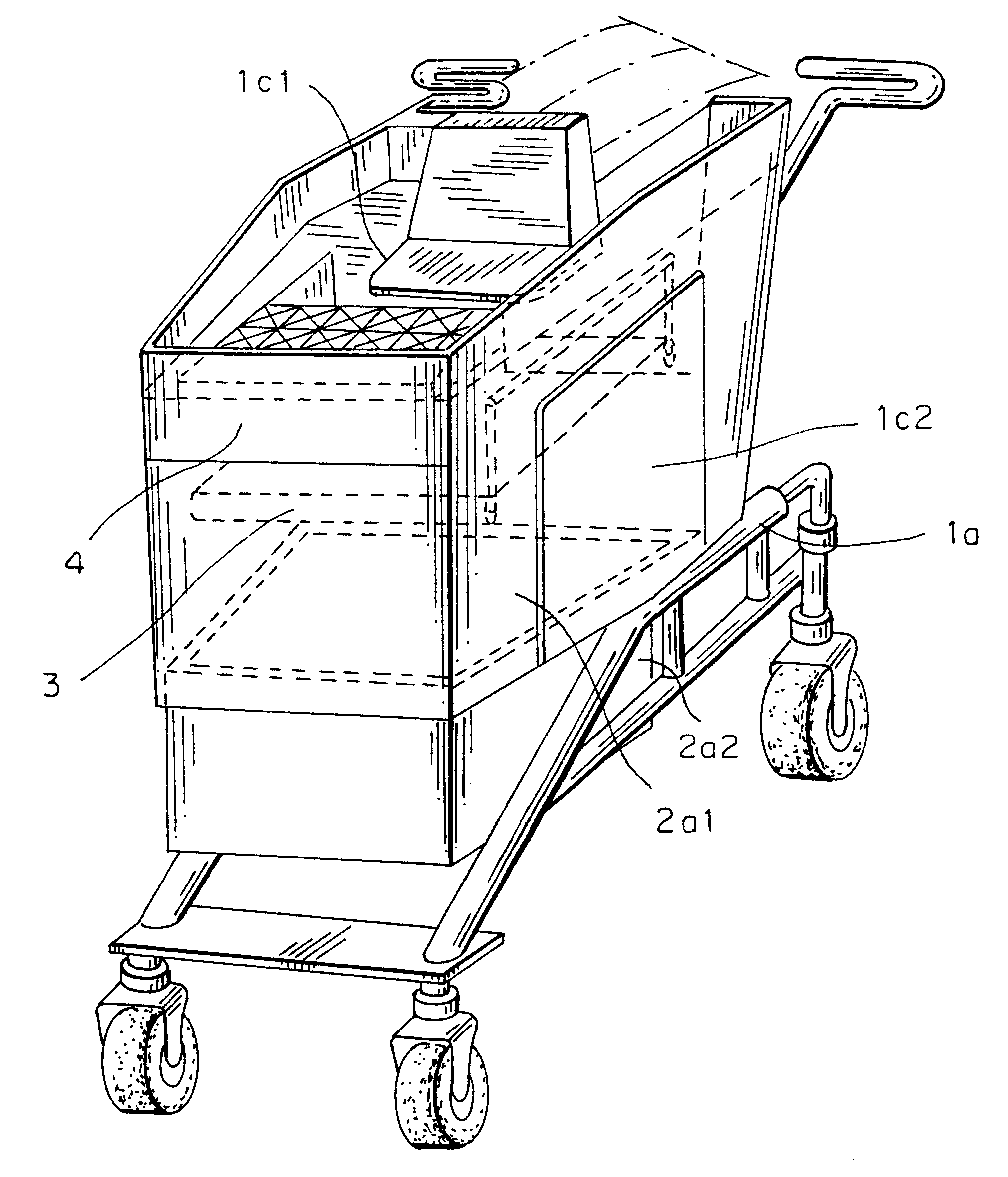
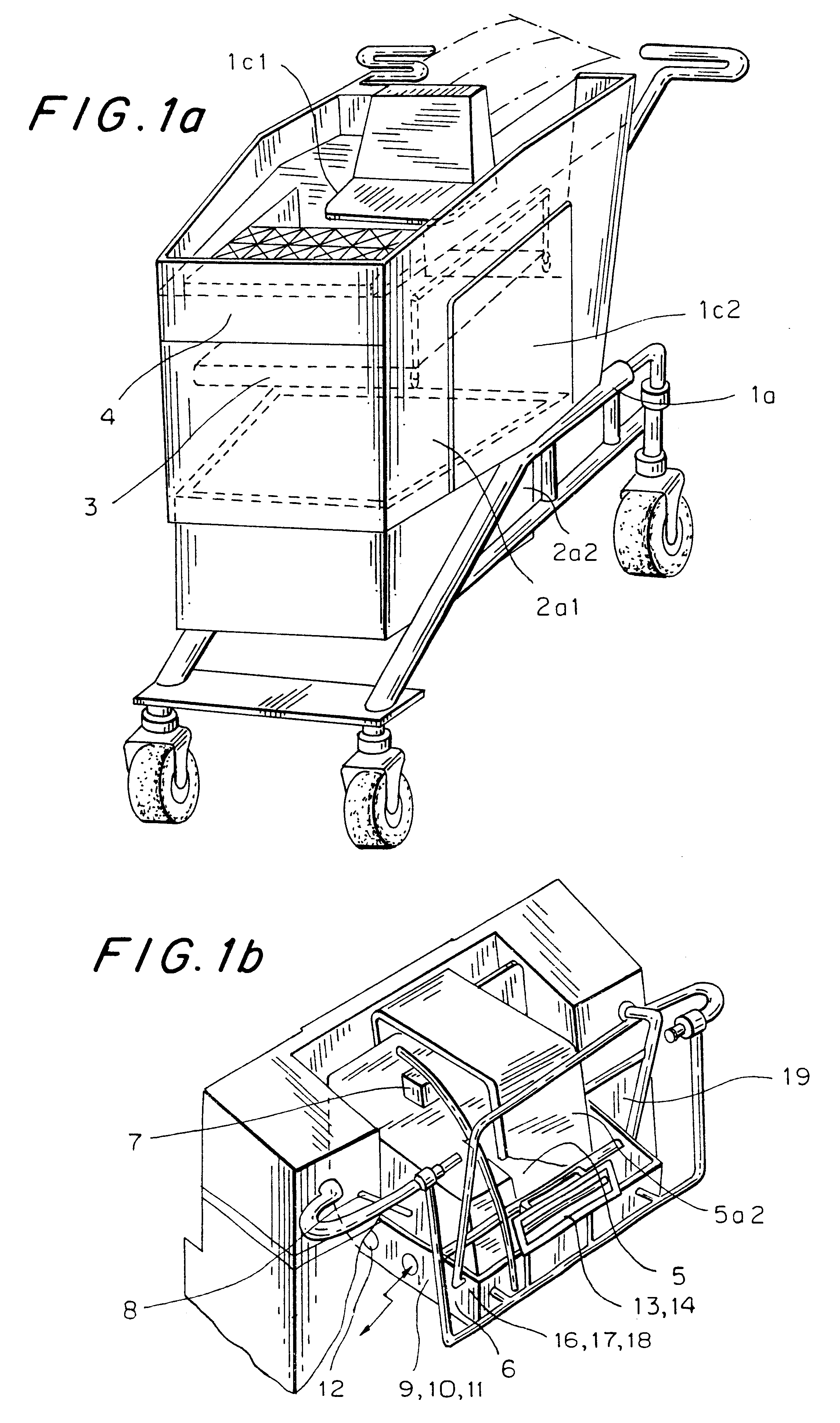
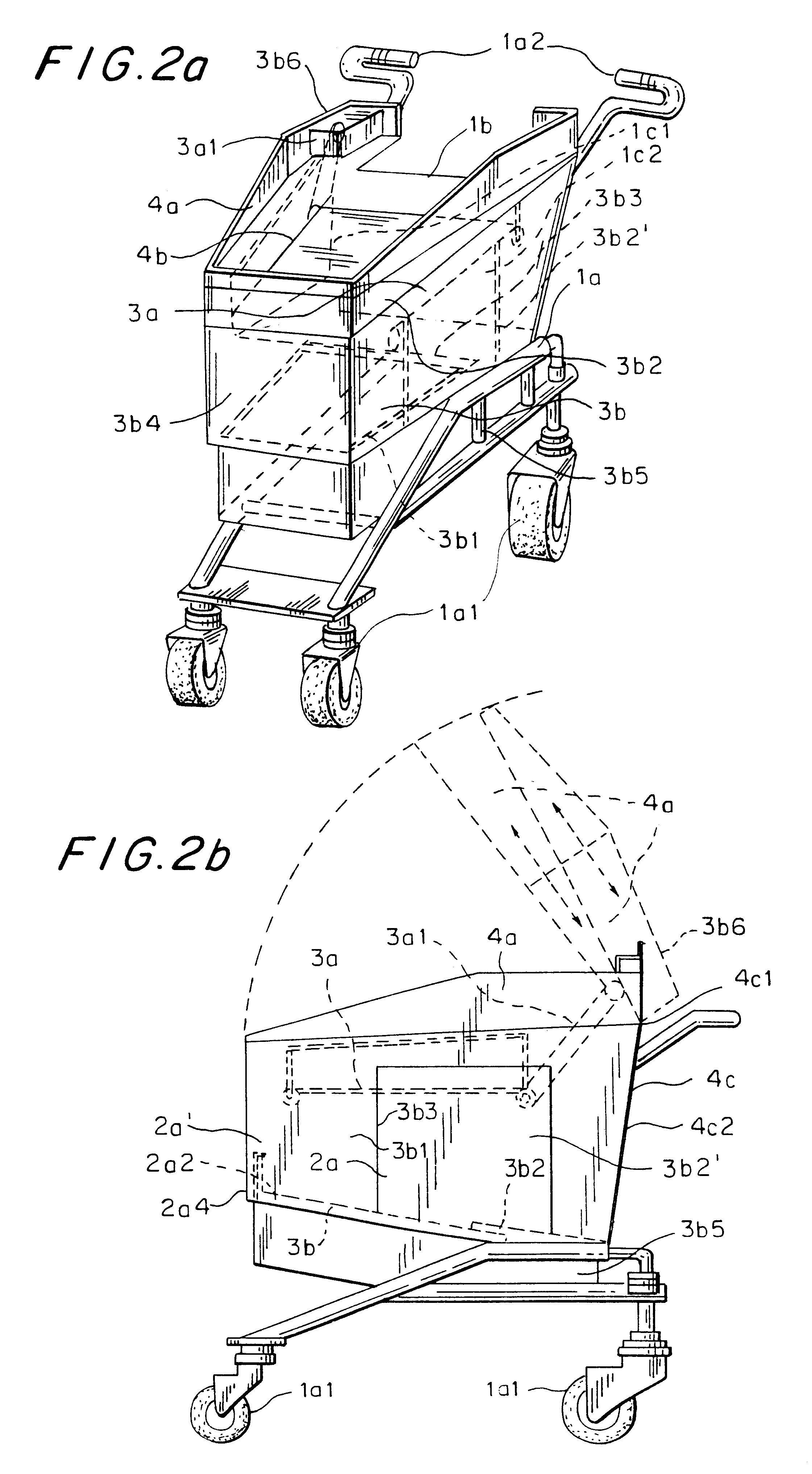
Computerized shopping cart with storage and distribution system, for supermarket use
InactiveUS6435407B1Soften aerial detachmentEase fitCredit registering devices actuationCo-operative working arrangementsDistribution systemBarcode
A system including a computerized shopping cart that includes a closed mechanical cart (1) which interconnects / disconnects to units for the insertion / storage of products upon manual command managed by a shopping computer aimed at managing shopping problems and controlling all electronic functions, i.e. automatic system for optically scanning (7) bar codes (UPC / EAN standard), one for checking (8) the correctness of the customer's operations, one emitting the intelligent multimedia commercial message, one for the wireless exchange of data with the outside, for use in supermarkets. The system provides shopping info (8) (list of products on sale, location, price, other), or of the product being purchased (price, whether it is on sale, partial groceries bill), or already purchased (list of purchased items) or to be purchased (remaining shopping list), guided by appropriate interactive messages (acoustic, visual.
Owner:FIORDELISI LUIGI
Methods and products for producing lattices of EMR-treated islets in tissues, and uses therefor
InactiveUS20060004347A1Big advantageSufficient amountElectrotherapySurgical instrument detailsBiomedical engineeringElectromagnetic radiation
Methods of treatment of tissue with electromagnetic radiation (EMR) to produce lattices of EMR-treated islets in the tissue are disclosed. Also disclosed are devices and systems for producing lattices of EMR-treated islets in tissue, and cosmetic and medical applications of such devices and systems.
Owner:PALOMAR MEDICAL TECH
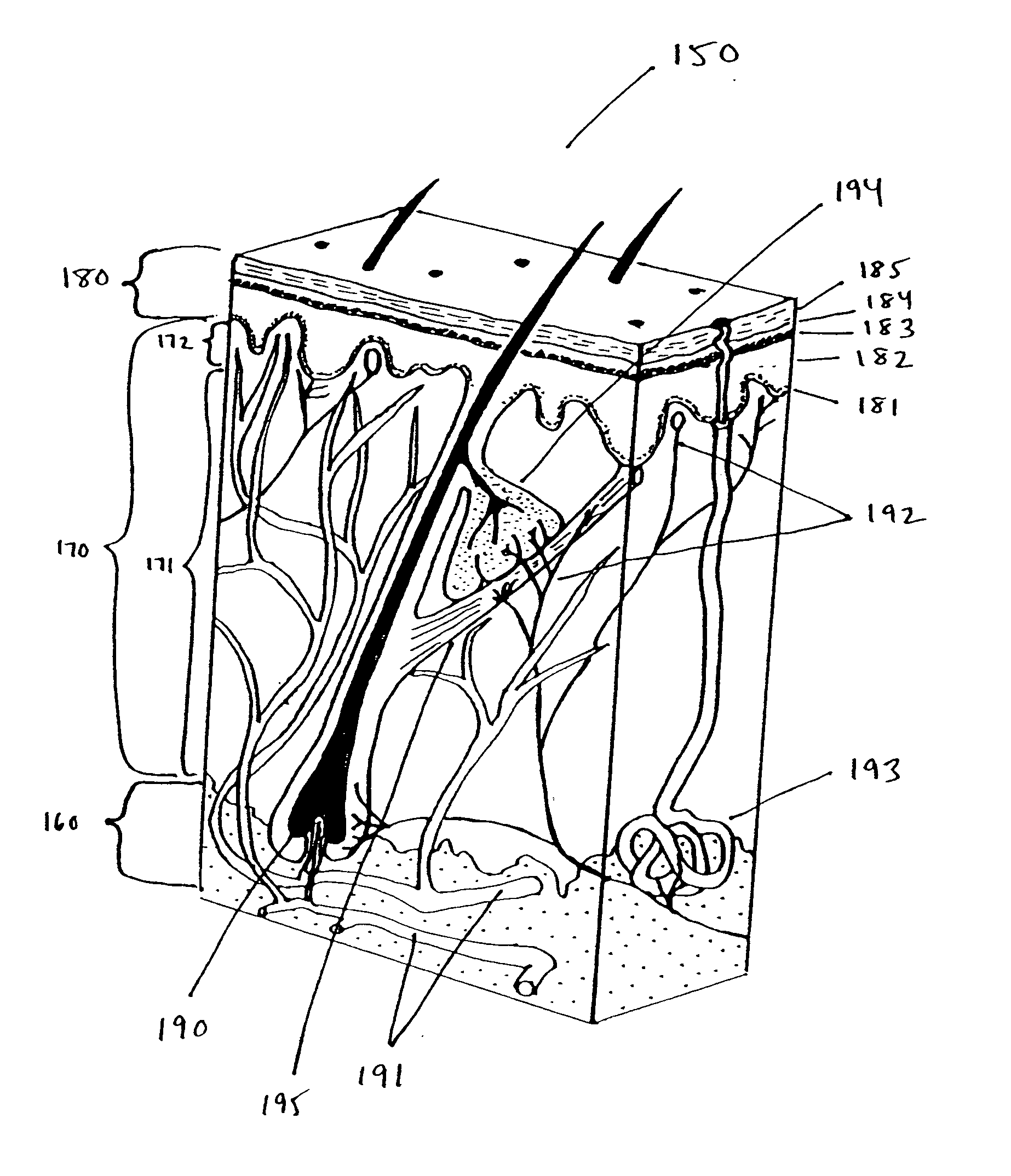
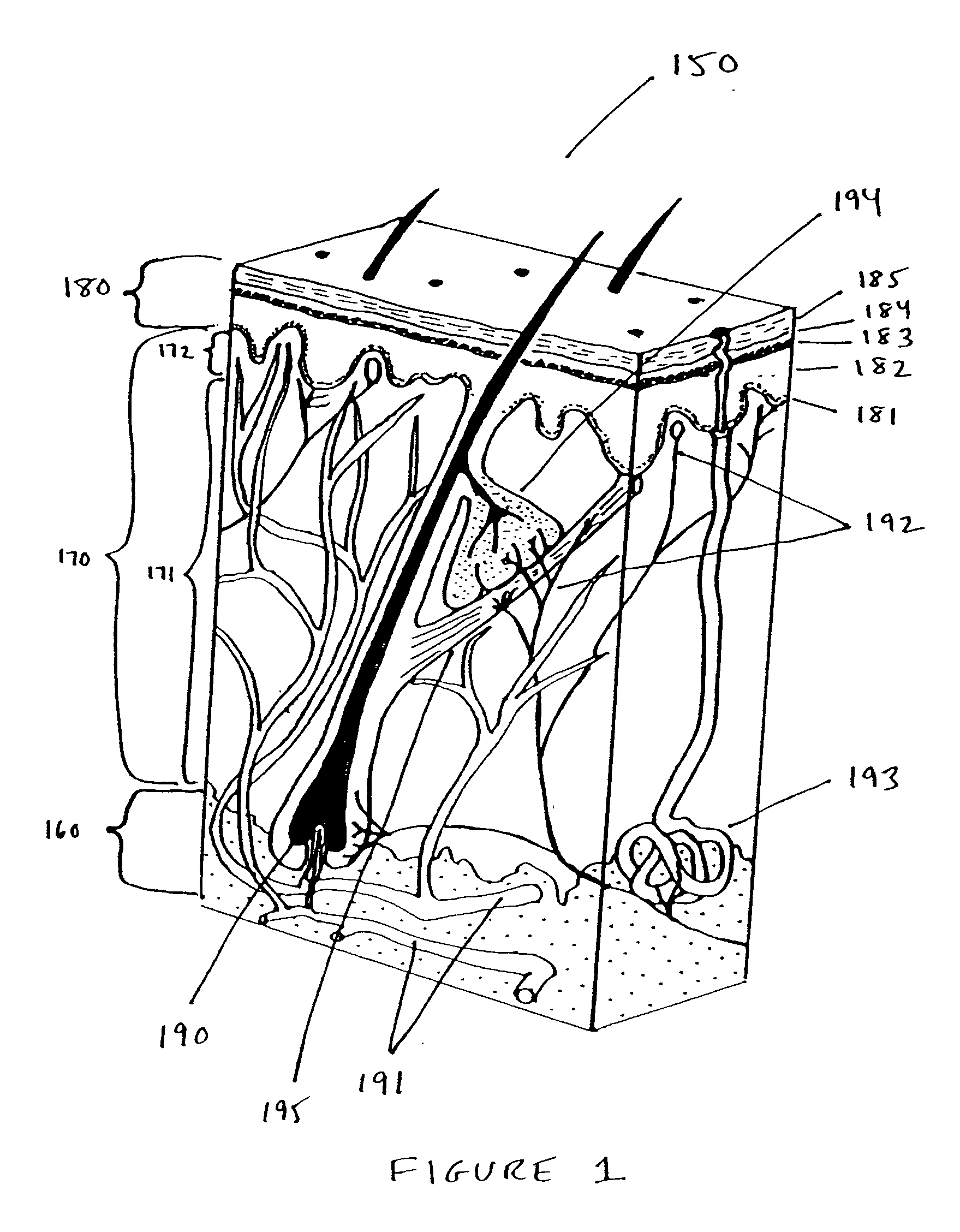
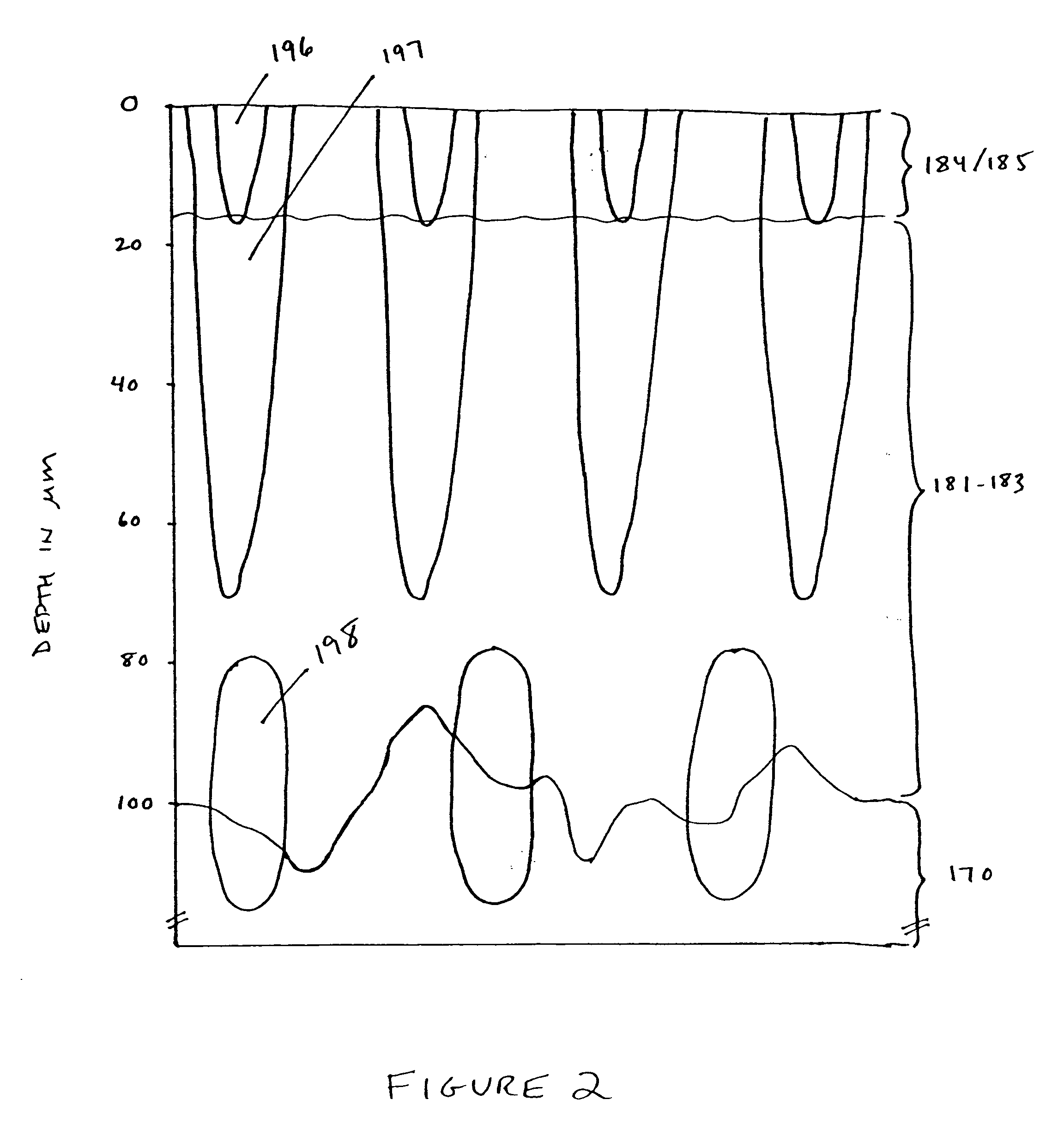
Interface for use between medical instrumentation and a patient
InactiveUS20050215901A1Obstruct passagePermit transmissionUltrasonic/sonic/infrasonic diagnosticsSurgeryULTRASOUND PROCEDURESThermoplastic elastomer
Disclosed herein are methods, devices, compositions, and systems for providing an interface between medical instrumentation and a patient. In various embodiments, the interface provides a sterile barrier, acoustic coupler, and thermal insulator between the patient and a medical instrument. In some embodiments, an acoustic coupler interface is used between an ultrasound instrument and a patient. In some embodiments, the acoustic coupler comprises a thermoplastic elastomer (“TPE”) and in particular oil-enhanced or gelatinous TPEs that can be used in diagnostic and therapeutic (HIFU) ultrasound procedures.
Owner:CERUS CORP +1
Implantable analyte sensor
ActiveUS8277713B2Improve convenienceMinimize movementImmobilised enzymesBioreactor/fermenter combinationsAnalyteMiniaturization
An implantable analyte sensor including a sensing region for measuring the analyte and a non-sensing region for immobilizing the sensor body in the host. The sensor is implanted in a precisely dimensioned pocket to stabilize the analyte sensor in vivo and enable measurement of the concentration of the analyte in the host before and after formation of a foreign body capsule around the sensor. The sensor further provides a transmitter for RF transmission through the sensor body, electronic circuitry, and a power source optimized for long-term use in the miniaturized sensor body.
Owner:DEXCOM INC
Gastric anchor
A swallowable medical treatment device is configured to initially assume a contracted state having a volume of less than 4 cm3. The device includes a gastric anchor, which initially assumes a contracted size, and which is configured to, upon coming in contact with a liquid, expand sufficiently to prevent passage of the gastric anchor through a round opening having a diameter of between 1 cm and 3 cm. The device also includes a duodenal unit, which is configured to pass through the opening, and which is coupled to the gastric anchor such that the duodenal unit is held between 1 cm and 20 cm from the gastric anchor. Other embodiments are also described.
Owner:RAINBOW MEDICAL LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com