Quetiapine fumarate film-controlled slow-release pellet capsule
A technology of quetiapine fumarate and sustained-release pellets, which can be used in medical preparations with non-active ingredients, organic active ingredients, nervous system diseases, etc., and can solve problems such as decreased release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The quetiapine fumarate sustained-release pellet capsule of embodiment 1 common ball core
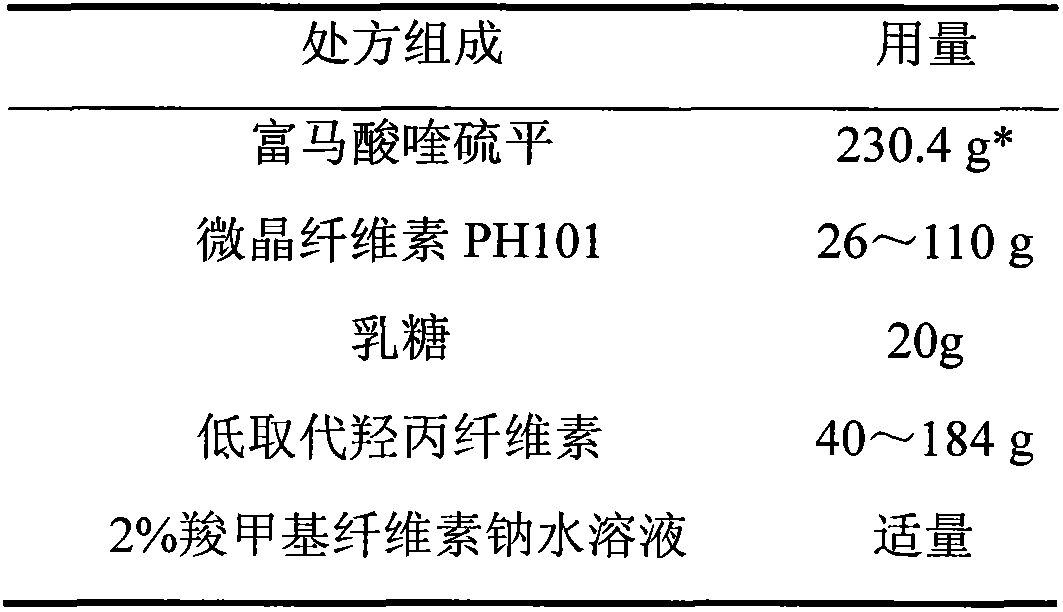
[0030] 1. Prescription
[0031] 1. Pill core prescription (1000 capsules)
[0032]
[0033] * Calculated as quetiapine is 200g.
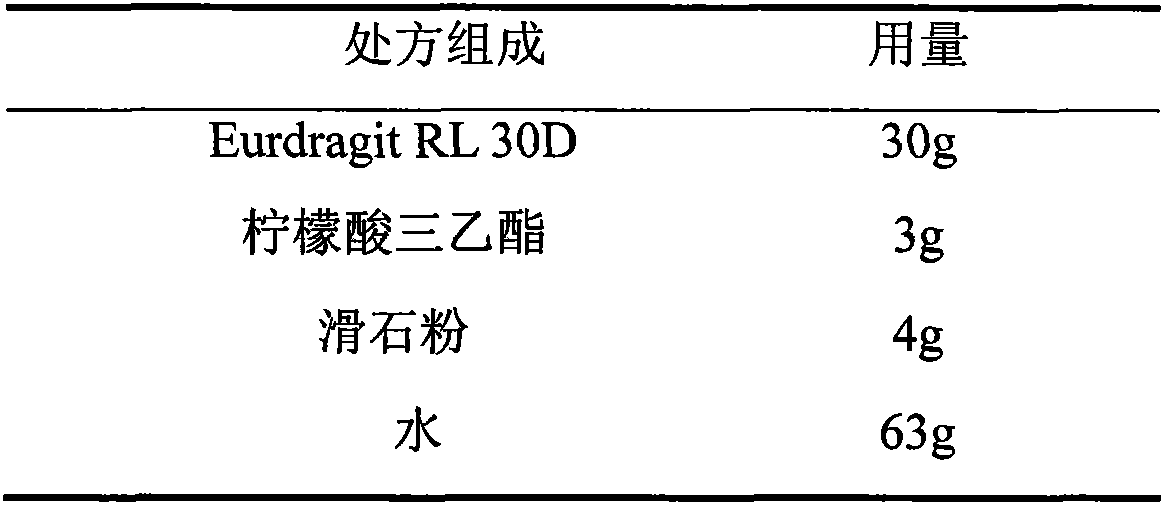
[0034] 2. Prescription of sustained-release film coating solution
[0035]
[0036] 3. No. 00 stomach-soluble gelatin capsule shell 1000 capsules
[0037] Second, the preparation process:
[0038] 1. Ball core preparation process:
[0039] (1) quetiapine fumarate is passed through a 60 mesh sieve;
[0040] (2) Take by weighing quetiapine fumarate, microcrystalline cellulose PH101, lactose of recipe quantity, put in the wet granulator and mix uniformly;
[0041] (3) soft material made of 2% sodium carboxymethyl cellulose aqueous solution;
[0042] (4) Extrude on the extruder, the screen aperture is 1.0mm, and the extrusion speed is 20-30rpm;
[0043] (5) spheronization, the spheronization speed is 900~1000rpm, drying in the fluidized bed;
...
Embodiment 2
[0071] Embodiment 2 quetiapine fumarate sustained-release pellet capsule containing 10% low-substituted hydroxypropyl cellulose
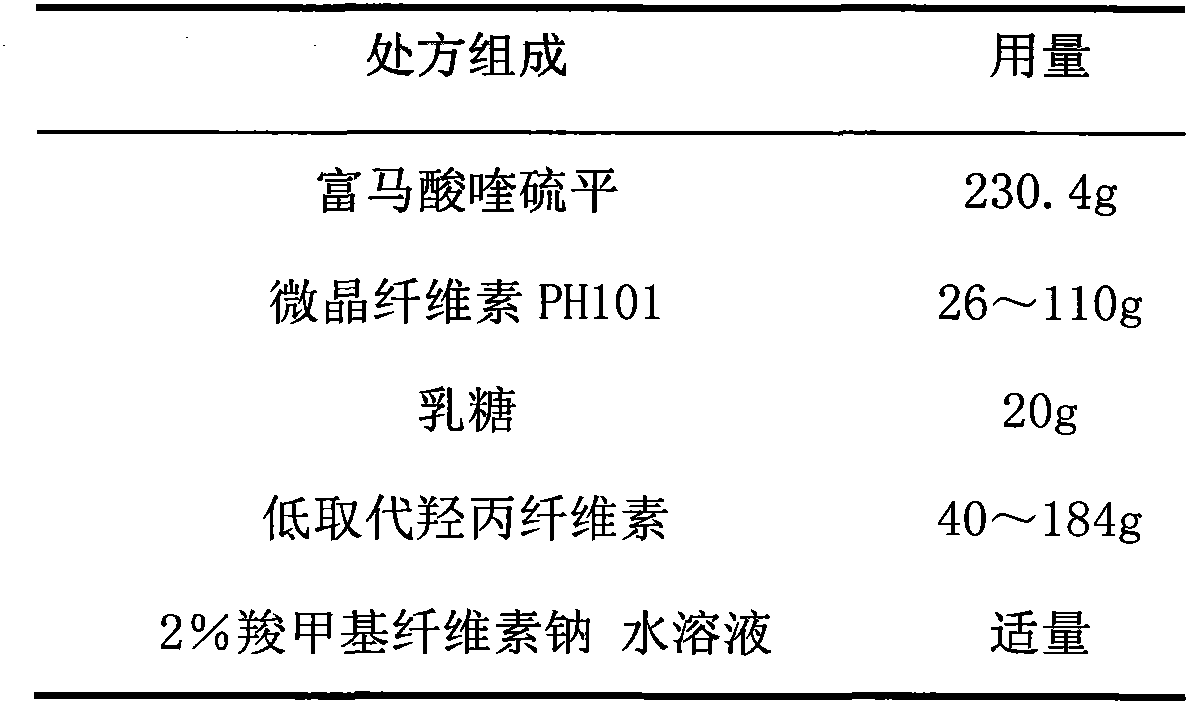
[0072] 1. Prescription
[0073] 1. Pill core prescription (1000 capsules)
[0074]
[0075]
[0076] 2. Prescription of sustained-release film coating solution: same as in Example 1
[0077] 3. No. 00 stomach-soluble gelatin capsule shell 1000 capsules
[0078] Second, the preparation process:
[0079] 1. Ball core preparation process:
[0080] (1) quetiapine fumarate is passed through a 60 mesh sieve;
[0081] (2) Take by weighing quetiapine fumarate, microcrystalline cellulose PH101, lactose, low-substituted hydroxypropyl cellulose of prescription quantity, put in wet granulator and mix evenly;
[0082] (3) soft material made of 2% sodium carboxymethyl cellulose aqueous solution;
[0083] (4) Extrude on the extruder, the screen aperture is 1.0mm, and the extrusion speed is 20-30rpm;
[0084] (5) spheronization, the spheronization spee...
Embodiment 3
[0103] Example 3 Quetiapine Fumarate Sustained-release Pellet Capsules Containing 20% Low-Substituted Hyprolose
[0104] 1. Prescription
[0105] 1. Pill core prescription (1000 capsules)
[0106]
[0107] 2. Prescription of sustained-release film coating solution: same as in Example 1
[0108] 3. No. 00 stomach-soluble gelatin capsule shell 1000 capsules
[0109] Second, the preparation process:
[0110] 1. Ball core preparation process: the same as in Example 2
[0111] 2. Preparation process of sustained-release film coating solution:
[0112] Same as Example 1
[0113] 3. Coating (sustained release film):
[0114] The ball core is placed in a fluidized bed for coating, and the weight gain of the coating film is controlled, and the weight gain of the coating is 23.5% and 26.7%.
[0115] 4, heat treatment: with embodiment 1
[0116] 5. Fill capsules
[0117] The coated pellets are filled into capsules to obtain the quetiapine fumarate sustained-release pellets an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com