Patents
Literature
79results about How to "Good release performance" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
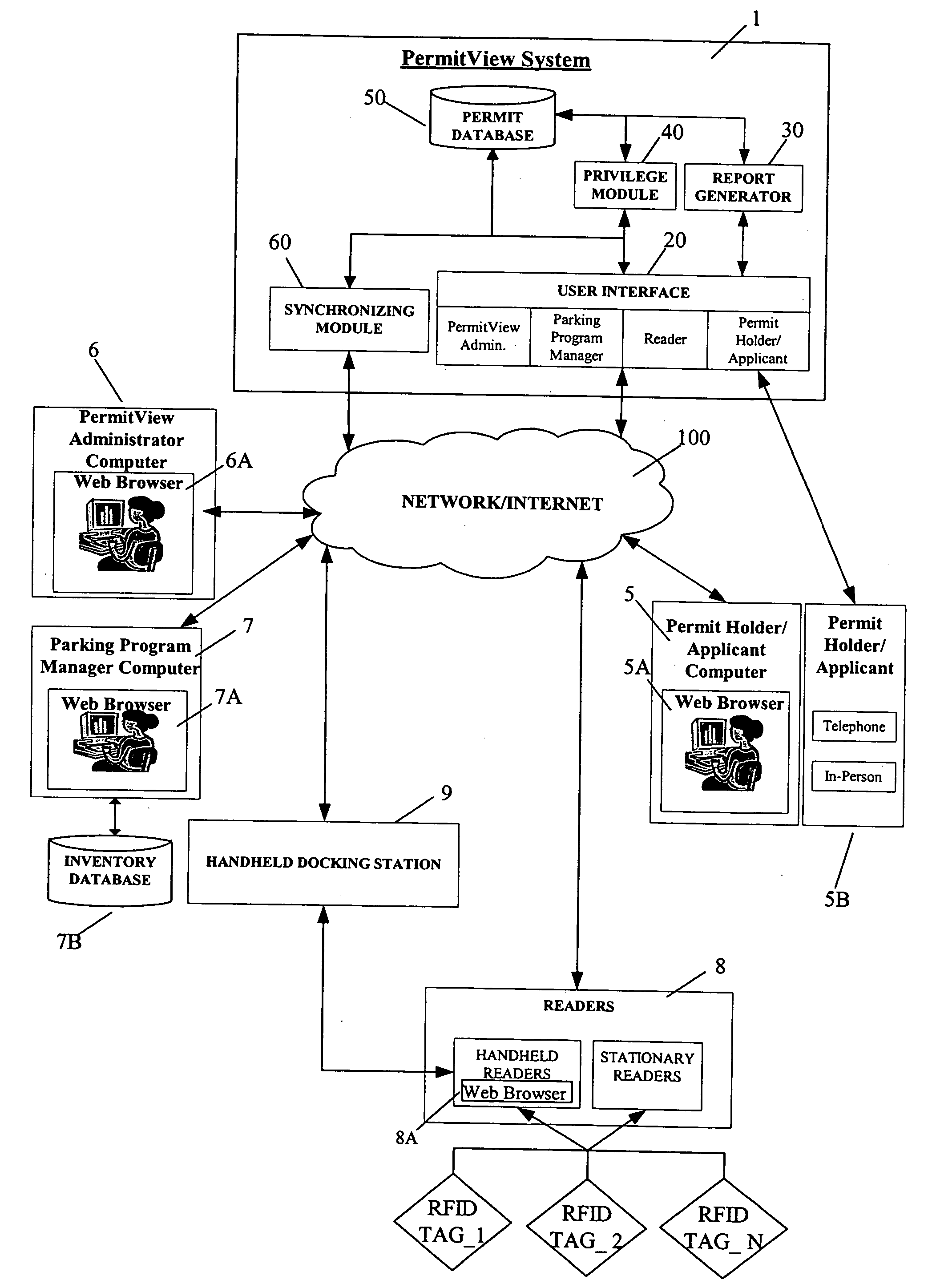
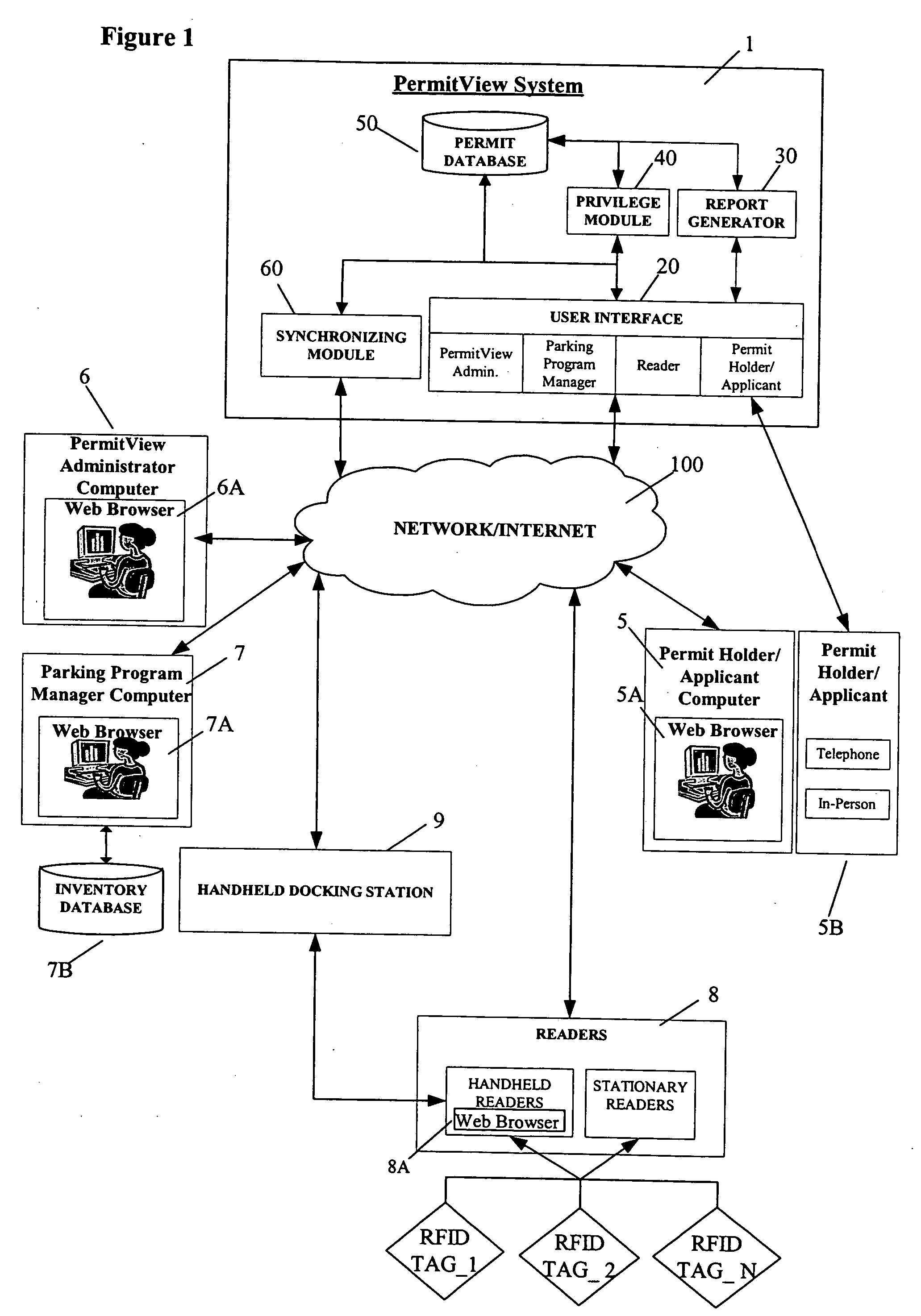
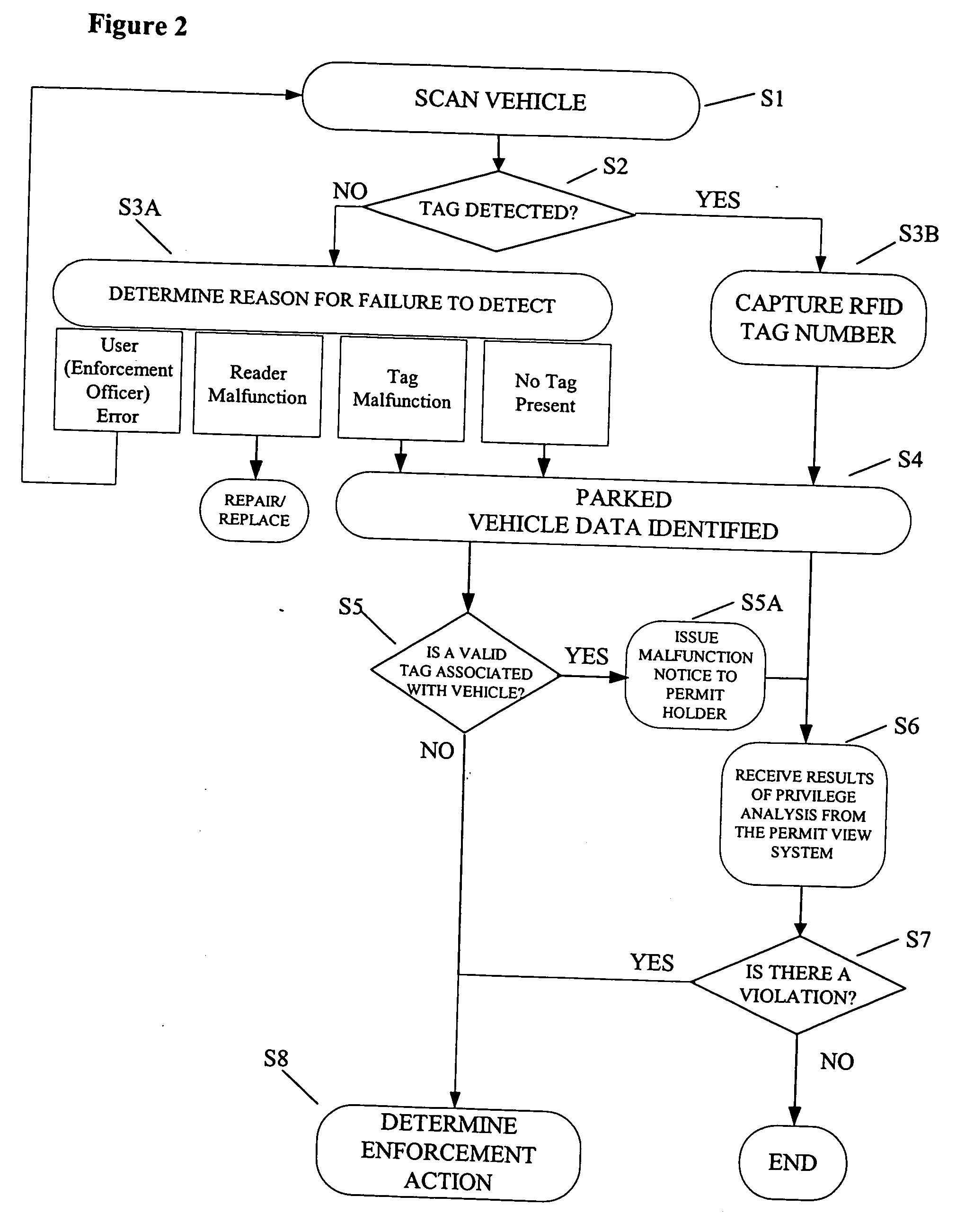
Parking environment management system and method
ActiveUS20060255119A1Realized benefitsGood release performanceAnti-theft cycle devicesBuilding locksProgram managementManagement system
A system and method for managing a permit-based parking environment governed by a parking program. The permit-based parking environment includes a number of parking permits each including a unique RFID tag and tag number. One or more RFID readers are used to scan the vehicles parked in the parking environment to determine if a RFID tag is associated with the parked vehicle. The results of the scan along with information related to the parked vehicle are provided to a permit management system to determine if the vehicle is parked within the scope of privileges pre-defined for that vehicle, pursuant to the parking program governing the parking environment. The permit management system stores, manages, and monitors data related to the permits controlled under the parking program.
Owner:IPT
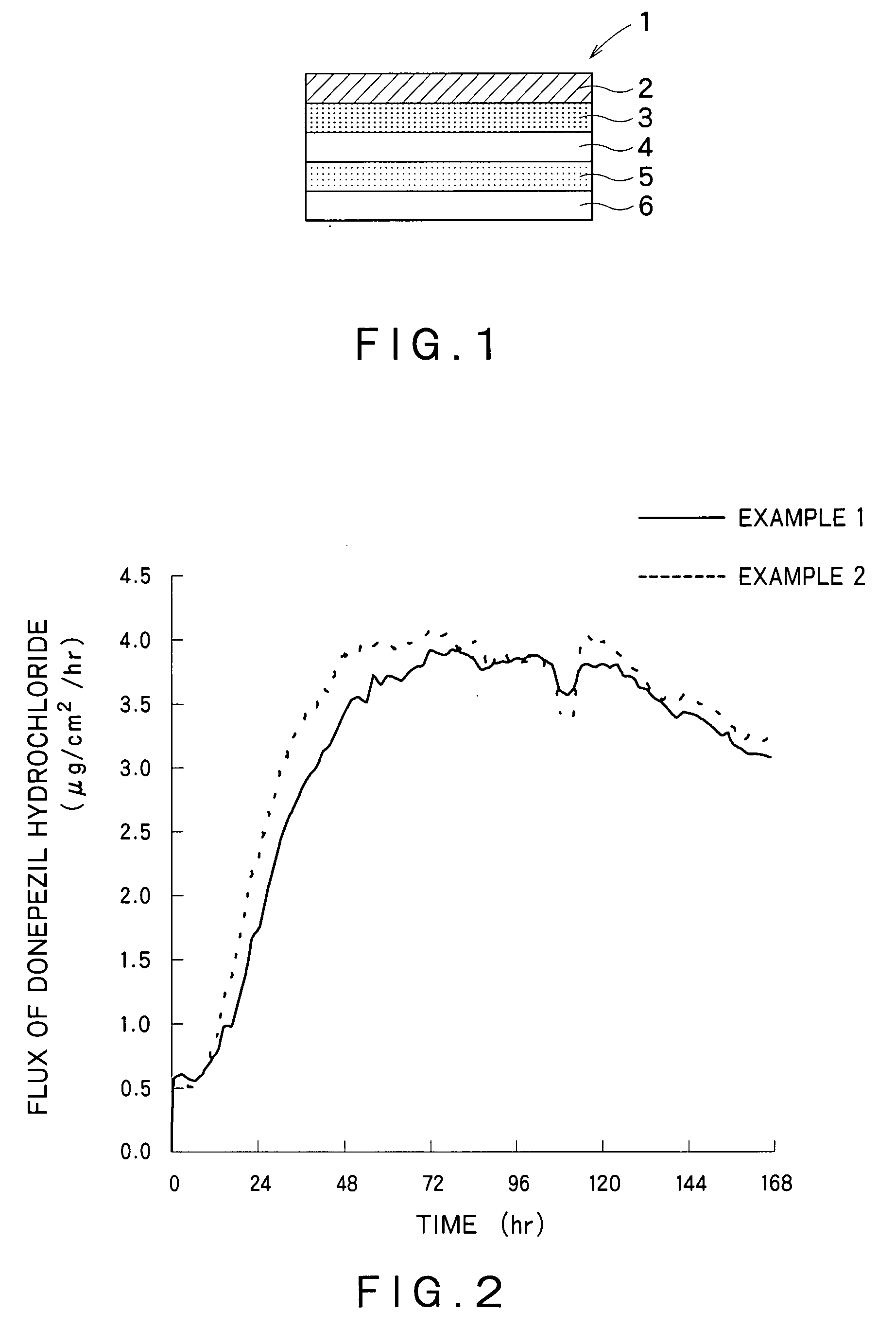
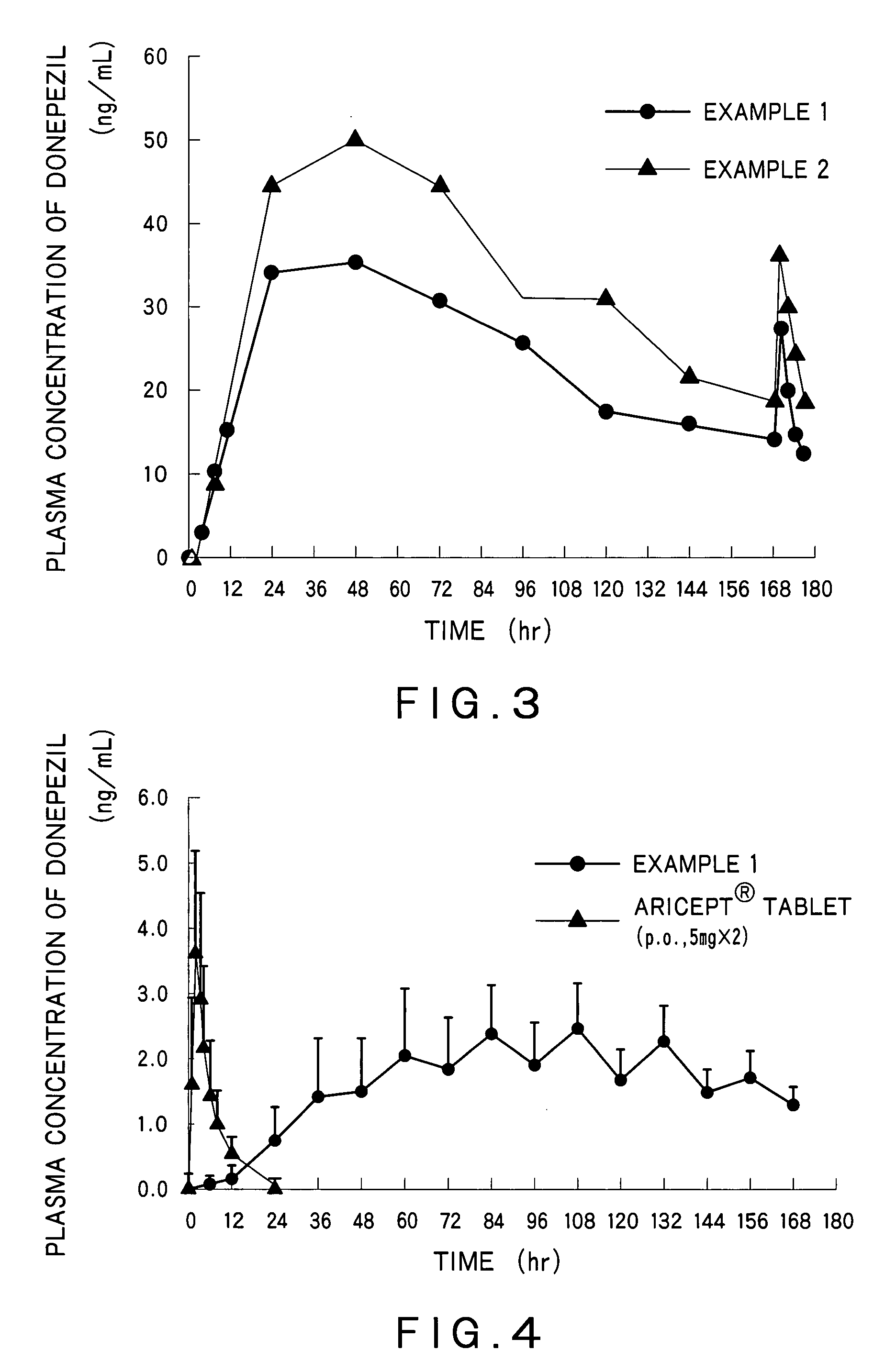
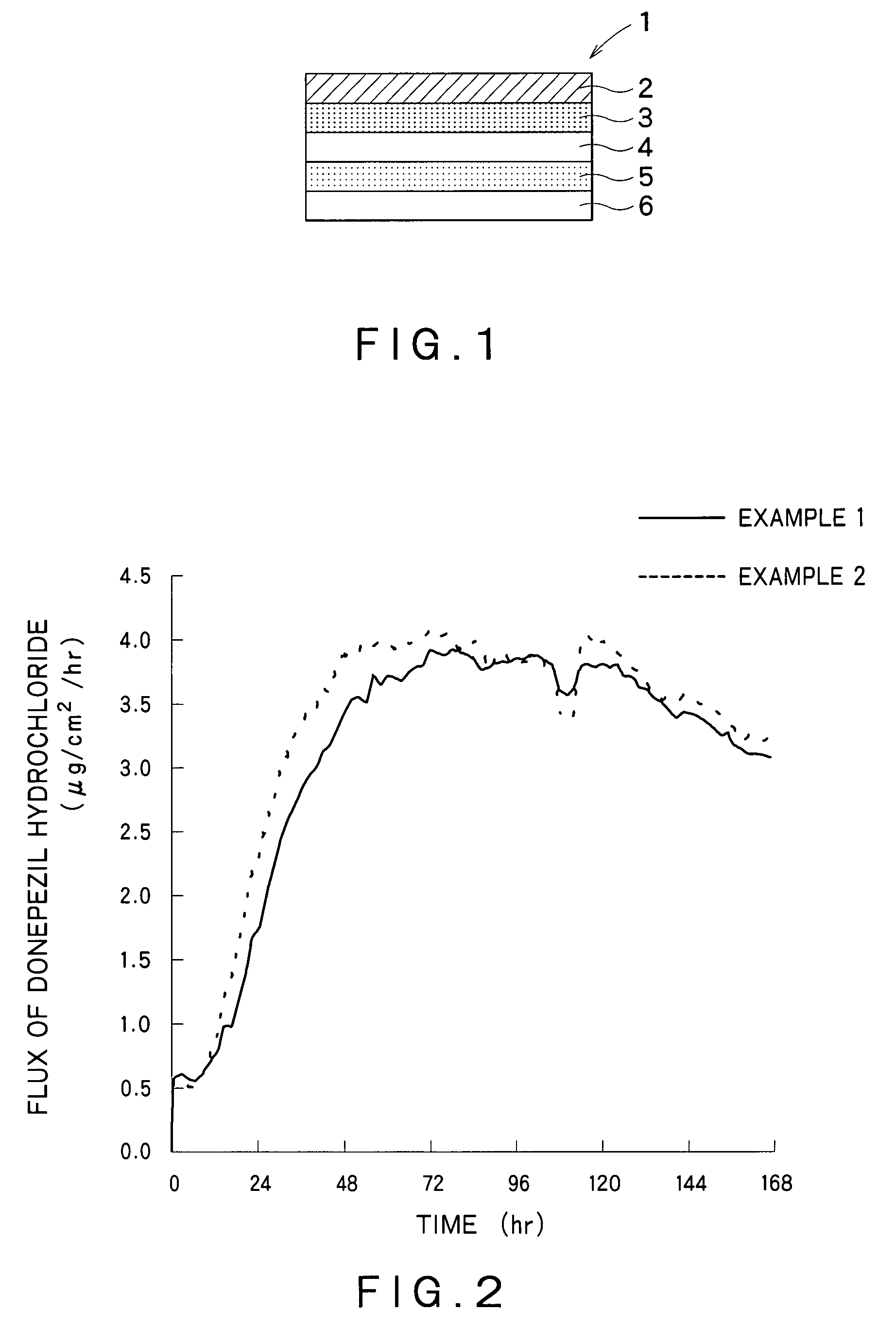
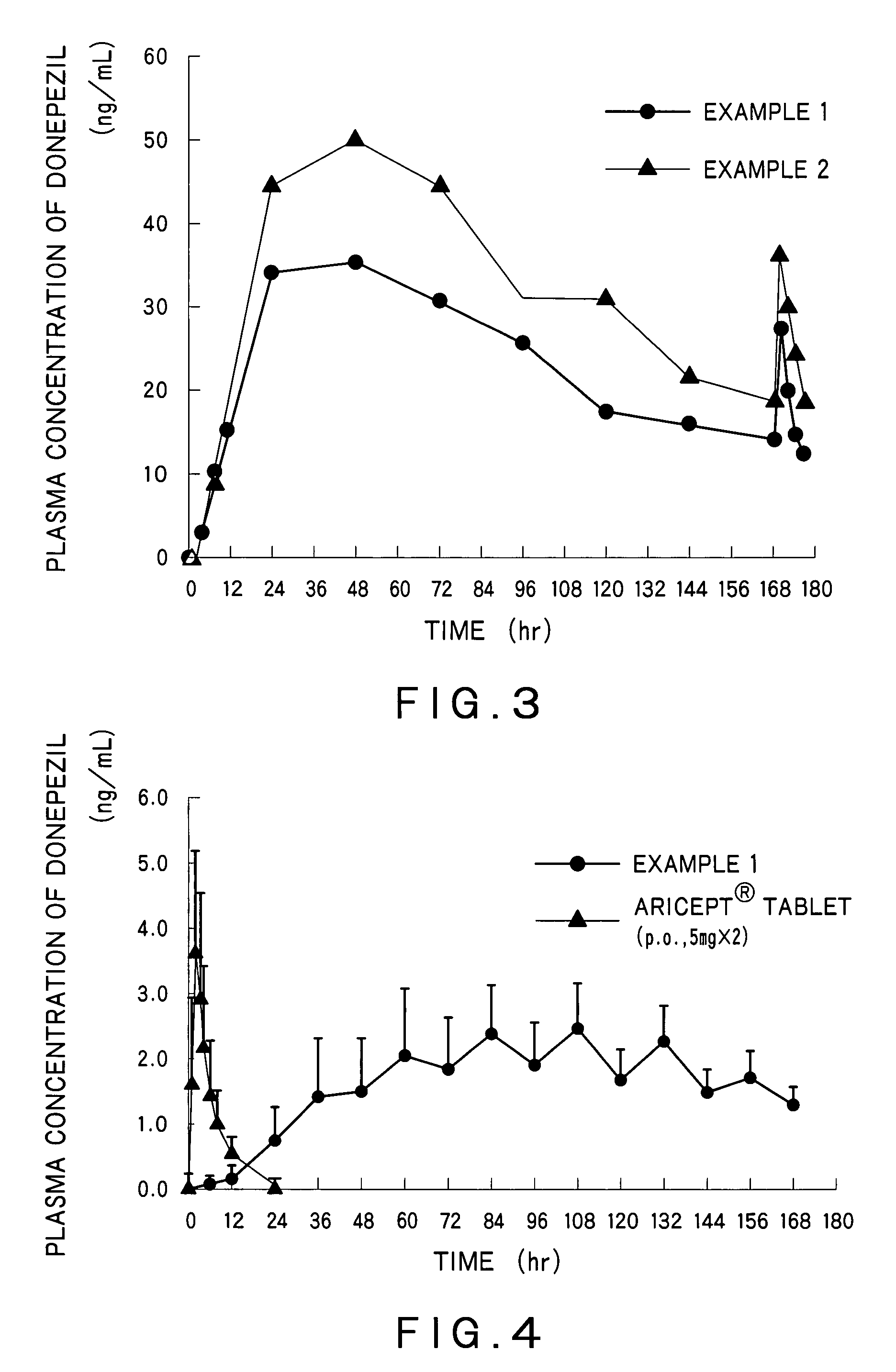
Percutaneous absorption preparations of antidementia drugs
InactiveUS20070259028A1Good release performanceStable drug releasabilityBiocideNervous disorderDrug reservoirAlcohol
Disclosed is a percutaneous absorption preparation which enables the stable administration of an antidementia drug over a long period of time. More particularly, the percutaneous absorption preparation of the antidementia drug which is used as a plaster on skin comprises at least an adherent layer, an intermediate membrane, and a drug reservoir layer sequentially from the side which is plastered on skin, wherein the drug reservoir layer comprises at least an antidementia drug, an aminated polymer, a polyhydric alcohol, and one or more carboxylic acid esters, the intermediate membrane enables the controlled permeation of the antidementia drug into the side of skin, the adherent layer enables the plastering of the percutaneous absorption preparation on skin, and is permeable to the antidementia drug.
Owner:TEIKOKU SEIYAKU KK TEIKOKU SEIYAKU CO LTD
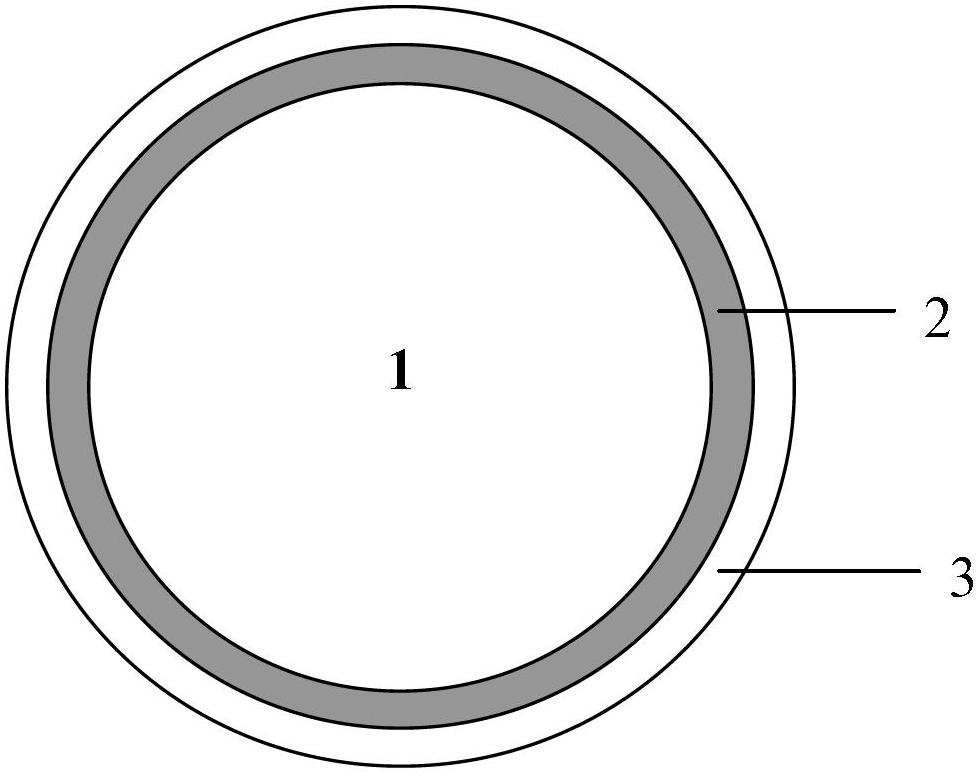
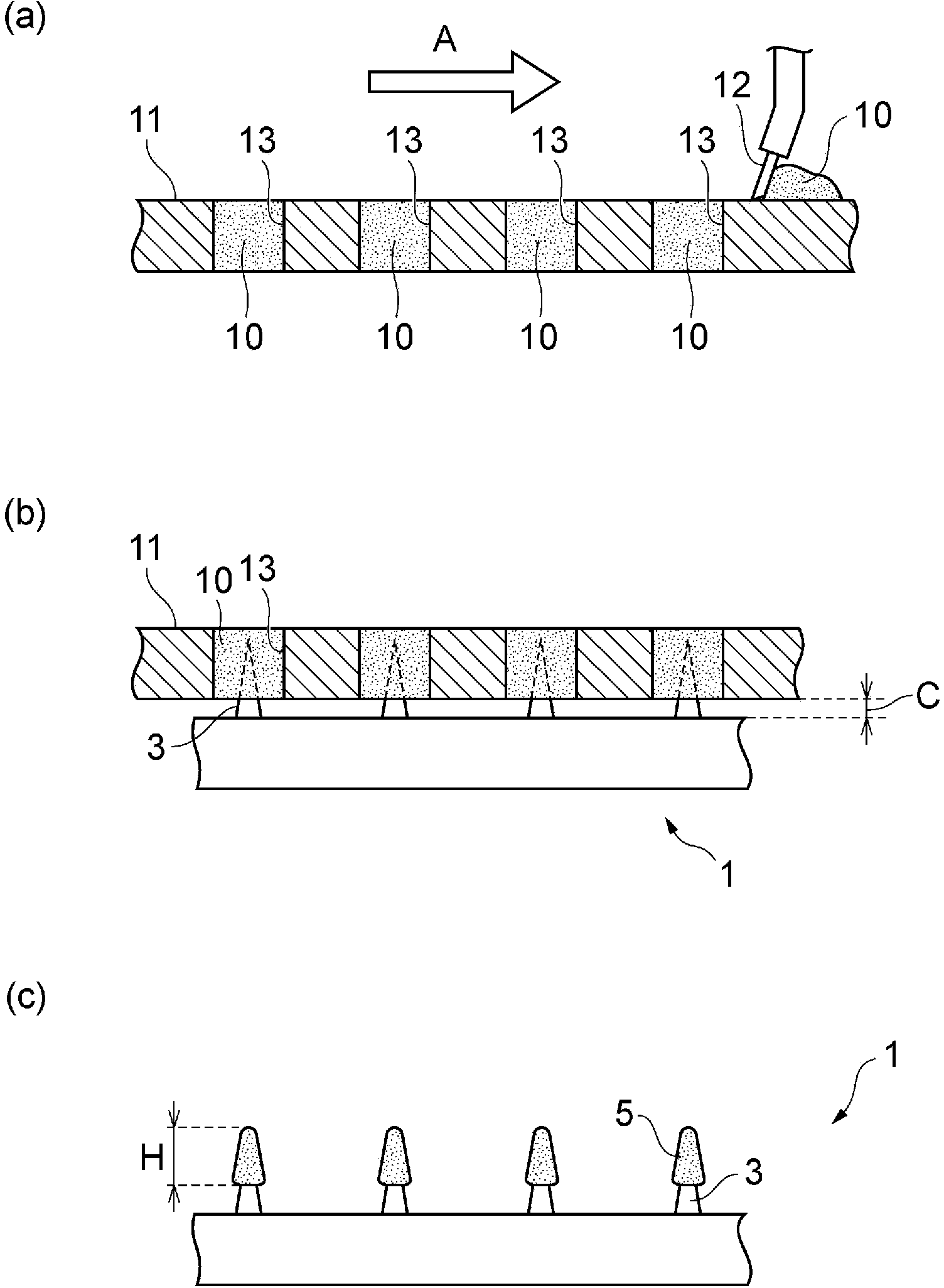
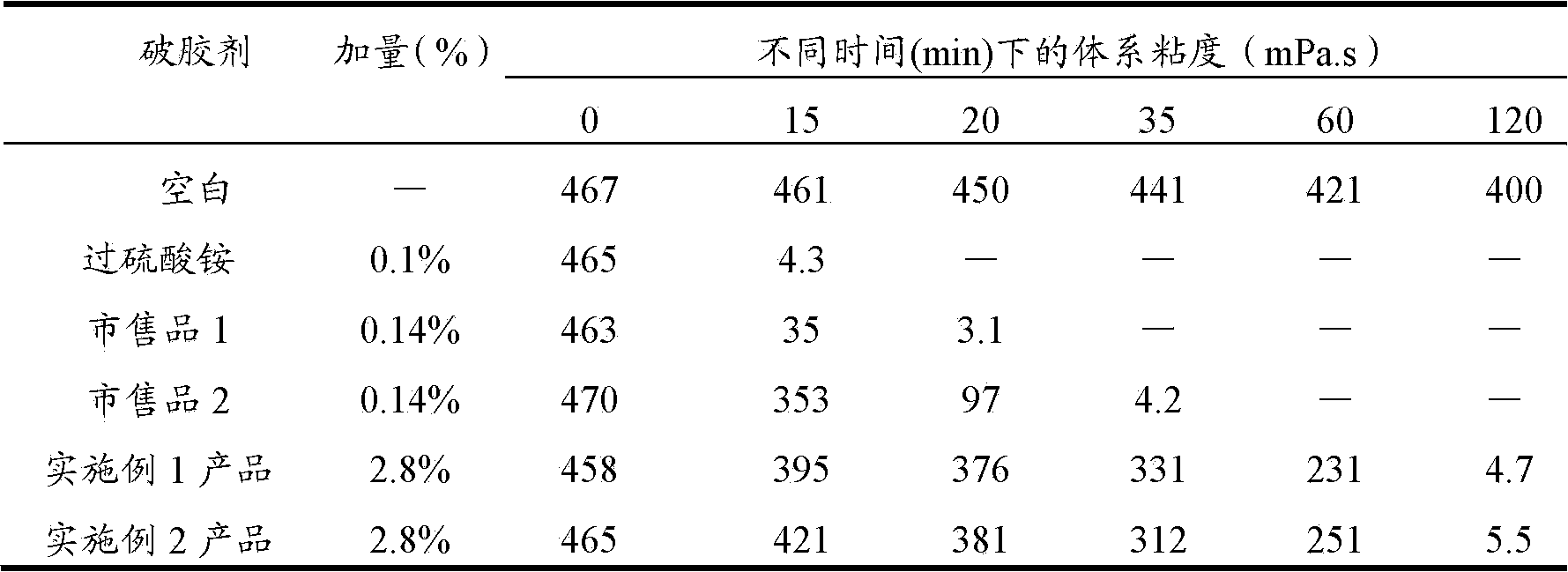
Sustained-release gel-breaking type fracturing propping agent and preparation method thereof
The invention discloses a sustained-release gel-breaking type fracturing propping agent for fracturing an oilfield. The propping agent sequentially comprises a base material, a medicament carrying layer and a sustained release layer from inside to outside, wherein the medicament carrying layer comprises a gel breaking agent and a filling agent, and the sustained release layer comprises a film forming material and a pore forming agent. The invention further provides a preparation method of the sustained-release gel-breaking type fracturing propping agent. In an actual application, the sustained-release gel-breaking type fracturing propping agent disclosed by the invention can enter the stratum along with fracturing fluid, and the gel breaking agent can be released slowly under the stratum conditions; and as the propping dosage is great, the gel breaking agent is distributed uniformly, and gel breaking can be realized to the greatest extent. Simultaneously, a chemical material coating the gel breaking agent can be stilly firmly coated on matrix particles, thus the problems of breaking, reflux and stratum embedding of the propping agent can be reduced.
Owner:CHINA NAT OFFSHORE OIL CORP +2
Silicone-filled casing for use with light-emitting unit and method of manufacturing the light-emitting unit
InactiveUS20050139850A1Improve cooling effectEfficient conductionPoint-like light sourceLighting heating/cooling arrangementsSilica gelLight-emitting diode
A light-emitting unit containing a substrate and light emitting diodes (LEDs) is housed inside a casing constituted by a first member and a second member which are joined together. The second member has a projecting piece which is inserted to the first member, and inside the projecting piece a passage is formed. When silicone is injected through an injection opening from an injector, the silicone starting from the projecting piece flows through in the order of the passage, first-member side space, connecting passage and second-member side space and it finally overflows from a discharge opening. By implementing this structure and process, the air or air bubbles inside the light-emitting unit is pushed outside completely.
Owner:TOKI
Percutaneous absorption preparations of antidementia drugs
InactiveUS7858114B2Good release performanceStable drug releasabilityBiocideNervous disorderDrug reservoirAlcohol
Disclosed is a percutaneous absorption preparation which enables the stable administration of an antidementia drug over a long period of time. More particularly, the percutaneous absorption preparation of the antidementia drug which is used as a plaster on skin comprises at least an adherent layer, an intermediate membrane, and a drug reservoir layer sequentially from the side which is plastered on skin, wherein the drug reservoir layer comprises at least an antidementia drug, an aminated polymer, a polyhydric alcohol, and one or more carboxylic acid esters, the intermediate membrane enables the controlled permeation of the antidementia drug into the side of skin, the adherent layer enables the plastering of the percutaneous absorption preparation on skin, and is permeable to the antidementia drug.
Owner:TEIKOKU SEIYAKU KK TEIKOKU SEIYAKU CO LTD
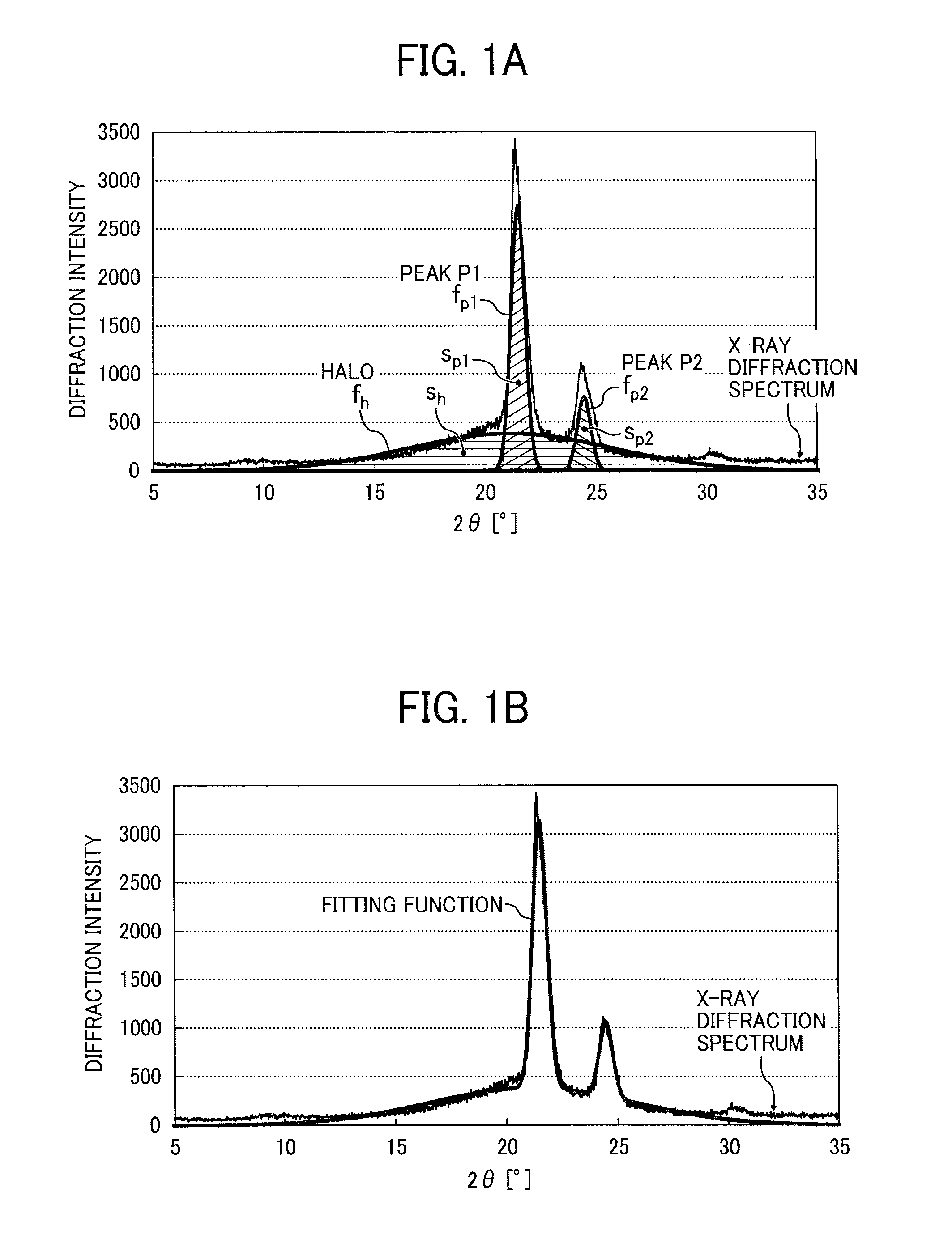
Toner, development agent, and image forming apparatus using the same
InactiveUS20130157185A1Excellent low temperature fixabilityNo pollutionElectrographic process apparatusDevelopersX-rayCrystallinity
A toner includes a binder resin, a coloring agent, and a releasing agent containing two or more kinds of alkyl monoester compounds having a different number of carbon atoms in a range of from 30 to 50 carbon atoms. The toner has a crystallinity of 10% or more as measured by x-ray diffraction and / or the binder resin is a crystalline resin in an amount of 50% by weight or more of the binder resin. The two or more kinds of alkyl monoester compounds include a component A accounting for the largest amount ranging from 30% by weight to less than 50% by weight of the releasing agent and a component B accounting for the second largest amount ranging from 10% by weight to less than 50% by weight of the releasing agent.
Owner:RICOH KK
Light release force solvent-free organic silicon release agent as well as preparation method and application
ActiveCN107936830AWith ultra-light peel forceLow peel force at room temperatureCoatingsFilm/foil adhesive release linersHydrogenSolvent free
The invention belongs to the field of release materials and discloses a light release force solvent-free organic silicon release agent as well as a preparation method and application. The light release force solvent-free organic silicon release agent disclosed by the invention comprises the following components in parts by mass: 100 parts of vinyl polysiloxane, 2-20 parts of hydrogen-containing polysiloxane, 2-10 parts of an anchoring agent, 2-10 parts of a fluorine-containing release force adjusting agent and 2-10 parts of a platinum catalyst, wherein the functional group ratio of vinyl polysiloxane to hydrogen-containing polysiloxane is H / Vi=1.0-5.0. The release agent disclosed by the invention is applied to release paper. The release paper prepared from the release agent has super lightrelease force, the normal-temperature release force of the release paper is as low as 1.3g / 25mm, the aging release force of the release paper is as low as 1.7g / 25mm, the release paper is stable in release property and can be applied to practical production processing, and the requirements of electronic processing industries on super light release force in application scenes are met.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI +3
Electrostatic image developing toner and image forming method
InactiveUS20070042285A1Improve the fixing strengthStable toner release performanceDevelopersEngineeringColoring agents
Owner:KONICA MINOLTA BUSINESS TECH INC
Silk-screen printing device
InactiveCN101045360AImprove yieldImprove printing qualityPrinted circuit assemblingScreen printersScreen printingBaseboard
Provided is a screen printing apparatus. In the forming of the protrude electrode using the screen printing method, resulting the adhesive force of the solder paste which is transferred by many opening-part groups and the metal mask, the departing of the printing board starts from the perimeter part, finally the middle part of the metal mask departs from the printing board, the occurring of this phenomenon is the main reason for the defective printing which concludes the thickness error of the printing film ,the printing deficiency and so on. Using the different thickness of the mentioned mask component sheet, or the different material, or the different elastic coefficient, connect every mask component sheet which is set on the accessory pattern of the printing baseboard or at all the patterns formed in the scheduled area, and the adjusting equipment which accords to the mentioned connection status and can reproduce random curve is also provided.
Owner:HITACHI LTD
Ultraviolet light cured non-silicon release agent and preparation method thereof
The invention discloses an ultraviolet light cured non-silicon release agent, which is composed of the following components in parts by weight: 50 to 80 parts of vinyl ether functionalized acrylic resin, 10 to 30 parts of dodecyl vinyl ether, 10 to 20 parts of triethyleneglycol divinyl ether, and 1 to 3 parts of photoinitiator. The preparation method comprises the following steps: step one, synthesizing vinyl ether functionalized acrylic resin; step two, adding 10 to 30 parts of dodecyl vinyl ether and 10 to 20 parts of triethyleneglycol divinyl ether into the 50-80 parts of vinyl ether functionalized acrylic prepolymer; step three, stirring and evenly mixing, then adding 1 to 3 parts of photoinitiator, stirring and evenly mixing so as to obtain ultraviolet light cured non-silicon release agent; step four, painting the obtained ultraviolet light cured non-silicon release agent on a film, and carrying out radiation curing by placing the film under an ultraviolet lamp. The invention aims to provide a non-mobility solvent-free ultraviolet light cured non-silicon release agent with a stable releasing performance and a preparation method thereof.
Owner:KUNSHAN BYE MACROMOLECULE MATERIAL CO LTD
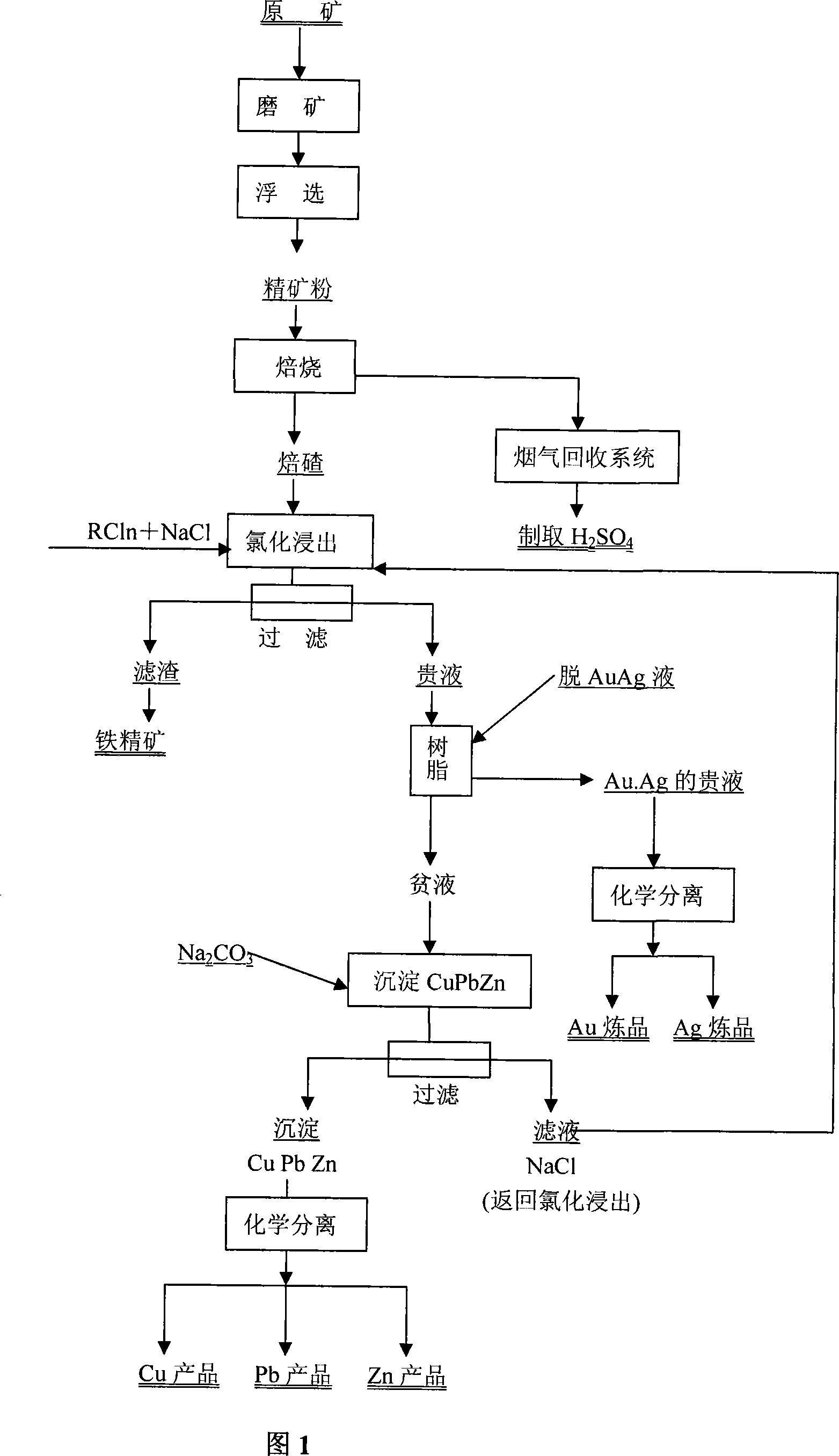
Integrated utilization technique for multi-metal ore containing gold and silver by chlorine carrier chlorination method
InactiveCN101215633AStrong leaching effectEffective soakingFlotationProcess efficiency improvementSlagChemical separation
The invention discloses a mineral processing and metallurgical technology which comprehensively uses multi-metallic ore through a chloride carrier chlorination process, which comprises calcining crude ore into slag through grinding, flotation and concentrate powder, separating out gold, silver liquid and barren liquor from slag through pitch absorption by penetrating through chloridization leach pregnant solution, preparing gold and silver through chemically separating pregnant solution, depositing and filtering barren liquor to recycle and separate copper, lead and zinc, and reusing filter solution which is sodium chloride passing through the steps of chlorination leaching above. The mineral processing and metallurgical technology of the invention has the characteristics of no toxic and environment-friendly, fast speed, low cost and multiple-kind comprehensive recycling products. The invention has high comprehensive recovering index, the recovery ratio of gold and silver all can be larger than 95%, the recovery ratio copper, lead and zinc and the like which are comprehensively recovered all can be larger than 90%, and the invention can change ferro-sulphur ore into iron-smelting raw material. The invention is superior to traditional, which has significant technology superiority, economic benefits superiority, environmental benefit superiority and high comprehensive utilization degree.
Owner:石嵩高
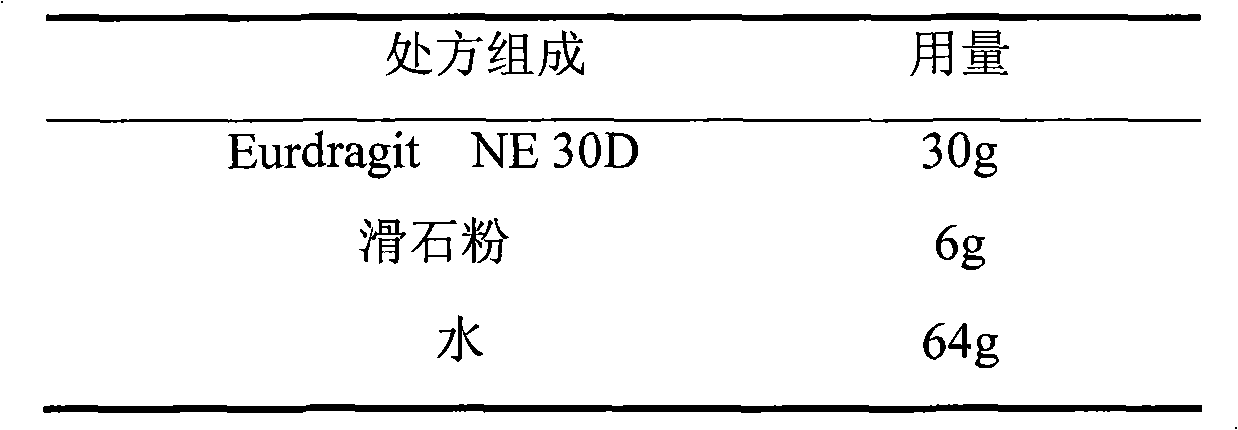
Acipimox film-controlled slow-release pellet capsule
ActiveCN103211785AAccelerated agingReduce permeabilityOrganic active ingredientsMetabolism disorderMedicineLactose
The invention relates to an acipimox film-controlled slow-release pellet capsule. A slow-release film of the acipimox film-controlled slow-release pellet utilizes Eurdragit NE 30D as a film-formation material. A pellet core of the acipimox film-controlled slow-release pellet contains low-substituted hydroxypropyl cellulose having high expansibility, and also contains pharmaceutically acceptable common excipients for the slow-release pellet, wherein preferably, the excipients comprise microcrystalline cellulose and lactose, and the pellet core comprises 10 to 40wt% of the low-substituted hydroxypropyl cellulose. The slow-release film comprises the Eurdragit NE 30D, triethyl citrate as a plasticizer and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit NE 30D to triethyl citrate to talcum powder is 30: 2: 4 and coating weight gain is in a range of 20 to 39%. The acipimox film-controlled slow-release pellet comprises the pellet core containing low-substituted hydroxypropyl cellulose having high water expansibility and thus after absorbing water, the acipimox film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the acipimox film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept prior to expiration date.
Owner:北京天衡药物研究院有限公司
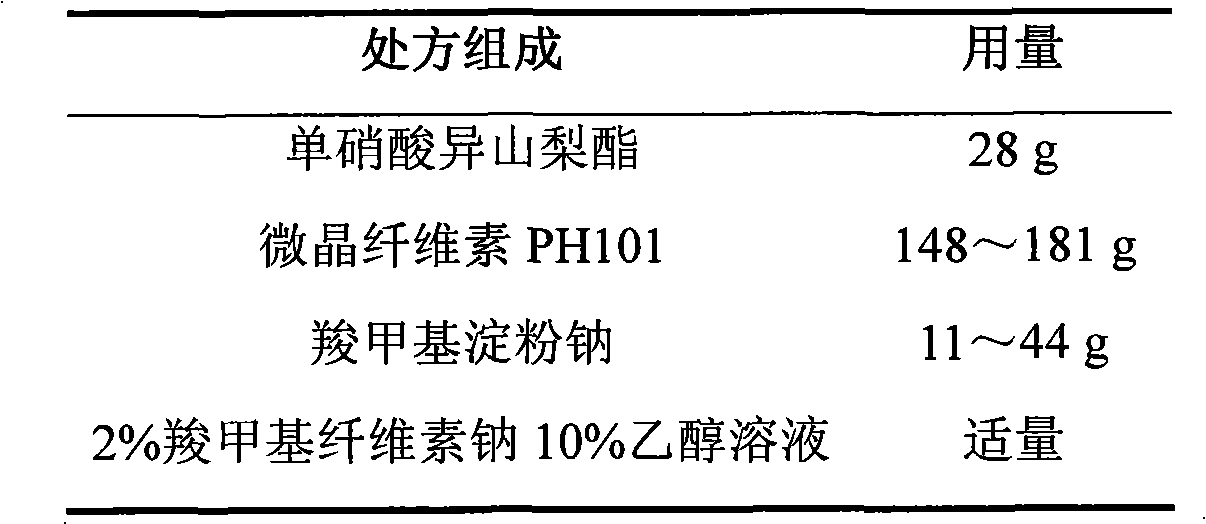
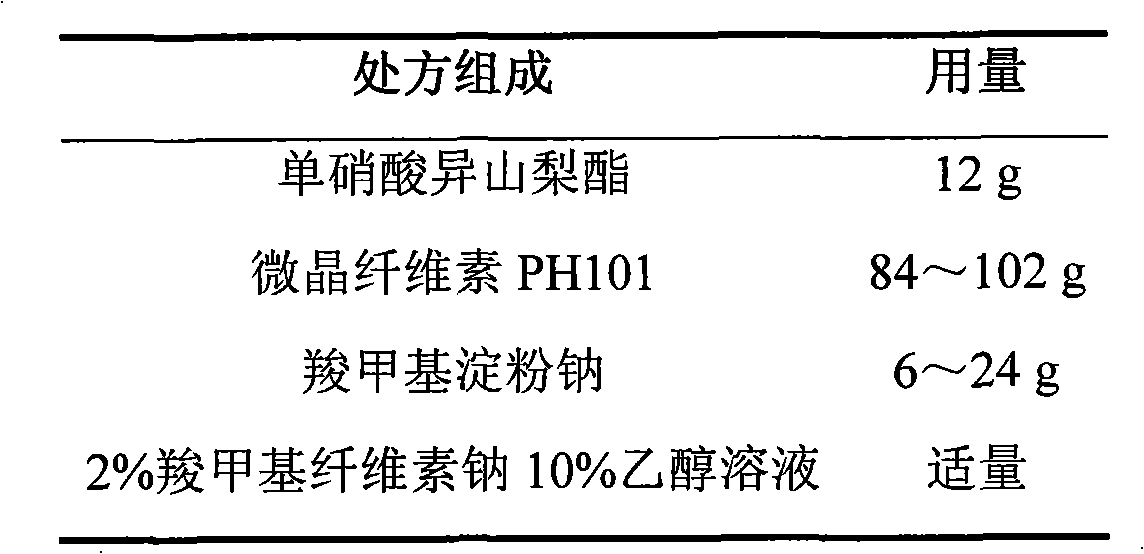
Isosorbide mononitrate sustained-release pellet, and isosorbide mononitrate quick-release and sustained-release pellet capsule adopting it
ActiveCN103211768AAccelerated agingReduce permeabilityPharmaceutical non-active ingredientsGranular deliverySustained release pelletsMedicine
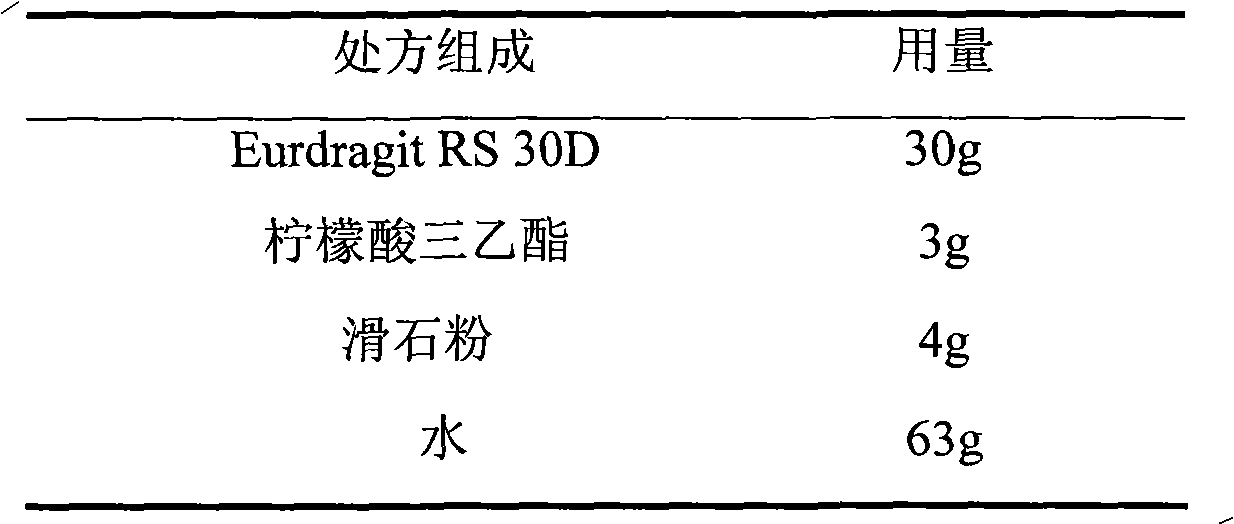
The invention relates to an isosorbide mononitrate sustained-release pellet, and an isosorbide mononitrate quick-release and sustained-release pellet capsule adopting it. The sustained-release film of the sustained-release pellet adopts Eurdragit RS 30D as a film forming material, the core of the sustained-release pellet contains high-expansibility sodium carboxymethyl starch and a pharmaceutically-acceptable excipient commonly used for sustained-release pellets, and optimally, the excipient is microcrystalline cellulose, wherein the weight percentage of sodium carboxymethyl starch in the core of the sustained-release pellet is 5-20%. The sustained-release film of the sustained-release pellet includes the Eurdragit RS 30D, a plasticizer triethyl citrate and an anti-adherent talcum powder, the optimal ratio of the Eurdragit RS 30D to triethyl citrate to the talcum powder is 30:3:4, and the optimal coating weight gain is 19-38%. The core will obviously expand after absorbing water because of the containment of sodium carboxymethyl starch highly expanding after contacting with water, so the sustained-release film is stretched, the thickness of the film is thinned, the apertures of water-pervious micro-pores are increased, the permeability is good, and the permeability decrease caused by film ageing is compensated, thereby the middle and later stage release speed is basically constant, the last stage residue is small, and a stable release performance is always maintained prior to the expiration date.
Owner:北京天衡药物研究院有限公司
Normal-temperature fast-curing silicone release agent and preparation method thereof
The invention belongs to the technical field of release materials, and discloses a solvent-free normal-temperature fast-curing silicone release agent and a preparation method thereof. The solvent-freenormal-temperature fast-curing silicone release agent provided by the invention comprises the following components in parts by mass: 100 parts of vinyl silicone oil, 3-10 parts of hydrogen-containingsilicone oil, 1-5 parts of an inhibitor and 1-3 parts of a catalyst. During the application of the release agent, various components of the release agent provided by the invention are mixed and stirred uniformly, and then the obtained mixture is coated on a substrate, wherein quick curing can be realized at 60 DEG C, and the curing time is 10s-2min. According to the difference of the usage amounts of the components and the varieties of the inhibitor in the release agent, the curing temperature can be adjusted (60-200 DEG C), and the curing time can also be adjusted. Moreover, the release paper sample obtained by using the release agent provided by the invention has stable release performance.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI +4
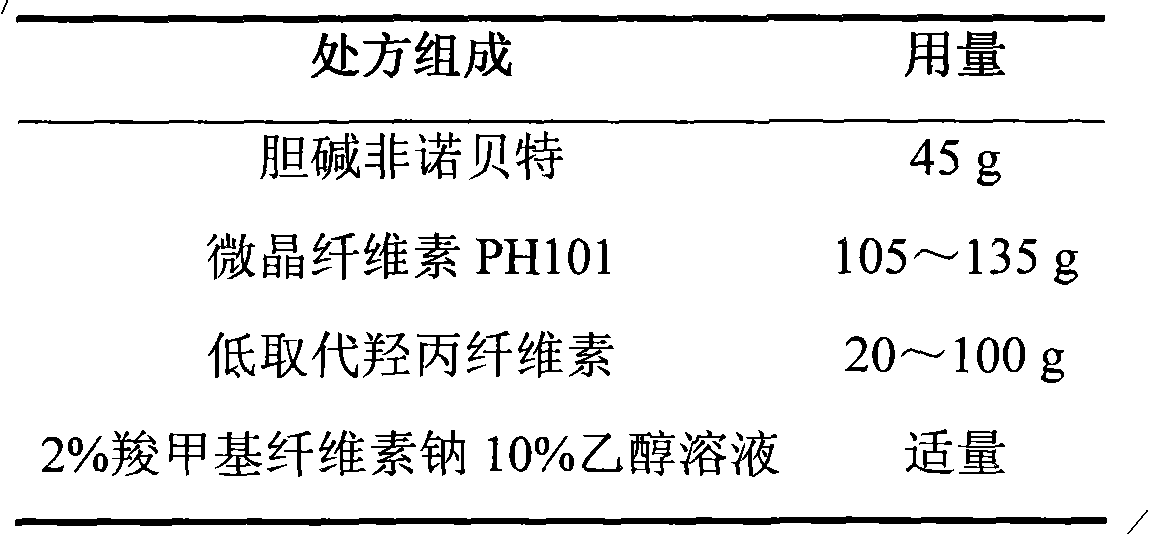
Choline fenofibrate film-controlled enteric slow-release pellet capsule
ActiveCN103211786AAccelerated agingReduce permeabilityOrganic active ingredientsMetabolism disorderSustained release pelletsMedicine
The invention relates to a choline fenofibrate film-controlled enteric slow-release pellet capsule. A slow-release film of the choline fenofibrate film-controlled enteric slow-release pellet utilizes Eurdragit RS 30D as a film-formation material. A pellet core of the choline fenofibrate film-controlled enteric slow-release pellet contains low-substituted hydroxypropyl cellulose having high expansibility, and also contains a pharmaceutically acceptable common excipient for the slow-release pellet, wherein preferably, the excipient comprises microcrystalline cellulose, and the pellet core comprises 10 to 40wt% of the low-substituted hydroxypropyl cellulose. The slow-release film comprises Eurdragit RS 30D, triethyl citrate as a plasticizer and talcum powder as an antiplastering aid, wherein preferably, a ratio of urdragit RS 30D to triethyl citrate to talcum powder is 30: 3: 4 and a film weight increasing ratio is in a range of 20 to 38%. The choline fenofibrate film-controlled enteric slow-release pellet comprises the pellet core containing low-substituted hydroxypropyl cellulose having high water expansibility and thus after absorbing water, the choline fenofibrate film-controlled enteric slow-release pellet expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the choline fenofibrate film-controlled enteric slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
High-elasticity silicone rubber lettering film and preparation method and application method thereof
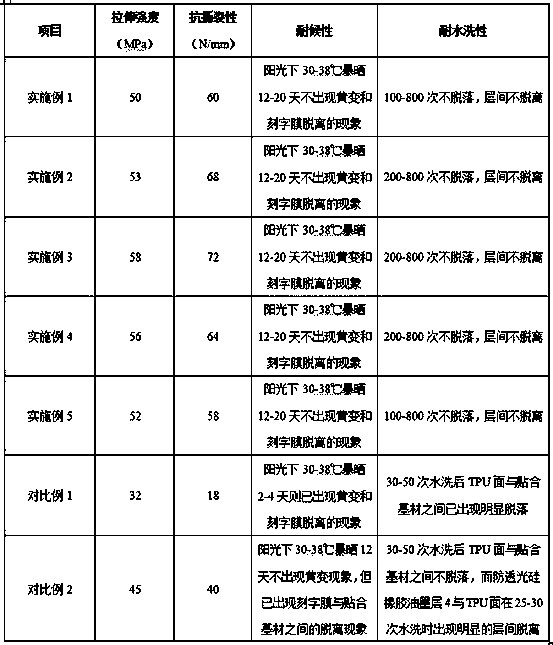
ActiveCN108610997AIncrease elasticityAvoid deformationPolyureas/polyurethane adhesivesInksRubber materialPolymer science
The invention relates to the technical field of thermal transfer printing lettering film, and in particular, relates to a high-elasticity silicone rubber lettering film and a preparation method and anapplication method thereof. The high-elasticity silicone rubber lettering film comprises a load-bearing substrate, a release layer, a surface layer silicone rubber ink layer, an anti-photopermeable silicone rubber ink layer, an adhesive interfacial agent layer and a TPU hot melt adhesive film which are connected from bottom to top successively; the adhesive interfacial agent layer is prepared from an adhesive interfacial agent, and the adhesive interfacial agent is an adhesive interfacial agent containing dual-resin components. The silicone rubber lettering film has the advantages of both thesilicone rubber material and the TPU material, has good elasticity, tearing strength, weatherability, yellowing resistance, water washing resistance and other comprehensive properties, has layers noteasy to separate, is not easy to discolor or fade by sunlight exposure, and has the water-washing fading rate not less than 0.5%, is not easy to dye when being mixed and washed with materials with other colors, and is high in stability, easy to bond with a variety of materials, high in bonding strength, not easy to peel off, good in stability, delicate in hand feeling, good in aesthetic degree, and long in service life.
Owner:卢汉军
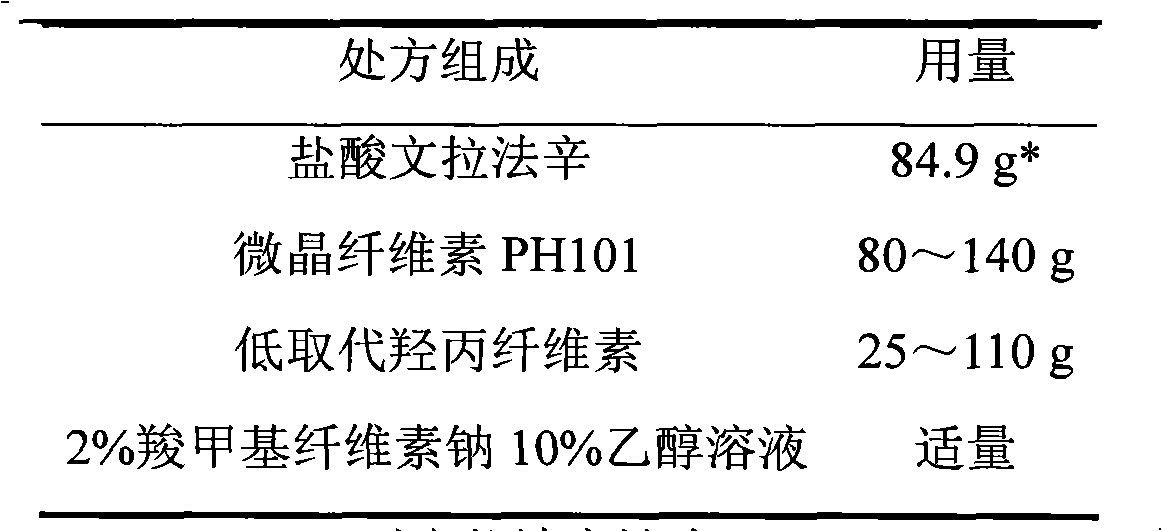
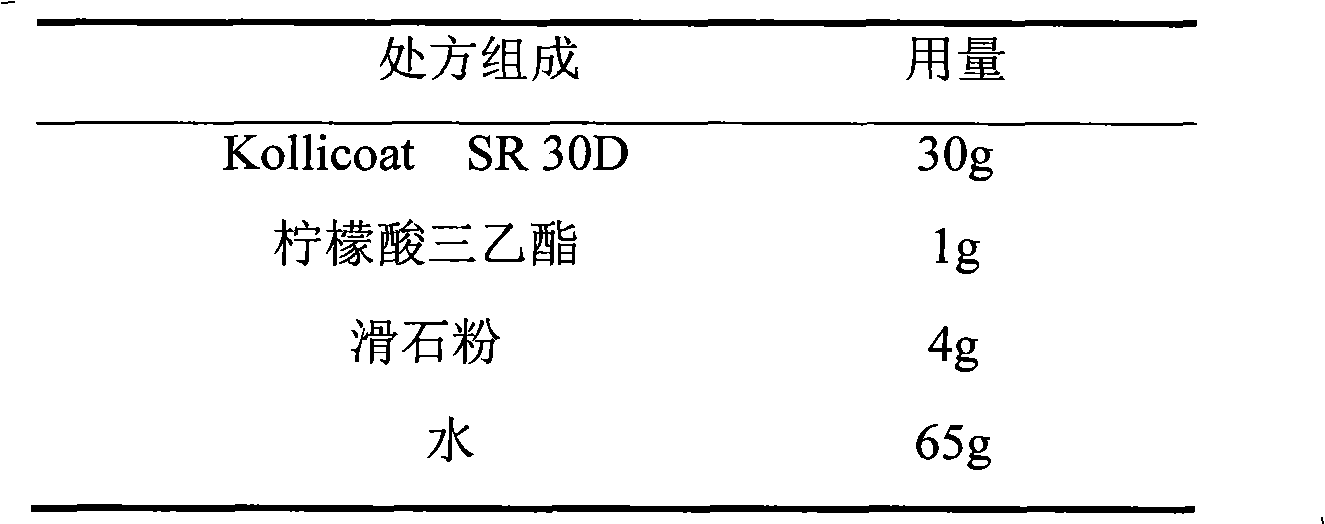
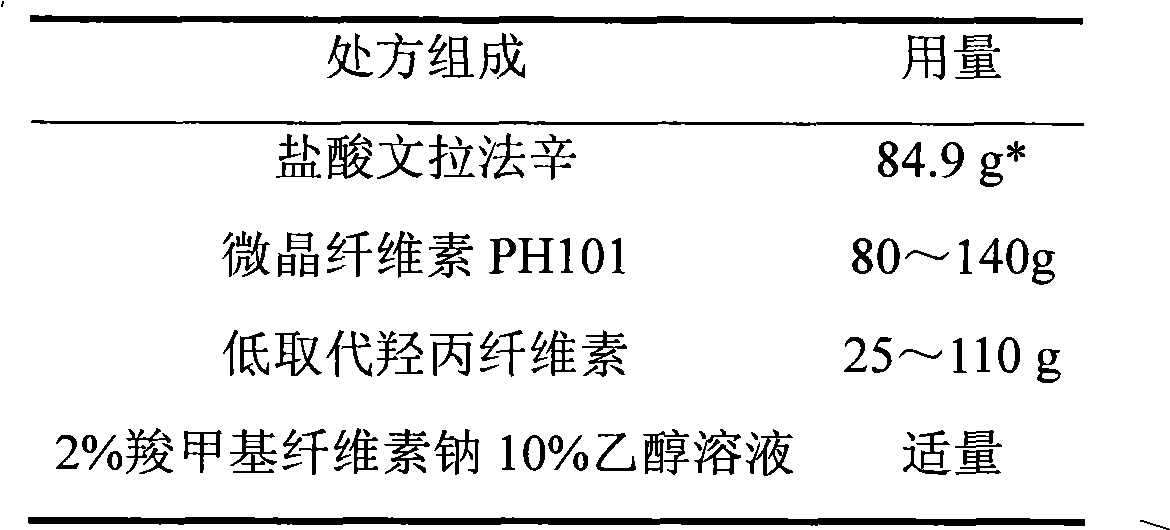
Venlafaxine hydrochloride film-controlled slow-release pellet capsule
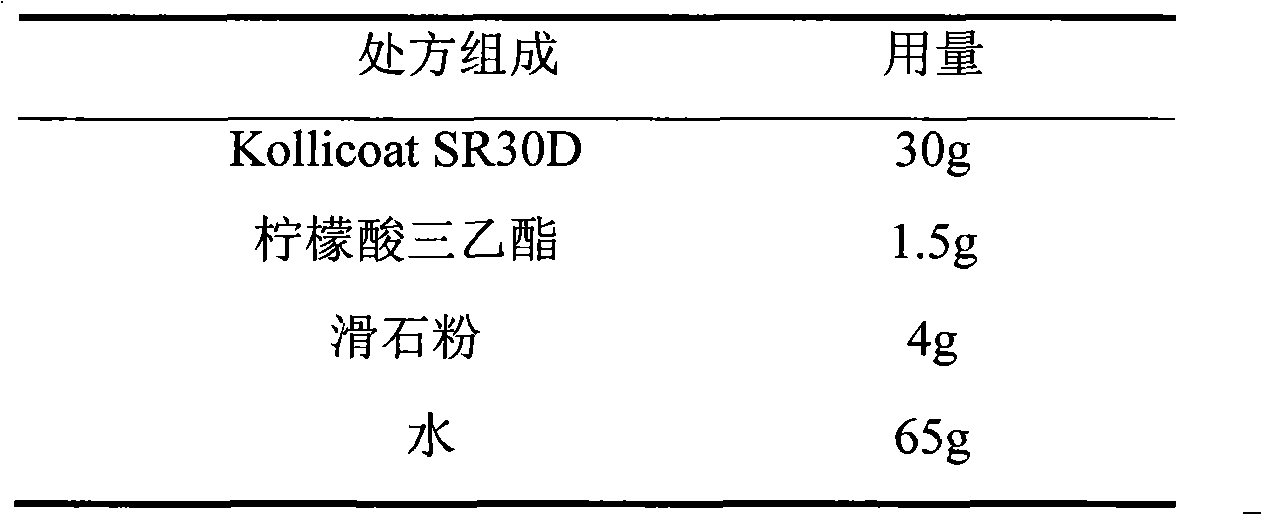
ActiveCN103211791AAccelerated agingReduce permeabilityOrganic active ingredientsNervous disorderSustained release pelletsLow-substituted hydroxypropylcellulose
The invention relates to a venlafaxine hydrochloride film-controlled slow-release pellet capsule. A slow-release film of the venlafaxine hydrochloride film-controlled slow-release pellet utilizes Kollicoat SR30D as a film-formation material. A pellet core of the venlafaxine hydrochloride film-controlled slow-release pellet contains low-substituted hydroxyprepyl cellulose having high expansibility, and also contains a pharmaceutically-acceptable common excipient for the slow-release pellet, wherein preferably, the excipient comprises microcrystalline cellulose, and the pellet core comprises 10 to 40wt% of low-substituted hydroxyprepyl cellulose. The slow-release film comprises Kollicoat SR30D, triethyl citrate as a plasticizer and talcum powder as an antiplastering aid, wherein preferably, a ratio of Kollicoat SR30D to triethyl citrate to talcum powder is 30: 1: 4 and a film weight increasing ratio is in a range of 21 to 39%. The venlafaxine hydrochloride film-controlled slow-release pellet comprises the pellet core containing low-substituted hydroxyprepyl cellulose having high water expansibility and thus after absorbing water, the venlafaxine hydrochloride film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the venlafaxine hydrochloride film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
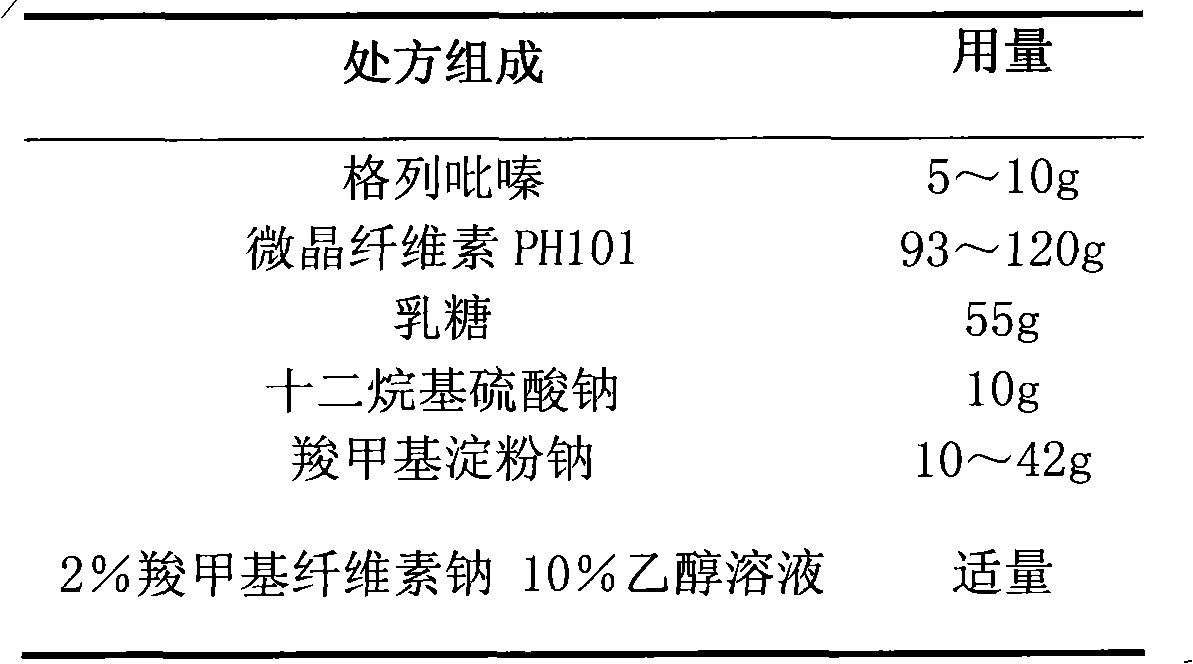
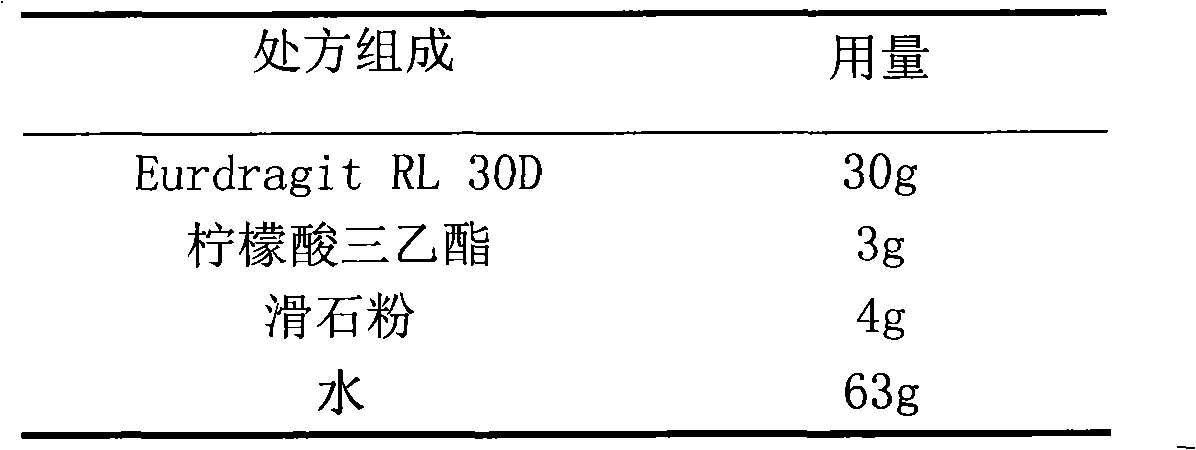
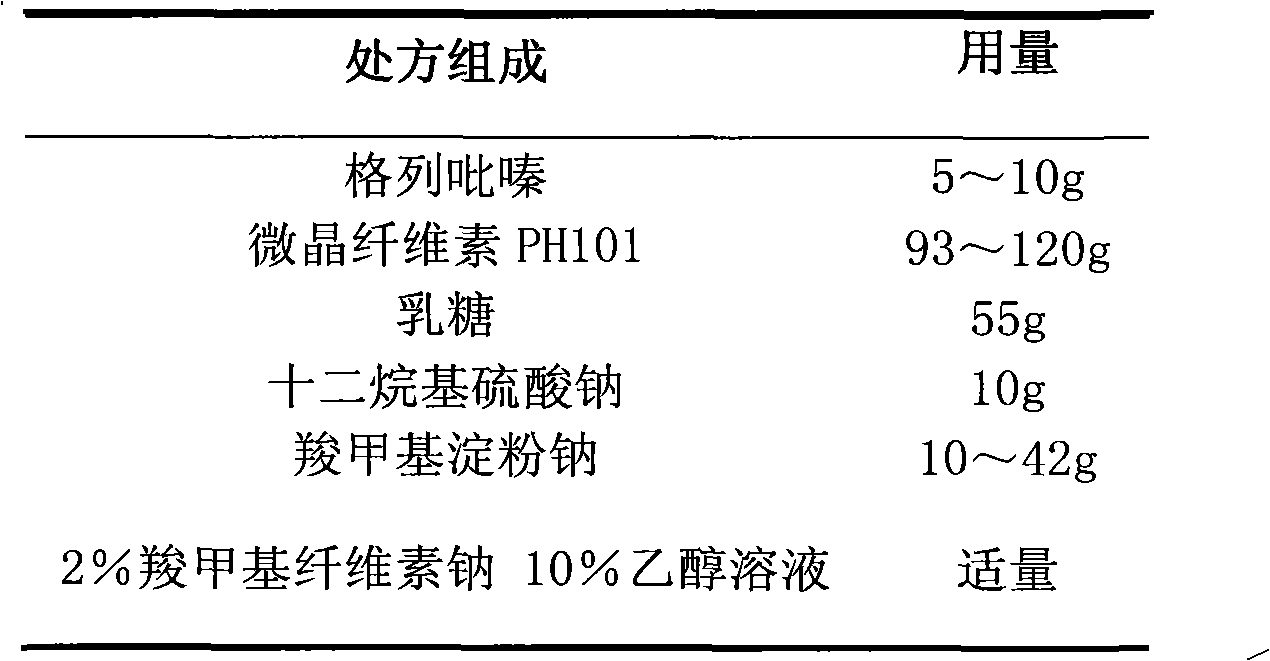
Glipizide film-controlled slow-release pellet capsule
ActiveCN103211787AAccelerated agingReduce permeabilityMetabolism disorderSulfonylurea active ingredientsSustained release pelletsMedicine
The invention relates to a glipizide film-controlled slow-release pellet capsule. A slow-release film of the glipizide film-controlled slow-release pellet utilizes Eurdragit RL 30D as a film-formation material. A pellet core of the glipizide film-controlled slow-release pellet contains sodium carboxymethyl starch having high expansibility, and also contains pharmaceutically-acceptable common excipients for the slow-release pellet, wherein preferably, the excipients comprise microcrystalline cellulose, lactose and sodium dodecyl sulfate, and the pellet core comprises 5 to 20wt% of sodium carboxymethyl starch. The slow-release film comprises Eurdragit RL 30D, triethyl citrate as a plasticizer and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit RL 30D to triethyl citrate to talcum powder is 30: 3: 4 and a film weight increasing ratio is in a range of 17 to 36%. The glipizide film-controlled slow-release pellet comprises the pellet core containing sodium carboxymethyl starch having high water expansibility and thus after absorbing water, the glipizide film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the glipizide film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:内蒙古天衡医院管理有限公司
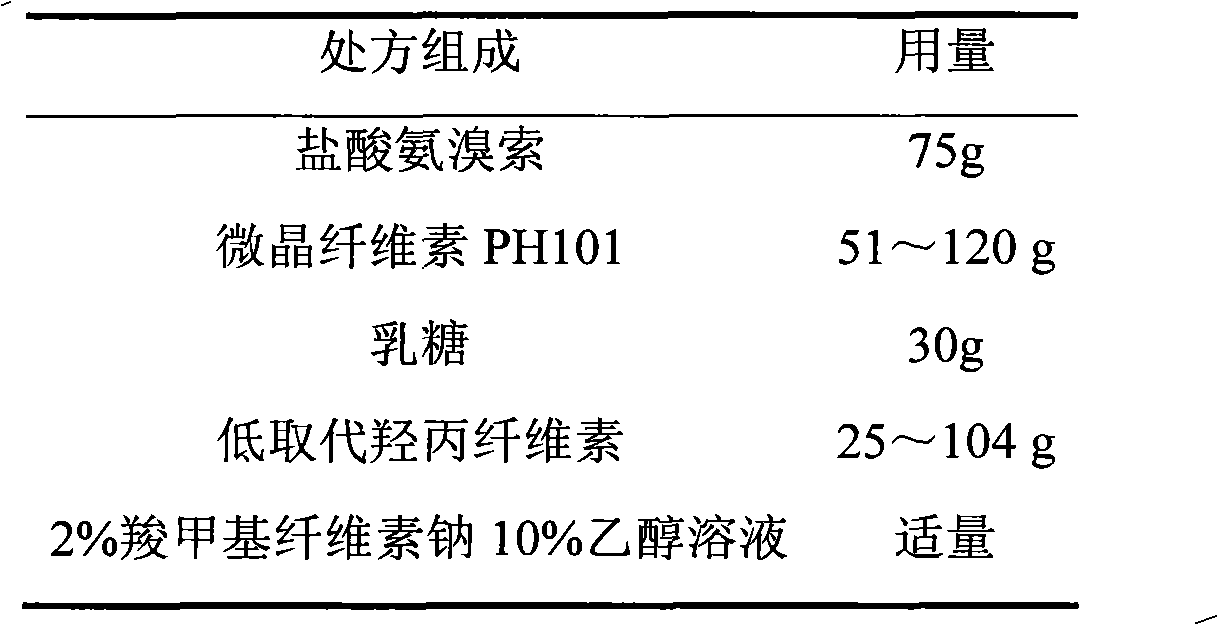
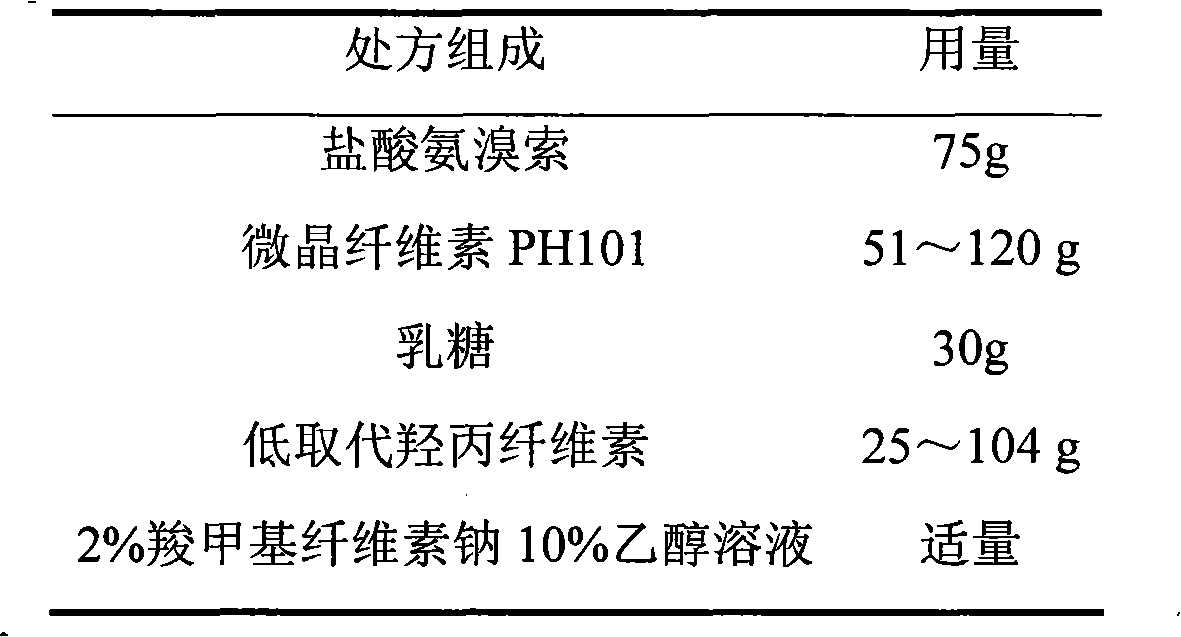
Ambroxol hydrochloride film-controlled slow-release pellet capsule
ActiveCN103211789AAccelerated agingReduce permeabilityOrganic active ingredientsPharmaceutical non-active ingredientsSustained release pelletsLactose
The invention relates to an ambroxol hydrochloride film-controlled slow-release pellet capsule. A slow-release film of the ambroxol hydrochloride film-controlled slow-release pellet utilizes Kollicoat SR 30D as a film-formation material. A pellet core of the ambroxol hydrochloride film-controlled slow-release pellet contains low-substituted hydroxyprepyl cellulose having high expansibility, and also contains pharmaceutically-acceptable common excipients for the slow-release pellet, wherein preferably, the excipients comprise microcrystalline cellulose and lactose, and the pellet core comprises 10 to 40wt% of low-substituted hydroxyprepyl cellulose. The slow-release film comprises Kollicoat SR 30D, triethyl citrate as a plasticizer, and talcum powder as an antiplastering aid, wherein preferably, a ratio of Kollicoat SR 30D to triethyl citrate to talcum powder is 30: 1.5: 4 and a film weight increasing ratio is in a range of 20 to 36%. The ambroxol hydrochloride film-controlled slow-release pellet comprises the pellet core containing low-substituted hydroxyprepyl cellulose having high water expansibility and thus after absorbing water, the ambroxol hydrochloride film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the ambroxol hydrochloride film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
GLP-1 analogue composition for microneedle devices
InactiveCN103391798AIncrease viscosityGood release performancePeptide/protein ingredientsMetabolism disorderPolyethylene glycolGlycerol
A GLP-1 analogue composition for microneedle devices, which contains a GLP-1 analogue and at least one kind of solvent that is selected from the group consisting of water, glycerol, propylene glycol, ethylene glycol, 1,3-butylene glycol and polyethylene glycol.
Owner:HISAMITSU PHARM CO INC
Liraglutide multivesicular liposome and preparation method and application thereof
ActiveCN110339166AAvoid problems prone to degradationHigh encapsulation efficiencyPeptide/protein ingredientsMetabolism disorderMedication costSide effect
The invention relates to the field of medicines, and particularly relates to a liraglutide multivesicular liposome and a preparation method and an application thereof. The liraglutide multivesicular liposome provided by the invention comprises liraglutide, a membrane material, an osmotic pressure regulator and a stabilizer. The liraglutide multivesicular liposome prepared by the invention has goodstability, high drug encapsulation rate, large drug loading capacity, slow and steady drug release rate and no burst release phenomenon, significantly improves the bioavailability of the drug, thereby improving the curative effect, reduces dose-related side effects of the drug and medication cost and has a higher application value. Experiments shows that the liposome provided by the present invention can continuously release the drug in vitro for about 432 hours, and provide a stable blood concentration in vivo, significantly prolongs in-vivo retention time compared to other injections, showsobvious pharmacokinetic characteristics of a sustained release preparation, can provide a normal steady blood glucose level, plays hypoglycemic effect for 312 hours, and has the relative bioavailability of 661% to injections.
Owner:SHENYANG PHARMA UNIVERSITY
Mold release agent and preparation method thereof
ActiveCN107570660AGood release performanceExtended service lifeFoundry mouldsFoundry coresCast ironCoal slurry
The invention relates to the technical field of ironmaking agents, in particular to a mold release agent. The mold release agent is prepared by mixing and stirring a dry material and water, the dry material comprises, by weight, 0.5-1.5% of carboxymethylcellulose, 10.0-15% of sodium bentonite, 50-60% of low carbon coal slurry, 15-20% of aluminum-magnesium refractory mortar, 10-15% of coal powder and the balance water, wherein the weight of the water is 9-11 times of the total weight of the dry material. The preparation method comprises the steps that when being poured, the mold release agent forms a sintered layer of 1-2 mm under the high temperature of molten iron, an isolation layer can be effectively formed between the molten iron and a cast iron mold, a cast iron block can smoothly fall off from the cast iron mold, the mold release performance of the mold release agent is good, the mold release rate is up to 100%, the cast iron mold can be protected and is not easily deformed, theservice life of the cast iron mold can be remarkably prolonged, the low carbon coal slurry is generally thrown away as waste, the low carbon coal slurry is used as the main component of the mold release agent, and waste using is achieved, so that the cost of the release agent is low, and popularization is facilitated.
Owner:PANGANG GROUP TITANIUM INDAL
Sustained-release gel-breaking type fracturing propping agent and preparation method thereof
Owner:CHINA NAT OFFSHORE OIL CORP +2
Trimetazine dihydrochloride sustained-release tablet and preparation method thereof
InactiveCN109908096AAvoid degradationGuaranteed stabilityOrganic active ingredientsSenses disorderTrimetazidine DihydrochlorideSustained Release Tablet
The invention discloses a trimetazine dihydrochloride sustained-release tablet which comprises trimetazidine dihydrochloride, a sustained-release matrix material, a filler, a binding agent and a lubricating agent. The sustained-release matrix material is a mixture of hydroxypropyl methyl cellulose with different viscosity. By the arrangement, the initial burst release of trimetazidine dihydrochloride in the early stage is stable, shortcomings of poor stability and easy degradation of trimetazidine dihydrochloride tablet are overcome, and the stability of the trimetazine dihydrochloride tabletis improved.
Owner:WUHAN WUYAO SCI & TECH
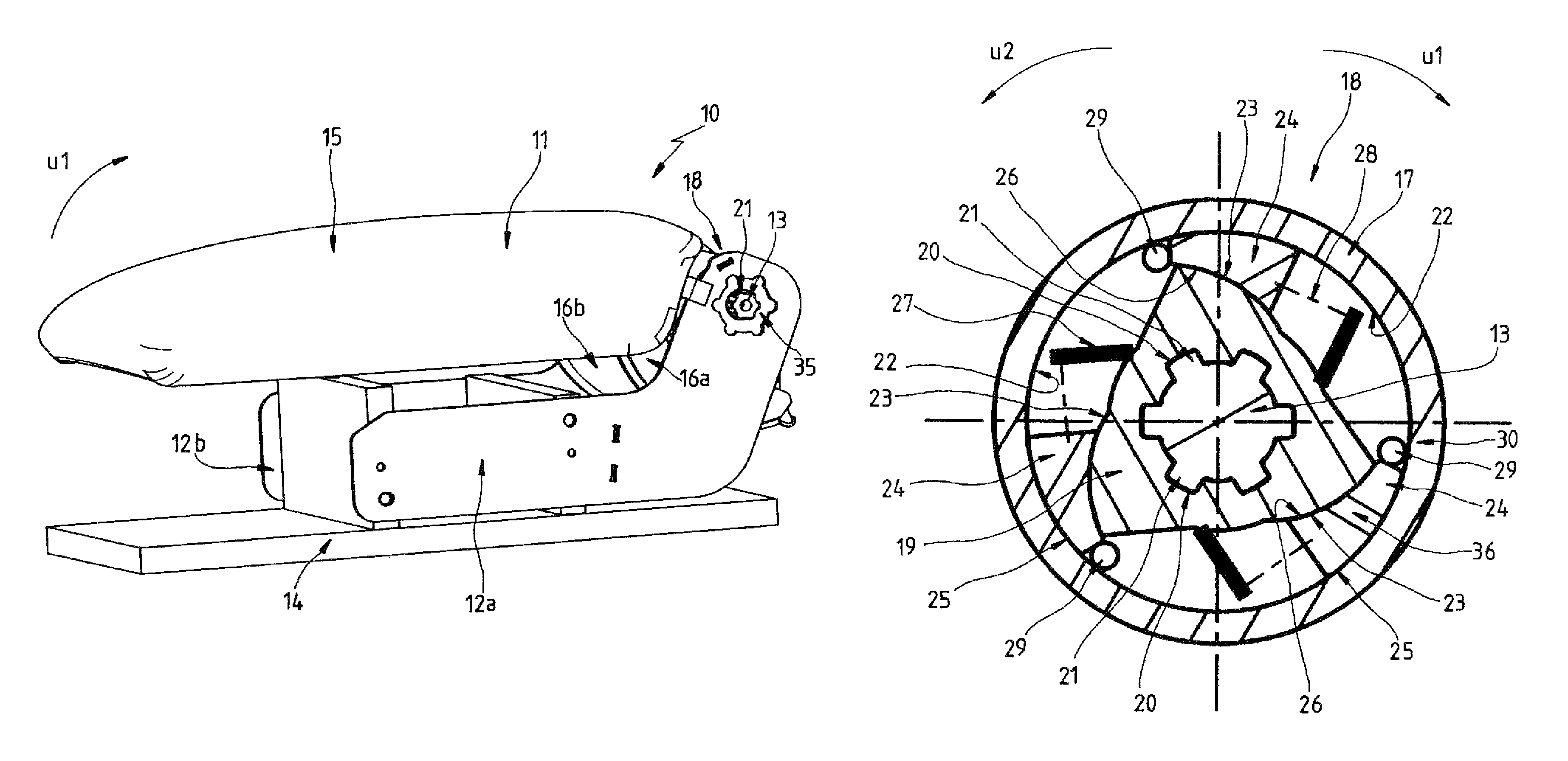
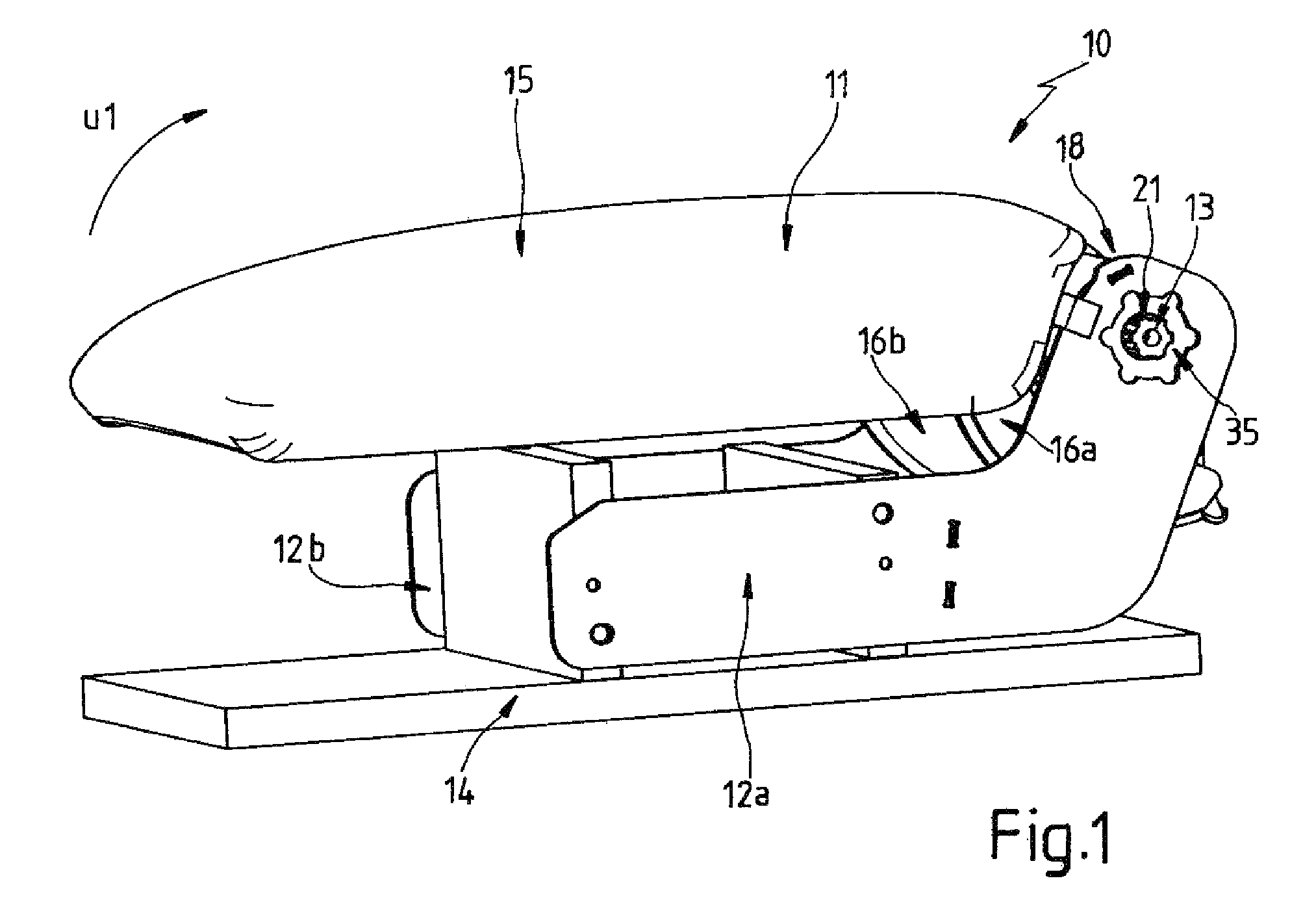
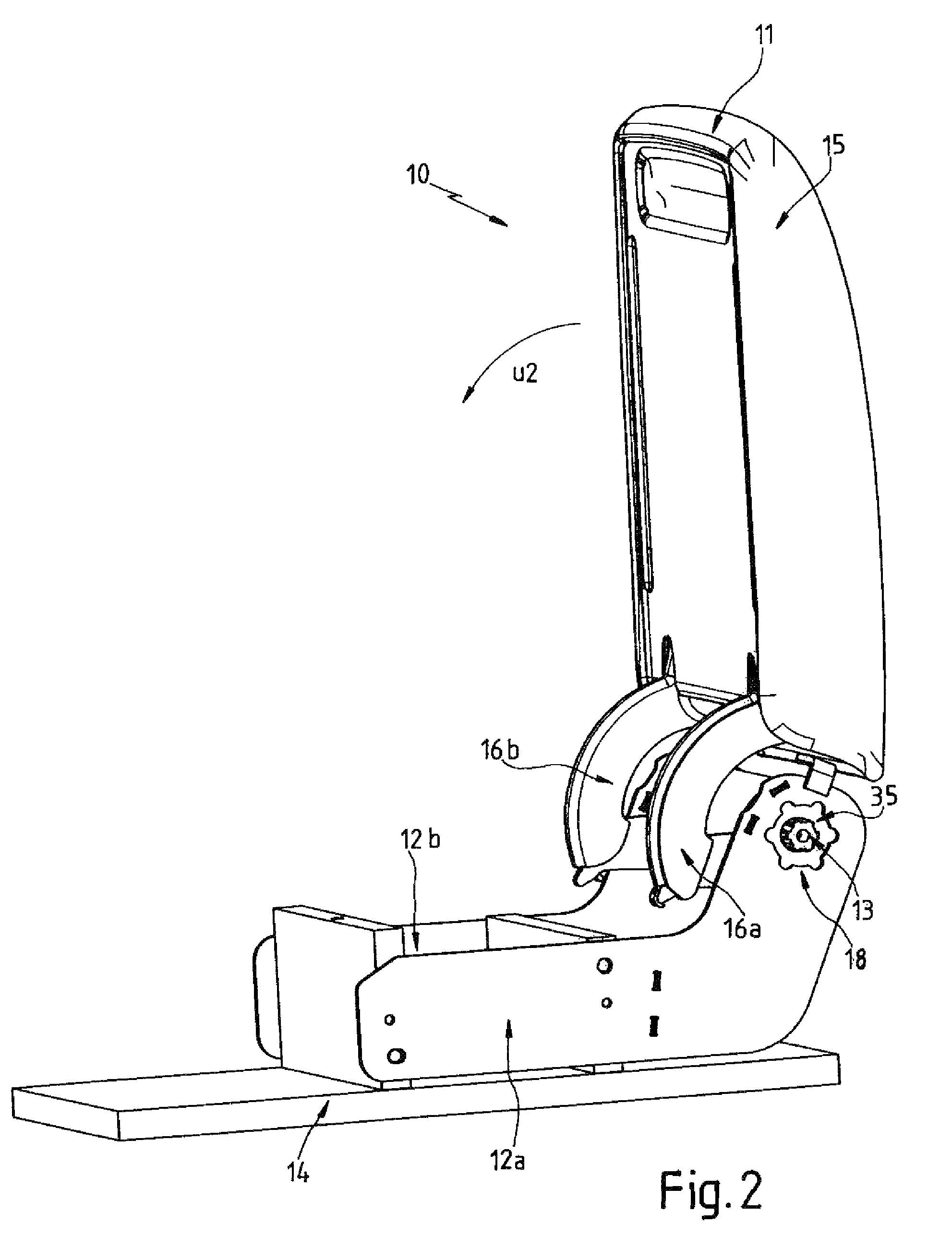
Accessory for a motor-vehicle interior, in particular an armrest for a motor-vehicle seat
ActiveUS8550505B2Improve locking performanceGood release performanceArm restsWing fastenersEngineeringMotorized vehicle
A motor-vehicle armrest has a base fixed on the vehicle and defining an axis and an armrest part pivotal on the base about the axis and having a first concave surface turned radially inward toward a second convex surface of the base. A wedge-shaped sprag is slidable between the surfaces generally angularly of the axis between a locking position in which the armrest part is arrested relative to the base and a release position in which pivoting of the armrest part with respect to the base is possible. The sprag has a first contact face cooperating with the first surface of the armrest part and a second nonparallel contact face cooperating with the second surface of the base. The sprag is biased into the locking position and can be moved into the release position.
Owner:GRAMMER AG
Microcapsule preparation for optimizing embedding
ActiveCN105560211AHigh embedding efficiencyImprove stabilityPharmaceutical non-active ingredientsFood shapingMonoglycerideFatty acid glycerol esters
The invention provides a microcapsule preparation for optimizing embedding. The microcapsule preparation adopts a wall material and wall material assistant mixture as a release material, the wall material assistant comprises fatty acid-based C12-22 saturated or unsaturated fatty acid glyceride, and the fatty acid glyceride is fatty acid monoglyceride or fatty acid diglyceride or a mixture containing fatty acid monoglyceride and fatty acid diglyceride according to an arbitrary ratio. The microcapsule preparation using the fatty acid glyceride has an excellent embedding promotion effect, can effectively reduce the surface core material content, and optimizes the active substance release behavior.
Owner:INNOBIO CORP LTD
Cefaclor film-controlled slow-release pellet capsule
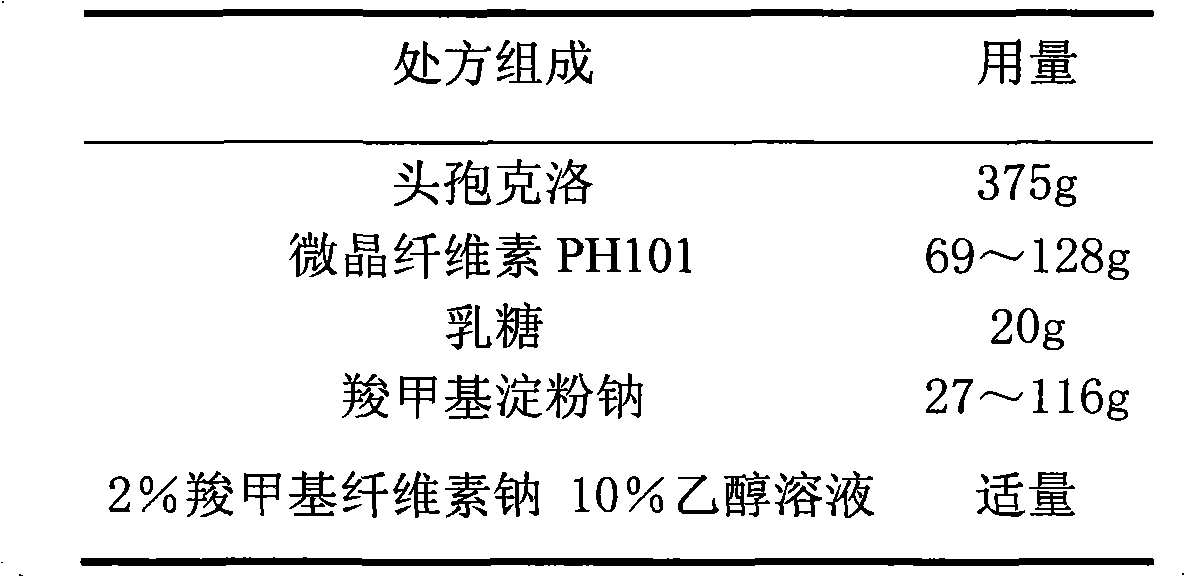
ActiveCN103211795AAccelerated agingReduce permeabilityAntibacterial agentsOrganic active ingredientsMedicineLactose
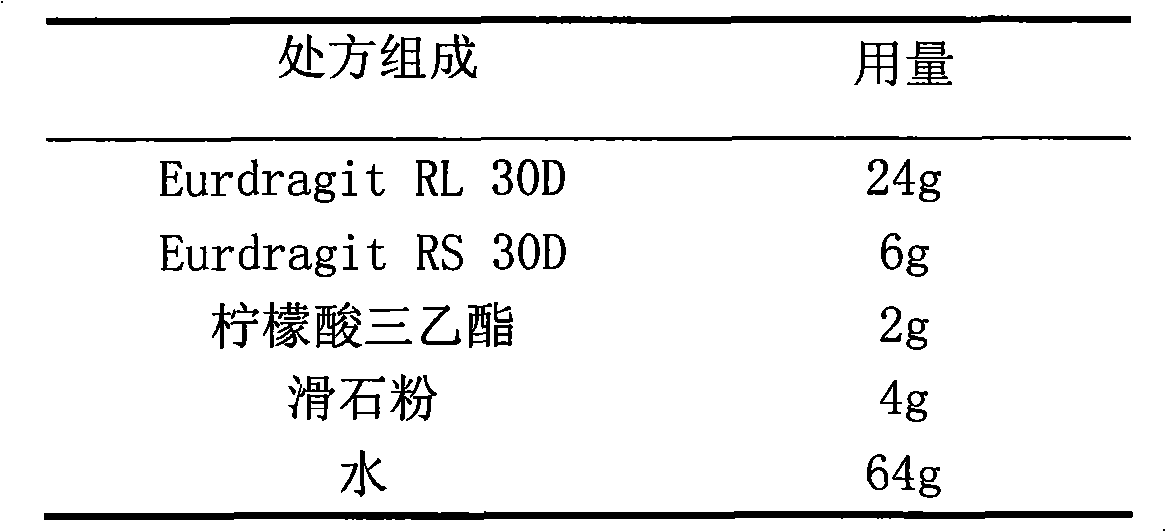
The invention relates to a cefaclor film-controlled slow-release pellet capsule. A slow-release film of the cefaclor film-controlled slow-release pellet utilizes a mixture of aqueous dispersion Eurdragit RL 30D and Eurdragit RS 30D as a film-formation material, wherein a weight ratio of Eurdragit RL 30D to Eurdragit RS 30D in the mixture is 4: 1. A pellet core of the cefaclor film-controlled slow-release pellet contains sodium carboxymethyl starch having high expansibility, and also contains pharmaceutically-acceptable common excipients for the slow-release pellet, wherein preferably, the excipients comprise microcrystalline cellulose and lactose, and the pellet core comprises 5 to 20wt% of the sodium carboxymethyl starch. The slow-release film comprises the mixture of Eurdragit RL 30D and Eurdragit RS 30D, triethyl citrate as a plasticizer, and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit RL 30D to Eurdragit RS 30D to triethyl citrate to talcum powder is 24: 6: 2: 4 and a film weight increasing ratio is in a range of 23 to 40%. The cefaclor film-controlled slow-release pellet comprises the pellet core containing sodium carboxymethyl starch having high water expansibility and thus after absorbing water, the cefaclor film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the cefaclor film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
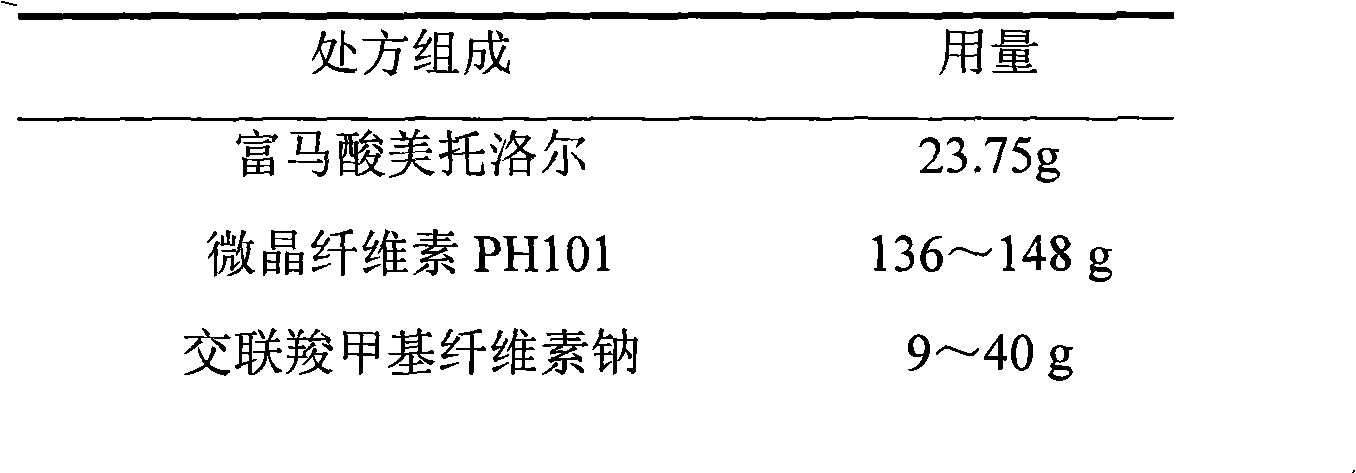
Metoprolol fumarate film-controlled slow-release pellet capsule
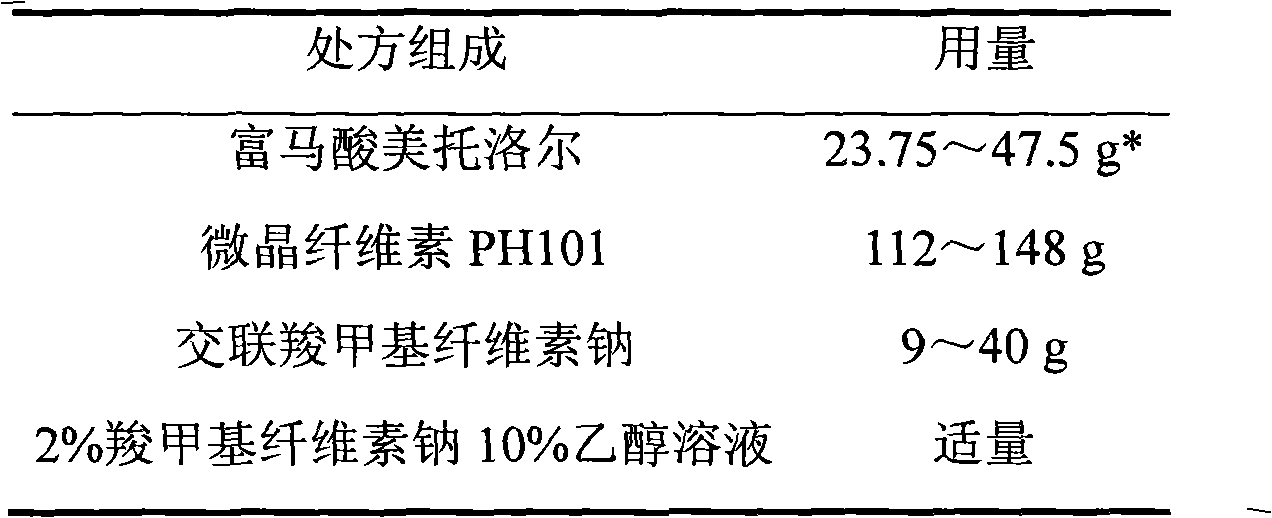
ActiveCN103211792AAccelerated agingReduce permeabilityOrganic active ingredientsNervous disorderMetoprolol FumarateMedicine
The invention relates to a metoprolol fumarate film-controlled slow-release pellet capsule. A slow-release film of the metoprolol fumarate film-controlled slow-release pellet utilizes Eurdragit RS 30D as a film-formation material. A pellet core of the metoprolol fumarate film-controlled slow-release pellet contains croscarmellose sodium having high expansibility, and also contains a pharmaceutically-acceptable common excipient for the slow-release pellet, wherein preferably, the excipient comprises microcrystalline cellulose, and the pellet core comprises 5 to 20wt% of croscarmellose sodium. The slow-release film comprises Eurdragit RS 30D, triethyl citrate as a plasticizer, and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit RS 30D to triethyl citrate to talcum powder is 30: 3: 4 and a film weight increasing ratio is in a range of 18 to 33%. The metoprolol fumarate film-controlled slow-release pellet comprises the pellet core containing croscarmellose sodium having high water expansibility and thus after absorbing water, the metoprolol fumarate film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the metoprolol fumarate film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
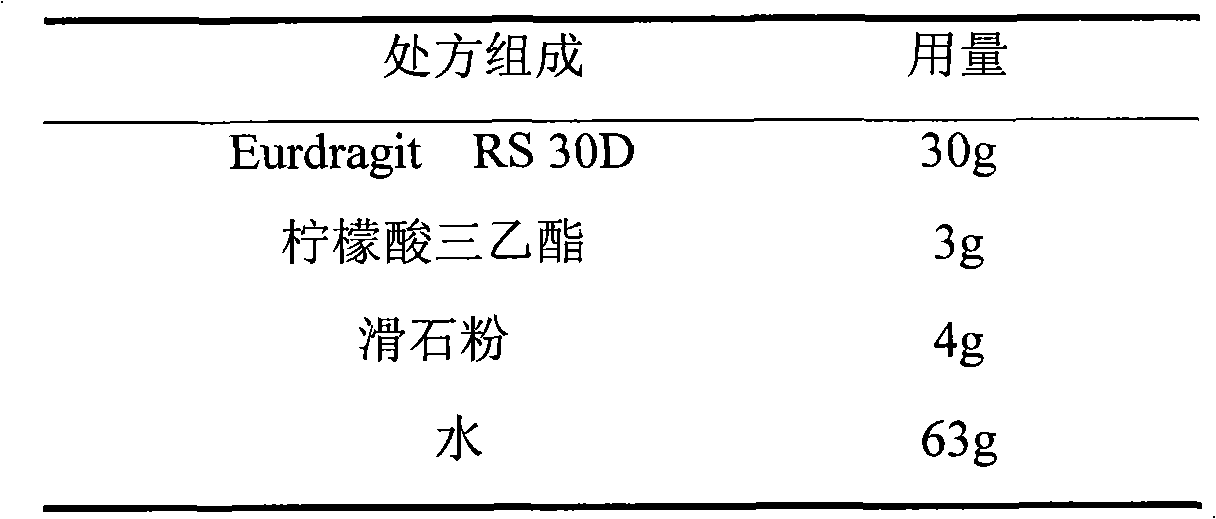
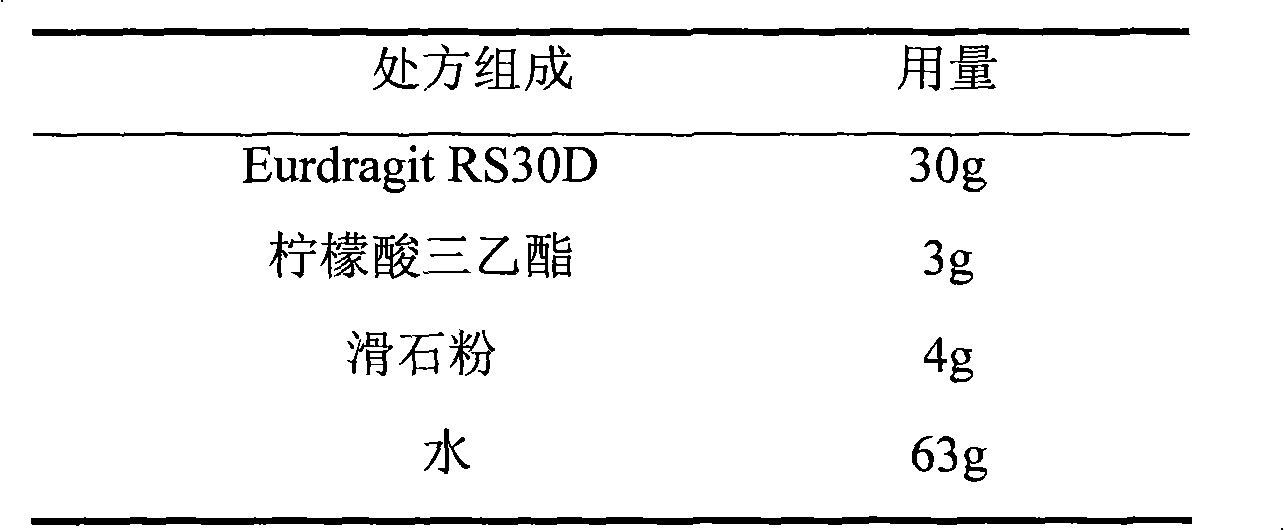
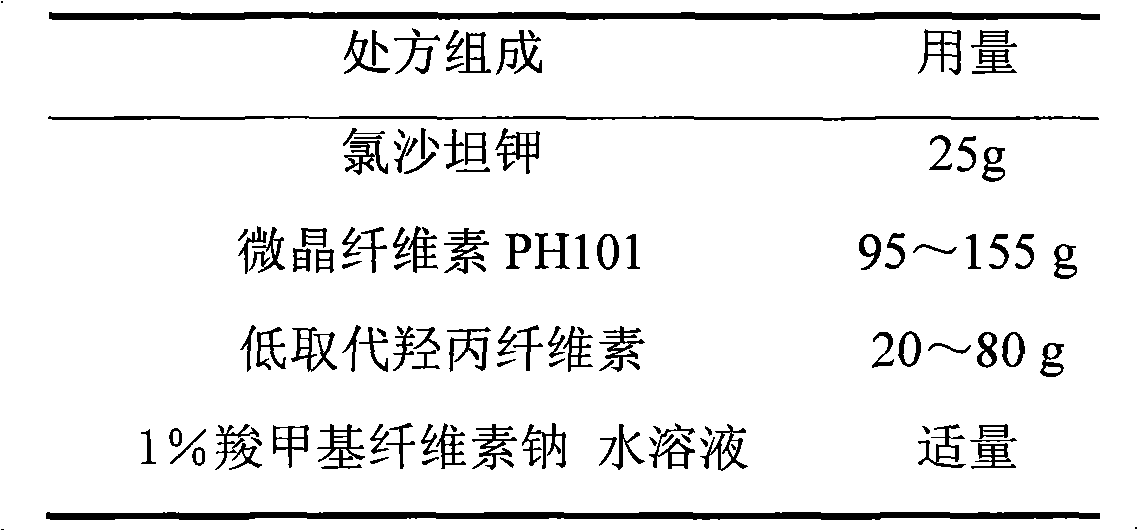
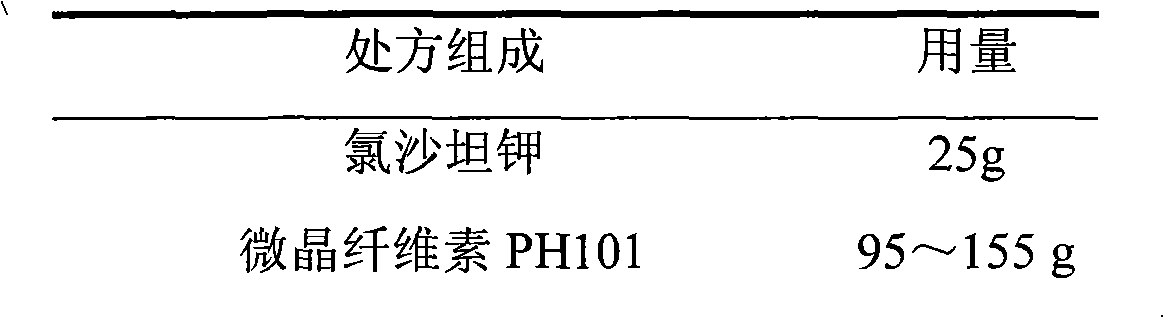
Losartan potassium membrane controlled-release pellet capsule
InactiveCN103211798AReduce permeabilityImprove permeabilityOrganic active ingredientsPharmaceutical delivery mechanismControlled releaseMedicine
The invention relates to a losartan potassium membrane controlled-release pellet capsule. The controlled-release film of the pellet adopts Eurdragit NE 30D as a film forming material, the core of the controlled-release pellet contains high-expansibility low-substituted hydroxypropylcellulose and a pharmaceutically-acceptable excipient commonly used for controlled-release pellets, and optimally, the excipient is microcrystalline cellulose, wherein the weight percentage of the low-substituted hydroxypropylcellulose in the core of the controlled-release pellet is 10-40%. The controlled-release film of the controlled-release pellet includes the Eurdragit NE 30D and an anti-adherent talcum powder, the optimal ratio of the Eurdragit NE 30D to the talcum powder is 30:6, and the optimal coating weight gain is 19-36%. The core will obviously expand after absorbing water because of the containment of the low-substituted hydroxypropylcellulose highly expanding after contacting with water, so the controlled-release film is stretched, the thickness of the film is thinned, the apertures of water-pervious micro-pores are increased, the permeability is good, and the permeability decrease caused by film ageing is compensated, thereby the middle and later stage release speed is basically constant, the last stage residue is small, and a stable release performance is always maintained prior to the expiration date.
Owner:内蒙古天衡医院管理有限公司
Preparation method of primary-taste modified rice fresh keeping agent material
InactiveCN109007017AMitigation release efficiencyGood slow releaseEdible seed preservationFood additiveHusk
The invention relates to a preparation method of a primary-taste modified rice fresh keeping agent material, and belongs to the technical field of food additive materials. The technical scheme adoptedby the invention lies in that the primary-taste modified rice fresh keeping agent material is prepared by using rice husks as raw materials; the rice husks contain rich antioxidation peptide, the rice husks are subjected to enzymolysis and collection, the rice husks are coated with essential oil, and a microcapsule structure is formed; the microcapsule structure can effectively relieve the releasing efficiency of the essential oil, so that the essential oil material has excellent slow release performance; in the storage course, due to massive release of the essential oil at the initial stage,after the essential oil disappears at the later stage, due to the effects of the antioxidation peptide, the fresh keeping agent material continues forming antioxygenic properties, so that the antioxidation durability of the rice fresh keeping agent material can be effectively improved; an adsorbent material is prepared by using the rice husks as raw materials, so that when the adsorbent materialis added to fresh keeping agent microspheres, the adsorbent material can effectively adsorb surplus essential oil smell; and besides, excellent pore canal structure of the fresh keeping agent materialcan be maintained, and the releasing properties and the durability of the fresh keeping agent material can be stable.
Owner:FOSHAN WANYANG BIOLOGICAL TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com