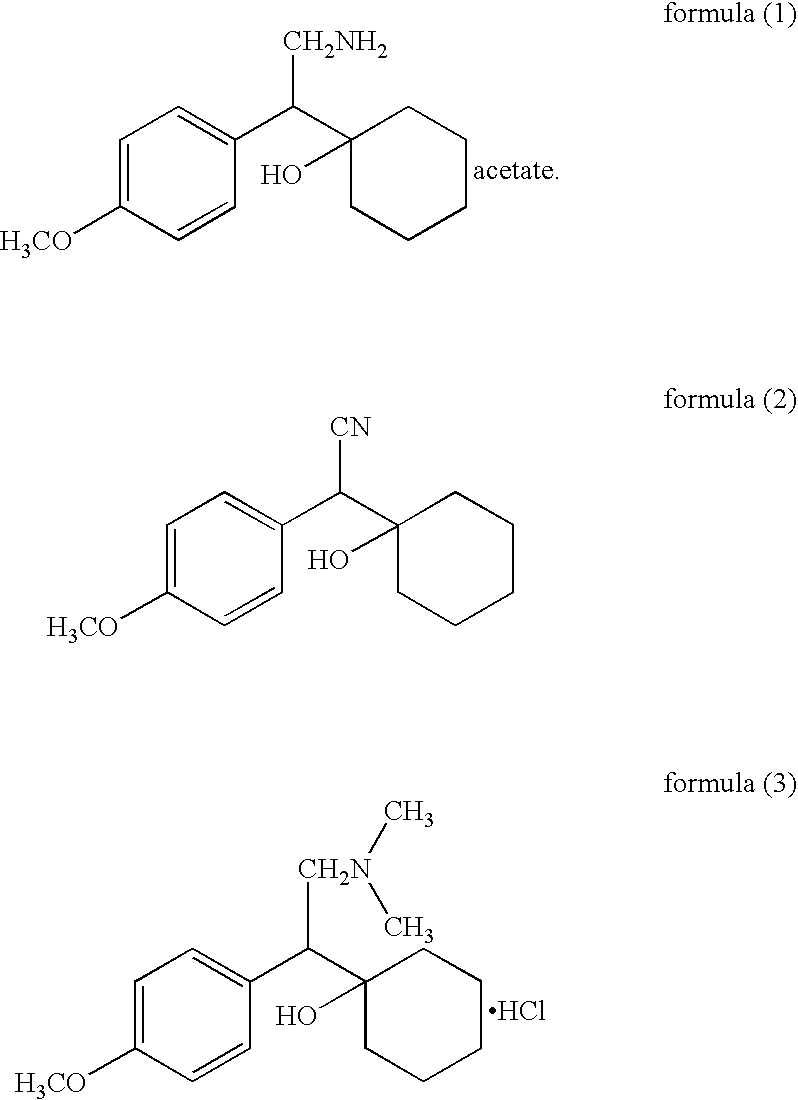
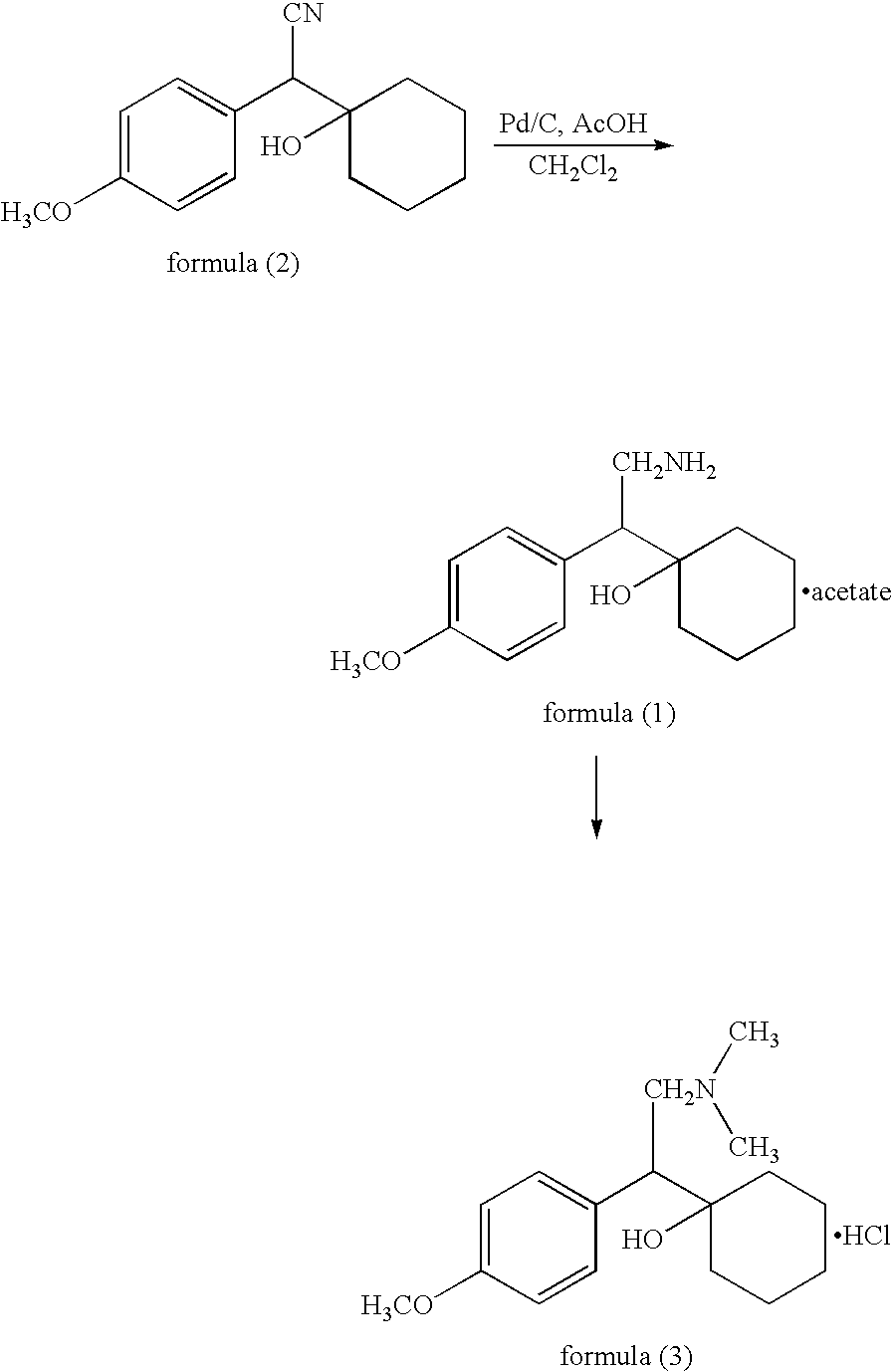
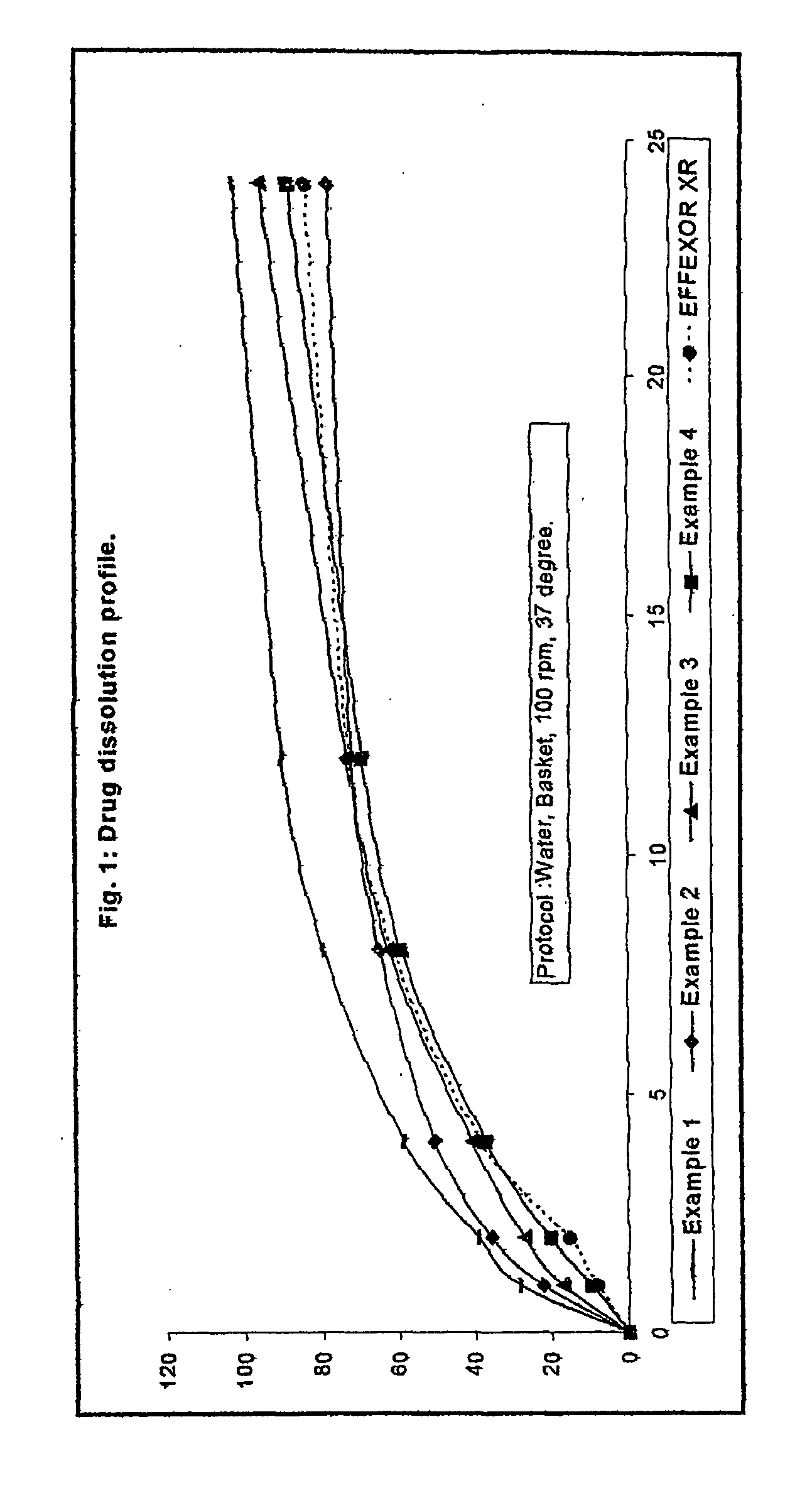
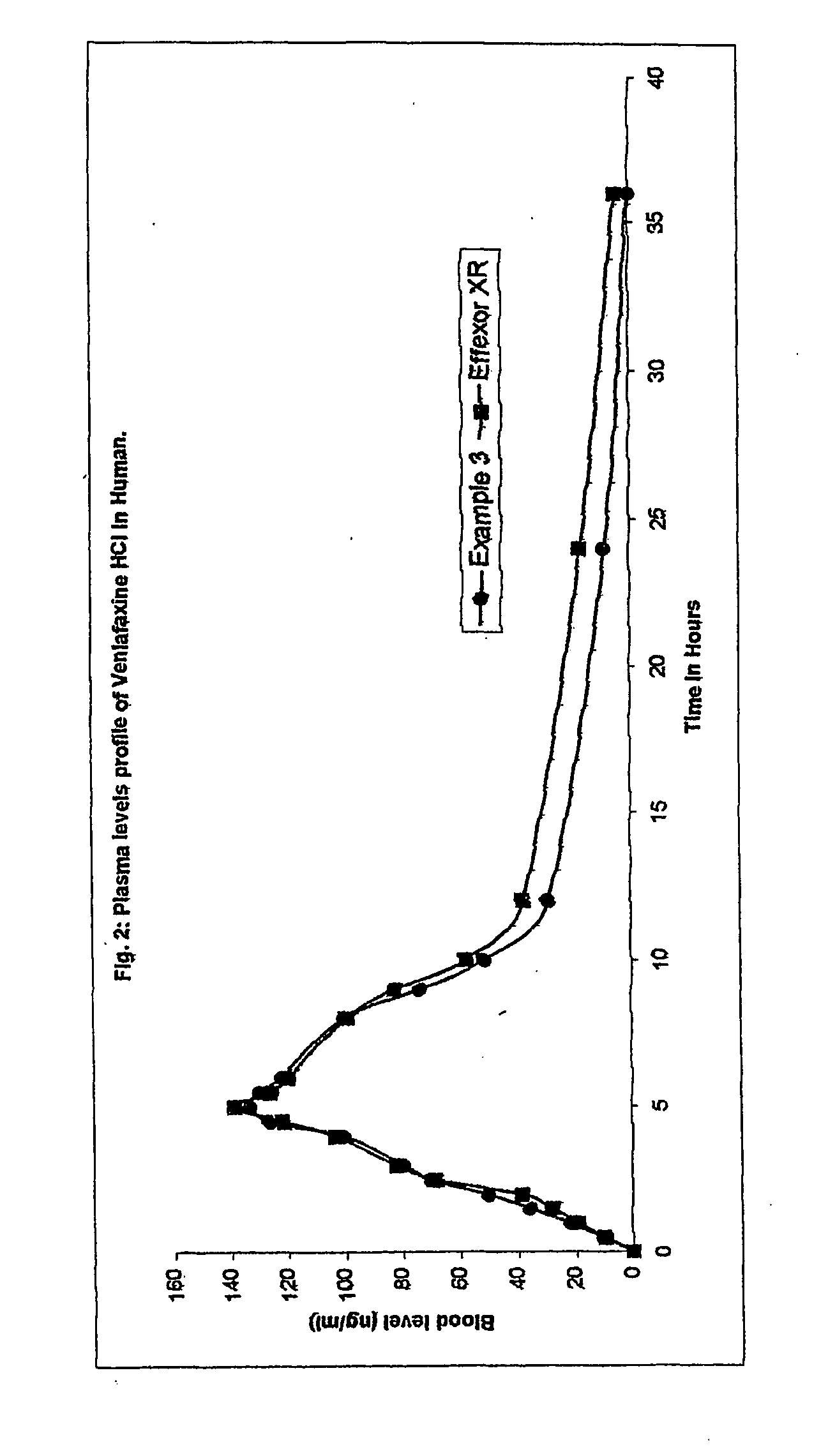
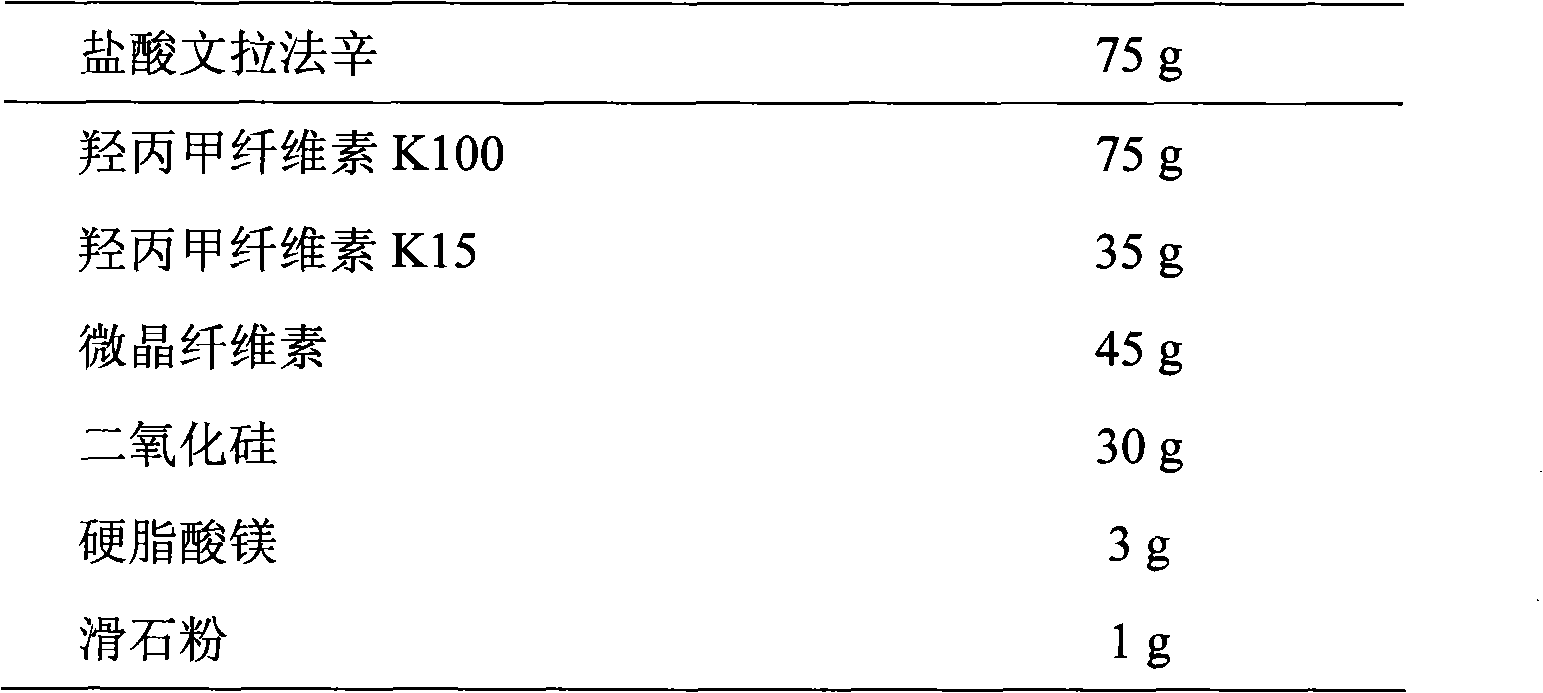
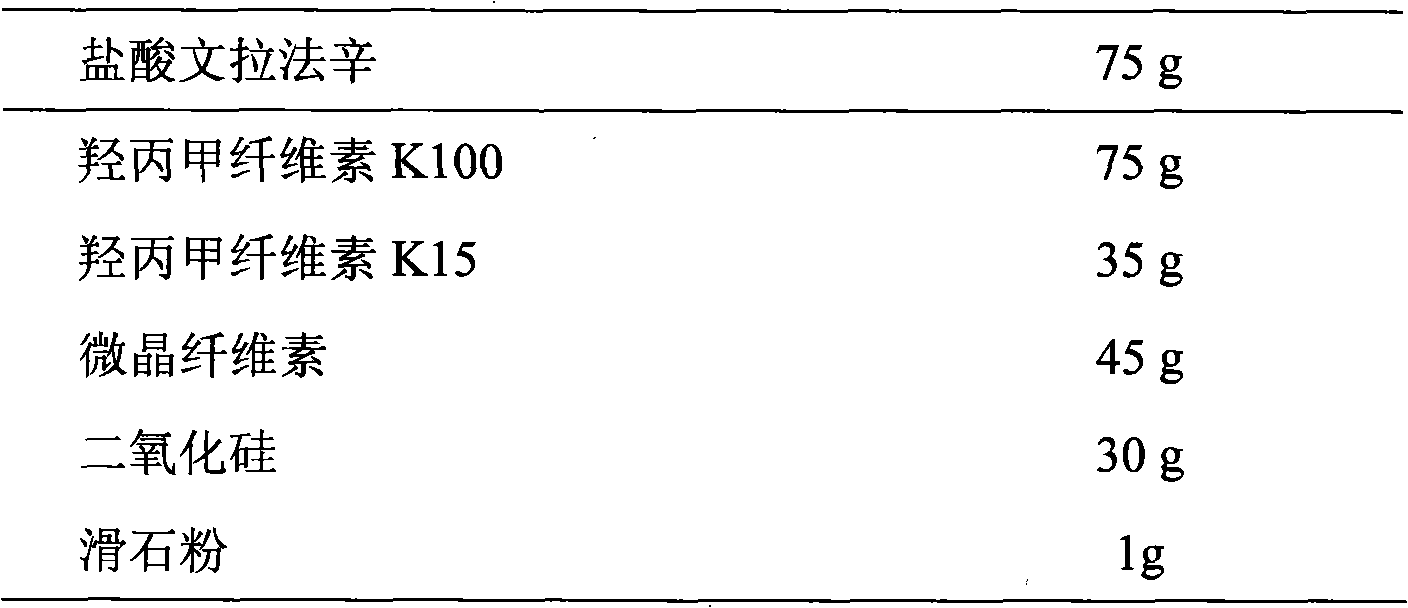
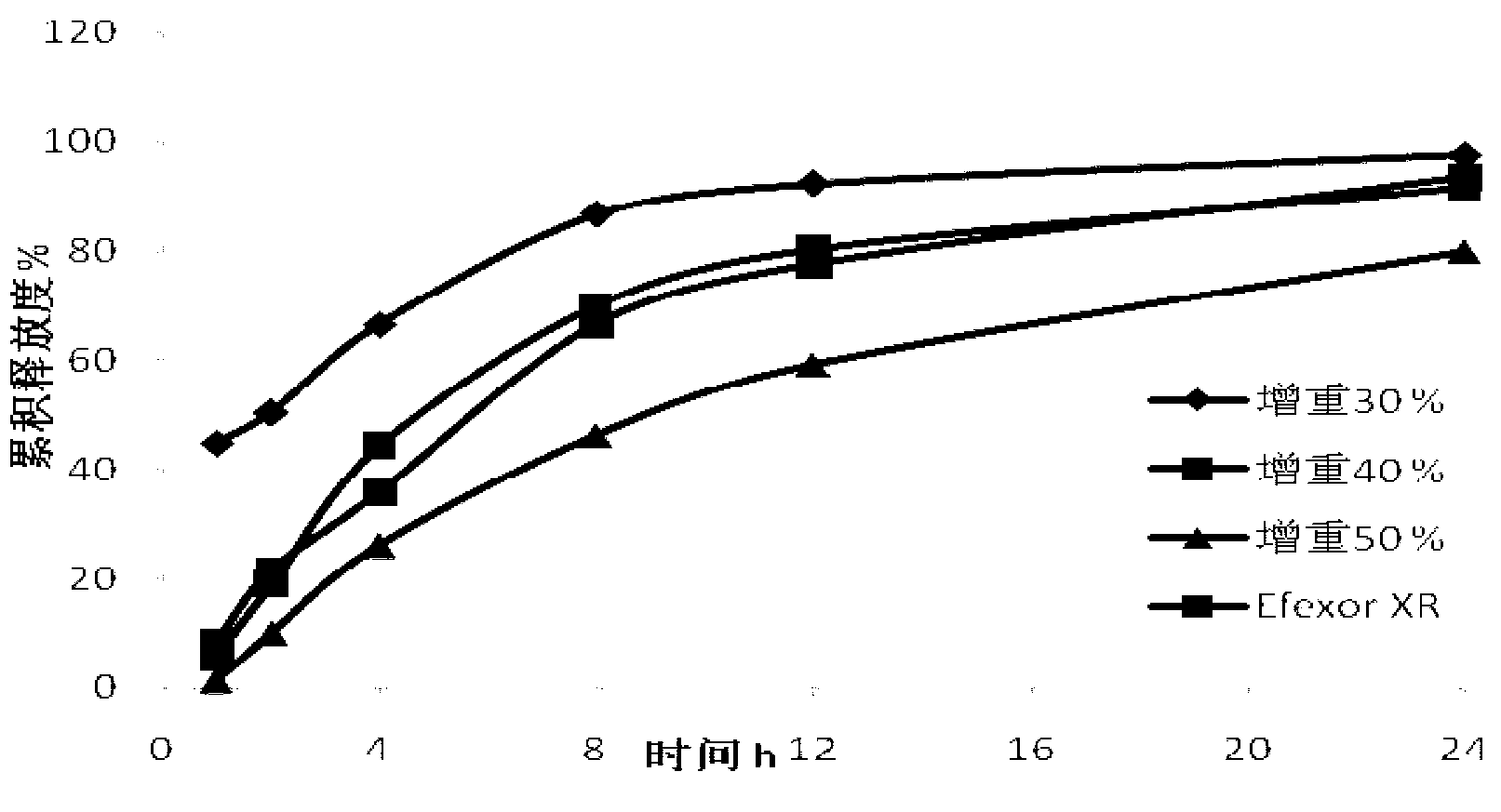
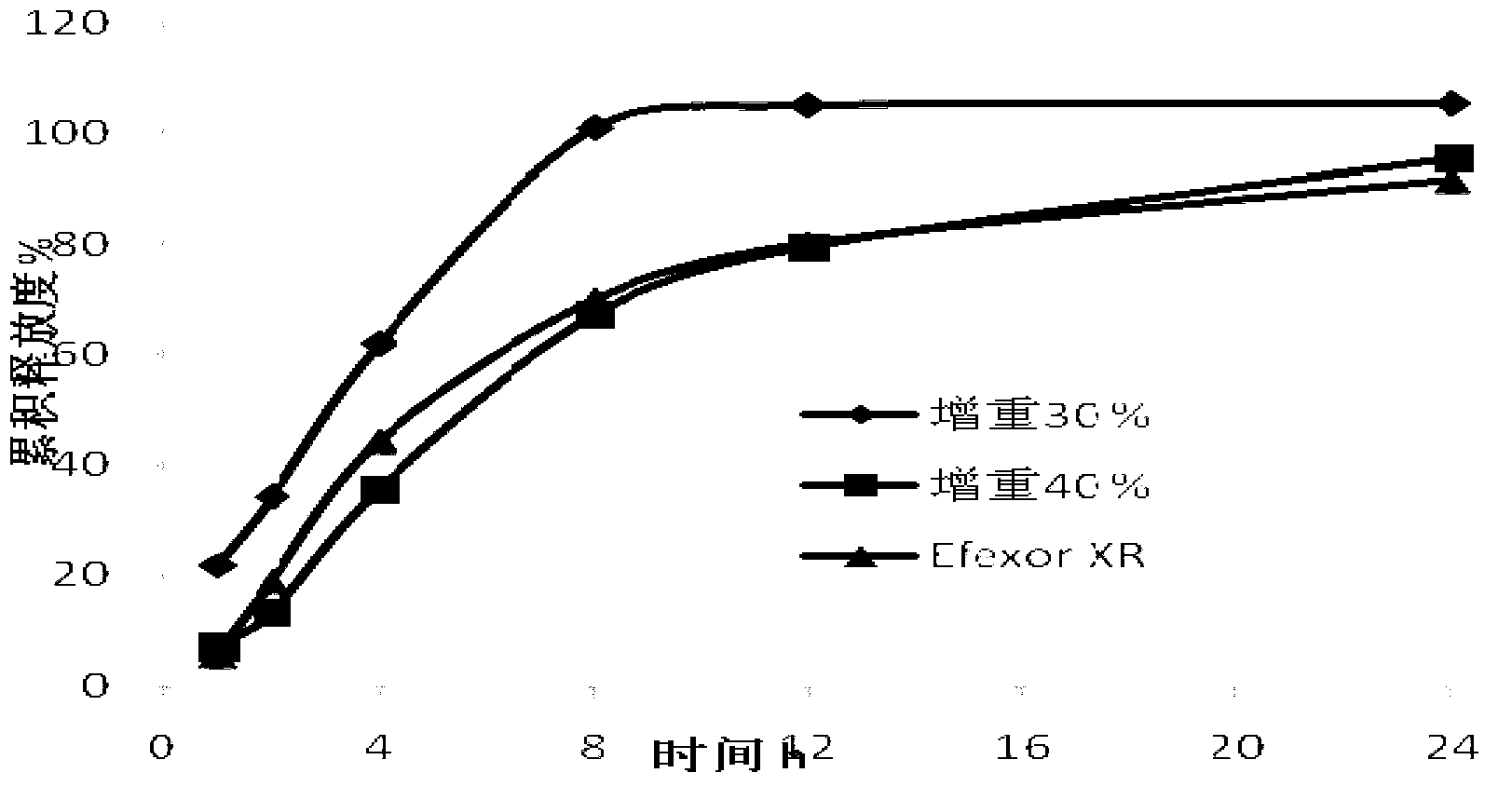
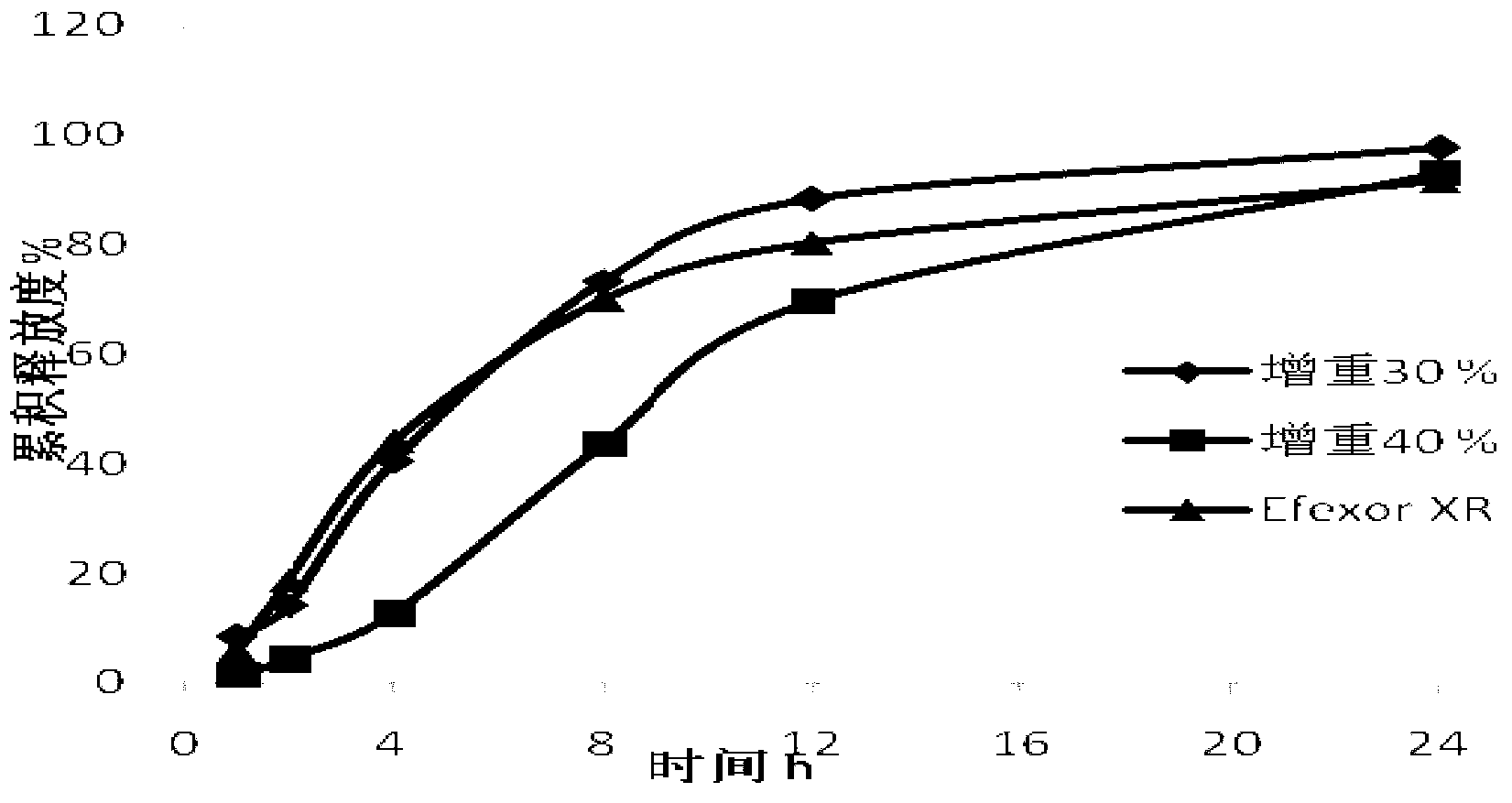
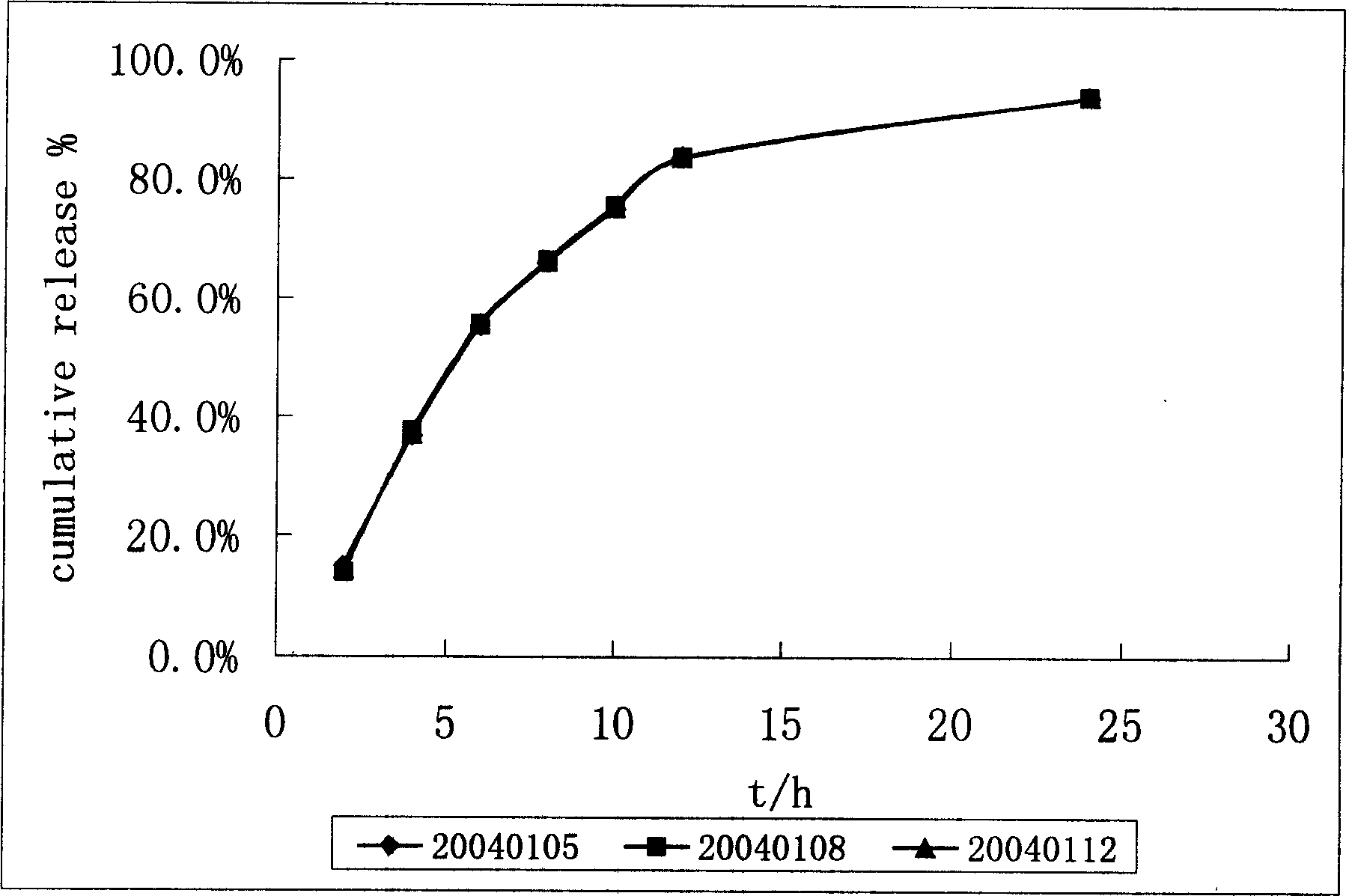
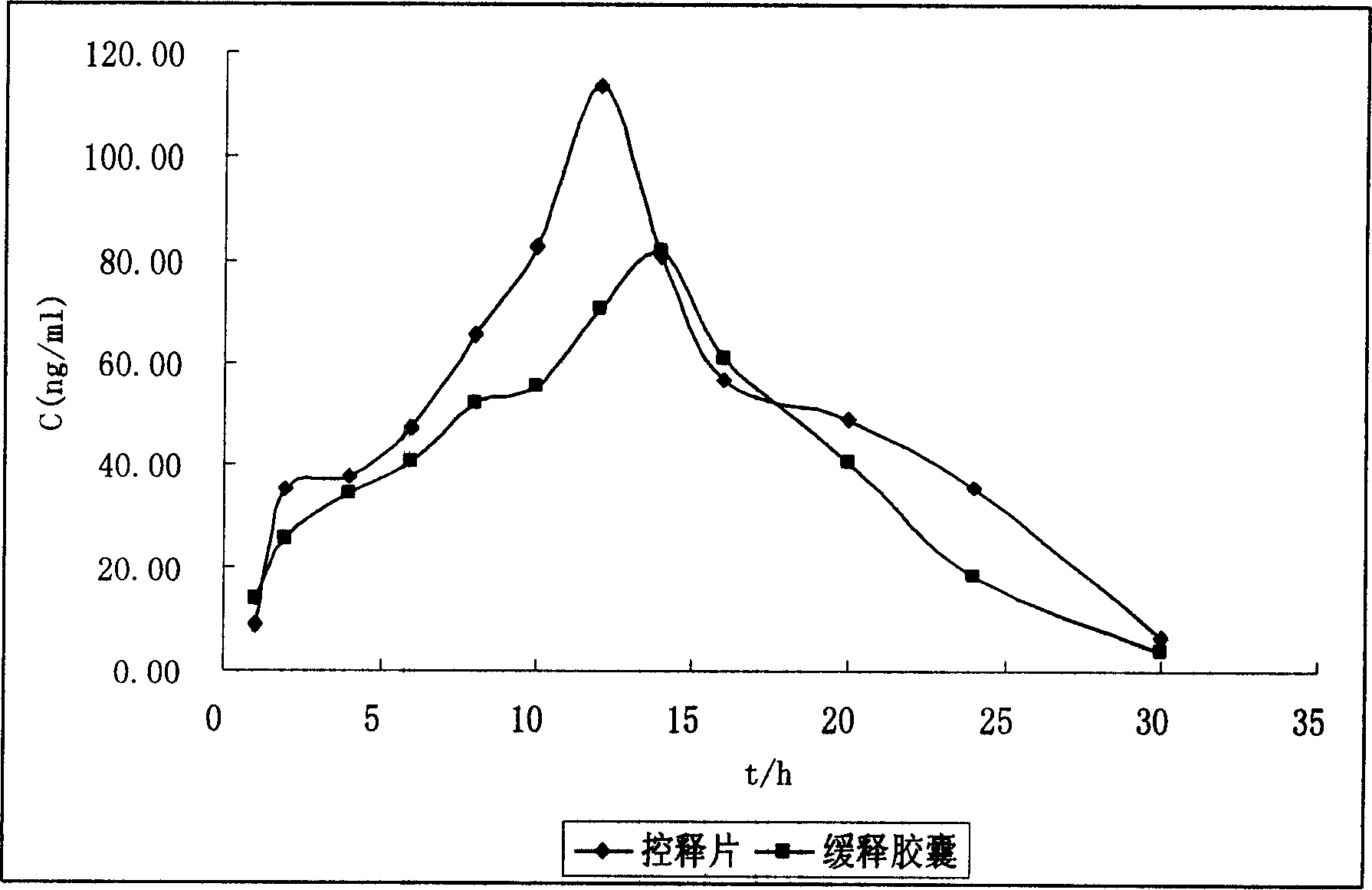
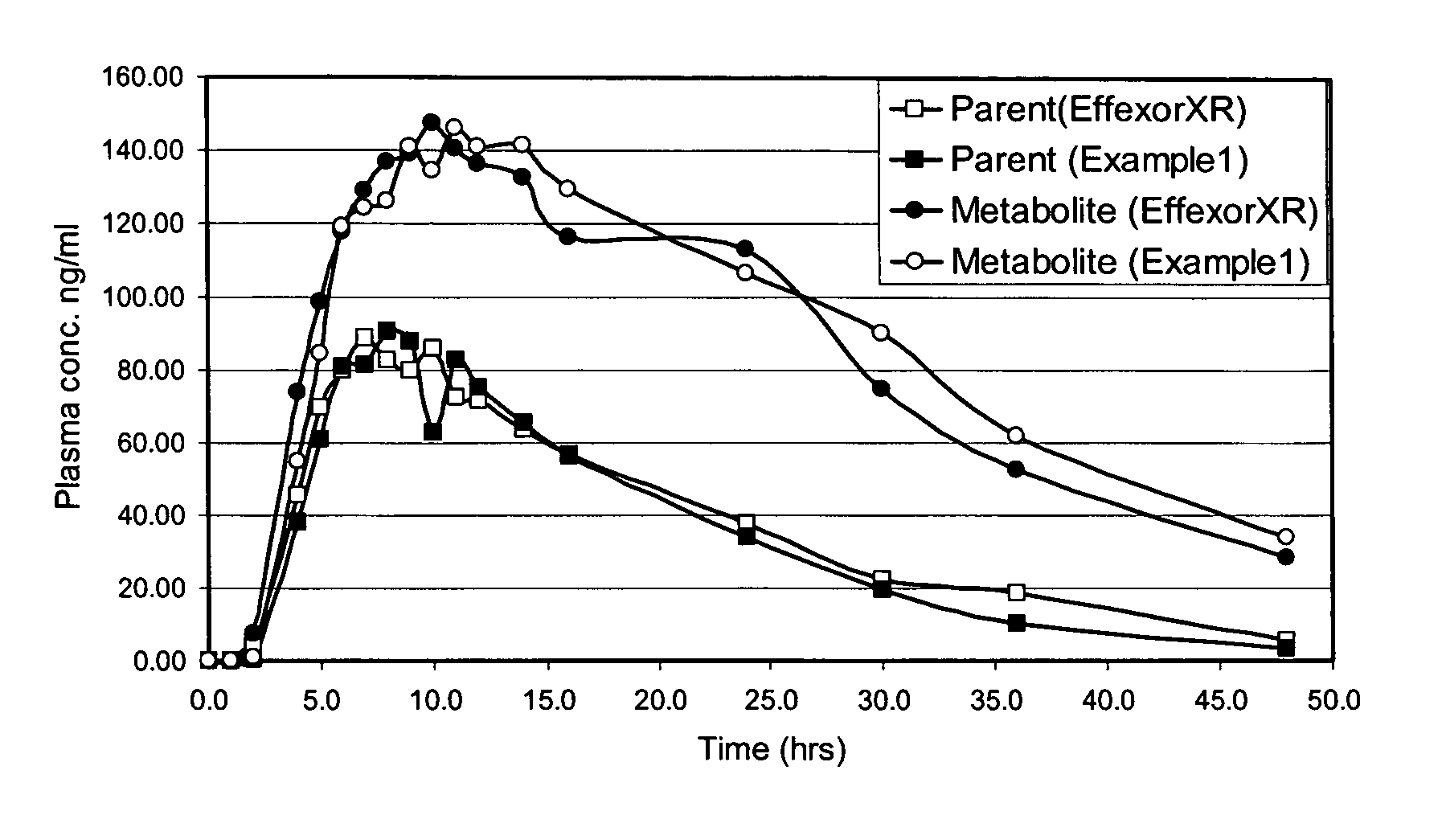
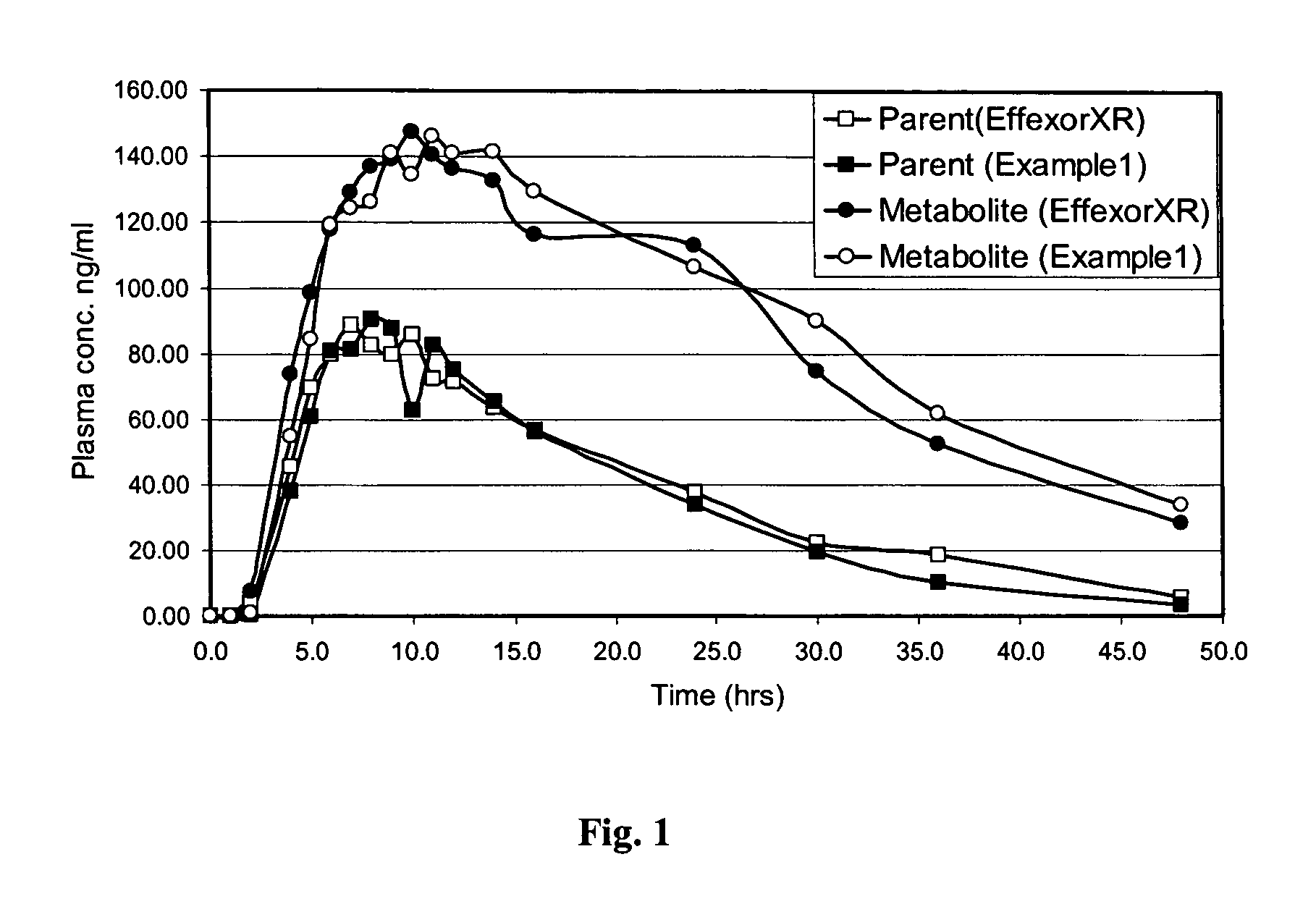
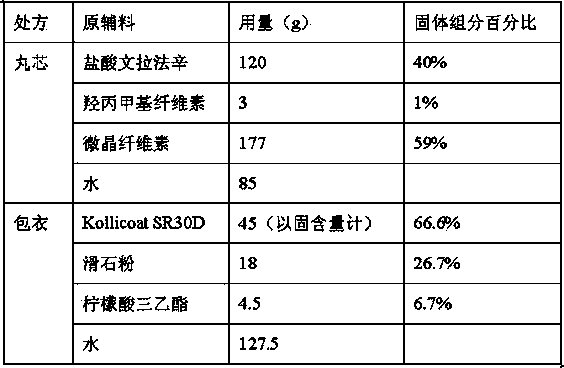
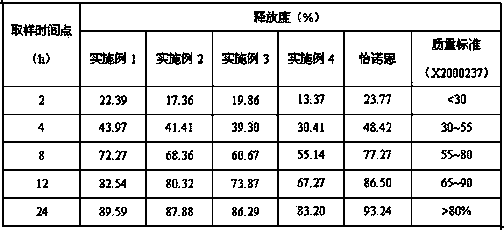
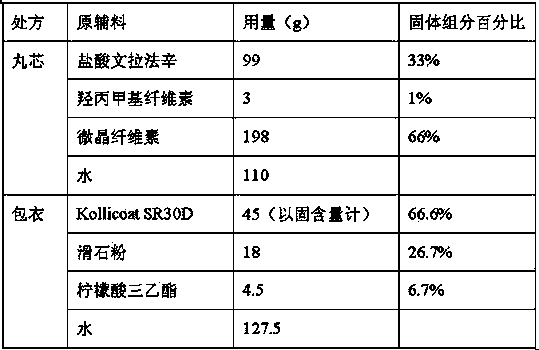
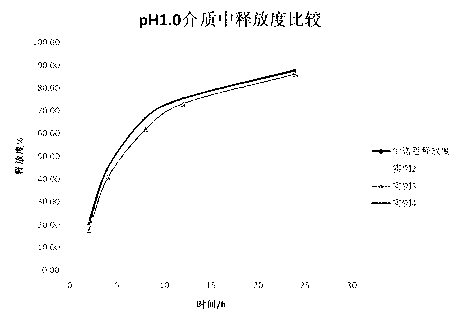
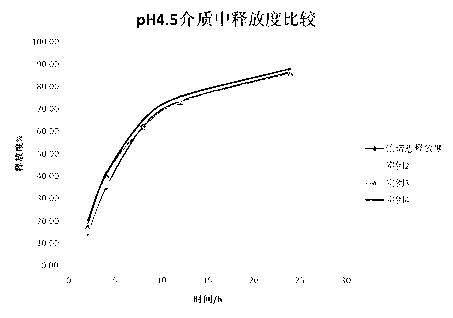
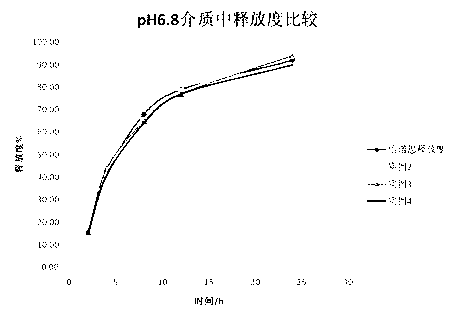
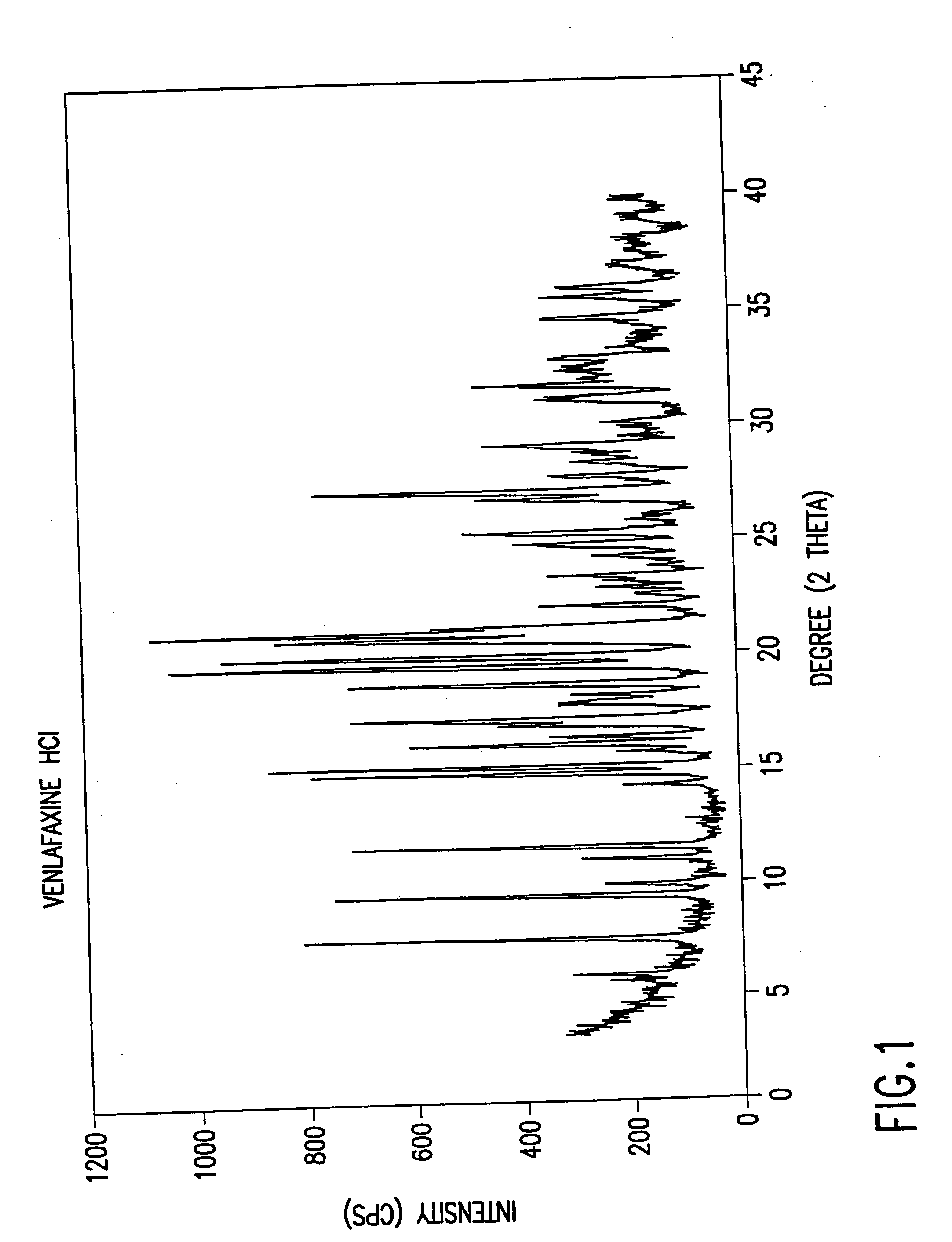
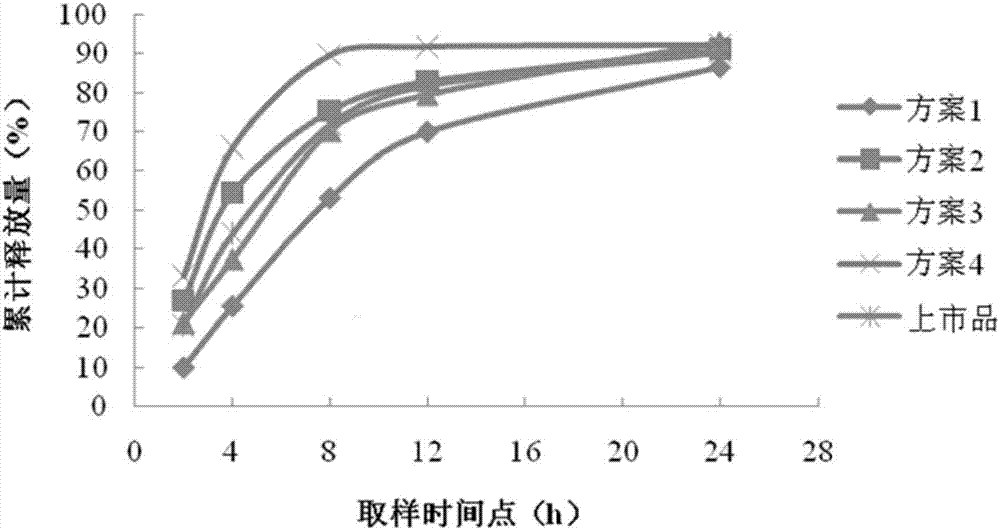
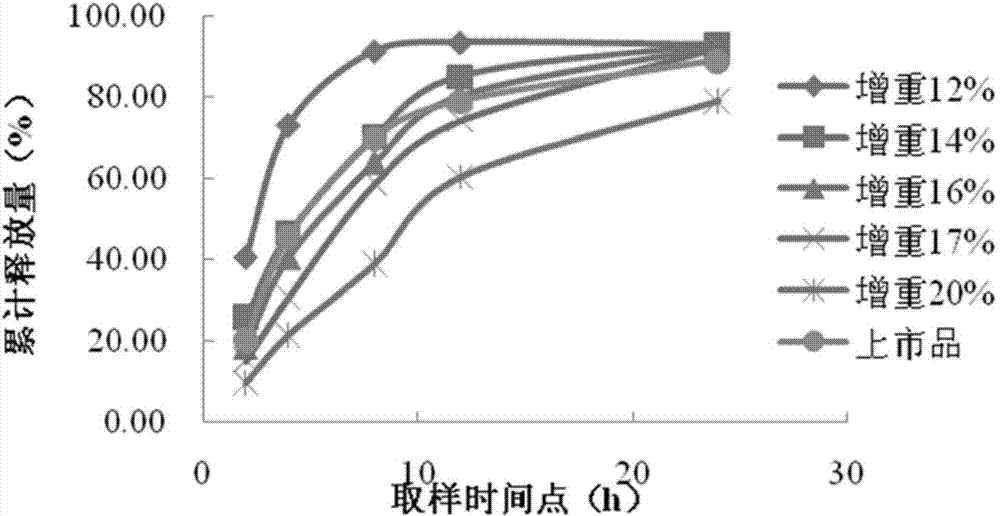
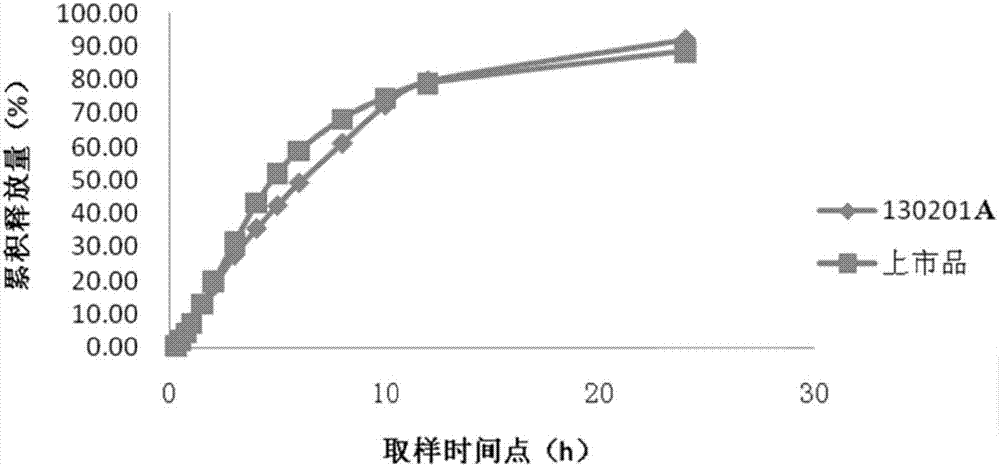
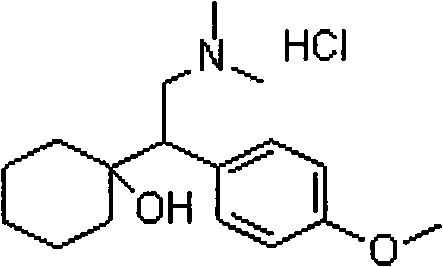
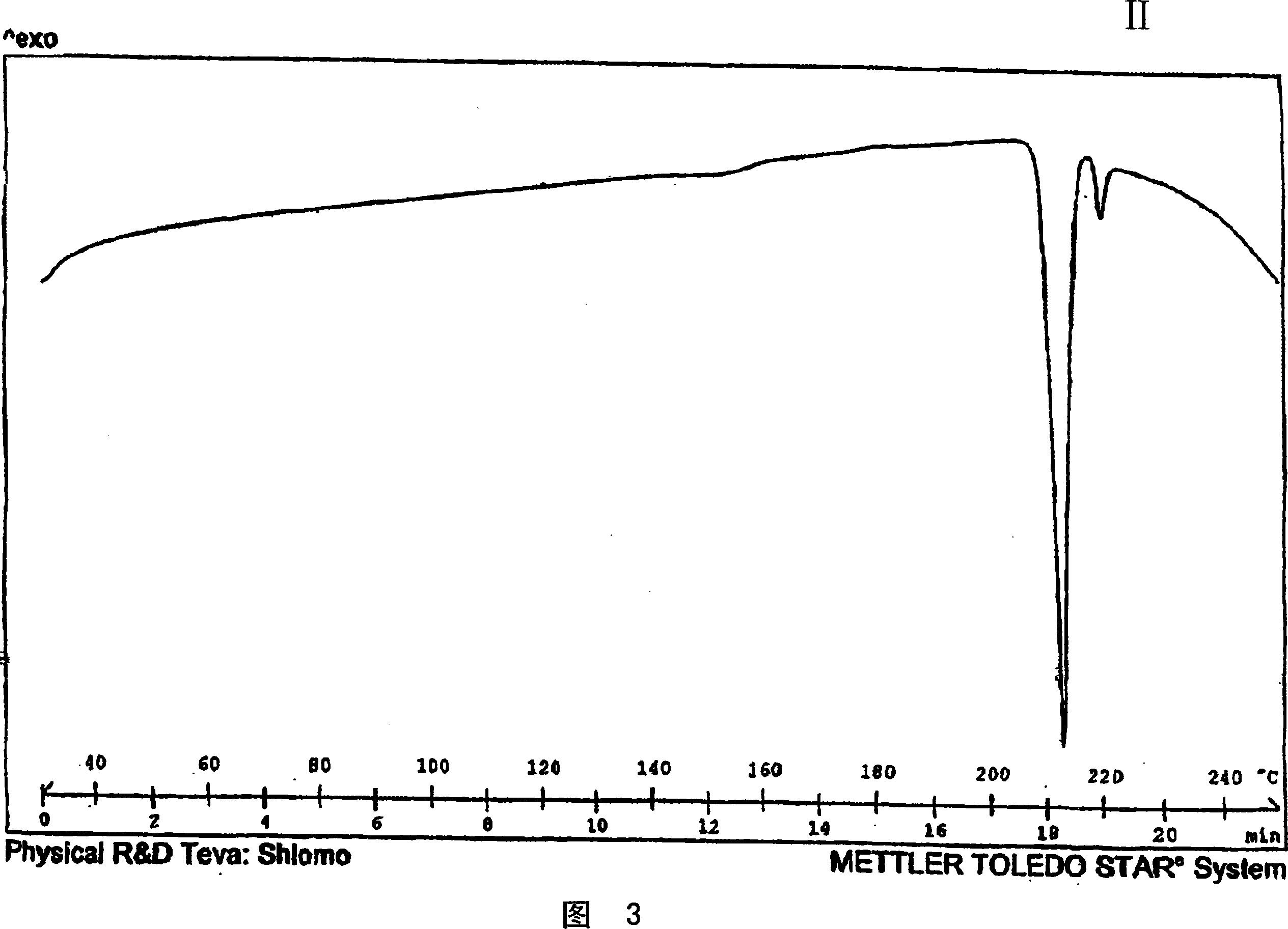
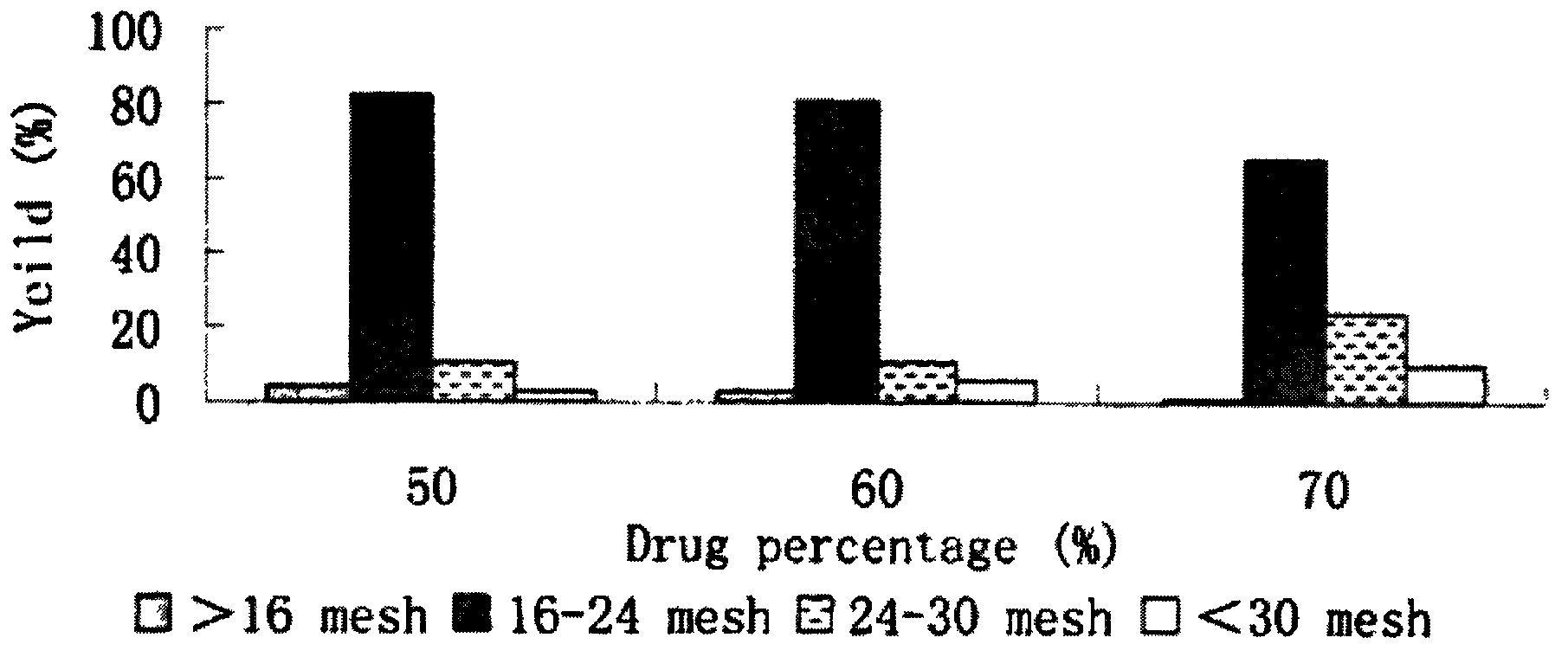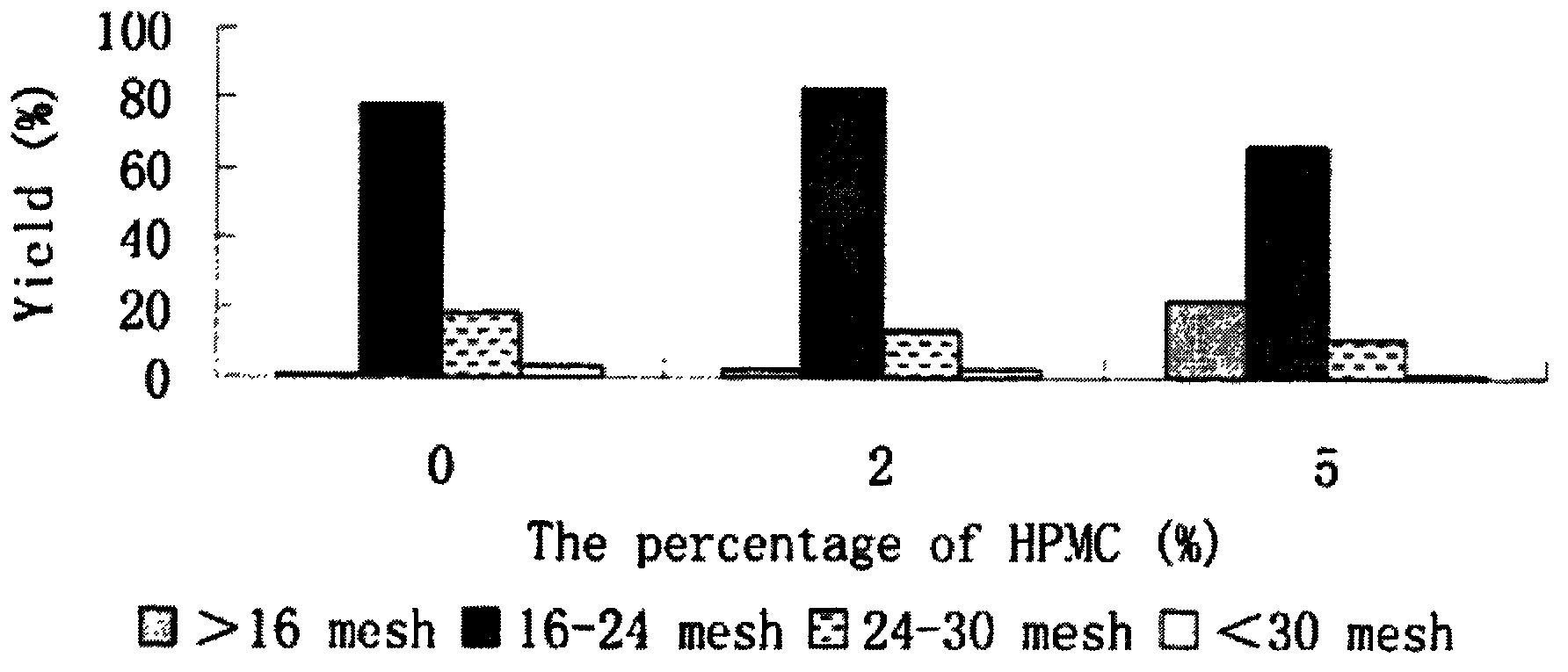Patents
Literature
72 results about "Venlafaxine Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
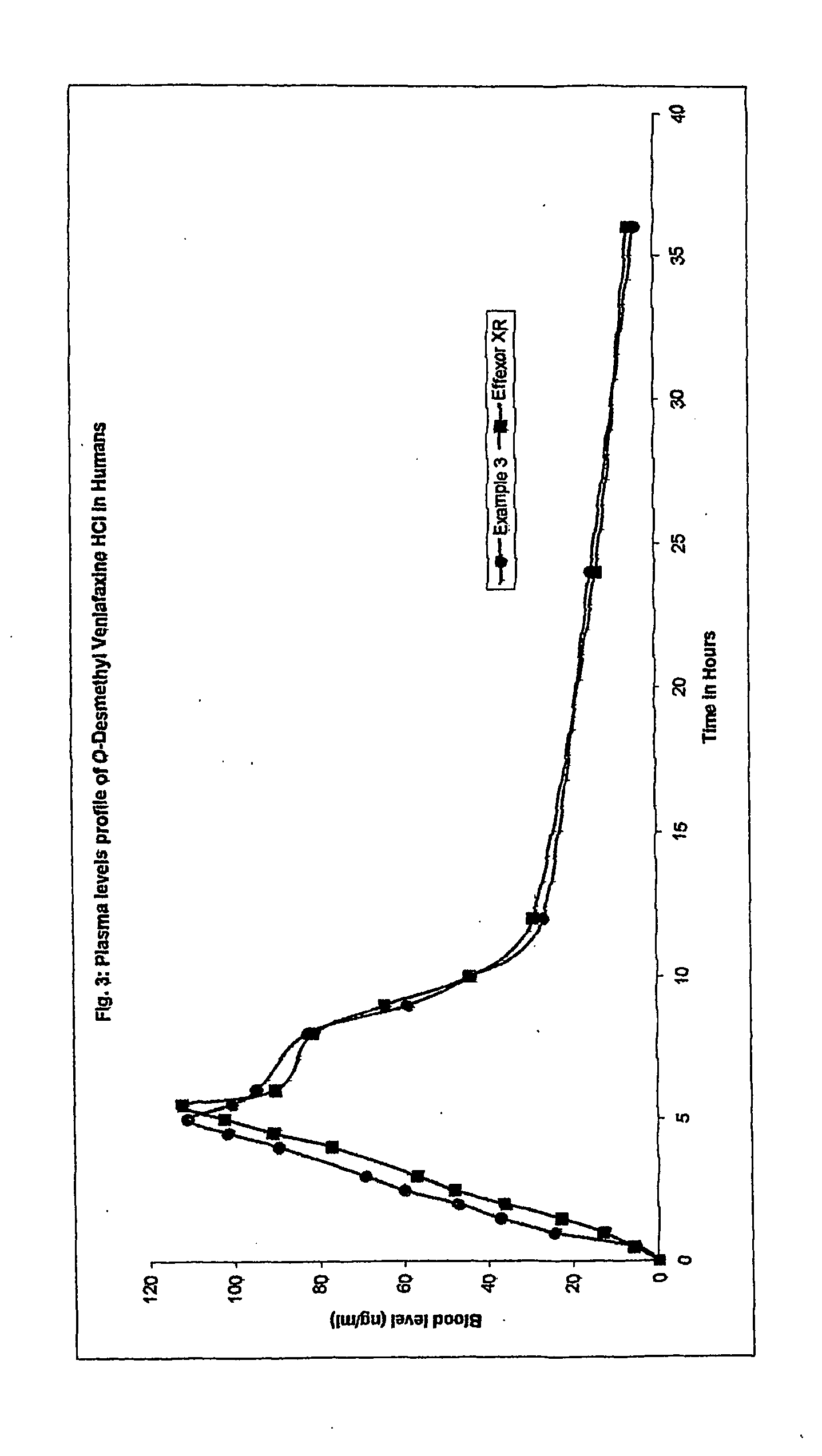
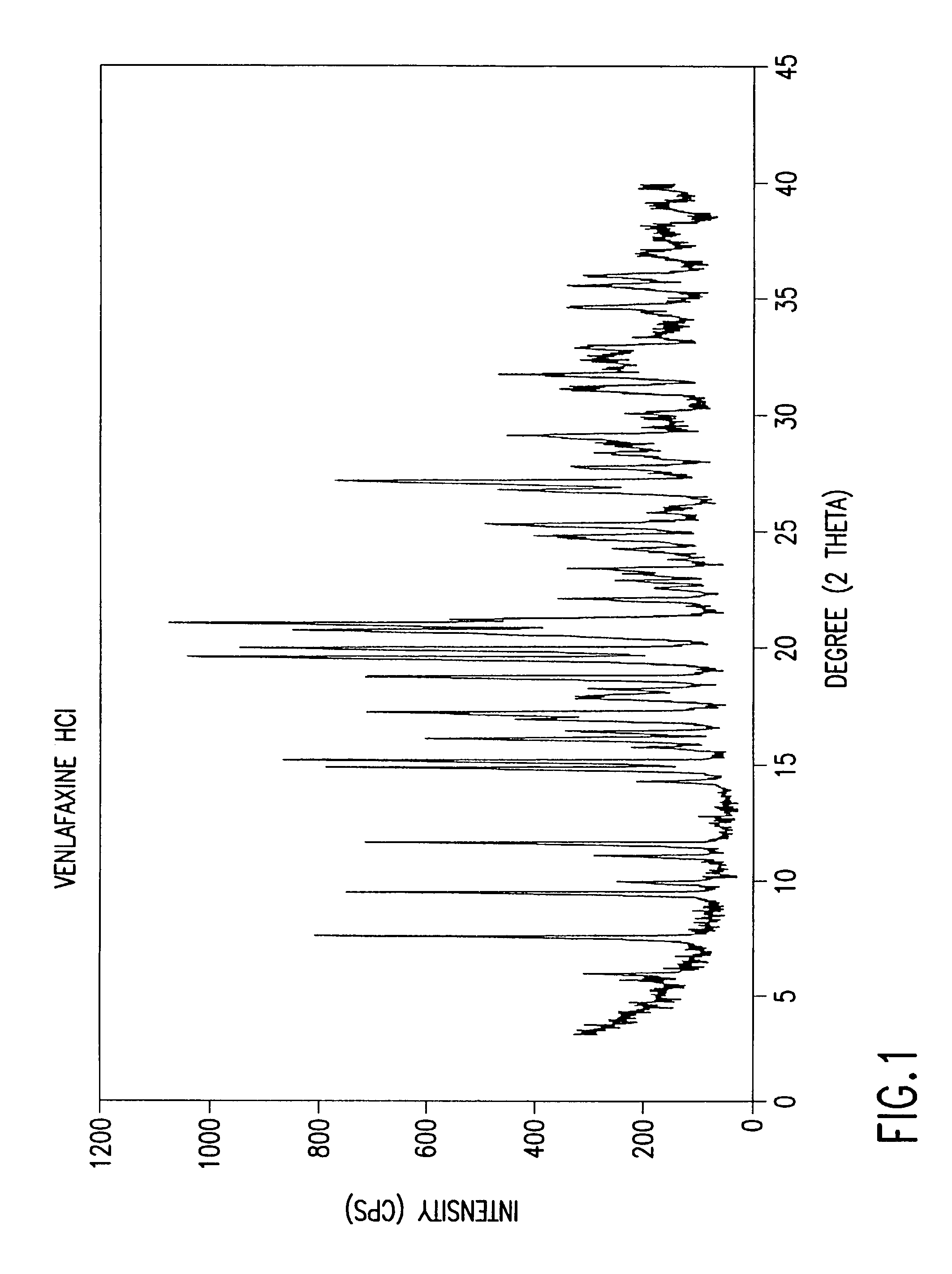
A synthetic ethyl-cyclohexanol derivative used for the treatment of depression, Venlafaxine Hydrochloride is metabolized to O-desmethylvenlafaxine, which potentiates CNS activity. Both venlafaxine and its active metabolite inhibit neuronal reuptake of norepinephrine, dopamine, and serotonin. (NCI04)
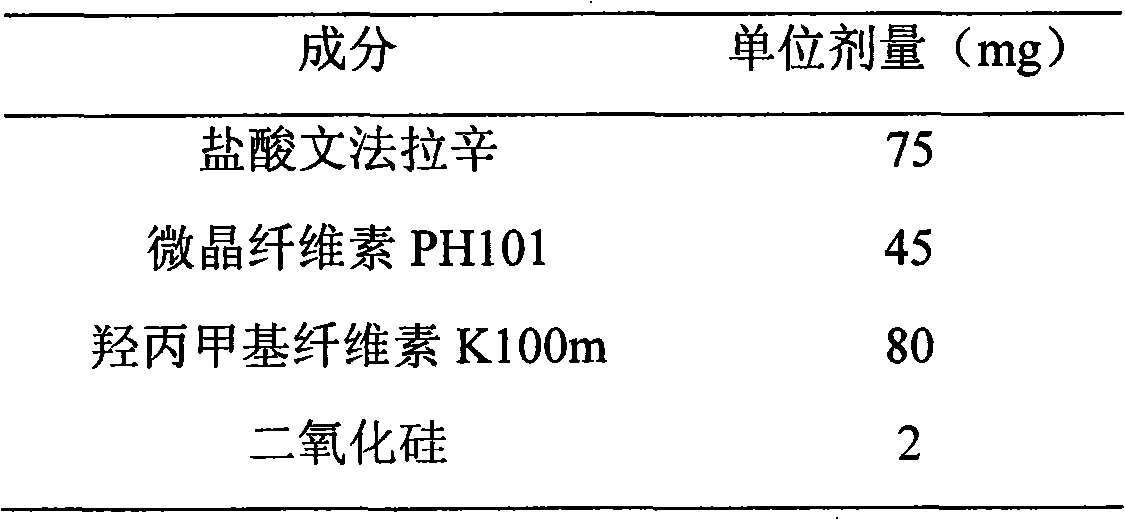
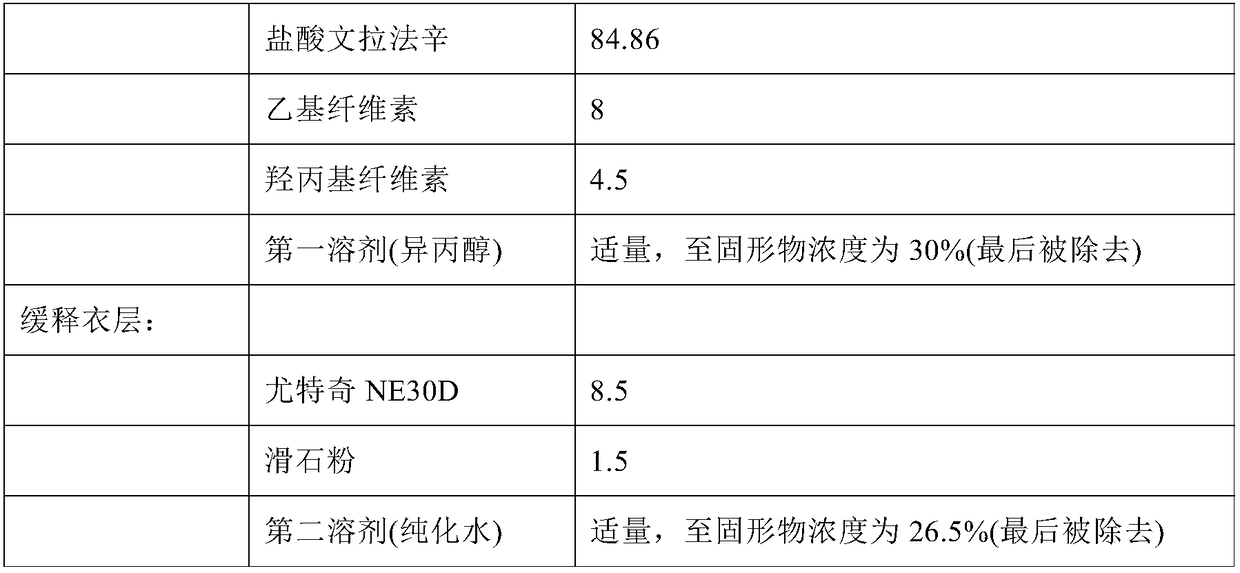
Extended release formulation of venlafaxine hydrochloride
InactiveUS20050169985A1Facilitated releaseReduce processing timeOrganic active ingredientsNervous disorderMini tabletsPharmaceutical formulation
The present invention relates to an extended release once daily pharmaceutical formulation comprising venlafaxine hydrochloride and pharmaceutically acceptable excipients. More particularly, the present invention relates to an extended release composition in the form of mini-tablets which are incorporated in hard gelatin capsules.
Owner:ALEMBIC LTD
Crystalline venlafaxine base and novel polymorphs of venlafaxine hydrochloride, processes for preparing thereof
InactiveUS6924393B2Organic compound preparationAmino-hyroxy compound preparationVenlafaxine HydrochlorideAcetone
The present invention relates to novel essentially pure venlafaxine and the process of preparation thereof. The present invention also relates to novel solvate forms of venlafaxine hydrochloride and the process of preparation thereof. Furthermore, the present invention provides a novel process for preparing venlafaxine hydrochloride from venlafaxine; the process comprises the steps of: i) preparing a mixture of venlafaxine with acetone; and ii) exposing the mixture in gaseous hydrochloric acid.
Owner:TEVA PHARMA IND LTD
Process for the preparation of phenethylamine derivative, an intermediate of Venlafaxine hydrochloride
InactiveUS20050033088A1High yieldOrganic compound preparationCarboxylic acid amides preparationPalladium on carbonPhenethylamine derivative
The present invention relates to an improved process for the preparation of phenethylamine derivatives or salts by hydrogenation of phenylacetonitriles in the presence of heterogeneous palladium on carbon catalyst.
Owner:DR REDDYS LAB LTD +1
Extended release tablet formulations of venlafaxine
This invention relates to an extended release tableted dosage formulation of the antidepressant venlafaxine hydrochloride or an optical form thereof having improved bioavailability.
Owner:WYETH LLC
Extended release osmo-microsealed formulation
InactiveUS20070110808A1Good water solubilityEffective controlBiocideOrganic active ingredientsParticulatesHydrophilic polymers
The extended release osmo-microsealed formulation includes three controlled release systems associated in series. First, there is an inner solid particulate phase containing Venlafaxine Hydrochloride (Active), and one or more hydrophobic polymers, one or more diluents required to increase the bulk one or more osmogen (agents which can generate osmotic pressure across the hydrophobic coating) and one or more binder polymers essentially to provide strength / hardness to the particle. Second, there is an outer solid continuous phase including one or more hydrophilic polymers, that is further compressed into a tablet. Third, there is an optional functional coat surrounding the tablet. The process / method for forming the Osmo-microsealed extended release delivery system and the process for using such system for treating human ailment / depression are also provided.
Owner:ALEMBIC LTD
Sutained release formulation for venlafaxine hydrochloride
InactiveUS20060182797A1Quick releaseOrganic active ingredientsNervous disorderSustained Release CapsuleMini tablets
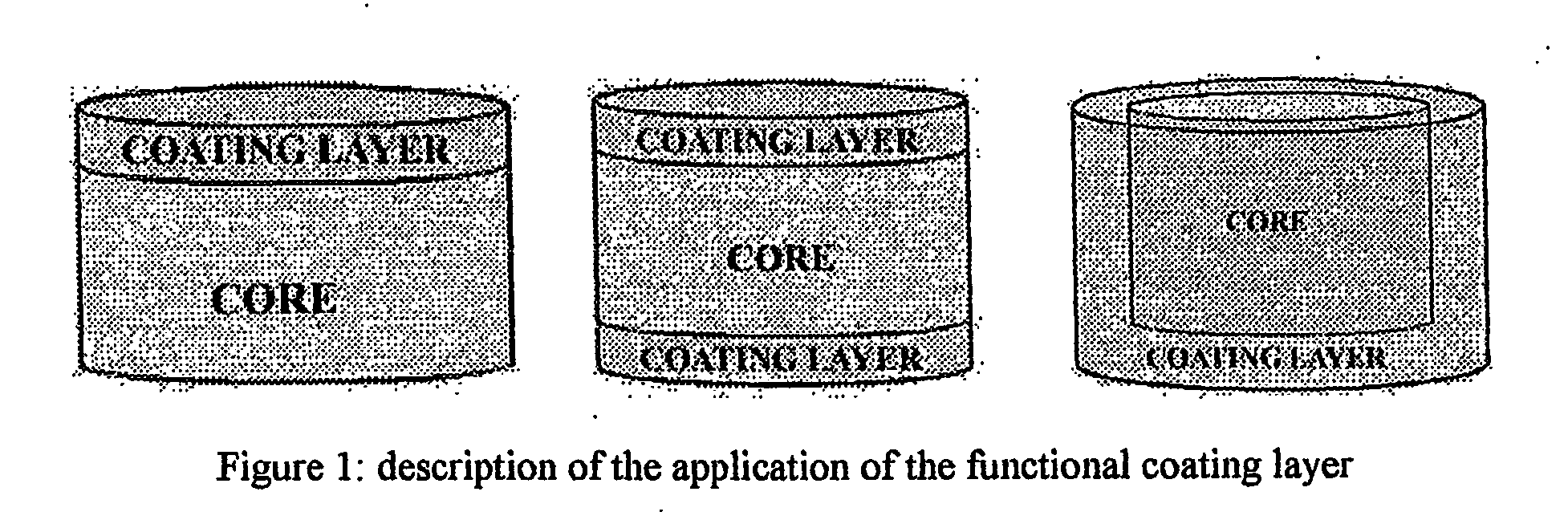
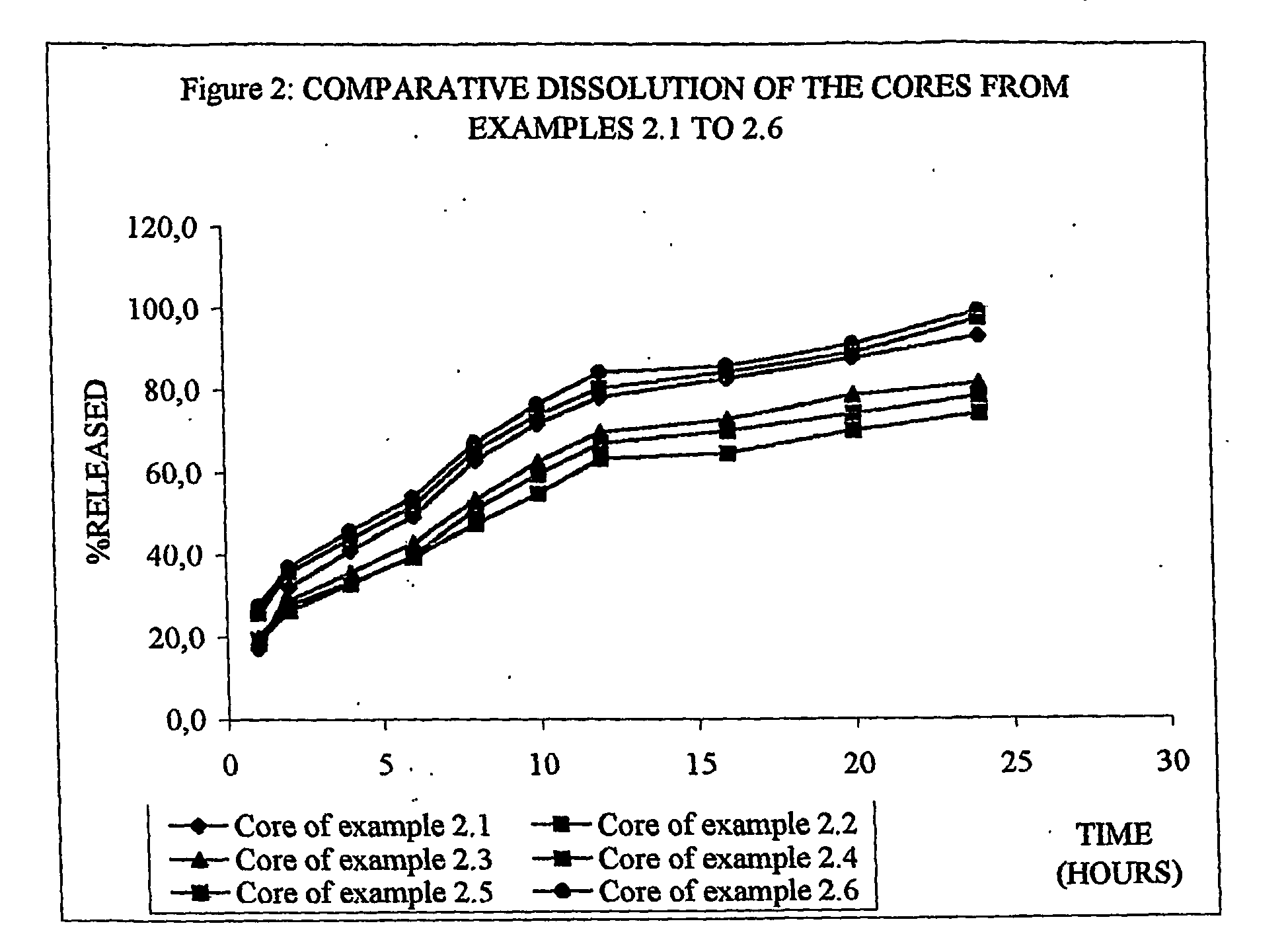
The invention provides a sustained release composition that; 1. Is free of initially increased drug delivery that occurs (in sustained release systems containing the water soluble drug venlafaxine HCl, known as burst phenomenon, by using a functional core partially or totally coated by a functional coating layer or film. 2. Delivers the drug substance within 24 hours and is therefore suitable for once daily administration of the said drug substance. 3. Exhibits linearity between the strength dosage form and the (total mass of the dosage form, by proportional increase of the amounts of the drug substance and the excipients in the formulation. 4. Is possible to be divided in smaller doses, without affecting the release of the drug substance. The invention provides a sustained release capsule formulation containing an appropriate number of functional complex mini tablets comprising of: I. A functional core comprising the active ingredient, especially the water-soluble drug Venlafaxine HCl and appropriate excipients. 2. A functional coating layer or film that reduces the initial surface of the core that is available for the release of the water-soluble drug Venlafaxine HClt phenomenon.
Owner:PHARMATHEN
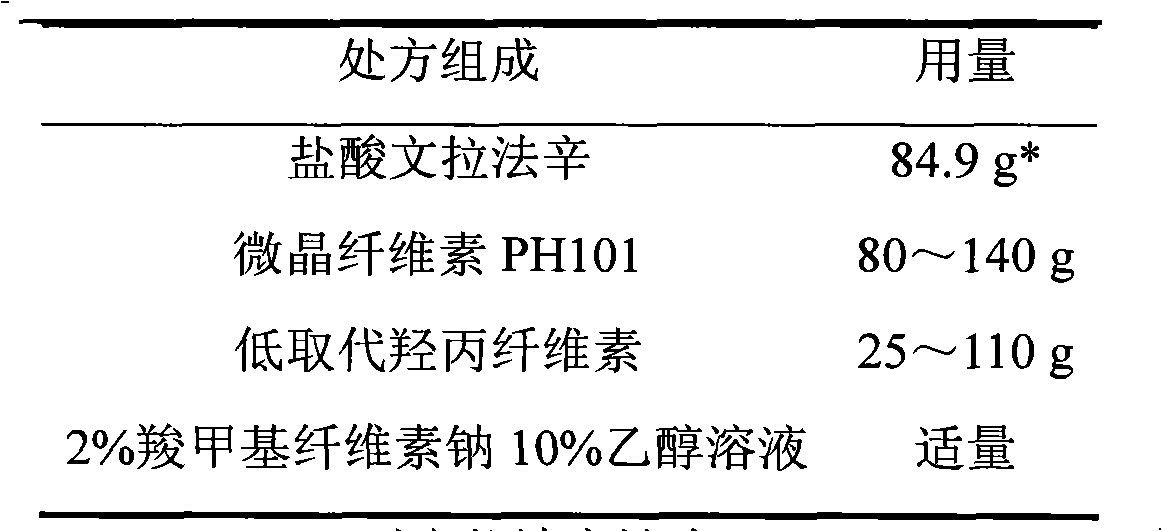
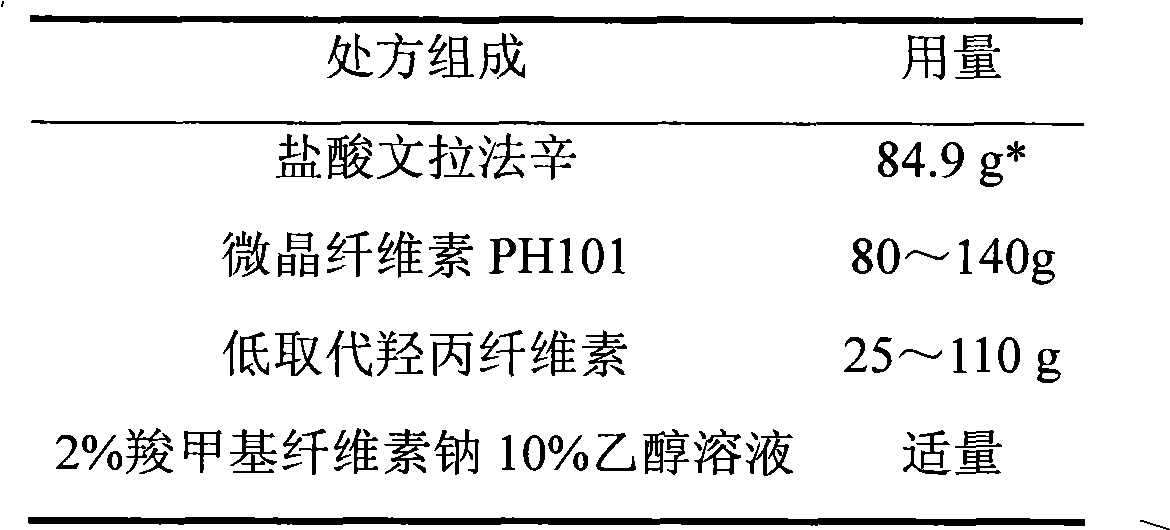
Venlafaxine hydrochloride sustained-release tablet preparation and preparation method thereof
InactiveCN101584674AGood sustained release effectOrganic active ingredientsNervous disorderSustained Release TabletAdhesive
The invention discloses a venlafaxine hydrochloride sustained-release tablet preparation which comprises venlafaxine hydrochloride, framework material, diluent, lubricant, adhesive and coating material. The experiments demonstrate that the venlafaxine hydrochloride sustained-release tablet achieves better sustained-release effect, can reduce the dosing frequency and is convenient for the patients to use. The invention also provides a preparation method thereof.
Owner:上海医药科技发展有限公司
Venlafaxine hydrochloride spansule and preparation method thereof
InactiveCN103191082AFix security issuesSolve the requestOrganic active ingredientsNervous disorderSolubilityControl layer
The invention discloses a venlafaxine hydrochloride spansule and a preparation method thereof. According to the existing method, aqueous dispersion is used for coating, and venlafaxine hydrochloride generates migration during the coating process due to high water solubility of venlafaxine hydrochloride, so that venlafaxine hydrochloride is possible to become a pore-forming agent to result in overquick release of venlafaxine hydrochloride if the thickness of a coating film is insufficient. The venlafaxine hydrochloride spansule disclosed by the invention is characterized in that the medicated micro-pill comprises a medicated pill core, an isolating layer coating the medicated pill core and a release control layer coating the isolating layer, wherein the medicated pill core is prepared from the following ingredients in percentage by weight: 30-60% of venlafaxine hydrochloride, 30-80% of filler, 1-20% of release skeleton material and 1-10% of adhesive. According to the invention, such technical problems as potential safety hazard to operators and high demands on equipment caused by utilizing organic solvents, namely dichloromethane and ethanol, for coating are completely solved.
Owner:浙江美华鼎昌医药科技有限公司 +1
Control released permeation bump tablet of venlafaxine hydrochloride and its prepn process
InactiveCN1846693ALittle side effectsGuaranteed curative effectOrganic active ingredientsNervous disorderPlasticizerControlled Release Tablet
The present invention discloses one kind of osmotic pump controlled release tablet of venlafaxine hydrochloride. The tablet consists of medicine core of venlafaxine hydrochloride and semipermeable film coating with small holes. The medicine core consists of venlafaxine hydrochloride in 75 weight portions, stuffing in 30-250 weight portions, osmotic pressure promoter in 1-50 weight portions, adhesive in 1-20 weight portions and lubricant in 0.1-20 weight portions. The semipermeable film coating consists of semipermeable film coating material in 5-15 weight portions and plasticizer in 1 weight portion. The tablet has excellent release controlling effect, high safety and good patient dependence. The present invention also discloses the preparation process of the osmotic pump controlled release tablet of venlafaxine hydrochloride.
Owner:潘卫三 +2
Modified release venlafaxine hydrochloride tablets
InactiveUS20050048118A1Organic active ingredientsNervous disorderVenlafaxine tabletExtended release tablets
A modified release tablet of venlafaxine hydrochloride is formed by a core containing a lipophilic matrix and venlafaxine hydrochloride and a water-insoluble, permeable coating thereover.
Owner:SYNTHON IP
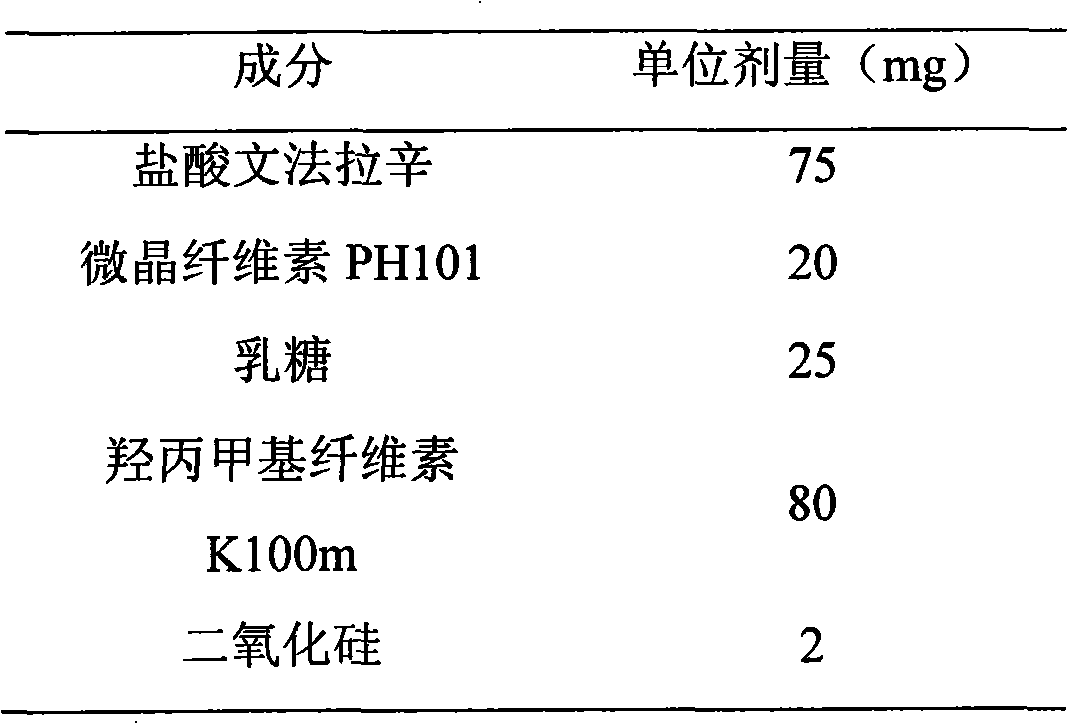
Extended release formulation of venlafaxine hydrochloride
InactiveUS7807195B2Facilitated releaseReduce processing timeOrganic active ingredientsNervous disorderMini tabletsPharmaceutical formulation
The present invention relates to an extended release once daily pharmaceutical formulation comprising venlafaxine hydrochloride and pharmaceutically acceptable excipients. More particularly, the present invention relates to an extended release composition in the form of mini-tablets which are incorporated in hard gelatin capsules.
Owner:ALEMBIC LTD
Venlafaxine hydrochloride sustained release capsule and preparation method thereof
ActiveCN103893151AHigh yieldRelease facilitates controlOrganic active ingredientsNervous disorderSustained release pelletsSocial benefits
The invention relates to a venlafaxine hydrochloride sustained release capsule. Sustained release pellets serve as contents of the sustained release capsule prepared by the invention, wherein the pellets comprise medicine-containing pill cores and sustained release coatings; the medicine-containing pill cores contain venlafaxine hydrochloride, hydroxypropyl methyl cellulose and microcrystalline cellulose. The venlafaxine hydrochloride sustained release capsule disclosed by the invention is stable to release medicines, free from a problem of organic solvent residue, safe to take and environment-friendly during producing. Furthermore, the invention provides a preparation method of the venlafaxine hydrochloride sustained release capsule. The method is high in production efficiency, simple in process, convenient to operate, low in cost and easy in industrial production; the method can be used for saving the cost for medicine production enterprises, and can bring about considerable economic and social benefits.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Venlafaxine hydrochloride liquid slow-release preparation and its preparation method
InactiveCN1634006AStable blood concentrationLong-lasting effectOrganic active ingredientsNervous disorderAdjuvantAcrylic resin
The invention discloses a Venlafaxine hydrochloride liquid slow-release preparation and its preparation method, wherein the preparation comprises venlafaxine hydrochloride 30-90%, adjuvant 10-70% for slow release action, and balancing adjuvant. The adjuvant with slow release action is cationic ion-exchange resin and acrylic resin and / or cellulose ethyl ether. The slow release preparation can sustain blood medicament concentration effectively within 24 hours.
Owner:SHENYANG PHARMA UNIVERSITY
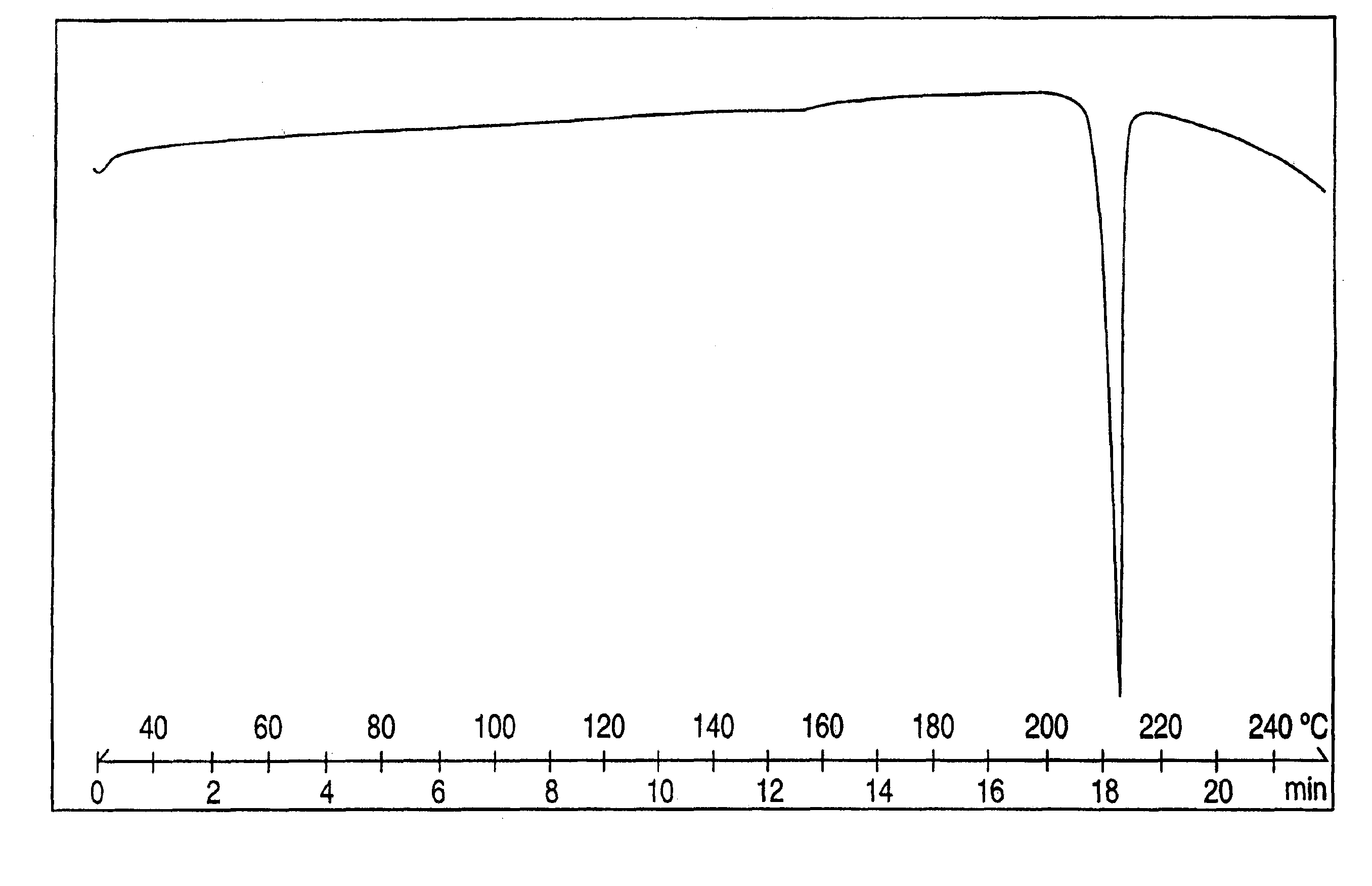
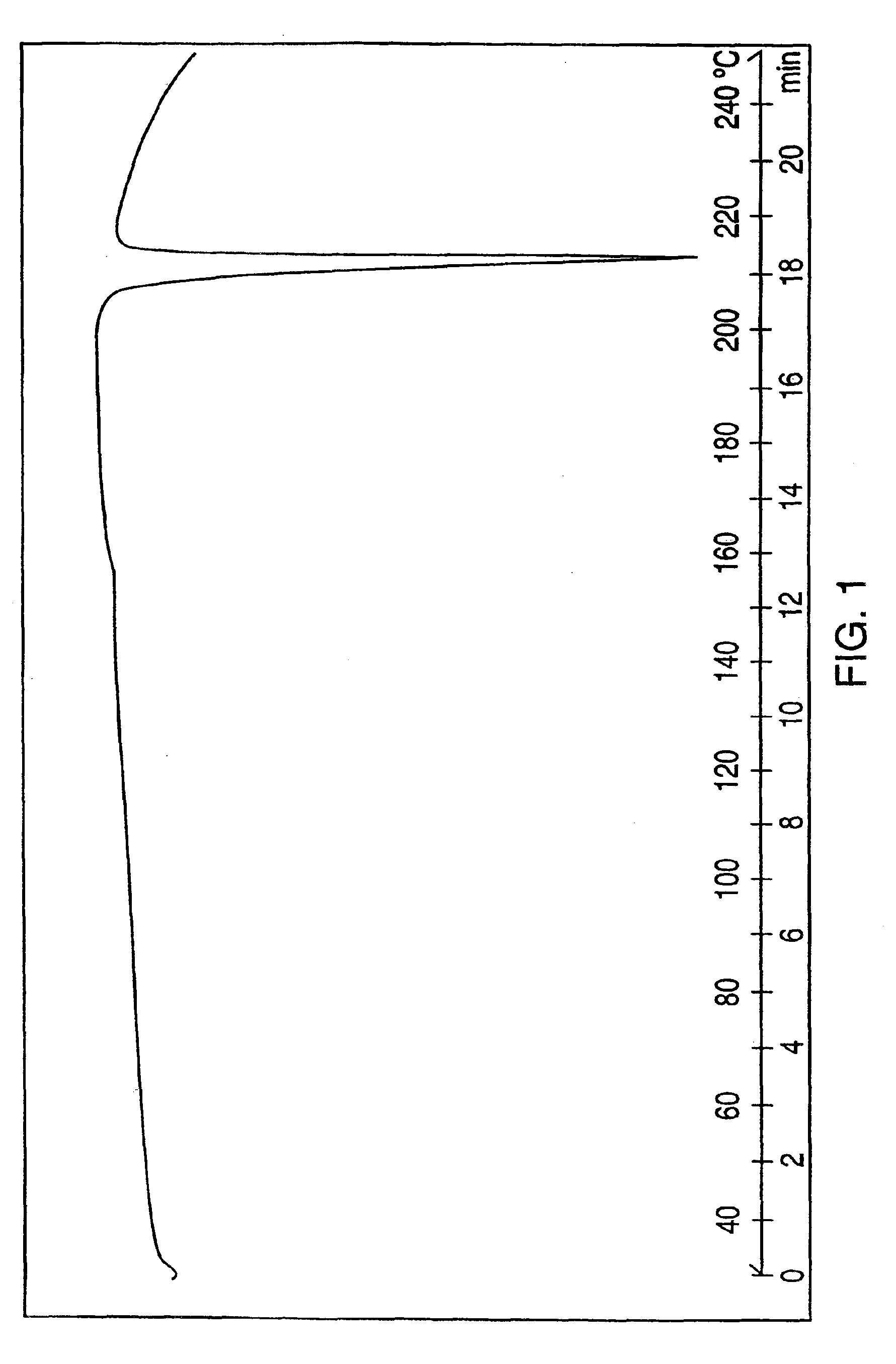
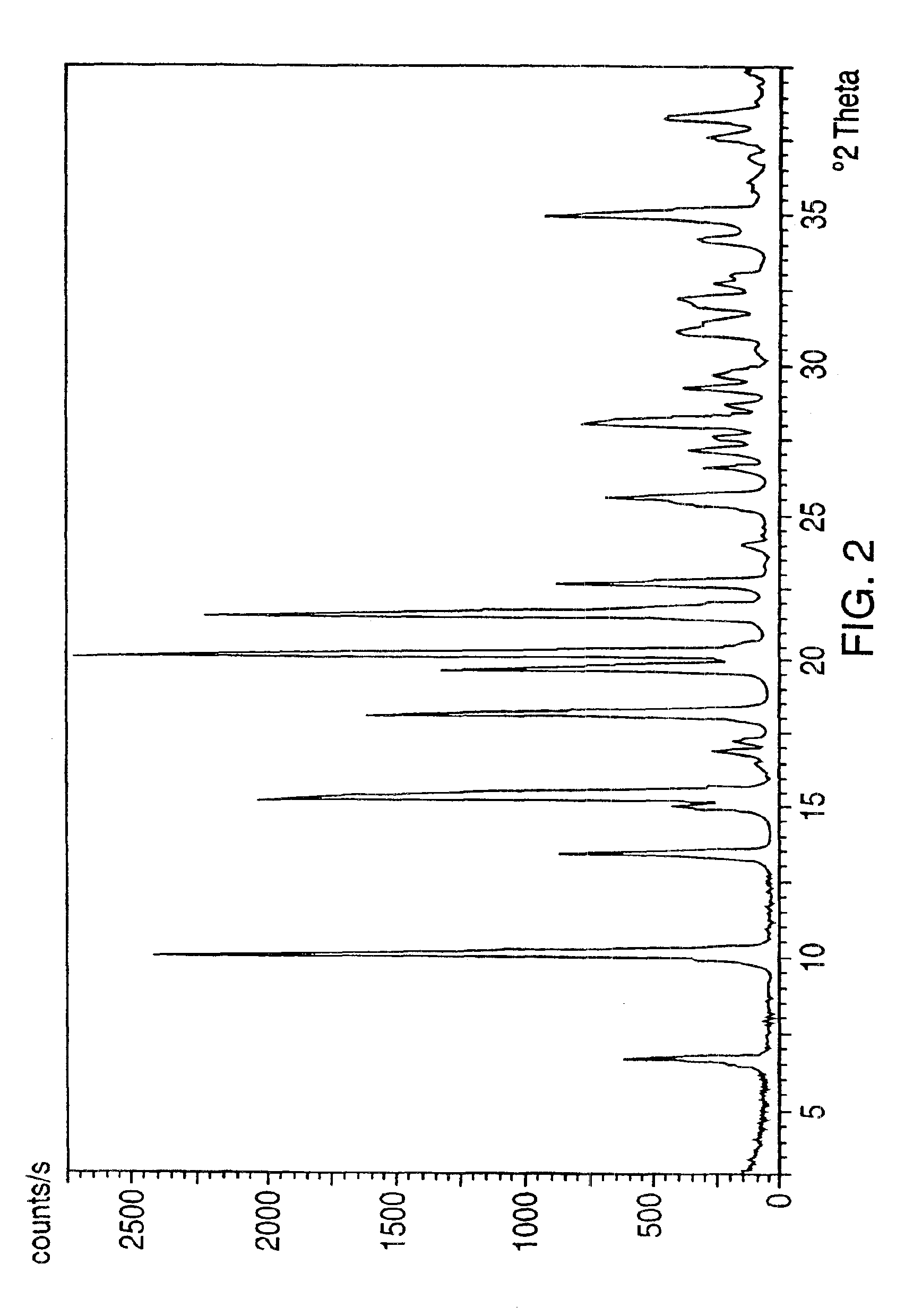
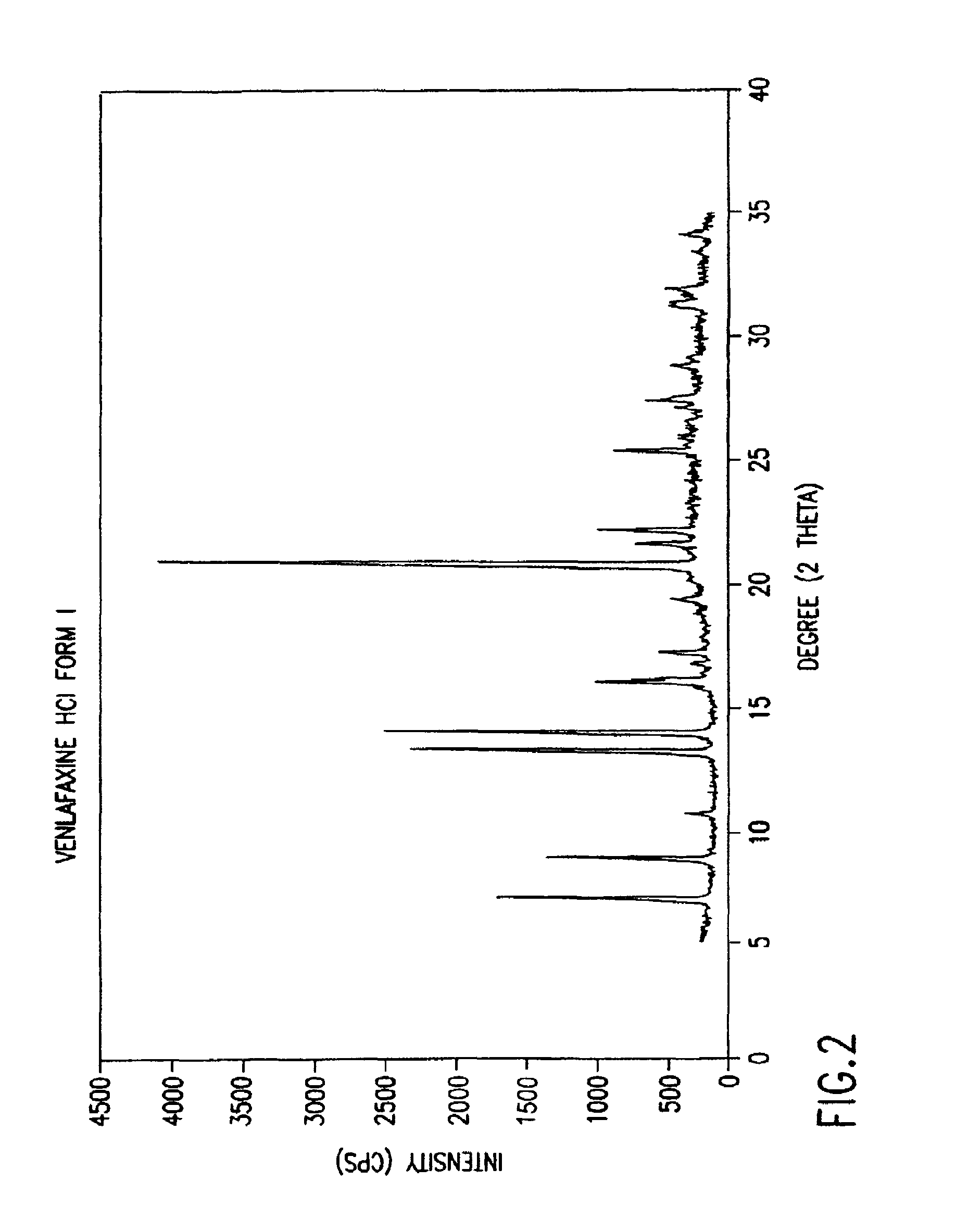
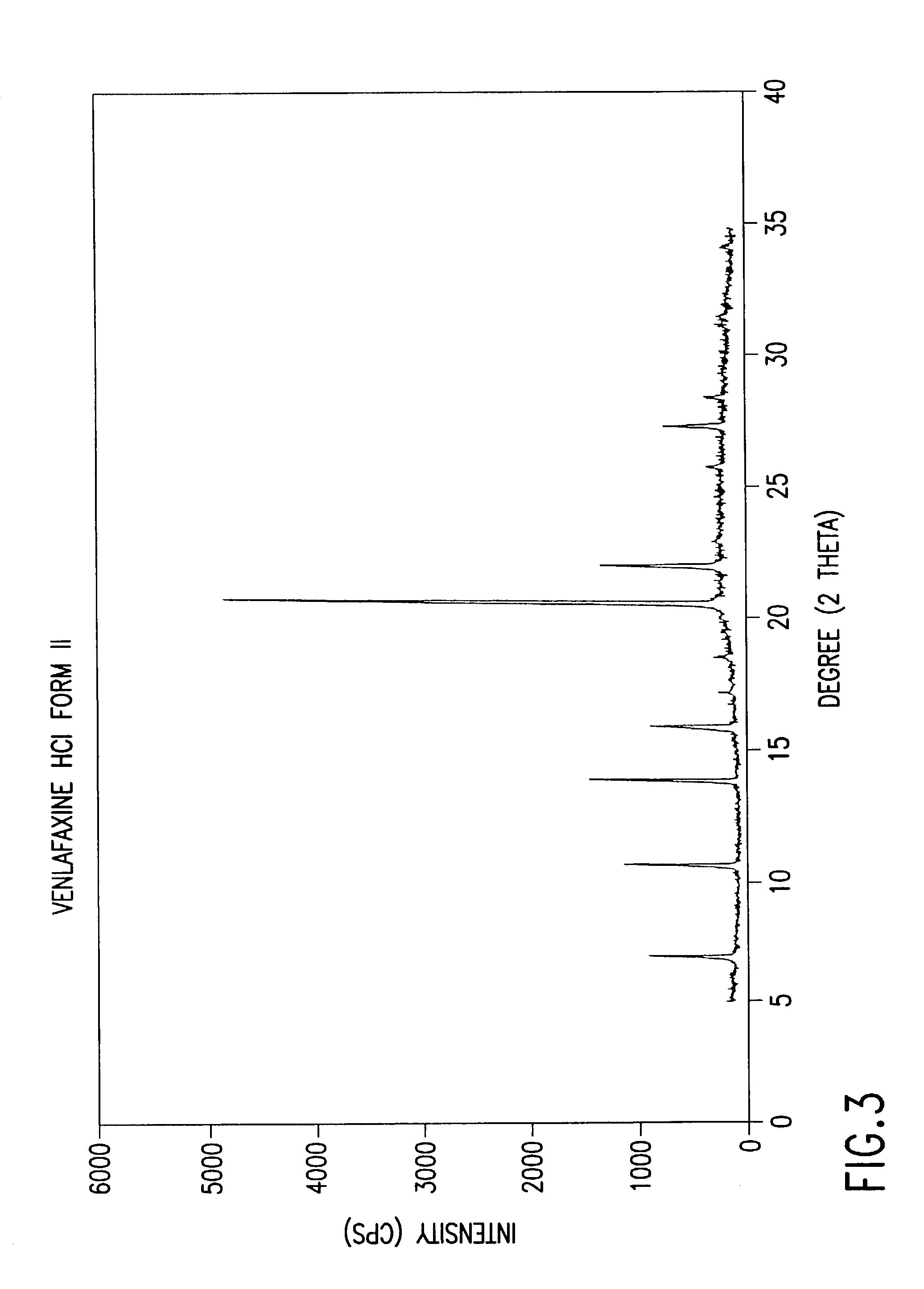
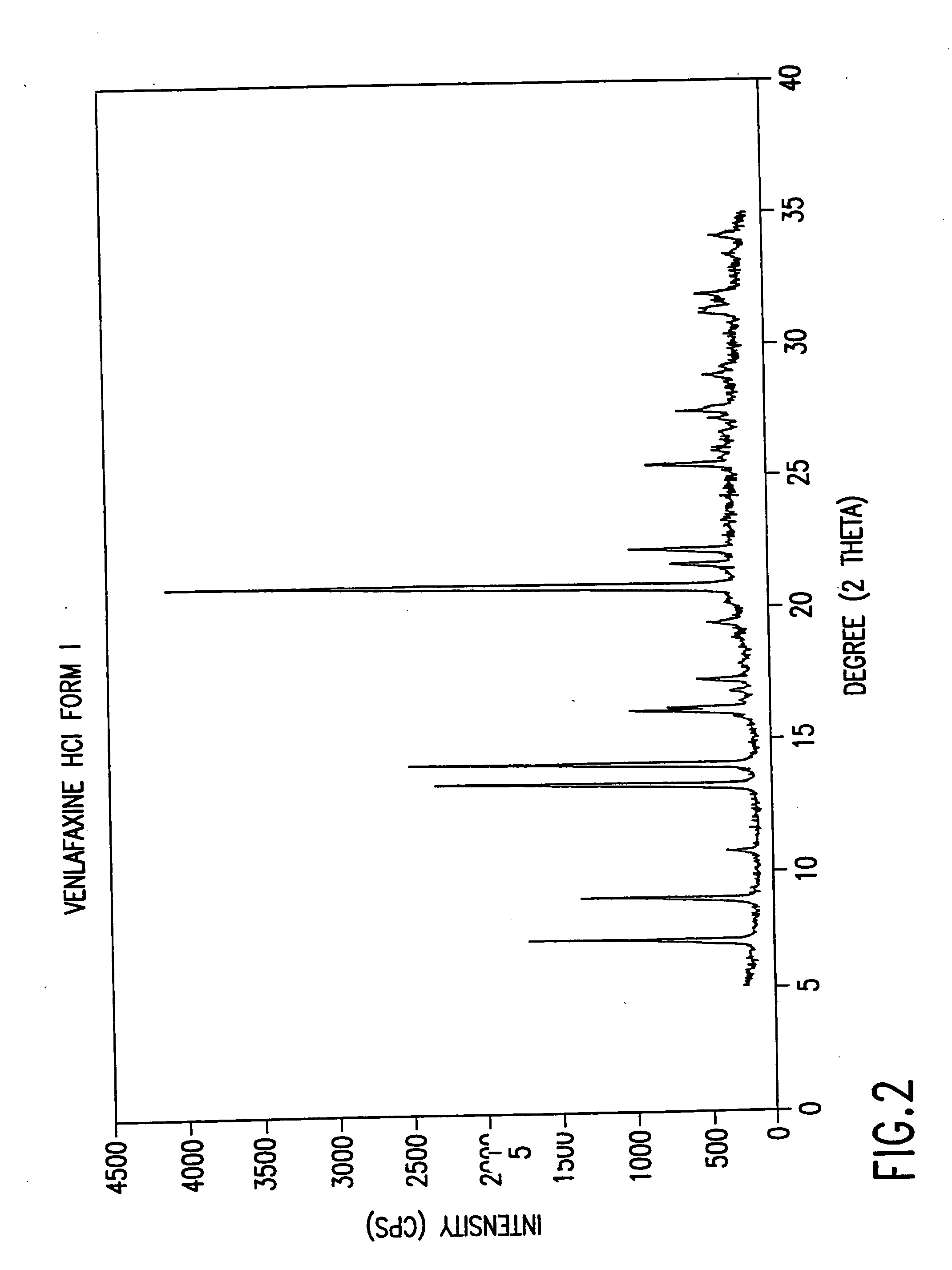
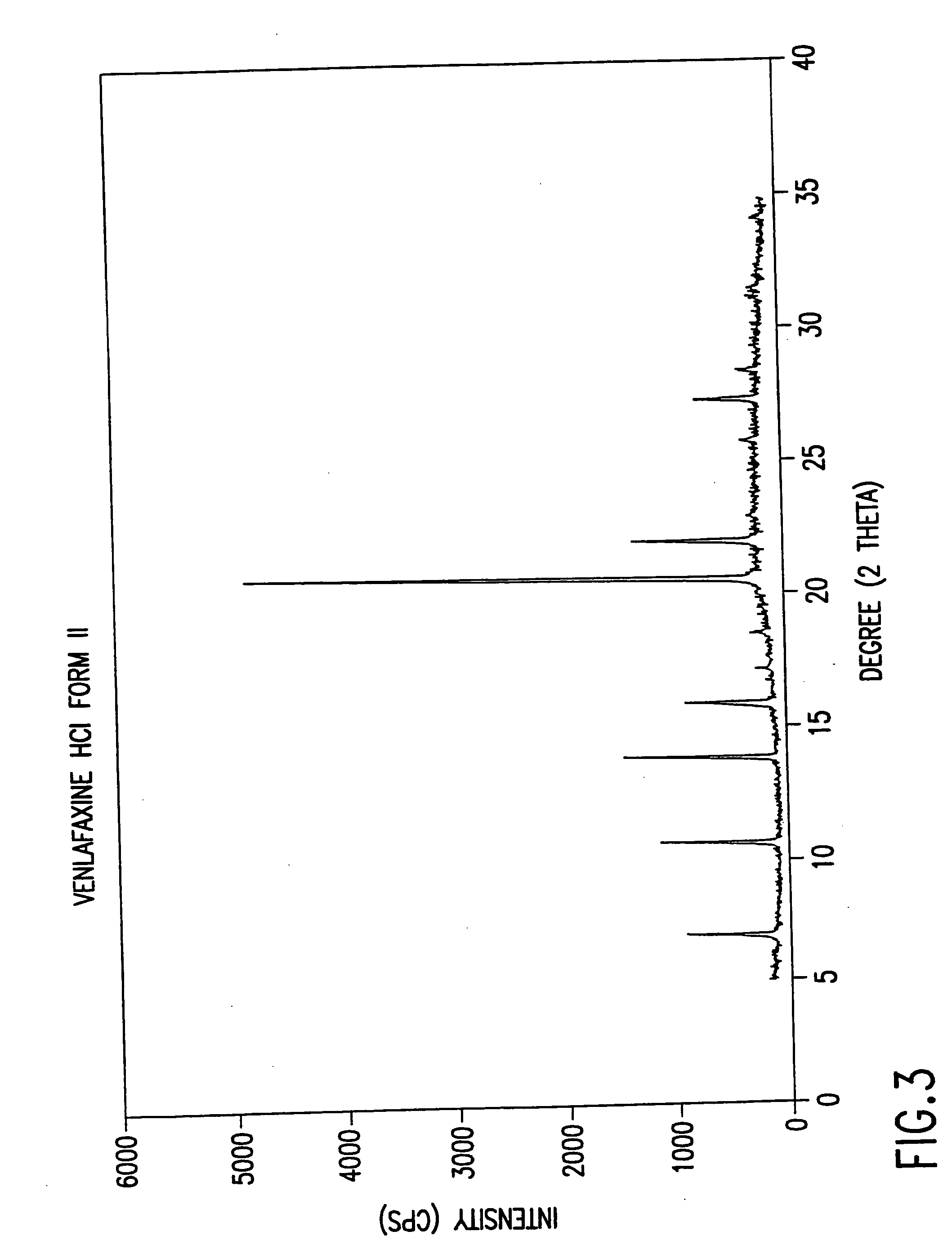
Crystalline polymorph of venlafaxine hydrochloride and methods for the preparation thereof
InactiveUS7030164B2Easy to disassembleOrganic active ingredientsBiocideVenlafaxine HydrochlorideCrystallization
This invention relates to a highly thermally stable novel anhydrous crystalline polymorphic form of venlafaxine hydrochloride, methods for the preparation thereof, and its use.
Owner:WYETH LLC
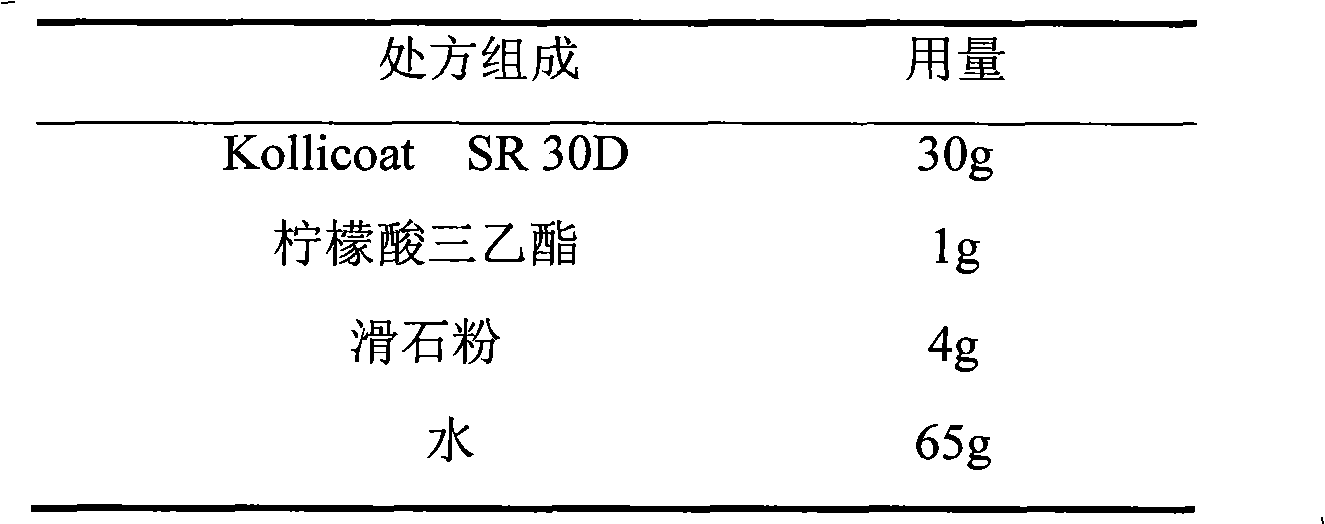
Venlafaxine hydrochloride film-controlled slow-release pellet capsule
ActiveCN103211791AAccelerated agingReduce permeabilityOrganic active ingredientsNervous disorderSustained release pelletsLow-substituted hydroxypropylcellulose
The invention relates to a venlafaxine hydrochloride film-controlled slow-release pellet capsule. A slow-release film of the venlafaxine hydrochloride film-controlled slow-release pellet utilizes Kollicoat SR30D as a film-formation material. A pellet core of the venlafaxine hydrochloride film-controlled slow-release pellet contains low-substituted hydroxyprepyl cellulose having high expansibility, and also contains a pharmaceutically-acceptable common excipient for the slow-release pellet, wherein preferably, the excipient comprises microcrystalline cellulose, and the pellet core comprises 10 to 40wt% of low-substituted hydroxyprepyl cellulose. The slow-release film comprises Kollicoat SR30D, triethyl citrate as a plasticizer and talcum powder as an antiplastering aid, wherein preferably, a ratio of Kollicoat SR30D to triethyl citrate to talcum powder is 30: 1: 4 and a film weight increasing ratio is in a range of 21 to 39%. The venlafaxine hydrochloride film-controlled slow-release pellet comprises the pellet core containing low-substituted hydroxyprepyl cellulose having high water expansibility and thus after absorbing water, the venlafaxine hydrochloride film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the venlafaxine hydrochloride film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:北京天衡药物研究院有限公司
Venlafaxine hydrochloride controlled-release pellet and preparation method thereof
ActiveCN102935071AReduce viscosityOvercome coating electrostatic problemsOrganic active ingredientsNervous disorderSpray coatingSolvent
The invention provides a venlafaxine hydrochloride controlled-release pellet and a preparation method thereof. The venlafaxine hydrochloride controlled-release pellet comprises a blank pellet core, a venlafaxine hydrochloride-containing layer and a controlled-release film, wherein the venlafaxine hydrochloride-containing layer comprises active components including venlafaxine hydrochloride and talcum powder which are in a weight ratio of 12:1-8:1, and the weight ratio of the venlafaxine hydrochloride to the blank pellet core is 4:10-8:10. The venlafaxine hydrochloride controlled-release pellet is prepared by adopting a fluidized bed bottom-spraying venlafaxine hydrochloride applying mode; by using 75 percent alcohol as a solvent, the viscosity of the venlafaxine hydrochloride in a water solution is remarkably lowered, the venlafaxine hydrochloride applying speed is increased; and the prepared venlafaxine hydrochloride containing pellet adopts a bottom-spraying coating process, thus the problem of static electricity generated because of the adoption of the pure organic solvent is overcome; the application process and the wrapping process can be continuously operated in the same device, thus the production efficiency is increased and the production cost is lowered; and no toxic organic solvent is adopted in the whole process, thus the venlafaxine hydrochloride controlled-release pellet is environmental-friendly and is good in stability, and almost no broken pellets or dust is produced.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Novel crystalline polymorph of venlafaxine hydrochloride and methods for the preparation thereof
InactiveUS20060074131A1Easy to disassembleBiocideOrganic active ingredientsMedicinal chemistryVenlafaxine Hydrochloride
This invention relates to a highly thermally stable novel anhydrous crystalline polymorphic form of venlafaxine hydrochloride, methods for the preparation thereof, and its use.
Owner:WYETH LLC
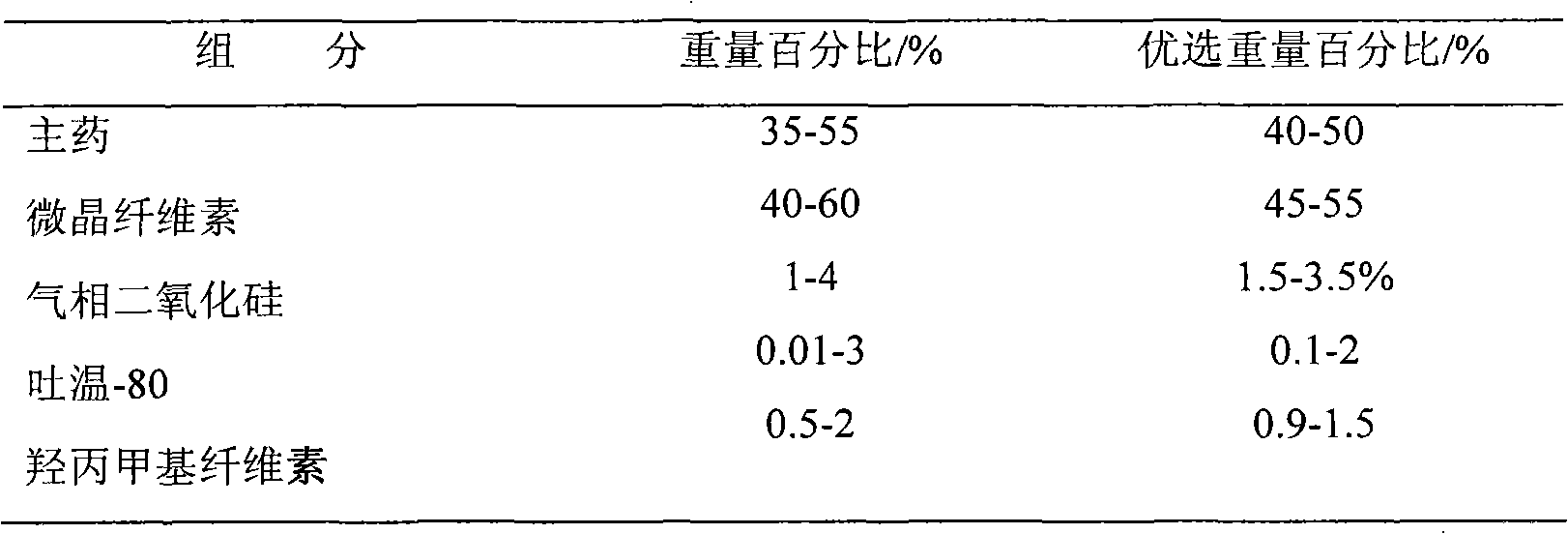
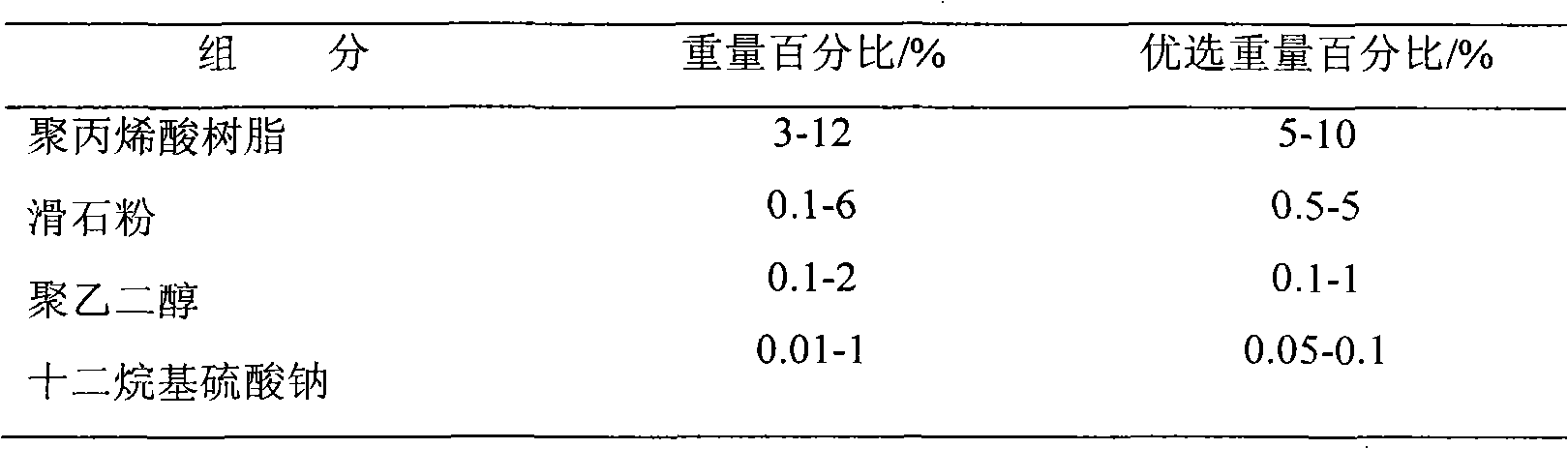
Venlafaxine hydrochloride slow-release capsule and preparation method thereof
InactiveCN103181916AGood sustained release effectAvoid stickingOrganic active ingredientsNervous disorderGas phasePolyethylene glycol
The invention relates to a venlafaxine hydrochloride slow-release capsule and a preparation method thereof. A drug-containing core of the slow-release capsule comprises venlafaxine hydrochloride, microcrystalline cellulose, gas phase silica, Tween-80, and hydroxypropyl methylcellulose; a slow-release coating comprises polyacrylic resin, talcum powder, polyethylene glycol and lauryl sodium sulfate. The invention realizes a pure water system for the capsule production by the addition of gas phase silica to change material flowing, and the selection of slow-release coating. The venlafaxine hydrochloride slow-release capsule prepared by the system is stable in drug release, has effective continuous release time of 24 hours, and is taken only once a day. The method is simple in process, mild in condition, free of any organic solvent, low in equipment requirements, and suitable for industrial production.
Owner:KUNMING JIDA PHARMA
Controlled release pharmaceutical composition of venlafaxine hydrochloride, and process for preparation thereof
InactiveCN101257893AOrganic active ingredientsNervous disorderControlled releaseVenlafaxine Hydrochloride
Herein is described a controlled release pharmaceutical composition of venlafaxine hydrochloride, an effective antidepressant, comprising an inert core, on which the active principle is uniformly layered, that in turn is coated with a layer comprising a hardening agent and a lipophilic agent; the process for the preparation of said pharmaceutical composition is also described.
Owner:VALPHARMA SA
Venlafaxine hydrochloride sustained-release capsule composition
ActiveCN106955276AAffect the sustained release effectAvoid stickingOrganic active ingredientsNervous disorderSustained release pelletsFoaming agent
The invention relates to the field of medicines and in particular to a venlafaxine hydrochloride sustained-release capsule composition. The content of the venlafaxine hydrochloride sustained-release capsule composition comprises a sustained-release micropill composed of a medicine carrying micropill and a sustained-release layer; the medicine carrying micropill is composed of 58-66 parts by weight of venlafaxine hydrochloride, 75-85 parts by weight of filler and 8-12 parts by weight of an adhesive; the sustained-release layer is composed of 3.5-6 parts by weight of a sustained-release coating material and 18-27 parts by weight of a pore-foaming agent; the mass of the sustained-release layer is 14-17% of that of the medicine carrying layer. The invention also provides a preparation method of the venlafaxine hydrochloride sustained-release capsule composition. The medicine carrying micropill is manufactured by using a fluidized bed and the micropill is coated by the sustained-release layer to obtain the sustained-release micropill. The venlafaxine hydrochloride sustained-release capsule composition prepared by the method does not contain auxiliary materials such as an antisticking agent and a plasticizer. The manufacturing condition can be met by adjusting the process of the fluidized bed to obtain a drug which is close to an original drug in effect; the cost is lowered and the process difficulty is reduced.
Owner:HAINAN HERUI PHARMA
Slow-release composition containing antidepressant
InactiveCN101669928AEasy to operateOrganic active ingredientsNervous disorderVenlafaxine HydrochlorideAntidepressant
The invention provides a slow-release composition containing antidepressant. The release speed of venlafaxine hydrochloride can be controlled by adding thinning agent and slow-release auxiliary materials with a certain proportion, so that composition form with the slow-release characteristic can be obtained.
Owner:BEIJING TRADE STAR MEDICAL TECH
Crystalline venlafaxine base and novel polymorphs of venlafaxine hydrochlorid, processes for preparing thereof
InactiveCN101092366AAmino compound purification/separationOrganic compound preparationVenlafaxine HydrochlorideAcetone
The present invention relates to novel essentially pure venlafaxine and the process of preparation thereof. The present invention also relates to novel solvate forms of venlafaxine hydrochloride and the process of preparation thereof. Furthermore, the present invention provides a novel process for preparing venlafaxine hydrochloride from venlafaxine; the process comprises the steps of: i) preparing a mixture of venlafaxine with acetone; and ii) exposing the mixture in gaseous hydrochoric acid.
Owner:TEVA PHARMA IND LTD
Preparation method for venlafaxine hydrochloride sustained-release pellet
InactiveCN104042570AFriability qualifiedImprove liquidityOrganic active ingredientsNervous disorderSustained release pelletsSlurry
The invention discloses a preparation method for a venlafaxine hydrochloride sustained-release pellet. The method comprises the steps of: subjecting MCC to centrifugal granulation, adjusting the guniting speed and powder feeding speed till the material flows in a flocculent state and maintains the flocculent flow state, feeding powder and controlling the powder-slurry ratio to prepare a microcrystalline cellulose blank pellet core; crushing bulk drug powder, mixing the powder with MCC and HPMC (3cp) evenly in proportion, employing a powder lamination technique, taking the MCC blank pellet core as a mother nucleus and adopting water as a wetting agent, adjusting the guniting speed and powder feeding speed, the rotational speed of a main engine, air jetting flow, air jetting pressure and air blast, at the end of powder feeding, polishing the pellet in a pot for 5min, and conducting drying for 24h in a 40DEG C drying oven after removal from the pot so as to obtain a venlafaxine hydrochloride pellet; conducting coating on the venlafaxine hydrochloride pellet, making Surelease, HPMC and TEC into a Surelease coating solution in proportion, and making Sureleae NG and TEC into a Surelease NG coating solution according to a proportion.
Owner:天津新济复兴药业科技有限公司
Preparation method of venlafaxine hydrochloride intermediate
ActiveCN106866434AImprove reaction efficiencyHigh yieldOrganic compound preparationChemical recyclingHydrogenSulfate
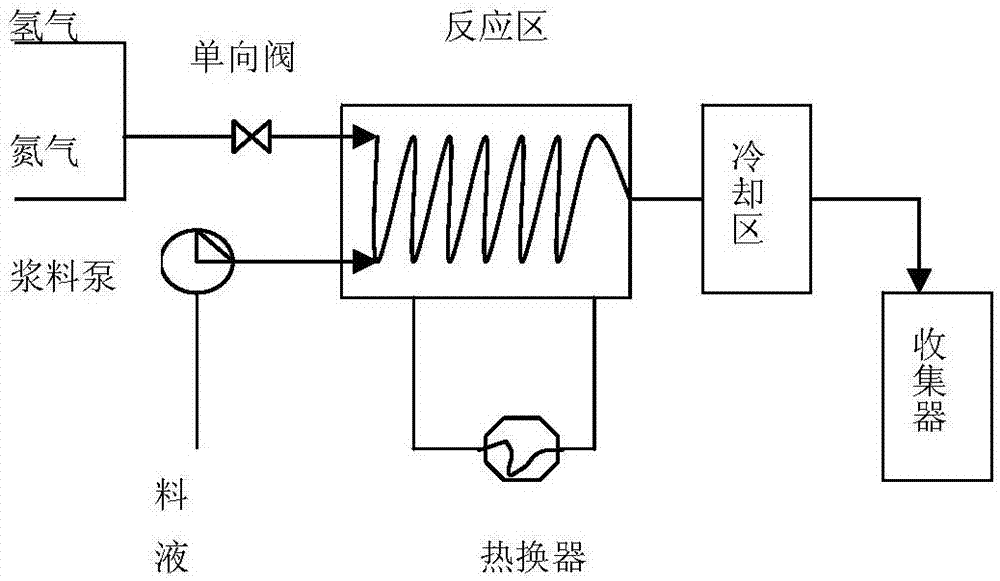
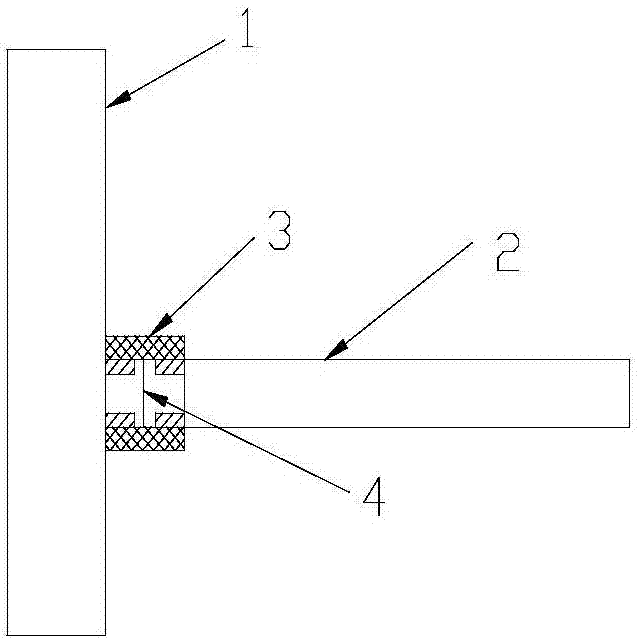
The invention discloses a preparation method of a venlafaxine hydrochloride intermediate. The preparation method comprises the following steps of in a micro-reactor loaded with a raney nickel screen net catalyst, enabling a compound I and hydrogen to react at the temperature of 20 to 40 DEG C, so as to obtain a compound II; adding the compound II into sulfuric acid, and forming salt, so as to obtain a target product, namely 1-[2-amino-1-(4-methoxyphenyl)ethyl]cyclohexanol sulfate (compound III). The preparation method has the advantages that on the basis of the original micro-reactor, the raney nickel screen net catalyst is creatively clamped to the connecting part of a pipeline, so that the catalyzing effect is enhanced, and the catalyst can be recycled; the damage to a slurry pump of the micro-reactor and the blockage of a reaction pipeline due to mixing of the catalyst into material liquid are avoided.
Owner:山东安信制药有限公司
Venlafaxine hydrochloride pellet capsule and preparation method thereof
InactiveCN104644615AMaintain therapeutic blood levelsMaintain stabilityOrganic active ingredientsNervous disorderOral medicationPlasma concentration
The invention relates to a venlafaxine hydrochloride slow release capsule and a preparation method thereof. The capsule comprises a capsule shell and a medicated pellet placed in the capsule shell, and the medicated pellet comprises a medicated pellet core and a slow release layer wrapped outside the medicated pellet core. The venlafaxine hydrochloride slow release capsule is an oral administration slow release pellet capsule preparation with venlafaxine hydrochloride as an active medicine component, can be administrated once a day to realize 24h slow release and maintain the therapeutic plasma concentration, and accords requirements of stability, industrial scale production and the like.
Owner:TIANJIN YIYAO SCI & TECH
Extended release formulation contg. venlafaxin
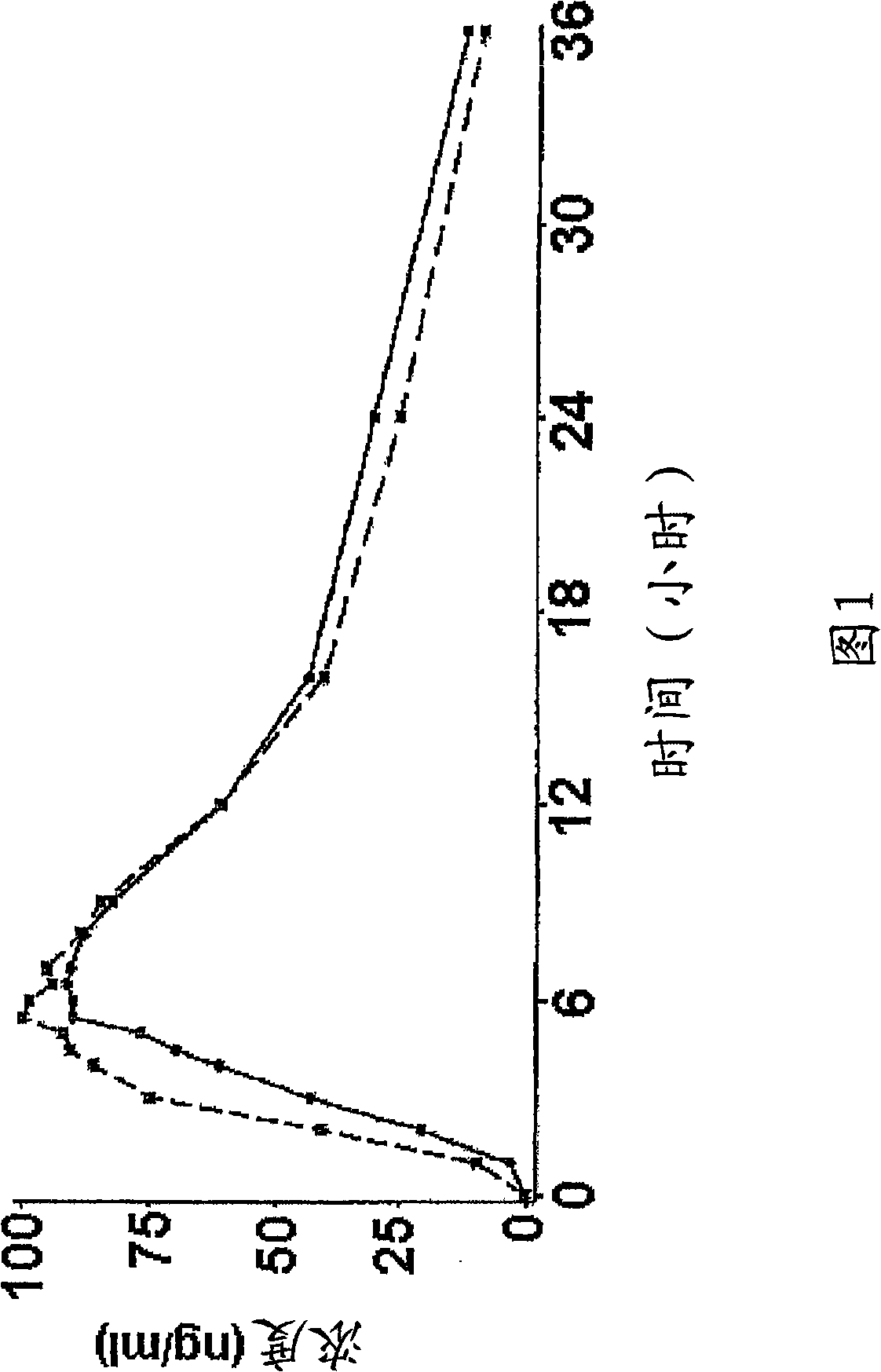
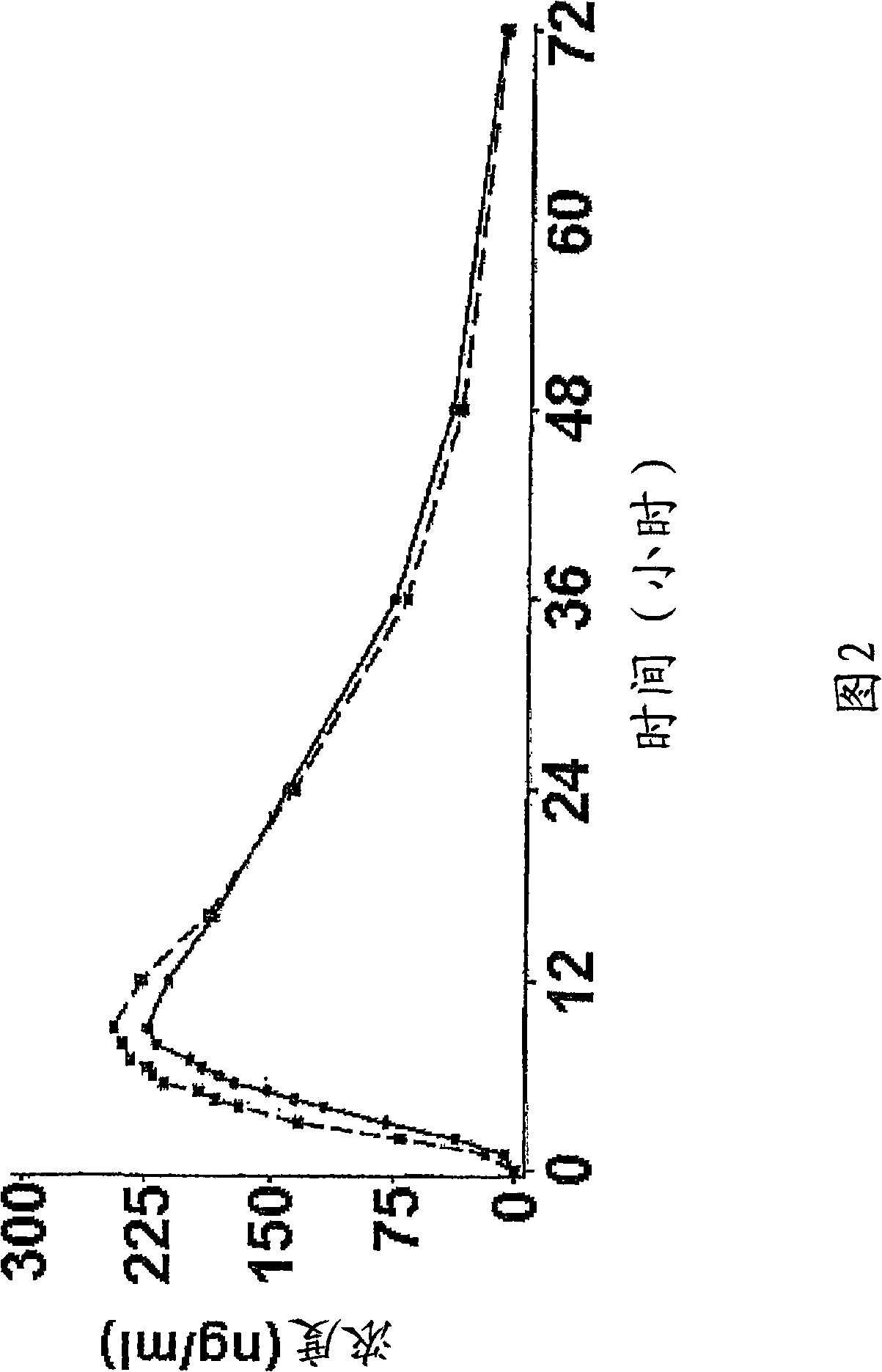
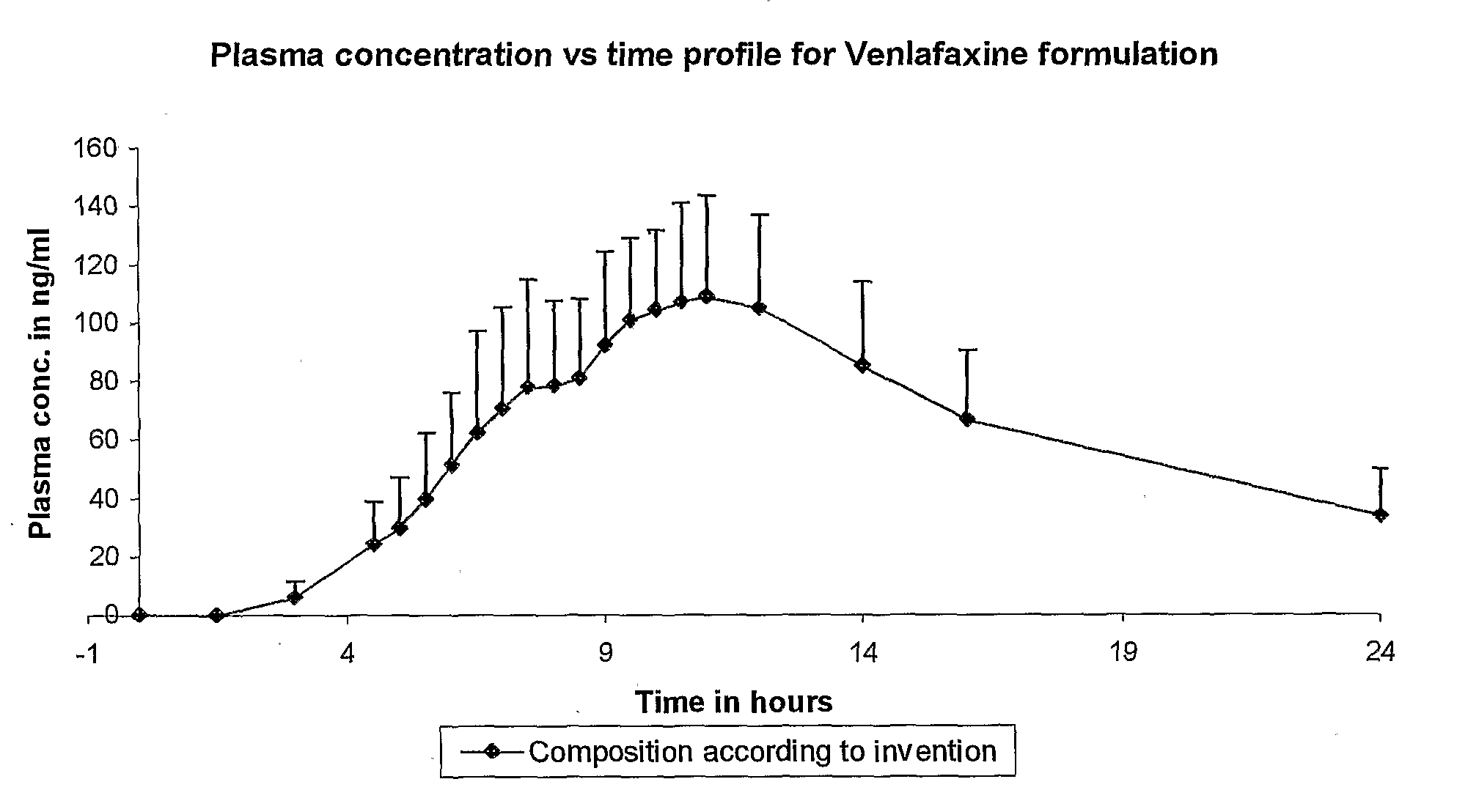
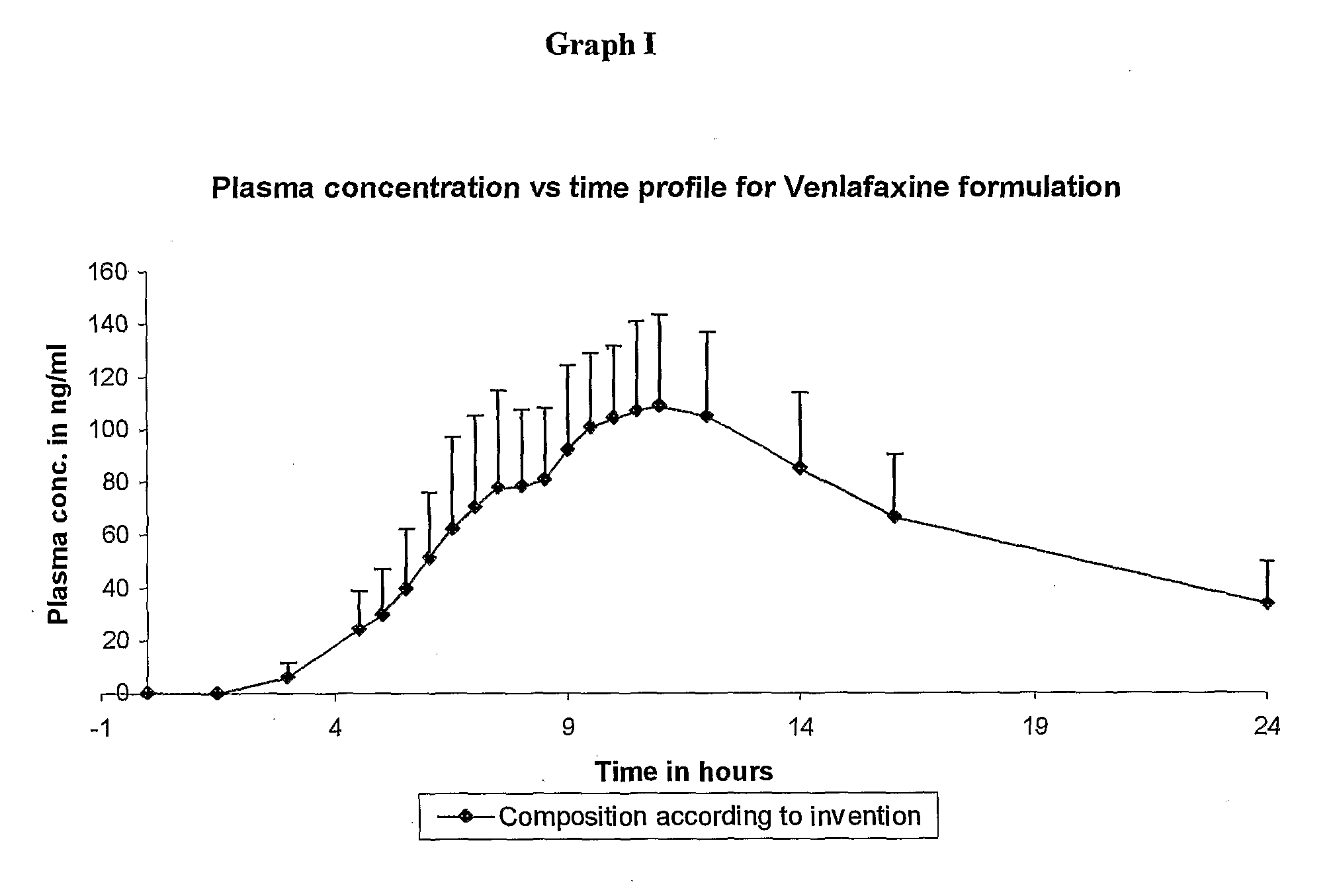
InactiveCN1278165ATight plasma therapeutic concentration range controlStatistically Significant ImprovementOrganic active ingredientsNervous disorderBlood plasmaPlasma concentration
This invention relates to a 24 hour extended release dosage formulation and unit dosage form thereof of venlafaxine hydrochloride, and antidepressant, which provides better control of blood plasma levels than conventional tablet formulations which must be administered two or more times a day and further provides a lower incidence of nausea and vomiting than the conventional tablets. In its primary aspect, this invention provides an improved core of the extended release spheroids comprised of venlafaxine hydrochloride and microcrystalline cellulose, that is, without the addition of hydroxypropylmethylcellulose.
Owner:AMERICAN HOME PRODUCTS CORPORATION
Extended Release Pharmaceutical Formulation of Venlafaxine and Method of Manufacturing the Same
InactiveUS20090175934A1Low toxicityCost efficientBiocideOrganic active ingredientsWater insolubleDiluent
Disclosed herein is an extended release pharmaceutical formulation suitable for once daily administration, comprising a highly water soluble core consisting essentially of about 30 to about 40% by weight of venlafaxine hydrochloride, about 50 to about 80% by weight of water soluble diluent and about 2 to about 10% of water soluble binder and a coating layer having an effective combination of rate controlling polymers comprising water-soluble polymer and water insoluble, water permeable polymer.
Owner:JUBILANT ORGANOSYS LTD
Novel Extended Release Composition of Venlafaxine Hydrochloride Containing Polyvinyl Acetate
Extended release pharmaceutical composition of Venlafaxine hydrochloride comprising a pharmaceutically acceptable capsule containing minitablets. The minitablets comprise from about 20% to about 70% by weight effective amount of Venlafaxine hydrochloride, polyvinyl acetate, one or more pharmaceutically acceptable excipients. The minitablets have diameter from about 1 mm to 5 mm and are coated with a release controlling composition.
Owner:LUPIN LTD
Related substances of venlafaxine hydrochloride and analysis and detection method of related substances
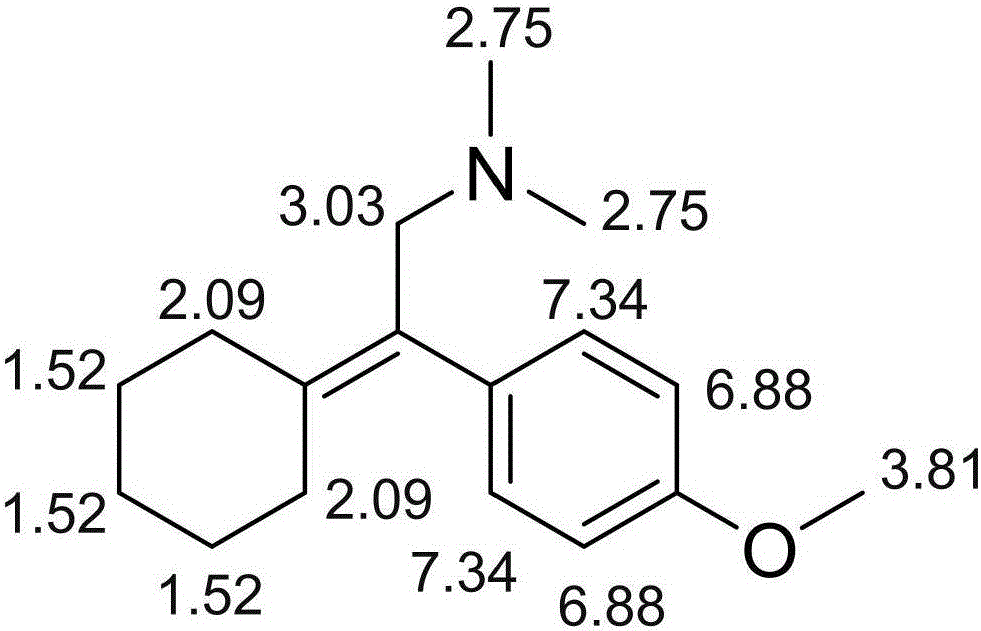
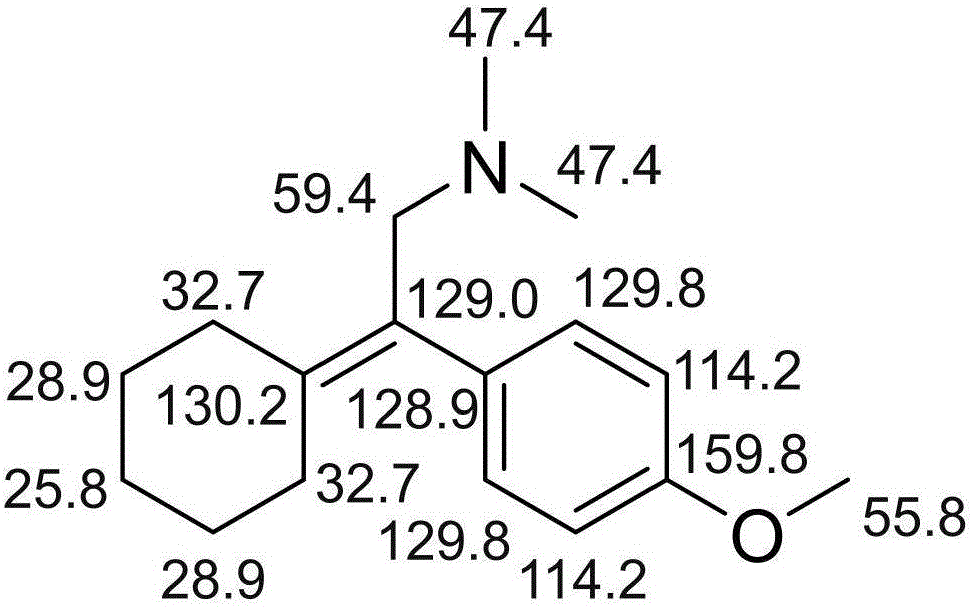
ActiveCN105884630ASimple manufacturing methodImprove the detection rateComponent separationOrganic compound preparationLength wavePhotodegradation
The invention discloses related substances of venlafaxine hydrochloride and an analysis and detection method of the related substances. A new venlafaxine hydrochloride photodegradation product is found, and the structure of the degradation product is reported for the first time. The preparation method of the photodegradation product is simple and easy to implement and can be used for preparing the degradation product on a large scale. The degradation product can be quickly detected with a liquid-phase analysis method, the maximum absorption wavelength of the degradation product is taken as a detection wavelength, the detection rate of the degradation product is increased, and results are accurate and reliable. The photodegradation product can be used for checking the related substances of venlafaxine hydrochloride and preparations of venlafaxine hydrochloride, the quality standards of venlafaxine hydrochloride and preparations of venlafaxine hydrochloride are further improved, and the safety and the controllability are improved.
Owner:ZHENJIANG GAOHAI BIOLOGICAL PHARMA CO LTD
High-drug-loading venlafaxine hydrochloride sustain release pellet composition, sustained-release capsule and preparation method thereof
ActiveCN109200032AOrganic active ingredientsNervous disorderSustained release pelletsSustained Release Capsule
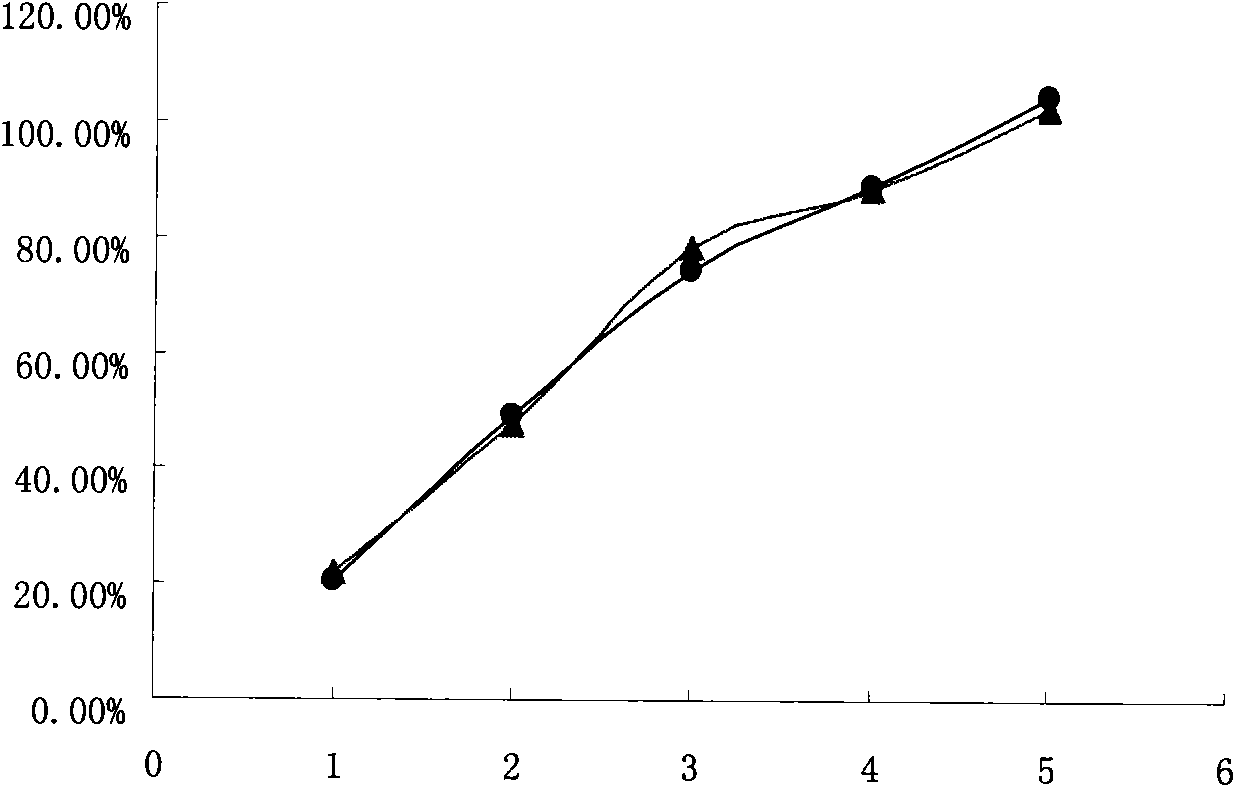
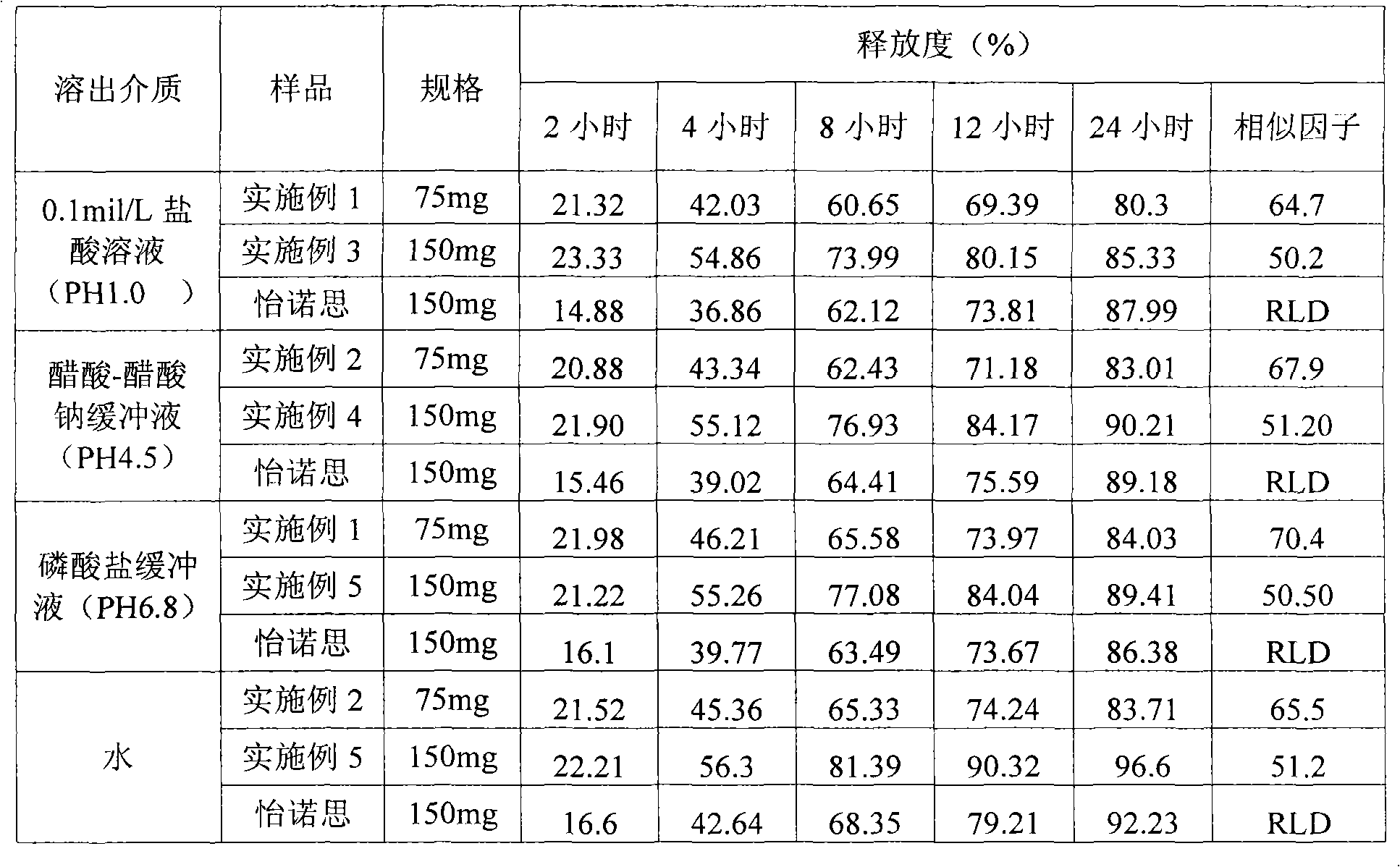
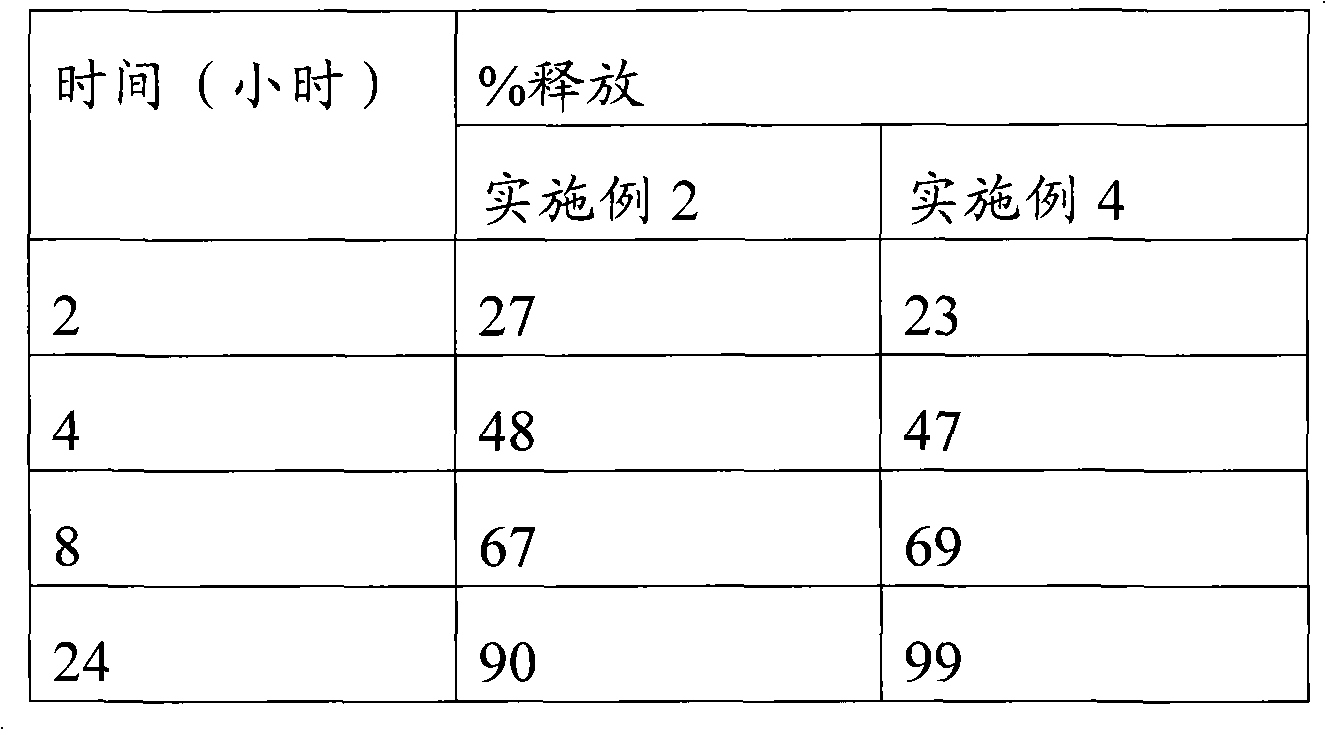
The invention relates to a high-drug-loadingvenlafaxine hydrochloride sustained-release pellet composition, a sustained-release capsule and a preparation method. Specifically, the venlafaxine hydrochloride sustained-release pellets comprise a blank pellet core, a drug-carrying coating layer coated on the outer surface of the blank pellet core, and a sustained-release coating layer coated on the outer surface of the drug-carrying coating layer, wherein the drug-carrying coating layer comprises venlafaxine hydrochloride as an active ingredient. 50 to 65 percent of venlafaxine hydrochloride active ingredient in that venlafaxine hydrochloride sustain release pellets, wherein the venlafaxine hydrochloride sustain release pellets can pass through a 14-mesh sieve but cannot pass through a 30-meshsieve, and the venlafaxine hydrochloride active ingredient in the venlafaxine hydrochloride sustain release pellets accounts for 50 to 65 percent of the weight of the sustained release pellets; Whichcan pass through a 16-mesh sieve but not a 25-mesh sieve; according to the dissolution determination method, the dissolution amount is not more than 30% in 2 hours, 40%-60% in 4 hours, 60%-80% in 8 hours, 70%-90% in 12 hours, and 85% in 24 hours. The venlafaxine hydrochloride sustained-release pellets and the sustained-release capsules of the invention exhibit excellent pharmaceutical properties.
Owner:HUNAN DONGTING PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com