Venlafaxine hydrochloride slow-release capsule and preparation method thereof
A technology for venlafaxine hydrochloride and sustained-release capsules, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., and can solve the problem of complex preparation equipment, long production process and lack of economic Environmental protection methods and other issues to achieve the effect of improving the sustained release effect, simple equipment, and avoiding adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
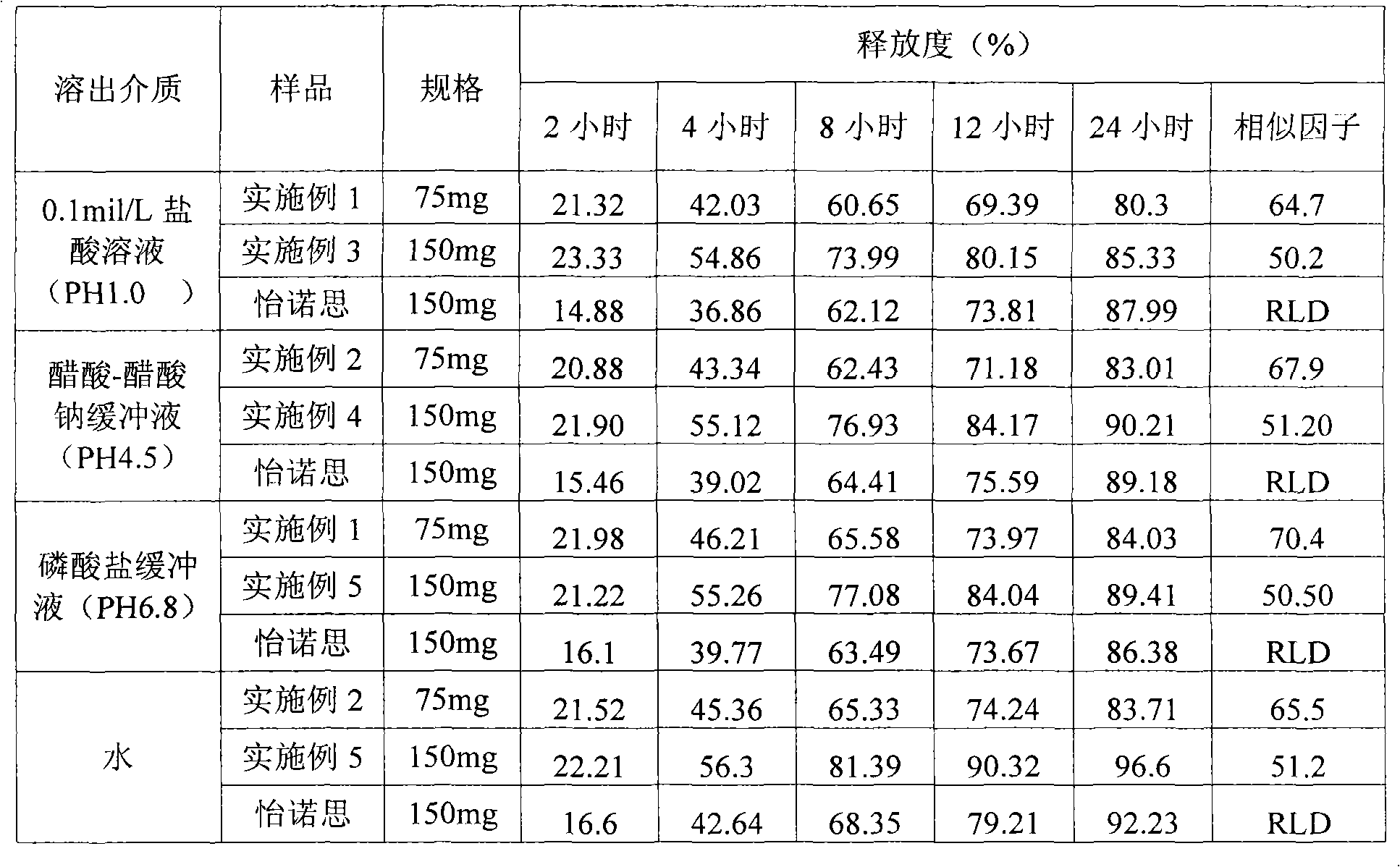
Examples
Embodiment 1
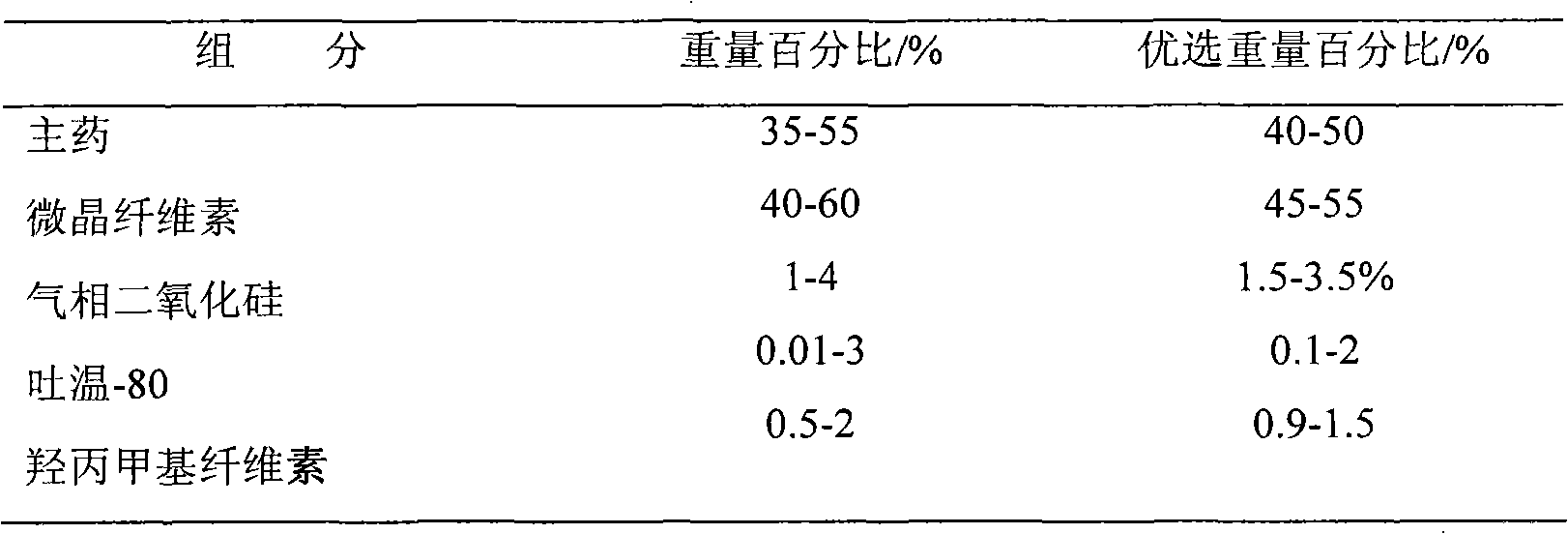
[0029] Composition of pill core:
[0030] Venlafaxine hydrochloride 35%, microcrystalline cellulose 60%, fumed silicon dioxide 3.5%, Tween-80 1%, hypromellose 0.5%.
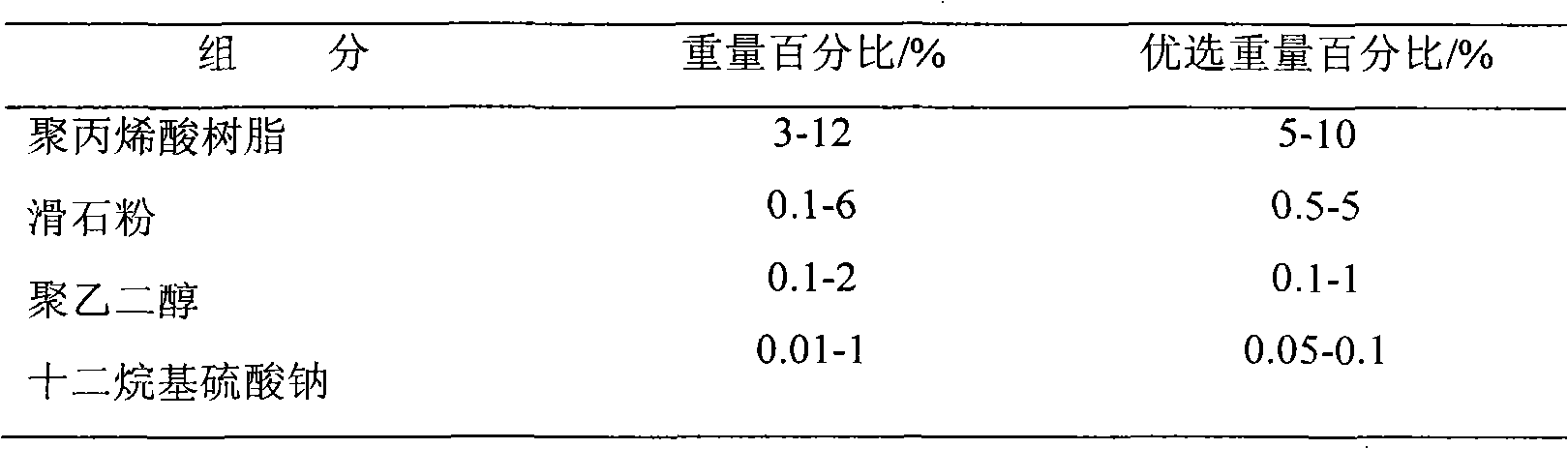
[0031] Sustained release coating composition:
[0032] Polyacrylic resin 3%, talc 6%, macrogol 6000 2%, sodium lauryl sulfate 1%.
[0033] The preparation method is:
[0034] (1) Venlafaxine hydrochloride, microcrystalline cellulose and fumed silicon dioxide are mixed;
[0035] (2) adding a mixed aqueous solution of Tween-80 and hypromellose to the resulting mixture;
[0036] (3) Extrude, spheronize, and dry to obtain a pill core;
[0037] (4) Dissolving polyethylene glycol and sodium lauryl sulfate in water, adding polyacrylic acid resin and talcum powder, to obtain a slow-release coating solution;
[0038] (5) coating the pill core with coating solution to obtain sustained-release pellets;
[0039] (6) Put the sustained-release pellets into hollow capsules to obtain venlafaxine hydrochloride sustained-rel...
Embodiment 2
[0042] Composition of pill core:
[0043] Venlafaxine hydrochloride 45%, microcrystalline cellulose 50%, fumed silicon dioxide 1.5%, Tween-80 2%, hypromellose E5 1.5%.
[0044] Sustained release coating composition:
[0045] Polyacrylic resin 5%, talc 5%, polyethylene glycol 6000 1%, sodium lauryl sulfate 0.1%.
[0046] The preparation method is:
[0047] (1) Venlafaxine hydrochloride, microcrystalline cellulose and fumed silicon dioxide are mixed;
[0048] (2) adding a mixed aqueous solution of Tween-80 and hypromellose to the resulting mixture;
[0049] (3) Extrude, spheronize, and dry to obtain a pill core;
[0050] (4) Dissolving polyethylene glycol and sodium lauryl sulfate in water, adding polyacrylic acid resin and talcum powder, to obtain a slow-release coating solution;
[0051](5) coating the pill core with coating solution to obtain sustained-release pellets;
[0052] (6) Put the sustained-release pellets into hollow capsules to obtain venlafaxine hydrochlorid...
Embodiment 3
[0055] Composition of pill core:
[0056] Venlafaxine hydrochloride 50%, microcrystalline cellulose 45%, fumed silicon dioxide 1%, Tween-80 3%, hypromellose 1%.
[0057] Sustained release coating composition:
[0058] Polyacrylic resin 12%, talc powder 0.5%, polyethylene glycol 6000 0.1%, sodium lauryl sulfate 0.05%.
[0059] The preparation method is:
[0060] (1) Venlafaxine hydrochloride, microcrystalline cellulose and fumed silicon dioxide are mixed;
[0061] (2) adding a mixed aqueous solution of Tween-80 and hypromellose to the resulting mixture;
[0062] (3) Extrude, spheronize, and dry to obtain a pill core;
[0063] (4) Dissolving polyethylene glycol and sodium lauryl sulfate in water, adding polyacrylic acid resin and talcum powder, to obtain a slow-release coating solution;
[0064] (5) coating the pill core with coating solution to obtain sustained-release pellets;
[0065] (6) Put the sustained-release pellets into hollow capsules to obtain venlafaxine hydro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com