Venlafaxine hydrochloride sustained release capsule and preparation method thereof
A technology of venlafaxine hydrochloride and sustained-release capsules, which is applied in the field of medicine, can solve the problems that the spray rate affects the drug application rate, unsatisfactory drug release performance, and poor sustained-release effect of pellets, etc., achieving considerable economic and social benefits , The production environment is friendly, and the drug release effect is stable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-4
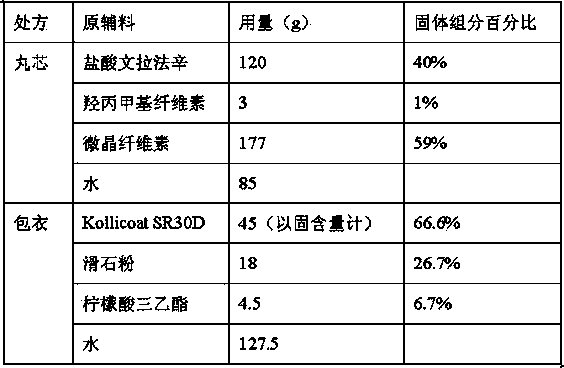
[0064] The inventor prepared pellet cores and coatings respectively by using the following prescriptions, only changing the mass percentage of coating (weight gain) relative to the pellet cores, prepared venlafaxine hydrochloride sustained-release capsules, and obtained products that meet the standards.
[0065]
[0066] The coating weight gains of Examples 1-4 were 13.0%, 14.5%, 16.5%, and 20.5%, respectively.
[0067] Preparation:
[0068] 1. Mix venlafaxine hydrochloride, microcrystalline cellulose, and hypromellose evenly;
[0069] 2. Add water to make soft materials;
[0070] 3. Add to the extruder (Nica E-140) for extrusion, equipment parameters: feeding speed 70rpm; extrusion speed 70rpm; screen diameter 0.8mm;
[0071] 4. The extruded product is spheronized in a spheronizer (CGC-350 multifunctional centrifugal granulation coating machine). Equipment parameters: ball rolling speed 1000rpm; blast frequency 20Hz; blast temperature 25°C; ball rolling time 8-10min;
...
Embodiment 5
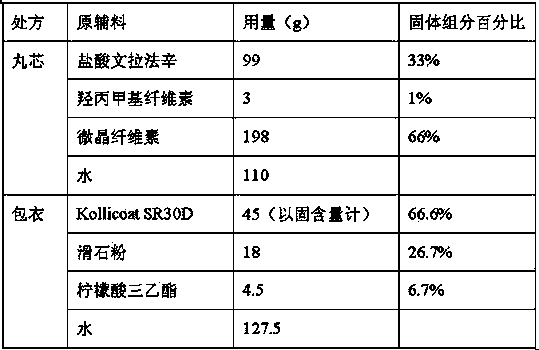
[0082] The inventor changed the ratio of the core components to prepare venlafaxine hydrochloride sustained-release capsules, and also obtained products that meet the requirements.
[0083]
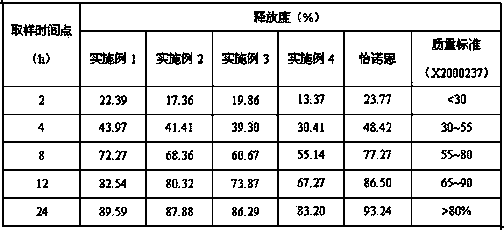
[0084] Coating weight gain was 15.2%. The preparation method is the same as in Example 1. The yield rate of the above prescription and method product is 85%.
[0085] Release measurement: with embodiment 1, the results are shown in the following table:
[0086] .
[0087] Conclusion: Changing the dosage ratio of venlafaxine hydrochloride and microcrystalline cellulose in the preparation of pellet cores, the in vitro release results meet the quality standards.
Embodiment 6
[0089] The inventor changed the ratio of the core components to prepare venlafaxine hydrochloride sustained-release capsules, and also obtained products that meet the requirements.
[0090]
[0091] Coating weight gain was 10.5%. The preparation method is the same as in Example 1. The product yield of the above prescription and preparation method is 86%.
[0092] Release measurement: with embodiment 1, the results are shown in the following table:
[0093] .
[0094] Conclusion: Changing the dosage ratio of venlafaxine hydrochloride and microcrystalline cellulose in the preparation of pellet cores, the in vitro release results meet the quality standards.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com