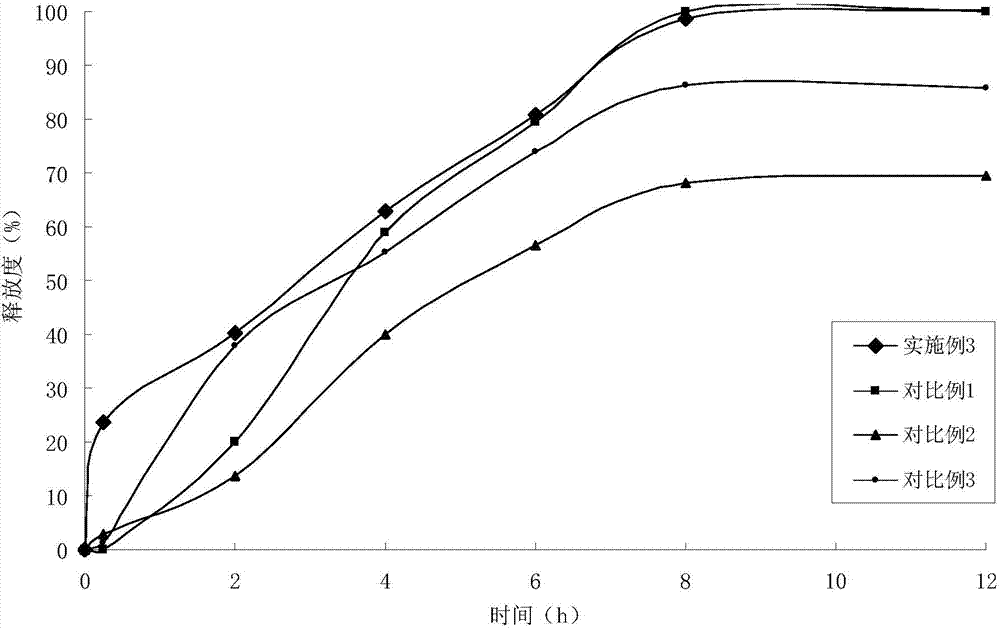
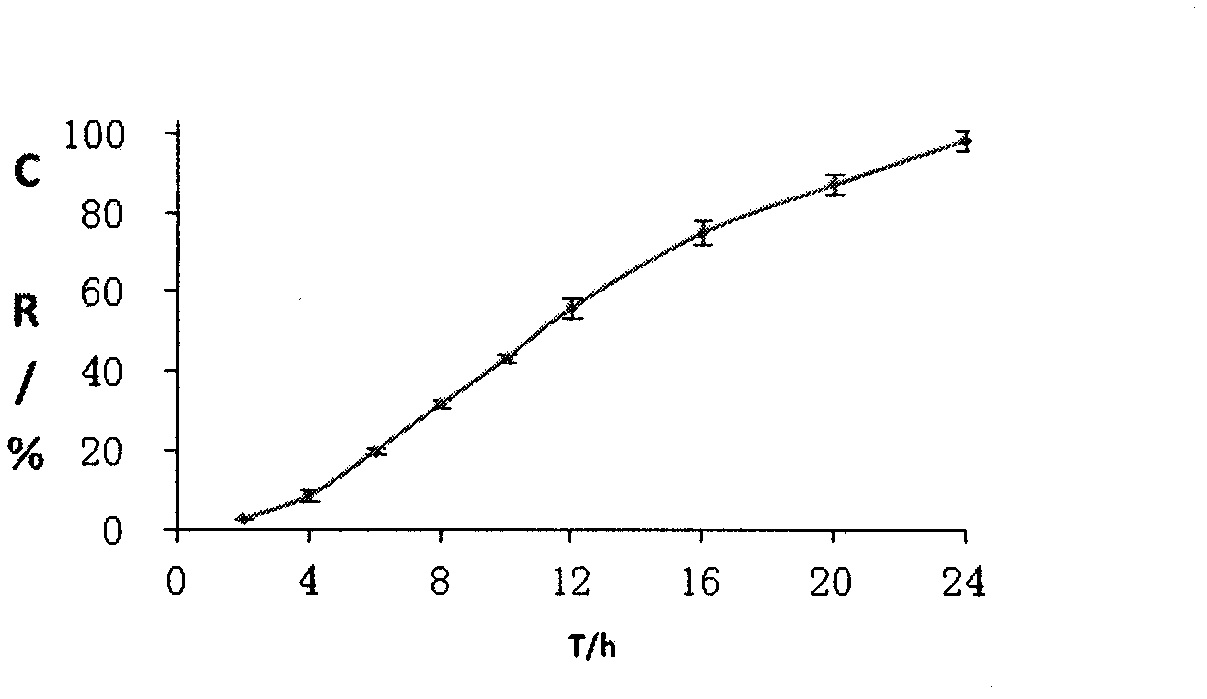
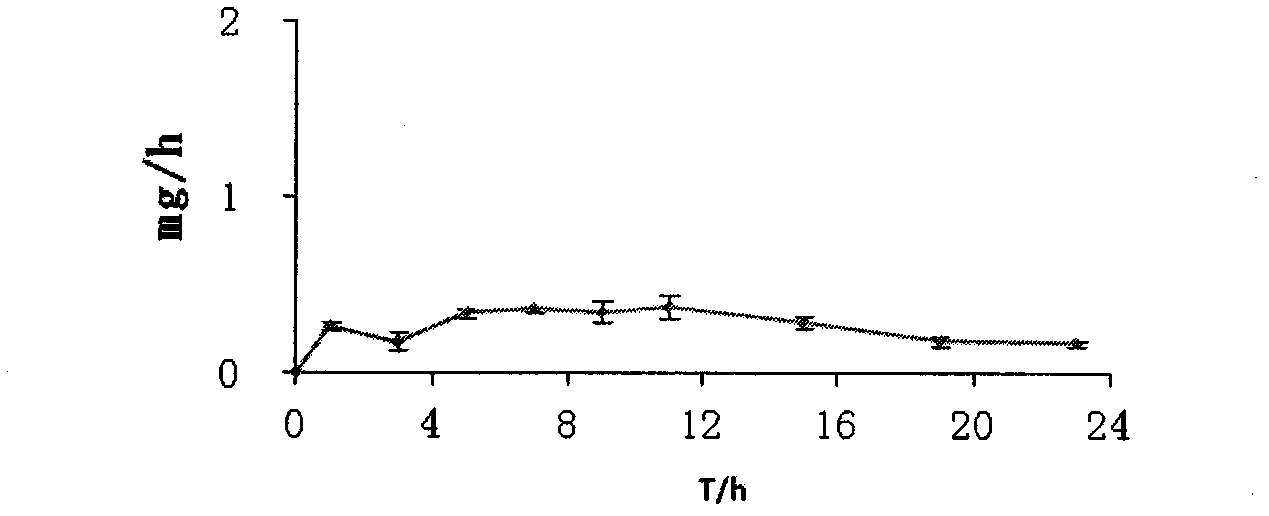
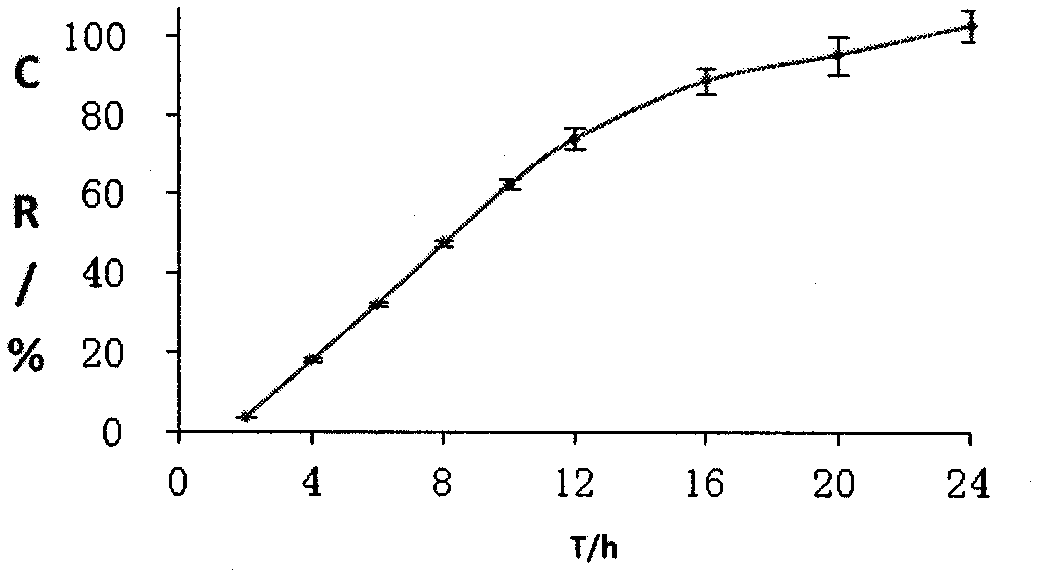
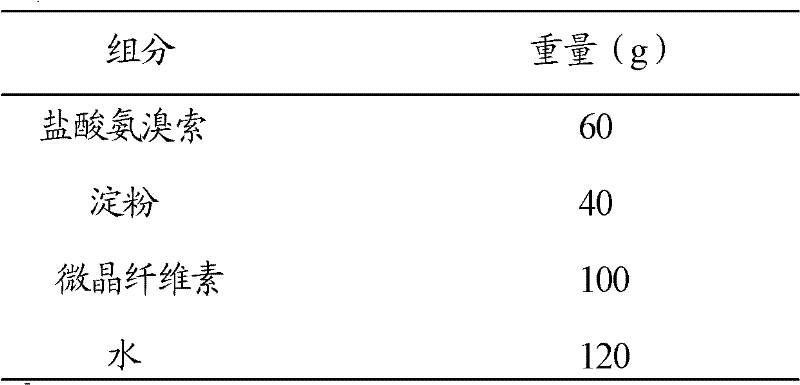
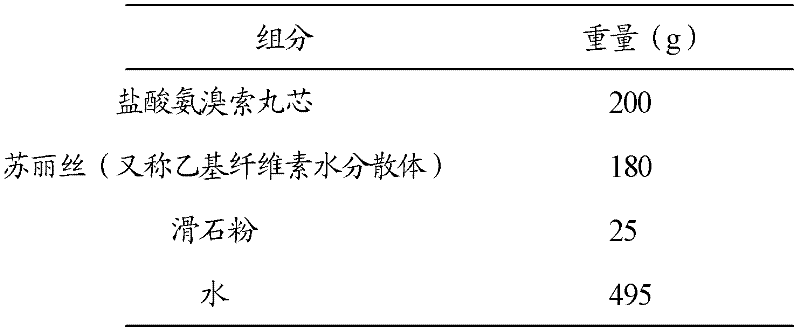
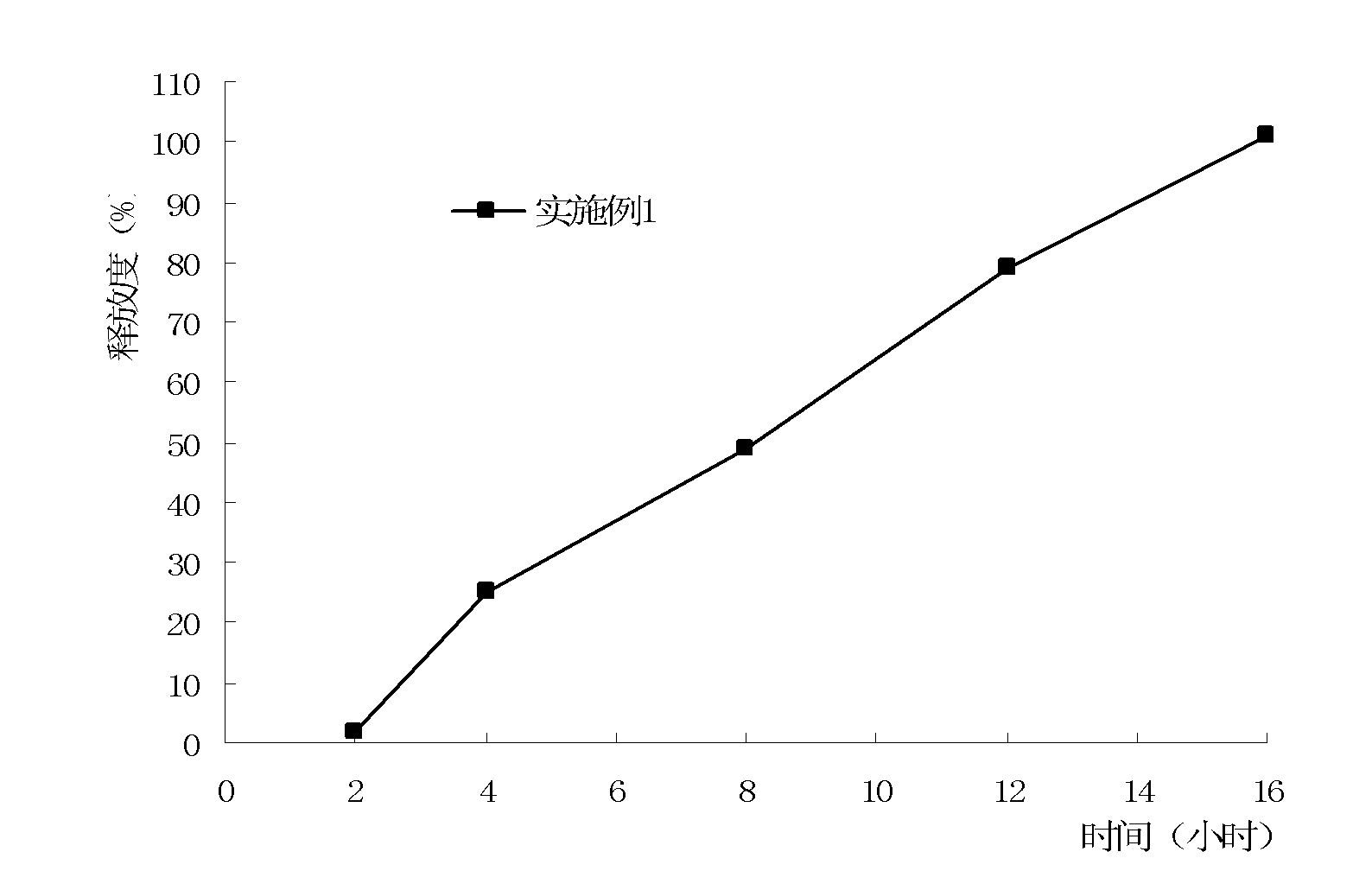
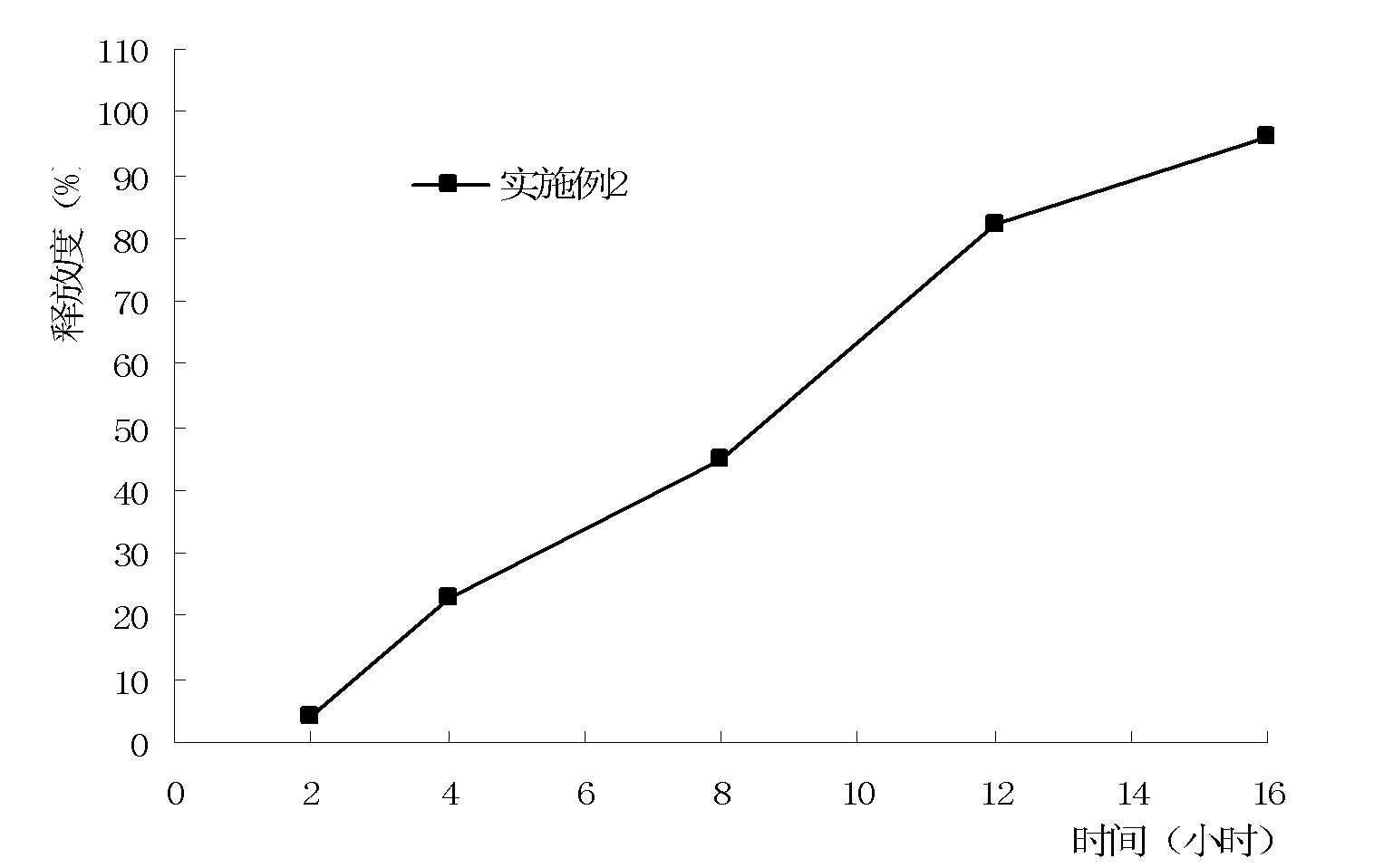
Patents
Literature
119results about How to "Stable drug release" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
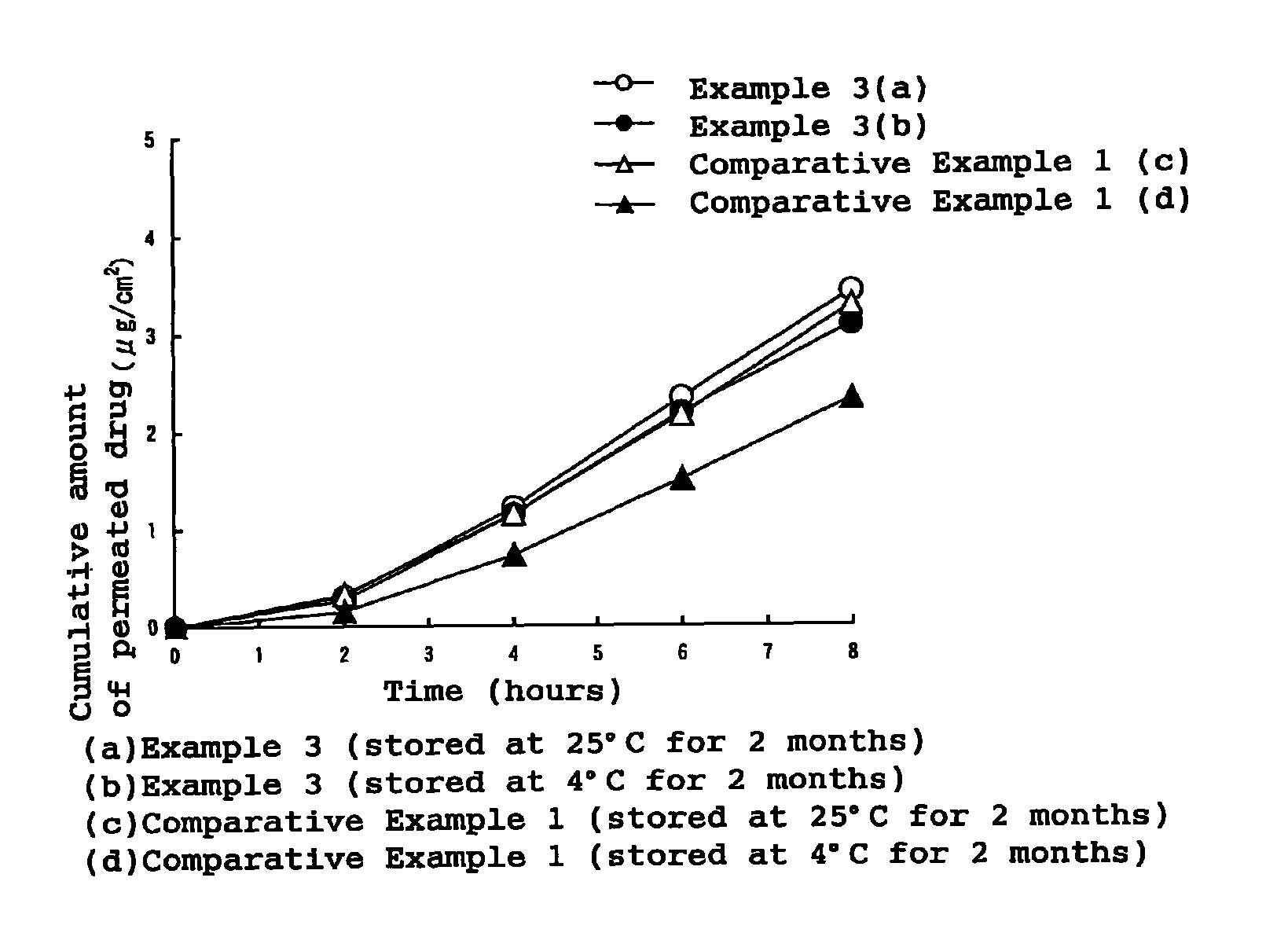
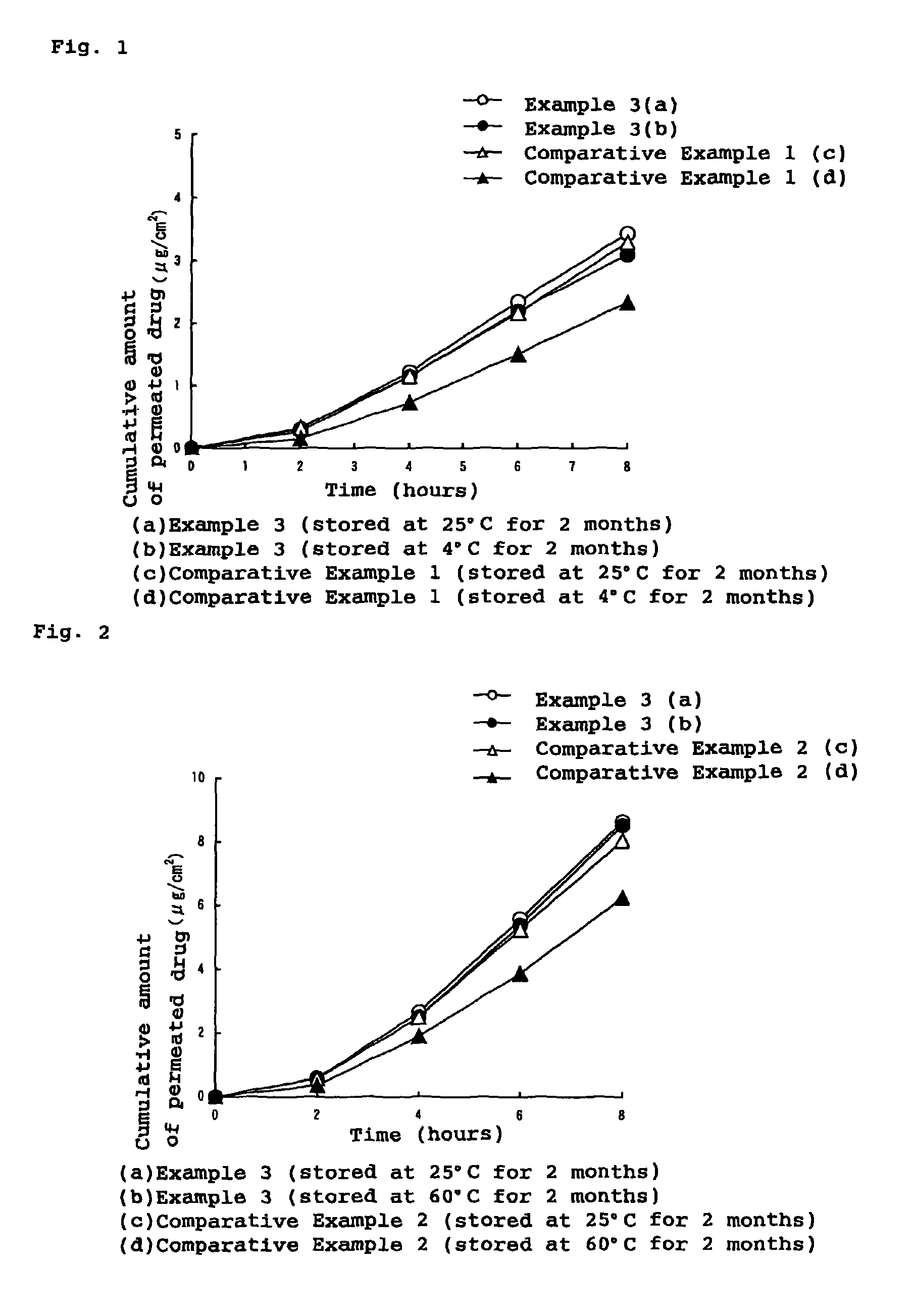
External patch containing estrogen and/or progestogen
InactiveUS8486442B2Stable drug releaseLess irritatingBiocideOrganic active ingredientsIrritationProlonged release
An external patch capable of stable prolonged release and transdermal absorption of active ingredient hormones (estrogens and / or progestogens) contained in a pressure sensitive adhesive layer, which external patch ensures low irritation on the skin. In particular, an external patch comprising a support and, superimposed thereon, a pressure sensitive adhesive layer, characterized in that the pressure sensitive adhesive layer comprises, as indispensable components, 5 to 50 wt. % of styrene / isoprene / styrene block copolymer, 20 to 70 wt. % of tackifier resin, 10 to 60 wt. % of softener and 1 to 20 wt. % of polyvinylpyrrolidone and contains, as an active ingredient, estrogen and / or progestogen.
Owner:TEIKOKU SEIYAKU KK TEIKOKU SEIYAKU CO LTD +1
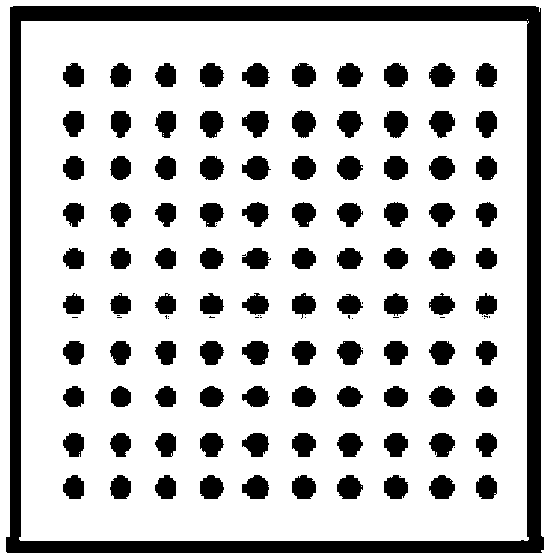
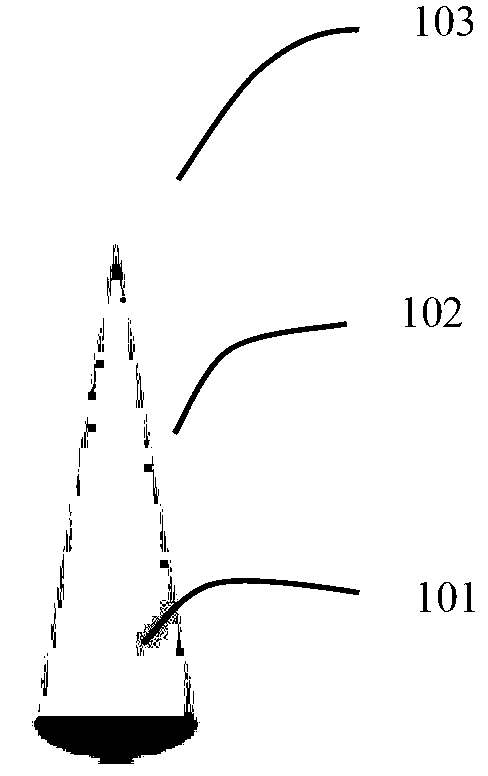
Soluble coaxial-cone multi-layer microneedle, microneedle array and preparation method of microneedle
ActiveCN103301563AEfficient drug releaseStable drug releaseMedical devicesMicroneedlesLayer wiseStructural material
The invention discloses a soluble coaxial-cone multi-layer microneedle. The microneedle is in a cone shape; an internal structure of the microneedle comprises a cone-shaped or conical-cylinder-shaped center layer, and one or more outer layers wrapping the center layer; the center layer and the outer layers are prepared by active drugs and / or structural materials; and a mass ratio of the active drugs to the structural materials is (10:1)-(1:100). The multi-layer structure of the microneedle can be dissolved layer by layer and step by step during intracutaneous contact and body fluid contact, so that the drug release time of one or more drugs and a procedure of drug release dosage can be controlled, and the microneedle can be used for partial skin treatment or whole body administration.
Owner:SUN YAT SEN UNIV
Core-shell type pre-charged chemotherapy medicine embolization microsphere and preparation method thereof
InactiveCN108721684ATargetedWith controlled releaseSurgical adhesivesTissue regenerationEmulsionArterial Embolization
The invention provides a core-shell type pre-charged chemotherapy medicine embolization microsphere and a preparation method thereof. The method comprises the following steps of using water / oil / water(W / O / W) complex emulsion type or oil / water / oil (O / W / O) complex emulsion type emulsified micro liquid drips as templates; coating tiny liquid drips containing medicine into outer layer liquid drips; high molecular polymers in the inner and outer layer liquid drips are converted through measures such as physical measures and chemical measures to form an inner layer core and an outer layer shell. Theformed core-shell structure medicine microsphere is formed by polymers of two-layer structures; the inner core contains medicine; the outer shell is favorable for maintaining the activity of the inner core medicine; the stable medicine release is realized; the sudden release is prevented; the safety is enhanced. The medicine carried microsphere prepared by the method has the advantages that the particle diameter range is 10 to 1000 micrometers; the sphere degree is good; the particle diameter is uniform; (the dimension deviation is 5 percent); the medicine encapsulation rate is high; the structure / carried medicine is controllable; meanwhile, the preparation method of the pre-charged chemotherapy medicine embolization microsphere can provide a novel idea for the clinic application study ofthe transcatheter arterial embolization.
Owner:ENERGY RES INST OF SHANDONG ACAD OF SCI
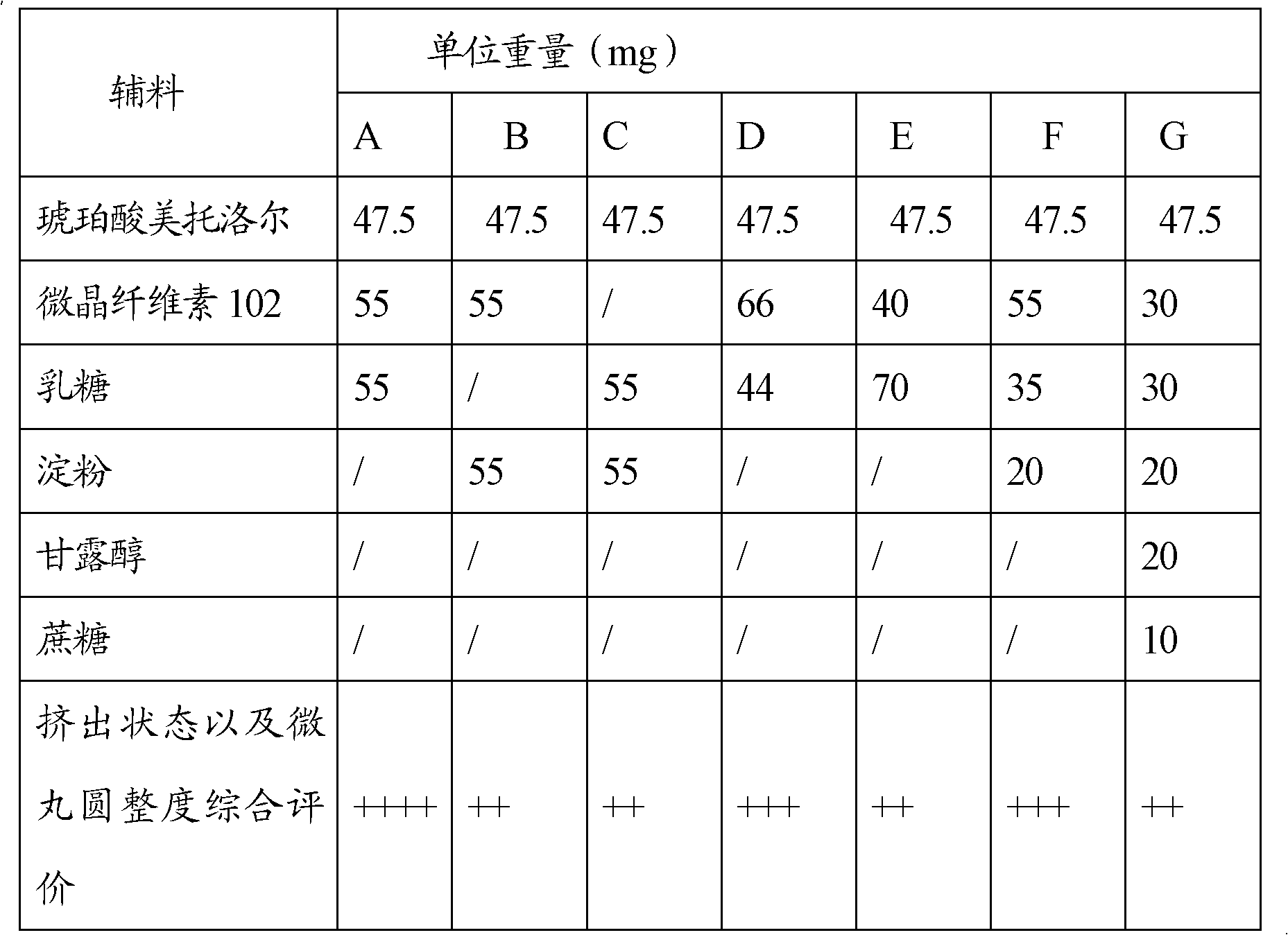
Sustained-release capsules of metoprolol succinate and preparation method thereof
ActiveCN102274205AStable drug releaseSimple processOrganic active ingredientsPharmaceutical delivery mechanismSustained release pelletsMedicine
The invention discloses a metoprolol succinate sustained-release capsule, consisting of a capsule shell and contents; the contents comprise a metoprolol succinate sustained-release pellet; the metoprolol succinate sustained-release pellet comprises a metoprolol succinate contained pill core and a sustained-release coating layer coated outside the metoprolol succinate contained pill core; the metoprolol succinate contained pill core is prepared by using metoprolol succinate as the active ingredient and mixing with an auxiliary material, wherein the weight ratio of the metoprolol succinate to the auxiliary material is 1:1.5 to 1:3. The metoprolol succinate sustained-release medicine disclosed by the invention is stable and the expected effects of all release behaviors in acid, alkaline and water can be achieved. The invention further discloses a method for preparing the metoprolol succinate sustained-release capsule; compared with the prior art, the process is simpler and has good operability and reproducibility.
Owner:佛山市隆信医药科技有限公司
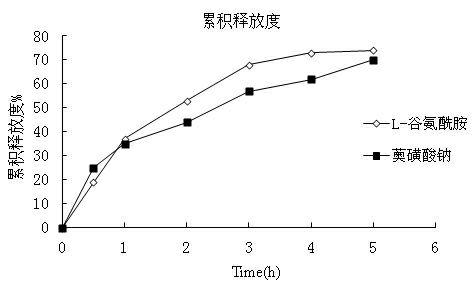
Paster for treating dental ulcer and preparation method thereof
InactiveCN102600122ASimple preparation processStable drug releaseDigestive systemAnhydride/acid/halide active ingredientsCurative effectL-Glutamin
The invention aims to provide a paster for treating dental ulcer and a preparation method thereof. Aiming at the defects of the existing dental ulcer preparation, the oral paster containing L-glutamine and sodium azulenesulfonate has the advantages of simple preparation process, stable drug release, durable action to ulcer position and remarkable curative effect.
Owner:沈阳长秀医药有限公司
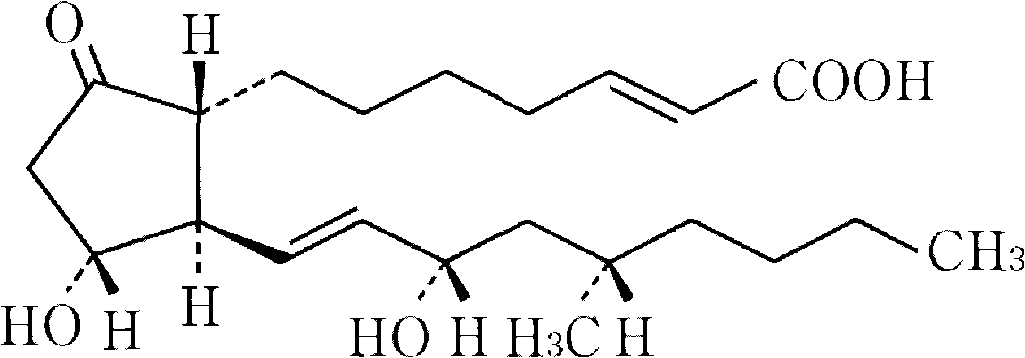
Drug composite containing limaprost and preparation method thereof
ActiveCN101862337ADoes not affect dissolutionDoes not affect disintegrationOrganic active ingredientsPharmaceutical product form changeCoated tabletsMANNITOL/SORBITOL
The invention provides a drug composite containing limaprost and a preparation method thereof, wherein the drug composite of the invention contains 0.01-1% (weight) of the cyclodextrin inclusion compound of limaprost, 0.5-10% (weight) of freeze-drying stabilizer and other pharmaceutically acceptable excipients, wherein the free-drying stabilizer contains mannitol. The drug composite adopts a freeze drying and dry granulating combined preparation technology, greatly overcomes the defects of easy moisture adsorption and extremely bad stability of limaprost which is the main drug, and simultaneously cannot influence the slaking characteristic and the dissolution of the main drug, in addition, the product is not a coated tablet, and thereby the invention prevents the defect of slower dissolution of the conventional coated tablets.
Owner:BEIJING TIDE PHARMA
Pantoprazole and its sodium salt enteric sustained-release pellet preparation
InactiveCN101428005AEliminate local irritationLarge distribution areaOrganic active ingredientsDigestive systemSustained release pelletsIsolation layer
The invention discloses pantoprazole and the sodium salt enteric sustained-release micropill preparation thereof. The preparation comprises pantoprazole-containing micropills, an isolation layer, a sustained-release layer, another isolation layer and an enteric layer from inside to outside in sequence; the weight of the first isolation layer is 0.5% to 40% of that of the pantoprazole-containing micropills, the weight of the sustained-release layer is 5% to 100% of that of the pantoprazole-containing micropills, the weight of the second isolation layer is 0.5% to 40% of that of the pantoprazole-containing micropills, and the weight of the enteric layer is 20% to 200% of that of the pantoprazole-containing micropills. The pantoprazole enteric sustained-release micropills can stably release the drug.
Owner:ZHEJIANG UNIV
Levetiracetam slow release medicinal composite and preparation method thereof
ActiveCN102379857ASimple preparation processPromote reproductionOrganic active ingredientsNervous disorderControl releaseBiocompatibility Testing
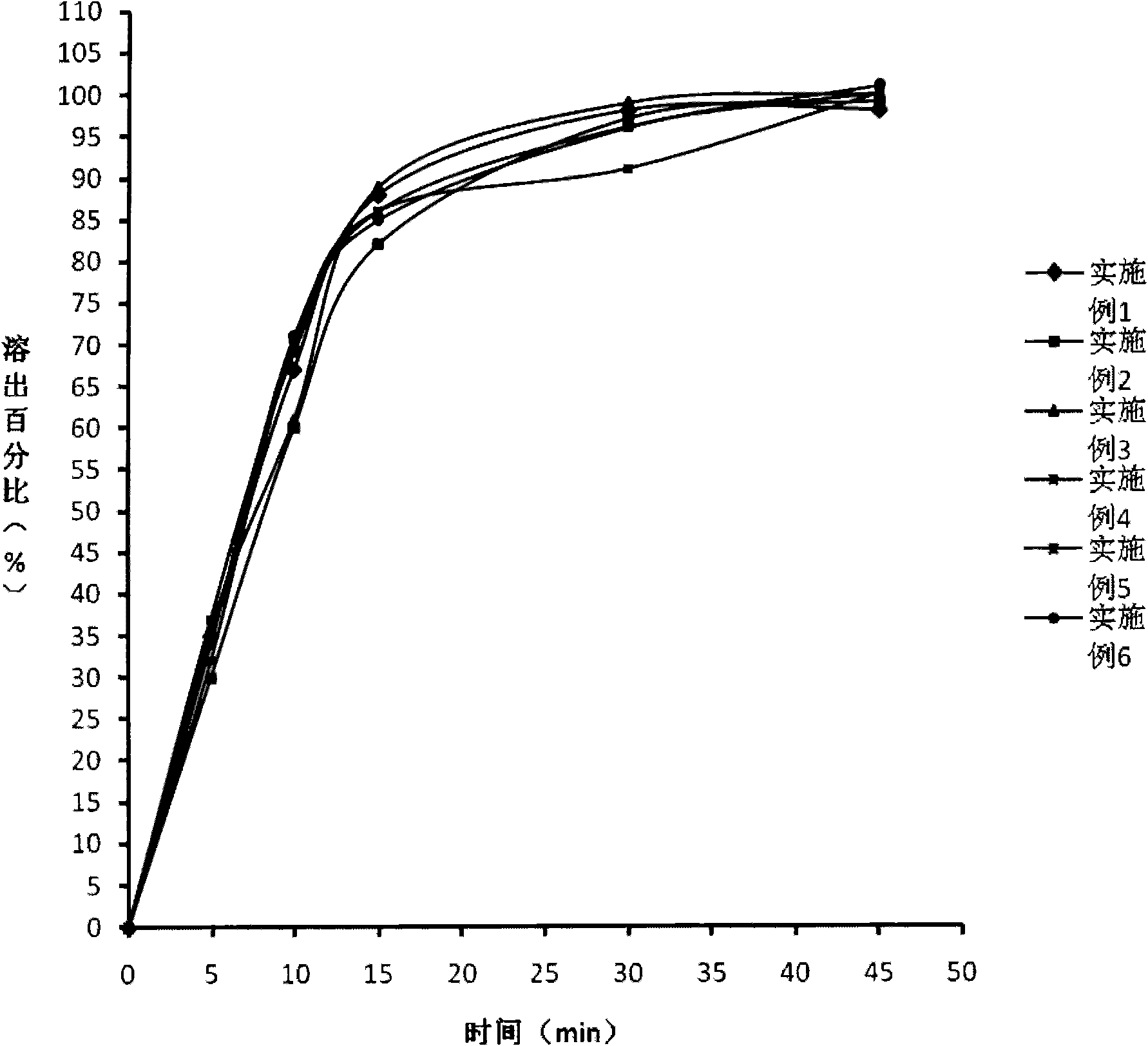
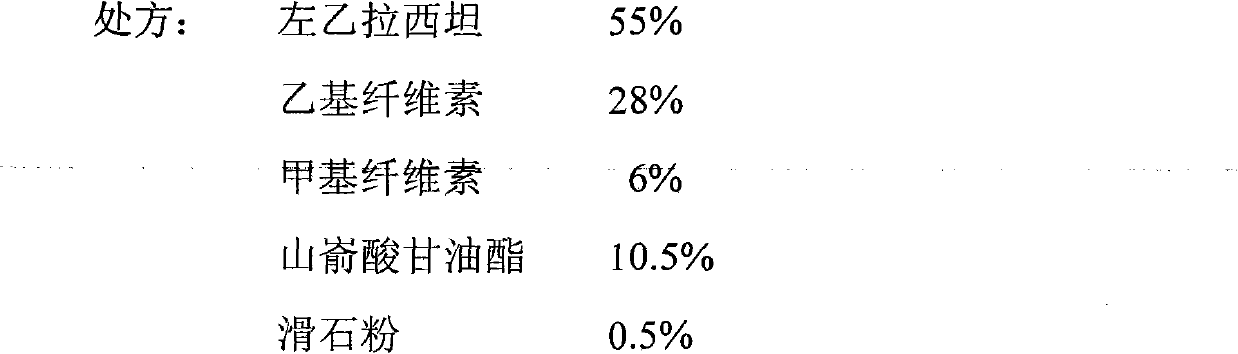
The invention relates to a levetiracetam slow release medicinal composite and a preparation method thereof. The tablet core of the medicinal composite is mainly composed by 55-65% of levetiracetam in weight percentage and 35-45% of non hydrophilia auxiliary material with the slow release function in weight percentage, the non hydrophilia auxiliary material with the slow release function is mixed by one, two or three of ethyl cellulose, methyl cellulose or behenic acid glyceride, and the slow release medicinal composite does not need a functional controlled release coating to adjust the drug release. The levetiracetam medicinal composite has stable drug release performance, good biocompatibility and dissolution performance; and the preparation technology is simple, is easy to reproduce, and is applicable to industrialization production.
Owner:SHENZHEN SALUBRIS PHARMA CO LTD
Gel patch for treating insomnia and method for preparing the same
InactiveCN101468138AOvercome the disadvantages of large side effectsLittle side effectsNervous disorderMacromolecular non-active ingredientsWater basedAdjuvant
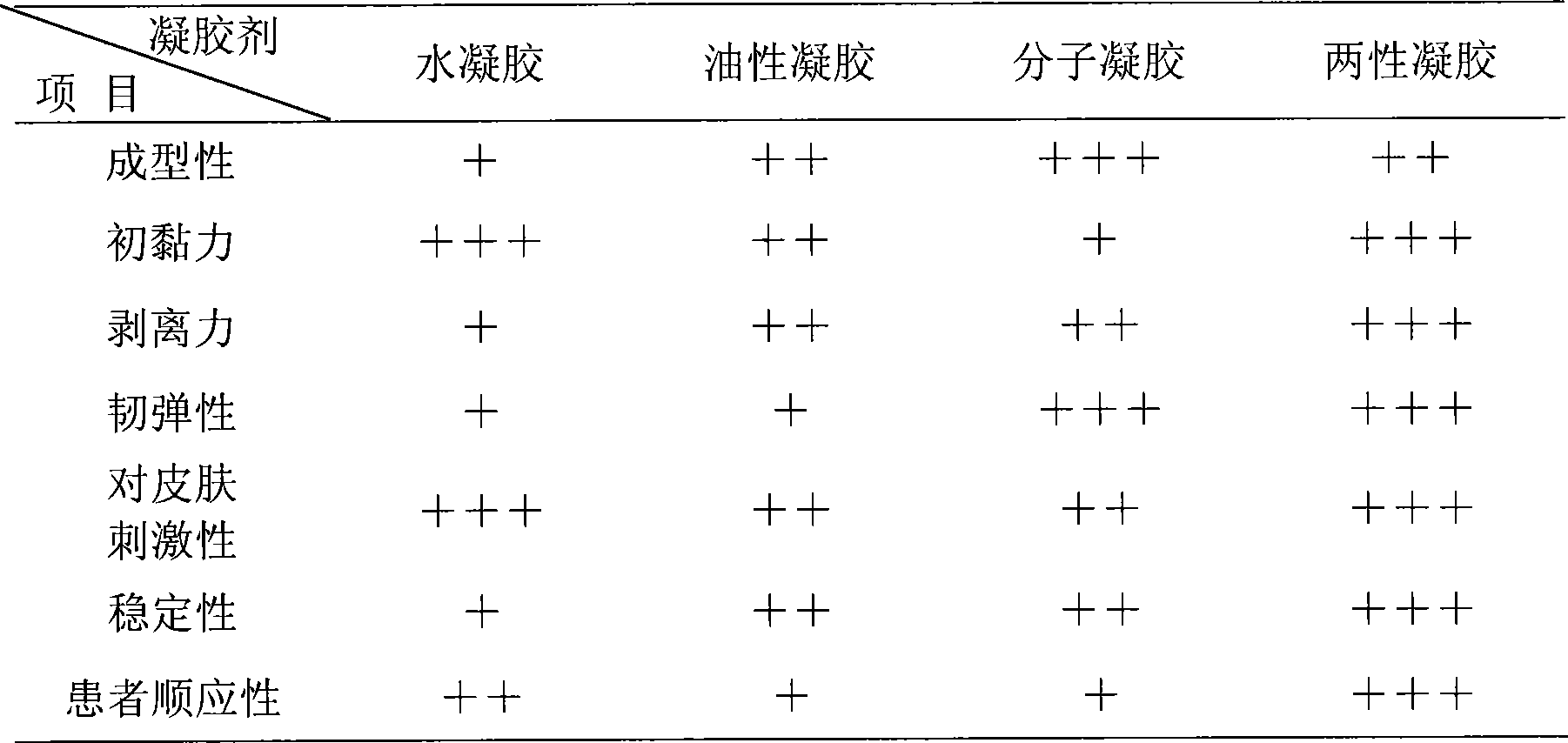
The present invention relates to a gel plaster for sleep disorder and its preparation mehtod thereof, composed of 2% -100% of extract of traditional Chinese medicine and 98% -0% of medicinal adjuvant, wherein the extracts of the lily, tuckahoe are the water-soluble extracts using water extraction method and the extracts of spiny jujube seed, polygala root are fat-soluble extracts using alcohol extraction method. The traditional Chinese medicine plaster is prepared by mixing the molecule gel with the hydrogel and the water-soluble medicine enters into the skin through a water-based channel and the fat-soluble medicine enters into the skin by a fat-soluble channel, thus the production technique prevents the separation of medicine and matrix, at the same time a certain repulsion force of oil-based matrix to the water-based medicine and a certain repulsion force of water-based matrix to the oil-based medicine can promote the medicine absorption and the medicine efficacy is increased.
Owner:TIANJIN ZHONGBAO PHARMACY
Organism degradable star-type structure poly (glycolide-lactide) medicine carrier microsphere and preparation method thereof
InactiveCN1927906AImprove poor wrapping abilityImprove drug loading and embedding efficiencyPharmaceutical non-active ingredientsLong actingDrug
The present invention relates to one kind of biodegradable medicine-carrying polyglycolide-lactide microsphere in star structure and its preparation process. The biodegradable medicine-carrying polyglycolide-lactide microsphere has coating material of star polyglycolide-lactide, coated protein polypeptide medicine of bovine serum albumin or leuteinizing hormone-releasing homone (LH-RH) analog, medicine carrying amount of 17.56-67.51 mcg / mg microsphere and embedding rate of 28.68-78.39 wt%. It is prepared through a W / O / W composite emulsifying process to coat protein polypeptide medicine into star polyglycolide-lactide to form microsphere. Compared with linear medicine-carrying polyglycolide-lactide microsphere, the present invention has obviously increased medicine carrying amount and embedding rate and obvious long-acting and slowly releasing effect.
Owner:NANKAI UNIV
Microsphere preparation encapsulating hydrophilic medicine and preparation method thereof
ActiveCN102266294BAvoid sudden releaseImprove hydrophilicityPharmaceutical non-active ingredientsGranular deliveryParaffin waxMicrosphere
The invention discloses a microsphere preparation encapsulating a hydrophilic medicine, and the microsphere preparation comprises the following components in percentage by weight: a hydrophilic medicine 0.1-40%, methoxy poly(ethylene glycol)-poly(lactic acid) block copolymers 0.1-99%, and poly(lactic acid) or poly(lactic acid-glycollic acid) copolymers 0.1-99%. The microsphere preparation has round and intact surface, uniform and controllable particle diameter, high encapsulation rate, and controllable drug release behaviors. The invention also discloses a preparation method of the microsphere preparation encapsulating the hydrophilic medicine, and the preparation method has simple process and good controllability and comprises the following steps: adding a water solution of the hydrophilic medicine into an organic solution of the methoxy poly(ethylene glycol)-poly(lactic acid) block copolymers, and vortexing to form water-in-oil type primary emulsion; adding the primary emulsion intoliquid paraffin containing emulsifier I, and vortexing to form water-in-oil type multiple emulsion; and adding the multiple emulsion into liquid paraffin containing emulsifier II, continuing stirringfor 4-24 h, centrifuging, collecting pellets, washing, and drying to obtain the microsphere preparation encapsulating the hydrophilic medicine.
Owner:ZHEJIANG UNIV
Pharmaceutical composition for parenteral administration, containing donepezil
InactiveCN105338966AStable drug releaseImprove Medication AdherencePowder deliveryNervous disorderDonepezilParenteral nutrition
The present invention relates to a composition for parenteral administration, containing donepezil as an active ingredient, and a preparation method therefor. Donepezil, which has been conventionally used for oral or transdermal administration, is prepared as microparticles comprising a biodegradable and biocompatible polymer and a release controller so as to be provided as a pharmaceutical composition for sustained release parenteral administration, thereby enabling in vivo sustained release continuously for 2-12 weeks or more. Therefore, it is possible to reduce the frequency of administration to a patient and maintain an effective concentration in the blood for a long time.
Owner:DONG KOOK PHARMA CO LTD
Oral ciclosporin A sustained-release agent and preparation method thereof
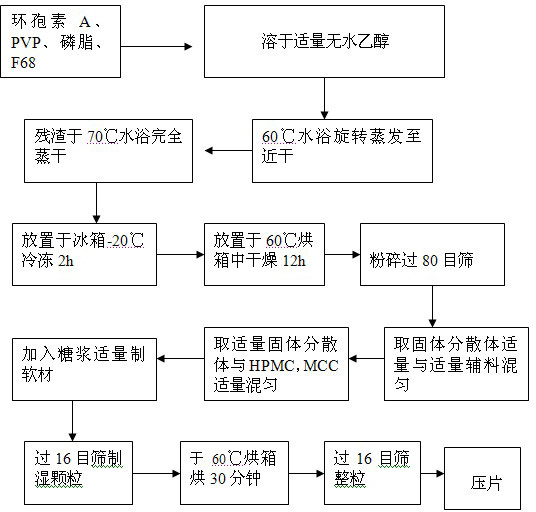
ActiveCN102166201AStable drug releaseImprove bioavailabilityPharmaceutical delivery mechanismCyclic peptide ingredientsCiclosporinSolubility
The invention provides an oral ciclosporin A sustained-release agent. The sustained-release agent comprises the following components in part by mass: 1 part of ciclosporin A, 2.5 to 7.5 parts of polyvidone-K30, 0.15 to 4.82 parts of hydroxypropyl methyl cellulose 4000cPa.s, 0.1 to 3.94 parts of microcrystalline cellulose, 0 to 2.57 parts of hydroxypropyl methyl cellulose 15000cPa.s and 0.1 to 1.0part of phospholipid. By combining a solid dispersing technology and a sustained-release hydrophilic gel matrix technology and based on the 'double release' principle of fast release first and slow release second, the method prepares the insoluble medicament of ciclosporin A sustained-release agent; and the method has the obvious advantages that the solubility of ciclosporin A is improved, and the oral agent can quickly play the effect first and then smoothly and slowly release drug. The ciclosporin A sustained-release tablets reduce the lowered blood concentration peak-valley phenomenon and lower the drug-taking frequency. The invention also discloses a method for preparing the oral ciclosporin A sustained-release agent.
Owner:JIANGSU UNIV +1
Felodipine sustained-release tablet and preparation technology thereof
ActiveCN104758266ARapid drug releaseStable drug releaseOrganic active ingredientsPharmaceutical delivery mechanismSustained release pelletsSustained Release Tablet
The invention discloses a felodipine sustained-release tablet and a preparation technology thereof. According to the preparation technology of the felodipine sustained-release tablet, fast-release solid dispersions, sustained-release micro-pills and pharmaceutically acceptable accessories are mixed and the mixture is compressed to obtain the preparation. The fast-release solid dispersions comprise felodipine, copovidone and polacrilin potassium; the sustained-release micro pills are obtained by coating the fast-release solid dispersions with the ethyl cellulose film with a pore-forming agent. The sustained-release tablet disclosed by the invention has the advantages of fast early-stage releasing speed and slow and stable later-stage releasing speed, so that the drug can be completely released and an effective blood drug concentration can be maintained for a long time; the felodipine sustained-release tablet is high in bioavailability, simple in preparation technology and applicable to industrial mass production.
Owner:CHANGZHOU NO 4 PHARMA FACTORY +1
Gel patch for treating motion sickness and preparation method thereof
InactiveCN101468187ALess irritatingReduce cohesionHydroxy compound active ingredientsDigestive systemMotion sicknessMedicinal herbs
The present invention discloses a gel patch for treating motion sickness and a preparing method thereof. The patch of the invention is composed of a back lining layer, an adhesive layer and a protecting layer. The invention is characterized in that the adhesive layer in the patch is mainly composed by mixing Chinese medicinal herbs, molecular gel, hydrogel and bleeding promoting agent. The composition and weight parts of Chinese medicinal herbs are as follows: 1-50 parts of fresh ginger, 1-25 parts of orange peel, 1-25 parts of agastache rugosa and 1-25 parts of bornyl alcohol. The weight part of each component in the adhesive layer is as follows: 1-30 parts of Chinese medicinal herb extract, 50-200 parts of molecular gel, 100-500 parts of hydrogel and 1-20 parts of bleeding promoting agent. The gel patch of the invention has the advantages of remarkable preventing and treating effect to motion sickness, little side effect, smooth medicine releasing, small excitation to skin, easy carrying, and convenience for the using of patients suffering from motion sickness.
Owner:TIANJIN ZHONGBAO PHARMACY
Isradipine controlled-release tablets and preparation method thereof
ActiveCN102349880AMedication safetyStable drug releaseOrganic active ingredientsPharmaceutical non-active ingredientsPharmaceutical preservativesPlasticizer
The invention provides a method for preparing isradipine controlled-release tablets. The isradipine controlled-release tablets comprise a double-layer tablet core composed of a medicament-containing layer and a promoting layer, a semi-permeable membrane, a drug-releasing small hole and a moistureproof coating layer, wherein the medicament-containing layer comprises active ingredients as isradipine and pharmaceutically acceptable excipients; the promoting layer at least comprises macromolecular osmoactive substances, water-insoluble polymer, penetration enhancer, colouring agent and others; and the semi-permeable membrane is composed of a film-forming material, a plasticizer and a porogenic agent.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
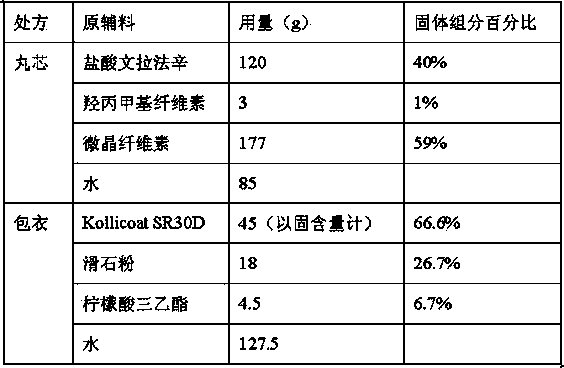
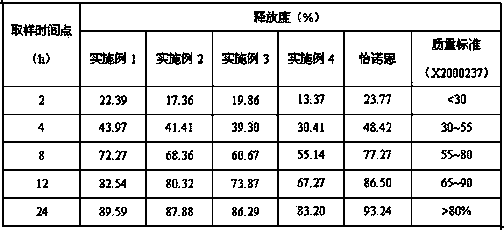
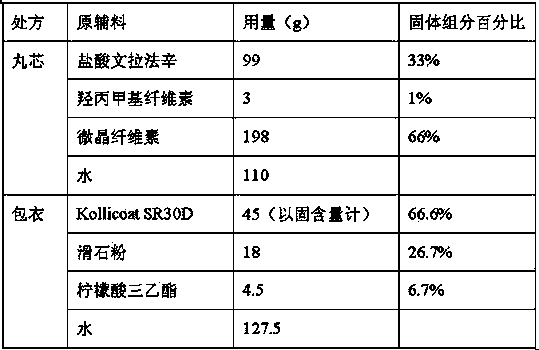
Venlafaxine hydrochloride sustained release capsule and preparation method thereof
ActiveCN103893151AHigh yieldRelease facilitates controlOrganic active ingredientsNervous disorderSustained release pelletsSocial benefits
The invention relates to a venlafaxine hydrochloride sustained release capsule. Sustained release pellets serve as contents of the sustained release capsule prepared by the invention, wherein the pellets comprise medicine-containing pill cores and sustained release coatings; the medicine-containing pill cores contain venlafaxine hydrochloride, hydroxypropyl methyl cellulose and microcrystalline cellulose. The venlafaxine hydrochloride sustained release capsule disclosed by the invention is stable to release medicines, free from a problem of organic solvent residue, safe to take and environment-friendly during producing. Furthermore, the invention provides a preparation method of the venlafaxine hydrochloride sustained release capsule. The method is high in production efficiency, simple in process, convenient to operate, low in cost and easy in industrial production; the method can be used for saving the cost for medicine production enterprises, and can bring about considerable economic and social benefits.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
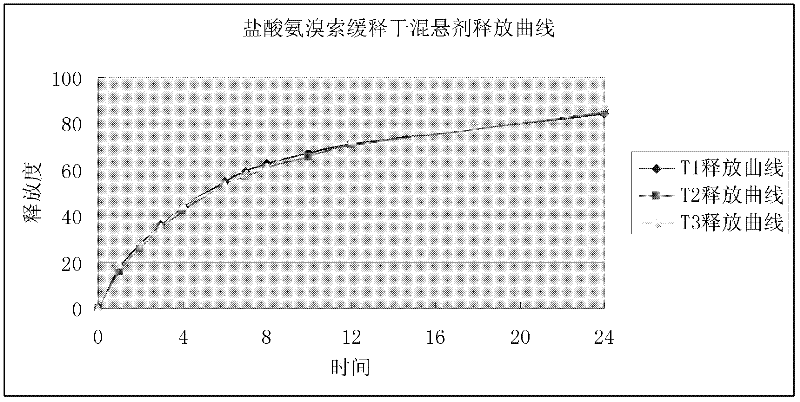
A kind of ambroxol hydrochloride sustained-release dry suspension and preparation method thereof
ActiveCN102266293AImprove complianceSolve residual problemsOrganic active ingredientsPharmaceutical non-active ingredientsSustained release pelletsWarm water
The invention discloses an ambroxol hydrochloride sustained-release dry suspension, which is prepared from the following components in percentage by weight: 20 to 60 percent of sustained-release micro-pills, 39.9 to 79.5 percent of suspending aid and 0.1 to 0.5 percent of flavoring agent, wherein the sustained-release micro-pills are prepared by wrapping a sustained-release coating layer outside medicine-carrying pill cores, and the medicine-carrying pill cores comprise 10 to 30 weight percent of ambroxol hydrochloride and 70 to 90 weight percent of pharmaceutic adjuvant. The ambroxol hydrochloride sustained-release dry suspension can be released continuously for 24 hours, can be taken by adding proper amount of warm water, is particularly suitable for treating phlegm eliminating treatment of acute and chronic respiratory tract diseases in olds, children and people who have difficulty in swallowing, and can increase the compliance of administration of patients. The invention also discloses a preparation method for the ambroxol hydrochloride sustained-release dry suspension.
Owner:佛山市隆信医药科技有限公司
Preparation method of melbinum osmotic pump controlled release tablets
ActiveCN102133204AHigh dissolution rateIntrinsic quality is stableOrganic active ingredientsMetabolism disorderIrritationControlled Release Tablet
The invention relates to a preparation method of melbinum osmotic pump controlled release tablets for treating type 2 diabetes. The preparation method comprises the steps of preparing tablet cores, coating semi-permeable membrane layers, perforating, drying and coating damp-proof layers, characterized in that when the tablet cores are prepared, a medicinal salt of melbinum and a nonionic surfactant are added, and the nonionic surfactant accounts for 2-20wt.% of the medicinal salt of melbinum. The medicament disclosed by the invention has stable release, no irritation on intestinal tracts and few times of administration, is acceptable by patients, and greatly improves the compliance of patients.
Owner:SHANDONG XINHUA PHARMA CO LTD
Nifedipine double-layer osmotic pump medicinal composition and preparation technology thereof
ActiveCN102178677AMedication safetyDrug safetyOrganic active ingredientsPill deliveryAdhesiveSolvent
The invention provides a nifedipine double-layer osmotic pump medicinal composition and a preparation technology thereof. The composition comprises a double-layer tablet core and a controlled release coating membrane thereof, wherein the double-layer tablet core is composed of pharmaceutically acceptable excipients and comprises one or more than one of a macromolecular suspending aid substance, an osmotic pressure active substance, a permeation enhancer, a dissolving aid substance, a colorant, a macromolecule swelling substance, a lubricant, a flow aid, an adhesive and a solvent; and the controlled release coating membrane is composed of one or more than one of a membrane forming material, a pore-forming agent and a plasticizer. The nifedipine double-layer osmotic pump medicinal composition prepared by the invention can effectively control a medicament to release in a specific time so that the medicament taking time can be decreased.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
Blood-nourishing and cephalocathartic micropills and its preparation technology
InactiveCN101053607AExcellent disintegration and solubilityThe curative effect is fastNervous disorderGranular deliveryBiotechnologyCassia
The invention relates to a micro-pill preparation and its producing craft, especially a micro-pill for tonifying blood and clearing liver heat and its producing craft. The material drug is produced according to following weight shares: angelica 250-420; ligusticus wallichii franchet 280-40; Paeonia lactiflora pallas 200-380; ripe radix rehmanniae 200-400; gambir plant 580-750; suberect spatholobus stem 550-780; brunella vulgaris linne 600-720; cassia seed 500-750; nacre 560-750; corydalis tuber 200-420; manchurian wildginger 30-100. The invention has a good resolvability, a smart curative effect, a stable medicine releasing, a high bio-availability, a small local thrill, a beautiful pill appearance, a small moisture absorption, and a good feeling for patients, further it is easy to transport, store and carry.
Owner:马鸿森
'Gushukang' micropills and its preparation technology
InactiveCN101053608AExcellent disintegration and solubilityThe curative effect is fastSkeletal disorderGranular deliverySalvia miltiorrhizaLongspur
The invention relates to a micro-pill preparation and its producing craft, especially the Gushukang micro-pill and its producing craft. The Gushukang micro-pill is produced according to following weight shares: longspur epimedium 100-1000; ripe radix rehmanniae 200-400; drynaria 60-660; astragalus root 130-1400; radix salviae miltiorrhizae 60-660; dried edible fungus 50-530; cucumber-seed 50-530. The invention has a good resolvability, a smart curative effect, a stable medicine releasing, a high bio-availability, a small local thrill, a beautiful pill appearance, a small moisture absorption, and a good feeling for patients, further it is easy to transport, store and carry.
Owner:杨义澄
Heart-benefiting Yixinshu micropill and its prepn process
InactiveCN101088546AExcellent disintegration and solubilityThe curative effect is fastPill deliveryCardiovascular disorderMANNITOL/SORBITOLMedicine
The present invention is heart-benefiting Yixinshu micropill and its preparation process. The heart-benefiting Yixinshu micropill is prepared with ginseng 100-500 weight portions, ophiopogon root 100-500weight portions, schisandra 100-300weight portions, astragalus root 150-450weight portions, red sage 200-600weight portions, Chuanxiong rhizome 100-300weight portions, haw 200-400weight portions, avicel 300-500weight portions, and mannitol 50-200weight portions. It has diameter smaller than 0.05mm, excellent disintegrating and dissolving performance, fast acting, high bioavailability, and other advantages, and possesses functions of promoting blood circulation to disperse blood clots, benefiting qi, nourishing yin, etc.
Owner:葛浩斌
Preparation method of injectable self-curing hypoglycemic hydrogel
InactiveCN103550139AAvoid harmDoes not affect normal lifeMetabolism disorderAerosol deliveryPolyethylene glycolDiabetic complication
The invention belongs to the field of biomedical science, and particularly relates to a preparation method of injectable self-curing hypoglycemic hydrogel. The preparation method is characterized in that dextran (Dex) and polyethylene glycol (PEG) are used as main raw materials and are subjected to chemical modification so as to obtain derivatives of the dextran and the polyethylene glycol, namely the furyl dextran derivative (DF) and the maleimide polyethylene glycol (Mal-PEG-Mal) derivative, the self-curing hydrogel material controlled in gelling time can be obtained through Diels-Alder reaction, and a hypoglycemic medicament comprising one or more of sulfonylureas, biguanides, insulin and the like is loaded on the hydrogel material. The purpose of stably controlling blood sugar for a long time is achieved by slowly releasing the hypoglycemic medicament, the morbidity of diabetic complications is reduced, and even the life span of patients is prolonged.
Owner:TONGJI UNIV
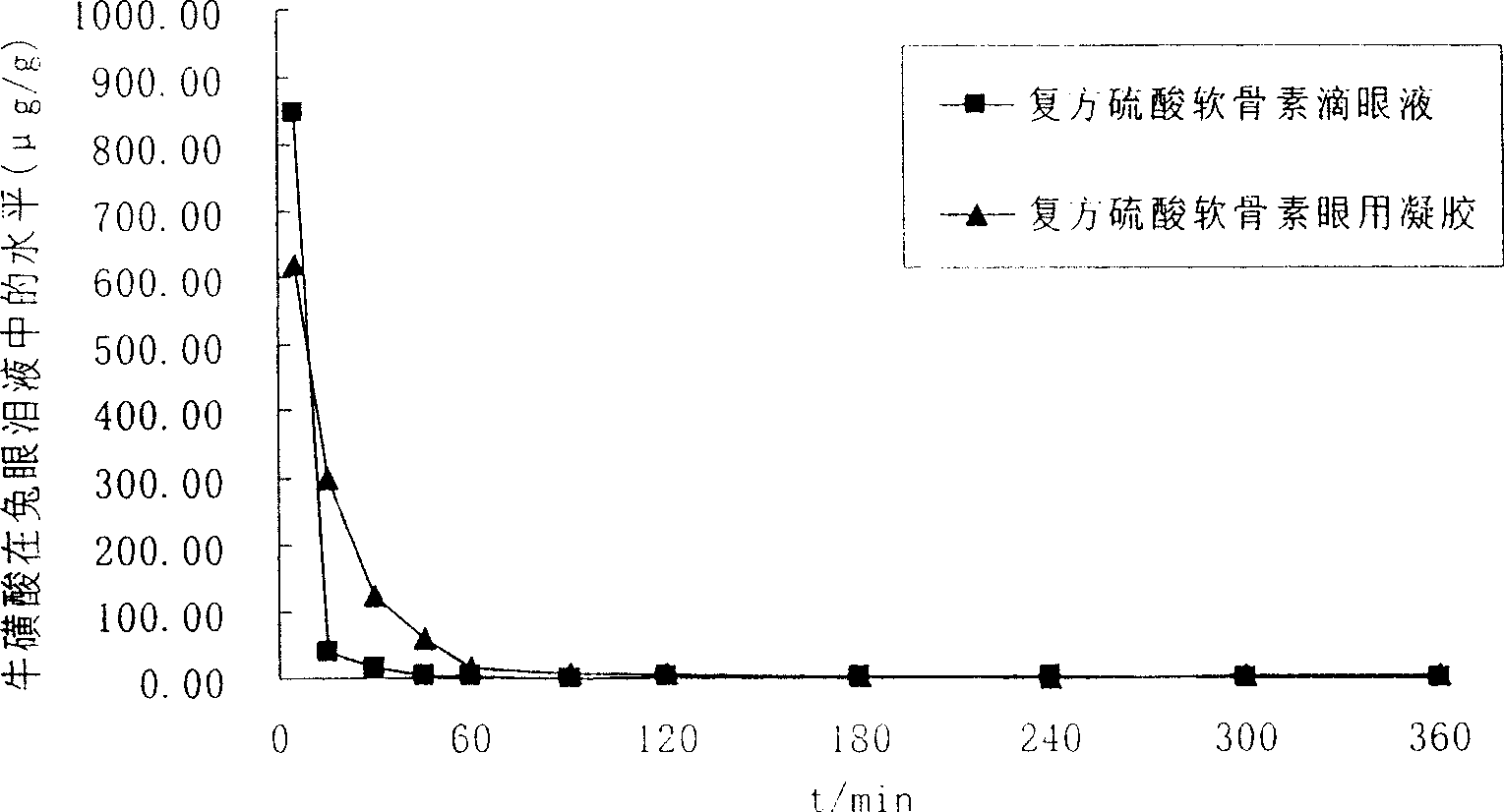
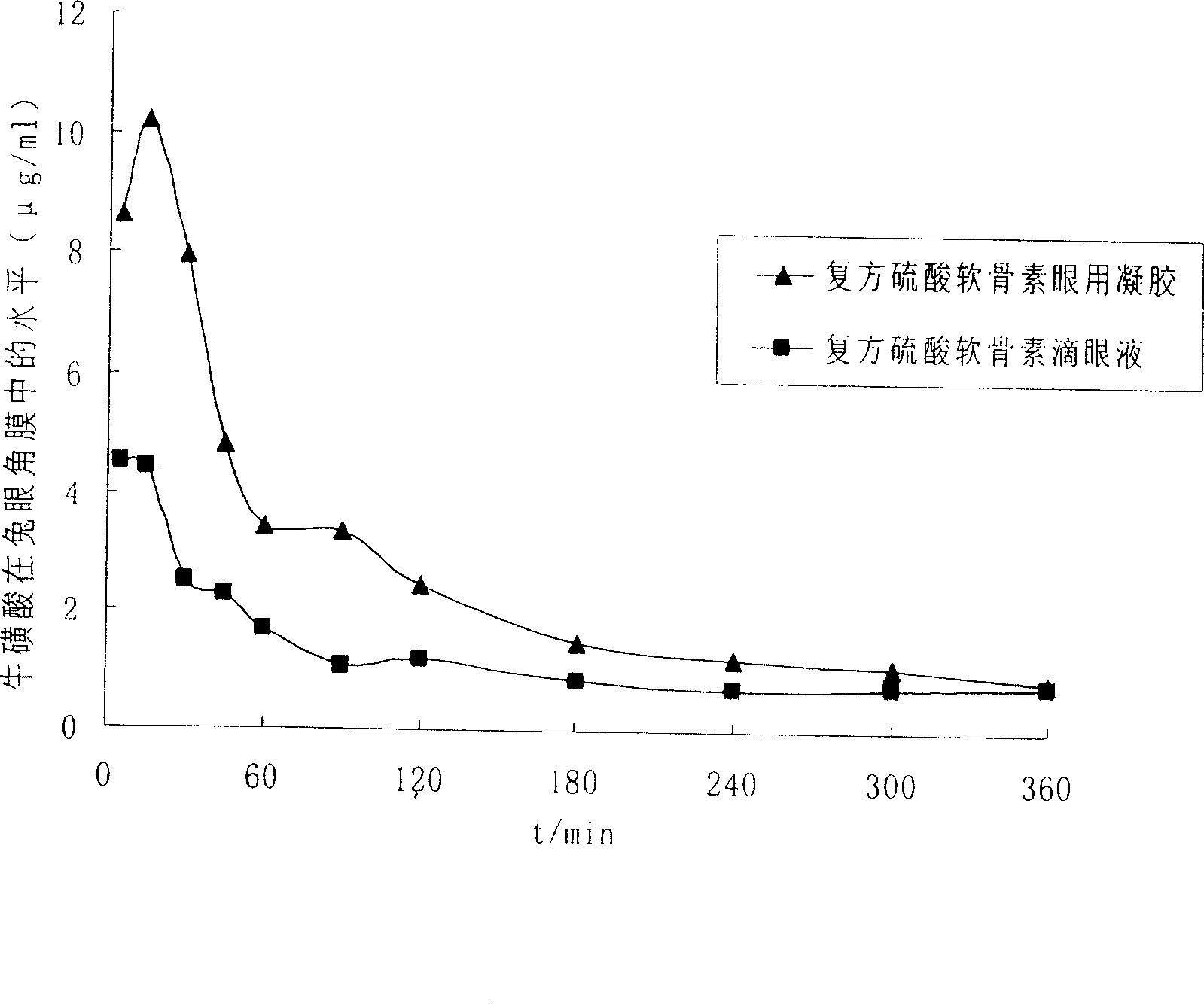
Ophthalmic gel containing chondroitin sulfate and method for preparing same
ActiveCN101543509AGood lookingSmooth appearanceSenses disorderPharmaceutical delivery mechanismIrritationBiocompatibility Testing
The invention relates to an ophthalmic gel containing chondroitin sulfate and a method for preparing the same. The ophthalmic gel comprises chondroitin sulfate and taurine in effective dose and an aqueous gel substrate in proper amount. Compared with eyedrop, the ophthalmic gel has the advantages of good biocompatibility, small stimulation, simple preparation process and the like.
Owner:SHENYANG XINGQI PHARM CO LTD
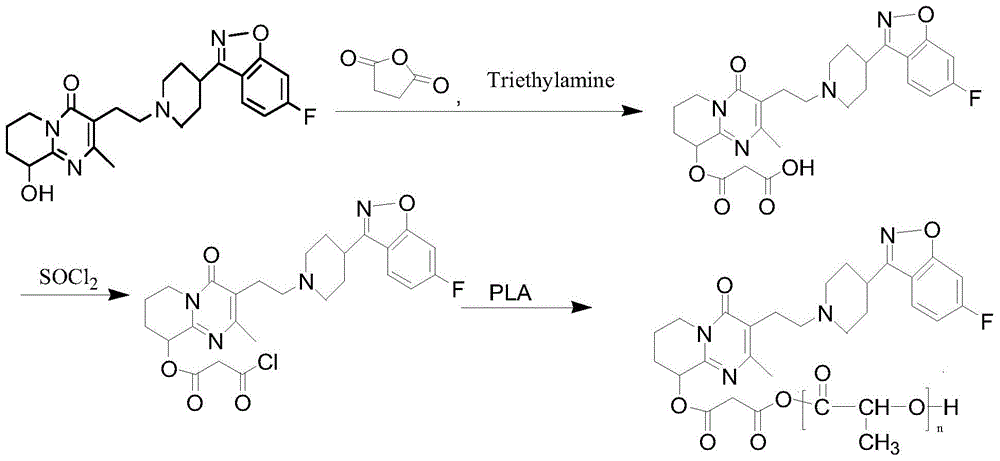
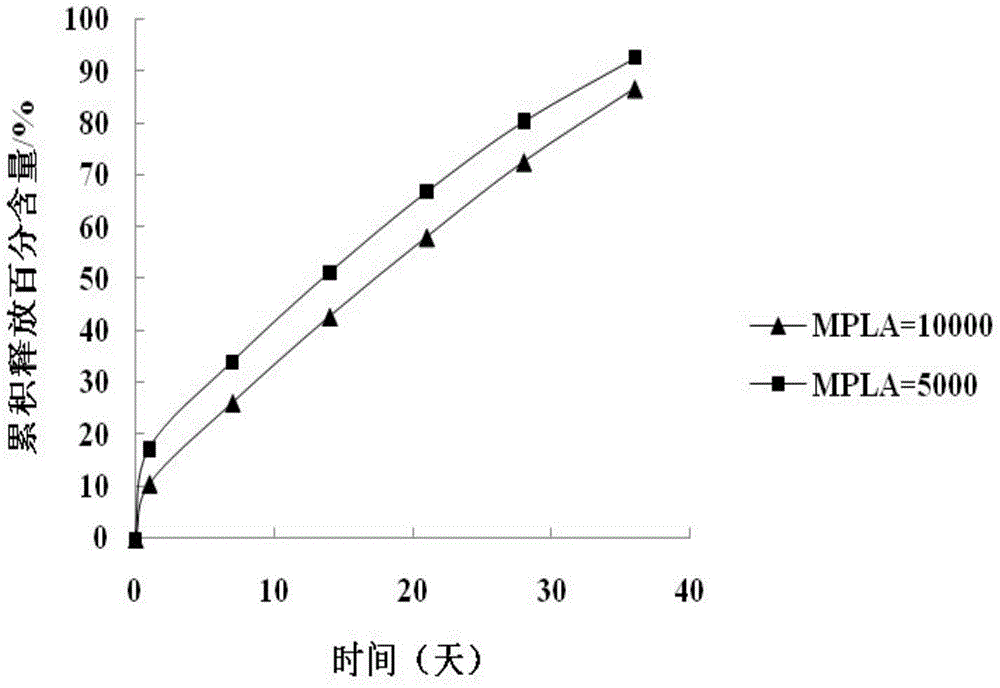
Preparation method of paliperidone sustained release microsphere injection
InactiveCN104940150AImprove toleranceStable blood concentrationPowder deliveryOrganic active ingredientsMicrosphereDrug release
The invention discloses a preparation method of a paliperidone sustained release microsphere injection. The preparation technology comprises the following steps: (1) paliperidone and an acid anhydrides reagent are respectively dissolved in a solvent to be subjected to esterification reaction under the effect of an alkaline catalyst; (2) the esterification product obtained in the step (1) is dissolved in anhydrous dichloromethane to be subjected to acylation reaction with the added acylation reagent with DMF (N, N- dimethylformamide) being the catalyst; (3) the acylated paliperidone is subjected to esterification reaction with polylactic acid (PLA) through chemical bond bonding; (4) paliperidone sustained release microspheres are prepared by adopting solvent evaporation method, and the paliperidone sustained release microspheres are prepared into the microsphere injection after being cooled and dried. The preparation method is safe to operate, mild in reaction condition and simple and clear in steps; the total yield reaches 50% or above; the particle sizes of the obtained paliperidone microspheres are 30-50 micron; the paliperidone sustained release microsphere injection provided by the invention is high in drug loading capacity, stable in drug release, long in drug release time and good in sustained release effect.
Owner:HEILONGJIANG UNIV
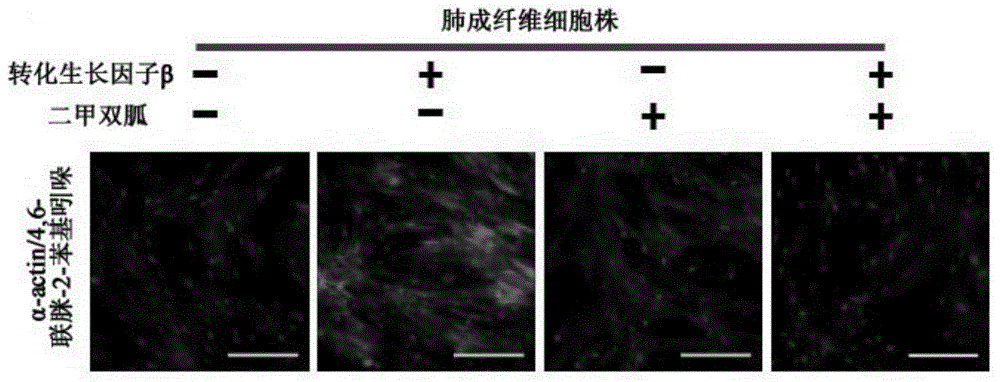
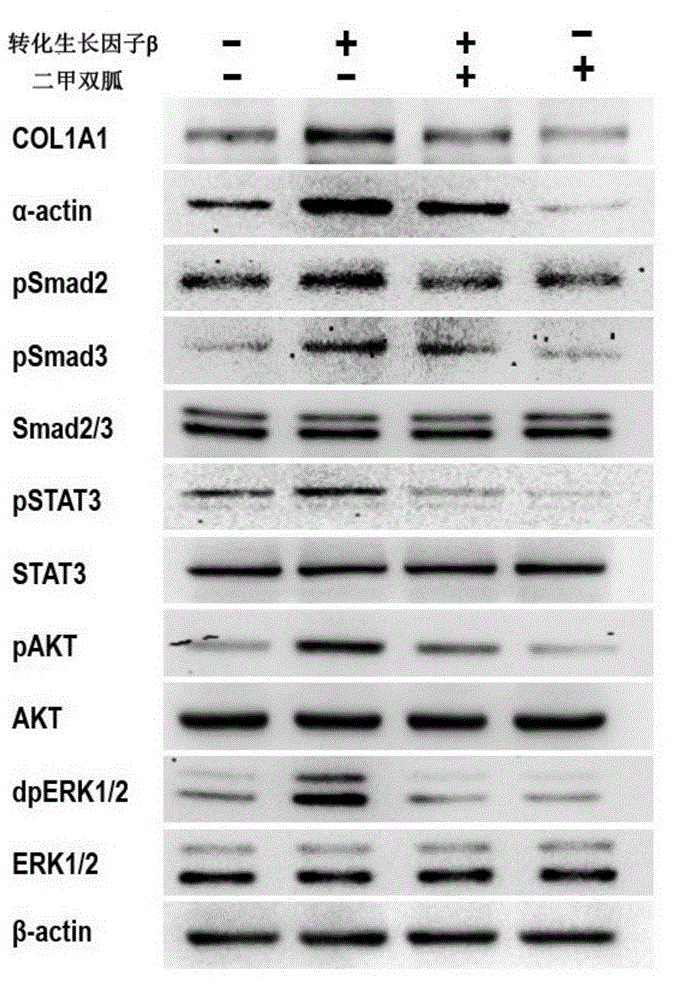
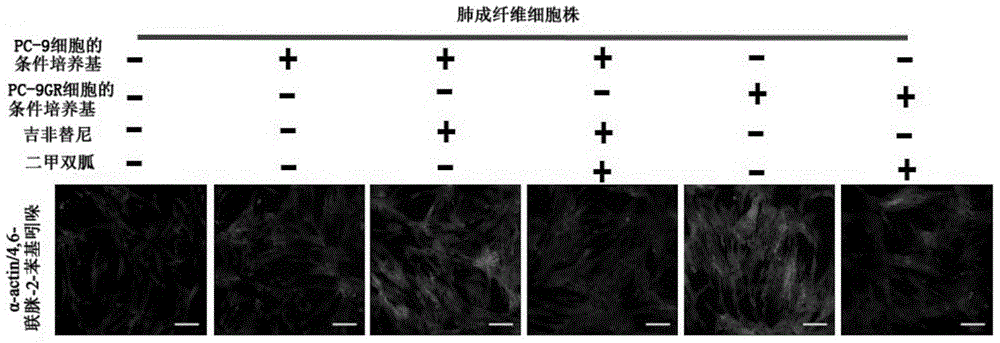
Application of biguanide drugs in preparation of drugs for preventing or weakening pulmonary fibrosis
InactiveCN106138020APrevent or slow downPrevent or slow the developmentOrganic active ingredientsRespiratory disorderTherapeutic effectNon small cell
The invention relates to new application of biguanide drugs, in particular to application of biguanide drugs in preparation of drugs for preventing or weakening pulmonary fibrosis. The pulmonary fibrosis is complications produced by EGFR-TKI or ALK-TKI in treatment of non-small cell lung cancers. The biguanide drugs can avoid the complications produced by the EGFR-TKI or ALK-TKI in treatment of non-small cell lung cancers, effectively improve an EGFR-TKI or ALK-TKI treatment effect and reduce occurrence and development of patients' complications.
Owner:THE THIRD AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIV OF PLA
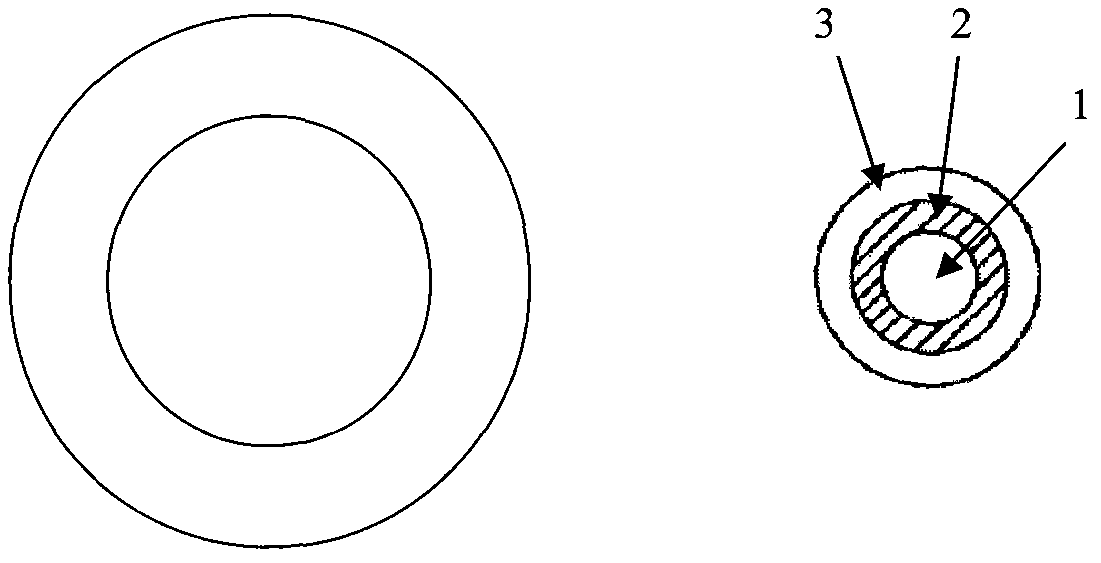
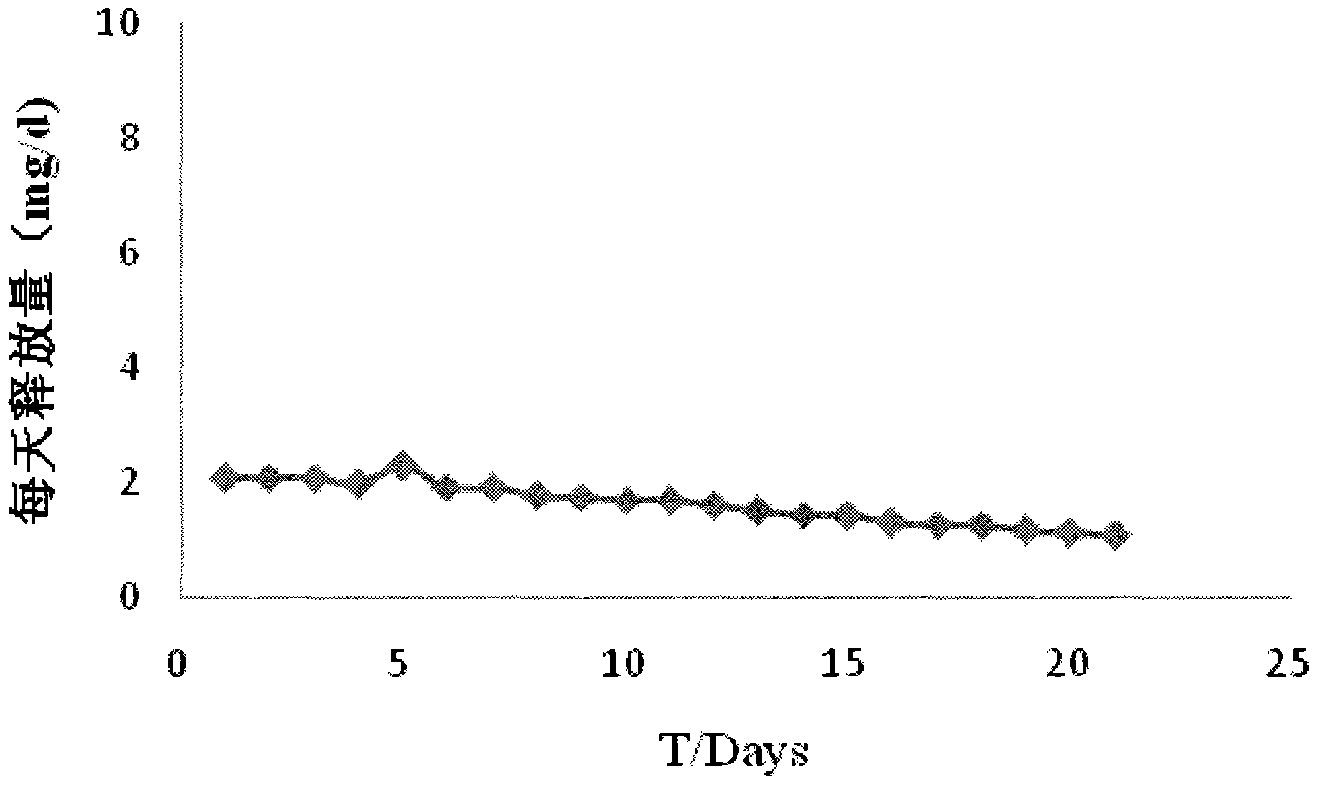
Mifepristone shell-type vaginal ring preparation and application
InactiveCN103505802AImprove solubilityReduce solubilityOrganic active ingredientsSurgeryPhysiologyGYNECOLOGIC DISORDERS
The invention relates to mifepristone shell-type vaginal ring preparation and application. A miferprisone shell-type vaginal ring comprises, by weight, 1.0-6.0% of mifepristone, 0.5-3.0% of medicine dispersion carriers, 0.3-2.0% of additives and 89.0-98.2% of medical polymer controlled release materials. Firstly, a solid dispersion technology is adopted for improving the solubility in water and release degree in vitro of the mifepristone, then a method of mold pressing vulcanization forming is adopted for preparing the mifepristone shell-type vaginal ring, the inner layer is a vacancy silicon rubber skeleton layer, the middle is a medicated layer, and the outmost layer is a controlled release film. By the implementation of the mifepristone shell-type vaginal ring preparation and application, the vaginal ring can release the mifepristone within 21 days at almost constant speed slowly and continuously for a long time, so that the medicine can be released more stably, the first-pass effect and gastrointestinal reaction caused by oral medication of the mifepriston are avoided, and the biological availability of the medicine and medication compliance of a patient are improved. The vaginal ring can be used for routine contraception and treating gynecological diseases such as the endometriosis and the uterine fibroid.
Owner:NAT RES INST FOR FAMILY PLANNING
Detection method of medicament for treating angina pectoris
InactiveCN102768247AExcellent disintegration and solubilityThe curative effect is fastComponent separationCardiovascular disorderAnginaCoronary heart disease
The invention provides a detection method of a medicament for treating angina pectoris. According to the detection method, ginsenoside Rg1, ginsenoside Re and ginsenoside Rb1 in the medicament are detected by high performance liquid chromatography. Conditions of the high performance liquid chromatography comprise: octadecylsilane bonded silica gel is used as a chromatographic column for a filler; and an acetonitrile solution is used as a mobile phase A and water is used as a mobile phase B for gradient elution. The gradient elution process is as follows: during the first 25 minutes, the mobile phase A acetonitrile solution is 15% by volume and the mobile phase B water is 85% by volume; from the 25th minute to the 70th minute, the mobile phase A acetonitrile solution is 15-22% by volume and the mobile phase B water is 85-78% by volume; from the 70th minute to the 80th minute, the mobile phase A acetonitrile solution is 22-30% by volume and the mobile phase B water is 78-70% by volume; from the 80th minute to 105th minute, the mobile phase A acetonitrile solution is 30-40% by volume and the mobile phase B water is 70-60% by volume; and from the 105th minute to 120th minute, the mobile phase A acetonitrile solution is 40-15% by volume and the mobile phase B water is 60-85% by volume.
Owner:梁丽
Nifedipine slow release tablet and preparation method thereof
ActiveCN103301083AImprove solubilityGood control releaseOrganic active ingredientsPharmaceutical delivery mechanismControlled releaseSolubility
The invention discloses a nifedipine slow release tablet. The nifedipine slow release tablet is formed through uniformly mixing and tabletting medicine-carrying particles and pharmaceutically acceptable auxiliary materials. The method for preparing the medicine-carrying particles comprises the following steps of: dissolving nifedipine and povidone in ethanol, evaporating an obtained mixed solution till the mixed solution becomes viscous, adding the viscous mixed solution into into docosanoic acid glyceride meltwater, fully stirring, cooling, smashing and sieving. The nifedipine slow release tablet provided by the invention improves the solubility of the nifedipine, well controls the release of the nifedipine, the process is simple, and the nifedipine slow release tablet is suitable for requirements of large-scale production.
Owner:ZHEJIANG ANGLIKANG PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com