Pharmaceutical composition for parenteral administration, containing donepezil
A technology of donepezil and composition, which is applied in the field of non-oral pharmaceutical compositions containing donepezil, can solve the problems of large drug loss and large dosage, and achieve the effects of improving medication compliance and stabilizing drug release properties for a long time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
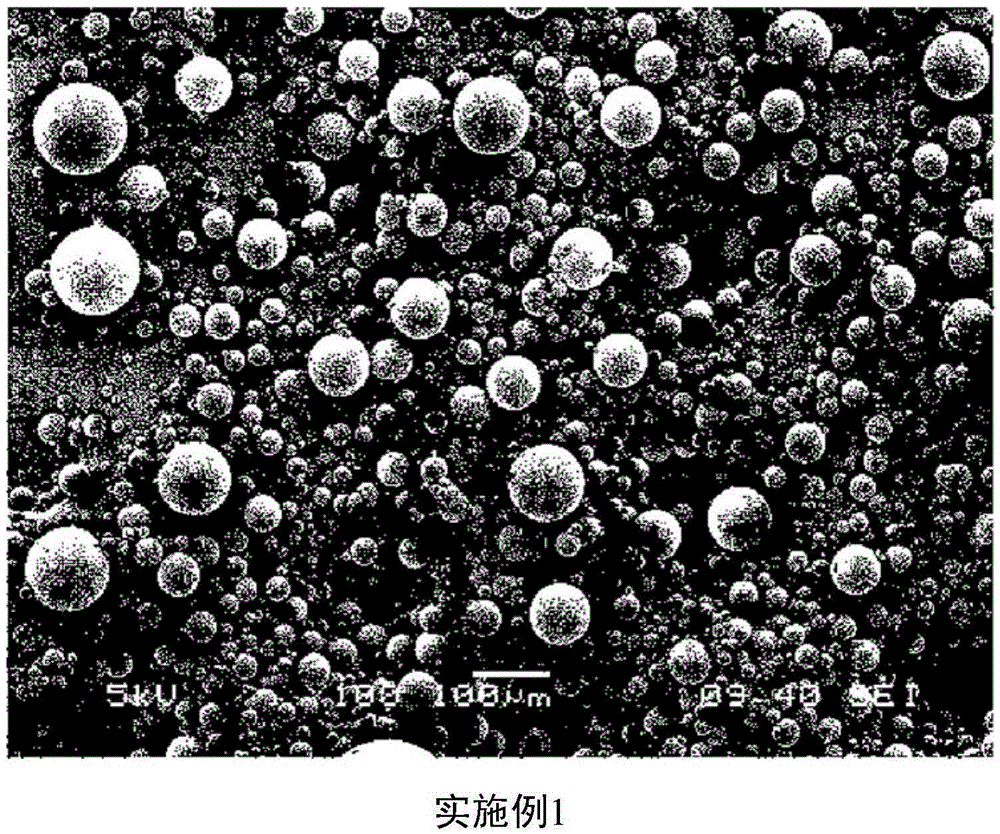
Embodiment 1
[0026] Example 1: Preparation of Donepezil Microspheres
[0027] To 1.4g of biocompatible polymer (RESOMERRG858S manufacturer: Boehringer Ingelheim) and 0.6g of donepezilbase (manufacturer: Megafine, India), add 12.6g of dichloride Methane (manufacturer: Merck) was stirred and completely dissolved to obtain a dispersion.
[0028] In the microsphere preparation reactor, add 500 mL of 0.5% polyvinyl alcohol (Mn=30,000~70,000; Sigma) aqueous solution, set it to 10°C, and stir at 3,000 times per minute while using a syringe Slowly add the previously prepared dispersion to prepare microspheres. Then, increase the temperature and volatilize the organic solvent for 2 hours, and then cool for 1 hour to 10°C.
[0029] After washing the prepared microspheres with water for injection several times, they were subjected to wet filtration using sieves of 25 μm and 150 μm, and freeze-dried for 64 hours.
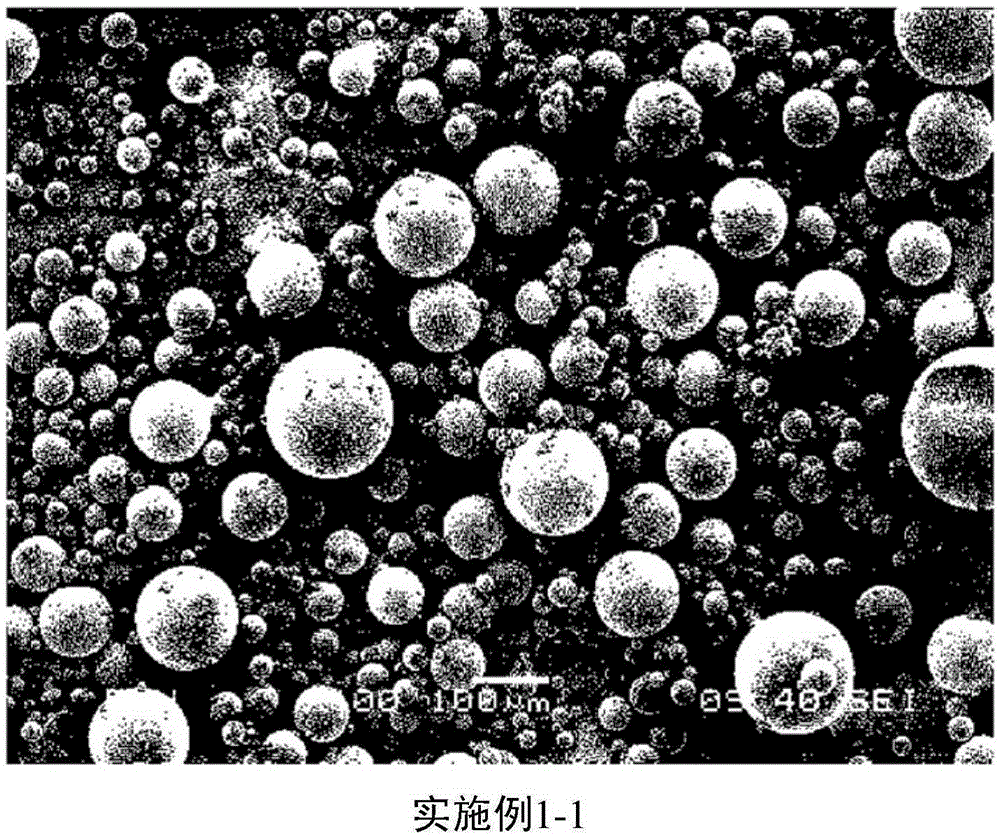
Embodiment 1-1
[0030] Example 1-1: Preparation of microspheres
[0031] A biocompatible polymer (RESOMERRG757S manufacturer: Boehringer Ingelheim) was used, and the rest of the preparation method was implemented in the same way as the method in Example 1, thereby preparing microspheres.
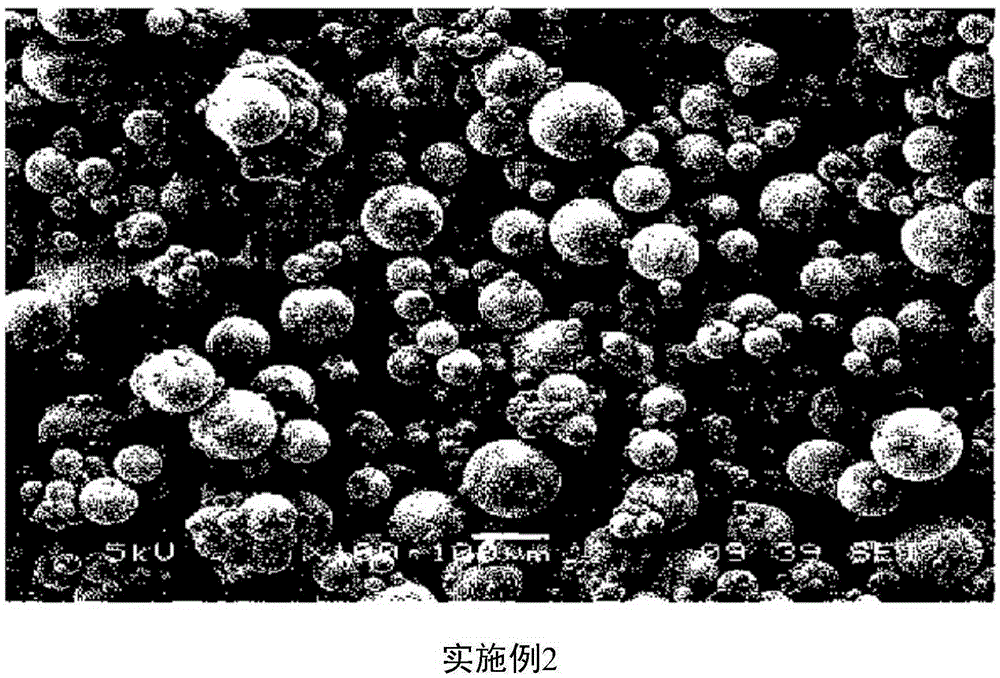
Embodiment 1-2
[0032] Example 1-2: Preparation of microspheres
[0033] In 0.8g of biocompatible polymer (RESOMERRG858S manufacturer: Boehringer Ingelheim) and 1.2g of donepezil base (manufacturer: Megafine, India), 12.6g of dichloromethane (manufacturer: Mo Gram Company) and stirring to make it completely dissolved to obtain a dispersion liquid. The rest of the preparation process was carried out according to the same process as in Example 1 to obtain microspheres.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com