Preparation method of paliperidone sustained release microsphere injection
A technology of paliperidone and sustained-release microspheres, which is applied in the directions of non-active ingredient medical preparations, medical preparations containing active ingredients, and pharmaceutical formulas to achieve high drug loading, good sustained-release effect, and reduced dosage. The effect of the number of doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
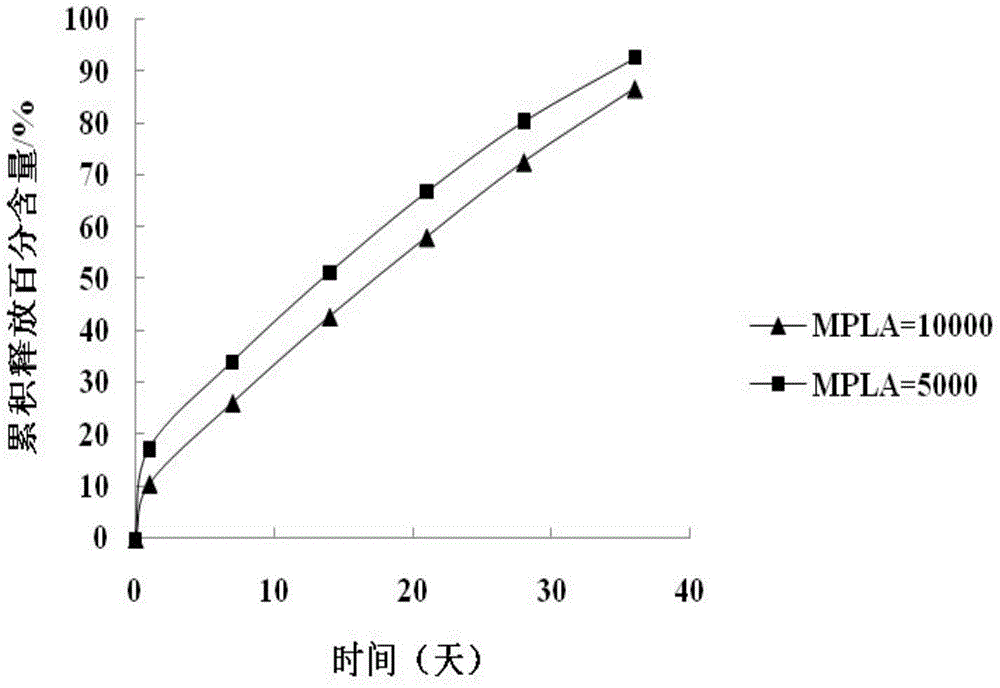
Image
Examples
Embodiment 1
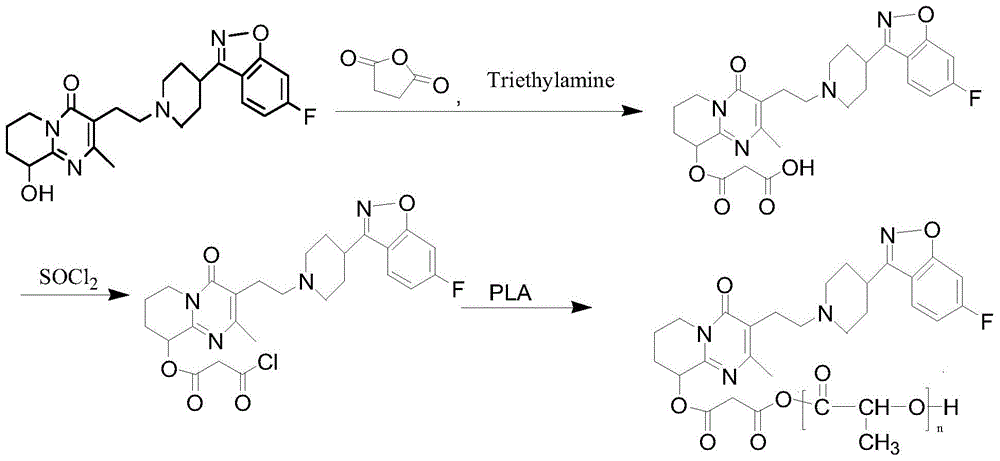
[0038] The preparation of the first step reaction intermediate: add 42g paliperidone (0.1mol) and 84mL acetone and 336mL chloroform in the three-neck round bottom flask, add 30g succinic anhydride (0.3mol) therein under the condition of sufficient stirring ), 20mL (0.15mol) of triethylamine, the temperature is controlled at 65°C during the feeding process, after the feeding is completed, continue to stir and reflux at this temperature for 24h, after the reaction is completed, add 200mL of water to the reaction, fully stir, separate layers, and the organic layer anhydrous NaSO 4 After drying, the solvent was removed by rotary evaporation to obtain 45.4 g of a light orange solid. The purity of the product analyzed by high performance liquid chromatography (HPLC) was 95.3%, and the yield was 86.2%.
[0039]Preparation of the second-step reaction intermediate: dissolve 26g (95.3%, 0.05mol) of the first-step reaction intermediate in 200mL of anhydrous dichloromethane, add 1-2 drops...
Embodiment 2
[0043] The preparation of the first step reaction intermediate: add 42g paliperidone (0.1mol) and 84mL acetone and 336mL chloroform in the three-neck round bottom flask, add 30g succinic anhydride (0.3mol) therein under the condition of sufficient stirring ), 20mL (0.15mol) of triethylamine, the temperature is controlled at 70°C during the feeding process, after the feeding is completed, continue to stir and reflux at this temperature for 24h, after the reaction is completed, add 200mL of water to the reaction, fully stir, separate layers, and the organic layer anhydrous NaSO 4 After drying, the solvent was removed by rotary evaporation to obtain 46.6 g of a light orange solid. The purity of the product analyzed by high performance liquid chromatography (HPLC) was 96.1%, and the yield was 88.6%.
[0044] Preparation of the second-step reaction intermediate: Dissolve 25g (96.1%, 0.05mol) of the first-step reaction intermediate in 200mL of anhydrous dichloromethane, add 1-2 drop...
Embodiment 3
[0048] The preparation of the first step reaction intermediate: add 42g paliperidone (0.1mol) and 84mL acetone and 336mL chloroform in the three-neck round bottom flask, add 30g succinic anhydride (0.3mol) therein under the condition of sufficient stirring ), 20mL (0.15mol) of triethylamine, the temperature was controlled at 75°C during the feeding process, and continued to stir and reflux at this temperature for 24h after the feeding was completed. anhydrous NaSO 4 After drying, the solvent was removed by rotary evaporation to obtain 44.7 g of a light orange solid. The purity of the product analyzed by high performance liquid chromatography (HPLC) was 95.8%, and the yield was 84.9%.
[0049] Preparation of the second-step reaction intermediate: dissolve 26g (95.8%, 0.05mol) of the first-step reaction intermediate in 200mL of anhydrous dichloromethane, add 1-2 drops of DMF dropwise, and add thionyl chloride dropwise at 55°C 7mL (0.1mol), reflux for 2h after the dropwise addit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com