Felodipine sustained-release tablet and preparation technology thereof
A felodipine and gentle technology, applied in the field of sustained-release tablets, can solve the problems of unfavorable environmental protection, complicated preparation process, gastrointestinal irritation, etc., and achieve the effect of slow and stable drug release, simple preparation process and rapid drug release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
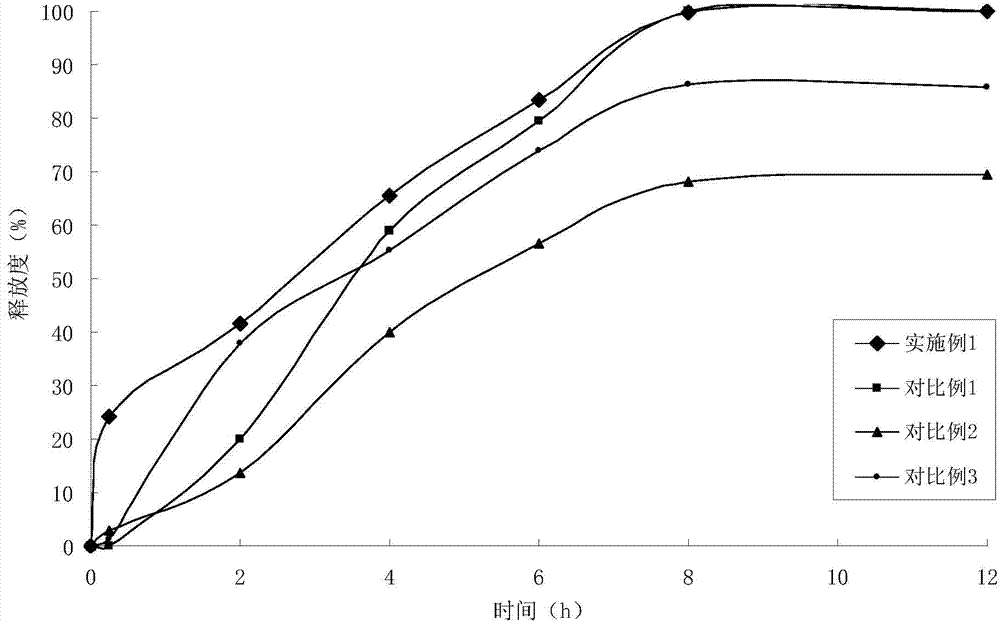
Embodiment 1
[0028] (1) Solid dispersion
[0029]
[0030] (2) Sustained-release pellets
[0031] Solid dispersion 75g
[0032] Ethylcellulose 15g
[0033] Hydroxypropyl Cellulose 1.5g
[0034] (3) Felodipine Sustained Release Tablets (4000 Tablets)
[0035]
[0036] Preparation Process:
[0037] ①Add felodipine and copovidone to absolute ethanol, stir to dissolve, add polacrilin potassium, stir, ultrasonically uniform, dry under reduced pressure to remove solvent, and obtain felodipine immediate-release solid dispersion;
[0038] 2. Get the felodipine immediate-release solid dispersion prepared in step 1. and add it in the fluidized bed, spray coating at the bottom with ethylcellulose ethanol solution containing porogen, to prepare slow-release coated pellets;
[0039] ③Weigh the felodipine immediate-release solid dispersion and sustained-release coated pellets in proportion, mix them evenly with microcrystalline cellulose, sodium carboxymethyl starch, and magnesium stearate, a...
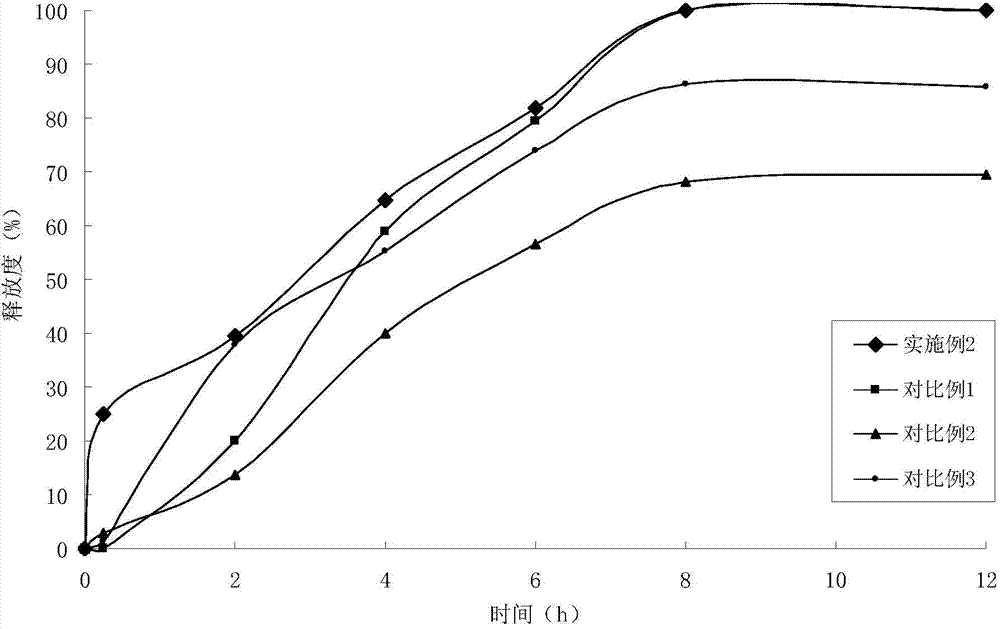
Embodiment 2
[0041] (1) Solid dispersion
[0042]
[0043]
[0044] (2) Sustained-release pellets
[0045] Solid dispersion 120g
[0046] Ethyl cellulose 30g
[0047] Hydroxypropyl Cellulose 6g
[0048] (3) Felodipine Sustained Release Tablets (4000 Tablets)
[0049]
[0050] Preparation Process:
[0051] ①Add felodipine and copovidone to absolute ethanol, stir to dissolve, add polacrilin potassium, stir, ultrasonically uniform, dry under reduced pressure to remove solvent, and obtain felodipine immediate-release solid dispersion;
[0052] 2. Get the felodipine immediate-release solid dispersion prepared in step 1. and add it in the fluidized bed, spray coating at the bottom with ethylcellulose ethanol solution containing porogen, to prepare slow-release coated pellets;
[0053]③ Weigh the felodipine immediate-release solid dispersion and sustained-release coated pellets in proportion, mix them evenly with lactose, pregelatinized starch, crospovidone, and magnesium stearate, ...
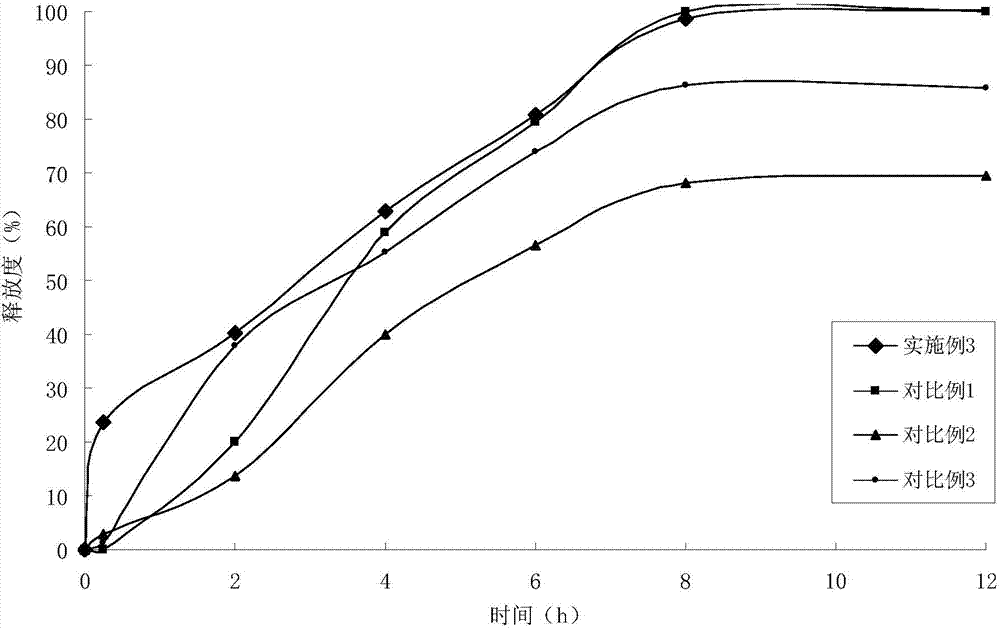
Embodiment 3
[0055] (1) Solid dispersion
[0056]
[0057] (2) Sustained-release pellets
[0058] Solid dispersion 165g
[0059] Ethylcellulose 39.5g
[0060] Hydroxypropyl Cellulose 7.8g
[0061] (3) Felodipine Sustained Release Tablets (4000 Tablets)
[0062]
[0063] Preparation Process:
[0064] ①Add felodipine and copovidone to absolute ethanol, stir to dissolve, add polacrilin potassium, stir, ultrasonically uniform, dry under reduced pressure to remove solvent, and obtain felodipine immediate-release solid dispersion;
[0065] 2. Get the felodipine immediate-release solid dispersion prepared in step 1. and add it in the fluidized bed, spray coating at the bottom with ethylcellulose ethanol solution containing porogen, to prepare slow-release coated pellets;
[0066] ③ Weigh the felodipine immediate-release solid dispersion and sustained-release coated pellets in proportion, mix them with microcrystalline cellulose, starch, croscarmellose sodium, and magnesium stearate, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com