Patents
Literature
106 results about "Carvedilol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
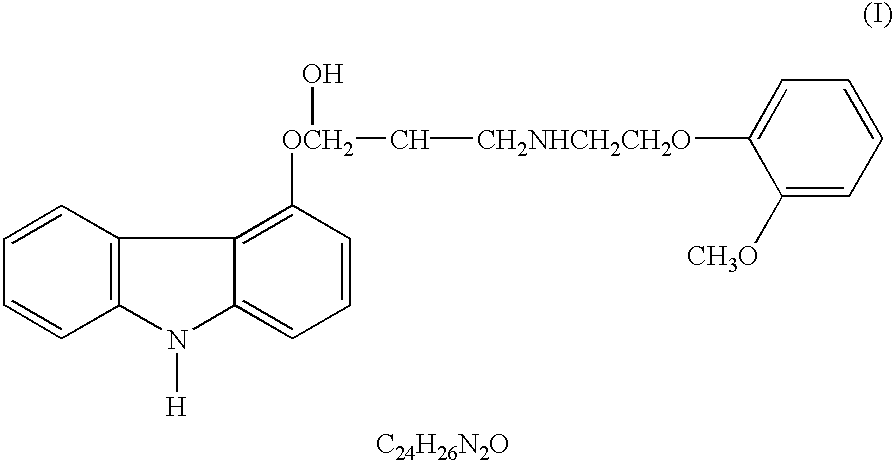
Carvedilol is used to treat high blood pressure and heart failure. It is also used after a heart attack to improve the chance of survival if your heart is not pumping well.
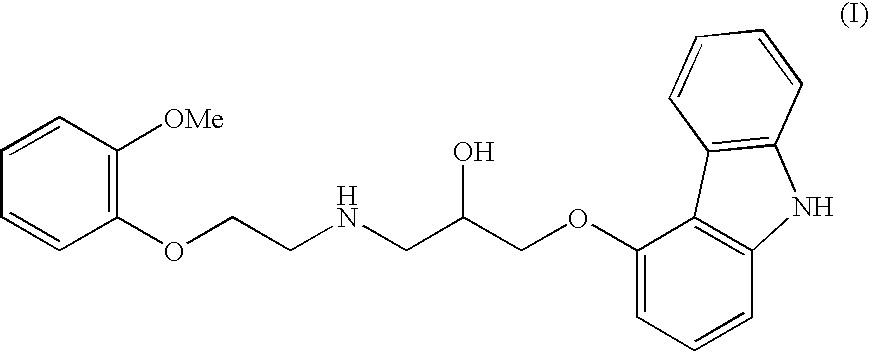
Carvedilol
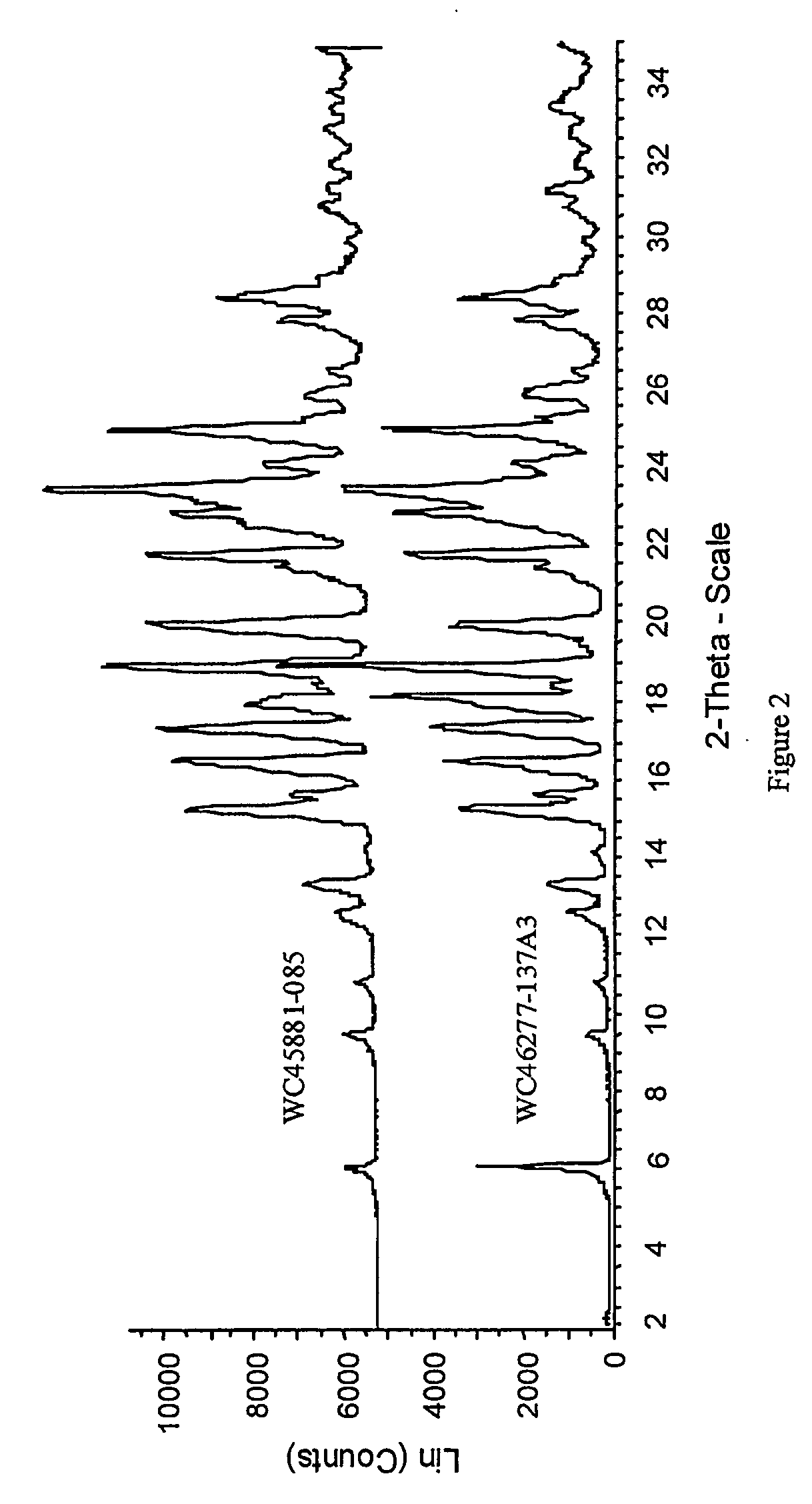
InactiveUS7056942B2Lower the temperature of the solutionBiocideOrganic chemistryCarvedilolMedicinal chemistry
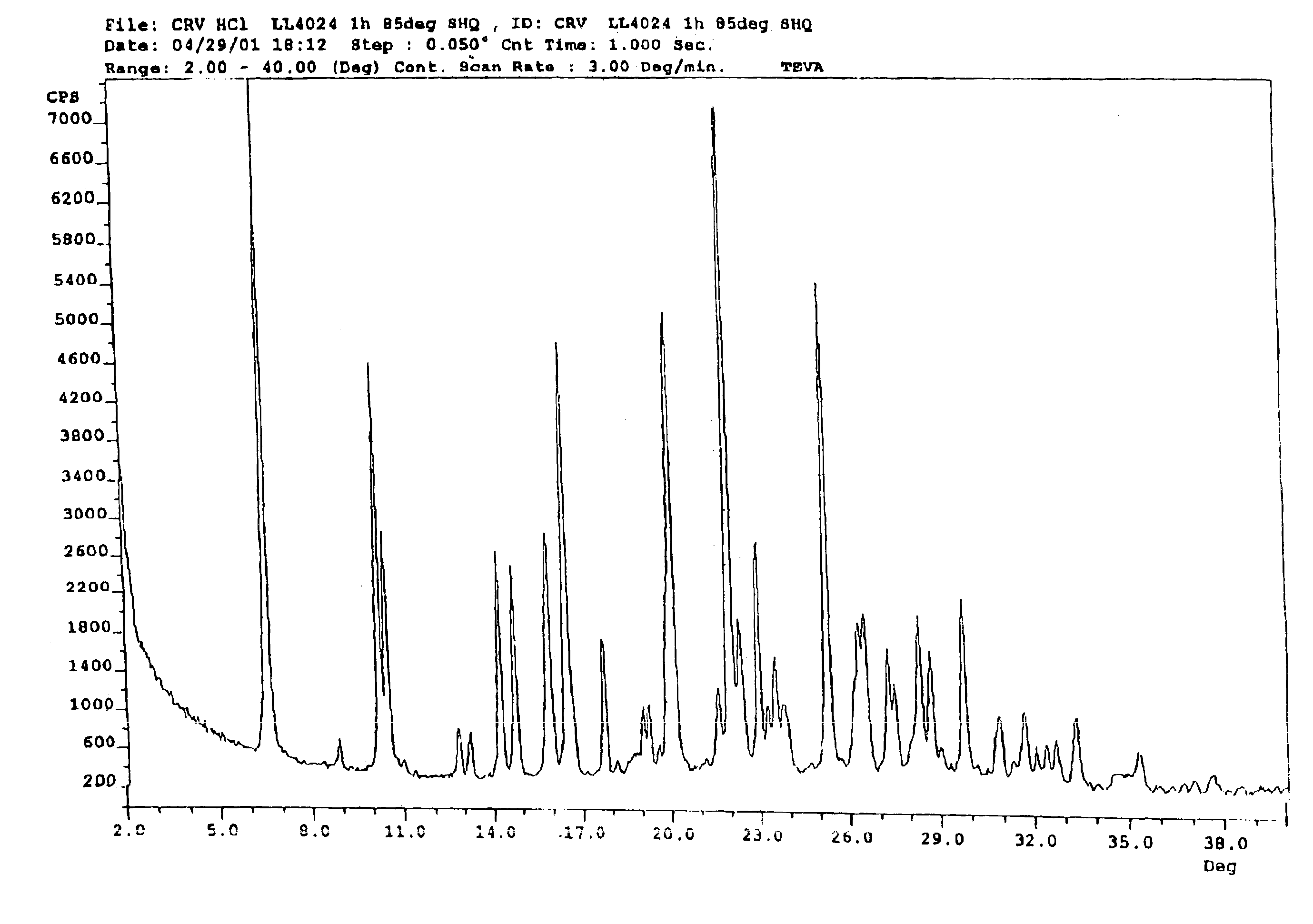
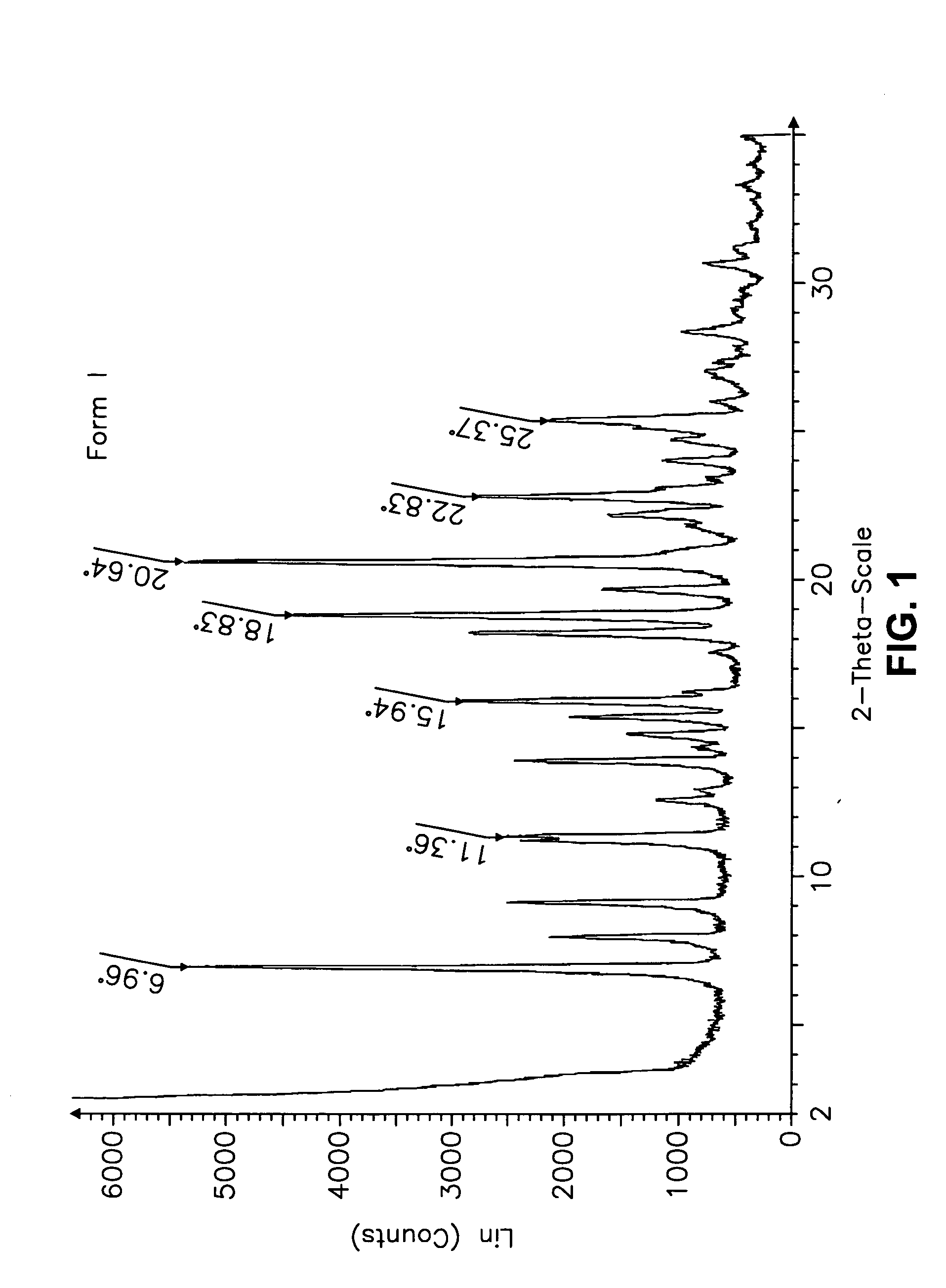
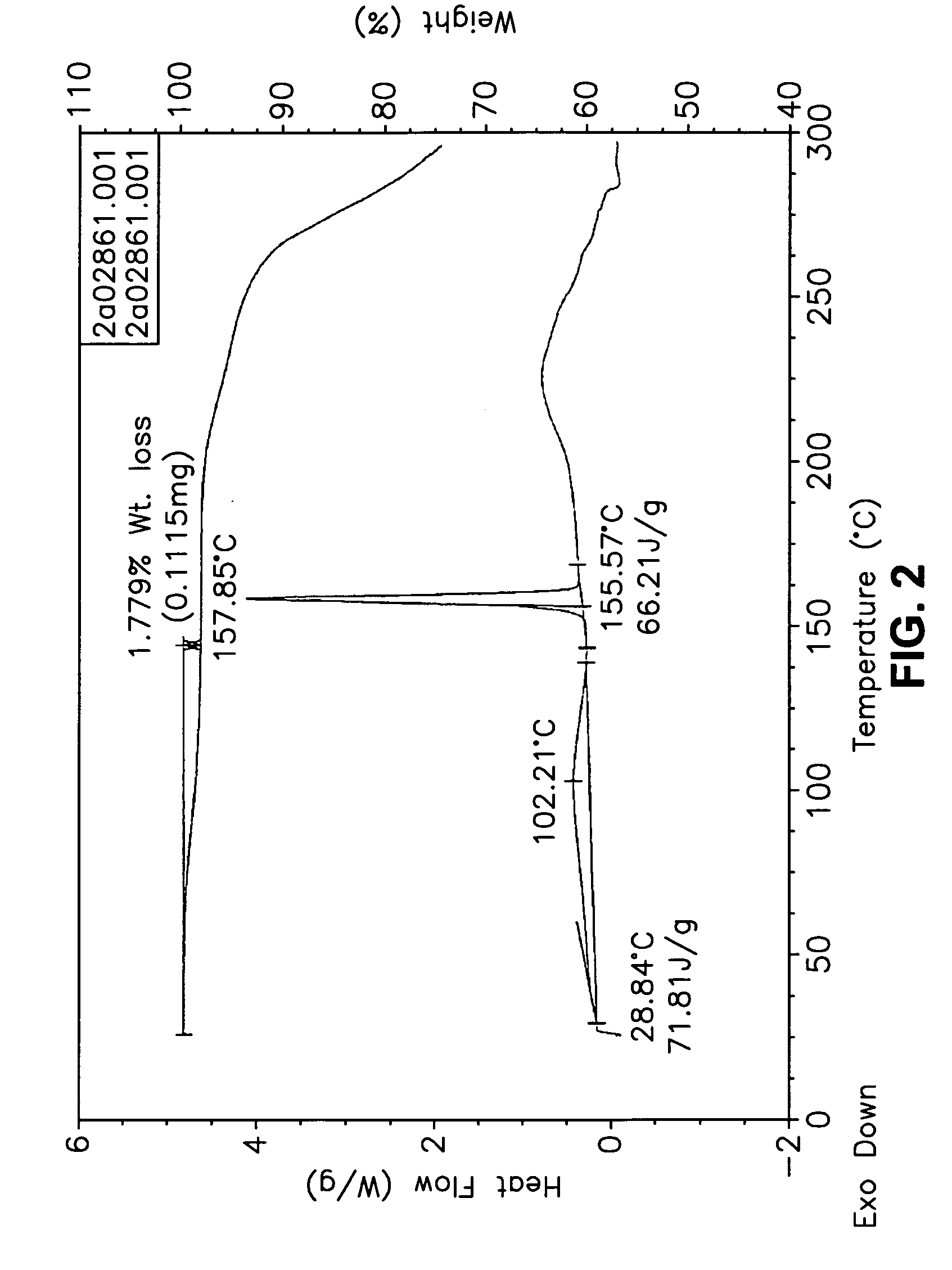
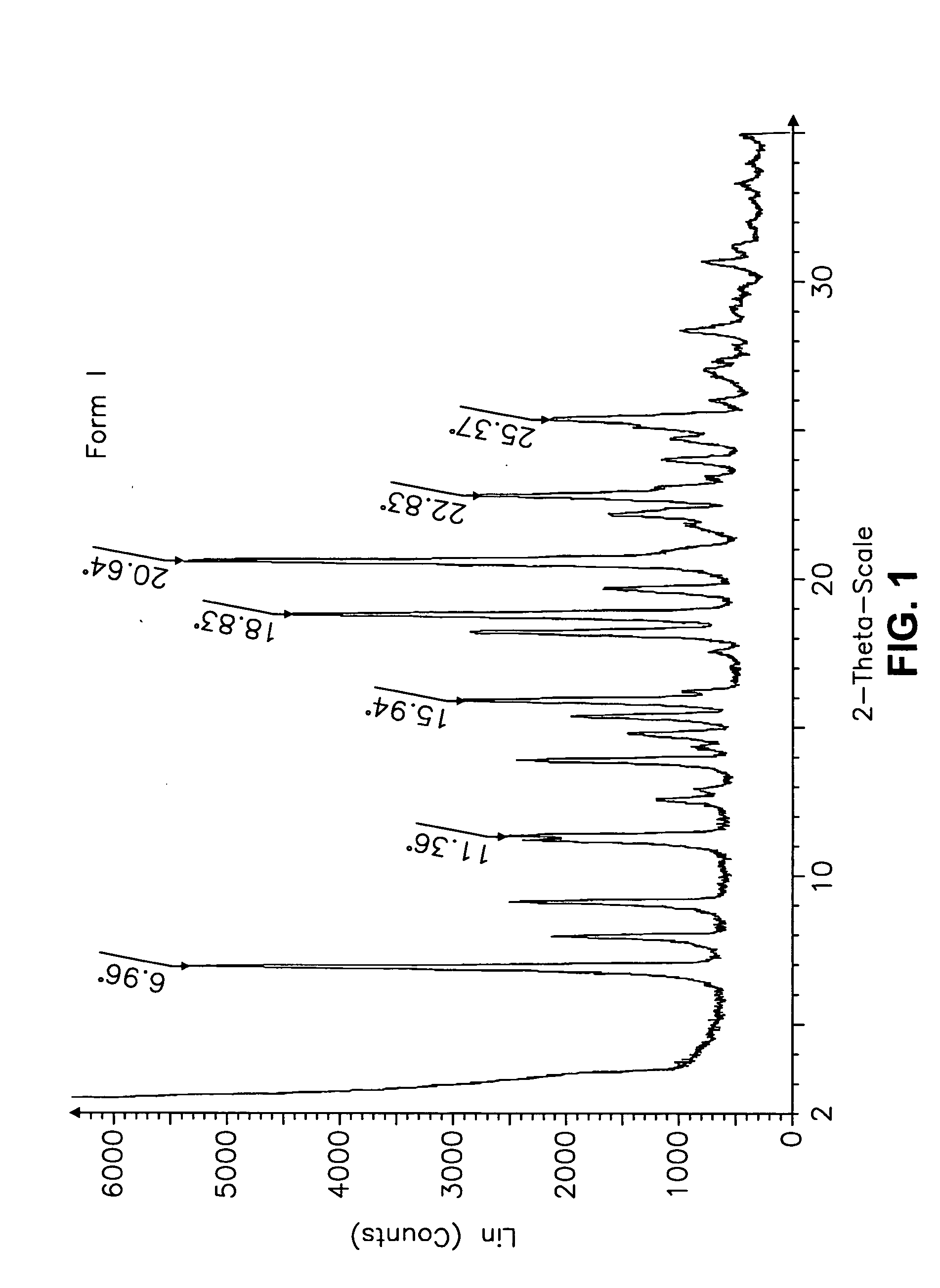
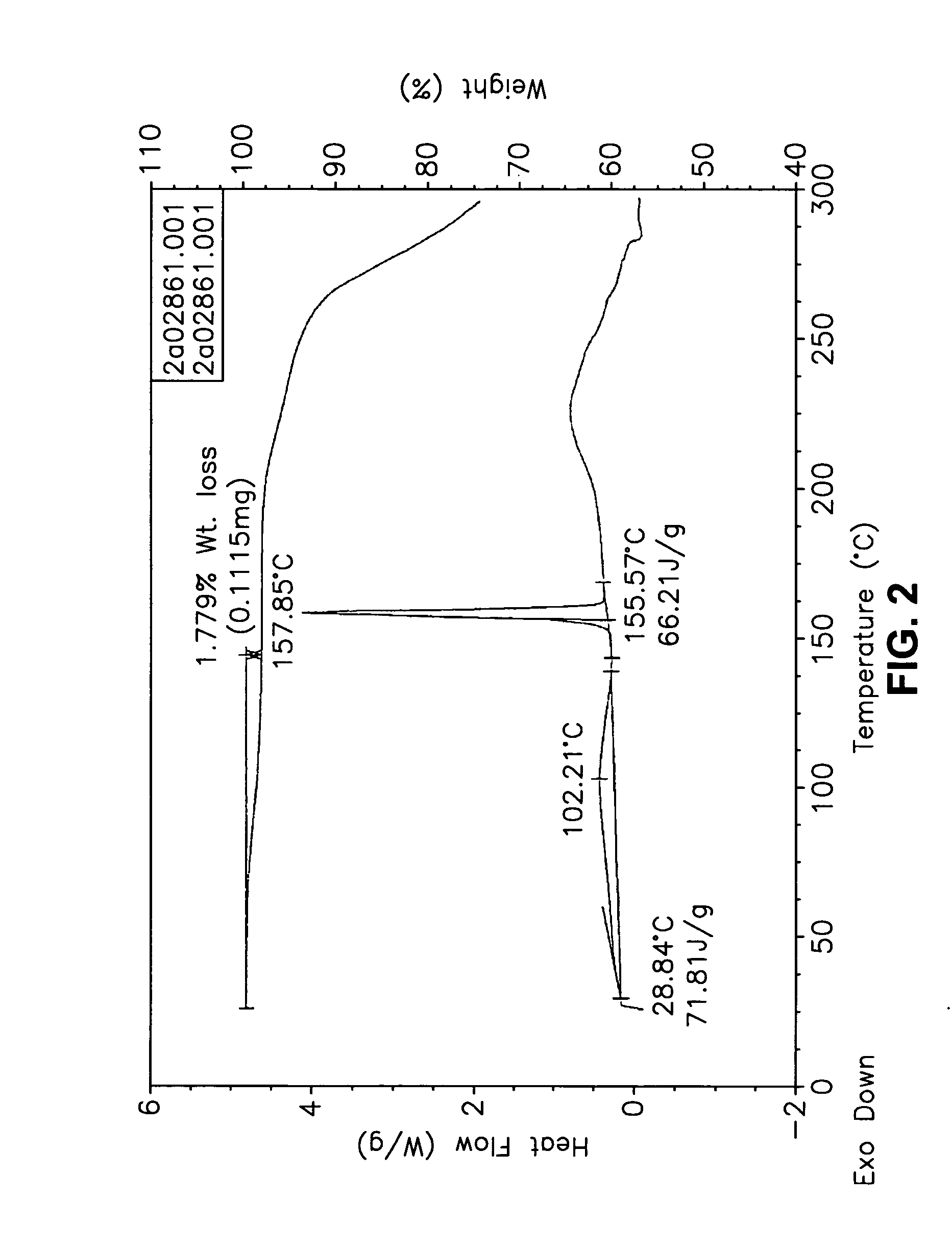
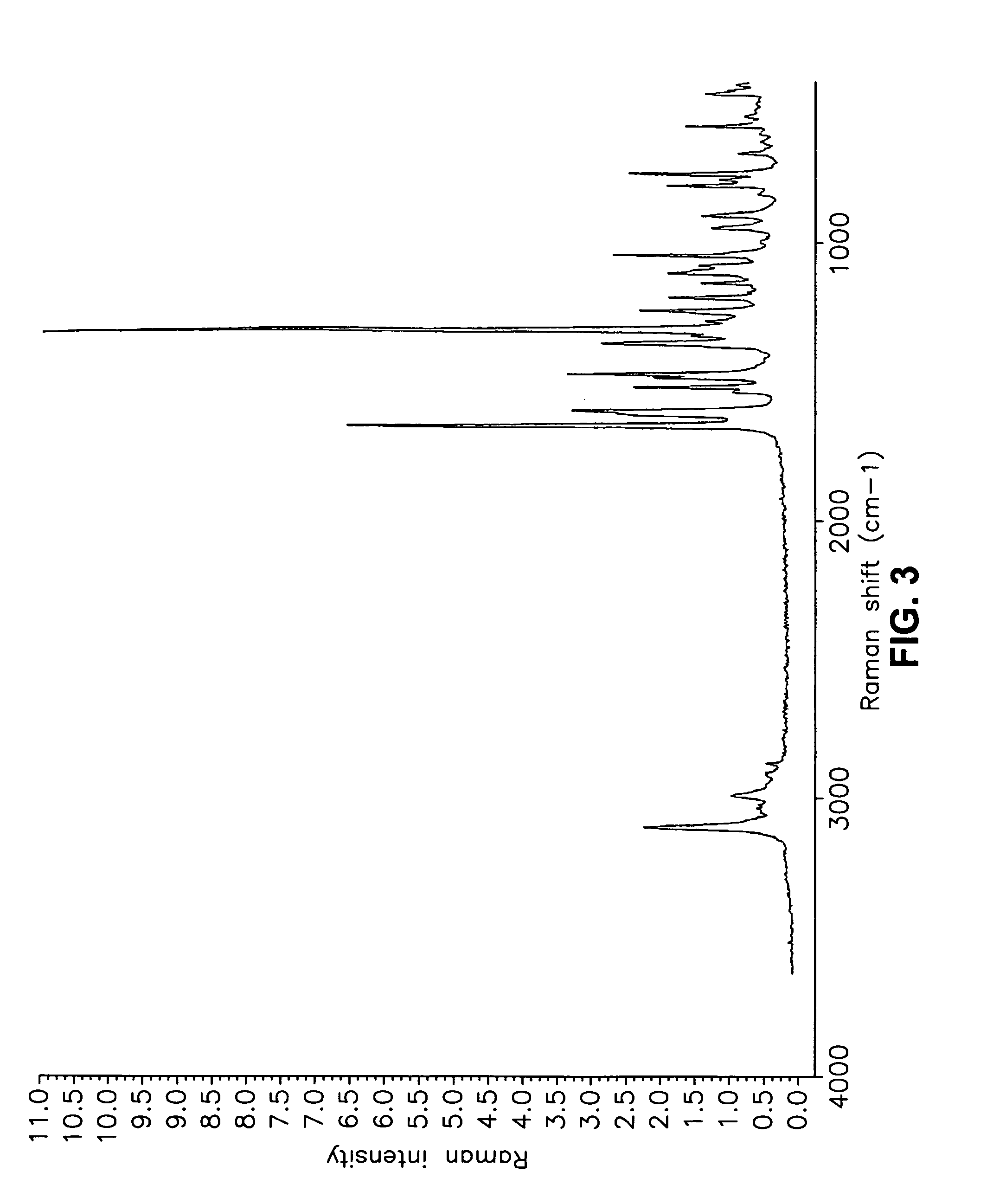
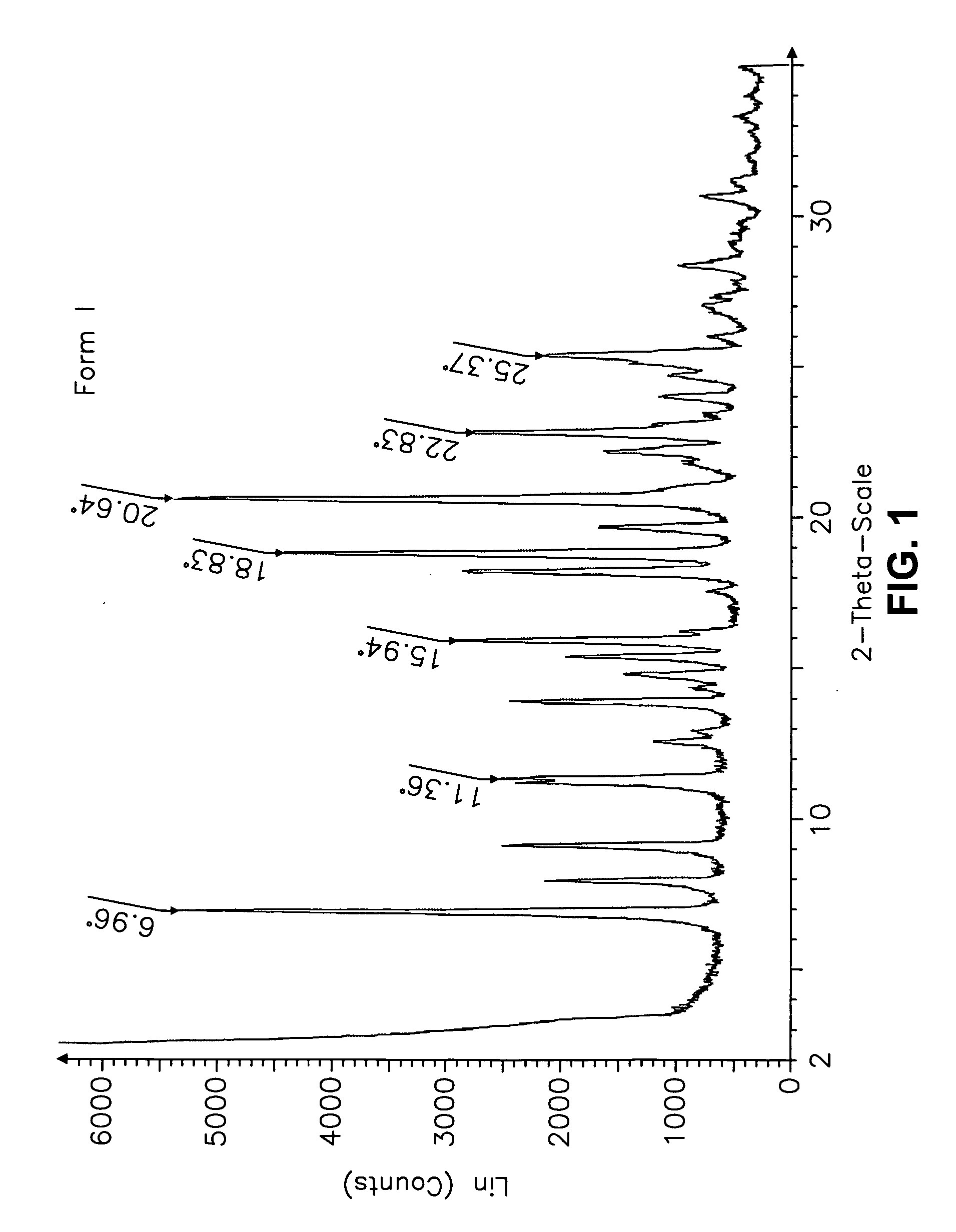
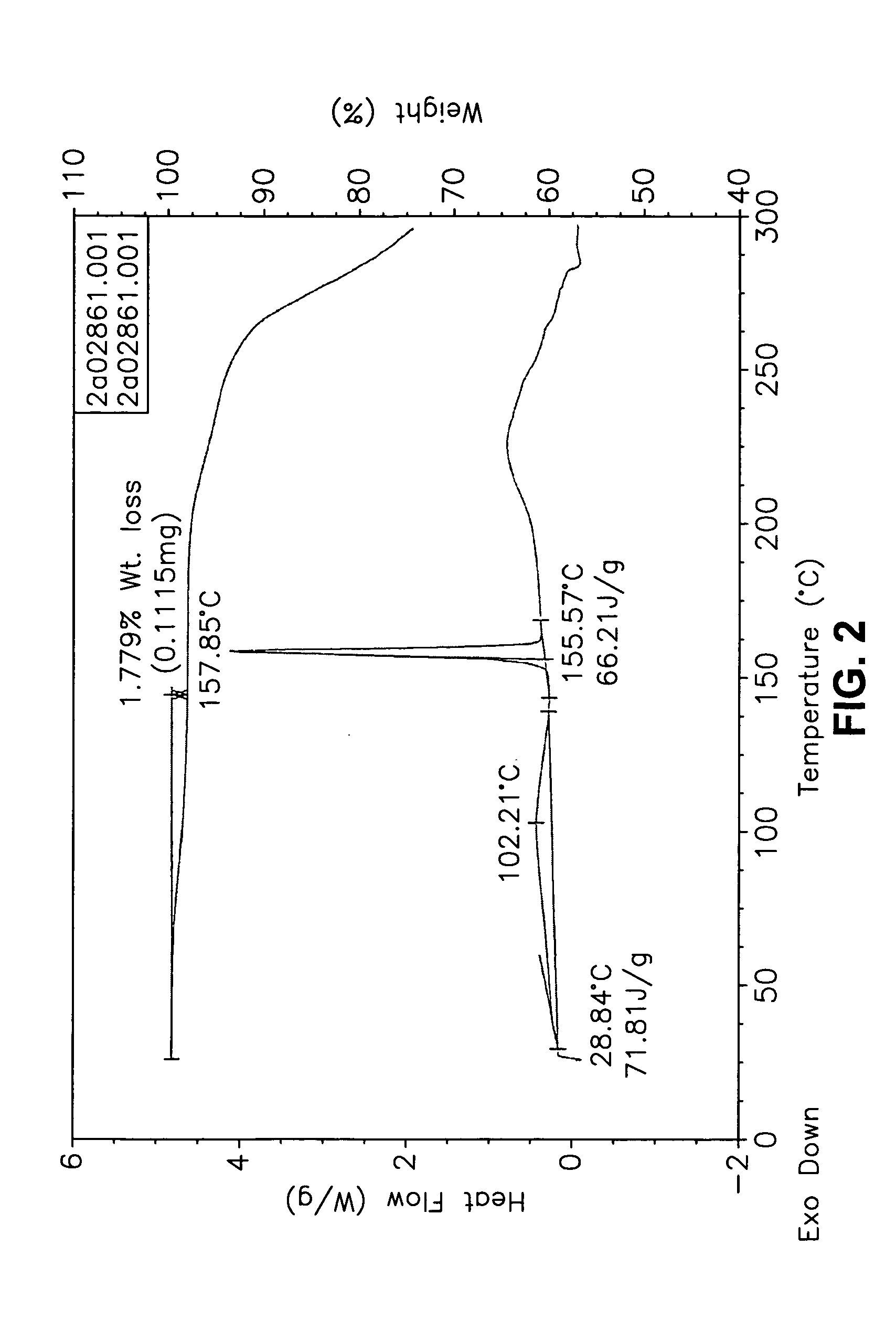
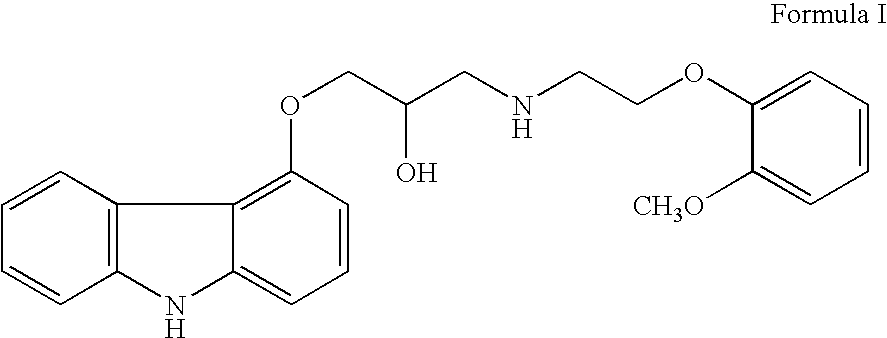
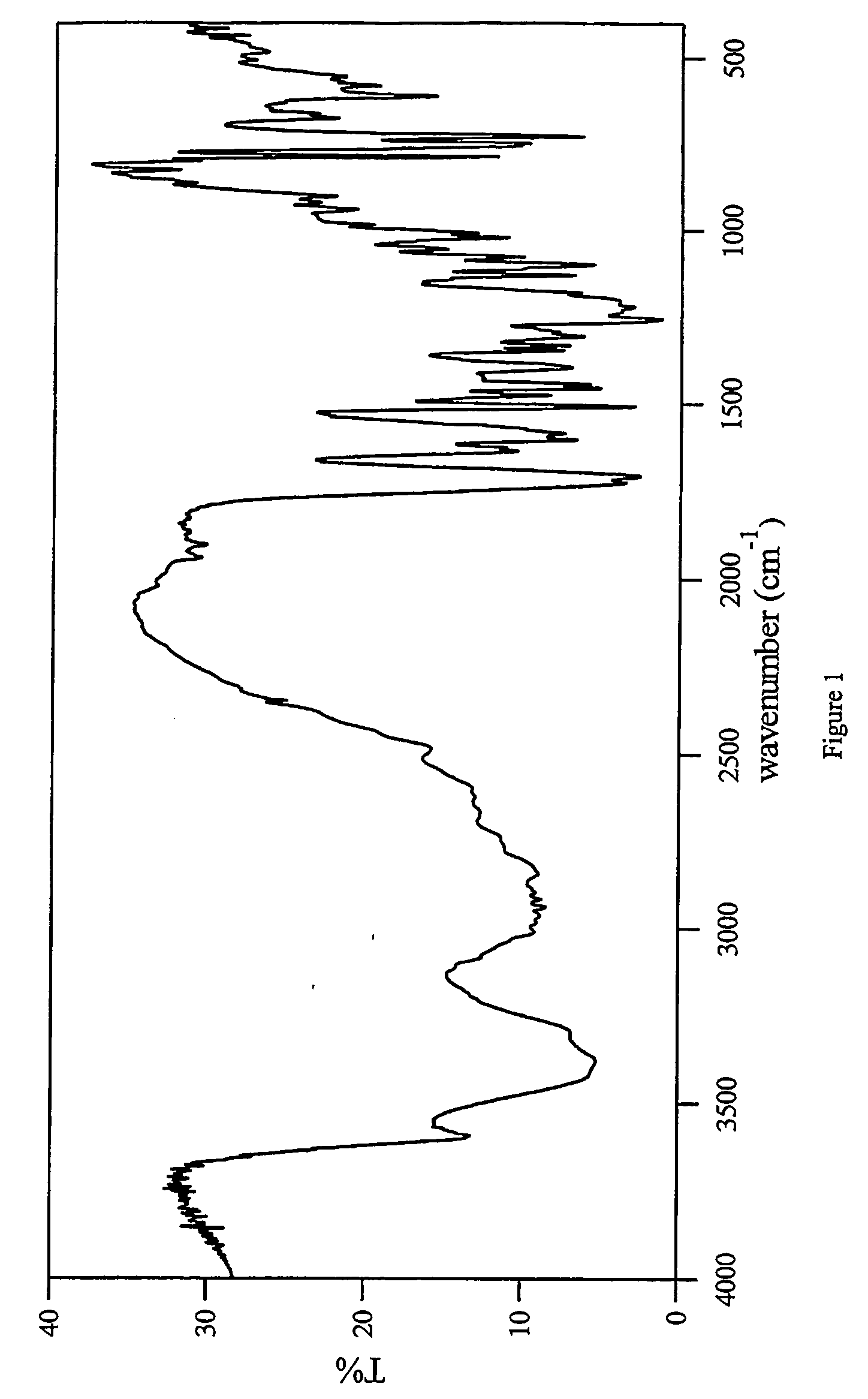
This invention relates to an improved process of preparing carvedilol, as well as a new crystalline hydrate and solvate and forms of carvedilol, processes for the manufacture thereof, and pharmaceutical compositions thereof.
Owner:TEVA PHARMA IND LTD
Controlled release solid dispersions
InactiveUS20050019399A1Suitable shelf-lifeImprove solubilityBiocideAnimal repellantsSolubilityPolyethylene oxide
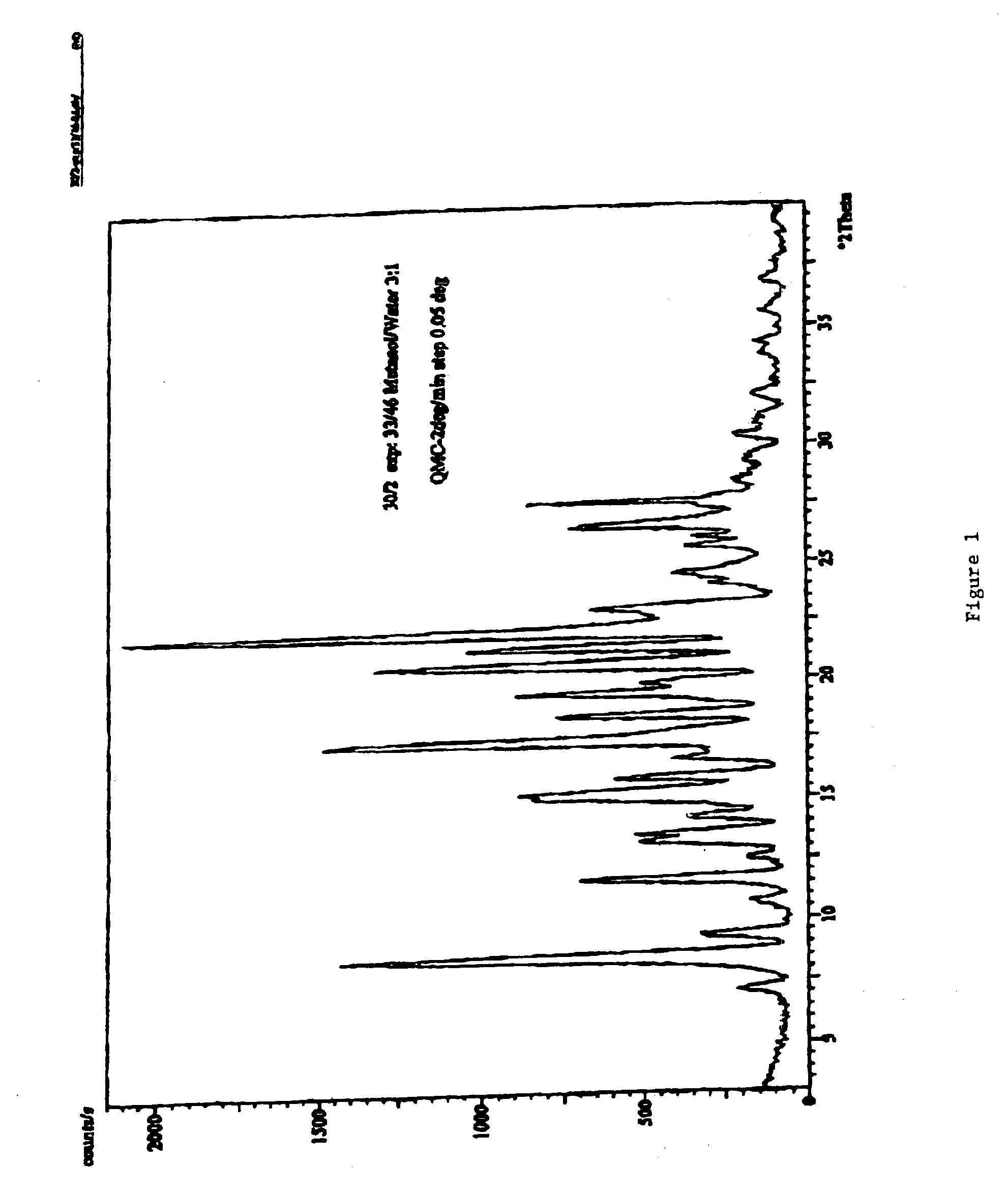
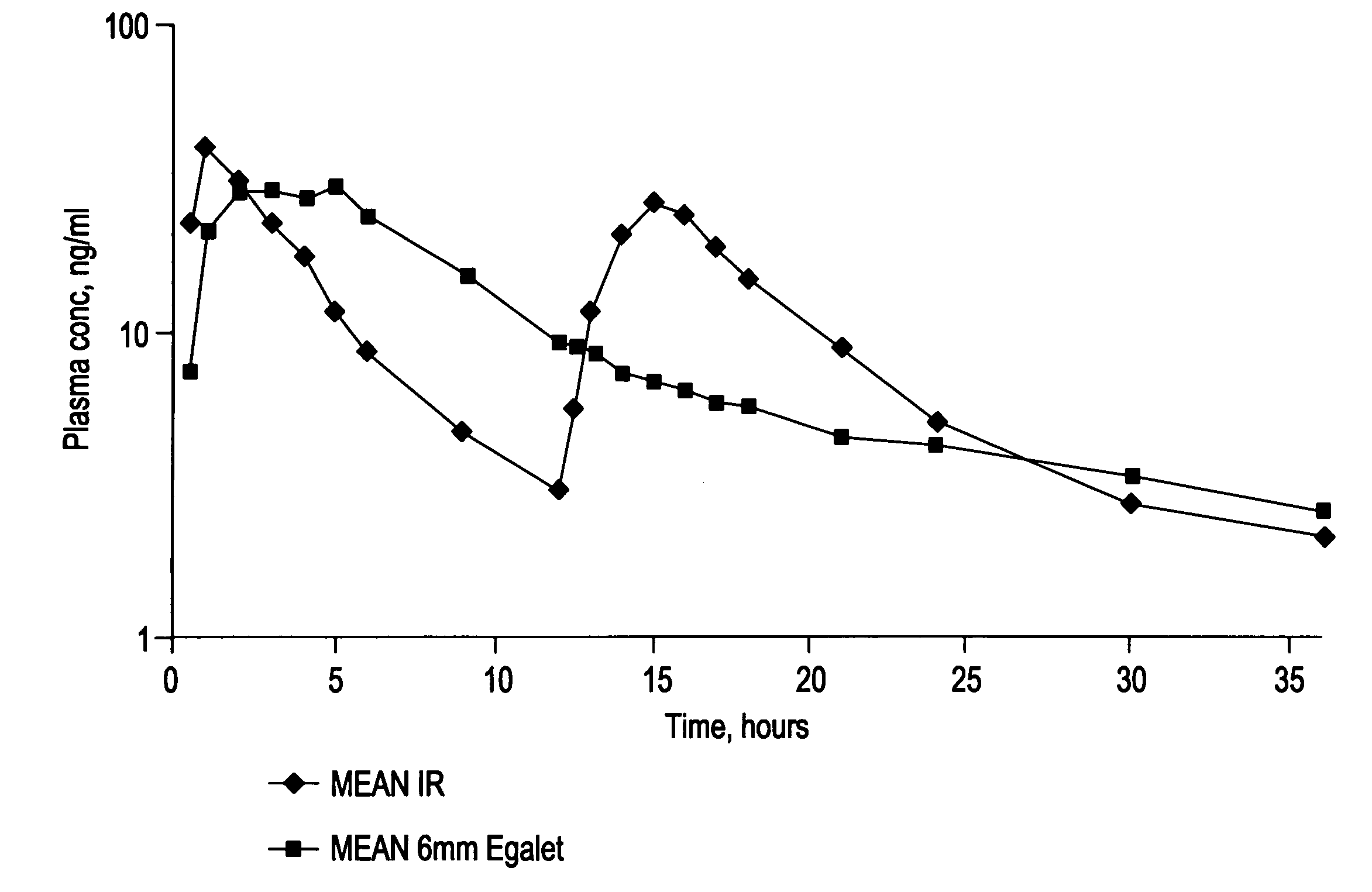
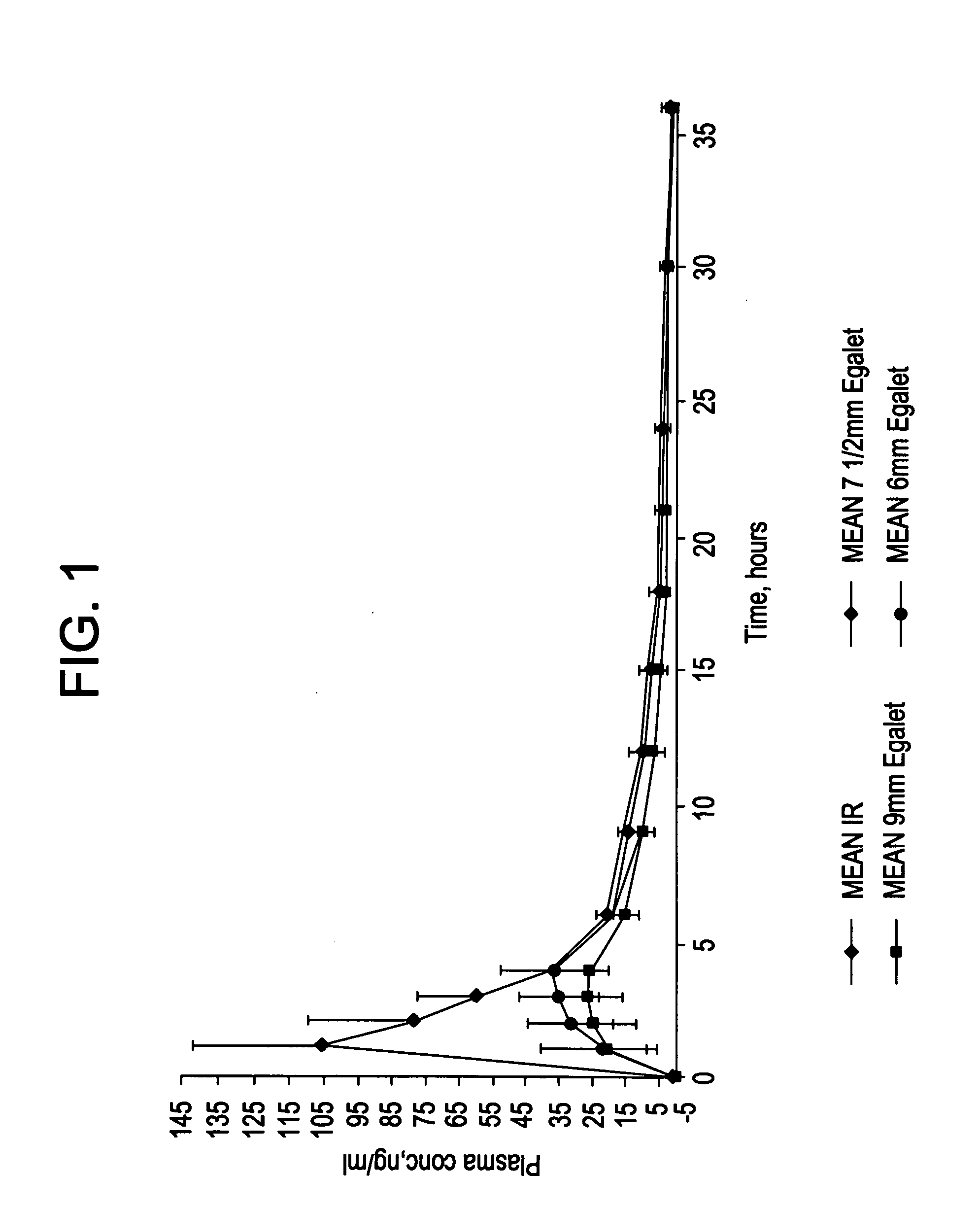
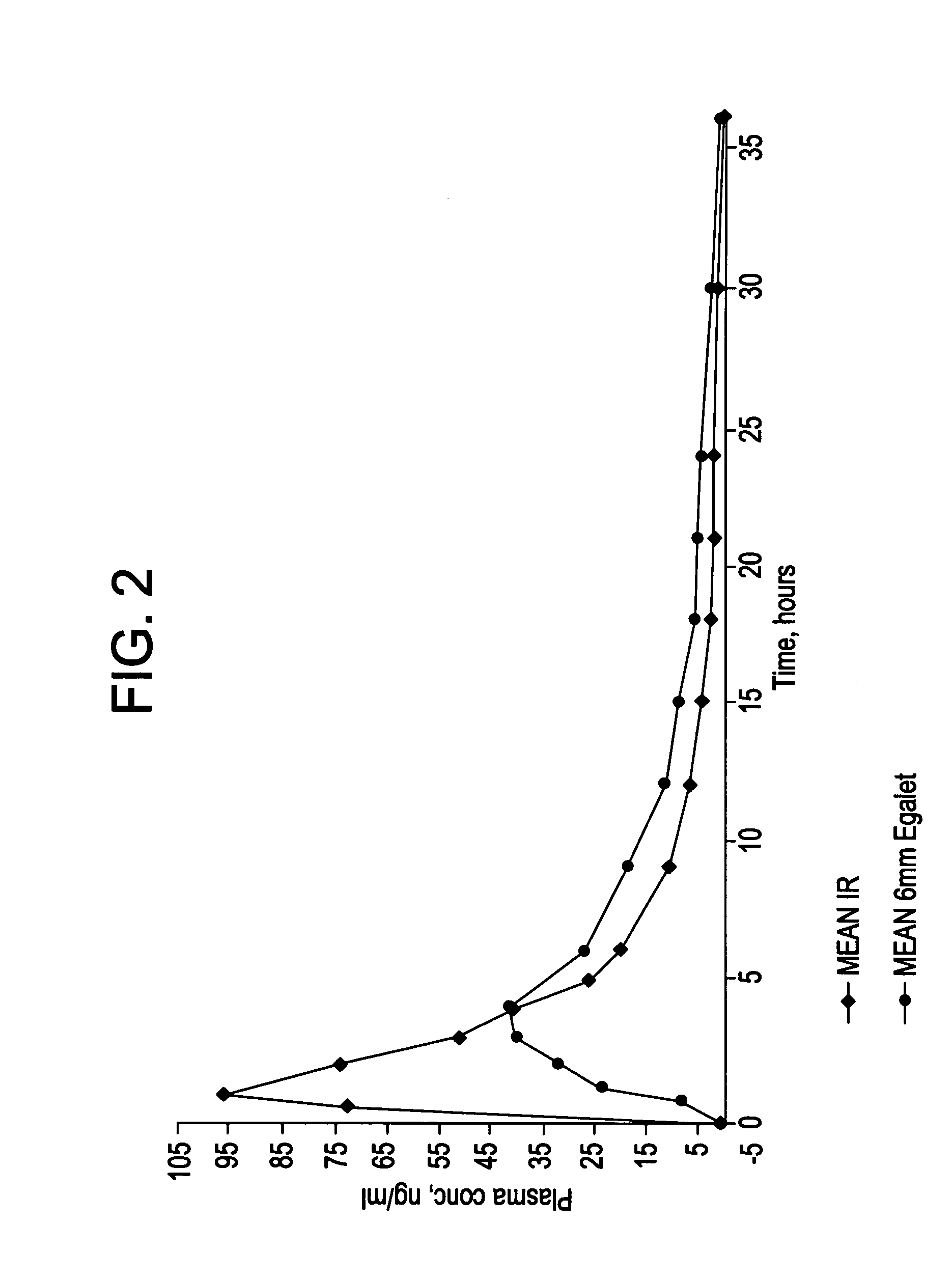
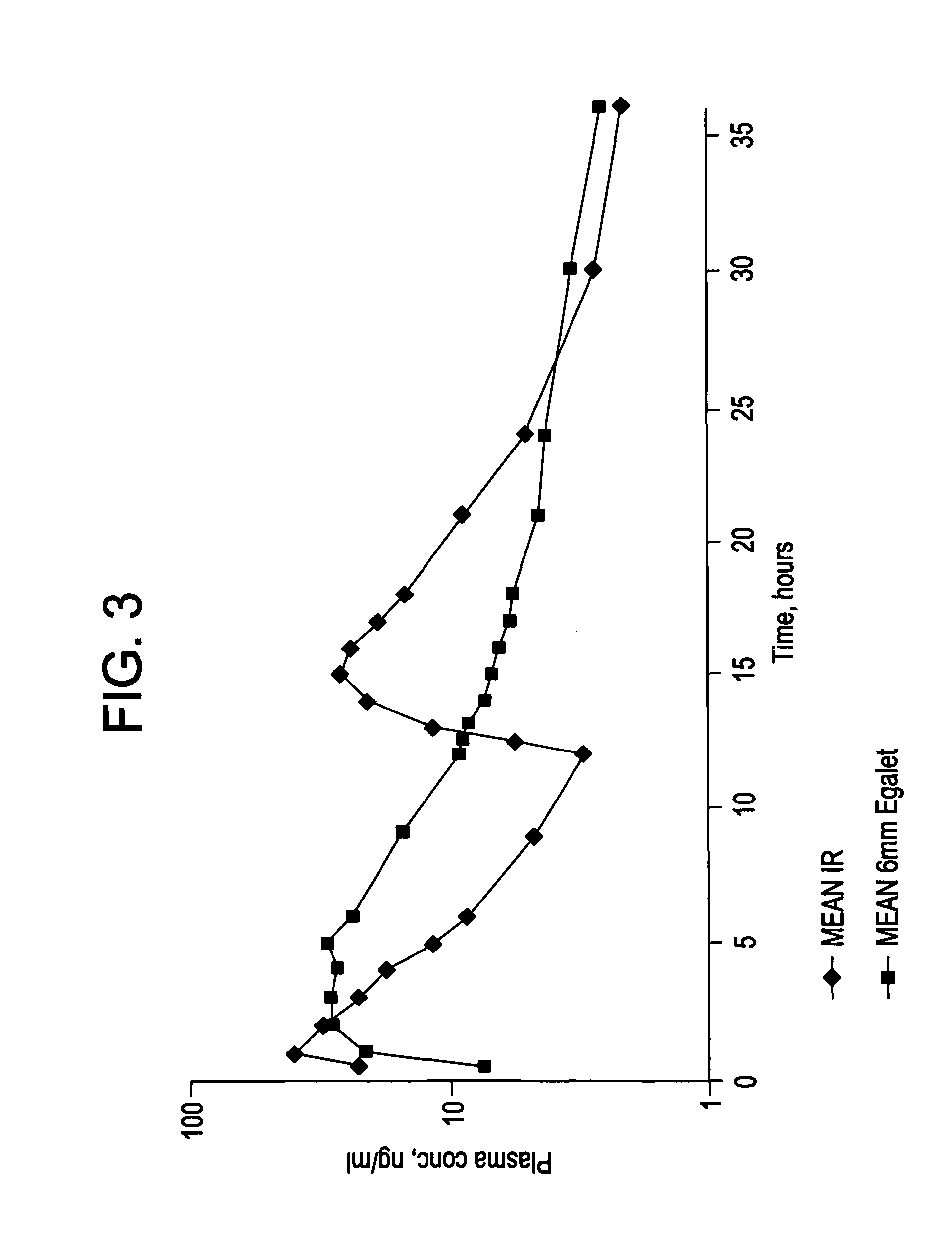
A controlled release pharmaceutical composition for oral use comprising a solid dispersion of: i) at least one therapeutically, prophylactically and / or diagnostically active substance, which at least partially is in an amorphous form, ii) a pharmaceutically acceptable polymer that has plasticizing properties, and iii) optionally, a stabilizing agent, the at least one active substance having a limited water solubility, and the composition being designed to release the active substance with a substantially zero order release. The polymer is typically a polyethylene glycol and / or polyethylene oxide having a molecular weight of at least about 20,000 in crystalline and / or amorphous form or a mixture of such polymers, and the active substance is typically carvedilol. The composition may comprise a coated matrix, the coating comprising a first cellulose derivative which is substantially insoluble in the aqueous medium, and at least one of a) a second cellulose derivative which is soluble or dispersible in water, b) a plasticizer, and c) a filler.
Owner:EGALET LTD
Nanoparticulate carverdilol formulations
The present invention is directed to nanoparticulate carvedilol compositions having improved pharmacokinetic profiles, improved bioavailability, dissolution rates and efficacy. In one embodiment, the nanoparticulate carvedilol composition has an effective average particle size of less than about 2000 nm.
Owner:ALKERMES PHARMA IRELAND LTD
Time-sustained-release formulations comprising a beta-blocker
InactiveUS20080131517A1Providing therapyAvoid problemsPowder deliveryOrganic active ingredientsCarteololBeta blocker
The present invention relates to compositions and methods of treating human subjects with a beta-adrenergic receptor blocking agent (“beta-blocker”) provided in a time-sustained-release delivery system. The time-sustained-release drug delivery systems includes at least three populations of beads, where each population of beads includes a beta-blocker. The beads may be selected from immediate-release beads, enteric-release beads, sustained-release beads, and time-sustained-release beads. The beta-blocker may be selected from acebutolol, atenolol, betaxolol, bisoprolol, esmolol, metoprolol, nebivolol, butoxamine, carteolol, carvedilol, labetalol, nadolol, oxprenolol, penbutolol, propranolol, pindolol, sotalol, and timolol. According to presently preferred embodiments, the beta-blocker is propranolol. The dosage forms of the present invention are useful for treating conditions including hypertension, angina pectoris due to coronary atherosclerosis, hypertrophic subaortic stenosis, congestive heart failure, arrhythmias, angina, anxiety, glaucoma, migraines, esophageal varices, alcohol withdrawal syndrome, irregular heartbeat, tachycardia, tremor, and neuroleptic-induced akathisia. They are also useful in the prophylaxis of migraine headaches.
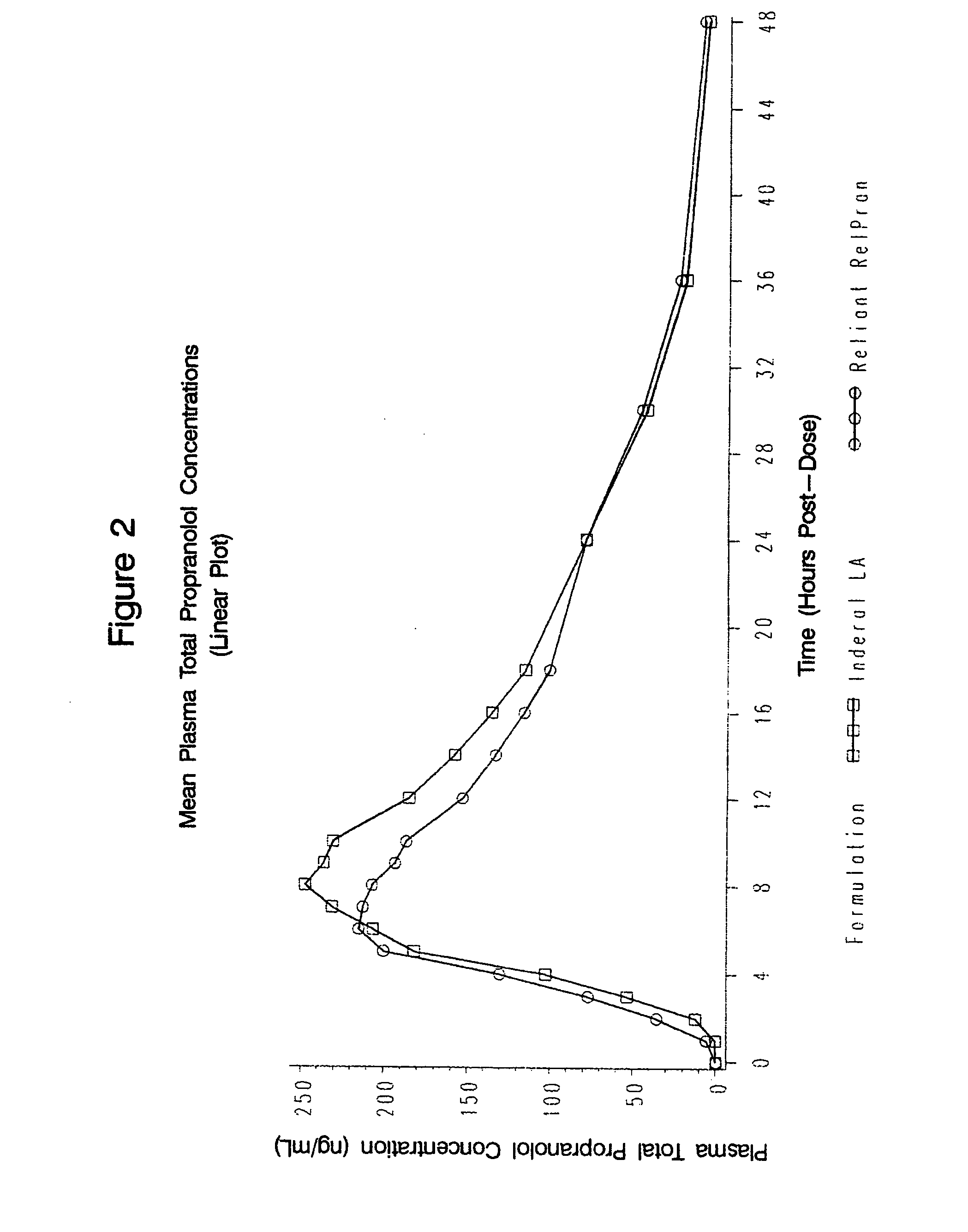
Owner:RELIANT PHARMACEUTICALS INC
Carvedilol free base, salts, anhydrous forms or solvates thereof, corresponding pharmaceutical compositions, controlled release formulations, and treatment or delivery methods
The present invention also relates to carvedilol free base, salts, anhydrous forms, or solvates thereof, corresponding pharmaceutical compositions or controlled release formulations, and methods delivery of carvedilol forms to the lower gastrointestingal tract or methods to treat cardiovascular diseases, which may include, but are not limited to hypertension, congestive heart failure, and angina. The present invention relates to control release formulations, which comprise various cavedilol forms, which may include, but are not limited to carvedilol free base and corresponding carvedilol salts, anhydrous forms or solvates thereof.
Owner:BURKE MATTHEW D +7
Carvedilol free base, salts, anhydrous forms or solvates thereof, corresponding pharmaceutical compositions, controlled release formulations, and treatment or delivery methods
The present invention also relates to carvedilol free base, carvedilol salts, anhydrous forms, or solvates thereof, corresponding pharmaceutical compositions or controlled release formulations, and delivery or dosing methods of carvedilol forms to the lower gastrointestingal tract or methods to treat cardiovascular diseases, which may include, but are not limited to hypertension, congestive heart failure, atherosclerosis, and angina. The present invention relates to controlled release formulations, which comprise various carvedilol forms, which may include, but are not limited to a carvedilol free base or corresponding carvedilol salts, anhydrous forms or solvates thereof.
Owner:FLAMEL TECHNOLOGIES +1
Carvedilol free base, salts, anhydrous forms or solvates thereof, corresponding pharmaceutical compositions, controlled release formulations, and treatment or delivery methods
The present invention also relates to carvedilol free base, salts, anhydrous forms, or solvates thereof, corresponding pharmaceutical compositions or controlled release formulations, and methods delivery of carvedilol forms to the lower gastrointestingal tract or methods to treat cardiovascular diseases, which may include, but are not limited to hypertension, congestive heart failure, and angina. The present invention relates to control release formulations, which comprise various cavedilol forms, which may include, but are not limited to carvedilol free base and corresponding carvedilol salts, anhydrous forms or solvates thereof.
Owner:SMITHKLINE BEECHAM (CORK) LTD
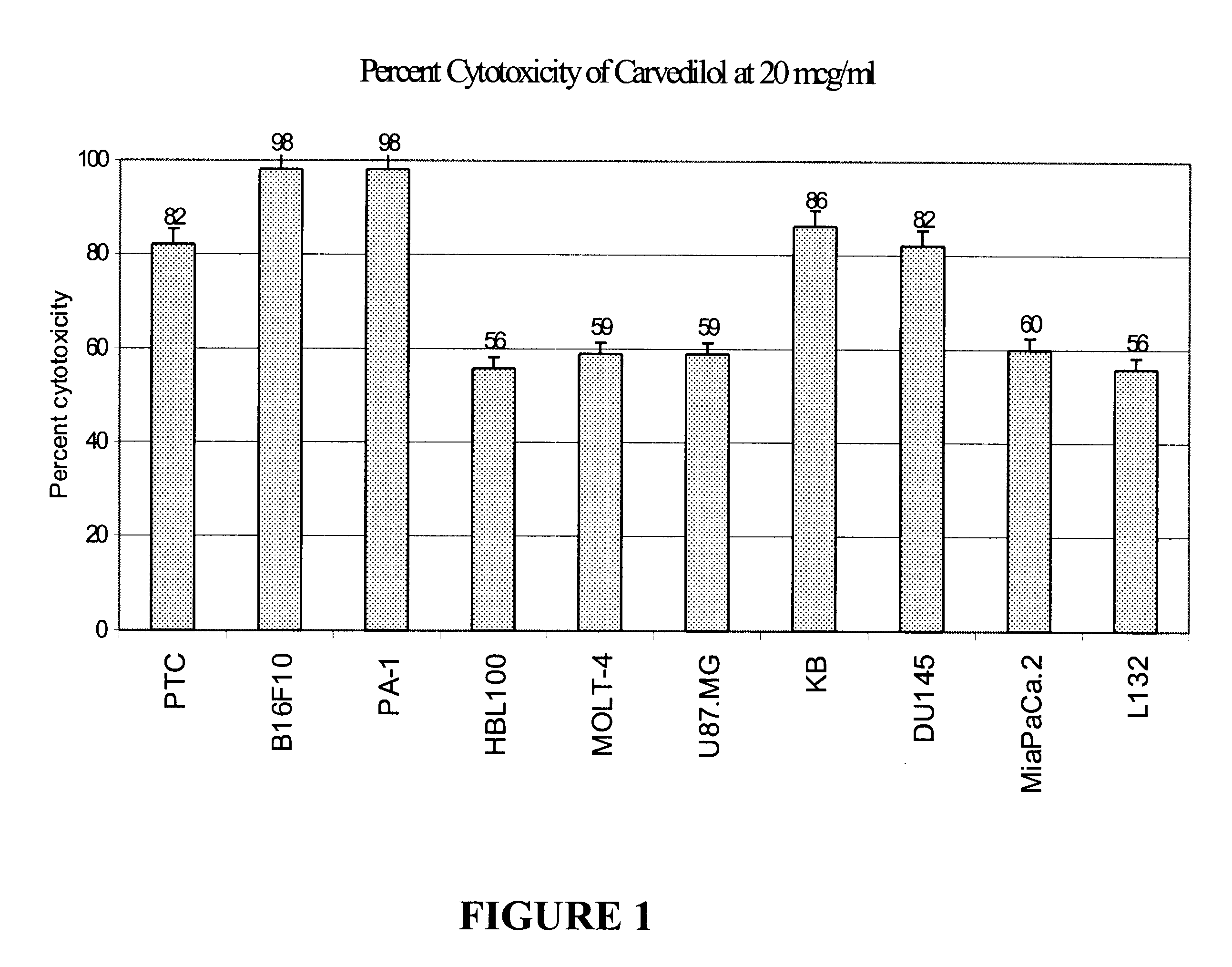
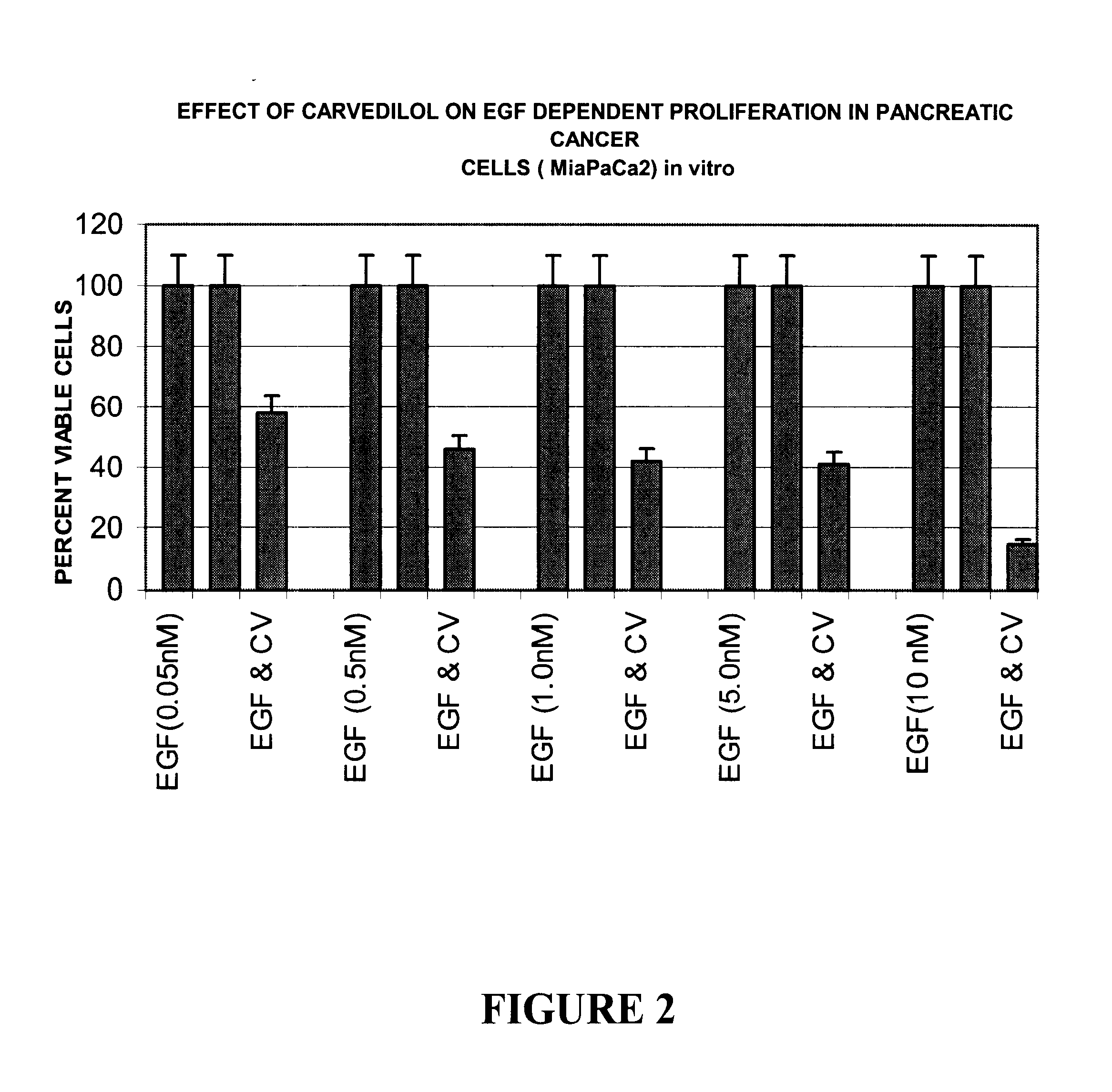
Anti-cancer activity of carvedilol and its isomers
InactiveUS6632832B1Inhibits COX activityPrevent and inhibit tumor growthBiocideArtificial cell constructsMelanomaProstate cancer
The present invention provides for pharmaceutical compositions comprising carvedilol for treatment of cancer. More particularly the invention relates to the use of carvedilol for treatment of cancers of the colon, ovary, breast, prostate, pancreas, lung, melanoma, glioblastoma, oral cancer and leukemias. Although not bound to any theory, the anticancer activity of carvedilol appears to be attributed to the inhibition of Epidermal Growth Factor and Platelet derived growth factor dependent proliferation of cancer cells. Further, carvedilol exerts anticancer effect by inhibition of the Protein kinase C (PKC) activity and that of the cyclooxygenase 2 enzyme. The invention also relates to the anticancer activity of the optically pure isomers S(-) and R(+) of carvedilol and the use of carvedilol and its isomers in pharmaceutical compositions for the treatment of cancer.< / PTEXT>
Owner:DABUR RESEARCH FOUNDATION
Carvedilol salts, anhydrates and/or solvate thereof, corresponding pharmaceutical compositions, controlled release formulations, and treatment or delivery methods
ActiveUS20050196459A1Increase in bioabsorption timeOrganic active ingredientsPill deliveryPharmacy medicineCarvedilol
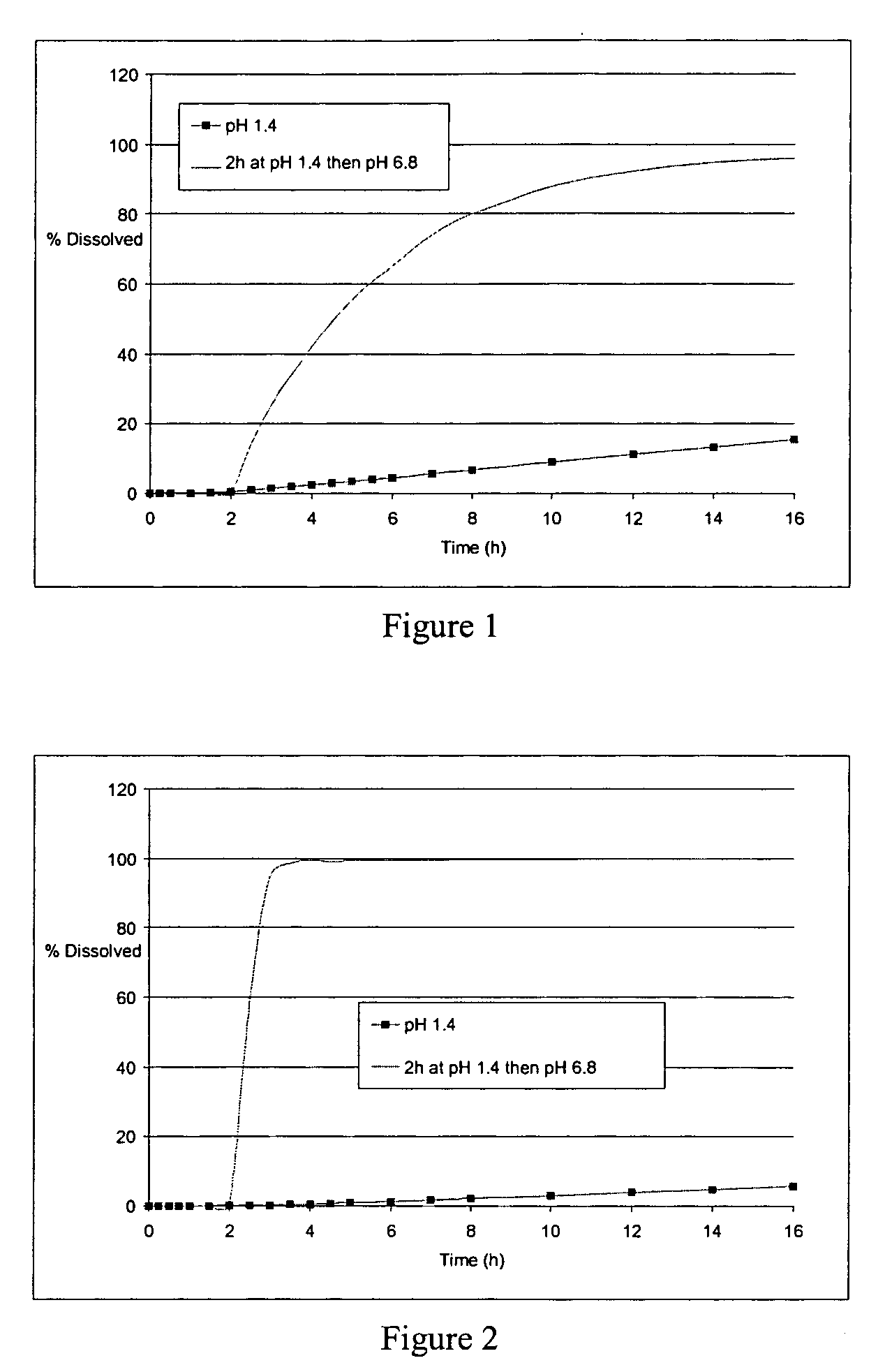
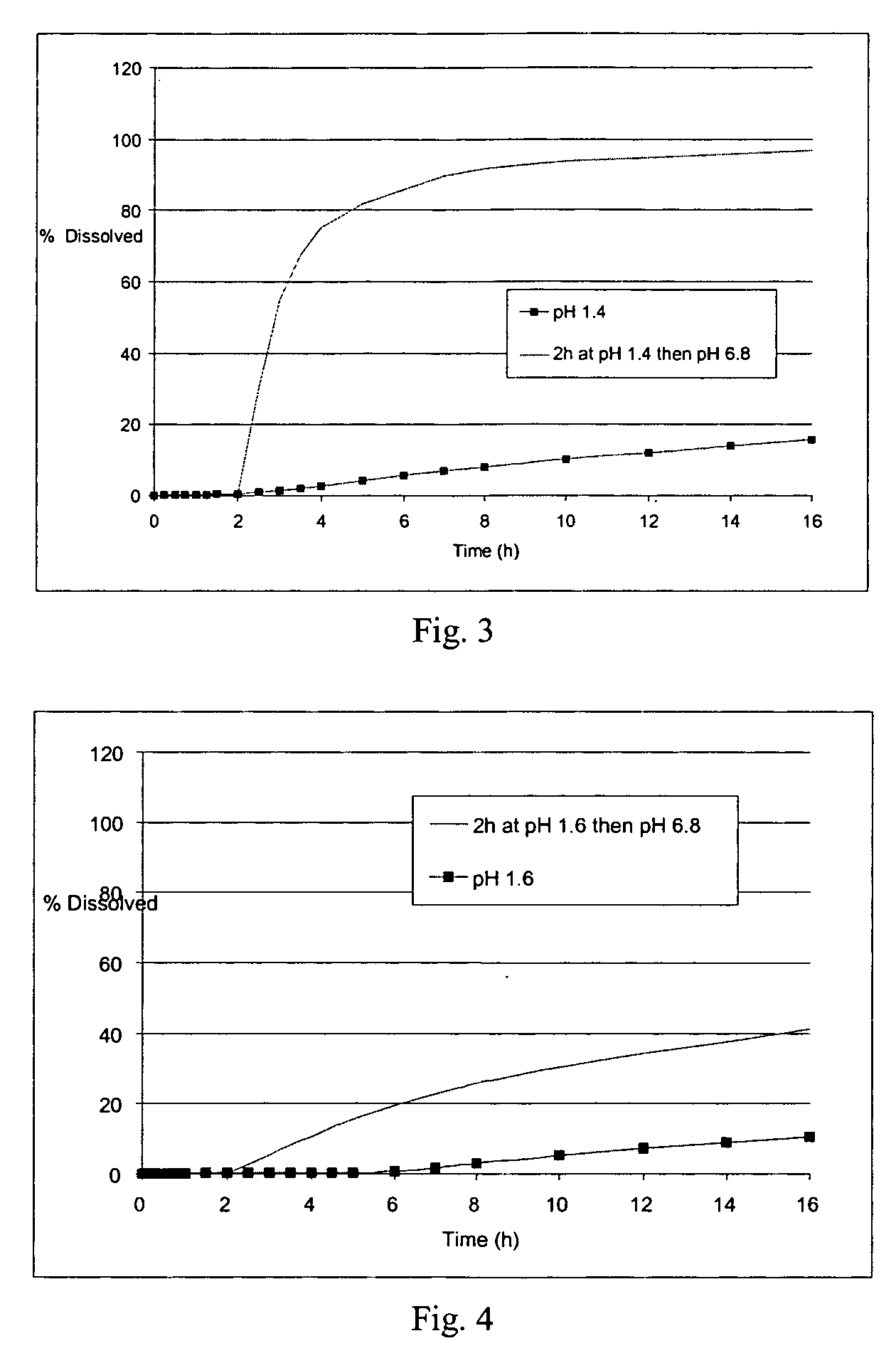
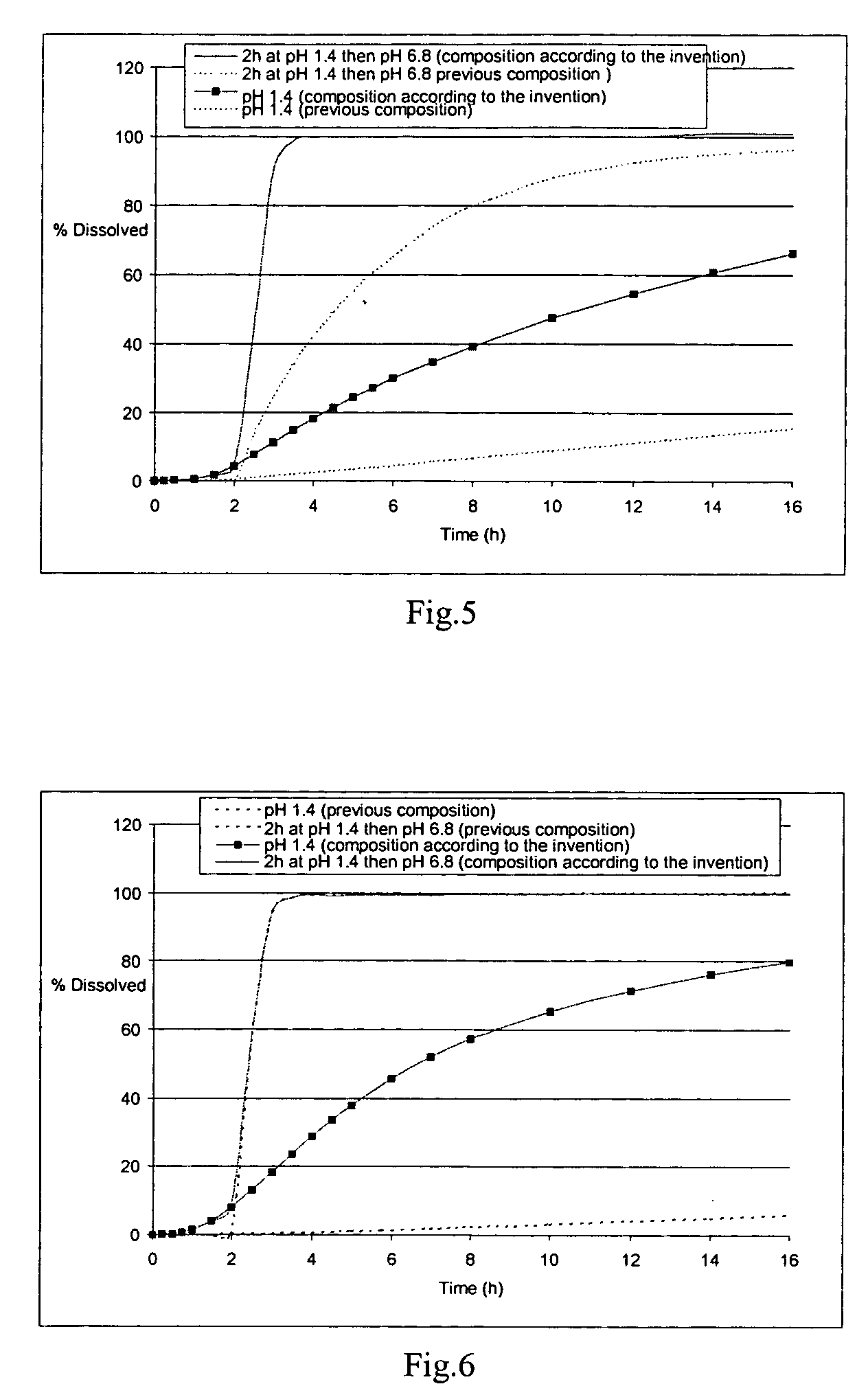
The field of the invention is that of oral pharmaceutical medicinal products or compositions, more particularly of the type of those comprising one or more active principles. The aim of the invention is to provide an improved oral medicinal product that can be administered in one or more daily doses, with modified release of active principle (in particular an active principle), for improving the prophylactic and therapeutic efficacy of such a medicinal product. This aim is achieved by means of the multimicrocapsular oral pharmaceutical form according to the invention in which the release of the AP is controlled by means of a double mechanism of triggering the release: “time triggering” and “pH triggering”. This medicinal product comprises microcapsules with modified release of active principle, each containing a core comprising the active principle and one or more swelling agents, and at least one coating making possible the modified release of the active principle.
Owner:FLAMEL IRELAND
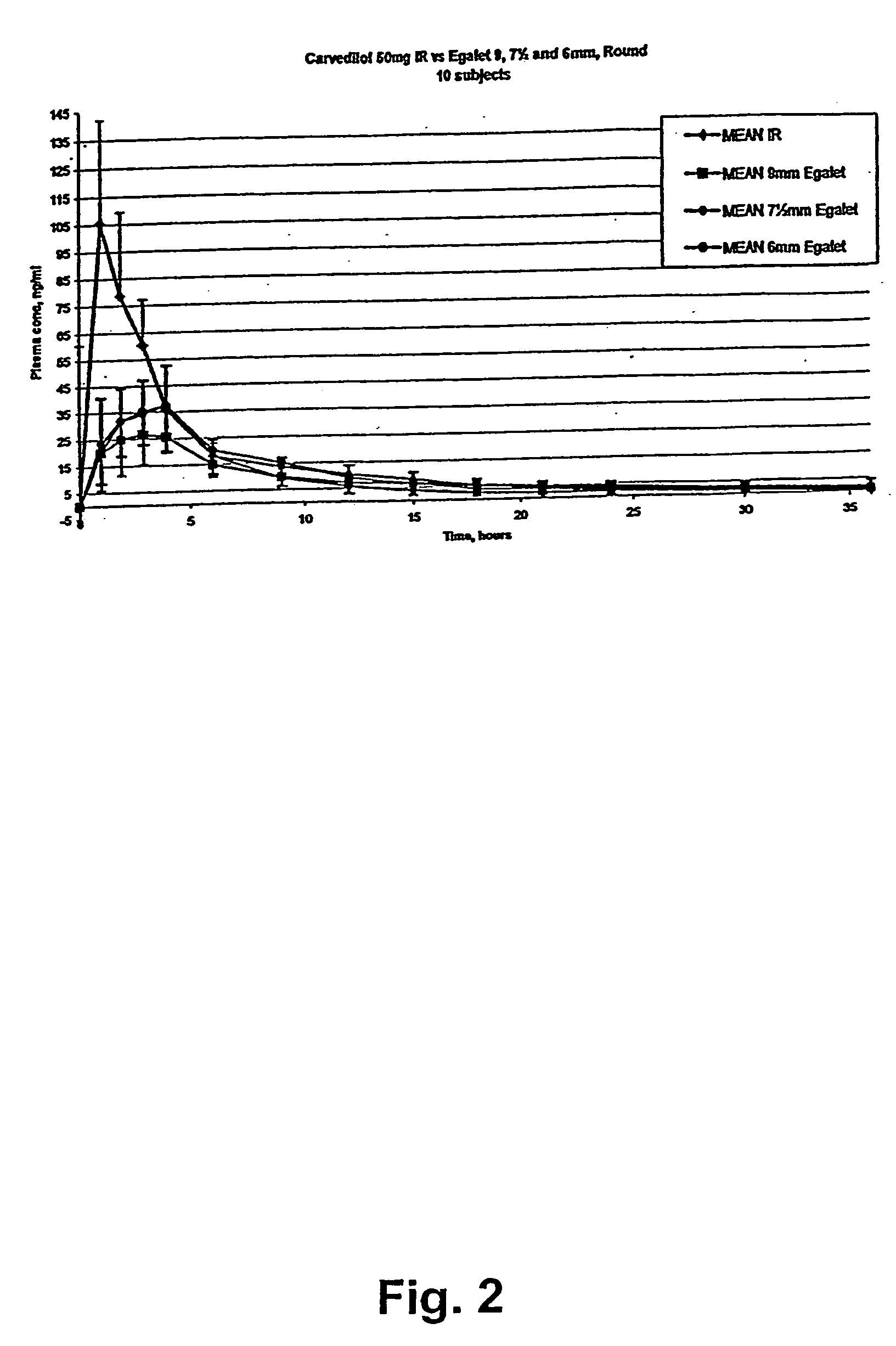
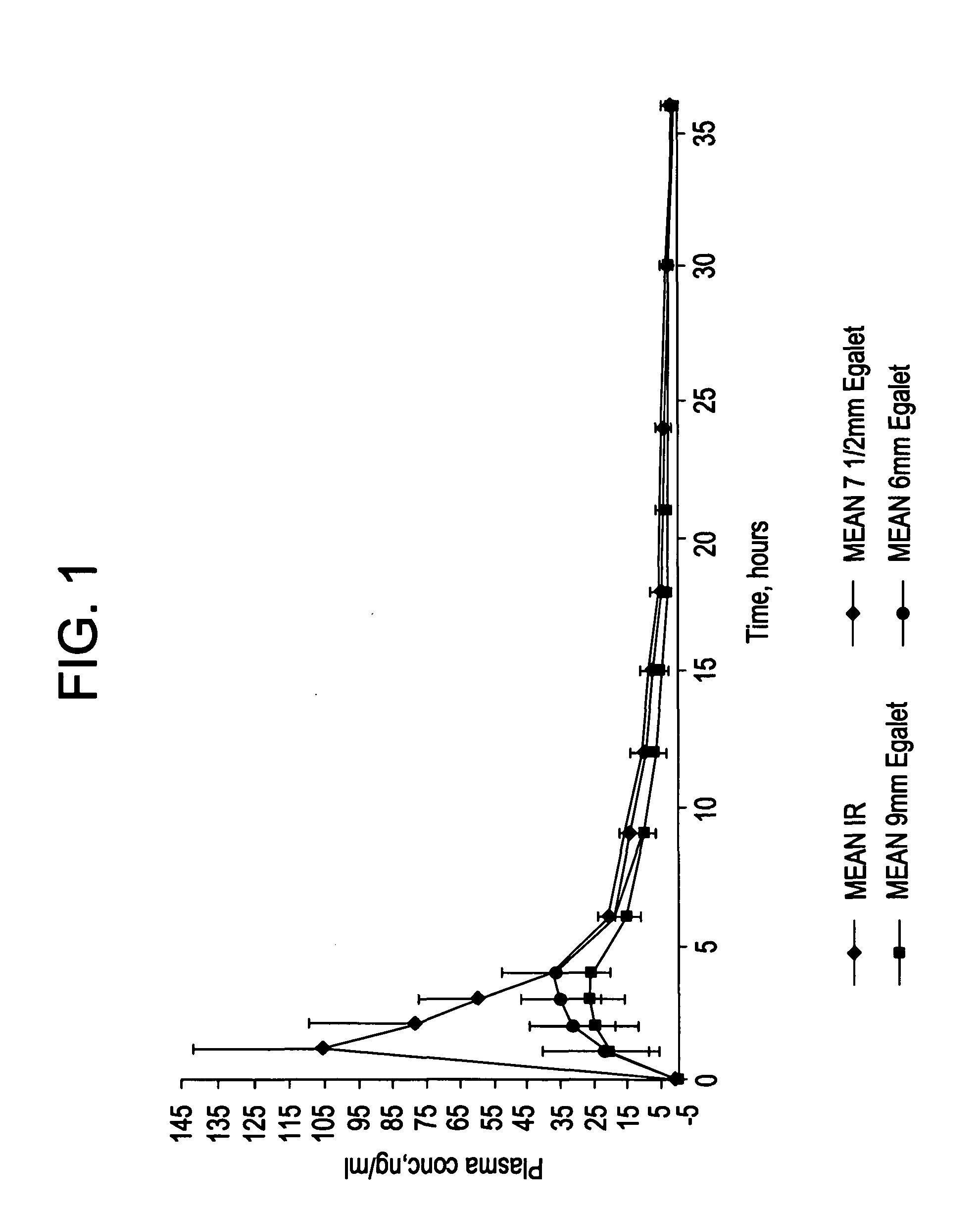
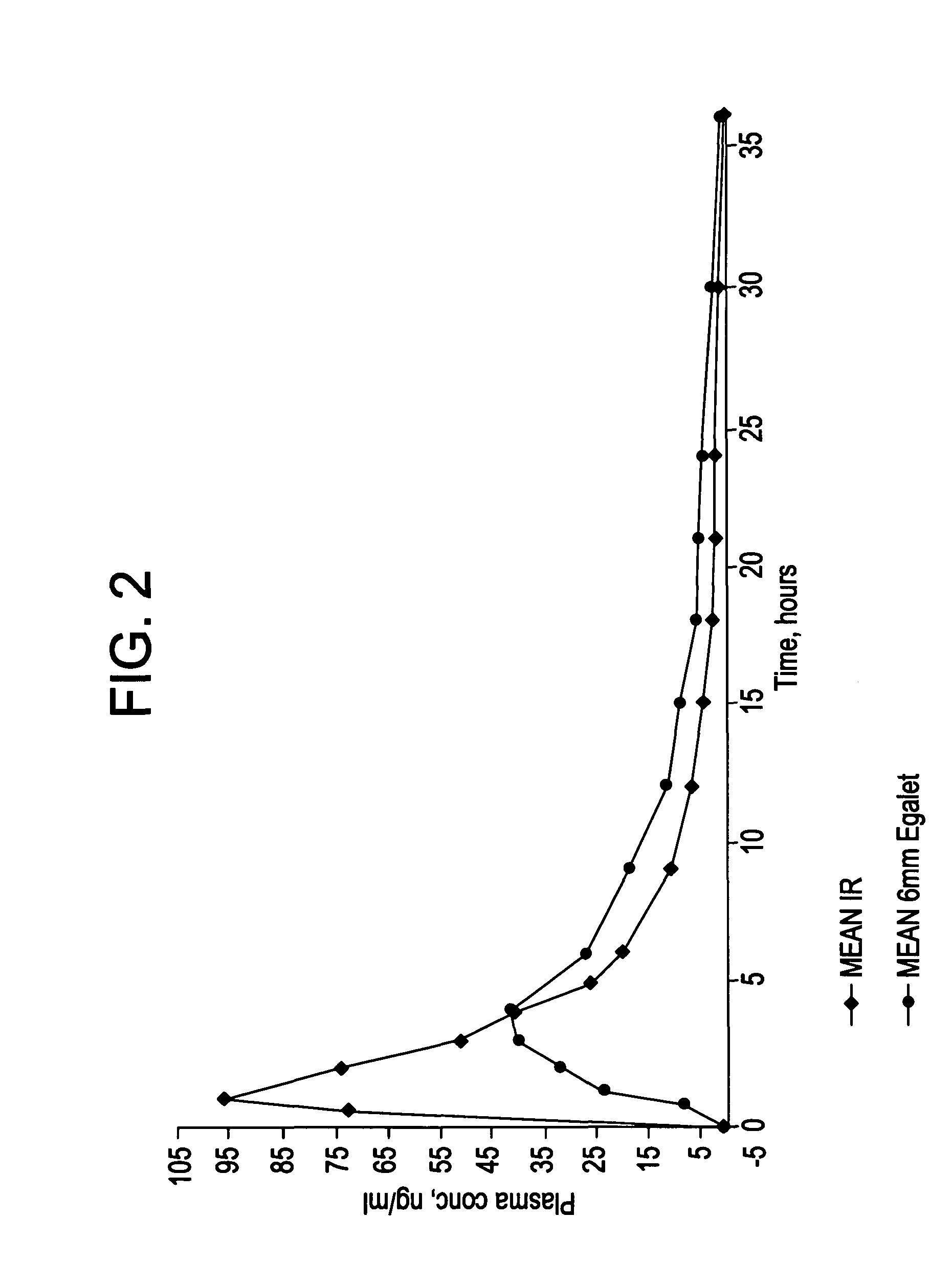
Controlled release carvedilol compositions
InactiveUS20080268057A1Low water solubilityImprove bioavailabilityPowder deliveryBiocideControlled releaseTreatment effect
A controlled release pharmaceutical composition for oral use comprising carvedilol. The composition releases carvedilol after oral administration to a mammal, including a human, in such a manner that a prolonged residence of carvedilol is obtained in the circulatory system compared with the known compositions of carvedilol. Furthermore, a composition according to the present invention makes available to the body a suitable plasma concentration of one or both of the enantiomeric species, namely R(+) and / or S(−) carvedilol for obtaining the desired therapeutic effect.
Owner:EGALET LTD
Process for preparation of carvedilol
InactiveUS20060167077A1High technical contentReduce contentBiocideOrganic chemistryCarvedilolSolvent
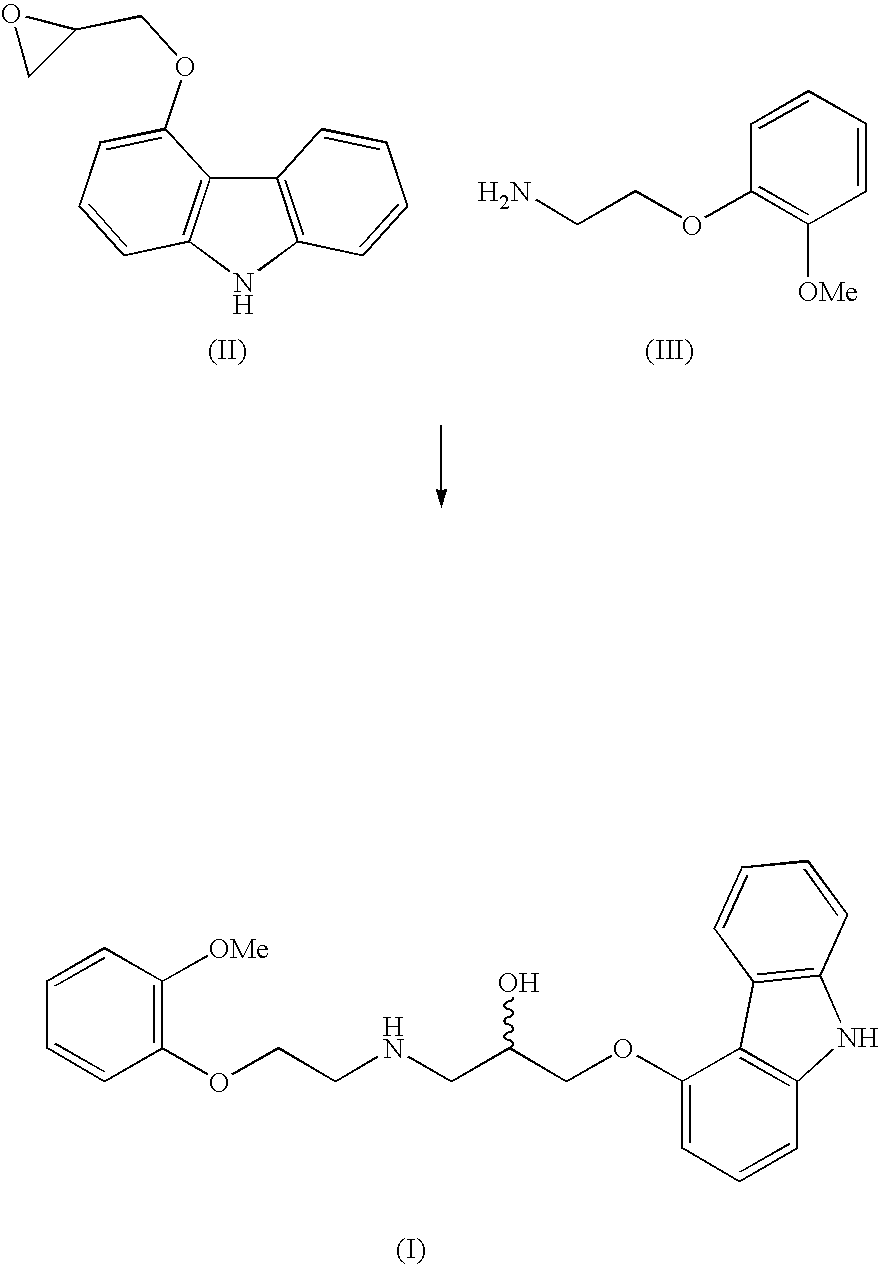
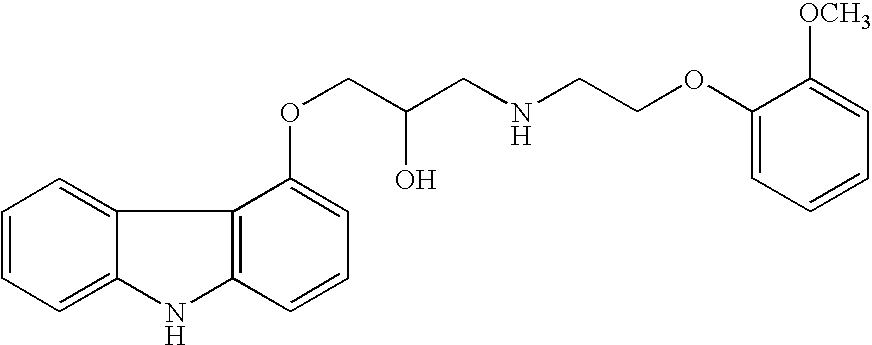
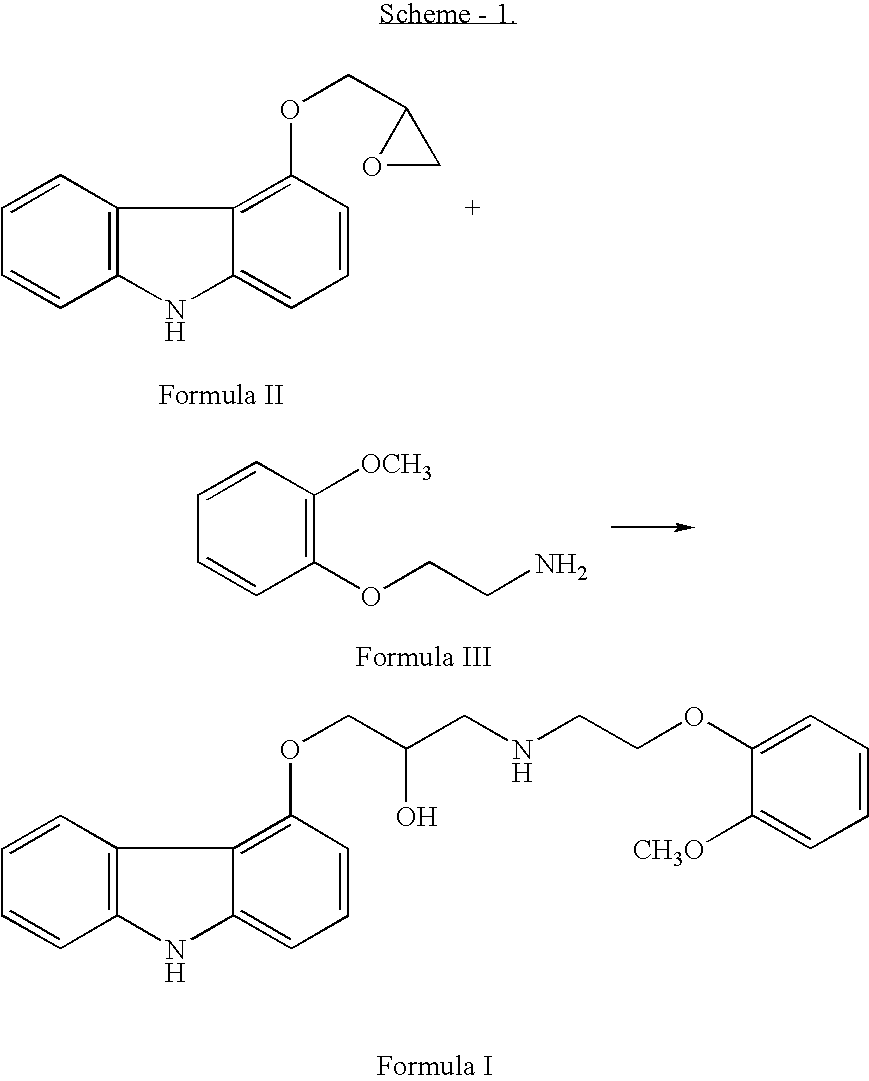
The invention solves a new method of preparation of Carvedilol for pharmaceutical use. In the synthesis of Carvedilol a reaction of 4-(oxirane-2-ylmethoxy)-9H-arbazole (II) with 2-(2-methoxyphenoxy)ethylamine salts (IV) in the presence of a base, in an alcohol having the number of carbons C2 to C5 as a solvent, at an elevated temperature, is used. After processing of the crude reaction mixture crude Carvedilol is obtained, which is purified by crystallization from ethylacetate with an addition of activated carbon and the final substance is formulated by crystallization from ethylacetate.
Owner:SANECA PHARMA
Method of treatment
This invention relates to a method of maintaining glycemic control in diabetic hypertensive patients which comprises administering carvedilol to a subject in need thereof.
Owner:SMITHKLINE BECKMAN CORP
Compounds and methods for carbazole synthesis
InactiveUS20070197797A1Reduce yieldPoor regioselectivityOrganic chemistryOrganic compound preparationCarbazoleHydroxamic acid
Compounds having bi-cyclic structure comprising a partially unsaturated 6-carbon first cyclic moiety interconnected to a 6-carbon second cyclic moiety second via a divalent linking moiety are provided. The compounds can be used as intermediates compounds in methods for the synthesis of carbazoles and derviatives thereof, including carvedilol, and tricyclic alkylhydroxamates, which do not require Fischer indole synthetic steps. Methods of preparing the compounds having bi-cyclic structures are also provided.
Owner:ROCHE COLORADO CORP
Carvedilol Monocitrate Monohydrate
This invention relates to carvedilol monocitrate monohydrate, compositions containing this salt of carvedilol and methods of using this compound to treat hypertension, congestive heart failure and angina.
Owner:SMITHKLINE BEECHAM (CORK) LTD
Process for the preparation of carvedilol and its salts
Disclosed herein is a process for preparation of carvedilol substantially free from its bis-impurity comprises the reaction of 4-(2,3-epoxypropoxy)carbazole and 2-(2-methoxyphenoxy)ethylamine in a polar aprotic solvent media; followed by isolation of carvediol from the reaction mass as an acid addition salt and subsequent conversion into pure carvedilol.
Owner:IPCA LAB LTD
Carvedilol monocitrate monohydrate
This invention relates to carvedilol monocitrate monohydrate, compositions containing this salt of carvedilol and methods of using this compound to treat hypertension, congestive heart failure and angina.
Owner:PHARMCO PUERTO RICO INC
Controlled release carvedilol compositions
InactiveUS8449914B2Improve solubilityPrevents and inhibits and delayPowder deliveryBiocideControlled releaseOral medication
A controlled release pharmaceutical composition for oral use comprising carvedilol. The composition releases carvedilol after oral administration to a mammal, including a human, in such a manner that a prolonged residence of carvedilol is obtained in the circulatory system compared with the known compositions of carvedilol. Furthermore, a composition according to the present invention makes available to the body a suitable plasma concentration of one or both of the enantiomeric species, namely R(+) and / or S(−) carvedilol for obtaining the desired therapeutic effect.
Owner:EGALET LTD
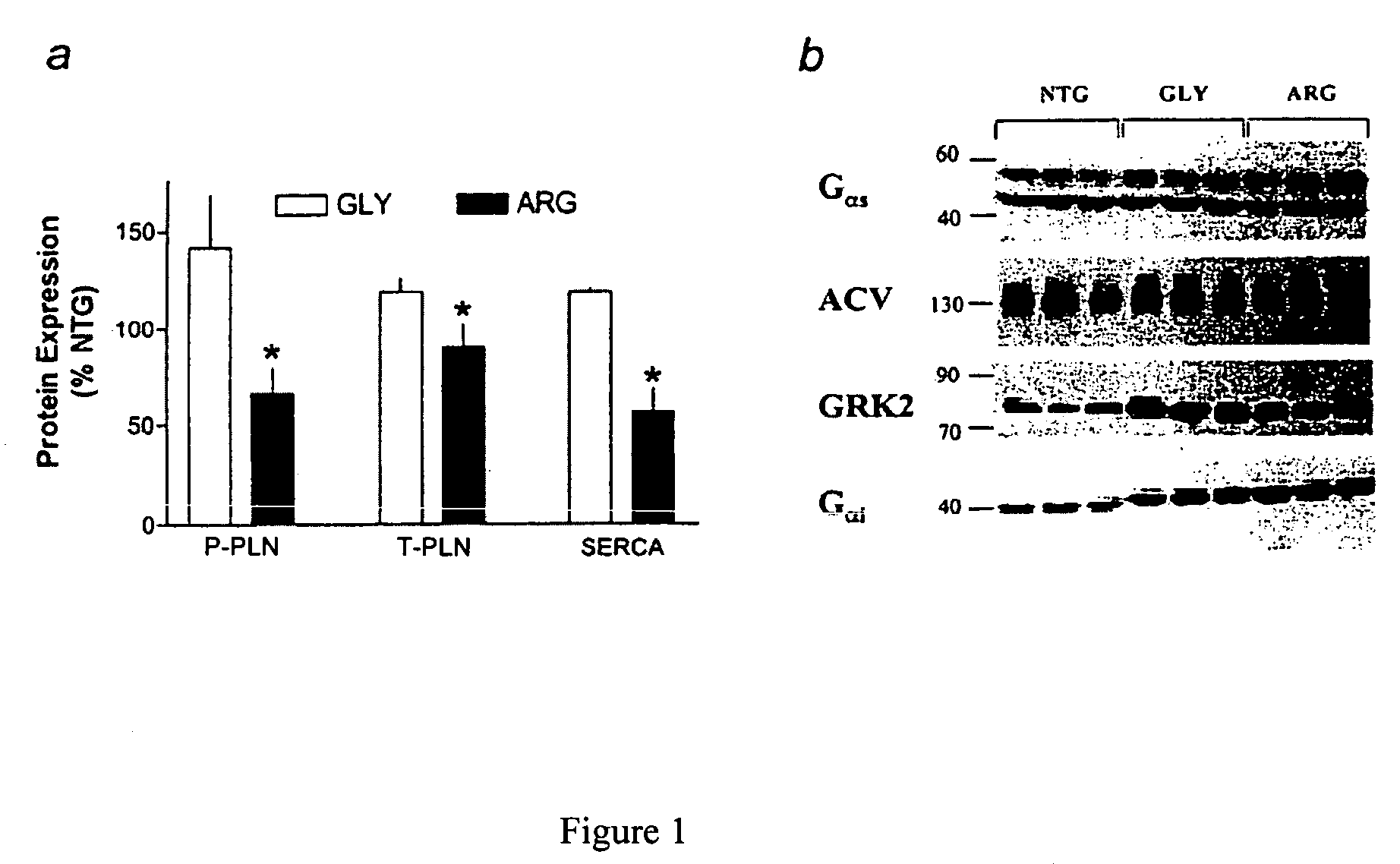
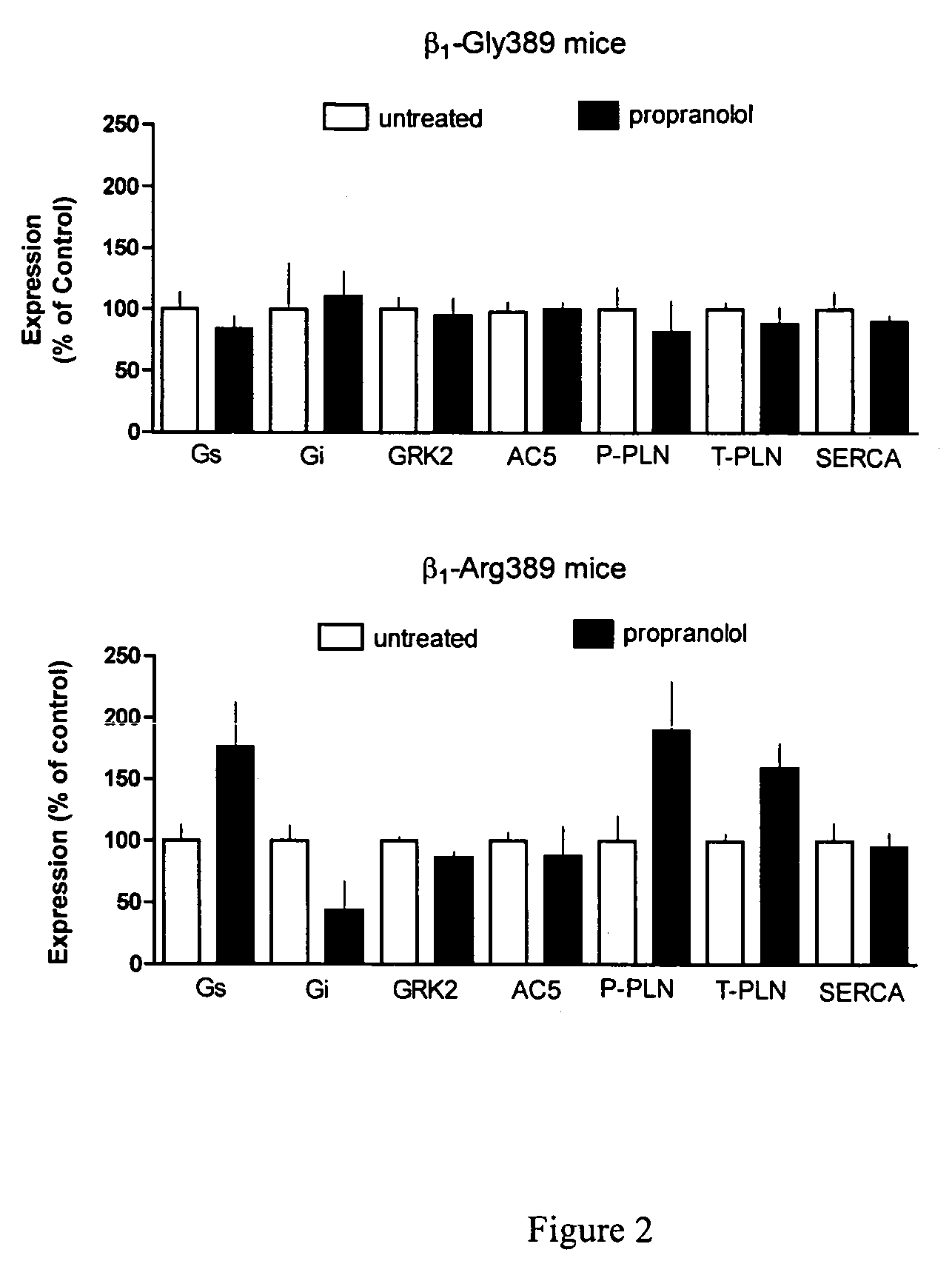
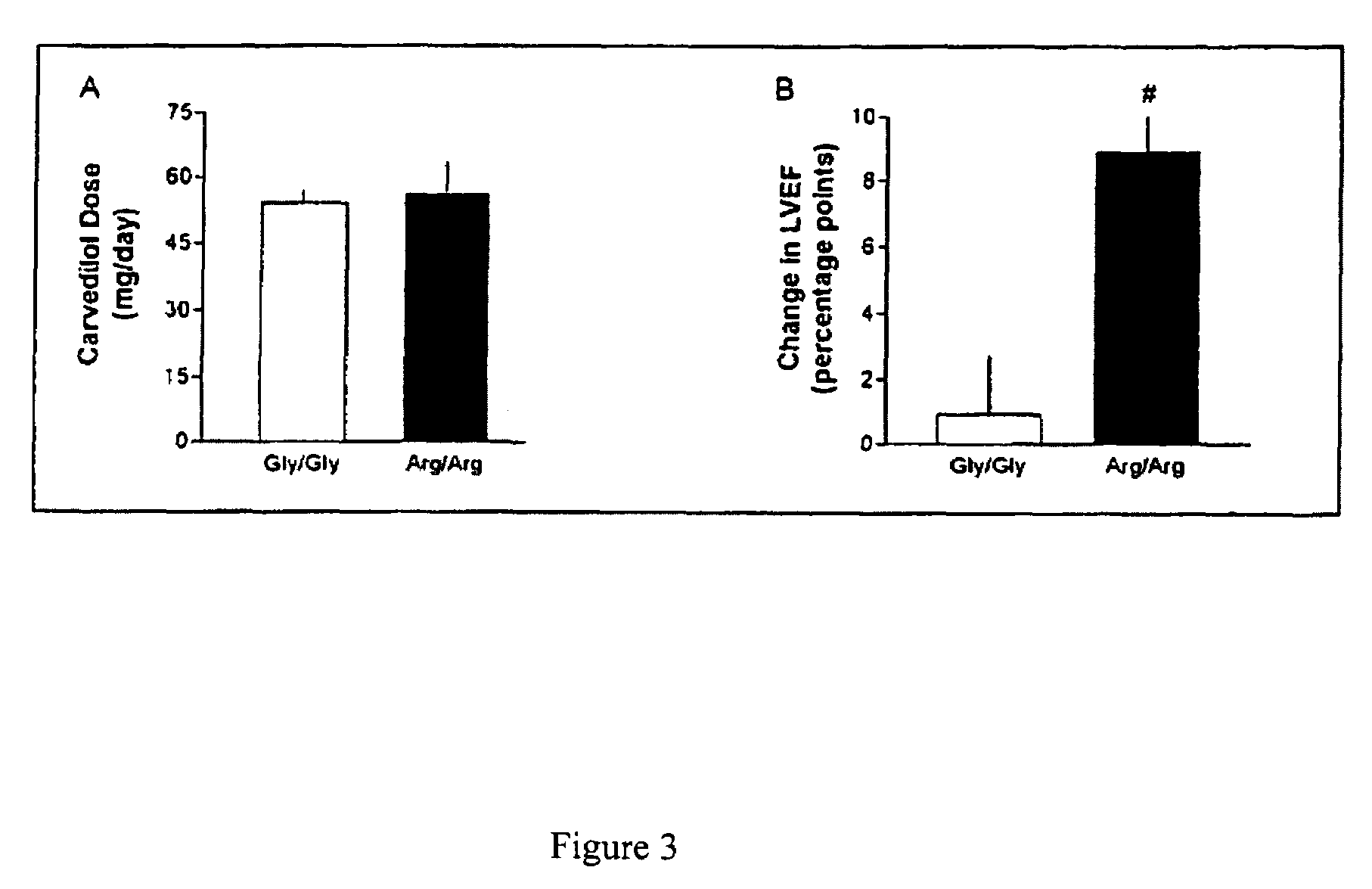
Methods for predicting relative efficacy of a beta blocker therapy based on a B1-adrenergic receptor polymorphism
InactiveUS7449292B2Sugar derivativesMicrobiological testing/measurementBeta blockerRelative efficacy
Methods and compositions for the detection, diagnosis, and prevention of cardiac conditions are provided. Polymorphisms of β1-adrenergic receptor are provided. The Gly389 β1-adrenergic receptor variants are not as responsive to treatment β blockers such as carvedilol, metoprolol or bisoprol. Thus, genotyping β1-adrenergic receptor polymorphisms is useful for predicting relative responsiveness to treatment with beta blockers. The Gly389 polymorphism also may be used, alone or in conjunction with other adrenergic receptor polymorphisms, to predict relative risk of developing cardiovascular diseases such as heart failure or to predict relative survival rate in patients with heart failure or other cardiovascular diseases. Also provided are transgenic mice and transgenic cells expressing the β1-adrenergic receptor polymorphisms, and their use in identifying therapeutic agents.
Owner:UNIVERSITY OF CINCINNATI
Simple preparation of expanded mesoporous silicon dioxide and dissolution promoting application of expanded mesoporous silicon dioxide
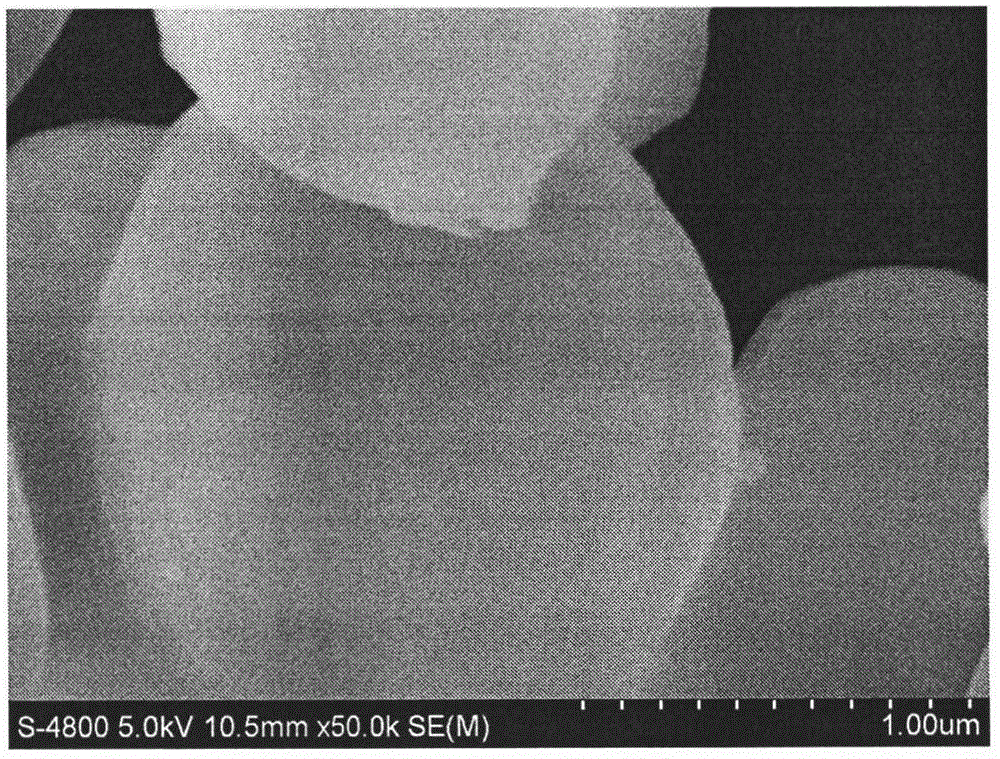
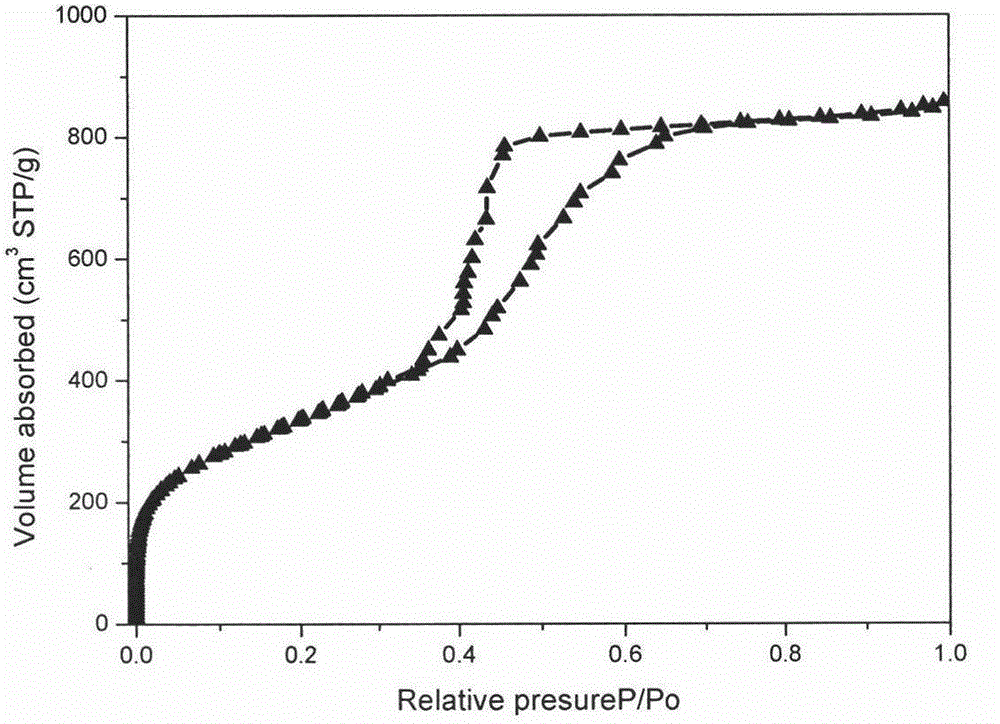
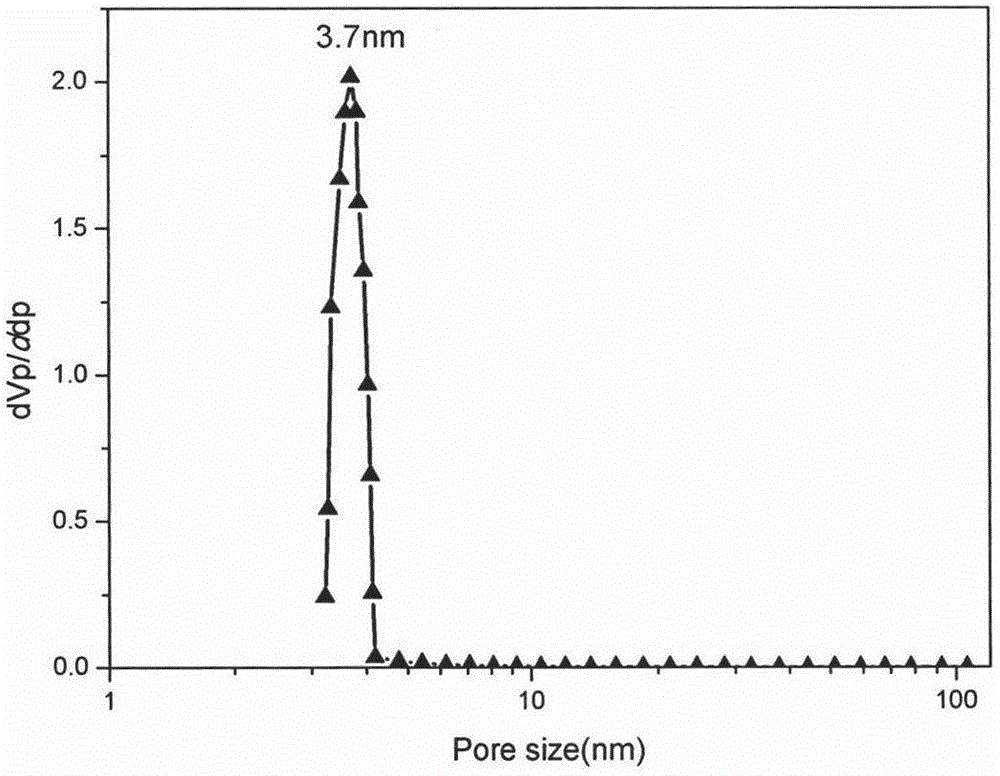
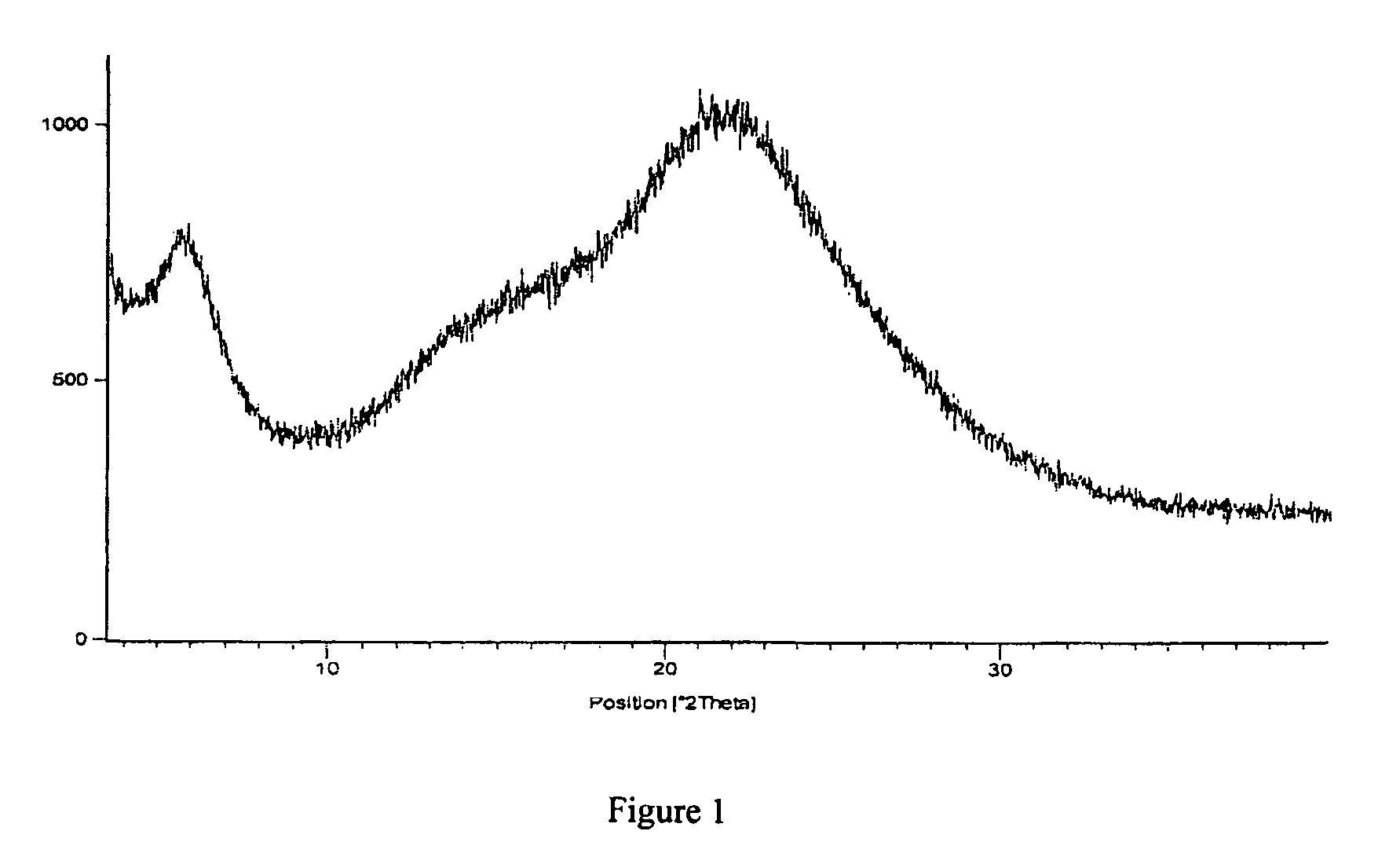
The invention discloses a simple synthetic method of expanded mesoporous silicon dioxide with a large specific surface area and a dissolution promoting application of expanded mesoporous silicon dioxide on an insoluble drug carvedilol. The method comprises the following steps: firstly dissolving a template in deionized water, dissolving a pore-enlarging agent in ethanol, then mixing the two solutions, adding sodium hydroxide and a silicon source into the mixed solution at 80 DEG C and filtering the reaction liquid and washing the filter cake after reaction for two hours; and finally, putting the filter cake in a hydrochloric acid / ethanol solution for refluxing for 3 hours to obtain an expanded silicon dioxide product with the large specific surface area. The preparation method is easy to operate, simple in process, mild in reaction and low in cost. The prepared expanded mesoporous silicon dioxide is in a mono-dispersive spherical shape, the specific surface area reaches up to 1234.4m<2> / g and the pore diameter is 3.7nm. Finally, the mesoporous silicon dioxide and carvedilol are used for preparing a solid dispersion, and dissolution of the medicinal raw material carvedilol is obviously improved, so that the dissolution rate of carvedilol within 15 minutes reaches near 80%.
Owner:CHINA PHARM UNIV
Stable amorphous form of carvedilol dihydrogen phosphate with stabilizer
The present invention provides a novel stable amorphous form of carvedilol dihydrogen phosphate and the process for its preparation that involves reaction of carvedilol base with ortho phosphoric acid in the presence of stabilizer in a suitable solvent or mixture of solvents followed by concentration and isolation. An alternate process for preparation of amorphous form of carvedilol dihydrogen phosphate involves addition of stabiliser to the solution of stable amorphous or crystalline carvedilol dihydrogen phosphate in a suitable solvent or mixture of solvents followed by concentration and isolation. The novel stable amorphous form of carvedilol dihydrogen phosphate is highly stable.
Owner:LUPIN LTD
Extended release formulations of carvedilol
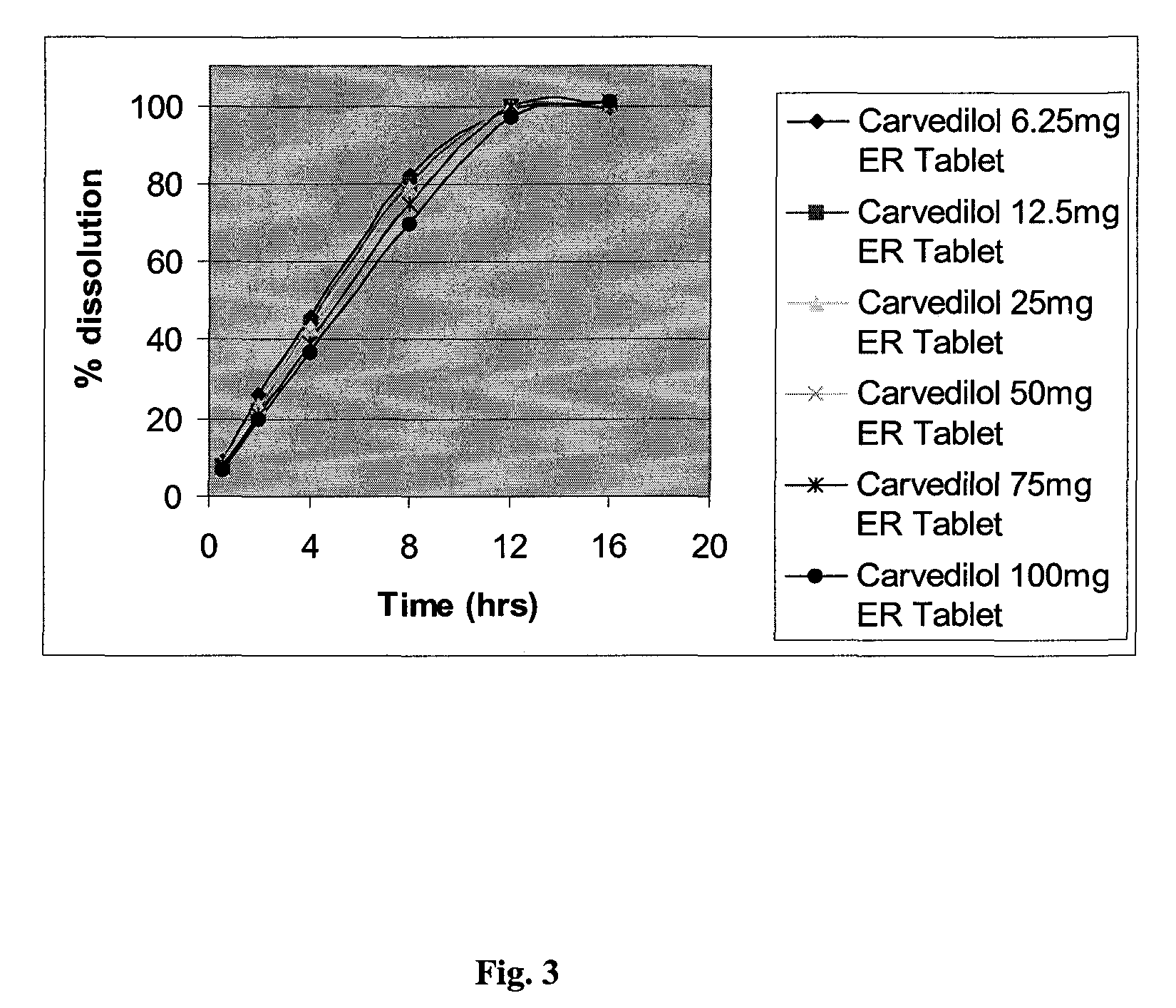
InactiveUS20080138404A1Dissolve fastEnhancing rate and extent of releaseBiocidePill deliveryHydrophilic polymersCarvedilol
An improved controlled release dosage form for once-daily administration of carvedilol is described. The controlled release dosage form comprises a therapeutically effective amount of carvedilol and / or a pharmaceutically acceptable salt thereof; one or more hydrophilic polymers; one or more pharmaceutically acceptable excipients; and a polyoxyalkylene block copolymer, a solid dispersion of carvedilol and an extrusion material or a combination of a polyoxyalkylene block copolymer, a solid dispersion of carvedilol and an extrusion material.
Owner:BIOVAIL LAB INT SRL
Use of medicine combination comprising carvedilol and angiotensin II recipient antagon in preparing medicine for treating kidney disease
ActiveCN101417132ASynergistic treatment effect is goodImpact on long-term survivalPowder deliveryMetabolism disorderNephrosisActive component
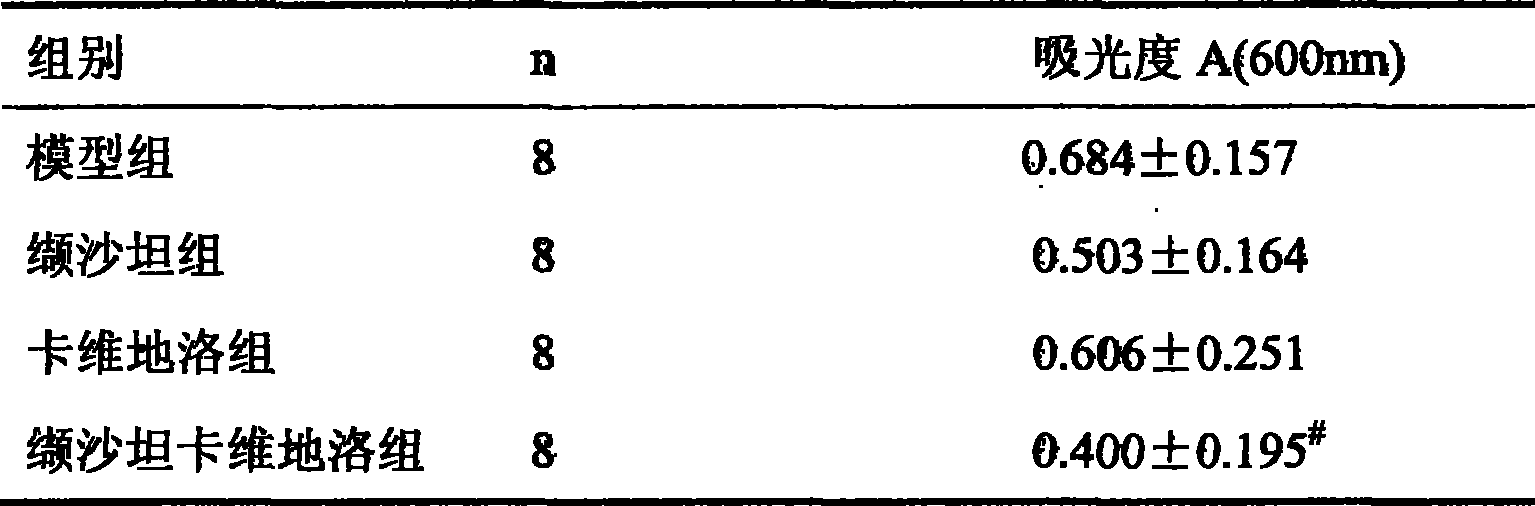
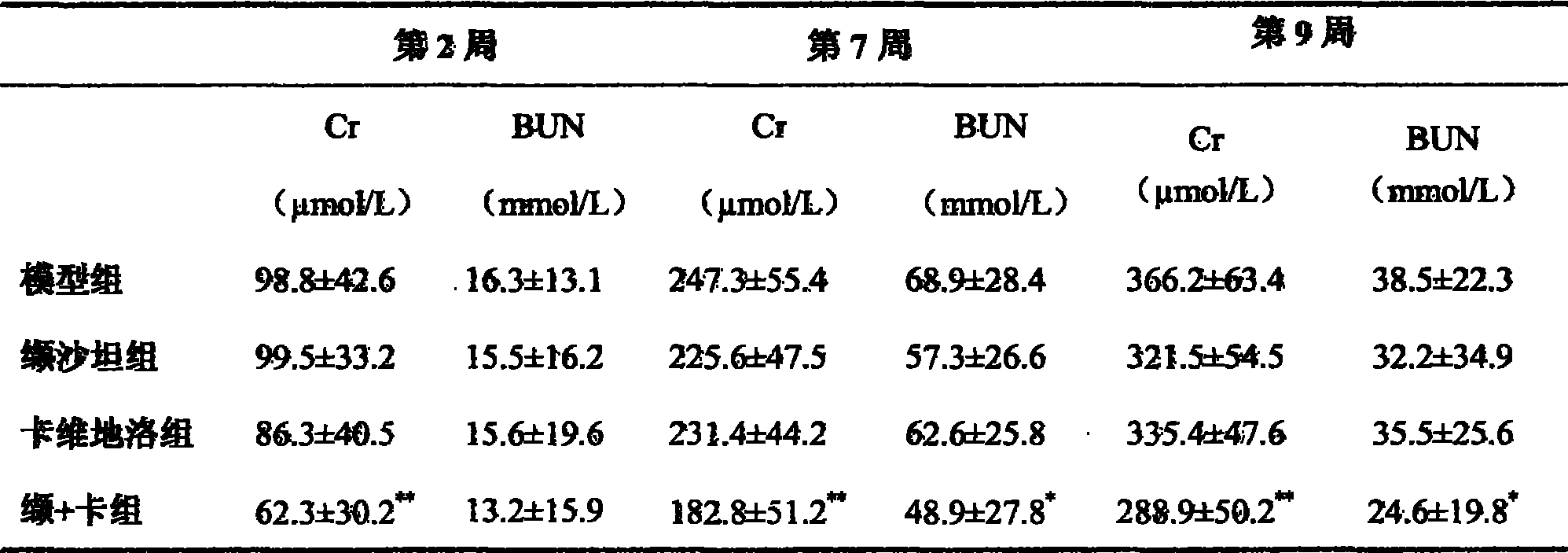
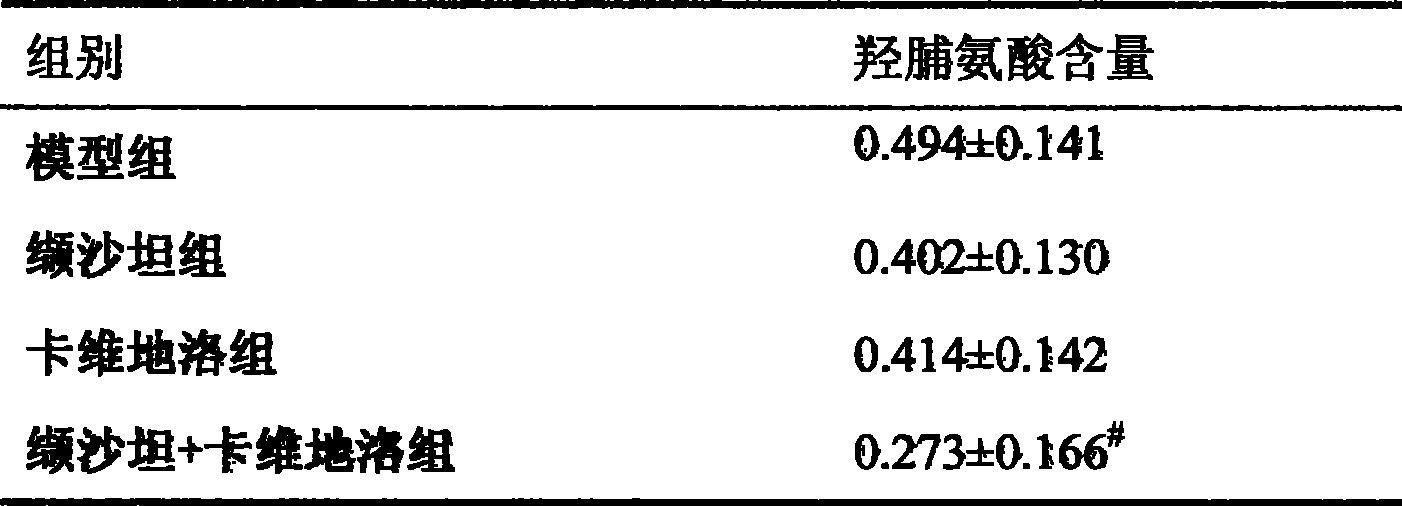
The invention provides the usages of a drug composition comprising the active components of carvedilol and an angiotensin II receptor antagonist for preparing a drug which can cure nephropathy. The cooperation effect of the carvedilol and the angiotensin II receptor antagonist are used for developing a method which is more effective in curing the nephropathy. The drug composition has remarkable effects when being used for curing nephropathy, diabetic nephropathy, hypertensive nephropathy, and the like.
Owner:LUNAN PHARMA GROUP CORPORATION
Method of treating airway diseases with beta-adrenergic inverse agonists
InactiveUS20060194882A1Improve securityReduce functionOrganic active ingredientsBiocideDiseaseAdrenergic
The use of β-adrenergic inverse agonists provides a new and highly efficient way of treating a number of pulmonary airway diseases, including asthma, emphysema, and chronic obstructive pulmonary diseases. In general, such a method comprises administering a therapeutically effective amount of a β-adrenergic inverse agonist to the subject to treat the pulmonary airway disease. Particularly preferred inverse agonists include nadolol and carvedilol. In addition, methods are described for long-term administration of such inverse agonists and for determining the suitability of patients for long-term inverse agonist therapy.
Owner:INVERSEON INC
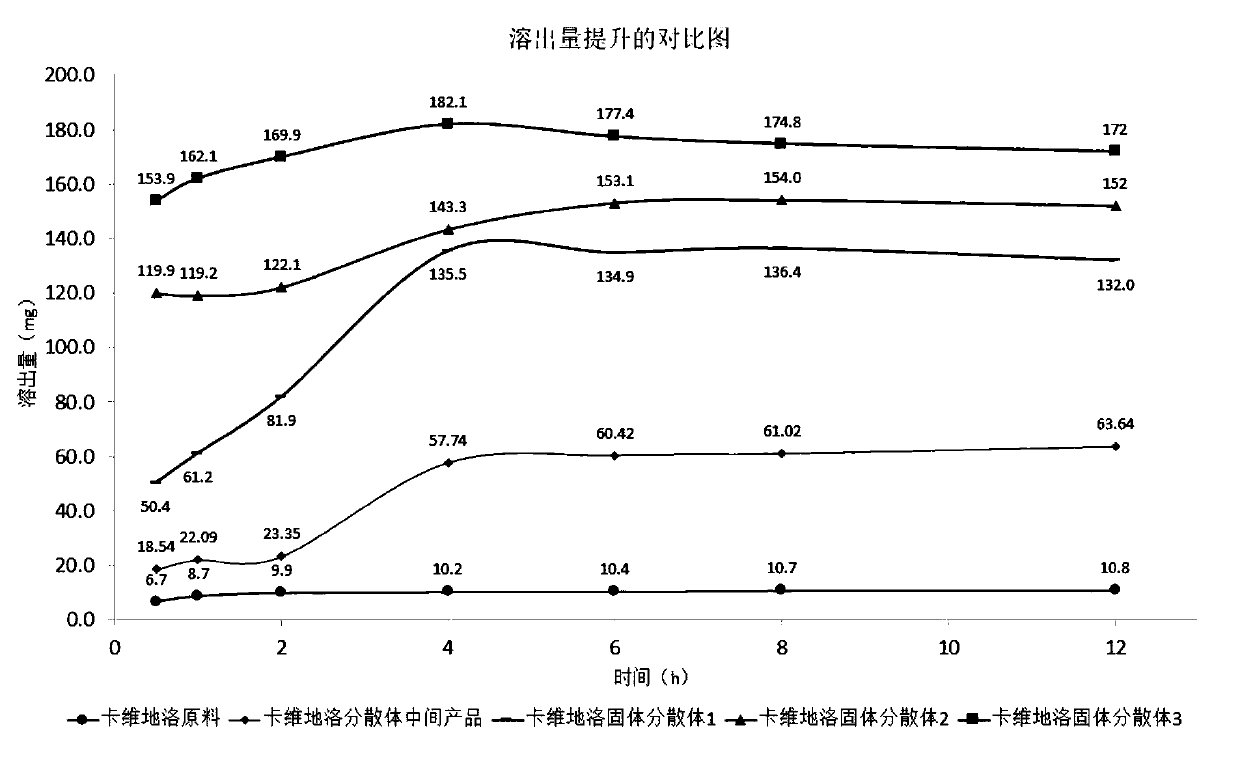
Method for preparing carvedilol solid dispersions by virtue of supercritical anti-solvent technique
ActiveCN106309434AImproved dissolution propertiesCarvedilol solid dispersion with significantly improved dissolution propertiesPowder deliveryOrganic active ingredientsSupercritical anti solventOrganic solvent
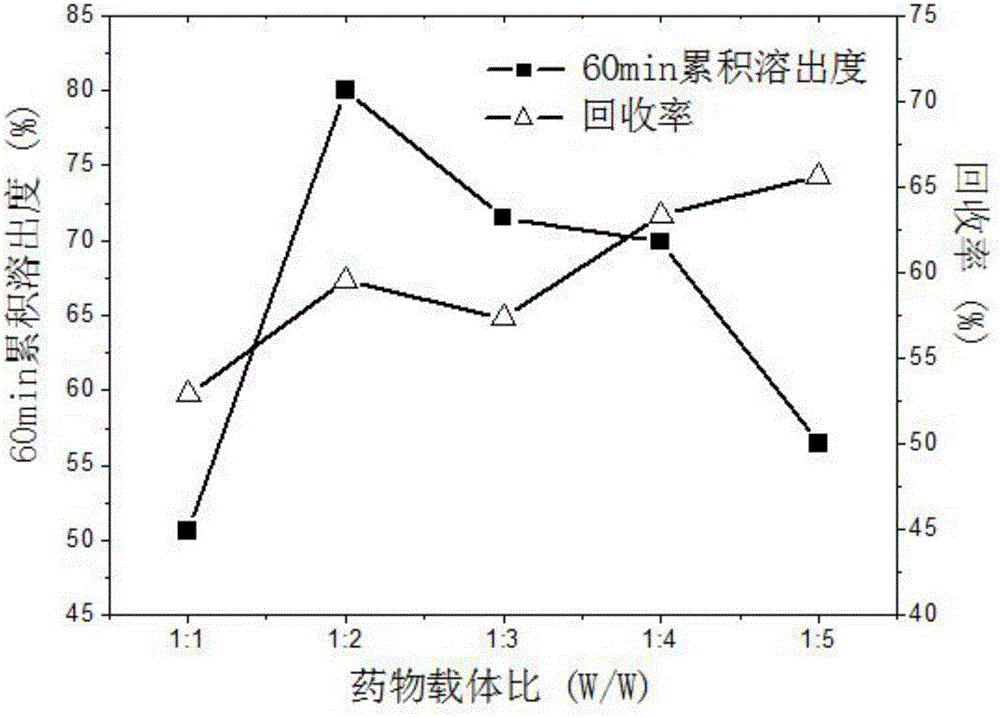
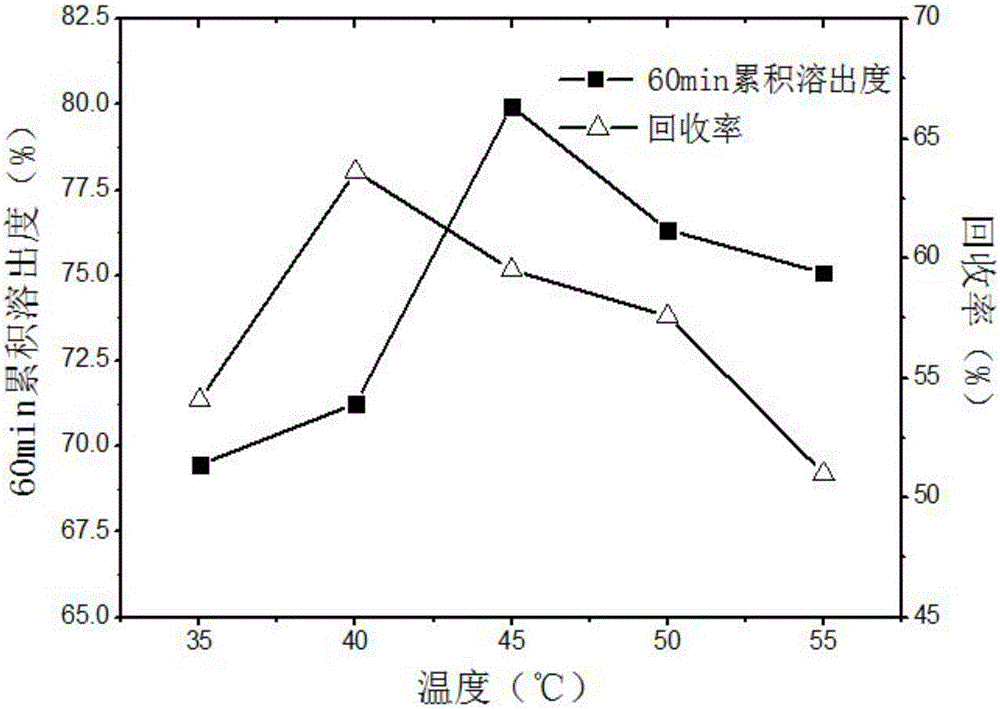
The invention discloses a method for preparing carvedilol solid dispersions by virtue of a supercritical anti-solvent technique. The method comprises the following steps: (S1) dissolving carvedilol and a water-soluble carrier into an organic solvent, so as to obtain a carvedilol-carrier mixed solution; (S2) introducing CO2 into a crystallization kettle, and regulating the temperature and pressure in the crystallization kettle; (S3) continuing to introduce CO2 to keep the temperature and pressure in the crystallization kettle constant, and simultaneously introducing the mixed solution prepared in the step (S1) into the crystallization kettle; and (S4) after the carvedilol-carrier mixed solution is introduced, continuing to introduce CO2 to keep the temperature and pressure in the crystallization kettle constant, finally relieving the pressure, and after the pressure in the crystallization kettle is decreased to the atmospheric pressure, opening the crystallization kettle, and collecting the carvedilol solid dispersions. By virtue of the method, the carvedilol solid dispersions with obviously improved dissolution characteristics can be prepared, and furthermore, the bioavailability of indissolvable drugs can be improved.
Owner:CHINA PHARM UNIV
Method of treating airway diseases with beta-adrenergic inverse agonists
InactiveUS7528175B2Improve securityReduce functionBiocideOrganic active ingredientsDiseaseAdrenergic
The use of β-adrenergic inverse agonists provides a new and highly efficient way of treating a number of pulmonary airway diseases, including asthma, emphysema, and chronic obstructive pulmonary diseases. In general, such a method involves administering a therapeutically effective amount of a β-adrenergic inverse agonist to the subject to treat the pulmonary airway disease. Particularly preferred inverse agonists include nadolol and carvedilol. In addition, methods are described for long-tern administration of such inverse agonists and for determining the suitability of patients for long-term inverse agonist therapy.
Owner:INVERSEON INC
Extended release matrix tablets
The present invention relates to extended release matrix tablets for oral administration that include a cationic polymer, a water-swellable polymer, and an alginic acid derivative to cause the release rate of the active ingredient of the tablets to be independent of pH and gastric residence time. The active pharmaceutical ingredient may be one or more of antibiotics, sympathomimetics, sympatholytic agents, cholinergic agents, antimuscarinics, gastro-intestinal drugs, gentio-urinary smooth muscle relaxants, cardiac drugs, anticonvulsants, tranquilizers and sedatives, and in particular may be an antibiotic, such as cefaclor, or may be a sympatholytic agent, such as carvedilol.
Owner:RANBAXY LAB LTD
Carvedilol push-pull osmotic pump type controlled release preparation and preparation method thereof
InactiveCN102670545AMaintain blood levelsGood curative effectOrganic active ingredientsPill deliveryPush pullCurative effect
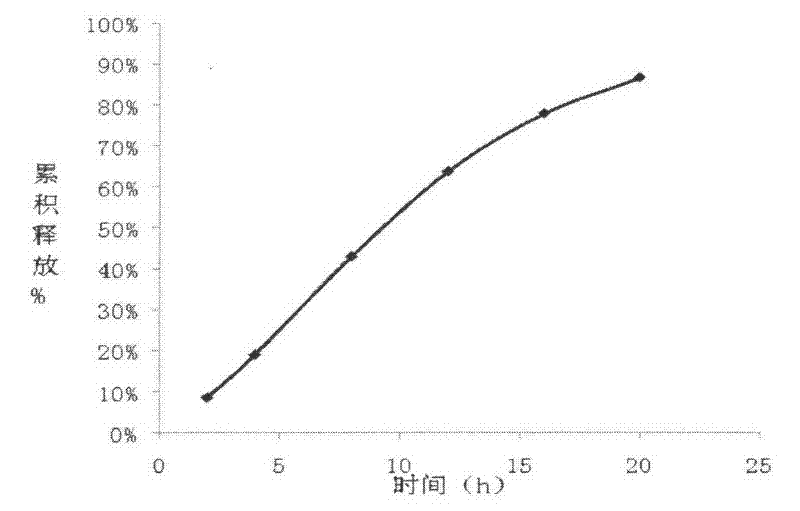
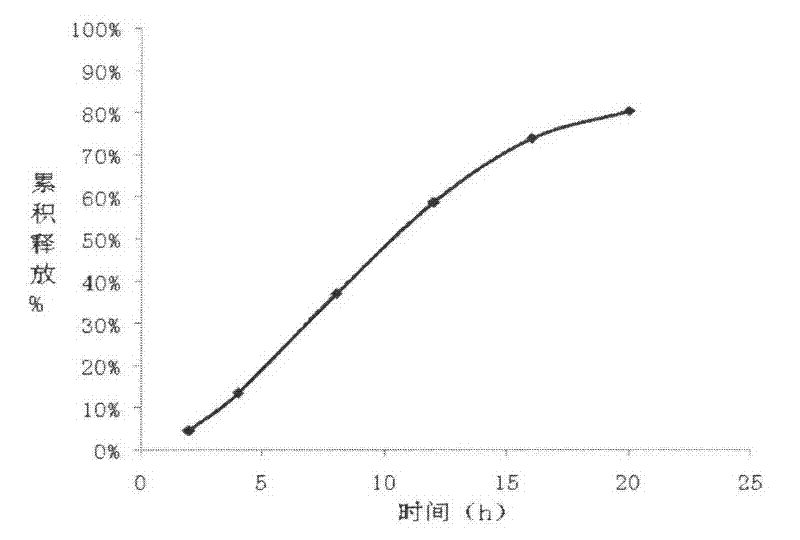
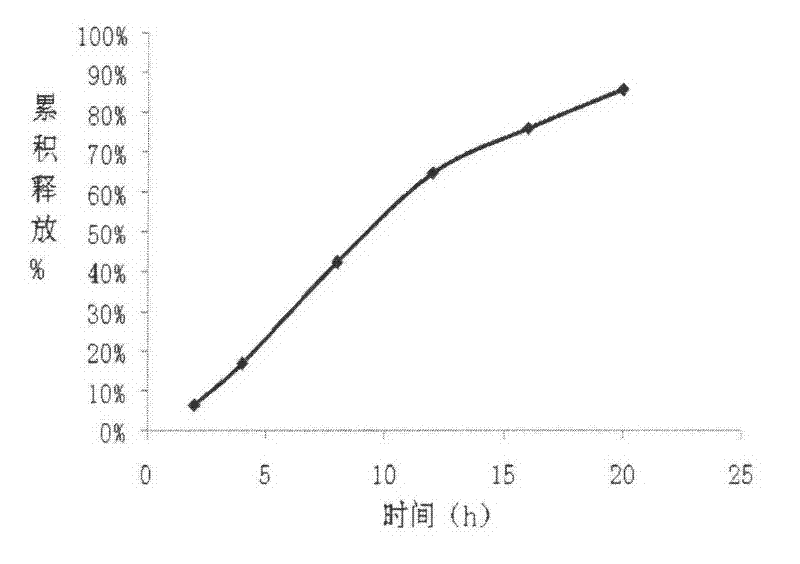
The invention relates to a carvedilol push-pull osmotic pump type controlled release preparation. The carvedilol push-pull osmotic pump type controlled release preparation comprises a medicine-containing layer tablet core, a boosting layer tablet core, a coating film and a single medicine releasing pore on the surface of a controlled release tablet on one side of the medicine-containing layer tablet core. According to the carvedilol push-pull osmotic pump type controlled release tablet disclosed by the invention, the medicine release accords with the zero-order release process and is basically complete, the administration frequencies of patients can be reduced and the peak-to-valley phenomenon which occurs after a general preparation is administrated is avoided; and plasma drug stability and durable curative effect in the release process of medicaments are obtained, and thus the safety and the effectiveness are improved.
Owner:CHINA PHARM UNIV
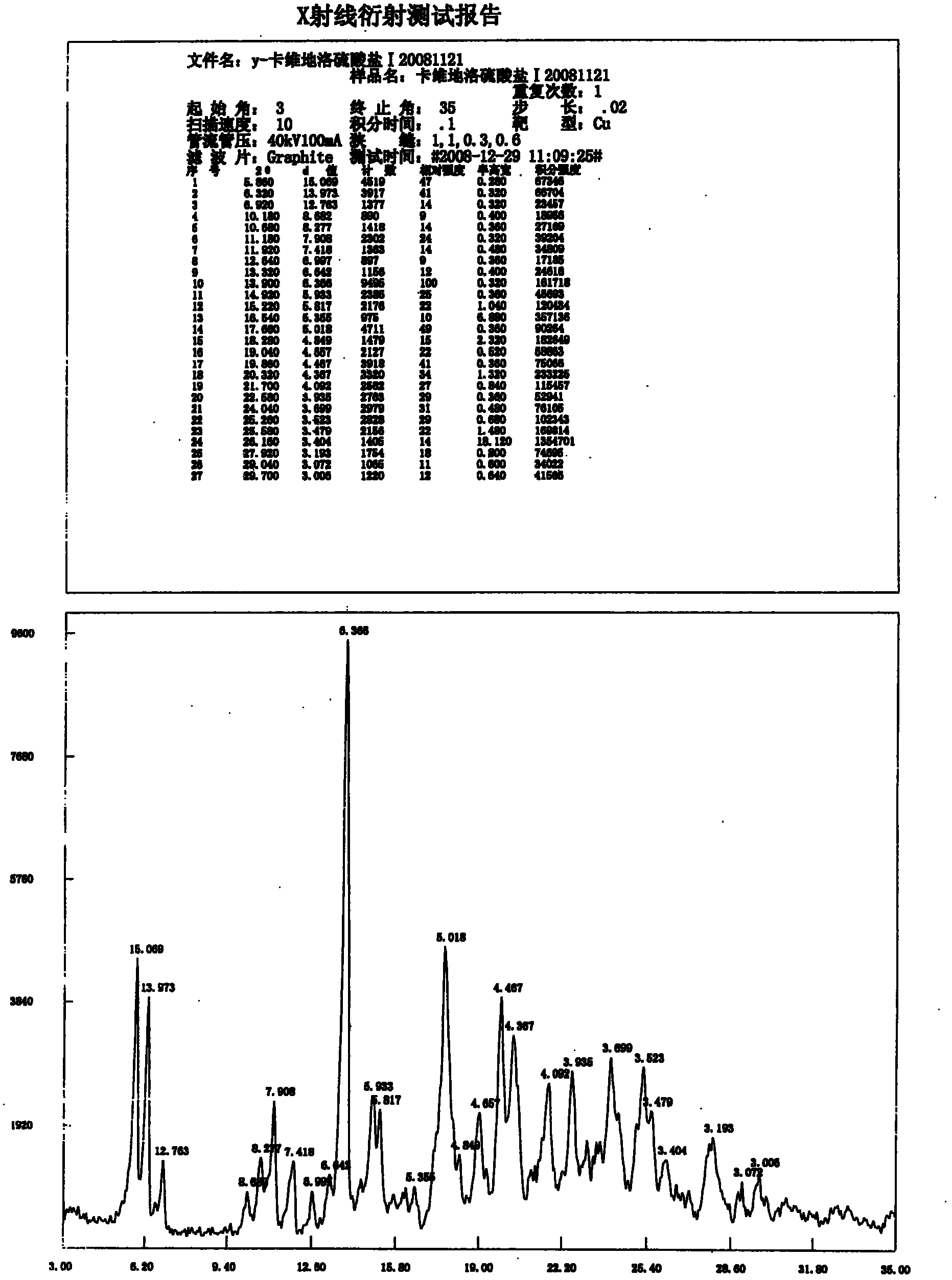
Carvedilol sulphate crystals, preparation method and application thereof in medicine
The invention relates to carvedilol sulphate crystals, a preparation method and application thereof in medicine, in particular to medicaments carvedilol sulphate type A, B, C and D crystals for treating hypertension, congestive heart failure and angina pectoris, a preparation method and application thereof. The preparation method comprises the following step: crystallizing a carvedilol sulphate solid in any crystal form or an amorphous carvedilol sulphate solid with conventional polar organic solvents and aqueous solutions thereof to obtain the type A, B, C or D crystal. The carvedilol sulphate crystals disclosed by the invention have favorable crystal form, and the used crystallizing solvents have the advantages of low toxicity and low residue. The carvedilol sulphate crystals prepared by the method can be better used for clinical treatment.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
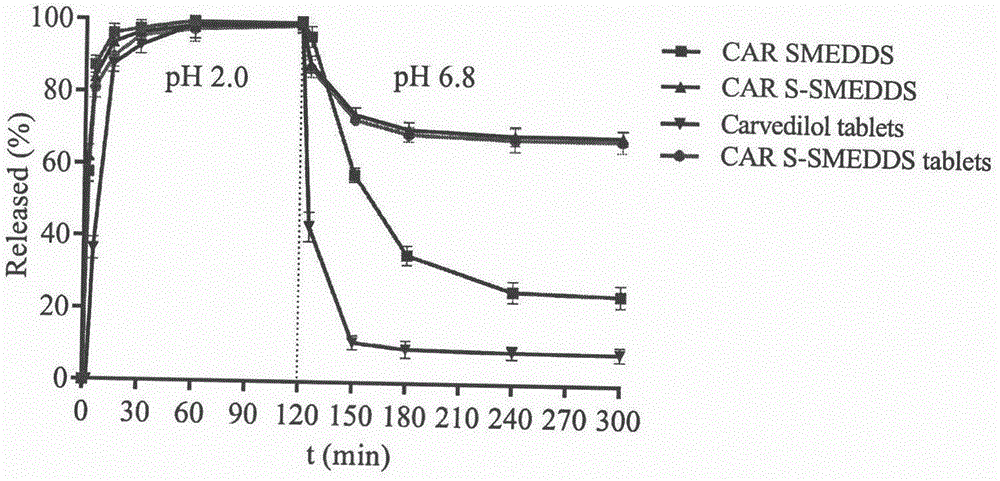
Carvedilol supersaturatable self-microemulsion dispersible tablets and preparing method thereof
InactiveCN106727382AImprove bioavailabilityImprove securityOrganic active ingredientsPill deliveryIrritationCarvedilol
The invention relates to the technical field of pharmaceutic preparations, in particular to carvedilol supersaturatable self-microemulsion dispersible tablets and a preparing method thereof. A carvedilol supersaturatable self-microemulsion preparation is prepared by combining an oil phase, a surfactant, a cosurfactant and a supersaturation promoter according to a certain ratio and mixed with other auxiliary materials after being adsorbed by an excipient, and then tabletting is conducted on powder directly to obtain the carvedilol supersaturatable self-microemulsion dispersible tablets. The prepared carvedilol supersaturatable self-microemulsion dispersible tablets can form microemulsion with particle size within 200 nm through spontaneous emulsification in the body after being orally taken. By the adoption of the supersaturatable self-microemulsion dispersible tablets, the dissolution rate of carvedilol in the gastrointestinal tract can be increased, bioavailability can be improved, usage of the surfactant and the cosurfactant in the prescription can be reduced, irritation to the gastrointestinal tract can be reduced, drug use safety can be improved, and market prospects are broad.
Owner:CHINA PHARM UNIV
Solid dispersoid of poorly soluble drug CVD (carvedilol), preparation method and application
PendingCN110279662AHigh Gibbs EnergyImprove solubilityOrganic active ingredientsPowder deliverySolubilityProtonation
The invention discloses a solid dispersoid of poorly soluble drug CVD (carvedilol), a preparation method and an application and relates to the technical field of medicines. With the adoption of a one-step solvent coprecipitation method, CVD and a protonation reagent are dissolved in a solvent, a dispersion material is added, a suspension is obtained for coprecipitation, and finally, the solid dispersoid is prepared after the solvent is removed. The one-step solvent coprecipitation method is adopted for in-situ production of the solid dispersoid. Detection proves that CVD is transformed into an amorphous form from an original crystal form and is dispersed in the solid dispersoid, besides, CVD is salified due to addition of the protonation reagent, thus, the solubility and the dissolution rate of free alkali drugs are improved from two aspects, and the bioavailability of the BCSII type poorly soluble drug CVD is effectively improved. a drug preparation with retention floating in the stomach and time-lag trip or release in different pH parts in a body is prepared from the CVD solid dispersoid according to a polymer coating material in cooperation with the dissolubility characteristic of pH dependency.
Owner:HEFEI COSOURCE PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com