Method for preparing carvedilol solid dispersions by virtue of supercritical anti-solvent technique
A supercritical anti-solvent, solid dispersion technology, applied in pharmaceutical formulations, organic active ingredients, medical preparations of non-active ingredients, etc., can solve the problems of obvious first-pass effect and low bioavailability, and achieve outstanding substantial Characteristics, optimization methods, effects of improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: single factor method determines the preferred value range of each key parameter
[0032] Instruments and materials
[0033] See Table 1 for experimental materials and Table 2 for experimental equipment.
[0034] Table 1 Experimental materials
[0035] material name Specification Manufacturer Carvedilol ≥99% Wuhan Xingyinhe Chemical Co., Ltd. CO 2
≥99% Nanjing Tongqi Gas Company Dichloromethane Analytical pure Nanjing Chemical Reagent Co., Ltd. Methanol Analytical pure Nanjing Chemical Reagent Co., Ltd. PVPK30 Premium Pure Shanghai Yuanye Biotechnology Co., Ltd.
[0036] Table 2 Experimental Instruments
[0037] device name model Manufacturer Supercritical Particle Preparation System Helix Applied Separations, USA Intelligent Dissolution Tester ZRS-8L Tianjin Tianda Tianfa Technology Co., Ltd. UV-visible spectrophotometer UV-1800 Shimadzu Corporatio...
Embodiment 2
[0066] Embodiment 2: Orthogonal experiment optimizes optimal parameters within the preferred range of each key parameter
[0067] Orthogonal experimental design and results
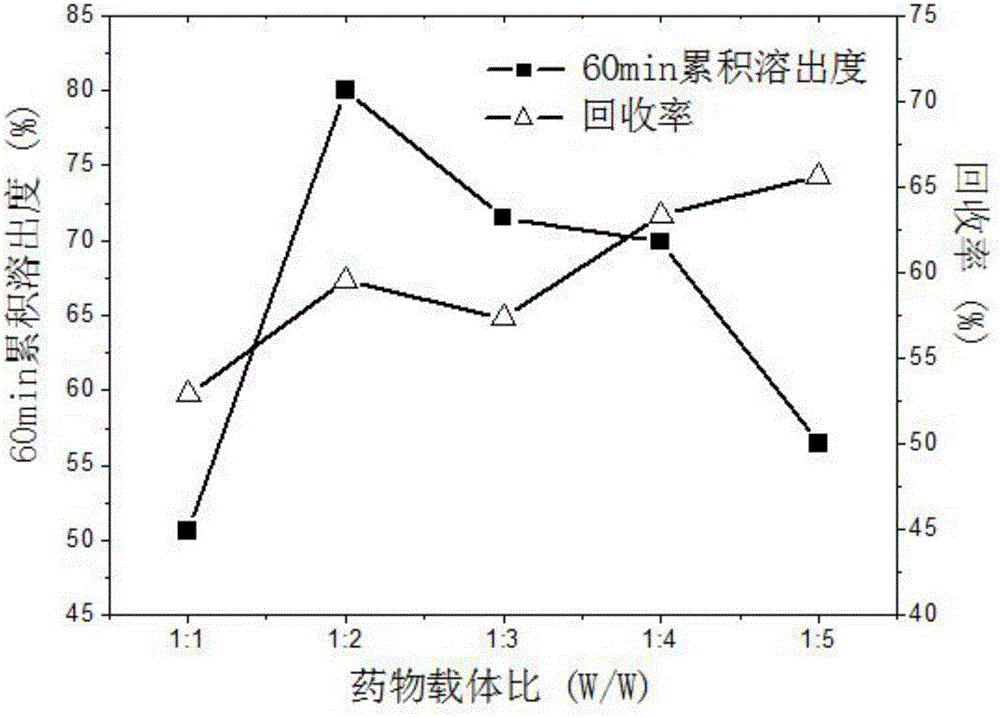
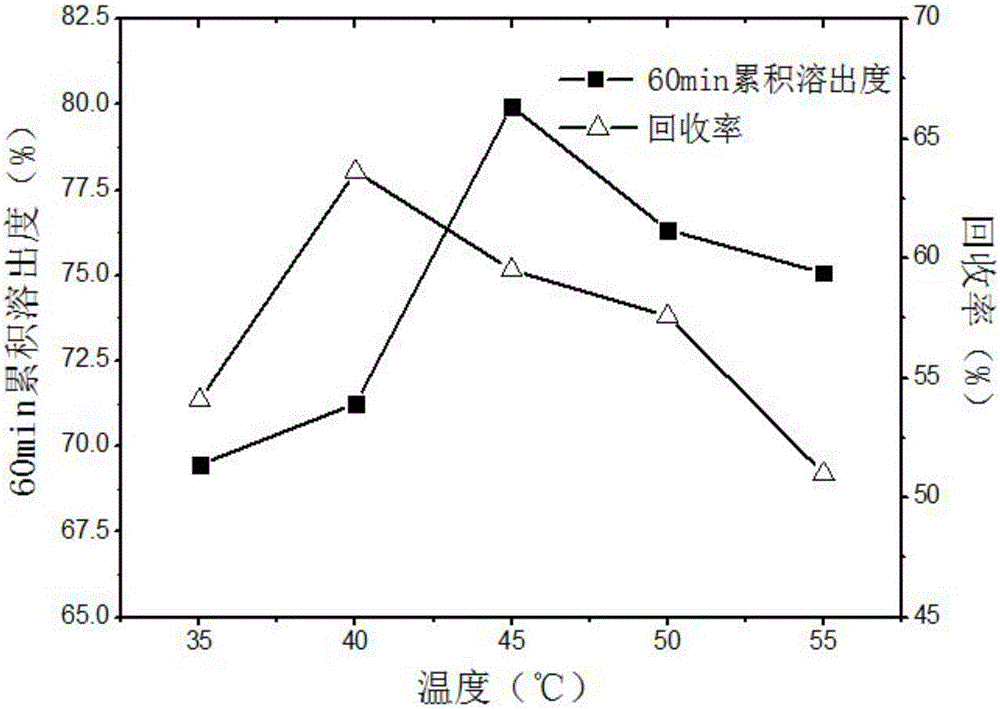
[0068] Carvedilol solid dispersion optimization process takes 60min cumulative dissolution rate as the main evaluation index, and selects three factors that have a more obvious impact on the index, crystallization pressure (A), crystallization temperature (B), and solution volume flow rate (C). To investigate, use L 9 (3 4 ) Orthogonal experimental design is carried out experiment, and its factor level has been determined by the single factor experiment of embodiment 1, see table 3. Other process conditions are CO 2 The exhaust volume flow rate is 3L / min, the ratio of carvedilol to PVP K30 is 1:2, and the organic solvent is dichloromethane. The experimental design and results are shown in Table 4.
[0069] Table 3 Orthogonal experiment factors and levels
[0070]
[0071] Table 4 Orthogonal exp...
Embodiment 3
[0083] A kind of supercritical antisolvent technology prepares the method for carvedilol solid dispersion, comprises the steps:
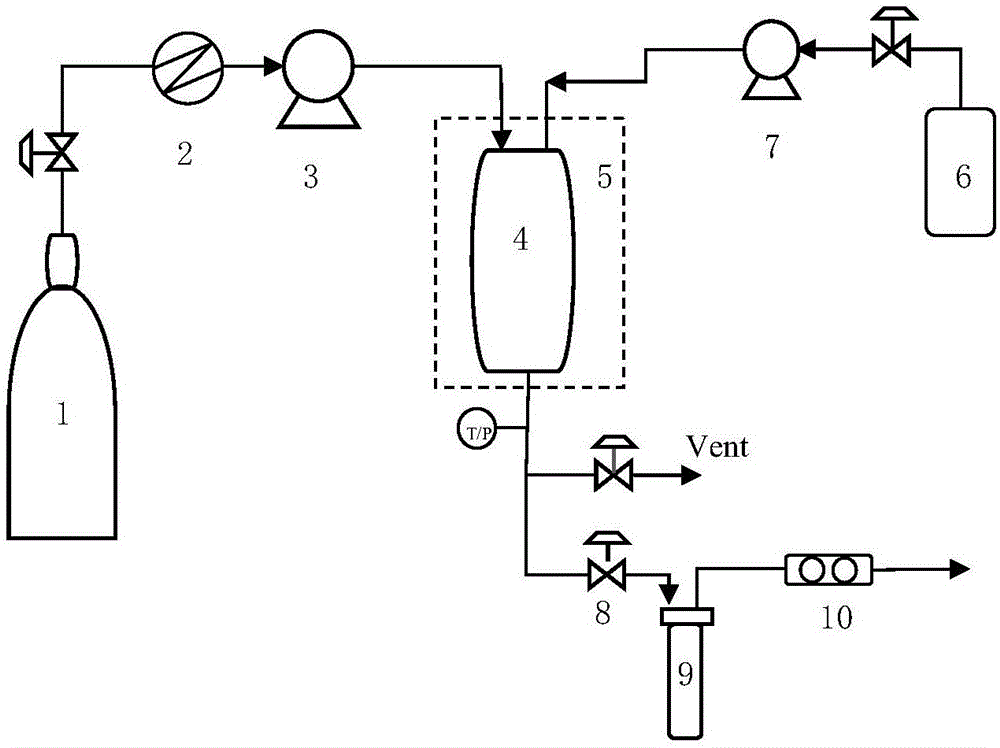
[0084] Step S1, dissolving carvedilol and a water-soluble carrier in an organic solvent to obtain a carvedilol-carrier mixed solution;
[0085] Step S2, the CO 2 Pass into the crystallization kettle, adjust the temperature and pressure in the crystallization kettle;
[0086] Step S3, continue to feed CO 2 , maintaining the temperature and pressure in the crystallization kettle constant, while passing the mixed solution prepared in step S1 into the crystallization kettle;
[0087] Step S4, after the carvedilol-carrier mixed solution is passed through, continue to pass through CO 2 , keep the temperature and pressure in the crystallization kettle constant, and finally release the pressure; when the pressure in the crystallization kettle drops to atmospheric pressure, open the crystallization kettle to collect carvedilol solid dispersion;
[0088] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com