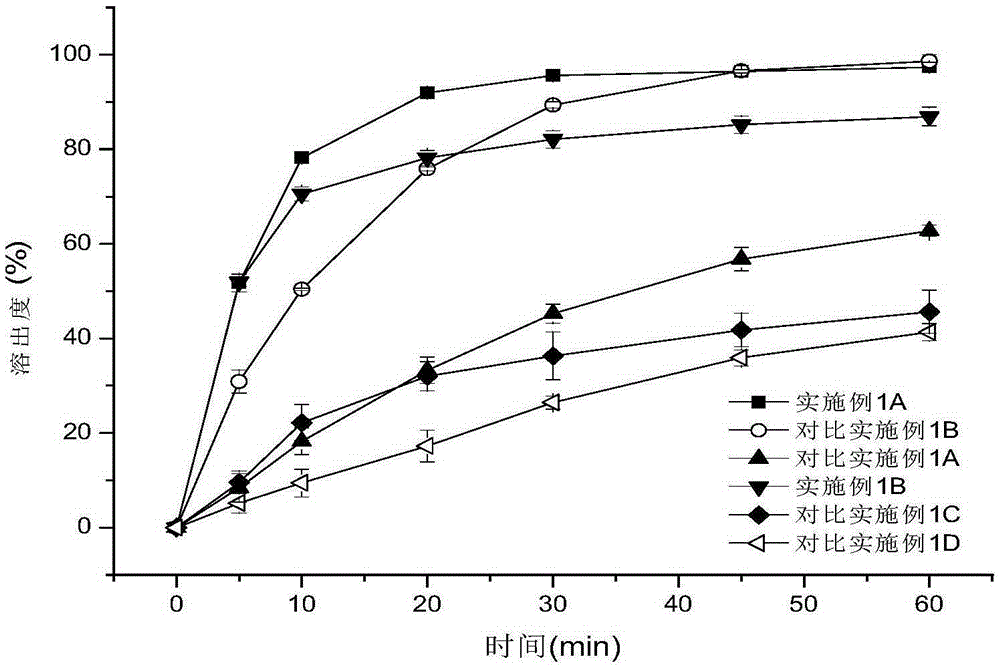
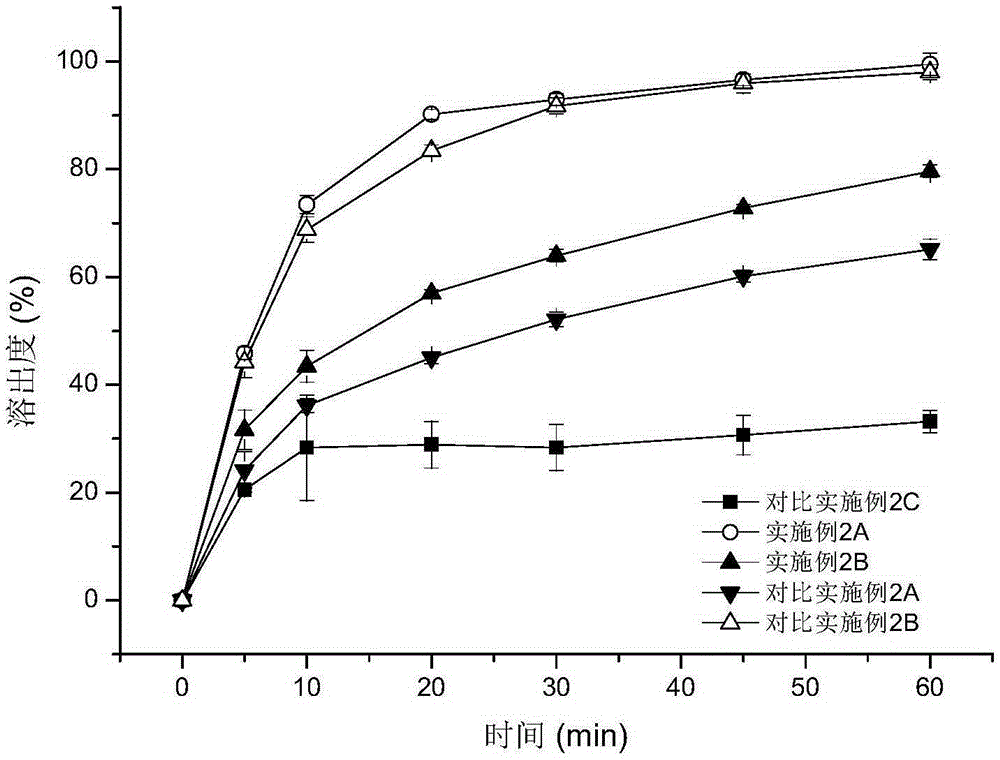
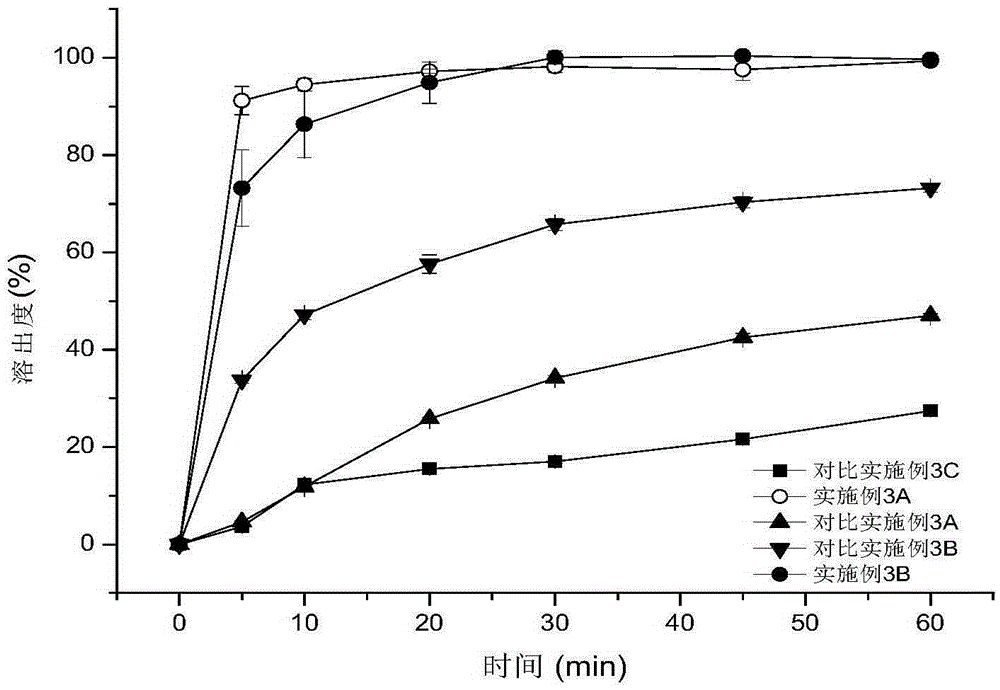
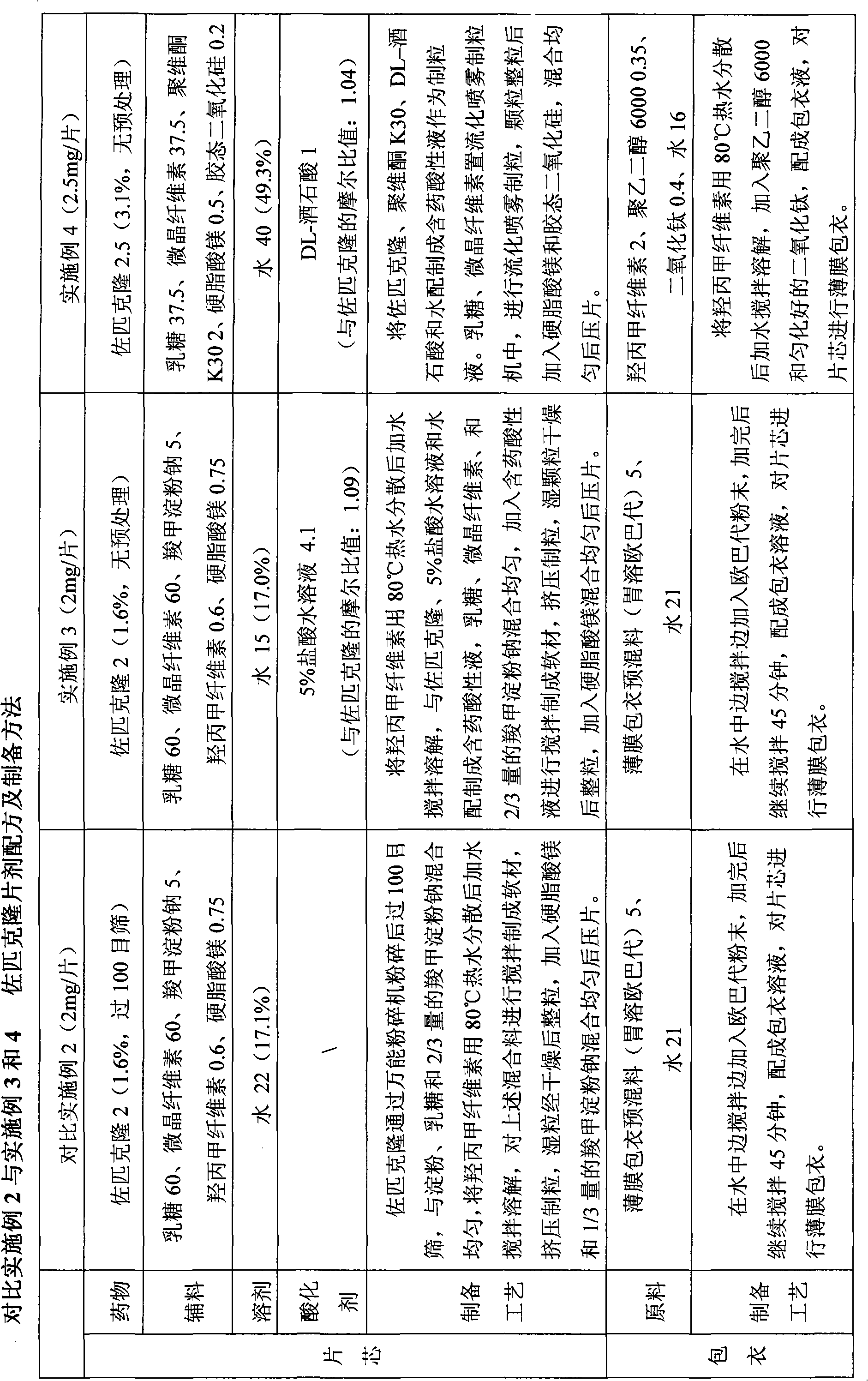
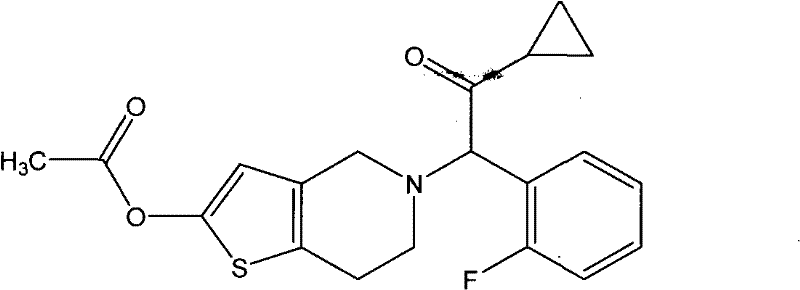
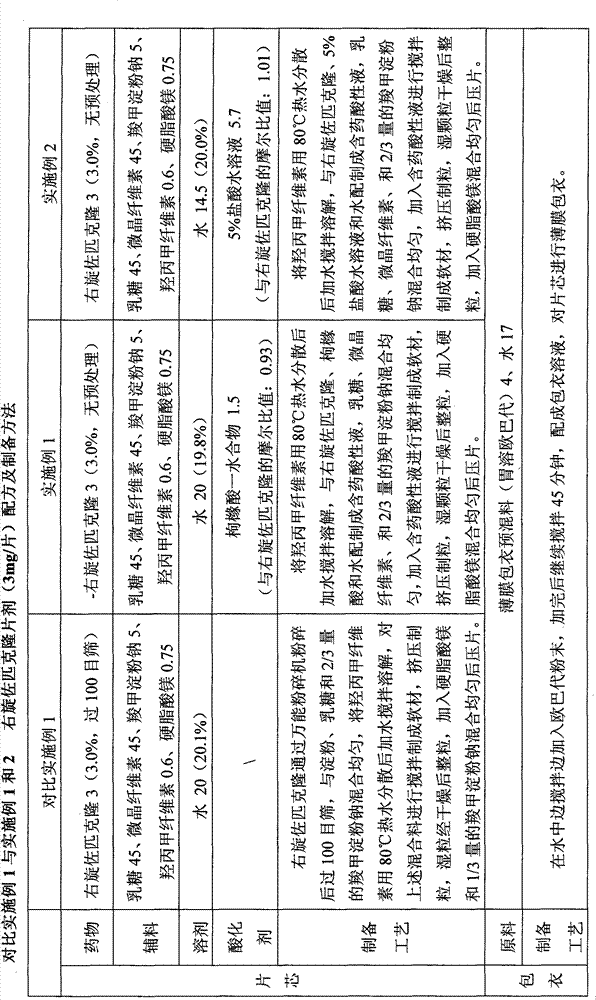
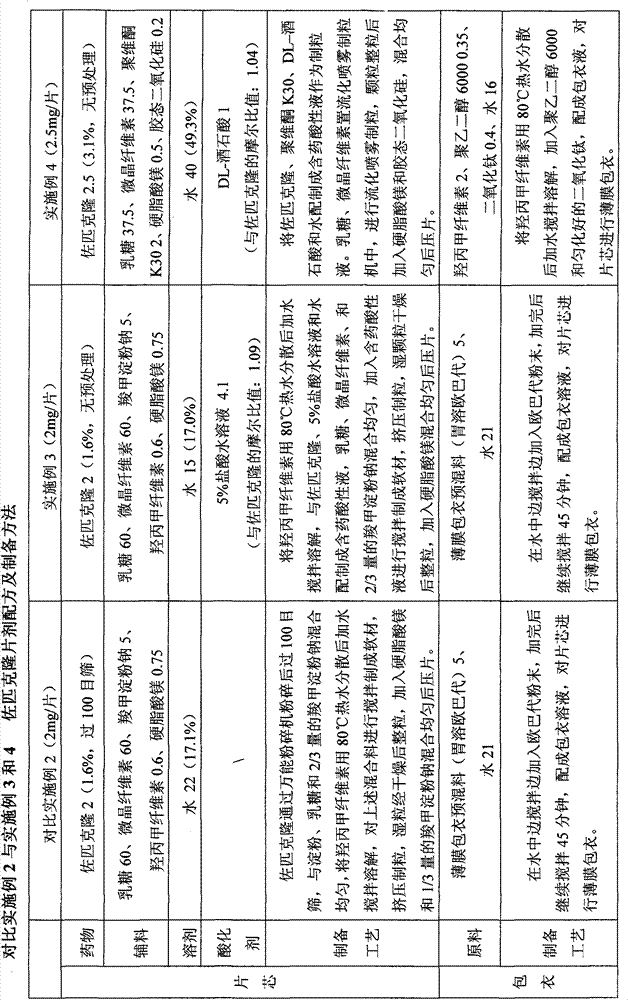
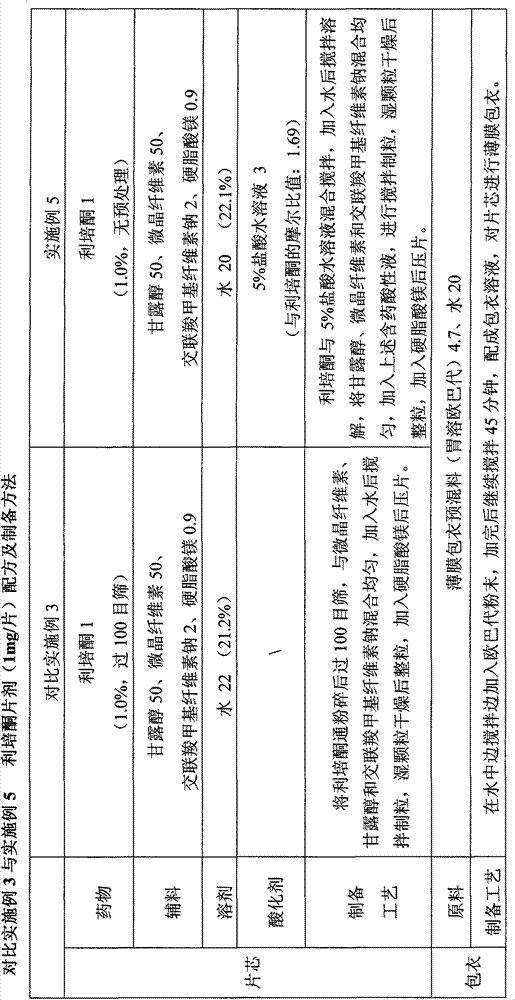
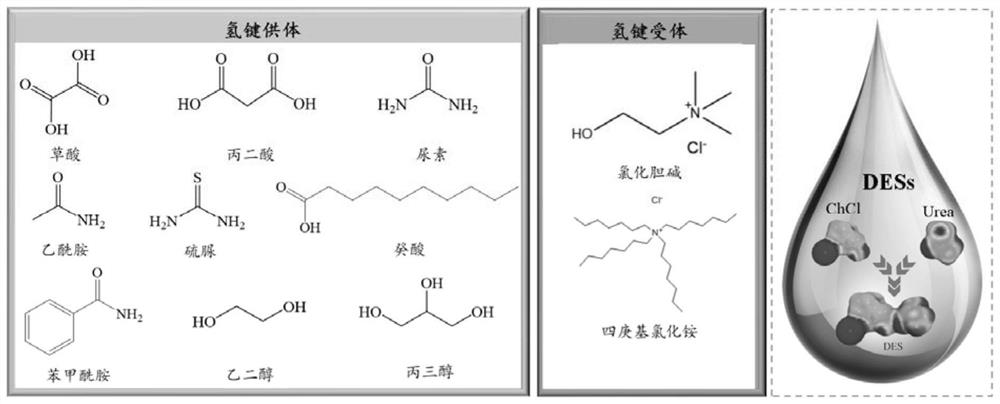
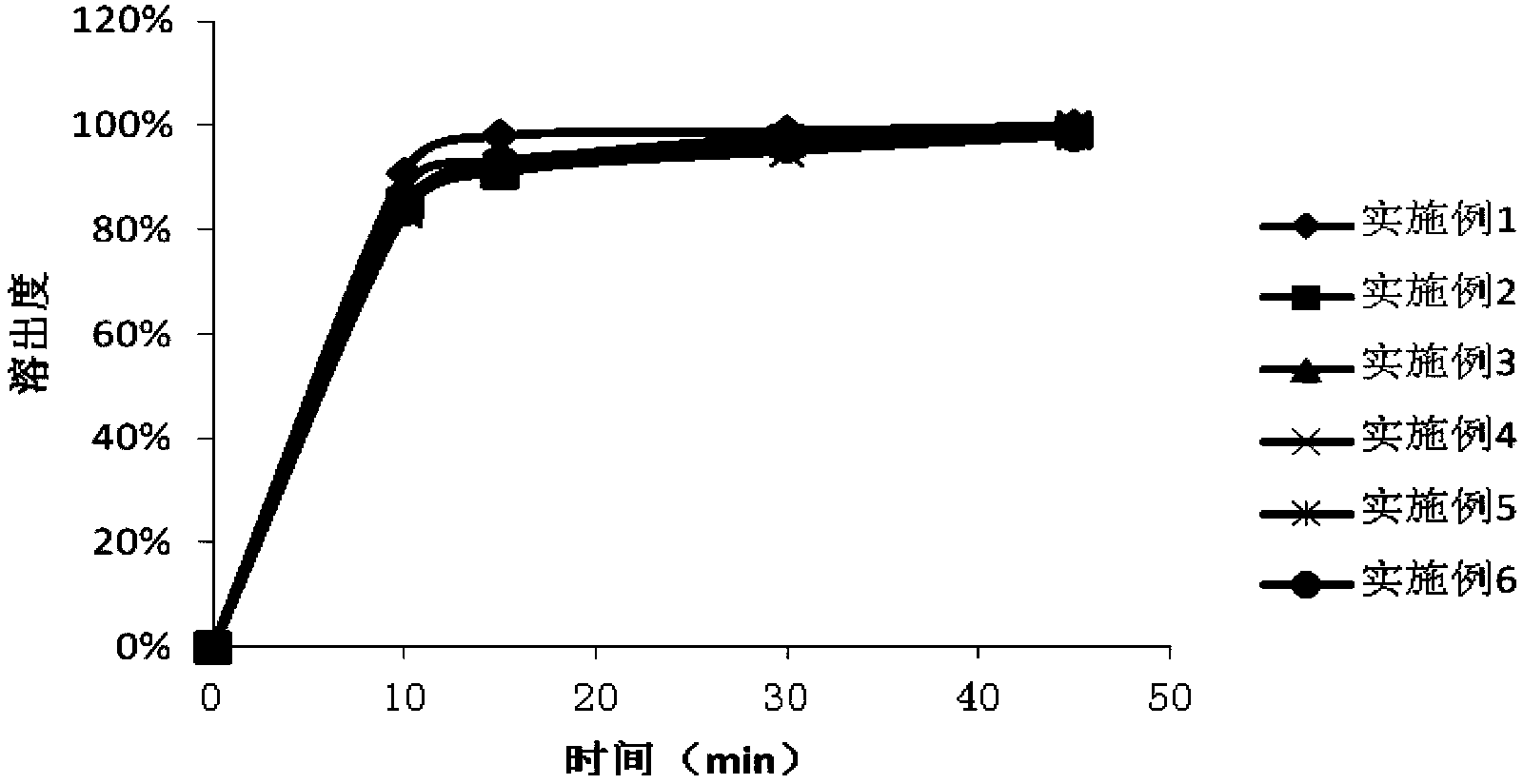
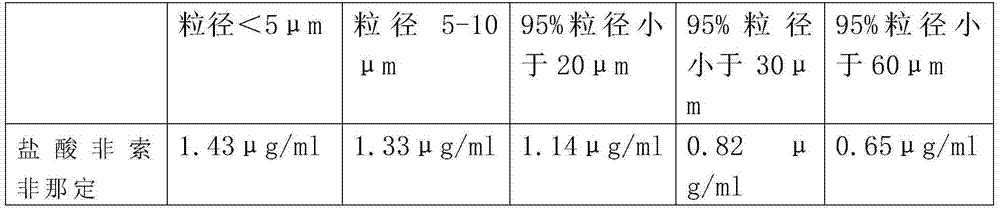
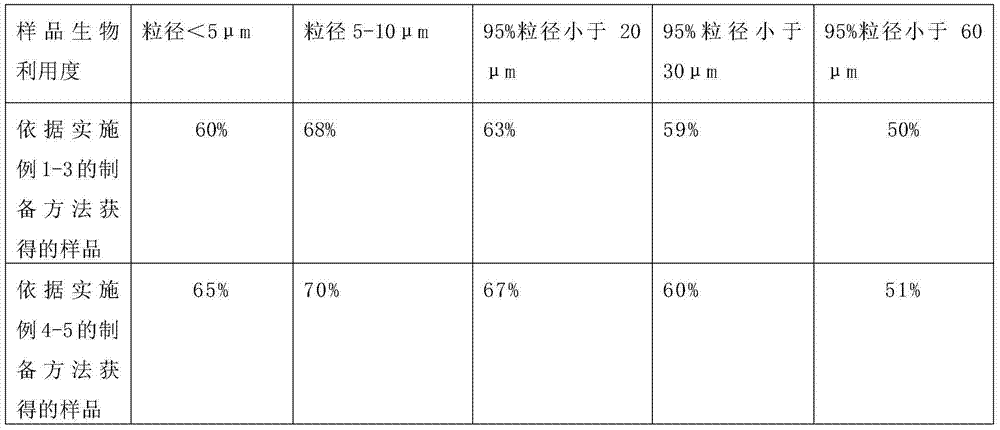
Patents
Literature
56results about How to "Improved dissolution properties" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
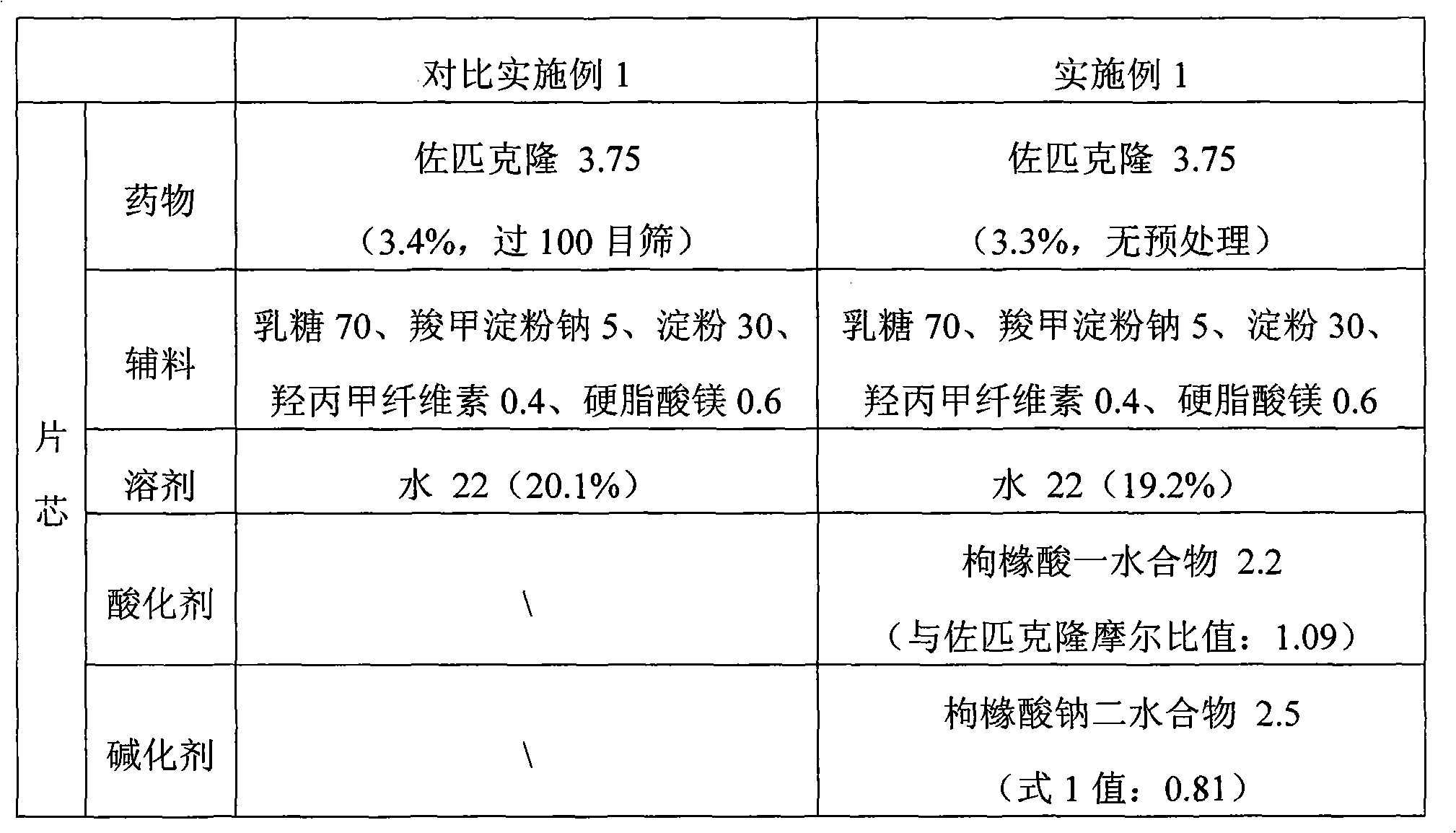
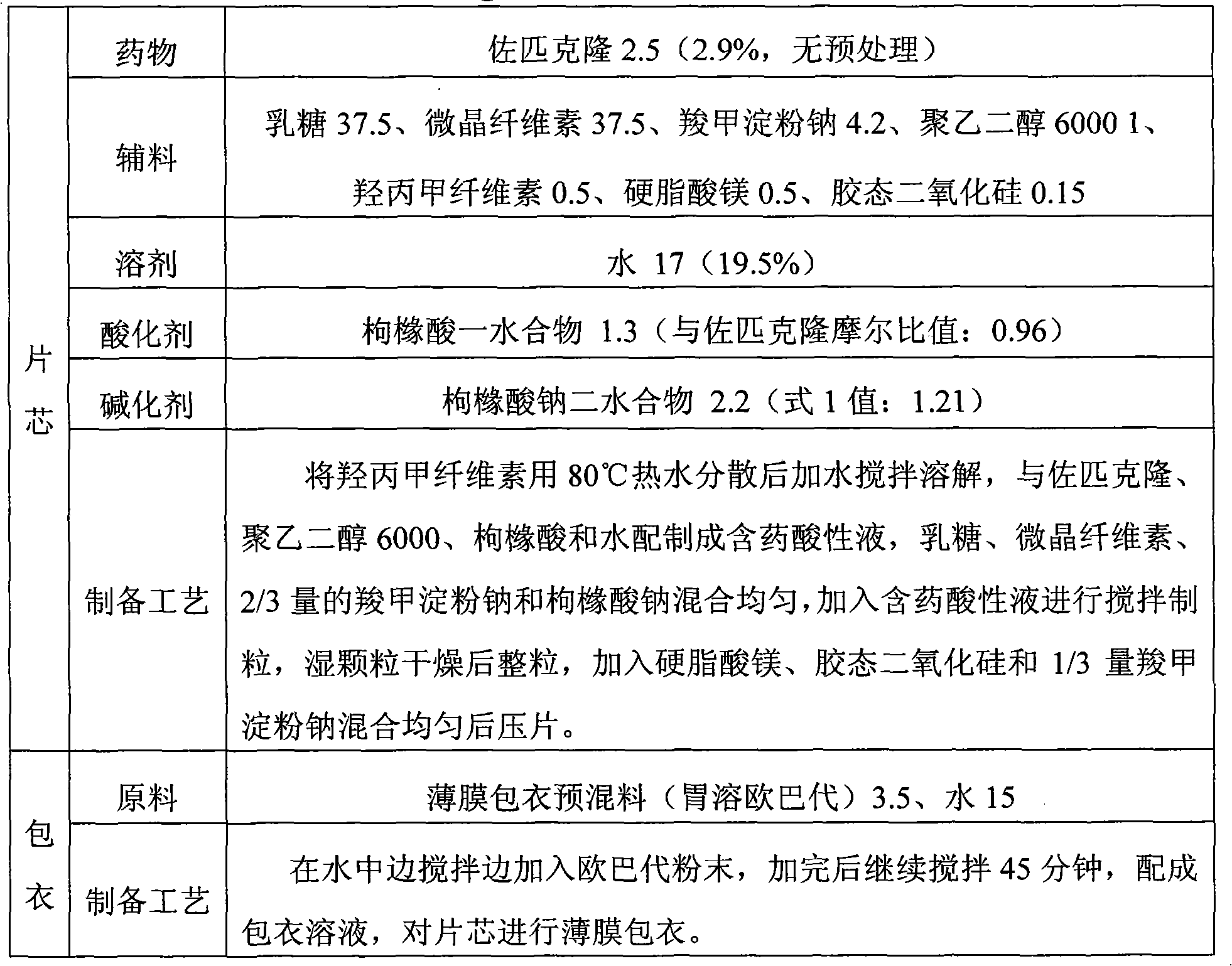
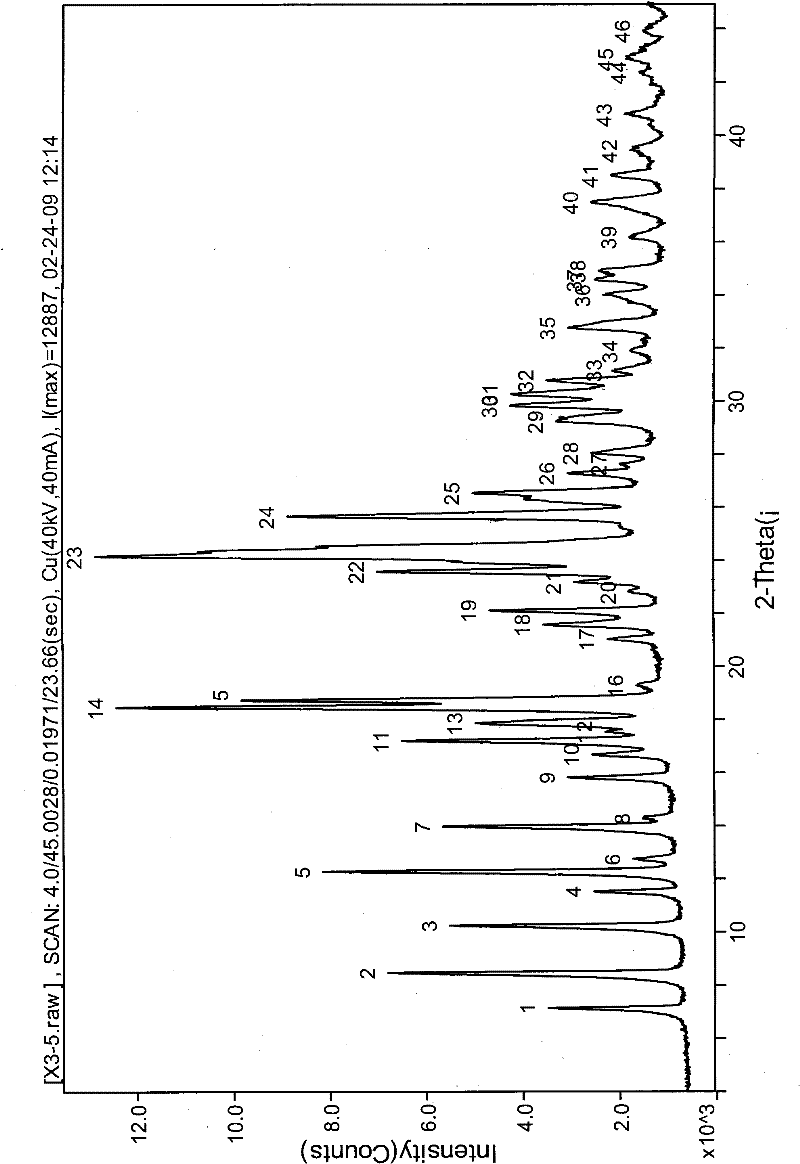
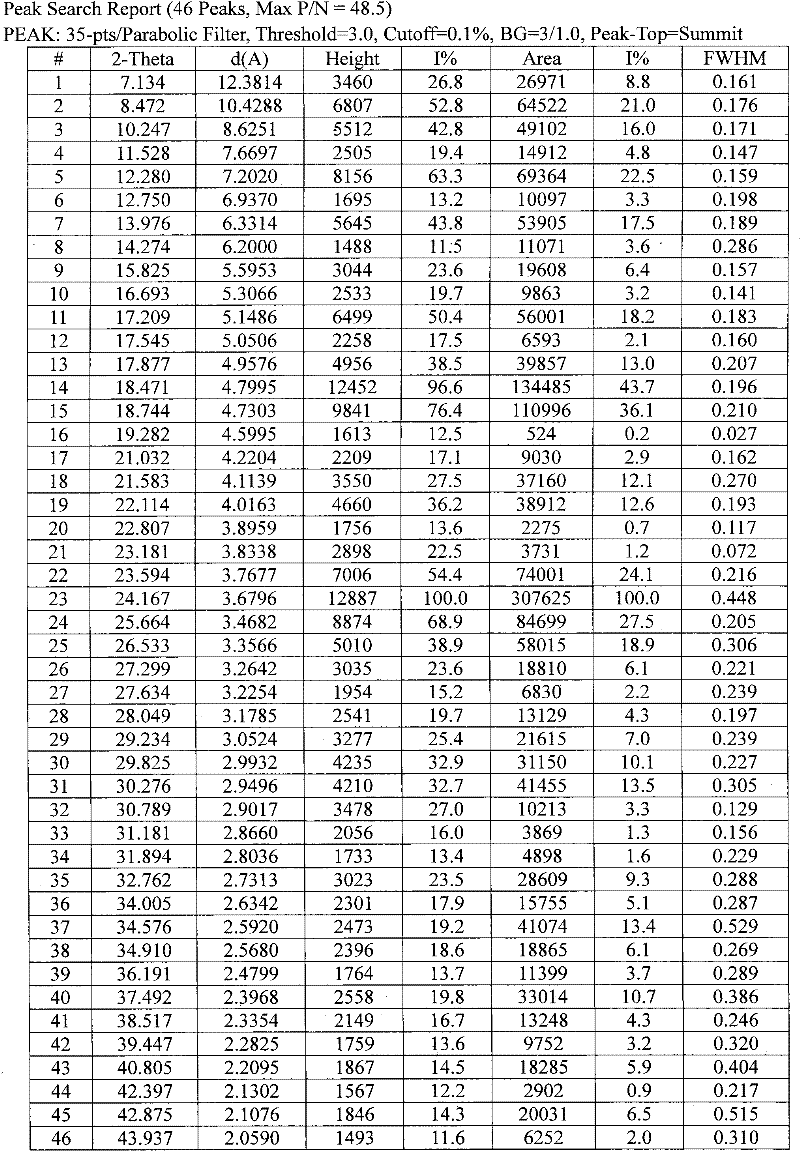
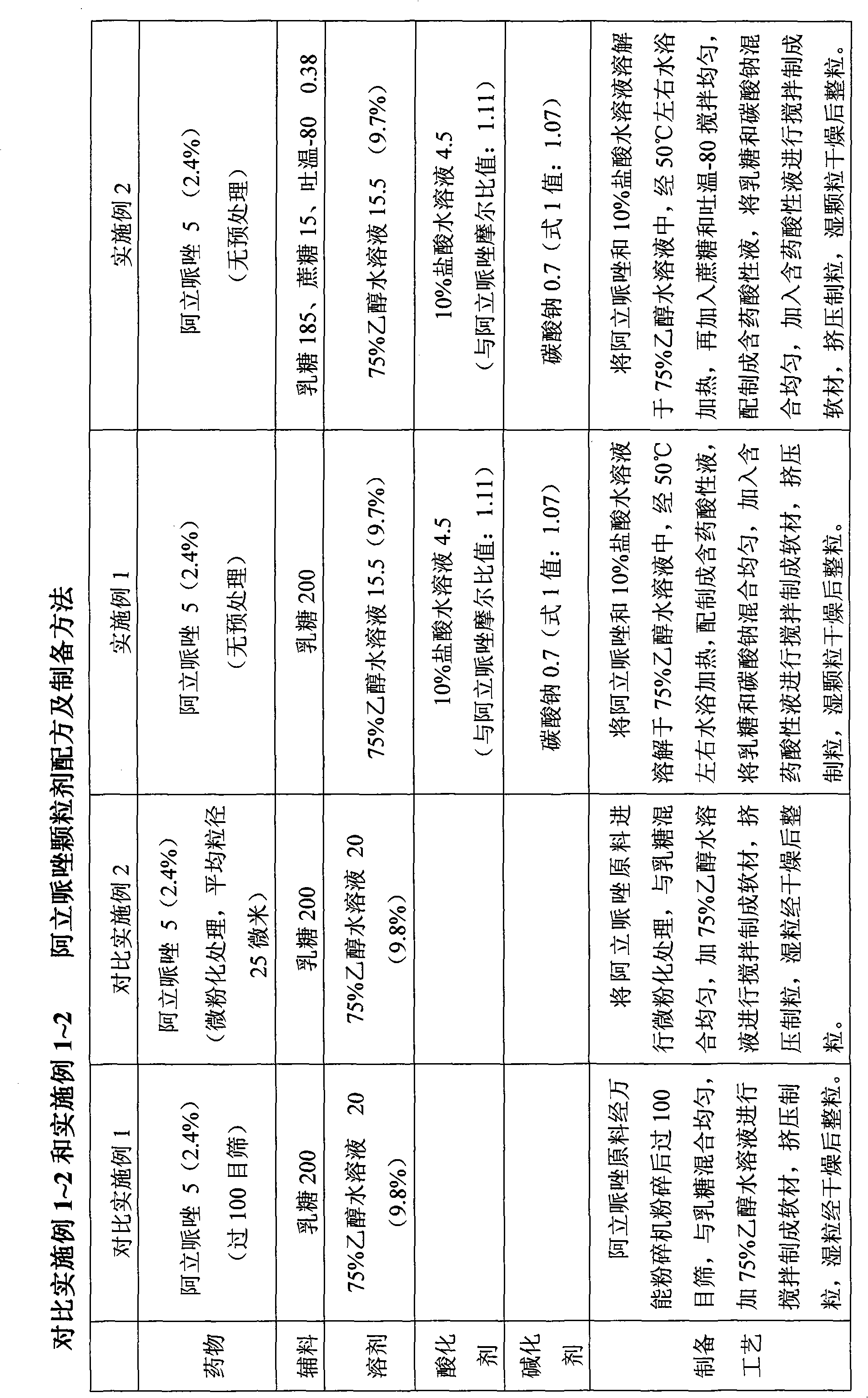
Sparingly soluble active component particle, particle preparation and preparation method thereof
ActiveCN105640890AChange natureChange releaseCosmetic preparationsPowder deliveryPolymer dissolutionActive component
The invention discloses a sparingly soluble active component particle, a particle preparation and a preparation method thereof. The preparation method of the sparingly soluble active component particle comprises the following steps: dissolving sparingly soluble active component and ionic polymer into an alkaline solution or an acidic solution, and then mixing the alkaline solution and acidic solution to change the pH value of solutions so as to precipitate the sparingly soluble active component and ionic polymer from the mixed solution to form particles; and the performance of the sparingly soluble active component particle is improved. According to the preparation method, sparingly soluble active component and ionic polymer are co-precipitated, at the same time, a preparation technology is adopted, and two technologies are tightly combined to prepare the sparingly soluble active component particle preparation. The prepared preparation has the advantages of excellent dissolving-out characteristic, high bioavailability, small individual difference, good stability, and good content uniformity.
Owner:SINOTHERAPEUTICS
Method for preparing solid preparation and solid preparation
ActiveCN102106806AEasy to operateImprove securityOrganic active ingredientsNervous disorderWater insolubleWater soluble
The invention discloses a method for preparing a solid preparation, which comprises the following steps of: dissolving active ingredients of water-insoluble and / or lowly water-soluble alkaline medicaments in acidulant-containing acid solution to prepare medicament-containing acid solution; and mixing the medicament-containing acid solution and auxiliary materials uniformly to perform wet-method pelletizing. The invention also discloses the solid preparation prepared by the method. By the method, the defects of serious pollution, large loss and serious potential safety hazard which are caused by mechanical pulverization are overcome, and the method is easy and convenient to operate, has high safety factors and is applied to industrial production easily. The solid preparation prepared by the method has the excellent dissolution characteristic, stability and content uniformity.
Owner:SHANGHAI ZHONGXI PHARMA +1
Method for preparing solid preparation and solid preparation
ActiveCN102106807AImprove bioavailabilityImproved dissolution propertiesOrganic active ingredientsInorganic non-active ingredientsWater insolubleWater soluble
The invention discloses a method for preparing a solid preparation. The method comprises the following steps of: dissolving active ingredients of a water-insoluble and / or slightly water-soluble alkaline medicament into acid solution containing an acidulant to obtain medicament-containing acid solution; and uniformly mixing a basifier, excipients and the medicament-containing acid solution, and performing wet granulation, wherein the basifier ensures that the acidity of mixed solution of the basifier and the medicament-containing acid solution is reduced compared with the acidity of the medicament-containing acid solution. The invention also discloses the solid preparation prepared by the method. The method overcomes the disadvantages of serious pollution, high loss and serious potential safety hazards caused by mechanical pulverization treatment, is easy and convenient to operate, has high safety factor, and is easily applied to industrialized production. The solid preparation prepared by the method has dissolution characteristic which is remarkably improved compared with that in the prior art, and has the stability and content uniformity which are equivalent to or better than those in the prior art.
Owner:SHANGHAI ZHONGXI PHARMACEUTICAL CO LTD +1
Aripiprazole solid preparation and preparation method thereof
ActiveCN102106826AEasy to operateImprove securityOrganic active ingredientsNervous disorderMechanical crushingAripiprazole
The invention discloses the preparation method of aripiprazole solid preparation. The method comprises the following steps of dissolving aripiprazole into acidic solution containing an acidifying agent to obtain medicine-containing acidic solution; and then uniformly mixing accessory with the medicine-containing acidic solution to granulate by a wet method. The invention also discloses aripiprazole solid preparation prepared by the method. According to the method disclosed by the invention, the defects of the serious pollution, high loss and serous potential safety hazards brought by mechanical crushing treatment are avoided; and the method is simple, convenient and feasible for operation and easy for industrial production and has high safety coefficient. The aripiprazole solid preparation prepared by the method has the advantages of excellent dissolution property, stability, and content uniformity.
Owner:SHANGHAI ZHONGXI PHARMA +1
Preparation method of medicament solid preparation and obtained medicament solid preparation
ActiveCN102552161ASmall particle sizeSmall individual differencesSulfonylurea active ingredientsPill deliveryMedicineBULK ACTIVE INGREDIENT
Owner:SHANGHAI ZHONGXI PHARMA
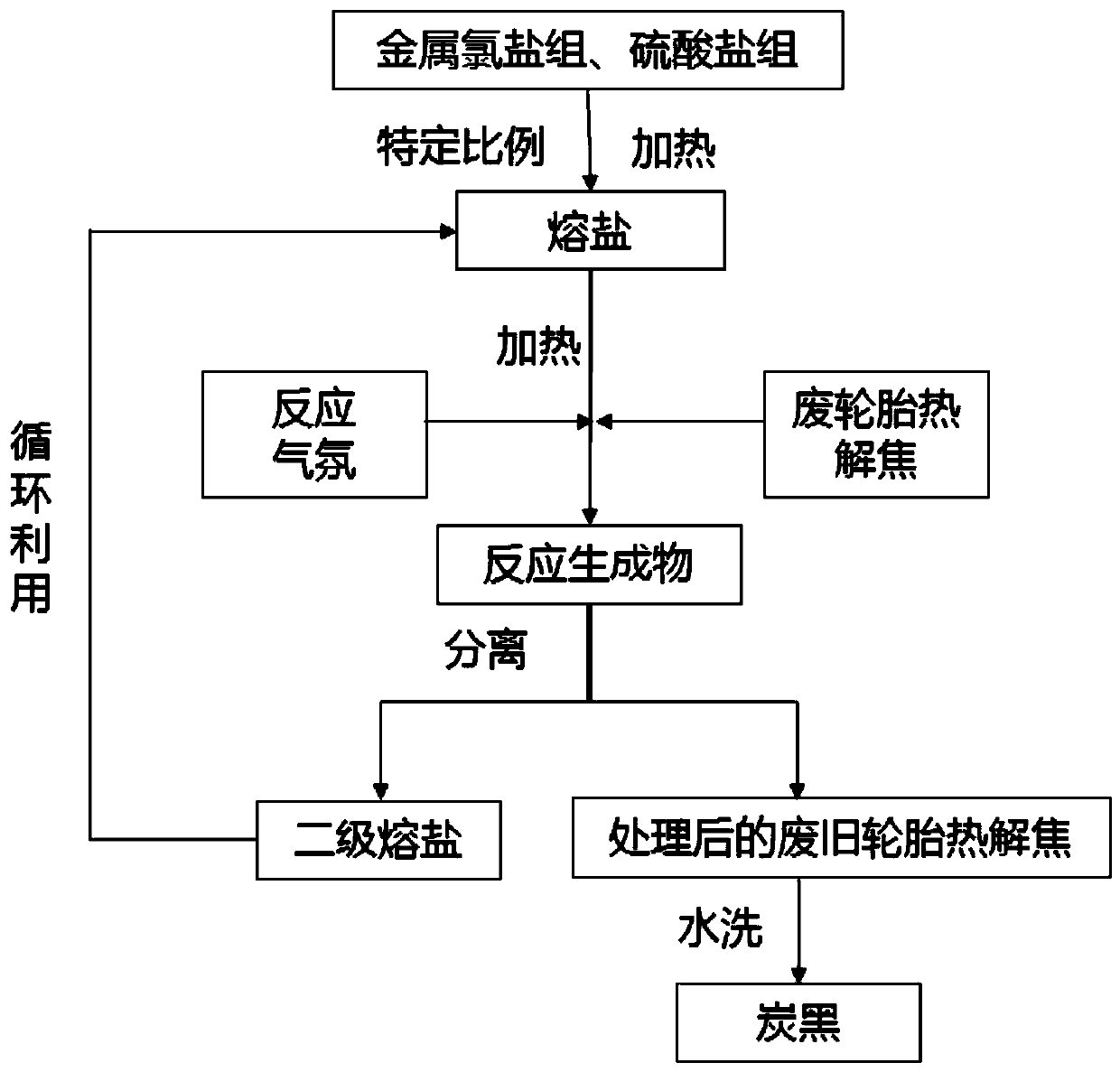
Method for preparing carbon black from pyrolysis coke of waste tire through molten salt heat treatment, and product prepared by using the same
ActiveCN110229543AEnhanced low temperature melting propertiesHigh activityPigmenting treatmentStrong acidsImpurity
The invention belongs to the technical field of resourceful utilization of organic solid wastes, and specifically discloses a method for preparing carbon black from pyrolysis coke of a waste tire through molten salt heat treatment, and a product prepared by using the same. The method comprises the following steps: heating one or two selected from the group consisting of a metal chloride salt groupand a metal sulfate salt group so as to obtain molten salt; adding the pyrolysis coke of the waste tire into the molten salt, and carrying out molten salt heat treatment under a preset reaction atmosphere; and after completion of a reaction, separating a reaction product into secondary molten salt and treated pyrolysis coke, washing treated pyrolysis coke with hot water, carrying out drying so asto obtain the carbon black, and recycling the secondary molten salt at the same time. According to the invention, by utilization of the melting characteristic of the molten salt, impurity componentsin the pyrolysis coke of the waste tire are dissolved out, so the use of strong acids and strong alkalies like nitric acid, hydrochloric acid and an alkali liquor is avoided; meanwhile, through addition of metal chloride salt, the low-temperature melting characteristic of the molten salt is reinforced; and by utilization of metal sulfate salt, acidic gases like hydrogen sulfide and hydrogen chloride are captured in situ at the same time.
Owner:HUAZHONG UNIV OF SCI & TECH
Method for storing color and luster and flavor of Chinese chestnut-lotus root composite ultramicro whole powder with easy gelatinization
The invention relates to a method for storing the color and luster and flavor of Chinese chestnut-lotus root composite ultramicro whole powder with easy gelatinization, and belongs to the field of the food processing of fruits and vegetables. The method comprises the following main processes of: peeling Chinese chestnuts and fresh lotus roots, cutting into slices, and keeping colors by using a composite color fixative prepared from citric acid, ascorbic acid and N-acetylcysteine; freeze-drying by microwave to prepare dried slices; and crushing the dried slices of the Chinese chestnuts and thelotus roots coarsely, mixing, and performing airflow type ultramicro full crushing to prepare the Chinese chestnut-lotus root composite ultramicro whole powder with the easy gelatinization. In the processing process, the change of the color and luster in the drying and crushing processes in the later period can be controlled by color-keeping processing; in the drying process, the change degree ofthe color and luster and flavor in the conventional hot-air drying process is reduced obviously by the microwave freeze-drying technology; and in the crushing process, the loss of excessive heat generated by the mechanical shearing crushing technology on the color and luster and flavor is prevented effectively by the airflow type ultramicro full crushing technology, and the flavor and nutrition of the Chinese chestnuts and the lotus roots can be better mixed by high-pressure air combination in the ultramicro full crushing process.
Owner:杭州建德天堂食品有限公司 +1
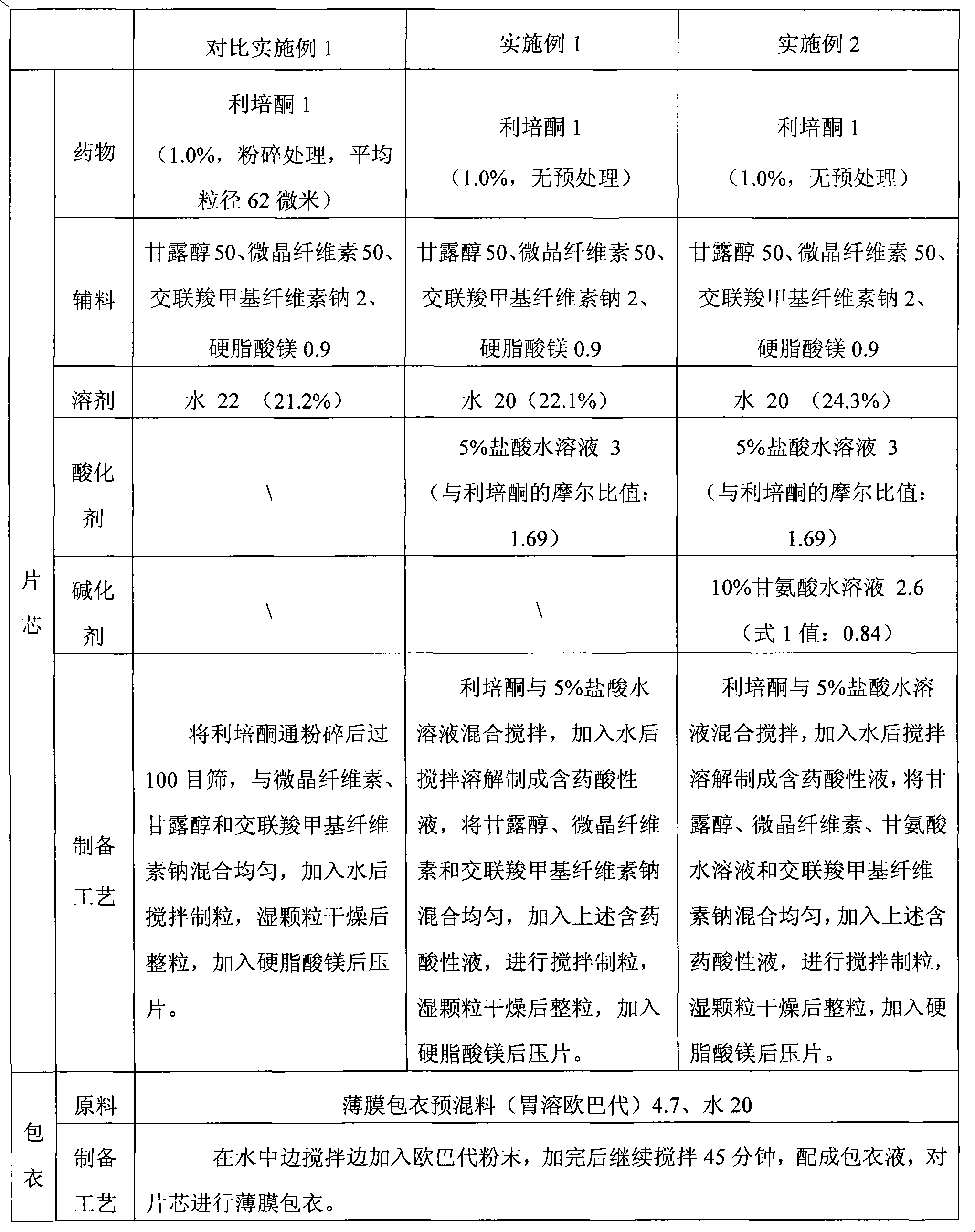
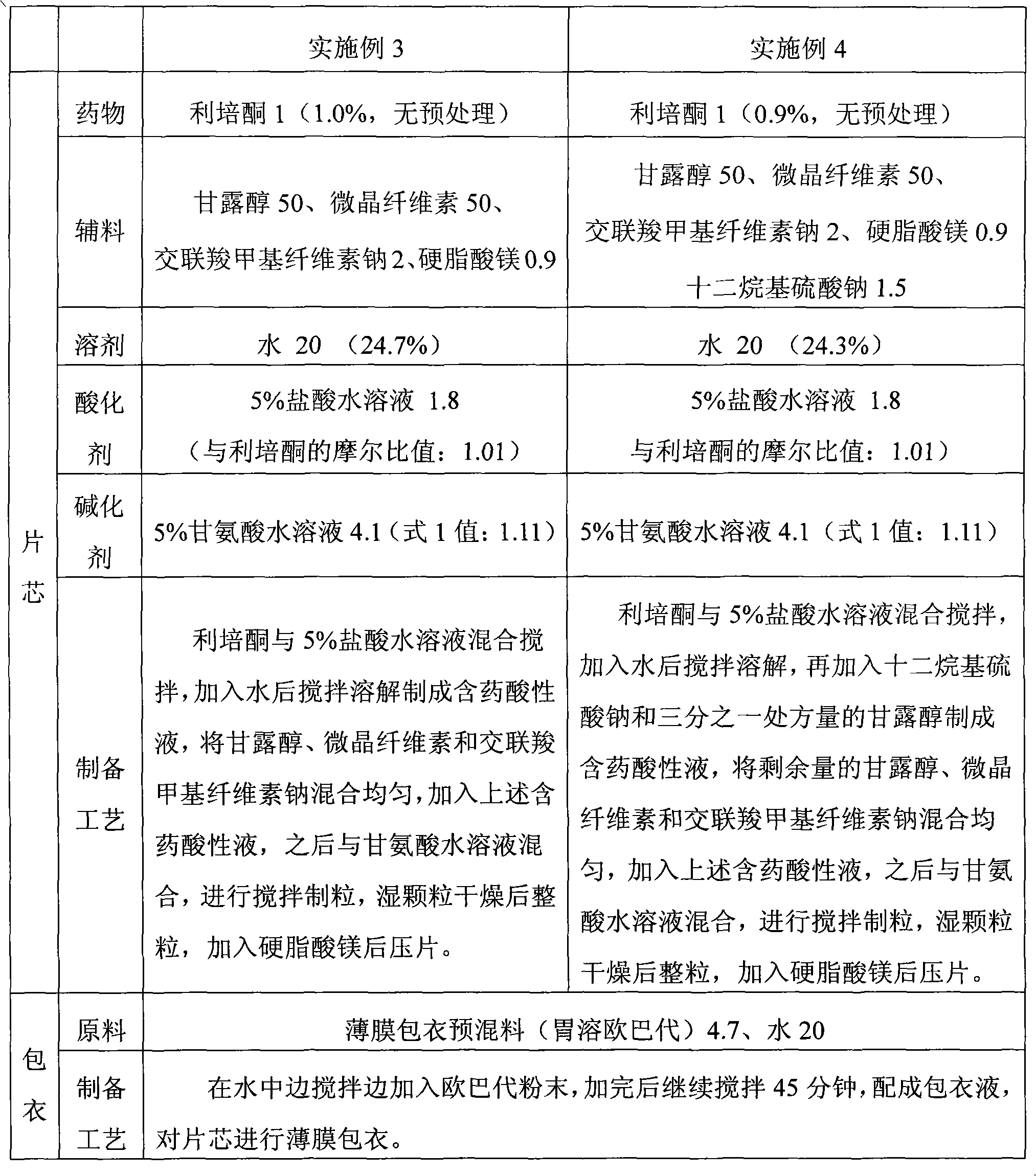
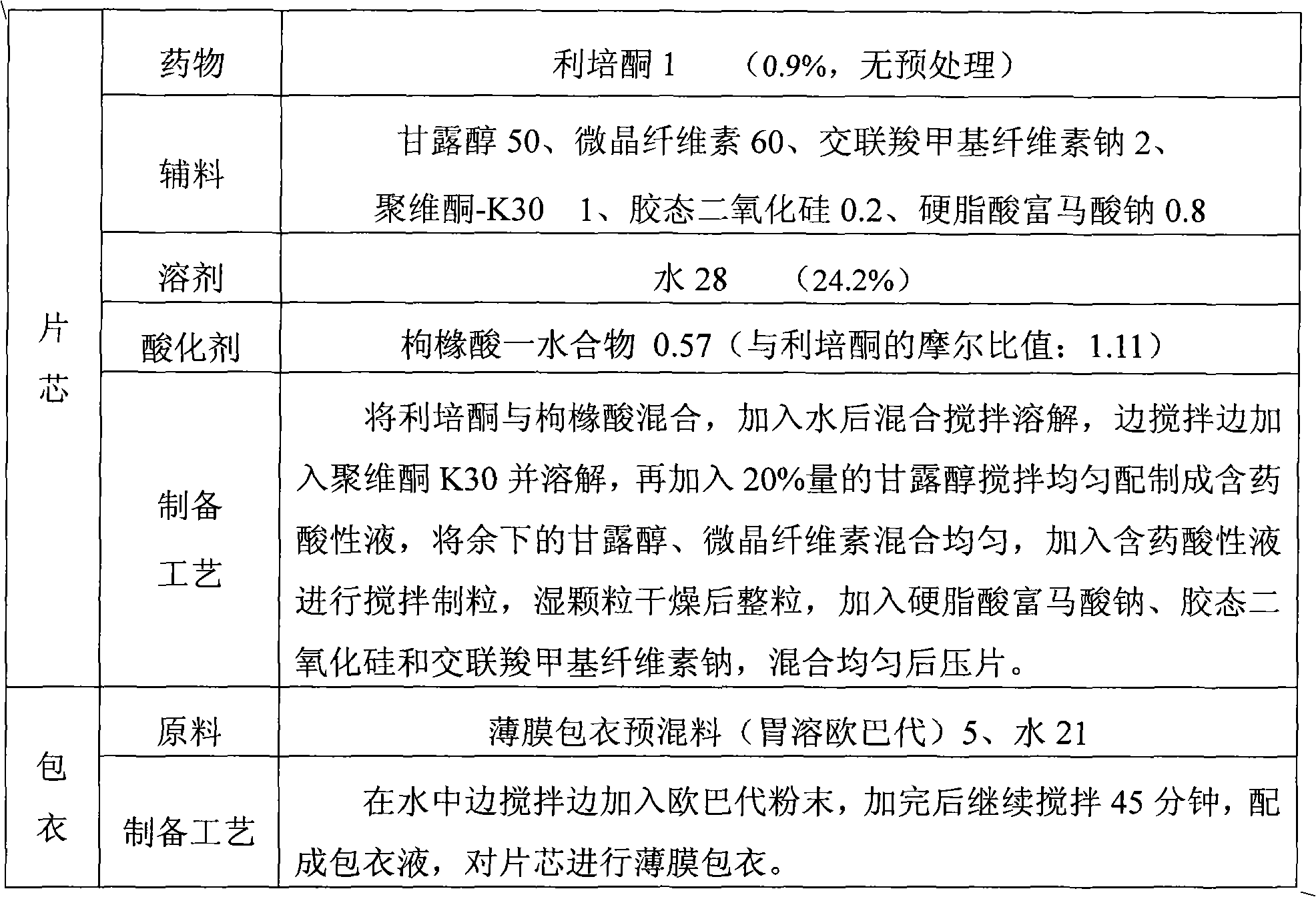
Solid preparation and preparation method thereof
ActiveCN102106808AAvoid heavy pollutionReduce usageOrganic active ingredientsNervous disorderAcidulantChemistry
The invention discloses a method for preparing a solid risperidone preparation. The method comprises the following steps of: dissolving risperidone into acid solution containing an acidulant to obtain medicament-containing acid solution; and uniformly mixing excipients and the medicament-containing acid solution, and performing wet granulation. The invention also discloses the solid risperidone preparation prepared by the method. The method overcomes the disadvantages of serious pollution, high loss and serious potential safety hazards caused by mechanical pulverization treatment, is convenient and easy to operate, has high safety factor, and is easily applied to industrialized production. The solid risperidone preparation prepared by the method has excellent dissolution characteristic, stability and content uniformity.
Owner:SHANGHAI ZHONGXI PHARMA +1
Solid zopiclone preparation and preparation method thereof
ActiveCN102106825AEasy to operateImprove securityOrganic active ingredientsNervous disorderChemistryExcipient
The invention discloses a method for preparing a solid zopiclone preparation. The method comprises the following steps of: dissolving zopiclone into acid solution containing an acidulant to obtain medicament-containing acid solution; and uniformly mixing a basifier, excipients and the medicament-containing acid solution, and performing wet granulation, wherein the basifier is a reagent which ensures that the acidity of mixed solution of the basifier and the medicament-containing acid solution is reduced compared with the acidity of the medicament-containing acid solution. The invention also discloses the solid zopiclone preparation prepared by the method. The method overcomes the disadvantages of serious pollution, high loss and serious potential safety hazards caused by mechanical pulverization treatment, is convenient and easy to operate, has high safety factor, and is easily applied to industrialized production. The solid zopiclone preparation prepared by the method has excellent dissolution characteristic, stability and content uniformity.
Owner:SHANGHAI ZHONGXI PHARMA +1
Pharmaceutical composition of prasugrel hydrobromide acetate compound
InactiveCN102342921ANeat appearanceComplete appearanceOrganic active ingredientsBlood disorderHydrobromideAnti platelet
The invention provides a pharmaceutical composition of a prasugrel hydrobromide acetate compound, which belongs to the medicine field. The pharmaceutical composition is an oral tablet, the tablet comprises the prasugrel hydrobromide acetate compound and auxiliary materials adapted to medicinal use, and is characterized in that the moisture content in the tablet accounts for 0.5-5.0% of total massof the tablet. The oral tablet can be used for treating anti-platelet.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Drug composition containing baricitinib and preparation method and application of drug composition
ActiveCN107334738AImprove mixing uniformityEasy to operateOrganic active ingredientsAntipyreticMANNITOL/SORBITOLFiller Excipient
The invention discloses a drug composition containing baricitinib and a preparation method and application of the drug composition. The drug composition comprises the baricitinib and an auxiliary material which can be received pharmaceutically, wherein the auxiliary material comprises a filling agent and a disintegrating agent. The preparation method of the drug composition comprises the steps that the filling agent and the disintegrating agent are evenly mixed, so that the mixed auxiliary material is obtained; and then a baricitinib crude drug and the mixed auxiliary material are evenly mixed or pelletizing liquor containing the baricitinib crude drug is evenly mixed with the mixed auxiliary material, and drug-carrying pellets of the solid drug composition containing the baricitinib are obtained through pelletizing. According to the drug composition containing the baricitinib and the preparation method and application of the drug composition, the solid drug composition which can be dissolved out rapidly in vitro and contains the baricitinib is prepared by controlling the particle size of the baricitinib, using microcrystalline cellulose, mannitol and the like in the hydrophilic auxiliary material as the filling agent, and using croscarmellose sodium and the like as the disintegrating agent; and the preparation technique is simple and suitable for industrialization.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Manufacturing method for high temperature-resistant high-strength flexible graphite material
ActiveCN102897754AGood dispersionEffectively control dosageCarbon compoundsGraphiteUltimate tensile strength
The invention provides a manufacturing method for a high temperature-resistant high-strength flexible graphite material. The manufacturing method comprises the following steps: with expandable graphite as a raw material, adding an additive solution into the expandable graphite to soak the expandable graphite; carrying out filtering; repeatedly flushing the soaked expandable graphite with a filtrate and controlling moisture content in the expandable graphite to be 18 to 40% of the total weight of the expandable graphite; drying the expandable graphite to obtain modified expandable graphite; and subjecting the modified expandable graphite to expansion, rolling compaction and molding so as to obtain the high temperature-resistant high-strength flexible graphite material which has low ignition loss and high tensile strength. According to the manufacturing method for the high temperature-resistant high-strength flexible graphite material in the invention, a phosphorus agent and a boron agent are added into the flexible graphite material, so inoxidizability and strength of the material are improved; and the method has the advantages of a simple process, low cost and easy popularization.
Owner:CHINA SCI HENGDA GRAPHITE
Method for Recovering Ethylene during the Process for Producing VAC and a Device thereof
ActiveUS20140366729A1Emission reductionLow production costGas treatmentUsing liquid separation agentAcetic acidSolvent
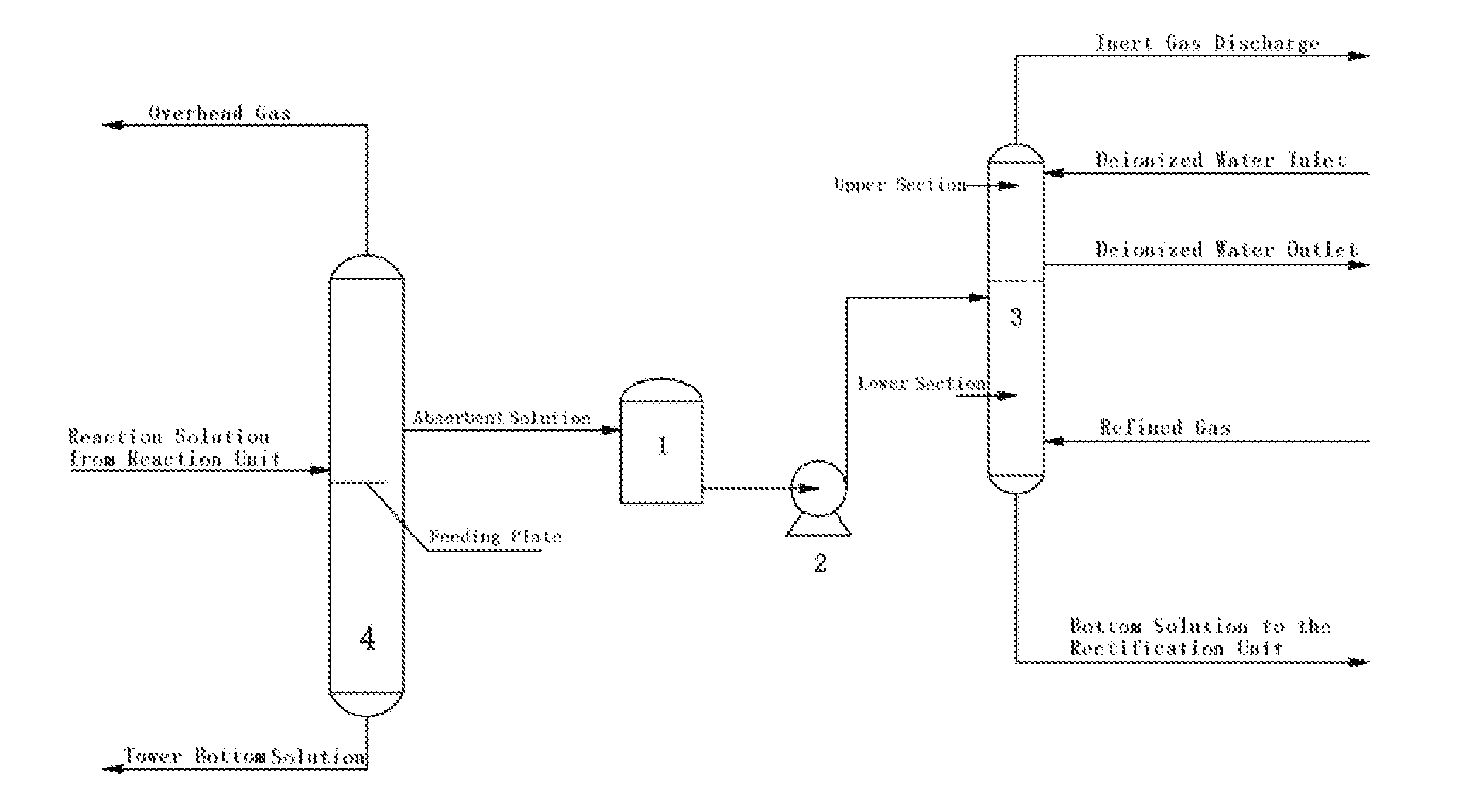
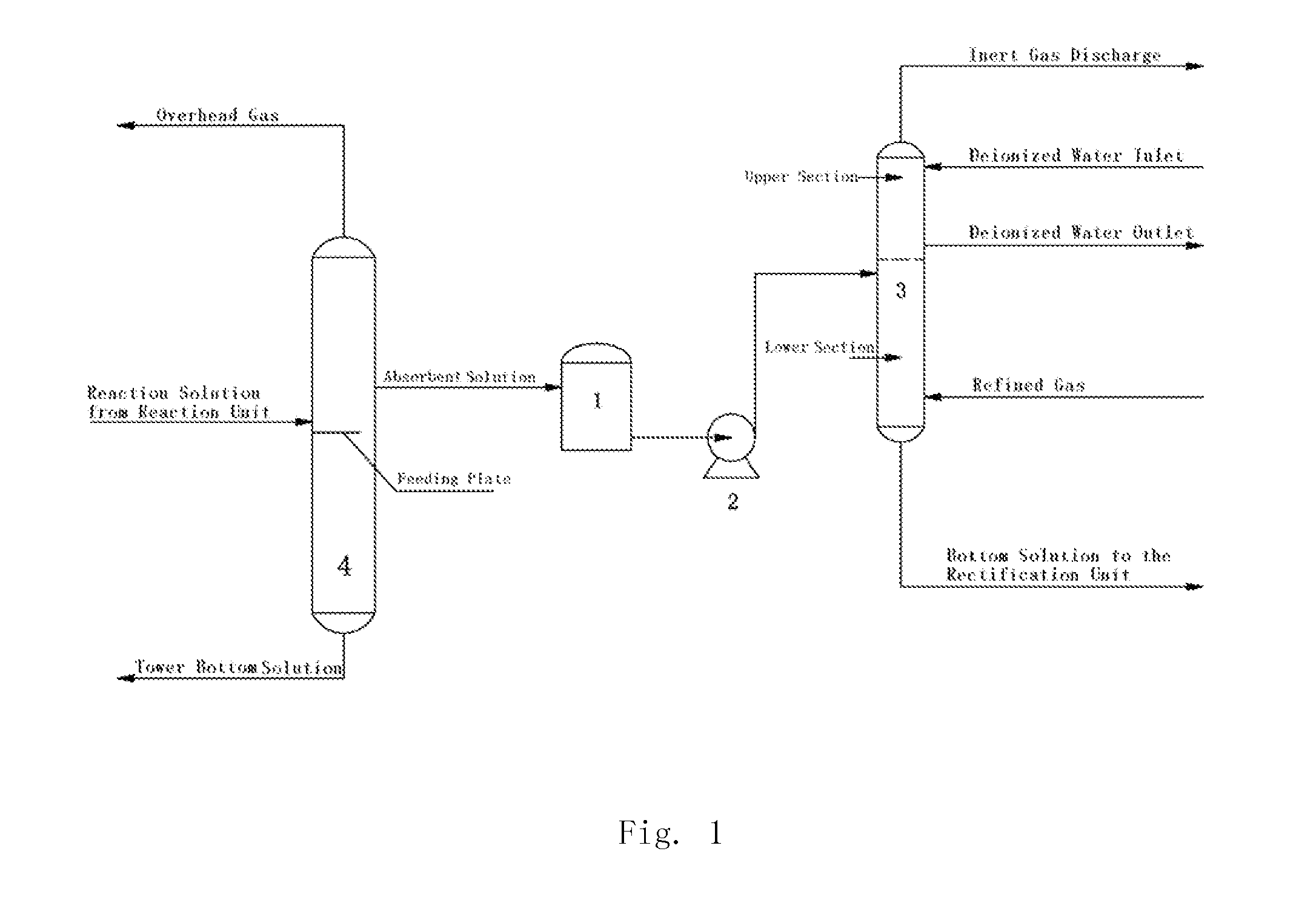
The present invention discloses a method and a device for recovering ethylene during the process for producing vinyl acetate, wherein a double solvent absorption method composed of an absorbent solution and deionized water is adopted. The method is as follows: introducing an outlet stream as an absorbent solution from the upper section of the feeding plate in an acetic acid tower during the rectification stage of vinyl acetate production; delivering same to the top of the lower section of the ethylene recovery tower by a delivery pump; charging refined gas from the tower bottom of the ethylene recovery tower for contacting with the absorbent solution in a counter-current; delivering the absorption bottom solution to the rectification stage for treatment; the gas continuing to rise to the upper section of the ethylene recovery tower and contacting with the deionized water introduced from the top of the tower in a counter-current; absorbing and removing the acetic acid therein; and discharging the residual inert gas, such as N2, from the top of the ethylene recovery tower. The absorbent solution is a mixture of acetic acid, vinyl acetate and water, and comprises by weight percentage of 50-85% acetic acid, 5-30% vinyl acetate and 5-20% water.
Owner:TIANJIN UNIV
Eszopiclone solid preparation and preparation method thereof
ActiveCN102106824AEasy to operateImprove securityOrganic active ingredientsNervous disorderMechanical crushingZopiclone
The invention discloses the preparation method of eszopiclone solid preparation, which comprises the following steps of dissolving eszopiclone into acidic solution containing an acidifying agent to obtain medicine-containing acidic solution; and uniformly mixing alkalinizing agent, accessories and the medicine-containing acidic solution to granulate by a wet method, wherein the alkalinizing agentis agent by which the acidity of mixed solution of the alkalinizing agent and the medicine-containing acidic solution is reduced relative to the acidity of the medicine-containing acidic solution. The invention also discloses eszopiclone solid preparation prepared by the method. According to the method disclosed by the invention, the defects of the serious pollution, high loss and serous potential safety hazards brought by mechanical crushing treatment are avoided; and the method is simple, convenient and feasible for operation and easy for industrial production and has high safety coefficient. The eszopiclone solid preparation prepared by the method has the advantages of excellent dissolution property, stability, and content uniformity.
Owner:SHANGHAI ZHONGXI PHARMA +1
Method for preparing myricetin/HP-beta-CD inclusion compound superfine granules through supercritical CO2 anti-solvent technique
InactiveCN109701032AImproved dissolution propertiesImprove stabilityOrganic active ingredientsMetabolism disorderChemistryWater soluble
The invention discloses a method for preparing myricetin / HP-beta-CD inclusion compound superfine granules through a supercritical CO2 anti-solvent technique. The method comprises the following steps of (1) compounding a myricetin-carrier mixed solution: weighing myricetin raw material medicines and a water-soluble carrier namely hydroxypropyl-beta-cyclodextrin, and enabling the weighed myricetin raw material medicines and the weighed water-soluble carrier namely hydroxypropyl-beta-cyclodextrin to dissolve in an organic solvent to obtain the myricetin-carrier mixed solution, wherein the organicsolvent is ethanol, and the molar ratio of the raw material medicines to the carrier is 1 to 1; (2) charging CO2 into a crystallization kettle at a certain flow rate, and regulating temperature and pressure in the crystallization kettle; (3) continuing charging the CO2, maintaining the temperature and the pressure in the crystallization kettle unchanged, and at the same time, spraying the myricetin-carrier mixed solution prepared in the step (1) into the crystallization kettle from the top of the crystallization kettle through a nozzle by a high-pressure liquid delivery pump; and (4) after completion of sample introduction, continuing charging the CO2 for some time, completely discharging remaining solvents, releasing pressure, opening the crystallization kettle, and collecting products.The myricetin / HP-beta-CD inclusion compound superfine granules obtained by the method can notably improve dissolving-out properties, and improvement of the biological availability of the myricetin isfacilitated.
Owner:CHINA PHARM UNIV
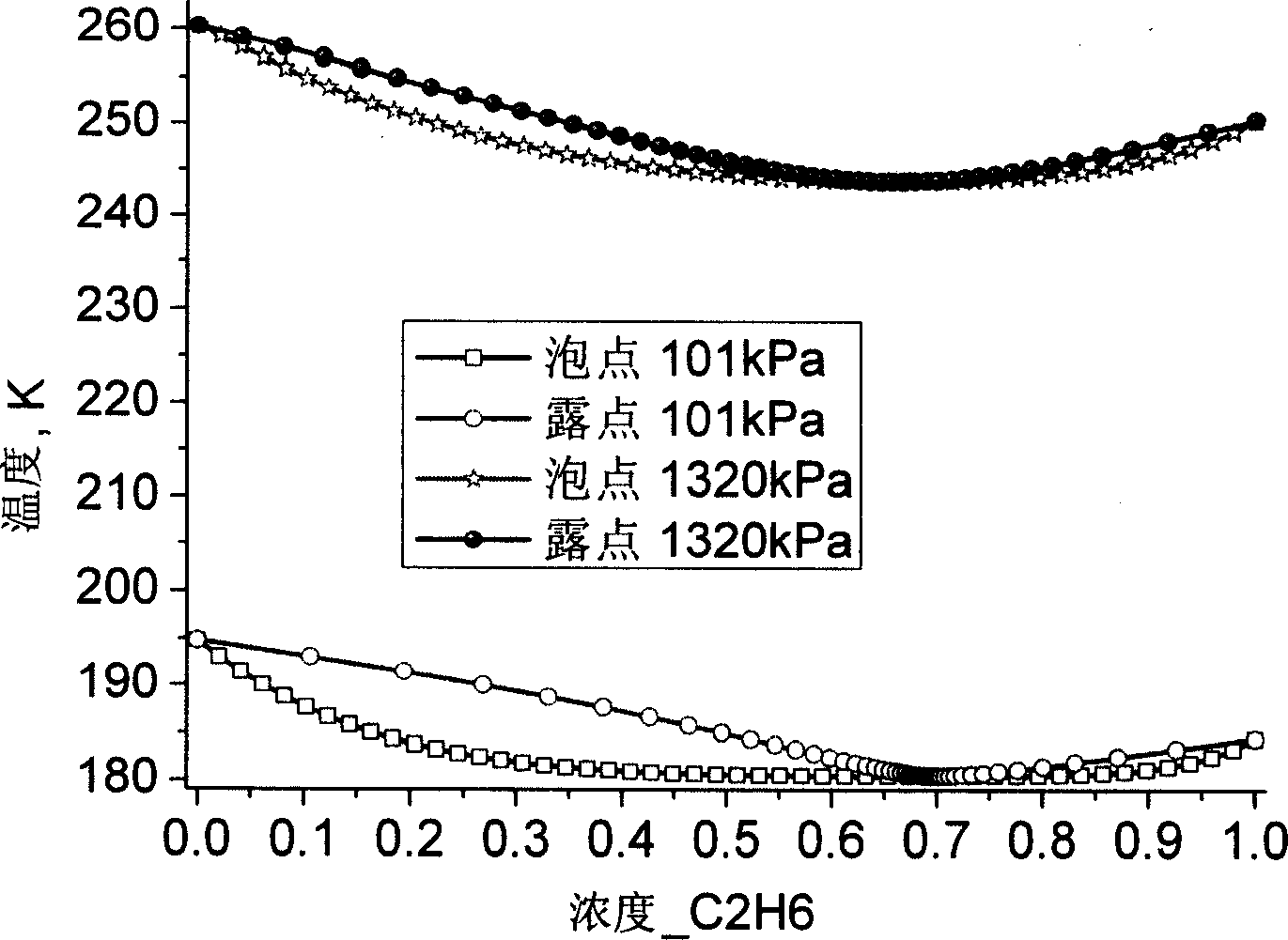
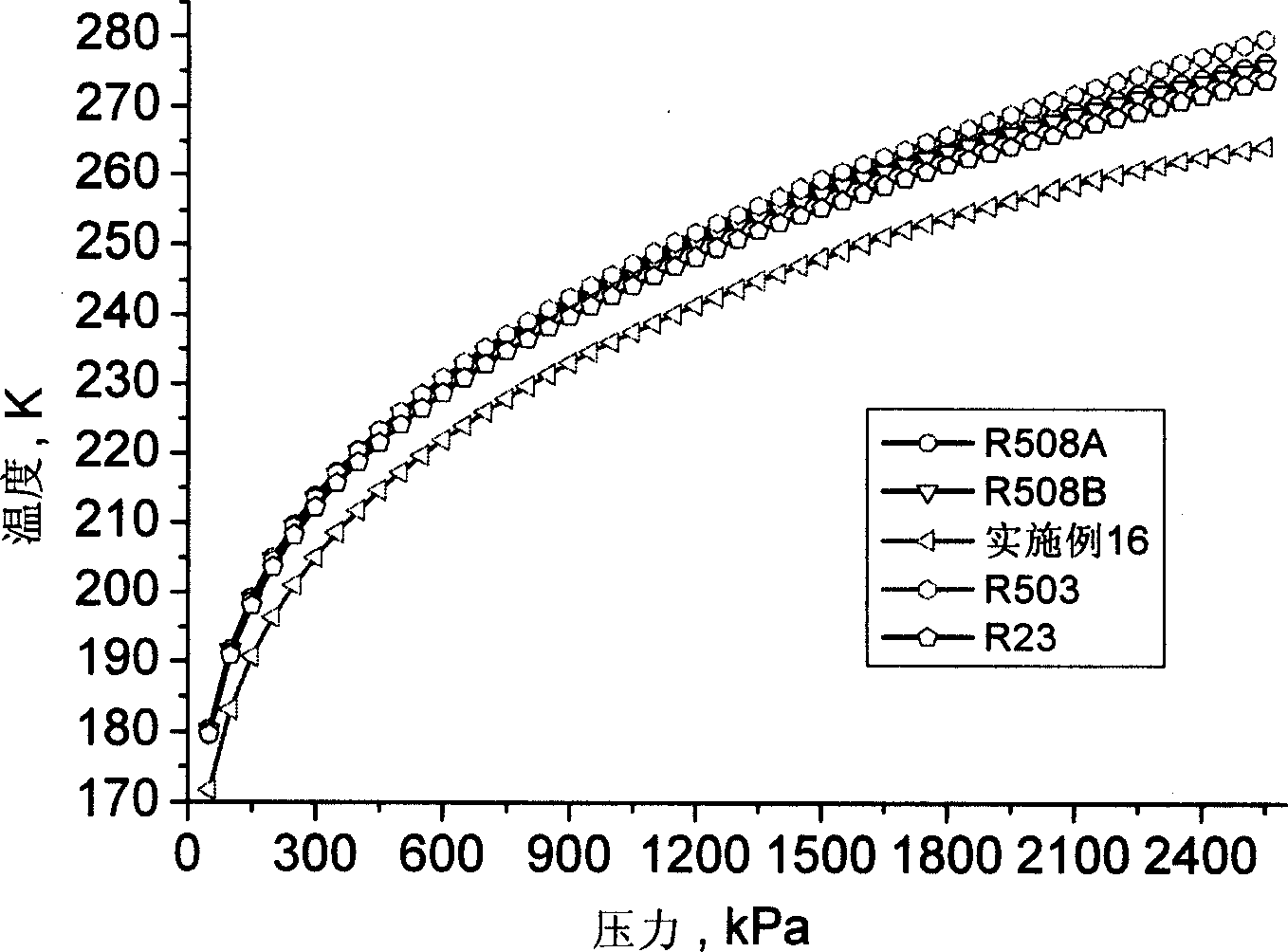
Cryogenic stage mixed refrigerant suitable in two-stage cascade refrigeration system
ActiveCN101284985AIncrease evaporating pressureIncrease condensing pressureHeat-exchange elementsPerfluoroethaneEvaporation
The invention relates to low temperature stage mixed refrigerant applicable to two-stage cascade refrigeration systems. The mixed refrigerant comprises ethane and monofluoro-methane, and is formed through physical mixture, wherein the molarity of ethane is 50 to 75 percent, and the balance is monofluoro-methane. The mixed refrigerant can also comprises ethane, monofluoro-methane and perfluoroethane, and is formed through physical mixture, wherein the molarity of ethane is 40 to 70 percent; the molarity of monofluoro-methane is 5 to 40 percent; the balance is perfluoroethane. The mixed refrigerant has high efficiency and high evaporation pressure, as well as good refrigeration capacity under the same compressor displacement, has zero ODP, greatly decreases GWP than R503 and R508B, and has good intersolubility with lubricating oil.
Owner:青岛中科未来健康研究院有限公司
Rasagiline or medicinal salt sublingual film agent thereof as well as preparation method and application thereof
PendingCN114469902AImprove complianceEasy to administerOrganic active ingredientsNervous disorderCyclodextrinPharmaceutical drug
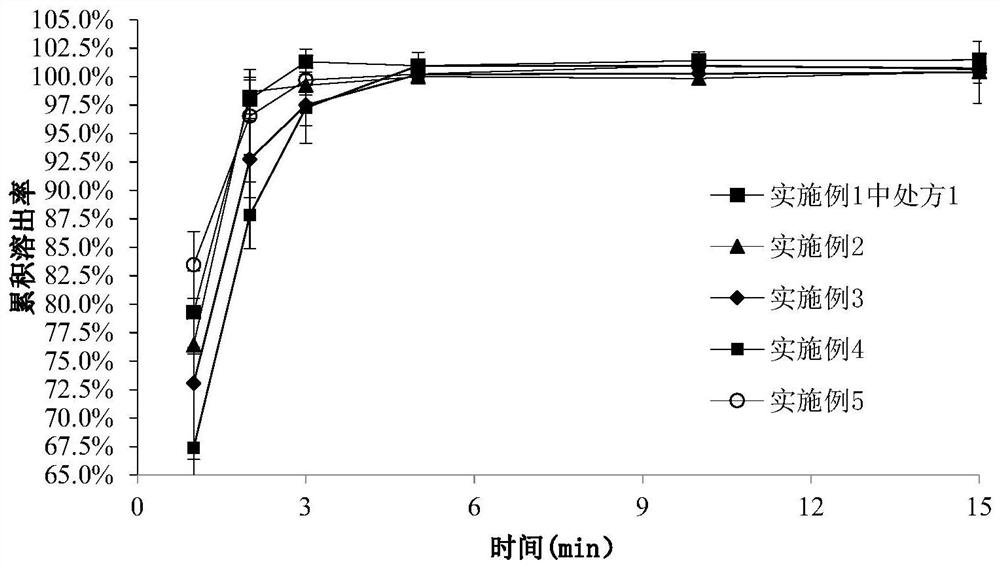
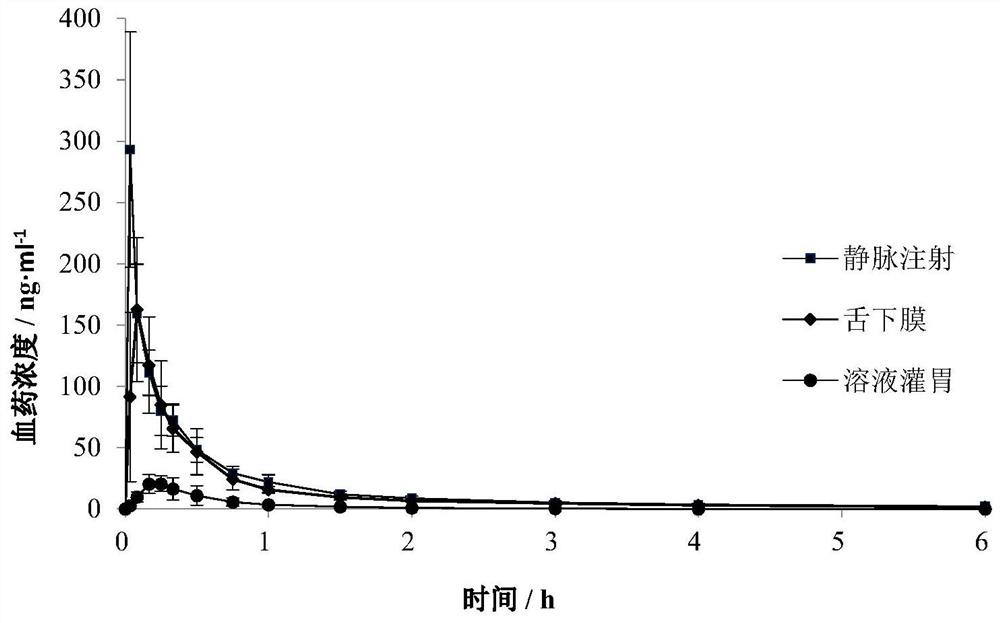
The invention discloses rasagiline or a medicinal salt sublingual film agent thereof as well as a preparation method and application of the rasagiline or the medicinal salt sublingual film agent. The sublingual film agent comprises the following prescription components: 0.5-15% of rasagiline or a pharmaceutical salt thereof, 30-85% of a polymer film-forming material, 5-40% of dextrin and 0-30% of other auxiliary materials (not 0), and the dextrin is one or more of maltodextrin, hydroxypropyl-beta-cyclodextrin, glucosyl-beta-cyclodextrin and sulfobutyl-beta-cyclodextrin; the percentage is the mass percentage of each component relative to the total components of the prescription of the sublingual film agent. The sublingual membrane disclosed by the invention can be adhered to an administration part and quickly dissolved without water, and a patient does not need to swallow, so that the sublingual membrane is good in compliance; through oral mucosa absorption, the first-pass effect of the liver is avoided, and the bioavailability is high; the stability is good, and the impurity content is still low after long-term storage; the pharmaceutical composition has a good dissolution characteristic, is rapid and complete in drug dissolution, is rapidly absorbed by sublingual mucosa and enters blood circulation, and takes effect rapidly.
Owner:SHANGHAI ZHONGXI PHARMA +1
Cymipristone solid preparation and preparation methods thereof
ActiveCN102106805AEasy to operateImprove securityOrganic active ingredientsGranular deliveryOrganic solventSolvent
The invention discloses preparation methods of a cymipristone solid preparation. A method 1 comprises the following steps: dissolving cymipristone in a solvent to obtain a drug-containing solution, then evenly mixing auxiliary materials with the drug-containing solution, and performing wet granulation, wherein, the solvent is an organic solvent or an aqueous solution containing more than 85% of an organic solvent by mass percent. A method 2 comprises the following steps: dissolving the cymipristone in an acid solution containing an acidulant to obtain a drug-containing acid solution, then evenly mixing the auxiliary materials with the drug-containing acid solution, and performing wet granulation. The invention further discloses the cymipristone solid preparation prepared by the methods. The preparation methods disclosed by the invention avoid the defects such as serious pollution, great loss and serious potential safety hazard caused by mechanical pulverization, and the methods are simple and feasible in operation and have a high safety factor, thus being applicable to industrial production. The cymipristone solid preparation prepared by the methods has the advantages of excellent leaching property, better stability and better content uniformity.
Owner:SHANGHAI ZHONGXI PHARMA +1
Pharmaceutical composition comprising PARP inhibitor
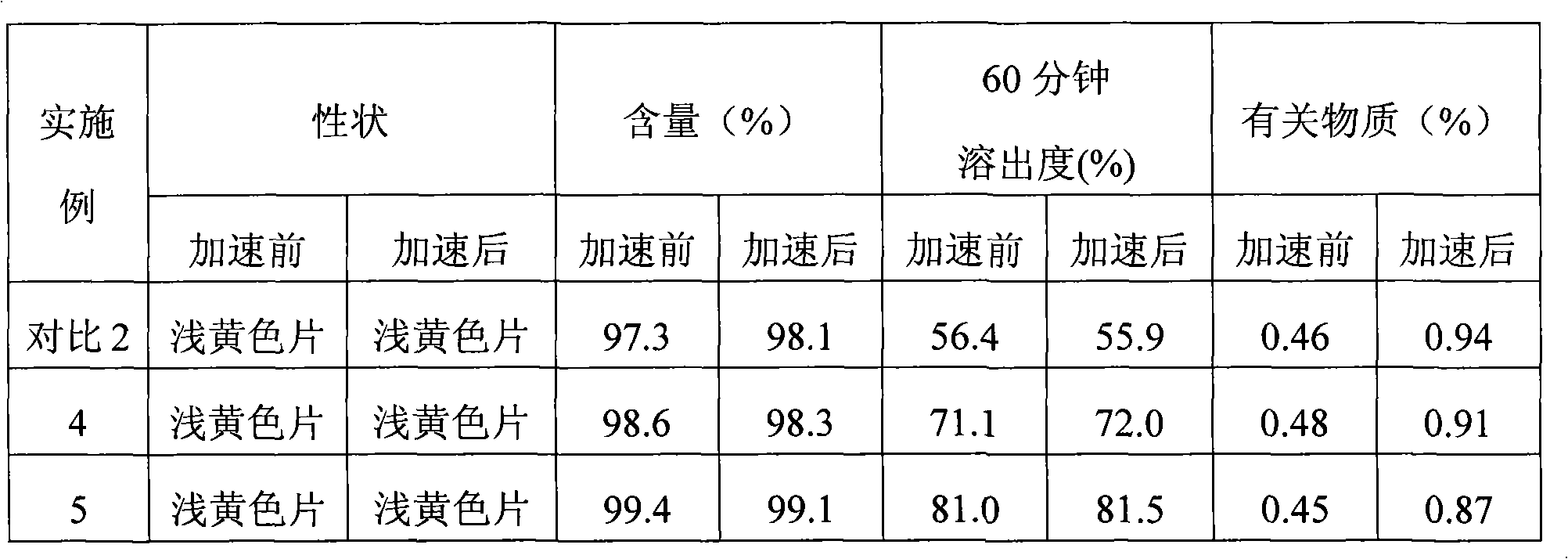
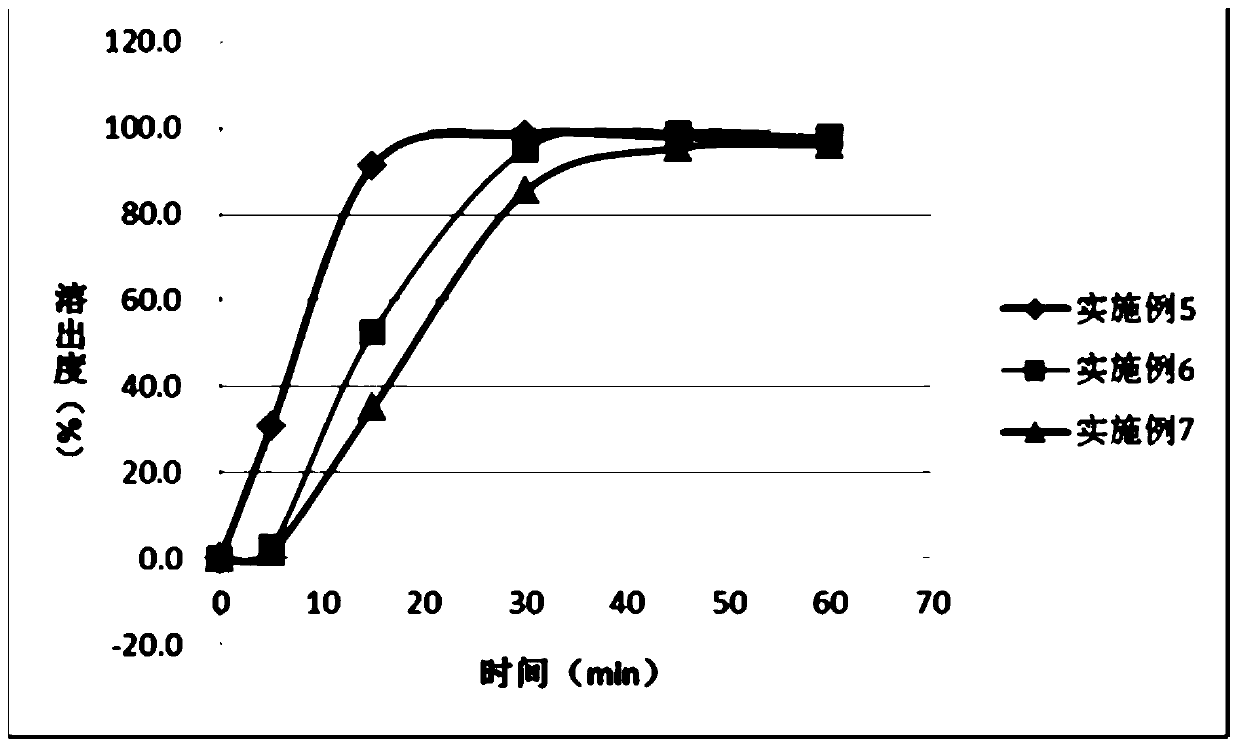
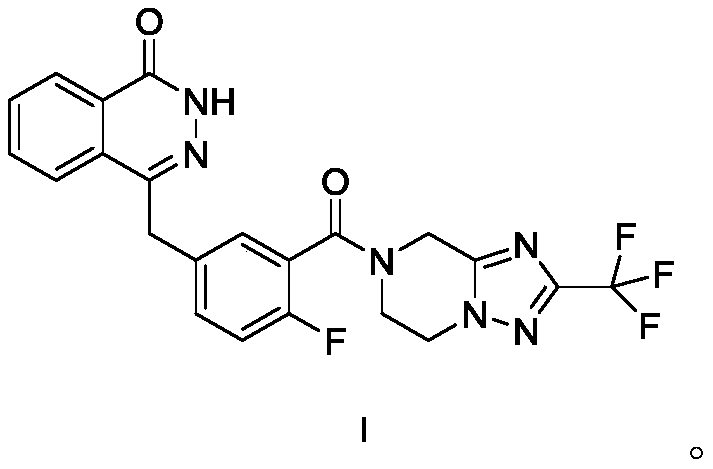
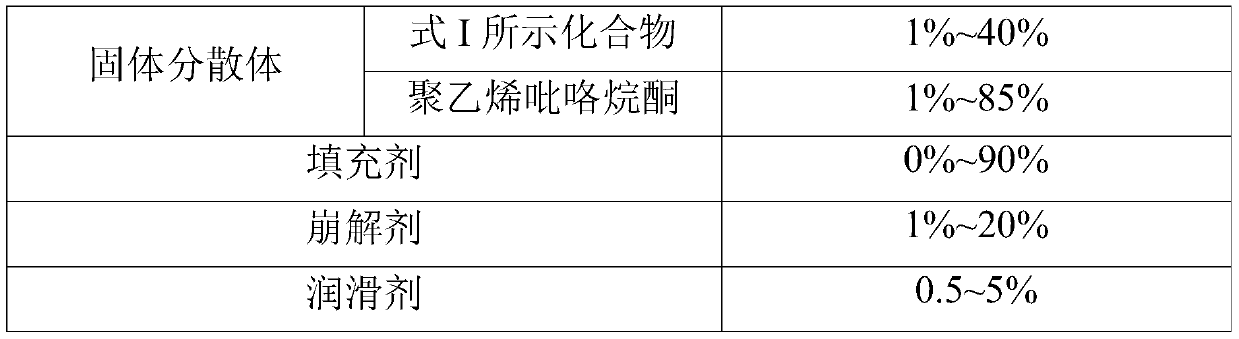
ActiveCN110840845AImproved dissolution propertiesImprove stabilityOrganic active ingredientsPowder deliveryPharmaceutical drugCombinatorial chemistry
The invention relates to a pharmaceutical composition comprising a PARP inhibitor. In particular, the invention relates to a pharmaceutical composition comprising a solid dispersion of a compound shown in a formula I. The composition has a good dissolution effect and excellent stability, and can be better applied to clinic.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Pharmaceutical Composition for Oral Administration Comprising Enzalutamide
PendingUS20200146977A1Improved dissolution propertiesImprove solubilityOrganic active ingredientsUrinary disorderOral medicationPolyvinyl alcohol
Provided is a pharmaceutical composition for oral administration in which the solubility and / or dissolution properties of enzalutamide are improved and supersaturation is maintained. Also provided is a pharmaceutical composition for oral administration in which the oral absorbability of enzalutamide is improved. The pharmaceutical composition for oral administration comprises enzalutamide and polyvinyl alcohol.
Owner:ASTELLAS PHARMA INC
Aripiprazole solid preparation and preparation method thereof
ActiveCN102106826BEasy to operateImprove securityOrganic active ingredientsNervous disorderAdjuvantOrganic chemistry
A production method of an aripiprazole solid preparation and the preparation produced by the method are disclosed. The method comprises: dissolving aripiprazole in the acid solution containing acidifying agent to obtain an acid liquid of the medicine; later, homogeneously mixing adjuvants and the acid liquid of the medicine, carrying out wet granulation.
Owner:SHANGHAI ZHONGXI PHARMA +1
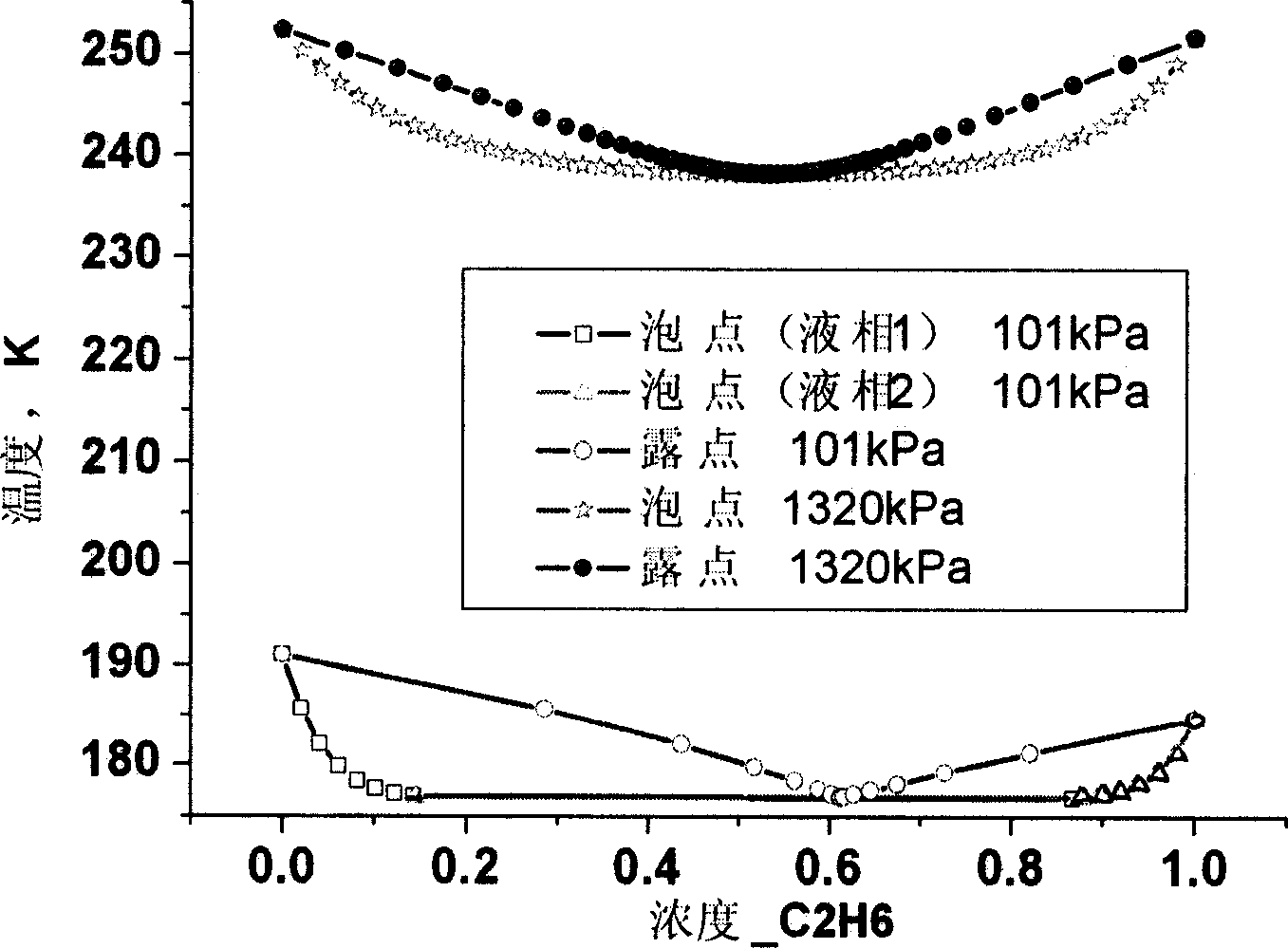
Low-temperature-level mixed refrigerant suitable for two-stage multiplex refrigerating system
InactiveCN1891781AIncrease evaporating pressureIncrease condensing pressureHeat-exchange elementsSolubilityEngineering
The invention relates to mixing refrigerant used in the low temperature stage of the two stage cascade system refrigeration system. The mixing refrigerant includes ethane, full fluorine ethane or / and fluoroform. It can be made up of ethane whose molar concentration is 25%-95% and full fluorine ethane whose is the rest. And it also can be made up of ethane whose is 45%-75% and fluoroform whose is the rest. It also can include all three of them whose respectively is 25%-90%, 5%-60%, and the rest. Its advantages are that it has high efficiency and evaporating pressure; its ODP is zero; the GWP is greatly reduced compared with the R503 and R5088; and it has good inter-solubility with lubricating oil.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Method for preparing solid preparation and solid preparation
ActiveCN102106806BEasy to operateImprove securityOrganic active ingredientsNervous disorderAdjuvantWater insoluble
A preparation method of solid formulations and the solid formulations prepared thereby are disclosed. The method comprises the steps of: dissolving insoluble or slightly soluble basic active ingredients in an acid solution comprising acidifiers, then, homogenously mixing the acidic solution of active ingredients with adjuvants, and granulating by wetting granulation method.
Owner:SHANGHAI ZHONGXI PHARMA +1
Multifunctional bioprotein-based aerogel material as well as preparation method and application thereof
ActiveCN114736421AImprove mechanical propertiesAchieve functionalizationGarment special featuresProtective garmentFiberFibril
According to the multifunctional bioprotein-based aerogel material as well as the preparation method and the application thereof provided by the invention, a biomass fiber fibril mixed component with a micro-nano size is extracted by adopting a deep eutectic solvent stripping technology, and an aerogel core material prepared by adopting a freeze drying process forms a fibril interpenetrating stable three-dimensional network structure; the mechanical property of the core material is improved, and the core material has excellent biocompatibility and degradability. Moreover, based on co-melting and vapor deposition schemes, the aerogel core material with the drug-loading function and the hydrophobic material are prepared, functionalization of the material is achieved, and the aerogel material with the drug-loading function can also be used for manufacturing detachable masks and plays a role in disease prevention. The drug-loaded multifunctional bioprotein-based aerogel core material provided by the invention has excellent biocompatibility and degradability, the raw materials are wide in source, and the core material can be self-degraded after being discarded without subsequent treatment cost.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY AND SCIENCE
Oral solid pharmaceutical composition in micronized form and preparation method thereof
InactiveCN110917200AImproved dissolution propertiesAvoid gatheringPowder deliveryPharmaceutical non-active ingredientsHomoharringtonineActive agent
The invention discloses an oral solid pharmaceutical composition in a micronized form by using a solid preparation with a micronized homoharringtonine derivative as an active component. The composition comprises a pharmacological active agent with an effective dosage, crospovidone or polyvinylpyrrolidone, and an oral solid pharmaceutical composition used for a pharmacological active agent. The oral solid pharmaceutical composition has an excellent in-vitro dissolution rate, and has good dissolution rates in a variety of pH conditions, so that good absorption of medicines in different populations can be effectively ensured, and the problems that an existing homoharringtonine derivative drug is not easily dissolved out in an in-vivo gastrointestinal environment and then in-vivo bioavailability of drugs is reduced and efficacy is influenced can be solved.
Owner:广州艾格生物科技有限公司
Pharmaceutical composition containing micronized fexofenadine hydrochloride
ActiveCN104257611AReduce in quantityExcellent in vitro dissolutionPowder deliveryPill deliveryCurative effectCrowds
The invention provides micronized fexofenadine hydrochloride. The particle size of fexofenadine hydrochloride at 95% cumulative volume is 60mu m or below. The invention also provides a method for preparing the micronized fexofenadine hydrochloride, a pharmaceutical composition containing the micronized fexofenadine hydrochloride, as well as a preparation method of the pharmaceutical composition. According to the invention, a solid preparation by taking the micronized fexofenadine hydrochloride as the active ingredient has excellent dissolution rate in vitro and has high dissolution characteristics under various pH conditions, the corresponding effects of the medicine in vivo in different crowds can be effectively guaranteed, and the problem that the bioavailability of the medicine in vivo is reduced so as to influence the curative effect because the conventional fexofenadine hydrochloride medicine is difficult to dissolve in an in-vivo gastrointestinal environment is solved.
Owner:KUSN ROTAM REDDY PHARMA
A pharmaceutical composition containing baricitinib and its preparation method and application
ActiveCN107334738BImprove bioavailabilityRapid dissolutionOrganic active ingredientsAntipyreticCarboxymethyl cellulosePharmaceutical medicine
The invention discloses a pharmaceutical composition containing baricitinib and its preparation method and application. The pharmaceutical composition comprises baricitinib and pharmaceutically acceptable auxiliary materials, wherein the auxiliary materials include fillers and disintegrants. The preparation method of the pharmaceutical composition comprises: uniformly mixing a filler and a disintegrant to obtain mixed excipients; uniformly mixing the baricitinib bulk drug with the mixed excipients or granulating the baricitinib bulk drug The liquid is uniformly mixed with the mixing excipients, and the drug-loaded granules of the solid pharmaceutical composition containing baricitinib are obtained by granulation. The present invention controls the particle size of baricitinib, uses hydrophilic auxiliary materials microcrystalline cellulose, mannitol, etc. The solid pharmaceutical composition containing baricitinib has a simple preparation process and is suitable for industrialization.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Preparation containing lurasidone hydrochloride and preparation method thereof
ActiveCN103006661BImproved dissolution propertiesSimple and fast operationOrganic active ingredientsNervous disorderLurasidone HydrochlorideActive component
Owner:YANGTZE RIVER PHARM GRP NANJING HAILING PHARM CO LTD
Veterinary five-flavored evergreen particle preparation method
InactiveCN107961330AImprove dissolution propertiesShort inclusion timePharmaceutical non-active ingredientsAntiparasitic agentsHerbChemistry
The invention relates to a veterinary five-flavored evergreen particle preparation method which includes the steps: 1 mixing sweet wormwood herbs with Chinese thorowax roots, and performing reflux extraction with water to obtain volatile oil, extracting solution A and sweet wormwood herb and Chinese thorowax root residues; 2 adding glycerol into the volatile oil, uniformly mixing the glycerol andthe volatile oil to obtain volatile oil and glycerol mixing solution; adding water into beta-cyclodextrin, heating and dissolving the beta-cyclodextrin to prepare beta-cyclodextrin water solution; slowly adding the volatile oil and glycerol mixing solution into the beta-cyclodextrin water solution, and adding alcohol after stirring, refrigeration, suction filtration and drying to prepare volatileoil inclusion particles; 3 adding lightyellow sophora roots, antifeverile dichroa roots and lalang grass rhizome into the sweet wormwood herb and Chinese thorowax root residues, performing extractionwith water twice, combining secondary extracting solution B and the extracting solution A, performing spray drying after concentration, sieving products and adding auxiliary material pellets to obtainmedicinal material particles; 4 uniformly mixing the volatile oil inclusion particles with the medicinal material particles. The products prepared by the method are small in volatile oil smell, highin content and stable in quality.
Owner:BAODING JIZHONG PHARMA
Pharmaceutical composition containing micronized fexofenadine hydrochloride
ActiveCN104257611BReduce in quantityExcellent in vitro dissolutionPowder deliveryPill deliveryCurative effectBULK ACTIVE INGREDIENT
The invention provides micronized fexofenadine hydrochloride. The particle size of fexofenadine hydrochloride at 95% cumulative volume is 60mu m or below. The invention also provides a method for preparing the micronized fexofenadine hydrochloride, a pharmaceutical composition containing the micronized fexofenadine hydrochloride, as well as a preparation method of the pharmaceutical composition. According to the invention, a solid preparation by taking the micronized fexofenadine hydrochloride as the active ingredient has excellent dissolution rate in vitro and has high dissolution characteristics under various pH conditions, the corresponding effects of the medicine in vivo in different crowds can be effectively guaranteed, and the problem that the bioavailability of the medicine in vivo is reduced so as to influence the curative effect because the conventional fexofenadine hydrochloride medicine is difficult to dissolve in an in-vivo gastrointestinal environment is solved.
Owner:KUSN ROTAM REDDY PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com