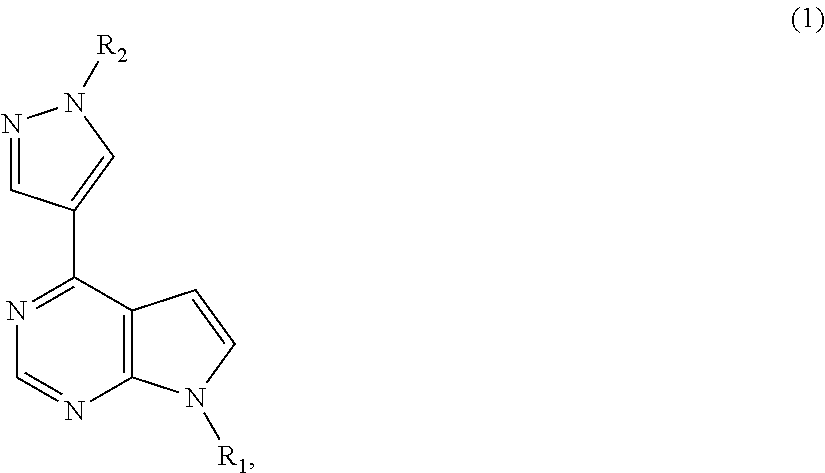
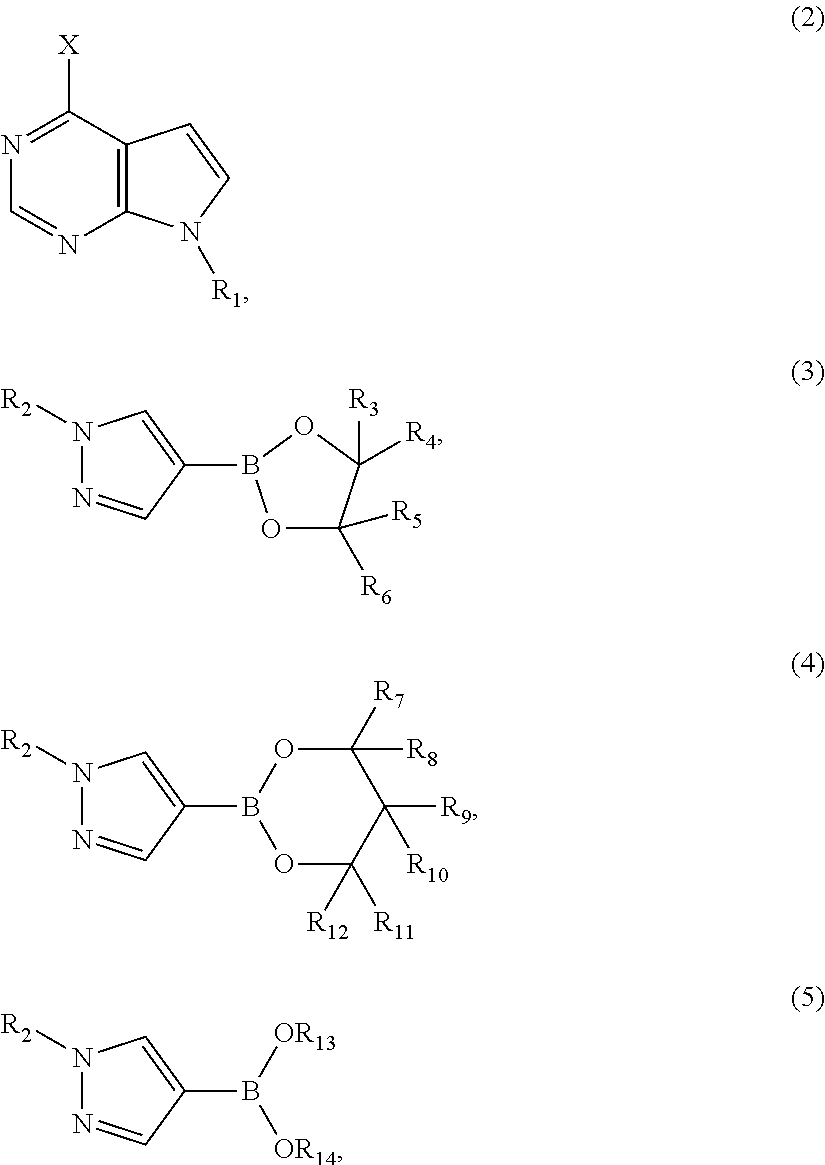
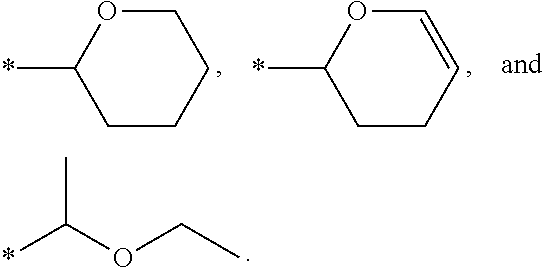
Patents
Literature
45 results about "Baricitinib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
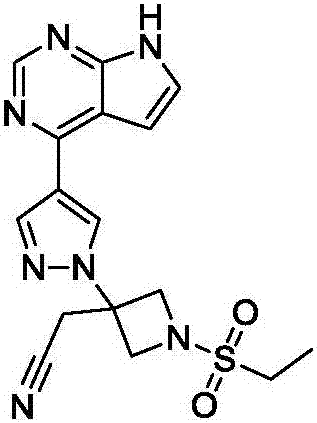
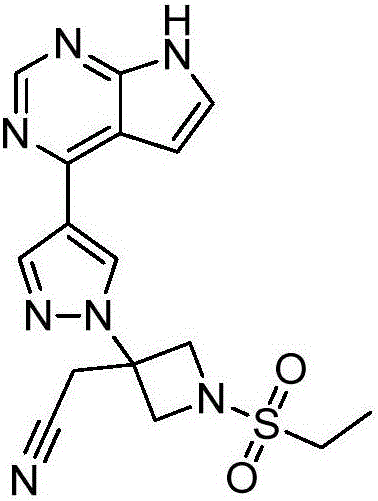
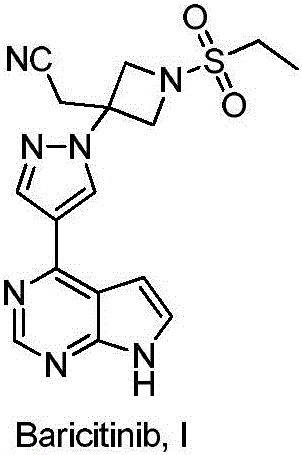
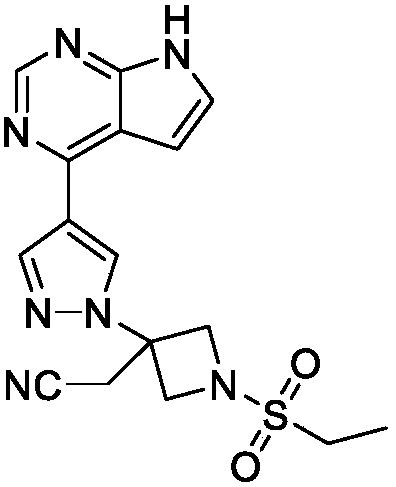
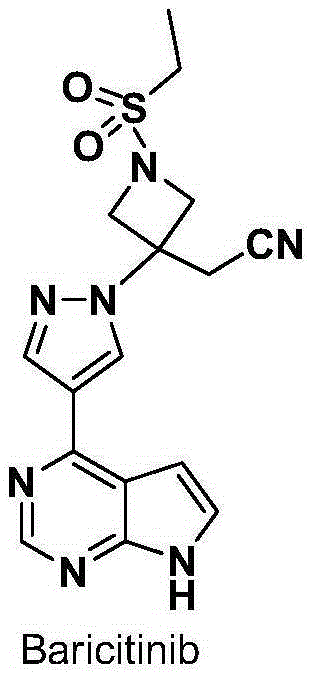
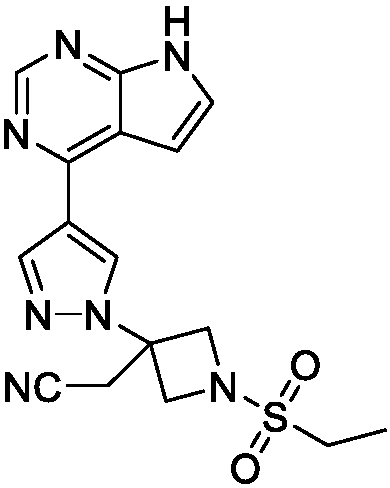
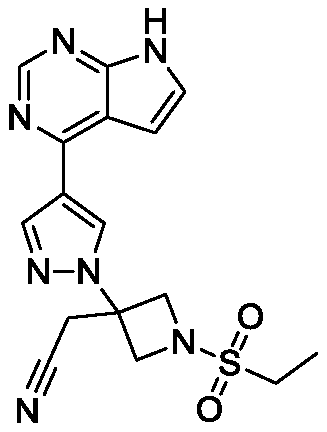
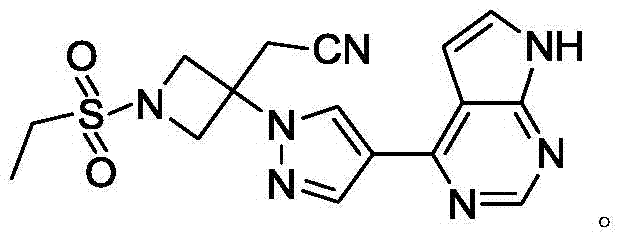
Baricitinib is used to treat rheumatoid arthritis.
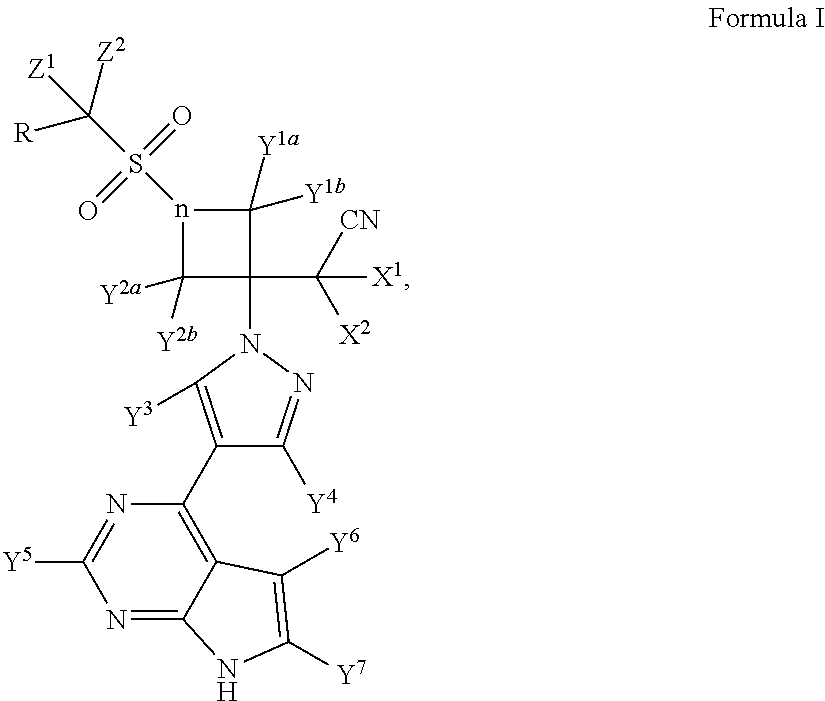
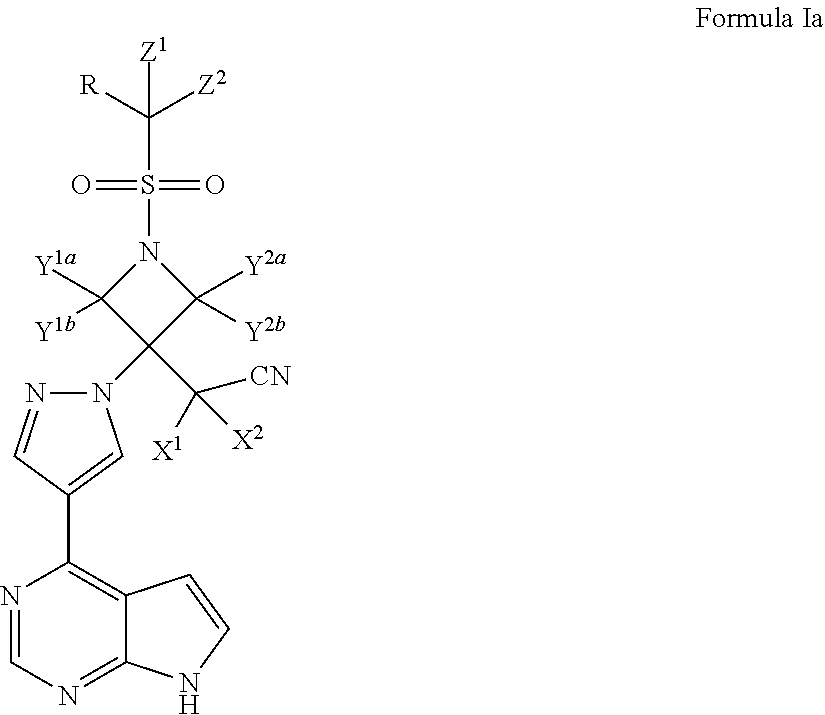
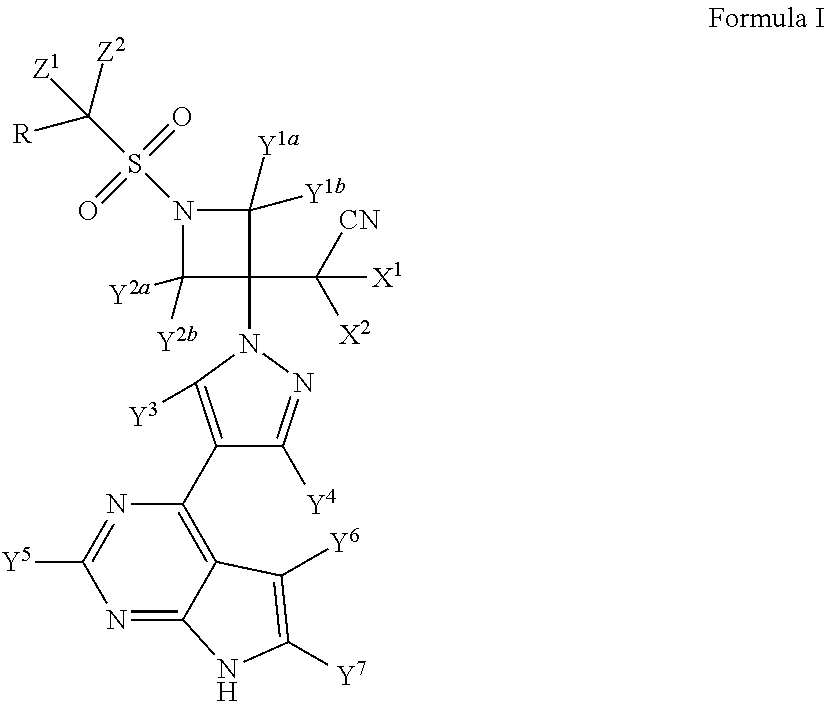
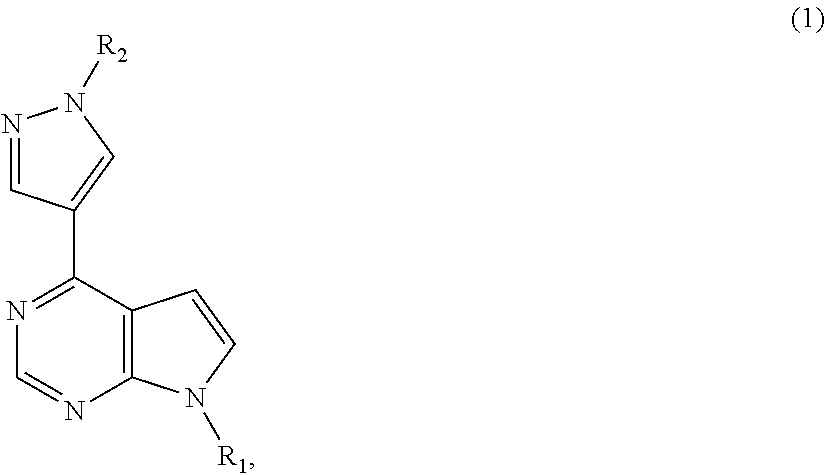
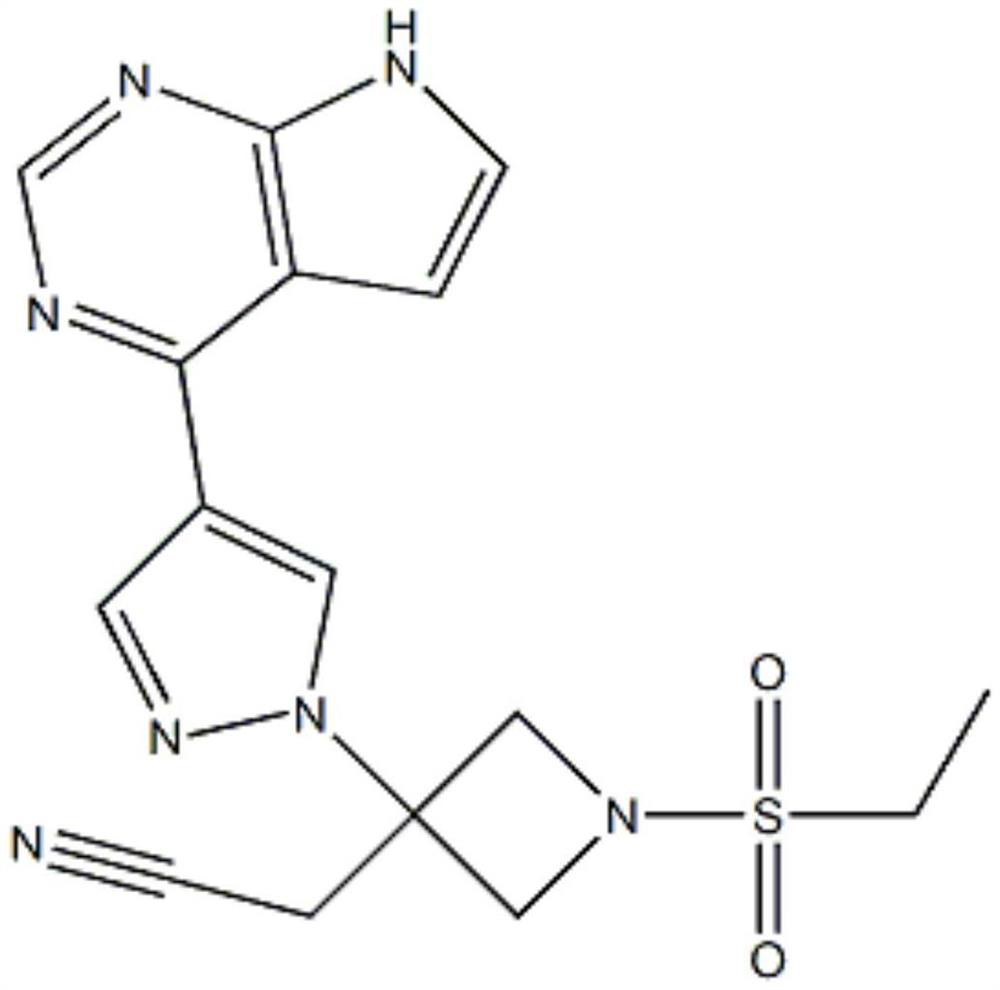
Deuterated baricitinib
ActiveUS9540367B2Organic active ingredientsIsotope introduction to heterocyclic compoundsBiochemistryBaricitinib
Owner:SUN PHARMA IND INC
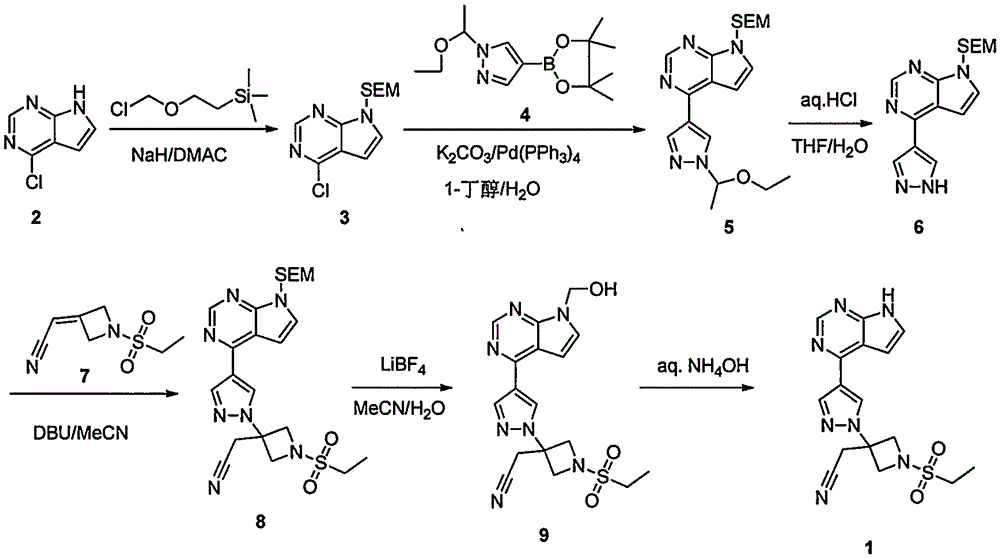
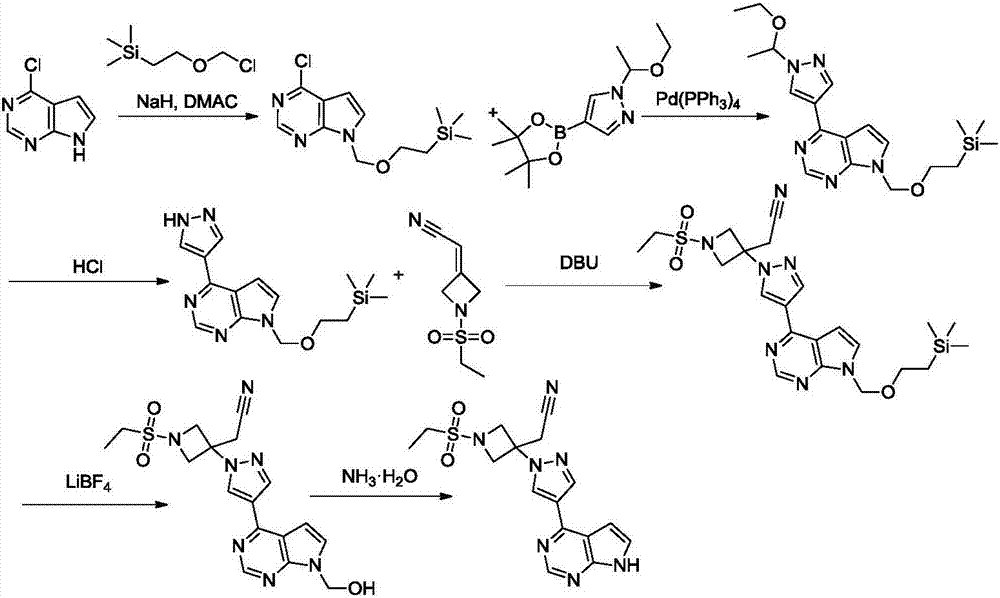
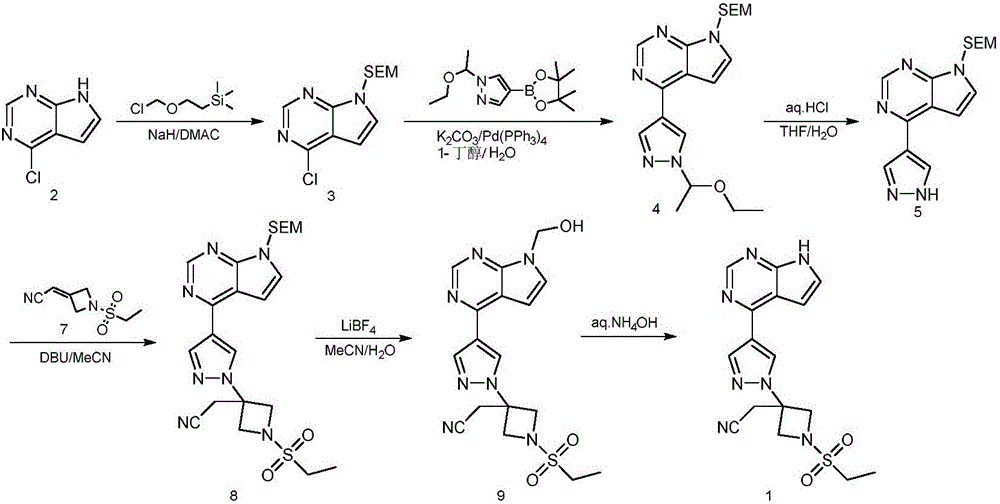
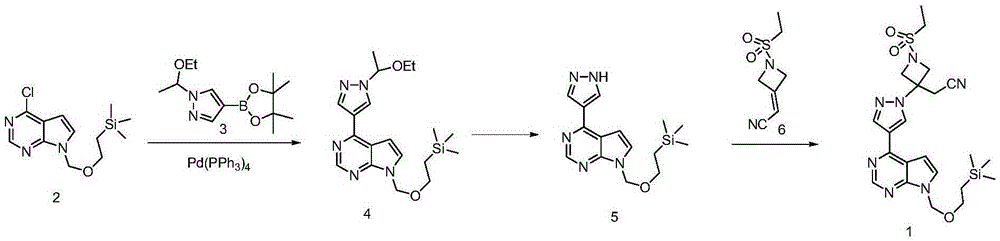
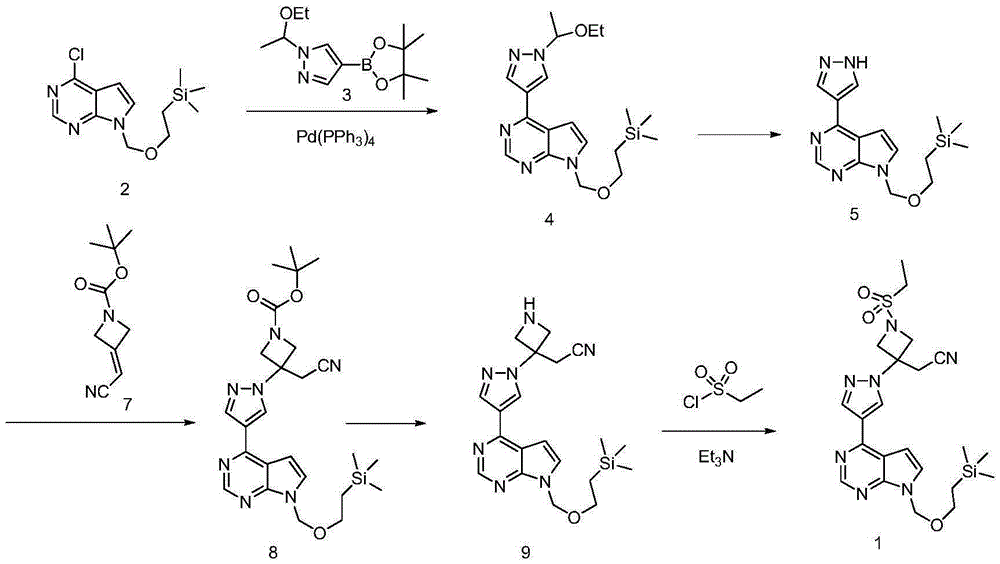
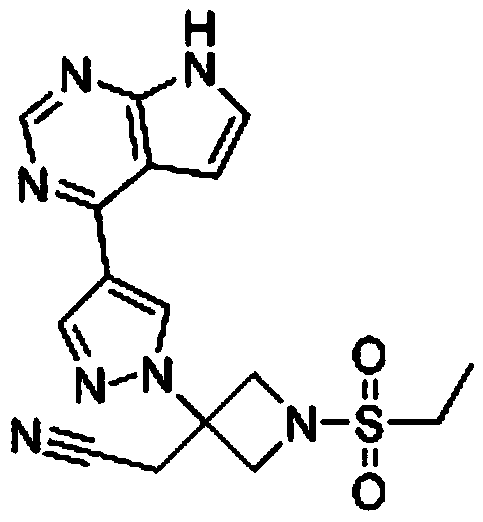
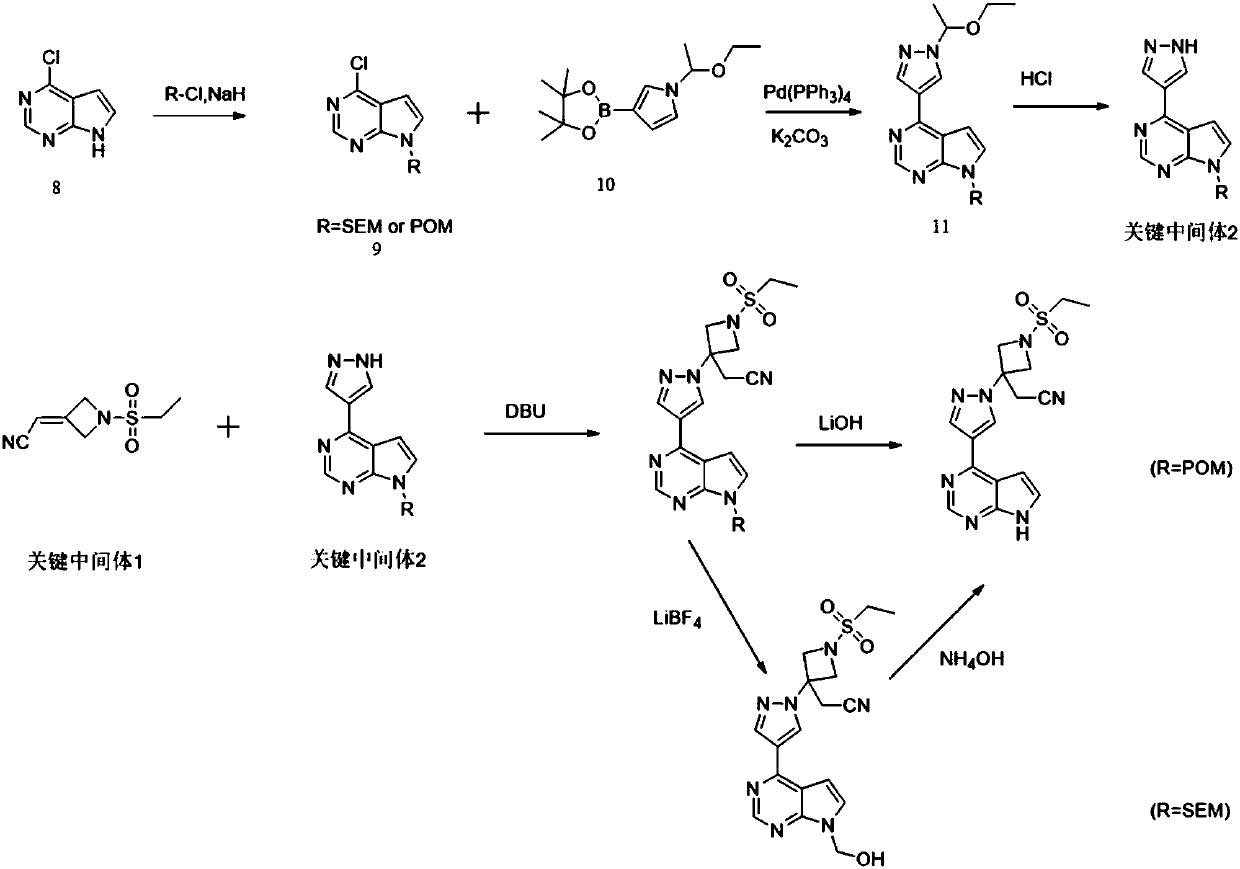
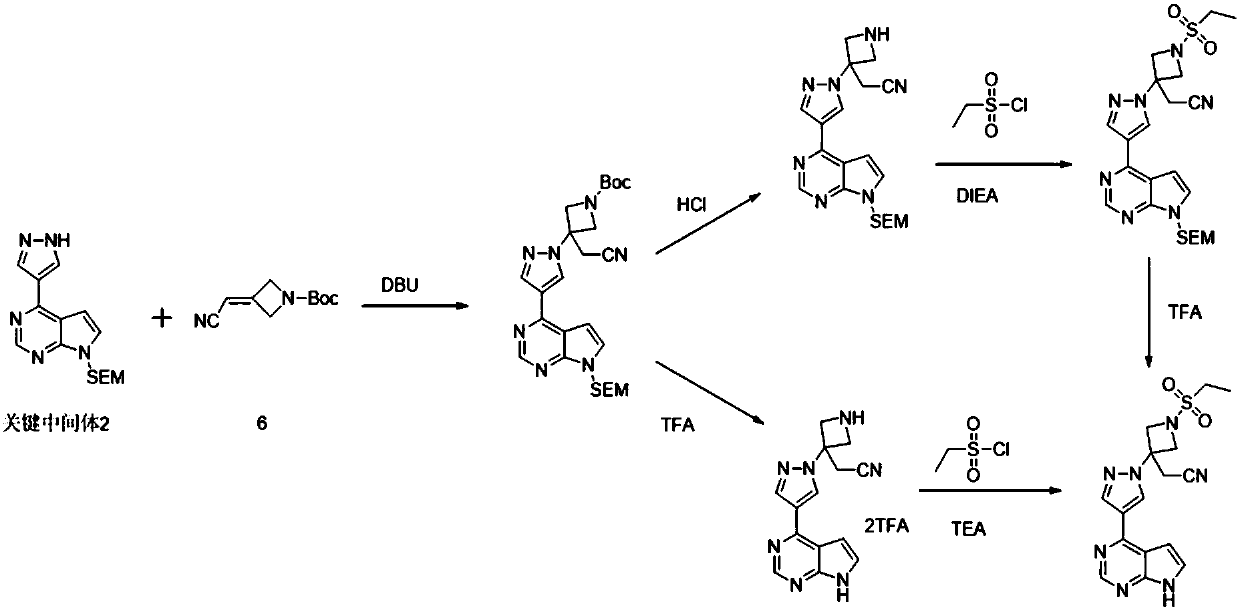
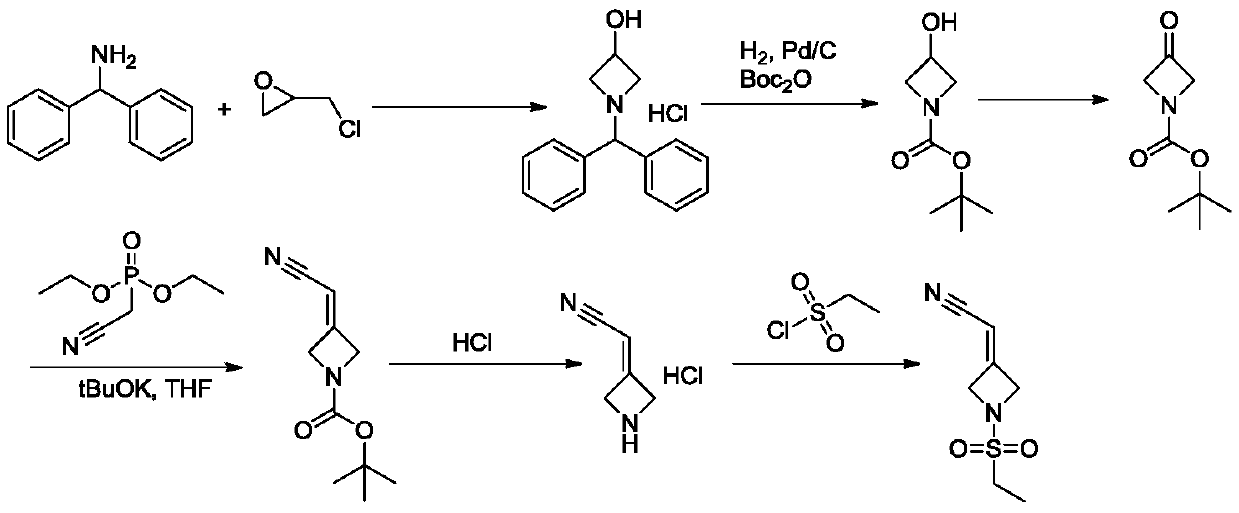
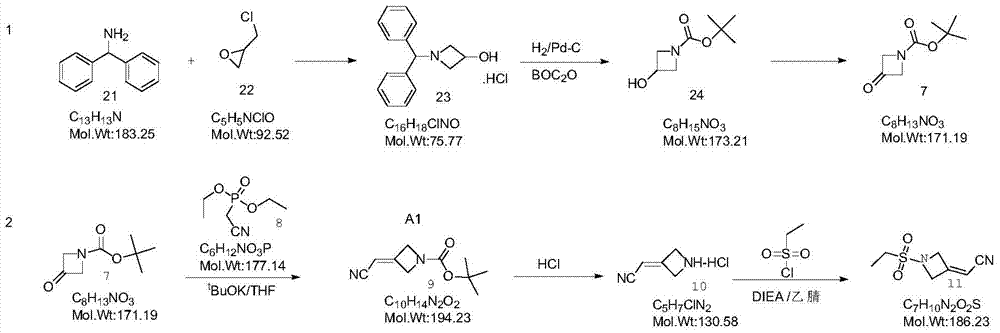
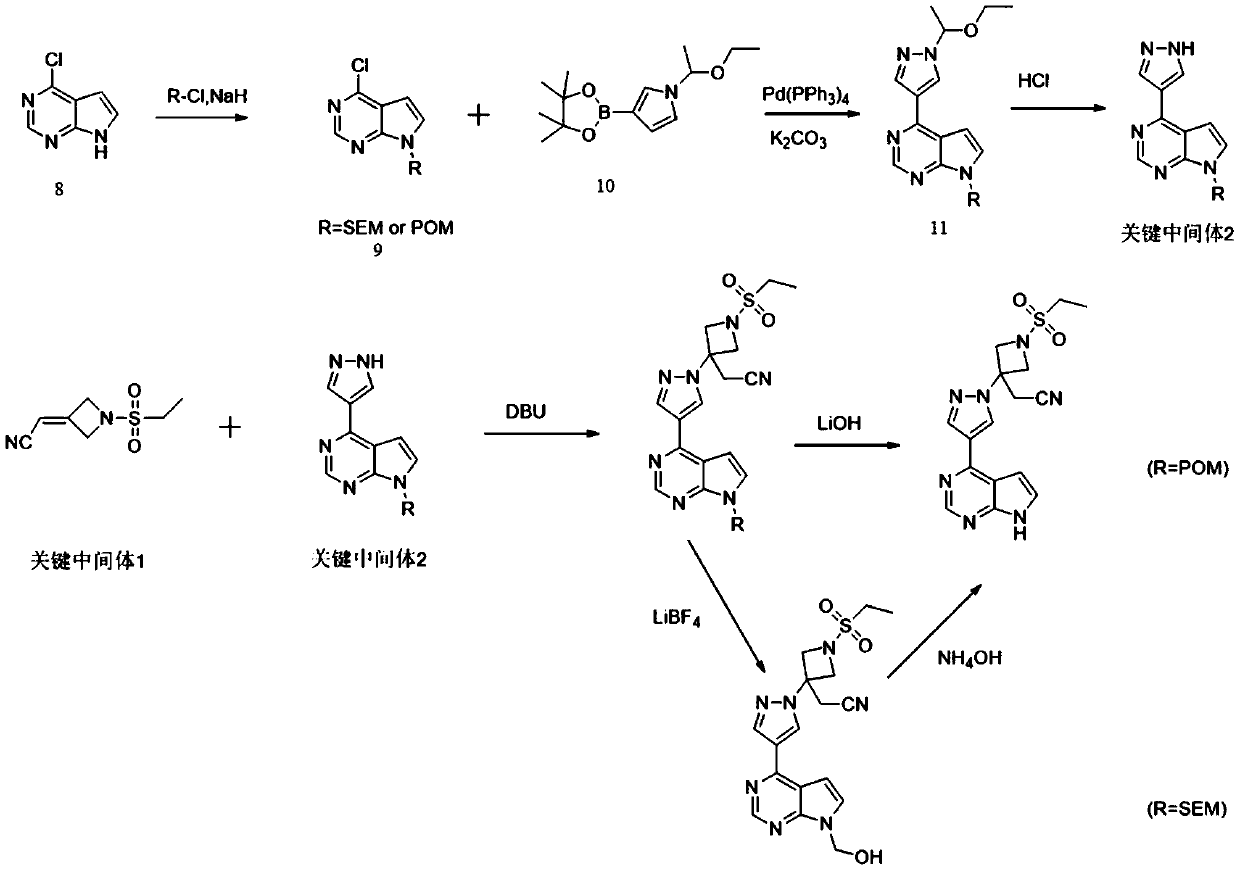
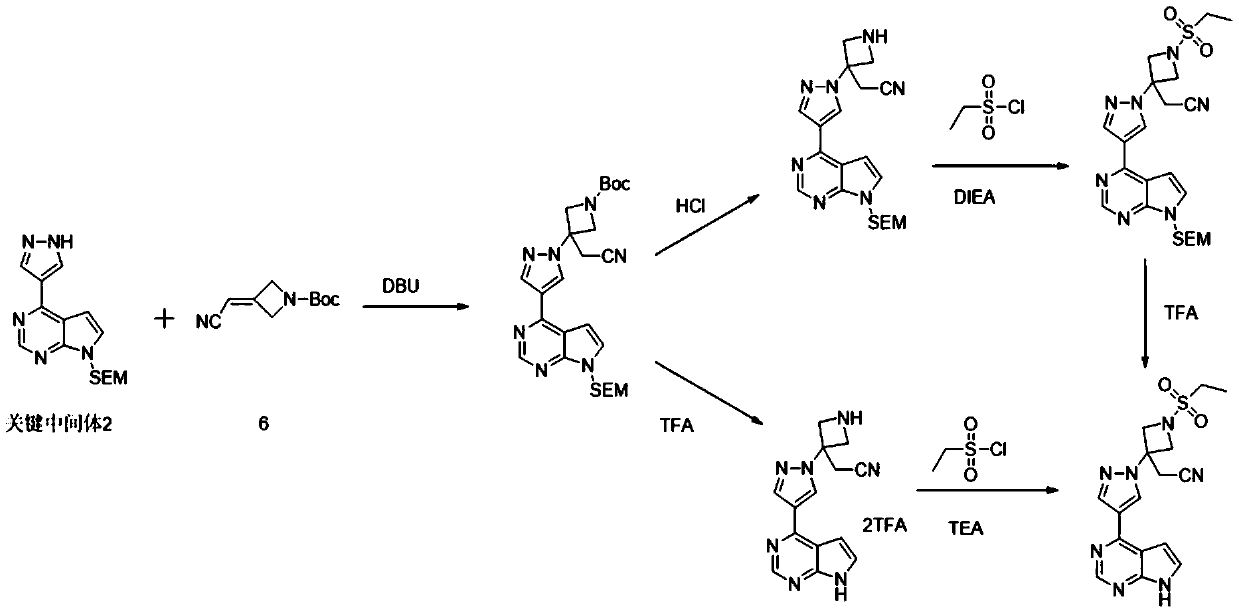
Method for preparing baricitinib
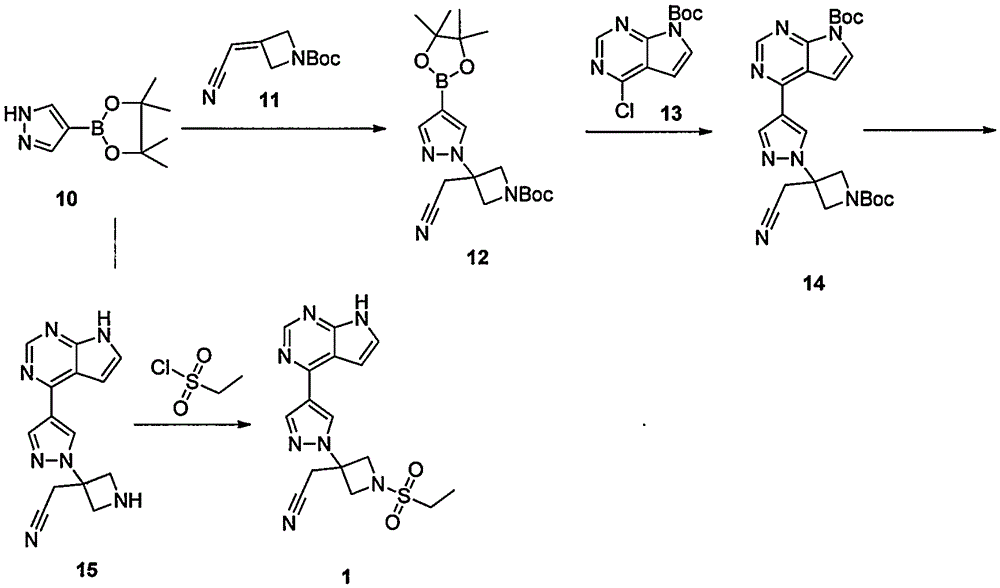
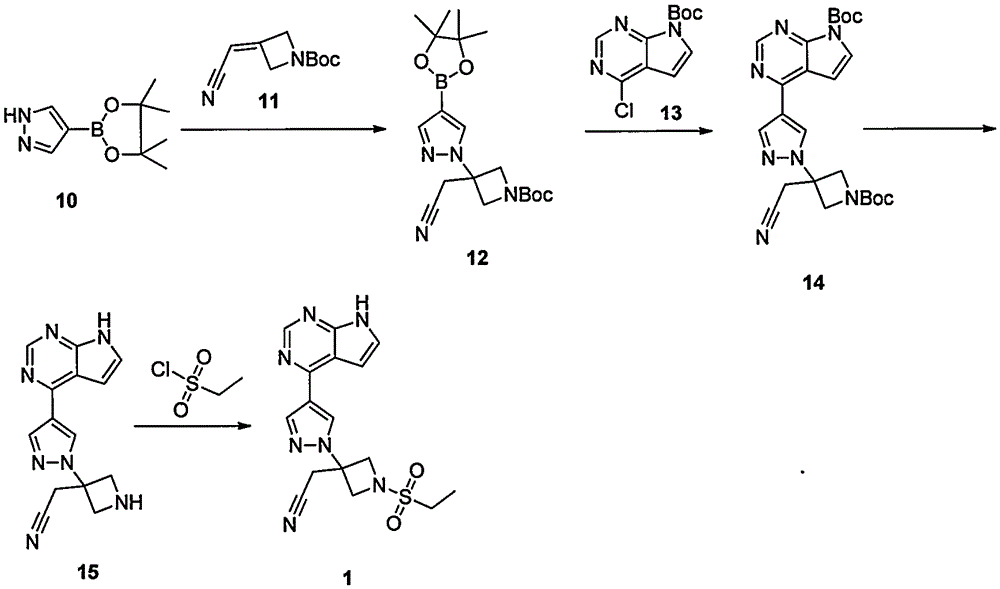
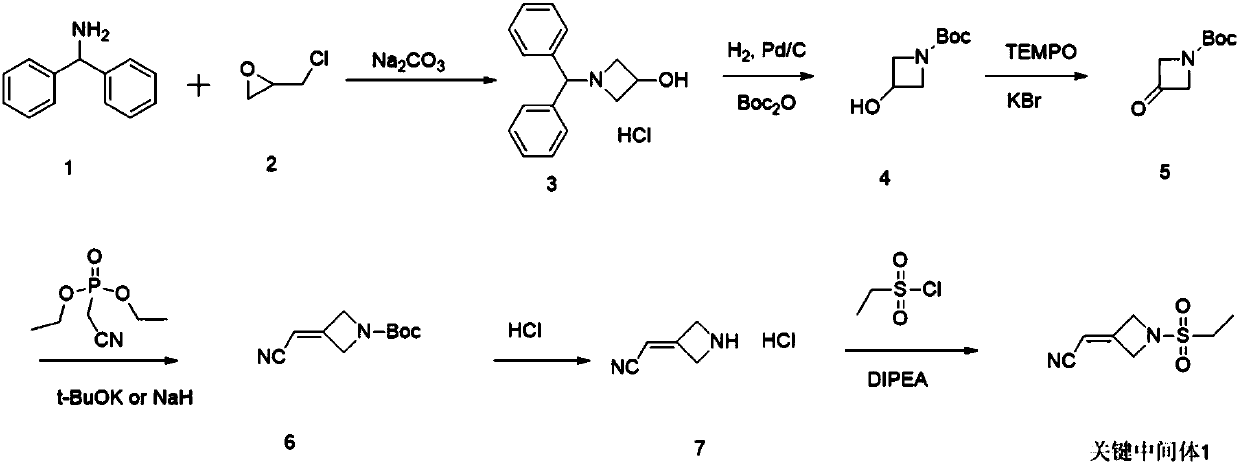
ActiveCN105294699ARaw materials are easy to getSimple processOrganic chemistryCyclobutaneCyanomethylidyne
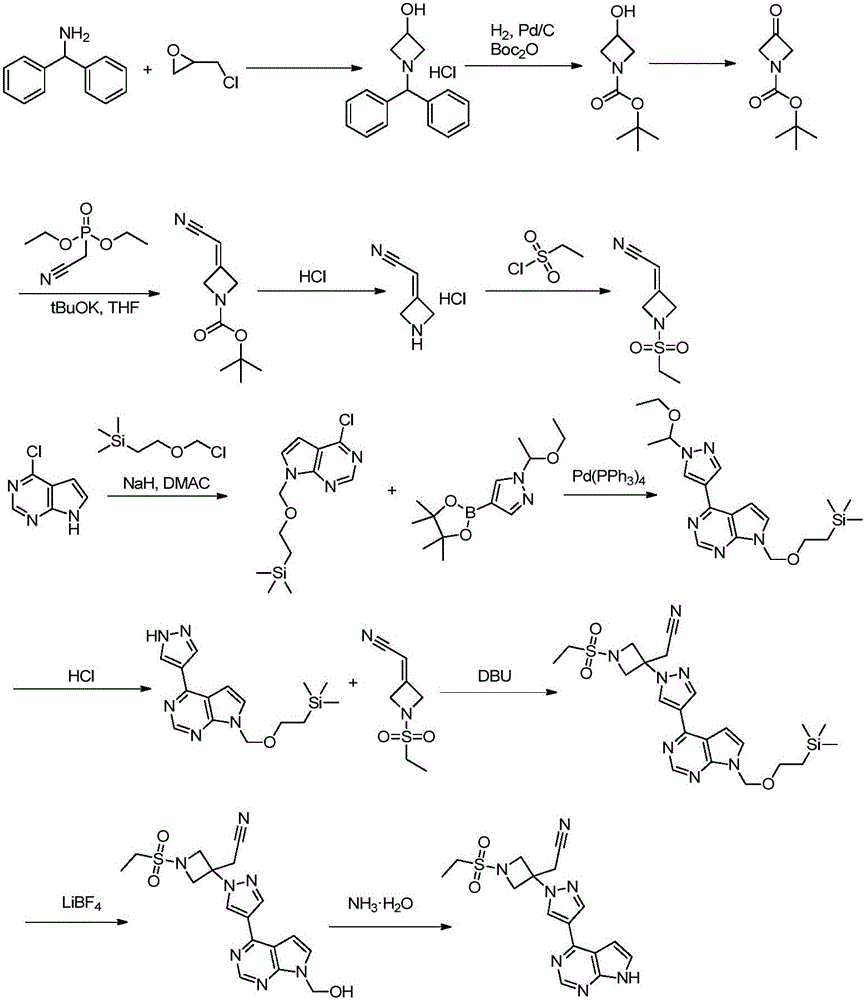
The invention provides a method for preparing baricitinib. The method comprises the following steps: by taking 4-pyrazol boric acid pinacol ester (10) as an initial raw material, performing Michael addition reaction on the initial raw material and 3-(icyanomethylene) azacyclo-cyclobutane-1-tert-butyl formate (11) so as to prepare an intermediate 12, and performing catalytic coupling reaction on 12 and 13, thereby preparing an intermediate 14; removing two-molecule tert-butyl formate of the intermediate 14, thereby preparing an intermediate 15; performing sulfamide reaction on the intermediate 15 and ethanesulfonyl chloride in an organic solvent, thereby obtaining a final product baricitinib (1). The method for preparing baricitinib has the advantages that the raw materials are easy to obtain, the process is simple, the operation is convenient, the reaction yield is high when being compared with that of document records, the atom utilization rate is high, industrial production can be easily achieved, and the like. The reaction general formula is as shown in the specification.
Owner:SHANGHAI XUNHE PHARMA TECH CO LTD
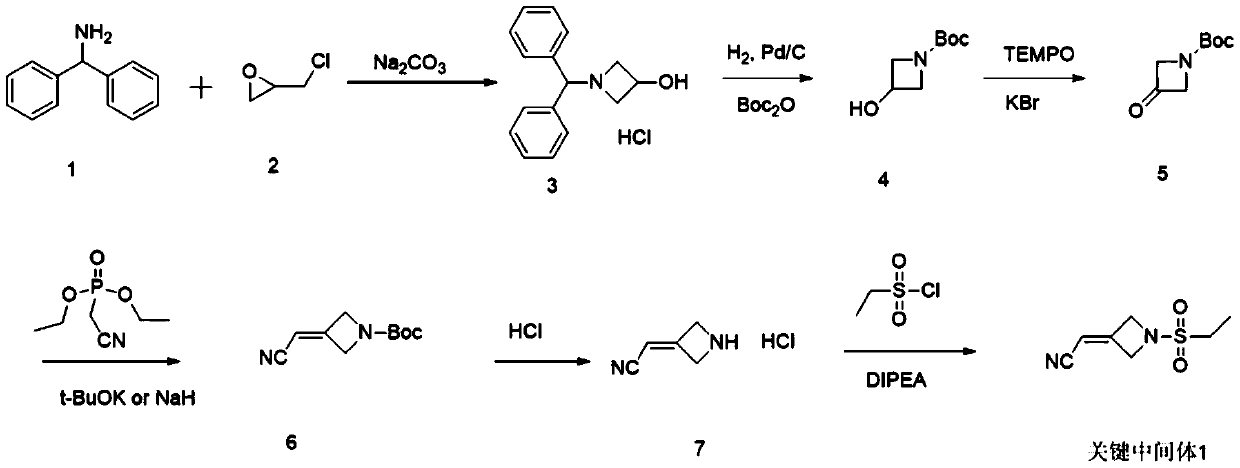
New synthesis methods of JCK inhibitor baricitinib and intermediate thereof
ActiveCN106946917AImprove efficiencyAtom economy is highGroup 3/13 element organic compoundsBulk chemical productionWittig reactionSynthesis methods
The present invention provides a new synthesis method of a baricitinib compound 11. According to the present invention, by using a compound 1 as a staring raw material, the amino group is protected by directly using ethanesulfonyl chloride, and direct cyclization is directly performed by using the effect of an alkali to obtain a key intermediate compound 3 so as to avoid the use of other protection groups and substantially improve the route efficiency and the atomic economy; during the compound 5 preparation, a Wittig reaction is performed by using triphenylphosphine acetonitrile so as to avoid the use of strong alkali and improve the reaction yield; the completely-new neopentyl glycol borate derivative compound 8 has good stability and good crystallinity so as to simplify the separation and purification process; and the route is simple to operate and has the high yield, the purity of the obtained product is high, and the synthesis method is suitable for amplification production. The formulas 1-11 are defined in the specification.
Owner:HANGZHOU CHEMINSPIRE TECH CO LTD
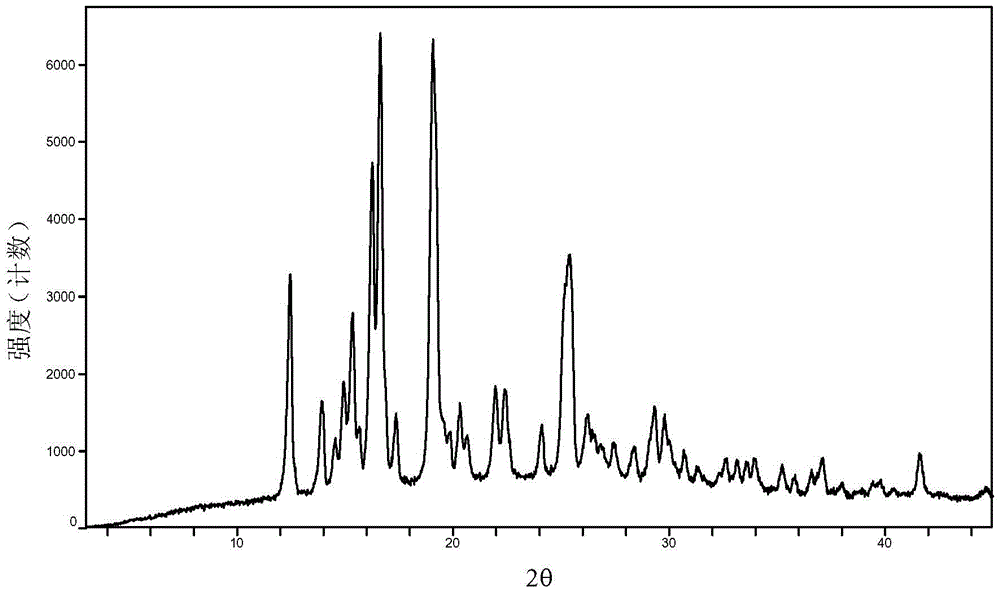
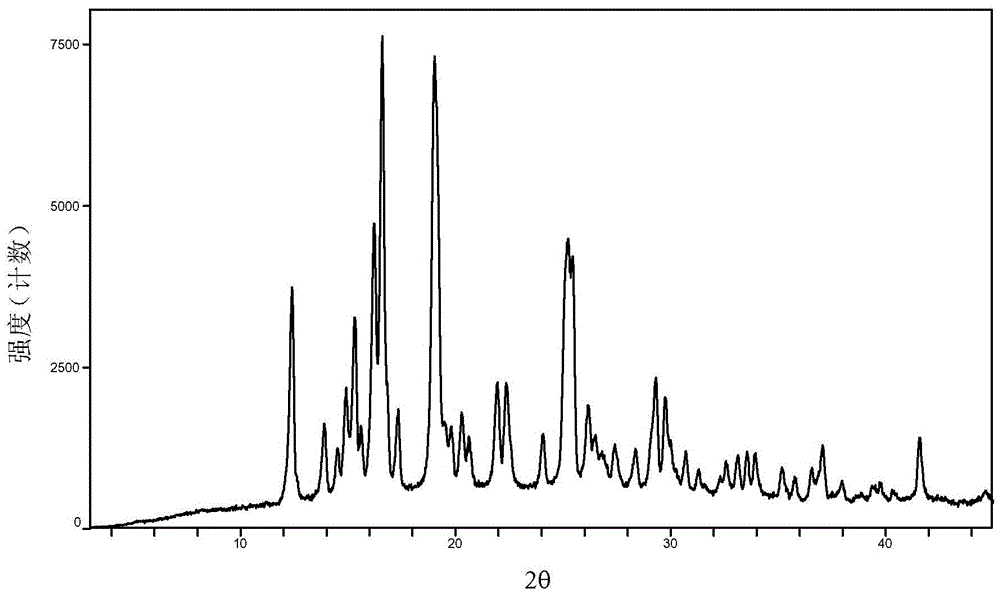
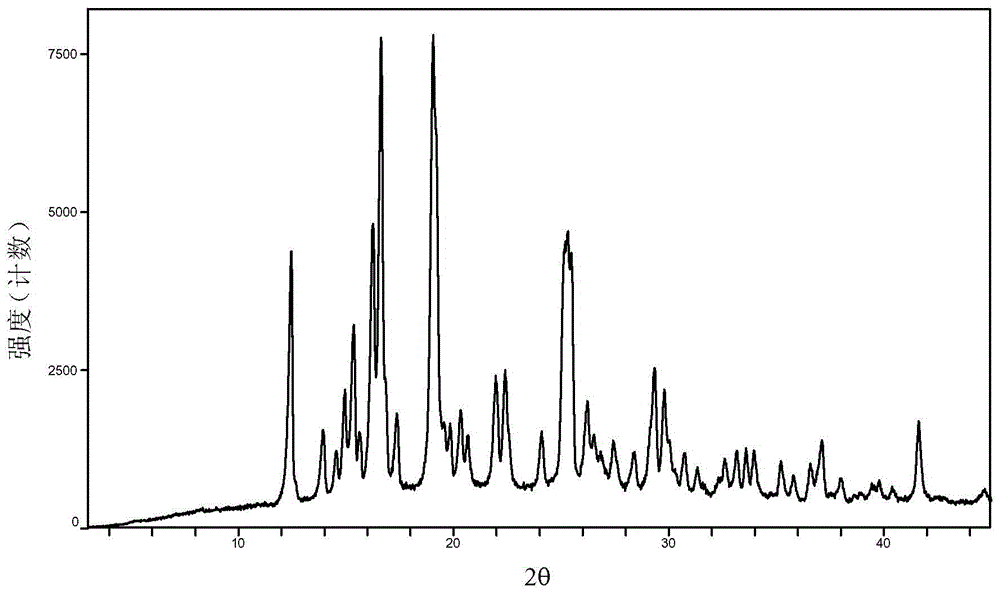
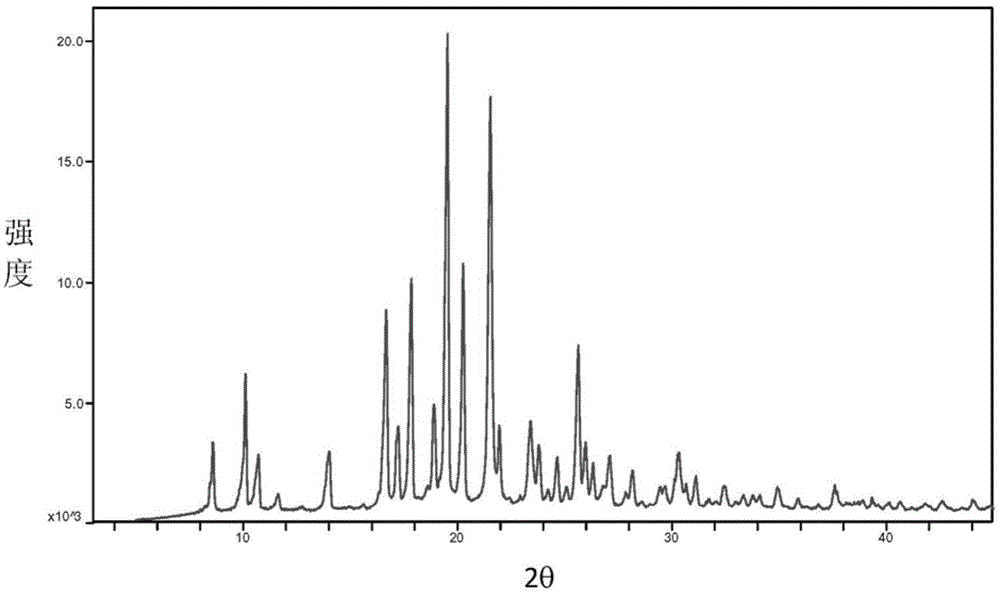
Baricitinib polymorph A and preparation method thereof
InactiveCN105693731AImprove high temperature stabilityImprove stabilityOrganic active ingredientsAntipyreticDiseaseHigh humidity
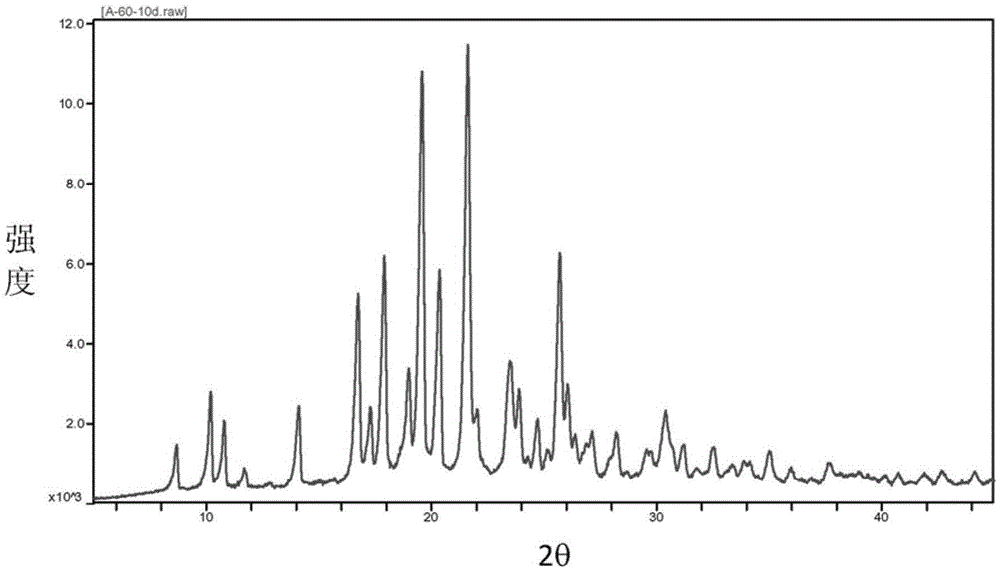
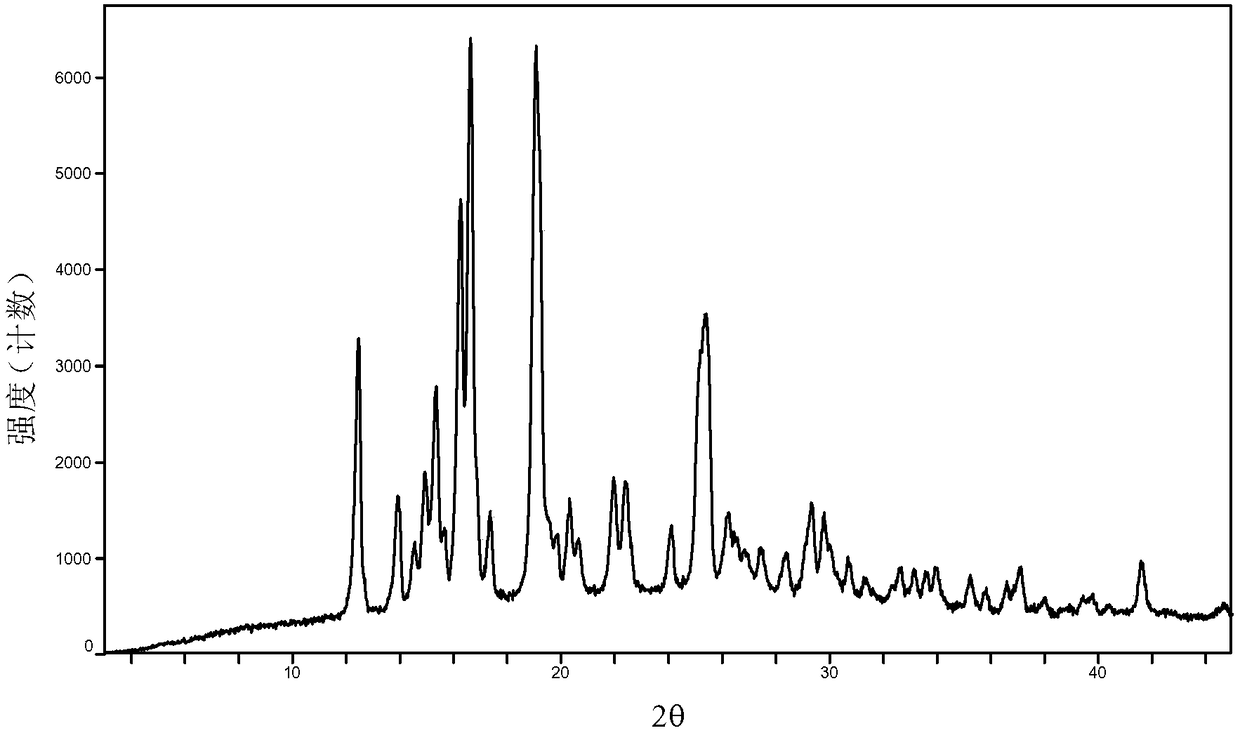
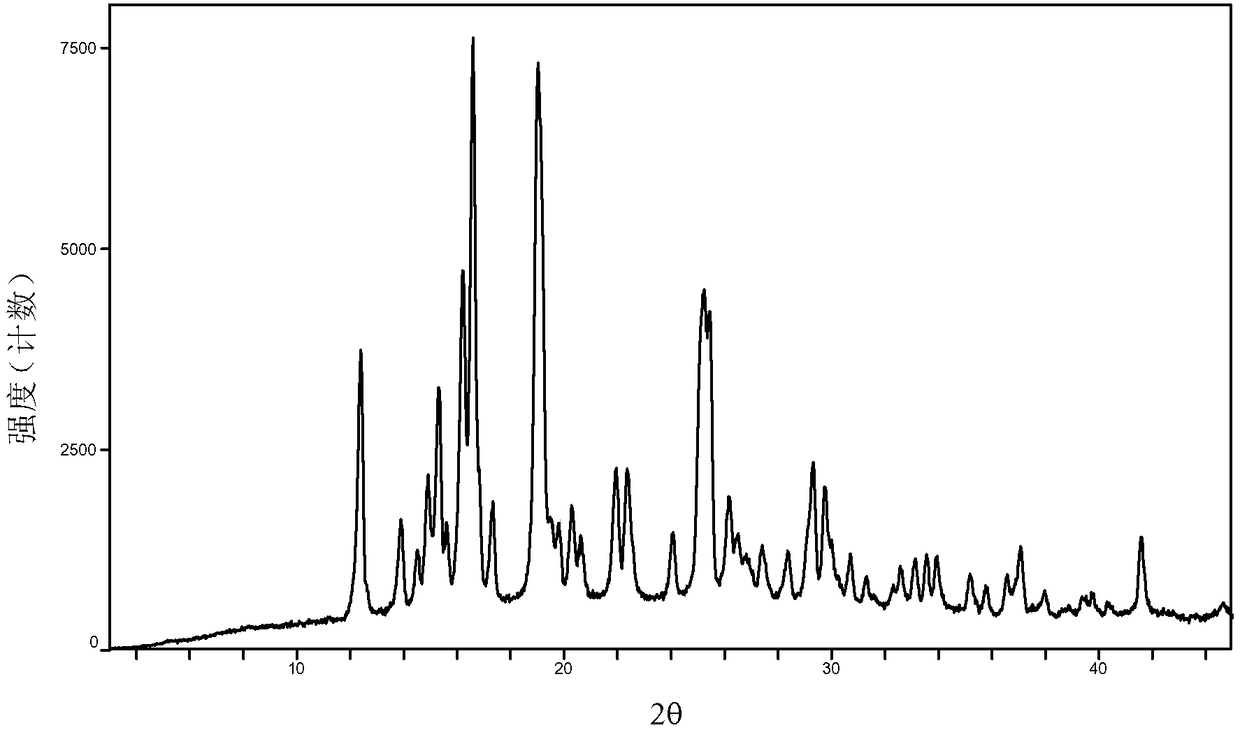
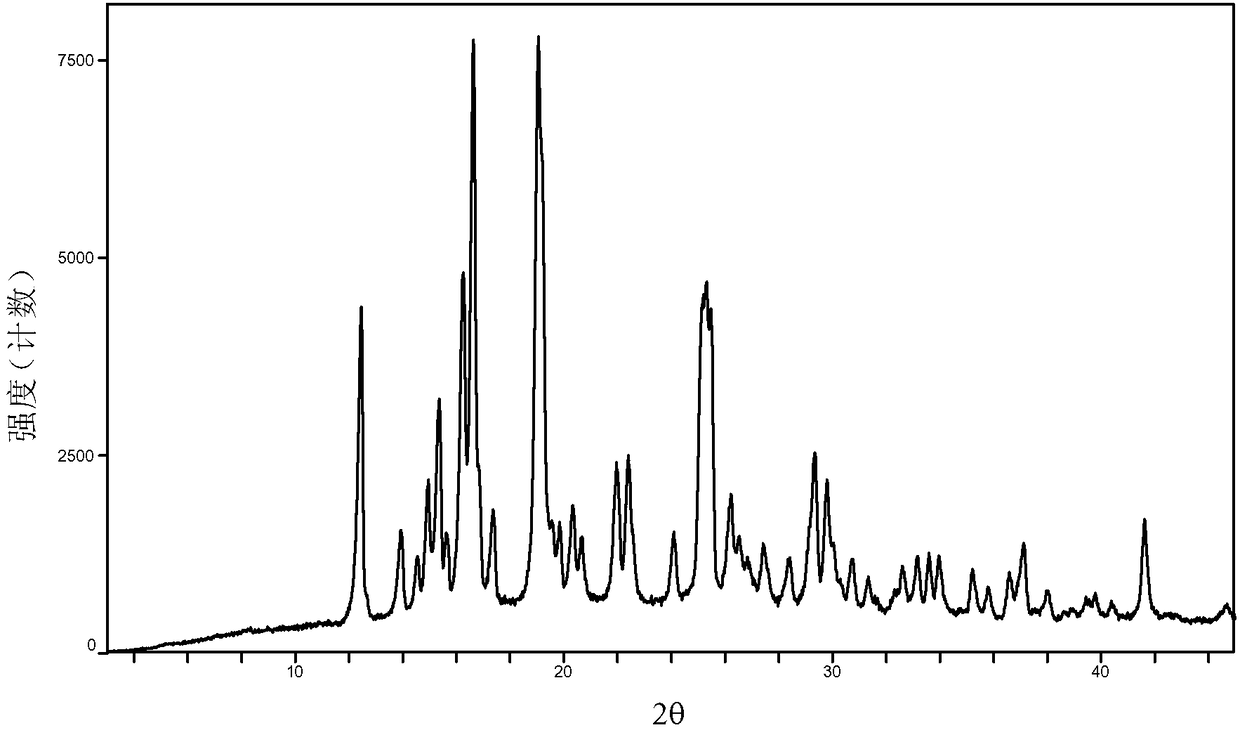
The invention provides a baricitinib polymorph A. The baricitinib polymorph A is characterized in that diffraction peaks are arranged on an XRPD (X Ray Powder Diffraction) map when values of 2 theta are equal to 12.46, 13.921, 14.94, 15.359, 16.26, 16.639, 17.36, 19.08, 20.321, 21.961, 22.381, 24.118, 25.42, 27.441, 28.381, 29.321, 29.799, 32.675, 33.14, 33.563, 33.923 and 41.6, wherein an error range of the values of the 2 theta is + / - 0.2. The baricitinib polymorph A provided by the invention has good high-temperature stability, good high-humidity stability and good illumination stability, can be applied to medicine for treating or preventing diseases related to JAK (Janus Kinase) and has better biological availability; meanwhile, provided qualitative and quantitative information has important significance in further studying the therapeutic effect of such solid medicine.
Owner:SHANGHAI SUNTRONG BIOTECH
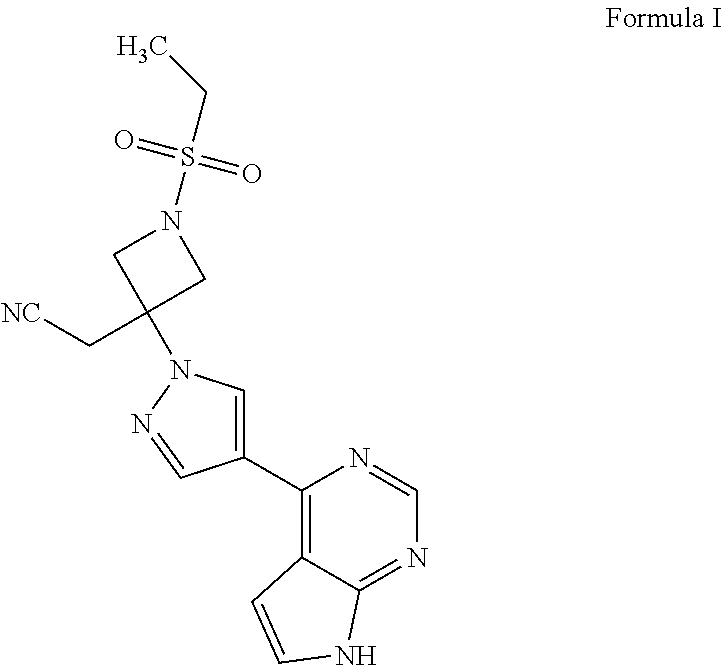
Pyrrolomiazine compound and preparation method and application thereof
ActiveCN106496233AImprove solubilityImprove bioavailabilityOrganic active ingredientsAntipyreticSolubilityCombinatorial chemistry
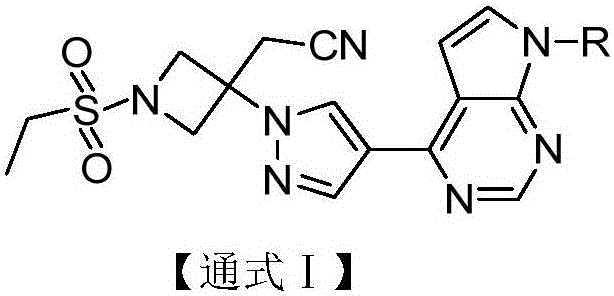
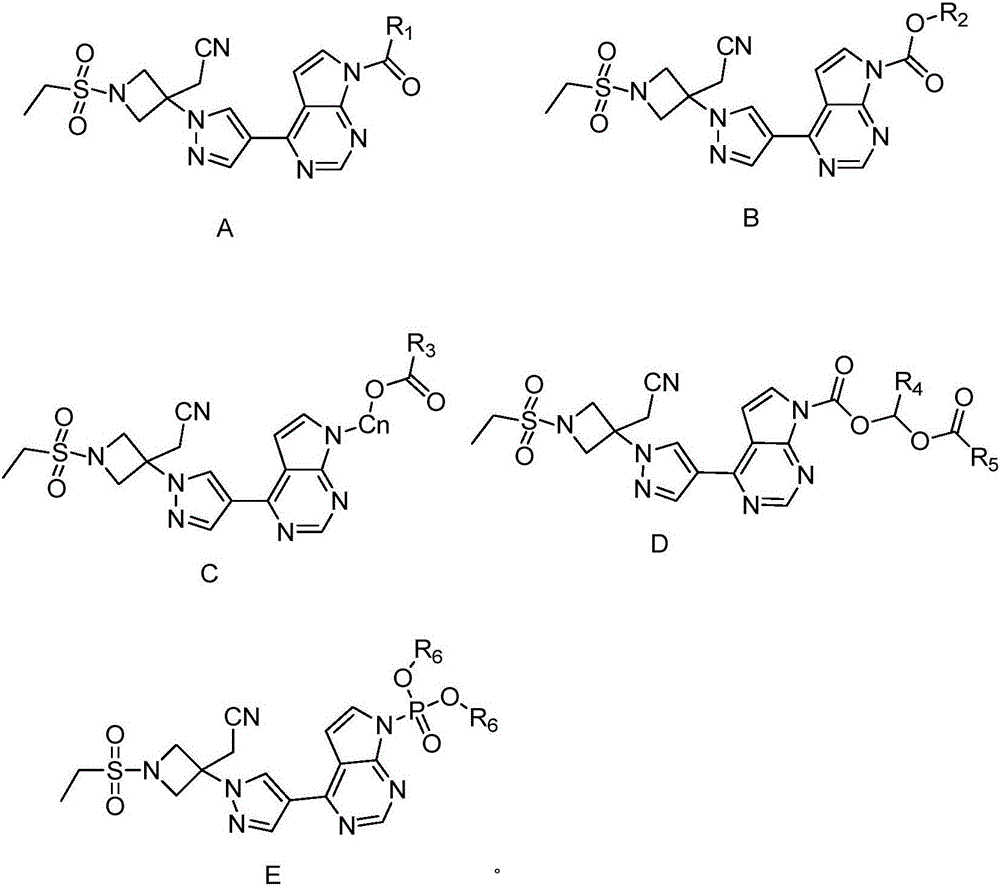
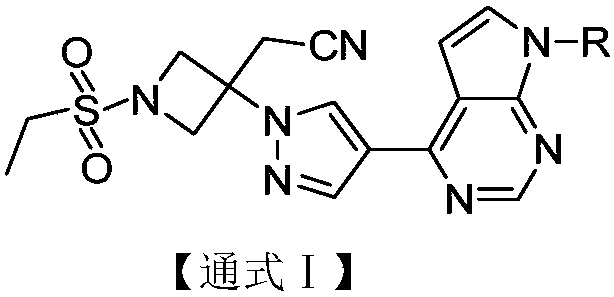
The invention discloses a pyrrolomiazine compound shown as the general formula I (please see the formula in the description) and pharmaceutically-acceptable salt or solvate of the pyrrolomiazine compound, and further discloses preparation methods and application of the pyrrolomiazine compound shown as the general formula I and the pharmaceutically-acceptable salt or solvate of the pyrrolomiazine compound. The prepared pyrrolomiazine compound can be quickly converted into a stock drug baricitinib in plasma and has the better solubility, the higher bioavailability and the enhanced drug efficacy compared with the stock drug.
Owner:SOUTHEAST UNIV
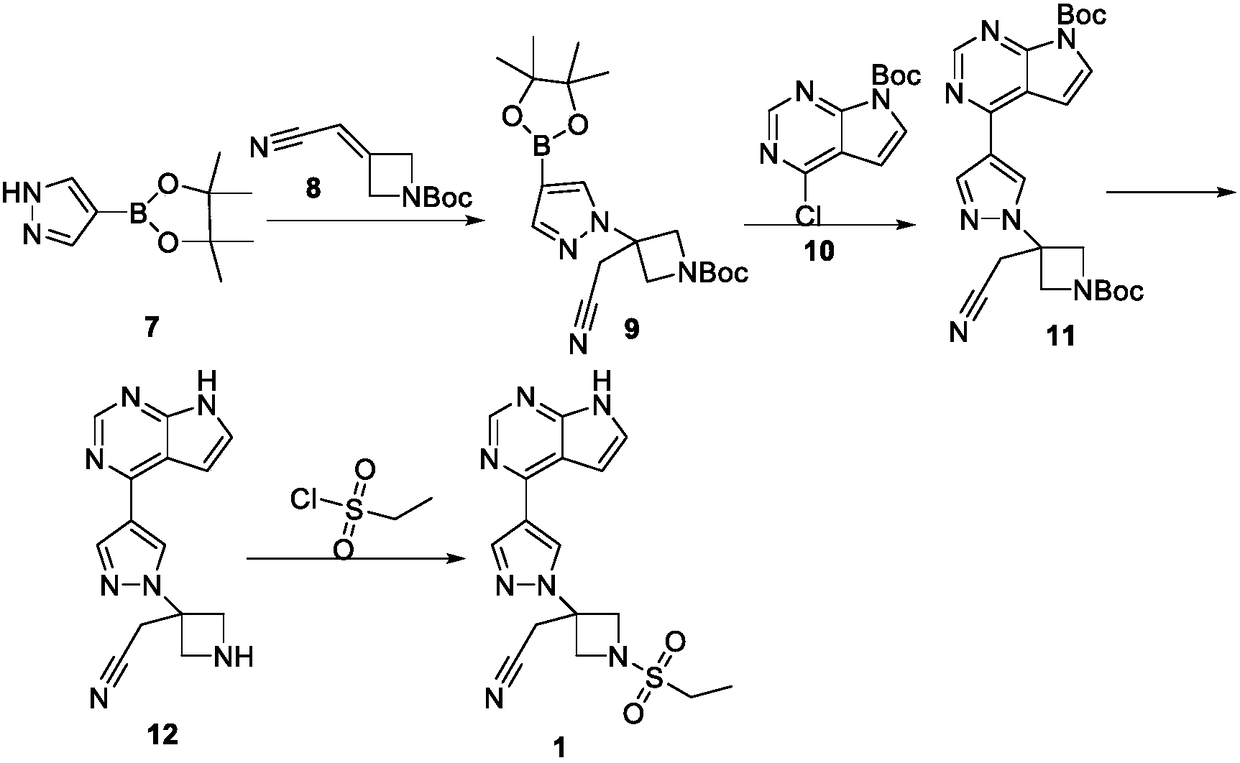
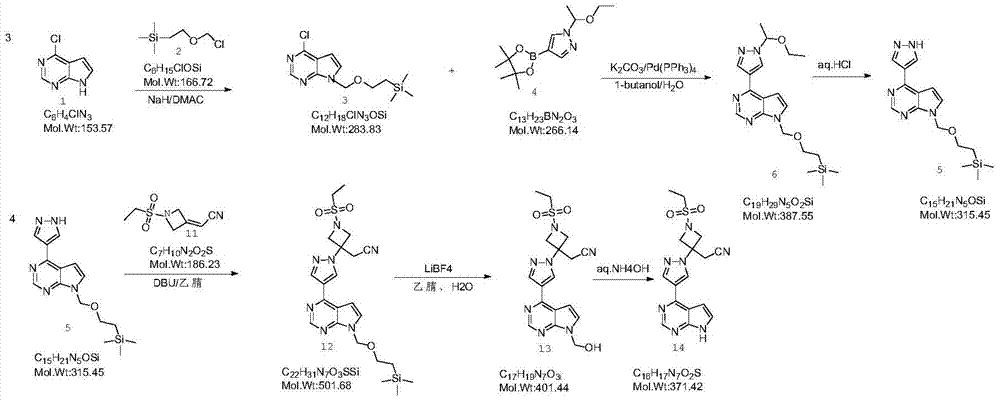
Novel synthetic method for baricitinib and intermediate thereof
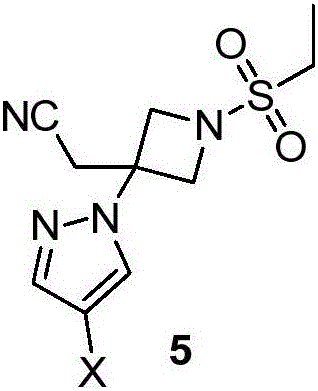
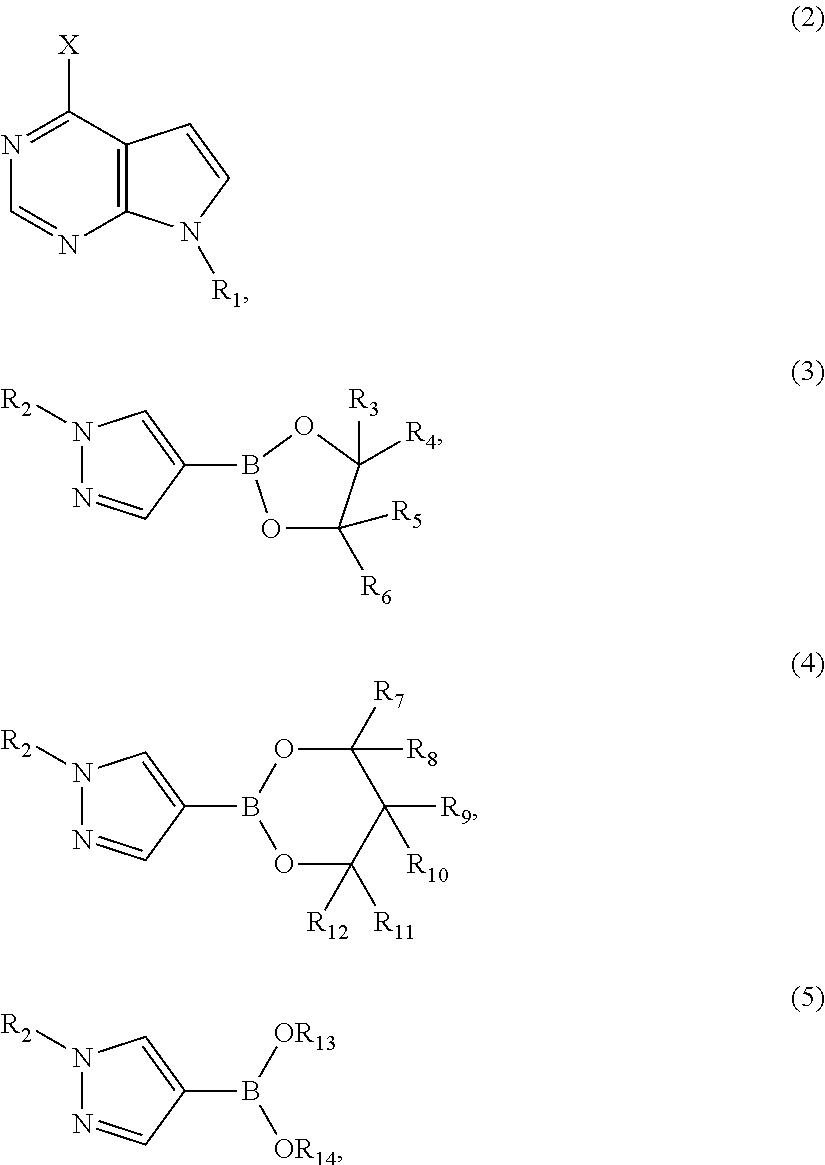
The invention discloses a novel intermediate compound 5 of baricitinib. The compound 5 has stable properties, which is favorable for separation and purification. The invention also discloses a preparation method for the compound 5 and two schemes for preparing baricitinib from the baricitinib. The two schemes are simple in operation, shortened in reaction steps, high in yield and product purity and suitable for enlarged production. The compound 5 has a structural formula as defined in the specification. In the formula, X represents halogen, e.g., chlorine, bromine or iodine.
Owner:HANGZHOU CHEMINSPIRE TECH CO LTD
Preparation method of baricitinib
ActiveCN107176955ARaw materials are easy to getSimple processOrganic chemistrySulfonyl chlorideBoronic acid
The invention discloses a preparation method of baricitinib. The method comprises the following steps: performing a substitution reaction on 4-chloro-7H-pyrrolo[2,3-d]pyrimidine (II) serving as a raw material and benzene sulfonyl chloride in the presence of an alkali to obtain an intermediate III; then, performing a Suzuki coupling reaction on the intermediate III and 4-pyrazole-4-boronic acid pinacol ester in the presence of a palladium catalytic system and an alkali to obtain an intermediate V; then performing a Michael addition reaction on the intermediate V and 3-(cyanomethylene)azetidine-1-tert-butyl formate in the presence of a catalyst to obtain an intermediate VII; then removing Boc protection from the intermediate VII under the action of hydrochloric acid to obtain an intermediate VIII; then performing a sulfoamidate reaction on the intermediate VIII and ethyl sulfonyl chloride in an organic solvent in the presence of an alkali to obtain an intermediate IX; lastly, removing benzenesulfonyl protection from the intermediate IX under the action of tetramethylammonium fluoride or tetrabutylammonium fluoride or a trihydrate of the tetramethylammonium fluoride or the tetrabutylammonium fluoride to obtain baricitinib (I). Compared with the prior art, the method has the advantages of adoption of readily-available raw materials, low cost, high product yield and easiness for industrial production.
Owner:NANJING YOKO PHARMA +2
Preparation method of baricitinib
InactiveCN108129482ALow costAvoid participating in the reactionOrganic chemistryBulk chemical productionDiethyl phosphatePurification methods
The invention discloses a preparation method of baricitinib and belongs to the technical field of drug preparation. The method comprises that 4-chloropyrrolopyrimidine as a starting raw material is subjected to amino protection, the product, hydrazine hydrate and acrolein undergo a one-pot displacement reaction and a cyclization reaction to produce an intermediate 4, a starting raw material 1, 3-dibromoacetone and ethylene glycol undergo a condensation reaction to produce an intermediate 5, the intermediate 5 and ethyl sulfonamide undergo a condensation reaction to produce an intermediate 6, the intermediate 6 and diethyl cyanomethylphosphonate undergo a reaction under action of a strong base to produce an intermediate 7, the intermediate 4 and the intermediate 7 undergo an addition reaction under the action of a catalyst, and the product undergoes a deprotection reaction to produce a desired product 1. The preparation method needs mild reaction conditions. The intermediate purification method is simple and easy, has a total yield of 40-55% and is suitable for industrial production.
Owner:JIANGSU ZHONGBANG PHARMA
Baricitinib intermediate, method for forming Baricitinib intermediate, and method for preparing Baricitinib or pharmaceutically acceptable salt thereof
The present disclosure provides a Baricitinib intermediate, a method for preparing the Baricitinib intermediate, and a method for preparing Baricitinib or a pharmaceutically acceptable salt thereof using the Baricitinib intermediate. The method for preparing the Baricitinib intermediate involves the use of a divalent palladium catalyst or a nickel catalyst and provides the Baricitinib intermediate in high yield.
Owner:FORMOSA LAB
Impurities of baricitinib and preparation and detection methods thereof
PendingCN108976233AGuaranteed safety and reliabilityHigh yieldOrganic active ingredientsOrganic chemistryQuality controlImpurity
The invention belongs to the field of drug synthesis, and in particular relates to three impurities of baricitinib: impurities A, B and C, a preparation method, structural characterization and a detection method of the three impurities and an application of controlling purity of a baricitinib raw material or a preparation as an impurity reference substance as well. The invention can provide a standard reference substance for quality control and clinical medication safety detection of baricitinib, so that the clinical medication safety and reliability can be guaranteed.
Owner:NANJING YOUKE BIOLOGICAL MEDICAL RES +3
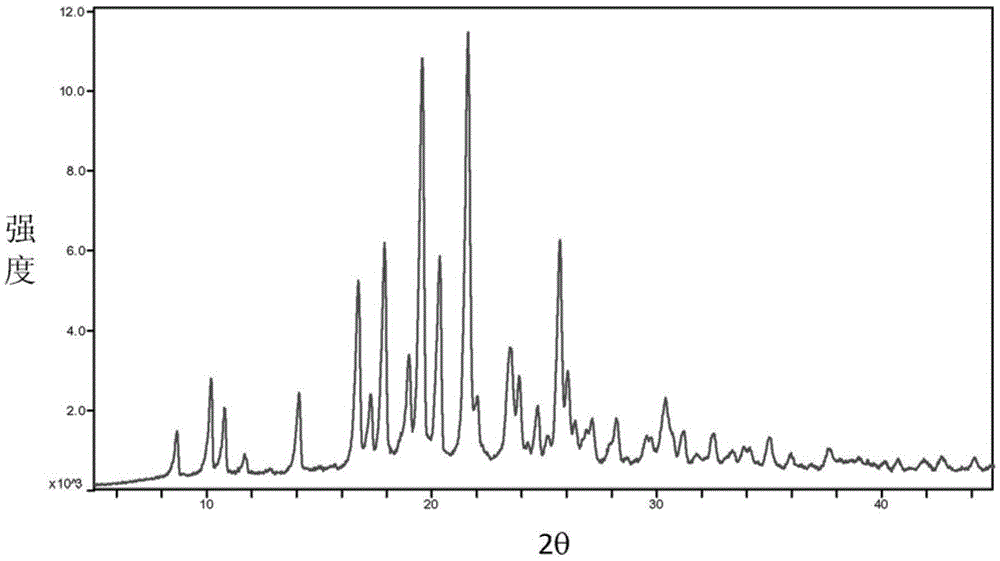
Baricitinib trifluoroacetate crystal forms A and B and preparation method thereof
ActiveCN105566332AEasy to processImprove stabilityOrganic chemistry methodsTreatment effectTrifluoroacetic acid
The invention provides Baricitinib trifluoroacetate crystal forms A and B in the formula (I) (please see the formula in the description). The XRPD atlas of the crystal form A has diffraction peaks at the positions representing that 2theta equals to 8.60, 10.12, 10.72, 14.02, 16.66, 17.24, 17.84, 18.90, 19.54, 20.26, 21.54, 21.94, 23.40, 23.78, 24.66, 25.64, 25.98, 26.32, 27.12, 28.16, 30.34 and 31.12, and the XRPD atlas of the crystal form B has diffraction peaks at the positions representing that 2theta equals to 5.34, 9.00, 10.08, 10.70, 16.12, 17.44, 17.80, 18.92, 19.48, 20.22, 21.56, 22.68, 23.42, 23.98, 25.84, 27.22, 28.76, 29.46 and 32.66, wherein the error range of 2theta is + / -0.2. The stability of the Baricitinib trifluoroacetate crystal forms A and B is better than that of a Baricitinib trifluoroacetate semi-crystal, and the medicine processing of the crystal forms A and B and the application of the crystal forms A and B in a medicine composition are facilitated. The Baricitinib trifluoroacetate crystal forms A and B can be applied in medicine for treating immunity diseases, inflammatory diseases or cancers; meanwhile, qualitative and quantitative information is provided, and important significance is achieved for further studying the treatment effect of such type of solid medicine.
Owner:SHANGHAI SUNTRONG BIOTECH
Drug composition containing baricitinib and preparation method and application of drug composition
ActiveCN107334738AImprove mixing uniformityEasy to operateOrganic active ingredientsAntipyreticMANNITOL/SORBITOLFiller Excipient
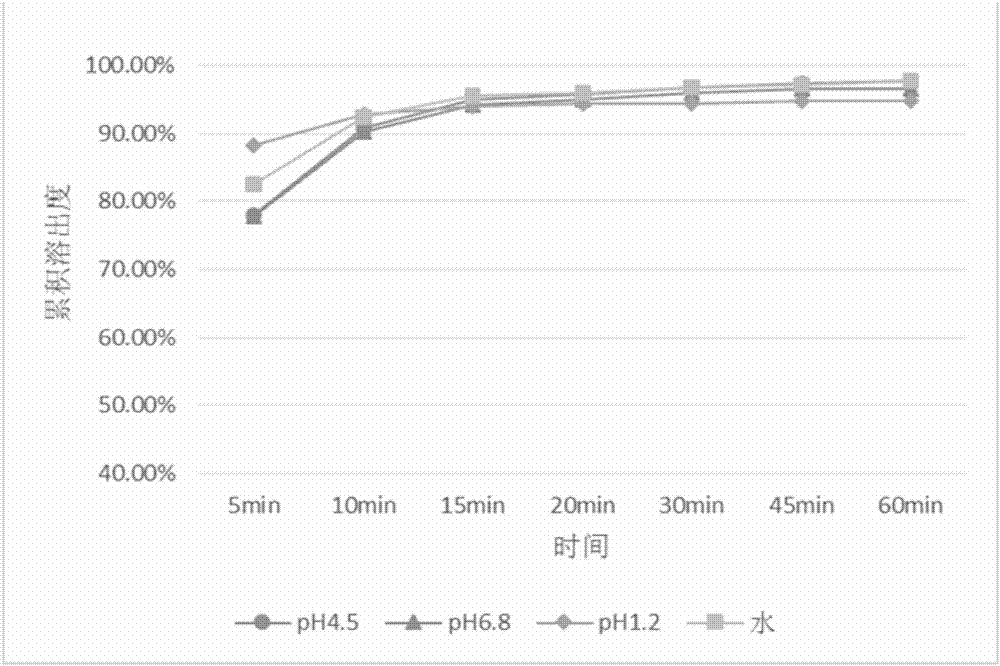
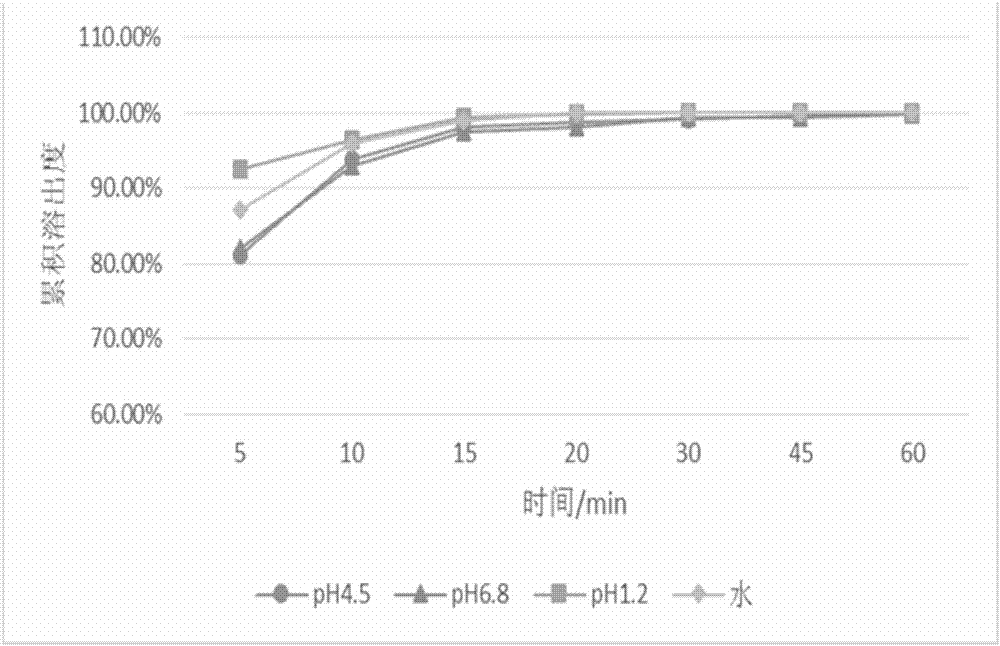
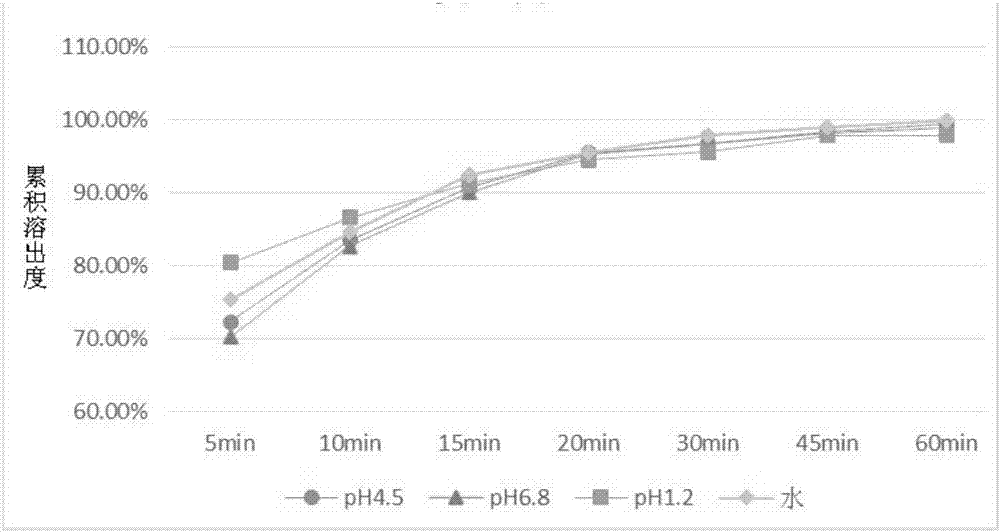
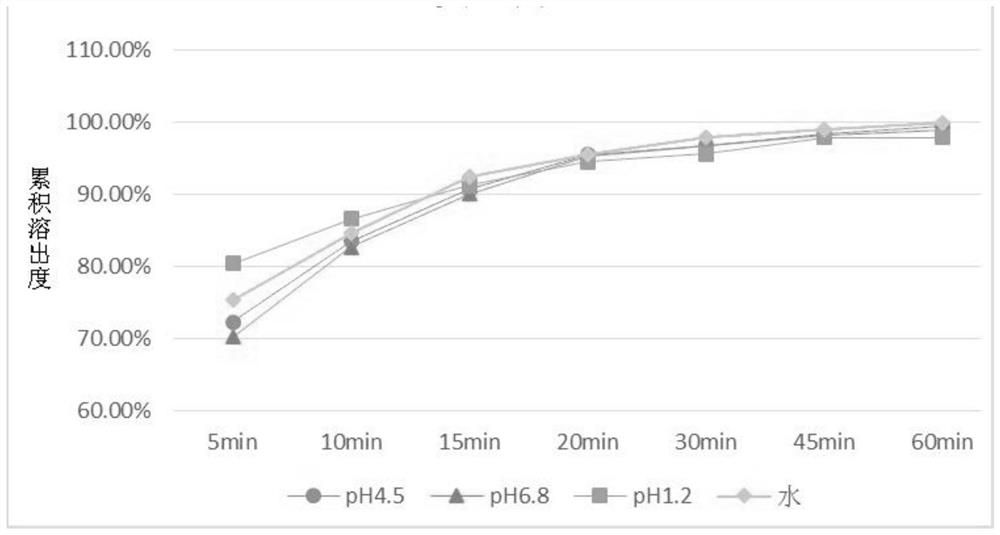
The invention discloses a drug composition containing baricitinib and a preparation method and application of the drug composition. The drug composition comprises the baricitinib and an auxiliary material which can be received pharmaceutically, wherein the auxiliary material comprises a filling agent and a disintegrating agent. The preparation method of the drug composition comprises the steps that the filling agent and the disintegrating agent are evenly mixed, so that the mixed auxiliary material is obtained; and then a baricitinib crude drug and the mixed auxiliary material are evenly mixed or pelletizing liquor containing the baricitinib crude drug is evenly mixed with the mixed auxiliary material, and drug-carrying pellets of the solid drug composition containing the baricitinib are obtained through pelletizing. According to the drug composition containing the baricitinib and the preparation method and application of the drug composition, the solid drug composition which can be dissolved out rapidly in vitro and contains the baricitinib is prepared by controlling the particle size of the baricitinib, using microcrystalline cellulose, mannitol and the like in the hydrophilic auxiliary material as the filling agent, and using croscarmellose sodium and the like as the disintegrating agent; and the preparation technique is simple and suitable for industrialization.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Preparation method for Baricitinib intermediate
ActiveCN106554363AShort reaction pathShort reaction timeGroup 4/14 element organic compoundsGroup 3/13 element organic compoundsBorideChemical industry
The invention belongs to the field of pharmaceutical and chemical industry, and particularly relates to a preparation method for a Baricitinib intermediate. The preparation method is characterized in that a key intermediate 2-[1-ethyl sulfonyl-3-[4-(4,4,5,5- tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-parazole-1-ethyl] azacyclobutyl-3-yl) acetonitrile is prepared through reaction of a 4-parazole boride and 2-[1-(ethyl sulfonyl-3-azacyclobutaneylidene) acetonitrile, and nitrogen on a pyrazolone ring of 4- parazole boride does not need to be protected, so that a follow-up step of removing a corresponding a protective group is avoided, and therefore, the whole reaction route is short, raw materials are obtained easier, the production cost is low, and the preparation method is especially suitable for industrial production.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Method for preparing baricitinib
ActiveCN108586465ALow costAvoid participating in the reactionOrganic chemistryBulk chemical productionDiethyl phosphatePurification methods
The invention discloses a method for preparing baricitinib and belongs to the technical field of drug preparation. The method comprises the following steps: taking chloro-1H-pyrrolopyrimidine as an initial raw material, performing amino protection, performing 'one-pot' substitution with hydrazine hydrate and acraldehyde, and performing ring closure to obtain an intermediate 4; performing condensation on an initial raw material 1,3-dibromoacetone and ethylene glycol so as to obtain an intermediate 5, performing condensation on the intermediate 5 and ethanesulfonamide to obtain an intermediate 6, reacting the intermediate 6 and diethyl cyanomethylphosphonate in the presence of strong base so as to an intermediate 7, carrying out an addition reaction on the intermediate 4 and the intermediate7 in the presence of a catalyst, and performing deprotection, so as to obtain multiple target products 1. The process is mild in reaction conditions and simple and feasible in intermediate purification method, reaches the total yield of 40-55%, and is suitable for industrialized production.
Owner:JIANGSU ZHONGBANG PHARMA
Application of Baricitinib in preparation of medicament for treating senile calcific heart valve disease
InactiveCN110327346ALower the targetGood conditionOrganic active ingredientsCardiovascular disorderDiseaseHeart valvular disease
The invention relates to new application of a medicament, in particular to application of Baricitinib in preparation of a medicament for treating senile calcific heart valve disease. The research of the invention shows that: by inhibiting a JAK pathway and selectively activating a substrate-STAT of the Baricitinib, the substrate-STAT is translocated to the nucleus so as to act on downstream targetgenes such as cyclin D1, BCL-xL, C-MYC, P21 WAF1 / CIP1 and the like, and the cell proliferation, differentiation and apoptosis is regulated, so that degeneration, fibrosis, thickening and calcification of heart valve connective tissues of a patient are obviously improved, and cardiomyocytes are protected. Compared with the prior art, the medicament can reduce various indexes of patients with thesenile calcific heart valve disease, improve the disease condition and improve the life quality of the patients with the senile calcific heart valve disease.
Owner:HUISHENG MEDICAL TECH XUZHOU CO LTD
Baricitinib intermediate, method for forming baricitinib intermediate, and method for preparing baricitinib or pharmaceutically acceptable salt thereof
ActiveUS20190202834A1Economical to useEasy to shapeOrganic chemistryNickel catalystPalladium catalyst
The present disclosure provides a Baricitinib intermediate, a method for preparing the Baricitinib intermediate, and a method for preparing Baricitinib or a pharmaceutically acceptable salt thereof using the Baricitinib intermediate. The method for preparing the Baricitinib intermediate involves the use of a divalent palladium catalyst or a nickel catalyst and provides the Baricitinib intermediate in high yield.
Owner:FORMOSA LAB
Preparation method for synthesizing key intermediate 1 of Baricitinib
ActiveCN107739328AReduce manufacturing costHigh yieldOrganic chemistryCombinatorial chemistryMethoxypropane
The invention relates to a preparation method for synthesizing a key intermediate 1 of Baricitinib. The preparation method comprises the following steps: generating ring closing reaction by virtue of1,3-dibromo-2,2-dimethoxypropane (SM) and ethyl sulfonamide (SM2) under an alkaline condition, and carrying out acetal deprotection under an acidic condition, so as to obtain an intermediate B; and generating witting reaction by virtue of the obtained intermediate B and diethyl cyanomethylphosphonate under the alkaline condition, so as to obtain the key intermediate 1. According to the preparationmethod, the ring closing reaction is generated by virtue of the commercial materials SM and ethyl sulfonamide under the alkaline condition, acetal deprotection is carried out under the acidic condition so as to obtain intermediate B, and witting reaction is carried out so as to obtain the key intermediate 1; the key intermediate 1 can be actually obtained only through two synthetic steps; and compared with the prior art, the preparation method has the advantages that the synthetic route is greatly shortened, the hydrogenation is avoided, and the production cost of the key intermediate 1 is lowered.
Owner:海化生命(厦门)科技有限公司
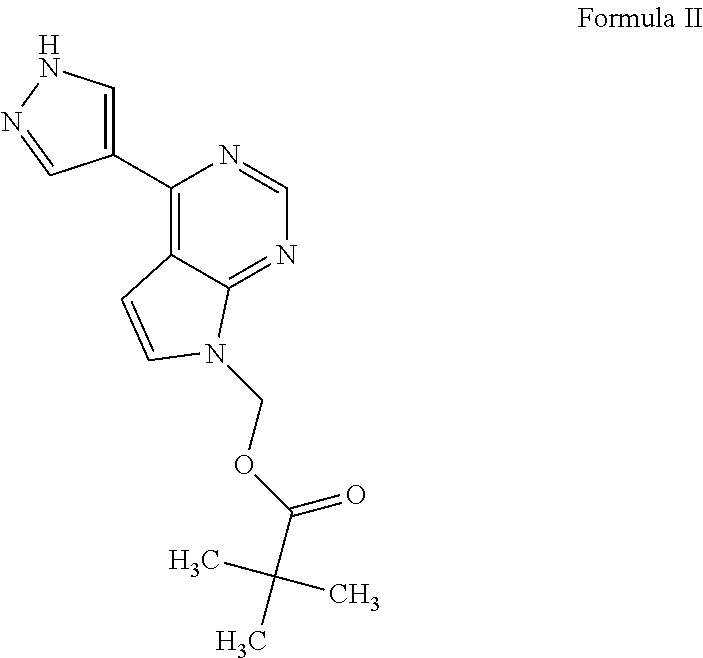
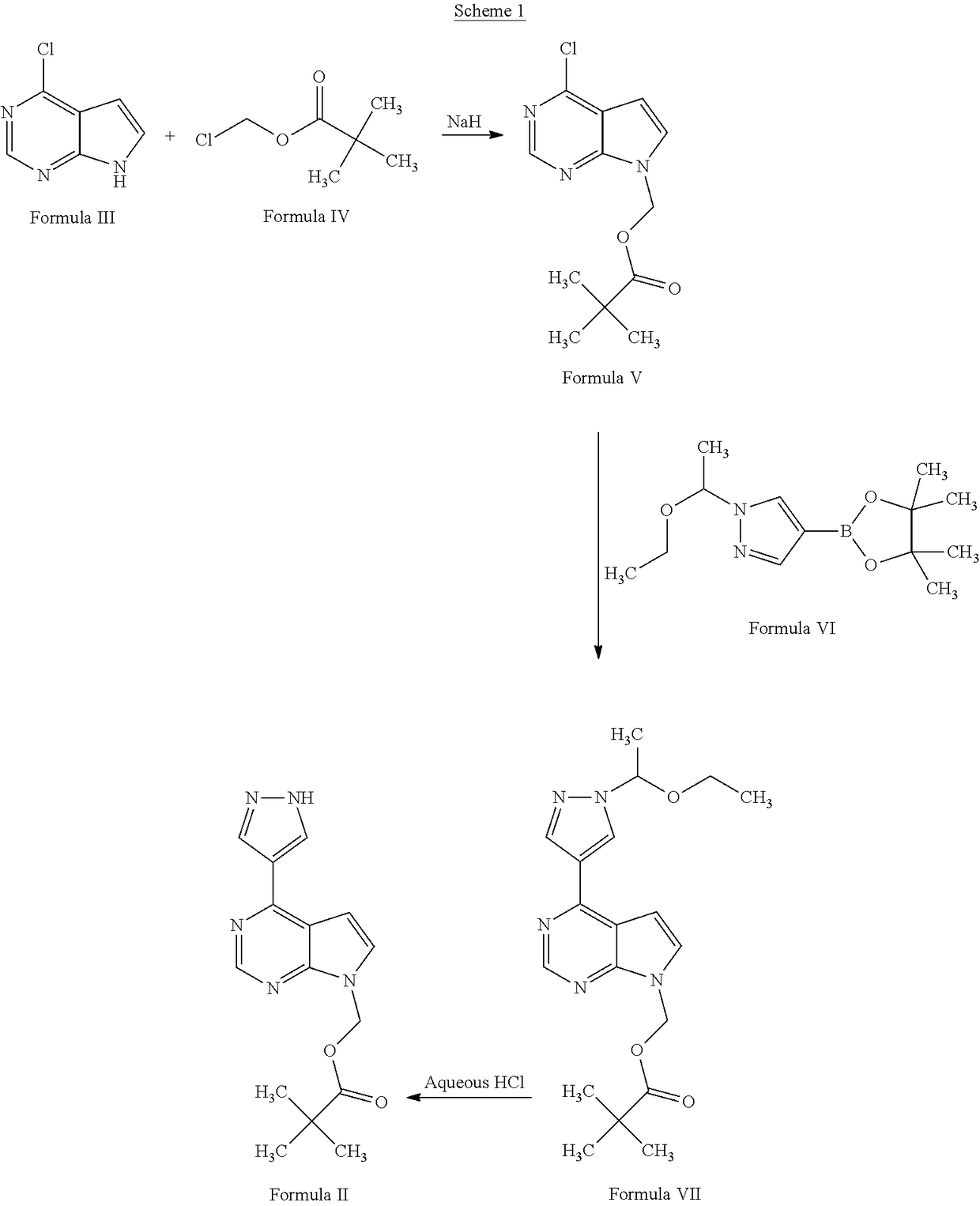
Process for the preparation of baricitinib and an intermediate thereof
The present invention provides a process for the preparation of baricitinib and an intermediate thereof. The present invention provides a convenient, economical, and industrially advantageous two-step process for the preparation of [4-(IH-pyrazol-4-yl)-7Hpyrrolo[2,3-d] pyrimidin-7-yl]methyl pivalate of Formula (II). The process of the present invention involves the use of an alkali or alkaline earth metal hydroxide, carbonate, or bicarbonate as a base for reacting 4-chloro-7H-pyrrolo[2,3-d]pyrimidine of Formula (III) with chloromethyl pivalate of Formula (IV), and the use of an unprotected pyrazole borolane of Formula (VIII) for the conversion of (4-chloro-7H-pyrrolo[2,3-d] pyrimidin-7-yl)methyl 2,2-dimethylpropanoate of Formula V into [4-(1H-pyrazol-4-yl)-7Hpyrrolo[2,3-d]pyrimidin-7-yl]methyl pivalate of Formula (II). The process of the present invention provides [4-(1H-pyrazol-4-yl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl]methyl pivalate of Formula (I) in high yield.
Owner:SUN PHARMA INDS
Pyrrolopyrimidine compounds, their preparation method and use
ActiveCN106496233BImprove solubilityImprove bioavailabilityOrganic active ingredientsAntipyreticSolubilityCombinatorial chemistry
The invention discloses a pyrrolomiazine compound shown as the general formula I (please see the formula in the description) and pharmaceutically-acceptable salt or solvate of the pyrrolomiazine compound, and further discloses preparation methods and application of the pyrrolomiazine compound shown as the general formula I and the pharmaceutically-acceptable salt or solvate of the pyrrolomiazine compound. The prepared pyrrolomiazine compound can be quickly converted into a stock drug baricitinib in plasma and has the better solubility, the higher bioavailability and the enhanced drug efficacy compared with the stock drug.
Owner:SOUTHEAST UNIV
Baricitinib cream and preparation method and application thereof
PendingCN112168774AMeet quality requirementsGood spreadabilityOrganic active ingredientsAntipyreticPolyethylene glycolDrugs preparations
The invention belongs to the field of pharmaceutical preparations, and particularly relates to a Baricitinib cream and a preparation method and application thereof. The Baricitinib cream comprises Baricitinib or pharmaceutically acceptable salt thereof and polyethylene glycol-7 stearate. In the preparation process of the Baricitinib cream, the Baricitinib cream which is fine and smooth, has the particle size meeting the quality requirement of Chinese pharmacopoeia for the cream and is good in coating performance can be prepared through low-speed stirring; and the cream has better high-temperature-resistant stability, and oil-water separation is not prone to occurring. Therefore, the Baricitinib cream is more suitable for industrial production and better in stability.
Owner:南京康川济医药科技有限公司
A pharmaceutical composition containing baricitinib and its preparation method and application
ActiveCN107334738BImprove bioavailabilityRapid dissolutionOrganic active ingredientsAntipyreticCarboxymethyl cellulosePharmaceutical medicine
The invention discloses a pharmaceutical composition containing baricitinib and its preparation method and application. The pharmaceutical composition comprises baricitinib and pharmaceutically acceptable auxiliary materials, wherein the auxiliary materials include fillers and disintegrants. The preparation method of the pharmaceutical composition comprises: uniformly mixing a filler and a disintegrant to obtain mixed excipients; uniformly mixing the baricitinib bulk drug with the mixed excipients or granulating the baricitinib bulk drug The liquid is uniformly mixed with the mixing excipients, and the drug-loaded granules of the solid pharmaceutical composition containing baricitinib are obtained by granulation. The present invention controls the particle size of baricitinib, uses hydrophilic auxiliary materials microcrystalline cellulose, mannitol, etc. The solid pharmaceutical composition containing baricitinib has a simple preparation process and is suitable for industrialization.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
New application of baricitinib in treatment of psychological abnormality and obesity
The invention relates to a novel application of baricitinib in treatment of psychological abnormality and obesity, in particular to application of baricitinib in preparation of a medicine used for treating psychological abnormality diseases and the obesity. The medicine comprises the baricitinib, at least one medicine carrier and at least one non-medicine carrier. The medicine comprises external auditory canal suspension medicine application, and the external auditory canal suspension medicine application is characterized in that the medicine disclosed by the invention is implanted into microporous slow-rebound sponge earplugs, then, the earplugs are plugged into external auditory canals of double ears, and the medicine is applied through ear canal wall ear hair cells and auditory tubes by medicine information induction or odor wave transmission, toxic and side effects of the medicine can be reduced, and safety is improved.
Owner:杨孟君
Method for preparing spherical baricitinib crystal
InactiveCN109384792AImprove liquidityNo stickinessOrganic chemistry methodsFiltrationCrystallization
The invention relates to a method for preparing a spherical baricitinib crystal. The method for preparing the spherical baricitinib crystal comprises the following steps: adding baricitinib into water, heating to 40-50 DEG C, dissolving, and filtering; keeping the filtrate at a temperature range of 5-15 DEG C, adding 95% ethanol which is 0.2-0.3 time of the volume of filtrate obtained at the firststep while stirring, culturing a crystal for 1-2.5 hours at a constant temperature, and stirring; continuously stirring, cooling to subzero 5 to 0 DEG C, continuously culturing the crystal for 1.5-2hours at the constant temperature, and stirring; carrying out suction filtration, washing filtrate cakes, and drying at 50-60 DEG C. By adopting the technical scheme of the invention, the spherical baricitinib crystal is obtained, and the spherical baricitinib crystal has very good flowability and is free of adhesion in the pressing process.
Owner:WEIHAI GUANBIAO INFORMATION TECH
A new synthetic method of JAK inhibitor baricitinib and its intermediate
ActiveCN106946917BImprove efficiencyAtom economy is highGroup 3/13 element organic compoundsBulk chemical productionWittig reactionSynthesis methods
The present invention provides a new synthesis method of a baricitinib compound 11. According to the present invention, by using a compound 1 as a staring raw material, the amino group is protected by directly using ethanesulfonyl chloride, and direct cyclization is directly performed by using the effect of an alkali to obtain a key intermediate compound 3 so as to avoid the use of other protection groups and substantially improve the route efficiency and the atomic economy; during the compound 5 preparation, a Wittig reaction is performed by using triphenylphosphine acetonitrile so as to avoid the use of strong alkali and improve the reaction yield; the completely-new neopentyl glycol borate derivative compound 8 has good stability and good crystallinity so as to simplify the separation and purification process; and the route is simple to operate and has the high yield, the purity of the obtained product is high, and the synthesis method is suitable for amplification production. The formulas 1-11 are defined in the specification.
Owner:HANGZHOU CHEMINSPIRE TECH CO LTD
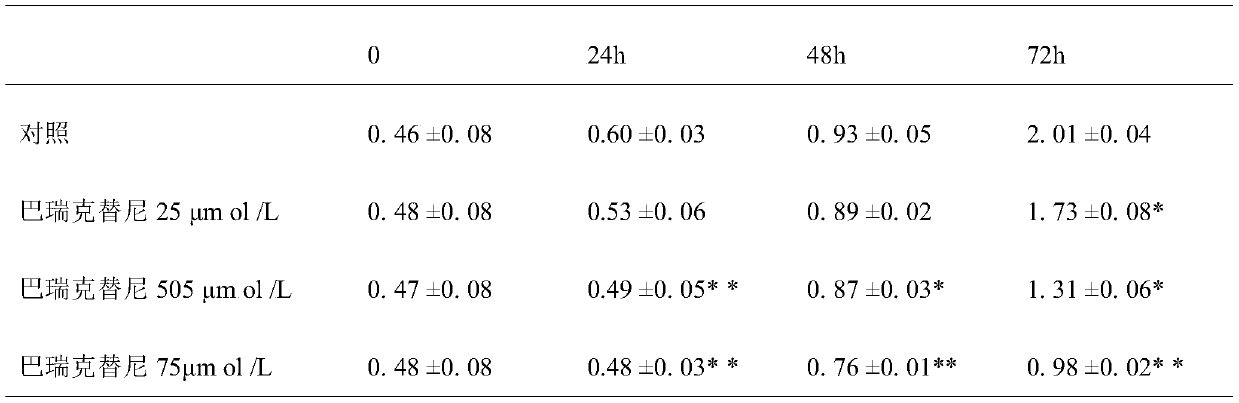
Novel drug application of baricitinib
ActiveCN112587530APromote proliferationOrganic active ingredientsSkeletal/connective tissue cellsPharmaceutical drugPharmaceutical medicine
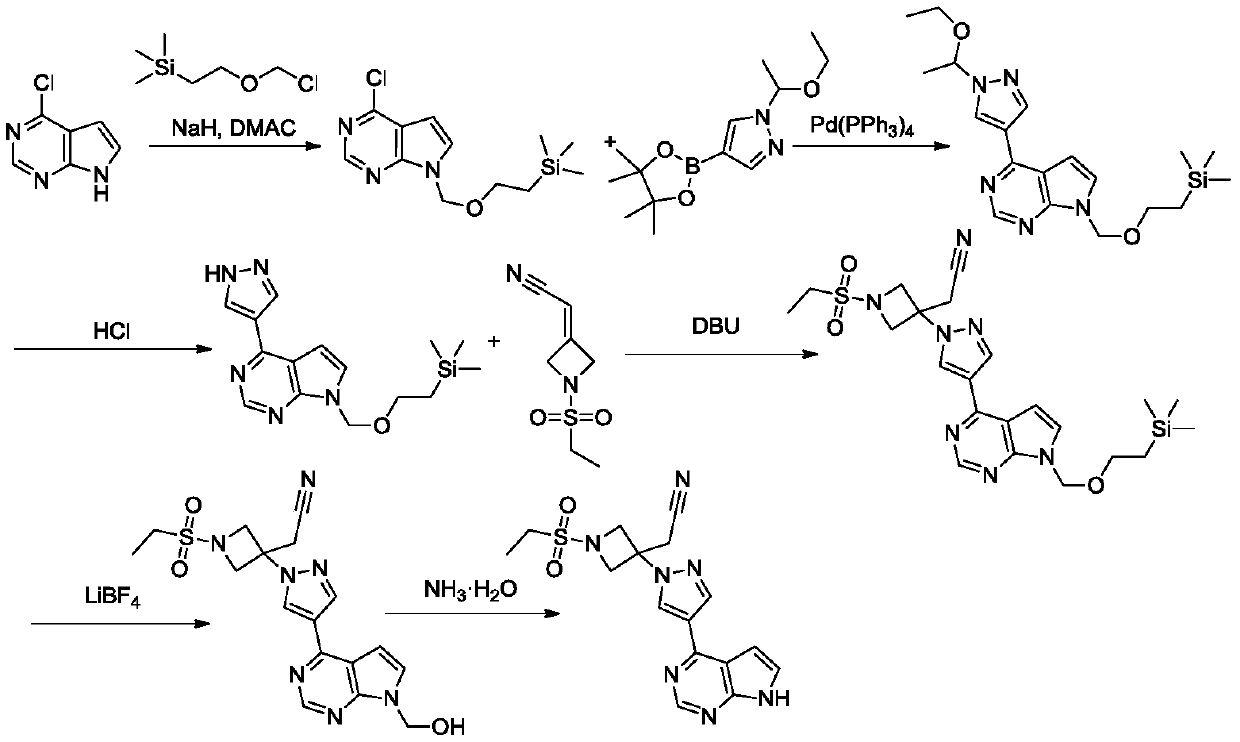
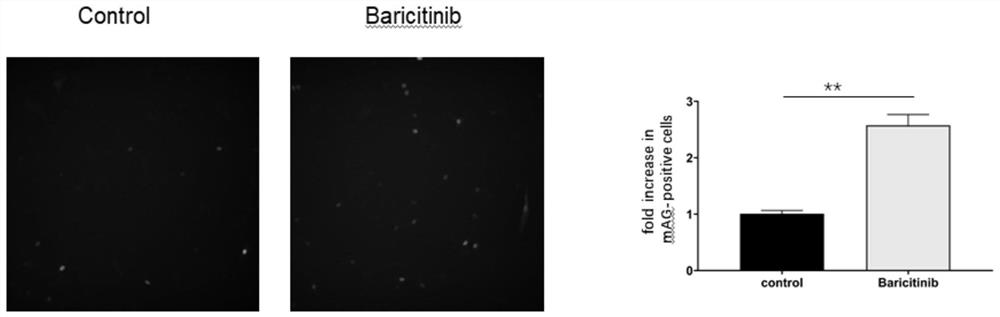
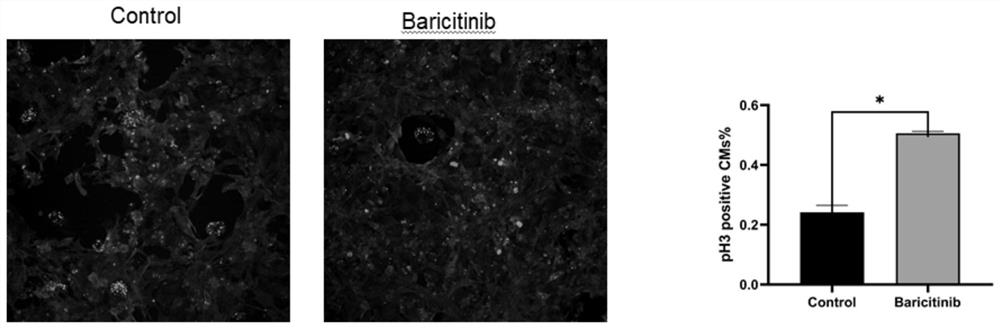
The invention discloses a novel drug application of baricitinib. More specifically, the application refers to the application of the baricitinib or pharmaceutically acceptable salt or ester thereof inpreparation of drugs for promoting the proliferation of cardiomyocytes. Experimental results show that the baricitinib can promote the proliferation of the cardiomyocytes of rats. Thus, the baricitinib can be used for the related fields in preparing the drugs for promoting the proliferation of the cardiomyocytes and the drugs for treating or preventing heart diseases; and a novel drug and treatment idea can be provided for treating or preventing the heart diseases such as myocardial infarction.
Owner:SYNOGENBIOPHARMACO LTD NANJING CHINA
A crystal form of baricitinib and preparation method thereof
InactiveCN108383846AImprove high temperature stabilityImprove stabilityOrganic active ingredientsAntipyreticDiseaseHigh humidity
The invention provides an A crystal form of baricitinib. Positions where 2-theta equals to 12.46, 13.921, 14.94, 15.359, 16.26, 16.639, 17.36, 19.08, 20.321, 21.961, 22.381, 24.118, 25.42, 27.441, 28.381, 29.321, 29.799, 32.675, 33.14, 33.563, 33.923 and 41.6 have diffraction peaks, wherein the error range of the 2-theta value is + / - 0.2. The provided A crystal form of the baricitinib has good high-temperature stability, high-humidity stability and illumination stability, can be applied to the medicine treating or preventing diseases related to JAK, has better bioavailability, and provided qualitative and quantitative information has very important significance for further studying the curative effect of the solid drugs.
Owner:SHANGHAI SUNTRONG BIOTECH
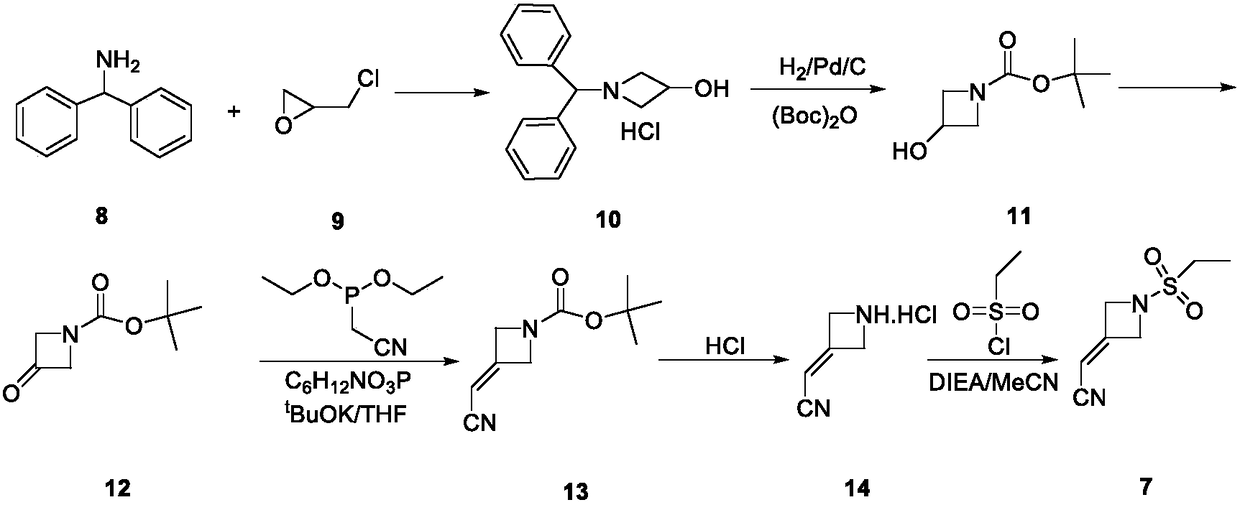
Baricitinib intermediate and preparation method thereof and method for preparing baricitinib from the intermediate
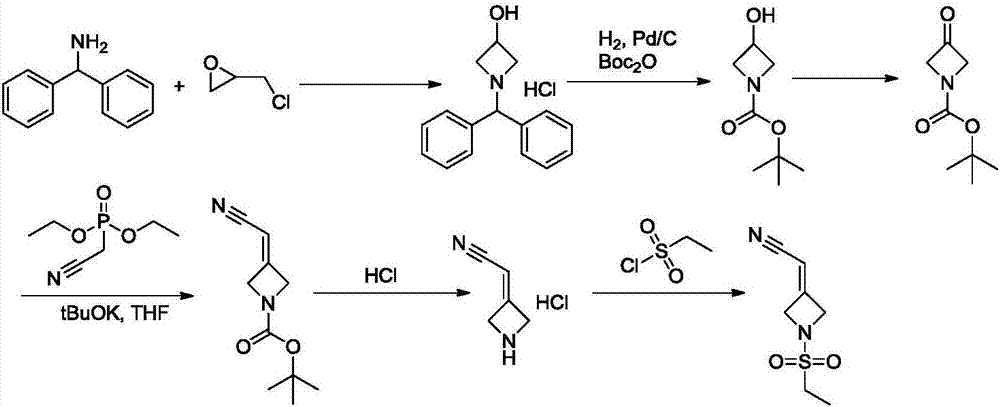
ActiveCN105541891BHigh yieldSimple process routeGroup 3/13 element organic compoundsDiethyl phosphateSulfonyl chloride
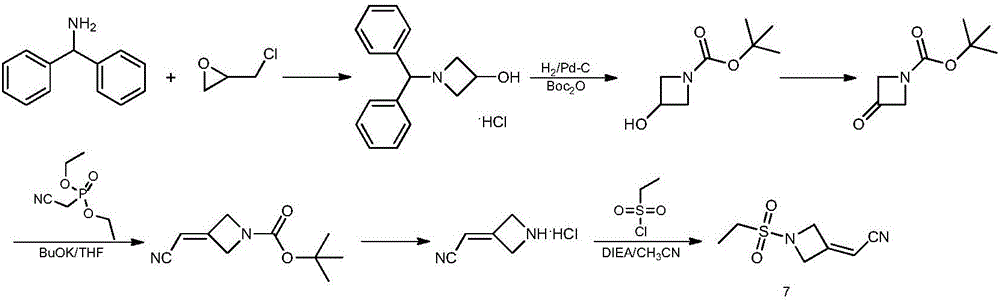
The invention discloses a baricitinib intermediate and a preparation method thereof, and a method for preparing baricitinib from the intermediate. The structure of the intermediate is disclosed as Formula (5). The preparation method of the intermediate comprises the following steps: carrying out reaction on diethyl cyanomethylphosphate and 1-boc-3-azacyclobutanone under the catalytic action of an alkali to obtain a compound 2 disclosed as Formula (2); removing the Boc group of the compound 2 to obtain a compound 3 disclosed as Formula (3); under alkaline conditions, carrying out reaction on the compound 3 and ethyl sulfonyl chloride to obtain a compound 4 disclosed as Formula (4); and in the presence of 1,8-diazabicyclo[5,4,0]hendecyne-7-ene, carrying out reaction on the compound 4 and pinacone 4-pyrazolylborate to obtain the intermediate. The method for preparing baricitinib from the intermediate comprises the following step: in the presence of a palladium catalyst and cesium fluoride, carrying out Suzuki coupling reaction on the intermediate and 6-chloro-7-deazapurine to obtain the baricitinib. The preparation method of baricitinib has the advantages of accessible raw materials and simple technique, and is suitable for industrial production.
Owner:SOUTHEAST UNIV
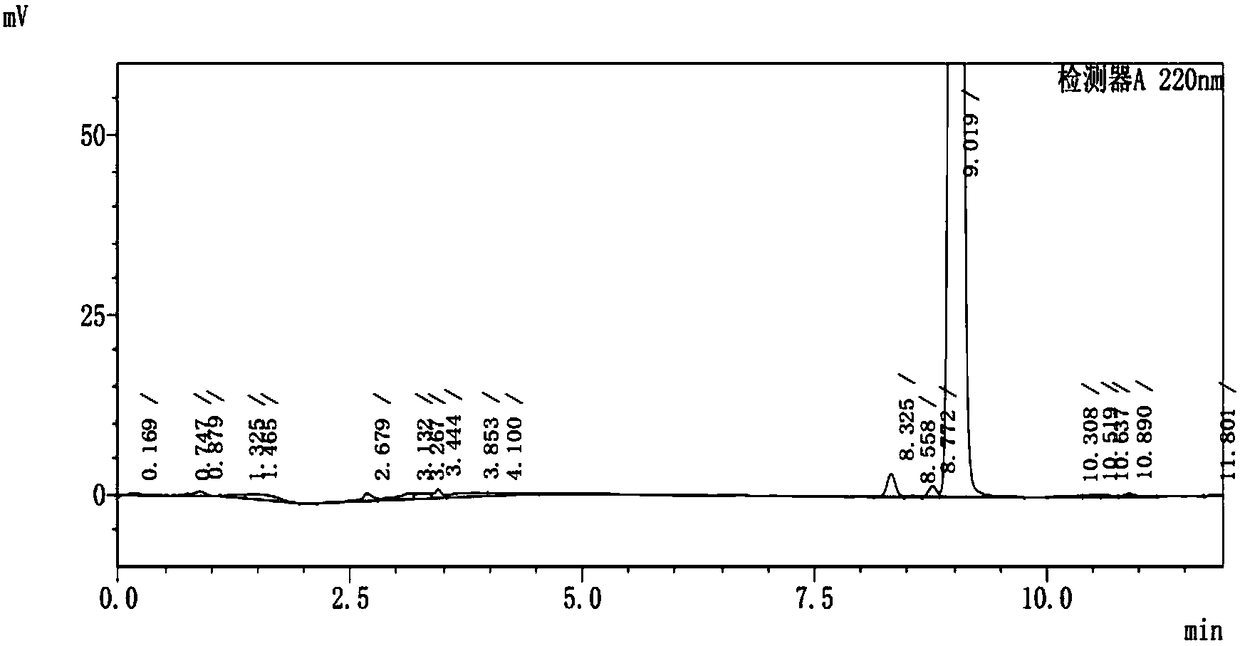
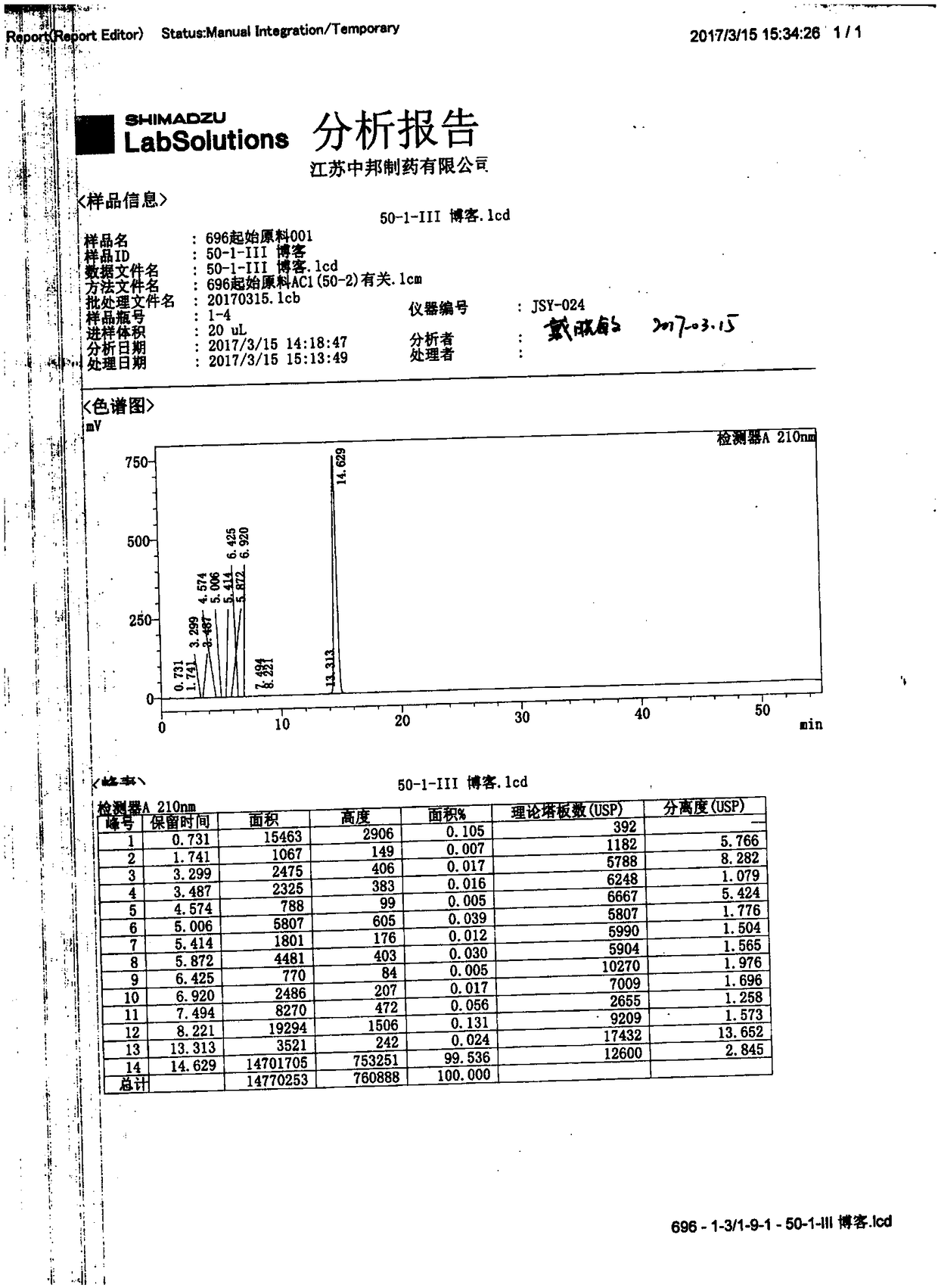
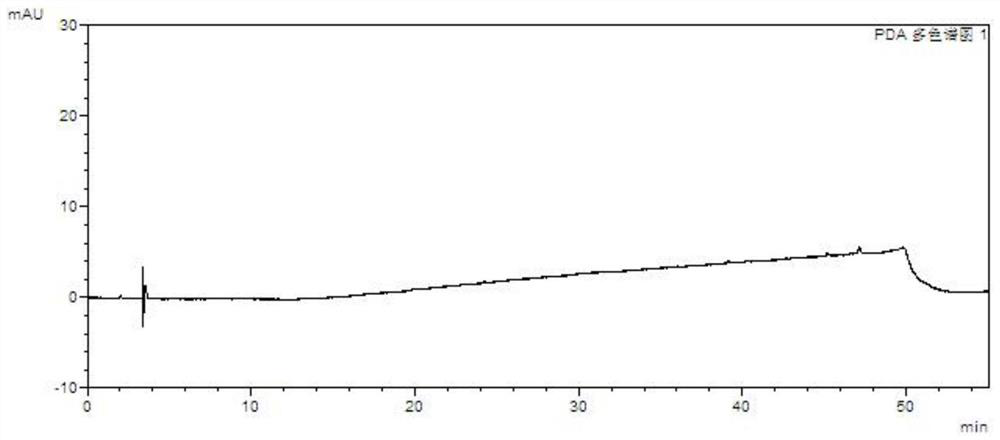
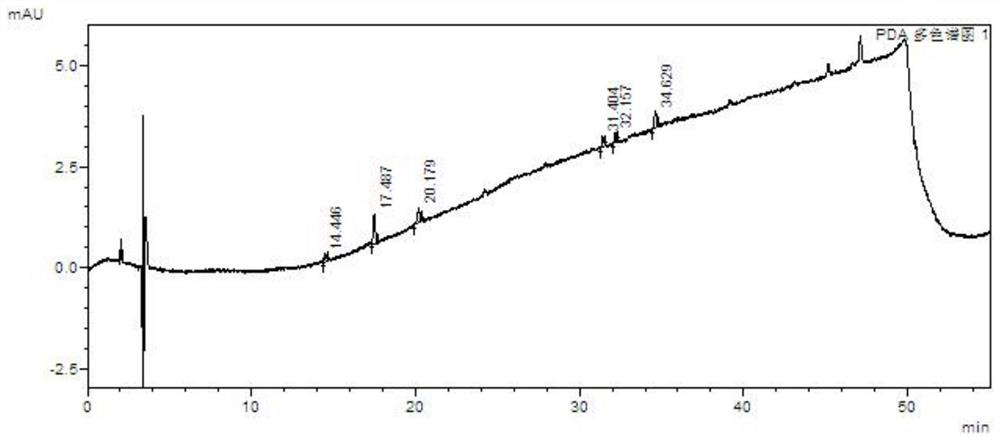
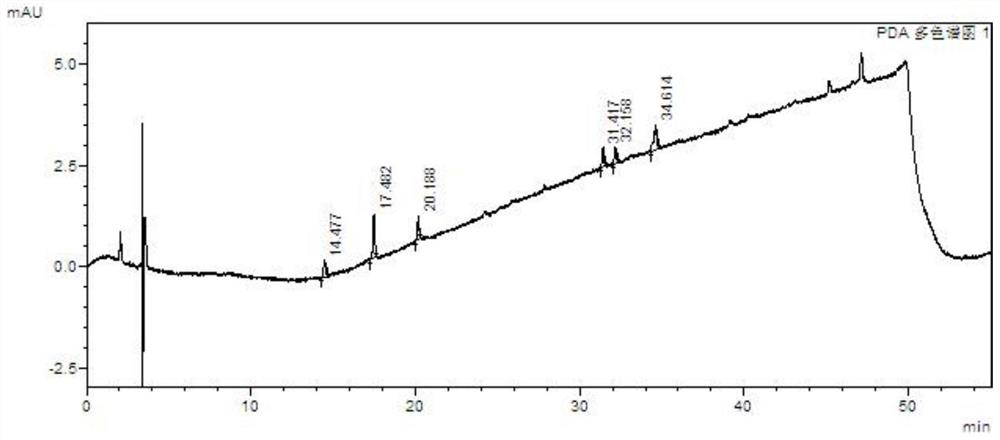
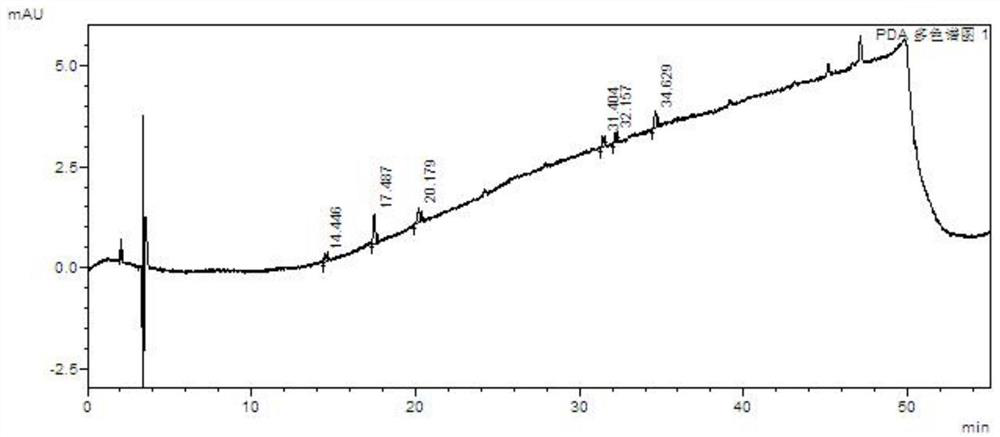
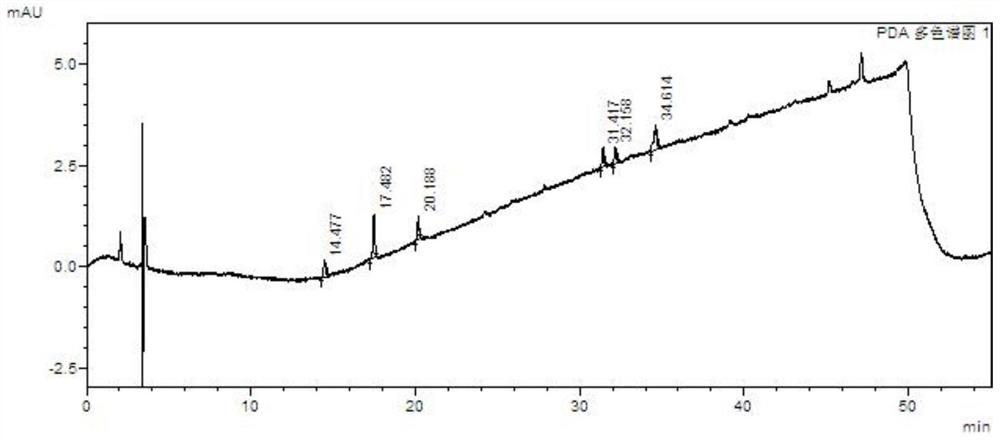
A kind of method for separating and measuring impurities of baricitinib bulk drug by hplc
The invention relates to a method for separating and measuring impurities of a baricitinib bulk drug by HPLC, comprising the following steps: Step 1, taking a baricitinib bulk drug sample, using octadecylsilane-bonded silica gel as a filler, and adding water Carry out gradient elution with mobile phase A and acetonitrile as mobile phase B, and take the eluent as the detection sample; step 2, prepare the detection solution, and take the baricitinib API test sample, baricitinib reference substance, and impurity A , impurity B, impurity C, impurity D, impurity E, prepare high performance liquid chromatography analysis liquid; Step 3, carry out high performance liquid chromatography analysis to the detection liquid prepared in step 2; Obtain the content of each impurity; The comprehensive effects of analytical column, mobile phase, gradient elution procedure, flow rate and column temperature on separation and detection have optimized the detection results. It has the advantages of rapidity, simplicity, high sensitivity, accuracy and reliability, and wide applicability. It is suitable for separation and determination. Impurity content of baricitinib drug substance.
Owner:安徽联创生物医药股份有限公司
The preparation method of the key intermediate 1 for the synthesis of baricitinib
ActiveCN107739328BReduce manufacturing costHigh yieldOrganic chemistryDiethyl methylphosphonateBiochemical engineering
The invention relates to a preparation method for synthesizing a key intermediate 1 of Baricitinib. The preparation method comprises the following steps: generating ring closing reaction by virtue of1,3-dibromo-2,2-dimethoxypropane (SM) and ethyl sulfonamide (SM2) under an alkaline condition, and carrying out acetal deprotection under an acidic condition, so as to obtain an intermediate B; and generating witting reaction by virtue of the obtained intermediate B and diethyl cyanomethylphosphonate under the alkaline condition, so as to obtain the key intermediate 1. According to the preparationmethod, the ring closing reaction is generated by virtue of the commercial materials SM and ethyl sulfonamide under the alkaline condition, acetal deprotection is carried out under the acidic condition so as to obtain intermediate B, and witting reaction is carried out so as to obtain the key intermediate 1; the key intermediate 1 can be actually obtained only through two synthetic steps; and compared with the prior art, the preparation method has the advantages that the synthetic route is greatly shortened, the hydrogenation is avoided, and the production cost of the key intermediate 1 is lowered.
Owner:海化生命(厦门)科技有限公司
Method for separating and determining baricitinib bulk drug impurities by using HPLC
ActiveCN111999400AQuality improvementEfficient separationComponent separationFluid phasePhysical chemistry
The invention relates to a method for separating and determining impurities in a baricitinib bulk drug by using HPLC (High Performance Liquid Chromatography). The method comprises the following steps:step 1, taking a baricitinib bulk drug sample, carrying out gradient elution by using octadecylsilane chemically bonded silica as a filler, water as a mobile phase A and acetonitrile as a mobile phase B, and taking eluent as a detection sample; step 2, performing preparation of a detection liquid: taking a baricitinib bulk drug detection sample, a baricitinib reference substance, an impurity A, an impurity B, an impurity C, an impurity D and an impurity E, and preparing a high performance liquid chromatography analysis liquid; and step 3, carrying out high performance liquid chromatography analysis on the detection liquid prepared in the step 2; obtaining the content of each impurity. According to the method, the comprehensive influence of an analysis column, a mobile phase, a gradient elution program, a flow rate and a column temperature on separation detection is comprehensively considered, so that a detection result is optimized; and the method has the advantages of rapidness, simplicity, convenience, high sensitivity, accuracy, reliability and wide applicability, and is suitable for separating and determining the impurity content of the baricitinib bulk drug.
Owner:安徽联创生物医药股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com