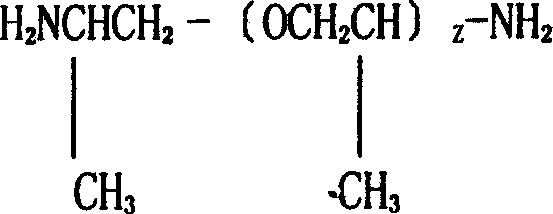Patents
Literature
1072 results about "Nickel catalyst" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nickel(II) precatalysts are a type of catalyst used in organic reactions. Many transformations are catalyzed by nickel in organometallic chemistry and in organic synthesis. Many of these transformations invoke a low valent (generally Ni(0)) species as the active catalyst.
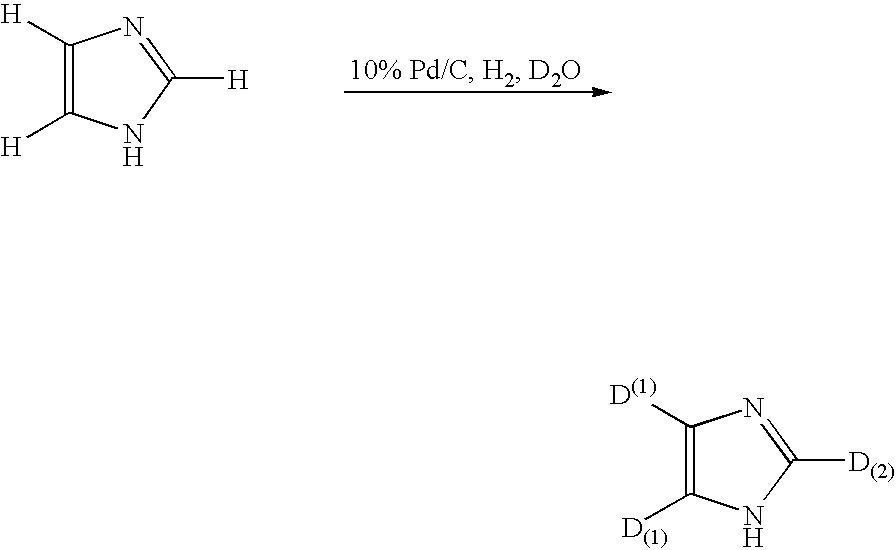
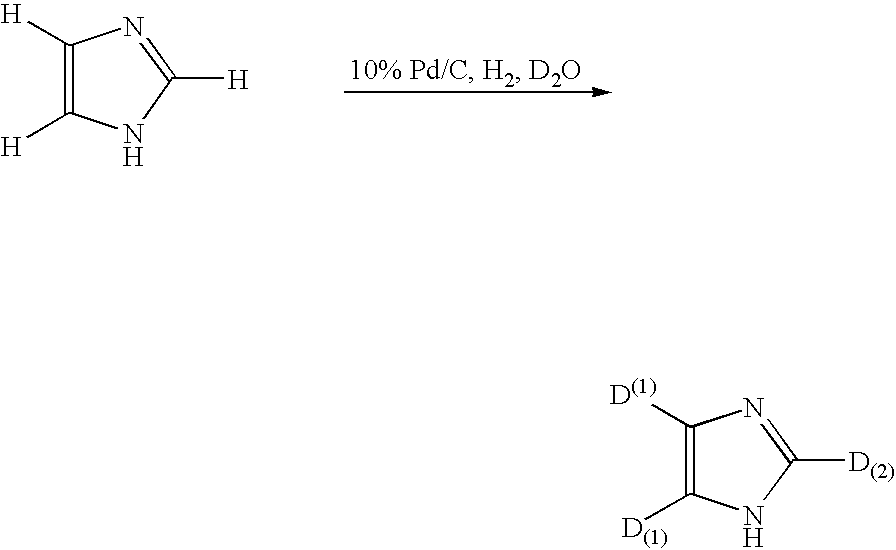
Method for deuteration of a heterocyclic ring
ActiveUS7517990B2Isotope introduction to heterocyclic compoundsSugar derivativesNickel catalystHydrogen atom
The present invention relates to a method for deuteration of a heterocyclic ring, which comprises subjecting a compound having a heterocyclic ring to sealed refluxing state in a deuterated solvent in the presence of an activated catalyst selected form a palladium catalyst, a platinum catalyst, a rhodium catalyst, a ruthenium catalyst, a nickel catalyst and a cobalt catalyst. In accordance with a method of the present invention, a hydrogen atom belonging to a heterocyclic ring of a compound having a heterocyclic ring can be very efficiently deuterated because temperature of deuteration reaction can be maintained at higher than boiling point of the solvent.Further, a method for deuteration of the present invention can be applied widely to deuteration of various compounds having a heterocyclic ring which are liable to decomposition under supercritical conditions or acidic conditions, leading to industrial and efficient deuteration of a compound having a heterocyclic ring.
Owner:FUJIFILM WAKO PURE CHEM CORP
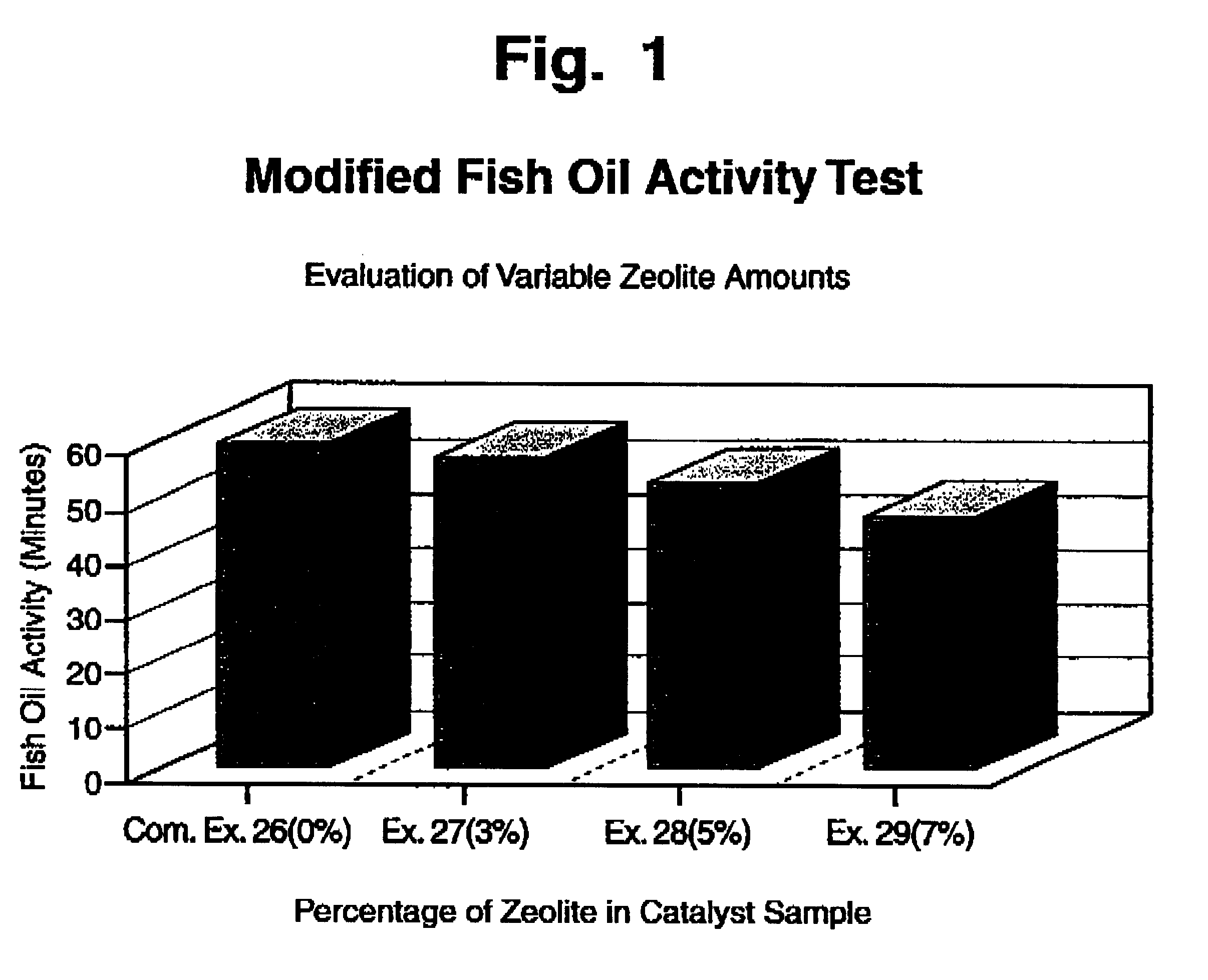
Combination sulphur adsorbent and hydrogenation catalyst for edible oils
An improved catalyst for edible oil hydrogenation produced by the incorporation of a sulphur adsorbing zeolite with a supported nickel hydrogenation catalyst. The zeolite is a cation-exchanged form of low silica faujasite with a silica to alumina ratio from about 1.8 to 2.1. The hydrogenation catalyst is a supported nickel catalyst. The zeolite is incorporated into the stabilization media with the reduced hydrogenation catalyst to form a physical blend of sulphur adsorbing zeolite and reduced nickel hydrogenation catalyst in a stabilization medium.
Owner:UNITED CATALYSTS INC
Fatty group end-amino polyether production method and special catalyzer preparation method
The invention discloses a fatty group end-amino polyether production method and special catalyzer preparation method, wherein the catalyst is special-purposed frame nickel catalyst, the custom made alloy is dissolved completely by sodium-hydroxide liquor under finite temperature and proportioning conditions, then distilled water is used for scouring to neutrality, then anhydrous alcohol is used for washing and sealing. The process for preparing the end-amino polyether comprises using the said special-purpose catalyst for reaction under finite concentration, temperature and proportioning conditions, thus the end-amino polyester can be obtained.wh
Owner:JIANGSU CHEM RES INST
Method for Deuteration of an Aromatic Ring
ActiveUS20070255076A1Improve working environmentAmino preparation from aminesOrganic compound preparationNickel catalystHydrogen
The present invention relates to a method for deuteration of a compound having an aromatic ring, using an activated catalyst, and the method comprises reacting a compound having an aromatic ring with heavy hydrogen source in the presence of an activated catalyst selected from a platinum catalyst, a rhodium catalyst, a ruthenium catalyst, a nickel catalyst and a cobalt catalyst.
Owner:FUJIFILM WAKO PURE CHEM CORP
Silicon tetrachloride hydrogenating process of producing trichloro hydrosilicon
InactiveCN1436725ANo pollution in the processEasy to controlSilicon hydridesHalogenated silanesNickel catalystHydrogen atmosphere
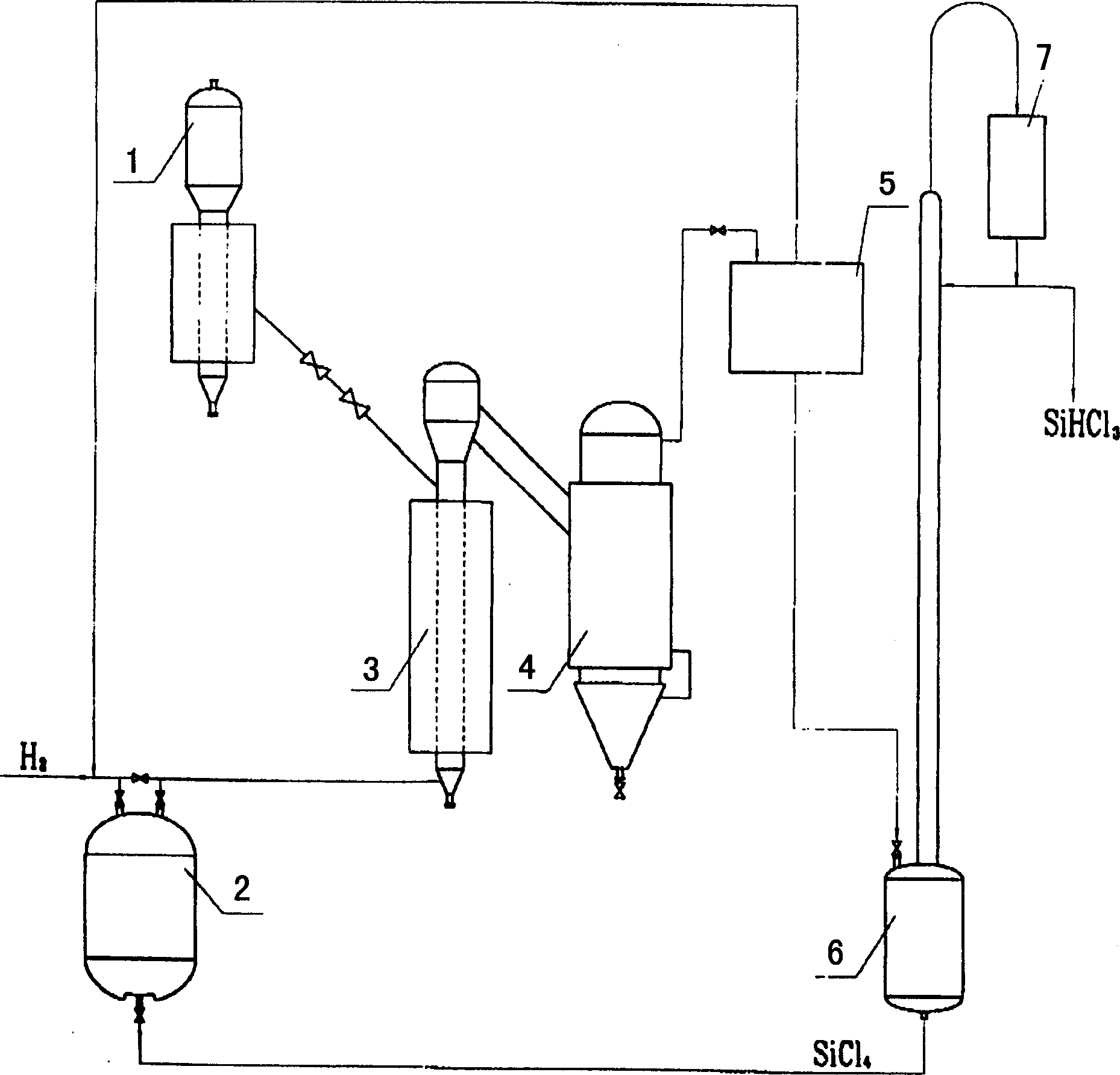
The silicon tetrachloride hydrogenating process of producing trichloro-hydrosilicon includes: mixing powdered nickel catalyst and silicon powder in the mass ratio of 1-10 and activating the mixture in hydrogen atmosphere and at the temperature of continuously change from 20 deg.C to 420 deg.c; and making mixed gas of hydrogen and SiCl4 in the molar ratio of 1-10 pass through the activated catalyst and silicon powder layer for hydrogenating SiCl4 continuously at 400-500 deg.c temperature and 1.2-1.5 MPa pressure. The equipment, system and operation of the present invention has high once SiCl4 converting rate and low power consumption and may be used in large-scale production.
Owner:CHINA ENFI ENG CO LTD
One-piece solid golf ball
InactiveUS20020052253A1Improve featuresIncreased durabilityGolf ballsSolid ballsVulcanizationPolymer science
The present invention provides a one-piece solid golf ball having excellent rebound characteristics and good shot feel, while maintaining excellent processability and durability. The present invention relates to a one-piece solid golf ball formed from a rubber composition comprising a mixture consisting of polybutadiene (a) synthesized using nickel-containing catalyst and polybutadiene (b) synthesized using cobalt-containing catalyst and hydroquinone or derivatives thereof as a vulcanization stabilizer, wherein a Mooney viscosity and a weight ratio of the polybutadienes (a) and (b), an amount of the vulcanization stabilizer, a center hardness (the minimum hardness in the golf ball) and surface hardness of the golf ball, and a difference between the maximum hardness and minimum hardness in the golf ball are adjusted to a specified range.
Owner:SUMITOMO RUBBER IND LTD
Nickel catalyst with composite pore structure used for selective hydrogenation
ActiveCN101191078AGood activity at low temperatureGood choiceRefining to eliminate hetero atomsAnti jammingRare earth
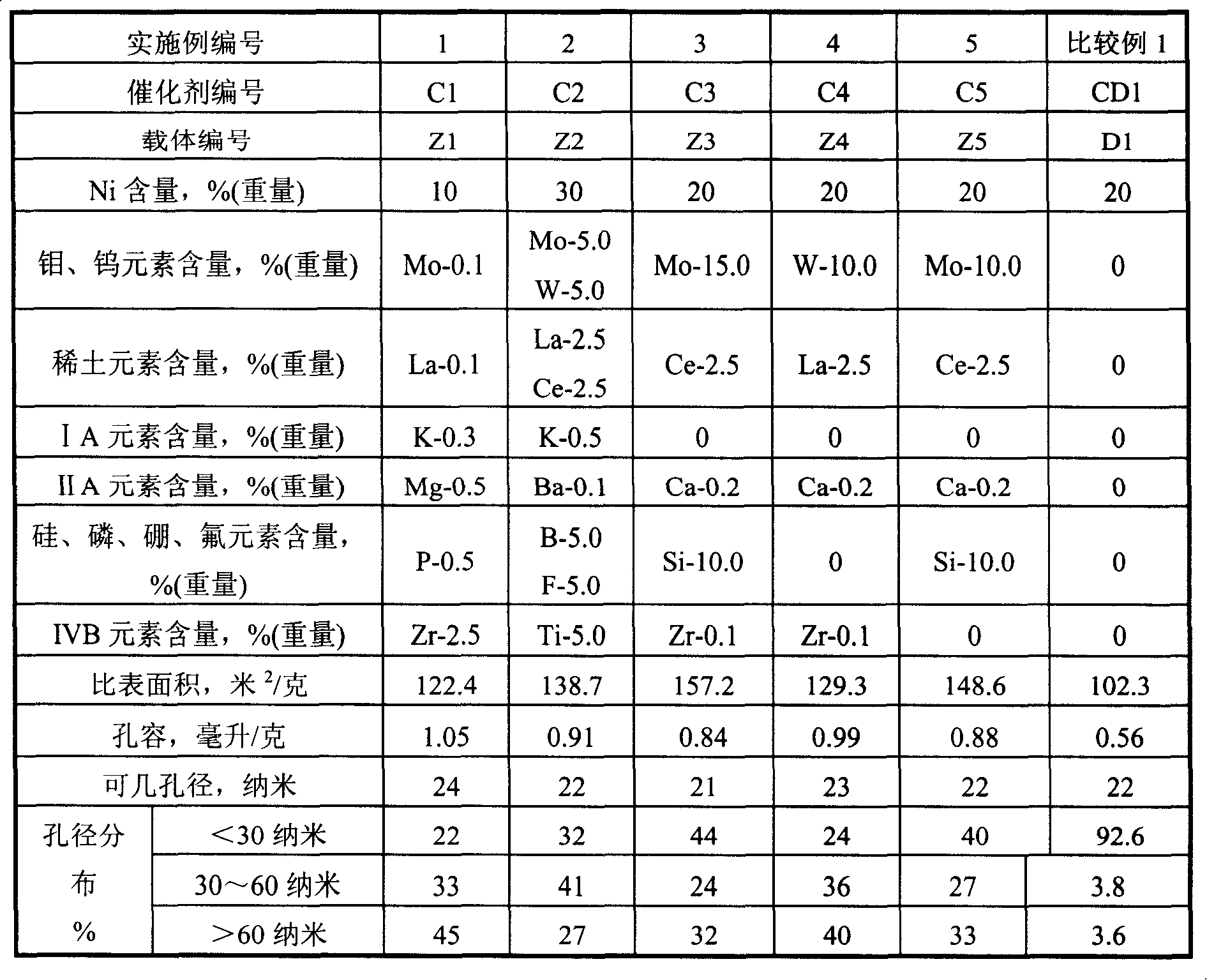
The invention relates to a nickel catalyst used for selective hydrogenation and provided with a complex hole structure and mainly solves the technical problems that the catalyst has low low-temperature activity, bad anti-jamming ability, low sol ability, bad stability and bad free water resistant performance existing in the prior art. The invention comprises the following compositions based on the weight percentage: (a) 5.0 percent to 40.0 percent of metal nickel or oxides of the metal nickel; (b) 0.01 percent to 20.0 percent of at least one element chosen from molybdenum or tungsten or the oxide thereof; (c) 0.01 percent to 10.0 percent of at least one element chosen from rare earth or the oxide thereof; (d) 0.01 percent to 2.0 percent of at least one element chosen from IA or IIA in Periodic Table of Elements or the oxide thereof; (e) 0 to 15.0 percent of at least one element chosen from silicon, phosphor, boron or fluorin or the oxide thereof; (f) 0 to 10.0 percent of at least one element chosen from IVB in Periodic Table of Elements or the oxide thereof; (g) the remaining alumina carrier, wherein, the technical proposal that the total pore volume of the carrier is between 0.5 and 1.2ml / g, the pore volume the pore diameter of which is less than 30 nanos accounts for 5 to 65 percent of the total pore volume, the pore volume the pore diameter of which is between 30 and 60 nanos accounts for 20 to 80 percent of the total pore volume, the pore volume the pore diameter of which is more than 60 nanos accounts for 20 to 50 percent of the total pore volume, better solves the problems and can be used in the industrial production of selective hydrogenation of cracking gasolilne.
Owner:CHINA PETROLEUM & CHEM CORP +1
Production process of aliphatic polyetheramine
The invention belongs to the technical field of high molecular materials and provides a production process of aliphatic polyetheramine. In the process, aliphatic polyetheramine is prepared by catalyzing, reducing and aminating polyether polyol of which the number-average molecular weight is over 100 and which serves as a raw material in the presence of hydrogen gas, an aminating agent and a framework nickel catalyst. The framework nickel catalyst consists of 85-95 percent by mass of metal nickel and 15-5 percent of metal aluminum. The framework nickel catalyst and the aminating agent are circularly applied mechanically after being treated. The entire process has the advantages of high route selectivity, high transformation ratio, low environmental pollution and convenience for post-treatment.
Owner:CHINA PETROCHEMICAL CORP +1
Nickel ethylene polymerization catalyzed system, preparation and use thereof
ActiveCN1603347AHigh polymerization activityIncrease the degree of branchingNickel catalystAluminoxane
This invention relates to a transition metal nickel catalyst component after ethylene polymerization. Component (A) is halogenated [N, N-(R bed -2-pyridine methylene)-substitution benzidine] nickel complexes, component (B) is alumina alkyl. The catalysis system is used to ethylene polymerization. There are high polymerization activity and high branching degree of complexes, and double peak feature of the complexes.
Owner:CHINA PETROLEUM & CHEM CORP +2
Binuclear acenaphthene (alpha-diimine) nickel/palladium catalysts for olefins, and preparation method and application thereof
ActiveCN102827311AHigh activityImprove stabilityNickel organic compoundsBulk chemical productionNickel catalystDiimine
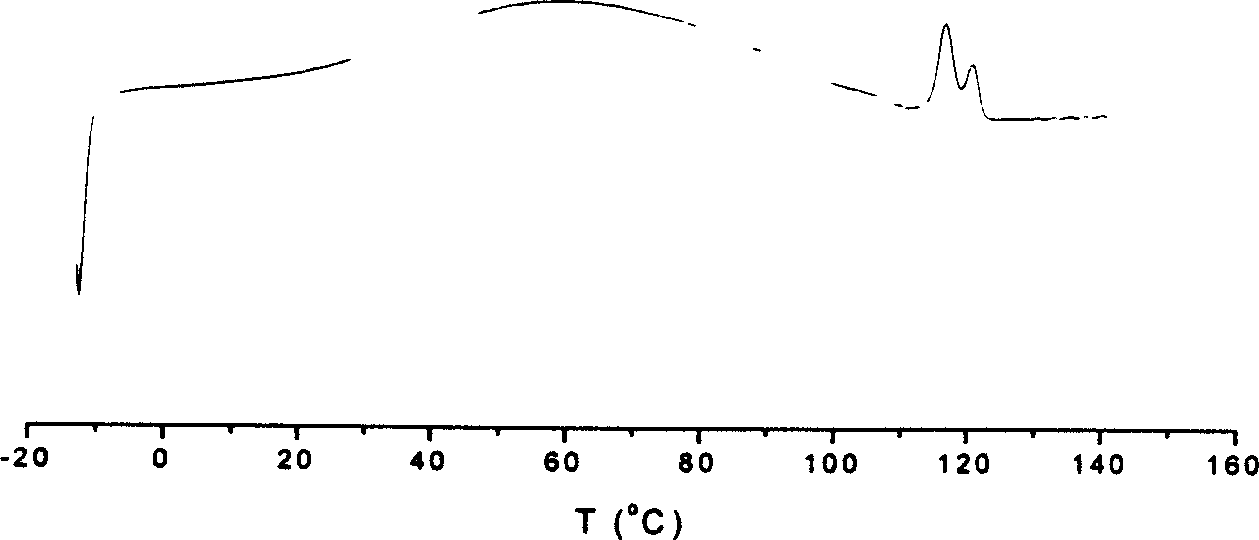
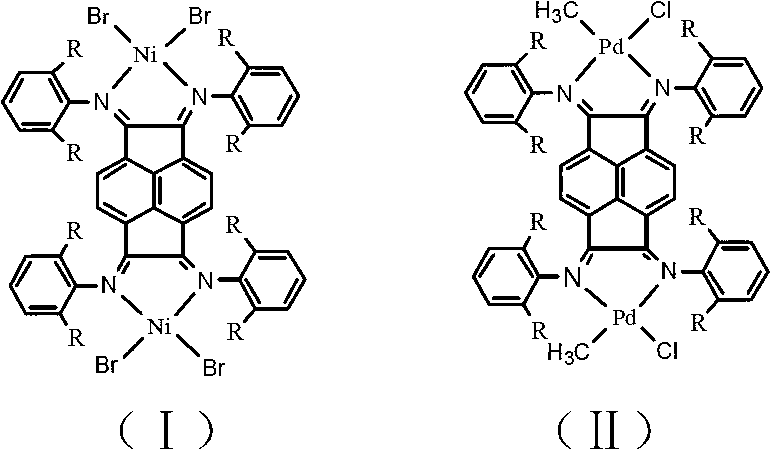
The invention discloses a preparation method for binuclear acenaphthene (alpha-diimine) nickel / palladium catalysts for olefins and application of the catalysts in catalysis of polymerization of olefins. The binuclear acenaphthene (alpha-diimine) nickel / palladium catalysts for olefins in the invention have structural formulas as represented by formula (I) and formula (II) in the specification. The binuclear acenaphthene (alpha-diimine) palladium catalyst for polymerization of olefins has high activity and good stability and can be used for preparing high-molecular-weight hyperbranched polyethylene with bimodal distribution; the binuclear acenaphthene (alpha-diimine) nickel catalyst for polymerization of olefins has high activity and good stability and can be used for preparing polypropylene with a weight-average molecular weight of more than 100,000 and thermoplastic elastomers with elasticity and a high molecular weight. The preparation method for the synthesized catalysts used for polymerization of olefins has the advantages of a simple process, a short synthetic route, low cost, high yield and easy industrialization.
Owner:ZHEJIANG UNIV
Full-cut fraction pyrolysis gasoline diolefin selective hydrogenation method
ActiveCN101429454AGood hydrogenation effectGood hydrogenation stabilityRefining by selective hydrogenationSilicon dioxideImpurity
The invention provides a selectivity hydrogenation method for diolefin of full-fraction pyrolysis gasoline, which comprises reduction and passivation of catalyst and application of technological conditions. The catalyst is nickel series hydrogenation catalyst which is used after being reduced or being subjected to reduction and passivation. The selectivity hydrogenation method is characterized in that the hydrogenation technological conditions are as follows: the volume space velocity of a liquid is less than or equal to 4h<-1>, the inlet temperature of a reactor is between 40 and 130 DEG C, the reaction pressure is more than or equal to 2 MPa, and the hydrogen / oil ratio is between 100 and 500 (v / v); the nickel series catalyst takes alumina as a carrier, is prepared by the immersion method, and contains 14 to 20 percent of nickel oxide, 1 to 8 percent of lanthanum oxide and / or cerium oxide, 1 to 8 percent of 4B oxide auxiliary agent, 2 to 8 percent of silicon dioxide and 1 to 8 percent of alkaline earth oxide as calculated by 100 weight percent of the catalyst; and the specific surface of the catalyst is between 60 and 150 square meters per gram, and the pore volume of the catalyst is between 0.4 and 0.6 milliliter per gram. The invention also provides a method for performing reduction and passivation on the catalyst on a hydrogenation unit. Under the conditions of the application method and the technological conditions, the nickel catalyst has good hydrogenation performance, and particularly has strong impurity and colloid resistance and good hydrogenation stability.
Owner:PETROCHINA CO LTD
Preparation process of amine terminated polyether
The invention belongs to the technical field of polymer materials and provides a preparation process of amine terminated polyether. In the process, polyether polyol with a number-average molecular weight of more than 100 is used as a raw material, and polyetheramine is prepared by catalytic reduction and amination in the presence of hydrogen, an aminating agent and a skeleton nickel catalyst, wherein the skeleton nickel catalyst comprises the following components by mass percent: 80-95% of metal nickel, 5-20% of metal aluminum and 0.5-5% of one, two or three of chromium, iron, copper or zinc; and the skeleton nickel catalyst and the aminating agent can be circularly applied after being processed. The whole preparation process has the advantages of good route selectivity, high conversion rate, low environmental pollution, and convenience in posttreatment.
Owner:CHINA PETROCHEMICAL CORP +1
Nickel catalyst for selective hydrogenation
ActiveCN101147871AGood activity at low temperatureGood choiceHydrocarbon oil crackingMetal/metal-oxides/metal-hydroxide catalystsRare-earth elementNickel catalyst
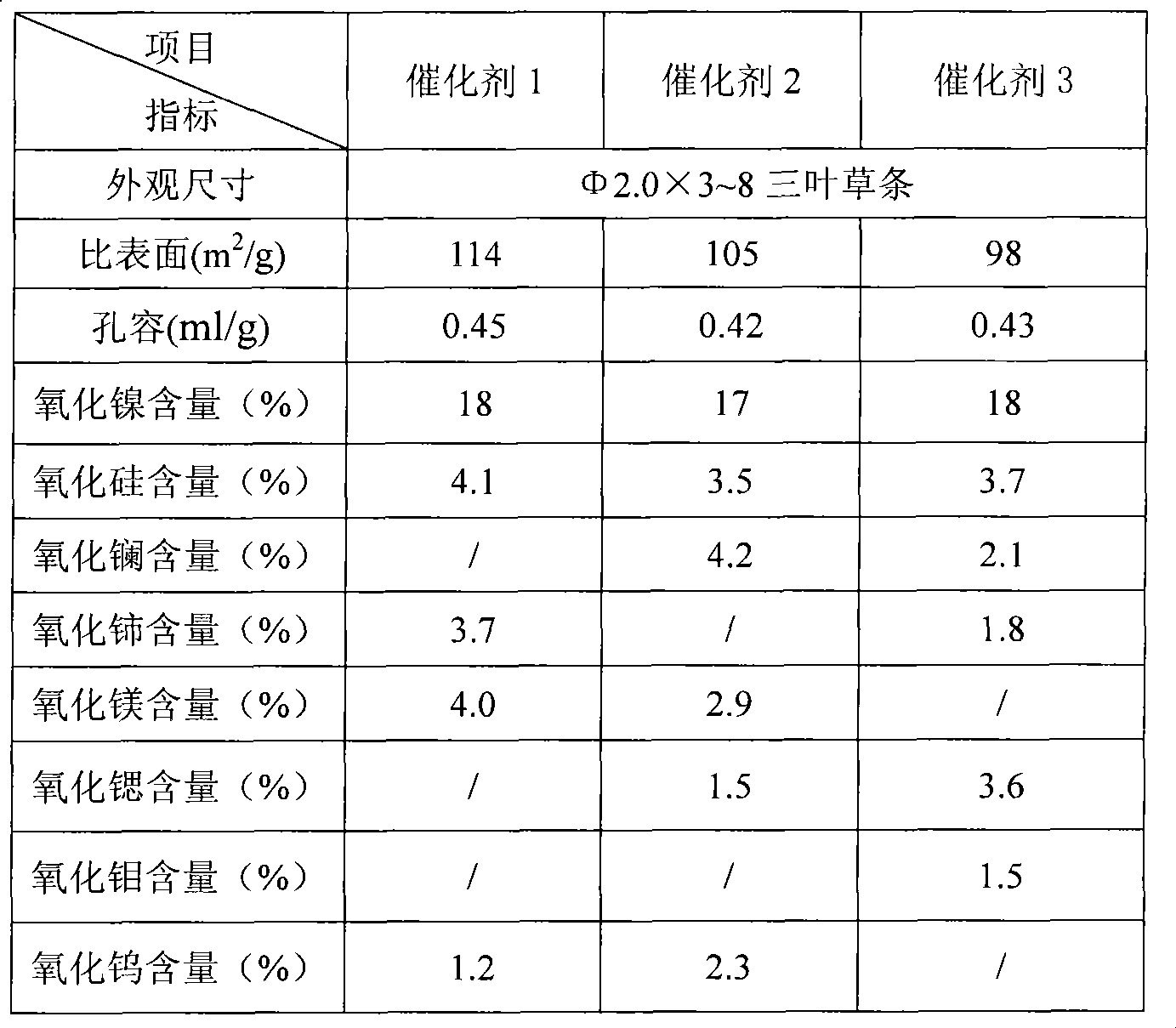
The present invention relates to a nickel catalyst for selective hydrogenation. The catalyst composition includes the following components: (by wt%) (a), 5.0-40.0% of metal nickel or its oxide; (b), 0.01-20.0% of at least one element selected from molybdenum or tungsten or its oxide; (c), 0.01-10.0% of at least one element selected from rare earth elements or its oxide; (d), 0.01-2.0% of at least one element selected from IA or IIA of periodic table of chemical elements or its oxide; (e), 0-15.0% of at least one element selected from Si, P, B or F or its oxide; (f), 0-10.0% of at least one element selected from IVB of periodic table of chemical elements or its oxide; and (g), the rest is aluminium oxide. Said catalyst can be used for pyrolysis gasoline hydrotreating production.
Owner:SHANGHAI RES INST OF PETROCHEMICAL TECH SINOPEC
Activator for producing glycol by catalytic hydrogenation method
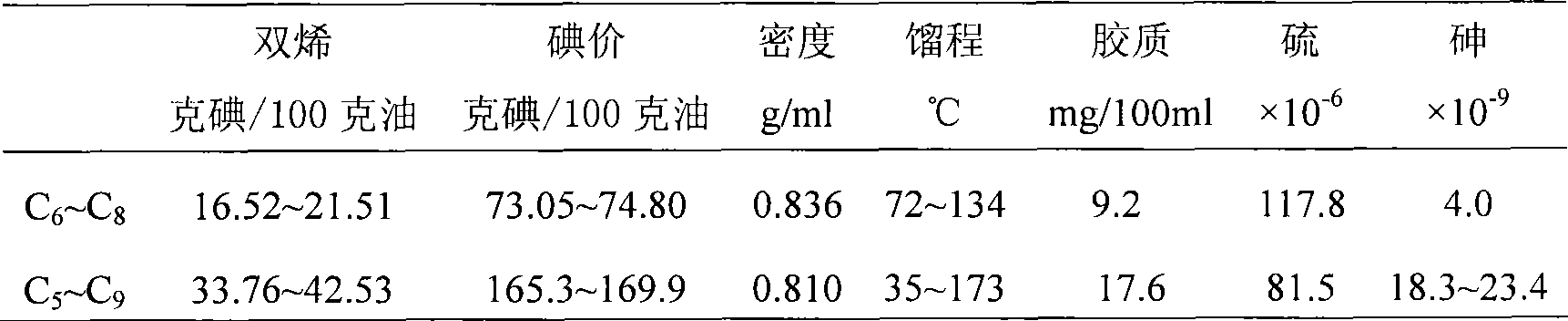
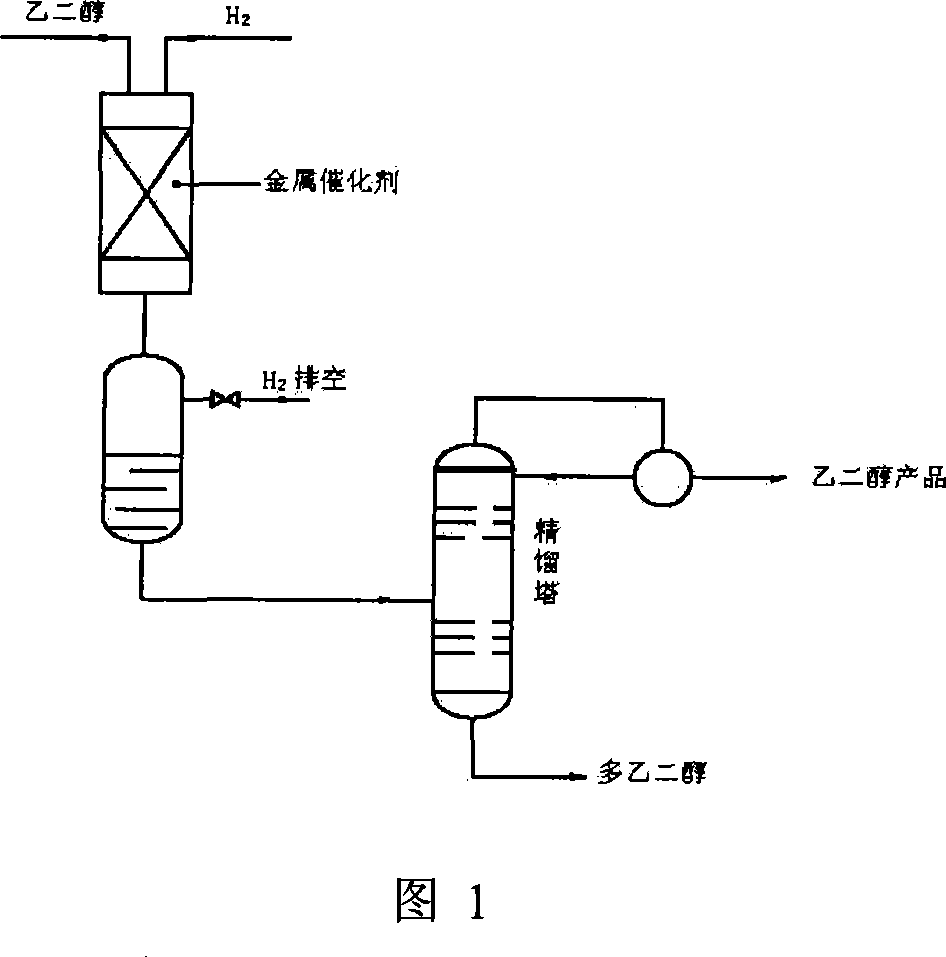
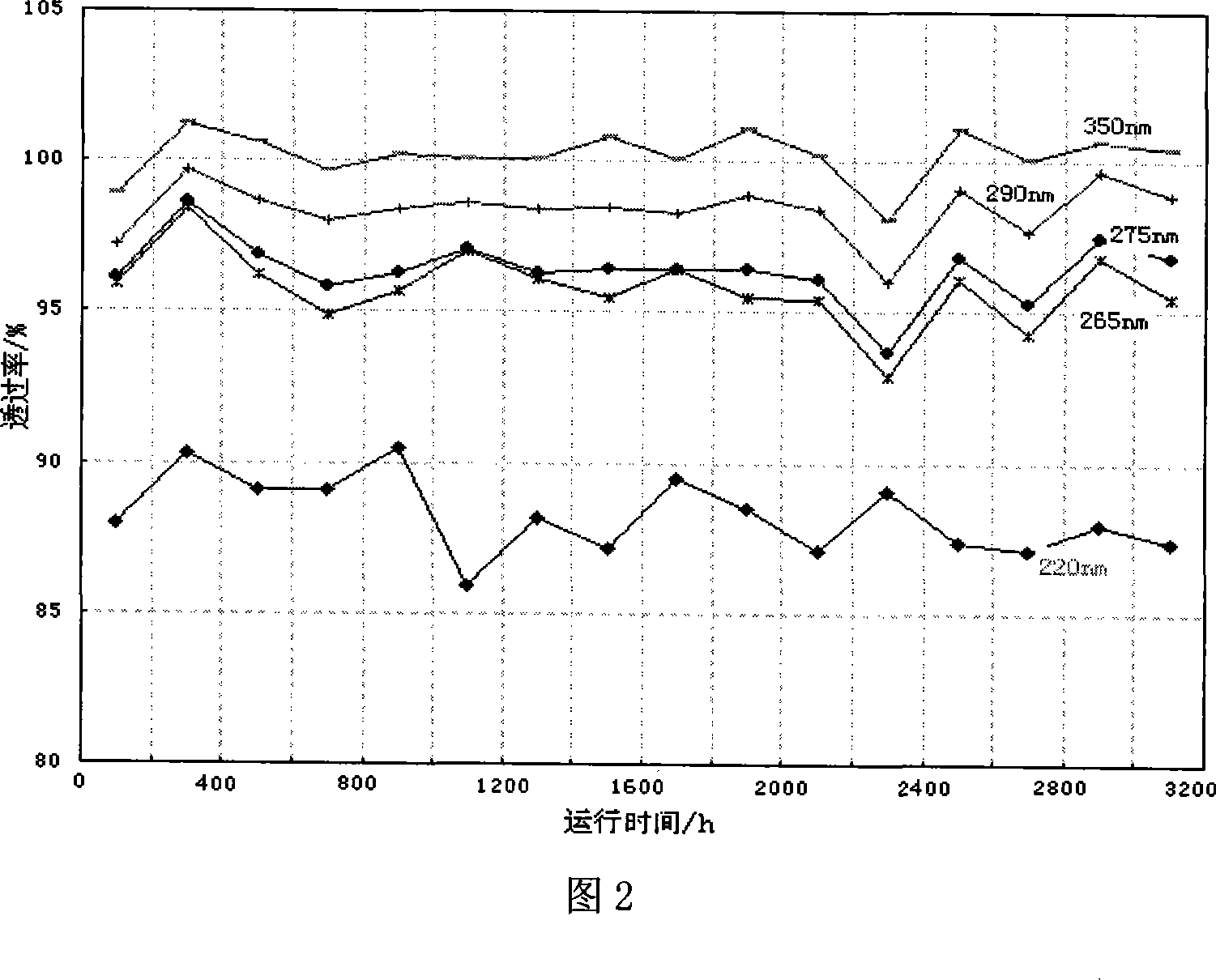
InactiveCN101032688AMetal/metal-oxides/metal-hydroxide catalystsPreparation by hydrolysisNickel catalystUltraviolet
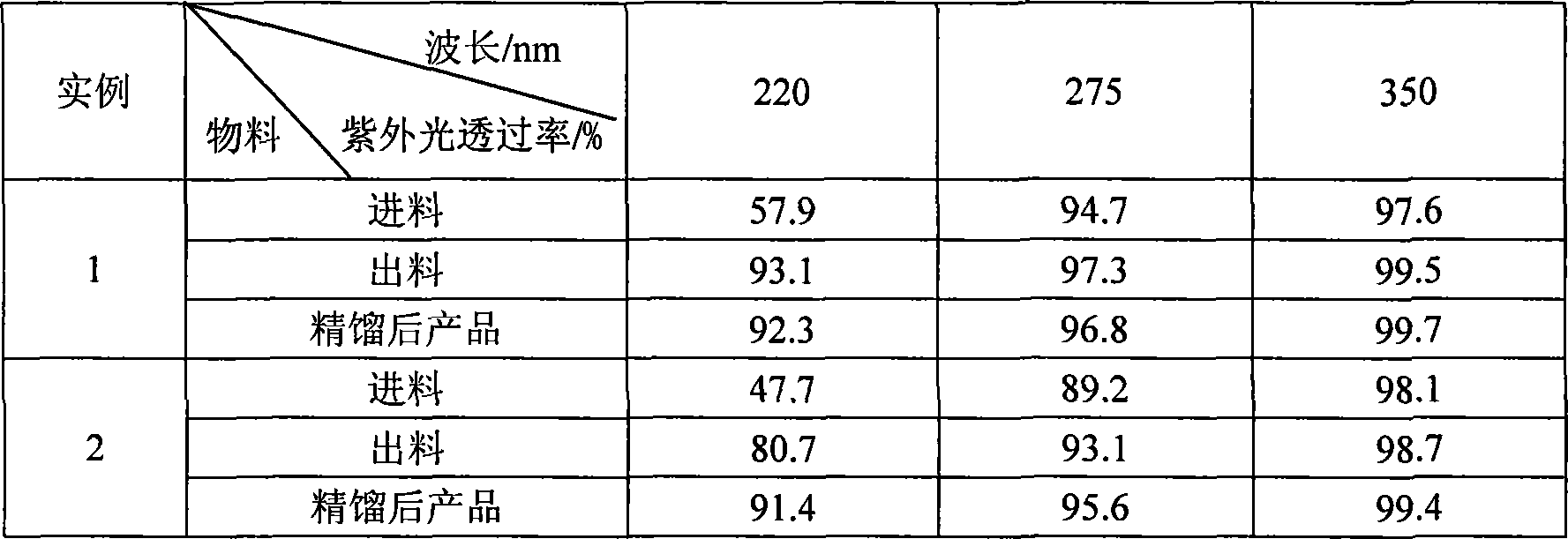
The present invention is Ni catalyst prepared with Ni-Al alloy as skeleton and through surface treatment with hot alkali solution to eliminate Al of 8-30 %. The Ni catalyst is used in catalytic hydrogenation of glycol material with low ultraviolet ray transmission rate to raise its ultraviolet ray transmission rate. The Ni catalyst has proper strength suitable for use in fixed bed reactor, high catalytic activity and long service life, and the rectified glycol product has low aldehyde content and high ultraviolet ray transmission rate.
Owner:JIANGSU POLYTECHNIC UNIVERSITY
Method for producing pale disproportionated rosin with high content dehydroabietic acid and P-camphogen simultinuously
InactiveCN1616570APrevent browningPrevent oxidationNatural resin purificationNickel catalystDistillation
The process of producing pale disproportionated rosin with high dehydroabietic acid content and paracymene simultaneously includes the following steps: rinsing turpentine to eliminate impurity, dissolving turpentine in turpentine oil to form turpentine solution, catalytic disproportionating turpentine in solution in the presence of Pd / C catalyst, filtering and decompression distillation to obtain pale disproportionated rosin with high dehydroabietic acid content and disproportionated turpentine oil containing paracymene in 20-60 %, and distilling disproportionated turpentine oil to obtain paracymene and pinane. Or, the rinsed turpentine as material may be re-crystallized to purify and catalytically disproportionated to prepare pale disproportionated rosin containing high dehydroabietic acid in 72-82 %. The Pd / C catalyst may be replaced with skeletal nickel catalyst.
Owner:GUANGXI UNIV
Method for recovering nickel and aluminum from waste aluminum based nickel-containing catalyst
InactiveCN1544666AEfficient separationEfficient recyclingProcess efficiency improvementDecompositionSodium aluminate
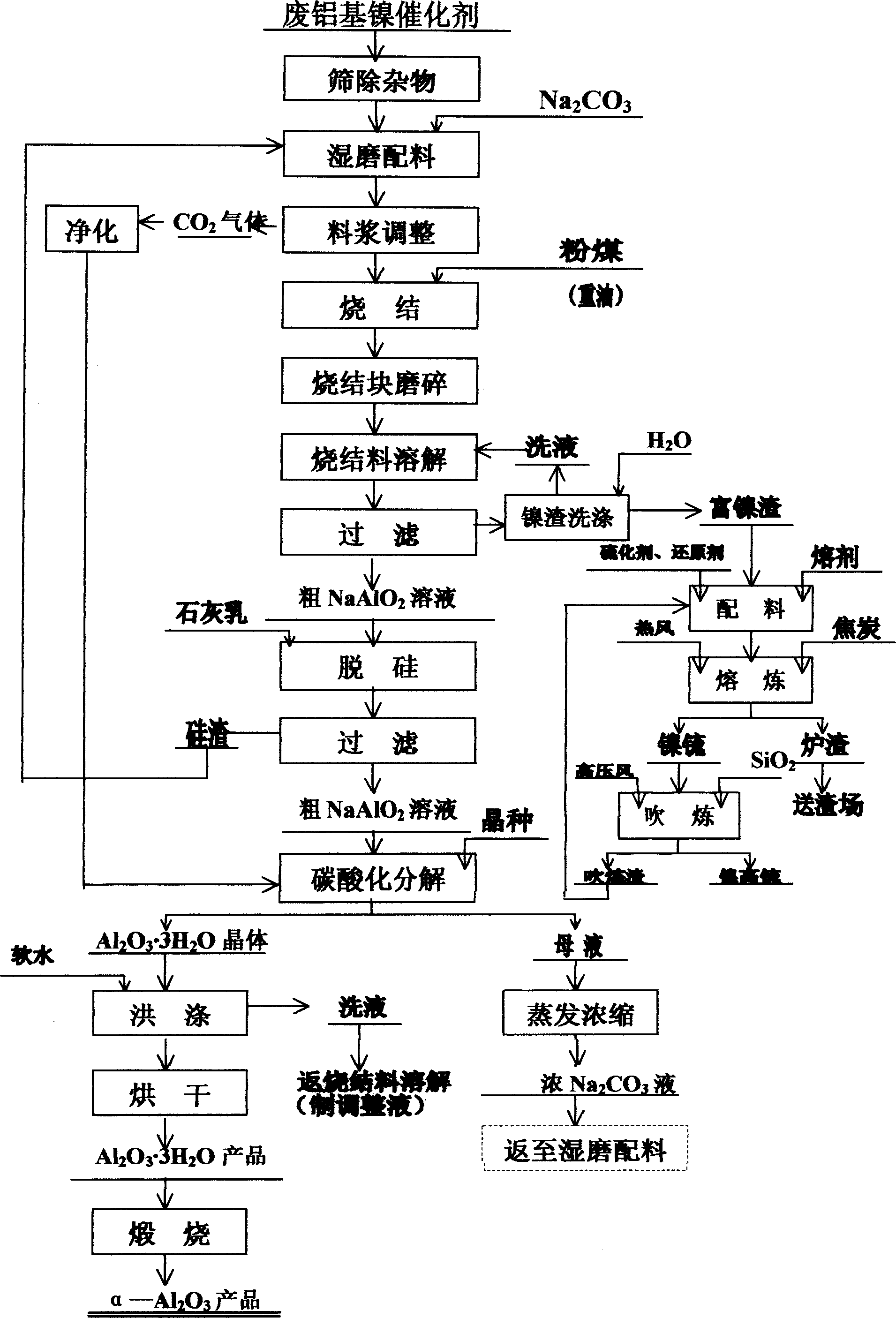
The invention is a method of recovering nickelic and aluminum from waste aluminum-based nickel catalyst, it has the characters of novel technique, reasonable flow, simple and convenient method and easy operation, and convenient scaled production, and benefits environmental protection. It includes the steps: sodium carbonate sintering and state-changing--boiling water dissolving sodium aluminate and separating aluminum--- making reducing-matte-making melting on nickel residues to obtain nickel matte Ni3S2-FeS-Ni-Fe alloy or copper-nickel matte Cu2S-Ni3S2-FeS alloy---blowing to obtain high-grade nickel matte Ni3S2 or high-grade copper-nickel matte Cu2S-Ni3S2-Cu-Ni alloy---desiliconizing crude NaAlO2 solution---making carbonated decomposition to obtain aluminum hydrate Al2O3íñ3H2O---calcining to obtain anhydrous aluminum oxide alpha-Al2O3. It is suitable to recover nickel and aluminum from the waste residue generated by extracting molybdenum and vanadium from waste aluminum-based nickelic catalysts and disabled catalysts containing nickel, aluminum, molybdenum and vanadium.
Owner:SHENYANG JIAHE METALLURGICAL FURNACE CHARGE
Method for recovering metallic oxide from waste aluminum base V-Mo-Ni catalyst
InactiveCN101457296AImprove the situationSimple processProcess efficiency improvementRecovery methodAluminium hydroxide
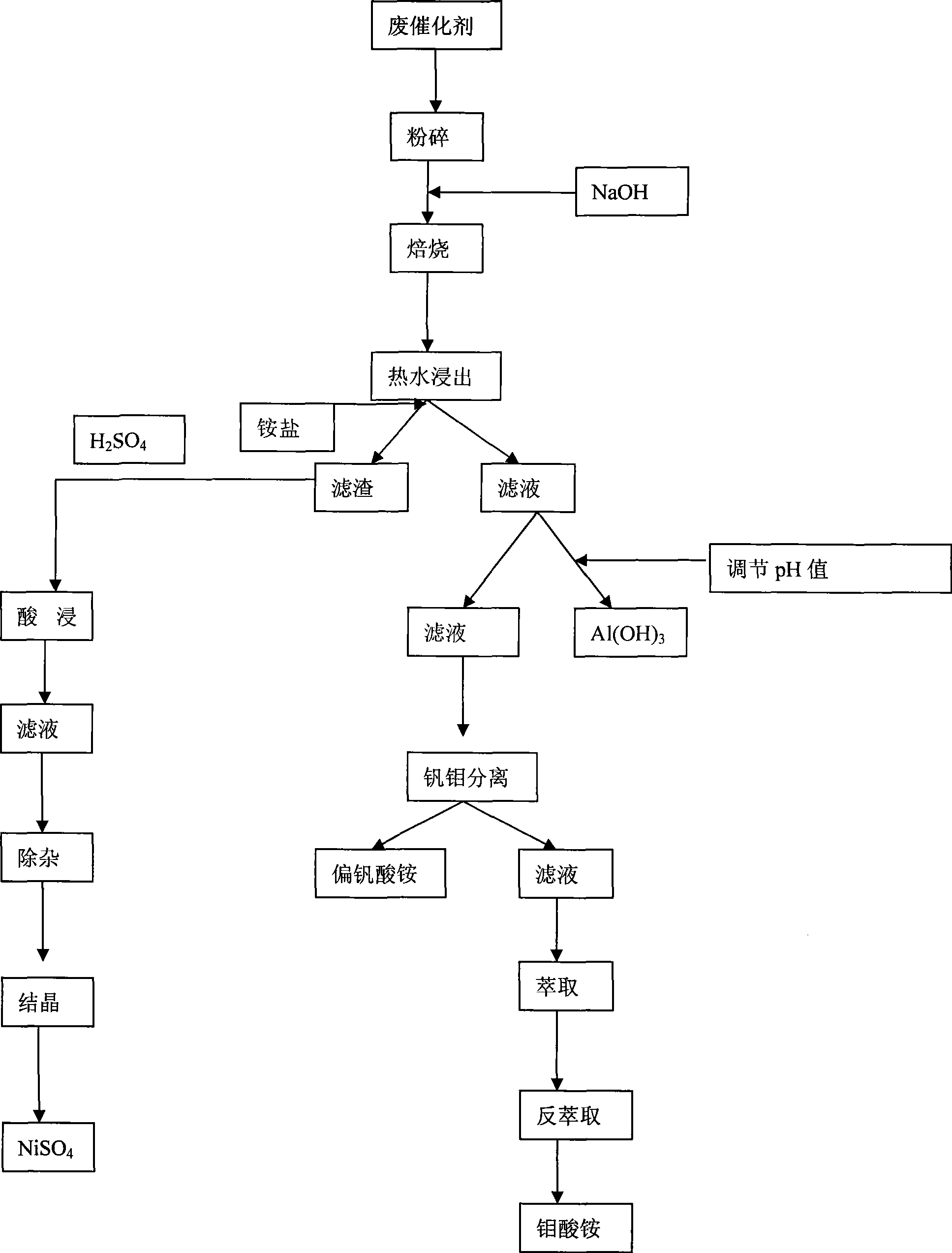
The invention discloses a method for recovering metal oxides from an aluminum scrap-based vanadium molybdenum nickel catalyst. The method comprises the following steps: crushing the aluminum scrap-based vanadium molybdenum nickel catalyst, evenly mixing with sodium hydroxide, calcining at high temperature of 700-1200 DEG C, maintaining a constant temperature for 3-6 hours, crushing sinter blocks, washing nickel slag with countercurrent hot water or washing aluminum hydroxide crystal with the weight ratio of liquid to solid being 5-10:1 at the temperature of 80-100 DEG C for 1.5-3 hours, acid leaching and precipitating and filtering to obtain Al(OH)3, adding ammonium salt to filtrate, filtering to obtain ammonium metavanadate, extracting molybdenum from the filtrate in which the ammonium metavanadate is filtered, recovering the nickel by acid leaching, hydrolyzing the filtrate for removing impurities from the nickel, condensing and drying to produce crystal nickel sulfate. Comprehensive recovery improves environment conditions, and creates good economic benefit; comprehensive use of secondary resources relieves the insufficient domestic resources, and protects the environment; and the recovery method has simple process and low recovery cost and is applicable to large-scale use.
Owner:WUHU RENBEN ALLOY
Method for synthesizing short-chain olefin by ethylene oligomerization
InactiveCN101200404AHigh activityDistribution has little effectOrganic-compounds/hydrides/coordination-complexes catalystsHydrocarbonsMass spectrometryHigh activity
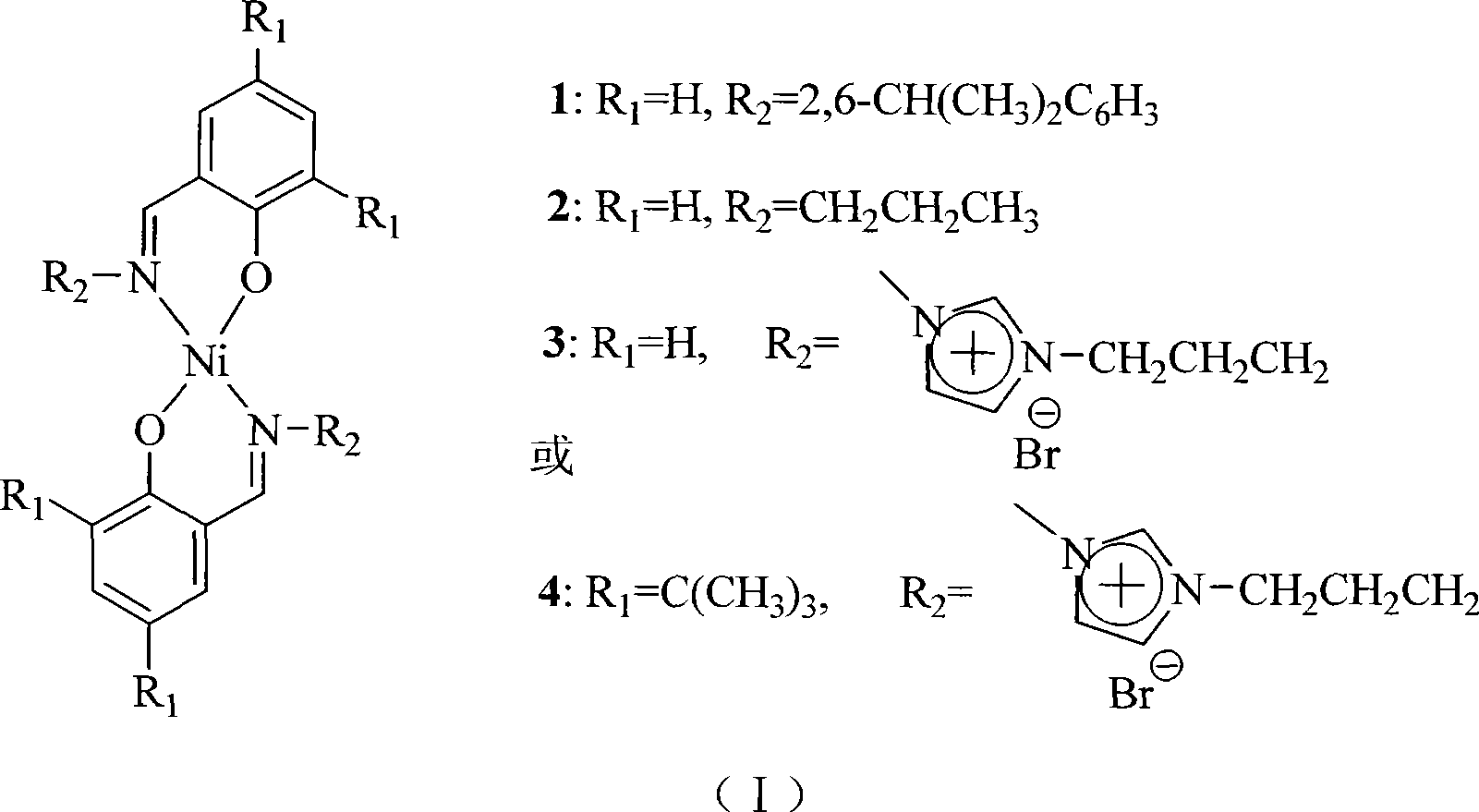
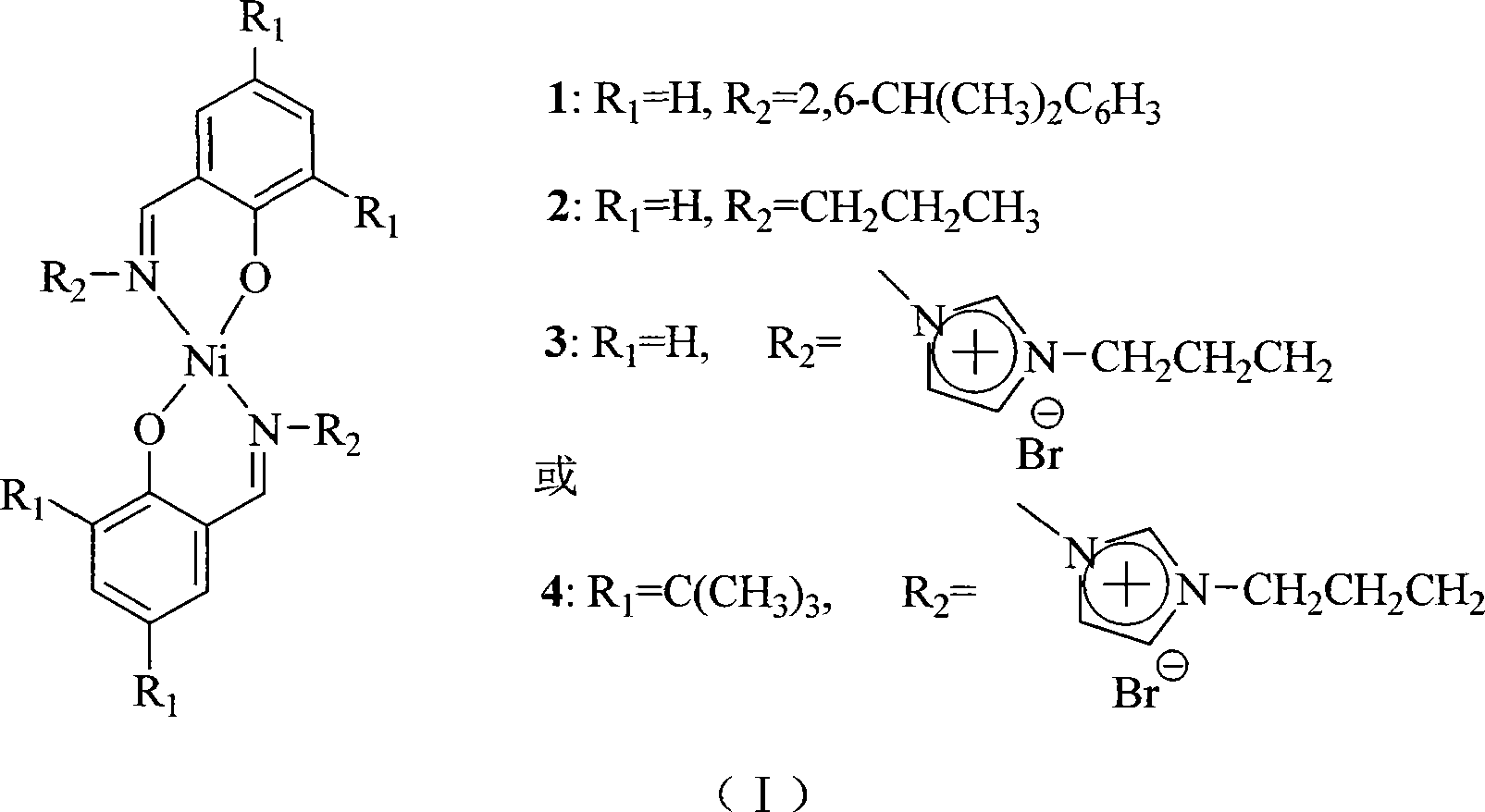
The invention discloses a method for a short chain olefin hydrocarbon through an ethylene oligomeric. The invention is characterized in that: the dual salicylaldehyde imine nickel catalyst is loaded in imidazole Al-ionic ionic liquid; under the assistant catalysis of AlEt2Cl, the ethylene oligosaccharide can be catalyzed in two phase in a mild reaction condition. Comparing with a homogeneous system, the activity of the catalyst system in the ionic liquid can be improved greatly; all generative products are ethylene oligomeric, and the production can be separated through a simple settlement. The ionic liquid of the catalyst can be recycled by being dissolved. The reaction condition has no great impact to the distribution of the oligomeric production. The test result of gas chromatography mass spectrum that the ethylene oligomeric production mainly is a short chain olefin hydrocarbon with the carbon atom of less than 10. Compared with the tradition of salicylaldehyde imine nickel catalyst 1 and 2, the catalyst 3 and 4 after the ion function has a higher activity and a better thermal stability. The test for atomic emission spectroscopy of the plasma shows that the recovery rate of catalyst 1 and 2 can be more than 98 percent, while that of the catalyst 3 and 4 after the ion function can reach 99.9 percent.
Owner:SUN YAT SEN UNIV
Process for preparing hydrogenated C5/C9 copolymerized petroleum resin
The invention discloses a preparing method of hydrogenated C5 / C9 copolymerized oil resin, which comprises the following steps: dissolving C5 / C9 copolymer oil resin in the hydrogenation solvent; decoloring through adsorbing agent to proceed catalytic hydrogenating reaction with hydrogen in the fixed bed reactor at 220-270 Deg C under 2.0-8.0Mpa; setting the bulk air-speed at 0.1-1.0h-1 and hydrogen and oil rate at 300-3000:1; allocating the catalyst as load-patterned nickel catalyst with 35-50% Ni; adopting gamma-Al2O3 as carrier with specific area at 150-300m2 / g and hole conpacitance at 0.2-0.4ml / g; decompressing hydrogenated product; distilling to recycle solvent to obtain the product.
Owner:SINOPEC YANGZI PETROCHEM
High dispersing supported type nickel catalyst prepared by lamellar precursor and preparation method thereof
InactiveCN1483513ACatalyst activation/preparationMetal/metal-oxides/metal-hydroxide catalystsNickel catalystSurface layer
The present invention provides a high-dispersion loaded type nickel catalyst prepared by using lamellar precursor and its preparation method. Said invention uses Al2O3 as carrier, in the surface layer internal hole of Al2O3 the lamellar precursor hydrotalcite (LDHs) containing Ni metal ions is assembled so as to obtain the NiAl-LDHs / Al2O3 composite material, then the composite material is roasted at high temp. and unverted into the correspondent composite metal oxide. Before application the composite metal oxide is undergone the process of reduction treatment so as to obtain the loaded type Ni base catalyst in which the metal Ni is highly dispersed in the surface of internal hole of Al2O3 carrier. Said catalyst has high activity and selectivity, and can be used in catalytic hydrogenation reaction.
Owner:BEIJING UNIV OF CHEM TECH
Method of preparation for carbon nanotube material
InactiveUS20060062714A1Overcomes drawbackSolve UtilizationMaterial nanotechnologyPigmenting treatmentPolymer sciencePolyolefin
The present invention relates to a method for preparing a carbon nanotube material, comprising the steps of: (a) preparing a modified montmorillonite by an ion exchange reaction comprising the substeps of: i) acidifying an alkylamine with equal mole of a concentrated HCl; ii) mixing the resulting acidified alkylamine with a montmorillonite dispersion in 1:1˜2 volume ratio of the acidified alkylamine to the montmorillonite dispersion; and iii) precipitating, filtering and pulverizing to obtain a modified montmorillonite; (b) preparing a catalyst by a hydrogenation reduction method, comprising the substeps of: i) mixing an aqueous solution of nickel nitrate and an alumina-silica hybrid in a weight ratio of 35-45 parts of nickel to 55-65 parts of alumina-silica hydrid, wherein the alumina-silica hydrid contains 10 wt % of alumina and has a particle size of 10-30 μm; ii) drying and calcining the resulting product; and iii) reducing the product with a reducing gas containing hydrogen to produce a nickel-supported catalyst; (c) preparing a polyolefin mixture of a polyolefin, the modified montmorillonite prepared in step a) and the catalyst prepared in step b) in a mixer in the weight ratio of 75˜97.5:0˜20:0˜5 provided that the amounts of the modified montmorillonite and the catalyst are not both 0; and (d) preparing and purifying a nanotube, comprising the substeps of: i) placing the polyolefin mixture obtained in step (c) in a crucible and heating the temperature inside crucible up to 550° C.˜650° C., wherein the heating time begins from the burning of the polymer and ends when no flame can be observed and cooling the polyolefin mixture to obtain a mixture of carbon nanotube, nickel catalyst and montmorillonite; ii) adding a hydrofluoric acid with a concentration of 20-50% to the mixture, mixing, and separating to obtain a carbon powder; and iii) adding a mixture of a concentrated sulfuric acid and a concentrated nitric acid, refluxing, and separating to obtain a purified carbon nanotube. The carbon source material used in the present invention was polyolefin or recovered polyolefin whose price was low and whose source was abundant. The manufacturing facilities involved for preparing supported catalyst and modified montmorillonite were simple. The mixer used was that of the conventional fabricating equipment for polymeric materials while the facilities used for synthesizing carbon nanotube material were porcelain crucible and common flame. The method could simultaneously solve the problem of recovery and utilization of waste plastics.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
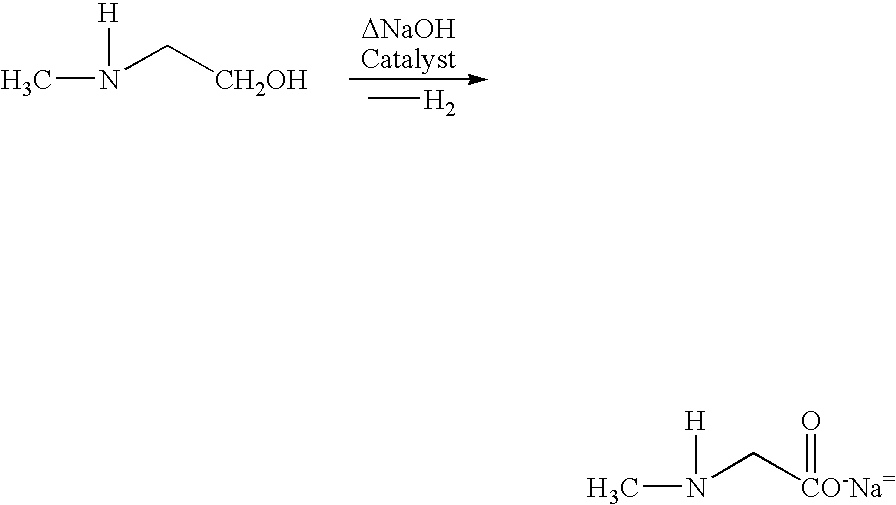
Process and catalyst for dehydrogenating primary alcohols to make carboxylic acid salts
InactiveUS20050043566A1Large specific surface areaReduce reduction reactionOrganic compound preparationGroup 5/15 element organic compoundsNickel catalystDehydrogenation
This invention is directed to a process for making a salt of a carboxylic acid. The process comprises contacting a catalyst with an alkaline mixture comprising a primary alcohol. In one embodiment, the catalyst comprises a metal supporting structure (preferably a metal sponge supporting structure comprising at least about 10% by weight nickel) having a copper-containing active phase at the surface thereof and iron as a catalyst modifier. The supporting structure is resistant to deformation under the conditions of the dehydrogenation reaction. This invention is also directed to novel nickel-containing catalysts having a copper-containing active phase and iron as a catalyst modifier which may, for example, be used in the above process. This invention is further directed to processes for making such catalysts.
Owner:MONSANTO TECH LLC
Method for deuteration or tritiation of heterocyclic ring
ActiveUS20060025596A1Isotope introduction to heterocyclic compoundsSugar derivativesDecompositionPalladium catalyst
The present invention relates to a method for deuteration of a heterocyclic ring, which comprises subjecting a compound having a heterocyclic ring to sealed refluxing state in a deuterated solvent in the presence of an activated catalyst selected form a palladium catalyst, a platinum catalyst, a rhodium catalyst, a ruthenium catalyst, a nickel catalyst and a cobalt catalyst. In accordance with a method of the present invention, a hydrogen atom belonging to a heterocyclic ring of a compound having a heterocyclic ring can be very efficiently deuterated because temperature of deuteration reaction can be maintained at higher than boiling point of the solvent. Further, a method for deuteration of the present invention can be applied widely to deuteration of various compounds having a heterocyclic ring which are liable to decomposition under supercritical conditions or acidic conditions, leading to industrial and efficient deuteration of a compound having a heterocyclic ring.
Owner:FUJIFILM WAKO PURE CHEM CORP
Nano-nickel catalyst loaded on grapheme and preparation method thereof
InactiveCN102350357AReduced activityExtended service lifeMetal/metal-oxides/metal-hydroxide catalystsNickel catalystHigh intensity
The invention discloses a nano-nickel catalyst loaded on grapheme and a preparation method thereof. The catalyst adopts grapheme as a carrier, which is loaded with 1 mass percent-10 mass percent of nickel particles with a particle size of 5-10nm. The preparation method comprises the steps of: preparing the purchased or self-made grapheme into an ethanol solution, preparing a precursor of grapheme-loaded nickel oxide with an ethanol solution of nickel nitrate hexahydrate and an ethanol solution of grapheme, reducing the precursor so as to obtain the nano-nickel catalyst loaded on grapheme. Grapheme with high strength, big specific surface area and stable structure is taken as the catalyst carrier, and the catalyst has high activity, long service life. Also nickel particles loaded on grapheme have a small size and centralized size distribution ranging from 5 to 10nm. And nickel nano-particles are uniformly dispersed on the grapheme carrier. The preparation method provided in the invention has the advantages of simple preparation process, easy operation, low cost, and less pollution.
Owner:TIANJIN UNIV
Process of synthesizing 1,4-cyclohexyl dione
InactiveCN101020627AReduce usageNo pollution in the processOrganic compound preparationCarbonyl compound preparationNickel catalystOrganic solvent
The present invention relates to environment friendly process of synthesizing 1, 4-cyclohexyl dione. The process includes the following steps: 1. preparing skeleton nickel catalyst; 2. preparing 1, 4-cyclohexyl diol with hydroquinone as material and through catalytic hydrogenation under the action of the skeleton nickel catalyst in a high pressure reactor; and 3. oxidizing with hydrogen peroxide or catalytic dehydrogenation to prepare 1, 4-cyclohexyl dione. The present invention is significant in industrial production of 1, 4-cyclohexyl dione, and has the advantages of easy-to-control mild reaction, low cost, environment friendship, easy-to-prepare catalyst with high stability and reusability, and high product yield.
Owner:HEBEI UNIVERSITY
Preparation method of supported nickel catalyst
InactiveCN101733106AOrganic reductionHydrocarbon by hydrogenationSupercritical dryingNickel catalyst
The invention discloses a preparation method of a supported nickel catalyst, which comprises the following steps of: reacting soluble nickel salt solution and a precipitator to obtain a green precipitate, wherein the precipitator is mixed solution of sodium silicate and sodium carbonate, the Na<+> concentration is 0.1-1 mol / L, the amount of the sodium silicate is calculated according to the content of SiO2 in a carrier, and the amount of the sodium carbonate is 10-30 percent more than that of nickel nitrate based on a stoichiometric ratio; washing the precipitate by distilled water and carrying out supercritical drying or azeotropic distillation drying to obtain a supported nickel catalyst precursor; roasting the supported nickel catalyst precursor for 2-5 hours under N2 atmosphere at 200-600 DEG C, changing the N2 atmosphere into H2 atmosphere, and reducing the supported nickel catalyst precursor for 2-4 hours under H2 atmosphere at 300-550 DEG C to obtain the supported nickel catalyst with the surface area of high active metal nickel. The surface area of the obtained catalyst is 250-450m<2> / g, the average aperture is 4-16nm, and the pore volume is 1.0 -1.9cm<3> / g. The surface area of the active metal nickel of the obtained catalyst is 40-70m<2> / g. The catalyst prepared by using the method can be used for catalyzing hydrogenation of a benzene ring.
Owner:NANJING UNIV
Preparation method and application of amido-imine nickel vinyl polymerization catalyst
ActiveCN102250152AThe synthesis method is simpleRaw materials are cheap and easy to getNickel organic compoundsAnilineBiological activation
The invention discloses a preparation method of an amido-imine nickel catalyst and application of the amido-imine nickel catalyst to catalyzing vinyl polymerization. The complex has the structures of a formula (I) and a formula (II) shown in the specification, wherein R1 is hydrogen or alkyl, R2 is hydrogen or alkyl, R3 is hydrogen or alkyl, R4 is hydrogen or alkyl, and X is halogen. The preparation method of the complex comprises the following steps of: carrying out condensation reaction on a diketone compound and phenylamine through ketoamine to obtain an diimine compound; then reacting with trimethyl aluminum, and hydrolyzing to obtain an amido-imine ligand; finally carrying out coordination reaction on the amido-imine ligand and (DME)NiX2 under the condition without water and oxygen to obtain a nickel complex. The complex disclosed by the invention has a specific ligand replacement structure and can be used for catalyzing the vinyl polymerization under the activation of modified methyl aluminium oxane or alkyl aluminum, showing the characteristics of living polymerization under specific conditions and obtaining the high-molecular-weight narrowly-distributed branched polyethylene.
Owner:SUN YAT SEN UNIV
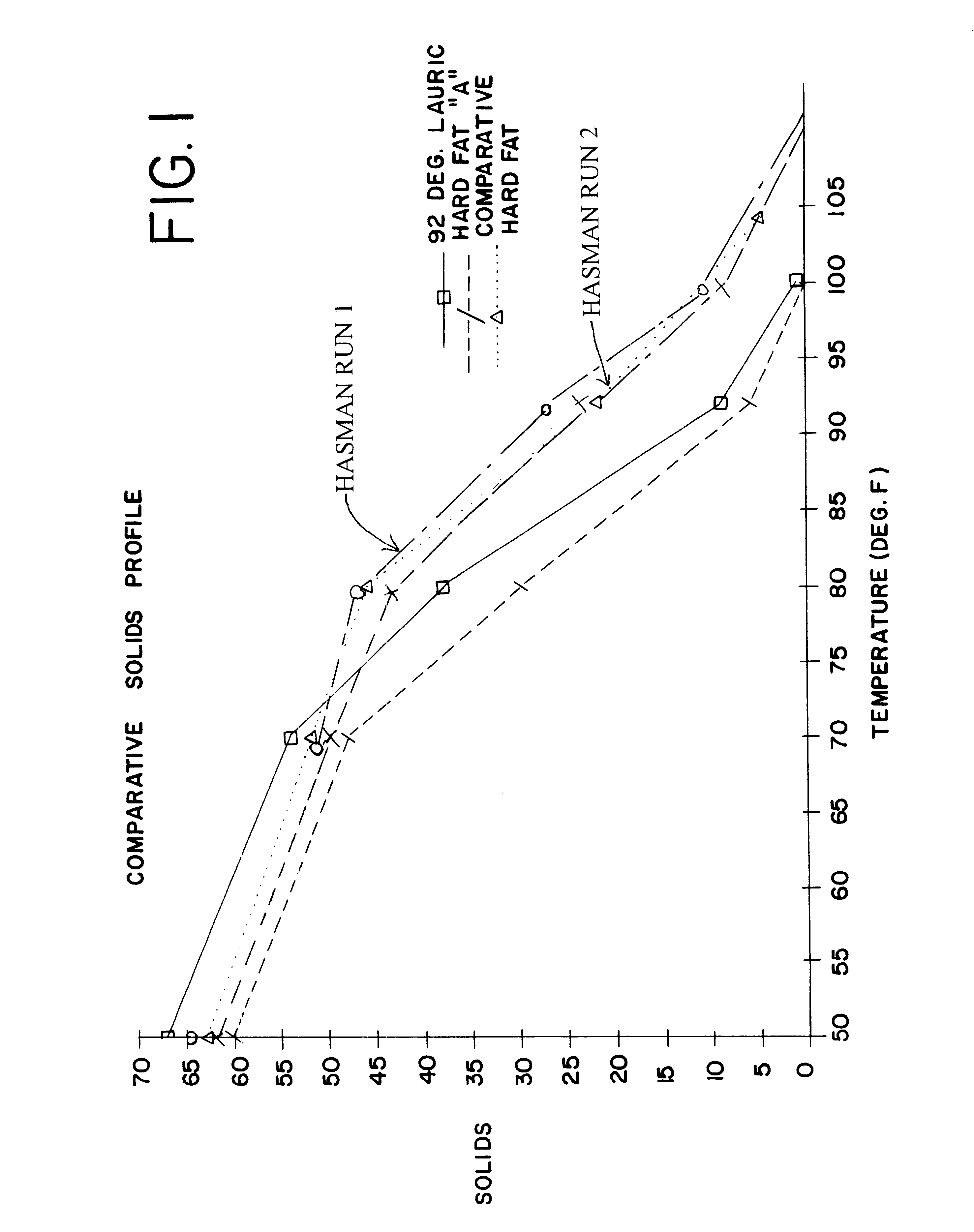
Selectively hydrogenated high oleic oil compositions and process
InactiveUS6391369B1High melting pointMinimizing developmentBiocideFatty acid hydrogenationNickel catalystVegetable oil
Butters are made from high oleic vegetable oils during a procedure by which a large percentage of the oleic acid is transformed into trans-configured elaidic acid, without significantly increasing the saturated fat present in the high oleic vegetable oil. The vegetable oils have an initial oleic acid content of at least about 75 weight percent, with the hard butter made from it being a high elaidic hard fat having at least about 65% trans-configured elaidic acid. The preferred process is a single-step procedure of hydrogenation in the presence of a deadened catalyst such as a sulfur-poisoned nickel catalyst.
Owner:BUNGE FOODS
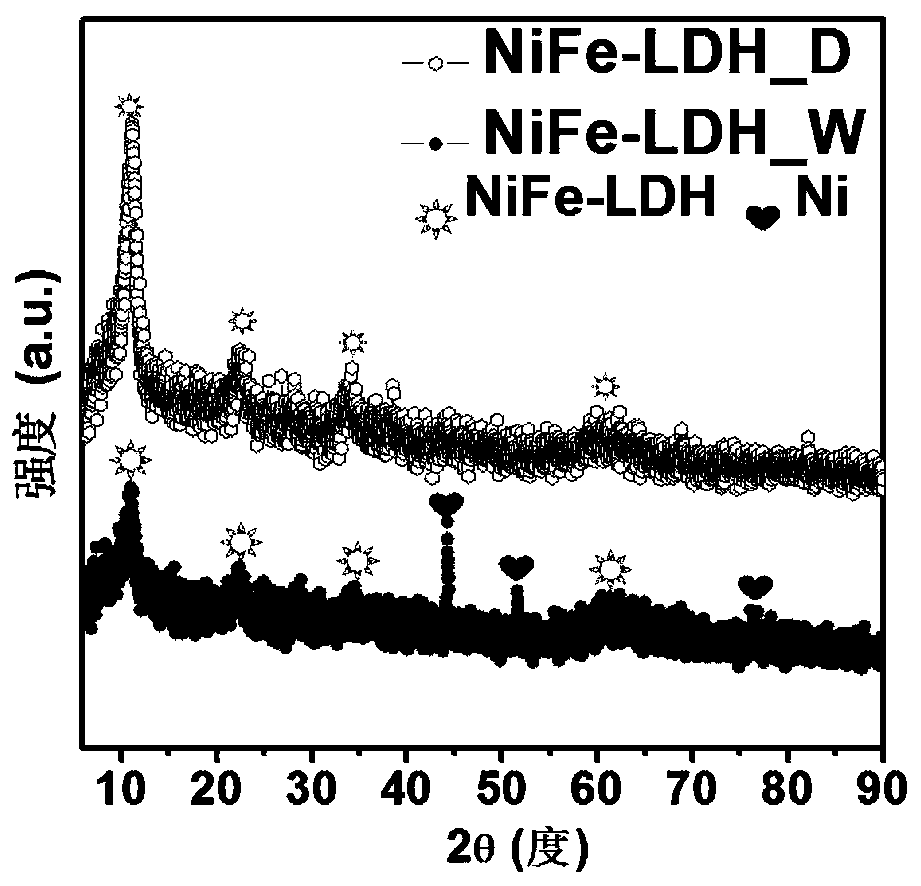
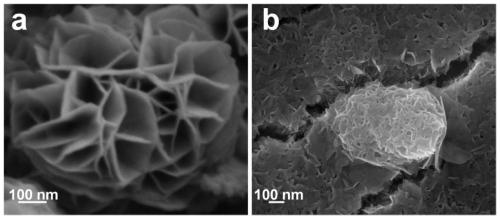
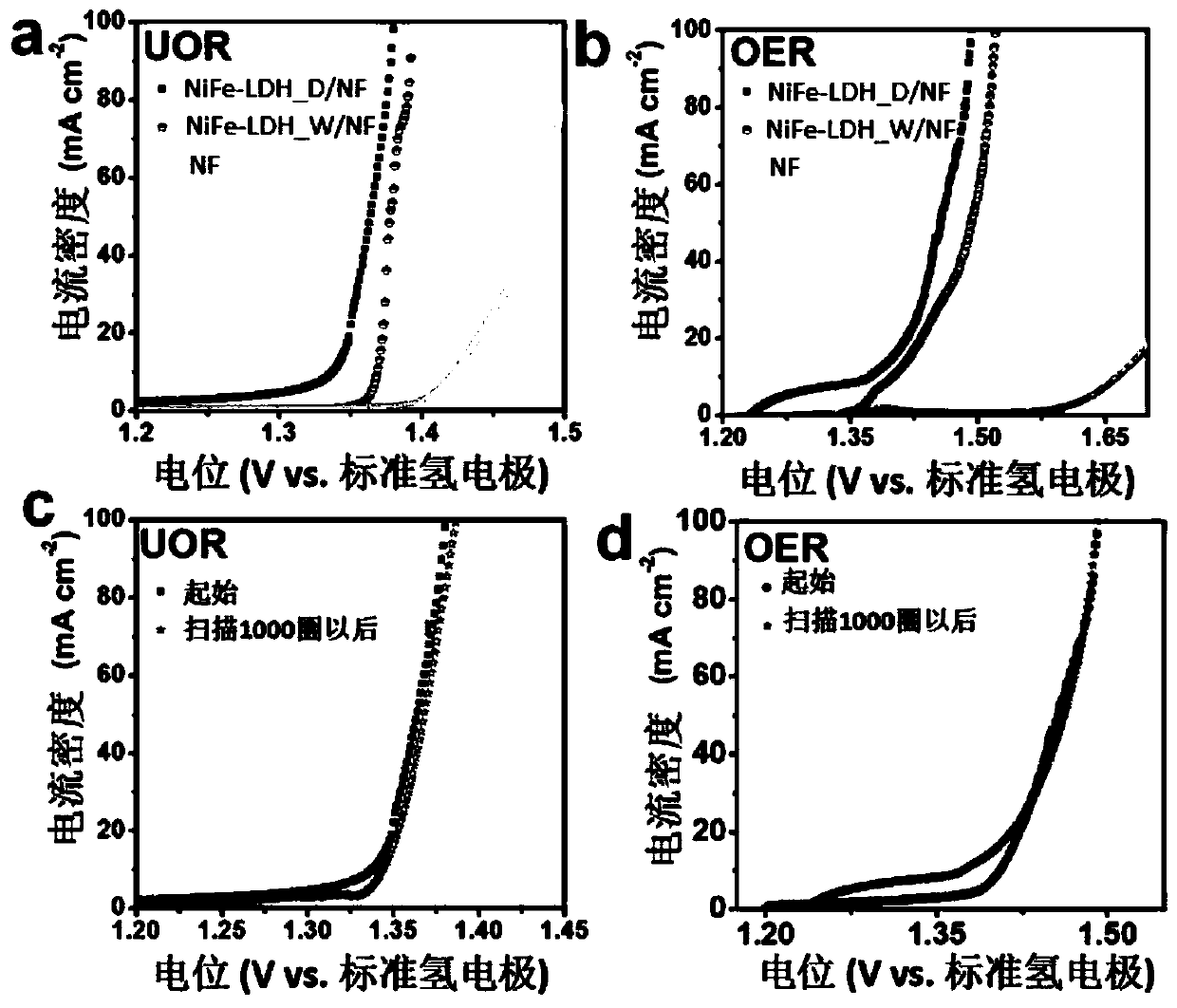
Nickel-iron double hydroxide/foamed nickel catalyst based on ferric chloride/urea eutectic solvent, and preparation method and application thereof
ActiveCN110201670ASimple processLow costCatalyst activation/preparationElectrode shape/formsElectrolysisSolvent
The invention provides a nickel-iron double hydroxide / foamed nickel catalyst based on a ferric chloride / urea eutectic solvent, and a preparation method and an application thereof. The nickel-iron double hydroxide / foamed nickel catalyst is prepared from the eutectic solvent which is prepared from ferric chloride hexahydrate and urea. The preparation method has the advantages of cheap and easily available raw materials, low cost, extremely simple operating process, easily realized reaction conditions, no high temperature, low energy consumption, short preparation period, and suitableness for industrial production. The obtained catalyst is nickel-iron double hydroxide loaded on foamed nickel, and the catalytic active component is nickel-iron double hydroxide (NiFe-LDH), and has a hierarchicalstructure that is a nanoflower structure composed of nanosheets. The catalyst has a good electrocatalytic electrolysis effect on water and urea.
Owner:SHANDONG UNIV
Method for preparing graphite paper with high thermal conductivity
ActiveCN103626172AImprove and enhance performanceImprove performanceMaterial nanotechnologyGraphiteCrack resistanceGas phase
The invention discloses a method for preparing graphite paper with high thermal conductivity. The method comprises the following steps: firstly preparing a nickel catalyst layer on a graphite sheet with a thickness of 0.2-1 mm by adopting a magnetron sputtering system, wherein the thickness of a nickel film is 10-500 nm; performing high temperature annealing on the prepared nickel catalyst layer to form nickel single crystal particles with diameters of 0.5-15 microns; then, preparing graphene on the graphite sheet plated with the nickel catalyst layer by using a chemical vapor deposition method; soaking the graphite sheet coated with a grapheme film in a catalyst solution ferric nitrate or ferric chloride or iron acetate aqueous solution for 10 minutes to 2 hours, taking out the graphite sheet, drying the graphite sheet at 120 DEG C, and putting the graphite sheet in a chemical vapor deposition system to grow a carbon nanotube; and finally, pressing the graphite sheet through a hydraulic press to obtain the graphite paper with high thermal conductivity. The graphite paper prepared by the method disclosed by the invention not only has high thermal conductivity, but also has excellent mechanical strength and cracking resistance, so that the graphite paper can be produced in a large area and can be widely applied to the field of heat conduction.
Owner:SHANGHAI LEVSON ENTERPRISE GRP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com