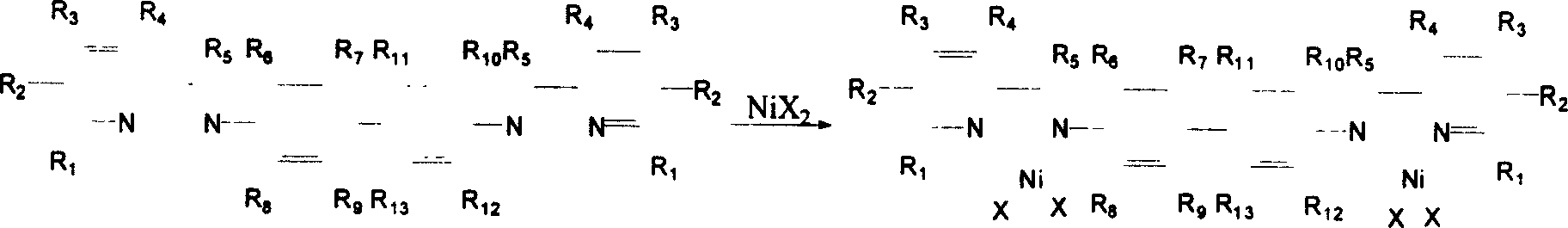Nickel ethylene polymerization catalyzed system, preparation and use thereof
An ethylene polymerization and catalyst technology, applied in the field of nickel-ethylene polymerization catalyst components, can solve the problems of difficult preparation, complicated catalyst synthesis steps and the like, and achieve the effects of simple preparation route, easy purification and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
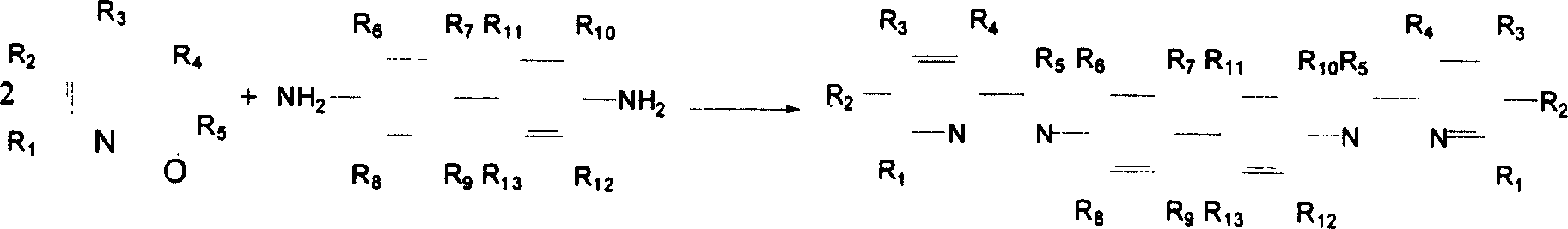
[0041] 1. Synthesis of ligand N, N'-(hydrogen-2-pyridine methylene)-3,3',5,5'-tetramethylbenzidine
[0042] Heat and dissolve 0.730g (3mmol) of 3,3',5,5'-tetramethylbenzidine in 10ml of absolute ethanol, add 0.810g (7.4mmol) of 2-pyridinecarbaldehyde dropwise under stirring, and heat to reflux for 10 hours , evaporated most of the solvent with a rotary evaporator, and recrystallized in absolute ethanol to obtain 1.116 g of bright yellow crystals, yield: 90%, m.p: 169-170 ° C. IR (cm -1 ): 3437 (NH), 1641 (C=N). EI-MS: m / z 418, 1 H NMR (CDCl3 ): δ=8.74(d, 2H), 8.40(d, 2H), 8.32(d, 2H), 7.86(td, 2H), 7.43(td, 2H), 7.34(s, 4H), 2.25(s, 12H).Anal.For C 28 h 26 N 4 (%): calculated value C 80.35, H 6.26, N 13.39; experimental value C 80.34, H 6.36, N 13.37.
[0043] 2. Preparation of [N,N'-(hydrogen-2-pyridylmethylene)-3,3',5,5'-tetramethylbenzidine]nickel complex
[0044] 0.226g ligand N,N'-(hydrogen-2-pyridyl methylene)-3,3',5,5'-tetramethylbenzidine (0.5mmol) was heated and...
Embodiment 2
[0048] 1. The synthesis of ligand N, N'-(hydrogen-2-pyridine methylene)-3,3',5,5'-tetramethylbenzidine is the same as
[0049] Example 1.
[0050] 2. The preparation of [N, N'-(hydrogen-2-pyridine methylene)-3,3',5,5'-tetramethylbenzidine] nickel complex is the same as in Example 1.
[0051] 3. Polymerization of ethylene under normal pressure: At normal temperature and pressure, the dried 250 mL three-neck flask was evacuated and replaced with nitrogen three times, and the catalyst complex (3.4 mg, 5 μmol) was added under the protection of nitrogen. Replaced with ethylene gas three times. 50 mL of freshly distilled toluene and cocatalyst methylaluminoxane (MAO) (3.6 mL, 1.4 M, Al / Ni=500) were added in sequence, and an automatic gas supply device was used to maintain a constant pressure of ethylene during the reaction. After reacting for 60 minutes, 5% acidified ethanol was added to terminate the reaction. Add 200mL of absolute ethanol, shake, and precipitate the polymer pro...
Embodiment 3
[0054] 1. The synthesis of ligand N, N'-(hydrogen-2-pyridine methylene)-3,3',5,5'-tetramethylbenzidine: the same
[0055] Example 1.
[0056] 2. Preparation of [N, N'-(hydrogen-2-pyridylmethylene)-3,3',5,5'-tetramethylbenzidine] nickel complex: same as Example 1.
[0057] 3. Polymerization of ethylene under normal pressure: Under normal temperature and pressure, the dried 250 mL three-neck flask was evacuated and replaced with nitrogen three times, and the catalyst complex (3.4 mg, 5 μmol) was added under nitrogen protection. Replaced with ethylene gas three times. Add 50 mL of freshly distilled toluene and cocatalyst methylaluminoxane (MAO) (5.8 mL, 1.4 M, Al / Ni=800) in sequence, and use an automatic gas supply device to maintain a constant pressure of ethylene during the reaction. After reacting for 60 minutes, 5% acidified ethanol was added to terminate the reaction. Add 200mL of absolute ethanol, shake, and precipitate the polymer product. After standing still for 24 h...
PUM
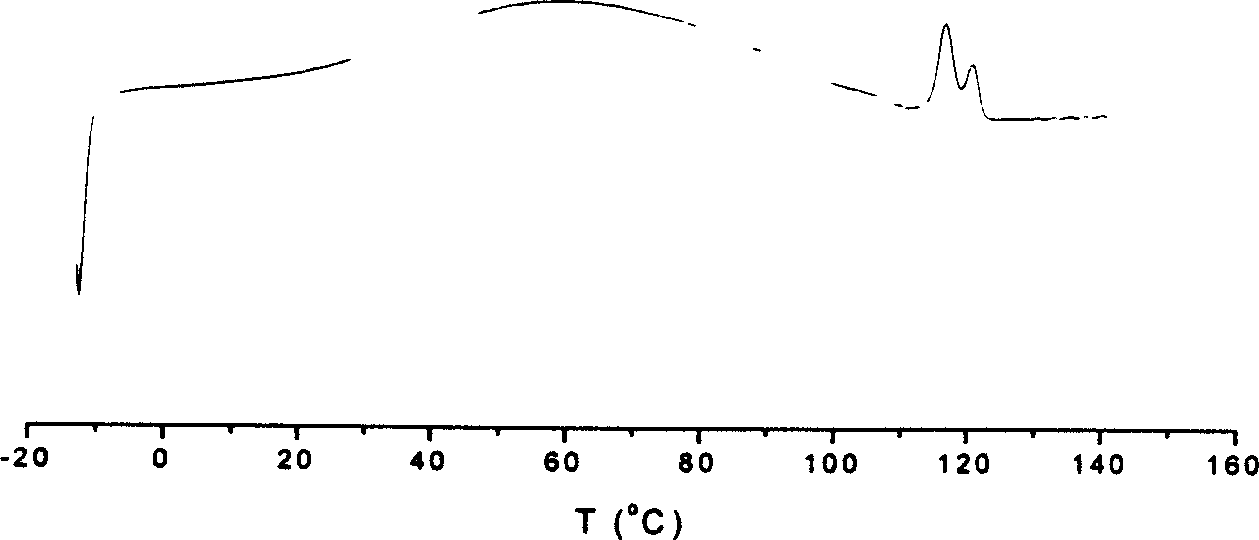
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Mw | aaaaa | aaaaa |
| The molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com