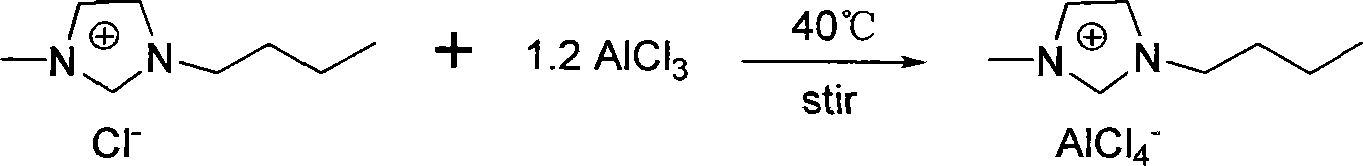Method for synthesizing short-chain olefin by ethylene oligomerization
A technology of ethylene oligomerization and oligomerization, which is applied in the field of olefin oligomerization, can solve the problems of difficult separation of catalyst and product, difficult synthesis of catalyst, poor stability, etc., and achieve the effects of improved catalytic activity, good thermal stability, and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: the synthesis of aluminate imidazolium ionic liquid
[0018] Under anhydrous and oxygen-free conditions, at 0°C, take 30.5g of anhydrous AlCl 3 (0.229mol) was added to 33.3g of 1-butyl-3-methylimidazole chloride (0.191mol), then the temperature was raised to 40°C, and stirred overnight to obtain an imidazolium-aluminate ionic liquid with a molar ratio of 1.2.
[0019]
Embodiment 2
[0020] Embodiment 2: the synthesis of double salicylaldamine nickel compound 1
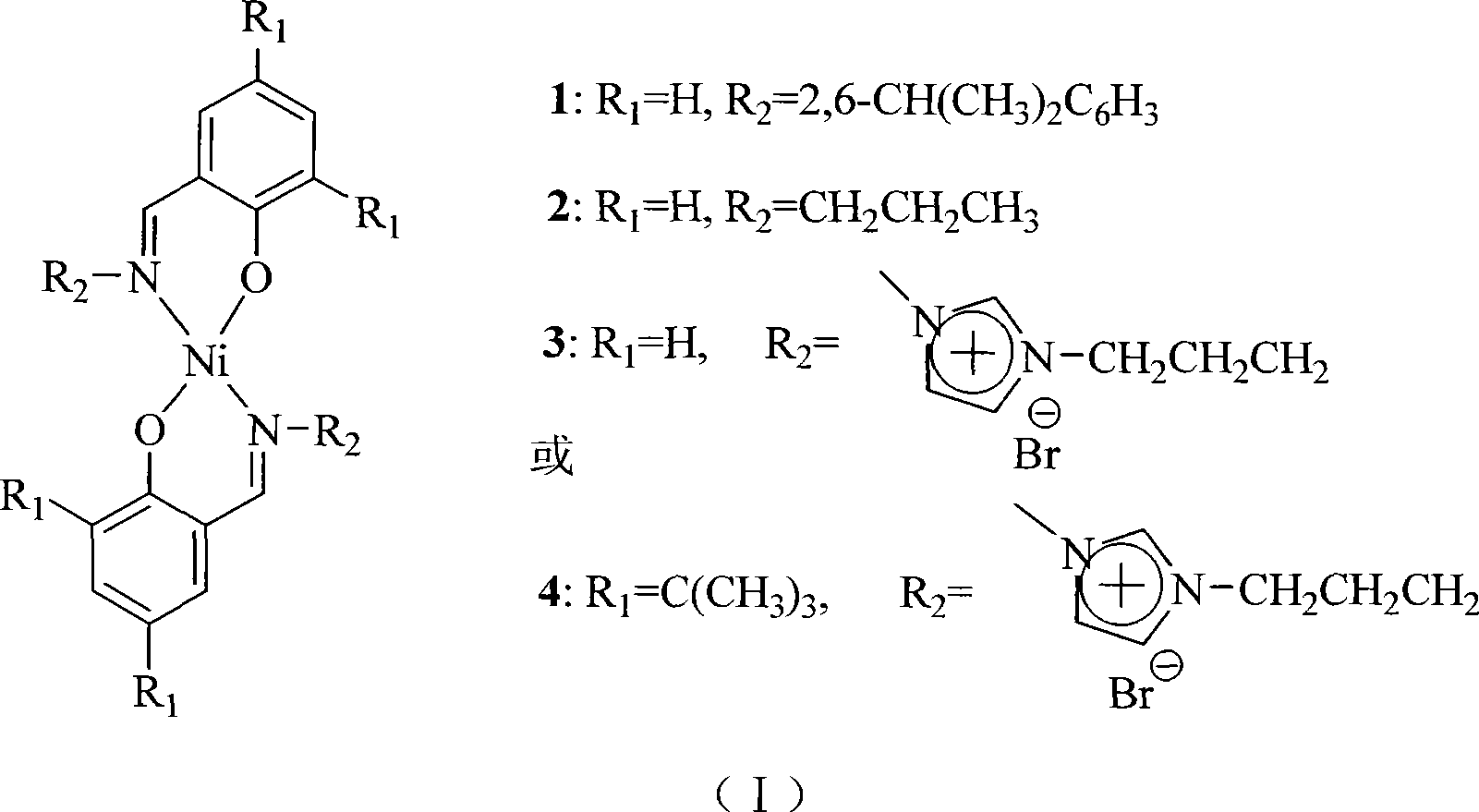
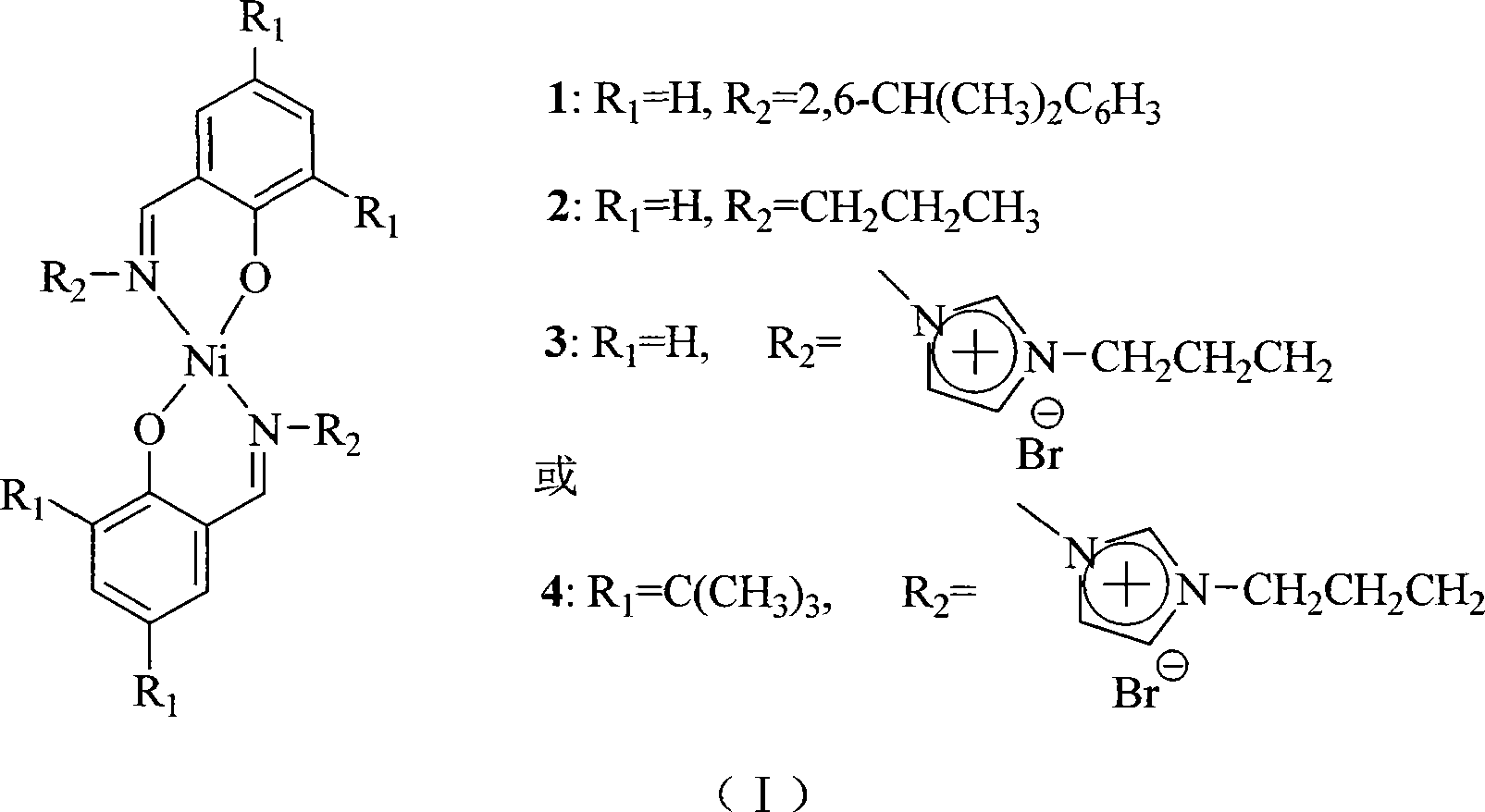
[0021] 1ml of salicylaldehyde (12.9mmol) was added to the solution containing 6.85g (38.7mmol) of 2,6-diisopropylaniline, 1.60g of Ni(OAc) 2 4H 2 O (6.45mmol) in 100ml ethanol solution, heated to reflux for 4h, cooled to room temperature, a large amount of green crystals were generated, filtered, and recrystallized in n-heptane to obtain fine granular green crystals, weighing 2.62g, yield 65.6 %. Elemental analysis (by C 38 h 44 N 2 NiO 2 Calculated, %), theoretical value: C, 73.68, H, 7.16, N, 4.52; found value: C, 73.66, H, 7.31, N, 4.43.
Embodiment 3
[0022] Embodiment 3: the synthesis of double salicylaldamine nickel compound 2
[0023] 1ml of salicylaldehyde (12.9mmol) was added to the solution containing 2.28g (38.7mmol) n-propylamine, 1.60gNi(OAc) 2 4·H 2 In 100ml ethanol solution of O (6.45mmol), heat and reflux for 4h, cool to room temperature, generate a large amount of green crystals, filter, recrystallize in n-heptane to obtain granular green crystals, weighing 1.55g, yield 62.8% . Elemental analysis (by C 20 h 24 N 2 NiO 2 Calculated, %), theoretical value: C, 62.70, H, 6.31, N, 7.31; found value: C, 62.68, H, 6.41, N, 7.29.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com