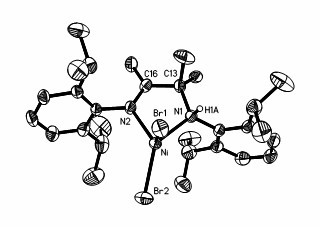
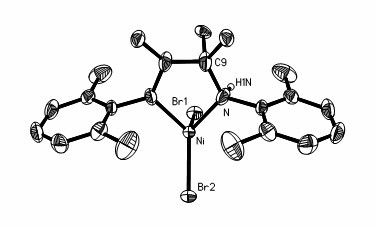
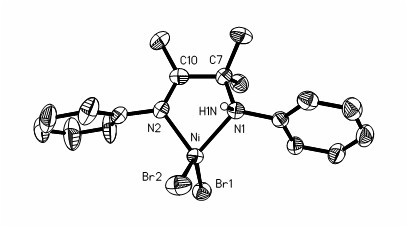
Preparation method and application of amido-imine nickel vinyl polymerization catalyst
A kind of amino imine nickel, when the amino imine nickel technology is applied in the field of catalyzing the active polymerization of ethylene and the preparation of the amino imine nickel complex, which can solve the problem of widening the dispersion coefficient of the polymer, worsening the solubility, reducing the Catalyst activity and other issues, to achieve the effect of simple synthesis method, wide variety and low cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Synthesis of α-diimine compound A1
[0062] Under nitrogen atmosphere and room temperature, 30 mL of absolute ethanol, 7.1 g (40 mmol) of 2,6-diisopropylaniline, 1.55 g (18 mmol) of diacetyl, and 0.5 mL of formic acid were added successively to a 100 mL branched flask. The reaction was stirred at ℃ for 12 h. After cooling to room temperature, the solvent was removed, and the crude product was recrystallized from ethanol to obtain 5.8 g of orange-yellow crystals with a yield of 79.8%. 1 H NMR (CDCl 3 , 500 MHz): 7.19-7.10 (m, 6H, Ph), 2.72 (septet, 4H, J = 6.87 Hz, CH), 2.10(s, 6H, CH 3 ), 1.19 (dd, 24H, J 1 = 2.61Hz, J 2 = 6.87 Hz, CH 3 ). 13 C NMR (CDCl 3 , 125 MHz): 168.15, 146.16, 135.03, 123.73, 122.99, 28.51, 23.00, 22.68, 16.57.
[0063]
Embodiment 2
[0065] Synthesis of α-diimine compound A2
[0066] According to the synthesis method of α-diimine compound A1 in Example 1, 2,6-dimethylaniline was used to replace 2,6-diisopropylaniline, and other operating conditions were the same to obtain 3.4g orange-yellow crystals, the yield 64.6%. 1 H NMR (CDCl 3 , 500 MHz): 7.08 (d, 4H, J = 7.50 Hz, Ph), 6.95 (t, 2H, J = 7.46 Hz, Ph), 2.05 (s, 6H, CH 3 ), 2.04(s, 12H, CH 3 ). 13 C NMR (CDCl 3 , 125 MHz): 167.96, 148.29, 127.86, 124.52, 123.18, 17.71, 15.73.
[0067]
Embodiment 3
[0069] Synthesis of α-diimine compound A3
[0070] According to the synthesis method of α-diimine compound A1 in Example 1, aniline was used instead of 2,6-diisopropylaniline, and other operating conditions were the same to obtain α-diimine compound A3. 1 H NMR (CDCl 3 , 500 MHz): 7.37(t, 4H, J = 7.95 Hz, Ph), 7.12(t, 2H, J = 7.45 Hz, Ph), 6.79(d, 4H, J = 7.34Hz, Ph), 2.16(s, 6H, Me). 13 C NMR (CDCl 3 , 125 MHz): 168.08, 150.85, 128.85, 123.68, 118.61, 15.23.
[0071]
PUM
| Property | Measurement | Unit |
|---|---|---|
| polydispersity index | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com