A crystal form of baricitinib and preparation method thereof
A baricitinib and crystal form technology, applied in the field of baricitinib crystal form A and its preparation, can solve the problem of unfavorable crystal form conversion, instability of baricitinib, and strong hygroscopicity easily occurring, etc. problem, to achieve the effect of excellent high temperature stability and good bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 to 6
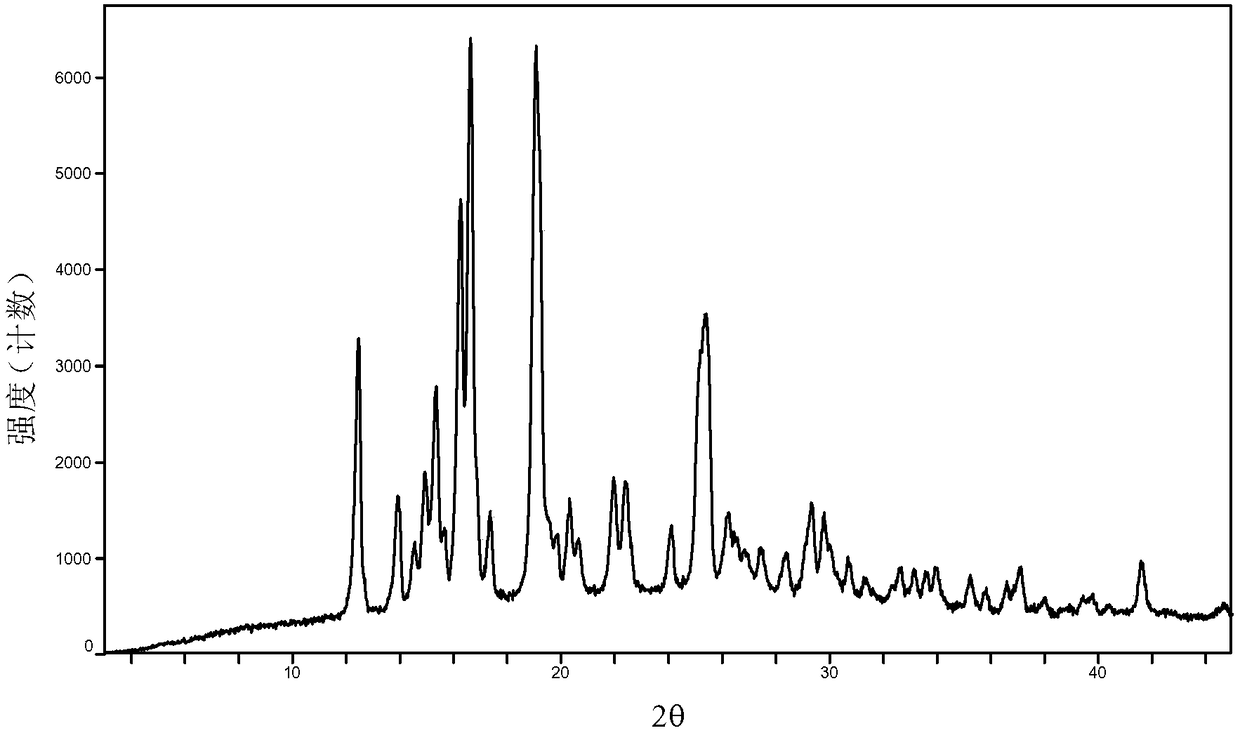
[0035] Embodiments 1 to 6 Preparation of Baricitinib A Crystal Form
[0036] Weigh 800 mg of baricitinib raw material into a container, add 1.5 mL of N,N dimethylformamide to completely dissolve it. Slowly add 50 mL of the solvent in Table 1 (analytical grade, water is deionized water) to the obtained solution respectively, let it stand overnight, filter, and vacuum-dry to obtain an off-white solid. Weigh and calculate its yield, and the results are shown in Table 1.
[0037] Table 1 Preparation of baricitinib A crystal form
[0038] Example
Embodiment 7 to 12
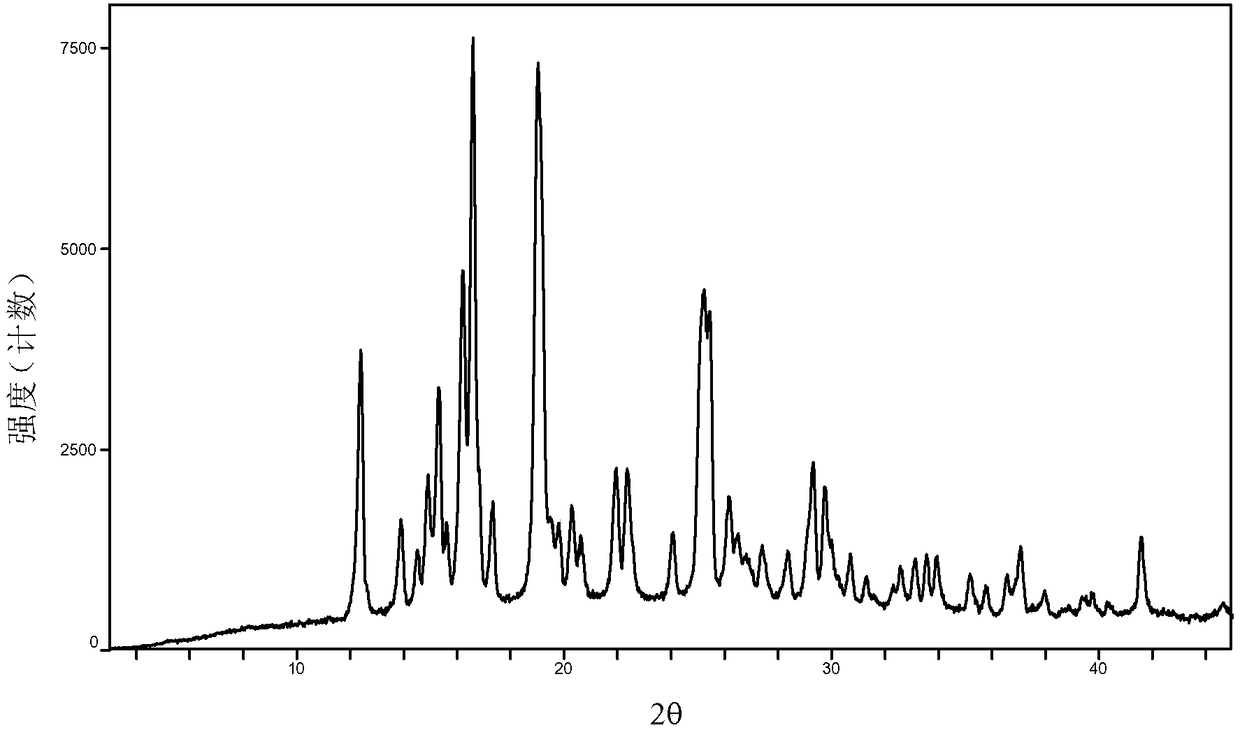
[0039] Examples 7 to 12 Preparation of crystal form A of baricitinib
[0040] Weigh 1.0 g of baricitinib raw material into a container, add 1.0 mL of N,N dimethylformamide to completely dissolve it. Slowly add 60 mL of the solvent in Table 1 (analytical grade, water is deionized water) to the obtained solution respectively, let it stand overnight, filter, and vacuum-dry to obtain an off-white solid.
Embodiment 13 to 18
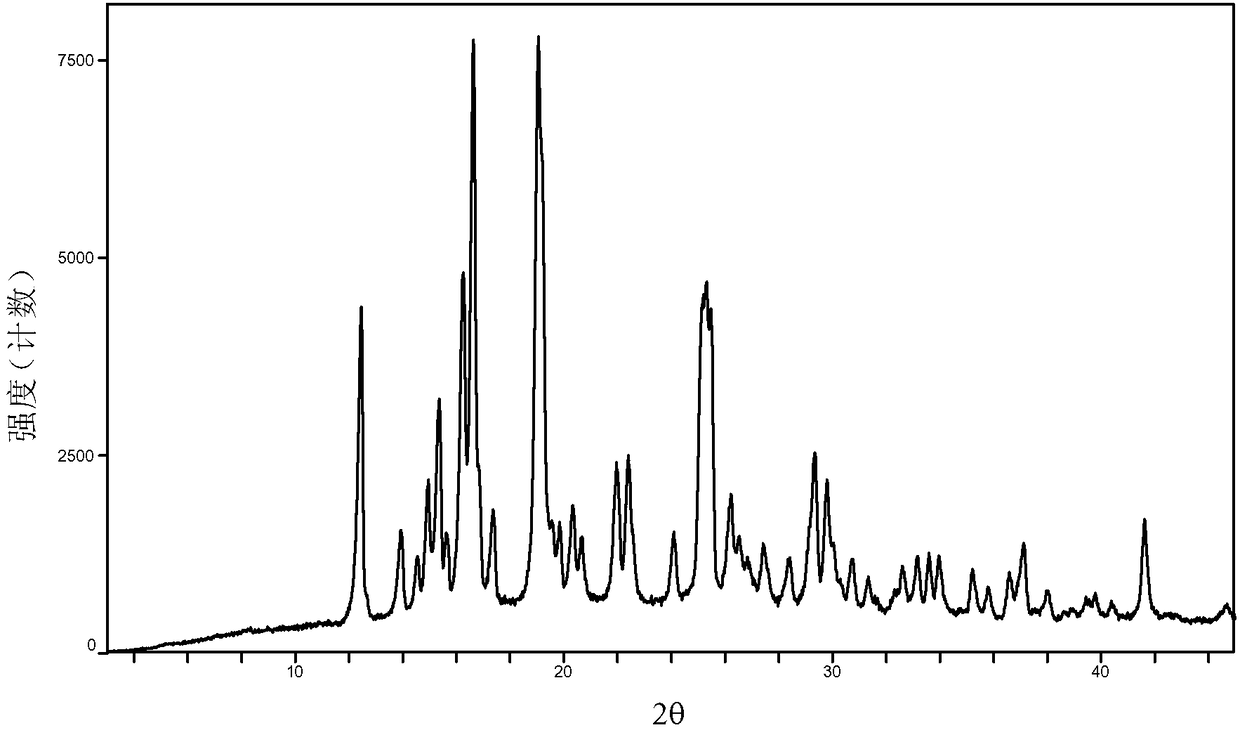
[0041] Examples 13 to 18 Preparation of crystal form A of baricitinib
[0042]Weigh 100mg of baricitinib raw material into a container, add 0.5mL N,N dimethylformamide to dissolve it completely. Slowly add 10 mL of the solvent in Table 1 (analytical grade, water is deionized water) to the obtained solution respectively, let it stand overnight, filter, and vacuum-dry to obtain an off-white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com