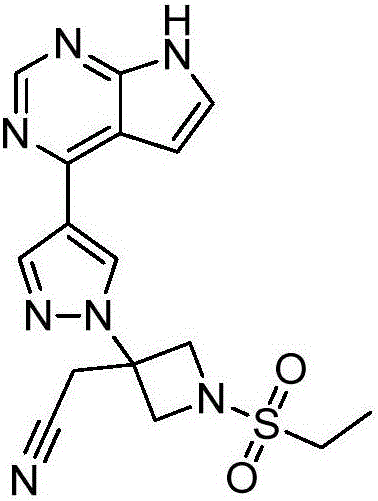
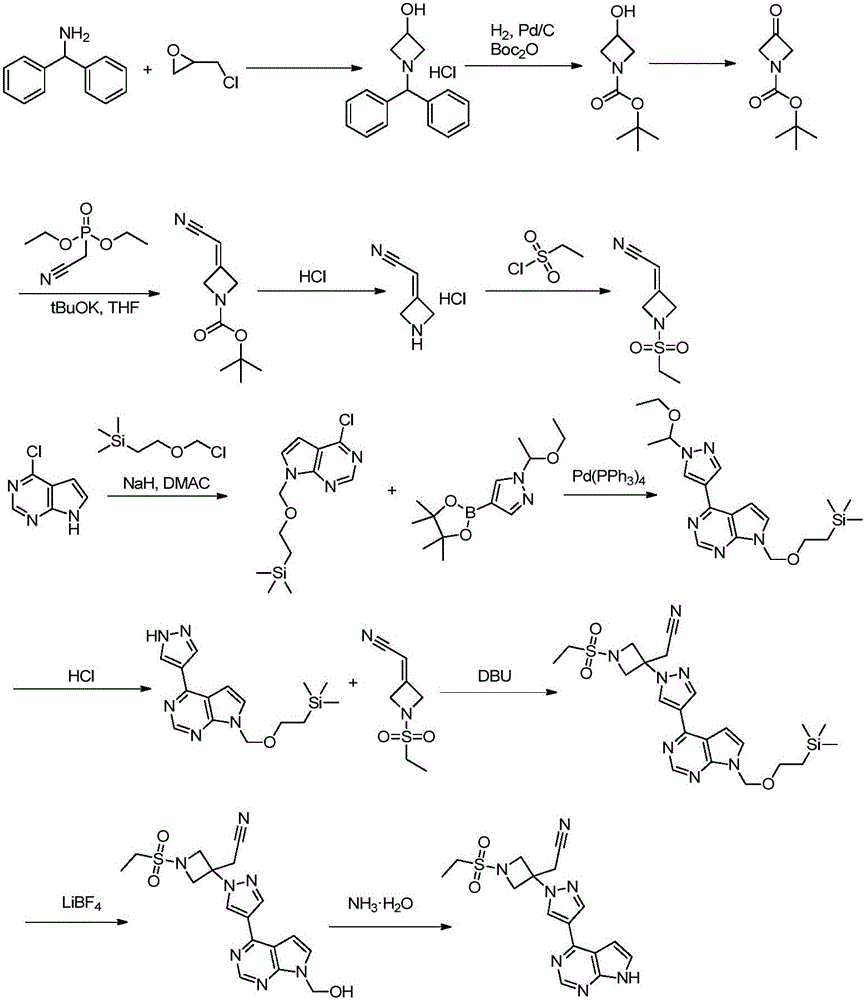
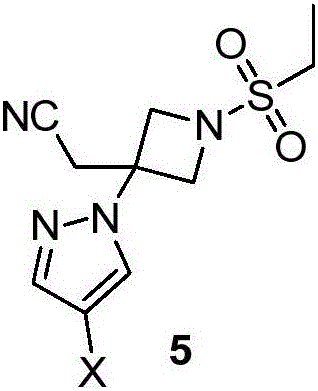
Novel synthetic method for baricitinib and intermediate thereof
A baricitinib and synthetic method technology, applied in the field of medicine and chemical industry, can solve the problems of high operation risk, high process cost and low efficiency, and achieve the effects of shortening the reaction steps, high product purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052]
[0053] Add compound 1 (10.75g, 100mmol) and dichloromethane (86mL) into a three-necked flask, stir evenly, cool to 0-5°C, add diisopropylethylamine (38.77g, 300mmol), stir for 5-5°C after adding For 10 minutes, add ethylsulfonyl chloride solution (15.43g, 120mmol, dissolved in 21mL of dichloromethane) dropwise, and after the dropping, warm up to room temperature and react for 3-4 hours, add 108mL of 0.5mol / L dilute hydrochloric acid to quench the reaction, stir and divide liquid, and the aqueous phase was extracted twice with 43 mL of dichloromethane, and the combined organic phase was washed once with saturated brine (108 mL), dried over sodium sulfate, concentrated and separated by column chromatography with petroleum ether ethyl acetate mixed solvent to obtain compound 2 (14.20 g ,87%).
[0054] ESI m / z=164.1(M+1), 1 H NMR (300MHz, CDCl3): δ4.08(d, J=2.4Hz, 2H), 3.94(d, J=2.6Hz, 2H), 3.35-3.10(m, 2H), 1.40-1.20(m, 3H )ppm.
[0055] The base diisopropylethylam...
Embodiment 2
[0057]
[0058] Add compound formula 2 (16.32g, 100mmol), cyanoacetic acid (17.01g, 200mmol) and toluene (82mL) into the three-necked flask, stir evenly, add piperidine (17.03g, 200mmol), after the addition, heat up to reflux reaction 16 -24 hours, after the reaction is completed, cool to room temperature and add 326 mL of 0.5 mol / L dilute hydrochloric acid to quench the reaction, separate the layers, extract the aqueous phase with ethyl acetate (163 mL) once, combine the organic phases and wash with saturated brine twice (163 mL), After drying over sodium sulfate and concentrating, compound 3 (15.08 g, 81%) was obtained by column chromatography with a mixed solvent of petroleum ether and ethyl acetate. ESI m / z=187.2(M+1), 1 HNMR (DMSO-d6, 400MHz) δ5.45 (t, J = 2.4Hz, 1H), 4.79 (t, J = 2.8Hz, 2H), 4.72 (t, J = 2.4Hz, 2H), 3.10-2.90 ( m,2H), 1.52-1.25(m,3H).
[0059] In embodiment 2, additive pyridine can be replaced by piperidine, acetic acid or ammonium acetate; solvent ...
Embodiment 3
[0061]
[0062] Add 3 (18.62g, 100mmol), 4-iodo-pyrazole 4a (19.40g, 100mmol) and acetonitrile (93mLmL) into the three-necked flask, stir well and then add DBU (15.20g, 100mmol), heat up to 35~ React overnight at 40°C. Part of the acetonitrile was spun off at the end of the reaction, the reaction was quenched by adding 0.5mol / L dilute hydrochloric acid (186mL), the aqueous phase was extracted three times with ethyl acetate (93mL), the combined organic phases were washed twice with saturated brine (93mL), dried over sodium sulfate, After concentration, compound 5a (29.66 g, 78%) was obtained by column chromatography with a mixed solvent of ethyl acetate and petroleum ether. ESI m / z=381.1(M+1), 1 HNMR(DMSO-d6,400MHz)δ8.10(s,1H),7.62(s,1H),4.40(d,J=8.0Hz,2H),4.24(d,J=8.4Hz,2H),3.63( s,2H), 3.34-3.15(m,2H), 1.44-1.24(m,3H)ppm.
[0063] In Example 3, 4-iodo-pyrazole can be replaced by 4-chloro-pyrazole, and the basic substance 1,8-diazabicyclo[5.4.0]undec-7-ene can be diisopr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com