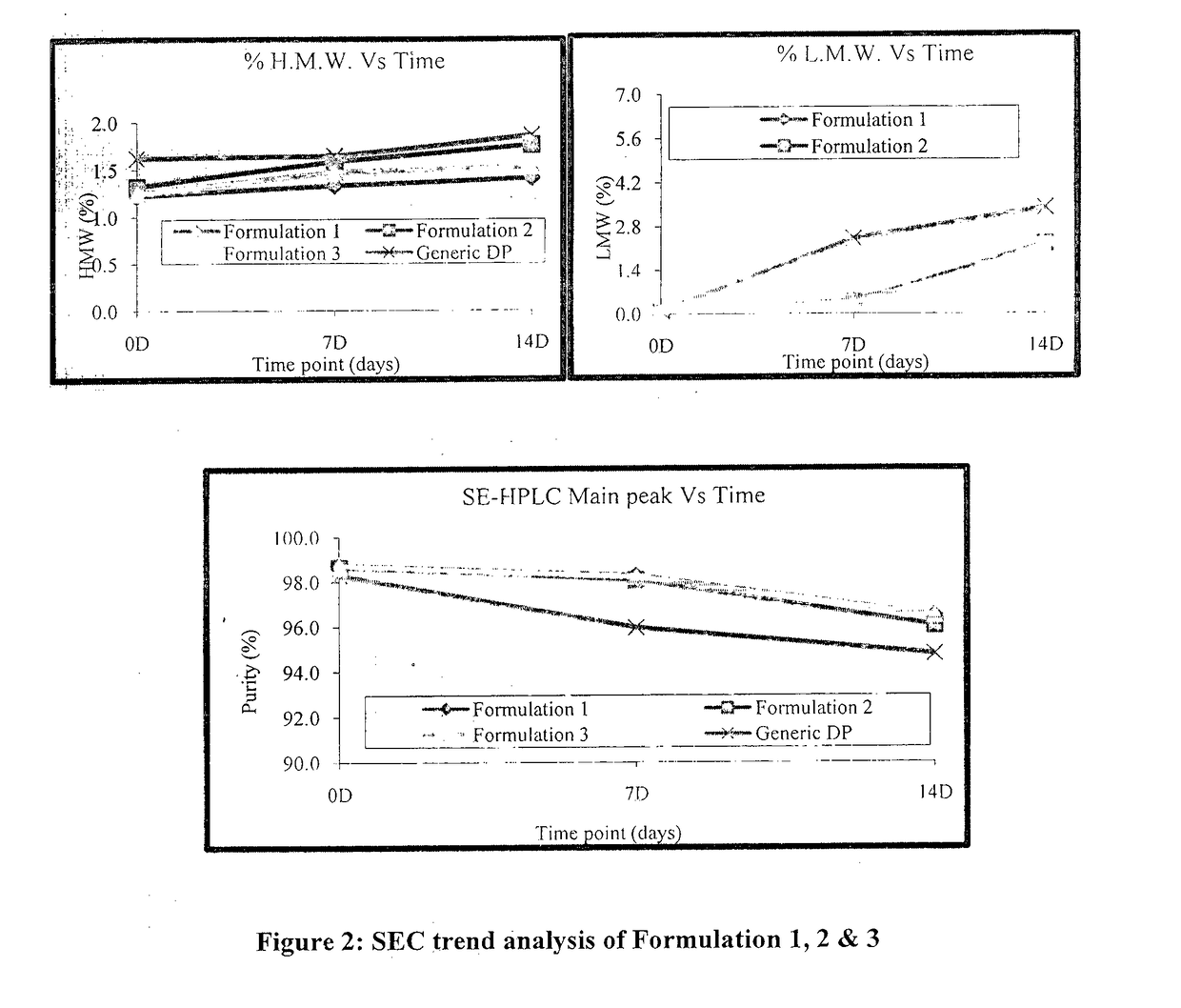
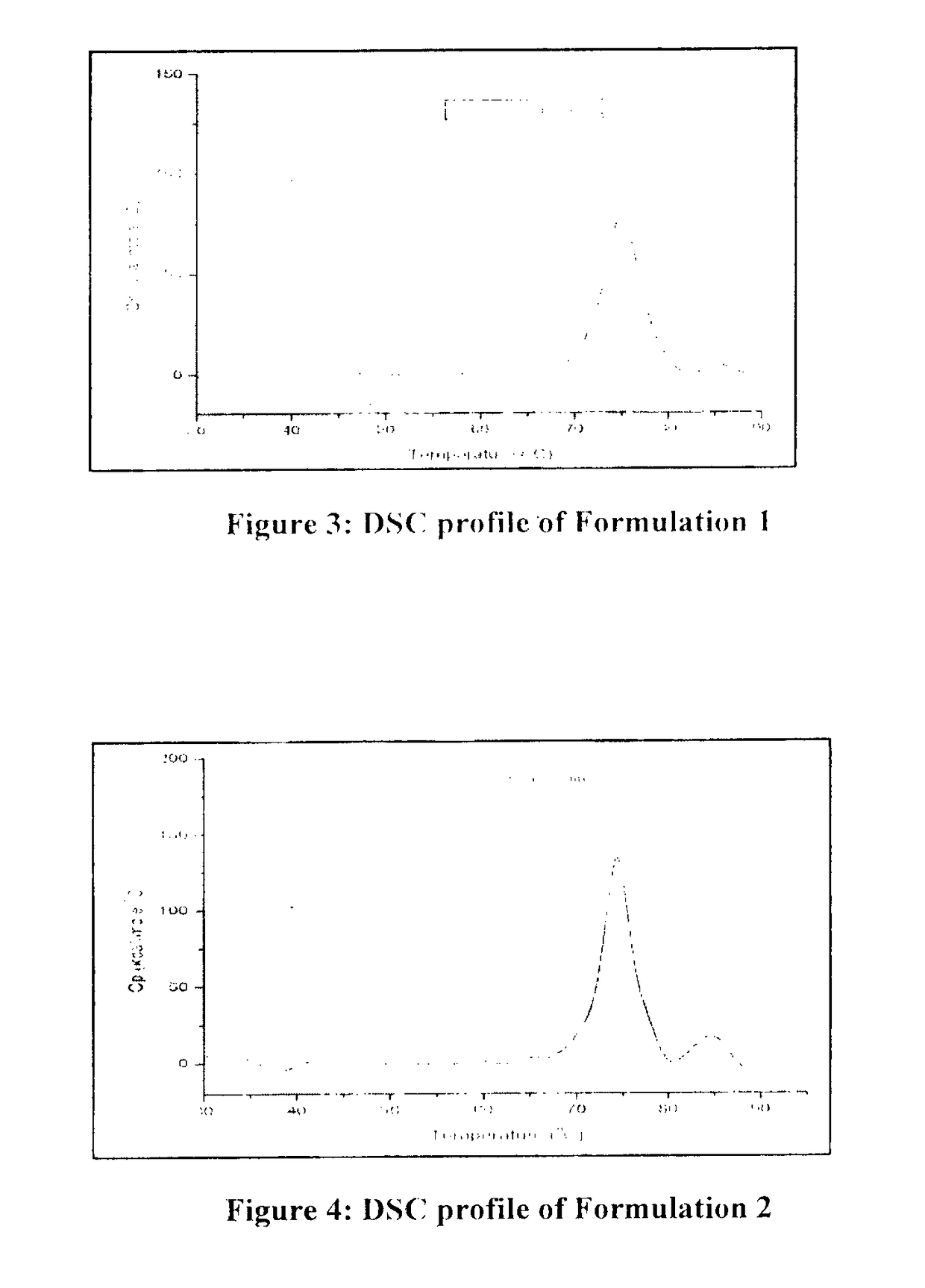
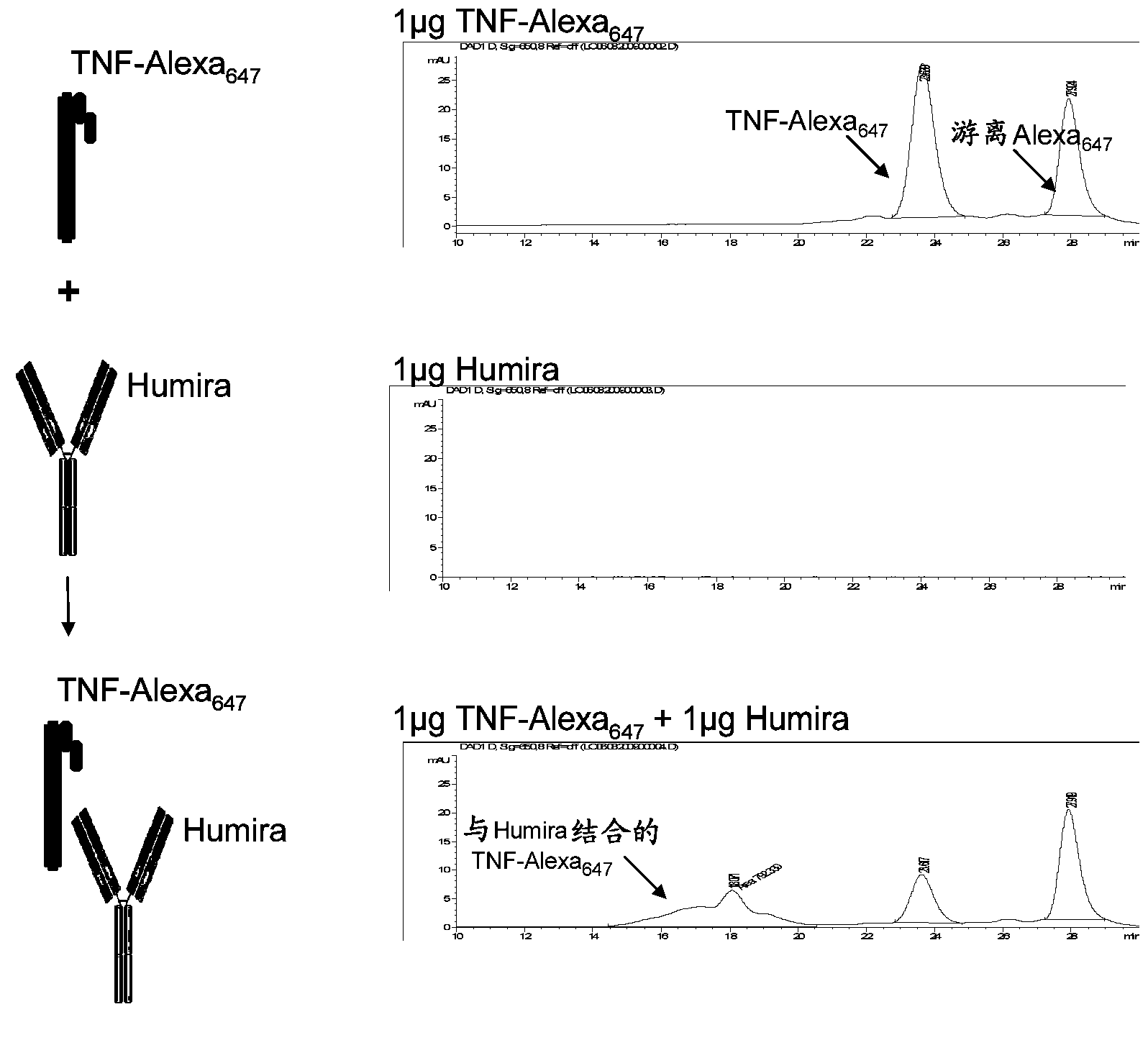
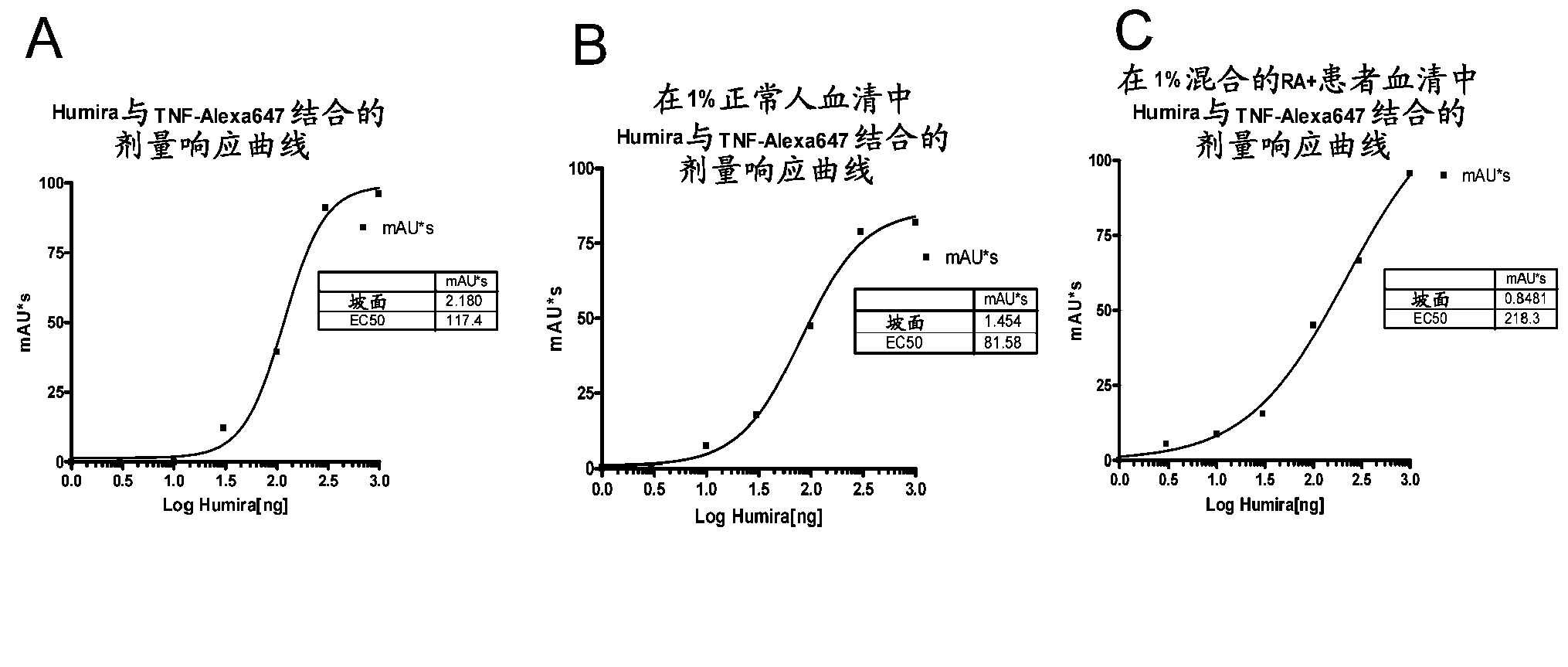
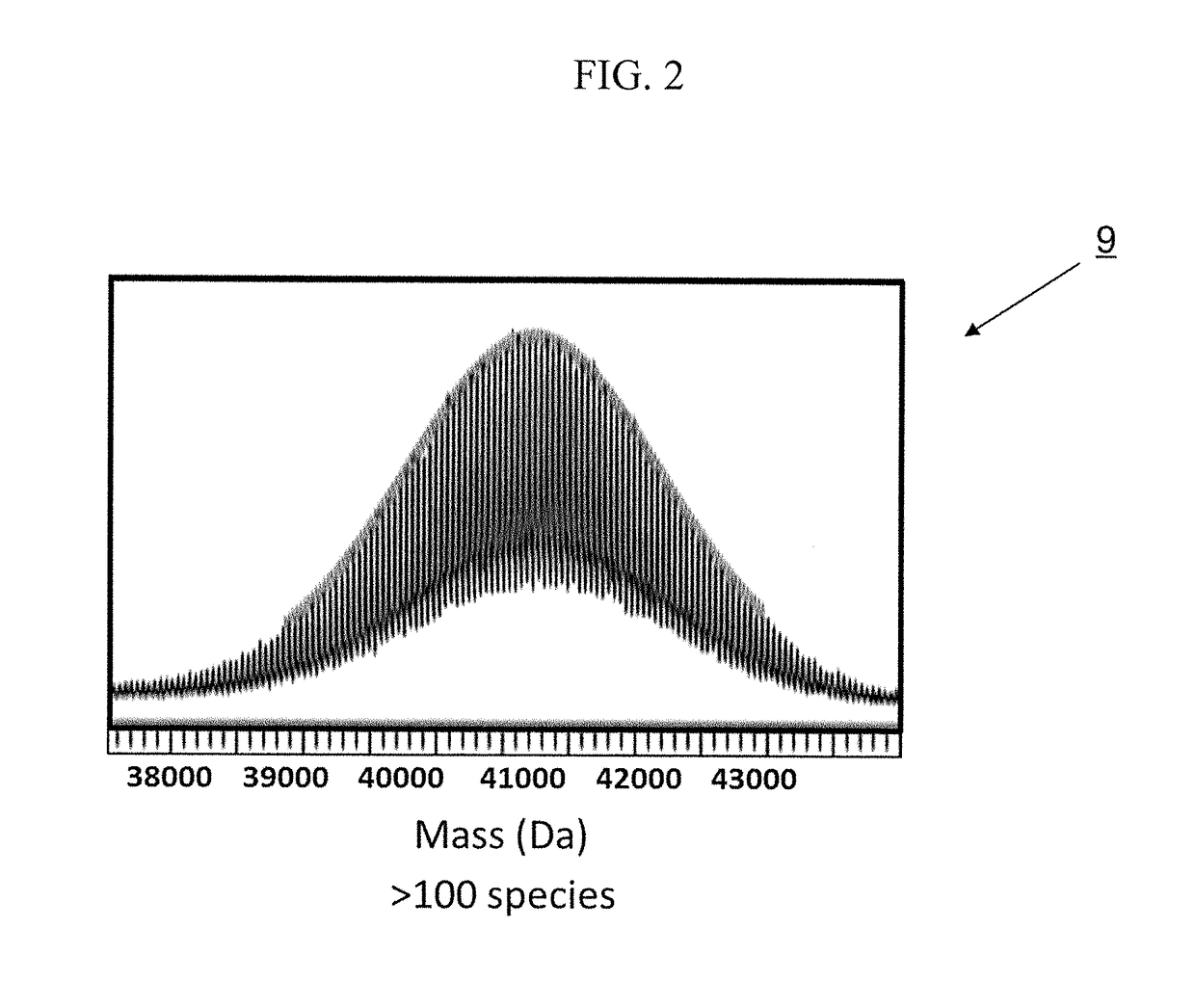
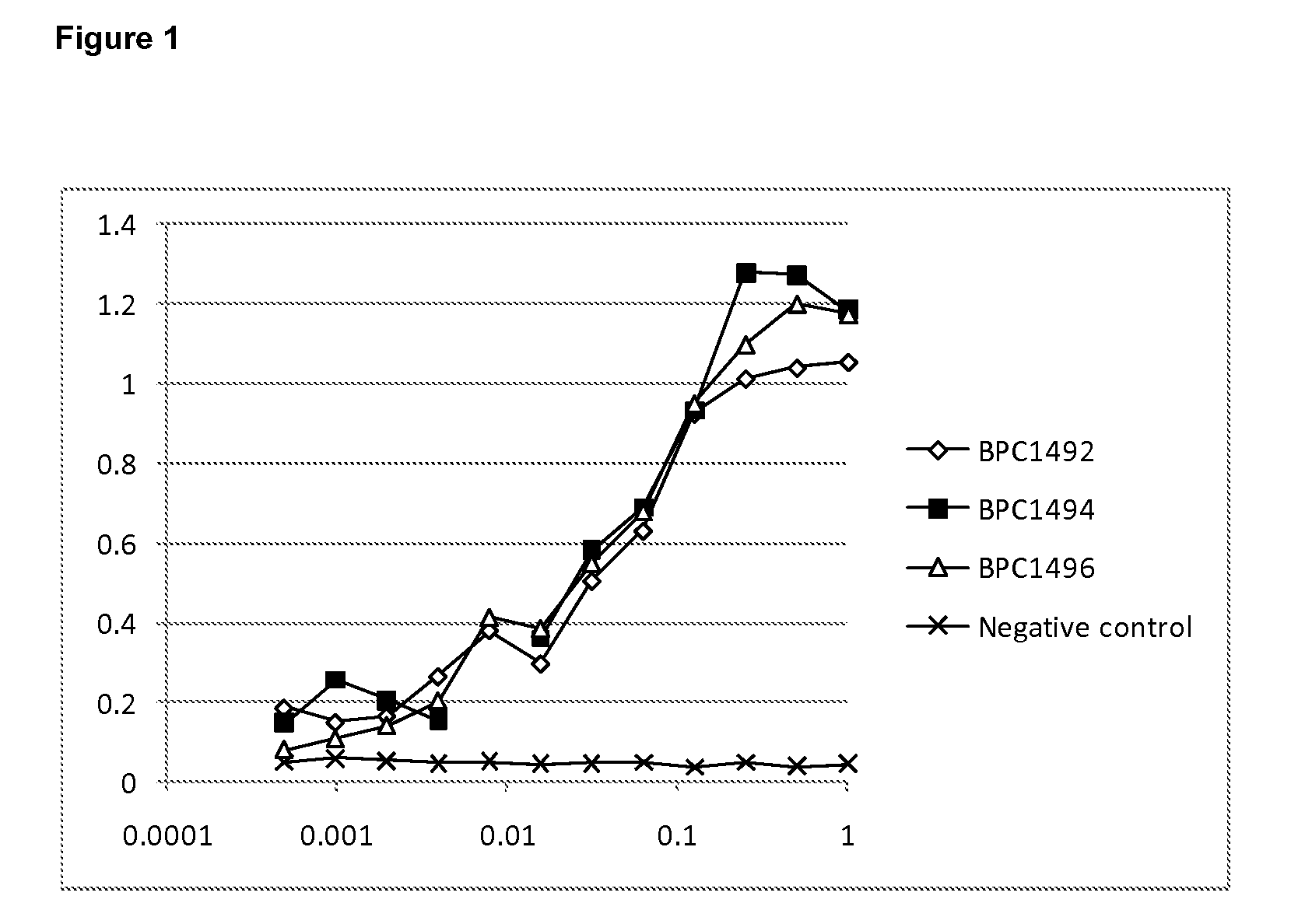
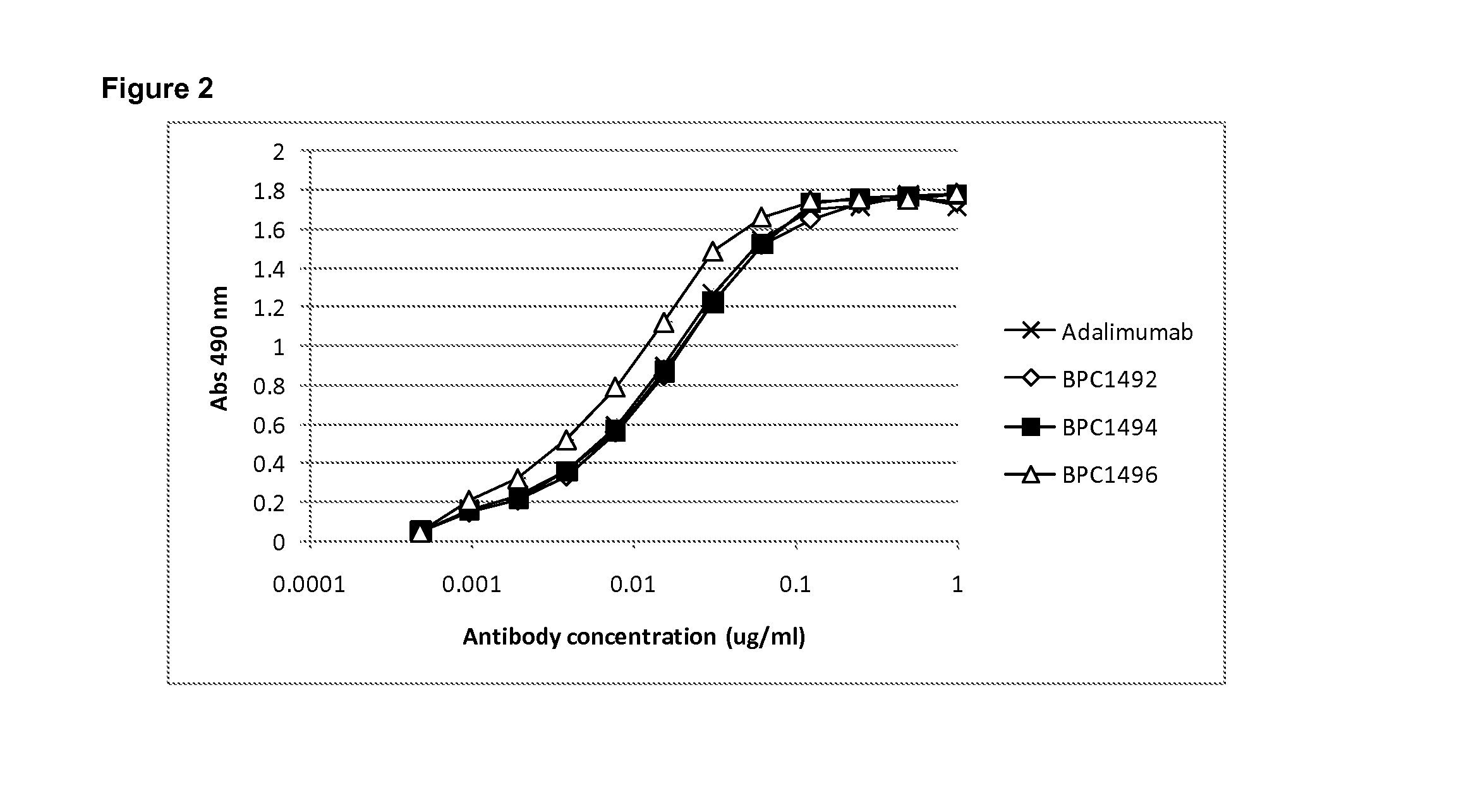
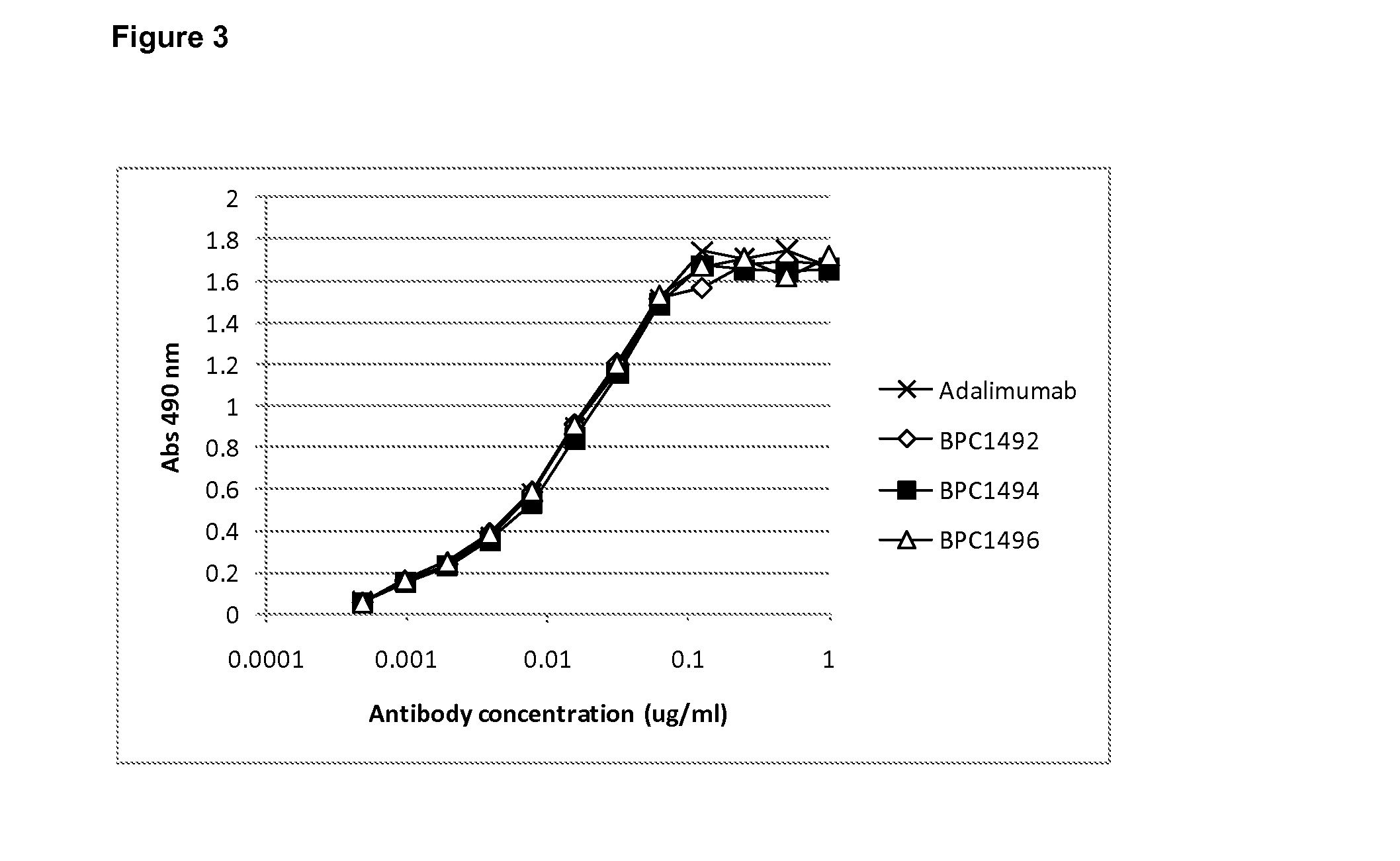
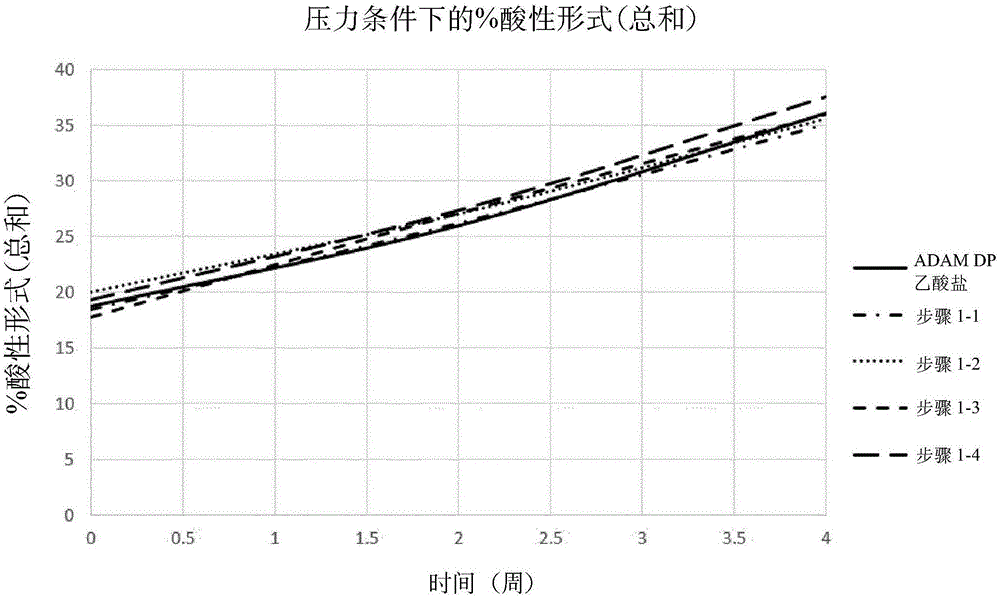
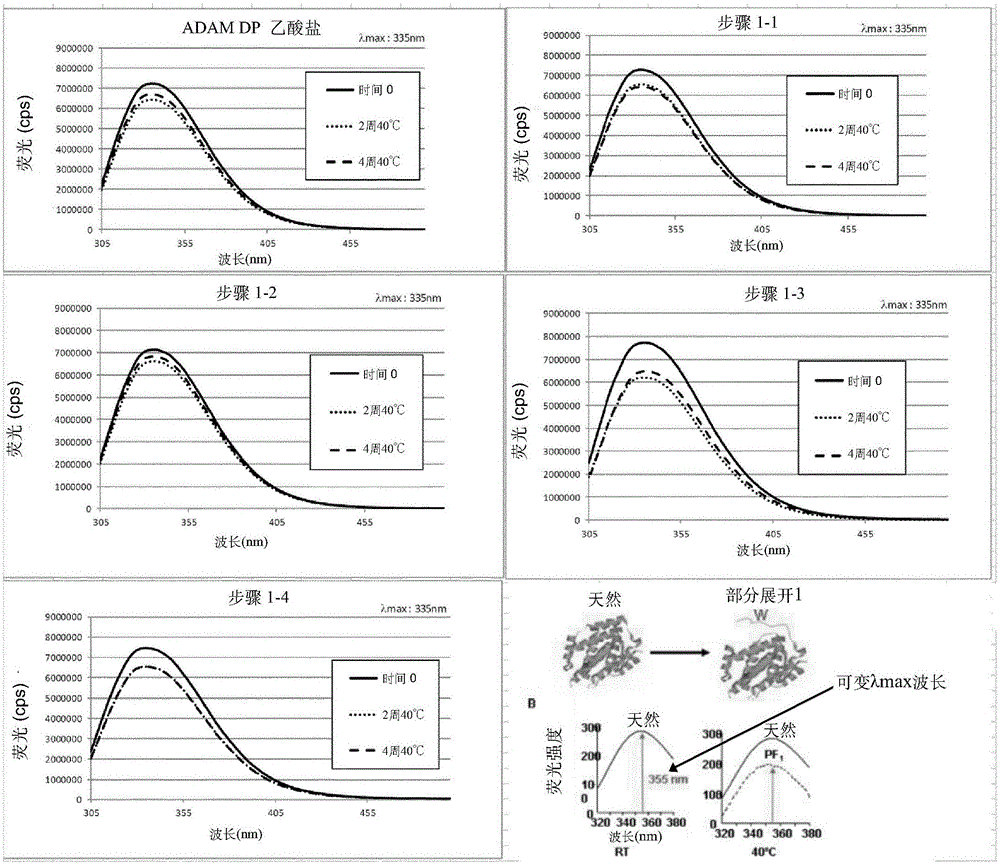
Patents
Literature
37 results about "Adalimumab" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Adalimumab is used to reduce pain and swelling due to certain types of arthritis (such as rheumatoid, psoriatic, juvenile idiopathic, ankylosing spondylitis). This medication is also used to treat certain skin disorders (such as plaque-type psoriasis, hidradenitis suppurativa).
Topical compositions for the treatment of chronic wounds
InactiveUS20060286108A1Avoid actionLow cytotoxicityBiocideAntibody ingredientsTace inhibitorEfalizumab
Methods for treating chronic wounds in a human are described by topically administering a dermatological composition comprising a TNF antagonist, a TACE inhibitor, a neutrophil antagonist, or a combination of a TNF antagonist and / or TACE inhibitor and a neutrophil antagonist. The TNF antagonist administered includes alefacept, efalizumab, etanercept, adalimumab, and onercept, while the neutrophil antagonist administered includes dapsone, colchicine, its analogs and prodrugs. The combination of TNF-antagonist and neutrophil antagonist administered includes sulfapyridine, sulfasalazine, mesalamine, and derivatives and prodrugs thereof. The topical compositions can be formulated to include the one or more of the antagonists in dissolved, semi-dissolved, and micro-particulate states.
Owner:BELL DERMATOLOGICS
Methods for determining Anti-drug antibody isotypes
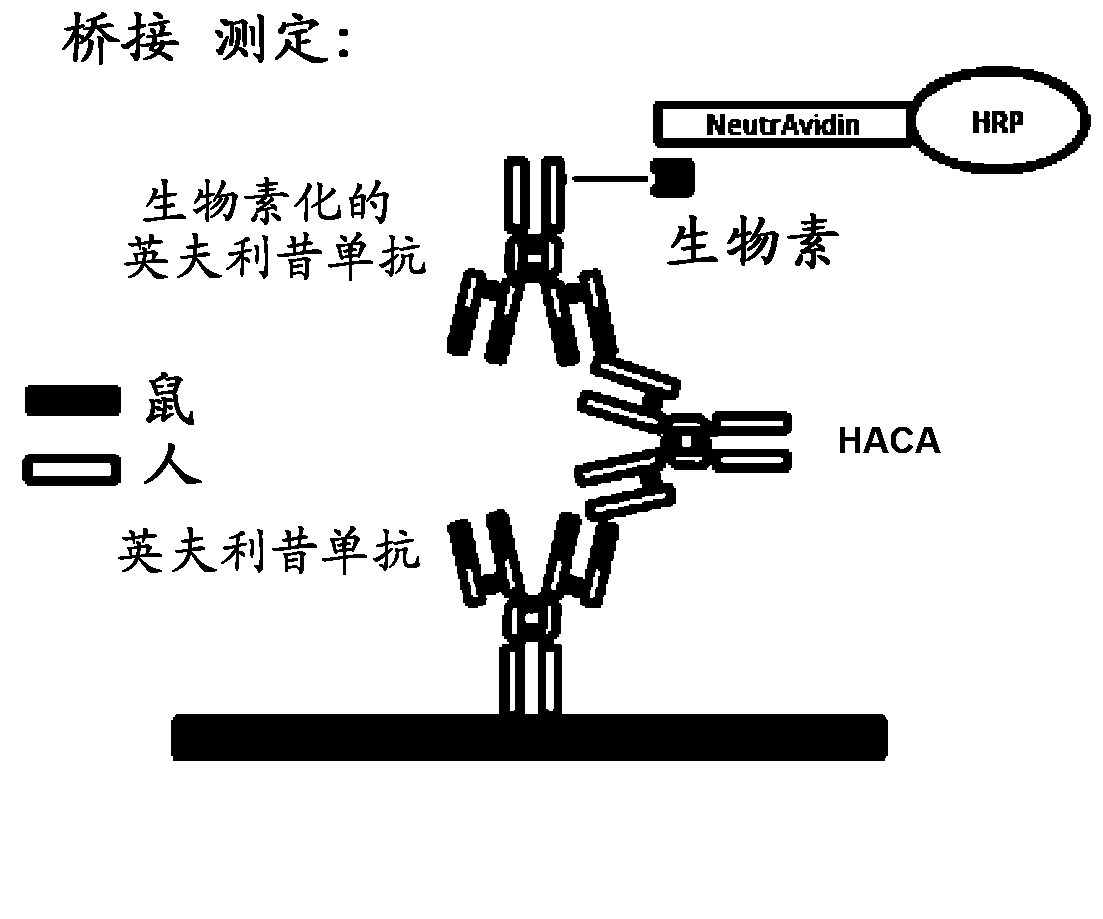
ActiveUS20130344621A1Convenient treatmentLow toxicityComponent separationBiological testingAntiendomysial antibodiesAssay
The present invention provides assay methods for the determination of one or more anti-drug antibody (ADA) isotypes in a sample. As a non-limiting example, the assays of the present invention are particularly useful for determining different ADA isotypes in samples from ADA-positive patients receiving an anti-TNFα drug such as REMICADE™ (infliximab) or HUMIRA™ (adalimumab). The present invention also provides methods for optimizing therapy and / or reducing toxicity in subjects receiving TNFα inhibitors for the treatment of TNFα-mediated disease or disorders.
Owner:PROMETHEUS LAB
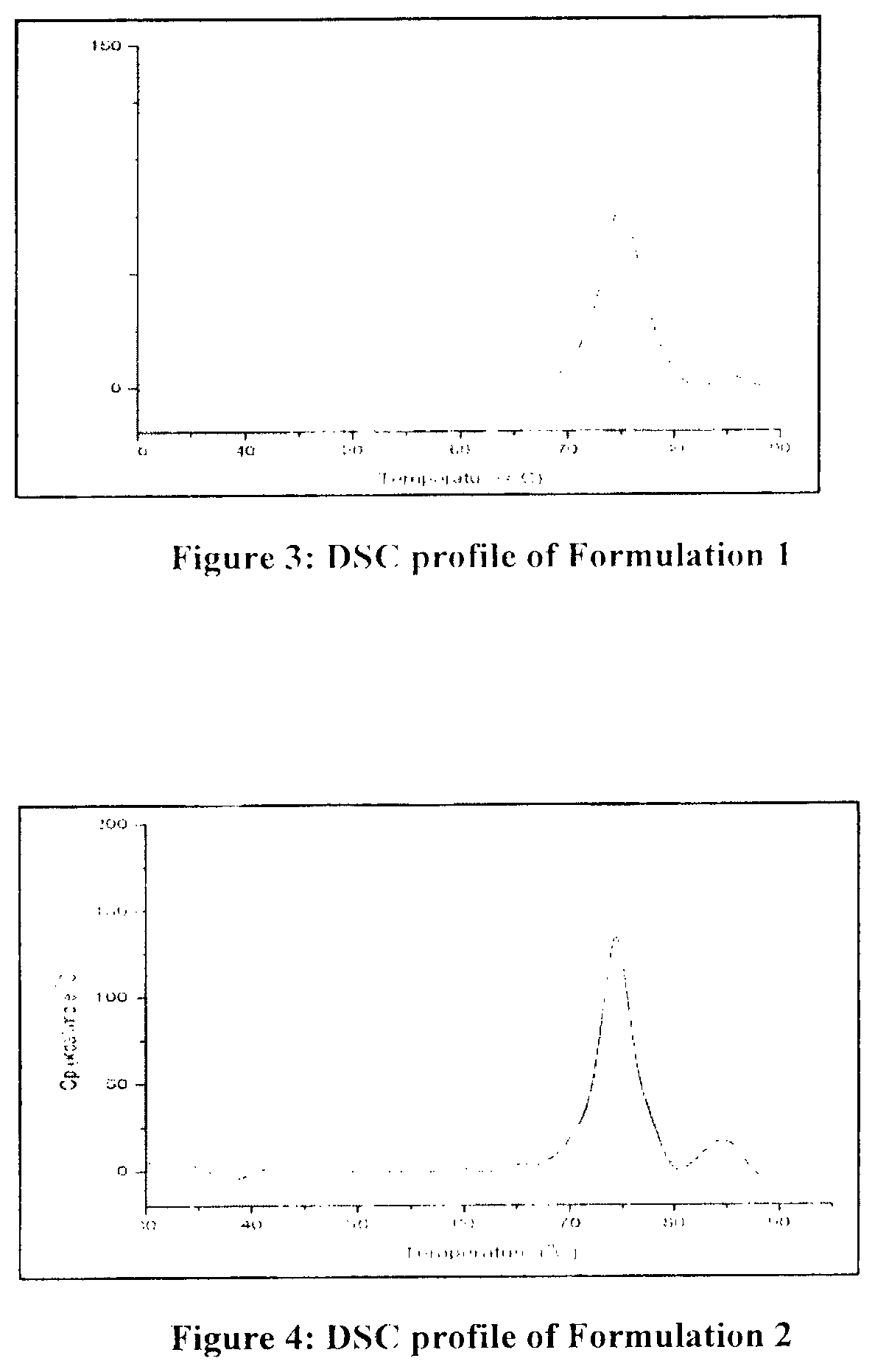
Adalimumab-containing pharmaceutical composition
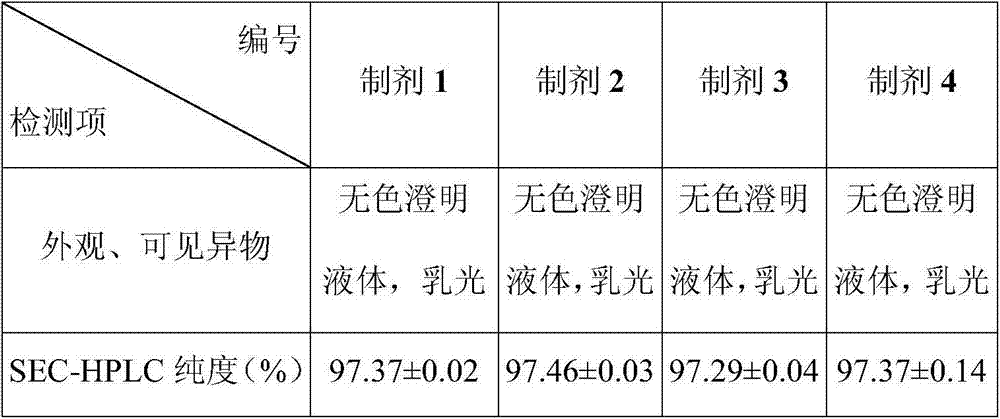
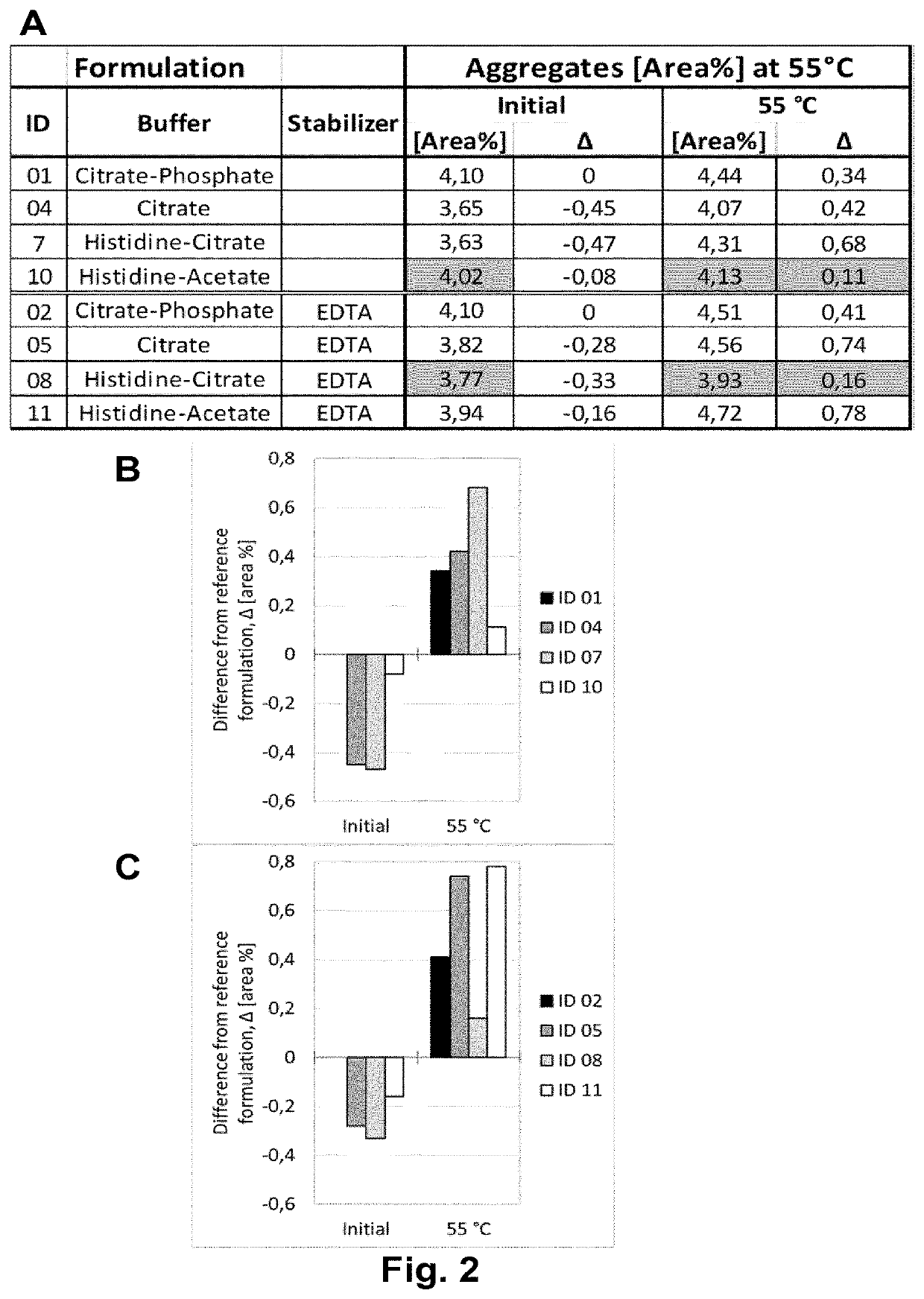
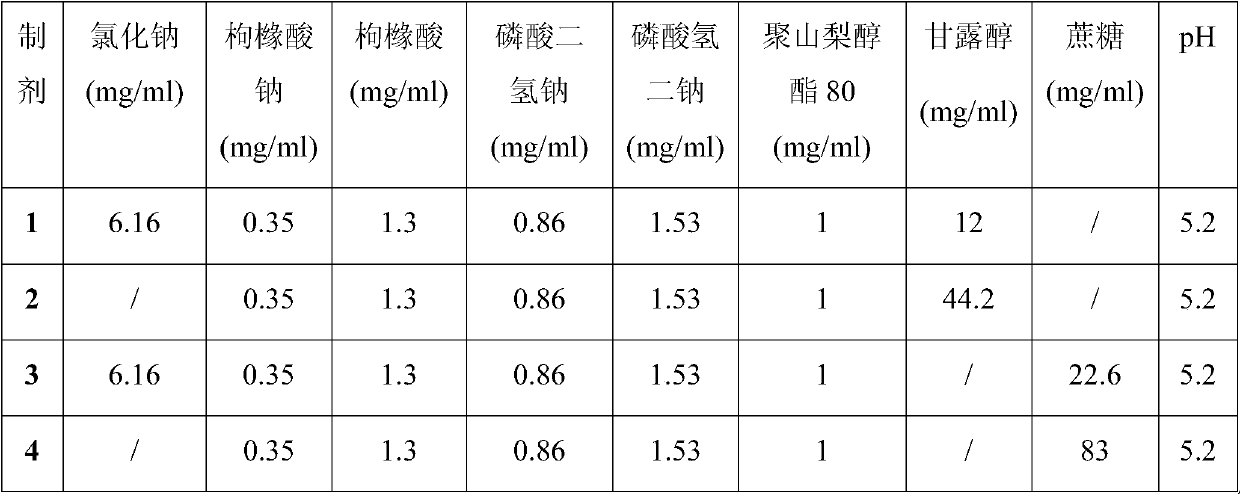
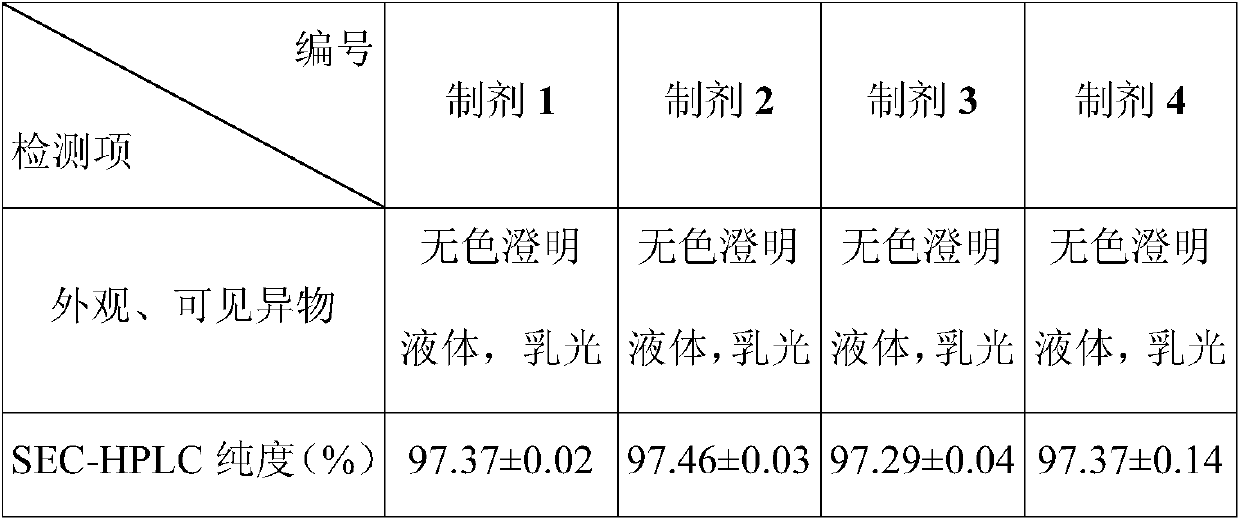
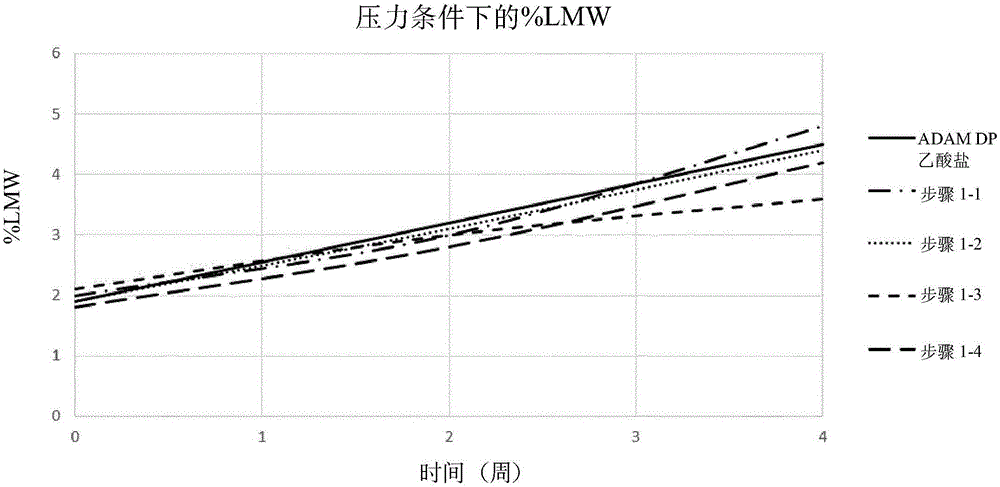
Disclosed is a pharmaceutical composition comprising adalimumab and a structural protective agent, wherein the structural protective agent is selected from sucrose or trehalose, and the pharmaceutical composition has a high stability.
Owner:HISUN BIOPHARMACEUTICAL CO LTD +1
Tnf-alpha antigen-binding proteins
The present invention provides liquid formulations comprising antigen binding proteins which bind specifically to TNF-alpha and histidine buffer. For example novel variants of anti-TNF antibodies such as adalimumab which show increased binding to the FcRn receptor or increased half life compared to adalimumab. Also provided are compositions comprising the antigen binding proteins and uses of such compositions in treatment of disorders and disease.
Owner:GLAXOSMITHKLINE INTPROP
Methods for determining anti-drug antibody isotypes
ActiveUS9784748B2Convenient treatmentLow toxicityComponent separationBiological testingAntiendomysial antibodiesAssay
The present invention provides assay methods for the determination of one or more anti-drug antibody (ADA) isotypes in a sample. As a non-limiting example, the assays of the present invention are particularly useful for determining different ADA isotypes in samples from ADA-positive patients receiving an anti-TNFα drug such as REMICADE™ (infliximab) or HUMIRA™ (adalimumab). The present invention also provides methods for optimizing therapy and / or reducing toxicity in subjects receiving TNFα inhibitors for the treatment of TNFα-mediated disease or disorders.
Owner:PROMETHEUS LAB
Liquid pharmaceutical composition of adalimumab
ActiveUS20170106090A1Composition is stableImmunoglobulins against cytokines/lymphokines/interferonsAntibody ingredientsMedicineAdalimumab
A liquid pharmaceutical composition having an anti-TNFα antibody, a buffer, a stabilizer, and a surfactant.
Owner:INTAS PHARM LTD
Methods for determining anti-drug antibody isotypes
The present invention provides assay methods for the determination of one or more anti-drug antibody (ADA) isotypes in a sample. As a non-limiting example, the assays of the present invention are particularly useful for determining different ADA isotypes in samples from ADA-positive patients receiving an anti-TNFa drug such as REMICADETM (infliximab) or HUMIRATM (adalimumab). The present invention also provides methods for optimizing therapy and / or reducing toxicity in subjects receiving TNFa inhibitors for the treatment of TNFa-mediated disease or disorders.
Owner:SOC DES PROD NESTLE SA
Highly galactosylated anti-TNF-α antibodies and uses thereof
InactiveUS10174110B2Strong cytotoxicityImprove the level ofAntipyreticMilk immunoglobulinsMethods of productionAnti tnf alpha
In one aspect, the disclosure relates to highly galactosylated anti-TNF-alpha antibodies and compositions thereof. In one aspect, the disclosure relates to populations of anti-TNF-alpha antibodies with a high level of galactosylation, and compositions thereof. In one aspect, the disclosure relates to methods of production and use of highly galactosylated anti-TNF-alpha antibodies and populations of anti-TNF-alpha antibodies with a high level of galactosylation. In some embodiments, the anti-TNF-alpha antibody is adalimumab.
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
Combined medium for expressing adalimumab
ActiveCN107904200AIncrease productionQuality improvementImmunoglobulins against cytokines/lymphokines/interferonsArtificial cell constructsMedicineAdalimumab
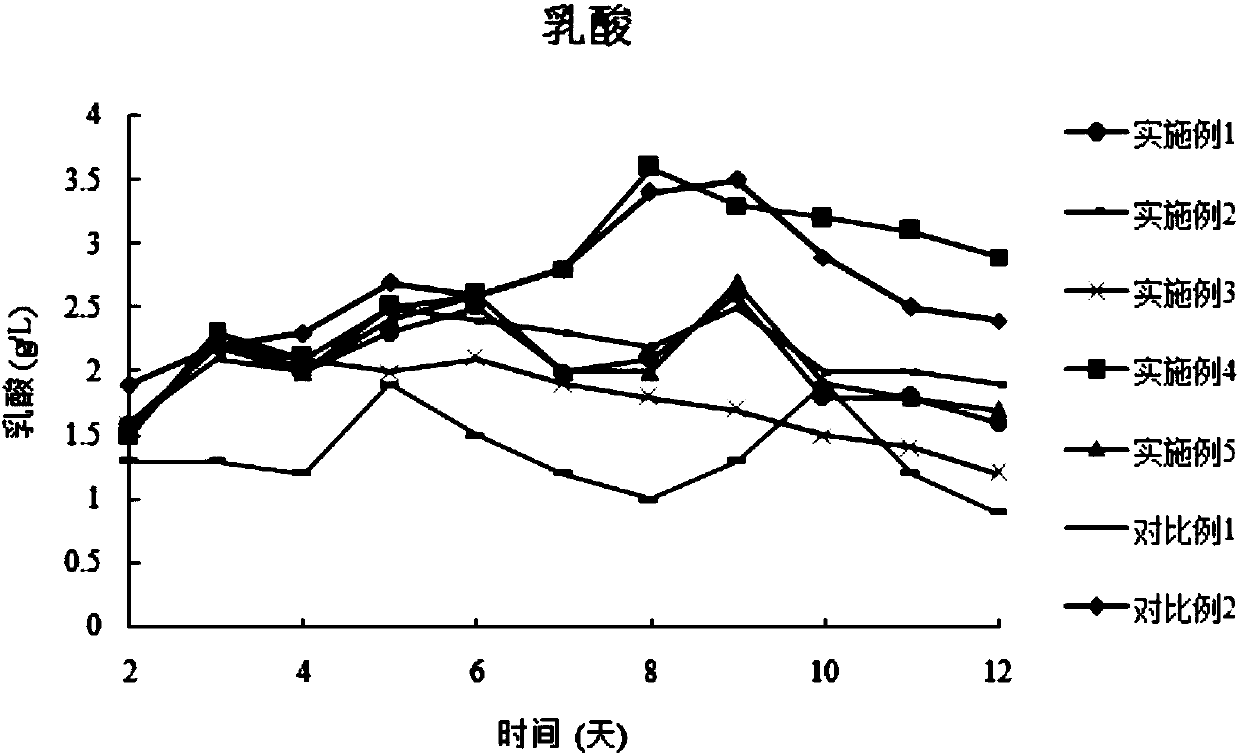
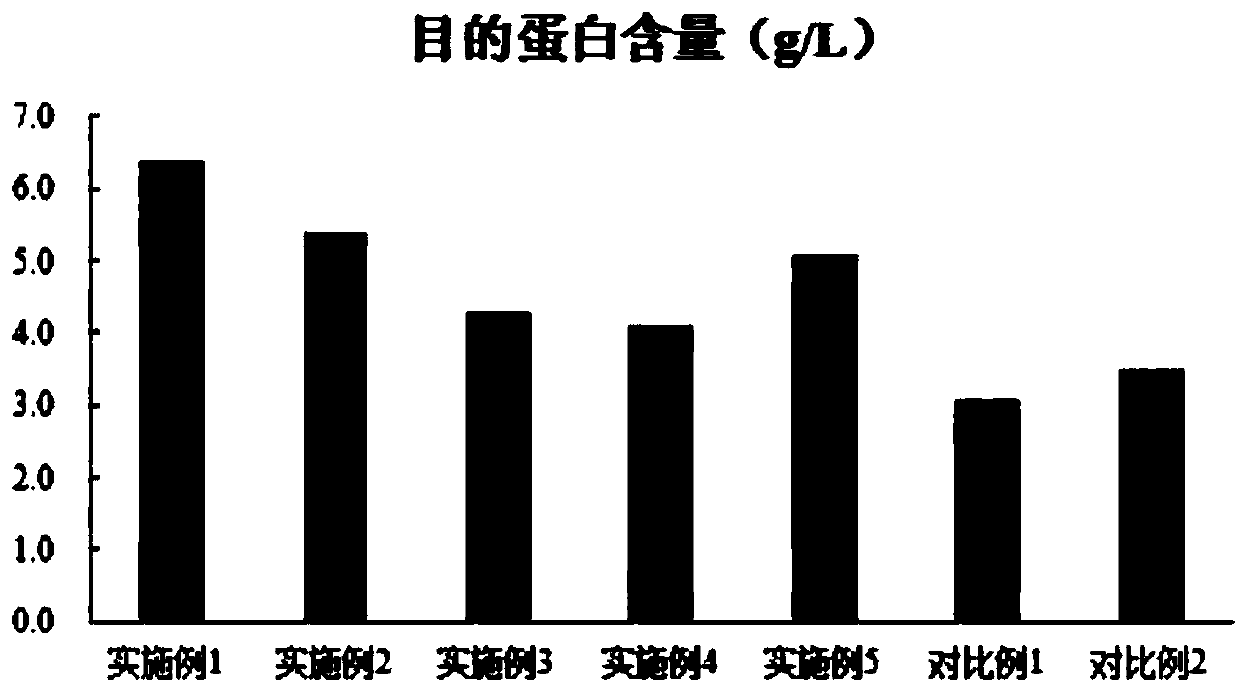
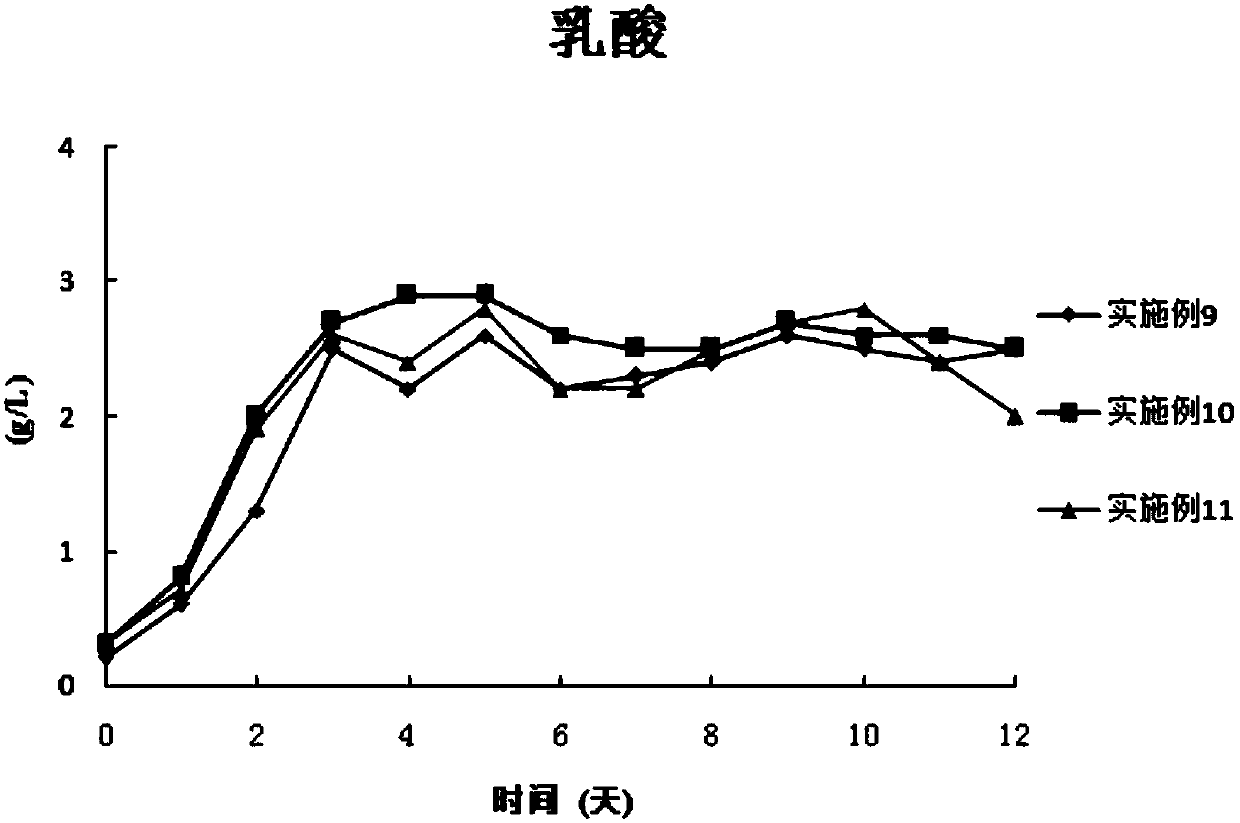
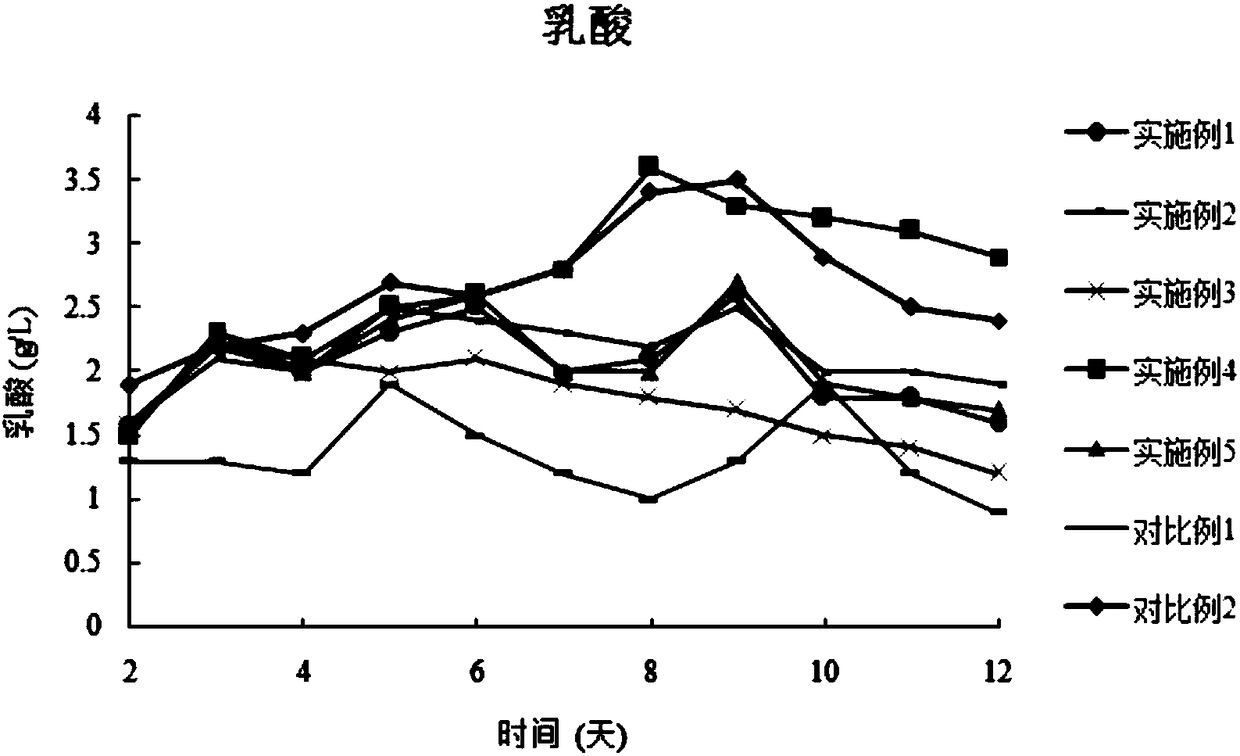
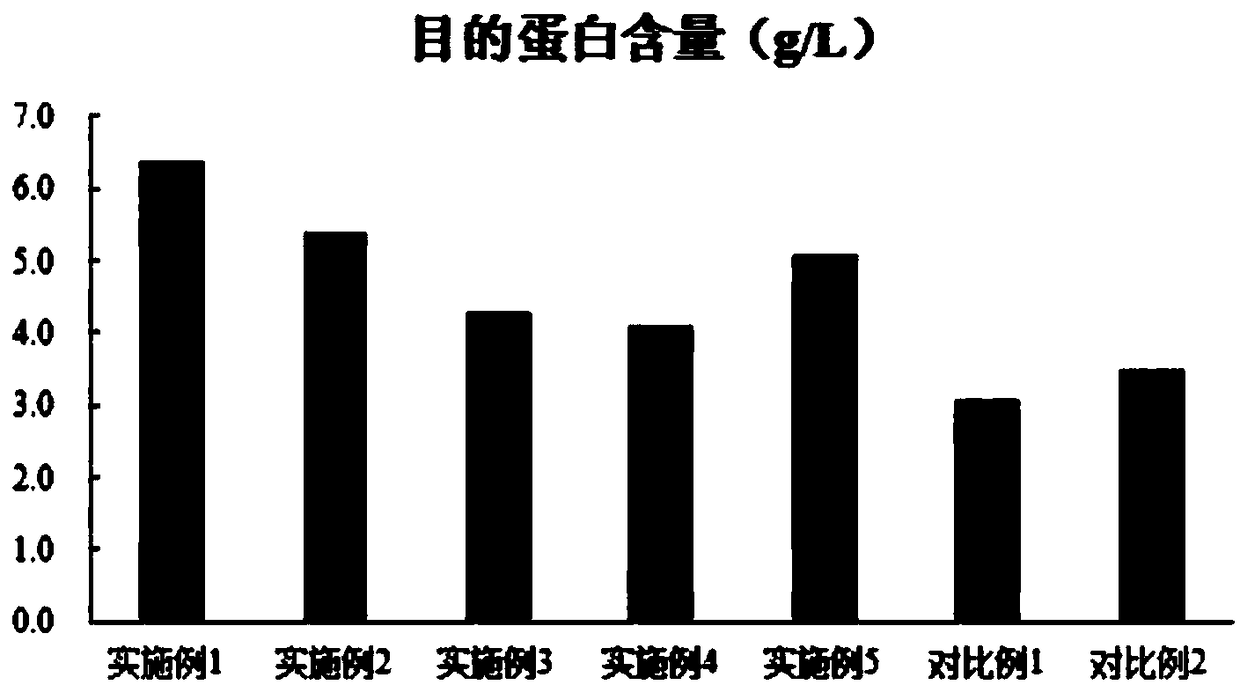
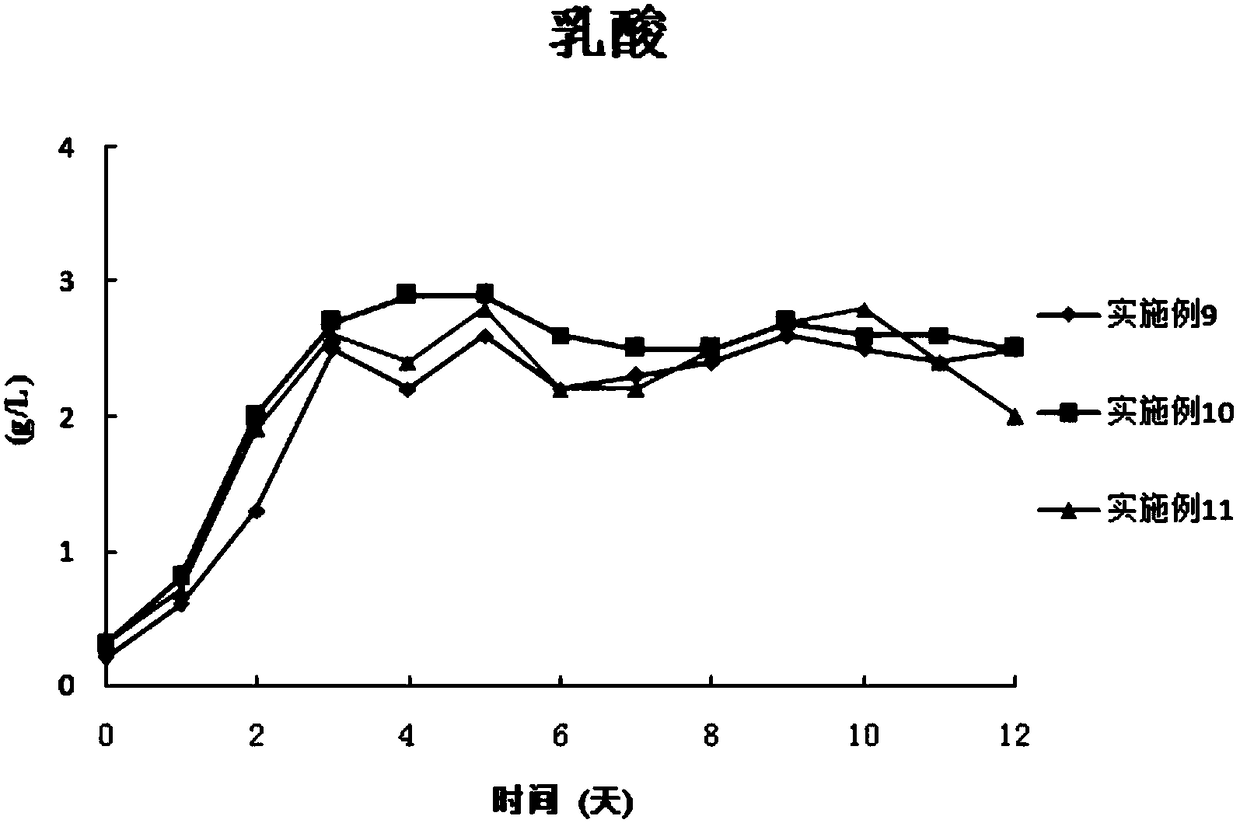
The invention discloses a combined medium for expressing adalimumab. The combined medium is composed of a basic medium and a fed medium, wherein the basic medium is composed of CD FortiCHO and M20A ina ratio of 1: 0.5-1.5; the fed medium consists of F001S and F001B in a ratio of 5-15: 1; and the above mediums are both commercial mediums. Individual usage of any of the mediums cannot produce high-yield stable adalimumab; but the combined medium of the basic medium and the fed medium can significantly increase the expression quantity of adalimumab and improve cell density and cell viability, stably overcomes the problem of accumulation of a large amount of lactic acid and low yield of target proteins in conventional adalimumab cell culture processes, and is beneficial for further improvement of the output and quality of adalimumab.
Owner:通化东宝生物科技有限公司
Primer pair and kit for detecting related gene polymorphism of adalimumab medication
ActiveCN112708669AEasy to handleSimple sequencing stepsMicrobiological testing/measurementDNA/RNA fragmentationReference genesNucleic acid detection
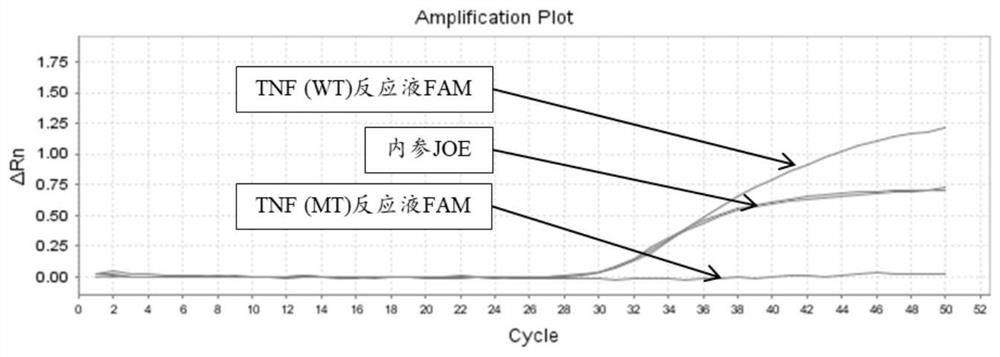
The invention relates to a primer pair and a kit for detecting related gene polymorphism of adalimumab medication, and belongs to the technical field of in-vitro nucleic acid detection. The primer pair comprises amplification primers aiming at TNFrs1800629, KLRC1rs7301582, FCGR2Ars1801274, PTPRCrs10919563, HLA-Ers1264457, TRAF1rs3761847 and KLRD1rs2302489 allelic genes and GAPDH reference genes respectively. The kit comprises a primer solution containing the amplification primers. The kit provided by the invention is high in sensitivity which can reach one ten- thousandth, and the lowest detection limit is only 1-2copies, so that the kit is particularly suitable for detecting low-content mutation samples; and compared with a sequencing method, a detection result can be observed in real time, a product does not need gel electrophoresis detection, tube closing operation is completely carried out, and the risk of PCR product pollution is effectively reduced; and in addition, the kit has the advantages of being high in detection speed and suitable for high-throughput sample detection.
Owner:南昌豪仕医学检验实验室有限公司
Increasing the half-life of a full-length or a functional fragment of variant anti-human TNF-alpha antibody
InactiveUS20180066049A1Low immunogenicityExtended half-lifePeptide/protein ingredientsAntibody mimetics/scaffoldsHalf-lifeInflammatory Bowel Diseases
Tumor Necrosis Factor-α (TNFα) promotes an inflammatory response resulting in many clinical problems associated with autoimmune disorders such as rheumatoid arthritis, ankylosing spondylitis, inflammatory bowel disease, psoriasis, hidradenitis suppurativa, and refractory asthma. Dysregulation of TNF production is implicated in a variety of human diseases including Alzheimer's disease, cancer, major depression, and inflammatory bowel disease. These disorders are treated with a TNFα inhibitor. Embodiments herein provide methods of preventing and / or treating acute and chronic inflammation, and autoimmune diseases by administering a prophylactic and / or therapeutic formulation containing an antibody fragment (Fab or F(ab′)2) of adalimumab modified by conjugation of natural amino acids such as proline, alanine and / or serine (PA / S) by PASylation®, and / or unnatural amino acids such as cysteine and other derivatives, thereby creating a polypeptide possessing none of the processing, preparation, formulation, cost, clinical performance, and other long-term issues of administering PEGylated drugs.
Owner:DNX BIOTECH LLC
Tnf-alpha antigen-binding proteins
InactiveUS20150368333A1Reduce adverse effectsEasy to prepareAntipyreticAnalgesicsHalf-lifeAntigen binding
The present invention provides antigen binding proteins which bind specifically to TNF-alpha. For example novel variants of anti-TNF antibodies such as adalimumab which show increased binding to the FcRn receptor or increased half life compared to adalimumab. Also provided are compositions comprising the antigen binding proteins and uses of such compositions in treatment of disorders and disease.
Owner:GLAXOSMITHKLINE INTPROP DEV LTD
ANTI-TNF-alpha FULLY HUMAN MONOCLONAL ANTIBODIES WITH LOW IMMUNOGENICITY AND APPLICATION THEREOF
ActiveUS20170327570A1Low immunogenicityLong half-livesImmunoglobulins against cytokines/lymphokines/interferonsAntibody ingredientsApoptosisMonoclonal antibody
Disclosed herein are low immunogenic human anti-TNF-α antibodies which can inhibit the apopotosis of cells induced by TNF-α. The invented low immunogenic human anti-TNF-α antibodies are capable of binding to TNF-α specifically. The invention presents the human anti-TNF-α antibodies which bind to TNF-α with similar affinities as Adalimumab. Most importantly, the invented human anti-TNF-α antibodies showed reduced immunogenicities in vivo, which made them safer candidate for antibody drug and other biotherapy. The invention also features method of de-immunogenicity of antibody drugs by identification, replacement of high immunogenic FR sequence(s) of the human antibody with low immunogenic FR sequences from other human IgGs, and significantly reduce the risk of human anti-human immunogenicity and improve the efficacy of antibody drugs.
Owner:ABMAX BIOTECHNOLOGY CO LTD
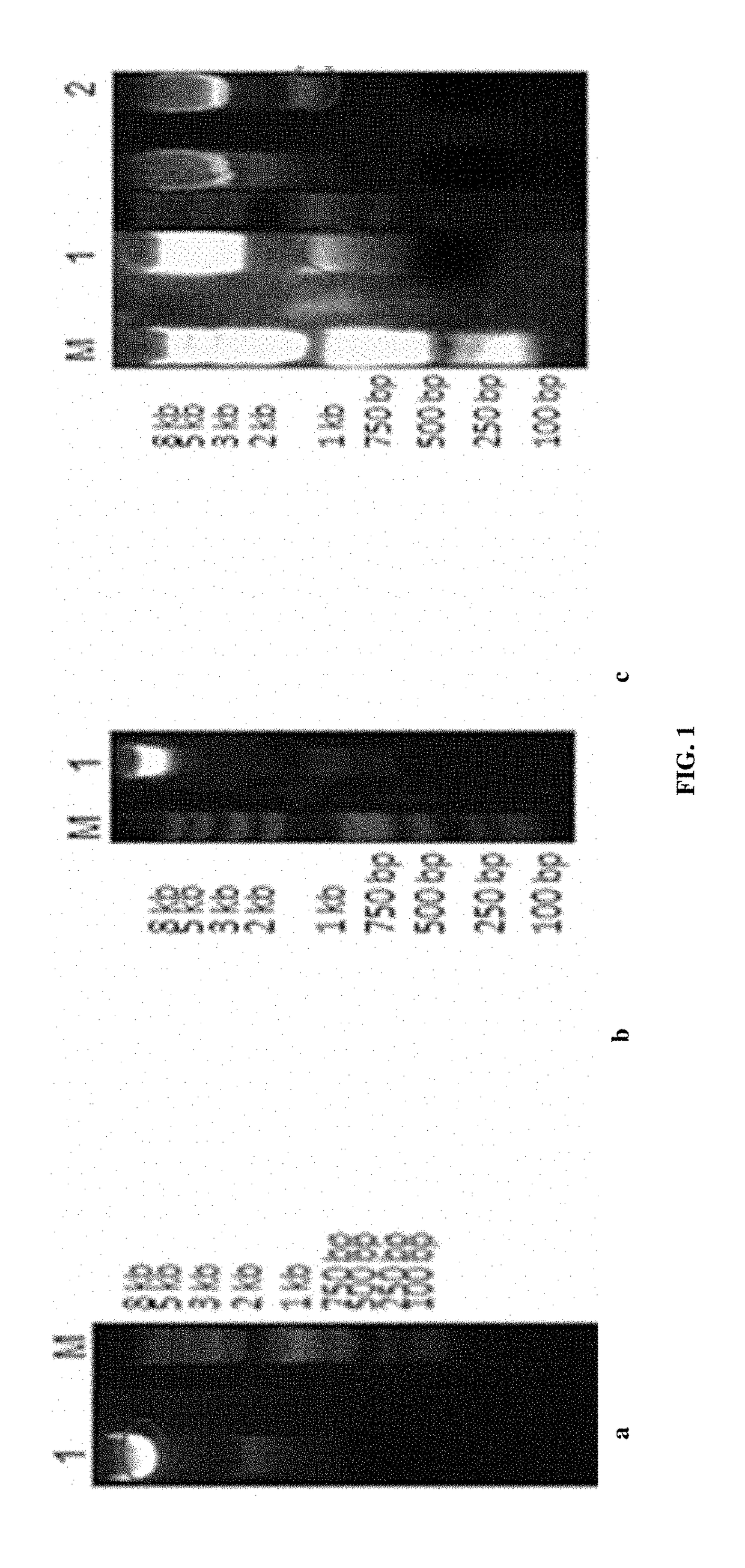
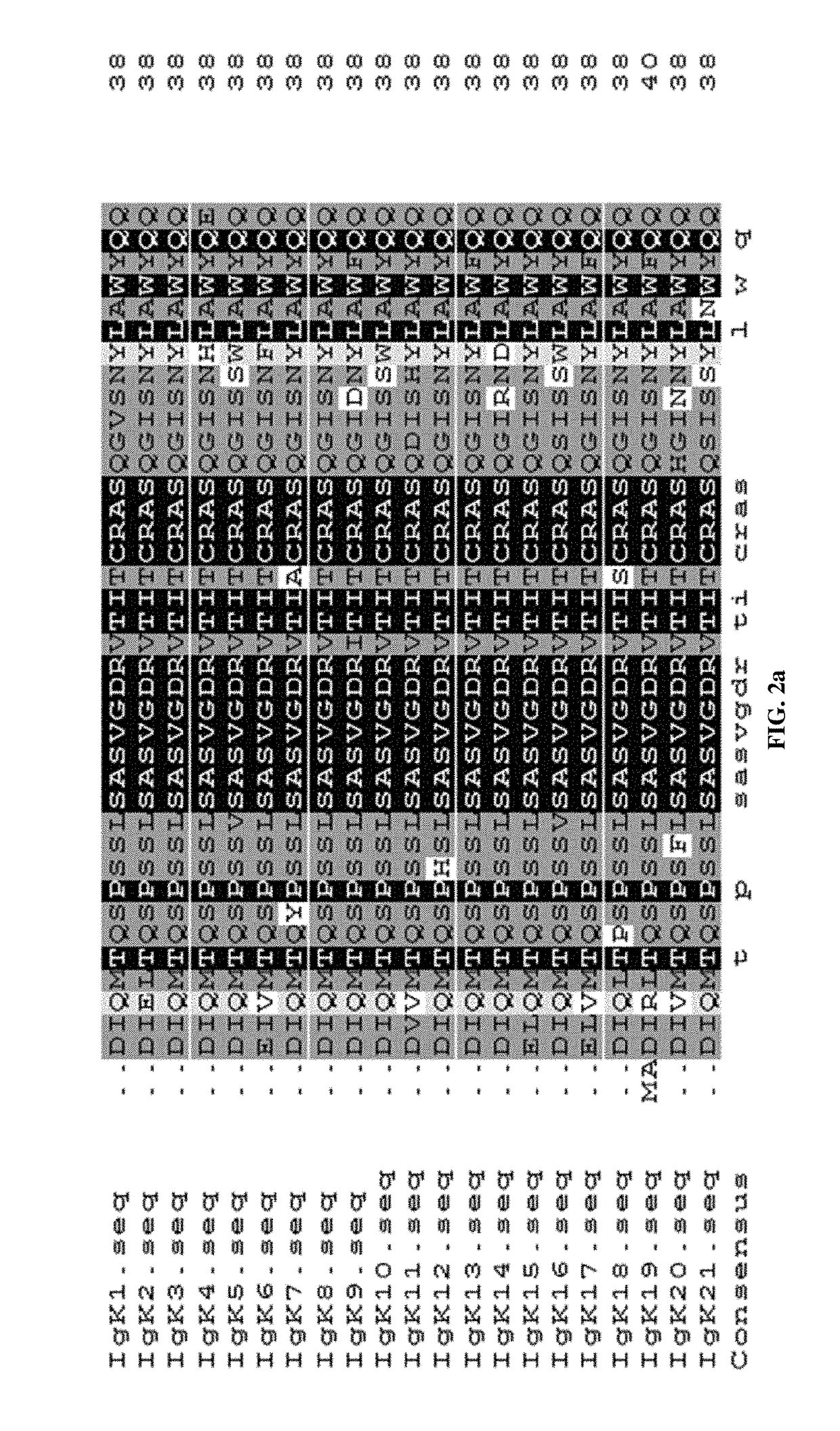
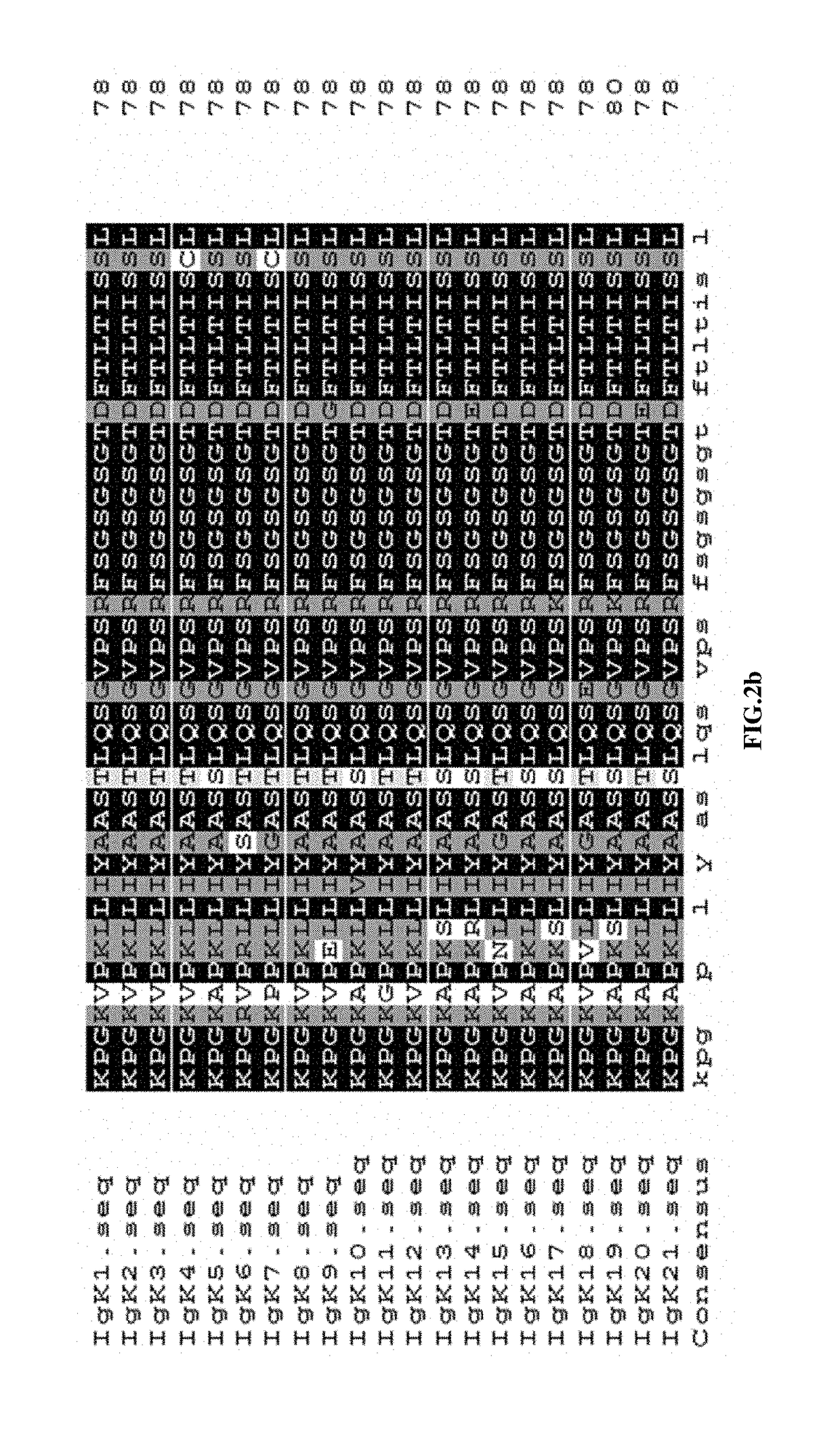
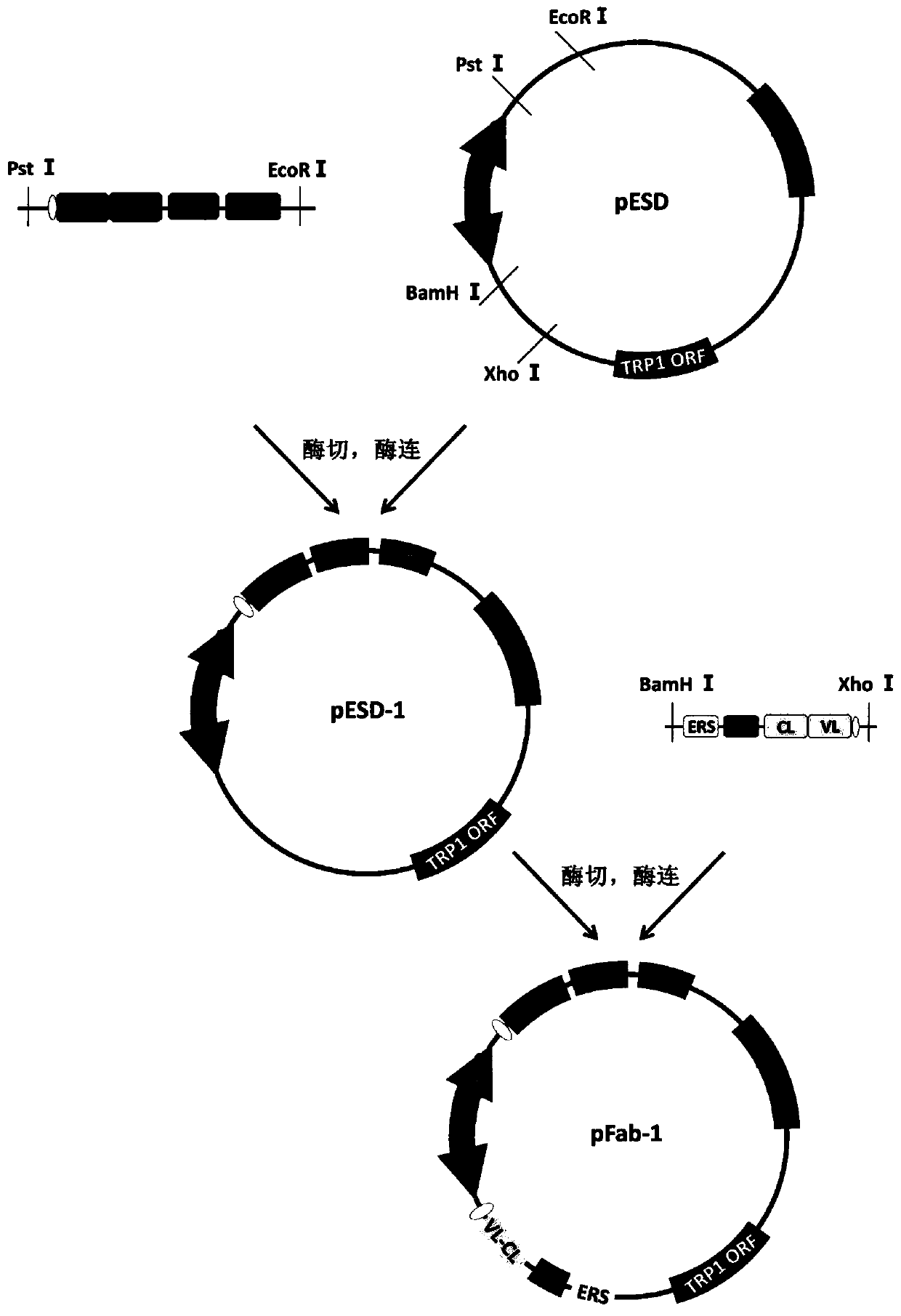
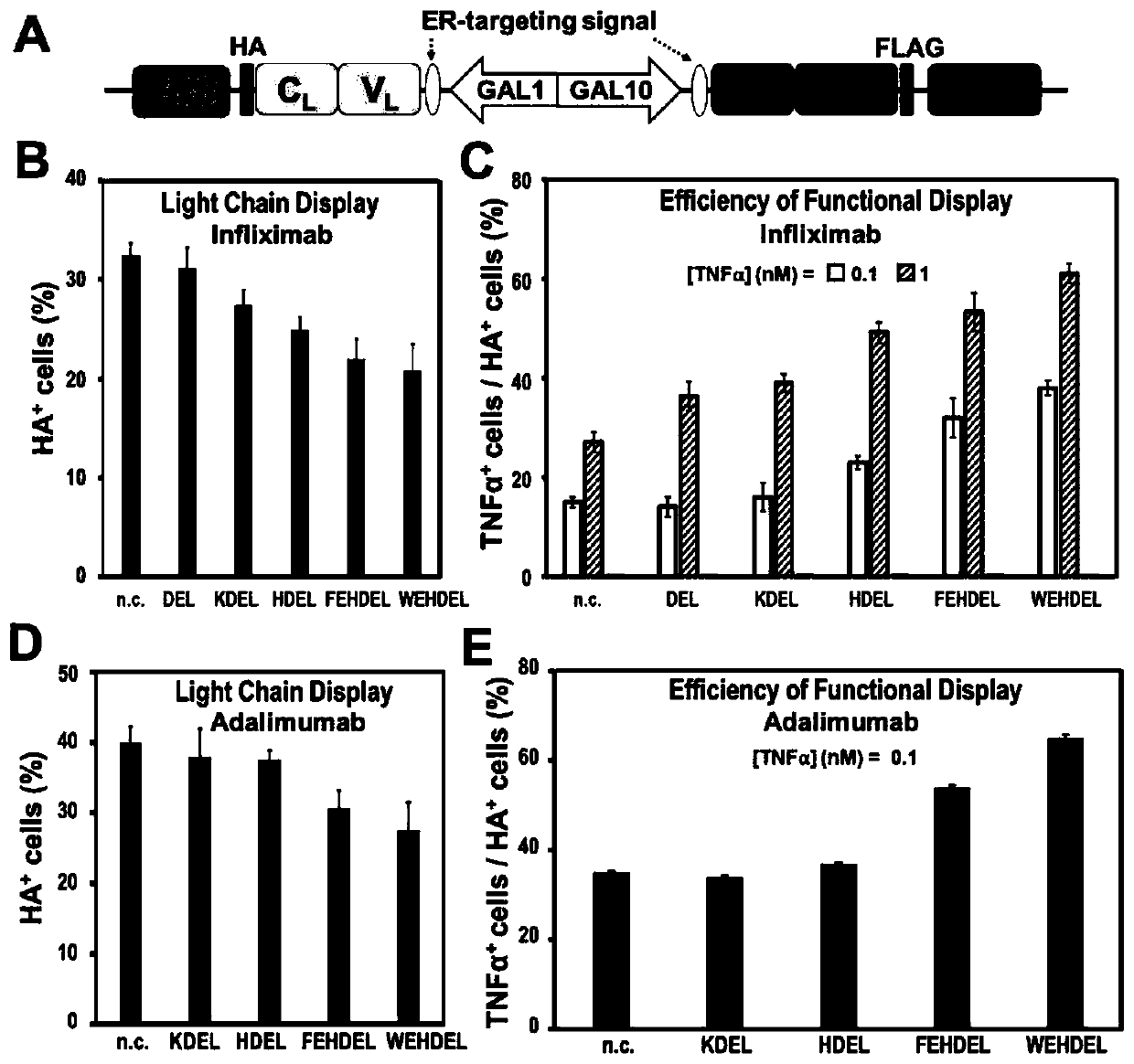
Recombinant vector for enhancing ability of displaying Fab fragment antigen binding on yeast cell surface by using endoplasmic reticulum retrieval signal sequence
ActiveCN110218737AImprove antigen binding abilityPromotes folding efficiencyPolypeptide with localisation/targeting motifMicroorganism based processesAntigenReticulum cell
The invention discloses a recombinant vector for enhancing the ability of displaying Fab fragment antigen binding on the yeast cell surface by using endoplasmic reticulum retrieval signal sequences, and belongs to the technical field of antibody engineering. A GAL1-GAL10 bidirectional promoter vector is used for expressing two structures domains at the same time, namely VH-CH1 and VL-CL, of Adalimumab or Infliximab, and the amino terminal endoplasmic reticulum retrieval signal sequences are located in an endoplasmic reticulum and assembled into natural Fab form fragments to be displayed on thecell surface through an Aga1-Aga2 yeast cell display system. Meanwhile, by fusing the saccharomyces cerevisiae retrieval signal sequences into the carboxyl terminal of the Fab fragment VL-CL structure domain, the ability of the yeast cell surface to display Fab fragment bonded antigens is greatly improved, displaying of functional Fab fragments is promoted, the vector is more suitable for displaying the Fab fragments on the yeast cell surface, and an optimization strategy is provided for Fab modification in antibody engineering.
Owner:HUBEI UNIV
Pharmaceutical formulations for adalimumab
InactiveUS20210070850A1Improve stabilityInorganic non-active ingredientsImmunoglobulins against cytokines/lymphokines/interferonsPolyolPharmaceutical formulation
A pharmaceutical composition comprises a buffer-less aqueous formulation of adalimumab, a salt, a polyol, and a polysorbate. The formulation is suitable for injection.
Owner:ALVOTECH HF
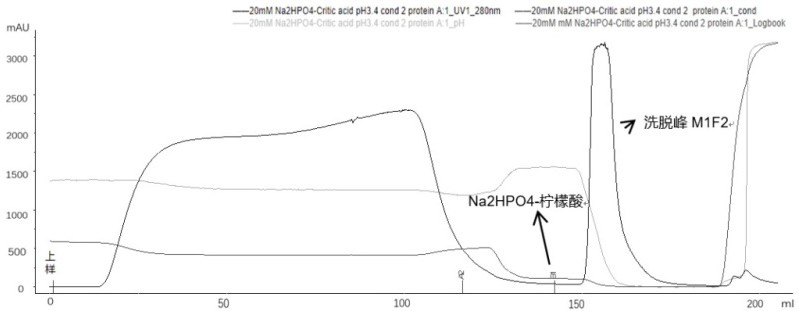
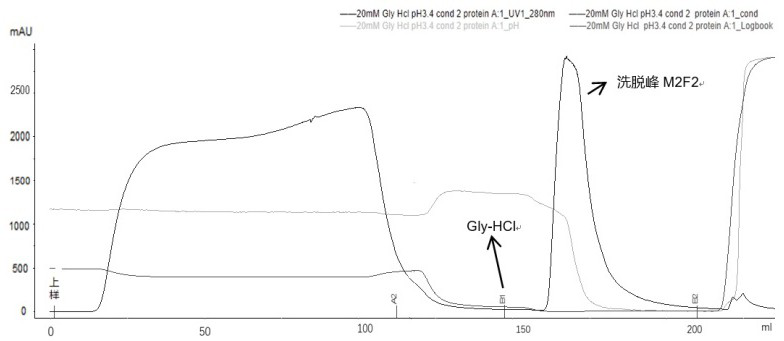
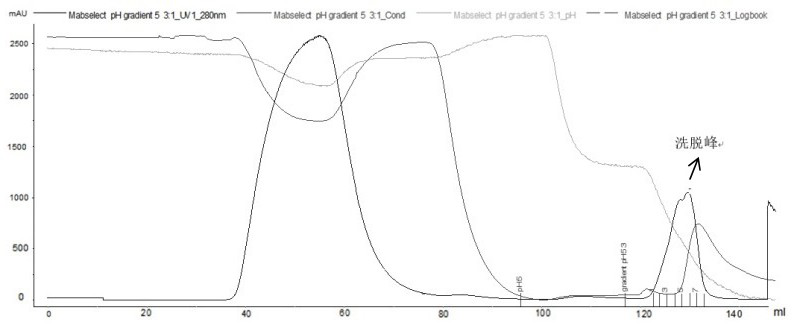
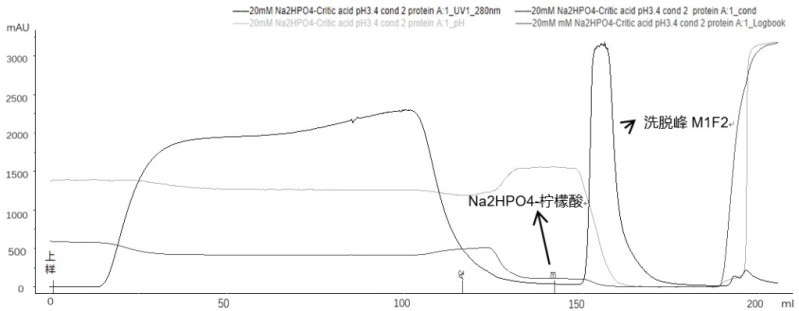
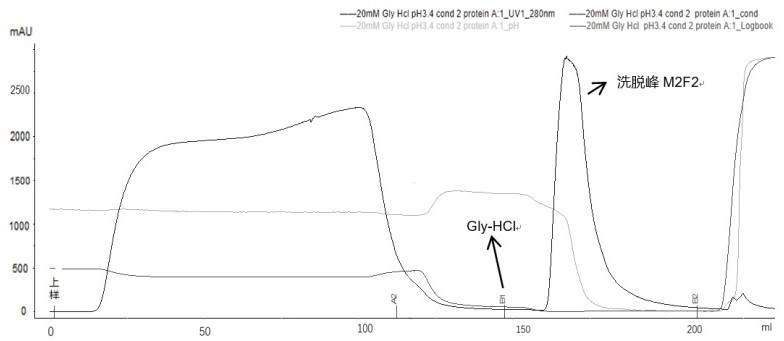
Method for purifying adalimumab by aid of cation exchange chromatography
InactiveCN105777903AImmunoglobulins against cytokines/lymphokines/interferonsPeptide preparation methodsHydrogenAnion-exchange chromatography
The invention discloses a method for purifying adalimumab by the aid of cation exchange chromatography. The method has the advantages that pollutants in mixtures with antibodies and the pollutants are removed by urea and betaine surfactants which are added in CEX (cation exchange) multi-step elution procedures, polymers are separated from the antibodies, then hydrophobic interaction chromatography is carried out, and accordingly good pollutant separation effects can be realized; samples are easy to regulate in chromatography procedures, drastic change of pH (potential of hydrogen) values can be prevented, and the contents of polymers in ultimately purified products can be obviously lowered.
Owner:SUNSHINE LAKE PHARM CO LTD
Anti-TNF-α fully human monoclonal antibodies with low immunogenicity and application thereof
ActiveUS10941196B2Low immunogenicityLong half-livesImmunoglobulins against cytokines/lymphokines/interferonsAntibody ingredientsAntiendomysial antibodiesEfficacy
Disclosed herein are low immunogenic human anti-TNF-αantibodies which can inhibit the apopotosis of cells induced by TNF-α. The invented low immunogenic human anti-TNF-α antibodies are capable of binding to TNF-α specifically. The invention presents the human anti-TNF-αantibodies which bind to TNF-α with similar affinities as Adalimumab. Most importantly, the invented human anti-TNF-α antibodies showed reduced immunogenicities in vivo, which made them safer candidate for antibody drug and other biotherapy. The invention also features method of de-immunogenicity of antibody drugs by identification, replacement of high immunogenic FR sequence(s) of the human antibody with low immunogenic FR sequences from other human IgGs, and significantly reduce the risk of human anti-human immunogenicity and improve the efficacy of antibody drugs.
Owner:ABMAX BIOTECHNOLOGY CO LTD
Liquid formulation of Anti-tnf alpha antibody
PendingCN110621303APharmacological effect is stableReduce formationPharmaceutical delivery mechanismAntibody ingredientsAntiendomysial antibodiesBiochemistry
The present invention relates to a liquid formulation of an anti-TNF alpha antibody, particularly adalimumab.
Owner:LG CHEM LTD
Method of removing recombinant expression antibody aggregates and degradation products
ActiveCN112010970AHigh purityHigh removal rateImmunoglobulins against cytokines/lymphokines/interferonsPeptide preparation methodsAntiendomysial antibodiesBiochemistry
The invention provides an affinity chromatography method for removing antibody aggregates and degradation fragments and improving the yield and purity of antibody single bodies. The purity of the antibody single body is improved by optimizing parameter conditions such as pH value, conductivity and buffer solution type in the affinity chromatography process of the recombinant adalimumab, and then the yield of the antibody singer body is improved by adding urea or a PEG additive into an elution buffer solution. In a preferred embodiment, the yield of the recombinant adalimumab single body can reach 90%, and the purity of the single body in the product exceeds 95%.
Owner:MABWELL (SHANGHAI) BIOSCIENCE CO LTD +1
Pharmaceutical Anti-tnf-alpha antibody formulation
PendingUS20220016244A1Improve stabilityImprove long-term stabilityOrganic active ingredientsAntipyreticAntiendomysial antibodiesPharmaceutical formulation
Provided are improved storage-stable liquid pharmaceutical antibody formulations. In particular, liquid aqueous pharmaceutical formulations of Adalimumab based on alternative buffer systems to citrate / phosphate and pharmaceutical containers like auto-injection devices containing the same are described.
Owner:RICHTER GEDEON NYRT
A kind of pharmaceutical composition containing adalimumab
The invention relates to a pharmaceutical composition containing adalimumab and a structure protecting agent. In the present invention, sucrose or trehalose is selected as the structural protection agent, so that the stability of the pharmaceutical composition is greatly improved, and has a very broad market application prospect.
Owner:HISUN BIOPHARMACEUTICAL CO LTD +1
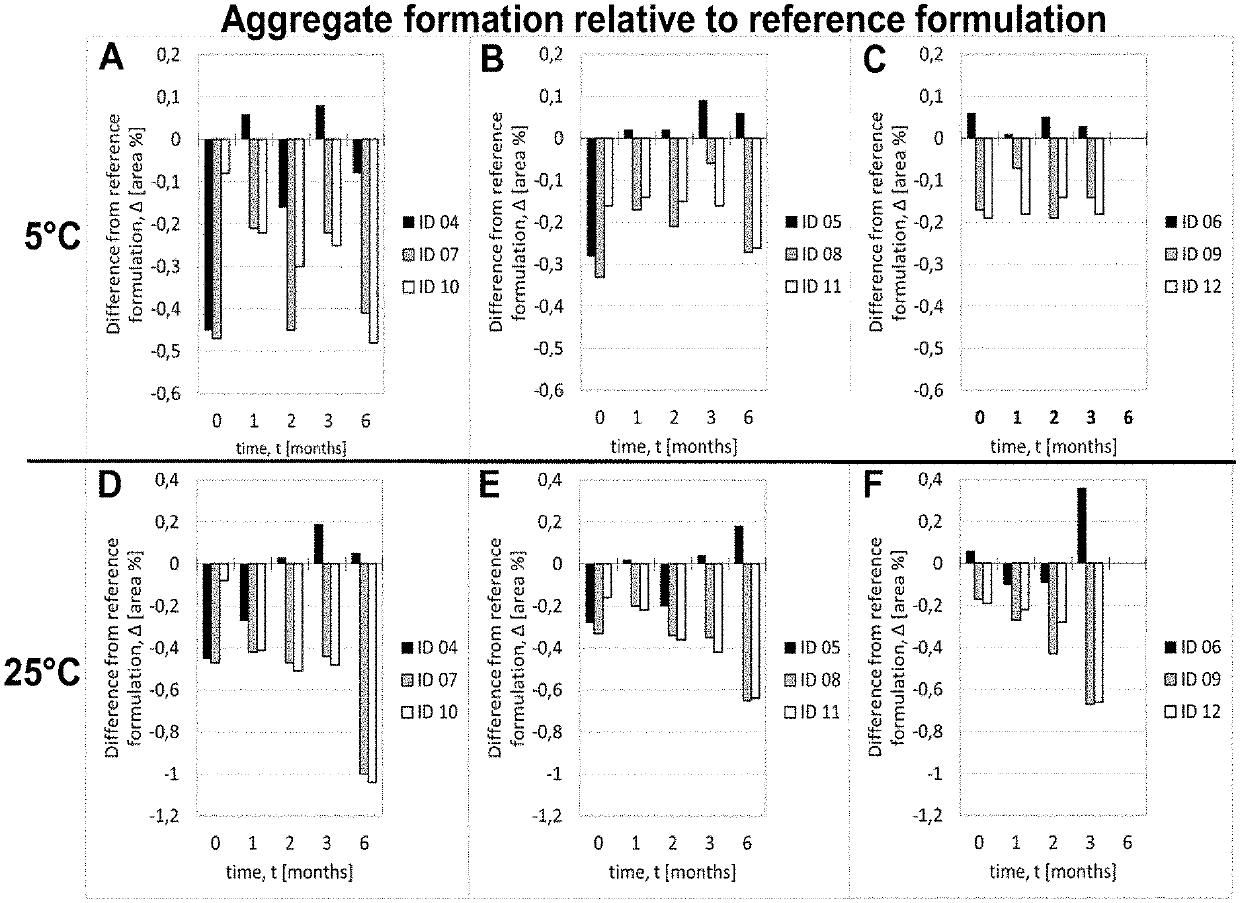
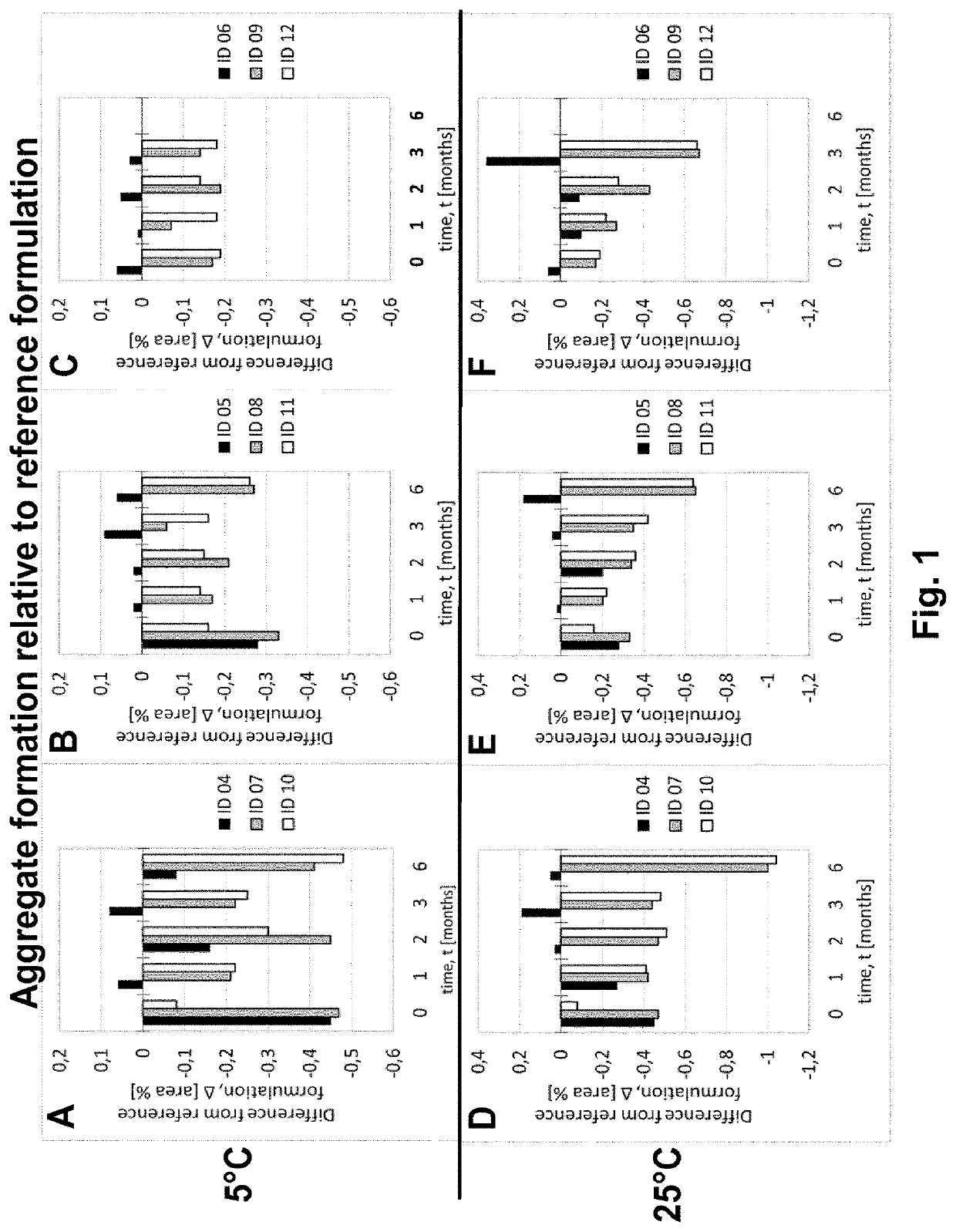
Liquid pharmaceutical composition
The present invention relates to novel liquid pharmaceutical compositions of adalimumab, which include adalimumab or a biosimilar thereof, and at least one component selected from the group consisting of: a polyvinylpyrrolidone (PVP) surfactant, an inositol sugar stabiliser, and a gluconate salt toncifier. Such a combination of components furnishes formulations having a stability (e.g. on storage and when exposed to stress) which is comparable to or an improvement upon those known in the art, and with fewer ingredients. Such advances will help adalimumab treatments to become more widely available at lower cost, and prolong the viability of pre-loaded delivery devices (e.g. pre-filled syringes) to reduce unnecessary waste of the drug.
Owner:FRESENIUS KABI DEUT GMBH
A kind of combination culture medium expressing adalimumab and application thereof
ActiveCN107904200BIncrease productionQuality improvementImmunoglobulins against cytokines/lymphokines/interferonsArtificial cell constructsProtein targetMedicine
The invention discloses a combined medium for expressing adalimumab. The combined medium is composed of a basic medium and a fed medium, wherein the basic medium is composed of CD FortiCHO and M20A ina ratio of 1: 0.5-1.5; the fed medium consists of F001S and F001B in a ratio of 5-15: 1; and the above mediums are both commercial mediums. Individual usage of any of the mediums cannot produce high-yield stable adalimumab; but the combined medium of the basic medium and the fed medium can significantly increase the expression quantity of adalimumab and improve cell density and cell viability, stably overcomes the problem of accumulation of a large amount of lactic acid and low yield of target proteins in conventional adalimumab cell culture processes, and is beneficial for further improvement of the output and quality of adalimumab.
Owner:通化东宝生物科技有限公司
Method for detecting adalimumab content in human plasma
PendingCN112213489AStrong specificityEasy to operateBiological material analysisBiological testingHuman tumorWhite blood cell
The invention relates to the technical field of adalimumab content detection, in particular to a method, instrument and material for detecting adalimumab content in human plasma, which comprise EDTA (ethylene diamine tetraacetic acid) plasma or serum, hTNF (human tumor necrosis factor) antigen, human IgG (H + L) HRP (immunoglobulin G + L), styrene 96-well plate, adalimumab fermentation liquor, blank cell strain fermentation liquor and auxiliary solution, according to the method, a corresponding antigen is used for coating, If the adalimumab is detected, the hTNF antigen is selected as a coating antigen, so that the specificity is very high, and meanwhile, the protein content measured by an immunological method and a physicochemical method is compared, so that the relevant information of the proportion of the protein with specific binding activity in the total protein can be obtained; however, in the process of determining the content of the monoclonal antibody in the monoclonal antibody product by the method, the content and activity of a standard substance need to be accurately calibrated, and the method is easy to operate, has good accuracy and precision, can be used for determining the content of the adalimumab, and provides methodological basis for rapid quantitative detection of the recombinant monoclonal antibody.
Owner:上海熙华检测技术服务股份有限公司
A method for removing recombinantly expressed antibody aggregates and degradation products
ActiveCN112010970BHigh purityHigh removal rateImmunoglobulins against cytokines/lymphokines/interferonsPeptide preparation methodsAntiendomysial antibodiesBiochemistry
The present disclosure provides an affinity chromatography method for removing antibody aggregates and degraded fragments, and improving the yield and purity of antibody monomers. The purity of the antibody monomer was improved by optimizing the pH value, conductivity, buffer type and other parameters in the affinity chromatography process of recombinant adalimumab, and the purity of the antibody monomer was improved by adding urea or PEG additives to the elution buffer. Antibody monomer yield. In a preferred embodiment, the yield of recombinant adalimumab monomer can reach 90%, and the purity of the monomer in the product exceeds 95%.
Owner:MABWELL (SHANGHAI) BIOSCIENCE CO LTD +1
Methods of Treating Interstitial Cystitis
The present invention relates to the use of adalimumab (Humira™), for the treatment of a pain and / or a lower urinary tract symptom(s) (LUTS) associated with interstitial cystitis and / or painful bladder syndrome and / or bladder pain syndrome.
Owner:BOSCH PHILIP
Dnmt3b gene deficient type CHO (Chinese hamster ovary) cell line, preparation method and application thereof and recombinant protein expression system
PendingCN110257340AImprove expression levelImprove stabilityHydrolasesImmunoglobulins against cytokines/lymphokines/interferonsProtein targetInstability
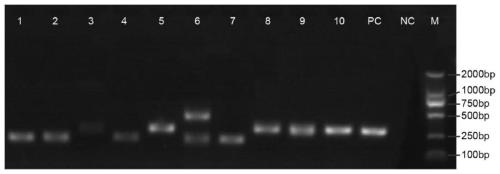
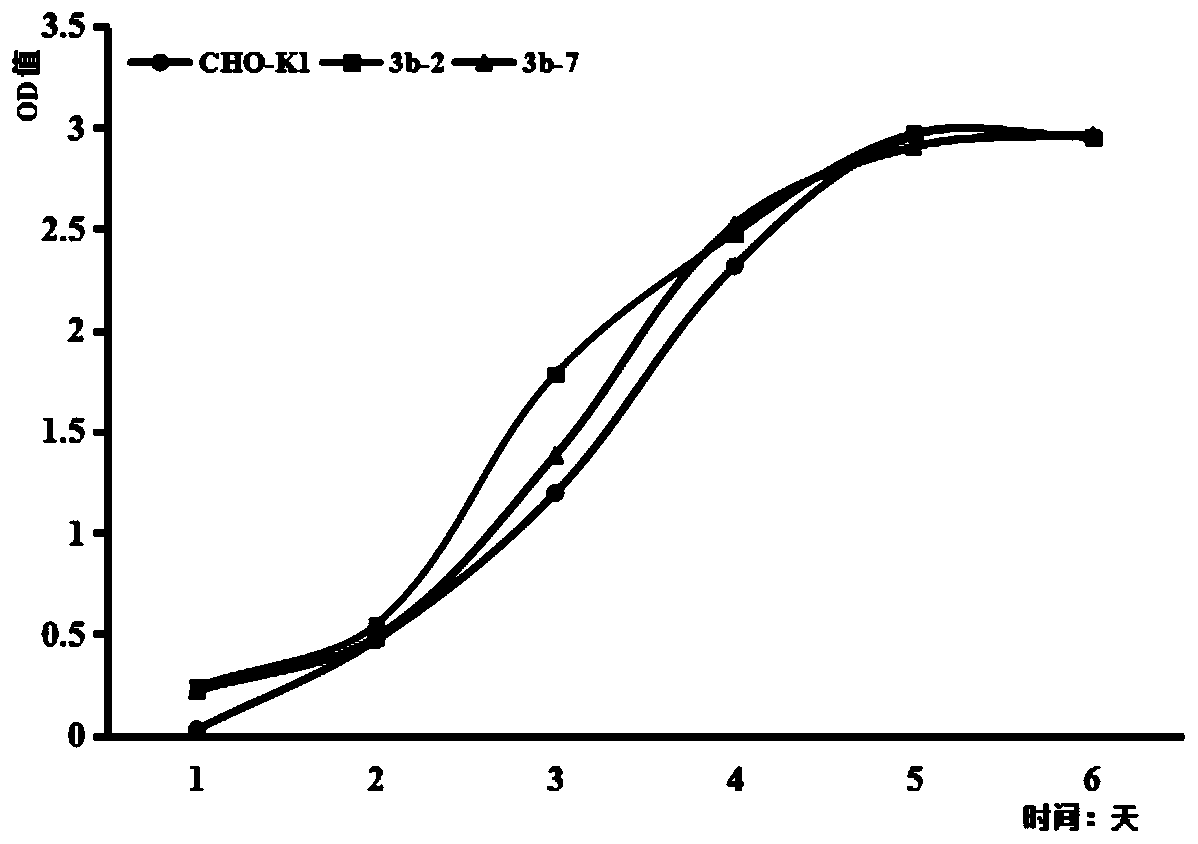
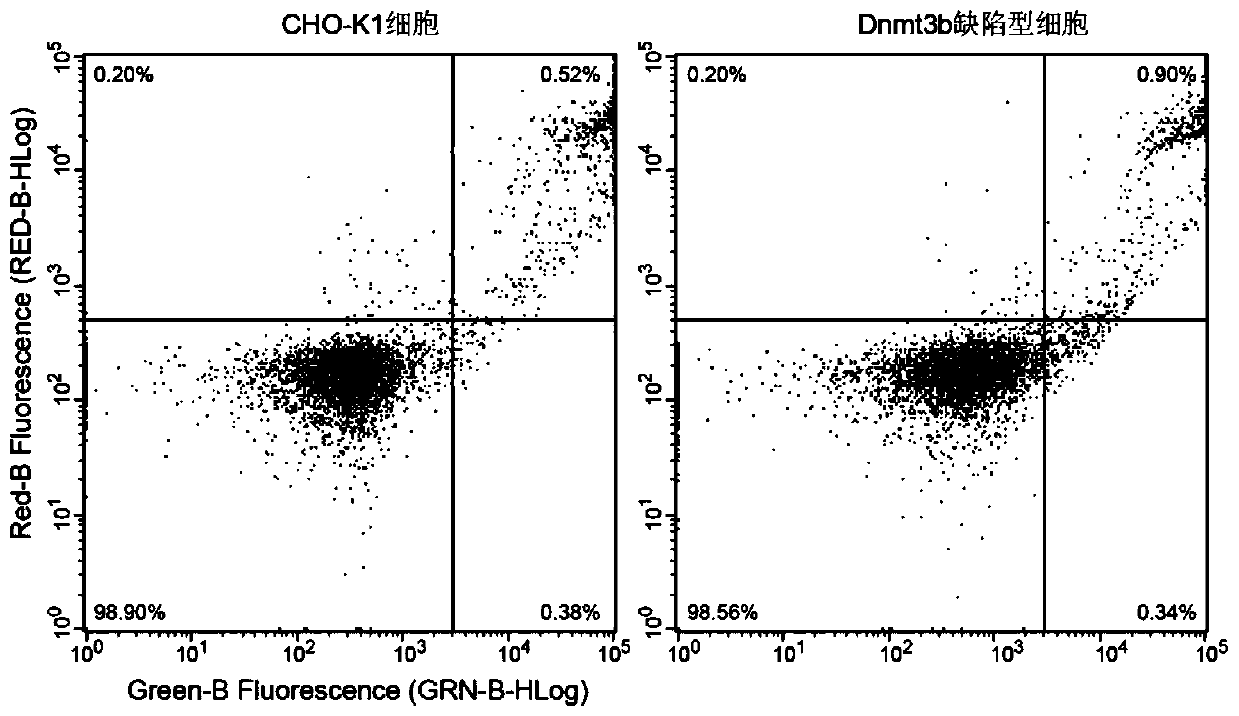
The invention relates to a DNA transmethylase Dnmt3b gene deficient type CHO (Chinese hamster ovary) cell line, a preparation method and application thereof and a recombinant protein expression system, and belongs to the technical field of genetic engineering. CHO cell Dnmt3b gene is knocked off by CRISPR / Cas9 gene editing technique so as to obtain the Dnmt3b gene deficient type CHO cell line is attained; the Dnmt3b gene deficient type CHO cell line can evidently increase the expression level and expression stability of a target gene in CHO cells, and the problem can be solved that existing CHO cell expression systems have low expression level and expression instability; upon expression of recombinant Adalimumab with the cell line herein, it is discovered that the expression level of the recombinant Adalimumab is increased evidently. Therefore, the cell line of the invention is widely applicable to the expression of target proteins.
Owner:XINXIANG MEDICAL UNIV
Clinical effect of pharmaceutical products using communication tool integrated with compound of several pharmaceutical products
InactiveUS20210233625A1Improve treatmentGood effectData processing applicationsDrug and medicationsSide effectBiomedical engineering
A method of treating a medical condition with a combination of substances, wherein one of the substances is adalimumab, in combination with a computer program configured for: providing a patient with sets of questions adapted to the combination, to adalimumab, and to the non-adalimumab substances, wherein the sets of questions serve to identify potential side effects; subjecting the answers to the sets of questions to functions, thereby generating patient specific feedback; providing the feedback to the patient, wherein the feedback is directed to making the patient aware of development of a possible side effect.
Owner:INTPROP ENABLER STOCKHOLM AB
Liquid pharmaceutical composition of adalimumab
ActiveUS10688187B2Composition is stablePowder deliveryImmunoglobulins against cytokines/lymphokines/interferonsAntiendomysial antibodiesActive agent
Owner:INTAS PHARM LTD
Clinical effect of pharmaceutical products using communication tool integrated with compound of several pharmaceutical products
InactiveUS20210233624A1Significant clinical effectImprove safety concernData processing applicationsDrug and medicationsTherapeutic effectBiomedical engineering
A method of treating a medical condition with a combination of substances, wherein one of the substances is adalimumab, in combination with a computer program configured for: providing a patient with sets of questions adapted to the combination, to adalimumab, and to the non-adalimumab substances, wherein at least one of the sets of questions is related to perceived and / or measured therapeutic effects; subjecting the answers to the sets of questions to functions, thereby generating patient specific feedback; and adjusting a dosage of at least one substance based at least in part on the patient-specific feedback.
Owner:INTPROP ENABLER STOCKHOLM AB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com