Liquid pharmaceutical composition
A liquid drug and composition technology, applied in drug combination, drug delivery, antibody, etc., can solve problems such as adverse interactions, damage to preparations, increased process and cost burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment
[0266] In certain embodiments, the liquid pharmaceutical composition comprises adalimumab and any one of the combinations of components 1, 2, and / or 3 listed in Table A below. In embodiments in which one or more components are left blank, the composition should be considered to require only those actually listed in components 1, 2, and / or 3, but it should be understood that these embodiments do not necessarily Compositions containing components not listed are excluded. For example, Example A.4 requires that the pharmaceutical composition comprises a PVP surfactant and a monocyclic sugar stabilizer, but not necessarily component 3.
[0267] Table A - Various examples of liquid pharmaceutical compositions comprising adalimumab in any combination with any of the listed components Example (A.1-A.25)
[0268]
[0269]
[0270] "PVP surfactant" = general PVP surfactant; "monocyclic SS" = general monocyclic sugar stabilizer; "metal carboxylic acid" = general metal (hydro...
Embodiment
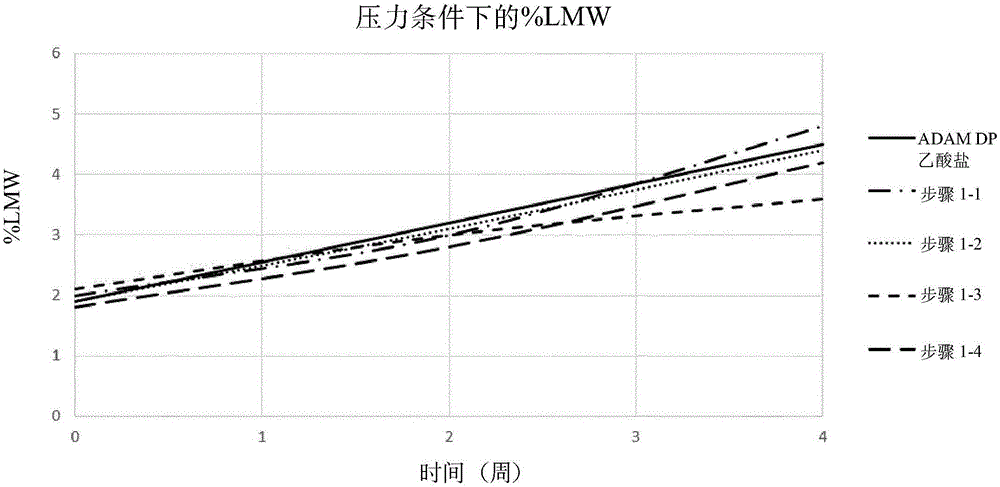
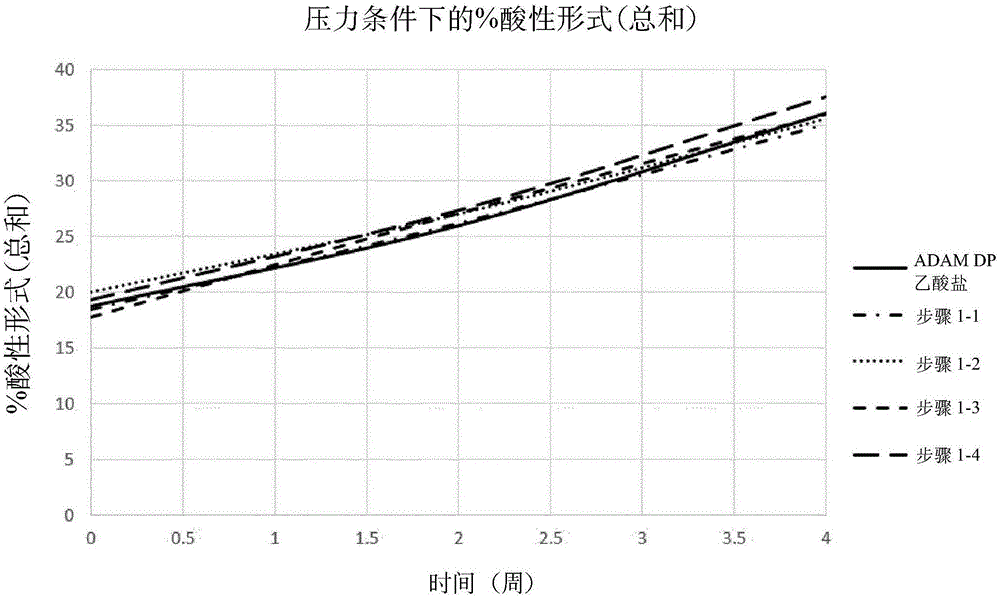
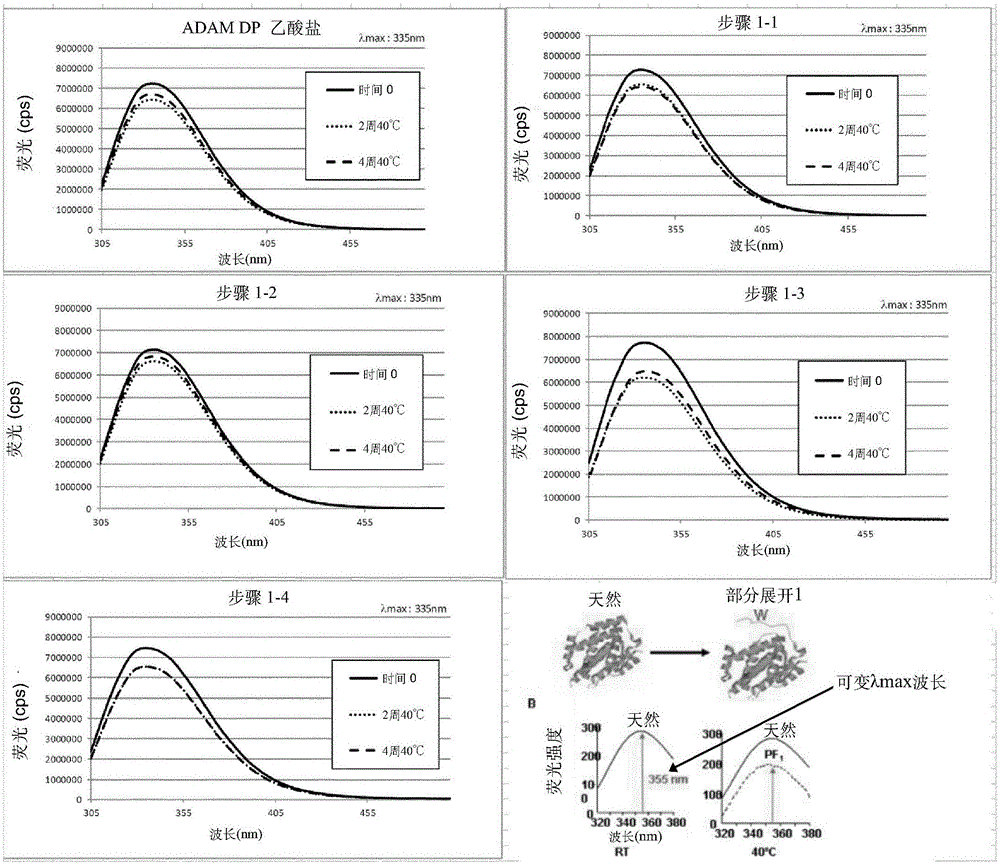
[3104] Stability studies performed on various liquid adalimumab formulations helped the inventors to identify some key parameters affecting the stability of adalimumab formulations, especially the stability of adalimumab itself. Such stability studies typically involve exposing the formulation to stress (eg heat, light, agitation, long term storage) and making appropriate time course measurements (ie before and after stress). For example, a bioanalyzer is commonly used for purity measurement; DSF is commonly used to measure development temperature; iCE280 is used to obtain isoform profiles; OD is used to assess protein content; SE-HPLC is used to determine protein aggregation; Other measurements, such as pH and osmolality, are made by known methods.
[3105] Based on the results of these stability studies, several formulations were selected as promising candidates for further study, including those shown in Table 1 below:
[3106] Table 1 - Promising candidate formulations ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com