Primer pair and kit for detecting related gene polymorphism of adalimumab medication
A technology of adalimumab and gene polymorphism, applied in the determination/testing of microorganisms, biochemical equipment and methods, DNA/RNA fragments, etc. problems, to achieve the effect of fast sequencing speed, easy operation, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0132] Embodiment 1: the preparation of kit (30 tests / box)
[0133] 1. Design and synthesis of primers and probes
[0134] For human TNF gene, KLRC1 gene, FCGR2A gene, PTPRC gene, HLA-E gene, TRAF1 gene and KLRD1 gene, select specific mutation sites rs1800629, rs7301582, rs1801274, rs10919563, rs1264457, rs3761847 and rs2302489, the selected primers and probes The needle is designed at the mutation site and the nearby conserved region to avoid SNPs in the primer binding region (search the SNP of the target gene sequence through the online NCBI website), perform Primer Blast through the online NCBI website, and design allele-specific PCR Amplify the primers to confirm the specific amplification of the primer pair. When there is a mismatch between the 3' terminal base of the PCR primer and its template DNA, the amplification efficiency will drop sharply. Only when the 3' base of the primer is paired with the template can it be A PCR amplification signal appears. The probe is l...
Embodiment 2
[0201] Embodiment 2: the use of kit
[0202] 1. Sample testing
[0203] Prepare the system according to the number of templates: take a PCR reaction tube, add the corresponding primer solution, PCR premix solution, sterilized purified water, add sample DNA, sterilized purified water or positive control as a template to form a PCR reaction system. Perform PCR amplification according to the PCR reaction procedure.
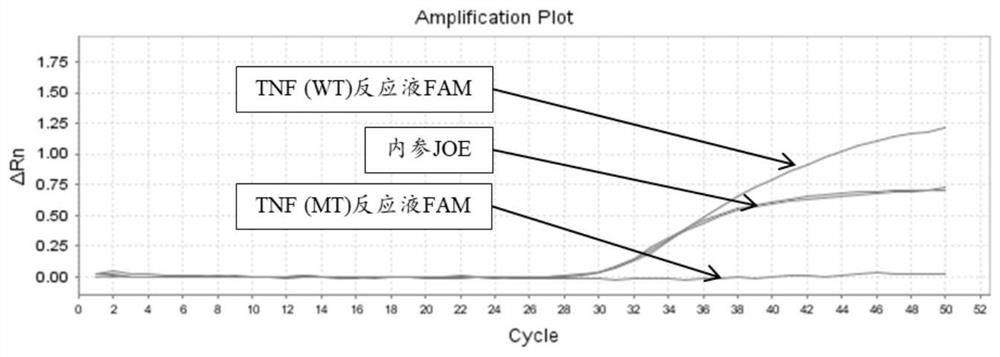
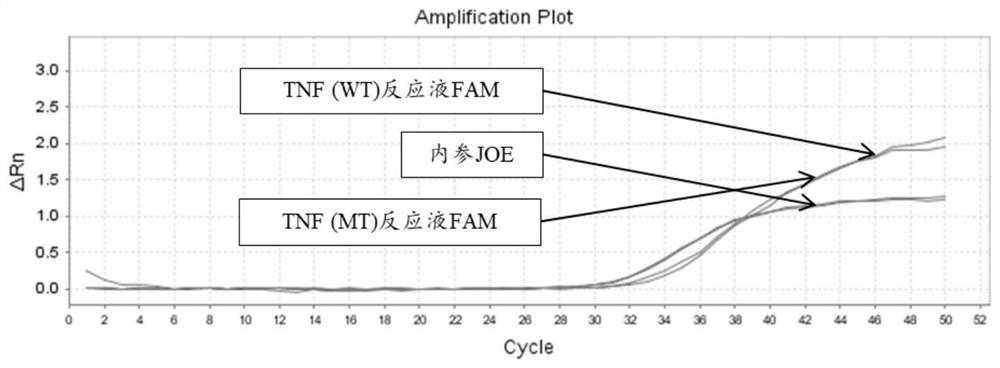
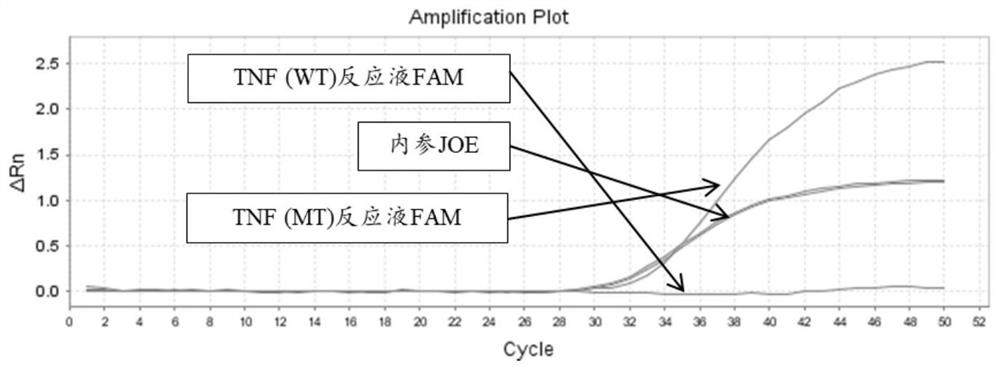
[0204] There are two kinds of reaction solutions for each site: wild (WT) and mutant (MT), and there are 14 kinds of reaction solutions for 7 sites. Each reaction solution was prepared as follows:
[0205] Table 18. Preparation composition of each reaction solution
[0206]
[0207]
[0208] The system reaction procedure is as follows:
[0209] Table 19. PCR reaction program
[0210]
[0211] 2. ABI7500 fluorescent quantitative PCR
[0212] Press the power button on the right to start ABI 7500. After starting up, the "power" indicator on the left end ...
Embodiment 3
[0249] Embodiment 3: Large sample verification of the kit
[0250] 1. According to the preparation method shown in Example 1, the relevant components of the kit were prepared and stored at -20°C for later use.
[0251] 2. Take 30 cases of whole blood samples with known genotypes, use "nucleic acid extraction or purification reagents" (record number: Xiangchang Machinery Equipment 20160167) to extract sample DNA, and use a nucleic acid protein analyzer to detect the concentration of DNA samples, 30 cases of samples A260 / 280 are all between 1.6 and 2.0.
[0252] 3. According to the steps shown in Example 2, add DNA samples and perform detection on an ABI 7500 fluorescent quantitative PCR instrument.
[0253] 4. According to the interpretation standard shown in embodiment 2, the results are interpreted and counted (the test result coincidence rate statistics), the sample coincidence rate is 100%; the test result specific information is as follows:
[0254]
[0255]
[02...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com