A kind of combination culture medium expressing adalimumab and application thereof
A technology of adalimumab and culture medium, which is applied in the field of biotechnology and fermentation, and can solve problems such as large errors, affecting the expression of cell growth products, and cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
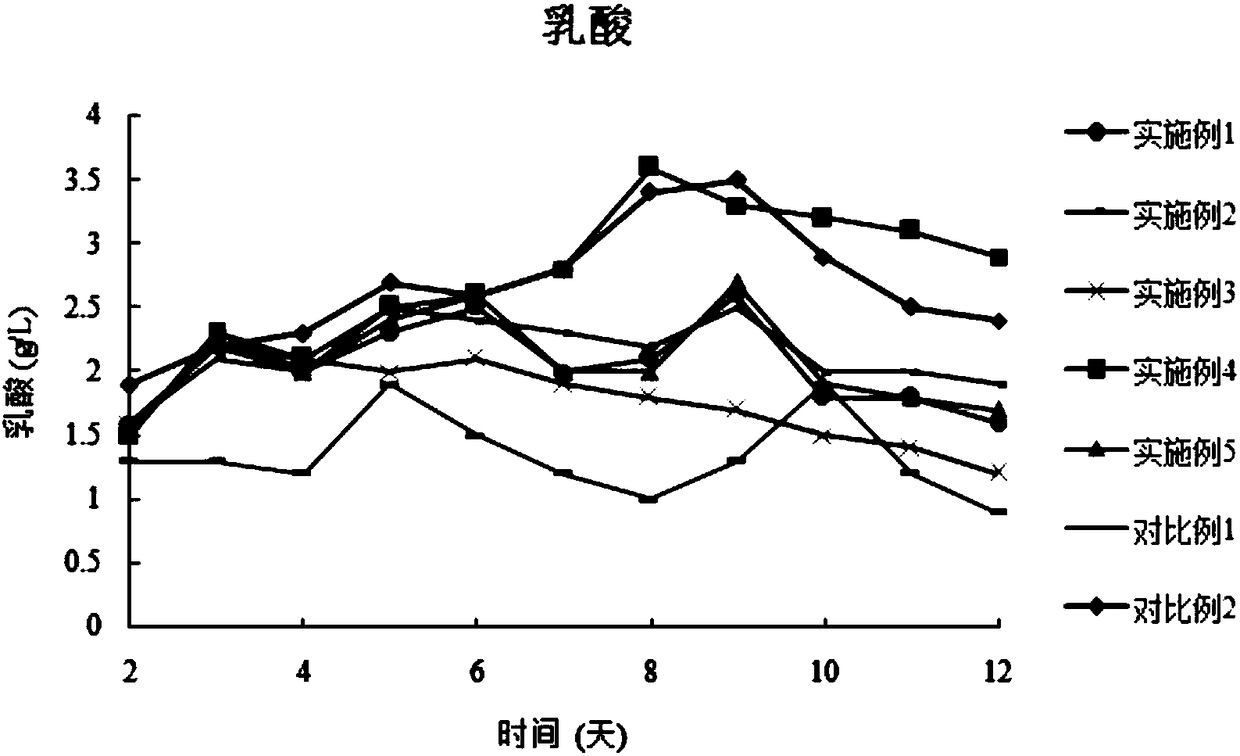
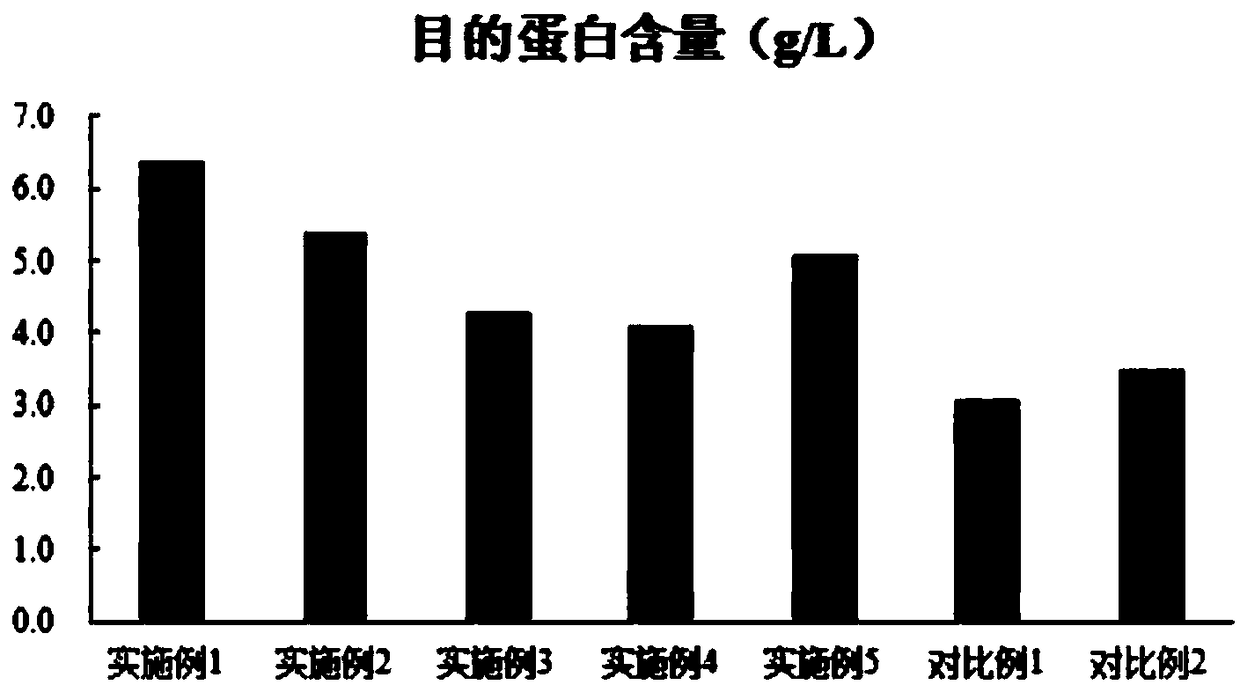
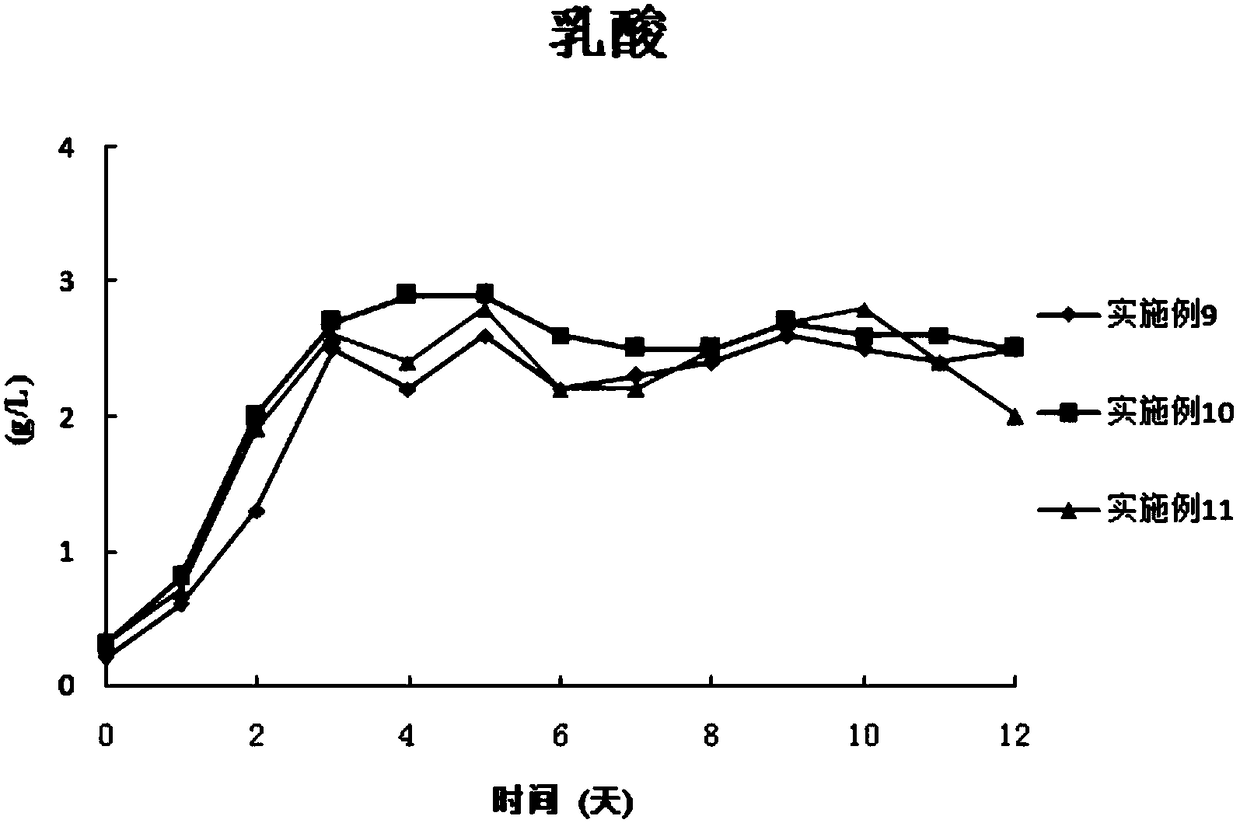
Examples
Embodiment 1
[0092] shake flask culture
[0093] Preparation of basal medium:
[0094] Take 0.86g CD FortiCHO, 0.175g glucose, and 0.035g poloxamer 188 to prepare solution A, in which the concentration of CD FortiCHO is 24.56g / L, the concentration of glucose is 5g / L, and the concentration of poloxamer 188 is 1g / L.
[0095] Take 0.96g M20A and prepare solution B whose concentration is 27.62g / L.
[0096] Mix 35ml of solution A with 35ml of solution B to obtain a basal medium.
[0097] Preparation of feed medium:
[0098] Take 4.45g of F001S and prepare solution C (27ml) with a concentration of 164.9g / L.
[0099] Take 0.2g of F001B to make solution D (2.7ml) with a concentration of 76g / L.
[0100] During the cell culture process, solution C and solution D were used as feed medium, and were poured into shake flasks at a volume ratio of 10:1.
[0101] Resuscitate cells, after expansion, dilute and inoculate the cells into CD FortiCHO:M20A=1:1 basal medium, the initial volume is 70ml, at 36...
Embodiment 2
[0111] shake flask culture
[0112] Preparation of basal medium:
[0113] Take 0.69g CD FortiCHO, 0.14g glucose, and 0.028g poloxamer 188 to prepare solution A, in which the concentration of CD FortiCHO is 24.56g / L, the concentration of glucose is 5g / L, and the concentration of poloxamer 188 is 1g / L.
[0114] Take 1.16g M20A and prepare solution B whose concentration is 27.62g / L.
[0115] Mix 28ml of solution A with 42ml of solution B to obtain a basal medium.
[0116] Preparation of feed medium:
[0117] Take 4.45g of F001S and prepare solution C (27ml) with a concentration of 164.9g / L.
[0118] Take 0.2g of F001B to make solution D (2.7ml) with a concentration of 76g / L.
[0119] During the cell culture process, solution C and solution D were used as feed medium, and were poured into shake flasks at a volume ratio of 10:1.
[0120] Resuscitate cells, after expansion, dilute and inoculate the cells into CD FortiCHO:M20A=1:1.5 basal medium, the initial volume is 70ml, at 3...
Embodiment 3
[0123] shake flask culture
[0124] Preparation of basal medium:
[0125] Take 1.075g CD FortiCHO, 0.22g glucose, and 0.044g poloxamer 188 to prepare solution A, in which the concentration of CD FortiCHO is 24.56g / L, the concentration of glucose is 5g / L, and the concentration of poloxamer 188 is 1g / L.
[0126] Take 0.725g M20A and prepare solution B whose concentration is 27.62g / L.
[0127] Mix 43.75ml of solution A with 26.25ml of solution B to obtain a basal medium.
[0128] Preparation of feed medium:
[0129] Take 4.12g of F001S and prepare solution C (25ml) with a concentration of 164.9g / L.
[0130] Take 0.38g of F001B and prepare solution D (5ml) with a concentration of 76g / L.
[0131] During the cell culture process, solution C and solution D were used as feed medium, and were poured into shake flasks at a volume ratio of 5:1.
[0132] Resuscitate cells, after expansion, dilute and inoculate the cells into CD FortiCHO:M20A=1:0.6 basal medium, the initial volume is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume ratio | aaaaa | aaaaa |
| coefficient of variation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com